Abstract
Background:
Sodium–glucose cotransporter 2 (SGLT2) inhibitors have important kidney and cardiovascular benefits in adults with chronic kidney disease. Among adults with diabetes, we characterized the prevalence of chronic kidney disease eligible for SGLT2 inhibitor treatment, based on definitions of eligibility from trials and diabetes guidelines, and assessed the predictors of SGLT2 inhibitor use.
Methods:
We conducted a cross-sectional study using linked administrative data from Alberta Health in adults with diabetes (2002–2019). Chronic kidney disease was defined as an estimated glomerular filtration rate (eGFR) less than 90 mL/min/1.73 m2 with severe or greater proteinuria (trial-based definition); or eGFR less than 60 mL/min/1.73 m2 or moderate or greater proteinuria regardless of eGFR (diabetes guideline–based definition). Predictors (sociodemographic characteristics, comorbidities and health care utilization) of SGLT2 inhibitor use were identified using logistic regression.
Results:
Of 446 315 adults with diabetes, 76 630 (17.2%, guideline-based definition; 12 867 [2.9%], trial-based definition) had chronic kidney disease eligible for SGLT2 inhibitor treatment. A total of 7.1% used SGLT2 inhibitors. Older age, lower hemoglobin A1c (HbA1c) levels, female sex, lower neighbourhood income, rural residence and hospital admission were among variables associated with nonuse of SGLT2 inhibitors (adjusted odds ratios [ORs] from 0.13 [age ≥ 85 yr] to 0.92 [rural residence], p < 0.05). Family physician visits were associated with higher SGLT2 inhibitor use (adjusted OR 4.01, p < 0.001 for > 4 visits/yr). Considering all adults, both with and without diabetes, 162 012 individuals with chronic kidney disease (5% of all Alberta adults) may benefit from treatment with SGLT2 inhibitors.
Interpretation:
Many adults with chronic kidney disease would derive heart and kidney benefits from treatment with SGLT2 inhibitors but had low SGLT2 inhibitor use as of 2019. Efforts will be needed to address lower use of SGLT2 inhibitors among female, older and lower-income adults, and to enhance primary care and promote awareness of the benefits of SGLT2 inhibitors independent of glycemic control.
Chronic kidney disease is a risk factor for end-stage renal disease and is associated with worse cardiovascular outcomes.1 The CREDENCE (Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation) and DAPA-CKD (Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease) trials showed that sodium–glucose cotransporter 2 (SGLT2) inhibitors reduced clinically meaningful kidney outcomes and cardiovascular events in adults both with2,3 and without diabetes.3 Diabetes guidelines now recommend SGLT2 inhibitors for end-organ protection in patients with chronic kidney disease.4–6 Similar recommendations are expected in adults without diabetes, particularly if similar results are observed in the forthcoming EMPA-KIDNEY trial of empagliflozin.7
As SGLT2 inhibitors are already indicated in adults with diabetes for glycemic control and prevention of atherosclerotic cardiovascular disease and heart failure, the incremental impact of the chronic kidney disease indication for SGLT2 inhibitors among those with diabetes is unknown. In this context, we define the “chronic kidney disease indication” for SGLT2 inhibitors specifically as meeting trial-and guideline-based criteria for cardiorenal benefit from SGLT2 inhibitors on the basis of estimated glomerular filtration rate (eGFR) or proteinuria.
The Alberta Kidney Health Strategic Clinical Network8 has identified improving SGLT2 inhibitor use in adults with chronic kidney disease as an emerging clinical priority. As a first step in these efforts, we examined a cross section of adults with diabetes, to answer the following questions:
What is the prevalence of adults with chronic kidney disease eligible for SGLT2 inhibitor treatment?
Among adults with chronic kidney disease, are sociodemographic factors, health status, diabetes status and health care utilization associated with SGLT2 inhibitor use? We examined predictors of SGLT2 inhibitor use to identify potential directions and opportunities to accelerate use in adults with chronic kidney disease.
What is the prevalence of adults with chronic kidney disease eligible for SGLT2 inhibitor treatment in the general Alberta population, both with and without diabetes? The prevalence of the chronic kidney disease indication in all Albertan adults, regardless of diabetes status, will foreshadow the magnitude of the knowledge translation challenge to come.
Methods
We performed a cross-sectional study using data from the Alberta Kidney Disease Network, composed of linked administrative databases of Alberta Health over the period Apr. 1, 2002, to Mar. 31, 2019 (Appendix 1, available at www.cmajopen.ca/content/11/1/E101/suppl/DC1).9
Defining SGLT2 inhibitor eligibility and the chronic kidney disease indication for SGLT2 inhibitors
Both dapagliflozin and canagliflozin have formal indications for chronic kidney disease; dapagliflozin’s indication covers adults both with and without diabetes. These indications are well supported by clinical trials and have been adopted by the most recent Canadian guidelines. Notably, the kidney trials of dapagliflozin and canagliflozin included exclusively patients with severe proteinuria, whereas diabetes guidelines,5,6 the KDIGO (Kidney Disease: Improving Global Outcomes) diabetic kidney disease guideline, 4 and the Health Canada indication apply to “chronic kidney disease” generally, presumably on the basis of evidence from cardiovascular trials showing renal benefits regardless of chronic kidney disease stage and proteinuria.10,11 Thus, we defined the chronic kidney disease indication as (A) eGFR of 25 mL/min/1.73 m2 or greater and less than 90 mL/min/1.73 m2 with evidence of severe or greater proteinuria (KDIGO A3, equivalent to urine albumin:creatinine ratio [UACR] ≥ 30 mg/mmol), reflecting the kidney trial inclusion criteria; and (B) eGFR of 25 mL/min/1.73 m2 or greater and less than 60 mL/min/1.73 m2, or evidence of moderate or greater proteinuria (KDIGO A2, equivalent to UACR ≥ 3 mg/mmol), reflecting the broader guideline-based definition of chronic kidney disease. Subindication (A) was nested in (B), the latter being more inclusive. The prevalence of adults with chronic kidney disease eligible for SGLT2 inhibitor treatment by both definitions is reported, with subsequent analyses (i.e., description of adults with chronic kidney disease, predictors of SGLT2 inhibitor use) using indication (B).
Inclusion and exclusion criteria
The base cohort included all Alberta adults with diabetes on Mar. 31, 2019, with at least 1 serum creatinine measurement between Apr. 1, 2002, and the index date. Diabetes status was determined using an established administrative data definition, 12,13 which continues to demonstrate good sensitivity (88%) and specificity (98%).14 We additionally classified patients with 1 or more hemoglobin A1c (HbA1c) measurements of 6.5% or greater,15 or 1 or more pharmacy dispensations for insulin, as having diabetes. Patients with most recent eGFR less than 25 mL/min/1.73 m2, end-stage renal failure on dialysis, diagnostic codes specifying type 1 diabetes, or no indicators of proteinuria since 2002 were excluded.
Identifying adults with chronic kidney disease
Estimated glomerular filtration rate was estimated from serum creatinine using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equations and categorized based on the most recent serum creatinine measurement, with at least 2 consecutive measurements meeting these criteria more than 90 days apart. Proteinuria was categorized using the most recent community UACR, urine protein:creatinine ratio, or semiquantitative urine dipstick interchangeably (Appendix 1, Supplement Table S1).
SGLT2 inhibitor use and other study variables
In Alberta, pharmacies report medication dispensations at the point of sale.16 Patients were considered current users of SGLT2 inhibitors if, from their most recent dispensation, the days supplied plus an additional 30 days for stockpile covered Mar. 31, 2019.
Other conditions for which SGLT2 inhibitors are indicated — coronary artery disease, stroke and heart failure — were defined in adults with chronic kidney disease using validated definitions (Appendix 1, Supplement Table S2).17 Explanatory variables were sociodemographic quantities, diabetes indicators, other comorbidities,17,18 Elixhauser Comorbidity Index summary scores19 and health care utilization (family physician visits, specialist visits and hospitalizations) in the preceding year.
Analysis
The characteristics of included adults were described with means and standard deviations (SDs), and counts and percentages. We reported the prevalence of adults with chronic kidney disease eligible for SGLT2 inhibitor treatment and their prevalence of SGLT2 inhibitor use. The association of various characteristics on current SGLT2 inhibitor use was determined using logistic regression in adults with chronic kidney disease and diabetes, with variables selected on the basis of a priori considerations that were based on our review of the literature20 and clinical experience. Finally, we broadened our focus to identify all adults in Alberta with 1 or more serum creatinine value since Apr. 1, 2002, who met the chronic kidney disease indication for SGLT2 inhibitors (indication [A] or [B]), including adults both with or without diabetes. The prevalence of adults with chronic kidney disease eligible for SGLT2 inhibitor treatment in Alberta was calculated using the census-derived adult population of Alberta (3.5 million) as a denominator. The analysis was performed in Stata version 17 (Stata Corp).
Ethics approval
This study was approved by the research ethics boards at the University of Alberta and University of Calgary.
Results
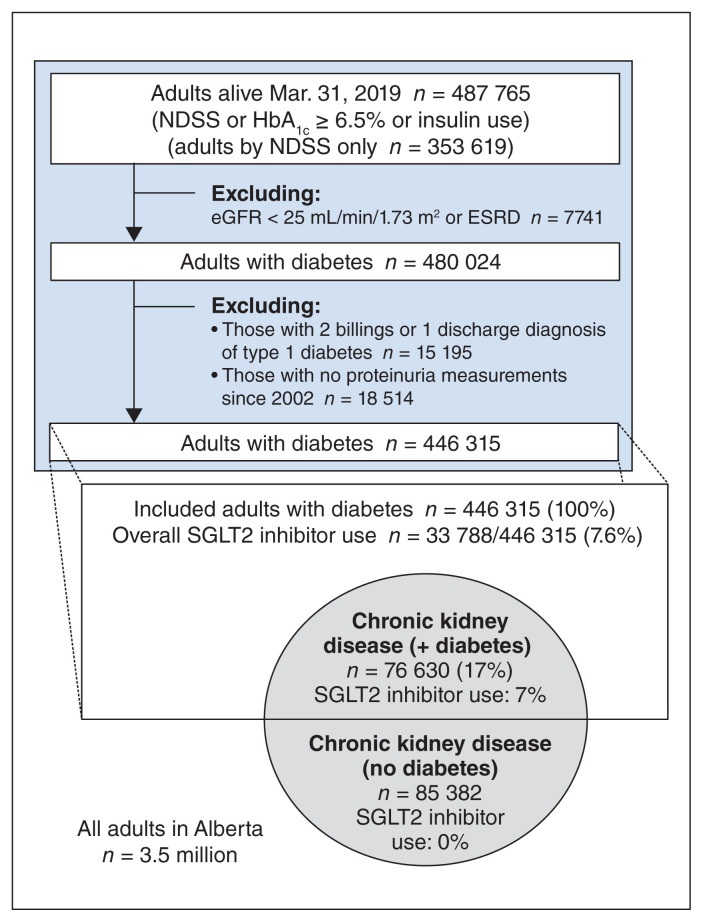
We identified 446 315 adults with diabetes (Figure 1). Whereas 12 867 adults (2.9%) with diabetes with renal indices met the inclusion criteria for the SGLT2 inhibitor renal outcome trials (indication [A]), a larger number of adults — 76 630 (17.2% of all adults with diabetes) — met the broader guideline-based definition of chronic kidney disease (indication [B]) used to guide initiation of SGLT2 inhibitors (Table 1).
Figure 1:
Adults with diabetes and prevalence of adults with chronic kidney disease eligible for SGLT2 inhibitor treatment (per definitions in Table 1). Note: eGFR = estimated glomerular filtration rate; ESRD = end-stage renal disease; HbA1c = hemoglobin A1c; NDSS = National Diabetes Surveillance System, referring to a well-accepted administrative-database case definition for diabetes; SGLT2 = sodium–glucose cotransporter 2. Encircled areas show SGLT2 inhibitor indications, in the style of a Venn diagram, and are not drawn to scale.
Table 1:
Indications for sodium–glucose cotransporter 2 inhibitors in adults with diabetes, focusing on chronic kidney disease*
| Indication | No. of adults with the indicated condition (proportion of all adults with diabetes) | No. of SGLT2 inhibitor users (proportion of SGLT2 inhibitor users, out of adults with the specified indication) |
|---|---|---|
| All adults with diabetes | 446 315 (100.0) | 33 788/446 315 (7.6) |
| Adults with chronic kidney disease eligible for SGLT2 inhibitors | ||
| (A) Chronic kidney disease stage ≥ 2† and at least severe proteinuria | 12 867 (2.9) | 988/12 867 (7.7) |
| (B) Chronic kidney disease stage ≥ 3† or at least moderate proteinuria | 76 630 (17.2) | 5460/76 630 (7.1) |
| Other conditions indicating SGLT2 inhibitor treatment (cardiovascular disease and heart failure) | ||
| Cardiovascular disease + heart failure | 126 453 (28.3) | 11 037/126 453 (8.7) |
| Cardiovascular disease + heart failure + chronic kidney disease | 165 617 (37.1) | 13 545/165 617 (8.2) |
Note: eGFR = estimated glomerular filtration rate, KDIGO = Kidney Disease: Improving Global Outcomes, SGLT2 = sodium–glucose cotransporter 2.
Two definitions of chronic kidney disease were considered, labelled (A) and (B). Indication (A) is a subset of (B). Indication (A) reflects trial inclusion criteria of the CREDENCE (Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation) and DAPA-CKD (Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease) trials, and indication (B) reflects a broader definition of chronic kidney disease for SGLT2 inhibitor eligibility adopted in the Diabetes Canada and KDIGO (Kidney Disease: Improving Global Outcomes) diabetic kidney disease guidelines. Cardiorenal benefits have been shown in kidney trials enrolling adults meeting criteria (A). Regarding criteria (B), cardiorenal benefits are extrapolated from the kidney trials and have also been shown directly in subgroup analyses of cardiovascular trials showing renal benefit in adults with chronic kidney disease not meeting criteria (A).
Chronic kidney disease stage ≥ 2 refers to eGFR < 90 mL/min/1.73 m2. Chronic kidney disease stage ≥ 3 refers to eGFR ≥ 30 and < 60 mL/min/1.73 m2.
As expected, adults with chronic kidney disease eligible for SGLT2 inhibitor treatment (definition [B]) were older, were more likely to have comorbidities and had more frequent health care utilization than the general population of adults with diabetes (not shown). Among adults with chronic kidney disease, there was a roughly even split of males and females, with a mean age of 74 (SD 12) years (Table 2). Mean eGFR was 55 mL/min/1.73 m2. Average frequency of family physician visits was 7.5 visits per year, and contact with medical specialists was less frequent (0.8 visits/yr). A substantial minority (15.2%) had been hospitalized in the previous year. Notably, most adults with chronic kidney disease eligible for SGLT2 inhibitor treatment had HbA1c levels of 7.0% or less (56.5%). Aside from HbA1c measurements, which were missing in about 12 000 individuals, missing data was less than 3%.
Table 2:
Characteristics of Alberta adults with diabetes and chronic kidney disease, stratified by use of sodium–glucose cotransporter 2 inhibitors
| Variable | No. (%)* | p value | ||
|---|---|---|---|---|
| Diabetes + chronic kidney disease n = 76 630 |
No SGLT2 inhibitor use n = 71 170 |
SGLT2 inhibitor use n = 5460 |
||
| Sociodemographic characteristics | ||||
| Age, yr, mean ± SD | 74 ± 12 | 74 ± 13 | 68 ± 9 | < 0.001 |
| Sex, female | 35 932 (46.9) | 34 085 (47.9) | 1847 (33.8) | < 0.001 |
| Residence, rural† | 10 961 (14.3) | 10 217 (14.4) | 744 (13.6) | 0.1 |
| Neighbourhood income quintile | n = 74 649 | n = 69 369 | n = 5280 | |
| 1 (lowest) | 18 721 (25.1) | 17 485 (25.2) | 1236 (23.4) | 0.02 |
| 2 | 16 678 (22.3) | 15 510 (22.4) | 1168 (22.1) | |
| 3 | 14 733 (19.7) | 13 669 (19.7) | 1064 (20.2) | |
| 4 | 13 425 (18.0) | 12 416 (17.9) | 1009 (19.1) | |
| 5 (highest) | 11 092 (14.9) | 10 289 (14.8) | 803 (15.2) | |
| Renal function | ||||
| Serum creatinine, μmol/L, mean ± SD | 108 ± 28 | 108 ± 29 | 105 ± 25 | < 0.001 |
| eGFR (CKD-EPI), mean ± SD | 55 ± 16 | 55 ± 16 | 61 ± 15 | < 0.001 |
| Chronic kidney disease stage by eGFR‡ | ||||
| None or stage 1 | 0 (0) | 0 (0) | 0 (0) | < 0.001 |
| Stage 2 | 29 085 (38.0) | 26 133 (36.7) | 2952 (54.1) | < 0.001 |
| Stage 3 | 46 297 (60.4) | 43 805 (61.5) | 2492 (45.6) | |
| Stage 4 | 1248 (1.6) | 1232 (1.7) | 16 (0.3) | |
| Proteinuria | ||||
| None or mild | 32 305 (42.2) | 30 759 (43.2) | 1546 (28.3) | < 0.001 |
| Moderate | 31 458 (41.1) | 28 532 (40.1) | 2926 (53.6) | |
| Severe | 12 038 (15.7) | 11 144 (15.7) | 894 (16.4) | |
| Nephrotic | 829 (1.1) | 735 (1.0) | 94 (1.7) | |
| Angiotensin-converting-enzyme inhibitor or angiotensin receptor blocker, current use | ||||
| Yes | 47 264 (61.7) | 42 604 (60.0) | 4660 (85.3) | < 0.001 |
| Diabetes | ||||
| HbA1c, %, mean ± SD | 7.2 ± 1.5 | 7.1 ± 1.5 | 1.5 ± 8.1 | < 0.001 |
| HbA1c, % | n = 64 477 | n = 59 017 | ||
| ≤ 7.0 | 36 425 (56.5) | 35 263 (59.8) | 1162 (21.3) | < 0.001 |
| 7.1–9.0 | 21 353 (33.1) | 18 152 (30.8) | 3201 (58.6) | |
| > 9.0 | 6699 (10.4) | 5602 (9.5) | 1097 (20.1) | |
| HbA1c missing | 12 153 (–) | 12 153 (–) | 0 (–) | – |
| Insulin intensity | ||||
| None | 60 365 (78.8) | 57 477 (80.8) | 2888 (52.9) | < 0.001 |
| Basal only | 7028 (9.2) | 5708 (8.0) | 1320 (24.2) | |
| Bolus ± basal | 9237 (12.1) | 7985 (11.2) | 1252 (22.9) | |
| Comorbidities | ||||
| Coronary artery disease | 25 896 (33.8) | 23 822 (33.5) | 2074 (38.0) | < 0.001 |
| Stroke§ | 15 006 (19.6) | 14 153 (19.9) | 853 (15.6) | < 0.001 |
| Heart failure | 15 127 (19.7) | 14 327 (20.1) | 800 (14.7) | < 0.001 |
| Elixhauser Comorbidity Index, mean ± SD | 16 ± 11 | 16 ± 11 | 13 ± 10 | < 0.001 |
| Health care utilization | ||||
| Family physician, no. of visits, mean ± SD | 6.9 ± 7.5 | 6.8 ± 7.6 | 7.2 ± 5.4 | 0.001 |
| Family physician visits, ≥ 1 | 68 675 (89.6) | 63 335 (89.0) | 5340 (987) | < 0.001 |
| Family physician visits, frequency, visits | ||||
| 0 | 7955 (10.4) | 7835 (11.0) | 120 (2.2) | < 0.001 |
| 1–4 | 25 728 (33.6) | 23 990 (33.7) | 1738 (31.8) | |
| > 4 | 42 947 (56.0) | 39 345 (55.3) | 3602 (66.0) | |
| Internal medicine physician, no. of visits, mean ± SD | 0.5 ± 1.3 | 0.4 ± 1.3 | 0.8 ± 1.6 | < 0.001 |
| Internal medicine physician visits, ≥ 1 | 16 415 (21.4) | 14 608 (20.5) | 1807 (33.1) | < 0.001 |
| Cardiologist, no. of visits, mean ± SD | 0.2 ± 0.7 | 0.2 ± 0.7 | 0.3 ± 0.8 | < 0.001 |
| Cardiologist visits, ≥ 1 | 8976 (11.7) | 8137 (11.4) | 839 (15.4) | < 0.001 |
| Endocrinologist, no. of visits, mean ± SD | 0 ± 0.3 | 0 ± 0.3 | 0.1 ± 0.7 | < 0.001 |
| Endocrinologist visits, ≥ 1 | 1158 (1.5) | 858 (1.2) | 300 (5.5) | < 0.001 |
| Nephrologist, no. of visits, mean ± SD | 0.1 ± 0.5 | 0.1 ± 0.5 | 0.1 ± 0.5 | 0.06 |
| Nephrologist visits, ≥ 1 | 6219 (8.1) | 5831 (8.2) | 388 (7.1) | < 0.001 |
| Hospitalization, ≥ 1 | 11 654 (15.2) | 11 018 (15.5) | 636 (11.6) | < 0.001 |
Note: CKD-EPI = Chronic Kidney Disease Epidemiology Collaboration, eGFR = estimated glomerular filtration rate, HbA1c = hemoglobin A1c, KDIGO = Kidney Disease: Improving Global Outcomes, SD = standard deviation, SGLT2 = sodium–glucose cotransporter 2.
Unless stated otherwise.
Rural residence determined by postal code linkage to Statistics Canada population centres or rural area classification. Rural areas defined by Statistics Canada as having a population less than 1000 or a population density of < 400 people per square kilometre. Owing to imprecision in postal code mapping, in some instances, mode of mail delivery may proxy for rural residence.
Chronic kidney disease stage definitions: none or stage 1 (eGFR ≥ 90), stage 2 (eGFR 60–89), stage 3 (eGFR 30–59), stage 4 (eGFR 15–29), with eGFR determined from serum creatinine measurements using CKD-EPI equations.
Stroke: includes hemorrhagic as well as ischemic intracerebral events (e.g., subarachnoid hemorrhage, ischemic stroke).
Though 17.2% of adults with diabetes met chronic kidney disease criteria for treatment with SGLT2 inhibitors, many of them already had cardiovascular disease or heart failure (Table 1). Among all adults with diabetes, the combination of cardiovascular disease and heart failure together identified 28.3% of adults with diabetes as having clinical benefit from SGLT2 inhibitors. The total prevalence of adults with diabetes with cardiorenal benefit from SGLT2 inhibitors after including chronic kidney disease (definition [B]) was 37.1%. Thus, chronic kidney disease increased eligibility for SGLT2 inhibitor treatment by an absolute increment of 8.8% in adults with diabetes.
Factors associated with use of SGLT2 inhibitors
The overall rate of SGLT2 inhibitor use was 7.1% in those with chronic kidney disease (7.6% among all adults with diabetes) (Figure 1, Table 1). The multivariable logistic regression model of predictors of SGLT2 inhibitor use included 62 823 individuals with complete data on all variables (Table 3). The strongest associations with SGLT2 inhibitor use were observed for HbA1c level, age and frequency of family physician contact. Those with HbA1c measurements of 7.0% or lower had lower odds of current use of SGLT2 inhibitors than those with HbA1c measurements between 7.1% and 9.0% (adjusted odds ratio [OR] 0.24). Insulin use was also associated with higher odds of SGLT2 inhibitor use. In terms of other indications for SGLT2 inhibitors, coronary artery disease increased the odds of use, but heart failure and stroke were associated with lower use of SGLT2 inhibitors.
Table 3:
Predictors of sodium–glucose cotransporter 2 inhibitor use in eligible adults with chronic kidney disease and diabetes*
| Variable | Crude OR (95% CI) | Adjusted OR† (95% CI) |
|---|---|---|
| Sociodemographic characteristics | ||
| Sex, female | 0.56 (0.52–0.59) | 0.75 (0.70–0.80) |
| Age, yr | ||
| ≤ 44 | 0.22 (0.17–0.30) | 0.61 (0.45–0.82) |
| 45–54 | 0.84 (0.75–0.94) | 1.08 (0.95–1.24) |
| 55–64 (Ref.) | 1.00 | 1.00 |
| 65–74 | 0.82 (0.76–0.88) | 0.79 (0.73–0.85) |
| 75–84 | 0.34 (0.31–0.37) | 0.39 (0.35–0.42) |
| ≥ 85 | 0.08 (0.07–0.09) | 0.13 (0.11–0.16) |
| Rural residence | 0.94 (0.87–1.02) | 0.92 (0.81–1.00) |
| Neighbourhood income quintile | ||
| 1 | 0.91 (0.83–0.99) | 0.82 (0.74–0.91) |
| 2 | 0.96 (0.88–1.06) | 0.93 (0.84–1.03) |
| 3 | 1.00 (0.91–1.10) | 0.94 (0.85–1.04) |
| 4 | 1.04 (0.95–1.15) | 0.98 (0.88–1.08) |
| 5 (Ref.) | 1.00 | 1.00 |
| Comorbidities and diabetes | ||
| Heart failure | 0.68 (0.63–0.74) | 0.84 (0.77–0.93) |
| Coronary artery disease | 1.24 (1.16–1.33) | 1.24 (1.16–1.33) |
| Stroke | 0.75 (0.69–0.80) | 0.91 (0.84–0.99) |
| Elixhauser Comorbidity Index (per 5 units) | 0.92 (0.90–0.93) | 0.92 (0.90–0.93) |
| HbA1c, % | ||
| ≤ 7.0 | 0.19 (0.17–0.20) | 0.24 (0.23–0.26) |
| > 7.0 and ≤ 9.0 (Ref.) | 1.00 | 1.00 |
| > 9.0 | 1.11 (1.03–1.20) | 0.92 (0.85–1.00) |
| Insulin | ||
| Basal insulin only | 4.60 (4.29–4.94) | 1.96 (1.81–2.12) |
| Bolus ± basal insulin | 3.12 (2.91–3.35) | 1.19 (1.10–1.29) |
| Health care utilization in the previous year | ||
| Family physician visits | ||
| No family physician visits (Ref.) | 1.00 | 1.00 |
| 2–4 | 4.73 (3.92–5.70) | 3.25 (2.67–3.97) |
| > 4 | 5.98 (4.98–7.18) | 4.01 (3.30–4.87) |
| Nephrologist, ≥ 1 visit | 0.86 (0.77–0.95) | 0.60 (0.53–0.67) |
| Cardiologist, ≥ 1 visit | 1.41 (1.30–1.52) | 1.25 (1.14–1.37) |
| Internist, ≥ 1 visit | 1.92 (1.81–2.03) | 1.63 (1.52–1.74) |
| Endocrinologist, ≥ 1 visit | 4.76 (4.17–5.45) | 2.64 (2.27–3.08) |
| Hospital admission, ≥ 1 admission | 0.72 (0.66–0.78) | 0.64 (0.58–0.71) |
Note: CI = confidence interval, HbA1c = hemoglobin A1c, OR = odds ratio, Ref. = reference group or level, SGLT2 = sodium–glucose cotransporter 2.
Logistic regression including 62 823 of 76 630 adults with diabetes and chronic kidney disease eligible for SGLT2 inhibitor treatment (definition [B] in Table 1).
Adjusted ORs from a multivariable logistic regression model adjusting for every other variable seen in this table. In crude analysis, all p < 0.001 except age 45–54 years (v. 55–64 yr, p = 0.004); rural residence (p = 0.1), neighbourhood income quintiles 2, 3 and 4 (v. quintile 5, p = 0.5, p > 0.9 and p = 0.4, respectively); HbA1c level > 9.0% (v. > 7.0 and ≤ 9.0%, p = 0.006); and ≥ 1 nephrology visit (p = 0.005). In multivariable analysis, all p < 0.001 except rural residence (p = 0.046); age 45–54 years (v. 55–64 yr, p = 0.2); neighbourhood income quintiles 2, 3 and 4 (v. quintile 5, p = 0.2, p = 0.2 and p = 0.6, respectively); stroke (p = 0.03); and HbA1c level > 9.0% (v. > 7.0 and ≤ 9.0%, p = 0.04).
The relation between age and use of SGLT2 inhibitors was nonlinear, with reduced use observed among young individuals and among patients aged 65 years or older, but particularly at the upper extreme of age, with ORs as low as 0.13 (age ≥ 84 yr v. 55–64 yr). Adults in lower quintiles of neighbourhood income also had lower odds of SGLT2 inhibitor use, with a gradient observed from highest to lowest income quintile, the lowest income quintile being associated with an adjusted 0.82-fold reduced odds of SGLT2 inhibitor use compared with the highest. Among other sociodemographic variables, female sex and rural residence were associated with reduced odds of SGLT2 inhibitor use (OR 0.75 and 0.92, respectively).
Patients were more likely to be using SGLT2 inhibitors with more frequent family physician exposure (OR 4.01 with > 4 visits). Having seen a nephrologist in the previous year was associated with reduced SGLT2 inhibitor use, and having seen a cardiologist, internist or endocrinologist increased the odds of use. Hospital admission was associated with lower odds of SGLT2 inhibitor use (OR 0.64). All of the above associations were significant at p < 0.001 and were similar in models featuring all adults with diabetes (Appendix 1, Supplement Table S3), suggesting that the trends identified here are not specific to chronic kidney disease.
Prevalence of chronic kidney disease (with and without diabetes) eligible for SGLT2 inhibitors among all Alberta adults
Among adults without diabetes, we identified 85 382 adults (Figure 1) who met the chronic kidney disease indication for treatment with SGLT2 inhibitors (indication [B]); 8716 of these individuals had severe proteinuria (indication [A]). Combined with the 76 630 similar adults with diabetes, the total number of Alberta adults who would have clinical benefits from SGLT2 inhibitors for chronic kidney disease was 162 012, representing about 5% of Alberta’s census-derived adult population of 3.5 million.
Interpretation
We examined a provincial cross section of adults with diabetes. Among them, 17.2% had chronic kidney disease and were eligible for SGLT2 inhibitor treatment (an increment of 8.8% when considered in addition to well-established cardiovascular disease and heart failure indications for SGLT2 inhibitors), yet only 7.1% of these individuals were using SGLT2 inhibitors. The CREDENCE trial had not yet been published. This study was therefore not meant to be evaluative but, rather, to identify and explore this gap between current prescribing and the emerging evidence of cardiorenal benefit in chronic kidney disease.
The extent to which the kidney benefits of SGLT2 inhibitors generalize to adults with less than severe proteinuria, who were not included in the kidney trials (CREDENCE, DAPA-CKD) of these agents, is unclear. A more limited definition based on kidney trial inclusion criteria produces a lower prevalence of adults with chronic kidney disease eligible for treatment with SGLT2 inhibitors. However, since secondary analyses of the cardiovascular trials indicate kidney benefit regardless of proteinuria, most guidelines use a broader definition of chronic kidney disease eligibility for SGLT2 inhibitors.
We observed a steady decline in SGLT2 inhibitor use beyond age 65 years, which includes most adults with chronic kidney disease. SGLT2 inhibitors are publicly funded in Alberta for all adults aged 65 years and older by special authorization, primarily for hyperglycemia.21 Indeed, meeting glycemic control targets (HbA1c level ≤ 7.0%) was associated with a fourfold reduced odds of SGLT2 inhibitor use, consistent with the origin of SGLT2 inhibitors as anti-hyperglycemic agents. A second explanation for lower SGLT2 inhibitor use in older adults may be the perception of increased risk of adverse events.22 SGLT2 inhibitors do increase the risk of euglycemic diabetic ketoacidosis and, debatably, lower limb amputations (hazard ratios ~2–3), though the absolute background risks of these events are low (< 5/1000 patient years).23–25 These adverse events are probably less of a barrier for adults with chronic kidney disease than concerns about orthostatic hypotension, acute kidney injury and urinary tract infections, despite evidence showing no association between SGLT2 inhibitor use and the latter two.26–28 It will be important not to shortchange older adults with cardiac and kidney comorbidities, who will benefit the most, in absolute terms, from SGLT2 inhibitors. Efforts will be needed to facilitate access to SGLT2 inhibitors for adults with chronic kidney disease, irrespective of baseline glycemic control, and to promote the understanding that these agents should be prescribed as kidney and heart medications.5,22
Individuals residing in lower income neighbourhoods were less likely to be users of SGLT2 inhibitors. Reasons for this may include less access to employment-derived drug benefits and competing acute issues.29 Those of rural residence were slightly less likely to receive SGLT2 inhibitors. Women were also less likely to be prescribed SGLT2 inhibitors. Sex-based disparities exist with other cardiovascular risk–reducing medications, 30 though for SGLT2 inhibitors, the disparity may simply be due to genital mycotic infections. Equitable access to SGLT2 inhibitors will be an important consideration for quality improvement.31
Family physician exposure was associated with higher SGLT2 inhibitor use, and family physician contacts were much more frequent than specialist contacts. Efforts to improve use of SGLT2 inhibitors in adults with a chronic kidney disease indication will largely depend on enhancing patient exposure to primary care providers alongside their specialist colleagues. Prescriber education and quality-improvement initiatives will be needed to accelerate the evidence-based uptake of SGLT2 inhibitors among those with chronic kidney disease.22 Hospital discharge may be an important opportunity to recommend or prescribe SGLT2 inhibitors for a substantial minority of adults.32,33 Notably, the above associations were similar in the wider group of all adults with diabetes, indicating general applicability beyond chronic kidney disease.
Limitations
First, missing HbA1c levels occurred in 15.9% of adults with diabetes and chronic kidney disease eligible for treatment with SGLT2 inhibitors. This likely represents a sizeable minority of adults with known diabetes who simply do not attend medical care. Their exclusion from the multivariable analysis of predictors of SGLT2 inhibitor use means that results may generalize best to adults who have at least minimal contact with medical care for diabetes management. Second, about one-third of proteinuria measurements were more than 2 years old. The prevalence of indications for SGLT2 inhibitors will depend on how conscientiously they are sought out and may be higher than estimated here. Third, our cross-sectional analysis examines numerous variables, raising the overall risk of type 2 error. The associations identified should be evaluated on their strength and plausibility, and provide a starting point from which to understand SGLT2 inhibitor prescribing. Finally, these data precede the formal indication of chronic kidney disease as an indication for SGLT2 inhibitors, but our study is intended to provide anticipatory insights relevant to the eventual rollout of SGLT2 inhibitors for as many as 5% of all adults.
Conclusion
Among adults with diabetes in Alberta, a substantial proportion (17.2%) met the chronic kidney disease indication for treatment with SGLT2 inhibitors. However, rates of SGLT2 inhibitor use were low (7.1%) during our study period. Barriers to SGLT2 inhibitor use will be important to address as SGLT2 inhibitors are recast as kidney and heart medications, indicated equally for adults with and without diabetes. In Alberta, at least 5% of the total adult population (162 012 individuals) met the chronic kidney disease indication and would benefit from treatment with SGLT2 inhibitors. Future efforts will need to address SGLT2 inhibitor use among older adults, women and those in lower income quintiles (including modifications to restrictive public drug insurance criteria), and promote the new understanding that SGLT2 inhibitors are indicated for end-organ protection regardless of diabetes status or glycemic control.
Supplementary Material
Acknowledgement
This work was produced with in-kind support for new investigators from the Alberta Kidney Disease Network.
Footnotes
Competing interests: Roseanne Yeung has previously received funding from the Novo Nordisk Alberta Diabetes Fund, a research grant administered by the University Hospital Foundation and Alberta Economic Development and Trade. Nairne Scott-Douglas has previously received research funding from Amgen and Bristol Myers Squibb. He has also received speaking honoraria and consulting fees from Amgen, Alexion, AstraZeneca, Janssen Pharmaceuticals, Novartis and Otsuka Pharmaceutical. Scott Klarenbach is co–scientific director of the Kidney Health Section of the Medicine Strategic Clinical Network, Alberta Health Services. No other competing interests were declared.
This article has been peer reviewed.
Contributors: Darren Lau (corresponding author) conceived the study, analyzed the data and wrote the manuscript. Scott Klarenbach obtained the data. All authors made substantial contributions to the analysis and interpretation of data, revised the work critically for important intellectual content, approved the final version of the manuscript, and agreed to be accountable for all aspects of the manuscript.
Funding: This study received funding from the Kidney Health Section of the Alberta Medicine Strategic Clinical Network.
Data sharing: The data used in this study are not available to others, and will not be made available to others, since it is governed by confidentiality and data-sharing agreements with Alberta Health via the Interdisciplinary Chronic Disease Collaboration and Alberta Kidney Disease Network. Further analyses of these data along the lines of this study can be discussed with the corresponding author.
Disclaimer: This study is based in part on data provided by Alberta Health and Alberta Health Services. The interpretation and conclusions contained herein are those of the researchers and do not necessarily represent the views of the Government of Alberta or Alberta Health Services. Neither the Government of Alberta nor Alberta Health or Alberta Health Services express any opinion in relation to this study.
Supplemental information: For reviewer comments and the original submission of this manuscript, please see www.cmajopen.ca/content/11/1/E101/suppl/DC1.
References
- 1.Thompson S, James M, Wiebe N, et al. Alberta Kidney Disease Network. Cause of death in patients with reduced kidney function. J Am Soc Nephrol. 2015;26:2504–11. doi: 10.1681/ASN.2014070714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perkovic V, Jardine MJ, Neal B, et al. CREDENCE Trial Investigators. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295–306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 3.Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al. DAPA-CKD Trial Committees and Investigators. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383:1436–46. doi: 10.1056/NEJMoa2024816. [DOI] [PubMed] [Google Scholar]
- 4.Navaneethan SD, Zoungas S, Caramori ML, et al. Diabetes management in chronic kidney disease: synopsis of the 2020 KDIGO clinical practice guideline. Ann Intern Med. 2021;174:385–94. doi: 10.7326/M20-5938. [DOI] [PubMed] [Google Scholar]
- 5.Diabetes Canada Clinical Practice Guidelines Expert Committee. Lipscombe L, Butalia S, Dasgupta K, et al. Pharmacologic glycemic management of type 2 diabetes in adults: 2020 update. Can J Diabetes. 2020;44:575–91. doi: 10.1016/j.jcjd.2020.08.001. [DOI] [PubMed] [Google Scholar]
- 6.American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: Standards of medical care in diabetes — 2021. Diabetes Care. 2021;44(Suppl 1):S111–24. doi: 10.2337/dc21-S009. [DOI] [PubMed] [Google Scholar]
- 7.Herrington WG, Preiss D, Haynes R, et al. The potential for improving cardio-renal outcomes by sodium-glucose co-transporter-2 inhibition in people with chronic kidney disease: a rationale for the EMPA-KIDNEY study. Clin Kidney J. 2018;11:749–61. doi: 10.1093/ckj/sfy090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pannu N, Gilmour L, Klarenbach S Kidney Health Strategic Clinical Network. Kidney Health Strategic Clinical Network: driving positive change to optimize kidney health in Alberta. CMAJ. 2019;191(Suppl):S39–41. doi: 10.1503/cmaj.190573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hemmelgarn BR, Clement F, Manns BJ, et al. Overview of the Alberta Kidney Disease Network. BMC Nephrol. 2009;10:30. doi: 10.1186/1471-2369-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323–34. doi: 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 11.Mosenzon O, Wiviott SD, Cahn A, et al. Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: an analysis from the DECLARE-TIMI 58 randomised trial. Lancet Diabetes Endocrinol. 2019;7:606–17. doi: 10.1016/S2213-8587(19)30180-9. [DOI] [PubMed] [Google Scholar]
- 12.Hux JE, Ivis F, Flintoft V, et al. Diabetes in Ontario: determination of prevalence and incidence using a validated administrative data algorithm. Diabetes Care. 2002;25:512–6. doi: 10.2337/diacare.25.3.512. [DOI] [PubMed] [Google Scholar]
- 13.Responding to the challenge of diabetes in Canada: first report of the National Diabetes Surveillance System (NDSS) 2003. Ottawa: Health Canada; 2003. [Google Scholar]
- 14.Lipscombe LL, Hwee J, Webster L, et al. Identifying diabetes cases from administrative data: a population-based validation study. BMC Health Serv Res. 2018;18:316. doi: 10.1186/s12913-018-3148-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diabetes Canada Clinical Practice Guidelines Expert Committee. Punthakee Z, Goldenberg R, Katz P. Definition, classification and diagnosis of diabetes, prediabetes and metabolic syndrome. Can J Diabetes. 2018;42(Suppl 1):S10–5. doi: 10.1016/j.jcjd.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Ye M, Vena JE, Johnson JA, et al. Validation of drug prescription records for senior patients in Alberta’s Tomorrow Project: assessing agreement between two population-level administrative pharmaceutical databases in Alberta, Canada. Pharmacoepidemiol Drug Saf. 2019;28:1417–21. doi: 10.1002/pds.4861. [DOI] [PubMed] [Google Scholar]
- 17.Tonelli M, Wiebe N, Fortin M, et al. Methods for identifying 30 chronic conditions: application to administrative data. BMC Med Inform Decis Mak. 2015;15:31. doi: 10.1186/s12911-015-0155-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tu K, Mitiku T, Lee DS, et al. Validation of physician billing and hospitalization data to identify patients with ischemic heart disease using data from the Electronic Medical Record Administrative data Linked Database (EMRALD) Can J Cardiol. 2010;26:e225–8. doi: 10.1016/s0828-282x(10)70412-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Walraven C, Austin PC, Jennings A, et al. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47:626–33. doi: 10.1097/MLR.0b013e31819432e5. [DOI] [PubMed] [Google Scholar]
- 20.Hao R, Myroniuk T, McGuckin T, et al. Underuse of cardiorenal protective agents in high-risk diabetes patients in primary care: a cross-sectional study. BMC Prim Care. 2022;23:124. doi: 10.1186/s12875-022-01731-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Interactive drug benefit list. Edmonton: Government of Alberta; [accessed 2021 Sept. 28]. Available: https://idbl.ab.bluecross.ca/idbl. [Google Scholar]
- 22.Milder TY, Stocker SL, Baysari M, et al. Prescribing of SGLT2 inhibitors in primary care: a qualitative study of general practitioners and endocrinologists. Diabetes Res Clin Pract. 2021;180:109036. doi: 10.1016/j.diabres.2021.109036. [DOI] [PubMed] [Google Scholar]
- 23.Douros A, Lix LM, Fralick M, et al. Canadian Network for Observational Drug Effect Studies (CNODES) Investigators. Sodium-glucose cotransporter-2 inhibitors and the risk for diabetic ketoacidosis: a multicenter cohort study. Ann Intern Med. 2020;173:417–25. doi: 10.7326/M20-0289. [DOI] [PubMed] [Google Scholar]
- 24.Ueda P, Svanström H, Melbye M, et al. Sodium glucose cotransporter 2 inhibitors and risk of serious adverse events: nationwide register based cohort study. BMJ. 2018;363:k4365. doi: 10.1136/bmj.k4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matthews DR, Li Q, Perkovic V, et al. Effects of canagliflozin on amputation risk in type 2 diabetes: the CANVAS Program. Diabetologia. 2019;62:926–38. doi: 10.1007/s00125-019-4839-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Donnan JR, Grandy CA, Chibrikov E, et al. Comparative safety of the sodium glucose co-transporter 2 (SGLT2) inhibitors: a systematic review and meta-analysis. BMJ Open. 2019;9:e022577. doi: 10.1136/bmjopen-2018-022577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lega IC, Bronskill SE, Campitelli MA, et al. Sodium glucose cotransporter 2 inhibitors and risk of genital mycotic and urinary tract infection: a population-based study of older women and men with diabetes. Diabetes Obes Metab. 2019;21:2394–404. doi: 10.1111/dom.13820. [DOI] [PubMed] [Google Scholar]
- 28.Iskander C, Cherney DZ, Clemens KK, et al. Use of sodium-glucose cotransporter-2 inhibitors and risk of acute kidney injury in older adults with diabetes: a population-based cohort study. CMAJ. 2020;192:E351–60. doi: 10.1503/cmaj.191283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hill-Briggs F, Adler NE, Berkowitz SA, et al. Social determinants of health and diabetes: a scientific review. Diabetes Care. 2020;44:258–79. doi: 10.2337/dci20-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao M, Woodward M, Vaartjes I, et al. Sex differences in cardiovascular medication prescription in primary care: a systematic review and meta-analysis. J Am Heart Assoc. 2020;9:e014742. doi: 10.1161/JAHA.119.014742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McAlister FA, Oreopoulos A, Norris CM, et al. Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease (APPROACH) Investigators. Exploring the treatment-risk paradox in coronary disease. Arch Intern Med. 2007;167:1019–25. doi: 10.1001/archinte.167.10.1019. [DOI] [PubMed] [Google Scholar]
- 32.Kosiborod M, Berwanger O, Koch GG, et al. Effects of dapagliflozin on prevention of major clinical events and recovery in patients with respiratory failure because of COVID-19: design and rationale for the DARE-19 study. Diabetes Obes Metab. 2021;23:886–96. doi: 10.1111/dom.14296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhatt DL, Szarek M, Steg PG, et al. SOLOIST-WHF Trial Investigators. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. 2021;384:117–28. doi: 10.1056/NEJMoa2030183. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.