Highlights
-
•
A rigorous evaluation of pre-analytical factors influencing dry plasma measurements of 5-methyltetrahydrofolic acid in combination with chloride normalization.
-
•
Identified hemolysis of blood during fingerstick collection would preclude successful chloride normalization.
-
•
Instability during uncontrolled ambient shipping was identified according to ISTA procedure 7D, but was resolved for overnight shipment with insulated, cold packaging.
Abbreviations: 5MTHF, 5-methyltetrahyrofolate; DBS, dried blood spot; ISTA, International Safe Transit Association; PCM, phase change material; LC-MS/MS, liquid chromatography tandem mass spectrometry; CLSI, Clinical Laboratory Standards Institute; FDA, Food and Drug Administration
Keywords: Folate, 5-Methyltetrahydrofolate, Mass spectrometry, Liquid chromatography, Microsample
Abstract
Introduction
Determination of folate insufficiency is of considerable interest given its importance in fetal development and red blood cell formation; however, access to blood tests may be limited due to the requirement for phlebotomy as well as controlled temperature shipping of blood specimens to laboratories for testing due to the inherent instability of folate and its vitamers.
Methods
An LC-MS/MS test was developed and validated for the measurement of 5-methyltetrahydrofolate (5MTHF) in dried plasma specimens collected from fingerstick blood using a laminar flow blood separation device, as well as liquid venous plasma for comparison. Two pre-analytical factors investigated influencing the measurement of 5MTHF in dried plasma were hemolysis of the fingerstick blood during collection and storage/shipment of the dried plasma.
Results
Although observed infrequently, hemolysis >10 % resulted in elevated 5MTHF measurements, but hemolysis >1 % resulted in elevated chloride measurements, which were necessary to normalize 5MTHF measurements for variation in volume of dried plasma specimens. Stability of 5MTHF was improved in dried plasma relative to liquid plasma at ambient temperatures, but not sufficiently to allow for uncontrolled temperature shipping despite controlling for humidity and light exposure. Shipping studies emulating ISTA procedure 7D were conducted with a reusable cold packaging solution. The packaging failed to stabilize 5MTHF in dried plasma specimens during a 2-day summer shipping evaluation, but did provide sufficient temperature control to stabilize 5MTHF during the overnight shipping evaluation.
Conclusion
Our studies provide boundary conditions with respect to hemolysis, storage, and shipping for successful analysis of 5MTHF from dried plasma specimens.
Introduction
Analysis of blood microsamples has been of significant interest given the ease of collection relative to standard phlebotomy [1], [2] Microsample applications have been used widely in support of drug measurements [3], [4], [5], [6] and are ubiquitous for newborn screening given the minimally invasive collection modality via heel stick [7], [8], [9]. With increased interest in at-home testing/collection, microsamples are also an attractive solution given the feasibility of self-collection [10]. When dried, blood microsamples also have the added advantage of increased analyte stability. Many analytes that have proven to be labile in liquid blood, serum or plasma have improved stability profiles when stored dry [11], [12], [13], allowing for long-term storage at moderate temperatures in biobanks and for stable shipment with minimal or no temperature control [14], [15]. However, drying of blood specimens does not guarantee analyte stability indefinitely or under all temperature conditions. As an example, characterization of amino acid stability in dried blood spots (DBS) has shown that some analytes have limited stability under ambient conditions for even short periods of time [16], [17]. As such, careful examination of the pre-analytical conditions for collection, shipment, and storage of dried blood microsamples is required for each measurand to ensure reliable application.
Blood and serum folate is a particularly unstable measurand that requires frozen or refrigerated shipment of specimens [18], [19]. Given its importance in fetal development [20], [21] and red blood cell formation [22], there is significant interest in evaluating folate status for personal and community health initiatives, which could be facilitated using at-home or remote collection of blood microsamples. Indeed, other groups have developed total folate measurements from both dried blood spots and dried plasma, as well as measurements of 5-methyltetrahydrofolate (5MTHF) – the primary form of folate [23] – from dried blood spots and dried plasma [24]. However, these works either did not evaluate, or had noted, instability of folate and 5MTHF in the dried microsamples under ambient temperature conditions that may be incurred during uncontrolled shipment. Further, volume of the dried microsamples – a known source of pre-analytical variability – was either uncontrolled (i.e., assumed) in these works, controlled through normalization with hemoglobin when analyzing dried blood, or controlled by volumetric sampling that may not be deployed without some level of training.
To that end, we developed an LC-MS/MS test for the measurement of 5MTHF from dried plasma microsamples that may be self-collected via a novel blood-plasma separation device. Although folate status can be determined readily from blood or plasma [25], the choice of dried plasma rather than dried blood afforded the ability to normalize for volume uncertainty using plasma chloride measurements, as previously described [26]. Consequently, we chose to measure 5MTHF by LC-MS/MS rather than total folate levels by a microbiological assay due to sensitivity limitations of the latter methodology for analysis of dried plasma, which inherently requires dilution of the specimen. 5MHTF measurements from both dried plasma and liquid plasma specimen types were fully validated according to CLSI guidance [27] and FDA Bioanalytical guidance [28] and a rigorous evaluation of the pre-analytical conditions was performed to ensure reliability of the results. Specifically, evaluations of storage and shipping conditions were performed, as well as the impact of hemolysis during collection of the dried plasma microsample, given the large amount of 5MTHF found in red blood cells relative to plasma.
Materials & methods
Chemicals
Bovine serum albumin (BSA), formic acid, ascorbic acid, and 5-methyltetrahydrofolic acid-(glutamic acid-13C5) ([13C5]-5MTHF) were from MilliporeSigma (St. Louis, MO). Calcium D,L-5-methyltetrahydrofolate reference standard was from United States Pharmacopeia (Rockville, MD). Water (Optima® LC/MS grade), acetonitrile (HPLC grade), and ammonium hydroxide were from Fisher Scientific (Hampton, NH), while charcoal stripped serum was from Golden West Diagnostics (Temecula, CA). Finally, trichloroacetic acid (TCA) was from G-Biosciences (St. Louis, MO).
Human samples
Volunteer donors provided blood via venipuncture and fingerstick with informed consent for the anonymous use of their blood specimens under an approved Institutional Review Board protocol (ASPIRE® Protocol #520100174). Venous blood was collected by standard phlebotomy using lithium heparin collection tubes (BD; Franklin Lakes, NJ). Following centrifugation, plasma was separated from the cells, aliquoted into light-protected amber tubes, and frozen at <-70 °C within 4 h of phlebotomy. Dried plasma samples were collected from fingerstick blood using the VelvetTM Blood Collection device (WEAVR Health; Cambridge, MA). The VelvetTM device contains 3 × 60 µL heparinized capillaries, which allowed for metered collection and, upon closure, metered dosing of finger stick blood onto a proprietary collection element [26]. Laminar flow of blood across the collection element allows for separation of plasma from red blood cells. Following collection, devices were immediately placed into the provided foil pouches (WEAVR Health) containing a desiccant and an oxygen scrubber to control the humidity and protect from light. Subsequently, devices within the sealed foiled pouches were allowed to dry overnight (16 – 20 h) at room temperature (20 – 25 °C) before analysis, unless otherwise noted.
5MTHF assay
Sixty microliters of liquid calibrators and liquid plasma samples were processed, along with six, 56.25 mm2 punches of dried plasma samples (equating to approximately 60 µL of plasma). Both liquid and dried samples were combined with 600 µL of 2 % BSA containing 2.5 ng/mL [13C5]-5MTHF and allowed to mix (200 rpm) for 1 h at room temperature, which served to both equilibrate the internal standard with the samples as well as extract the dried plasma components from the collection elements. Subsequently, 125 µL of the BSA-extracts were mixed (2:1, v/v) with 10 % TCA for 5 min, followed by centrifugation for 10 min prior to transferring the supernatant to a 96-well plate for LC-MS/MS analysis.
Acid-precipitated samples were analyzed for 5MTHF by LC-MS/MS on an ARIATM TLX4 system (Thermo Scientific; San Jose, CA) coupled to a 7500 Triple Quadrupole (SCIEX; Framingham, MA) with an OptiFlowTM Pro ESI source operated in positive ion mode for detection of 5MTHF and its internal standard by selected reaction monitoring (SRM). Separation was performed on a 2.1 × 50 mm (5 µm) Zorbax XDB-C18 column (Agilent Technologies; Santa Clara, CA) at 1 mL/min where mobile phase A and B consisted of 0.1 % formic acid in water and acetonitrile, respectively. Samples were loaded for 10 s at 0 % B, eluted with a linear gradient from 0 to 25 % B over 25 s, followed by column washing for 45 s at 100 % B, and column re-equilibration for 60 s at 0 % B (total cycle time: 150 s). The SRM transitions of 5MTHF and [13C5]-5MTHF used for quantitation were 460.2/313.2 and 465.2/313.2, respectively, both with a collision energy (CE) of 30 V. Qualifying transitions were 460.2/194.0 and 465.2/194.0, respectively, both with a CE of 45 V. All transitions were acquired with a 40 msec dwell time with a 5 msec pause time (total scan time: 0.18 sec). Curtain gas (CUR), nebulizer gas (GS1), and heating gas (GS2), and collision gas (CAD) were set to 20, 70, 40, and 6, respectively. Source temperature (TEM) was 300C, spray voltage was 2500 V, and Q0 was operated in Simple Mode at 30 V. Both Q1 and Q3 were operated at unit resolution under the Low Mass (LM) settings. Q2 entrance potential (EP) and exit potential (CXP) of 10 and 12 V, respectively.
To accurately estimate the volume of dried plasma analyzed and correct for variability in extraction from the collection element, chloride was measured in the BSA-extracts as previously described [26]. 5MTHF measurements from dried plasma specimens were normalized as follows using the matching chloride measurement and expected chloride concentration in plasma (100.28 mmol/L):
Absolute 5MTHF measurement / absolute chloride measurement × 100.28 mmol/L
Each analytical run was calibrated with a series of liquid standards (0.5, 1, 2, 4, 10, 25, 100, and 200 ng/mL 5MTHF) prepared in charcoal stripped serum containing 50 mM ascorbic acid. Results for both dried and liquid specimens were obtained using the calibration curve generated from the liquid standards, which used a quadratic fit with 1/x2-weighting. Each level was prepared from a common stock solution of 5MTHF in 0.1 % ammonium hydroxide and value assigned spectrophotometrically (ε290nm = 31700 L/mol/cm) [29] and accuracy of the calibration was demonstrated relative to NIST SRM®3949 and SRM®1950 (see supplemental material, Table S26).
Each batch was qualified for liquid and dried specimens. Liquid controls comprised two levels of serum folate controls (CDC Nutritional Biomarkers Branch), with approximate 5MTHF concentrations of 7 and 24 ng/mL (inter-assay coefficient of variance (CV, n = 20) of 7.2 % and 5.5 %, respectively); however, target values for 5MTHF were not provided for these controls by the CDC – only total folate concentrations. Dried plasma controls were produced from two pools of venous lithium heparin plasma with approximate 5MTHF concentrations of 25 ng/mL and 100 ng/mL, which were stored as frozen aliquots and dosed (160 µL) onto the collection elements (matching those contained within the VelvetTM device). After 2 h of drying at room temperature, the ‘dosed-elements’ were placed into foil storage bags (containing a desiccant and an oxygen scrubber) and refrigerated up to 7 days until analysis (inter-assay CV (n = 20) of 6.7 % and 10.2 %, respectively, with chloride-normalization). Additional validation result for both liquid and dried specimen types may be found in the supplemental material.
Insulated packaging
Shipping stability studies were conducted using an 8.5 in × 6.5 in × 4.5 in styrofoam box (Akuratemp; Arden, NC) containing two phase change material (PCM) pouches with 2 g HS22P, each (Akuratemp). Given that HS22P freezes below room temperature, the PCM pouches were stored in a refrigerator (2 – 8 °C) overnight in order to solidify/freeze the material prior to use. The VelvetTM device (contained within the supplied foil bag) was sandwiched between the two PCM pouches, which fit securely into the styrofoam box for shipment (Fig. 1).
Fig. 1.
(Left) Shown is the insulated packaging comprised of a 1″ thick styrofoam box and 2 PCM pouches. The test tube is shown for perspective. (Right) The packaging was filled by placement of one PCM pouch in the bottom of the box, followed by up to three VelvetTM devices contained within their respective sealed foil bags. The second PCM pouch was placed on top of the devices followed by the styrofoam lid. Finally, the styrofoam box containing the PCM pouches and VelvetTM devices was placed into a cardboard box of the same geometry.
Results
Impact of hemolysis
Given the presence of 5MTHF within red blood cells, the impact of hemolysis was first evaluated in liquid plasma samples. Matching lithium heparin tubes of venous blood were collected from 37 donors – one tube from each individual was immediately processed/separated to produce an unhemolyzed plasma specimen, while the matching tube was first placed into an ultra-low freezer for ∼20 min to induce gross (but not complete) hemolysis in the plasma fraction. Direct comparison of matching plasma and grossly hemolyzed plasma specimens collected from venous blood of 37 donors showed marked elevation in plasma 5MTHF levels due to hemolysis (Fig. 2). The mean bias was +42 % (range: −19 to +181 %).
Fig. 2.
Shown is the bias of 5MTHF measurements due to hemolysis established by direct comparison of matching hemolyzed and unhemolyzed plasma specimens. Matching specimens were each analyzed in singlicate in a single run and 5MTHF measurements in hemolyzed samples were compared to those of the unhemolyzed sample to calculate the bias due to hemolysis. Dashed black lines represent acceptable bias limits (±15 %).
In using the VelvetTM Blood Collection device, some degree of hemolysis was also observable in the dried plasma fraction in approximately 3 % of specimens, indicating hemolysis could also be a pre-analytical factor to consider for dried plasma specimens. To determine the impact of hemolysis on the quantification of 5MTHF from dried plasma, a freshly drawn lithium heparin whole blood specimen was collected and split into two aliquots. One aliquot remained as-is (0 % hemolysis), while the second aliquot was frozen for 2 h at <2−70 °C (100 % hemolysis). The un-hemolyzed and hemolyzed aliquots were mixed at different ratios to create a series of matched specimens with 0 (baseline), 1, 5, 10, 20, 50, and 100 % hemolysis (Fig. 3). Each specimen was dosed onto collection elements, dried, and then assayed as four replicates.
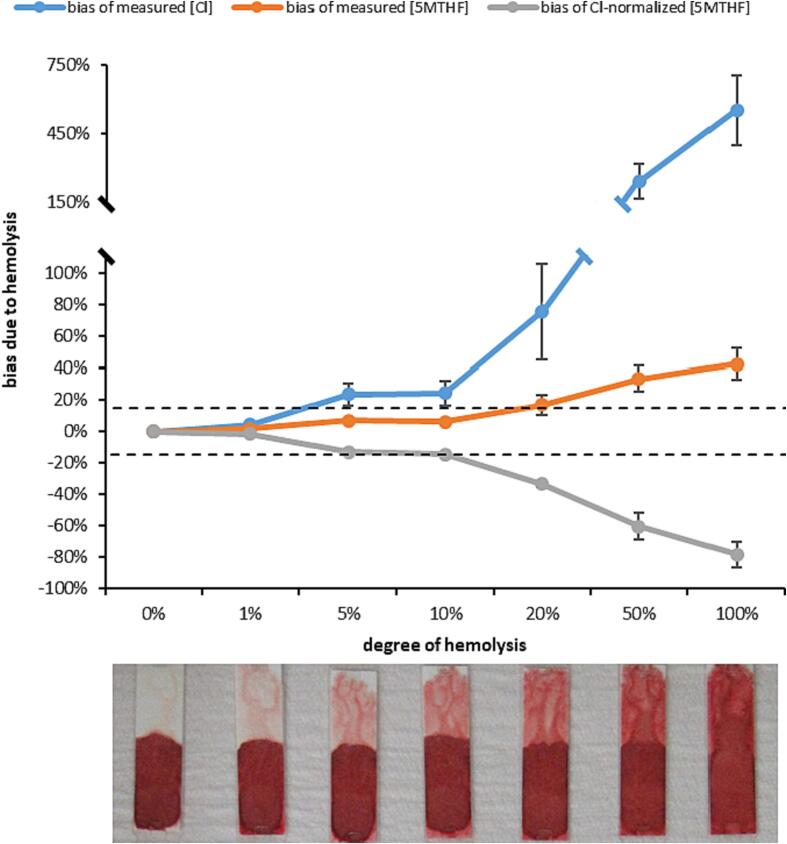
Fig. 3.
Shown is the bias for absolute measurements of 5MTHF and chloride (Cl) due to increasing levels of hemolysis in dried plasma. The resulting bias of Cl-normalized 5MTHF results are also shown. Each data point is the average of 4 replicate measures (error bars +/- 1SD; error bars < 2 % in magnitude are not visible due to scaling), with bias calculated to the average result in the matching unhemolyzed (0 %) sample. Dashed black lines represent acceptable bias limits (±15 %).
Dried plasma specimens showed increasing chloride results with increasing degrees of hemolysis. Chloride results were at least 25 % higher in plasma specimens with at least 5 % hemolysis or higher, but no substantial interference (<5% bias) was observed at 1 % hemolysis (Fig. 3). Absolute measurements of 5MTHF from dried plasma also increased with increasing hemolysis, though no appreciable interference was observed (<7% bias) with up to 10 % hemolysis. At 20 % or higher hemolysis, absolute 5MTHF measurements were markedly elevated (>16 %), but to a lesser degree than the chloride measurements. As such, chloride-normalized 5MTHF results were lower with increasing levels of hemolysis. Allowing for up to 15 % bias due to hemolysis, chloride-normalized 5MTHF results were acceptable with up to 10 % hemolysis.
Stability
Stability of liquid and dried plasma specimens obtained from 3 donors was evaluated for room temperature (20 to 25 °C), refrigerated (2 to 8 °C), and frozen (−25 to −15 °C) storage. Additionally, dried plasma specimens were subjected to elevated storage conditions (40 – 50 °C) given the potential for uncontrolled temperature shipment via standard postal services. For each time point and condition tested, each specimen was analyzed as six replicates. Venous lithium heparin blood was collected fresh from three individual donors, and the resulting plasma aliquoted. On the same day of collection (day 0), matching aliquots were placed into the respective storage conditions while another aliquot was analyzed to establish a baseline target concentration.
Given the number of time points and conditions to be tested, it was impractical to have individual donors provide the requisite number of matching dried plasma specimens using fingerstick blood. As such, matching dried plasma specimens were produced from 3 individual donors by fresh collection of venous lithium heparin blood, which was then added to VelvetTM Blood Collection devices containing capillaries without a lithium heparin coating. Devices were placed into storage bags (containing a desiccant and an oxygen scrubber) and allowed to dry overnight before placement into the respective storage conditions or analysis to establish a baseline (day 1) value. Freeze-thaw stability was also evaluated by freezing (-25 to −15 °C) matching specimens for at least 12 h followed by at least 4 h thawing at room temperature for each cycle.
Allowing for 15 % bias relative to the baseline value, liquid plasma specimens only exhibited acceptable stability when tested after 1 day storage at room temperature (Fig. 4). After 3, 7, and 14 days of room temperature storage, liquid plasma specimens produced 5MTHF measurements that were −20.0 %, −61.9 %, and −79.8 % biased on average versus baseline targets, respectively. Consistent with previous studies [30], liquid plasma specimens were stable when stored up to 14 days refrigerated, 14 days frozen or following 3 freeze/thaw cycles, producing 5MTHF measurements that were biased less than or equal to 8.0 % from baseline values on average for each time point and condition tested.
Fig. 4.
Shown is the stability profile of 5MTHF in liquid (orange) and dried (blue) plasma specimens stored under a variety of conditions. Profiles for each specimen type were established using specimens collected from three unique donors. Notably, the same 3 donors were not used for both dried and liquid specimens. The dashed black lines represent acceptable bias limits (±15 %). Note, stability of 5MTHF in liquid plasma was not tested at elevated temperatures. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Although the rate of degradation was slower at room temperature, dried plasma specimens still exhibited unacceptable degradation when stored for>1 day at room temperature (Fig. 4). After 3, 7, and 14 days room temperature storage, 5MTHF measurements that were −15.4 %, −27.4 %, and −34.4 % biased on average, respectively, in dried plasma specimens relative to baseline targets. Similar to liquid plasma specimens, dried plasma specimens showed improved stability with refrigerated and frozen storage, producing 5MTHF measurements biased less than or equal to 11.2 % from baseline values on average after 14 days refrigeration, 7 days frozen, or following 3 freeze/thaw cycles. At elevated temperature conditions, deleterious degradation (absolute bias > 65 %) was observed even after 1 day of storage.
Shipping stability
Stability of 5MTHF in dried plasma specimens during shipment was evaluated according to a modified ISTA procedure 7D [31], wherein the length of specific incubation steps was increased so that the temperature profile could be executed practically without a programmable incubator. Initially, both 62-hour summer and winter profiles were conducted to emulate a 2-day shipment (Fig. 5). For each study, six matching specimens were collected into VelvetTM devices by fingerstick from 18 individual donors and immediately placed into storage bags (containing a desiccant and an oxygen scrubber). One device from each donor was allowed to dry overnight at room temperature, as per the standard protocol, then assayed to establish baseline values from the dried plasma specimens. Two devices from each donor were immediately subjected to the summer profile – one without additional packaging (‘exposed’) and one with insulated packaging (‘insulated’). Likewise, matching devices from each donor were immediately subjected to the winter profile with and without the additional packaging. The final device from each donor remained at room temperature for the duration of the profiles to serve as a control.
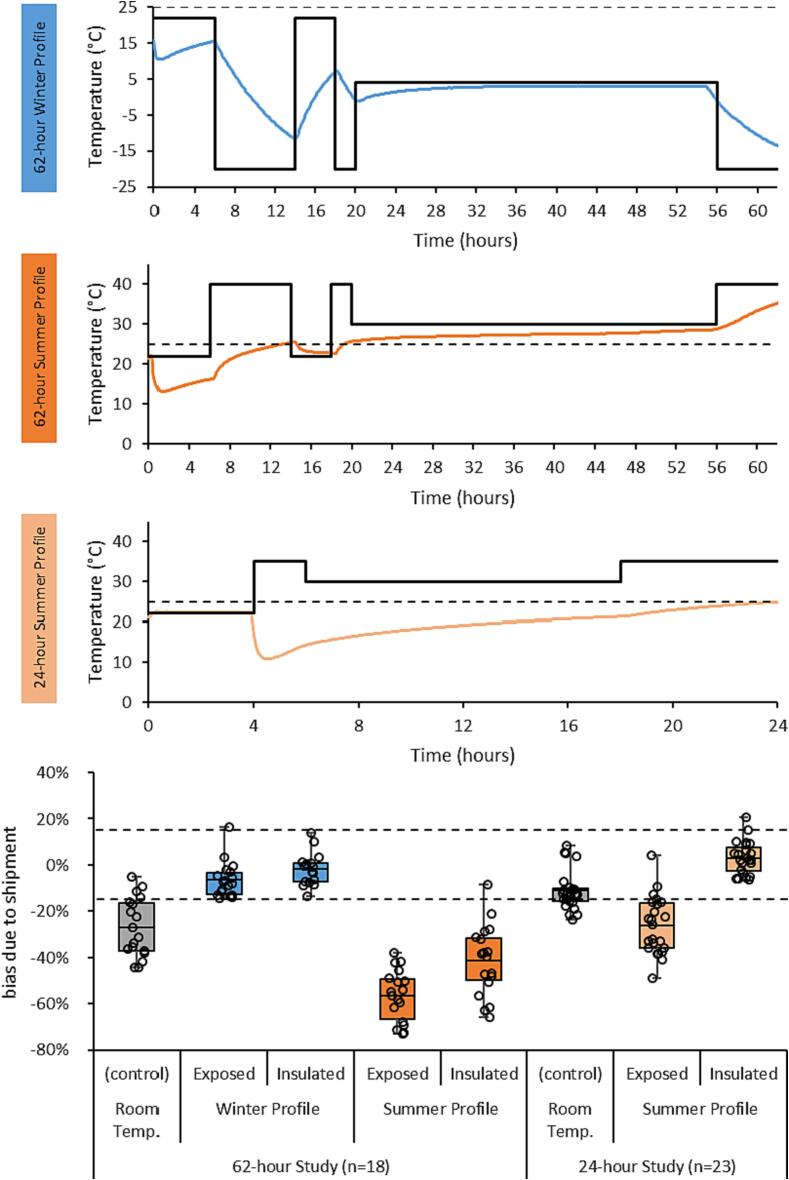
Fig. 5.
Shown are the temperature profiles (black line) executed for the three shipping studies along with the temperature inside the insulated packaging (colored lines). The dotted black line on the temperature profiles denote 25 °C – the upper limit for room temperature. The bottom figure shows the bias of the Cl-normalized 5MTHF measurements under each condition relative to baseline, where the dashed black lines represent acceptable bias limits (±15 %).
5MTHF results obtained in specimens subjected to the winter excursion profile showed a mean bias of + 1.9 % and −6.5 % relative to their baseline values with and without the insulated packaging, respectively (Fig. 5). By contrast, 5MTHF results obtained in specimens subjected to the summer excursion profile showed a mean bias of −41.3 % and −56.5 % relative to their baseline values with and without insulated packaging, respectively. Even the control specimens stored for the same duration at room temperature exhibited a bias of −26.9 % on average relative to baseline, which was consistent with the instability observed after 72 h of room temperature storage observed in the prior study (Fig. 4). The internal temperature of the insulated packaging was monitored for the duration of the profiles and showed that 25 °C was consistently exceeded after the first 19 h of the summer profile, exposing the devices to elevated temperature for the remaining 43 h (Fig. 5). The mean kinetic temperature inside the packaging was calculated to be 25.7 °C for the duration of the profile, but only 21.6 °C for the initial 24 h.
As such, the same study design was repeated with a summer profile emulating a 24-hour (i.e., overnight) shipment of the dried plasma specimens (Fig. 5). 5MTHF results obtained with control specimens subjected to room temperature storage for the duration of the 24-hour study showed a mean bias of −11.1 %, corroborating the results of the previous room temperature study (Fig. 4). A mean bias of −26.3 % was observed when devices were exposed to the 24-hour summer profile, while a mean bias of + 3.1 % was observed with devices stored within the insulated packaging (Fig. 5). Monitoring of the insulated packaging’s internal temperature confirmed the temperature was maintained below 25 °C with a mean kinetic temperature of 20.0 °C across the 24-hour profile.
Discussion
Folate levels are routinely measured in both whole blood and serum to assess folate status [32] and, consequently, there is a desire to facilitate these measurements using blood microsamples. Multiple groups have demonstrated the feasibility to measure folate from both DBS and dried plasma [33], [34], but instability of folate [35], as well as systematic errors due to variable hematocrit and volume uncertainty have limited the utility of DBS [36]. Volume uncertainty of the dried plasma specimens herein was addressed as previously described using normalization with chloride given its exceptionally low biological variability within and between individuals [26]. However, the present work expands on previous studies through careful examination of the pre-analytical factors known to adversely impact serum folate measurements – namely, hemolysis and instability.
Given the comparatively high concentration of folate in red blood cells relative to plasma [37], hemolysis represents a significant challenge to the measurement of folate in plasma and serum. In our usage of the VelvetTM Blood Collection device, visible hemolysis (>1%) was observable in approximately 3 % of dried plasma specimens. This hemolysis resulted in interference of both the 5MTHF measurement, but was more impactful to the chloride measurements used for normalization. Although chloride is also found in red blood cells [38], the magnitude of interference was greater than could be explained by the relative concentration of chloride in plasma (∼100 nmol/L) and red blood cells (∼90 nmol/L) – indicating the colorimetric measurement of chloride was likely the source of interference. Given the relative interference was larger for the chloride measurement than for 5MTHF, chloride-normalized 5MTHF results decreased. Unacceptable interference (>15 % bias) to the chloride-normalized 5MTHF results was only observed above 10 % hemolysis, which was not a common level of hemolysis observed in our experience with the VelvetTM device. However, a more conservative cut-off of 1 % hemolysis – above which chloride measurements alone demonstrated unacceptable interference – would potentially preclude a significant portion of specimens collected with the VelvetTM device from analysis. To that end, it may be appropriate to measure markers of red blood cells (i.e., hemoglobin, potassium, or glutathione) in the dried plasma fraction to identify unacceptable levels of hemolysis and determine the reliability of the chloride measurement without subjectivity.
A common assumption of dried specimens is improved pre-analytical stability. However, Zimmerman et al [35] showed folate measurements from DBS to be unstable during long-term storage, except when stored frozen (-80 °C). Our results indicate that 5MTHF has a similar instability in both liquid plasma and dried plasma collected with the VelvetTM device – requiring refrigerated storage (or colder) in order to stabilize specimens beyond 24 h. Exposure to light as well as high humidity has been a noted issue with storage of dried blood samples [17], [39]. These factors were easily mitigated in the present studies because the dried plasma specimens were always stored in the dark and with low relative humidity through the use of the vendor-supplied foil pouches, which contained both an oxygen scrubber and desiccant.
Given the gross instability of 5MTHF in dried plasma specimens at elevated and room temperature conditions, it was necessary to verify a means to stably ship specimens following potential at-home/remote collection. A basic packaging solution was devised to cool and insulate the VelvetTM device (Fig. 1). Although the insulated packaging did provide some reduction in the level of degradation that 5MTHF experienced during the 62-hour summer profile, unacceptable degradation (>15 % bias on average) still persisted given thermal control was lost after ∼ 30 h. Nonetheless, stability of 5MTHF in the dried plasma specimens was demonstrated during a 24-hour summer profile using the insulated packaging, making overnight shipping with this packaging a viable option following at-home collection.
To mitigate the need for controlled temperature shipping, others have suggested addition of antioxidants, such as ascorbic acid, to collection devices may improve stability of folates in dried specimens [24]; however, this was not feasible in our work because many antioxidants interfered with the chloride measurement (data not shown). Alternatively, others have suggested it may be feasible to obtain a ‘total 5MTHF’ that would comprise 5MTHF and its oxidized form(s), 4α-Hydroxy-5-methyltetrahydrofolic acid and its pyrazino-s-triazine derivative, MeFox [19], [30]. A ‘total 5MTHF’ measure would be resistant to instability due to oxidation, although these measurements would ideally require stable isotope-labeled compounds for these oxidation products that are not readily available. Perhaps a simpler approach would be forced oxidation of 5MTHF during the dried sample extraction step, whereby 5MTHF would only be detected in its oxidized form whether or not the oxidation occurred during storage or extraction of the dried specimen.
Conclusion
This work highlights two pre-analytical artifacts influencing the analysis of 5MTHF in dried plasma specimens – hemolysis and instability. Boundary conditions were established empirically to guide when 5MTHF could be successfully analyzed from dried plasma without interference from hemolysis or degradation due to storage or shipping. This work may serve as a framework for consideration of other analyte measurements from dried plasma.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank Brain Brooks for his contributions to generation of the data.
Footnotes
Peer review under responsibility of “MSACL”.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jmsacl.2023.01.003.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Lei B.U.W., Prow T.W. A review of microsampling techniques and their social impact. Biomed. Microdevices. 2019;21:81. doi: 10.1007/s10544-019-0412-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freeman J.D., Rosman L.M., Ratcliff J.D., Strickland P.T., Graham D.R., Silbergeld E.K. State of the science in dried blood spots. Clin. Chem. 2018;64:656–679. doi: 10.1373/clinchem.2017.275966. [DOI] [PubMed] [Google Scholar]
- 3.Patel P., Mulla H., Tanna S., Pandya H. Facilitating pharmacokinetic studies in children: a new use of dried blood spots. Arch. Dis. Child. 2010;95(6):484–487. doi: 10.1136/adc.2009.177592. [DOI] [PubMed] [Google Scholar]
- 4.Wilhelm A.J., den Burger J.C.G., Swart E.L. Therapeutic drug monitoring by dried blood spot: progress to date and future directions. Clin. Pharmacokinet. 2014;53(11):961–973. doi: 10.1007/s40262-014-0177-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verhaeghe T., Dillen L., Stieltjes H., Zwart L.d., Feyen B., Diels L., Vroman A., Timmerman P. The application of capillary microsampling in GLP toxicology studies. Bioanalysis. 2017;9(7):531–540. doi: 10.4155/bio-2016-0297. [DOI] [PubMed] [Google Scholar]
- 6.Guerra Valero Y., Dorofaeff T., Parker L., Coulthard M.G., Sparkes L., Lipman J., et al. Microsampling to support pharmacokinetic clinical studies in pediatrics. Pediatr Res. Springer, US. 2022;91:1557–1561. doi: 10.1038/s41390-021-01586-4. [DOI] [PubMed] [Google Scholar]
- 7.Guthrie R., Susi A. A simple phenylalanine method for detecting phenylketonuria in large populations of newborn infants. Pediatrics. 1963;32:338–343. [PubMed] [Google Scholar]
- 8.George R.S., Moat S.J. Effect of dried blood spot quality on newborn screening analyte concentrations and recommendations for minimum acceptance criteria for sample analysis. Clin. Chem. 2016;62:466–475. doi: 10.1373/clinchem.2015.247668. [DOI] [PubMed] [Google Scholar]
- 9.Moat S.J., George R.S., Carling R.S. Use of dried blood spot specimens to monitor patients with inherited metabolic disorders. Int. J. Neonatal Screen. 2020;6:1–17. doi: 10.3390/ijns6020026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Uytfanghe K., Heughebaert L., Stove C.P. Self-sampling at home using volumetric absorptive microsampling: coupling analytical evaluation to volunteers’ perception in the context of a large scale study. Clin. Chem. Lab. Med. 2021;59 doi: 10.1515/cclm-2020-1180. e185–7. [DOI] [PubMed] [Google Scholar]
- 11.Alfazil A.A., Anderson R.A. Stability of benzodiazepines and cocaine in blood spots stored on filter paper. J. Anal. Toxicol. 2008;32(7):511–515. doi: 10.1093/jat/32.7.511. [DOI] [PubMed] [Google Scholar]
- 12.Boy R.G., Henseler J., Mattern R., Skopp G. Determination of morphine and 6-acetylmorphine in blood with use of dried blood spots. Ther. Drug Monit. 2008;30:733–739. doi: 10.1097/FTD.0b013e31818d9fdb. [DOI] [PubMed] [Google Scholar]
- 13.D’Arienzo C.J., Ji Q.C., Discenza L., Cornelius G., Hynes J., Cornelius L., Santella J.B., Olah T. DBS sampling can be used to stabilize prodrugs in drug discovery rodent studies without the addition of esterase inhibitors. Bioanalysis. 2010;2(8):1415–1422. doi: 10.4155/bio.10.94. [DOI] [PubMed] [Google Scholar]
- 14.Grecsó N., Zádori A., Szécsi I., Baráth Á., Galla Z., Bereczki C., Monostori P., Lomonaco T. Storage stability of five steroids and in dried blood spots for newborn screening and retrospective diagnosis of congenital adrenal hyperplasia. PLoS One. 2020;15(5):e0233724. doi: 10.1371/journal.pone.0233724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaduskar O., Bhatt V., Prosperi C., Hayford K., Hasan A.Z., Deshpande G.R., Tilekar B., Vivian Thangaraj J.W., Kumar M.S., Gupta N., Murhekar M.V., Moss W.J., Mehendale S.M., Sangal L., Sapkal G., Pasetti M.F. Optimization and stability testing of four commercially available dried blood spot devices for estimating measles and rubella IgG antibodies. mSphere. 2021;6(4) doi: 10.1128/mSphere.00490-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adam B.W., Hall E.M., Sternberg M., Lim T.H., Flores S.R., O'Brien S., Simms D., Li L.X., De Jesus V.R., Hannon W.H. The stability of markers in dried-blood spots for recommended newborn screening disorders in the United States. Clin. Biochem. 2011;44(17-18):1445–1450. doi: 10.1016/j.clinbiochem.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han J., Higgins R., Lim M.D., Lin K., Yang J., Borchers C.H. Short-term stabilities of 21 amino acids in dried blood spots. Clin. Chem. 2018;64:400–402. doi: 10.1373/clinchem.2017.278457. [DOI] [PubMed] [Google Scholar]
- 18.O’Broin S.D., Kelleher B.P., Davoren A., Gunter E.W. Field-study screening of blood folate concentrations: Specimen stability and finger-stick sampling. Am. J. Clin. Nutr. 1997;66:1398–1405. doi: 10.1093/ajcn/66.6.1398. [DOI] [PubMed] [Google Scholar]
- 19.Hannisdal R., Ueland P.M., Eussen S.J.P.M., Svardal A., Hustad S. Analytical recovery of folate degradation products formed in human serum and plasma at room temperature. J. Nutr. 2009;139:1415–1418. doi: 10.3945/jn.109.105635. [DOI] [PubMed] [Google Scholar]
- 20.Scholl TO, Johnson WG. Folic acid: Influence on the outcome of pregnancy. Am J Clin Nutr. 2000;71. [DOI] [PubMed]
- 21.Ray J.G., Laskin C.A. Folic acid and homocyst(e)ine metabolic defects and the risk of placental abruption, pre-eclampsia and spontaneous pregnancy loss: A systematic review. Placenta. 1999;20(7):519–529. doi: 10.1053/plac.1999.0417. [DOI] [PubMed] [Google Scholar]
- 22.Morris M.S., Jacques P.F., Rosenberg I.H., Selhub J. Folate and vitamin B-12 status in relation to anemia, macrocytosis, and cognitive impairment in older Americans in the age of folic acid fortification. Am. J. Clin. Nutr. 2007;85:193–200. doi: 10.1093/ajcn/85.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pfeiffer C.M., Sternberg M.R., Fazili Z., Lacher D.A., Zhang M., Johnson C.L., Hamner H.C., Bailey R.L., Rader J.I., Yamini S., Berry R.J., Yetley E.A. Folate status and concentrations of serum folate forms in the US population: National Health and Nutrition Examination Survey 2011–2. Br. J. Nutr. 2015;113(12):1965–1977. doi: 10.1017/S0007114515001142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verstraete J, Boffel L, Stove C. Dried blood microsample-assisted determination of vitamins: recent developments and challenges. TrAC – Trends Anal Chem. Elsevier Ltd; 2020;132:116057.
- 25.WHO. Serum and Red Blood Cell Folate Concentrations for Assessing Folate Status in Populations. Vitamin and Mineral Nutrition Information System. 2012.
- 26.Crawford M.L., Collier B.B., Bradley M.N., Holland P.L., Shuford C.M., Grant R.P. Empiricism in microsampling: utilizing a novel lateral flow device and intrinsic normalization to provide accurate and precise clinical analysis from a finger stick. Clin. Chem. 2020;66:821–831. doi: 10.1093/clinchem/hvaa082. [DOI] [PubMed] [Google Scholar]
- 27.Guideline C.L.S.I. 2nd ed. Clinical and Laboratory Standards Institute; 2022. C62: Liquid Chromatography-Mass Spectrometry Methods. [Google Scholar]
- 28.Bioanalytical Method Validation Guidance for Industry. U.S. Department for Health and Human Services Food and Drug Administration; 2018.
- 29.Gupta V.S., Huennekens F.M. Preparation and properties of crystalline 5-methyl tetrahydrofolate and related compounds. Arch. Biochem. Biophys. 1967;120(3):712–718. [Google Scholar]
- 30.Fazili Z, Sternberg MR, Paladugula N, Whitehead RD, Chen H, Pfeiffer CM. The Loss of 5-Methyltetrahydrofolate in Human Serum under Suboptimal Preanalytical Conditions Can Only Partially Be Recovered by an Oxidation Product. J Nutr. 2014;144:1873–9. [DOI] [PMC free article] [PubMed]
- 31.ISTA Procedure 7D 2007: Temperature Test for Transport Packaging. East Lansing, Michigan: International Safe Transit Association; 2013.
- 32.Bailey L.B. Folate status assessment. J. Nutr. 1990;120:1508–1511. doi: 10.1093/jn/120.suppl_11.1508. [DOI] [PubMed] [Google Scholar]
- 33.Croff J., Tan C., Al C., Ml H., Ek C., Tk T. Vitamins & minerals erythrocyte and serum folate collection techniques : a multi-method study of folate status. Vitam Miner. 2020;9:1–4. [Google Scholar]
- 34.Kopp M., Rychlik M., Mudiam M.K.R. Quantitation of 5-methyltetrahydrofolic acid in dried blood spots and dried plasma spots by stable isotope dilution assays. PLoS One. 2015;10(11):e0143639. doi: 10.1371/journal.pone.0143639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zimmerman R.K., Slater M.E., Langer E.K., Ross J.A., Spector L.G. Long-term stability of folate in dried blood spots stored in several conditions. J Pediatr. Elsevier Ltd. 2013;163(2):596–597.e1. doi: 10.1016/j.jpeds.2013.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Denniff P., Spooner N. The effect of hematocrit on assay bias when using DBS samples for the quantitative bioanalysis of drugs. Bioanalysis. 2010;2(8):1385–1395. doi: 10.4155/bio.10.103. [DOI] [PubMed] [Google Scholar]
- 37.Sobczyńska-Malefora A., Harrington D.J., Voong K., Shearer M.J. Plasma and red cell reference intervals of 5-methyltetrahydrofolate of healthy adults in whom biochemical functional deficiencies of folate and vitamin B 12 had been excluded. Adv Hematol. Hindawi Publishing Corporation. 2014;2014:1–7. doi: 10.1155/2014/465623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pfortmueller C.A., Uehlinger D., von Haehling S., Schefold J.C. Serum chloride levels in critical illness—the hidden story. Intensive Care Med Exp. Intensive Care Medicine Experimental. 2018;6(1) doi: 10.1186/s40635-018-0174-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carpentieri D., Colvard A., Petersen J., Marsh W., David-Dirgo V., Huentelman M., Pirrotte P., Sivakumaran T.A. Mind the quality gap when banking on dry blood spots. Biopreserv Biobank. 2021;19(2):136–142. doi: 10.1089/bio.2020.0131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.