OBJECTIVE:
To ascertain the association between cholesterol and triglyceride levels on ICU admission and mortality in patients with sepsis.
DATA SOURCES:
Systematic review and meta-analysis of published studies on PubMed and Embase.
STUDY SELECTION:
All observational studies reporting ICU admission cholesterol and triglyceride levels in critically ill patients with sepsis were included. Authors were contacted for further data.
DATA EXTRACTION:
Eighteen observational studies were identified, including 1,283 patients with a crude overall mortality of 33.3%. Data were assessed using Revman (Version 5.1, Cochrane Collaboration, Oxford, United Kingdom) and presented as mean difference (MD) with 95% CIs, p values, and I2 values.
DATA SYNTHESIS:
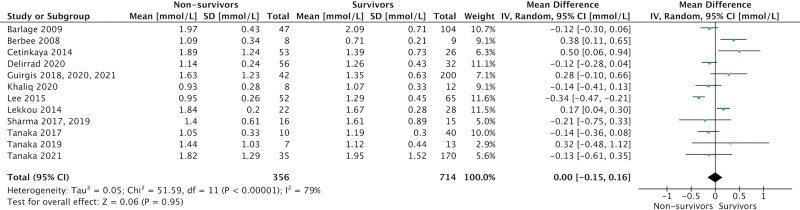
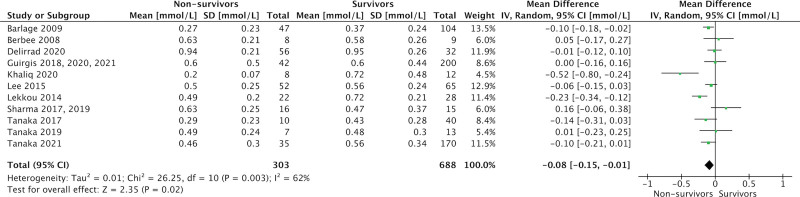
Admission levels of total cholesterol (17 studies, 1,204 patients; MD = 0.52 mmol/L [0.27–0.77 mmol/L]; p < 0.001; I2 = 91%), high-density lipoprotein (HDL)-cholesterol (14 studies, 991 patients; MD = 0.08 mmol/L [0.01–0.15 mmol/L]; p = 0.02; I2 = 61%), and low-density lipoprotein (LDL)-cholesterol (15 studies, 1,017 patients; MD = 0.18 mmol/L [0.04–0.32 mmol/L]; p = 0.01; I2 = 71%) were significantly lower in eventual nonsurvivors compared with survivors. No association was seen between admission triglyceride levels and mortality (15 studies, 1,070 patients; MD = 0.00 mmol/L [–0.16 to 0.15 mmol/L]; p = –0.95; I2 = 79%).
CONCLUSIONS:
Mortality was associated with lower levels of total cholesterol, HDL-cholesterol, and LDL-cholesterol, but not triglyceride levels, in patients admitted to ICU with sepsis. The impact of cholesterol replacement on patient outcomes in sepsis, particularly in at-risk groups, merits investigation.
Keywords: cholesterol levels, intensive care unit, lipids, sepsis, triglycerides
KEY POINTS
Question: Does an association exist between cholesterol and triglyceride levels on ICU admission and mortality in patients with sepsis?
Findings: In a systematic review and meta-analysis, admission levels of total cholesterol, HDL-cholesterol, and LDL-cholesterol significantly differed between eventual nonsurvivors and survivors. No association was seen between admission triglyceride levels and mortality.
Meaning: Cholesterol levels are associated with mortality in sepsis. Effects of cholesterol replacement in sepsis need to be determined in future studies, particularly in at-risk groups.
Cholesterol is integral to several key physiologic processes including physical properties of the cell membrane, maintenance of cell membrane integrity, signaling pathways, immunity, and as a precursor for the synthesis of hormones, Vitamin D, and bile acids (1). The association between sepsis—the life-threatening organ dysfunction caused by a dysregulated host response to infection (2)—and hypocholesterolemia was first recognized a century ago (3). Many subsequent studies have demonstrated an association between the magnitude of decrease in serum cholesterol in sepsis and mortality, in particular the component of cholesterol bound to high-density lipoprotein (HDL-C) (4–7). The association between outcome and serum levels of cholesterol bound to low-density lipoprotein (LDL-C) or triglyceride (TG) appears less consistent.
Little mechanistic work has been performed to date to understand the pathophysiological mechanisms underlying hypocholesterolemia, nor clinical implications beyond the association with poor outcomes (1). Nonetheless, the therapeutic possibilities of lipoprotein administration or modifying the cholesterol pathway in sepsis are generating increasing interest. A recent review article noted that high-density lipoproteins (HDLs) display antioxidant, antiapoptotic, antithrombotic, anti-inflammatory, and anti-infectious properties (8). Improved organ function and survival were noted in animal models of sepsis infused with reconstituted HDL or an apolipoprotein A1 mimetic (apolipoprotein A1 being the major lipoprotein in HDL) (9, 10). A small pilot study of a cholesterol-sphingomyelin liposome given to patients with pneumococcal pneumonia showed good safety and tolerability and an encouraging reduction in organ dysfunction (11). A clinical trial with an anti-inflammatory intravenous fish oil emulsion is ongoing (12).
It is, thus, timely to perform a systematic review and meta-analysis on the association between mortality and ICU admission levels of cholesterol (total, HDL, and low-density lipoprotein [LDL]) and TG levels in critically ill patients with sepsis and septic shock. This systematic review was registered on the PROSPERO (International prospective register of systematic reviews, National Institute for Health Reasearch NIHR, United Kingdom) database (registration number: CRD42021286120).
MATERIALS AND METHODS
Search Strategy
A systematic search of PubMed and Embase was conducted on July 20, 2022. Controlled vocabulary (MeSH) and key words were used when possible. Date restrictions were not applied. Our Boolean search strategy included the following search terms: (cholesterol OR triglyceride OR HDL OR high-density lipoprotein OR LDL OR low-density lipoprotein OR lipoprotein) AND (sepsis OR septic OR septicaemia OR intensive care OR critical care OR critical illness OR intensive care unit OR ICU). Where cholesterol levels were not stratified according to mortality status, the corresponding author was contacted for this information in order to reduce reporting bias.
Eligibility Criteria
Inclusion and exclusion criteria were determined a priori. All observational studies reporting ICU admission serum cholesterol (including total cholesterol, HDL-C, and LDL-C) and TG levels in critically ill adult patients (≥18 yr old) with sepsis were considered.
Study Selection
Both titles and abstracts were independently screened by two investigators (D.A.H., P.A.). A designated third author (N.A.) resolved discrepancies. Applying the predefined inclusion criteria, all relevant full-text publications were subsequently analyzed for eligibility.
Data Collection and Analysis
Two investigators (D.A.H., P.A.) independently extracted information from the selected publications using a standardized data collection form. Data were collected on year of publication, total number of included patients, ICU admission cholesterol (total cholesterol, HDL-C, and LDL-C) and TG levels, origin of sepsis, mortality, and preadmission statin use. Where reported, normal laboratory ranges cited for these variables were also collected. When not reported in metric units, cholesterol and TG units were converted from mg/dL to mmol/L (multiplication by 0.02586 and 0.01129 for cholesterol and TG units, respectively). Unclear data were not processed. As studies were observational, no risk of bias assessment was performed.
Outcome
Associations were sought between serum levels of cholesterol, its constituents (HDL-C and LDL-C) and TG at ICU admission, and subsequent mortality. ICU mortality was primarily analyzed, where available. Otherwise, 28 or 30-day mortalities were used, unless stated otherwise.
Statistical Analysis
Mean and sd of the relevant variables were collected for outcome analysis. Where data were reported as median and interquartile range with CIs, we followed published and online Cochrane recommendations to approximate the values of mean and sd. Outcome differences were analyzed using an inverse variance model with 95% CIs. Summary values are reported using mean difference (MD). The association between cholesterol and TG levels and survival was assessed using area under the receiver operating characteristic curve (AUROC). Statistical analyses were conducted using Review Manager (“Revman”) for Mac (Version 5.1, Cochrane Collaboration, Oxford, United Kingdom) and GraphPad Prism (Version 9.0, GraphPad Software, San Diego, CA). Statistical heterogeneity was assessed using the I2 methodology. I2 values greater than 50% and greater than 75% were considered to indicate moderate and significant heterogeneities among studies, respectively. All p values were two-tailed and considered statistically significant if below 0.05. Data are presented as MD with 95% CIs, p values, and I2 values.
No subgroup or sensitivity analyses were performed.
RESULTS
Included Trials
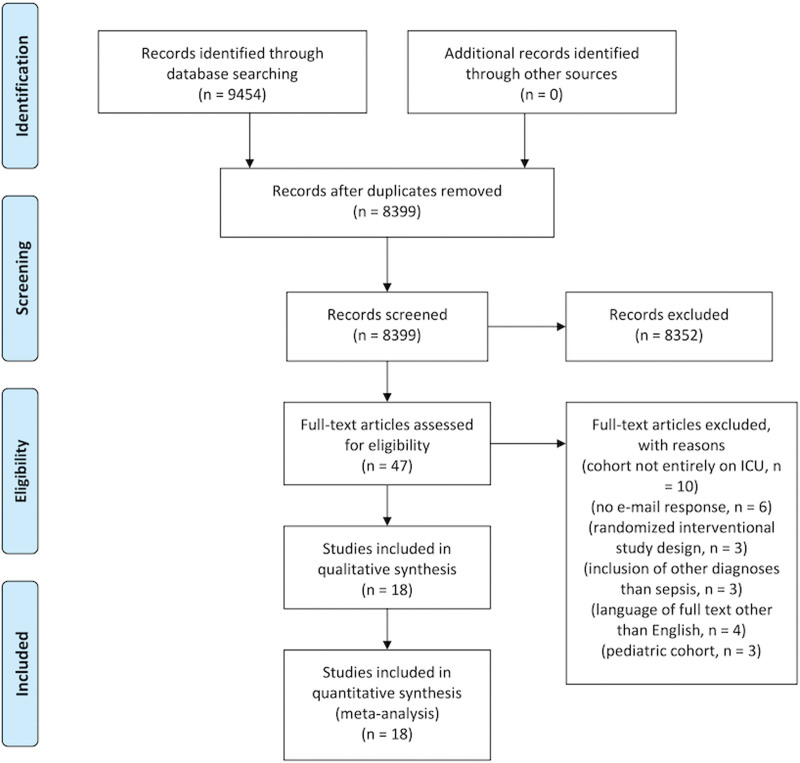
The search strategy identified 9,454 titles and abstracts, 1,055 of which were duplicates (Fig. 1). Following screening of the remaining 8,399 titles and abstracts, 47 articles were identified for full-text review. Thirteen studies included data on cholesterol but were not stratified by mortality (5, 6, 13–23). Of these studies, the corresponding authors were contacted by e-mail, of whom seven responded and provided the relevant data (13–19). The remaining six studies were excluded (5, 6, 20–23). Of the remaining 34 studies, a further 23 were excluded as 10 studies included patients outside the ICU (24–33), three had a randomized, interventional design investigating selenium infusions, glycemic control, and antithrombin III application possibly confounding analysis (34–36), three included diagnoses other than sepsis (37–39), four were in a language other than English (40–43), and three were obtained from a pediatric cohort (44–46). Eighteen studies were, therefore, included in the final analysis (7, 13–19, 47–56).
Figure 1.
PRISMA flowchart.
Study Characteristics
Eighteen observational studies including 1,283 patients with sepsis reporting ICU admission cholesterol and/or TG levels were identified. Sources of sepsis varied among studies and are presented in groups in Supplemental Table 1 (http://links.lww.com/CCX/B143). Mortality was reported as ICU mortality in 15 studies, and 28- or 30- day mortalities in three studies. Crude overall mortality was 33.3%. Preadmission statin use was reported in six studies according to eventual ICU nonsurvivors and survivors (Supplemental Table 1, http://links.lww.com/CCX/B143).
ICU Admission Cholesterol and Triglyceride Levels in Septic Nonsurvivors and Survivors
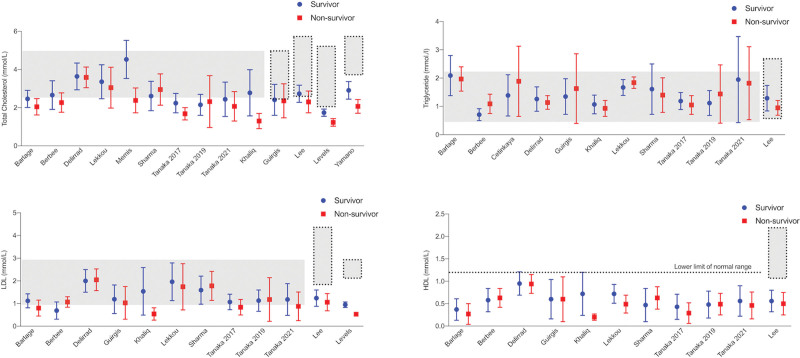
Figure 2 demonstrates overall mean and sd of admission cholesterol and TG levels in septic nonsurvivors and survivors for the included studies.
Figure 2.
Comparison of total cholesterol, high-density lipoprotein (HDL)-cholesterol, low-density lipoprotein (LDL)-cholesterol, and triglyceride levels in eventual survivors and nonsurvivors by study. Dotted lines represent normal laboratory ranges, where reported in the studies. Horizontal gray bars represent normal laboratory ranges of the author’s institution. All values are demonstrated as mean and sd.
Overall, total admission cholesterol levels were 2.7 mmol/L (sd, 0.94 mmol/L) in survivors and 2.44 mmol/L (sd, 0.89 mmol/L) in nonsurvivors. Overall HDL-C was 0.56 mmol/L (sd, 0.36 mmol/L) in survivors and 0.54 mmol/L (sd, 0.36 mmol/L) in nonsurvivors. LDL-C was 1.24 mmol/L (sd, 0.63 mmol/L) in survivors and 1.22 mmol/L (sd, 0.74 mmol/L) in nonsurvivors. TG levels were 1.58 mmol/L (sd, 0.96 mmol/L) in survivors and 1.51 mmol/L (sd, 0.89 mmol/L) in nonsurvivors. Individual lipid admission levels were available for a total number of 518 patients (13–19). Of these 518 patients, HDL-C at admission was below the lower limit of normal in 476 patients (91.9%). Of these, 376 patients (79%) eventually survived. In contrast, corresponding numbers with admission levels below the lower limit of normal were less pronounced for total cholesterol values, where only 58.1% (301/518 patients) had levels below the normal range at admission. Of these 301 patients, 232 patients (77%) eventually survived. Admission TGs were below the normal laboratory range in three of 518 (0.6%) patients. Calculations were not performed for LDL-C, as differing cardiovascular risk profiles influence target ranges in individual patients.
Mean Difference of ICU Admission Cholesterol and Triglyceride Levels Between Nonsurvivors and Survivors
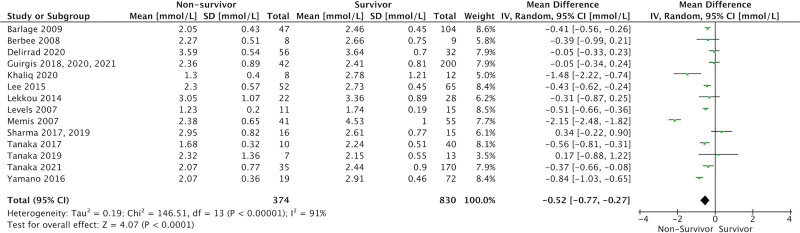
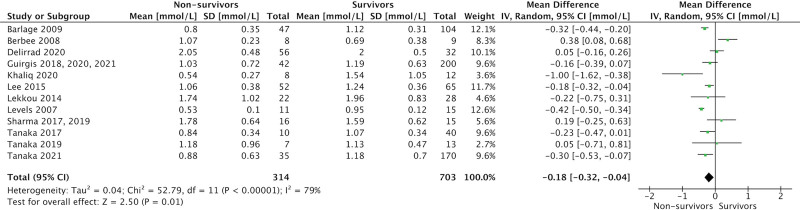
ICU admission levels of total cholesterol (17 studies, 1,204 patients; MD = 0.52 mmol/L [0.27–0.77 mmol/L]; p < 0.001; I2 = 91%), HDL-cholesterol (14 studies, 991 patients; MD = 0.08 mmol/L [0.01–0.15 mmol/L]; p = 0.02; I2 = 61%), and LDL-cholesterol (15 studies, 1,017 patients; MD = 0.18 mmol/L [0.04–0.32 mmol/L]; p = 0.01; I2 = 71%) were significantly lower in eventual nonsurvivors compared with survivors (Figs. 3–5). There was no association between admission TG levels and mortality (15 studies, 1,070 patients; MD = 0.00 mmol/L [–0.16 to 0.15 mmol/L]; p = –0.95; I2 = 79%) (Fig. 6).
Figure 3.
Forest plot—total cholesterol by study. Forest plots representing observational studies and effects on lipid alterations. Horizontal bars represent 95% CIs.
Figure 5.
Forest plot—low-density lipoprotein cholesterol by study. Forest plots representing observational studies and effects on lipid alterations. Horizontal bars represent 95% CIs.
Figure 6.
Forest plot—triglycerides by study. Forest plots representing observational studies and effects on lipid alterations. Horizontal bars represent 95% CIs.
Figure 4.
Forest plot—high-density lipoprotein cholesterol by study. Forest plots representing observational studies and effects on lipid alterations. Horizontal bars represent 95% CIs.
Area Under the Receiver Operating Characteristic Curves
Supplemental Figure 1 (http://links.lww.com/CCX/B143) depicts AUROCs showing the association between ICU admission cholesterol and TG levels and survival combined for all studies where individual patient parameters were available and provided by the corresponding authors.
DISCUSSION
Our meta-analysis shows an association between a greater degree of hypocholesterolemia (applying to total cholesterol, HDL-C, and LDL-C) at ICU admission and eventual mortality in patients with sepsis, but no association between mortality and admission TG levels.
Alterations in serum lipids are a well-characterized feature in critical illness including sepsis, trauma, and burns (1, 57). A large review by Golucci et al (4) including patients with systemic inflammatory response syndrome and sepsis highlighted changes in lipid metabolism of greater than 30,000 patients and demonstrated profound alterations in total cholesterol, and HDL-C and LDL-C levels. However, this article did not stratify according to survivor status and also included diagnoses other than sepsis.
A recently published meta-analysis analyzed lipid levels in COVID-19 patients and did seek associations with disease severity and outcomes (58). Comparable with our findings, the authors were able to demonstrate that patients with severe disease or eventual nonsurvival exhibited lower cholesterol (total, HDL-C, and LDL-C) but not TG levels, with effect sizes similar to our study. These findings highlight possible similarities in the host response toward bacterial and viral triggers.
From a pathophysiological point of view, diverse biological mechanisms leading to hypocholesterolemia in sepsis have been proposed, including impaired cholesterol synthesis and reverse cholesterol transport (1). Decreased intake and intestinal absorption and upregulation of the scavenger receptor class B type 1 have also been characterized during critical illness (1). Given the association between low cholesterol and critical illness and, in our present analysis, with sepsis-related mortality, it can be postulated that elevating cholesterol levels could be a beneficial adjunct treatment in critical illness. Clearly, this relationship may simply be epiphenomenal. However, the multiple roles played by cholesterol in the body do suggest otherwise. A cholesterol-containing liposomal preparation has been trialed in patients with pneumococcal pneumonia as a toxin-binding agent (11), whereas an ongoing clinical study of a fish oil-containing lipid emulsion is being conducted in septic patients with the rationale that the constituents provide substrate for cholesterol synthesis and may, thus, stabilize cholesterol levels (12). Although a large randomized study failed to detect reduced mortality or organ dysfunction after a phospholipid infusion containing phosphatidylcholine, soybean oil, and cholate (but containing no cholesterol) (59), a secondary analysis suggested possible benefits in patients with low HDL levels (60). This work follows on from encouraging laboratory studies, showing various strategies aimed at increasing cholesterol levels in animal, and cell models of infection or sepsis offer outcome benefits. Such strategies include liposome administration (61), pharmacological inhibition of cholesterol ester transfer protein (CETP) (33), and intracellular delivery of cholesterol using nanocarriers (62). As important effects of cholesterol might also be mediated by lipoproteins, which are often dysfunctional in inflammatory states, application of functional lipoproteins might be an interesting approach during sepsis. Preclinical studies applying recombinant HDL without containing cholesterol have shown convincing results (63, 64). Clearly, we await large randomized trials demonstrating safety, tolerability, and efficacy, with identification of subgroups of patients who may benefit most from cholesterol and/or lipoprotein supplementation.
A limitation of our study is the infrequent reporting of preillness use of statins and/or other lipid-lowering agents. One could speculate that preillness statin use may be deleterious because of their lipid-lowering properties. However, statins themselves have anti-inflammatory properties and, notably, also increase both HDL-cholesterol and apolipoprotein A1 to a modest degree, an effect probably mediated by reductions in CETP activity (65). Possible outcome benefits in septic patients have been suggested from preexisting statin use and continuation throughout the septic episode (66–68). Although a randomized trial in patients with acute respiratory distress syndrome showed no overall benefit (69), in a post hoc analysis, the subset of patients with a hyperinflammatory phenotype showed a significant mortality reduction (70). The difficulty to detect reliable results from statin use in sepsis may be largely due to significant patient heterogeneity. Other limitations include possible publication bias with studies failing to report a lack of association between lipid alterations in sepsis and outcome. Different healthcare settings and patient demographics could also reduce the generalizability of our findings, and preillness baseline levels are not reported. However, we consider our obtained I2 values (especially for HDL-C and LDL-C) adequate, adding strength to this study. Any confounding impact from concurrent use of nutrition and lipid-containing drugs such as the sedative agent propofol has not been reported.
Finally, it is worth reflecting on the utility of the results from this meta-analysis and possible clinical applications. Although these findings sit alongside many other clinical and biochemical prognosticators of poor outcomes in septic patients (71), the baseline serum cholesterol level (total, HDL, or LDL) could be used to enrich trial design by prestratifying patients into higher and lower mortality risk groups. The underlying rationale is that those with low cholesterol levels are most at risk of poor outcomes and would likely benefit most from augmentation of cholesterol levels. A further theranostic application may be achieved by titrating therapy to an optimal serum level. Fixed dosing regimens of immunomodulatory and other interventions have been traditionally used in prospective randomized controlled trials without cognizance of the impact on the individual patient whose baseline pathobiology and response to the therapy will be highly heterogeneous. Arguably, the consistently disappointing results of these studies are due in large part to a combination of nonspecific patient selection and inadequate titration to prime effect.
CONCLUSIONS
In conclusion, our study demonstrated that mortality was associated with lower total cholesterol, HDL, and LDL, but not TG levels among patients admitted to ICU with sepsis. The impact of cholesterol replacement on patient outcomes in sepsis has yet to be determined but merits study, especially in at-risk groups.
Supplementary Material
Footnotes
The authors have disclosed that they do not have any potential conflicts of interest.
The review protocol and raw data are available from the corresponding author upon reasonable request.
Registration: PROSPERO International Prospective Register of Systematic Reviews (registration number: CRD42021286120).
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
REFERENCES
- 1.Hofmaenner DA, Kleyman A, Press A, et al. : The many roles of cholesterol in sepsis: A review. Am J Respir Crit Care Med 2022; 205:388–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singer M, Deutschman CS, Seymour CW, et al. : The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016; 315:801–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Macadam W, Shiskin C: The cholesterol content of the blood in relation to genito-urinary sepsis. Proc R Soc Med 1924; 17:53–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Golucci APBS, Marson FAL, Ribeiro AF, et al. : Lipid profile associated with the systemic inflammatory response syndrome and sepsis in critically ill patients. Nutrition 2018; 55-56:7–14 [DOI] [PubMed] [Google Scholar]
- 5.Cirstea M, Walley KR, Russell JA, et al. : Decreased high-density lipoprotein cholesterol level is an early prognostic marker for organ dysfunction and death in patients with suspected sepsis. J Crit Care 2017; 38:289–294 [DOI] [PubMed] [Google Scholar]
- 6.Walley KR, Boyd JH, Kong HJ, et al. : Low low-density lipoprotein levels are associated with, but do not causally contribute to, increased mortality in sepsis. Crit Care Med 2019; 47:463–466 [DOI] [PubMed] [Google Scholar]
- 7.Lee SH, Park MS, Park BH, et al. : Prognostic implications of serum lipid metabolism over time during sepsis. Biomed Res Int 2015; 2015:1789298–1789298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanaka S, Couret D, Tran-Dinh A, et al. : High-density lipoproteins during sepsis: From bench to bedside. Crit Care 2020; 24:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moreira RS, Irigoyen M, Sanches TR, et al. : Apolipoprotein A-I mimetic peptide 4F attenuates kidney injury, heart injury, and endothelial dysfunction in sepsis. Am J Physiol Regul Integr Comp Physiol 2014; 307:R514–R524 [DOI] [PubMed] [Google Scholar]
- 10.Datta G, Gupta H, Zhang Z, et al. : HDL mimetic peptide administration improves left ventricular filling and cardiac output in lipopolysaccharide-treated rats. J Clin Exp Cardiolog 2011; 2:1000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laterre PF, Colin G, Dequin PF, et al. : CAL02, a novel antitoxin liposomal agent, in severe pneumococcal pneumonia: A first-in-human, double-blind, placebo-controlled, randomised trial. Lancet Infect Dis 2019; 19:620–630 [DOI] [PubMed] [Google Scholar]
- 12.Guirgis FW, Black LP, Rosenthal MD, et al. : LIPid Intensive Drug therapy for Sepsis Pilot (LIPIDS-P): Phase I/II clinical trial protocol of lipid emulsion therapy for stabilising cholesterol levels in sepsis and septic shock. BMJ Open 2019; 9:e029348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guirgis FW, Black LP, DeVos E, et al. : Lipid intensive drug therapy for sepsis pilot: A Bayesian phase I clinical trial. J Am Coll Emerg Physicians Open 2020; 1:1332–1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guirgis FW, Black LP, Henson M, et al. : A hypolipoprotein sepsis phenotype indicates reduced lipoprotein antioxidant capacity, increased endothelial dysfunction and organ failure, and worse clinical outcomes. Crit Care 2021; 25:341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guirgis FW, Dodani S, Leeuwenburgh C, et al. : HDL inflammatory index correlates with and predicts severity of organ failure in patients with sepsis and septic shock. PLoS One 2018; 13:e0203813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharma NK, Ferreira BL, Tashima AK, et al. : Lipid metabolism impairment in patients with sepsis secondary to hospital acquired pneumonia, a proteomic analysis. Clin Proteomics 2019; 16:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma NK, Tashima AK, Brunialti MKC, et al. : Proteomic study revealed cellular assembly and lipid metabolism dysregulation in sepsis secondary to community-acquired pneumonia. Sci Rep 2017; 7:15606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanaka S, Diallo D, Delbosc S, et al. : High-density lipoprotein (HDL) particle size and concentration changes in septic shock patients. Ann Intensive Care 2019; 9:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanaka S, Stern J, Bouzid D, et al. : Relationship between lipoprotein concentrations and short-term and 1-year mortality in intensive care unit septic patients: Results from the HIGHSEPS study. Ann Intensive Care 2021; 11:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li H, Liu W, Su W, et al. : Changes in plasma HDL and its subcomponents HDL2b and HDL3 regulate inflammatory response by modulating SOCS1 signaling to affect severity degree and prognosis of sepsis. Infect Genet Evol 2021; 91:104804. [DOI] [PubMed] [Google Scholar]
- 21.Novak F, Borovska J, Vecka M, et al. : Plasma phospholipid fatty acid profile is altered in both septic and non-septic critically III: A correlation with inflammatory markers and albumin. Lipids 2017; 52:245–254 [DOI] [PubMed] [Google Scholar]
- 22.Vavrova L, Rychlikova J, Mrackova M, et al. : Increased inflammatory markers with altered antioxidant status persist after clinical recovery from severe sepsis: A correlation with low HDL cholesterol and albumin. Clin Exp Med 2016; 16:557–569 [DOI] [PubMed] [Google Scholar]
- 23.Chien JY, Jerng JS, Yu CJ, et al. : Low serum level of high-density lipoprotein cholesterol is a poor prognostic factor for severe sepsis. Crit Care Med 2005; 33:1688–1693 [DOI] [PubMed] [Google Scholar]
- 24.Karahan I, Cifci A: “Are lipoprotein levels and ratios able to predict mortality due to sepsis?”. J Coll Physicians Surg Pak 2020; 30:272–275 [DOI] [PubMed] [Google Scholar]
- 25.Alvarez C, Ramos A: Lipids, lipoproteins, and apoproteins in serum during infection. Clin Chem 1986; 32:142–145 [PubMed] [Google Scholar]
- 26.Pizzini A, Kurz K, Orth-Hoeller D, et al. : The impact of bacteremia on lipoprotein concentrations and patient’s outcome: A retrospective analysis. Eur J Clin Microbiol Infect Dis 2019; 38:1279–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ngaosuwan K, Houngngam N, Limpisook P, et al. : Apolipoprotein A-V is not a major determinant of triglyceride levels during human sepsis. J Crit Care 2015; 30:727–731 [DOI] [PubMed] [Google Scholar]
- 28.Genga KR, Cirstea CLM, Zhou G, et al. : Two-year follow-up of patients with septic shock presenting with low HDL: The effect upon acute kidney injury, death and estimated glomerular filtration rate. J Intern Med 2017; 281:518–529 [DOI] [PubMed] [Google Scholar]
- 29.Zou G, He J, Ren B, et al. : The delta high-density lipoprotein cholesterol ratio: A novel parameter for Gram-negative sepsis. Springerplus 2016; 5:1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kanchana N, Natnicha H, Pichapa L, et al. : Apolipoprotein A-V is not a major determinant of triglyceride levels during human sepsis. J Crit Care 2015; 30:727–731 [DOI] [PubMed] [Google Scholar]
- 31.Grion CM, Cardoso LT, Perazolo TF, et al. : Lipoproteins and CETP levels as risk factors for severe sepsis in hospitalized patients. Eur J Clin Invest 2010; 40:330–338 [DOI] [PubMed] [Google Scholar]
- 32.Trinder M, Genga KR, Kong HJ, et al. : Cholesteryl ester transfer protein influences high-density lipoprotein levels and survival in sepsis. Am J Respir Crit Care Med 2019; 199:854–862 [DOI] [PubMed] [Google Scholar]
- 33.Trinder M, Wang Y, Madsen CM, et al. : Inhibition of cholesteryl ester transfer protein preserves high-density lipoprotein cholesterol and improves survival in sepsis. Circulation 2021; 143:921–934 [DOI] [PubMed] [Google Scholar]
- 34.Brodska H, Valenta J, Malickova K, et al. : Biomarkers in critically ill patients with systemic inflammatory response syndrome or sepsis supplemented with high-dose selenium. J Trace Elem Med Biol 2015; 31:25–32 [DOI] [PubMed] [Google Scholar]
- 35.Cappi SB, Noritomi DT, Velasco IT, et al. : Dyslipidemia: A prospective controlled randomized trial of intensive glycemic control in sepsis. Intensive Care Med 2012; 38:634–641 [DOI] [PubMed] [Google Scholar]
- 36.van Leeuwen HJ, Heezius EC, Dallinga GM, et al. : Lipoprotein metabolism in patients with severe sepsis. Crit Care Med 2003; 31:1359–1366 [DOI] [PubMed] [Google Scholar]
- 37.Chien YF, Chen CY, Hsu CL, et al. : Decreased serum level of lipoprotein cholesterol is a poor prognostic factor for patients with severe community-acquired pneumonia that required intensive care unit admission. J Crit Care 2015; 30:506–510 [DOI] [PubMed] [Google Scholar]
- 38.Bonville DA, Parker TS, Levine DM, et al. : The relationships of hypocholesterolemia to cytokine concentrations and mortality in critically ill patients with systemic inflammatory response syndrome. Surg Infect 2004; 539–49 [DOI] [PubMed] [Google Scholar]
- 39.Tsai MH, Peng YS, Chen YC, et al. : Low serum concentration of apolipoprotein A-I is an indicator of poor prognosis in cirrhotic patients with severe sepsis. J Hepatol 2009; 50:906–915 [DOI] [PubMed] [Google Scholar]
- 40.Bai J, Lin J, Zhuang H, et al. : [Changes in plasma cholesterol level and risk factors of death in patients with sepsis]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2016; 28:164–168 [DOI] [PubMed] [Google Scholar]
- 41.Barati M, Nazari MR, Talebi Taher M, et al. : Comparison of lipid profile in septic and non-septic patients. Iranian J Clin Infect Dis 2011; 6:144–148 [Google Scholar]
- 42.Bentz MH, Magnette J: Hypocholesterolemia during the acute phase of an inflammatory reaction of infectious origin. 120 cases. Rev Med Interne 1998; 19:168–172 [DOI] [PubMed] [Google Scholar]
- 43.Xu Y, Wang D, Liu H, et al. : [The relationship between the lipid profiles and inflammation in patients with sepsis]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2022; 34:127–132 [DOI] [PubMed] [Google Scholar]
- 44.Bermudes ACG, de Carvalho WB, Zamberlan P, et al. : Changes in lipid metabolism in pediatric patients with severe sepsis and septic shock. Nutrition 2018; 47:104–109 [DOI] [PubMed] [Google Scholar]
- 45.Atreya MR, Whitacre BE, Cvijanovich NZ, et al. : Proprotein convertase subtilisin/kexin type 9 loss-of-function is detrimental to the juvenile host with septic shock. Crit Care Med 2020; 48:1513–1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vermont CL, den Brinker M, Kâkeci N, et al. : Serum lipids and disease severity in children with severe meningococcal sepsis. Crit Care Med 2005; 33:1610–1615 [DOI] [PubMed] [Google Scholar]
- 47.Barlage S, Gnewuch C, Liebisch G, et al. : Changes in HDL-associated apolipoproteins relate to mortality in human sepsis and correlate to monocyte and platelet activation. Intensive Care Med 2009; 35:1877–1885 [DOI] [PubMed] [Google Scholar]
- 48.Berbée JF, van der Hoogt CC, de Haas CJ, et al. : Plasma apolipoprotein CI correlates with increased survival in patients with severe sepsis. Intensive Care Med 2008; 34:907–911 [DOI] [PubMed] [Google Scholar]
- 49.Cetinkaya A, Erden A, Avci D, et al. : Is hypertriglyceridemia a prognostic factor in sepsis? Ther Clin Risk Manag 2014; 10:147–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Delirrad M, Gharebaghi N, Mobarhan S, et al. : Relationship between lipid profile and sepsis outcome in intensive care unit. Arch Clin Infect Dis 2020; 15:e93533 [Google Scholar]
- 51.Lekkou A, Mouzaki A, Siagris D, et al. : Serum lipid profile, cytokine production, and clinical outcome in patients with severe sepsis. J Crit Care 2014; 29:723–727 [DOI] [PubMed] [Google Scholar]
- 52.Levels JH, Pajkrt D, Schultz M, et al. : Alterations in lipoprotein homeostasis during human experimental endotoxemia and clinical sepsis. Biochim Biophys Acta 2007; 1771:1429–1438 [DOI] [PubMed] [Google Scholar]
- 53.Memiş D, Gursoy O, Tasdogan M, et al. : High C-reactive protein and low cholesterol levels are prognostic markers of survival in severe sepsis. J Clin Anesth 2007; 19:186–191 [DOI] [PubMed] [Google Scholar]
- 54.Tanaka S, Labreuche J, Drumez E, et al. : Low HDL levels in sepsis versus trauma patients in intensive care unit. Ann Intensive Care 2017; 7:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamano S, Shimizu K, Ogura H, et al. : Low total cholesterol and high total bilirubin are associated with prognosis in patients with prolonged sepsis. J Crit Care 2016; 31:36–40 [DOI] [PubMed] [Google Scholar]
- 56.Khaliq W, Großmann P, Neugebauer S, et al. : Lipid metabolic signatures deviate in sepsis survivors compared to non-survivors. Comput Struct Biotechnol J 2020; 18:3678–3691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chiarla C, Giovannini I, Giuliante F, et al. : Severe hypocholesterolemia in surgical patients, sepsis, and critical illness. J Crit Care 2010; 25:361.e7–361.e12 [DOI] [PubMed] [Google Scholar]
- 58.Zinellu A, Paliogiannis P, Fois AG, et al. : Cholesterol and triglyceride concentrations, COVID-19 severity, and mortality: A systematic review and meta-analysis with meta-regression. Front Public Health 2021; 9:705916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dellinger RP, Tomayko JF, Angus DC, et al. : Efficacy and safety of a phospholipid emulsion (GR270773) in Gram-negative severe sepsis: Results of a phase II multicenter, randomized, placebo-controlled, dose-finding clinical trial. Crit Care Med 2009; 37:2929–2938 [DOI] [PubMed] [Google Scholar]
- 60.Parker TS, Levine DM, Gordon BR, et al. : Subgroup analysis of the lipid infusion and patient outcomes in sepsis trial (LIPOS) reveals benefit in a subgroup not treated with stress replacement doses of corticosteroids. Crit Care 2014; 18:P62 [Google Scholar]
- 61.Henry BD, Neill DR, Becker KA, et al. : Engineered liposomes sequester bacterial exotoxins and protect from severe invasive infections in mice. Nat Biotechnol 2015; 33:81–88 [DOI] [PubMed] [Google Scholar]
- 62.Kammann T, Hoff J, Yildirim I, et al. : Intracellularly released cholesterol from polymer-based delivery systems alters cellular responses to pneumolysin and promotes cell survival. Metabolites 2021; 11:821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tanaka S, Genève C, Zappella N, et al. : Reconstituted high-density lipoprotein therapy improves survival in mouse models of sepsis. Anesthesiology 2020; 132:825–838 [DOI] [PubMed] [Google Scholar]
- 64.McDonald MC, Dhadly P, Cockerill GW, et al. : Reconstituted high-density lipoprotein attenuates organ injury and adhesion molecule expression in a rodent model of endotoxic shock. Shock 2003; 20:551–557 [DOI] [PubMed] [Google Scholar]
- 65.McTaggart F, Jones P: Effects of statins on high-density lipoproteins: A potential contribution to cardiovascular benefit. Cardiovasc Drugs Ther 2008; 22:321–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hackam DG, Mamdani M, Li P, et al. : Statins and sepsis in patients with cardiovascular disease: A population-based cohort analysis. Lancet 2006; 367:413–418 [DOI] [PubMed] [Google Scholar]
- 67.Kruger P, Fitzsimmons K, Cook D, et al. : Statin therapy is associated with fewer deaths in patients with bacteraemia. Intensive Care Med 2006; 32:75–79 [DOI] [PubMed] [Google Scholar]
- 68.Liappis AP, Kan VL, Rochester CG, et al. : The effect of statins on mortality in patients with bacteremia. Clin Infect Dis 2001; 33:1352–1357 [DOI] [PubMed] [Google Scholar]
- 69.McAuley DF, Laffey JG, O’Kane CM, et al. : Simvastatin in the acute respiratory distress syndrome. N Engl J Med 2014; 371:1695–1703 [DOI] [PubMed] [Google Scholar]
- 70.Calfee CS, Delucchi KL, Sinha P, et al. : Acute respiratory distress syndrome subphenotypes and differential response to simvastatin: Secondary analysis of a randomised controlled trial. Lancet Respir Med 2018; 6:691–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pierrakos C, Velissaris D, Bisdorff M, et al. : Biomarkers of sepsis: Time for a reappraisal. Crit Care 2020; 24:287. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.