Abstract
Background
Outbreaks in healthcare facilities played a pivotal role in the course of the coronavirus (COVID-19) pandemic.
Aim
To investigate severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) outbreaks in hospitals, outpatient care, and rehabilitation facilities in Germany from March 2020 to May 2022.
Methods
Data from the German mandatory notification system were used to describe outbreaks by number of cases and case fatality ratio (CFR), and outbreak cases by age and gender. Using Pearson correlation, the dynamics of cases in the general population were compared with cases in healthcare-associated infection (HAI) SARS-CoV-2 outbreaks before and after the start of the vaccination campaign. Additionally, a counterfactual scenario was used to estimate numbers of prevented HAI cases, using the phase before vaccination as baseline.
Findings
By the end of May 2022, 8941 healthcare-associated outbreaks were observed with 73,626 cases: 51,504 in hospitals, 15,524 in outpatient care, and 6598 in rehabilitation facilities. Median number of cases per outbreak was 4 (range: 2–342) and cases were more frequently reported in women with 46,818 (63.6%). Overall CFR was 8.1%, higher in men (12.4%) than in women (5.7%). After the vaccination campaign was fully introduced, the association between increasing incidence in the general population and consecutive outbreak cases was decreased by a factor of 10. Furthermore, our counterfactual analysis suggests that more than 55,000 outbreak cases could have been prevented until the end of 2021.
Conclusion
The vaccination campaign in combination with non-pharmaceutical measures was key to reduce number, size and CFR of healthcare-associated outbreaks.
Keywords: COVID-19, Outbreaks, Healthcare-associated infection, Healthcare facilities, Hospitals, Outpatient care and rehabilitation facilities
Introduction
More than 26 million cases of COVID-19 were documented in the national surveillance system in Germany on May 31st, 2022 [1]. With an estimated basic reproduction number R 0 of 2.8–3.8, each infected individual transmits the virus on average to about three other people if no measures are in place [2]. However, it has been reported that only about 20% of infected individuals may be responsible for 80% of infections, as SARS-CoV-2, like other infectious diseases, spreads through so-called superspreading events [3]. Therefore, outbreak clusters of COVID-19 play an important role in the transmission of SARS-CoV-2.
Hospitals and other medical facilities represent relevant settings for transmission as healthcare providers, infected persons and others – often vulnerable patients – get in close proximity [4,5]. SARS-CoV-2 outbreaks in hospitals have been described and can cause a serious threat to patients [[6], [7], [8], [9]]. Healthcare workers (HCWs) in this setting are both at increased risk of getting infected as well as relevant possible contributors to the spread of disease [10,11]. Infection prevention and control (IPC) measures and early vaccination campaigns for HCWs and high-risk populations therefore aimed to reduce the risk of healthcare-associated infection (HAI) and nosocomial SARS-CoV-2 outbreaks.
This study analysed all healthcare-associated outbreaks and associated cases that were notified in Germany since the beginning of the pandemic in 2020 until May 2022. These outbreaks were characterized and compared across different high-incidence periods such as the ‘Delta-wave’ (where lineage B.1.617.2 was predominant) from August to December 2021 and the first ‘Omicron wave’ (where lineages BA.1 and BA.2 were predominant) from January to May 2022 [[12], [13], [14]]. Furthermore, differences were investigated between healthcare-associated outbreaks in hospitals, in outpatient care, and in rehabilitation facilities. The aim was also to analyse the correlation between SARS-CoV-2 cases in the general population and these outbreak cases. Our results will help to better understand the dynamics of outbreaks in healthcare facilities and their contribution to the overall spread and burden of COVID-19.
Methods
Healthcare-associated SARS-CoV-2 outbreaks (SARS-CoV-2 HAI outbreaks) were defined by at least two SARS-CoV-2 cases with an epidemiological link that were notified by local health authorities as ‘healthcare-associated outbreaks’ or with setting ‘healthcare facilities’. It was not always clear where the index case in each outbreak was infected with SARS-CoV-2; however, all consecutive cases in the dataset were epidemiologically linked to HAI outbreaks by the responsible local health authority. The study included all polymerase chain reaction (PCR)-confirmed SARS-CoV-2 cases that occurred in outbreaks and that were reported through the German mandatory notification surveillance (SurvNet/DEMIS) between calendar week (CW) 9/2020 (March 2020) and CW 22/2022 (May 2022) [15]. To better understand the differences between healthcare settings, SARS-CoV-2 HAI outbreaks were differentiated between hospitals, outpatient care, and rehabilitation facilities. The setting outpatient care included ambulatory clinics, general practitioners, as well as specialists working in practices. Rehabilitation clinics that notified outbreaks were predominantly larger inpatient care facilities.
This study focuses on characteristics and size of SARS-CoV-2 HAI outbreaks: number of outbreaks, number of outbreak cases, median size of outbreaks and range of outbreak cases per outbreak, as well as severity of outbreaks with proportion of deaths among outbreak cases (cases fatality ration, CFR) and notified symptoms. ‘Severe’ symptoms (or diagnosis) were defined as one or more of the following: pneumonia, dyspnoea, acute respiratory distress syndrome (ARDS), and cases needing artificial respiration or ventilators. Remaining cases had either one or more ‘mild’ symptoms such as runny nose, cough, or fever, were asymptomatic or no information on their symptom status was available (it was not possible to differentiate between truly asymptomatic and missing symptom information in the used surveillance data).
SARS-CoV-2 HAI outbreaks were analysed by gender (men and women) and 10-year age groups: <20, 20–29, 30–39, 40–49, 50–59, 60–69, 70–79, and ≥80 years. These stratifications were used to describe CFR and severe symptoms. Trends of CFR over months were described with two age stratifications <70 and ≥70 years; this cut-off was chosen to exclude HCWs and other personnel from the latter group.
In this study, SARS-CoV-2 HAI outbreak cases in HCWs were defined as outbreak cases with information on occupational status according to the German Infection Protection Act.
To further analyse size, severity, and dynamic of outbreaks through the course of the pandemic, four phases were defined as follows: phase 1 (CW 9/2020 to CW 53/2020) for the time period where there were limited pharmaceutical interventions; phase 2 (CW 1/2021 to CW 15/2021) for the start of the vaccination campaign; phase 3 (CW 16/2021 to CW 52/2021) for increasing vaccination coverage or recovery status; phase 4 (CW 1/2022 to CW 22/2022) for high population immunity due to vaccination and/or natural immunity during Omicron wave.
The association between SARS-CoV-2 HAI outbreak cases and cases in the general population was analysed with Pearson correlations and regression slopes from a linear model. The Pearson correlations and regression slopes aimed to explain the correlation of occurrences of outbreak cases after the increase of cases in the general population. Confidence intervals (95% CI) for regression slopes were calculated with 0 as the lowest cut-off. We used cases in the general population as predictor (x) and outbreak cases as outcome (y). This association was analysed and compared across the four phases including a two-week lag to the occurrence of cases in the general population. It was investigated for all HAI outbreak cases, as well as for each facility setting (hospitals, outpatient care and rehabilitation facilities) seperately, including a two-week lag to the occurrence of cases in the general populations.
Additionally, a counterfactual analysis was performed for all healthcare settings by using the linear regression equations in the first phase as the baseline. The ‘observed’ cases were defined as the actual number of outbreak cases, ‘scenario’ as hypothetical numbers of outbreak cases with cases in the general population as a predictor, and ‘prevented’ as number of outbreak cases that have been prevented during the second, third, and fourth phases.
Further detailed information on method of correlations, linear model and counterfactual scenario used in this study have been described elsewhere [16].
All descriptive analyses were conducted with R (Version x64 4.0.3).
Results
Epidemiology of SARS-CoV-2 HAI outbreaks
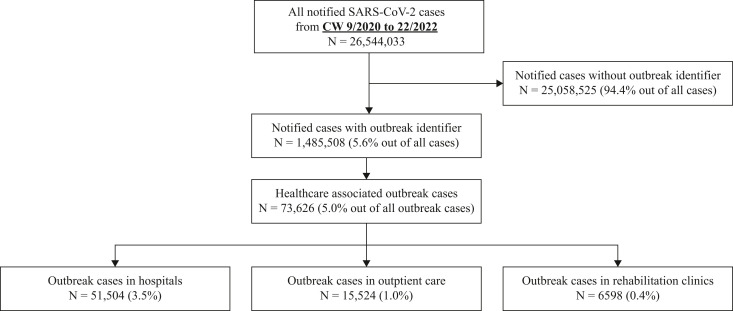
From March 2020 (CW 9/2020) to May 2022 (22/2022), a total of 26,544,033 SARS-CoV-2 cases were notified (data status: July 8th, 2022), with 5.6% (N = 1,485,508) of all notified SARS-CoV-2 cases associated with outbreaks (Figure 1 ). Out of all outbreak cases, 73,626 outbreak cases (5.0%) were notified as HAI outbreaks (N = 8941 outbreaks). Median number of HAI outbreak cases per outbreak was 4 (range: 2–342). Most HAI outbreaks occurred in hospitals with 5830 outbreaks and 51,504 outbreak cases (median: 5; range: 2–342), followed by 2369 outbreaks in outpatient care (15,524 outbreak cases, median (range): 4 (2–279)) and 742 outbreaks in rehabilitation facilities (6598 outbreak cases; median (range): 4 (2–201)). HAI outbreak cases were more frequently reported in women (46,818, 63.6%) than in men (26,466, 35.9%). For 341 cases (0.5%) no information on gender was supplied and one case was notified as gender diverse.
Figure 1.
SARS-CoV-2 cases in Germany from March 2020 (calendar week (CW) 9/2020) to May 2022 (CW 22/2022). Source of data: mandatory notification data with last update on July 8th, 2022.
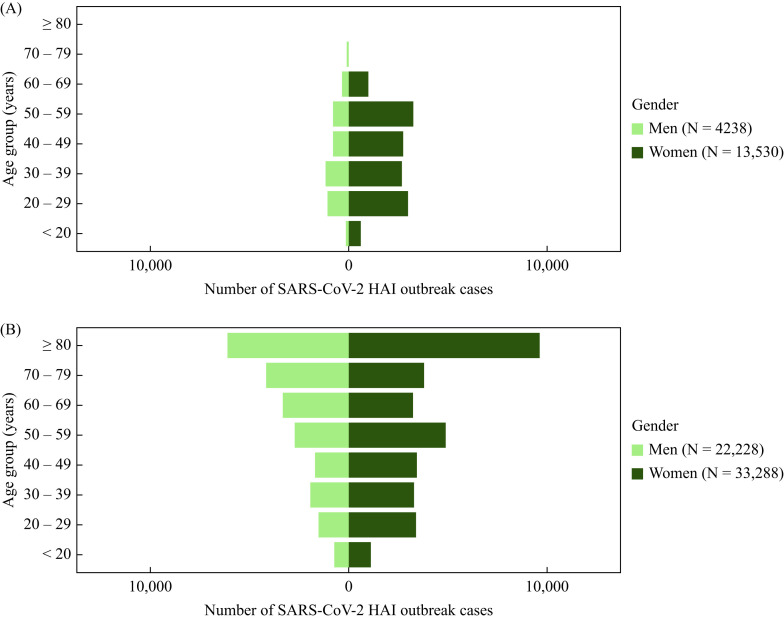
Out of 73,626 outbreak cases, 17,849 outbreak cases were notified among HCWs (24.2%), and 55,777 cases (75.8%) among patients linked to these outbreaks. The majority of cases were female among both HCWs (13,530, 75.8%) and patients (33,288, 59.7%) (Figure 2 ). This was true for all age groups for both HCWs and patients with the exception of age groups 60–69 years for patients and 70–79 years for both categories.
Figure 2.
SARS-CoV-2 healthcare-associated infection (HAI) outbreak cases among healthcare workers (A) and patients (B) stratified with age and gender. Dark green represents women; light green represents men.
Out of 73,284 outbreak cases in all healthcare facilities with information on genders male and female, 5349 cases (7.3%) were notified with severe symptoms, 33,154 cases (45.2%) with mild symptoms, and 34,781 cases (47.5%) with no symptoms or no information on symptoms. Severe symptoms were more frequent in men than in women for age groups ≥50 but vice versa for younger age groups. For both genders, symptom severity increased with age. The highest proportion of severe symptoms with 15.3% was found in men ≥80 years.
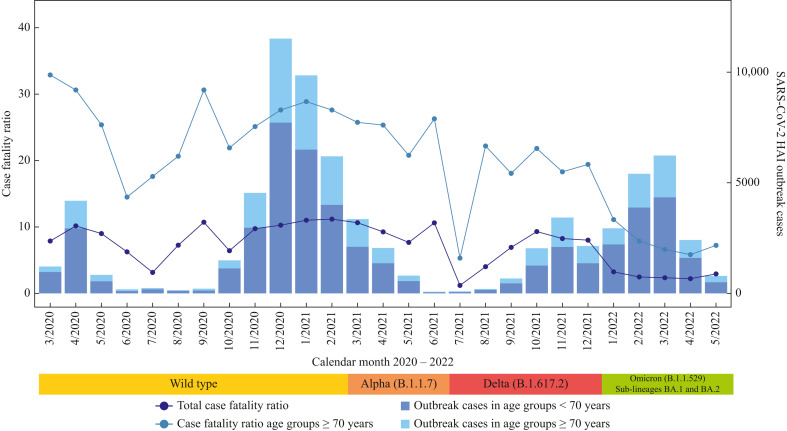
In total, 5944 deaths were observed among outbreak cases (CFR: 8.1%), with most deaths notified in elderly ≥70 years (N≥70: 5265 deaths; CFR≥70: 22.0%). The quantile range (Q25, Q75) of CFR per outbreak was 0-8.3% for all HAI outbreaks and CFR≥70 was 0–33.3%. Death cases were reported more frequently from men (3284 cases; CFR: 12.4%) than from women (2650; CFR: 5.7%) (Table I ). Of 341 outbreak cases with no information on gender, 10 deaths (CFR: 2.9%) were notified. The CFR for age group >70 years overall decreased across the months with some fluctuations in part due to small case numbers from a maximum of around 33.0% at the beginning of the pandemic in March 2020 to around 7.0% in May 2022 (Figure 3 ).
Table I.
Number of outbreak cases and deaths in SARS-CoV-2 HAI outbreaks for hospitals, outpatient care and rehabilitation facilities (data status July 8th, 2022)
| Gender and age group (years) | Total no. of outbreak cases | Total no. of deaths in outbreaks (%) | Hospitals |
Outpatient care |
Rehabilitation facilities |
|||
|---|---|---|---|---|---|---|---|---|
| N | Death (%) | N | Death (%) | N | Death (%) | |||
| Men | 26,466 | 3284 (12.4) | 18,835 | 2657 (14.1) | 5337 | 509 (9.5) | 2294 | 120 (5.2) |
| <20 | 858 | 0 (0) | 485 | 0 (0) | 294 | 0 (0) | 79 | 0 (0) |
| 20–29 | 2612 | 2 (0.1) | 1972 | 2 (0.1) | 416 | 0 (0) | 224 | 0 (0) |
| 30–39 | 3074 | 4 (0.1) | 2309 | 4 (0.2) | 492 | 0 (0) | 273 | 0 (0) |
| 40–49 | 2442 | 23 (0.9) | 1609 | 19 (1.2) | 585 | 3 (0.5) | 248 | 1 (0.4) |
| 50–59 | 3531 | 94 (2.7) | 2299 | 74 (3.2) | 811 | 17 (2.1) | 421 | 3 (0.7) |
| 60–69 | 3630 | 315 (8.7) | 2397 | 243 (10.1) | 825 | 54 (6.5) | 408 | 18 (4.4) |
| 70–79 | 4261 | 881 (20.7) | 3198 | 721 (22.5) | 735 | 123 (16.7) | 328 | 37 (11.3) |
| ≥80 | 6058 | 1965 (32.4) | 4566 | 1592 (34.9) | 1179 | 312 (26.5) | 313 | 61 (19.5) |
| Women | 46,818 | 2650 (5.7) | 32,380 | 2072 (6.4) | 10,155 | 469 (4.6) | 4283 | 112 (2.6) |
| <20 | 1834 | 1 (0.1) | 1294 | 1 (0.1) | 408 | 0 (0) | 132 | 0 (0) |
| 20–29 | 6469 | 1 (0) | 4969 | 1 (0) | 1083 | 0 (0) | 417 | 0 (0) |
| 30–39 | 6083 | 6 (0.1) | 4284 | 4 (0.1) | 1218 | 2 (0.2) | 581 | 0 (0) |
| 40–49 | 6283 | 4 (0.1) | 4188 | 2 (0) | 1403 | 1 (0.1) | 692 | 1 (0.1) |
| 50–59 | 8225 | 62 (0.8) | 5434 | 49 (0.9) | 1814 | 12 (0.7) | 977 | 1 (0.1) |
| 60–69 | 4329 | 166 (3.8) | 2808 | 143 (5.1) | 1018 | 19 (1.9) | 503 | 4 (0.8) |
| 70–79 | 3891 | 474 (12.2) | 2729 | 374 (13.7) | 800 | 75 (9.4) | 362 | 25 (6.9) |
| ≥80 | 9704 | 1936 (20.0) | 6674 | 1495 (22.4) | 2411 | 360 (14.9) | 619 | 81 (13.1) |
HAI, healthcare-associated infection.
Figure 3.
Monthly case fatality ratio (%) in SARS-CoV-2 healthcare-associated infection (HAI) outbreaks from March 2020 to May 2022. Data status July 8th, 2022. Approximate time periods of dominant variants are displayed below the graph [[17], [18], [19]].
SARS-CoV-2 HAI outbreaks in different healthcare facilities: hospitals, outpatient care, and rehabilitation facilities
Key numbers of SARS-CoV-2 HAI outbreaks in the three types of healthcare facilities are reported in Table I, stratified by gender and age. Among women, the CFR ranged from 2.6% in rehabilitation facilities to 6.4% in hospitals, compared to 5.2% and 14.1%, respectively, in men. Higher CFR was observed in men for all age groups in all settings and CFR increased with age in general, with highest CFR among elderly patients aged ≥80 years.
SARS-CoV-2 HAI outbreaks before and after the start of vaccination campaign
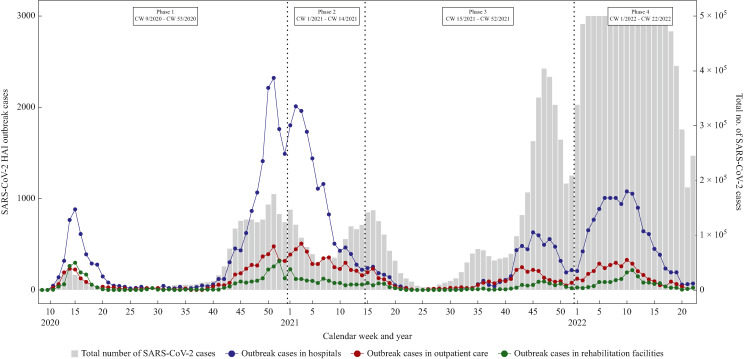
The dynamic of SARS-CoV-2 HAI outbreak cases in relation to the overall number of SARS-CoV-2 cases in the general population during the pandemic is shown in Figure 4 . There were more outbreak cases in hospitals than in the other two facility settings throughout the four pandemic phases. For the purpose of visualization, the number of cases in the general population was cut off at 500,000 cases. Full data without cut-off can be found in Supplementary Figure S1.
Figure 4.
SARS-CoV-2 healthcare-associated infection (HAI) outbreak cases in hospitals, outpatient care and rehabilitation facilities from CW 9/2020 to CW 22/2022. Cut-off of 500,000 (5 × 105) COVID-19 cases was set for the purpose of visualization. COVID-19 cases from CW 4/2022 to CW 13/2022 were higher than 1,000,000. Data status 8 July 2022.
Additional information on the characteristics across the different phases for all HAI outbreaks together as well as each healthcare facility setting individually are shown in Table II . In all three different healthcare settings, highest numbers of outbreaks and related outbreak cases were observed before the start of the vaccination campaign (phase 1). The median size of outbreaks, maximum number of outbreak cases and deaths declined after the start of the vaccination campaign (phase 2) and afterwards (phase 3 and phase 4) in all three settings. CFR also decreased from phase 1 to phase 4 for outpatient care and rehabilitation facilities, but increased slightly for hospitals in phase 2 before also gradually decreasing in this setting.
Table II.
SARS-CoV-2 healthcare-associated infection (HAI) outbreaks in hospitals, outpatient care and rehabilitation facilities in four different phases (data status July 8th, 2022)
| Variables | Total (CW 9/2020 to 22/2022) | Phase 1 (CW 9/2020 to 52/2020) | Phase 2 (CW 53/2020 to 14/2021) | Phase 3 (CW 15/2021 to 52/2021) | Phase 4 (CW 1/2022 to 22/2022) |
|---|---|---|---|---|---|
| COVID-19 cases in general population | 26,544,033 | 1,783,688 | 1,229,518 | 4,211,449 | 19,319,378 |
| No. of HAI outbreaks (outbreak cases) | 8941 (N = 73,626) | 2715 (N = 30,751) | 1996 (N = 15,080) | 1831 (N = 10,994) | 2399 (N = 16,801) |
| Death cases (case fatality ratio (%)) | 5949 (8.1) | 3018 (9.8) | 1652 (11.0) | 853 (7.8) | 426 (2.5) |
| Outbreak cases two weeks after increase of 10,000 COVID-19 cases in general population (95% CI) | – | 150 (126–171) | NAa | 15 (8–22) | 7 (2–12) |
| Healthcare workers in HAI outbreaks (%) | 17,849 (24.2) | 8778 (28.5) | 3052 (20.2) | 2530 (23.0) | 3489 (20.8) |
| Hospitals | |||||
| No. of outbreaks (outbreak cases) | 5830 (N = 51,504) | 1753 (N = 22,401) | 1214 (N = 10,204) | 1095 (N = 6861) | 1768 (N = 12,038) |
| Outbreak cases ≥70 years | 17,225 | 6962 | 3781 | 2577 | 3905 |
| Median outbreak size (range cases/outbreak) | 5 (2–342) | 6 (2–342) | 4 (2–126) | 4 (2–94) | 4 (2–173) |
| Death cases (case fatality ratio (%)) | 4738 (9.2) | 2367 (10.6) | 1306 (12.8) | 677 (9.9) | 388 (3.2) |
| Death cases ≥70 years (case fatality ratio (%) ≥70 years) | 4195 (24.4) | 2107 (30.3) | 1162 (30.7) | 583 (22.6) | 343 (8.8) |
| Outbreak cases two weeks after increase of 10,000 COVID-19 cases in general population (95% CI) | – | 113 (96–130) | NAa | 11 (7–16) | 5 (2–9) |
| Outpatient care | |||||
| No. of outbreaks (outbreak cases) | 2369 (N = 15,524) | 715 (N = 5186) | 631 (N = 3868) | 575 (N = 3159) | 448 (N = 3311) |
| Outbreak cases ≥70 years | 5134 | 1781 | 1402 | 1011 | 940 |
| Median outbreak size (range cases/outbreak) | 4 (2–279) | 4 (2–146) | 4 (2–62) | 3 (2–70) | 3 (2–279) |
| Death cases (case fatality ratio (%)) | 979 (6.3) | 466 (9.0) | 317 (8.2) | 161 (5.1) | 35 (1.1) |
| Death cases ≥70 years (case fatality ratio (%) ≥70 years) | 871 (17.0) | 417 (23.4) | 277 (19.8) | 146 (14.4) | 31 (3.3) |
| Outbreak cases two weeks after increase of 10,000 COVID-19 cases in general population (95% CI) | – | 24 (21–28) | NAa | 2 (0–4) | 1 (0–2) |
| Rehabilitation facilities | |||||
| No. of outbreaks (outbreak cases) | 742 (N = 6598) | 247 (N = 3164) | 151 (N = 1008) | 161 (N = 974) | 183 (N = 1452) |
| Outbreak cases ≥70 years | 1622 | 983 | 254 | 179 | 206 |
| Median outbreak size (range cases/outbreak) | 4 (2–201) | 6 (2–201) | 3 (2–40) | 3 (2–81) | 4 (2–57) |
| Death cases (case fatality ratio (%)) | 232 (3.5) | 185 (5.8) | 29 (2.9) | 15 (1.5) | 3 (0.2) |
| Death cases ≥70 years (case fatality ratio (%) ≥70 years) | 204 (12.6) | 165 (16.8) | 23 (9.1) | 15 (8.4) | 1 (0.5) |
| Outbreak cases two weeks after increase of 10,000 COVID-19 cases in general population (95% CI) | – | 117 (8–16) | NAa | 2 (1–2) | 1 (0–1) |
HAI, healthcare-associated infection; CW, calendar week.
For phase 2, no correlation was calculated as the assumption of a linear relationship between cases in HAI outbreaks and the general population was not met.
Association between SARS-CoV-2 HAI outbreak cases with SARS-CoV-2 cases in the general population
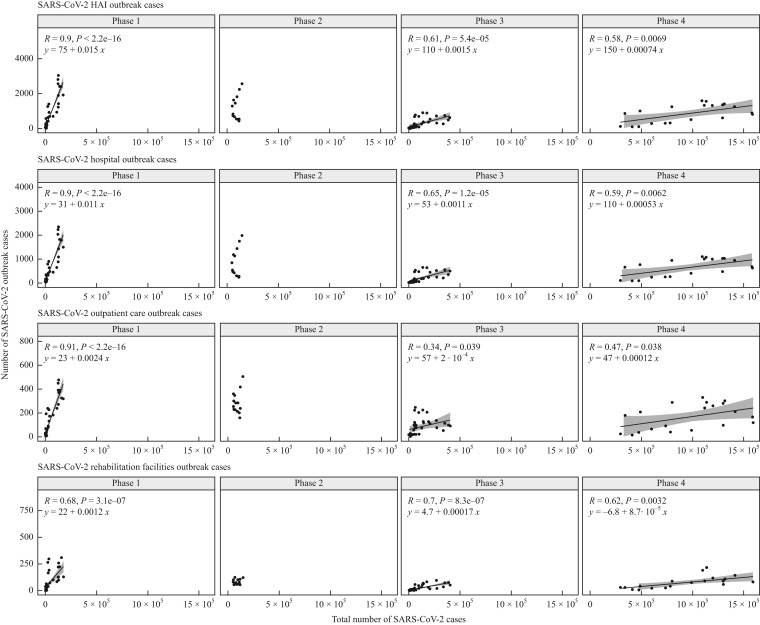
Strong and significant correlations between SARS-CoV-2 HAI outbreak cases with SARS-CoV-2 cases in the general population were observed in phase 1 for all three settings combined and individually (Figure 5 ). In this phase, on average, 150 outbreak cases (95% CI: 126–171) for all HAI outbreaks were expected two weeks after an increase of 10,000 SARS-CoV-2 cases in the general population with the majority of outbreak cases (113 outbreak cases; 95% CI: 96–130) estimated in hospitals (Table II).
Figure 5.
Correlations between SARS-CoV-2 healthcare-associated infection (HAI) outbreak cases and SARS-CoV-2 cases in the general population in four different phases for all healthcare facilities, hospitals, outpatient care and rehabilitation facilities. Correlation coefficients with P-value (R and P) along with linear regression equations are described for each phase. y-Axes represent outbreak cases as outcome and x-axes the total number of SARS-CoV-2 cases in general populations as predictor. For phase 2, no correlation was calculated since the assumption of a linear relationship between cases in HAI outbreaks and the general population was not suited.
In phases 3 and 4, the numbers of outbreak cases significantly correlated (P < 0.05) again for overall HAI outbreaks and for each setting individually (Figure 5). In comparison to phase 1, the correlations for overall HAI outbreaks, hospitals and outpatient care were weaker in phase 3 and phase 4, while in rehabilitation facilities they remained stable. Moreover, the estimated numbers of outbreak cases per 10,000 cases in the general population were considerably lower than in phases 1 and 2 in all three healthcare settings on average, 11 outbreak cases (95% CI: 7–16) in hospitals, 2 outbreak cases (95% CI: 0–4) in outpatient setting and 2 outbreak cases (95% CI: 1–2) in rehabilitation facilities (Table II). These estimated numbers continued decreasing in phase 4 (Table II, Figure 5), while the number of SARS-CoV-2 cases in the general populations sharply increased (Figure 4).
Counterfactual analysis of SARS-CoV-2 HAI outbreak cases
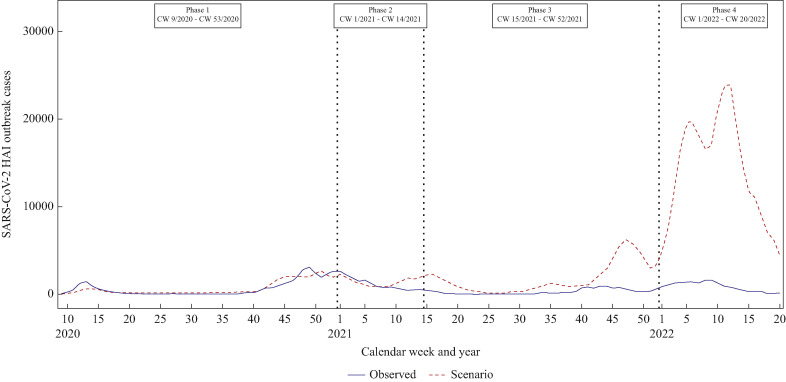
Trend of ‘observed’ and ‘scenario’ of SARS-CoV-2 HAI outbreak cases are displayed in Figure 6 . For the purpose of the counterfactual scenario, the regression lines from phase 1 were used as baseline for HAI outbreak cases (Figure 5). The number of observed SARS-CoV-2 outbreak cases (blue solid line) was lower than in the scenario of SARS-CoV-2 outbreak cases (red dashed line) at the end of phase 2 and in phases 3 to 4 for all three healthcare facility settings. In phase 2, 3389 outbreak cases (17.4% out of 19,493 hypothetical outbreak cases) could have been prevented in SARS-CoV-2 HAI outbreaks, while in phase 3, 55,339 outbreak cases (83.4% out of 66,021 hypothetical outbreak cases) and in phase 4, 267,875 outbreak cases (94.1% out of 284,812 hypothetical outbreak cases) (Supplementary Table S1) could have been prevented.
Figure 6.
SARS-CoV-2 healthcare-associated infection (HAI) outbreak cases in four different phases. Blue solid line represents observed SARS-CoV-2 outbreak cases, while red dashed line for scenario outbreak cases (the hypothetical number of outbreak cases if association with cases in the general population had remained constant from phase 1 onwards).
Discussion
The present study offers the most comprehensive overview of SARS-CoV-2-outbreaks in healthcare facilities in Germany to date. Outbreaks in various healthcare settings showed different characteristics, and numbers and sizes of outbreaks decreased over the course of the pandemic, in particular after the start of the vaccination campaign. Due to regular screening procedures of personnel and patients in hospitals and other analysed healthcare facilities, mild and asymptomatic cases were also found and notified. Therefore, we are confident that the presented data reflect the population in these settings very well in regards to COVID-19 incidence, demographics, and CFR.
There was an overall CFR of 8.1% in HAI SARS-CoV-2 outbreaks, which remained relatively stable until the end of 2021. This is in line with a systematic review and meta-analysis published in May 2021 that included 33 studies from five different countries and found a mortality ratio of 11.5% among general hospital-admitted patients [20]. In contrast the CFR among all cases in Germany was only 1.7% during the same time period [21]. Patients in hospitals in rehabilitation and in outpatient care may be frailer and therefore at higher risk of death due to an infection with SARS-CoV-2 because of their underlying disease. This highlights the necessity to properly protect this population from COVID-19 infections.
From January 2022, the CFR for all HAI outbreak cases and the sub-group of >70-year-olds declined sharply and remained stable for the remaining study period. Over the turn of the year 2021/2022, Omicron (B.1.1.529 with sub-lineages BA.1 and BA.2) became the dominant variant in Germany and replaced Delta (lineage B.1.617.2) [14]. Additionally, more than 70% of the German population had already received two doses of the COVID-19 vaccine [21]. It has been argued that it is hard to infer the intrinsic severity of Omicron because this variant encountered populations that were to some extent already protected [22]. Regardless of the underlying causes, available evidence suggests that severe outcomes from infections with Omicron strains BA.1 and BA.2 were less likely than with previous strains [19].
Overall, there were considerably more cases in women than in men. This observation was relatively stable over time and the proportion of women decreased only slightly from phase 1 to phase 4 from 66% to 61% (data not shown). Among HCWs, female gender was predominant across all age groups. In part, this may be explained by the fact that typically more women work in healthcare in Germany [23]. Among patients, women were also more often affected than men for the majority of age groups. Higher hospitalization rates due to pregnancy, birth, and postpartum can explain only parts of this observation, as otherwise in Germany men were more often hospitalized than women before the pandemic [24]. More research on this observed gender difference will be necessary to explore possible underlying factors.
In all three investigated healthcare settings, the CFR was substantially higher in men than in women. This observation was stable across all phases and is in line with many other studies that found higher case fatality in men [[25], [26], [27], [28], [29]]. When looking into symptom severity, women especially presented in outbreaks less often with severe outcomes such as ARDS, pneumonia, or dyspnoea, and were less likely to need a respiratory ventilator. Studies suggest that oestrogens downregulate the renin–angiotensin–aldosterone system (RAAS) including the angiotensin-converting enzyme 2 (ACE2), which has been found to be more often expressed in men than in women, especially under pathological conditions [29,30]. This may explain why women are both less likely to develop severe symptoms and consequently die from a SARS-CoV-2 infection. Due to systematic screening in outbreaks we consider the number of missed cases to be negligible in the analysed data; thus the CFR and symptom differences between genders should indicate the actual situation and an underlying biological reason for the gender difference appears plausible.
HCWs were at the forefront in battling the pandemic. Especially in the first phase of the investigated time period, more than 25% of cases in HAI outbreaks were among HCWs. In the following phases and with the introduction of the vaccination campaign, the proportion of HCWs in outbreaks declined slightly but remained above 20%. In Germany, as in many other countries, HCWs were prioritized by the Standing Committee on Vaccination (STIKO) and in the first group to receive COVID-19 vaccines [31]. Overall acceptance among HCWs was high and by CW 14/2021 already 83% of hospital personnel had received at least one vaccine dose [32,33]. COVID-19 vaccines have been proven not only to prevent infection but also to prevent transmission, at least for a limited amount of time [34]. Therefore, early vaccination of HCWs in combination with enhanced IPC measures – including use of personal protective equipment, heightened surveillance, and screening of HCWs and patients – may have contributed to the reduction in case numbers in the studied outbreak settings.
Most outbreaks and respective outbreak cases were found in hospitals (51,504), followed by ambulatory settings (15,524) and rehabilitation facilities (6598). However, the overall number of healthcare episodes was highest in ambulatory settings (∼550 million episodes per year), followed by hospitals (∼17 million episodes) and rehabilitation facilities (∼1.6 million episodes) [[35], [36], [37]]. This discrepancy may be explained by the fact that in ambulatory settings the length of stay, and therefore the risk of being involved in a SARS-CoV-2 outbreak, is lowest. Additionally, detection rates were likely higher in hospitals and rehabilitation facilities as screening in these settings was mandatory, leading to higher numbers in HAI outbreak cases compared to lower overall patient numbers.
Hospitals are at the centre of care for COVID-19 patients. Until the end of 2021, more than 380,000 SARS-CoV-2 cases in Germany were hospitalized, equalling a proportion of 5.4% of all cases [21]. Patients with severe COVID-19 symptoms are usually admitted to hospitals to receive the best available care. Deficiencies in IPC measures may then cause outbreaks in hospitals despite usage of surgical and particle-filtering half masks [38]. At the beginning of the pandemic, and before the implementation of strict IPC measures, up to 65% of screened hospital patients were infected with SARS-CoV-2, as shown in a recent scoping review [39]. In this phase of the pandemic, observed CFRs among patients aged >70 years were highest in this setting, with a proportion of 30% deceased patients. After vaccines and improved medical treatment became available the CFR among elderly was reduced and numbers of outbreaks and outbreak sizes in hospitals decreased. At the end of 2021 and in spring 2022, comparatively small increases were observed in hospital outbreak cases when case numbers in the overall population reached unprecedented levels. This showed that even in vaccinated populations, when the infection pressure was very high, hospital outbreaks could not be completely prevented.
In Germany, outpatient care and ambulatory services with a strong emphasis on practices with only one doctor are typically the first contact point of patients with any kinds of symptoms with primary healthcare [40]. Especially in the beginning of the pandemic, when wearing face or surgical masks was subject to shortage of supplies, general practitioners themselves or patients in waiting rooms may have caused outbreaks [41]. However, due to small contact networks, on average, outbreak sizes in these facilities were small (about four cases per outbreak) and remained relatively stable.
Rehabilitation facilities are a important setting as therapists and patients come into close contact and patients share common rooms. Even though in absolute numbers outbreaks in this setting were least frequent, there was a number of large outbreaks during the first wave of the pandemic. This might be explained by the fact that the introduction of SARS-CoV-2 into rehabilitation facilities was less frequent than into hospitals and ambulatory services, but once introduced the transmission among patients and personnel was relatively easy, especially before IPC measures were in place. Over the course of the pandemic precautionary recommendations were developed and implemented into rehabilitation guidelines, as summarized in a scoping review [42]. Developed measures were similar to measures in hospitals and outpatient care but may have been harder to implement due to the less clinical character of rehabilitation facilities. These setting-specific IPC measures, in combination with vaccination, most likely contributed to the reduction in outbreak numbers and cases we observed in phases 3 and 4 of the pandemic.
Before the start of the vaccination campaign, a strong correlation was observed between the total number of SARS-CoV-2 cases in Germany and the number of outbreak cases in all three investigated healthcare-facility settings. High infection pressure in the general population directly led to outbreaks in hospitals, and in ambulatory and rehabilitation facilities. In phase 2, in the beginning of 2021, the numbers started to become detached from one another, which coincides with the introduction of vaccinations. When in spring 2021 the Alpha (lineage B.1.1.7) strain caused another increase in cases in Germany, outbreak case numbers continued to decline. In phase 3, after the vaccination campaign had been implemented and most vulnerable populations protected, outbreak case numbers showed again medium-strong correlations with total case numbers in hospitals and rehabilitation facilities. However, in all three settings the slope was reduced at least by a factor of 10, meaning that at least ten times more cases in the general population were needed to cause one additional HAI outbreak case. In phase 4, with Omicron being the dominant variant, this development continued even further and again twice as many cases were necessary to cause an HAI outbreak case than during phase 3.
In a counterfactual scenario, we calculated how many cases may have been prevented through vaccination and improved IPC practices. For that, we assumed that outbreak case numbers in phase 3 were still as dependant on overall case numbers as in phase 1. In healthcare facilities, about 55,000 cases more would have been expected without preventive measures in 2021. This is very much in line with our previous analysis, where we modelled prevented cases within outbreaks in long-term care facilities and hospitals in Germany until September 2021 [16]. Especially in autumn and winter 2021, when case numbers all over the country increased sharply due to the Delta variant, a lot more outbreak cases would have been expected in the counterfactual scenario. Even though vaccines may offer only limited protection against infection and immunity waning over time, this observation demonstrates the pivotal role the vaccination campaign had in reducing outbreaks in different healthcare settings in Germany.
In phase 4, with Omicron replacing all previous variants since the beginning of 2022, the counterfactual scenario predicted an even higher number of prevented cases (more than 250,000) based on comparisons to the pre-vaccination phase. At this stage of the pandemic and based on serological studies of blood donors, almost 90% of the German population already had antibodies against SARS-CoV-2, due either to natural immunity or to vaccination [43]. Severe infectious courses were reported less frequently and therefore less stringent measures for the population were in place during this phase, leading to the maximum number of overall cases in Germany since the beginning of the pandemic [19]. That the infectious pressure in the general population did not lead to corresponding surges of outbreak cases highlights that high immunization rates will be crucial to prevent SARS-CoV-2 HAI outbreaks and corresponding deaths in winter 2022/2023 and beyond.
The kind of surveillance data that we used for our study is prone to a couple of biases. The notification of positive SARS-CoV-2 PCR results is mandatory [15]. However, lateral flow/antigen tests for SARS-CoV-2 are not notifiable and cases might not have been registered due to missing or late confirmatory PCR tests. Also, testing strategies have changed over time and potentially cases, who did not show any symptoms, were not involved in routine testing scenarios (e.g. schools), and were not identified as contact persons (according to changing definitions) were not tested at all and remained therefore undetected. Thus, the case numbers in this study are most likely underestimated as data from sero-epidemiological studies from Germany suggest [44]. However, with screening measures in place in most healthcare facilities and among HCWs, we consider the underestimation to be smaller than in the general population. Increasing workload and shortfall of human resources in local health authorities may have led to incompleteness on data entries, predominantly during case surges. Our ecological study design may not prove causality and should therefore be interpreted with caution. It cannot be excluded that (under-)reporting of cases, outbreaks, and/or fatalities may have changed over time and therefore contributed to observed differences between pandemic phases.
In conclusion, outbreaks of SARS-CoV-2 in hospitals, outpatient care and rehabilitation facilities pose a great threat for patients and personnel. Observed CFRs in HAI outbreaks were high, especially among hospital patients and elderly, and only decreased slightly over the course of the pandemic. High-quality surveillance data are necessary to facilitate investigation of outbreaks by local health authorities. In addition, comprehensive genomic surveillance allowing the linkage of genomic pathogen data to epidemiological case data may enable better differentiation between outbreak cases and non-cases, better understanding of transmission chains, and help more effectively to control pathogen spread. Obstacles that remain include a lack of financing, data protection, and supportive cooperation of interdisciplinary teams involved.
Prevention of outbreaks in healthcare facility settings through IPC was very important but nevertheless extremely challenging during the ongoing SARS-CoV-2 pandemic. High vaccination coverage, especially in elderly and HCWs, in combination with setting-specific IPC measures are vital to reduce HAI outbreaks and as a consequence the overall mortality due to COVID-19.
Acknowledgements
We would like to thank all German health authorities, both at the county and federal levels, that actively supported containment of the COVID-19 pandemic in Germany and submitted the data to Robert Koch Institute. Moreover, we would like to thank our colleagues in Robert Koch Institute, especially for M. an der Heiden, for preparing the data.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhin.2023.01.011.
Conflict of interest statement
None declared.
Funding sources
B. Suwono was funded by the containment scout initiative from the German Federal Ministry of Health (1368–1830). Other authors were funded internally from RKI.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Robert Koch Institute. Coronavirus disease 2019 (COVID-19) daily situation report 31/05/2022. Available at: https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Situationsberichte/Mai_2022/2022-05-31-en.pdf?__blob=publicationFile [last accessed July 2022].
- 2.Robert Koch Institute. Epidemiologischer Steckbrief zu SARS-CoV-2 und COVID-19 (Stand: 14.7.2021). Available at: https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Steckbrief.html;jsessionid=CFDB368529E2D0B228A6E184093C9465.internet091?nn=13490888 [last accessed September 2021].
- 3.Adam D.C., Wu P., Wong J.Y., Lau E.H.Y., Tsang T.K., Cauchemez S., et al. Clustering and superspreading potential of SARS-CoV-2 infections in Hong Kong. Nat Med. 2020;26 doi: 10.1038/s41591-020-1092-0. 1714-19. [DOI] [PubMed] [Google Scholar]
- 4.Bhattacharya A., Collin S.M., Stimson J., Thelwall S., Nsonwu O., Gerver S., et al. Healthcare-associated COVID-19 in England: a national data linkage study. J Infect. 2021;83:565–572. doi: 10.1016/j.jinf.2021.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lumley S.F., Constantinides B., Sanderson N., Rodger G., Street T.L., Swann J., et al. Epidemiological data and genome sequencing reveals that nosocomial transmission of SARS-CoV-2 is underestimated and mostly mediated by a small number of highly infectious individuals. J Infect. 2021;83:473–482. doi: 10.1016/j.jinf.2021.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang P.Y., Wu T.S., Cheng C.W., Chen C.J., Huang C.G., Tsao K.C., et al. A hospital cluster of COVID-19 associated with a SARS-CoV-2 superspreading event. J Microbiol Immunol Infect [Wei Mian Yu gan Ran Za Zhi] 2022;55:436–444. doi: 10.1016/j.jmii.2021.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rickman H.M., Rampling T., Shaw K., Martinez-Garcia G., Hail L., Coen P., et al. Nosocomial transmission of coronavirus disease 2019: a retrospective study of 66 hospital-acquired cases in a London teaching hospital. Clin Infect Dis. 2021;72:690–693. doi: 10.1093/cid/ciaa816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang X., Zhou Q., He Y., Liu L., Ma X., Wei X., et al. Nosocomial outbreak of COVID-19 pneumonia in Wuhan, China. Eur Respir J. 2020;55 doi: 10.1183/13993003.00544-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borges V., Isidro J., Macedo F., Neves J., Silva L., Paiva M., et al. Nosocomial outbreak of SARS-CoV-2 in a “non-COVID-19” hospital ward: virus genome sequencing as a key tool to understand cryptic transmission. Viruses. 2021;13:604. doi: 10.3390/v13040604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wratil P.R., Schmacke N.A., Osterman A., Weinberger T., Rech J., Karakoc B., et al. In-depth profiling of COVID-19 risk factors and preventive measures in healthcare workers. Infection. 2022;50:381–394. doi: 10.1007/s15010-021-01672-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lucey M., Macori G., Mullane N., Sutton-Fitzpatrick U., Gonzalez G., Coughlan S., et al. Whole-genome sequencing to track severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmission in nosocomial outbreaks. Clin Infect Dis. 2021;72 doi: 10.1093/cid/ciaa1433. e727–e735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schilling J., Buda S., Fischer M., Goerlitz L., Grote U., Haas W., et al. 2021. Retrospektive Phaseneinteilung der COVID-19-Pandemie in Deutschland bis Februar. [DOI] [Google Scholar]
- 13.Tolksdorf K., Buda S., Schilling J. 2021. Aktualisierung zur “Retrospektiven Phaseneinteilung der COVID-19-Pandemie in Deutschland”. [DOI] [Google Scholar]
- 14.Schilling J., Buda S., Tolksdorf K. 2022. Zweite Aktualisierung der “Retrospektiven Phaseneinteilung der COVID-19-Pandemie in Deutschland”. [Google Scholar]
- 15.Robert Koch Institute . 2020. Falldefinition Coronavirus Disease 2019 (COVID-19) (SARS-CoV-2), Stand: 23.12.https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Falldefinition.pdf?__blob=publicationFile Available at: [Google Scholar]
- 16.Suwono B., Steffen A., Schweickert B., Schönfeld V., Brandl M., Sandfort M., et al. SARS-CoV-2 outbreaks in hospitals and long-term care facilities in Germany: a national observational study. Lancet Regional Health – Europe. 2022:14. doi: 10.1016/j.lanepe.2021.100303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robert Koch Institute. Wöchentlicher Lagebericht des RKI zur Coronavirus-Krankheit-2019 (COVID-19) 05/08/2021. Berlin.
- 18.Robert Koch Institute. Bericht zu Virusvarianten von SARS-CoV-2 in Deutschland 14/07/2021. Berlin.
- 19.Sievers C., Zacher B., Ullrich A., Huska M., Fuchs S., Buda S., et al. SARS-CoV-2 Omicron variants BA.1 and BA.2 both show similarly reduced disease severity of COVID-19 compared to Delta, Germany, 2021 to 2022. Euro Surveill. 2022;27 doi: 10.2807/1560-7917.ES.2022.27.22.2200396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Macedo A., Gonçalves N., Febra C. COVID-19 fatality rates in hospitalized patients: systematic review and meta-analysis. Ann Epidemiol. 2021;57:14–21. doi: 10.1016/j.annepidem.2021.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robert Koch Institute https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Situationsberichte/Dez_2021/2021-12-30-en.pdf?__blob=publicationFile Coronavirus disease 2019 daily situation report 30/12/2021. Available at:
- 22.Bhattacharyya R.P., Hanage W.P. Challenges in inferring intrinsic severity of the SARS-CoV-2 Omicron variant. N Engl J Med. 2022;386:e14. doi: 10.1056/NEJMp2119682. [DOI] [PubMed] [Google Scholar]
- 23.Statista. Anteil von Frauen und Männern in verschiedenen Berufsgruppen in Deutschland am 30. Juni 2021. 2022. Available at: https://de.statista.com/statistik/daten/studie/167555/umfrage/frauenanteil-in-verschiedenen-berufsgruppen-in-deutschland/[last accessed June 2022].
- 24.Brandl M., Hoffmann A., Willrich N., Reuss A., Reichert F., Walter J., et al. Bugs that can resist antibiotics but not men: gender-specific differences in notified infections and colonisations in Germany, 2010–2019. Microorganisms. 2021;9:894. doi: 10.3390/microorganisms9050894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fortunato F., Martinelli D., Lo Caputo S., Santantonio T., Dattoli V., Lopalco P.L., et al. Sex and gender differences in COVID-19: an Italian local register-based study. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2021-051506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Punjani N., Ha A., Caputo J., Wang V., Wiechmann L., Chiasson M.A., et al. Outcome disparities among men and women with COVID-19: an analysis of the New York City population cohort. J Drugs Dermatol. 2020;19:960–967. doi: 10.36849/JDD.2020.5590. [DOI] [PubMed] [Google Scholar]
- 27.Forsblom E., Silén S., Kortela E., Ahava M., Kreivi H.R., Holmberg V., et al. Male predominance in disease severity and mortality in a low Covid-19 epidemic and low case-fatality area – a population-based registry study. Infect Dis (Lond) 2021;53:789–799. doi: 10.1080/23744235.2021.1936157. [DOI] [PubMed] [Google Scholar]
- 28.Alkhouli M., Nanjundappa A., Annie F., Bates M.C., Bhatt D.L. Sex differences in case fatality rate of COVID-19: insights from a multinational registry. Mayo Clin Proc. 2020;95:1613–1620. doi: 10.1016/j.mayocp.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gebhard C., Regitz-Zagrosek V., Neuhauser H.K., Morgan R., Klein S.L. Impact of sex and gender on COVID-19 outcomes in Europe. Biol Sex Diff. 2020;11:29. doi: 10.1186/s13293-020-00304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu J., Ji H., Zheng W., Wu X., Zhu J.J., Arnold A.P., et al. Sex differences in renal angiotensin converting enzyme 2 (ACE2) activity are 17β-oestradiol-dependent and sex chromosome-independent. Biol Sex Diff. 2010;1:6. doi: 10.1186/2042-6410-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vygen-Bonnet S., Koch J., Bogdan C., Harder T., Heininger U., Kling K., et al. 2020. Beschluss und Wissenschaftliche Begründung der Ständigen Impfkommission (STIKO) für die COVID-19-Impfempfehlung; pp. 3–63. 2. [Google Scholar]
- 32.Holzmann-Littig C., Braunisch M.C., Kranke P., Popp M., Seeber C., Fichtner F., et al. COVID-19 vaccination acceptance and hesitancy among healthcare workers in Germany. Vaccines. 2021;9(7) doi: 10.3390/vaccines9070777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robert Koch Institute . Ergebnisbericht Erste Welle; 2021. Kroco – die Krankenhausbasierte Online-Befragung zur COVID-19-Impfung.https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Projekte_RKI/Kroco-Report150721.pdf?__blob=publicationFile Available at: [Google Scholar]
- 34.Bouton T.C., Lodi S., Turcinovic J., Schaeffer B., Weber S.E., Quinn E., et al. Coronavirus disease 2019 vaccine impact on rates of severe acute respiratory syndrome coronavirus 2 cases and postvaccination strain sequences among health care workers at an urban academic medical center: a prospective cohort study. Open Forum Infect Dis. 2021;8:ofab465. doi: 10.1093/ofid/ofab465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kassenärztliche Bundesvereinigung (KBV). Kennzahlen der ambulanten Versorgung auf einen Blick. Available at: https://www.kbv.de/html/zahlen.php [last accessed January 2023].
- 36.Statistisches Bundesamt (Destatis). Krankenhäuser: Einrichtungen, Betten und Patientenbewegung. Available at: https://www.destatis.de/DE/Themen/Gesellschaft-Umwelt/Gesundheit/Krankenhaeuser/Tabellen/gd-krankenhaeuser-jahre.html [last accessed January 2023].
- 37.Statistisches Bundesamt (Destatis). Anzahl der Einrichtungen, der Betten und Patientenbewegungen der Vorsorge- oder Rehabilitationseinrichtungen. Available at: https://www.destatis.de/DE/Themen/Gesellschaft-Umwelt/Gesundheit/Vorsorgeeinrichtungen-Rehabilitationseinrichtungen/Tabellen/gd-vorsorge-reha-jahre.html [last accessed January 2023].
- 38.Hetemaki I., Kaariainen S., Alho P., Mikkola J., Savolainen-Kopra C., Ikonen N., et al. An outbreak caused by the SARS-CoV-2 Delta variant (B.1.617.2) in a secondary care hospital in Finland. Euro Surveill. 2021;26(30) doi: 10.2807/1560-7917.ES.2021.26.30.2100636. May 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ngandu N.K., Mmotsa T.M., Dassaye R., Thabetha A., Odendaal W., Langdown N., et al. Hospital acquired COVID-19 infections amongst patients before the rollout of COVID-19 vaccinations, a scoping review. BMC Infect Dis. 2022;22:140. doi: 10.1186/s12879-022-07128-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hahn U., Baulig C., Brzoska P. [Structures of outpatient medical care: Germany and other decentrally organized healthcare systems] Gesundheitswesen (Bundesverband der Arzte des Offentlichen Gesundheitsdienstes (Germany)) 2021;83:337–344. doi: 10.1055/a-1390-3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boender T.S., Bender J.K., Krüger A., Michaelis K., Buchholz U. Factors preventing SARS-CoV-2 transmission during unintentional exposure in a GP practice: a cohort study of patient contacts; Germany, 2020. Epidemiol Infect. 2021;149:e161. doi: 10.1017/S0950268821001503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Negm A.M., Salopek A., Zaide M., Meng V.J., Prada C., Chang Y., et al. Rehabilitation care at the time of coronavirus disease-19 (COVID-19) pandemic: a scoping review of health system recommendations. Front Aging Neurosci. 2022;13 doi: 10.3389/fnagi.2021.781271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robert Koch Institute . 2022. Serologische Untersuchungen von Blutspenden auf Antikörper gegen SARS-CoV-2 (SeBluCo-Studie)https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Projekte_RKI/SeBluCo_Zwischenbericht.html Available at: [Google Scholar]
- 44.Robert Koch Institute. SARS-CoV-2-Seroprävalenz in der Allgemeinbevölkerung in Deutschland – Aktualisierung Februar 2022. Available at: https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/AK-Studien/Factsheet.pdf?__blob=publicationFile [last accessed April 2022].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.