Abstract
Background and study aims Accurate assessment of the lymph node (LN) status is crucial in resectable perihilar cholangiocarcinoma (pCCA) to prevent major surgery in patients with extraregional metastatic LNs (MLNs). This study investigates the added value of preoperative endoscopic ultrasound (EUS) with or without tissue acquisition (TA) for the detection of MLNs in patients with resectable pCCA.
Patients and methods In this retrospective, multicenter cohort study, patients with potentially resectable pCCA who underwent EUS preoperatively between 2010–2020, were included. The clinical impact of EUS-TA was defined as the percentage of patients who did not undergo surgical resection due to MLNs found with EUS-TA. Findings of cross-sectional imaging were compared with EUS-TA findings and surgery.
Results EUS was performed on 141 patients, of whom 107 (76 %) had suspicious LNs on cross-sectional imaging. Surgical exploration was prevented in 20 patients (14 %) because EUS-TA detected MLNs, of which 17 (85 %) were extraregional. Finally, 74 patients (52 %) underwent surgical exploration followed by complete resection in 40 (28 %). MLNs were identified at definitive pathology in 24 (33 %) patients, of which 9 (38 %) were extraregional and 15 (63 %) regional.
Conclusions EUS-TA may be of value in patients with potentially resectable pCCA based on preoperative cross-sectional imaging, regardless of lymphadenopathy at cross-sectional imaging. A prospective study in which a comprehensive EUS investigation with LN assessment and EUS-TA of LNs is performed routinely should confirm this promise.
Introduction
Perihilar cholangiocarcinoma (pCCA) is a rare malignancy originating from the second-degree bile ducts up to the insertion of the cystic duct 1 . Survival of all pCCA patients is dismal, with diagnoses often in an advanced stage. The resectability of pCCA depends on biliary tumor extension and vascular involvement. Prognosis however mainly depends on the presence of regional metastatic lymph nodes (MLNs) and distant metastases 2 3 .
MLNs are present in about half of patients at presentation and associated with poor survival 2 3 4 . In patients with extraregional MLNs, the limited oncological advantage of surgery may not outweigh the substantial surgical risks 4 5 6 . Therefore, accurate preoperative lymph node (LN) assessment, according to The American Joint Committee on Cancer (AJCC) staging system, is crucial 7 8 9 . In current practice, computed tomography (CT) is recommended to identify MLNs and in some guidelines also magnetic resonance imaging (MRI) 10 11 . Accurate identification of MLNs preoperatively on both CT and MRI is challenging because sensitivity and specificity are 61 % and 88 %, respectively, for CT and 64 % and 68 %, respectively, for MRI 12 13 . More accurate detection of MLNs preoperatively saves more patients from unnecessary invasive surgical treatments.
To improve LN staging, endoscopic ultrasound with tissue acquisition (EUS-TA) of the LN through fine-needle aspiration (FNA) or fine-needle biopsy (FNB) may show benefit 14 . In a study of 47 pCCA patients screened for liver transplantation as curative treatment option, EUS-FNA identified malignant LNs in eight (17 %) patients, of which only two patients had suspicious LNs on cross-sectional imaging 15 . On the other hand, a study from the USA showed that CT was more often able to detect malignant LNs (4 of 22 patients, 18 %) compared to EUS-FNA (2 of 23 patients, 9 %) 16 . In patients with positive extraregional LNs, surgical exploration is almost always precluded. But in patients with only regional MLNs, surgery is still an option and only in selected cases is surgery precluded.
In conclusion, data is scarce and inconsistent about the added value and impact of EUS-TA for clinical decision-making in the setting of pCCA 14 15 16 . Therefore, the aim of this study is determine the yield of EUS-TA and the subsequent change in clinical management.
Patients and methods
Study population
A retrospective, multicenter cohort study was performed at three Dutch tertiary referral centers for pancreato-biliary diseases. All consecutive patients with potentially resectable pCCA, who underwent EUS preoperatively (with or without EUS-TA) and who were discussed at a multidisciplinary meeting between January 2010 and June 2020, were eligible for inclusion. EUS was not part of the Dutch preoperative surgical work-up guidelines, but at the discretion of the local management team. Exclusion criteria were surgically treated pCCA or unresectable pCCA at time of diagnosis. This study was conducted according to the guidelines in the Helsinki Declaration and institutional review board approval was obtained in participating centers.
Regional and extraregional LN locations
Regional LN consisted of LNs at the liver hilum, cystic duct, common bile duct, hepatic artery and portal vein. Extraregional LNs consisted of LNs at the peri-aortic region, peri-caval region, superior mesenteric artery and celiac trunk. LNs that were not covered by these locations were noted separately and were interpreted as extraregional when located distal to the hepatoduodenal ligament, as described by the EUS. The most important difference between the 7 th and the newer 8 th edition AJCC staging system is the location of the regional (N1) versus extraregional LN (N2) in the 7 th edition, while in the 8 th edition extraregional LN locations are considered M1 metastases and the number of MLN determines the N stage ( Table 1 ) 7 8 9 .
Table 1. N staging of the 7 th and 8 th edition of the AJCC staging system.
| N1 | N2 | M1 | |
| AJCC 7 th edition | ≥ 1 MLN in the regional LNs (H, CD, CBD, HA or PV LNs) | ≥ 1 MLN in the regional LNs (PA, PC, SMA or CO LNs) | Distant metastasis |
| AJCC 8 th edition | 1–3 MLN in the regional LNs (H, CD, CBD, HA, PPD or PV LNs) | ≥ 4 MLN in the regional LNs described for N1 | Distant metastasis (includes MLN distant to the HDL) |
For both 7th and 8th edition of the AJCC staging system, Nx is defined as “Regional LNs cannot be assessed” and N0 as “No regional MLN”.
H, hilar; CD, cystic duct; CBD, common bile duct; HA, hepatic artery; PV, portal vein; PPD, posterior pancreato-duodenal; PA, peri-aortic; PC, peri-caval; SMA, superior mesenteric artery; CO, celiac; HDL, hepato-duodenal ligament.
EUS procedure and work-up for surgery
EUS procedures were performed using a linear ultrasound endoscope (Olympus GF-UCT-160 or GF-UCT-180 and Pentax EG-3870 UTK, EG-3270 UK or EG38-J10 UT). For FNA and FNB 19-, 20-, 22- or 25-G needles were used (Cook Medical). The indication for EUS was categorized in: assessment of suspicious bile duct mass, LN assessment due to suspicious LNs on cross-sectional imaging (e. g., necrotic center and/or short axis >10mm), liver transplantation screening, and resectability assessment (e. g., ductal extension). Comprehensive LN assessment and EUS-TA of LNs were not an integral part of the procedure. Both LN assessment due to (a specific) suspicious LNs on cross-sectional imaging and liver transplantation screening were considered as EUS indications specific for LN assessment. LNs were defined as suspicious on EUS based on appearance (short axis diameter > 5 mm, hypoechoic, round shape and clear margins) according to the opinion of the endosonographer. TA was performed in a low threshold manner, but whenever multiple suspicious LN were identified in a single patient in one EUS procedure, the endosonographer could decide to perform EUS-TA in one or a subset of these LNs. Whenever a specific LN was suspicious on cross-sectional imaging, this was often targeted with EUS-TA. Rapid on-site evaluation was not routinely performed. After EUS, patients were re-discussed at multidisciplinary team meetings. For patients with regional MLN surgery could be precluded whenever the patient also had significant comorbidities, negatively affecting post-surgical outcomes. For patients with extraregional MLN surgery is almost always precluded, excluding young patients without comorbidities. Pathology results of the LN were categorized by the pathologist into malignant, suspicious for malignancy, no malignancy and non-diagnostic. LNs were considered as positive for malignancy if EUS-TA results were rated as suspicious for malignancy or malignant. Both regional and extraregional LNs removed during surgery were evaluated by the pathologist.
Outcomes
The primary outcome of this study was the yield of EUS-TA and subsequent change in clinical management, as defined as the number of patients in whom surgery was withheld because of MLNs found by EUS-TA.
The secondary outcome was the accuracy of cross-sectional imaging, defined as the number of patients that had confirmed MLNs by EUS-TA or surgery, for patients with and without suspicious LNs on cross-sectional imaging. Actual accuracy of cross-sectional imaging could not be determined, since LN status after surgery cannot be considered the gold standard. This is because not all patients undergo surgery, and during surgery in pCCA patients very few extraregional LNs are usually assessed and removed.
Data collection
Data were collected on patient demographics, cross-sectional imaging, EUS, surgery and clinical outcomes. On cross-sectional imaging, LNs were defined as suspicious based on location, heterogeneity and size criteria according to the objective assessment of reporting radiologists. LNs identified at surgical procedures were collected, at staging laparoscopy, explorative laparotomy, and surgical resection.
Statistical analysis
The statistical analysis contained descriptive statistics using medians (with interquartile ranges (IQR)) for continuous not normally distributed variables and using frequencies and proportions for categorical and dichotomous variables. Categorical and dichotomous variables were analyzed using the Chi-square test. The Fisher exact test was used if any categories have a frequency < 5. Subgroup analysis was performed for two patient groups: with or without an EUS with LN assessment as indication. A two-sided P < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS Statistics version 25.
Results
Baseline patient characteristics
In total, 141 patients were included with potentially resectable pCCA who underwent EUS preoperatively. Table 2 lists the baseline characteristics of the study population.
Table 2. Baseline characteristics of study population.
| Variable | All resectable pCCA patients with preoperative EUS performed (n = 141) |
| Age at diagnosis, median [IQR], years | 63 [55 – 71] |
| Male gender – n (%) | 93 (66 %) |
| PSC – n (%) | 27 (19 %) |
| ASA physical status score – n (%) 1 | |
|
30 (21 %) |
|
77 (55 %) |
|
33 (24 %) |
| WHO performance status – n (%) 2 | |
|
74 (55 %) |
|
49 (37 %) |
|
10 (8 %) |
|
1 (1 %) |
| Imaging performed prior to EUS – n (%) | |
|
28 (20 %) |
|
5 (4 %) |
|
108 (77 %) |
pCCA, perihilar cholangiocarcinoma; EUS, endoscopic ultrasound; PSC, primary sclerosing cholangitis; ASA, American Society of Anesthesiologists; WHO, World Health Organization; CT, computed tomography; MRI, magnetic resonance imaging; MRCP, magnetic resonance cholangiopancreatography.
Missing in one patient.
Missing in seven patients.
EUS characteristics
A total of 169 EUS procedures were performed in these 141 patients ( Table 3 ). In 77 of 141 patients (55 %), at least one EUS with LN assessment was performed.
Table 3. Characteristics of 169 EUS procedures across 141 patients.
| Variable | Total no. EUS (n = 169) |
| Location of EUS – n (%) 1 | |
|
125 (74 %) |
|
44 (26 %) |
| Drainage prior to EUS – n (%) | |
|
65 (39 %) |
|
10 (6 %) |
|
1 (1 %) |
| Cholangitis < 1 month prior to EUS – n (%) | 8 (5 %) |
| Indication for EUS – n (%) | |
|
74 (44 %) |
|
68 (40 %) |
|
17 (10 %) |
|
10 (6 %) |
| Any LN described at EUS – n (%) | 117 (69 %) |
EUS, endoscopic ultrasound; ERCP, endoscopic retrograde cholangiopancreatography; PTCD, percutaneous drain; LN, lymph node.
A total of three EUS procedures were performed in four patients (3 %), two EUS procedures in 20 patients (14 %) and only one EUS procedure in 117 patients (83 %).
Across 96 of 141 patients (68 %), a total of 161 LN locations were described as shown in Table 4 . EUS-TA was successfully performed in 67 of 96 patients (70 %) across a total of 88 of 161 (55 %) LNs. In suspicious LNs identified with EUS, EUS-TA was successfully performed in 81 of 130 LNs (62 %) and not successful in nine LNs (7 %) due to scope position, intermediary tissue or patient unrest. In non-suspicious LNs, EUS-TA was successfully performed in seven of 31 LNs (23 %). Of these 88 biopsied LNs, 23 (26 %) were classified as malignant, 53 (60 %) as non-malignant, 11 (13 %) as non-diagnostic, and one (1 %) was missing. FNA was performed in 75 of 88 (85 %), confirming malignancy in 20 of 75 (27 %), FNB in 12 of 88 (14 %) with confirmed malignancy in three of 12 (25 %) and both techniques in one LN (1 %) showing no malignancy. Cholangitis prior to EUS was not associated with a lower yield (2 of 8 (25 %) vs 21 of 161 (13 %), P = 0.3).
Table 4. Characteristics of all described LNs by EUS.
| Described LN | # | Successful EUS-TA | EUS-TA not possible | Pathology results | |||||
| FNA | FNB | Both | Malignant | Non-malignant | Non-diagnostic | Missing | |||
| Regional | |||||||||
|
52 | 20 | 5 | 1 | 2 × | 5 | 16 | 5 | 0 |
|
12 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | |
| Extraregional | |||||||||
|
78 | 48 | 7 | 0 | 7 × | 17 | 31 | 6 | 1 |
|
19 | 6 | 0 | 0 | 1 | 5 | 0 | 0 | |
| Total | 161 | 75 | 12 | 1 | 9 × | 23 | 53 | 11 | 1 |
LN, lymph node; EUS, endoscopic ultrasound; EUS-TA, endoscopic ultrasound with tissue acquisition; FNA, fine-needle aspiration; FNB, fine-needle biopsy.
Impact of EUS-TA on clinical decision-making
In the 141 patients, there were 65 (46 %) in whom one or more extraregional LNs were described at EUS, 31 (22 %) in whom only regional LNs were described and 45 (32 %) in whom no LNs were described. In 27 of 65 patients (42 %) in whom one or more extraregional LNs were described, at the same time one or more regional LNs were also described. EUS-TA was performed in 61 of 97 extraregional LNs (63 %), showing malignancy in 18 of 61 (30 %). Regarding regional LNs, EUS-TA was performed in in 27 of 64 regional LNs (42 %), showing malignancy in five of 27 (19 %). In two patients EUS-TA proved malignancy in multiple LNs. Surgical exploration was precluded due to positive EUS-TA in 20 patients (14 %); due to extraregional MLNs in 17 patients and due to regional MLNs in three patients. PSC diagnosis was not associated with a higher preclusion rate (1 of 27 (4 %) vs 19 of 114 (17 %), P = 0.1).
After EUS, six patients (4 %) were treated in another hospital and were recorded as loss to follow-up and 41 (29 %) were precluded from surgery due to other reasons ( Fig. 1 ). In the remaining patients, the probable diagnosis of resectable pCCA prevailed for which radical resection was the only curative treatment option. Two patients proceeded to surgical exploration, despite extraregional MLNs at EUS-TA in one and regional MLNs at EUS-TA in the other. Finally, explorative surgery was performed in 74 patients (52 %), with complete resection in 40 patients (28 %). In 34 patients (24 %) only explorative surgery was performed, due to regional or extraregional MLNs in 13 (38 %), advanced or metastatic disease in 13 (38 %) and other reasons in eight (24 %). The median period from last EUS to first surgical procedure in 74 patients was 49 days [IQR:31–76]. One patient died during explorative laparotomy and one patient had a missing pathology report. In 24 of 72 patients (33 %) without preoperative confirmation of MLNs by EUS-TA, MLNs were identified during explorative surgery or in surgical resection specimens. These were only regional in 15 (63 %) and at one or more extraregional MLNs were described in nine patients (38%). These extraregional LNs were located at the celiac trunk in three (33 %), peri-aortic region in two (22 %), around the pancreatic tail and body in two (22 %), periduodenal in one (11 %) and the aorta-caval region in one (11 %). In the patients undergoing complete resection without preoperative EUS-TA-proven MLNs, benign disease was identified in surgical specimens in 11 patients (30 %).
Fig. 1 .
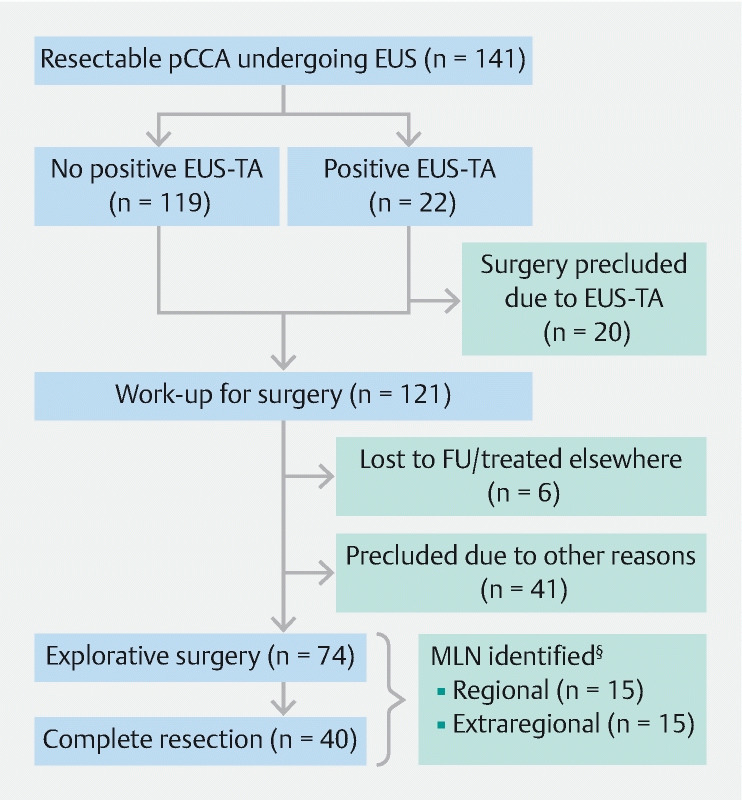
Flowchart of patients included in this study. § In 72 patients, because of missing pathology in one patient and one patient with cardiac arrest during surgery, before any resection. Two patients had preoperative confirmation of MLN by EUS-TA and underwent surgery. The first patient underwent a diagnostic laparoscopy which showed locally advanced disease. The second patient underwent left hemi-hepatectomy with regional MLN. These patients are not taken into account.
Differences between the two indication groups
Comparing the clinical impact between the group in which at least one EUS was performed for LN assessment specifically and the group in which EUS was performed for other reasons, a higher clinical impact was found for the former (15 of 77 (20 %) vs 5 of 64 (12 %), P = 0.048). There was no difference regarding identification of MLNs at surgery in these two groups (14 of 36 (39 %) vs 10 of 34 (29 %), P = 0.4).
Comparison with cross-sectional imaging
In the entire group of 141 patients, cross-sectional imaging showed suspicious LN in 107 patients (76 %) and no suspicious LNs in 34 patients (24 %) ( Fig. 2 ). In these 34 patients, EUS-TA was performed in six patients; five extraregional LNs and in one regional LN. Malignancy was identified in two of 34 patients (6%), both in extraregional LNs. In 16 patients (47 %) surgery was performed, of whom four patients (25 %) had MLNs identified. Of these four patients, two had extraregional MLNs at the celiac trunk and aorta-caval region respectively, both identified at explorative surgery. Benign disease was confirmed in four patients (12 %) in surgical specimens.
Fig. 2 .
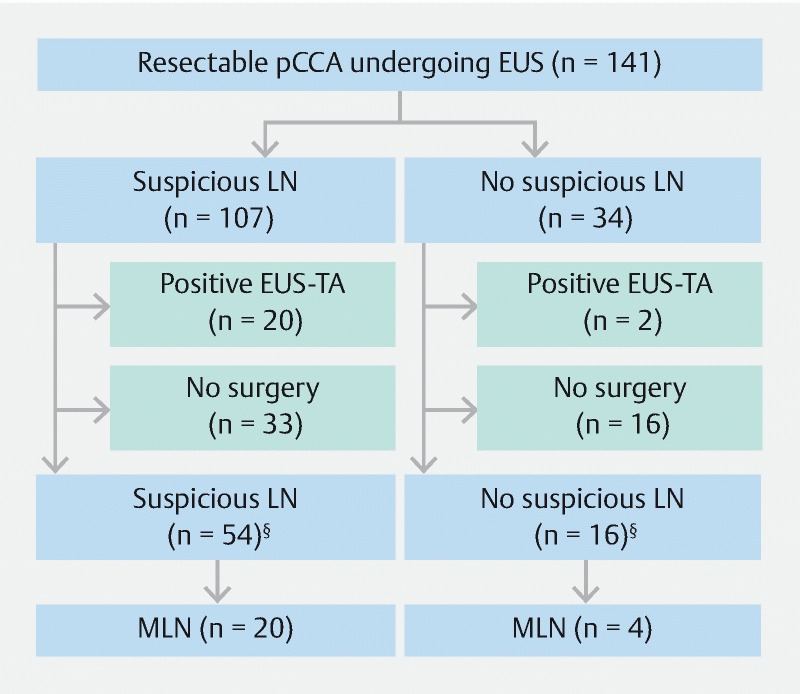
Flowchart of patients included in this study, according to imaging findings. § For patients without preoperative confirmation of MLN by EUS-TA.
In the 107 patients with suspicious LNs at cross-sectional imaging, EUS-TA was performed in 61 patients (57 %); 37 extraregional LNs, 20 regional LNs and in four patients both. Malignancy was identified by EUS-TA in 20 of 107 patients (19 %), of which 16 (80 %) were extraregional. In 54 patients (51 %) surgery was performed without preoperative confirmation of MLN, of whom 20 patients (37 %) had MLN identified. In eight patients (40 %) these were extraregional LN. The yield of EUS-TA was similar for patients with and without suspicious LNs on cross-sectional imaging (19 % vs 6 %, P = 0.1). In the patients undergoing surgery, MLNs were identified in both groups (37 % vs 25 %, P = 0.37). Benign disease was confirmed in seven patients (7 %).
Discussion
In this multicenter cohort study, we demonstrated that in 14 % of patients eligible for surgery with presumed resectable pCCA undergoing EUS, MLNs were identified by EUS-TA that prevented surgery. This was primarily influenced by already suspicious LNs identified on cross-sectional imaging investigations. Unfortunately, we were unable to confirm nodal status in patients not undergoing any form of surgery, limiting the interpretation on the missed LNs with EUS. The accuracy of cross-sectional imaging pertaining to LN involvement was limited, as only in 37 % of the patients that had suspicious LNs on cross-sectional imaging, MLNs were confirmed by EUS-TA or surgery. Whenever cross-sectional imaging detected no suspicious LNs, EUS-TA still affected clinical decision-making in 6 % of the patients.
The primary aim of our study was to assess the influence of EUS on clinical decision-making in patients with presumed resectable pCCA. We found that in 14 % of the patients, surgery was precluded due to positive EUS-TA. As expected, EUS performed to assess LNs yielded more MLNs than for other EUS indications (20 % vs 12 %, P = 0.048). Two retrospective studies from the Mayo Clinic reported similar findings 14 15 . In the study by Gleeson et al, 47 liver transplantation candidates underwent EUS-TA of all identified LN 15 . FNA of 70 LNs identified MLNs in nine LNs in eight patients (17 %), precluding all of them from transplantation. Similarly, in the more recent study by Malikowski et al, 20 of 124 patients (16 %) with pCCA had MLNs precluding them from surgery 14 . This differed from our clinical practice, as for most patients with only regional MLNs we find resection a feasible option. Also, we did not check all LN locations systematically. In addition, we did not perform EUS-TA routinely whenever the LN was not suspicious.
MLN were identified during surgery in one of three patients, more often in patients with lymphadenopathy on cross-sectional imaging. Although not directly comparable, this “miss” rate was higher than in the two studies from the Mayo Clinic. Gleeson et al. described 22 patients without preoperative confirmation of MLN by EUS-TA that underwent explorative laparotomy, with identification of MLN in only two patients (9 %) 15 . Malikowski et al. did not report the number of missed LNs for pCCA, but for all cholangiocarcinoma types 14 . Of the 130 patients without MLNs by EUS-TA, 80 (62 %) proceeded to staging laparotomy, with identification of MLNs in four patients (5 %). This can be due to the non-systematic method we used and high threshold of EUS-TA. Another explanation is that for patients enrolled in pCCA LT work-up at the Mayo Clinic, both regional and extraregional LNs are targeted while in patients worked-up for radical resection, primarily extraregional LNs are important. It is important to assess LN status preoperatively, as in a recent meta-analysis, MLNs were associated with poorer disease-free survival 17 .
Cross-sectional imaging has limited accuracy regarding LN involvement. In the patients with suspicious LNs on cross-sectional imaging, confirmation of MLN was found in 37 % during EUS-TA and/or surgery, in comparison to 18 % patients without suspicious LNs on cross-sectional imaging. This is line with the results from Malikowski et al describing that presence of lymphadenopathy on cross-sectional imaging was significantly associated with MLNs at EUS-TA 14 . In patients without clear lymphadenopathy on cross-sectional imaging, EUS-TA identified MLNs in 11 % for all cholangiocarcinoma subtypes. Unfortunately, cross-sectional imaging has limited performance to adequately define LN involvement in pCCA 14 15 . Positron emission tomography/CT is not recommended as standard procedure for preoperative LN assessment because the sensitivity is only 33 % with a specificity of 97 % 18 . In daily practice, radiologists define lymphadenopathy in the upper abdomen primarily on size criteria, a short axis of > 10 mm. However, there are various reports that the size-criterion is not specific enough for various cancers 19 20 . Cross-sectional imaging could potentially assist in locating LNs to facilitate a more targeted approach for EUS-TA. This strategy is probably less useful in patients with PSC, as enlarged benign LNs are often identified at cross-sectional imaging.
Our study is the largest retrospective multicenter study on the value of preoperative EUS in patients with suspected resectable pCCA. By including all patients with potentially resectable pCCA, instead of pathologically proven pCCA, the actual impact of preoperative EUS is assessed. However, our study has some limitations. First, due to the retrospective nature, data regarding specific LN locations and characteristics were often reported with little details. Because of this, we were unable to use the 8 th AJCC classification as the number of LNs were often inadequately described. Also due to the retrospective nature, LN evaluation and subsequent EUS-TA was performed differently. In some patients multiple suspicious LNs were identified and EUS-TA was only performed in one LN. In some patients with only suspicious regional LNs no EUS-TA was performed, as resection was still considered an option for these patients regardless of regional LN status. The patients included in our study were discussed in three different multidisciplinary team meetings, potentially affecting the clinical decision-making success rate. Second, because our study focused on the role of EUS and, therefore, only patients who had undergone an EUS were included, the accuracy of cross-sectional imaging for LN detection may have been overestimated. Possibly, we have performed EUS more often in patients with advanced disease so patients that were not included in our study had a lower prevalence of suspicious LNs on cross-sectional imaging. We were unable to report the total number and/or findings in patients with pCCA not undergoing EUS. Third, the yield of EUS may increase with systematic EUS-TA of all LNs, which was not clinical practice at the time of this study. Therefore, our results most likely underrepresent the role of EUS-TA in these patients. In good clinical practice, all LN locations should be routinely evaluated, sampled, and described in the report.
Conclusions
Our study combined with previous evidence shows that in patients with presumed resectable pCCA, clinical decision-making can be influenced significantly by EUS-TA with far-reaching consequences for individual patients. Also, our study supports further prospective evaluation of routine implementation of EUS in patients with potentially resectable pCCA.
Footnotes
Competing interests Dr. Bruno received research funding for industry-initiated studies from Boston Scientific and Cook Medical. He received research funding for investigator initiated studies from Boston Scientific, Cook Medical, Pentax Medical, Interscope, Mylan and ChiRoStim. He is a consultant to Boston Scientific, Cook Medical, and Pentax Medical. Dr. Voermans received research funding for investigator initiated studies from Boston Scientific and Prion Medical. He is a consultant with speakers fee for Boston Scientific.
References
- 1.Blechacz B, Komuta M, Roskams T et al. Clinical diagnosis and staging of cholangiocarcinoma. Nat Rev Gastroenterol Hepatol. 2011;8:512–522. doi: 10.1038/nrgastro.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blechacz B. Cholangiocarcinoma: Current knowledge and new developments. Gut Liver. 2017;11:13–26. doi: 10.5009/gnl15568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Radtke A, Konigsrainer A. Surgical therapy of cholangiocarcinoma. Visc Med. 2016;32:422–426. doi: 10.1159/000452921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khan S A, Davidson B R, Goldin R D et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: an update. Gut. 2012;61:1657–1669. doi: 10.1136/gutjnl-2011-301748. [DOI] [PubMed] [Google Scholar]
- 5.Banales J M, Cardinale V, Carpino G et al. Expert consensus document: Cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA) Nat Rev Gastroenterol Hepatol. 2016;13:261–280. doi: 10.1038/nrgastro.2016.51. [DOI] [PubMed] [Google Scholar]
- 6.Cillo U, Fondevila C, Donadon M et al. Surgery for cholangiocarcinoma. Liver Int. 2019;39:143–155. doi: 10.1111/liv.14089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amin M B, Gress D M, Meyer Vega LR . New York: Springer International Publishing; 2017. AJCC Cancer Staging Manual, Eighth Edition; pp. 295–301. [Google Scholar]
- 8.Edge S B, Compton C C, Fritz A G . New York: Springer International Publishing; 2010. Trotti. American joint committee on cancer: Cancer staging manual, 7 ed; pp. 201–234. [Google Scholar]
- 9.Gaspersz M P, Buettner S, van Vugt J LA et al. Evaluation of the new american joint committee on cancer staging manual 8th edition for perihilar cholangiocarcinoma. J Gastrointest Surg. 2020;24:1612–1618. doi: 10.1007/s11605-019-04127-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cai Y, Cheng N, Ye H et al. The current management of cholangiocarcinoma: A comparison of current guidelines. Biosci Trends. 2016;10:92–102. doi: 10.5582/bst.2016.01048. [DOI] [PubMed] [Google Scholar]
- 11.[Anonymous]. Galweg- en galblaascarcinoom, landelijke richtlijn [Internet]. Integraal Kankercentrum Nederland; c2003–2013Available from (Accessed June 1 2022)https://richtlijnendatabase.nl/richtlijn/galweg_en_galblaascarcinoom/algemeen.html
- 12.Ruys A T, van Beem B E, Engelbrecht M R et al. Radiological staging in patients with hilar cholangiocarcinoma: a systematic review and meta-analysis. Br J Radiol. 2012;85:1255–1262. doi: 10.1259/bjr/88405305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanninen E L, Pech M, Jonas S et al. Magnetic resonance imaging including magnetic resonance cholangiopancreatography for tumor localization and therapy planning in malignant hilar obstructions. Acta Radiol. 2005;46:462–470. doi: 10.1080/02841850510021625. [DOI] [PubMed] [Google Scholar]
- 14.Malikowski T, Levy M J, Gleeson F C et al. Endoscopic ultrasound/fine needle aspiration is effective for lymph node staging in patients with cholangiocarcinoma. Hepatology. 2020;72:940–948. doi: 10.1002/hep.31077. [DOI] [PubMed] [Google Scholar]
- 15.Gleeson F C, Rajan E, Levy M J et al. EUS-guided FNA of regional lymph nodes in patients with unresectable hilar cholangiocarcinoma. Gastrointest Endosc. 2008;67:438–443. doi: 10.1016/j.gie.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 16.Mohamadnejad M, DeWitt J M, Sherman S et al. Role of EUS for preoperative evaluation of cholangiocarcinoma: a large single-center experience. Gastrointest Endosc. 2011;73:71–78. doi: 10.1016/j.gie.2010.08.050. [DOI] [PubMed] [Google Scholar]
- 17.Liang L, Li C, Jia H D et al. Prognostic factors of resectable perihilar cholangiocarcinoma: a systematic review and meta-analysis of high-quality studies. Ther Adv Gastrointest Endosc. 2021;14 doi: 10.1177/2631774521993065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furukawa H, Ikuma H, Asakura-Yokoe K et al. Preoperative staging of biliary carcinoma using 18F-fluorodeoxyglucose PET: prospective comparison with PET+CT, MDCT and histopathology. Eur Radiol. 2008;18:2841–284719. doi: 10.1007/s00330-008-1062-2. [DOI] [PubMed] [Google Scholar]
- 19.Loch F N, Asbach P, Haas M et al. Accuracy of various criteria for lymph node staging in ductal adenocarcinoma of the pancreatic head by computed tomography and magnetic resonance imaging. World J Surg Oncol. 2020;18:213. doi: 10.1186/s12957-020-01951-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhuang Z, Zhang Y, Wei M et al. Magnetic resonance imaging evaluation of the accuracy of various lymph node staging criteria in rectal cancer: a systematic review and meta-analysis. Frontier Oncology. 2021;11 doi: 10.3389/fonc.2021.709070. [DOI] [PMC free article] [PubMed] [Google Scholar]


