Sir,
Since COVID-19 has commenced, we are trying to learn its impact on individuals with different comorbidities and coinfections; however, coinfection of COVID-19 and tuberculosis (TB) is still less travelled ground. The epidemiological data on coinfection of COVID-19 and TB is available;[1,2] however, there is limited data on the clinical course of these patients.[3-5] There are a few published case series and reports, which suggest that patients infected with COVID-19 and TB are likely to have severe disease and poorer outcomes[5,6] and there can be reactivation of latent TB with COVID infection.[7] However, all these studies involve heterogeneous populations, i.e. stable as well as critical patients.[3-5] The present retrospective study describes the clinical characteristics, course and outcome of critically ill patients coinfected with COVID-19/TB admitted in the intensive care unit (ICU) of a tertiary care COVID centre of the Indian subcontinent, a high endemic area for TB.
A total of 32 patients with COVID-19 and current or previous diagnoses of TB were admitted to the ICU from August 2020 to January 2021. The demographic data and clinical features of the patients are tabulated in Table 1. The patients were young with a median age of 40 years (interquartile range (IQR) 31.5–50.5) and most (69%) had one or more comorbidities similar to other studies.[3-5] The most common comorbidity was chronic kidney disease (CKD) followed by hypertension and diabetes mellitus in our group of patients. HIV infection was also prevalent accounting for 12% of patients. The majority of patients (84.3%) had previously been diagnosed with TB due to high endemicity of TB in our country. Of 32 patients, 15 had only pulmonary TB, 12 had extrapulmonary TB and 5 had TB involving more than one site. There was a significant number of patients with extrapulmonary TB in our study as compared to other cohorts,[3,4] reflecting the high percentage (15%–20%) of extrapulmonary TB in our country.[8] The most common presenting symptom amongst our patients was shortness of breath (56.2%), followed by cough (46.8%), fever (37.5%) and altered sensorium (37.5%), whereas fever was the most common presenting complaint in other cohorts.[3,5] This could be due to the late presentation of patients to the hospital in our setup. Though TB is highly prevalent in India, there is a delay in reaching hospitals due to poor socioeconomic status and lack of education.
Table 1.
Demographic data, clinical characteristics and laboratory parameters of study patients [n=32]
| Characteristic | Patients |
|---|---|
| Age median in years (IQR) | 40 ( 31.5-50.5) |
| Male/female | 23/9 |
|
| |
| Comorbidities | No. of patients (%) |
|
| |
| Chronic kidney disease | 6 (18.5) |
| Diabetes mellitus | 3 (9.38) |
| Hypertension | 4 (12.50) |
| Coronary artery disease | 3 (9.38) |
| HIV | 4 (12.50) |
| Chronic liver disease | 3 (9.38) |
| Others | 11 (34.37) |
| Status of ATT | |
| ATT completed | 8 (25) |
| On ATT treatment | 19 (59.37) |
| ATT started after the COVID diagnosis | 5 (15.62) |
| Site of TB | |
| Pulmonary (previous treatment) | 7 (21.87) |
| Pulmonary (current) | 8 (25) |
| Tubercular meningitis | 9 (28.12) |
| Abdominal TB | 3 (9.3) |
| TB involving more than one site | 5 (15.62) |
| Presenting complaints [n=32] | No. (%) |
| Shortness of breath | 18 (56.25) |
| Cough | 15 (46.88) |
| Fever | 12 (37.50) |
| Altered sensorium | 12 (37.50) |
| Seizure | 4 (12.50) |
| Others | 13 (40.63) |
| Initial respiratory support | No. (%) |
| Room air | 8 (25) |
| Oxygen by face mask | 8 (25) |
| Oxygen by HFNC | 7 (21.8) |
| Invasive ventilation | 9 (28.1) |
| Highest respiratory support | |
| Oxygen by face mask | 8 (25) |
| Oxygen by HFNC | 5 (15.6) |
| Invasive ventilation | 19 (59.3) |
| Chest X-ray findings | No. (%) |
| Active TB | 12 (37.50) |
| Post TB sequelae | 10 (31.2) |
| Features of COVID | 23 (71.8) |
|
| |
| Severity indices and inflammatory markers | Median (IQR) |
|
| |
| Initial SOFA (n=32) | 4 (3-7) |
| IL6 (pg/mL) (n=26) | 53.5 (19-105) |
| Ferritin (µg/L ) (n=24) | 405 (216-940) |
| CRP (mg/dL) (n=22) | 9.3 (4.05-20.7) |
| Procalcitonin (ng/mL) (n=16) | 2.8 9 (0.4-6.6) |
| SOFA at 48 h (n=29) | 5 (4-6) |
| Duration of hospital stay (days) (n=32) | 11.5 (7-19.5) |
Data are presented as median [IQR] or number (percentages). IQR=Interquartile range, HIV=Human immunodeficiency virus, ATT=Anti tubercular treatment, TB=Tuberculosis, HFNC=High frequency nasal cannula, SOFA=Sequential Organ Failure Assessment, IL-6=Interleukin- 6, CRP=C-reactive protein
Most patients (59.3%) developed severe COVID and patients were sicker as compared to other studies. The median initial sequential organ failure assessment (SOFA) score was 4 (IQR 3–7) and at 48 h was 5 (IQR 4-6). Various initial lab parameters and radiological findings are tabulated in Table 1. The chest roentgenogram and CT findings are depicted in Figure 1. The patients were treated according to the institutional protocol. Of 32 patients, 19 patients received remdesivir and 30 patients received steroids, none received convalescent plasma or immunomodulators. Remdesivir was discontinued if alanine transaminase levels increased to > five times the upper normal. The dosages of steroids were based on the clinical response and lab values of inflammatory markers.
Figure 1.
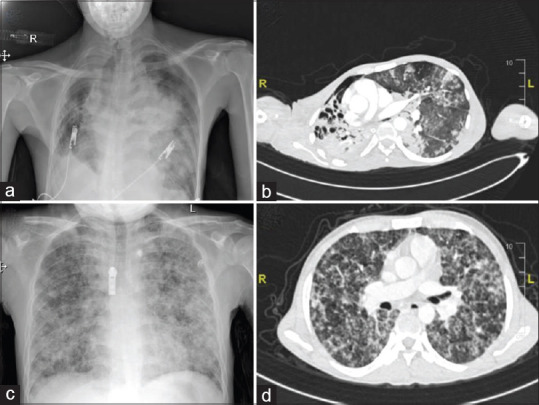
(a) The chest roentgenogram of patient 1 shows decreased right lung volume with cavitary lesions in upper and mid zones, and the left mid zone shows a small area of sub-segmental consolidation with preserved lung volume suggestive of TB and COVID. (b) Computed tomography of the thorax of the same patient [patient 1] shows shifting of the upper mediastinum towards the right side with multiple cavitary lesions in the right upper lobe. A few atelectatic bands were noted in the left upper lobe and left lower lobe with sub-segmental collapse. (c) Chest roentgenogram of patient 2 shows areas of reticulonodular opacities in the bilateral lung parenchyma (left > right) with a few areas of fluffy opacities suggestive of military tuberculosis. (d) Corresponding computed tomography of the thorax of patient 2 shows the thickening of interlobular septa and small nodules. Ground glass opacities were seen in the lower lobe (image not in set)
Out of 32 patients, 5 patients developed pneumothorax, 1 patient developed empyema and 1 had massive hemoptysis. The high incidence of pneumothorax was possibly due to barotrauma to the diseased lungs. Deranged liver function tests were seen in 10 patients and a similar number of patients developed AKI. The mortality was 46.8% and the most common cause of death was sepsis with MODS (67%). The mortality was significantly higher in our study as compared to available published data understandably due to the inclusion of critically ill patients in our cohort.
Non-survivors were compared with survivors [Supplementary table]. There were no significant differences between the two groups in the demographic features; however, the SOFA score at admission and at 48 h was significantly higher in non-survivors. The laboratory parameters were also similar except for initial ferritin levels, which were significantly higher in the non-survivors indicating a higher inflammatory state and severe disease. However, in a study by Song et al.,[5] the non-survivors were of higher age and more likely to have hypertension, dyspnea, bilateral lesions and higher Total leukocyte count (TLC) counts.
Supplementary Table.
Comparisons of survivors and non-survivors [n=32]
| Variable | Survivors [n=17] Mean±SD Median (IQR) No. (%) | Non survivors [n=15] Mean±SD Median (IQR) No. (%) | P |
|---|---|---|---|
| Age in years | 40.1±17.48 | 47.0±16.3 | 0.25 |
| M: F | 13:4 | 10:5 | 0.69 |
| Pulmonary TB | 7 (41.1) | 7 (46.6) | 0.75 |
| Tubercular meningitis | 7 (41.1) | 5 (33.3) | 0.64 |
| Initial invasive ventilation | 2 (11.76) | 7 (46.6) | 0.03* |
| Initial SOFA | 3 ( 2-5) | 7 (4-9) | 0.008* |
| SOFA at 48 h | 4 (3-5) | 6 (5.5-8) | 0.006* |
| Initial inflammatory markers | |||
| IL6 (pg/mL) | 66.02 (18.4-105.7) | 30.5 (19-253) | 0.81 |
| Ferritin (µg/L) | 287.8 (98-567) | 844 (404 - 1500) | 0.04* |
| CRP (mg/dL) | 7.7 (2.3-18.1) | 14.5 (7.8-21.7) | 0.23 |
| Procalcitonin (ng/mL) | 4.4 (0.8-10) | 1.4 (0.2-6.4) | 0.31 |
| Complications | |||
| Pneumothorax | 1 (5.88) | 4 (26.67) | 0.16 |
| Deranged LFT | 5 (29.41) | 5 (33.33) | 1.00 |
| AKI | 4 (23.53) | 6 (40.0) | 0.45 |
| Duration of hospital stay (days) | 14 (9-20) | 9 (4-19) | 0.17 |
*- significant; TB: Tuberculosis, SOFA: Sequential Organ Failure Assessment; IL-6: Interleukin- 6; CRP: C-reactive protein; LFT: liver function test; AKI: acute kidney injury. Data is presented as mean ±SD; median (IQR); No. (%)
In conclusion, in this study of critically ill patients with COVID-19 infection and previous or current TB, the majority were young males with comorbidities. Though most had pulmonary TB, there were significant numbers of patients with extrapulmonary TB. Most patients had severe COVID and the mortality was significantly high.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Sy KTL, Haw NJL, Uy J. Previous and active tuberculosis increases risk of death and prolongs recovery in patients with COVID-19. Infect Dis (Lond) 2020;52:902–7. doi: 10.1080/23744235.2020.1806353. [DOI] [PubMed] [Google Scholar]
- 2.Davies MA. HIV and risk of COVID-19 death: A population cohort study from the Western Cape Province, South Africa. medRxiv. 2020 2020.07.02.20145185. [Google Scholar]
- 3.Tadolini M, Codecasa LR, García-García J-M, Blanc F-X, Borisov S, Alffenaar J-W, et al. Active tuberculosis, sequelae and COVID-19 co-infection: First cohort of 49 cases. Eur Respir J. 2020;56:2001398. doi: 10.1183/13993003.01398-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stochino C, Villa S, Zucchi P, Parravicini P, Gori A, Raviglione MC. Clinical characteristics of COVID-19 and active tuberculosis co-infection in an Italian reference hospital. Eur Respir J. 2020;56:2001708. doi: 10.1183/13993003.01708-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song WM, Zhao JY, Zhang QY, Liu SQ, Zhu XH, An QQ, et al. COVID-19 and tuberculosis coinfection: An overview of case reports/case series and meta-analysis. Front Med (Lausanne) 2021;8:657006. doi: 10.3389/fmed.2021.657006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Visca D, Ong CWM, Tiberi S, Centis R, D’Ambrosio L, Chen B, et al. Tuberculosis and COVID-19 interaction: A review of biological, clinical and public health effects. Pulmonology. 2021;27:151–65. doi: 10.1016/j.pulmoe.2020.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crisan-Dabija R, Grigorescu C, Pavel C-A, Artene B, Popa IV, Cernomaz A, et al. Tuberculosis and COVID-19: Lessons from the past viral outbreaks and possible future outcomes. Can Respir J. 2020;2020:1401053. doi: 10.1155/2020/1401053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma SK, Mohan A. Extrapulmonary tuberculosis. Indian J Med Res. 2004;120:316–53. [PubMed] [Google Scholar]


