Abstract
Background
The objective of the present study was to estimate the effectiveness of the BBIBP-CorV vaccine (VE) in preventing SARS-CoV-2 infection, related hospitalization, and death among people living with multiple sclerosis (PLWMS).
Methods
In this population-based retrospective observational study, data on all PLWMS, vaccination, SARS-CoV-2 tests, hospitalization, and deaths were collected in Isfahan, Iran between February 9, 2021, and November 4, 2021. We estimated the hazard ratio between vaccinated (partially and fully) and unvaccinated groups using the Andersen-Gill extension of the Cox proportional hazards model. We also performed Cox proportional hazards analysis to identify risk factors for breakthrough infection and COVID-19-related hospitalization in fully-immunized group.
Results
Of the 9869 PLWMS, 1368 were in partially-vaccinated group, 4107 were in the fully-vaccinated group, and 3794 were in the unvaccinated group. In the partially-vaccinated group, the estimated VE against COVID-19 infection was 39.3% (16%, 56.1%), hospitalization was 64.9% (1.3%, 87.5%), and mortality was 92.7% (88.8%, 100%). The respective results for the fully-vaccinated group were 63.9% (56%, 70.3%), 75.7% (57.5%, 86.1%), and 100%. Progressive MS was independently associated with a greater risk of breakthrough infection (HR=1.952, 95%CI: 1.174–3.246, p = 0.010). Older adults (≥50 years vs. 18–49 years, HR=3.115, 95%CI: 1.145–8.470, p = 0.026) and those on rituximab (HR=7.584; 95% CI: 1.864–30.854; p = 0.005) were at an increased risk of COVID-19-related hospitalization.
Conclusion
This study showed that two doses of the BBIBP-CorV vaccine can effectively prevent COVID-19 infection and hospitalization among PLWMS. Old PLWMS and those who treating with rituximab are at increased risk of hospitalization after receiving two doses of the vaccine.
Keywords: Multiple sclerosis, COVID-19, SARS-CoV-2, Vaccine, BBIBP-CorV
1. Introduction
The coronavirus disease 2019 (COVID-19) has posed significant challenges for people living with multiple sclerosis (PLWMS). As PLWMS are mostly immunocompromised, they are more susceptible to develop severe viral and bacterial infections (Marrodan et al., 2019). In addition, infections are linked to an increased risk of MS relapse (Marrodan et al., 2019). According to a systematic review of 87 studies, the COVID-19 mortality rate was 3.0% among PLWMS, which was marginally higher than the 2.2% rate in the general population (Barzegar et al., 2021a). Notably, the majority of infected MS patients in this review were female and young, which protects PLWMS from severe COVID-19. A recent study from Italy showed higher risk of hospitalization, intensive care unit (ICU) admission, and mortality in MS patients compared to age- and sex-matched controls (Sormani et al., 2022a). Aging, comorbidity, greater disability, and anti-CD20 agents increase the severity of SARS-CoV-2 infection (Salter et al., 2021). Current evidence suggests that COVID-19 could trigger exacerbation of MS (Barzegar et al., 2021b; Garjani et al., 2021).
Vaccination is the best strategy to lessen the morbidity and mortality burden of COVID-19. The WHO has authorized the emergency use of BBIBP-CorV, an inactivated whole-virus vaccine that was developed by China National Pharmaceutical Group Corporation (Sinopharm). This vaccine has been administered in over fifty Asian, African, and European countries. On February 9, 2021, the Iranian Food and Drug Administration authorized the emergency use of BBIBP-CorV, which is currently the most widely administered vaccine in Iran, with over 50 million doses.
Immune system dysregulation in PLWMS treated with immunosuppressive agents can result in vaccine hyporesponsiveness (Otero-Romero et al., 2021). A growing body of literature showed a diminished humoral immune response following COVID-19 vaccine among MS patients receiving anti-CD20 agents and fingolimod (Apostolidis et al., 2021; Capuano et al., 2022; Etemadifar et al., 2022; Holroyd et al., 2022; Maniscalco et al., 2022; Ozakbas et al., 2022; Satyanarayan et al., 2022; Tallantyre et al., 2022; Yeo et al., 2022). This finding was also observed after the booster dose of the vaccine (Maniscalco et al., 2022; Wallach et al., 2022). Despite a low seroconversion rate, these studies have found a robust T-cell response. Therefore, the level of protection conferred by vaccination in PLWMS with insufficient humoral immunity, particularly those on anti-CD20 agents is unknown. Concerns exist regarding the effectiveness of SARS-CoV-2 vaccines in PLWMS in light of these findings and a slightly decreased protection against SARS-CoV-2 reinfection in this population (Barzegar et al., 2022).
This study aimed to assess the effectiveness of the BBIBP-CorV vaccine against confirmed SARS-COV2 infection, hospitalization, and mortality in PLWMS residing in Isfahan province, Iran. In addition, we sought to identify risk factors for breakthrough COVID-19 infection and infection-related hospitalization among fully-immunized group.
2. Methods
2.1. Data sources
This retrospective population-based observational study was conducted in Isfahan, Iran, between February 9, 2021, and November 4, 2021, using four datasets from the Isfahan University of Medical Sciences also known as Medical University of Isfahan (MUI). MUI is responsible for providing healthcare to all 5.2 million residents of Isfahan province, excluding those who reside in Kashan. Since the first case of COVID-19 was identified in Iran, the MUI launched the Isfahan COVID-19 Registry (I-CORE) to collect data on all individuals who underwent a SARS-CoV-2 polymerase chain reaction (PCR) or rapid antigen test at any public or private laboratory in the province (Javanmard et al., 2020). The SARS-CoV-2 PCR and rapid antigen test results were captured regardless of the testing reason and symptoms associated with COVID-19. I-CORE database also included the date of hospitalization of all patients who were hospitalized in for-profit or non-profit hospitals across the province with a suspected or confirmed COVID-19 diagnosis. Nearly all hospitalized patients suspected of having COVID-19 were tested for SARS-CoV-2. The community health center stores the death records of Isfahan province residents who die of any cause.
In Iran, PLWMS were given priority for COVID-19 vaccination, which began on February 9, 2021. Vaccine regimens in Iran included BBIBP-CorV, ChAdOx1 nCoV-19, Gam-COVID-Vac, Covaxin, and COVIran Barekat, each was administered in two doses. Isfahan MS society recommended the use of the BBIBP-CorV, since this vaccine was safe, well-tolerated, and easily available in the province. Individual-level data on the date and type of SARS-CoV-2 vaccination were stored in the Integrated Health System's electronic health records.
The electronic health record (EHR) of the Vice-Chancellery for Clinical Affairs contains the information of nearly all confirmed PLWMS residing in Isfahan province (n = 10,639), except for those residing in Kashan. PLWMS in Isfahan province are registered with the Vice-Chancellery to alleviate the disease's financial burden. The demographical and clinical data included age, gender, MS duration, MS courses (clinically isolated syndrome [CIS], relapsing-remitting MS [RRMS], and progressive MS [PMS]), and disease-modifying therapy (DMT).
On January 5–15, 2022, data on the demographic and clinical characteristics of PLWMS, testing for SARS-CoV-2, hospitalization, mortality, and vaccination status were extracted from the datasets and linked using their national identification numbers.
This study is part of a larger project investigating the effect of SARS-CoV-2 vaccination on SARS-CoV-2 infection, hospitalization, and mortality in the Isfahan population. The study was approved by the regional bioethics committee of Isfahan University of Medical Sciences (IR.MUI.MED.REC.1400.483).
2.2. Study population
PLWMS who resided in Isfahan province and were 18 years or older were included in the study. PLWMS with a positive PCR or rapid antigen test for SARS-CoV-2 before or on February 9, 2022, were excluded from the study. We focused on the effectiveness of BBIBP-CorV since the majority of PLWMS had received this vaccine in the current study.
2.3. Vaccine and outcomes
PLWMS were categorized into three groups based on their vaccination status: (a) partially-immunized (≥14 days after receiving the first dose), (b) fully-immunized (≥14 days after receiving the second dose), and (c) unvaccinated. We set a cutoff of 14 days because BBIBP- CorV's immunogenicity is not fully realized until 13 days after receiving the vaccine (Al Kaabi et al., 2021; Pérez et al., 2021). In Iran, the recommended time between the first and second doses of BBIBP-CorV was 28 days. PLWMS who received the second BBIBP-CorV dose less than 24 days before (≥4 days earlier than the recommended interval) or more than 34 days after (≥7 days more than the recommended interval) the first dose were excluded from the vaccinated group (Pawlowski et al., 2021). We used this interval since the reason for delaying vaccination was not documented. However, PLWMS who received the vaccine outside of this window were considered unvaccinated until the date of their first vaccination. The unvaccinated group also included PLWMS who had received no COVID-19 vaccine during study and those who had received vaccines rather than BBIBP-CorV.
We defined three primary outcomes: (a) incidence of infection after vaccination as determined by positive SARS-CoV-2 PCR or rapid antigen test, (b) incidence of COVID-19-associated hospitalization in infected PLWMS, and (c) incidence of COVID-19-associated mortality in infected PLWMS. The date of the first positive PCR or rapid antigen test result was considered the infection date. Hospitalization associated with COVID-19 was defined as hospitalization within 15 days of a positive SARS-CoV-2 PCR or rapid antigen test (Ioannou et al., 2020). We defined this interval to identify hospitalized patients with confirmed COVID-19. COVID-19-associated mortality was defined as death occurring within 30 days of a positive SARS-CoV-2 PCR or rapid antigen test (Ioannou et al., 2020; King et al., 2020). We identified factors associated with COVID-19 breakthrough infection after BBIBP-CorV vaccine and COVID-19-related hospitalization among fully-immunized group as secondary outcomes.
2.4. Statistical analysis
Our analysis model was largely based on Jara et al. (2021) models for estimating the effectiveness of the CoronaVac vaccine in Chile. The Cox regression model was utilized to calculate the hazard ratio between the vaccinated and unvaccinated groups. We employed the Andersen-Gill extension of the Cox proportional hazards model, which allowed us to account for the time-varying vaccination status. Using Kaplan-Meier analysis, we estimated the cumulative incidence of positive SARS-CoV-2 testing among the unvaccinated group, PLWMS who received only one dose of BBIBP-CorV vaccine, and PLWMS who received both doses of the BBIBP-CorV vaccine.
The date of vaccine availability for PLWMS (February 09, 2021) was considered the baseline date. All included subjects were followed until the end of the study (November 4, 2021), occurring primary outcomes, or death, whichever occurred first. PLWMS who received a vaccine rather than BBIBP-CorV were considered unvaccinated and were followed up until the date of receiving the first dose of vaccine or other end-points. To assess the effectiveness of the initial vaccine dose, PLWMS with a positive COVID-19 test within 13 days of vaccination were excluded from the partially-vaccinated group. To analyze the effectiveness of the second dose, those who had a positive test up to 13 days after receiving the second dose were excluded from the fully-vaccinated group. Vaccine effectiveness (VE) (%) was calculated as 100 * (1 - adjusted hazard ratio). The models were adjusted for potential confounding variables such as age, gender, MS course, and MS duration.
We used Cox proportional hazards analysis to determine the association between clinical factors and breakthrough infection and COVID-19-related hospitalization among the fully-immunized group. Age (younger adults: 18–49 years vs. older adults: ≥50 years), gender, MS course (RRMS vs. progressive MS [PMS]), DMTs, and disease duration were identified as possible risk factors. To examine the association between MS treatment and the study outcomes, DMTs were categorized as interferon, fingolimod, rituximab, and others (all other PLWMS). Inequality in the sample size of each DMTs (higher use of interferon and rituximab) could be problematic. Therefore, we categorized all PLWMS except those on interferon, fingolimod, and rituximab as a group. We selected this group as the reference category due to its high frequency. The significant factors associated with outcomes in the univariate model were incorporated into the final multivariate model using the backward stepwise selection method. P values <0.05 (two-tailed) were considered significant in all analyses. The statistical analysis was conducted with SPSS version 23.0. (SPSS Inc., Chicago, IL).
3. Results
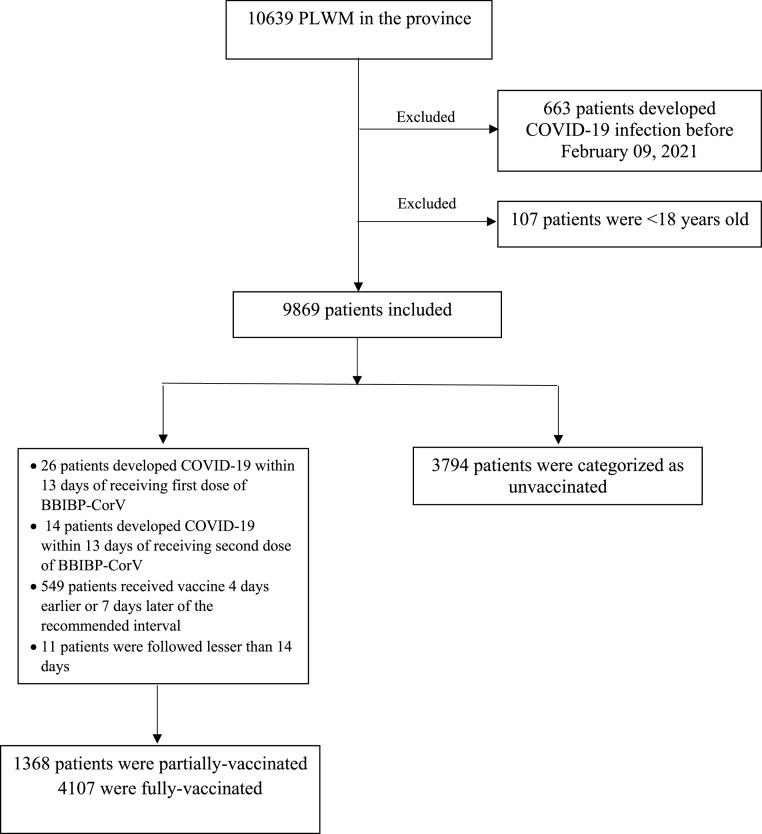
We excluded PLWMS who developed COVID-19 before receiving the COVID-19 vaccine (n = 663) and those who were 18 years or younger (n = 107), leaving 9869 as study subjects. Fig. 1 illustrates the study's flowchart. In total, there were 1368 PLWMS in the partially-vaccinated group and 4107 in the fully-vaccinated group. During the study period, 3794 PLWMS did not receive a COVID-19 vaccine. Table 1 summarizes the characteristics of the study groups.
Fig. 1.
Flowchart of the study.
Table 1.
Characteristics of PLWMS.
| Variables | Unvaccinated N = 3794 | Partially vaccinated N = 1368 | Fully vaccinated N = 4107 | ||
|---|---|---|---|---|---|
| Sex; n (%) | Female | 2891 (76.2) | 1090 (79.7) | 3220 (78.4) | |
| Male | 903 (23.8) | 278 (20.3) | 887 (21.6) | ||
| Age; mean (SD) | 38.95 (9.75) | 38.54 (9.05) | 40.93 (10.11) | ||
| MS type; n (%) | CIS | 172 (5) | 93 (7.5) | 146 (3.9) | |
| RRMS | 3075 (88.9) | 1103 (89) | 3377 (89.6) | ||
| Progressive MS | 213 (6.2) | 43 (3.5) | 244 (6.5) | ||
| MS drugs; n (%) | Interferon | 1702 (44.9) | 604 (44.2) | 1649 (40.2) | |
| Glatiramer acetate | 316 (8.3) | 127 (9.3) | 319 (7.8) | ||
| Fingolimod | 236 (6.2) | 107 (7.8) | 381 (9.3) | ||
| Rituximab | 679 (17.9) | 209 (15.3) | 859 (20.9) | ||
| Dimethyl fumarate | 364 (9.6) | 157 (11.4) | 440 (10.7) | ||
| Teriflunomide | 172 (4.5) | 63 (4.6) | 255 (6.2) | ||
| Natalizumab | 27 (0.7) | 4 (0.3) | 22 (0.5) | ||
| Others | 173 (4.5) | 51 (3.7) | 141 (3.4) | ||
| No treatment | 13 (0.3%) | 2 (1) | 6 (0.1) | ||
| Disease duration; mean (SD) | 7.66 (5.19) | 7.22 (4.97) | 8.33 (5.76) | ||
| Follow up time; mean (SD) | 251 (53.68) | 263.2 (23.65) | 265.3 (15.04) | ||
PLWMS: people living with multiple sclerosis; CIS: clinically isolated syndrome RRMS: relapsing-remitting multiple sclerosis.
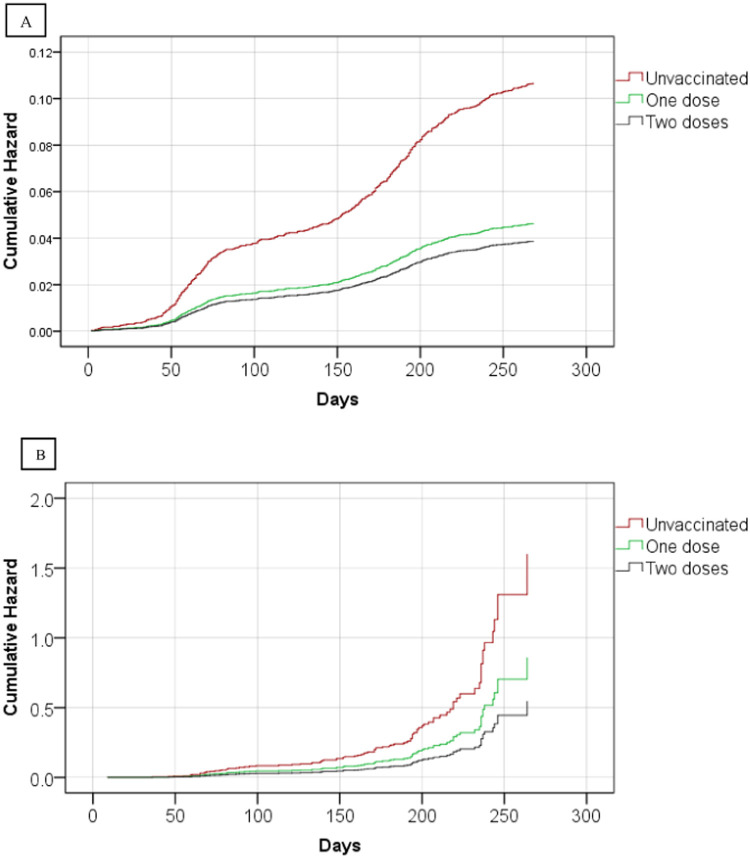
During the follow-up period, we recorded 377 (number of events/10,000 person-days: 3.95) infections among the unvaccinated, 67 (number of events/10,000 person-days: 1.72) infections among the partially-vaccinated, and 157 (number of events/10,000 person-days: 1.44) infections among the fully-vaccinated. There were 56 (number of events/10,000 person-days: 13.41), 7 (number of events/10,000 person-days: 8.82), and 27 (number of events/10,000 person-days: 8.62) hospitalizations related to COVID-19 in the unvaccinated, partially-vaccinated, and fully-vaccinated groups, respectively. Six of the seven PLWMS who died from COVID-19 were unvaccinated, while one was partially-immunized. Fig. 2 depicts the crude cumulative incidence of COVID-19 infection and associated hospitalization after vaccination with BBIBP-CorV by vaccination status. In the partially-vaccinated group, the estimated effectiveness against COVID-19 infection was 39.3% (16%, 56.1%), hospitalization was 64.9% (1.3%, 87.5%), and mortality was 92.7% (88.8%, 100%). The respective results for the fully-vaccinated group were 63.9% (56%, 70.3%), 75.7% (57.5%, 86.1%), and 100%.
Fig. 2.
Cumulative incidence of SARS-CoV-2 infection (A) and COVID-19-related hospitalization (B).
The cumulative incidence of COVID-19 infection at 3 and 6 months were 0.037 and 0.071 among the unvaccinated, 0.017 and 0.032 among the partially-vaccinated, 0.013 and 0.026 among the fully-vaccinated, respectively. The cumulative incidence of COVID-19-related hospitalization at 3 and 6 months were 0.1 and 0.26 among the unvaccinated, 0.03 and 0.15 among the partially-vaccinated, and 0.01 and 0.11 among the fully-vaccinated, respectively.
According to the Cox univariate regression model, fully-immunized female PLWMS had a lower risk of infection (HR=0.686, 95%CI: 0.486–0.967, p = 0.032) (Table 2 ). Progressive MS was also associated with breakthrough COVID-19 infection (HR=1.952, 95%CI: 1.174–3.246, p = 0.010). Only the association between progressive MS and breakthrough infection remained statistically significant in the multivariate model (HR=1.952, 95%CI: 1.174–3.246, p = 0.010).
Table 2.
Factors associated with breakthrough SARS-CoV-2 infection after vaccination among fully-immunized group.
| Variables | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Sex (Ref.=Male) | 0.686 (0.486–0.967) | 0.032 | 0.760 (0.526–1.099) | 0.145 | |
| Age (Ref.=less than 50) | 1.003 (0.804–1.250) | 0.981 | – | – | |
| MS type (Ref.=RRMS) | 1.952 (1.174–3.246) | 0.010 | 1.952 (1.174–3.246) | 0.010 | |
| DMTs (Ref.= All other patients) |
RTX | 1.349 (0.894,2.036) | 0.153 | – | – |
| FNG | 0.526 (0.248, 1.113) | 0.093 | – | – | |
| IFN | 0.914 (0.622, 1.344) | 0.651 | – | – | |
| Disease duration | 1.002 (0.976–1.029) | 0.872 | – | – | |
Ref: reference; RRMS: relapsing-remitting multiple sclerosis; DMTs: disease-modifying therapies, RTX: rituximab; FNG: fingolimod; IFN: interferon.
Age (HR=1.049, 95%CI: 1.011–1.090, p = 0.012), disease duration (HR=1.055, 95%CI: 1.001–1.113, p = 0.048), and rituximab (HR=4.149, 95%CI: 1.197, 14.492, p = 0.025) were linked with an increased risk of COVID-19-related hospitalization (Table 3 ). In the multivariate model, a one-year increase in age was connected with a 6.4% increase in the risk of COVID-19-related hospitalization (HR=1.064, 95%CI: 1.022–1.108, p = 0.003). Those treated with rituximab had a 5.81-fold increased risk of hospitalization compared to those in the reference group (HR=5.813; 95% CI: 1.636, 20.833; p = 0.006).
Table 3.
Factors associated with COVID-19-related hospitalization after vaccination among fully-immunized patients.
| Variables | Univariate | Multivariable | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Sex (Ref.=Male) | 1.422 (0.613–3.298) | 0.412 | – | – | |
| Age (Ref.=less than 50) | 2.504 (1.045–6.003) | 0.040 | 3.115 (1.145–8.470) | 0.026 | |
| MS type (Ref.=RRMS) | 2.271 (0.933–5.529) | 0.071 | – | – | |
| DMTs (Ref.=All other patients) |
RTX | 4.149 (1.197, 14.492) | 0.025 | 7.584 (1.864–30.854) | 0.005 |
| FNG | 2.564 (0.424, 15.384) | 0.305 | – | – | |
| IFN | 1.718 (0.442, 6.666) | 0.434 | – | – | |
| Disease duration | 1.055 (1.001–1.113) | 0.048 | 1.041 (0.971–1.117) | 0.258 | |
Ref: reference; RRMS: relapsing-remitting multiple sclerosis; DMTs: disease-modifying therapies, RTX: rituximab; FNG: fingolimod; IFN: interferon.
4. Discussion
Despite numerous clinical trials and real-world studies investigating the effectiveness of SARS-CoV-2 vaccination, there is still a significant knowledge gap regarding the effectiveness of vaccines in the MS population. This population-based observational study provided strong evidence that the full dose of BBIBP-CorV vaccine can effectively prevent COVID-19 infection and hospitalization among PLWMS. We observed an increased risk of hospitalization in fully-immunized COVID-19 people who were treated with rituximab.
A single dose of the vaccine was estimated to be 39% effective in preventing infection, which is lower than the estimate of 85.4% in Serbian adults aged ≥60 (Petrović et al., 2022) but higher than the estimate of 15.5% after the first dose of CoronaVac reported by Jara et al. (2021). A partial vaccination was associated with a 64.9% (1.3%−87.5%) reduction in the risk of hospitalization. Although this is strong statistical evidence that a single dose of the vaccine reduced the risk of COVID-19-related hospitalization, the large confidence interval resulting from the small sample size and the low number of events reduces the estimate's accuracy. Inactive vaccines induce a weak immune response after the initial dose, necessitating additional doses to provide adequate protection against infection (Pérez et al., 2021; Xia et al., 2020). As a result, precautions should be taken until the second dose is received.
As anticipated, administering the second dose of BBIBP-CorV increased the VE against COVID-19 infection and hospitalization. Among those who received the full vaccination, 64% were protected from contracting the disease. This is comparable to the 68.7% estimate reported in Hungary (Vokó et al., 2022) but lower than some earlier studies. The interim analysis of a phase-3 randomized clinical trial conducted in the United Arab Emirates and Bahrain demonstrated 78.1% efficacy against symptomatic COVID-19 infection (Al Kaabi et al., 2021). In an Iranian retrospective cohort study, the VE against the infection was 79.9% (Mirahmadizadeh et al., 2022). In our study, the VE against hospitalization was 75.7%, which is lower than the 100% reported in clinical trials for preventing COVID-19-related hospitalization and severe infection (Al Kaabi et al., 2021; Tanriover et al., 2021). However, this finding is consistent with a recent meta-analysis of nine real-world studies that reported the pooled effectiveness of inactivated vaccines to be 79.10% (95% CI: 71.69 to 86.51) (Fu et al., 2022).
In this study, only one death was detected in the vaccinated group (partially-vaccinated group), possibly leading to an overestimation of the VE against death. During the follow-up, however, there were six deaths in the unvaccinated group. We, therefore, hypothesized that two doses of BBIBP-CorV could prevent mortality in the MS population. Our estimate falls within the 85% to 97% range reported by real-world studies for the VE against COVID-19-related mortality (Al Kaabi et al., 2022; AlHosani et al., 2022; Mirahmadizadeh et al., 2022).
Our results showed that people with progressive MS were at a greater risk for breakthrough COVID-19 infection. This may be due to advanced age and multiple comorbidities in progressive MS patients (Sun et al., 2022). A greater disability could also contribute to severe COVID-19 outcomes after receiving the vaccine. Another possible explanation is the poor humoral and cellular immune response in progressive MS patients, as observed in convalescent COVID-19 patients (Zabalza et al., 2022). Antibody level following the second dose of mRNA vaccine was the only significant factor for breakthrough infection in the CovaXiMS study from Italy (Sormani et al., 2022b). Studies on the general population found that a prior COVID-19 infection, being male, obesity, and smoking may have an association with an increased risk of vaccine breakthrough infection (Basso et al., 2022; Stouten et al., 2022).
We found an association between aging and increased hospitalization risk. This is supported by previous studies with unvaccinated individuals indicating that aging contributes to an adverse COVID-19 outcome (Li et al., 2021; Sun et al., 2022). Studies showed that the efficacy of SARS-CoV-2 vaccines varied by age. We observed an increased odd of hospitalization among older PLWMS compared to the younger adult people (50–79 years vs. 18–49 years). In a prospective nested case control study, compared to people aged 18–64 years, those who were 65–84 and ≥80 years had a greater risk of hospitalization and death from COVID-19 after receiving first dose of BNT162b2 or ChAdOx1 vaccines (Agrawal et al., 2021). A study from the United Kingdom found an increased risk of severe COVID-19 outcome after receiving two doses of mRNA-1273 or BNT162b2 in patients who were 50 years or older compared to younger patients (Agrawal et al., 2022). This suggests that the effect of age on COVID-19 outcomes after vaccination in MS people might be similar to what was observed among the general population. However, due to the small sample size, we were unable to identify the age threshold for severe COVID-19 outcomes after vaccination.
Rituximab was another factor that was independently associated with an increased risk of COVID-19-related hospitalization in fully-immunized group. Our result supports the growing evidence that rituximab may impair the vaccine-induced immune response in MS patients (Apostolidis et al., 2021; Ciampi et al., 2022; Etemadifar et al., 2022; Tallantyre et al., 2022). Studies on rituximab-treated patients with other autoimmune disorders such as inflammatory rheumatic diseases and musculoskeletal diseases showed impaired immune responses after SARS-CoV-2 vaccination (Batıbay et al., 2022; Furer et al., 2021; Kroon et al., 2022; Moor et al., 2021; Zheng et al., 2022). However, some found suitable immunity after vaccination among rituximab-treated patients (Takai et al., 2022). The protective response following SARS-CoV-2 in rituximab-treated patients is related to the interval between the last rituximab exposure and receiving the vaccine, rituximab concentration, and/or B cell counts in peripheral blood (Ammitzbøll et al., 2022; Spiera et al., 2021; Takai et al., 2022; Woopen et al., 2022).
The strength of this study lies in its use of a rich population-based dataset, which included information on all SARS-CoV-2 tests, COVID-19-related hospitalization, vaccination, and death in the province. In addition, our survey included a population-based database of nearly all PLWMS in the province. Second, with five COVID-19 peaks and over 700,000 SARS-CoV-2 tests, an eight-month follow-up, and a large vaccine campaign, we could estimate VE for three outcomes: COVID-19 infection, hospitalization, and mortality.
There are limitations to this study. The absence of data on comorbidity, the severity of MS, and socioeconomic status, which are risk factors for acquiring COVID-19 and its severity, could influence the analyses. A systematic distinction exists between the vaccinated and unvaccinated groups. PLWMS who received SARS-CoV-2 vaccines could be more likely to follow the precautions to prevent catching COVID-19. These patients might have a more tendency to perform the SARS-CoV-2 tests when they developed respiratory symptoms. This can lead to an overestimation of VE. We attempted to reduce the differences by adjusting for age, gender, MS duration, and MS course. Our database did not contain information on the severity of COVID-19, such as the need for oxygen therapy or intensive care admission. The reason for not receiving the second dose of the vaccine in partially-vaccinated group was not documented. To identify hospitalization due to COVID-19, we established a positive test timeframe. A hospitalization for COVID-19 infection near the end of this period may lead to misclassification. However, the risk of bias is low because the majority of hospitalizations occurred within two weeks of a positive test result (Nyberg et al., 2021). Not all individuals who died during the follow-up period were tested for COVID-19. Consequently, if some people died of COVID-19 but were not tested for COVID-19, this would go undetected and skew our mortality estimates. Our database did not include the SARS-CoV-2 variant. We were unable to include a representative control group of the general population because mass vaccination of the general population began after the study's conclusion. We compared VE against hospitalization and mortality with previous real-world studies. Direct comparisons between our results and those of other studies should be made with caution due to differences in health policy, vaccine coverage, infection prevalence, predominant variant, research methodology, and follow-up period.
Overall, to the best of our knowledge, this is one of the first studies to examine the effectiveness of any COVID-19 vaccine in the MS population. According to the present study, two doses of the BBIBP-CorV vaccine were associated with a 75.7% reduction in hospitalization risk and a 100% reduction in mortality risk. After vaccination, elderly PLWMS and those receiving rituximab are more likely to develop a severe infection. Further research with a control group is required to compare the effectiveness of SARS-CoV-2 vaccines between PLWMS and the general population.
Data sharing and data accessibility
Anonymized data not published within this article will be made available by request from any qualified investigator.
Funding
No fund.
CRediT authorship contribution statement
Mahdi Barzegar: Conceptualization, Data curation, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing. Amirreza Manteghinejad: Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. Alireza Afshari-Safavi: Data curation, Formal analysis, Validation, Writing – review & editing. Omid Mirmosayyeb: Investigation, Writing – review & editing. Maryam Nasirian: Formal analysis, Writing – review & editing. Sara Bagherieh: Investigation, Writing – review & editing. Shahrbanoo Mazaheri: Writing – review & editing. Maryam Rahimi: Investigation, Writing – review & editing. Aram zabeti: Writing – review & editing. Shaghayegh Haghjooy Javanmard: Conceptualization, Methodology, Project administration, Supervision, Writing – review & editing. Vahid Shaygannejad: Conceptualization, Methodology, Project administration, Supervision, Writing – review & editing.
Declaration of Competing Interest
All authors declare no conflict of interest relevant to study.
References
- Agrawal U., Bedston S., McCowan C., Oke J., Patterson L., Robertson C., Akbari A., Azcoaga-Lorenzo A., Bradley D.T., Fagbamigbe A.F. Severe COVID-19 outcomes after full vaccination of primary schedule and initial boosters: pooled analysis of national prospective cohort studies of 30 million individuals in England, Northern Ireland, Scotland, and Wales. Lancet. 2022;400(10360):1305–1320. doi: 10.1016/S0140-6736(22)01656-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal U., Katikireddi S.V., McCowan C., Mulholland R.H., Azcoaga-Lorenzo A., Amele S., Fagbamigbe A.F., Vasileiou E., Grange Z., Shi T. COVID-19 hospital admissions and deaths after BNT162b2 and ChAdOx1 nCoV-19 vaccinations in 2· 57 million people in Scotland (EAVE II): a prospective cohort study. Lancet Respir. Med. 2021;9(12):1439–1449. doi: 10.1016/S2213-2600(21)00380-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AlHosani F.I., Stanciole A.E., Aden B., Timoshkin A., Najim O., Zaher W.A., AlDhaheri F.A., Al Mazrouie S., Rizvi T.A., Mustafa F. Impact of the Sinopharm's BBIBP-CorV vaccine in preventing hospital admissions and death in infected vaccinees: results from a retrospective study in the emirate of Abu Dhabi, United Arab Emirates (UAE) Vaccine. 2022;40(13):2003–2010. doi: 10.1016/j.vaccine.2022.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Kaabi N., Oulhaj A., Ganesan S., Al Hosani F.I., Najim O., Ibrahim H., Acuna J., Alsuwaidi A.R., Kamour A.M., Alzaabi A. Effectiveness of BBIBP-CorV vaccine against severe outcomes of COVID-19 in Abu Dhabi, United Arab Emirates. Nat. Commun. 2022;13(1):1–9. doi: 10.1038/s41467-022-30835-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Kaabi N., Zhang Y., Xia S., Yang Y., Al Qahtani M.M., Abdulrazzaq N., Al Nusair M., Hassany M., Jawad J.S., Abdalla J. Effect of 2 inactivated SARS-CoV-2 vaccines on symptomatic COVID-19 infection in adults: a randomized clinical trial. JAMA. 2021;326(1):35–45. doi: 10.1001/jama.2021.8565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammitzbøll C., Kragh Thomsen M., Bøgh Andersen J., Jensen J.M.B., From Hermansen M.-.L., Dahl Johannsen A., Larsen M.L., Mistegaard C.E., Mikkelsen S., Szabados F. Rituximab-treated rheumatic patients: b-cells predict seroconversion after COVID-19 boost or revaccination in initial vaccine non-responders. Rheumatology. 2022 doi: 10.1093/rheumatology/keac666. [DOI] [PubMed] [Google Scholar]
- Apostolidis S.A., Kakara M., Painter M.M., Goel R.R., Mathew D., Lenzi K., Rezk A., Patterson K.R., Espinoza D.A., Kadri J.C. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat. Med. 2021;27(11):1990–2001. doi: 10.1038/s41591-021-01507-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzegar M., Manteghinejad A., Bagherieh S., Sindarreh S., Mirmosayyeb O., Javanmard S.H., Shaygannejad V., Nasirian M. Risk and severity of SARS-CoV-2 reinfection among patients with multiple sclerosis vs. the general population: a population-based study. BMC Neurol. 2022;22(1):1–10. doi: 10.1186/s12883-022-02907-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzegar M., Mirmosayyeb O., Gajarzadeh M., Afshari-Safavi A., Nehzat N., Vaheb S., Shaygannejad V., Maghzi A.-.H. COVID-19 among patients with multiple sclerosis: a systematic review. Neurol. Neuroimmunol. Neuroinflamm. 2021;8(4) doi: 10.1212/NXI.0000000000001001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzegar M., Vaheb S., Mirmosayyeb O., Afshari-Safavi A., Nehzat N., Shaygannejad V. Can coronavirus disease 2019 (COVID-19) trigger exacerbation of multiple sclerosis? A retrospective study. Mult. Scler. Relat. Disord. 2021;52 doi: 10.1016/j.msard.2021.102947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso P., Negro C., Cegolon L., Larese Filon F. Risk of vaccine breakthrough SARS-CoV-2 infection and associated factors in healthcare workers of Trieste Teaching Hospitals (North-Eastern Italy) Viruses. 2022;14(2):336. doi: 10.3390/v14020336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batıbay S., Ulucaköy R.K., Günendi Z., Fidan I., Bozdayı G., Göğüş F.N. Immunogenicity and safety of the CoronaVac and BNT162b2 Covid-19 vaccine in patients with inflammatory rheumatic diseases and healthy adults: comparison of different vaccines. Inflammopharmacology. 2022:1–8. doi: 10.1007/s10787-022-01089-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuano R., Bisecco A., Conte M., Donnarumma G., Altieri M., Grimaldi E., Franci G., Chianese A., Galdiero M., Coppola N. Six-month humoral response to mRNA SARS-CoV-2 vaccination in patients with multiple sclerosis treated with ocrelizumab and fingolimod. Mult. Scler. Relat. Disord. 2022;60 doi: 10.1016/j.msard.2022.103724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciampi E., Uribe-San-Martin R., Soler B., García L., Guzman J., Pelayo C., Jürgensen L., Guzman I., Vera F., Galleguillos L. Safety and humoral response rate of inactivated and mRNA vaccines against SARS-CoV-2 in patients with Multiple Sclerosis. Mult. Scler. Relat. Disord. 2022;59 doi: 10.1016/j.msard.2022.103690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etemadifar M., Sedaghat N., Nouri H., Lotfi N., Chitsaz A., Khorvash R., Zolfaghari H., Movaghar A.G., Pourabbas M., Salari M. SARS-CoV-2 serology among people with multiple sclerosis on disease-modifying therapies after BBIBP-CorV (Sinopharm) inactivated virus vaccination: same story, different vaccine. Mult. Scler. Relat. Disord. 2022;57 doi: 10.1016/j.msard.2021.103417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y., Zhao J., Wei X., Han P., Yang L., Ren T., Zhan S., Li L. Effectiveness and cost-effectiveness of inactivated vaccine to address COVID-19 pandemic in China: evidence from randomized control trials and real-world studies. Front. Public Health. 2022;10 doi: 10.3389/fpubh.2022.917732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furer V., Eviatar T., Zisman D., Peleg H., Paran D., Levartovsky D., Zisapel M., Elalouf O., Kaufman I., Meidan R. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann. Rheum. Dis. 2021;80(10):1330–1338. doi: 10.1136/annrheumdis-2021-220647. [DOI] [PubMed] [Google Scholar]
- Garjani A., Middleton R.M., Hunter R., Tuite-Dalton K.A., Coles A., Dobson R., Duddy M., Hughes S., Pearson O.R., Rog D. COVID-19 is associated with new symptoms of multiple sclerosis that are prevented by disease modifying therapies. Mult. Scler. Relat. Disord. 2021;52 doi: 10.1016/j.msard.2021.102939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd K.B., Healy B.C., Conway S., Houtchens M., Bakshi R., Bhattacharyya S., Bose G., Galetta K., Kaplan T., Severson C. Humoral response to COVID-19 vaccination in MS patients on disease modifying therapy: immune profiles and clinical outcomes. Mult. Scler. Relat. Disord. 2022;67 doi: 10.1016/j.msard.2022.104079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannou G.N., Locke E., Green P., Berry K., O'Hare A.M., Shah J.A., Crothers K., Eastment M.C., Dominitz J.A., Fan V.S. Risk factors for hospitalization, mechanical ventilation, or death among 10 131 US veterans with SARS-CoV-2 infection. JAMA Netw. Open. 2020;3(9) doi: 10.1001/jamanetworkopen.2020.22310. -e2022310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jara A., Undurraga E.A., González C., Paredes F., Fontecilla T., Jara G., Pizarro A., Acevedo J., Leo K., Leon F. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N. Engl. J. Med. 2021;385(10):875–884. doi: 10.1056/NEJMoa2107715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javanmard S.H., Nasirian M., Ataei B., Vaseghi G., Vaezi A., Changiz T. Isfahan COvid-19 REgistry (I-CORE): design and methodology. J. Res. Med. Sci. 2020;25 doi: 10.4103/jrms.JRMS_271_20. : The Official Journal of Isfahan University of Medical Sciences. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King J.T., Jr, Yoon J.S., Rentsch C.T., Tate J.P., Park L.S., Kidwai-Khan F., Skanderson M., Hauser R.G., Jacobson D.A., Erdos J. Development and validation of a 30-day mortality index based on pre-existing medical administrative data from 13,323 COVID-19 patients: the Veterans Health Administration COVID-19 (VACO) Index. PLoS ONE. 2020;15(11) doi: 10.1371/journal.pone.0241825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroon F.P., Najm A., Alunno A., Schoones J.W., Landewé R.B., Machado P.M., Navarro-Compán V. Risk and prognosis of SARS-CoV-2 infection and vaccination against SARS-CoV-2 in rheumatic and musculoskeletal diseases: a systematic literature review to inform EULAR recommendations. Ann. Rheum. Dis. 2022;81(3):422–432. doi: 10.1136/annrheumdis-2021-221575. [DOI] [PubMed] [Google Scholar]
- Li Y., Ashcroft T., Chung A., Dighero I., Dozier M., Horne M., McSwiggan E., Shamsuddin A., Nair H. Risk factors for poor outcomes in hospitalised COVID-19 patients: a systematic review and meta-analysis. J. Glob. Health. 2021;11 doi: 10.7189/jogh.11.10001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniscalco G.T., Liotti A., Ferrara A.L., Prestipino E., Salvatore S., Di Battista M.E., Moreggia O., Cesare D.D.G., Vastano R., Belardo M. Humoral efficacy of the third SARS-CoV-2 vaccine dose in Multiple Sclerosis subjects undergoing different disease-modifying therapies. Mult. Scler. Relat. Disord. 2022;68 doi: 10.1016/j.msard.2022.104371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrodan M., Alessandro L., Farez M.F., Correale J. The role of infections in multiple sclerosis. Mult. Scler. J.l. 2019;25(7):891–901. doi: 10.1177/1352458518823940. [DOI] [PubMed] [Google Scholar]
- Mirahmadizadeh A., Heiran A., Bagheri Lankarani K., Serati M., Habibi M., Eilami O., Heiran F., Moghadami M. Effectiveness of coronavirus disease 2019 vaccines in preventing infection, hospital admission, and death: a historical cohort study using iranian registration data during vaccination program. Open Forum Infect. Dis. 2022:ofac177. doi: 10.1093/ofid/ofac177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moor M.B., Suter-Riniker F., Horn M.P., Aeberli D., Amsler J., Möller B., Njue L.M., Medri C., Angelillo-Scherrer A., Borradori L. Humoral and cellular responses to mRNA vaccines against SARS-CoV-2 in patients with a history of CD20 B-cell-depleting therapy (RituxiVac): an investigator-initiated, single-centre, open-label study. Lancet Rheumatol.y. 2021;3(11):e789–e797. doi: 10.1016/S2665-9913(21)00251-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg T., Twohig K.A., Harris R.J., Seaman S.R., Flannagan J., Allen H., Charlett A., De Angelis D., Dabrera G., Presanis A.M. Risk of hospital admission for patients with SARS-CoV-2 variant B. 1.1. 7: cohort analysis. BMJ. 2021;373 doi: 10.1136/bmj.n1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero-Romero S., Ascherio A., Lebrun-Frénay C. Vaccinations in multiple sclerosis patients receiving disease-modifying drugs. Curr. Opin. Neurol. 2021;34(3):322–328. doi: 10.1097/WCO.0000000000000929. [DOI] [PubMed] [Google Scholar]
- Ozakbas S., Baba C., Dogan Y., Cevik S., Ozcelik S., Kaya E. Comparison of SARS-CoV-2 antibody response after two doses of mRNA and inactivated vaccines in multiple sclerosis patients treated with disease-modifying therapies. Mult. Scler. Relat. Disord. 2022;58 doi: 10.1016/j.msard.2022.103486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlowski C., Lenehan P., Puranik A., Agarwal V., Venkatakrishnan A., Niesen M.J., O'Horo J.C., Virk A., Swift M.D., Badley A.D. FDA-authorized mRNA COVID-19 vaccines are effective per real-world evidence synthesized across a multi-state health system. Med. 2021;2(8):979–992.e978. doi: 10.1016/j.medj.2021.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez C.A., Zhang G.Q., Li X., Huang Y., Lincoln J.A., Samudralwar R.D., Gupta R.K., Lindsey J.W. COVID-19 severity and outcome in multiple sclerosis: results of a national, registry-based, matched cohort study. Mult. Scler. Relat. Disord. 2021;55 doi: 10.1016/j.msard.2021.103217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrović V., Vuković V., Marković M., Ristić M. Early Effectiveness of Four SARS-CoV-2 Vaccines in Preventing COVID-19 among Adults Aged≥ 60 Years in Vojvodina, Serbia. Vaccines. 2022;10(3):389. doi: 10.3390/vaccines10030389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter A., Fox R.J., Newsome S.D., Halper J., Li D.K., Kanellis P., Costello K., Bebo B., Rammohan K., Cutter G.R. Outcomes and risk factors associated with SARS-CoV-2 infection in a North American registry of patients with multiple sclerosis. JAMA Neurol. 2021;78(6):699–708. doi: 10.1001/jamaneurol.2021.0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satyanarayan S., Safi N., Sorets T., Filomena S., Zhang Y., Klineova S., Fabian M., Horng S., Tankou S., Miller A. Differential antibody response to COVID-19 vaccines across immunomodulatory therapies for multiple sclerosis. Mult. Scler. Relat. Disord. 2022;62 doi: 10.1016/j.msard.2022.103737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormani M.P., Schiavetti I., Carmisciano L., Cordioli C., Filippi M., Radaelli M., Immovilli P., Capobianco M., De Rossi N., Brichetto G. COVID-19 severity in multiple sclerosis: putting data into context. Neurol. Neuroimmunol. Neuroinflamm. 2022;9(1) doi: 10.1212/NXI.0000000000001105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormani M.P., Schiavetti I., Inglese M., Carmisciano L., Laroni A., Lapucci C., Visconti V., Serrati C., Gandoglia I., Tassinari T. Breakthrough SARS-CoV-2 infections after COVID-19 mRNA vaccination in MS patients on disease modifying therapies during the Delta and the Omicron waves in Italy. EBioMedicine. 2022;80 doi: 10.1016/j.ebiom.2022.104042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiera R., Jinich S., Jannat-Khah D. Rituximab, but not other antirheumatic therapies, is associated with impaired serological response to SARS-CoV-2 vaccination in patients with rheumatic diseases. Ann. Rheum. Dis. 2021;80(10):1357–1359. doi: 10.1136/annrheumdis-2021-220604. [DOI] [PubMed] [Google Scholar]
- Stouten V., Hubin P., Haarhuis F., van Loenhout J.A., Billuart M., Brondeel R., Braeye T., Van Oyen H., Wyndham-Thomas C., Catteau L. Incidence and risk factors of COVID-19 vaccine breakthrough infections: a prospective cohort study in Belgium. Viruses. 2022;14(4):802. doi: 10.3390/v14040802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Zheng Q., Madhira V., Olex A.L., Anzalone A.J., Vinson A., Singh J.A., French E., Abraham A.G., Mathew J. Association between immune dysfunction and COVID-19 breakthrough infection after SARS-CoV-2 vaccination in the US. JAMA Intern Med. 2022;182(2):153–162. doi: 10.1001/jamainternmed.2021.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai S., Nishida H., Ito H., Fukuhara H., Nawano T., Narisawa T., Kanno H., Yagi M., Yamagishi A., Sakurai T. Immunogenicity and safety of two doses of SARS-CoV-2 mRNA vaccine in kidney transplant recipients with low-dose rituximab. Int. J. Urol. 2022;29(11):1279–1286. doi: 10.1111/iju.14978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallantyre E.C., Vickaryous N., Anderson V., Asardag A.N., Baker D., Bestwick J., Bramhall K., Chance R., Evangelou N., George K. Covid-19 vaccine response in people with multiple sclerosis. Ann. Neurol. 2022;91(1):89–100. doi: 10.1002/ana.26251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanriover M.D., Doğanay H.L., Akova M., Güner H.R., Azap A., Akhan S., Köse Ş., Erdinç F.Ş., Akalın E.H., Tabak Ö.F. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. 2021;398(10296):213–222. doi: 10.1016/S0140-6736(21)01429-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vokó Z., Kiss Z., Surján G., Surján O., Barcza Z., Pályi B., Formanek-Balku E., Molnár G.A., Herczeg R., Gyenesei A. Nationwide effectiveness of five SARS-CoV-2 vaccines in Hungary—The HUN-VE study. Clin. Microbiol. Infect. 2022;28(3):398–404. doi: 10.1016/j.cmi.2021.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallach A.I., Schiebel M., Picone M.A. Antibody response to SARS-CoV-2 vaccination following typical and three-dose dosing schedules in multiple sclerosis patients treated with disease modifying therapies. Mult. Scler. Relat. Disord. 2022 doi: 10.1016/j.msard.2022.103856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woopen C., Dunsche M., Haase R., Raposo C., Pedotti R., Akgün K., Ziemssen T. Timing of SARS-CoV-2 vaccination matters in people with multiple sclerosis on pulsed Anti-CD20 treatment. Neurol. Neuroimmunol. Neuroinflamm. 2022;9(6) doi: 10.1212/NXI.0000000000200031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia S., Duan K., Zhang Y., Zhao D., Zhang H., Xie Z., Li X., Peng C., Zhang Y., Zhang W. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. JAMA. 2020;324(10):951–960. doi: 10.1001/jama.2020.15543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo T., Quek A.M.L., Yong K.P., Tye J.S.N., Ratnagopal P., Soon D.T.L., Tan K. COVID-19 infection after two doses of SARS-CoV-2 mRNA vaccine in multiple sclerosis, AQP4-antibody NMOSD and MOGAD. Mult. Scler. Relat. Disord. 2022;65 doi: 10.1016/j.msard.2022.104003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabalza A., Arrambide G., Tagliani P., Cárdenas-Robledo S., Otero-Romero S., Esperalba J., Fernandez-Naval C., Campuzano J.T., Gallo M.M., Castillo M. Humoral and cellular responses to SARS-CoV-2 in convalescent COVID-19 patients with multiple sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2022;9(2) doi: 10.1212/NXI.0000000000001143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y.-.Q., Li H.-.J., Chen L., Lin S.-.P. Immunogenicity of inactivated COVID-19 vaccine in patients with autoimmune inflammatory rheumatic diseases. Sci. Rep. 2022;12(1):1–6. doi: 10.1038/s41598-022-22839-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published within this article will be made available by request from any qualified investigator.