Abstract
Background and Aim
A potential solution to the deceased organ shortage is to include live organ donations and to identify patients with lower rates of HCC recurrence to fairly allocate liver grafts. Our aims were to detect the long-term outcomes of LDLT versus DDLT for HCC and predictors of recurrence after transplantation.
Methods
PubMed, Scopus, Web of Science, Cochrane library were searched for eligible studies from inception to July 2021 and a systematic review and meta-analysis were done.
Results
35 studies with a total of 7822 patients were included. The 1-, 3-, 4 year-OS showed trivial improvement for LDLT recipients. However, the two modalities had similar 5-, 6- and 10-year OS. A significant improvement in the ITT-OS was observed for LDLT recipients. Regarding the DFS and recurrence after transplantation, no significant difference was observed between LDLT and DDLT. In addition to that, the pooled hazard ratio of the included studies showed that Milan criteria, level of AFP, presence of vascular invasion, tumor differentiation were significant predictors of recurrence.
Conclusion
The cancer biology (not the graft type) is the most important determinant of recurrence and survival after LT. However, LDLT provided much better survival benefits to HCC patients especially in regions that suffer from low deceased organ availability.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12072-022-10435-3.
Keywords: Liver transplantation, Living donor, Living donor liver transplantation, Deceased donor, Deceased donor liver transplantation, Hepatocellular carcinoma, Cancer liver, Liver tumor, LT, Hepatobiliary surgery
Introduction
Liver cancer remains a global health problem and its incidence is rising worldwide [1, 2]. It is estimated that, by 2025, > 1 million people will be diagnosed with liver cancer annually [3]. Hepatocellular carcinoma (HCC) is the most common form of primary liver cancer and accounts for ~ 90% of cases [4].
Therapeutic treatment options are available for patients with the local disease and include ablation, resection, and liver transplantation (LT) [5]. LT is a recognized treatment choice for patients with cirrhosis of the liver and HCC [6].
The greatest obstacle in liver transplant is the shortage of donors which has contributed to a remarkable increase in the waiting lists. Therefore, there is an increase in the time from the decision of transplantation to the LT itself. During this period, the HCC may progress and drop out from the waiting list [7–9].
Several strategies have been evaluated to reduce this risk: increasing the pool of donors by including live donors, treatment of HCC upon enlistment, and priority policies by identifying patients with lower rates of HCC recurrence and higher rates of survival to fairly allocate liver grafts. However, the long-term outcomes of LDLT versus DDLT for HCC are still controversial. Several studies demonstrate that LDLT was associated with better intention to treat overall survival (ITT-OS) when compared to DDLT [10, 11]. While some studies illustrated that HCC patients undergoing LDLT would result in worse DFS and recurrence rate [12, 13], other studies reported equal recurrence rate for the two modalities [14]. Moreover, some studies showed equal overall survival and DFS between the two modalities [10]. In addition to that, there are many predictors of recurrence other than the type of the graft such as level of AFP, vascular invasion and tumor grade that could be used to fairly allocate graft to those with lower incidence of recurrence [15–17].
Patients and methods
Search strategy
The protocol for this meta-analysis was registered to PROSPERO (CRD42021281670). The search was directed through PubMed, Scopus, Web of science, and the Cochrane Library for information from May 1963 to July 1, 2021 with a combination of the following terms: liver donor liver transplant, hepatocellular carcinoma, LDLT and HCC. More searches by Google Scholar have been used to supplement the search with the sites mentioned above. All studies were reviewed and evaluated by two authors (Elkomos, B. E.& Abdelaal, A.) according to the eligibility process. Abstract-based eligibility studies were obtained, and the manuscripts were fully reviewed.
Inclusion and exclusion criteria
The eligible studies included the following: (1) randomized controlled trials and prospective or retrospective cohort studies; (2) target population were patients diagnosed with HCC; (3) studies designating a comparison of LDLT and DDLT as a primary aim; and (4) the primary outcomes were overall survival (OS), intention-to-treat overall survival (ITT-OS), disease-free survival (DFS) or recurrence of HCC for both LDLT and DDLT patients. Exclusion criteria: (1) reviews, case reports and case series; (2) studies designed to analyses information from the United Network for Organ Sharing database or the Scientific Registry of Transplant Recipients database; (3) studies missing a comparison group (DDLT recipients).
Outcomes of interest
We assessed 4 primary outcomes of LDLT and DDLT for HCC patients in this meta-analysis, including patient long-term overall survival from the time of transplant (1-, 2-, 3-, 4-,5-, 6- and 10-year OS), patient long-term overall survival from the time of listing to transplantation (1-, 2-, 3-, 4-,5-, 6- and 10-year ITT-OS), disease-free survival (1-, 2-, 3-, 4-,5-, 6- and 10-year DFS) and recurrence rate. In addition to that, our secondary outcomes were to detect the effect of age of recipient, sex of recipient, level of AFP and tumor biology (presence of vascular invasion and tumor grade) on the survival and recurrence of HCC after transplantation.
Quality assessment and data extraction
A modification of the Newcastle–Ottawa scale was used to assess the quality of all cohort studies included in this meta-analysis [18]. Only studies with seven or more stares were included (Table 2).
Table 2.
Newcastle–Ottawa scale for included studies
| Study | Representativeness of expose of cohort | Selection of the non-exposed cohort | Ascertainment of exposure | Demonstration that outcome of interest was not present at start of study | Comparability | Assessment of outcome | Adequate follow-up length | Adequacy of followup | Score |
|---|---|---|---|---|---|---|---|---|---|
| Gondolesi GE (2004) USA | * | * | * | * | ** | * | * | * | 9 |
| Roayaie S (2004) USA | * | * | * | * | * | * | * | 7 | |
| Hwang S (2005) Korea | * | * | * | * | ** | * | * | * | 9 |
| Karakayali H (2006) Turkey | * | * | * | * | ** | * | 7 | ||
| Sotiropoulos GC (2007) Germany | * | * | * | * | ** | * | * | * | 9 |
| Fisher RA (2007) USA | * | * | * | * | ** | * | * | * | 9 |
| Terrault NA (2007) USA | * | * | * | * | ** | * | 7 | ||
| Allam N (2008) KSA | * | * | * | * | * | * | * | * | 8 |
| Di Sandro S (2009) Italy | * | * | * | * | * | * | * | * | 9 |
| Vakili K (2009) USA | * | * | * | * | * | * | * | 7 | |
| Berg CL (2011) USA | * | * | * | * | * | * | * | * | 8 |
| Bhangui P (2011) France | * | * | * | * | ** | * | * | * | 9 |
| Azzam AZ (2011) KSA | * | * | * | * | * | * | * | 7 | |
| Kulik LM (2012) USA | * | * | * | * | ** | * | * | * | 9 |
| Sandhu L (2012) Canada | * | * | * | * | ** | * | * | * | 9 |
| Li C (2013) China | * | * | * | * | ** | * | * | * | 9 |
| Lei J (2013) China | * | * | * | * | ** | * | * | * | 9 |
| Xiao GQ (2014) China | * | * | * | * | * | * | * | 7 | |
| Chen J (2014) China | * | * | * | * | * | * | * | 7 | |
| Park MS (2014) Korea | * | * | * | * | ** | * | * | * | 9 |
| Wan P (2014) China | * | * | * | * | ** | * | * | * | 9 |
| Bonadio I (2014) Belgium | * | * | * | * | * | * | * | 7 | |
| Ninomiya M (2015) Japan USA | * | * | * | * | * | * | * | * | 8 |
| Chen LP (2015) China | * | * | * | * | ** | * | * | 8 | |
| Azoulay D (2017) France | * | * | * | * | ** | * | * | * | 9 |
| Goldaracena N (2019) Canada | * | * | * | * | * | * | * | * | 8 |
| Wong TCL (2019) China | * | * | * | * | ** | * | * | * | 9 |
| Lee S (2020) Korea | * | * | * | * | ** | * | * | * | 9 |
| Rahatli S (2020) Turkey | * | * | * | * | * | * | * | 7 |
* stands for one point
** stands for two points
We extracted data on study characteristics (author, year of publication, country of transplant, number of institutes included in the study, the follow-up of the patients), patient characteristics (type of graft, sample size, age, gender ratio, wait-time on the listing to transplantation, number of tumor nodules, size of the largest one, Child score, tumor differentiation, vascular invasion, pre-transplant treatment), study primary outcomes and study secondary outcomes. The data were extracted by 2 investigators (Elkomos, B. E.& Abdelaal, A.) independently.
Statistical analysis
The meta-analysis was performed according to Cochrane Handbook for Systematic Reviews of Interventions [19], which is recommended by the Cochrane Collaboration. Regarding the primary outcomes (OS, DFS, ITT-OS, recurrence of HCC), the pooled risk ratios (RRs) and their corresponding 95% confidence intervals (CIs) were calculated with fixed effects models. However, if there was moderate or considerable heterogeneity (I2 > 40), random effects models were used to solve the heterogeneity between studies. Nevertheless, pooled hazard ratio were calculated for secondary outcomes (predictors of recurrence and prognostic facts after transplantation). All calculations for the current meta-analysis were performed with Review Manager 5.4 for Windows (Cochrane Collaboration, Oxford, United Kingdom).
Assessment of publication bias and heterogeneity
Funnel plots were generated so that we could visually inspect for publication bias. Statistical heterogeneity was assessed with forest plots and the inconsistency statistic (I2). An I2 value of 40% or less corresponded to low heterogeneity. Statistical significance was considered at p < 0.05.
Results
Characteristics and quality assessment of eligible studies
As shown in the flow diagram (Fig. 1), 1584 articles were revealed using the following search string: living donor liver transplantation or LDLT and hepatocellular carcinoma or HCC. After careful selection according to our eligibility criteria, 35 controlled clinical trials with 7822 participants were included in the meta-analysis. These trials included 34 retrospective cohort studies and 1 prospective study. However, none of the included studies were randomized studies.
Fig. 1.
PRISMA flow diagram
Recipients baseline data [including number, age, sex and waiting time], follow-up time and the tumor-related baseline variables [including percentage of patients beyond the Milan or UCSF criteria, number of tumors, tumor differentiation, the size of largest tumor, vascular invasion, MELD score, Child-Pugh class, and treatment before LT] were comparable between groups in all studies (Table 1). The quality assessment was conducted according to a modification of the Newcastle–Ottawa scale (Table 2). Most of the cohort studies included in this analysis demonstrated sufficient quality with reasonable selection criteria, comparable patient characteristics, and adequate follow-up of the subjects.
Table 1.
Basic data of the included studies
| Studies: author, year, country | Study design | Study period | No. of centers | Arm | Sample size (n) | Age (years) | Gender: male/female (n) | Waiting time (days) | Follow-up period (years) | Serology: HBV/HCV/both/none (n) | Child–Pugh class: A/B/C(n) | Within/beyond Milan criteria (n) | Within/beyond UCSF criteria (n) | No/micro/macro vascular invasion (n) | No. of tumor nodules (1/2/3 or more) (n) | Size of the largest nodule (cm) | Differentiation: well/moderate/poor (n) | Pretreatment: yes/none (n) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gondolesi GE (2004) USA [20] | Retrospective cohort | 1998–2002 | 1 | LDLT | 36 | 56.17 ± 7.56a | 29/7 | 62 | 1.25 ± 0.84a | 9/24/0/3 | 12/16/8 | N/A | N/A | 15/15/6 | 15/8/12 | N/A | 15/15/6 | 13/23 |
| 1992–2002 | DDLT | 165 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |||
| Roayaie S (2004) USA [21] | Retrospective cohort | 1988–2002 | 1 | LDLT | 36 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| DDLT | 275 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | ||||
| Hwang S (2005) Korea [22] | Retrospective cohort | 1992–2002 | 4 | LDLT | 237 | 50 ± 8a | 196/41 | N/A | 3.75 (0.3–8.4)b | 215/13/8/1 | 29/70/138 | 173/64 | N/A | N/A | N/A | N/A | N/A | N/A |
| DDLT | 75 | 49 ± 7a | 60/15 | N/A | 2.2 (0.3–6.7)b | 68/6/0/1 | 4/13/55 | 53/22 | N/A | N/A | N/A | N/A | N/A | N/A | ||||
| Karakayali H (2006) Turkey [23] | Retrospective cohort | 2004–2005 | 1 | LDLT | 11 | 31.8 ± 24.9a | N/A | N/A | N/A | 2/4/0/5 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 9/2 |
| DDLT | 6 | 55 ± 4.7a | N/A | N/A | N/A | 5/0/0/1 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 3/3 | ||||
| Sotiropoulos GC (2007) Germany [24] | Retrospective cohort | 1998–2006 | 1 | LDLT | 45 | 55.0 ± 10.1a | 33/12 | N/A | N/A | 12/18/2/13 | 10/24/11 | 23/22 | 25/20 | N/A | 20/8/1/16 | N/A | N/A | N/A |
| DDLT | 55 | 53.4 ± 9.1a | 42/13 | N/A | N/A | 15/27/0/13 | 19/23/13 | 25/30 | 2728 | N/A | 26/9/2/18 | N/A | N/A | N/A | ||||
| Fisher RA (2007) USA [25] | Retrospective cohort | 1998–2003 | 9 | LDLT | 58 | 54.6 ± 9a | 45/13 | 95 | 4 | N/A | N/A | 21/37 | 28/28 | 43/9/3 | N/A | N/A | N/A | 26/32 |
| DDLT | 34 | 52.1 ± 10a | 25/9 | 353 | 3.4 | N/A | N/A | 20/14 | 24/10 | 27/3/0 | N/A | N/A | N/A | 14/20 | ||||
| Terrault NA (2007) USA [26] | Retrospective cohort | 1998–2003 | 9 | LDLT | 36 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| DDLT | 27 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | ||||
| Allam N (2008) KSA [27] | Retrospective cohort | 2001–2007 | 1 | LDLT | 8 | 55.14 ± 8.1a | 6/3 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| DDLT | 15 | 48.78 ± 17.5a | 10/4 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | ||||
| Di Sandro S (2009) Italy [28] | Retrospective cohort | 2000–2007 | 1 | LDLT | 25 | N/A | N/A | 107 (11–385)b | N/A | N/A | N/A | 15/10 | N/A | N/A | N/A | N/A | N/A | 21/4 |
| DDLT | 154 | N/A | N/A | 404 (3–1704)b | N/A | N/A | N/A | 106/48 | N/A | N/A | N/A | N/A | N/A | 107/47 | ||||
| Vakili K (2009) USA [29] | Retrospective cohort | 1999–2007 | 1 | LDLT | 28 | 56 (47–67)b | 21/7 | N/A | 3.4 (0.25–8.7)b | 15/2/0/11 | N/A | 21/7 | 26/2 | N/A | 18/7/2/1 | 3.4 ± 1.0a | 6/19/3 | 5/23 |
| DDLT | 74 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | ||||
| Giacomoni A (2009) Italy [30] | Retrospective cohort | 2000–2007 | 1 | LDLT | 25 | 57 | N/A | 264 | N/A | N/A | N/A | 15/10 | N/A | N/A | N/A | N/A | N/A | 21//4 |
| DDLT | 154 | 54 | N/A | 404 | N/A | N/A | N/A | 107/47 | N/A | N/A | N/A | N/A | N/A | 107/47 | ||||
| Hsieh TH (2010) USA [31] | Retrospective cohort | 1999–2008 | 1 | LDLT | 15 | 56 | N/A | N/A | N/A | N/A | N/A | 11//4 | N/A | N/A | N/A | N/A | N/A | 4/11 |
| DDLT | 121 | 56 | N/A | N/A | N/A | N/A | N/A | 90/31 | N/A | N/A | N/A | N/A | N/A | 88/33 | ||||
| Sharr WW (2011) China [32] | Retrospective cohort | 1995–2005 | 1 | LDLT | 90 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 90/0 | N/A | N/A | N/A | N/A | N/A |
| DDLT | 34 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 34/0 | N/A | N/A | N/A | N/A | N/A | ||||
| Kornberg A (2011) Germany [33] | Retrospective cohort | N/A | 1 | LDLT | 12 | N/A | N/A | 120 | N/A | N/A | N/A | 6/6 | N/A | N/A | N/A | N/A | N/A | N/A |
| DDLT | 78 | N/A | N/A | 365 | N/A | N/A | N/A | 51/27 | N/A | N/A | N/A | N/A | N/A | N/A | ||||
| Berg CL (2011) USA [34] | Retrospective cohort | 2002–2009 | 9 | LDLT | 49 | N/A | N/A | 50.08 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| DDLT | 65 | N/A | N/A | 72.65 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | ||||
| Bhangui P (2011) France [35] | Retrospective cohort | 2000–2009 | 1 | LDLT | 36 | 54 ± 7a | 32/4 | 78 ± 72a | 4.8 ± 3a | N/A | N/A | 26/10 | 31/5 | N/A | N/A | 2.9 ± 1.1a | N/A | 12/24 |
| DDLT | 120 | 56 ± 8a | 100/20 | 237 ± 270a | 4.2 ± 2.6a | N/A | N/A | 94/26 | 104/16 | N/A | N/A | 3 ± 2.3a | N/A | 45/75 | ||||
| Azzam AZ (2011) KSA [36] | Retrospective cohort | 2001–2011 | 1 | LDLT | 18 | N/A | N/A | N/A | N/A | N/A | N/A | 18/0 | N/A | N/A | N/A | N/A | N/A | N/A |
| DDLT | 34 | N/A | N/A | N/A | N/A | N/A | N/A | 34/0 | N/A | N/A | N/A | N/A | N/A | N/A | ||||
| Kulik LM (2012) USA [37] | Retrospective cohort | 1998–2009 | 9 | LDLT | 100 | 55.2 ± 8a | 75/25 | 77.7 ± 106a | 5.9 | 78 (HCV) | N/A | 41/59 | 65/35 | N/A | 2.4 ± 1.8a | 4.3 ± 2.5a | N/A | 59/41 |
| DDLT | 97 | 53.9 ± 8.5a | 76/21 | 180.5 ± 258a | 4.3 | 78 (HCV) | N/A | 71/26 | 83/14 | N/A | 2.1 ± 1.7a | 3.5 ± 1.9a | N/A | 73/24 | ||||
| Sandhu L (2012) Canada [38] | Retrospective cohort | 1996–2009 | 1 | LDLT | 58 | 54.5 ± 8.8a | 46/12 | 93 (6–753)b | 2.5 (0.2–7.3)b | 8/38/0/12 | N/A | 42/16 | N/A | N/A | 1 (0–11) | 3.9 (0.5–22)b | 49 W&M/3 p | 29/29 |
| DDLT | 287 | 55.8 ± 7.1a | 246/41 | 159 (0–1071)b | 3.2 (0–13.4)b | 81/137/0/69 | N/A | 189/91 | N/A | N/A | 1 (0–12) | 3.8 (0.5–15.4)b | 199 W&M/33 p | 159/128 | ||||
| Li C (2013) China [39] | Retrospective cohort | 2004–2012 | 1 | LDLT | 60 | 45.23 ± 8.18a | 54/6 | N/A | N/A | N/A | N/A | N/A | N/A | 35/25/0 | N/A | N/A | 7/41/12 | N/A |
| DDLT | 156 | 47.99 ± 9.60a | 138/18 | N/A | N/A | N/A | N/A | N/A | N/A | 111/45/0 | N/A | N/A | 25/106/25 | N/A | ||||
| Lei J (2013) China [40] | Retrospective cohort | 2002–2009 | 1 | LDLT | 31 | 44.4 ± 9.7a | 18/13 | N/A | N/A | 26/1/1/3 | 15/3/3 | N/A | N/A | N/A | 14/8/9 | N/A | 10/15/8 | 31/0 |
| DDLT | 52 | 44.0 ± 8.21a | 31/21 | N/A | N/A | 39/3/3/7 | 14/6/3 | N/A | N/A | N/A | 22/16/14 | N/A | 20/20/12 | 52/0 | ||||
| Xiao GQ (2014) China [41] | Retrospective cohort | 1999–2012 | 1 | LDLT | 84 | 44.3 | 6/78 | 27 | N/A | N/A | 43/34/7 | 22/58 | 28//52 | N/A | 44.3 | N/A | N/A | 17/69 |
| DDLT | 276 | 47.3 | 29/247 | 48 | N/A | N/A | 137/119/20 | 69/199 | 84/184 | N/A | 47.3 | N/A | N/A | 101/175 | ||||
| Chen J (2014) China [42] | Retrospective cohort | 2007–2010 | 1 | LDLT | 47 | N/A | 44/3 | 22 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 30 W&M/17 p | N/A |
| DDLT | 94 | N/A | 88/6 | 91 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 55 W&M/39 p | N/A | ||||
| Park MS (2014) Korea [12] | Retrospective cohort | N/A | 1 | LDLT | 166 | 52.5 ± 7.7a | 131/35 | N/A | N/A | 146/12/0/8 | N/A | N/A | N/A | N/A | 1.4 ± 0.6a | N/A | N/A | 96/70 |
| DDLT | 50 | 54.3 ± 9.6a | 29/21 | N/A | N/A | 39/6/0/5 | N/A | N/A | N/A | N/A | 1.5 ± 0.7a | N/A | N/A | 33/17 | ||||
| Wan P (2014) China [43] | Retrospective cohort | 2007–2010 | 1 | LDLT | 40 | 48.6 ± 9.7a | N/A | N/A | 1.75 (0.08–6.25)b | 40/0/0/0 | 12/18/10 | 24/16 | N/A | N/A | 48.6 ± 9.7a | N/A | N/A | 12/28 |
| DDLT | 80 | 49.5 ± 8.9a | N/A | N/A | 24 (0.08–6.3)b | 76/0/0/4 | 25/38/17 | 48/32 | N/A | N/A | 49.5 ± 8.9a | N/A | N/A | 29/51 | ||||
| Bonadio I (2014) Belgium [44] | Retrospective cohort | 2000–2007 | 1 | LDLT | 28 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| DDLT | 48 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | ||||
| Ninomiya M (2015) Japan/USA [14] | Retrospective cohort | 2002–2010 | 2 | LDLT | 133 | 57.6 ± 7.1a | 78/55 | 44 (4–236)b | 6.3 | 21/100/12 | N/A | N/A | N/A | N/A | 4.8 ± 7.9a | 2.4 ± 1.1a | 7.5%/63.9%/28.6% | N/A |
| DDLT | 362 | 58.3 ± 7.4a | 285/77 | 196 (0–3996)b | 5.6 | 51/212/99 | N/A | N/A | N/A | N/A | 2.6 ± 2.2a | 2.8 ± 1.8a | 40.8%/49.7%/9.7% | N/A | ||||
| Chen LP (2015) China [45] | Retrospective cohort | 2005–2013 | 1 | LDLT | 66 | 45.82 ± 7.72a | 60//6 | 23.37 ± 16.32 | N/A | N/A | N/A | 34/32 | 42/24 | N/A | N/A | 5.22 ± 2.31a | 13/45/8 | N/A |
| DDLT | 163 | 47.93 ± 9.51a | 144/19 | 46.88 ± 32.12a | N/A | N/A | N/A | 72/91 | 95/68 | N/A | N/A | 5.24 ± 2.24a | 27/110/26 | N/A | ||||
| Tomiyama K (2016) Canada [46] | Retrospective cohort | 2000–2004 | 1 | LDLT | 106 | N/A | N/A | 264 (189–450)b | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| DDLT | 434 | N/A | N/A | 465 (210–891)b | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | ||||
| Fonseca E (2016) Brazil [47] | Retrospective cohort | 2000–2009 | 1 | LDLT | 43 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| DDLT | 23 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | ||||
| Azoulay D (2017) France [48] | Retrospective cohort | 2000–2009 | 5 | LDLT | 75 | 54.2 ± 7.6a | 62/13 | 78 ± 69a | 8.5 ± 1.9a | N/A | 46 A&B/ 28 C | 44/31 | N/A | N/A | N/A | N/A | N/A | 53/22 |
| DDLT | 576 | 56.3 ± 7.4a | 499/77 | 183 ± 219a | 5.6 ± 13.4a | N/A | 441/76 | 333/243 | N/A | N/A | N/A | N/A | N/A | 366/205 | ||||
| Goldaracena N (2019) Canada [11] | Retrospective cohort | 2000–2015 | 1 | LDLT | 118 | N/A | N/A | 108 (75–195)b | 4 (1.7–8.2)b | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 64/54 |
| DDLT | 527 | N/A | N/A | 189 (96–336)b | 4.3 (2–9)b | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 380/147 | ||||
| Wong TCL (2019) China [10] | Retrospective cohort | 1995–2014 | 1 | LDLT | 161 | 55 (30–73)b | 129/32 | N/A | N/A | 125 (HBV) 28 (HCV) | 81/48/32 | 113/48 | 141/20 | N/A | 85/47/20 | 2.9 (0.90–8.80)b | 45/91/10 | N/A |
| DDLT | 85 | 57 (41–68)b | 72/13 | N/A | N/A | 76 (HBV) 6 (HCV) | 32/31/22 | 72/13 | 85/0 | N/A | 57/17/11 | 2.4 (0.70–0.60)b | 16/46/6 | N/A | ||||
| Lee S (2020) Korea [49] | Retrospective cohort | 2005–2015 | 1 | LDLT | 829 | 53.7 ± 6.2a | 709/120 | N/A | N/A | 728 (HBV) 45 (HCV) | 346/319/164 | N/A | N/A | N/A | N/A | 1.5 ± 1.6a | N/A | 635/194 |
| DDLT | 67 | 54.7 ± 7.7a | 51/16 | N/A | N/A | 58 (HBV) 4 (HCV) | 8/20/39 | N/A | N/A | N/A | N/A | 1.7 ± 2.0a | N/A | 53/14 | ||||
| Rahatli S (2020) Turkey [50] | Retrospective cohort | 1988–2018 | 1 | LDLT | 29 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| DDLT | 20 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
aThe results are presented as means and standard deviation
bThe results are presented as median and range
Primary outcome
Overall survival
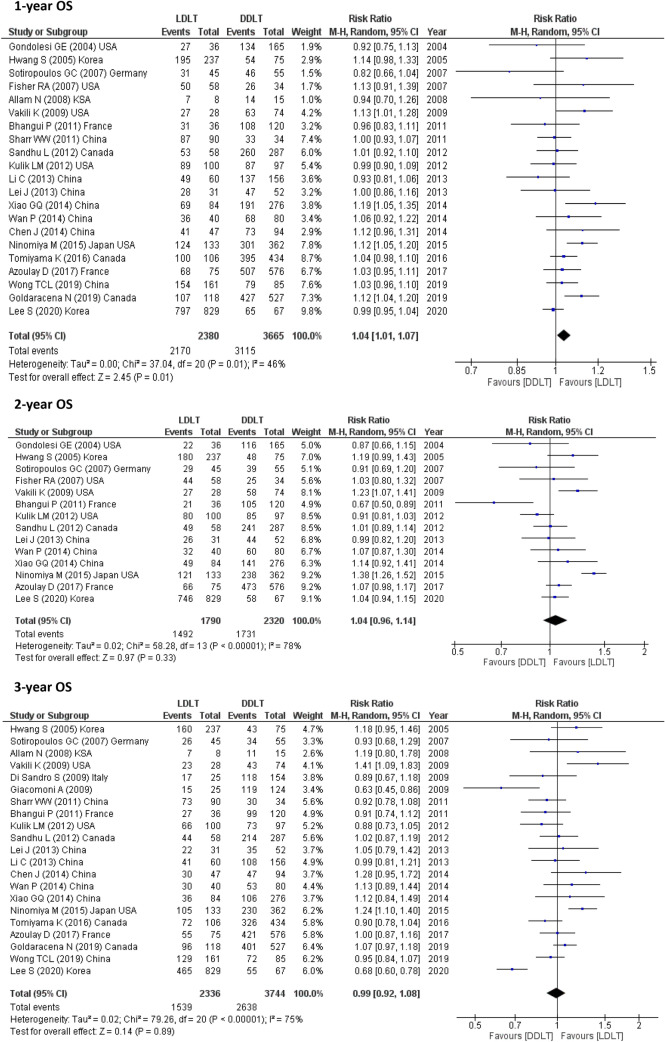
21 studies (6045 participants) assessed 1-year OS, 19 studies (5859) reported 3-year OS and 12 studies (3817) calculated 4 year-OS. The pooled results from these studies showed possible improvement for LDLT recipients as follows (1-year OS, RR = 1.04, 95% CI 1.01–1.07, p = 0.01; I2 = 46%) and (3-year OS, RR = 1.07, 95% CI 1.01–1.13, p = 0.02; I2 = 63%) However, this meta-analysis showed that LDLT recipients and DDLT recipients had similar 5-, 6- and 10-year OS (5-year OS, RR = 0.99, 95% CI 0.92–1.08, p = 0.89; I2 = 75%) and (10-year OS, RR = 1.24, 95% CI 0.92–1.67, p = 0.16; I2 = 90%) as shown in 21 studies (6080) for 5-year OS, 5 studies (2002) for 6-year OS and 2 studies (1391) for 10-year OS (Fig. 2).
Fig. 2.
OS for LDLT and DDLT recipients
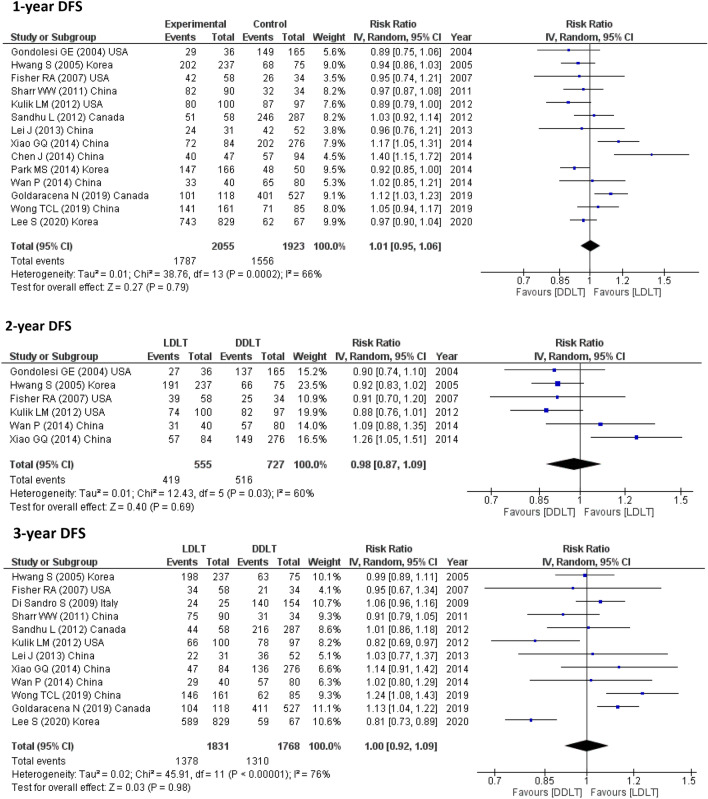
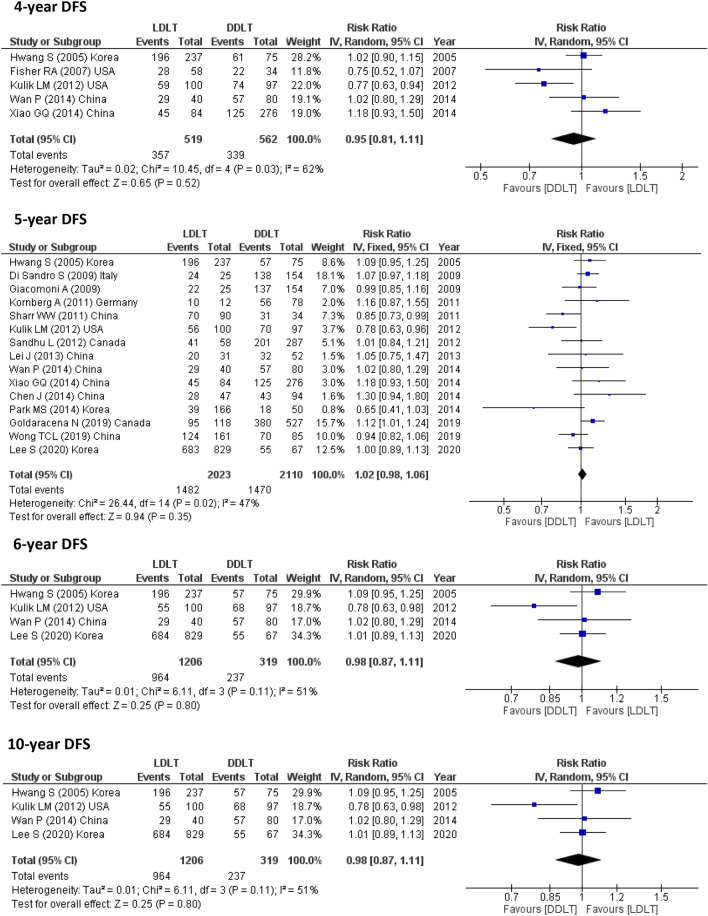
RFS
14 studies (3978 participants) reported 1-year RFS, 6 studies (1282 participants) assessed 2-year DFS, 12 studies (3599 participants) reported 3-year RFS, 5 studies (1081 participants) calculated 4-year DFS, 15 studies (4133 participants) assessed 5-year DFS, 4 studies (1525 participants) reported 6-year DFS and only one study assessed 10-year DFS (896 participants). The pooled results from these studies showed no significant difference between LDLT and DDLT (Fig. 3).
Fig. 3.
DFS for LDLT and DDLT recipients
ITT-OS
While 1-, 3- and 5-year ITT-OS were reported in 5 studies (2934 participants) and 2-, 4-year ITT-OS were assessed in 3 studies (1419), no study calculated the 6-, 10-year ITT-OS. Significant improvement was observed for LDLT recipients especially for 5-year ITT-OS. (1-year, RR = 1.14, 95% CI 1.01–1.28, P = 0.03; I2 = 88%), (2-year, RR = 1.23, 95% CI 1.00–1.50, P = 0.05; I2 = 85%), (3-year, RR = 1.26, 95% CI 1.08–1.47, P = 0.004; I2 = 84%), (4-year, RR = 1.46, 95% CI 1.07–1.99, P = 0.02; I2 = 87%) and (5-year, RR = 1.37, 95% CI 1.09–1.72, P = 0.006; I2 = 89%) (Fig. 4).
Fig. 4.
ITT-OS for LDLT and DDLT recipients
Recurrence rates
The number of HCC recurrence was pooled from 16 studies (3617 participants) and showed comparable recurrence between recipients after LDLT and DDLT (RR = 1.07, 95% CI 0.77–1.48, P = 0.70; I2 = 62%) (Fig. 5).
Fig. 5.
Recurrence for LDLT and DDLT recipients
Subgroup analysis
To investigate the source of heterogeneity among studies, a subgroup analysis was carried out by stratifying the analysis according to Milan criteria and region of transplant.
Milan criteria
We performed an additional comparative analysis of LDLT and DDLT in patients with HCC meeting or exceeding the Milan criteria regarding 0S and DFS (Table 3). For those meeting the Milan criteria, no significant difference in OS and DFS could be detected between LDLT and DDLT recipients. On the other hand, OS for those exceeding Milan criteria was better after LDLT. However, there were insufficient data to detect DFS for patients beyond Milan criteria. Notably, the outcome for those exceeding Milan criteria should be carefully interpreted because of the limited data (Tables 3, 4).
Table 3.
OS for LDLT and DDLT within and beyond Milan criteria
| Subgroup | Outcome | Studies (n) | Patient (n) | Effect estimate [RR (95% CI)] | Heterogeneity | Test for overall effect | Favour group |
|---|---|---|---|---|---|---|---|
| Within Milan | 1 year OS | 5 | 1593 | 1.04 [0.96, 1.12] | I2 = 72% (p = 0.006) | Z = 1.02 (p = 0.31) | None |
| 2 year OS | 4 | 1502 | 1.06 [0.97, 1.16] | I2 = 70% (p = 0.02) | Z = 1.23 (p = 0.22) | None | |
| 3 year OS | 5 | 1580 | 1.01 [0.88, 1.16] | I2 = 81% (p = 0.0002) | Z = 0.15 (p = 0.88) | None | |
| 4 year OS | 4 | 1502 | 1.07 [0.92, 1.25] | I2 = 83% (p = 0.0005) | Z = 0.85 (p = 0.39) | None | |
| 5 year OS | 5 | 1593 | 1.10 [0.93, 1.29] | I2 = 83% (p = 0.0001) | Z = 1.10 (p = 0.27) | None | |
| 6 year OS | 3 | 1271 | 1.22 [0.97, 1.52] | I2 = 88% (p = 0.0002) | Z = 1.70 (p = 0.09) | None | |
| 10 year OS | 2 | 1078 | 1.23 [0.83, 1.84] | I2 = 96% (p < 0.00001) | Z = 1.04 (p = 0.30) | None | |
| Beyond Milan | 1 year OS | 4 | 501 | 1.02 [0.94, 1.10] | I2 = 0% (p = 0.73) | Z = 0.50 (p = 0.62) | None |
| 2 year OS | 4 | 501 | 1.06 [0.95, 1.18] | I2 = 0% (p = 0.83) | Z = 0.98 (p = 0.33) | None | |
| 3 year OS | 4 | 501 | 1.16 [1.01, 1.32] | I2 = 0% (p = 0.60) | Z = 2.16 (p = 0.03) | LDLT | |
| 4 year OS | 4 | 501 | 1.20 [1.04, 1.38] | I2 = 32% (p = 0.22) | Z = 2.44 (p = 0.01) | LDLT | |
| 5 year OS | 3 | 420 | 1.32 [1.13, 1.54] | I2 = 0% (p = 0.79) | Z = 3.44 (p = 0.0006) | LDLT | |
| 6 year OS | 2 | 313 | 1.30 [1.03, 1.64] | I2 = 0% (p = 0.75) | Z = 2.25 (p = 0.02) | LDLT | |
| 10 year OS | 2 | 313 | 1.42 [1.07, 1.87] | I2 = 34% (p = 0.22) | Z = 2.47 (p = 0.01) | LDLT |
Table 4.
DFS for LDLT and DDLT within and beyond Milan criteria
| Subgroup | Outcome | Studies (n) | Patient (n) | Effect estimate [RR (95% CI)] | Heterogeneity | Test for overall effect | Favour group |
|---|---|---|---|---|---|---|---|
| Within Milan | 1 year DFS | 2 | 853 | 0.99 [0.89, 1.10] | I2 = 64% (p = 0.10) | Z = 0.18 (p = 0.86) | None |
| 2 year DFS | 1 | 762 | N/A | N/A | N/A | N/A | |
| 3 year DFS | 2 | 853 | 0.93 [0.89, 0.97] | I2 = 6% (p = 0.30) | Z = 3.42 (p = 0.0006) | DDLT | |
| 4 year DFS | 1 | 762 | N/A | N/A | N/A | N/A | |
| 5 year DFS | 2 | 853 | 0.96 [0.83, 1.11] | I2 = 47% (p = 0.17) | Z = 0.61 (p = 0.54) | None | |
| 6 year DFS | 1 | 762 | N/A | N/A | N/A | N/A | |
| 10 year DFS | 1 | 762 | N/A | N/A | N/A | N/A | |
| Beyond Milan | 1 year DFS | 1 | 134 | N/A | N/A | N/A | N/A |
| 2 year DFS | 1 | 134 | N/A | N/A | N/A | N/A | |
| 3 year DFS | 1 | 134 | N/A | N/A | N/A | N/A | |
| 4 year DFS | 1 | 134 | N/A | N/A | N/A | N/A | |
| 5 year DFS | 1 | 134 | N/A | N/A | N/A | N/A | |
| 6 year DFS | 1 | 134 | N/A | N/A | N/A | N/A | |
| 10 year DFS | 1 | 134 | N/A | N/A | N/A | N/A |
Region (Asia, America and Europe)
Another comparison was done to detect the OS and DFS between LDLT and DDLT recipients according to the region of transplant (Asia, America and Europe). No remarkable difference in OS between LDLT and DDLT could be detected according to the region (Tables 5, 6).
Table 5.
OS for LDLT and DDLT according to the region of transplantation
| Subgroup | Outcome | Studies (n) | Patient (n) | Effect estimate [RR (95% CI)] | Heterogeneity | Test for overall effect | Favour group |
|---|---|---|---|---|---|---|---|
| Asia | 1 year OS | 11 | 2722 | 1.03 [0.98, 1.07] | I2 = 44% (p = 0.06) | Z = 1.14 (p = 0.25) | None |
| 2 year OS | 5 | 1771 | 1.07 [1.00, 1.14] | I2 = 0% (p = 0.56) | Z = 1.82 (p = 0.07) | None | |
| 3 year OS | 8 | 2357 | 1.09 [0.98, 1.21] | I2 = 68% (p = 0.003) | Z = 1.64 (p = 0.10) | None | |
| 4 year OS | 5 | 1771 | 1.08 [1.00, 1.18] | I2 = 0% (p = 0.81) | Z = 1.89 (p = 0.06) | None | |
| 5 year OS | 9 | 1625 | 1.03 [0.95, 1.12] | I2 = 22% (p = 0.25) | Z = 0.82 (p = 0.41) | None | |
| 6 year OS | 2 | 1208 | 1.06 [0.95, 1.18] | I2 = 0% (p = 0.83) | Z = 0.97 (p = 0.33) | None | |
| 10 year OS | 1 | 896 | N/A | N/A | N/A | N/A | |
| America | 1 year OS | 8 | 2434 | 1.06 [1.02, 1.10] | I2 = 31% (p = 0.18) | Z = 3.08 (p = 0.002) | LDLT |
| 2 year OS | 5 | 937 | 1.01 [0.89, 1.15] | I2 = 67% (p = 0.02) | Z = 0.21 (p = 0.83) | None | |
| 3 year OS | 6 | 1921 | 1.05 [0.93, 1.18] | I2 = 77% (p = 0.0005) | Z = 0.75 (p = 0.45) | None | |
| 4 year OS | 3 | 644 | 1.11 [0.93, 1.34] | I2 = 54% (p = 0.11) | Z = 1.13 (p = 0.26) | None | |
| 5 year OS | 5 | 1829 | 1.02 [0.90, 1.16] | I2 = 68% (p = 0.01) | Z = 0.31 (p = 0.76) | None | |
| 6 year OS | 2 | 299 | 1.17 [0.61, 2.23] | I2 = 93% (p = 0.0002) | Z = 0.48 (p = 0.63) | None | |
| 10 year OS | 0 | 0 | N/A | N/A | N/A | N/A | |
| Europe | 1 year OS | 5 | 1420 | 0.99 [0.91, 1.09] | I2 = 44% (p = 0.13) | Z = 0.13 (p = 0.90) | None |
| 2 year OS | 3 | 907 | 0.88 [0.64, 1.22] | I2 = 85%(p = 0.001) | Z = 0.77 (p = 0.44) | None | |
| 3 year OS | 4 | 1086 | 1.03 [0.95, 1.13] | I2 = 0% (p = 0.72) | Z = 0.75 (p = 0.46) | None | |
| 4 year OS | 3 | 907 | 0.99 [0.90, 1.10] | I2 = 0% (p = 0.50) | Z = 0.18 (p = 0.86) | None | |
| 5 year OS | 4 | 1079 | 0.87 [0.71, 1.06] | I2 = 58% (p = 0.07) | Z = 1.35 (p = 0.18) | None | |
| 6 year OS | 1 | 197 | N/A | N/A | N/A | N/A | |
| 10 year OS | 0 | 0 | N/A | N/A | N/A | N/A |
Table 6.
DFS for LDLT and DDLT according to the region of transplantation
| Subgroup | Outcome | Studies (n) | Patient (n) | Effect estimate [RR (95% CI)] | Heterogeneity | Test for overall effect | Favour group |
|---|---|---|---|---|---|---|---|
| Asia | 1 year DFS | 9 | 2498 | 1.02 [0.95, 1.09] | I2 = 69% (p = 0.001) | Z = 0.52 (p = 0.60) | None |
| 2 year DFS | 3 | 792 | 1.07 [0.87, 1.31] | I2 = 78% (p = 0.010) | Z = 0.61 (p = 0.54) | None | |
| 3 year DFS | 7 | 2141 | 1.00 [0.88, 1.14] | I2 = 79% (p < 0.0001) | Z = 0.03 (p = 0.98) | None | |
| 4 year DFS | 3 | 792 | 1.04 [0.95, 1.15] | I2 = 0% (p = 0.53) | Z = 0.85 (p = 0.39) | None | |
| 5 year DFS | 10 | 2843 | 1.00 [0.93, 1.08] | I2 = 38% (p = 0.11) | Z = 0.00 (p = 1.00) | None | |
| 6 year DFS | 3 | 1328 | 1.04 [0.95, 1.13] | I2 = 0% (p = 0.68) | Z = 0.82 (p = 0.41) | None | |
| 10 year DFS | 1 | 896 | N/A | N/A | N/A | N/A | |
| America | 1 year DFS | 5 | 1480 | 0.99 [0.89, 1.09] | I2 = 68% (p = 0.01) | Z = 0.28 (p = 0.78) | None |
| 2 year DFS | 3 | 490 | 0.89 [0.80, 0.99] | I2 = 0% (p = 0.95) | Z = 2.14 (p = 0.03) | DDLT | |
| 3 year DFS | 4 | 1279 | 0.99 [0.84, 1.16] | I2 = 74% (p = 0.008) | Z = 0.18 (p = 0.85) | None | |
| 4 year DFS | 2 | 289 | 0.77 [0.64, 0.91] | I2 = 0% (p = 0.86) | Z = 2.99 (p = 0.003) | DDLT | |
| 5 year DFS | 3 | 1187 | 0.97 [0.79, 1.19] | I2 = 78% (p = 0.01) | Z = 0.27 (p = 0.78) | None | |
| 6 year DFS | 1 | 197 | N/A | N/A | N/A | N/A | |
| 10 year DFS | 0 | 0 | N/A | N/A | N/A | N/A | |
| Europe | 1 year DFS | 0 | 0 | N/A | N/A | N/A | N/A |
| 2 year DFS | 0 | 0 | N/A | N/A | N/A | N/A | |
| 3 year DFS | 1 | 179 | N/A | N/A | N/A | N/A | |
| 4 year DFS | 0 | 0 | N/A | N/A | N/A | N/A | |
| 5 year DFS | 3 | 448 | 1.06 [0.98, 1.14] | I2 = 0% (p = 0.55) | Z = 1.35 (p = 0.18) | None | |
| 6 year DFS | 0 | 0 | N/A | N/A | N/A | N/A | |
| 10 year DFS | 0 | 0 | N/A | N/A | N/A | N/A |
Secondary outcome
A secondary outcome was to detect the prognostic valves and predictors of recurrence after liver transplantation for HCC patients other than the type of graft.
Prognostic factors and predictor values of recurrence
The age and the sex of the recipient could not be used as a prognostic factor or as a predictive value of recurrence after liver transplantation. On the other hand, a remarkable decrease in survival and increase in the recurrence rate are associated with tumors which are beyond Milan criteria, number, size of the tumor, high levels of AFP (> 400 ng), the presence of vascular invasion and the poorly differentiated tumors (Tables 7, 8).
Table 7.
Prognostic factors after liver transplantation
| Variable | Studies (n) | Effect estimate [HR (95% CI)] | Heterogeneity | Test for overall effect | Reference |
|---|---|---|---|---|---|
| Recipient male sex | 2 | 0.97 [0.74, 1.27] | I2 = 38% (p = 0.20) | Z = 0.21 (p = 0.83) | Female sex |
| Recipient age, years | 2 | 1.01 [0.99, 1.03] | I2 = 24% (p = 0.27) | Z = 1.17 (p = 0.24) | Per 1 year increase |
| Beyond Milan criteria | 2 | 1.89 [1.19, 3.00] | I2 = 71% (p = 0.03) | Z = 2.69 (p = 0.007) | Within Milan |
| AFP > 400 | 0 | N/A | N/A | N/A | N/A |
| No. of tumor nodules | 2 | 1.04 [1.01, 1.07] | I2 = 70% (p = 0.03) | Z = 2.40 (p = 0.02) | Per 1 nodule increase |
| Largest tumor diameter, cm | 2 | 1.09 [1.05, 1.12] | I2 = 33% (p = 0.22) | Z = 4.91 (p < 0.00001) | Per 1 cm increase |
| Microscopic vascular invasion | 2 | 1.89 [1.52, 2.36] | I2 = 0% (p = 0.44) | Z = 5.64 (p < 0.00001) | No |
| Macroscopic vascular invasion | 1 | N/A | N/A | N/A | N/A |
| Poor differentiation | 2 | 1.65 [1.21, 2.25] | I2 = 0% (p = 0.69) | Z = 3.16 (p = 0.002) | Well/mod differentiated |
Table 8.
Predictor values of recurrence after liver transplantation
| Variable | Studies (n) | Effect estimate [HR (95% CI)] | Heterogeneity | Test for overall effect | Reference |
|---|---|---|---|---|---|
| Recipient male sex | 3 | 1.02 [0.70, 1.48] | I2 = 0% (p = 0.83) | Z = 0.09 (p = 0.93) | Female sex |
| Recipient age, years | 4 | 0.99 [0.97, 1.01] | I2 = 0% (p = 0.57) | Z = 1.12 (p = 0.26) | Per 1 year increase |
| Beyond Milan criteria | 4 | 2.81 [1.69, 4.69] | I2 = 73% (p = 0.005) | Z = 3.98 (p < 0.0001) | Within Milan |
| AFP > 400 | 3 | 3.70 [2.11, 6.47] | I2 = 33% (p = 0.22) | Z = 4.58 (p < 0.00001) | AF P < 400 |
| No. of tumor nodules | 4 | 1.14 [1.08, 1.20] | I2 = 46% (p = 0.12) | Z = 4.83 (p < 0.00001) | Per 1 nodule increase |
| Largest tumor diameter, cm | 4 | 1.19 [1.06, 1.32] | I2 = 86% (p < 0.00001) | Z = 3.04 (p = 0.002) | Per 1 cm increase |
| Microscopic vascular invasion | 4 | 3.73 [2.78, 5.01] | I2 = 26% (p = 0.25) | Z = 8.77 (p < 0.00001) | No |
| Macroscopic vascular invasion | 3 | 3.88 [2.64, 5.70] | I2 = 53% (p = 0.12) | Z = 6.91 (p < 0.00001) | No |
| Poor differentiation | 2 | 2.69 [1.29, 5.61] | I2 = 62% (p = 0.07) | Z = 2.63 (p = 0.008) | Well/mod differentiated |
Publication bias assessment
No evidence of publication bias could be detected. The funnel plot analysis showed a symmetrical appearance.
Discussion
Regarding the overall survival after liver transplantation, while some studies reported equal OS after LDLT and DDLT, some papers reported better survival after LDLT. In our study, pooled patient OS showed trivial improvement in LDLT recipients especially the 3-year OS. However, the long-term OS (5- and 10-year OS) did not show any significant difference between the two types of transplant. In addition to that, according to the pooled results of five studies, our subgroup analysis showed equal long-term OS between LDLT and DDLT for those who are within MC. Nevertheless, beyond Milan criteria, there was a better prognosis, could be detected for the patients who underwent LDLT but this data should be treated cautiously due to the small sample size.
On the other hand, according to a French study, the OS from the time of listing was similar for both LDLT and DDLT. However, this has been explained by a Canadian study by the small sample size that failed to address the better outcome after LDLT. This meta-analysis illustrated that the patients listed for LDLT showed a dramatic increase in the OS (ITT-OS) than those listed for DDLT. This could be attributed to the short waiting time and the low dropout rate. Thus it can be said that if dropout was taken into consideration, LDLT provided much better survival benefits to HCC patients especially in regions that suffer from low deceased organ availability as it provides an endless source of donors and eliminate the probability of progression while waiting [10].
Whether HCC recurrence is more frequent in LDLT remains controversial. Some studies attributed the high levels of recurrence after LDLT in their studies to the growth factors that are released during the natural course of liver regeneration of a partial liver graft [29] and according to Fisher et al. [25], the technique of living donor transplant is the determent factor for recurrence due to greater manipulation of the native liver and preservation of the native vena cava, as well as more hepatic artery and bile duct length that results in leaving residual tumor or violating tumor capsule and tumor embolization through the hepatic veins.
However, in our study, no remarkable difference could be detected between LDLT and DDLT recipients in the DFS. Moreover, our subgroup analysis showed equal DFS between the two groups for those who are within MC. In addition to that no difference in the recurrence rate could be detected between LDLT and DDLT receipts and according to Bhangui et al. [35], there was no difference in the severity of recurrence at presentation in the two groups.
Moreover, the high incidence of recurrence in LDLT recipients that was mentioned in some studies could be explained by two reasons; first, the fast tracking to LDLT may not allow sufficient time for evaluation of the biological aggressiveness of tumors [29, 37]. Secondly, the presence of other factors related to the biology of the tumor, not the graft type. For instance, in the Fisher et al. [25] study, while 15% of the patients in the LDLT group had poorly differentiated tumors, only 3% of DDLT had poorly differentiated tumors and in the study by Vakili et al. [29] 46% of the tumors in the LDLT group had microvascular invasion. In other words, Macrovascular invasion, preoperative serum alpha-fetoprotein (AFP) level, tumor size, histopathologic grading were significant factors for survival and tumor-free survival by univariate analysis [28, 38].
Our secondary outcome was to detect these factors that affect the survival and recurrence after liver transplantation for HCC. To begin with, according to four of the included studies, the age and the sex of the receipt is not considered prognostic factor after transplantation.
Nevertheless, the MC have been well adopted worldwide as a set of guidelines for listing patients for LT [5]. However, these criteria are criticized for being too stringent, since many patients beyond the criteria could still have reasonable post-LT survival [51–53]. Nevertheless, according to the pooled hazard ratio, a significant increase in the recurrence of HCC could be detected for those who were beyond Milan criteria.
In addition to that the biological markers could be used as a predictive value after liver transplantation. In other words, a high AFP level has been shown to be associated with poorer outcomes but the exact consensual cut-off value remains undefined [12, 38]. According to some recent studies, an AFP level of 54 ng/mL was associated with disease recurrence, and AFP level of 105 ng/mL was found to decrease overall survival [15]. In addition to that, using an AFP level > 1000 ng/mL as an exclusion criterion for LT within the MC may further improve posttransplant outcomes [54, 55]. In this meta-analysis, the pooled results from three studies showed that an AFP level > 400 IU/mL at the time of transplantation was associated with a significant increase in the recurrence rate [12, 36, 38].
Additionally, this study illustrates that the presence of MVI increases the recurrence and mortality rate after transplantation. In addition to that, according to Lim et al. [16], HCC patients exceeding the MC without MVI could achieve comparable overall survival rates after surgical resection, relative to patients within Milan. In other words, to improve survival and decrease recurrence after transplantation, radiological tools are needed to predict the presence of MVI before liver transplantation [56, 57]. Moreover, macrovascular invasion of hepatic or portal veins has been documented in up to one-third of patients with hepatocellular carcinoma (HCC) [58]. According to AASLD Guidelines it is considered a contraindication to liver transplantation [59]. In our study, the presence of macrovascular invasion is associated with a dramatic increase in the recurrence rate and a significant decrease in survival. Thus it can be said that those patients could benefit from down staging [60].
Moreover, tumor grade of differentiation had a statistically significant effect on the long-term prognosis of HCC after LT. This is explained by Pawlik et al. that the grade was the most powerful predictor of occult vascular invasion [17]. Therefore, the role of percutaneous biopsy for grading prior to transplantation requires study as a way to improve outcomes.
To our knowledge, it is the first time for 2-, 4-, 6-, 10-year outcomes and predictors of recurrence after liver transplantation to be included in a meta-analysis. In addition to that, all studies designed to compare the outcome between LDLT and DDLT for HCC patients were included to increase the statistical power of the results.
However, we have to acknowledge some limitations in our study. First, all the studies included were cohort studies because no randomized controlled trials could be found. Second, the existence of significant heterogeneity in several outcomes could not be explained well enough by subgroup analysis. Third, included studies were conducted in different regions where policies and ethics about LT were different, and this might cause potential bias.
Conclusion
This study is in consonance with the view that cancer biology (not the graft type) is the most important determinant of recurrence and survival after LT. However, LDLT provided much better survival benefits to HCC patients especially in regions that suffer from low deceased organ availability.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This research would not have been possible without the exceptional support and effort of my supervisor, Prof. Dr Amr Abdelaal and I would also like to show my gratitude to Ass. Prof. Dr Mostafa Abdo, and Dr Remon Mamdouh for sharing their pearls of wisdom.
Abbreviations
- AASLD
American association of study of liver disease
- AFP
Alfa fetoprotein
- DDLT
Deceased donor liver transplant
- DFS
Disease free survival
- HBV
Hepatitis B virus
- HCC
Hepatocellular carcinoma
- HCV
Hepatitis C virus
- HDV
Hepatitis D virus
- ITT-OS
Intention to treat overall survival
- LDLT
Living donor liver transplant
- LT
Liver transplant
- MC
Milan criteria
- N/A
Not applicable or not available
- OS
Overall survival
- VI
Vascular invasion
- MVI
Microvascular invasion
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Declarations
Conflict of interest
Beshoy Effat Elkomos, Mostafa Abdo, Remon Mamdouh and Amr Abdelaal declare no competing interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Beshoy Effat Elkomos, Email: Beshoyafet0100304@med.asu.edu.eg, Email: beshoy3ft@gmail.com.
Mostafa Abdo, Email: Mostafa.abdo@med.asu.edu.eg.
Remon Mamdouh, Email: Remonmamdouh@gmail.com.
Amr Abdelaal, Email: aaabdelaal@med.asu.edu.eg.
References
- 1.Llovet JM, Zucman-Rossi J, Pikarsky E, et al. Hepatocellular carcinoma. Nat Rev Dis Prim. 2016;2:16018. doi: 10.1038/nrdp.2016.18. [DOI] [PubMed] [Google Scholar]
- 2.Carcinoma Villanueva A. Hepatocellular. N Engl J Med. 2019;380:1450–1462. doi: 10.1056/nejmra1713263. [DOI] [PubMed] [Google Scholar]
- 3.International Agency for Research on Cancer, editor. WHO classification of tumours of the digestive system. International Agency for Research on Cancer, Lyon; 2019
- 4.Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu MA, Allen C, Al-Raddadi R, Alvis-Guzman N, Amoako Y, Artaman A, Ayele TA. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the global burden of disease study 2015. JAMA Oncol. 2017;3(12):1683–1691. doi: 10.1001/jamaoncol.2017.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334(11):693–700. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 6.Xu X, Lu D, Ling Q, Wei X, Wu J, Zhou L, Yan S, Wu L, Geng L, Ke Q, Gao F. Liver transplantation for hepatocellular carcinoma beyond the Milan criteria. Gut. 2016;65(6):1035–1041. doi: 10.1136/gutjnl-2014-308513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology. 1999;30(6):1434–1440. doi: 10.1002/hep.510300629. [DOI] [PubMed] [Google Scholar]
- 8.Yao FY, Bass NM, Nikolai B, Merriman R, Davern TJ, Kerlan R, Ascher NL, Roberts JP. A follow-up analysis of the pattern and predictors of dropout from the waiting list for liver transplantation in patients with hepatocellular carcinoma: implications for the current organ allocation policy. Liver Transpl. 2003;9(7):684–692. doi: 10.1053/jlts.2003.50147. [DOI] [PubMed] [Google Scholar]
- 9.Freeman RB, Edwards EB, Harper AM. Waiting list removal rates among patients with chronic and malignant liver diseases. Am J Transplant. 2006;6(6):1416–1421. doi: 10.1111/j.1600-6143.2006.01321.x. [DOI] [PubMed] [Google Scholar]
- 10.Wong TC, Ng KK, Fung JY, Chan AA, Cheung TT, Chok KS, Dai JW, Lo CM. Long-term survival outcome between living donor and deceased donor liver transplant for hepatocellular carcinoma: intention-to-treat and propensity score matching analyses. Ann Surg Oncol. 2019;26(5):1454–1462. doi: 10.1245/s10434-019-07206-0. [DOI] [PubMed] [Google Scholar]
- 11.Goldaracena N, Gorgen A, Doyle A, Hansen BE, Tomiyama K, Zhang W, Ghanekar A, Lilly L, Cattral M, Galvin Z, Selzner M. Live donor liver transplantation for patients with hepatocellular carcinoma offers increased survival vs. deceased donation. J Hepatol. 2019;70(4):666–673. doi: 10.1016/j.jhep.2018.12.029. [DOI] [PubMed] [Google Scholar]
- 12.Park MS, Lee KW, Suh SW, You T, Choi Y, Kim H, Hong G, Yi NJ, Kwon CH, Joh JW, Lee SK. Living-donor liver transplantation associated with higher incidence of hepatocellular carcinoma recurrence than deceased-donor liver transplantation. Transplantation. 2014;97(1):71–77. doi: 10.1097/TP.0b013e3182a68953. [DOI] [PubMed] [Google Scholar]
- 13.Lo CM, Fan ST, Liu CL, Chan SC, Ng IO, Wong J. Living donor versus deceased donor liver transplantation for early irresectable hepatocellular carcinoma. J Br Surg. 2007;94(1):78–86. doi: 10.1002/bjs.5528. [DOI] [PubMed] [Google Scholar]
- 14.Ninomiya M, Shirabe K, Facciuto ME, Schwartz ME, Florman SS, Yoshizumi T, Harimoto N, Ikegami T, Uchiyama H, Maehara Y. Comparative study of living and deceased donor liver transplantation as a treatment for hepatocellular carcinoma. J Am Coll Surg. 2015;220(3):297–304. doi: 10.1016/j.jamcollsurg.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 15.She WH, Chan AC, Cheung TT, Lo CM, Chok KS. Survival outcomes of liver transplantation for hepatocellular carcinoma in patients with normal, high and very high preoperative alpha-fetoprotein levels. World J Hepatol. 2018;10(2):308. doi: 10.4254/wjh.v10.i2.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim KC, Chow PK, Allen JC, Chia GS, Lim M, Cheow PC, Chung AY, Ooi LL, Tan SB. Microvascular invasion is a better predictor of tumor recurrence and overall survival following surgical resection for hepatocellular carcinoma compared to the Milan criteria. Ann Surg. 2011;254(1):108–113. doi: 10.1097/SLA.0b013e31821ad884. [DOI] [PubMed] [Google Scholar]
- 17.Pawlik TM, Delman KA, Vauthey JN, Nagorney DM, Ng IO, Ikai I, Yamaoka Y, Belghiti J, Lauwers GY, Poon RT, Abdalla EK. Tumor size predicts vascular invasion and histologic grade: implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transplant. 2005;11(9):1086–1092. doi: 10.1002/lt.20472. [DOI] [PubMed] [Google Scholar]
- 18.Peterson J, Welch V, Losos M, Tugwell PJ. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa: Ottawa Hospital Research Institute. 2011;2(1):1–2
- 19.Higgins JP, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions. New York: Wiley; 2019. [Google Scholar]
- 20.Gondolesi GE, Roayaie S, Muñoz L, Kim-Schluger L, Schiano T, Fishbein TM, Emre S, Miller CM, Schwartz ME. Adult living donor liver transplantation for patients with hepatocellular carcinoma: extending UNOS priority criteria. Ann Surg. 2004;239(2):142. doi: 10.1097/01.sla.0000109022.32391.eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roayaie S, Schwartz JD, Sung MW, Emre SH, Miller CM, Gondolesi GE, Krieger NR, Schwartz ME. Recurrence of hepatocellular carcinoma after liver transplant: patterns and prognosis. Liver Transplant. 2004;10(4):534–540. doi: 10.1002/lt.20128. [DOI] [PubMed] [Google Scholar]
- 22.Hwang S, Lee SG, Joh JW, Suh KS, Kim DG. Liver transplantation for adult patients with hepatocellular carcinoma in Korea: comparison between cadaveric donor and living donor liver transplantations. Liver Transpl. 2005;11(10):1265–1272. doi: 10.1002/lt.20549. [DOI] [PubMed] [Google Scholar]
- 23.Karakayali H, Moray G, Sozen H, Dalgic A, Emiroglu R, Haberal M. Expanded criteria for liver transplantation in patients with hepatocellular carcinoma. In: Transplantation proceedings 2006 Mar 1, vol. 38, no. 2, pp. 575–578. Elsevier. [DOI] [PubMed]
- 24.Sotiropoulos GC, Lang H, Nadalin S, Neuhäuser M, Molmenti EP, Baba HA, Paul A, Saner FH, Weber F, Hilgard P, Frilling A. Liver transplantation for hepatocellular carcinoma: University Hospital Essen experience and metaanalysis of prognostic factors. J Am Coll Surg. 2007;205(5):661–675. doi: 10.1016/j.jamcollsurg.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 25.Fisher RA, Kulik LM, Freise CE, Lok AS, Shearon TH, Brown RS, Jr, Ghobrial RM, Fair JH, Olthoff KM, Kam I, Berg CL. Hepatocellular carcinoma recurrence and death following living and deceased donor liver transplantation. Am J Transplant. 2007;7(6):1601–1608. doi: 10.1111/j.1600-6143.2007.01802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Terrault NA, Shiffman ML, Lok AS, Saab S, Tong L, Brown RS, Jr, Everson GT, Reddy KR, Fair JH, Kulik LM, Pruett TL. Outcomes in hepatitis C virus-infected recipients of living donor vs. deceased donor liver transplantation. Liver Transplant. 2007;13(1):122–129. doi: 10.1002/lt.20995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allam N, Khalaf H, Fagih M, Al-Sebayel M. Liver transplant for hepatocellular carcinoma: experience in a Saudi population. Exp Clin Transplant. 2008;6(1):14–24. [PubMed] [Google Scholar]
- 28.Di Sandro S, Slim AO, Giacomoni A, et al. Living donor liver transplantation for hepatocellular carcinoma: long-term results compared with deceased donor liver transplantation. Transplant Proc. 2009;41(4):1283–1285. doi: 10.1016/j.transproceed.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 29.Vakili K, Pomposelli JJ, Cheah YL, Akoad M, Lewis WD, Khettry U, Gordon F, Khwaja K, Jenkins R, Pomfret EA. Living donor liver transplantation for hepatocellular carcinoma: increased recurrence but improved survival. Liver Transpl. 2009;15(12):1861–1866. doi: 10.1002/lt.21940. [DOI] [PubMed] [Google Scholar]
- 30.Giacomoni A, Slim A, Lauterio A, Di Sandro S, De Carlis L. Liver transplantation for hepatocellular carcinoma with graft coming from deceased or living donors. A comparative analysis. Liver Transplant. 2009;15(7):S73–S73. [Google Scholar]
- 31.Hsieh TH, Byrne T, Carey E, et al (2010) Living-and deceased-donor liver transplantation for hepatocellular carcinoma at a single center: comparison of clinical parameters and outcomes. In: Abstract of American transplant congress 2010;10:23. 10.1111/j.1600-6143.2010.03108.x.
- 32.Sharr WW, Lo CM, Chan SC, et al. Primary treatment for hepatocellular carcinoma within UCSF criteria: comparing outcomes of deceased and living donor liver transplantation. In: Abstracts of the 2011 joint international congress of ILTS, ELITA, and LICAGE liver transplant 2011;17. 10.1002/lt.22457.
- 33.Kornberg A, Küpper B, Thrum K, Wilberg J, Sappler A, Gottschild D. Recurrence-free long-term survival after liver transplantation in patients with 18F-FDG non-avid hilar cholangiocarcinoma on PET. Am J Transplant. 2009;9(11):2631–2636. doi: 10.1111/j.1600-6143.2009.02821.x. [DOI] [PubMed] [Google Scholar]
- 34.Berg CL, Merion RM, Shearon TH, Olthoff KM, Brown RS, Jr, Baker TB, Everson GT, Hong JC, Terrault N, Hayashi PH, Fisher RA. Liver transplant recipient survival benefit with living donation in the model for endstage liver disease allocation era. Hepatology. 2011;54(4):1313–1321. doi: 10.1002/hep.24494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhangui P, Vibert E, Majno P, Salloum C, Andreani P, Zocrato J, Ichai P, Saliba F, Adam R, Castaing D, Azoulay D. Intention-to-treat analysis of liver transplantation for hepatocellular carcinoma: living versus deceased donor transplantation. Hepatology. 2011;53(5):1570–1579. doi: 10.1002/hep.24231. [DOI] [PubMed] [Google Scholar]
- 36.Azzam AZ, Hegab B, Khalaf H, Al Bahili H, Mohammed H, Kamel Y, Khail FA, Al-hamoudi W, Al Sofayan M, Al SM. Liver transplantation in patients with hepatocellular carcinoma: a single-center experience. Exp Clin Transplant. 2011;9(5):323–328. [PubMed] [Google Scholar]
- 37.Kulik LM, Fisher RA, Rodrigo DR, Brown RS, Jr, Freise CE, Shaked A, Everhart JE, Everson GT, Hong JC, Hayashi PH, Berg CL. Outcomes of living and deceased donor liver transplant recipients with hepatocellular carcinoma: results of the A2ALL cohort. Am J Transplant. 2012;12(11):2997–3007. doi: 10.1111/j.1600-6143.2012.04272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sandhu L, Sandroussi C, Guba M, Selzner M, Ghanekar A, Cattral MS, McGilvray ID, Levy G, Greig PD, Renner EL, Grant DR. Living donor liver transplantation versus deceased donor liver transplantation for hepatocellular carcinoma: comparable survival and recurrence. Liver Transplant. 2012;18(3):315–322. doi: 10.1002/lt.22477. [DOI] [PubMed] [Google Scholar]
- 39.Li C, Wen TF, Yan LN, Li B, Yang JY, Xu MQ, Wang WT, Wei YG. Scoring selection criteria including total tumour volume and pretransplant percentage of lymphocytes to predict recurrence of hepatocellular carcinoma after liver transplantation. PLoS One. 2013;8(8):e72235. doi: 10.1371/journal.pone.0072235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lei J, Yan L, Wang W. Comparison of the outcomes of patients who underwent deceased-donor or living-donor liver transplantation after successful downstaging therapy. Eur J Gastroenterol Hepatol. 2013;25(11):1340–1346. doi: 10.1097/MEG.0b013e3283622743. [DOI] [PubMed] [Google Scholar]
- 41.Xiao GQ, Song JL, Shen S, Yang JY, Yan LN. Living donor liver transplantation does not increase tumor recurrence of hepatocellular carcinoma compared to deceased donor transplantation. World J Gastroenterol WJG. 2014;20(31):10953. doi: 10.3748/wjg.v20.i31.10953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen J, Xu X, Wu J, Ling Q, Wang K, Wang W, Zhang M, Shen Y, Zhou L, Xie H, Zheng S. The stratifying value of Hangzhou criteria in liver transplantation for hepatocellular carcinoma. PLoS One. 2014;9(3):e93128. doi: 10.1371/journal.pone.0093128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wan P, Zhang JJ, Li QG, Xu N, Zhang M, Chen XS, Han LZ, Xia Q. Living-donor or deceased-donor liver transplantation for hepatic carcinoma: a case-matched comparison. World J Gastroenterol WJG. 2014;20(15):4393. doi: 10.3748/wjg.v20.i15.4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bonadio I, Colle I, Geerts A, Smeets P, Berardi G, Praet M, Rogiers X, de Hemptinne B, Van Vlierberghe H, Troisi RI. Liver transplantation for hepatocellular carcinoma comparing the Milan, UCSF, and Asan criteria: long-term follow-up of a Western single institutional experience. Clin Transplant. 2015;29(5):425–433. doi: 10.1111/ctr.12534. [DOI] [PubMed] [Google Scholar]
- 45.Chen LP, Li C, Wen TF, Yan LN, Li B, Yang JY. Can living donor liver transplantation offer similar outcomes to deceased donor liver transplantation using expanded selection criteria for hepatocellular carcinoma? Pak J Med Sci. 2015;31(4):763. doi: 10.12669/pjms.314.7523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tomiyama K, Goldaracena N, Barbas A, Dib M, Levy G, Lilly L, Ghanekar A, McGilvray I, Renner E, Bhat M, Selzner M. A Comparative intention-to-treat analysis of liver transplantation for HCC-living donor liver transplant vs. deceased donor liver transplant. Am J Transplant. 2016;16:461–461. [Google Scholar]
- 47.Fonseca E, Seda J, Benavides M, et al. Living donor versus deceased donor liver trans- plantation for hepatocellular carcinoma. In: Abstracts of the ILTS 22nd annual international congress annual international congress. Transplantation 2016;100. 10.1097/01.tp.0000483259.57907.d4.
- 48.Azoulay D, Audureau E, Bhangui P, Belghiti J, Boillot O, Andreani P, Castaing D, Cherqui D, Irtan S, Calmus Y, Chazouillères O. Living or brain-dead donor liver transplantation for hepatocellular carcinoma: a multicenter, western, intent-to-treat cohort study. Ann Surg. 2017;266(6):1035–1044. doi: 10.1097/SLA.0000000000001986. [DOI] [PubMed] [Google Scholar]
- 49.Lee S, Song GW, Kim KW, et al. Living donor liver transplantation versus deceased donor liver transplantation for hepatocellular carcinoma within or beyond the Milan criteria: comparable long-term outcomes. Transplant Proc. 2021;53(1):92–97. doi: 10.1016/j.transproceed.2020.10.012. [DOI] [PubMed] [Google Scholar]
- 50.Rahatli S, Ayvazoglu Soy EH, Oguz A, Altundag O, Moray G, Haberal M. Single-center experience of recurrence patterns and survival analyses of patients with hepatocellular carcinoma and liver transplant. Exp Clin Transplant. 2020;18(2):201–205. doi: 10.6002/ect.2020.0046. [DOI] [PubMed] [Google Scholar]
- 51.Onaca N, Davis GL, Goldstein RM, Jennings LW, Klintmalm GB. Expanded criteria for liver transplantation in patients with hepatocellular carcinoma: a report from the International Registry of Hepatic Tumors in Liver Transplantation. Liver Transpl. 2007;13(3):391–399. doi: 10.1002/lt.21095. [DOI] [PubMed] [Google Scholar]
- 52.Toso C, Trotter J, Wei A, Bigam DL, Shah S, Lancaster J, Grant DR, Greig PD, Shapiro AJ, Kneteman NM. Total tumor volume predicts risk of recurrence following liver transplantation in patients with hepatocellular carcinoma. Liver Transpl. 2008;14(8):1107–1115. doi: 10.1002/lt.21484. [DOI] [PubMed] [Google Scholar]
- 53.Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, Camerini T, Roayaie S, Schwartz ME, Grazi GL, Adam R. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10(1):35–43. doi: 10.1016/S1470-2045(08)70284-5. [DOI] [PubMed] [Google Scholar]
- 54.Duvoux C, Roudot-Thoraval F, Decaens T, Pessione F, Badran H, Piardi T, Francoz C, Compagnon P, Vanlemmens C, Dumortier J, Dharancy S. Liver transplantation for hepatocellular carcinoma: a model including α-fetoprotein improves the performance of Milan criteria. Gastroenterology. 2012;143(4):986–994. doi: 10.1053/j.gastro.2012.05.052. [DOI] [PubMed] [Google Scholar]
- 55.Hameed B, Mehta N, Sapisochin G, Roberts JP, Yao FY. Alpha-fetoprotein level > 1000 ng/mL as an exclusion criterion for liver transplantation in patients with hepatocellular carcinoma meeting the Milan criteria. Liver Transpl. 2014;20(8):945–951. doi: 10.1002/lt.23904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Renzulli M, Brocchi S, Cucchetti A, Mazzotti F, Mosconi C, Sportoletti C, Brandi G, Pinna AD, Golfieri R. Can current preoperative imaging be used to detect microvascular invasion of hepatocellular carcinoma? Radiology. 2016;279(2):432–442. doi: 10.1148/radiol.2015150998. [DOI] [PubMed] [Google Scholar]
- 57.Wang WT, Yang L, Yang ZX, Hu XX, Ding Y, Yan X, Fu CX, Grimm R, Zeng MS, Rao SX. Assessment of microvascular invasion of hepatocellular carcinoma with diffusion kurtosis imaging. Radiology. 2018;286(2):571–580. doi: 10.1148/radiol.2017170515. [DOI] [PubMed] [Google Scholar]
- 58.Lee YH, Hsu CY, Huang YH, Hsia CY, Chiou YY, Su CW, Lin HC, Huo TI. Vascular invasion in hepatocellular carcinoma: prevalence, determinants and prognostic impact. J Clin Gastroenterol. 2014;48(8):734–741. doi: 10.1097/MCG.0b013e3182a8a254. [DOI] [PubMed] [Google Scholar]
- 59.Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–380. doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- 60.Mehta N, Guy J, Frenette CT, Dodge JL, Osorio RW, Minteer WB, Roberts JP, Yao FY. Excellent outcomes of liver transplantation following down-staging of hepatocellular carcinoma to within Milan criteria: a multicenter study. Clin Gastroenterol Hepatol. 2018;16(6):955–964. doi: 10.1016/j.cgh.2017.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.