Abstract
Background and study aims Texture and color enhancement imaging (TXI) is a new image-enhanced endoscopy that augments subtle tissue differences and color changes in gastric mucosa. This study aimed to compare the usefulness of TXI and white light imaging (WLI) for diagnosing Helicobacter pylori gastritis.
Patients and methods We retrospectively prepared one image set with 22 endoscopic images acquired by WLI and TXI from 60 consecutive patients individually. Five endoscopists independently reviewed the randomly displayed image sets and assessed the H. pylori infection status on endoscopy according to the Kyoto Classification of Gastritis. The primary endpoints were the accuracies of WLI and TXI in diagnosing H. pylori -active gastritis. The correlation of the endoscopic features with the three H. pylori infection statuses (current infection, past infection, and noninfection) was also evaluated.
Results Diagnostic accuracy for active gastritis was significantly higher in TXI than in WLI (85.3 % vs. 78.7 %; P = 0.034). All the specific endoscopic features associated with H. pylori infection statuses had a higher odds ratio with TXI than with WLI. Additionally, interobserver agreement among the five reviewers was higher in TXI than in WLI, except for one pair.
Conclusions TXI may improve the endoscopic diagnosis accuracy for H. pylori infection.
Introduction
Helicobacter pylori infection is closely associated with gastric carcinogenesis 1 2 3 4 5 . The International Agency for Research on Cancer Working Group Report recommends that all countries should consider the introduction of population-based H. pylori screening and treatment programs as a strategy for gastric cancer prevention 6 . In Japan, the National Health Insurance system covers the H. pylori test and eradication therapy for patients with endoscopically confirmed H. pylori -active gastritis 7 . Endoscopic diagnosis of H. pylori infection in a patient indicates a high risk of developing gastric cancer, and facilitates provision of H. pylori eradication therapy to reduce morbidity of gastric cancer. In 2014, the Kyoto Classification of Gastritis was published according to the endoscopic characteristics of gastritis associated with H. pylori infection to diagnose H. pylori infection accurately and assess the risk factors of gastric cancer 8 9 . However, it is often difficult with white light imaging (WLI) to access various endoscopic features accurately.
Compared with WLI, image-enhanced endoscopy (IEE) improves the accuracy of the endoscopic diagnosis of H. pylori -active or inactive gastritis. Under thorough examination of the gastric mucosal pattern, magnifying endoscopy with narrow-band imaging can accurately diagnose mucosal atrophy and intestinal metaplasia 10 11 . Linked color imaging is also useful for diagnosing intestinal metaplasia, diffuse redness, and map-like redness during routine endoscopy 12 13 14 15 . However, there is no report to investigate the usefulness of IEE for diagnosing H. pylori gastritis based on the Kyoto Classification of Gastritis.
In 2020, Olympus Medical Systems Corporation (Tokyo, Japan) developed a new IEE called texture and color enhancement imaging (TXI), which enhances three image factors in WLI (texture, brightness, and color), improves brightness, and expands color range; thus, TXI enables easier recognition of slight differences in mucosal color 16 . Given these features, the visibility of endoscopic findings associated with H. pylori infection during routine endoscopy may be improved by TXI. In this study, we aimed to analyze the efficacy of TXI to diagnose H. pylori -active gastritis in comparison with WLI based on the Kyoto Classification of Gastritis.
Patients and methods
Patients
This retrospective single-center pilot study was conducted at Chiba Cancer Center in Chiba, Japan. It included 60 consecutive patients who underwent esophagogastroduodenoscopy with a TXI system at Chiba Cancer Center between January 2021 and June 2021. All were asymptomatic screening patients. Patients who had a history of prior gastric cancer or gastrectomy were excluded.
The ethics committee of Chiba Cancer Center approved the study protocol, which was displayed on the notification board for inpatients and outpatients (R02–252). This study conformed to the World Medical Association’s Declaration of Helsinki. All patients had provided informed consent to undergo endoscopy.
Endoscopic procedure
Before the endoscopic examination, all patients received a mixture of 100 mL of water, 20,000 U of Pronase (Kaken Pharmaceutical, Tokyo, Japan), 1 g of sodium bicarbonate, and 10 mL of dimethicone (20 mg/mL; Kissei Pharmaceutical Co., Ltd., Nagano, Japan). After the mucus and bubbles were rinsed away, the endoscopic examination was initiated.
We used the new endoscopic system employing the CV-1500 light source equipped with a TXI system, as well as the GIF-H290Z endoscope (Olympus Medical Systems, Tokyo, Japan). TXI has two settings: mode 1 (with color enhancement) and mode 2 (without color enhancement, which appears closer to WLI color tone). This study used the TXI mode 1. One expert (YK) took all the endoscopic images. Based on the systematic screening protocol for the stomach (SSS), endoscopic screening was performed with both WLI and TXI 17 .
H. pylori infection status
The H. pylori infection status was evaluated by using the urea breath test (UBT; cutoff value < 2.5 %, UBIT, Otsuka, Tokyo, Japan) and the serum antibody test (HpAb; cutoff < 10 U/mL, E-plate, Eiken, Tokyo, Japan). When the UBT or HpAb was positive, the H. pylori infection status was considered as current infection. When both the UBT and HpAb were negative, the patient had no H. pylori infection. When a H. pylori -negative patient had a definite history of eradication therapy, the H. pylori infection status was regarded as past infection. Absence of H. pylori infection after H. pylori eradication therapy was confirmed using the UBT. Among H. pylori -negative patients, those without a history of eradication therapy were classified as the noninfection group. Proton pump inhibitor or histamine blocker was stopped at least 2 weeks prior to the UBT.
Review of endoscopic images
For evaluating endoscopic gastritis, we prepared one image set per patient; each set had 22 endoscopic images acquired by both WLI and TXI, including the antegrade and retroflex views of each part of the stomach, according to the SSS 17 . The image sets of WLI and TXI were randomly displayed and independently reviewed by five skilled endoscopists. These professionals were board certified by the Japanese Gastrointestinal Endoscopy Society and had over 5 years’ experience using IEE. The endoscopists were blinded to the H. pylori test results and did not view any of these images before this study. According to the Kyoto Classification of Gastritis 8 9 , the reviewers assessed the H. pylori infection status as either current infection, past infection, or noninfection on endoscopy.
We previously had some consensus meetings, and the endoscopic findings of Kyoto Classification of Gastritis with each modality were confirmed using an atlas of representative endoscopic images ( Fig. 1 ).
Fig. 1 .
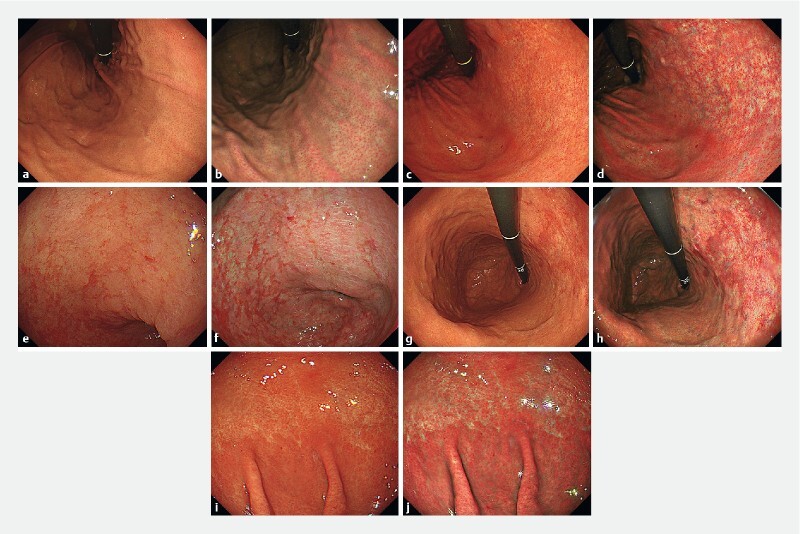
Representative endoscopic images. Endoscopic images of the regular arrangement of collecting venules (RAC) in the stomach. a WLI. Venular accumulation is noted with an increase in minute points, and it is regularly distributed in the body of the stomach. b TXI. Regular tiny veins are depicted more clearly. Endoscopic images of the atrophic border in the stomach. c WLI. An atrophic border is found at the lesser curvature of the gastric body. d TXI. The atrophic border is more identified. Endoscopic images of intestinal metaplasia in the stomach. e WLI. Slightly elevated whitish patches are observed in the antrum of the stomach. f TXI. Whitish patches become more noticeable. Endoscopic images of map-like redness in the stomach. g WLI. Reddish depressed area is observed in the atrophic area of the gastric body. h TXI. The reddish depressed area appears more vivid. Endoscopic images of diffuse redness in the stomach. i WLI. A uniform redness is observed on the non-atrophic mucosa of the fundic gland in the gastric body. j TXI. Visualization of the reddish area is enhanced.
Outcomes
The primary endpoints were the diagnostic accuracies of WLI and TXI for H. pylori -active gastritis. In addition, the sensitivity, specificity, positive predict value (PPV), and negative predict value (NPV) were calculated.
We also evaluated the correlation of the endoscopic features with the three H. pylori infection statuses (current infection, past infection, and noninfection) and calculated the diagnostic odds ratio (OR). It was determined using the following formula: the diagnostic OR = (true positives/false negatives)/(false positives/true negatives) 18 .
Statistical analysis
The diagnostic accuracies of WLI and TXI for active gastritis were compared using the McNemar test. The interobserver agreement among the five reviewers for each group was evaluated using Fleiss’ kappa statistics. We used the arbitrary interpretation of Landis and Koch 19 . All statistical tests were two-tailed, and P values of less than 0.05 were considered significant. All statistical data were analyzed using the SPSS software, version 17.0 (SPSS Inc., Chicago, Illinois, United States).
Results
Table 1 lists patient characteristics. The median patient age was 73 (45–84) years, with male prevalence (41, 68.3 %). Atrophic gastritis was present in 48 patients (closed type, 5; open type, 43). Furthermore, 24, 24, and 12 patients had current infection, past infection, and no infection, respectively.
Table 1. Baseline tumor characteristics.
| Patient characteristics | (n = 60) |
| Age, median (IQR), years | 73 (68–78) |
| Sex | |
|
41/19 |
| Atrophy of gastric mucosa, n (%) | |
|
12 (20.0) |
|
5 (8.3) |
|
43 (71.7) |
| Helicobacter pylori , n (%) | |
|
24 (40.0) |
|
24 (40.0) |
|
12 (20.0) |
| Acid therapy, n (%) | |
|
45 (75.0) |
|
13 (21.7) |
|
2 (3.3) |
| NSAIDs, n (%) | |
|
53 (88.3) |
|
7 (11.7) |
IQR, interquartile range; C, closed; O, open; PPI, proton pump inhibitor; H 2 blocker, Histamine H 2 -receptor antagonist; NSAIDs, nonsteroidal anti-inflammatory drugs.
The relationship between endoscopic diagnosis and H. pylori infection status is shown in Table 2 . The diagnostic performance from each reviewer is shown in Supplementary Table 1.
Table 2. Relationship between endoscopic diagnosis and H. pylori infection status .
| Helicobacter pylori infection status | |||||
| WLI | Current infection | Past infection | Non-infection | Total | |
| Endoscopic diagnosis | Current infection | 63 | 7 | 0 | 70 |
| Past infection | 56 | 105 | 7 | 168 | |
| Noninfection | 1 | 8 | 53 | 62 | |
| Total | 120 | 120 | 60 | 300 | |
| TXI | Current infection | Past infection | Non-infection | Total | |
| Endoscopic diagnosis | Current infection | 83 | 6 | 1 | 90 |
| Past infection | 37 | 107 | 4 | 148 | |
| Noninfection | 0 | 7 | 55 | 62 | |
| Total | 120 | 120 | 60 | 300 | |
WLI, white light imaging; TXI, texture and color enhancement imaging.
Table 3 summarizes the diagnostic accuracies of endoscopy with WLI and TXI for H. pylori -active gastritis. The accuracy, sensitivity, specificity, PPV, and NPV were 78.7 %, 52.5 %, 96.1 %, 90.0 %, and 75.2 % for WLI diagnosis, and 85.3 %, 69.2%, 96.1 %, 92.2 %, and 82.4 % for TXI diagnosis, respectively. Hence, TXI was significantly more accurate and sensitive than WLI ( P = 0.034 and P = 0.012, respectively).
Table 3. Diagnostic accuracy of endoscopy with WLI and TXI for Helicobacter pylori -active gastritis.
| Accuracy | Sensitivity | Specificity | PPV | NPV | |
| WLI (95 % CI) | 78.7 (74.9–81.0) | 52.5 (47.7–55.4) | 96.1 (92.9–98.0) | 90.0 (81.9–94.9) | 75.2 (72.7–76.7) |
| TXI (95 % CI) | 85.3 (81.6–87.6) | 69.2 (64.5–72.0) | 96.1 (93.0–98.0) | 92.2 (86.0–96.0) | 82.4 (79.7–84.0) |
| P value | 0.034 | 0.012 | 1.000 | 0.779 | 0.081 |
CI, confidence interval; PPV, positive predict value; NPV, negative predict value; WLI, white light imaging; TXI, texture and color enhancement imaging.
For current infection, atrophy, intestinal metaplasia, and diffuse redness had a high OR using both WLI and TXI ( Table 4 ). However, atrophy and intestinal metaplasia also had a high OR for past infection. Thus, diffuse redness was the only specific observation for current infection using WLI and TXI (OR, 22.0 and 56.1, respectively). A high OR using WLI and TXI was specifically observed for map-like redness in past infection (OR 6.3 and 11.0, respectively) and the regular arrangement of collecting venules (RAC) in noninfection (OR 25.2 and 42.3, respectively). All the specific endoscopic features associated with H. pylori infection statuses had a higher OR with TXI than with WLI.
Table 4. Diagnostic odds ratio of the endoscopic features.
| Current infection | Past infection | Noninfection | ||
| WLI | RAC (95 % CI) | 0.13 (0.07–0.25) | 0.70 (0.42–1.16) | 25.24 (11.70–54.33) |
| Atrophy (95 % CI) | 64.70 (11.11–375.14) | 5.81 (2.69–12.50) | 0.00 (0.00–0.01) | |
| Intestinal metaplasia (95 % CI) | 2.69 (1.66–4.35) | 1.39 (0.86–2.24) | 0.00 (0.00–0.08) | |
| Map-like redness (95 % CI) | 0.65 (0.40–1.05) | 6.29 (3.74–10.50) | 0.00 (0.00–0.08) | |
| Diffuse redness (95 % CI) | 22.00 (9.31–52.45) | 0.13 (0.06–0.31) | 0.00 (0.00–0.19) | |
| Current infection | Past infection | Noninfection | ||
| TXI | RAC (95 % CI) | 0.04 (0.01–0.11) | 0.74 (0.44–1.26) | 42.25 (18.61–95.57) |
| Atrophy (95 % CI) | 69.75 (11.98–404.29) | 6.20 (2.76–13.90) | 0.00 (0.00–0.01) | |
| Intestinal metaplasia (95 % CI) | 3.05 (1.90–4.91) | 1.54 (0.96–2.45) | 0.01 (0.00–0.07) | |
| Map-like redness (95 % CI) | 0.39 (0.24–0.64) | 10.97 (6.35–18.98) | 0.02 (0.00–0.11) | |
| Diffuse redness | 56.21 (20.35–154.50) | 0.04 (0.01–0.13) | 0.04 (0.01–0.22) | |
CI, confidence interval; WLI, white light imaging; TXI, texture and color enhancement imaging; RAC, regular arrangement of collecting venules.
The kappa values of interobserver agreement among the five reviewers were fair to substantial for WLI, and moderate to substantial for TXI ( Table 5 ). Additionally, TXI had higher kappa values than the WLI among the five reviewers, except for one pair.
Table 5. Interobserver agreement among the five reviewers.
| Kappa values for TXI | Kappa values for WLI | Endoscopist |
| 0.52 | 0.30 | A to B |
| 0.68 | 0.71 | A to C |
| 0.77 | 0.28 | A to D |
| 0.65 | 0.44 | A to E |
| 0.57 | 0.53 | B to C |
| 0.66 | 0.41 | B to D |
| 0.62 | 0.46 | B to E |
| 0.67 | 0.55 | C to D |
| 0.71 | 0.51 | C to E |
| 0.72 | 0.57 | D to E |
WLI, white light imaging; TXI, texture and color enhancement imaging.
Discussion
This study revealed that TXI significantly improved endoscopic diagnostic accuracy for active gastritis compared with WLI. Additionally, all the specific endoscopic features associated with H. pylori infection statuses had a higher OR with TXI than with WLI.
The recently developed Kyoto Classification of Gastritis provides 16 endoscopic findings that are useful for differentiating H. pylori infection statuses 8 9 . Among the endoscopic features, diffuse redness, which pathologically correlates with neutrophil and mononuclear cell inflammation, is an important feature of active H. pylori infection 12 14 20 .
In the present study, diffuse redness showed a high OR using both WLI and TXI in patients with current infection. It was also strongly related to current infection using TXI compared with using WLI. This result can be explained by the imaging principle of TXI, which enhances color to greatly accentuate color tone differences of mucosal surfaces, thereby enhancing the mucosal redness. In addition, TXI is more useful for visualizing gastric mucosal atrophy 21 because it strengthens the contrast between the non-atrophic mucosa showing diffuse redness and the atrophic mucosa. Considering the relationship between endoscopic diagnosis and H. pylori infection status, we speculate that H. pylori -active cases were misdiagnosed as past infections with WLI, because of the possibility that WLI could miss diffuse redness. TXI can detect diffuse redness accurately, thereby improving diagnostic sensitivity and accuracy for active H. pylori infection compared with WLI. Good accuracy for current infection is vital; those who are not diagnosed as having current infection may miss the opportunity of H. pylori eradication therapy and surveillance for gastric cancer.
In a multicenter observation study conducted by Kawamura et al., endoscopic atrophy, invisible RAC, intestinal metaplasia, and map-like redness were high-risk endoscopic findings in the Kyoto Classification of Gastritis 22 . In our study, map-like redness correlated with past infection, whereas RAC correlated with noninfection. As with diffuse redness, these endoscopic features were strongly associated with H. pylori infection statuses using TXI compared with using WLI. The reason is that TXI improves the visibility of map-like redness and RAC by enhancing the reddish color, resulting in a stronger contrast.
Among the five reviewers, the interobserver agreement with WLI was fair to substantial (0.28–0.71), whereas that with TXI was moderate to substantial (0.52–0.77). Hence, TXI seems a more reliable modality for diagnosing H. pylori infection than WLI. Therefore, through TXI, the endoscopists could assess various endoscopic features of H. pylori -related gastritis more precisely.
However, the present study has some limitations. The diagnostic performance of the two modalities was compared using stored images; thus, this study did not assess real-time diagnostic performance, and the possibility of selection bias remains. Considering that all image sets were reviewed in a single session, another bias is possible because of the previous display of the same images in different modalities. The proportion of current infection was higher than that normally seen in the clinical practice. Although the UBT or stool antigen test would be better to evaluate the H. pylori infection status, some cases with current infection were determined based on the HpAb. We could not perform the histologic examination for atrophy and intestinal metaplasia. However, additional examinations could not be performed because the endoscopic procedures were part of routine screening. Interobserver agreement between one pair was higher in WLI than in TXI. Furthermore, this study has a small sample size, was conducted in a single center, and is retrospective in design. Thus, a prospective, randomized, multicenter study is required to validate our findings.
Conclusions
Nonetheless, the present study demonstrated the efficacy of TXI in accurately diagnosing H. pylori infection on endoscopy under the same conditions.
In conclusion, TXI can be a useful modality for the accurate endoscopic diagnosis of H. pylori infection.
Footnotes
Competing interests The authors declare that they have no conflict of interest.
Supplementary material :
References
- 1.Nomura A, Stemmermann G N, Chyou P-H et al. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N Engl J Med. 1991;325:1132–1136. doi: 10.1056/NEJM199110173251604. [DOI] [PubMed] [Google Scholar]
- 2.Parsonnet J, Friedman G D, Vander-steen D P et al. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 3.Uemura N, Okamoto S, Yamamoto S et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784–789. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- 4.Fukase K, Kato M, Kikuchi S et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomized controlled trial. Lancet. 2008;372:392–397. doi: 10.1016/S0140-6736(08)61159-9. [DOI] [PubMed] [Google Scholar]
- 5.Choi I J, Kook M C, Kim Y I et al. Helicobacter pylori therapy for the prevention of metachronous gastric cancer. N Engl J Med. 2018;378:1085–1095. doi: 10.1056/NEJMoa1708423. [DOI] [PubMed] [Google Scholar]
- 6.International Agency for Research on Cancer . IARC working group reports; Helicobacter pylori eradication as a strategy for preventing gastric cancer. http://www.iarc.fr/en/publications/pdfs-online/wrk/wrk8/Helicobacter_pylori_Eradication.pdf
- 7.Asaka M, Kato M, Sakamoto N et al. Roadmap to eliminate gastric cancer with Helicobacter pylori eradication and consecutive surveillance in Japan. J Gastroenterol. 2014;49:1–8. doi: 10.1007/s00535-013-0897-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haruma K, Kato M, Inoue K . Tokyo: Nihon Medical Center, Inc; 2014. Kyoto Classification of Gastritis. [Google Scholar]
- 9.Sugano K, Tack J, Kuipers E J et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64:1353–1367. doi: 10.1136/gutjnl-2015-309252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamasaki Y, Uedo N, Kanzaki H et al. Investigation of mucosal pattern of gastric antrum using magnifying narrow-band imaging in patients with chronic atrophic fundic gastritis. Ann Gastroenterol. 2017;30:302–308. doi: 10.20524/aog.2017.0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uedo N, Ishihara R, Iishi H et al. A new method of diagnosing gastric intestinal metaplasia: Narrow-band imaging with magnifying endoscopy. Endoscopy. 2006;38:819–824. doi: 10.1055/s-2006-944632. [DOI] [PubMed] [Google Scholar]
- 12.Dohi O, Yagi N, Onozawa Y et al. Linked color imaging improves endoscopic diagnosis of active Helicobacter pylori infection. Endosc Int Open. 2016;04:E800–E805. doi: 10.1055/s-0042-109049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ono S, Kato M, Tsuda M et al. Lavender color in linked color imaging enables noninvasive detection of gastric intestinal metaplasia. Digestion. 2018;98:222–230. doi: 10.1159/000489454. [DOI] [PubMed] [Google Scholar]
- 14.Ono S, Dohi O, Yagi N et al. Accuracies of endoscopic diagnosis of Helicobacter pylori-gastritis: Multicenter prospective study using white light imaging and linked color imaging. Digestion. 2020;101:624–630. doi: 10.1159/000501634. [DOI] [PubMed] [Google Scholar]
- 15.Majima A, Dohi O, Takayama S et al. Linked color imaging identifies important risk factors associated with gastric cancer after successful Helicobacter pylori eradication. Gastrointest Endosc. 2019;90:763–769. doi: 10.1016/j.gie.2019.06.043. [DOI] [PubMed] [Google Scholar]
- 16.Sato T. TXI: Texture and color enhancement imaging for endoscopic image enhancement. Health Eng. 2021;2021:5.518948E6. doi: 10.1155/2021/5518948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yao K, Uedo N, Muto M et al. Development of an e-learning system for teaching endoscopists how to diagnose early gastric cancer: Basic principles for improving early detection. Gastric Cancer. 2017;20:28–38. doi: 10.1007/s10120-016-0680-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glas A S, Lijmer J G, Prins M H et al. The diagnostic odds ratio: a single indicator of test performance. J Clin Epidemiol. 2003;56:1129–1135. doi: 10.1016/s0895-4356(03)00177-x. [DOI] [PubMed] [Google Scholar]
- 19.Landis J R, Koch G G. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 20.Hattori T. On cell proliferation and differentiation of fundic mucosa of the golden hamster. Fractographic study combined with microscopy and 3H-thymidine autoradiography. Cell Tissue Res. 1974;148:213–226. doi: 10.1007/BF00224583. [DOI] [PubMed] [Google Scholar]
- 21.Ishikawa T, Matsumura T, Okimoto K et al. Efficacy of texture and color enhancement imaging in visualizing gastric mucosal atrophy and gastric neoplasms. Sci Rep. 2021;11:6910. doi: 10.1038/s41598-021-86296-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawamura M, Uedo N, Koike T et al. Kyoto classification risk scoring system and endoscopic grading of gastric intestinal metaplasia for gastric cancer: Multicenter observation study in Japan. Dig Endosc. 2022;34:508–516. doi: 10.1111/den.14114. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


