Abstract
Trypanocidal resistance is a major cause of treatment failure. This study evaluated the sensitivity of Trypanosoma evansi field isolates collected from Marsabit and Isiolo counties, Kenya. A total of 2,750 camels were screened using parasitological tests for trypanosomes. Of the screened camels, 113 tested positive from which 40 T. evansi isolates were tested using the single dose mice sensitivity test. Five treatment groups each comprising of 6 mice were inoculated intraperitoneally with 1x105 trypanosomes of each isolate and treated 24 hours later with isometamidium chloride at 1 mg/kg, homidium chloride at 1mg/kg, diminazene aceturate at 20 mg/kg and quinapyramine sulphate & chloride at 1 mg/kg. The fifth group was left untreated (positive control). The mice were monitored daily for 60 days. A survey on camel owners’ practices that influence development of resistance to trypanocidal drugs was then conducted. Results indicated presence of drug resistance in all the 7 study sites that had infected camels. Seven of the isolates tested were resistant to diminazene aceturate whereas, 28, 33 and 34 were resistant to isometamidium chloride, quinapyramine sulphate & chloride and homidium chloride, respectively. Seven (17.5%) isolates of the 40 tested were sensitive to all 4 drugs, whereas, 7.5%, 10%,55% and 10% were resistant to 1,2,3 and 4 drugs, respectively. The prevalence of multiple drug resistance was 75%. Survey data indicated that camel management practices influenced the prevalence and degree of drug resistance. In conclusion, the multiple drug resistance observed in the two counties may not be an indication of total trypanocidal drug failure. Judicious treatment of confirmed trypanosomiasis cases with correct dosage would still be effective in controlling the disease since the observed resistance was at the population and not clonal level. However, integrated control of the disease and the vectors using available alternative methods is recommended to reduce drug use.
Introduction
Trypanosomiasis (Surra) caused by T. evansi is one of the most important diseases of camels in the camel keeping belt in the horn of Africa. Surra is endemic in areas where camels are kept and occurs with varying degrees of prevalence causing significant economic losses with far reaching implications on food and nutrition security of pastoral communities [1]. T. evansi is transmitted mechanically by biting flies of the genera Tabanus, Lyperosia, Stomoxys, Atylotus [2] and Hipobosca [3–6]. Surra presents with clinical signs such as fluctuating fever, anemia, weight loss, oedema of ventral regions, lymphadenopathy and sudden death [2,7,8]. It is the most widely distributed of the pathogenic animal trypanosomiasis [9,10] and is the most important single cause of economic losses in camels causing morbidity of up to 30% and mortality of around 3% [11,12]. In Kenya, trypanosomiasis has been identified as the most important disease of camels [13]. The disease is endemic in camel-keeping areas where it occurs with varying degrees of prevalence [14,15]. Camels are susceptible to the different types of trypanosome species; T. evansi, T. brucei and T. congolense. While T. evansi infections, transmitted by non-tsetse biting flies occur beyond the limits of tsetse fly distribution, the latter two trypanosome species are found mainly in tsetse infested areas [10]. Existence of different levels of virulence in T. evansi isolates from Kenya has been observed [16].
Management of T. evansi largely depend on treatment with different trypanocidal drugs; polysulfonated naphthylamine, quinapyramine sulphate & chloride, melarsomine, homidium salts and diminazene aceturate [1]. In areas where T. congolense infections occur, isometamidium chloride is the preferred drug of choice. The therapeutic efficacy of polysulfonated naphthylamine, quinapyramine, diminazene and isometamedium has been evaluated in naturally T. evansi-infected buffaloes by Singh and Joshi [17]. Comparative evaluation of all the four drugs indicated that quinapyramine and isometamedium were good curative agents but prophylactically quinapyramine proved better than isometamidium [17]. However, due to high cost, inaccessibility and unavailability of these trypanocidals in remote areas where camels are kept, the majority of the pastoralists resort to use of an assortment of trypanocidals, antibiotics and ethno-veterinary (herbal) products in the treatment of Surra. Trypanocidals have been in use for more than half a century and there have been increased reports of development of drug resistance (see Kasozi et al. [18]). Geerts & Holmes [19] estimated that 35 million doses of trypanocides are administered every year in sub-Saharan Africa, with isometamidium, ethidium bromide and diminazene aceturate representing 40%, 26% and 33% respectively.
Melarsomine has been found to mitigate the problem of drug resistance to polysulfonated naphthylamine and quinapyramine [20] but costs more than these traditional drugs [21] which has limited its adoption. A recent survey conducted among some camel keepers in the Laisamis and Loglogo areas in Marsabit County in Kenya showed that 71.4% of camel owners used conventional antibiotics, while 37.1% used herbal concoctions as alternative drugs and remedy for the treatment of Surra owing to the high cost of melarsomine [15]. The second phase of the survey undertaken in the neighboring camel keeping areas in Isiolo showed that isometamidium chloride and homidium chloride were also used to treat the disease, while 61.4% and 68.2% of the pastoralists from Kulamawe (Isiolo) used quinapyramine and homidium chloride, respectively, to treat camel trypanosomiasis [15]. Consistent findings of high trypanocidal drug use were also reported in a similar survey conducted in Isiolo and Marsabit counties [22]. The high trypanocidal drug use is taking place against a background of privatized veterinary services that resulted in inadequate professional veterinary service providers and increase of unskilled community animal health service providers in the remote areas [23–25]. Therefore, the wide use of trypanocidal drugs by unskilled persons as well as treatment undertaken by camel keepers [26] could potentially compromise the efficacy of treatments and contribute to the development of drug resistance.
It is important to understand that drug resistance does not imply an end to chemotherapy. Studies have shown that resistant trypanosome populations vary greatly in the sensitivity of individual trypanosomes in that population [27–29]. In addition, Ndung’u et al. [30] demonstrated the existence of variations in virulence and sensitivity of T. b. rhodesiense cloned stabilates from one isolate. This suggests that drug resistant populations can be effectively treated by increasing the dosage or through multiple treatment [17,31], or through combination of different synergistic trypanocides [32]. Several factors have been considered as contributing to development of drug resistance such as prolonged exposure of trypanosomes to sub-therapeutic drug levels, thereby favoring the selection of drug-resistant populations [33,34], immunological status of the host [35], high incidence of trypanosomiasis [36] and erratic treatments with prophylactics among others [37–39]. Exposure of trypanosomes to sub therapeutic drug levels has been attributed to incorrect body weight estimation, failure to calculate correct dosage, deliberate under dosing, poor drug preparation and administration [26]. Inappropriate use of trypanocidals by farmers such as self-treatment of sick camels by farmers, wrong route of administration, wrong reconstitution and dosages have been associated with development of drug resistance which has been reported for diminazene aceturate, Isometadidium chloride and other trypanocides in East Africa (See Kasozi et al. [40] for detailed review of farmers’ practices contributing to trypanocidal resistance).
Instability of resistance to trypanocidal compounds in trypanosome populations has been reported [34,38,41]. However, Mulugeta et al. [42] showed that the phenotype of multiple drug-resistant T. congolense remained stable over a period of four years. In T. evansi, drug resistance has been reported in several type A strains originating from Africa, Asia and Latin America [43,44]. Some Chinese strains appear to be innately resistant to the phenanthridine class of drugs [45,46]. Decrease in virulence and loss of fitness in drug resistant T. evansi has been reported [47,48]. This loss of fitness has been suggested to contribute immensely in the epidemiology of drug resistance [36,49].
Earlier studies in Marsabit and Isiolo counties of Kenya revealed that 73.2% of the isolates collected from camels showed resistance to isometamidium chloride, 31.6% of the isolates showed resistance to diminazene aceturate while 89.5% of the isolates exhibited resistance to quinapyramine sulphate & chloride at the respective doses tested [15]. In this same study the prevalence of multiple drug resistance (89.0%) was more than two fold the prevalence of resistance (31.5%) observed in T. evansi isolates collected 15 years earlier [50] in the same region. This indicated that the prevalence of drug resistance is not static and changes with time and in space, perhaps due to a number of socioeconomic factors associated with drug use by different communities. Therefore, the present study aimed to determine variation of sensitivity of T. evansi population in selected sites in Marsabit and Isiolo counties and effect of various management practices and disease prevalence on this variation.
Materials and methods
Ethical approval and informed consent
The study was approved by the Kenya Agricultural and Livestock Research Organization (KALRO)-Institutional Animal Care and Use Committee (KALRO-IACUC) while the survey of camel owners was approved by Kenya Agricultural and Livestock Research Organization Scientific Review Committee. The camel keepers who participated in this study signed a written informed consent form before camel sampling and questionnaire administration. The process entailed providing the camel keepers with adequate information about the study, outlining the possible benefits and consequences of participating in the study, responding to the farmers’ questions, making it clear that they were free to discontinue the interview at any point, providing adequate time for them to make informed decision and then getting their permission before proceeding with the study.
Sample collection sites
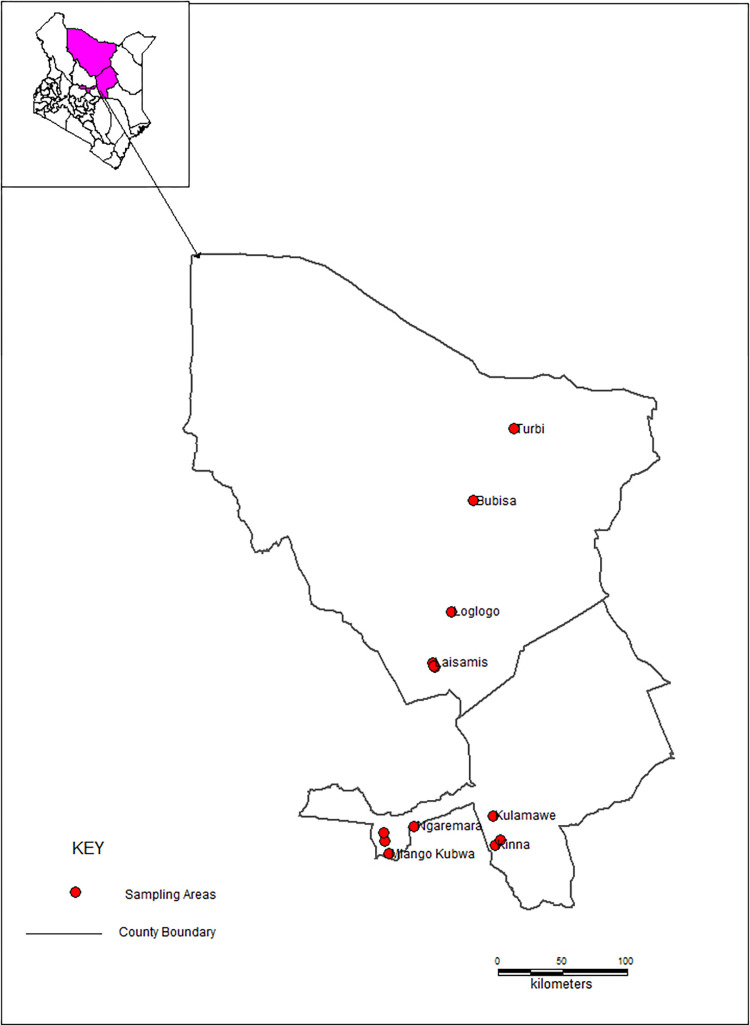
The study was conducted in Isiolo and Marsabit counties, Kenya. Isiolo County covers an area of about 25,349 square km land size. The county has a human population of 268,002, with a population density of about 11 persons/km2 according to the 2019 population and household census [51]. It is inhabited by four ethnic communities that include Meru, Borana, Somali and Turkana. Four study sites were selected in Isiolo namely Kulamawe (Borana-inhabited), Kinna (Borana-inhabited), Ngaremara (Turkana-inhabited) and the Somali-inhabited Livestock Marketing Division (LMD) based on ethnic composition of the camel owners and proximity to administrative centers as proxies to access to veterinary services. Marsabit County covers an area of about 70,944 square kilometers. The county borders the country of Ethiopia to the north. Marsabit is home to 459,785 people with a population density of 6 persons/km2 [51]. The county is inhabited by various ethnic communities including the Cushitic Rendille, Gabbra and Borana as well as the Nilotic Samburu and Turkana. Four study sites were selected namely Bubisa (Gabbra-inhabited), Turbi (Gabbra-inhabited), Logologo (Rendille-inhabited) and Laisamis (Rendille-inhabited) based on ethnic composition and proximity to administrative centers as proxies to access veterinary services. Camel production is an important source of livelihood for the ethnic communities in the present study. Map of Kenya showing the study sites in Isiolo and Marsabit counties is presented in Fig 1.
Fig 1. A map of Kenya showing the sampling sites in Isiolo and Marsabit counties (made with Natural Earth).
Animal screening, collection and storage of samples
An epidemiological survey was carried out to screen for Surra in 8 preselected sites in Marsabit (Laisamis. Loglogo, Bubisa, Turbi) and Isiolo (Kulamawe, Ngaremara, Kinna, LMD) counties. A total of 2,750 camels from 160 herds belonging to different individuals were examined using buffy coat technique and Giemsa-stained dry smears for trypanosomes. The camels were examined at the beginning of dry season, at peak of dry season and during the wet season between September 2018 and April 2020. A total of 113 (4.1%) of the screened camels tested positive for T. evansi infection of which 100 isolates were used in the sensitivity study. Blood from all the camels infected with T. evansi were collected from the jugular vein into heparinized vacutainer tubes containing 20% glycerol and EDTA and cryopreserved in liquid nitrogen at -196°C as a stabilate.
Infectivity of donor mice
One hundred (100) cryo-preserved T. evansi isolates were thawed in two batches of 60 and 40 respectively, and inoculated intraperitoneally (i.p) into outbred Swiss White adult (23–25 days old) female mice. Each stabilate was administered into two mice (24 hours after last dose of cyclophosphamide administered at 100 mg/kg via intraperitoneal route for 3 consecutive days as immunosuppressant) at a rate of 1 x 105 trypanosomes/mouse via i.p route. The mice were monitored daily by collecting blood from the tail, expressing a drop on a microscope slide and examining it under dark field magnification X400 in a Leitz microscope. At peak parasitemia, blood from donor mice that developed an infection was collected and diluted appropriately with EDTA Buffer for inoculation into the experimental mice. Confirmation of trypanosome species was based on movement of the parasites under the microscope and morphological structures on dry stained smears as described by [1] (Giemsa-stained dry blood smears were prepared from all trypanosome isolates for trypanosome species identification). The dry smears were fixed with absolute methanol for 1 minute and stained with Giemsa (10%) for 45 minutes, dried and examined under oil immersion at X1000 magnification [52]. T. vivax was ruled out through mice inoculation as it rarely establishes infection in mice [53].
Test drugs
Four trypanocides were tested at the recommended dosages: isometamidium chloride at 1mg/kg bwt, diminazene aceturate at 20mg/kg bwt, homidium bromide at 1mg/kg and quinapyramine sulphate & chloride at 1mg/kg bwt.
Infectivity of experimental mice (In vivo mice sensitivity test)
In a randomized controlled trial (RCT) of a single-dose test as described by Eisler et al. [53], mice were randomly allocated into five treatment groups each containing 6 mice. Briefly; each mouse in all the treatment groups was injected with an inoculum containing T. evansi (0.2 ml of 5x104 trypanosomes/ml) from the donor mice via intraperitoneal route. The mice were weighed before administration of treatments to an accuracy of 1 gram. All the body weights varied by less than 10%. A single differentiating dose for each drug was used to treat groups of mice 24 hours after inoculum injection at the following dosage rates:
| Treatment group | Treatment given | Dosage | Manufacturer | Batch no. |
|---|---|---|---|---|
| Group 1 | Isometamidium chloride | 1 mg/kg | Ceva Sante’ Animale France | TFP711A |
| Group 2 | Diminazene aceturate | 20 mg/kg | Ceva Sante’ Animale France | 360A1 (lot) |
| Group 3 | Homidium bromide | 1 mg/kg | Laprovet France | |
| Group 4 | Quinapyramine sulphate & chloride | 1 mg/kg | Vetoquinol India animal Health Pvt. Ltd, India | STQ18008 |
| Group 5 | Distilled water (positive control) | 0.2 ml | N/A | N/A |
All the drugs were reconstituted so that the final drug dosage received by each mouse was contained in 0.2 ml of the final solution. After treatment, screening for trypanosomes in mice in all the treatment groups was done daily for the first 14 days and thereafter twice a week until day sixty (60) using dark ground/phase contrast buffy coat technique (magnification x250) of wet smear prepared from tail blood as described by Eisler et al. [53]. Results were recorded as negative or positive and scoring of parasitaemia carried out as described by Herbert and Lumsden [54]. Only experiments where at least 5 out of the 6 control mice became parasitaemic were considered valid [53]. Blood for parasitemia assessment was collected via tail nips which induces minimal pain thus does not require use of analgesia. There was no intrusive procedure conducted on the mice warranting use of analgesia or anesthesia. The mice were humanely euthanized when the parasitemia score remained at least 7.5 (Herbert and Lumsden score [54]) for five consecutive days through cervical dislocation. All the surviving mice were euthanized after day 60 through cervical dislocation.
Interpretation of results
Trypanosome isolates were considered drug-sensitive if at least 5 out of the 6 treated mice got cured (remained aparasitaemic until the end of the 60-day observation period). If fewer than 5 mice were cured, the isolate was considered to express resistance to the dosage used. In case one test mouse died prior to detection of parasitaemia, the isolate was considered as drug-sensitive if at least 4 out of the remaining 5 mice were cured; if fewer than 4 were cured, the isolate was considered to express resistance to the dosage used. However, if more than one test mouse died prior to detection of parasitaemia, the test was either repeated or dropped [53].
Survey of camel owners’ practices around Surra prevention, control and treatment
In order to explore the management practices among camel keeping ethnic communities in Marsabit and Isiolo counties that influence drug resistance, a cross-sectional survey was undertaken to 1) determine camel management practices across the communities, 2) evaluate the level of knowledge of Surra and its vectors across the communities and 3) identify specific measures applied by the communities in management of Surra and its vectors. The survey was undertaken in the same eight sites where animal screening was undertaken. A structured questionnaire administered by trained enumerators was used to capture relevant variables across the communities. A total of 340 respondents were interviewed distributed as follows: Somali (111) from LMD, Rendille (69) from Laisamis and Logologo, Gabbra (84) from Bubisa and Turbi, Borana (35) from Kulamawe and Kinna, and Turkana (41) from Ngaremara. The questionnaire captured the following aspects:
Camel husbandry practices of different communities. The respondents were asked to respond to husbandry related questions focusing on pasturing practices. The questions asked whether the farmers avoided infested areas during grazing and if they applied insecticides on camels among other aspects. The complete questionnaire is given as S1 Appendix.
Knowledge of camel trypanosomiasis, vectors and its management. Knowledge of Surra was assessed by evaluating camel owners’ awareness of the main clinical signs as explained in Desquesnes et al. [10], (see S2 Appendix for the list of clinical signs assessed). However, since these signs are not unique to Surra, but can manifest in other camel diseases present in the area [55], herders were required to give possible source of the clinical signs by describing the vectors as well as the options available for treatment of the symptoms. Clinical signs used were the 12 symptoms listed by Kula et al. [56]. The ability of the farmers to mention six clinical signs was arbitrarily selected as cut off point in the computation of knowledge level so as to get an understanding of herders’ knowledge of symptoms associated with both acute and chronic forms of Surra. Vector awareness was guided by herders’ pictorial identification of any of the vectors listed by Desquesnes et al. [1], including description of their habitat and seasonality. Assessment of Surra treatment knowledge entailed camel owners’ correct description of use of any of the drugs listed in Giordani et al. [21]. Herders were scored on each of the three areas and those adjudged to have good knowledge of Surra had to describe at least six correct clinical signs, identify at least one vector and describe proper use of at least one therapeutic or prophylactic drug.
Management of Surra and its vectors by the different communities. In assessing Surra management options used by different communities, respondents were required to state the time (date and month) the last case of Surra occurred in their herds; whether the case was treated, who undertook the treatment, approach used to treat the last case; name of product used in treating the case, how the product was prepared during the treatment, if multiple trypanocides were used and treatment outcome (S1 Appendix). The drug sensitivity results were interpreted together with epidemiological prevalence data and socioeconomic data on trypanocidal drug use, insecticidal use, level of education, herd dynamics and use of ethno-veterinary products in the study sites.
Data analysis
The data from mice sensitivity tests, prevalence and survey of camel owners’ practices were collated and inputted in Microsoft Excel® sheet and imported into STATA version 14 for analysis of frequencies, descriptive statistics, and association tests. The descriptive statistics were done and presented as mean ± standard deviation (mean ±SD). The prevalence of drug resistance in each study site was determined as a percentage of the number of isolates considered to be resistant to a particular drug (as per interpretation of mice results above) over the number of isolates tested from that study site. The prevalence of T. evansi was the number of camels that tested positive for T. evansi over the total number of camels sampled in the study sites. A one way and two-way ANOVA was conducted to determine mean differences in age of household heads, years of formal education and average household size by community orientation. Multivariate regression model was used to determine the effect of drug use on drug resistance and trypanosomiasis prevalence and the effect of insecticide use, use of ethno veterinary products and treatment of camels by the pastoralists on the prevalence of T. evansi infection in camels. Socioeconomic data on use of trypanocidal drugs and insecticides, level of education, herd dynamics and use of ethno-veterinary products in the study sites were converted to proportion (%) of respondents in each study site who answered ‘Yes’ to the questions relating to the various variables considered as independent variable in the multivariate regression analysis while the prevalence of T. evansi (%) in each study site was considered as the dependent variable. All significant levels were stated at p<0.05.
Results
Demographic characteristics of the interviewed camel keeping households
A total of 340 household heads comprising of 83.5% men and 16.5% women were interviewed in the study. Distribution of respondents by ethnic community was as follows: Somali (111), Turkana (41), Borana (46), Gabbra (83) and Rendille (72). The main source of income for majority of the households was livestock production (63.5%), followed by business (13.6%), formal employment (2.9%), while 2.4% of the households practiced crop farming. Up to 17.6% of respondents derived their livelihoods from other sources such as informal employment and pension. Disaggregation of the camel farmers by ethnic community, years of formal education, size of household and age of household head is presented in Table 1.
Table 1. Disaggregation of the camel farmers by ethnic community, years of formal education, size of household and age of household head.
| Demographic characteristic | Ethnic community | |||||
|---|---|---|---|---|---|---|
| Somali (N = 111) | Rendille (N = 72) | Gabbra (N = 83) | Borana (N = 46) | Turkana (N = 41) | Total (N = 340) | |
| Household head with formal education (n) | 73 | 67 | 72 | 22 | 34 | 268 |
| Years of formal education of household head (mean ±SD) | 2.59±4.0a | 0.32±2.0 | 1.1±3.5 | 2.7±4.6 a | 1.3±3.2 | 1.6±3.6 |
| Age of household head (years) (mean ± SD) | 54.8±15.1 | 52.9±12.3 | 55.5±14.8 | 47.6±15.5 | 46.6±15.6 | 52.9±14.9 |
| Size of household (number) (mean ± SD) | 9.9±4.4 | 10.0±5.5 | 7.6±2.7 | 9.3±3.7 | 10.5±5.1 | 9.4±4.4 |
Values across rows with the same superscript were significantly different at p<0.05.
Practices of the camel keepers around prevention, control and treatment of Surra
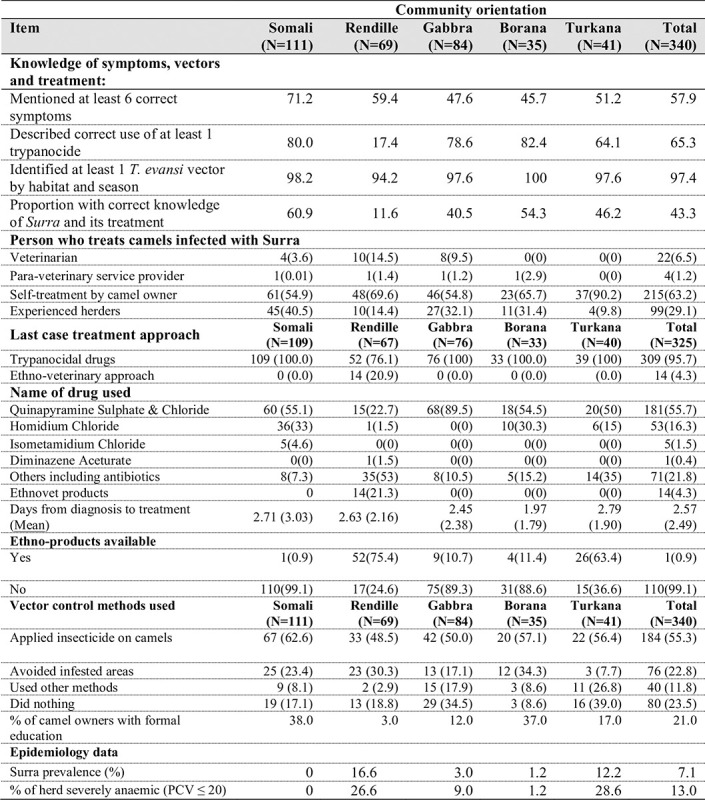
Data from camel owners’ practices revealed that knowledge of Surra was highest among Somali and lowest within Rendille ethnic communities, being significantly different across the 5 communities (p<0.00; χ2 = 44.26; df = 4). There were no significant differences across communities in the proportion of herders who were able to mention at least six correct clinical signs of the disease. Majority of the camel keepers carried out self-treatment (63.2%) of Surra infected camels whereas 29.1% and 7.7% procured services of experienced herders and veterinary professionals, respectively, in treating the sick camels. A high proportion of the camel keepers used trypanocidals (95.7%) to treat Surra while 4.3% used herbal remedies. Quinapyramine sulphate & chloride was the most commonly used trypanocide (55.7%) followed by homidium chloride (16.3%). Detailed characterization of community knowledge and practices around Surra is given in Fig 2.
Fig 2. Characterization of community knowledge and practices around camel Surra.
Sensitivity of the isolates to trypanocides
A total of nineteen (19) of the Isolates tested were collected from Marsabit County (Laisamis, Loglogo, Bubisa and Turbi) and 21 from Isiolo County (Ngaremara, Kulamawe and Kinna). All camels screened at LMD sampling site in Isiolo tested negative for T. evansi and therefore there was no isolate from this site. Table 2 shows the distribution of tested isolates by location and sensitivity results.
Table 2. Number of tested isolates that were sensitive or resistant to various drugs used for Surra treatmet in Marsabit and Isiolo counties.
| County | Sampling site | Sensitivity | HOM | ISMM | QPY | DIM |
|---|---|---|---|---|---|---|
| Isiolo | Kinna | Resistant | 2/2 | 2/2 | 2/2 | 0/2 |
| Sensitive | 0/2 | 0/2 | 0/2 | 2/2 | ||
| Ngaremara | Resistant | 9/10 | 6/10 | 8/10 | 0/10 | |
| Sensitive | 1/10 | 4/10 | 2/10 | 10/10 | ||
| Kulamawe | Resistant | 8/9 | 8/9 | 8/9 | 2/9 | |
| Sensitive | 1/9 | 1/9 | 1/9 | 8/9 | ||
| Marsabit | Laisamis | Resistant | 8/10 | 6/10 | 8/10 | 2/10 |
| Sensitive | 2/10 | 4/10 | 2/10 | 8/10 | ||
| Turbi | Resistant | 4/6 | 2/6 | 3/6 | 1/6 | |
| Sensitive | 2/6 | 4/6 | 3/6 | 5/6 | ||
| Logologo | Resistant | ½ | ½ | ½ | 0/2 | |
| Sensitive | ½ | ½ | ½ | 2/2 | ||
| Bubisa | Resistant | 1/1 | 1/1 | 1/1 | 0/1 | |
| Sensitive | 0/1 | 0/1 | 0/1 | 1/1 |
Numerator = number of isolates either sensitive or resistant to the drug.
Denominator = total number of isolates tested from the location.
HOM = Homidium chloride/bromide.
ISMM = Isometamidium Chloride.
DIM = Diminazene aceturate.
QPY = Quinapyramine sulphate & chloride.
Of the 40 isolates tested, 33(82.5%) were resistant to the trypanocidal drugs used in the study site. Isolates resistance to all the four drugs tested were observed in 2 (Kulamawe and Laisamis) of the 7 sites sampled and isolates sensitive to all the 4 drugs tested were observed in 5 of the 7 sites sampled. Five (12.5%) of the 40 isolates tested were resistant to diminazene aceturate whereas, 25(62.5%), 30(75%) and 31(77.5%) were resistant to isometamidium chloride, quinapyramine sulphate & chloride and homidium chloride, respectively. Seven (17.5%) isolates of the 40 tested were sensitive to all 4 drugs, whereas, 7.5%, 10%, 55% and 10% were resistant to 1,2,3 and 4 drugs respectively. Therefore, the overall prevalence of multiple drug resistance (resistance of an isolate to more than one drug) in the two counties was 75.0% as presented in Table 3.
Table 3. The number of tested isolates from study sites in Marsabit and Isiolo that were sensitive or resistant to one, two, three or all the four drugs tested.
| County | Sites | Level of resistance | Number of isolates (n) | Percentage of isolates (%) |
|---|---|---|---|---|
| Isiolo | Kinna | S1/R3 | 2 | 100 |
| Ngaremara | S1/R3 | 6 | 60 | |
| S2/R2 | 1 | 10 | ||
| S3/R1 | 2 | 20 | ||
| S4 | 1 | 10 | ||
| Marsabit | Laisamis | R4 | 2 | 20 |
| S1/R3 | 4 | 40 | ||
| S2/R2 | 2 | 20 | ||
| S4 | 2 | 20 | ||
| Loglogo | S1/R3 | 1 | 50 | |
| S4 | 1 | 50 | ||
| Bubisa | S1/R3 | 1 | 100 | |
| Turbi | S1/R3 | 2 | 20 | |
| S2/R2 | 1 | 20 | ||
| S3/R1 | 1 | 20 | ||
| S4 | 2 | 40 |
S = Sensitive, R = Resistant, S4 = Sensitive to 4 drugs, S3/R1 = Resistant to 1 drug; S2/R2 = Resistant to 2 drugs; S1/R3 = resistant to 3 drugs; R4 = Resistant to all 4 drugs.
Of the 21 isolates from Isiolo County tested 19(90.5%) were resistant to the trypanocidal drugs used in the study counties. Two (9.5%) isolates were resistant and 2 were sensitive to all the four drugs tested. Whereas, 2 (9.5%), 16 (76.2%), 18 (85.7%) and 18(85.7%) isolates were resistant to diminazene aceturate, isometamidium chloride, quinapyramine sulphate & chloride and homidium chloride, respectively (Table 2). Fourteen (66.7%), 1(4.8%) and 2(9.5%) were resistant to 3, 2 and 1 drug, respectively (Table 3). Consequently, the prevalence of multiple drug resistance in Isiolo was 81.0%. Prevalence of resistance against diminazene aceturate was 22.2%, whereas resistance against isometamidium chloride, homidium and quinapyramine sulphate & chloride varied from 60% to 100%. Tables 4 and 5 presents summarized resistance results from the different sampling sites.
Table 4. Number of isolates resistant to a drug per site.
| County | Site | HOM | ISM | QPY | DIM |
|---|---|---|---|---|---|
| Isiolo | Kinna | 2 (100%) | 2 (100%) | 2 (100%) | 0 |
| Ngaremara | 8 (80%) | 6 (60%) | 8 (80%) | 0 | |
| Kulamawe | 8 (88.9%) | 8 (88.9%) | 8 (88.9%) | 2 (22.2%) | |
| Prevalence | 85.70% | 76.20% | 85.70% | 9.50% | |
| Marsabit | Laisamis | 8 (80%) | 6 (60%) | 8 (80%) | 2 (20%) |
| Turbi | 4 (66.7%) | 2 (33.3%) | 3 (50%) | 1 (16.7%) | |
| Loglogo | 1 (50%) | 1 (50%) | 1 (50%) | 0 | |
| Bubisa | 1 (100%) | 1 (100%) | 1 (100%) | 0 | |
| Prevalence | 73.70% | 52.60% | 68.40% | 15.80% | |
| Overall prevalence | 80.0% | 65.0% | 77.5% | 12.5% | |
HOM = Homidium chloride.
ISM = Isometamidium Chloride.
DIM = Diminazene aceturate.
QPY = Quinapyramine sulphate & chloride.
Table 5. Prevalence of trypanocidal drug resistance in the study sites in Marsabit and Isiolo counties.
| County | Sites | Isolates resistant to drugs | Prevalence of drug resistance |
|---|---|---|---|
| Isiolo | Kinna | 2 | 100% |
| Ngaremara | 9 | 90% | |
| Kulamawe | 8 | 88.9% | |
| Marsabit | Laisamis | 8 | 80.0% |
| Loglogo | 1 | 50.0% | |
| Bubisa | 1 | 100.0% | |
| Turbi | 4 | 66.7% |
Within the three sites in Isiolo county prevalence of trypanocidal resistance varied from 88.9% in Kulamawe to 100% in Kinna (Table 5). One isolate in Ngaremara and Kulamawe respectively was sensitive to all the four drugs tested, while 2 (22.2%) isolates in Kulamawe were resistant to all the drugs tested. Therefore, the prevalence of multiple drug resistance was 100% in Kinna, 70% in Ngaremara and 88.9% in Kulamawe (Table 3).
Of the isolates from Marsabit County tested, 73.7% were resistant to the trypanocidal drugs used in the study counties. Two (10.5%) Isolates were resistant while 5 (26.3%) were sensitive to all four drugs tested. Three (15.8%), 10 (52.6%), 13 (68.4%) and 14 (73.7%) isolates were resistant to diminazene aceturate, isometamidium chloride, quinapyramine sulphate/chloride and homidium respectively (Table 2), whereas 8(42.1%), 3(15.8%) and 1(5.3%) were resistant to 3, 2 and 1 drug respectively (Table 3). Consequently, the prevalence of multiple drug resistance in Marsabit was 73.7%. Within the 4 sites in Marsabit County, prevalence of trypanocidal resistance varied from 50.0% in Loglogo to 100.0% in Bubisa (Table 5). Prevalence of resistance against diminazene aceturate varied from a minimum of 16.7% resistance (in Turbi), to a maximum of 20% (in Laisamis). Prevalence of resistance against ISM, HOM and QPY varied between 33.3% to 100%, 66.7% to 100% and 50% to 100% for ISM, HOM and QPY respectively (Table 4). Two (22.2%), 1 (50%) and 2 (40%) isolates in Laisamis, Loglogo and Turbi respectively were sensitive to all the 4 drugs tested, while 2 (20%) isolates in Laisamis were resistant to all the drugs tested. Therefore, the prevalence of multiple drug resistance was 80% in Laisamis, 50% in Loglogo, 100% in Bubisa and 60% in Turbi (Table 3).
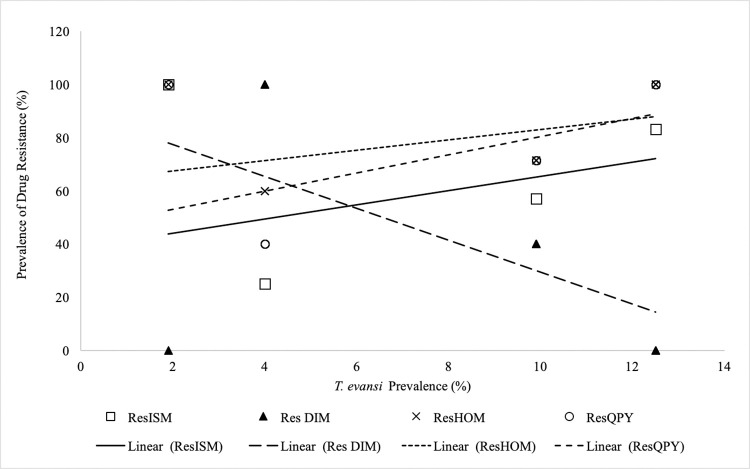
The prevalence of camel Surra in the study area affected the prevalence of isolates resistant to various drugs differently (Fig 3).
Fig 3. Variation of various drug resistance with prevalence of T. evansi in Isiolo and Marsabit counties.
The prevalence of isolates resistant to HOM (p = 0.049), ISM (p = 0.073) and QPY (p = 0.010) increased with prevalence of T. evansi infections. However, prevalence of isolates resistant to DIM decreased significantly (P = 0.006) with increase of prevalence of T. evansi infections. Trypanocidal drug use by the different ethnic communities affected the prevalence and the degree of drug resistance isolates in the counties. The increase in HOM, QPY and DIM use in the counties significantly (p = 00001) increased the prevalence of HOM, QPY and DIM resistance, respectively. However, use of isometamidium by pastoralists had no effect (p = 0.888) on the prevalence of isometamidium drug resistance in the study area. Mixing of trypanocidal drugs with other drugs or use of ethno-veterinary compounds had no effect (p = 0.314) on the prevalence of drug resistance. Increase in insecticidal use by pastoralists in the study area increased (p = 0.0001) the prevalence of drug resistant isolates. The proportion of pastoralist with no formal education in an area had a significant (p = 0.013) effect on the prevalence of drug resistance of isolates from the area. The higher the lack of formal education among the pastoralist the lower the prevalence of drug resistant isolates recorded. In contrast, the proportion of pastoralists with formal education had no significant effect on the prevalence of drug resistant isolates in an area. Herd dynamics influenced by inflows such as new births, purchases, gifts exchanges had no effect (p = 0.445) on the prevalence of drug resistance in the study area.
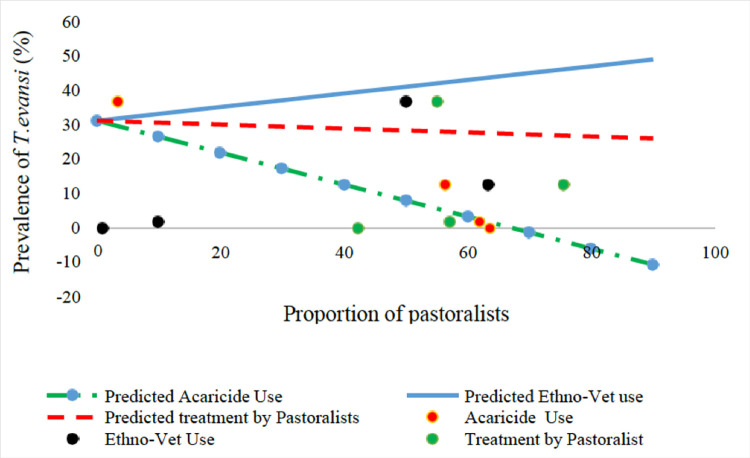
The herd prevalence of T. evansi in camels decreased with the increase in insecticidal use (p = 0.065), homidium use (p = 0.005), avoiding of suspected high fly density areas (p-0.082) and increase in female to male ratio of camels in the study area. In contrast, the herd prevalence of T. evansi increased significantly (p = 0.007) with the increase in the use of Ethnoveterinary products (Fig 4) and increase in the knowledge of clinical signs of Surra. In contrast, the proportion of pastoralist treating their own camels had no significant (p = 0.405) effect on the prevalence of T. evansi in camels despite frequent treatment (52%) by the pastoralists (Fig 4).
Fig 4. The effect of insecticide use, ethno veterinary compound use and treatment of camels by the pastoralists on the prevalence of T. evansi infection in camels in Isiolo and Marsabit counties.
Discussion
The overall prevalence of drug resistance was 82.5% indicating the circulating T. evansi populations exhibit reduced sensitivity (resistance) to the available trypanocidal drugs in the two counties. However, this also indicates there are still T. evansi populations circulating that would respond to treatment if administered correctly. Instability of drug resistance in bacteria and trypanosome populations has been observed in response to withdrawal of antibiotic or trypanocidal due to selective pressure [57]. This implies that the observed drug resistance in the two counties is not permanent and may vary with time due to inconsistency of the selective drug pressure which is dependent on availability of the drugs to the pastoralist communities and their usage. From a similar sensitivity study carried out more than six years earlier in the two study counties, the prevalence of resistance to diminazene, isometamidium and quinapyramine decreased following withdrawals of the drugs [15]. Resistance by field isolates to ISM, HOM, DIM, quinapyrammine have been previously demonstrated in other studies [34,58–62]. It is imperative then that trypanocidal drugs should only be used upon accurate disease diagnosis so as to reduce drug pressure on the trypanosome populations. However, majority of the camel owners in the present study carried out self-treatment of sick camels instead of using qualified veterinarians, a practice that may be contributing to development of drug resistance. Self-treatment of sick camels by unqualified farmers has previously been associated with wrong route of administration, wrong reconstitution and wrong dosages all of which may lead to development of drug resistance as has been reported for diminazene aceturate and Isometadidium chloride [40].
Despite the resistant isolates being observed in all the seven sites where trypanosome isolates were collected, only two sites had circulating trypanosome populations that were resistant to all the four drugs being used in the region, whereas five of the seven sites had circulating trypanosome populations that were sensitive to all the drugs used in the region. This is an indication that control of Surra using drugs would still be effective in the region if used properly according to the manufactures prescription. Previous studies showed that increasing dosage and repeat application of some trypanocidal drugs can be effective in eliminating the infection as long as the therapeutic index is observed [58–60,63]. A single dose test was used in the present study and it may be interesting to know how the resistant isolates may respond to increased trypanocidal doses. The level of intensity of trypanocidal drug use by the communities was associated with increase in prevalence of drug resistance especially for the drugs that were in frequent use (QPY, ISM and HOM). The intensive use of trypanocidal drugs for the control of trypanosomiasis has been associated with appearance of drug resistant trypanosomes [33,64,65]. This suggests that rational use of trypanocides where treatment is administered correctly (right dose and route of administration) in animals definitively diagnosed to be trypanosome infected would reduce the incidence of drug resistance.
Despite ready availability of diminazene aceturate in the two counties, majority of the isolates (90%) were sensitive to this drug. This may be attributed to the limited use of this drug in treatment of Surra by pastoralists in the two counties relative to other trypanocides as our survey revealed. diminazene aceturate in powder formulation has been observed to be toxic to camels at 10mg/kg and therefore not recommended for use in camels [66]. Also, diminazene aceturate is rapidly eliminated from the system after administration which minimizes development of drug resistance compared to other more persistent trypanocidals [67,68]. However, a liquid formulation of diminazene aceturate with phenazone and procaine hydrochloride has been recommended for Surra treatment in camels [69,70] and is available in the study counties, although its efficacy in treatment of T. evansi infections is questionable [71].
The 12% isolates that exhibited resistance to diminazene aceturate were collected from Marsabit county in areas inhabited by the Rendille community whose knowledge of use of drugs was limited and only used quinapyramine sulphate & chloride in treatment of Surra. It is likely that the observed diminazene aceturate resistance is as a result of cross resistance from quinapyramine sulphate & chloride. Early studies by whiteside [38,72] concluded that induction of resistance to quinapyramine under field conditions appeared to result in cross-resistance to homidium chloride, isometamidium chloride and diminazene aceturate. In the present study, a significant difference in the prevalence of multiple drug resistance between isolates from the two counties was observed which may be attributed to differences in trypanocidal drug usage between pastoralist communities in the two counties. In a previous study, a high proportion of pastoralist in isiolo used homidium chloride and quinapyramine sulphate & chloride respectively whereas the majority of pastoralist in Marsabit county used either antibiotics or herbal concoctions as an alternative treatment of what they perceived to be Surra [26].
Extensive use of trypanocidal drugs and under dosing of drugs has been indicated as the main contributor to development of drug resistance due to exposure of trypanosomes to sub-therapeutic drug levels, thereby favoring the selection of drug-resistant populations [33]. The presence of multiple drug resistant T. evansi populations circulating in the two study counties is of concern. However, the fact that these populations are sensitive to diminazene aceturate suggests that use of ‘sanative pair’ of drugs may still be applicable in the control of Surra in Marsabit and Isiolo. The ‘Sanative pair’ are pairs of drugs that do not induce resistance to each other and can be used alternatively in the field when resistance to either drug appears [36,72]. Whiteside [72] proposed that homidium and diminazene or isometamidium and diminazene should be used together as ‘sanative’ pairs of drugs in areas where drug resistance has been observed. In addition, the multiple drug resistance observed in this study is at the population level and most likely not at the individual trypanosome level where treatment using a “sanative pair” would have been impossible [42]. Ndungu et al. [30] demonstrated that several clones derived from an isolate that exhibited resistance to trypanocidal drugs in mice showed variable sensitivity to treatment. Whether the clones from the multiple resistant isolates from this study could have variable sensitivity to diminazene, homidium and isometamidium was not investigated.
However, the observation in the present study that trypanosome populations circulating in the study region that demonstrated resistance to the four drugs tested were located in only two of the seven sites further suggests multiple drug resistance is not widely spread in the region at present. Therefore, control of Surra using drugs would still be effective if used rationally as per label prescription. It is well known that–although there is a quite good correlation of the mouse test with the test in ruminants–the curative dose in mice cannot be extrapolated to livestock [19]. However, the standard mouse test (used in this study) has been used effectively as a single dose test for epidemiological purposes in studying of area-wide occurrence of drug resistance in various regions of Africa [73] allowing spatial and temporal comparisons or as a multi-dose test, which measures the degree of resistance in individual trypanosome isolates [53]. The present study established high drug resistance levels in areas with high T. evansi prevalence. The association between prevalence and resistance to trypanocides has been recorded elsewhere [61]. High prevalence of treatment failure of trypanocides in cattle that was attributed to drug resistance has been observed in seasons of high tsetse and trypanosomosis challenge in the coastal areas of Kenya [32]. Since the herd prevalence decreased with use of insecticides, avoiding vector infested areas and Surra knowledge, these practices should be integrated in Surra control strategies. The relatively lower prevalence recorded in herds with more female camels can be attributed to the comparatively better management accorded to female camels by pastoral communities due their economic importance [74].
In conclusion, the multiple drug resistance observed in the two counties is not an indication of total trypanocidal drug treatment failure. Use of “sanative pair” of drugs as the commonly accepted method for delaying the development of drug resistance may most likely be still applicable in the region since there were a number of isolates that were resistant (10%) to a single drug and sensitive (17.5%) to all the drugs from five out of the seven sites sampled. In addition, judicious treatment of confirmed cases of Surra with adequate amount of drug would still be able to effectively control the disease in the region especially since the observed resistance appears to be at the population level and not at the clonal (individual trypanosome) level. However, cloning and the individual characterization of trypanosomes from the multiple drug resistant isolates may be necessary to clarify whether or not the sanative pair can still be used. Trypanosome isolates collected from the field as was the case in the present study have been shown to consist of a panmictic population of trypanosomes resistant to a single drug or a homogenous population of multi-resistant trypanosomes or a mixture of both together. In each of these situations different measures will have to be taken to avoid further worsening of the situation. To achieve that, further molecular characterization is required. A moderately high trypanosomiasis prevalence was recorded using buffy coat technique and Giemsa-stained dry smears despite their moderate sensitivities implying the prevalence could be even higher. Consequently, strategic use of integrated control of the disease and the vector using available alternative methods to reduce drug use is necessary to avoid rapid development of drug resistance. Integrated approaches have been shown to lead to reduced disease prevalence under reduced drug use. Such a strategy would comprise of (i) judicious use of trypanocides in definitively diagnosed tsetse positive animals, (ii) control of vectors by targeted bi-monthly insecticidal spraying of the animals, (iii) strategic deworming in the beginning and the end of the rainy season and, (iv) strategic use of prophylaxis when grazing through highly vector infested areas.
Supporting information
(PDF)
(PDF)
Acknowledgments
The management of the Biotechnology Research Institute of Kenya Agricultural and Livestock Research Organization is acknowledged for their support. The support and guidance of the Project Principal Investigator and Project Coordinator is highly appreciated. The authors are indebted to all project team members and technical staff for their input in assuring the success of this study.
Data Availability
Data files used in the paper are available from the Mendeley data and can be accessed here: https://data.mendeley.com/datasets/b64fg8zbyg/1 and https://tinyurl.com/ynnk7ans.
Funding Statement
This project (Grant No. AURG-II-1-179-2016) was funded by the African Union (AU-IBAR). However, the views expressed herein do not necessarily represent those of African Union (AU). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Desquesnes M, Dargantes A, Lai DH, Lun ZR, Holzmuller P, Jittapalapong S. Trypanosoma evansi and Surra: a review and perspectives on transmission, epidemiology and control, impact, and zoonotic aspects. BioMed Res Int. 2013;2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brun R, Hecker H, Lun ZR. Trypanosoma evansi and T. equiperdum: distribution, biology, treatment and phylogenetic relationship (a review). Vet Parasitol [Internet]. 1998. Oct 1 [cited 2022 Mar 1];79(2):95–107. Available from: doi: 10.1016/s0304-4017(98)00146-0 [DOI] [PubMed] [Google Scholar]
- 3.Kidambasi KO, Masiga DK, Villinger J, Carrington M, Bargul JL. Detection of blood pathogens in camels and their associated ectoparasitic camel biting keds, Hippobosca camelina: the potential application of keds in xenodiagnosis of camel haemopathogens. AAS Open Res [Internet]. 2020. May 20 [cited 2022 Mar 2];2:164. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7243205/. doi: 10.12688/aasopenres.13021.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Odeniran PO, Macleod ET, Ademola IO, Welburn SC. Molecular identification of bloodmeal sources and trypanosomes in Glossina spp., Tabanus spp. and Stomoxys spp. trapped on cattle farm settlements in southwest Nigeria. Med Vet Entomol [Internet]. 2019. [cited 2022 Mar 2];33(2):269–81. Available from: https://tinyurl.com/2p9cyedw. doi: 10.1111/mve.12358 [DOI] [PubMed] [Google Scholar]
- 5.Baldacchino F, Muenworn V, Desquesnes M, Desoli F, Charoenviriyaphap T, Duvallet G. Transmission of pathogens by Stomoxys flies (Diptera, Muscidae): a review. Parasite [Internet]. 2013. [cited 2022 Mar 2];20:26. Available from: https://tinyurl.com/5ez7bzct. doi: 10.1051/parasite/2013026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baldacchino F, Desquesnes M, Mihok S, Foil LD, Duvallet G, Jittapalapong S. Tabanids: Neglected subjects of research, but important vectors of disease agents! Infect Genet Evol [Internet]. 2014. Dec 1 [cited 2022 Mar 2];28:596–615. Available from: https://www.sciencedirect.com/science/article/pii/S1567134814001221. doi: 10.1016/j.meegid.2014.03.029 [DOI] [PubMed] [Google Scholar]
- 7.Touratier L. Eleventh international meeting on Trypanosoma evansi: report of the Working Group. Paris, 17 May 1990. Rev Sci Tech Int Off Epizoot [Internet]. 1992 Mar 1 [cited 2022 Mar 1];11(1):275–304. Available from: 10.20506/rst.11.1.605. [DOI] [PubMed] [Google Scholar]
- 8.Aquino LPCT de Machado RZ, Alessi AC Marques LC, Castro MB de Malheiros EB. Clinical, parasitological and immunological aspects of experimental infection with Trypanosoma evansi in dogs. Mem Inst Oswaldo Cruz. 1999;94(2):255–60. doi: 10.1590/s0074-02761999000200025 [DOI] [PubMed] [Google Scholar]
- 9.Lun ZR, Fang Y, Wang CJ, Brun R. Trypanosomiasis of domestic animals in China. Parasitol Today [Internet]. 1993. Feb 1 [cited 2022 Mar 1];9(2):41–5. Available from: https://www.sciencedirect.com/science/article/pii/016947589390029F. doi: 10.1016/0169-4758(93)90029-f [DOI] [PubMed] [Google Scholar]
- 10.Desquesnes M, Holzmuller P, Lai DH, Dargantes A, Lun ZR, Jittaplapong S. Trypanosoma evansi and Surra: a review and perspectives on origin, history, distribution, taxonomy, morphology, hosts, and pathogenic effects. BioMed Res Int. 2013;2013:194176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pacholek X, Gamatie D, Franck SGV, Tibayrenc R. Prevalence of Trypanosoma evansi trypanosomiasis in young camels in West Niger. Rev DÉlevage Médecine Vét Pays Trop [Internet]. 2000. [cited 2022 Mar 2];53(2):177–82. Available from: https://tinyurl.com/558fk5na. [Google Scholar]
- 12.Njiru ZK, Brett B, Ole-Mapeny IM, Githiori JB, Ndung’u JM. Trypanosomosis and helminthosis in camels: comparison of ranch and traditional camel management systems in Kenya. J Camel Pract Res. 2002;9(1):67–71. [Google Scholar]
- 13.Njiru ZK, Ole-Mapeny IM, Ouma JO, Ndung’u JM, Olaho Mukani W. Prevalence of trypanosomosis in camel calves: A pilot study in Laikipia District of Kenya. Rev Elev Médecine Vét Pays Trop [Internet]. 2000. [cited 2022 Mar 1]; Available from: https://agritrop.cirad.fr/477505/. [Google Scholar]
- 14.Dolan R, Wilson AJ, Schwartz HJ, Newson RM, Field CR. Camel production in kenya and its constraints. Trop Anim Health Prod [Internet]. 1983. Sep 1 [cited 2022 Mar 1];15(3):179–85. Available from: 10.1007/BF02239930. [DOI] [PubMed] [Google Scholar]
- 15.Mdachi RE, Murilla GA, Bateta R, Munga LK. Sensitivity and virulence of Trypanosoma evansi isolates from camels in Marsabit County, Kenya. The 31st Meeting of the International Scientific Council for Trypanosomiasis Research and control; 2011; Bomoko, Mali. [Google Scholar]
- 16.Kamidi CM, Auma J, Mireji PO, Ndungu K, Bateta R, Kurgat R, et al. Differential virulence of camel Trypanosoma evansi isolates in mice. Parasitology [Internet]. 2018. Aug [cited 2022 Mar 1];145(9):1235–42. Available from: https://tinyurl.com/2n9k992r. doi: 10.1017/S0031182017002359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joshi SS, Singh B. Evaluation of therapeutic and chemo prophylactic efficacy of certain drugs against clinical Surra in buffaloes. Indian Vet J [Internet]. 2000. [cited 2022 Mar 2];77(10):895–7. Available from: https://tinyurl.com/yeyujpb3. [Google Scholar]
- 18.Kasozi KI, MacLeod ET, Ntulume I, Welburn SC. An Update on African Trypanocide Pharmaceutics and Resistance. Front Vet Sci [Internet]. 2022. [cited 2022 May 19];9. Available from: https://www.frontiersin.org/article/10.3389/fvets.2022.828111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geerts S, Holmes PH. Drug management and parasite resistance in bovine trypanosomiasis in Africa. Drug Manag Parasite Resist Bov Trypanos Afr [Internet]. 1998. [cited 2022 Mar 2];(No. 1). Available from: https://tinyurl.com/3tpuc5vd. [Google Scholar]
- 20.Luckins AG. Epidemiology of Surra: Unanswered Questions. J Protozool Res [Internet]. 1998. [cited 2022 Mar 1];8(3):106–19. Available from: https://tinyurl.com/khpm4pwf. [Google Scholar]
- 21.Giordani F, Morrison LJ, Rowan TG, Koning HPD, Barrett MP. The animal trypanosomiases and their chemotherapy: a review. Parasitology [Internet]. 2016. Dec [cited 2022 Mar 1];143(14):1862–89. Available from: https://tinyurl.com/2p9s26vd. doi: 10.1017/S0031182016001268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wanjala K B, Mugunieri GL, Alusi Phyllis M, Kurgat R K, Mdachi R E, Chemuliti J K, et al. 2021. Management of Camel Trypanosomiasis (Surra) among Pastoralists of Isiolo and Marsabit Counties, Kenya. World Journal of Agricultural Research. 2021, 9(1), 15–23. doi: 10.12691/wjar-9-1-3 [cited 2022 May 19];9(1):15–23. [DOI] [Google Scholar]
- 23.Jaime G, Hobeika A, Figuié M. Access to Veterinary Drugs in Sub-Saharan Africa: Roadblocks and Current Solutions. Front Vet Sci [Internet]. 2022. [cited 2022 May 19];8. Available from: https://www.frontiersin.org/article/10.3389/fvets.2021.558973. doi: 10.3389/fvets.2021.558973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sen A, Chander M. Privatization of veterinary services in developing countries: a review. Trop Anim Health Prod. 2003;35(3):223–36. doi: 10.1023/a:1023343429498 [DOI] [PubMed] [Google Scholar]
- 25.Oruko LO, Upton M, McLeod A. Restructuring of Animal Health Services in Kenya: Constraints, Prospects and Options. Dev Policy Rev [Internet]. 2000. [cited 2022 May 19];18(2):123–38. Available from: https://tinyurl.com/4xajbcvr. [Google Scholar]
- 26.Mdachi RE, Muinde M, Holmes PH, Eisler MC. Evidence of improper use of trypanocidal drugs by farmers and field personnel in one district in Kenya. In: Proceedings of the 27th Meeting of the ISCTRC, Pretoria, South Africa. 2003. [Google Scholar]
- 27.Burudi EME, Peregrine AS, Majiwa PAO, Mbiuki SM, Murphy NB. Response of diminazene-resistant and diminazene-susceptible Trypanosoma congolense to treatment with diminazene when occurring as a mixed infection in goats. Ann Trop Med Parasitol [Internet]. 1994. Jan 1 [cited 2022 Mar 2];88(6):595–606. Available from: doi: 10.1080/00034983.1994.11812910 [DOI] [PubMed] [Google Scholar]
- 28.Mamman M, Gettinby G, Murphy NB, Kemei S, Peregrine AS. Frequency of diminazene-resistant trypanosomes in populations of Trypanosoma congolense arising in infected animals following treatment with diminazene aceturate. Antimicrob Agents Chemother [Internet]. 1995. May [cited 2022 Mar 1]; Available from: https://tinyurl.com/2p99bvrv. doi: 10.1128/AAC.39.5.1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mamman M, Williams DJL, Murphy NB, Peregrine AS. Apparent rarity of diminazene-resistant trypanosomes in goats infected with a diminazene-resistant population of Trypanosoma congolense. Res Vet Sci [Internet]. 1995. Mar 1 [cited 2022 Mar 1];58(2):113–8. Available from: https://tinyurl.com/4zbkusp4. doi: 10.1016/0034-5288(95)90062-4 [DOI] [PubMed] [Google Scholar]
- 30.Ndung’u K, Murilla GA, Thuita JK, Ngae GN, Auma JE, Gitonga PK, et al. Differential virulence of Trypanosoma brucei rhodesiense isolates does not influence the outcome of treatment with anti-trypanosomal drugs in the mouse model. PLOS ONE [Internet]. 2020. Nov 5 [cited 2022 Mar 1];15(11):e0229060. Available from: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0229060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mdachi RE, Murilla GA, Omukuba JN, Cagnolati V. Disposition of diminazene aceturate (Berenil) in trypanosome-infected pregnant and lactating cows. Vet Parasitol Neth [Internet]. 1995. [cited 2022 Mar 2]; Available from: https://tinyurl.com/2p89yxhc. doi: 10.1016/0304-4017(94)00722-o [DOI] [PubMed] [Google Scholar]
- 32.Bacchi CJ, Nathan HC, Yarlett N, Goldberg B, McCann PP, Sjoerdsma A, et al. Combination chemotherapy of drug-resistant Trypanosoma brucei rhodesiense infections in mice using DL-alpha-difluoromethylornithine and standard trypanocides. Antimicrob Agents Chemother. 1994. Mar;38(3):563–9. doi: 10.1128/AAC.38.3.563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leach TM, Roberts CJ. Present status of chemotherapy and chemoprophylaxis of animal trypanosomiasis in the eastern hemisphere. Pharmacol Ther [Internet]. 1981. Jan 1 [cited 2022 Mar 2];13(1):91–147. Available from: https://tinyurl.com/28pr3ztr. doi: 10.1016/0163-7258(81)90069-3 [DOI] [PubMed] [Google Scholar]
- 34.Peregrine AS. Chemotherapy and delivery systems: haemoparasites. Vet Parasitol [Internet]. 1994. Aug 1 [cited 2022 Mar 2];54(1):223–48. Available from: https://www.sciencedirect.com/science/article/pii/0304401794900922. doi: 10.1016/0304-4017(94)90092-2 [DOI] [PubMed] [Google Scholar]
- 35.Osman AS, Jennings FW, Holmes PH. The rapid development of drug-resistance by Trypanosoma evansi in immunosuppressed mice. Acta Trop [Internet]. 1992. Feb 1 [cited 2022 Mar 1];50(3):249–57. Available from: https://tinyurl.com/2rsv2ck8. doi: 10.1016/0001-706x(92)90081-8 [DOI] [PubMed] [Google Scholar]
- 36.Mdachi RE. Impact of drug resistance in disease control in coastal Kenya in Epidemiological Evaluation of Trypanosomiasis the control in Cattle. Germany: Lambert Academic Publishing; 2014. [Google Scholar]
- 37.Davey DG. The Chemotherapy of Animal Trypanosomiasis Wim Particular Reference to the Trypanosomal Diseases of Domestic Animals In Africa. Entry No Jfpounds∼∼ OATE Ri¢ 71. 1957;25. [Google Scholar]
- 38.Whiteside EF. Interactions between drugs, trypanosomes and cattle in the field. Drugs Parasites Hosts. 1962;116:141. [Google Scholar]
- 39.Holmes PH, Torr SJ. The control of animal trypanosomiasis in Africa: current methods and future trends. Outlook on Agriculture. 1988. Jun;17(2):54–60. [Google Scholar]
- 40.Kasozi KI, MacLeod ET, Waiswa C, Mahero M, Ntulume I, Welburn SC. Systematic Review and Meta-Analysis on Knowledge Attitude and Practices on African Animal Trypanocide Resistance. Tropical medicine and infectious disease. 2022. Aug 23;7(9):205. doi: 10.3390/tropicalmed7090205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maman M, Katende J, Moloo SK, Peregrine AS. Variation in sensitivity of Trypanosoma congolense to diminazene during the early phase of tsetse-transmitted infection in goats. Vet Parasitol [Internet]. 1993. Oct 1 [cited 2022 Mar 1];50(1):1–14. Available from: https://www.sciencedirect.com/science/article/pii/0304401793900025. [DOI] [PubMed] [Google Scholar]
- 42.Mulugeta W, Wilkes J, Mulatu W, Majiwa PAO, Masake R, Peregrine AS. Long-term occurrence of Trypanosoma congolense resistant to diminazene, isometamidium and homidium in cattle at Ghibe, Ethiopia. Acta Trop [Internet]. 1997. Apr 15 [cited 2022 Mar 1];64(3):205–17. Available from: https://tinyurl.com/3v4rr68e. [DOI] [PubMed] [Google Scholar]
- 43.El Rayah IE, Kaminsky R, Schmid C, El Malik KH. Drug resistance in Sudanese Trypanosoma evansi. Vet Parasitol [Internet]. 1999. Jan 28 [cited 2022 Mar 1];80(4):281–7. Available from: https://www.sciencedirect.com/science/article/pii/S0304401798002210. doi: 10.1016/s0304-4017(98)00221-0 [DOI] [PubMed] [Google Scholar]
- 44.Boid R, Jones TW, Luckins AG. 3. Protozoal diseases of camels. Br Vet J [Internet]. 1985. Jan 1 [cited 2022 Mar 1];141(1):87–105. Available from: https://tinyurl.com/5n7u7t7b. [DOI] [PubMed] [Google Scholar]
- 45.Brun R, Lun ZR. Drug sensitivity of Chinese Trypanosoma evansi and Trypanosoma equiperdum isolates. Vet Parasitol [Internet]. 1994. Mar 1 [cited 2022 Mar 1];52(1):37–46. Available from: https://www.sciencedirect.com/science/article/pii/0304401794900337. doi: 10.1016/0304-4017(94)90033-7 [DOI] [PubMed] [Google Scholar]
- 46.Zhou J, Shen J, Liao D, Zhou Y, Lin J. Resistance to drug by different isolates Trypanosoma evansi in China. Acta Trop [Internet]. 2004. May 1 [cited 2022 Mar 1];90(3):271–5. Available from: https://tinyurl.com/4pbskwcf. doi: 10.1016/j.actatropica.2004.02.002 [DOI] [PubMed] [Google Scholar]
- 47.Berger BJ, Carter NS, Fairlamb AH. Characterisation of pentamidine-resistant Trypanosoma brucei brucei. Mol Biochem Parasitol. 1995;69(2):289–98. doi: 10.1016/0166-6851(94)00215-9 [DOI] [PubMed] [Google Scholar]
- 48.Mutugi MW, Boid R, Luckins AG. Differences in cloning and sub-cloning success rates in four stocks of Trypanosoma evansi and variation in suramin resistance of the clones. Vet Parasitol [Internet]. 1995. Dec 1 [cited 2022 Mar 1];60(3):213–20. Available from: https://www.sciencedirect.com/science/article/pii/0304401795007874. doi: 10.1016/0304-4017(95)00787-4 [DOI] [PubMed] [Google Scholar]
- 49.Mutugi MW, Boid R, Luckins AG. Experimental induction of suramin-resistance in cloned and uncloned stocks of Trypanosoma evansi using immunosuppressed and immunocompetent mice. Trop Med Parasitol. 1994. Sep 1;45(3):232–6. [PubMed] [Google Scholar]
- 50.Mutugi MW. Studies on suramin resistance in Kenyan stocks of Trypanosoma evansi (Steel, 1885 Balbiani, 1888). 1993. [cited 2022 Mar 1]; Available from: https://era.ed.ac.uk/handle/1842/29291. [Google Scholar]
- 51.Kenya National Bureau of Statistics. Volume iv: Distribution of population by socio-economic characteristics. Vol. 4. Kenya National Bureau of Statistics; 2019. [Google Scholar]
- 52.Hilali M, Abdel-Gawad A, Nassar A, Abdel-Wahab A. Hematological and biochemical changes in water buffalo calves (Bubalus bubalis) infected with Trypanosoma evansi. Vet Parasitol [Internet]. 2006. Jun 30 [cited 2022 Mar 1];139(1):237–43. Available from: https://www.sciencedirect.com/science/article/pii/S030440170600118X. doi: 10.1016/j.vetpar.2006.02.013 [DOI] [PubMed] [Google Scholar]
- 53.Eisler MC, Brandt J, Bauer B, Clausen PH, Delespaux V, Holmes PH, et al. Standardised tests in mice and cattle for the detection of drug resistance in tsetse-transmitted trypanosomes of African domestic cattle. Vet Parasitol [Internet]. 2001. Jun 12 [cited 2022 Mar 1];97(3):171–83. Available from: https://tinyurl.com/yeykxbay. doi: 10.1016/s0304-4017(01)00415-0 [DOI] [PubMed] [Google Scholar]
- 54.Herbert WJ, Lumsden WHR. Trypanosoma brucei: A rapid “matching” method for estimating the host’s parasitemia. Exp Parasitol [Internet]. 1976. Dec 1 [cited 2022 Mar 2];40(3):427–31. Available from: https://tinyurl.com/2p99suu4. doi: 10.1016/0014-4894(76)90110-7 [DOI] [PubMed] [Google Scholar]
- 55.Dirie MF, Abdurahman O. Observations on little known diseases of camels (Camelus dromedarius) in the Horn of Africa. Revue Scientifique et Technique (International Office of Epizootics). 2003. Dec 1;22(3):1043–9. doi: 10.20506/rst.22.3.1456 [DOI] [PubMed] [Google Scholar]
- 56.Jilo K, Abdela N, Dabasa G, Elias M. Camel trypanosomiasis: a review on past and recent research in Africa and Middle East. American-Eurasian J Sci Res. 2017;12(1):13–20. [Google Scholar]
- 57.Anene BM, Onah DN, Nawa Y. Drug resistance in pathogenic African trypanosomes: what hopes for the future? Vet Parasitol [Internet]. 2001. Mar 20 [cited 2022 Mar 1];96(2):83–100. Available from: https://tinyurl.com/yckzaph2. doi: 10.1016/s0304-4017(00)00427-1 [DOI] [PubMed] [Google Scholar]
- 58.Chitambo H, Arakawa A. Trypanosoma congolaise: Manifestation of Resistance to Berenil and Samorin in Cloned Trypanosomes Isolated from Zambian Cattle. Zentralblatt Für Bakteriol [Internet]. 1992. Oct 1 [cited 2022 Mar 1];277(3):371–81. Available from: https://www.sciencedirect.com/science/article/pii/S0934884011809163. [DOI] [PubMed] [Google Scholar]
- 59.Peregrine AS, Mamman M. Pharmacology of diminazene: a review. Acta Trop [Internet]. 1993. Sep 1 [cited 2022 Mar 1];54(3):185–203. Available from: https://www.sciencedirect.com/science/article/pii/0001706X9390092P. doi: 10.1016/0001-706x(93)90092-p [DOI] [PubMed] [Google Scholar]
- 60.Sutherland IA, Peregrine AS, Lonsdale-Eccles JD, Holmes PH. Reduced accumulation of isometamidium by drug-resistant Trypanosoma congolense. Parasitology [Internet]. 1991. Oct [cited 2022 Mar 1];103(2):245–51. Available from: https://tinyurl.com/3f57899d. doi: 10.1017/s0031182000059527 [DOI] [PubMed] [Google Scholar]
- 61.Clausen PH, Sidibe I, Kabore´ I, Bauer B. Development of multiple drug resistance of Trypanosoma congolense in Zebu cattle under high natural tsetse fly challenge in the pastoral zone of Samorogouan, Burkina Faso. Acta Trop [Internet]. 1992. Aug 1 [cited 2022 Mar 1];51(3):229–36. Available from: https://tinyurl.com/yeymapu3. [DOI] [PubMed] [Google Scholar]
- 62.Codjia V, Mulatu W, Majiwa PAO, Leak SGA, Rowlands GJ, Authié E, et al. Epidemiology of bovine trypanosomiasis in the Ghibe valley, southwest Ethiopia. 3. Occurrence of populations of Trypanosoma congolense resistant to diminazene, isometamidium and homidium. Acta Trop [Internet]. 1993. Apr 1 [cited 2022 Mar 1];53(2):151–63. Available from: https://www.sciencedirect.com/science/article/pii/0001706X93900268. doi: 10.1016/0001-706x(93)90026-8 [DOI] [PubMed] [Google Scholar]
- 63.Biswas RK, Hunter AG. Effect of stage of infection with Trypanosoma evansi on cymelarsan therapy. Trop Anim Health Prod U K [Internet]. 1993. [cited 2022 Mar 1]; Available from: https://tinyurl.com/2vtmzus6. doi: 10.1007/BF02250872 [DOI] [PubMed] [Google Scholar]
- 64.Pinder M, Authie E. The appearance of isometamidium resistant Trypanosoma congolense in West Africa. Acta Trop. 1984. Sep 1;41(3):247–52. [PubMed] [Google Scholar]
- 65.Rowlands GJ, Mulatu W, Authié E, d’Ieteren GDM, Leak SGA, Nagda SM, et al. Epidemiology of bovine trypanosomiasis in the Ghibe valley, southwest Ethiopia. 2. Factors associated with variations in trypanosome prevalence, incidence of new infections and prevalence of recurrent infections. Acta Trop [Internet]. 1993. Apr 1 [cited 2022 Mar 1];53(2):135–50. Available from: https://tinyurl.com/ypyh4z6n. doi: 10.1016/0001-706x(93)90025-7 [DOI] [PubMed] [Google Scholar]
- 66.Homeida AM, El Amin EA, Adam SEI, Mahmoud MM. Toxicity of diminazene aceturate (Berenil) to camels. J Comp Pathol [Internet]. 1981. Jul 1 [cited 2022 Mar 1];91(3):355–60. Available from: https://www.sciencedirect.com/science/article/pii/0021997581900050. doi: 10.1016/0021-9975(81)90005-0 [DOI] [PubMed] [Google Scholar]
- 67.Aliu YO, Odegaard S, Søgnen E. Diminazene/Berenil: bioavailability and disposition in dairy goats. Acta Vet Scand. 1984;25(4):593–6. doi: 10.1186/BF03546926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rushigajiki PKB, Mayende JSP, Guloba A, Wilson AJ. Maintenance of a herd of breeding cattle in an area of trypanosome challenge. Bull Anim Health Prod Afr. 1986;34:149–55. [Google Scholar]
- 69.Bourdichon AJ. Report on the Use of the Trypanocidal Drug ″TRYPAN ″. J Protozool Res. 1998;8(4):258–62. [Google Scholar]
- 70.Nneka Chizoba Enwezor F, Kojo Bedu Sackey A. Camel trypanosomosis—a review. Vet Arh [Internet]. 2005. Oct 20 [cited 2022 Mar 2];75(5):439–52. Available from: https://hrcak.srce.hr/clanak/50720. [Google Scholar]
- 71.Maina N, Ngotho JM, Njiru ZK, Karanja WM, Gem CO, Karanja S, et al. Efficacy of Trypan® (diminazene di-aceturate) in camels infected with trypanosoma evansi. J Camel Pract Res. 2003. Jun 1;10:51–5. [Google Scholar]
- 72.Whiteside EF. The maintenance of cattle in tsetse-infested country. A summary of four years’ experience in Kenya. Maint Cattle Tsetse-Infested Ctry Summ Four Years Exp Kenya [Internet]. 1960. [cited 2022 Mar 1];(41). Available from: https://www.cabdirect.org/cabdirect/abstract/19601000443. [Google Scholar]
- 73.Mamoudou A, Delespaux V, Chepnda V, Hachimou Z, Andrikaye JP, Zoli A, et al. Assessment of the occurrence of trypanocidal drug resistance in trypanosomes of naturally infected cattle in the Adamaoua region of Cameroon using the standard mouse test and molecular tools. Acta Trop [Internet]. 2008. May 1 [cited 2022 Mar 1];106(2):115–8. Available from: https://www.sciencedirect.com/science/article/pii/S0001706X08000466. doi: 10.1016/j.actatropica.2008.02.003 [DOI] [PubMed] [Google Scholar]
- 74.Elhadi YA, Nyariki DM, Wasonga OV. Role of camel milk in pastoral livelihoods in Kenya: contribution to household diet and income. Pastoralism [Internet]. 2015. Apr 20 [cited 2022 Mar 2];5(1):8. Available from: 10.1186/s13570-015-0028-7. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
Data Availability Statement
Data files used in the paper are available from the Mendeley data and can be accessed here: https://data.mendeley.com/datasets/b64fg8zbyg/1 and https://tinyurl.com/ynnk7ans.