Abstract
The primary goal of this review is to provide a compilation of the complex architectural features of staphylococcal cell walls and of some of their unusual morphogenetic traits including the utilization of murosomes and two different mechanisms of cell separation. Knowledge of these electron microscopic findings may serve as a prerequisite for a better understanding of the sophisticated events which lead to penicillin-induced death. For more than 50 years there have been controversial disputes about the mechanisms by which penicillin kills bacteria. Many hypotheses have tried to explain this fatal event biochemically and mainly via bacteriolysis. However, indications that penicillin-induced death of staphylococci results from overall biochemical defects or from a fatal attack of bacterial cell walls by bacteriolytic murein hydrolases were not been found. Rather, penicillin, claimed to trigger the activity of murein hydrolases, impaired autolytic wall enzymes of staphylococci. Electron microscopic investigations have meanwhile shown that penicillin-mediated induction of seemingly minute cross wall mistakes is the very reason for this killing. Such “morphogenetic death” taking place at predictable cross wall sites and at a predictable time is based on the initiation of normal cell separations in those staphylococci in which the completion of cross walls had been prevented by local penicillin-mediated impairment of the distribution of newly synthesized peptidoglycan; this death occurs because the high internal pressure of the protoplast abruptly kills such cells via ejection of some cytoplasm during attempted cell separation. An analogous fatal onset of cell partition is considered to take place without involvement of a detectable quantity of autolytic wall enzymes (“mechanical cell separation”). The most prominent feature of penicillin, the disintegration of bacterial cells via bacteriolysis, is shown to represent only a postmortem process resulting from shrinkage of dead cells and perturbation of the cytoplasmic membrane. Several schematic drawings have been included in this review to facilitate an understanding of the complex morphogenetic events.
Microbiologists are highly interested in the sophisticated, unique architecture and morphogenesis of the cell wall of staphylococci which make these bacteria suitable for exploring the reason for penicillin-induced death during defined morphogenetic steps (48, 50, 53). More detailed knowledge of those structural “weak points” in the staphylococcal wall, which turned out to be the main sites of penicillin action, is an important prerequisite not only for attempts to enhance the efficiency of beta-lactam antibiotics but also for efforts to attack even staphylococci that are highly resistant to this type of antibiotic (the so-called methicillin-resistant Staphylococcus aureus [MRSA] strains). Great attention is being paid to structural and chemical variations in the cell walls of such highly resistant strains (23, 24, 74, 87) and to factors involved in the biosynthesis of those staphylococcal cell walls, both of which might be suitable as novel targets in the combat against MRSA (70). Such extremely drug-resistant strains of S. aureus are already posing major public health problems (21). Many physicians are gravely concerned about such antibiotic resistance, and they are highly interested in any attempts to overcome this problem (6).
Therefore, this review shall serve several aims. First, we want to compile our current knowledge of the macromolecular wall architecture and wall morphogenesis of staphylococci. On the basis of our last review (42) and new data we will discuss some recent concepts including even some speculative considerations on staphylococcal cell wall morphogenesis, wall degradation, and the combination of both these processes during cell separation. In particular, we have paid great attention to all the morphologic and morphogenetic details of the staphylococcal cell walls which, some day, might serve as new targets for the badly needed progress in our therapeutic efforts. However, since recent reviews are available concerning biochemical data (see references 78 and 118), only the most relevant findings will be mentioned and all details are purposely omitted.
Second, our contribution will deal with penicillin-induced structural variations during staphylococcal cell wall morphogenesis and degradation. Since it has been shown that staphylococci do not die from bacteriolysis but from very characteristic cross wall defects (50–53), we will focus our interest (i) on those morphogenetic wall variations which regularly lead to death and (ii) on attempts of the staphylococci to survive in spite of such morphological handicaps.
In order to update the review, some electron micrographs from rather early publications have been replaced by more recent high-resolution pictures and several other, unpublished ones have been included (from our archive, which now holds about 60,000 electron micrographs of staphylococci). In order to prevent fixation artifacts, electron microscopic pictures of unfixed, freeze-fractured staphylococci are included as often as possible. They are regarded as “images of latent living bacteria.”
We hope that the ample schematic drawings will help give an idea of the highly differentiated dynamic processes of wall morphogenesis, wall degradation, and fatal wall variations. These simplified, line art illustrations are not only a visual aid for the reader but they also give reliable information to those scientists who do not have time enough to read all the details of this review. That is why we made every effort to include in the schematic drawings all of the data which seem to be essential for an overview.
Furthermore, recent unpublished findings on staphylococcal wall morphology and morphogenesis have been included here with a view to presenting state of the art knowledge about a fascinating field: the staphylococcal cell wall.
Since detailed knowledge of wall morphogenesis is the most important prerequisite for analyzing the very complex sequences of the penicillin-induced killing process, this review is divided in two parts: “Morphogenesis of the staphylococcal cell wall” and “Penicillin-induced death.”
MORPHOGENESIS OF THE STAPHYLOCOCCAL CELL WALL
Chemical Composition of the Staphylococcal Cell Wall
Chemical structure of the cell wall of S. aureus.
The cell wall of S. aureus shows the typical features of gram-positive bacterial cell walls. Under the electron microscope it appears as a relatively thick (about 20 to 40 nm) homogeneous structure.
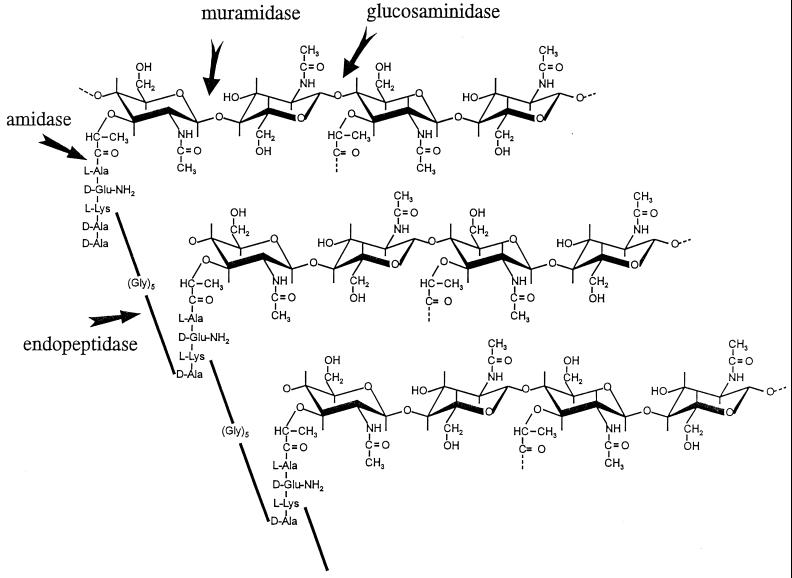
The chemical structure of its major component, the peptidoglycan, has been known for a long time (see reference 115). This heteropolymer consists of a disaccharide backbone formed by alternating β-1-4-N-acetylglucosamines and N-acetylmuramic acids. The average chain length is in the range of 10 disaccharides (119). Tetrapeptides consisting of l-alanine, d-glutamine, l-lysine, and d-alanine are attached to the N-acetylmuramic acid. About 90% of these stem peptides are cross-linked to the stem peptides of another glycan chain by a pentaglycine group (74). This pentaglycine is a characteristic feature of the staphylococcal peptidoglycan and connects the ɛ-amino group of the l-lysine of one stem peptide to the d-alanine of the other one. The stem peptides which are not cross-linked carry an additional d-alanine which is cleaved during the cross-linking reaction. The structure of the staphylococcal peptidoglycan is summarized in a schematic drawing (Fig. 1).
FIG. 1.
The structure of peptidoglycan and the sites where peptidoglycan may be attacked by cell wall hydrolases. Three glycan strands of peptidoglycan, consisting of alternating N-acetylmuramic acid and N-acetylglucosamine are depicted. The tetrapeptides (stem peptides), branching from N-acetylmuramic acid, are interconnected by pentaglycine bridges. The sites where cell wall hydrolases may attack peptidoglycan are indicated by arrows, but staphylococci contain only three of these wall hydrolases (amidase, glucosaminidase, and endopeptidase).
The process of cell wall cross-linking is catalyzed by transpeptidases, the penicillin-binding proteins (PBPs) (74). Knowledge about the exact functions of the four staphylococcal PBPs is not as detailed as is what is known about the PBPs in Escherichia coli, but there is evidence that the function of PBP 1 is the most important one for the survival of staphylococci exposed to beta-lactams (8, 112). PBP 4, in contrast, seems to be responsible for secondary cross-linking, as can be deduced from a low cross-linking rate in PBP 4-defective S. aureus mutants (58).
The so-called PBP 2a, the reason for methicillin resistance in staphylococci, seems to need proper pentaglycine interpeptide bridges to perform cross-linking reactions (70). Mutant strains with shortened interpeptide bridges (femA-femB, containing significantly increased amounts of mono-, di-, and triglycine residues) showed a drastically reduced resistance level (24, 25, 58, 70, 87) and a reduced level of cross-linking when grown in the presence of beta-lactam antibiotics (23, 74).
O acetylation of the muramic acid is another important feature of the staphylococcal peptidoglycan (114). Due to this, staphylococcal cell walls are rarely degraded by lysozyme, which is sterically hindered in its action (62).
About 50% of the total mass of the cell wall consist of teichoic acid, a polymer covalently linked to the muramic acid via phosphodiester bonds. Teichoic acids consist of long chains of ribitol phosphate units (114); they are usually replaced by ester-linked d-alanine (28). The degree of such substitution seems to have a very great effect on the activity of autolytic enzymes (29).
Cell wall hydrolases of S. aureus.
The necessity that bacteria with a compact peptidoglycan network have their own cell wall hydrolases is quite evident. In order to divide and separate, the cells must cleave certain parts of their walls in a highly regulated manner (for a review, see reference 118). Disturbance of these control mechanisms usually leads to cell lysis; this is the reason why endogenous cell wall hydrolases are called wall autolysins or autolytic wall enzymes. Cell wall hydrolases are also a prerequisite for cell wall morphogenesis and turnover, a problem to be discussed in more detail in a following section.
S. aureus has three different autolytic enzymes: an N-acetylglucosaminidase, an N-acetylmuramidase, and an endopeptidase (114, 123). The sites where these enzymes attack the staphylococcal peptidoglycan are shown in Fig. 1.
However, examination of cell wall hydrolases by the so-called zymogram method has shown (via Triton-mediated reactivation of autolysins) that several bands are capable of hydrolyzing peptidoglycan (61, 63, 90), indicating that these autolytic activities must be represented by more than three enzymes. The number of bacteriolytic enzymes, however, decreases when staphylococcal cells reach the stationary phase (69). The overall rate of the murein hydrolase activity seems mainly to be regulated genetically (by the lytS-lytR regulatory locus) (16).
Recently, the atl gene encoding an autolytic enzyme with bifunctional activities was cloned and sequenced (102). The two domains contain an N-acetylmuramyl-l-alanine-amidase (AM) and an N-acetylglucosaminidase (GL). A gene for an additional amidase, encoding a polypeptide with a molecular weight of 23,000, was cloned earlier (61).
The two cell wall lytic enzymes AM and GL proved to be capable of acting as cluster-dispersing enzymes (see “Inhibition of cell separation results in the formation of pseudomulticellular staphylococci”) when externally added to cluster-forming mutant strains of S. aureus (20, 122).
Purification and production of antibodies against these autolysins enabled immunoelectron microscopic investigations revealing the exact localization of these enzymes. They were shown to be arranged in a circumferential double ring at the surface of the peripheral cell wall (146); after penicillin treatment these enzymes could be detected at the strictly localized perforations of the peripheral wall (123) which initiate cell separation (see “Initiation of cell separation via murosomes”).
The physiological role associated with the staphylococcal endopeptidase activity is still unclear. It has been speculated that endopeptidase activity is needed for the completion of cell separation (58).
Lysostaphin, an endopeptidase produced by Staphylococcus simulans subsp. staphylolyticus is known to be an effective agent for the complete lysis of S. aureus cell walls. Whether the endopeptidases, possibly for example the gene product of lytM (110), of S. aureus are capable of performing similar actions or whether they are only needed for localized alterations of the peptidoglycan remains to be resolved.
Cell Division in Staphylococci
Differentiation of three consecutive division planes.
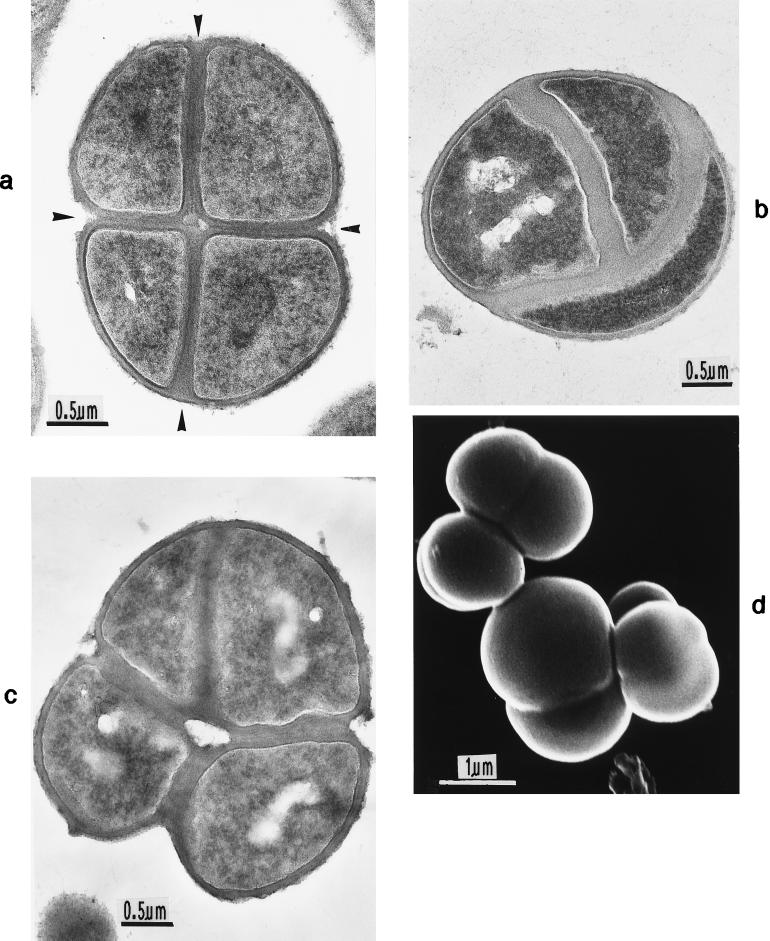
Different types of cross wall formation have been reported for bacterial cocci. (i) In the division of Streptococcus cells only simple pairs or chains are formed, like in rod-like bacteria, indicating the existence of one single division plane. (ii) In other cocci, for instance, in Pediococcus (34), Thiopedia (104), Lampropedia (97), and possibly also Deinococcus (99), successive division always leads to four cells being arranged in two-dimensional tetrads. Later on even, square tablets of 16 to 64 cells are formed, indicating the existence of two division planes which during subsequent cell divisions must regularly alternate their direction at right angles to each other. (iii) In Sarcina (18) and the cyanobacterium Synechocystis (113), eight cells are arranged in three-dimensional, cuboidal packets via stringent alteration of three consecutive division planes.
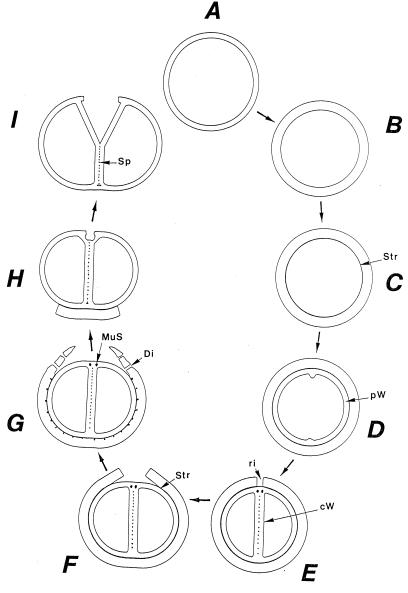
For a long time it had not been evident to which of these three types of cell division the staphylococci would belong, because complete cell separation normally takes place right after cell division, resulting in groups of individual cells; the very name staphylococci (“bunch of cocci”) already points to this characteristic formation of cell groups. Only by scanning electron microscopy did it became evident that the staphylococci do not belong to organisms with only two division planes, as had been assumed earlier (42), but must be ascribed to the Sarcina type. Their three-dimensional arrangements to eight-cell packets was demonstrated in some strains of S. aureus (71); in most strains, however, this was only possible after experimentally retarded cell separation (Fig. 2a).
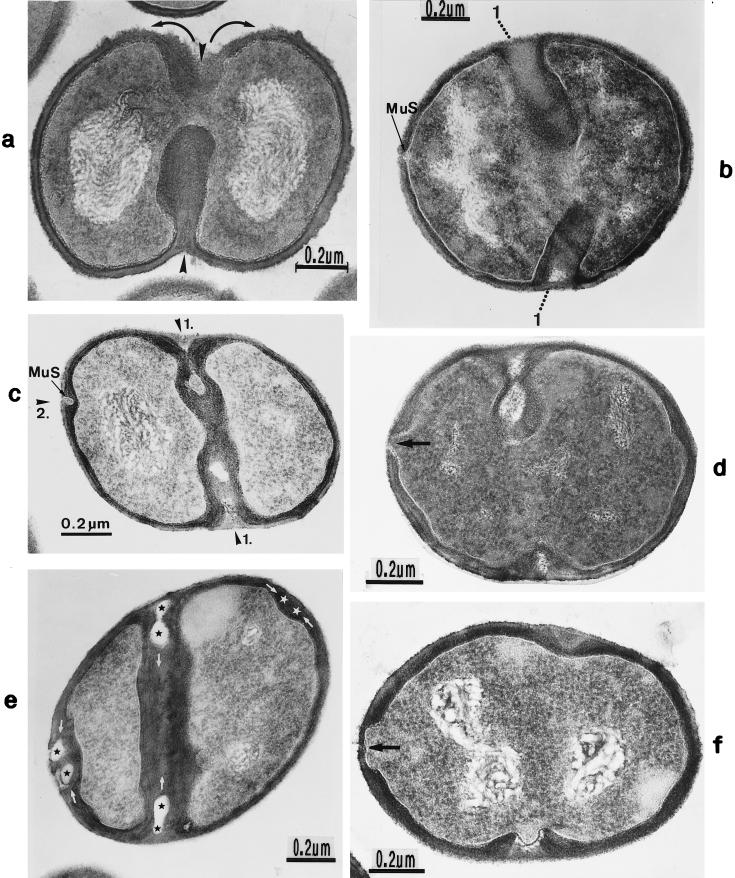
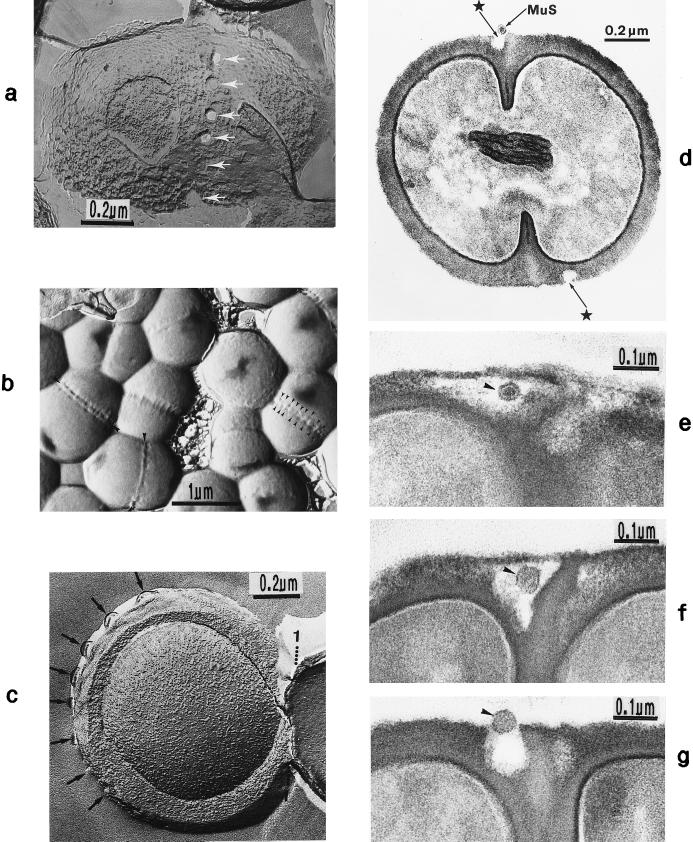
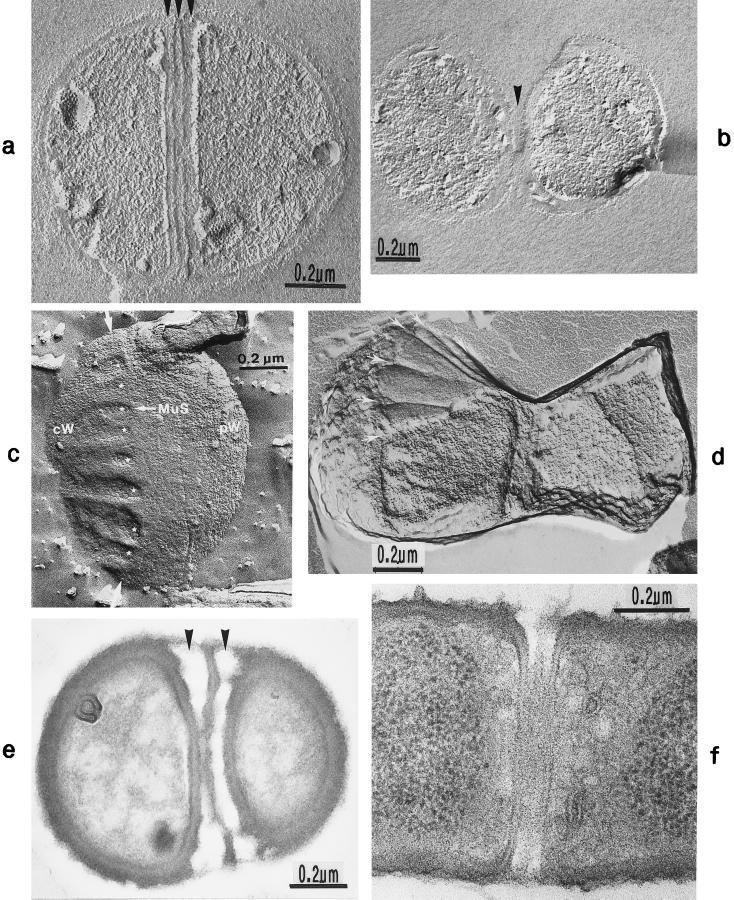
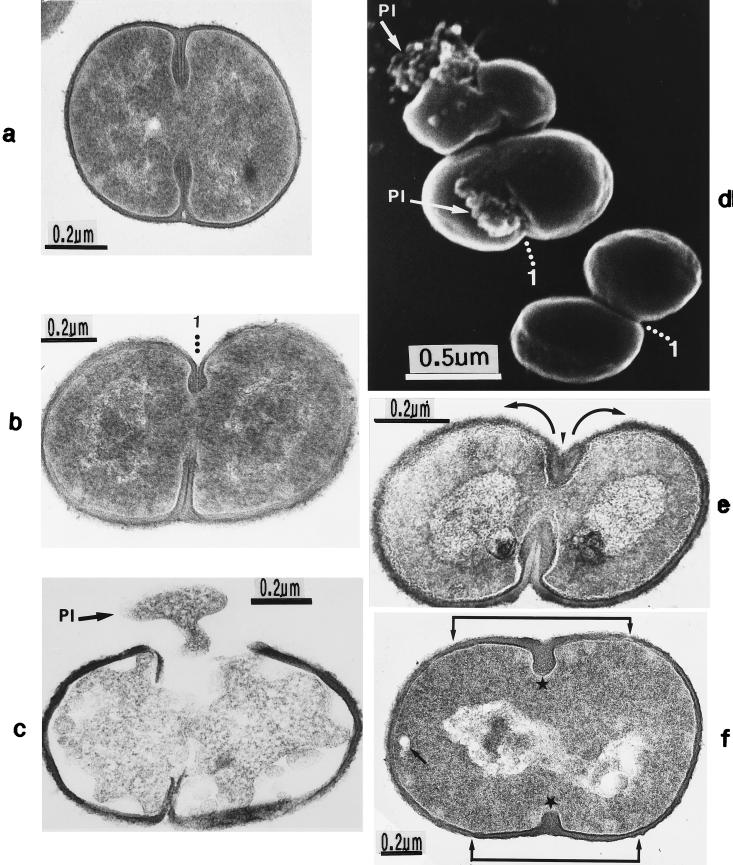
FIG. 2.
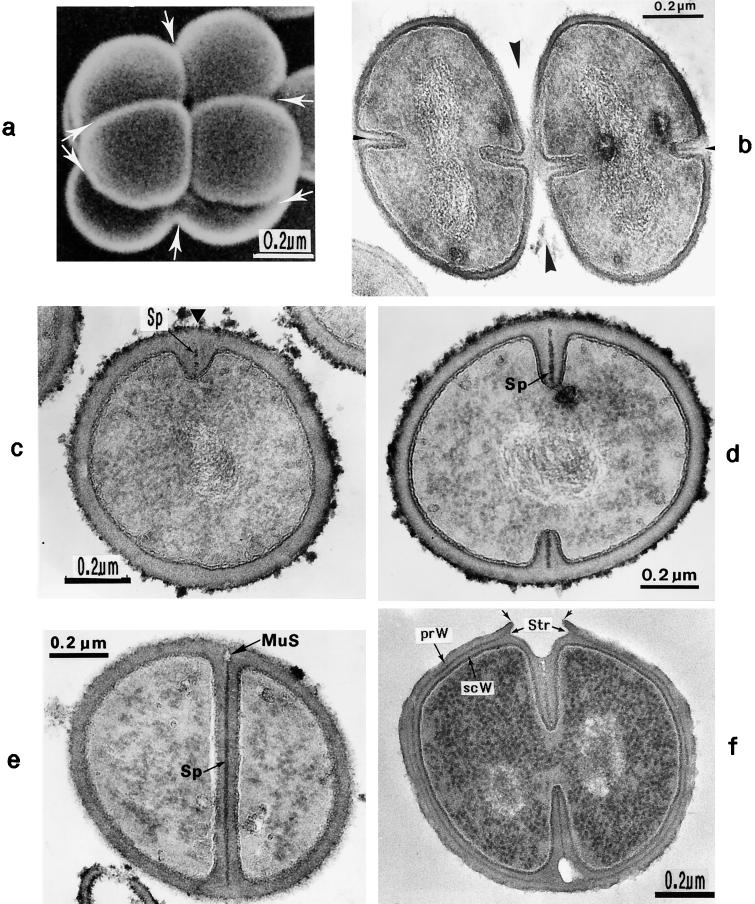
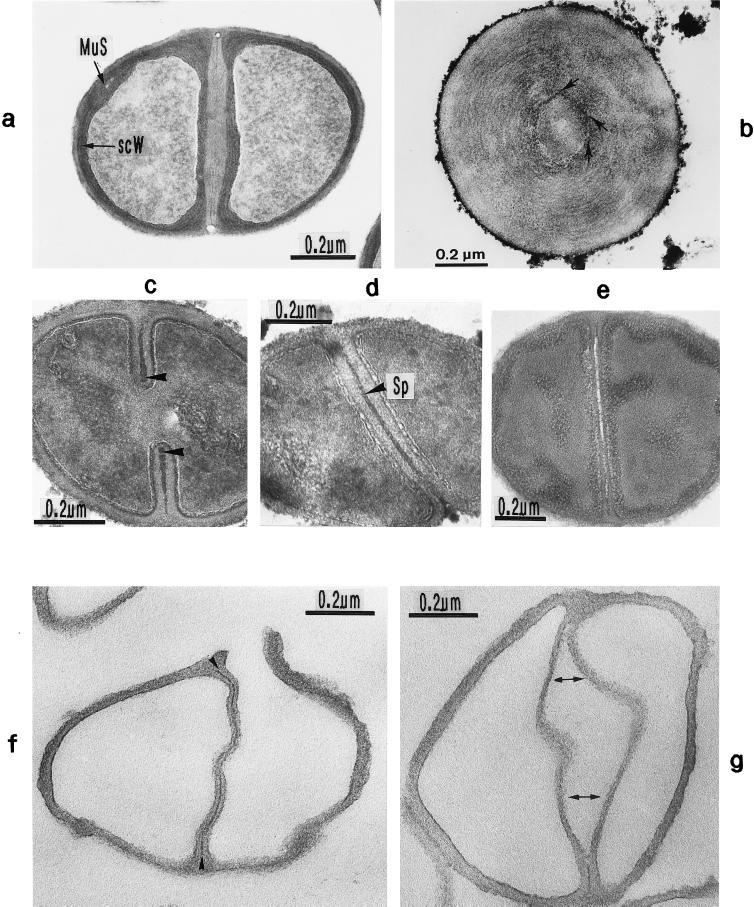
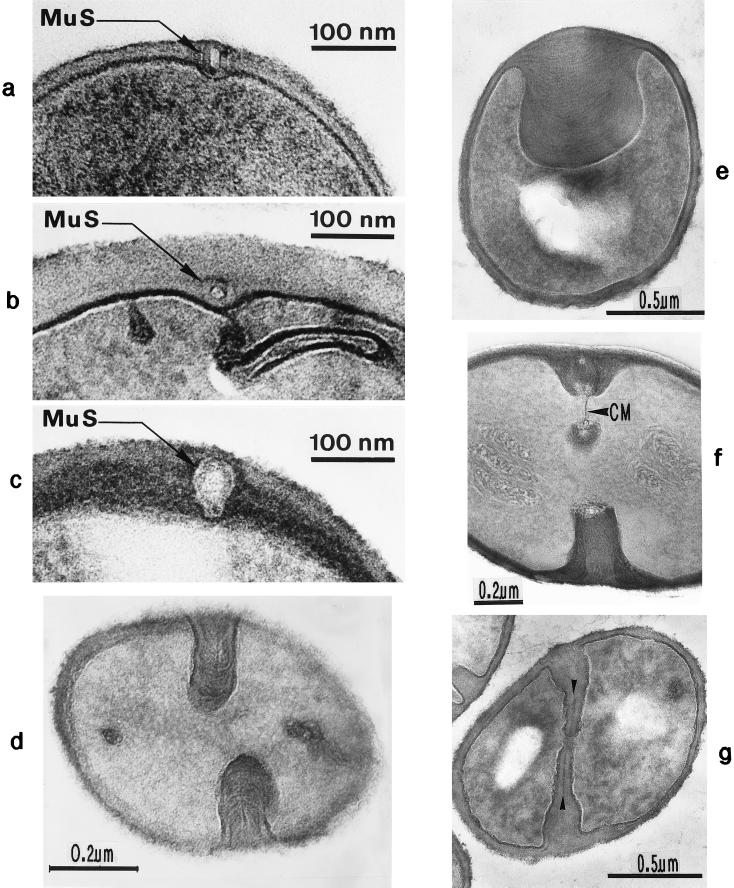
Scanning electron micrograph (a) and thin sections of staphylococcal cells (b to f). (a) A packet of eight staphylococcal cells was induced by liquoid; this packet is derived from one bacterium by three consecutive cell divisions, each having changed its direction at an angle of 90° to the preceding division plane. The three division planes are indicated by arrows (reproduced with permission from reference 139). (b) Characteristic alternation of consecutive division planes (arrowheads) (reproduced with permission from reference 41). (c) Asymmetrical initiation of cross wall formation (arrowhead), Sp, splitting system (reproduced with permission from reference 41). (d) Centripetal growth of the closing cross wall. The splitting system (Sp) appears as a darker central cross wall layer. (e) At the cell periphery, above the closed cross wall with its splitting system (Sp), there is one of the murosomes (MuS) (reproduced with permission from reference 50). (f) After a 2-h exposure to the antibiotic batumin (1 μg/ml) the peripheral wall appears to be differentiated into an outer layer, the so-called primary wall (prW), and an inner layer, the so-called secondary wall (scW). The dark line between these two layers represents the so-called stripping system (Str) of the staphylococcal cell wall which is involved in cell wall turnover. The remnants of the cutting through of the primary wall, the so-called clefts, are marked by arrows.
The characteristic alteration of consecutive division planes was demonstrated in thin sections (Fig. 2b). However, the reason for this astonishing alternation is far from being understood.
Neoformation of the cross wall.
Cross wall formation is initiated asymmetrically (Fig. 2c) at one single starting point beneath the peripheral cell wall (41). Like in other prokaryotic cells, cross wall formation proceeds via centripetal growth resembling a closing iris (Fig. 2d), until the tips of the ingrowing cross wall eventually fuse in the center of the cell (Fig. 2e).
After closure of the cross wall, the peripheral cell wall in most cases appears as a rather compact, homogeneous-looking structure (cf. Fig. 2e and 3A). Treatment with various antibiotics revealed, however, that it consists of two layers (Fig. 2f): (i) an outer layer, the so-called primary wall, and (ii) an inner layer, the so-called secondary wall (42). The secondary wall continues into the cross wall (Fig. 2f and 3B). Sandwiched between the primary and the secondary walls is the so-called stripping system that is involved in cell wall turnover (see “Wall thickening via underlayering processes”). Sometimes, characteristic “clefts” are left behind on the cell surface after the cutting through of the primary wall during early stages of cell separation (cf. Fig. 2f, and 3C and see “Cell separation in staphylococci”). If they are not turned over (Fig. 13b), clefts are capable of marking, even during later stages of the cell cycle, the site where the primary wall had first been cut through (135).
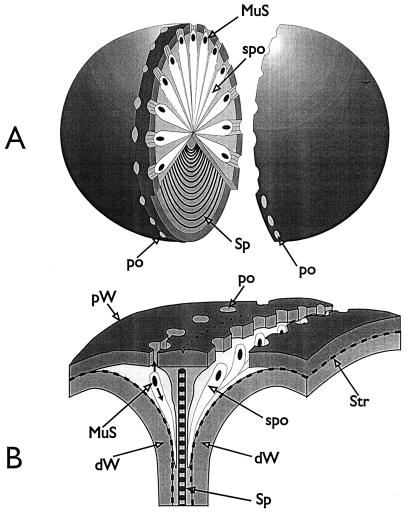
FIG. 3.
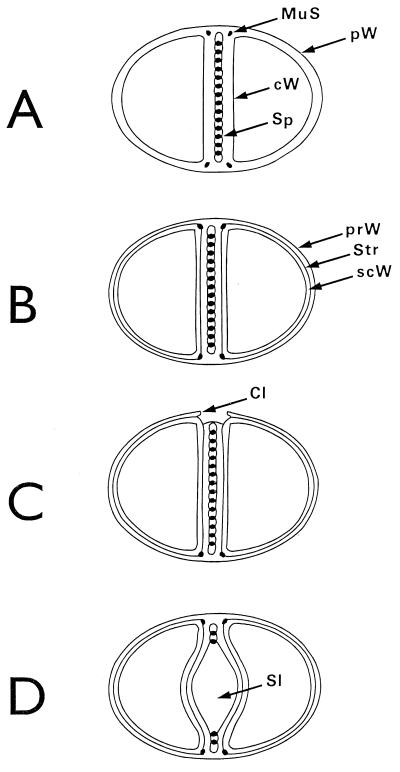
Nomenclature of the different parts of the staphylococcal cell wall. Schematic overview of the common parts of the cell wall, as seen in the electron microscope by investigating thin sections of fixed staphylococci. (For more details see Fig. 18). (A) Cell wall, splitting system, and murosomes. A highly elastic peripheral cell wall (pW) protects the protoplast against the extremely high turgor of the cytoplasm. The cross wall (cW) contains the splitting system (Sp) consisting of concentrically arranged ring-shaped tubuli. The splitting system is involved in cell separation. Minute, vesicular, extraplasmatic wall organelles, the murosomes (MuS), are located in two circumferential rows above the closed cross wall. They are engaged in lytic processes during cross wall formation and initiation of cell separation. Reference figure, Fig. 2e. (B) Primary and secondary walls. The seemingly homogeneous peripheral cell wall is, in fact, differentiated into an outer layer (the so-called primary wall [prW]) and an inner layer (the so-called secondary wall [scW]). The secondary wall, which continues into the cross wall, is deposited beneath the primary wall in connection with the formation of a new cross wall. The dark line between the primary and the secondary wall represents the so-called stripping system (Str), which is involved in wall turnover. Reference figures, Fig. 2f and Fig. 4a. (C) Clefts. During early stages of cell separation the lytic capacity of the murosomes is activated. The murosomes perforate and, subsequently, cut through the primary wall, sometimes leaving behind characteristic clefts (Cl) on the cell surface. If such clefts are not turned over, they mark the site of cutting through even during later stages in the cell cycle. Reference figures, Fig. 2f and 13b (D) Longitudinal slit of the cross wall. Sandwiched between layers of the cross wall, a preformed, longitudinal slit (Sl) is found in the center of the cross wall, which contains the concentrically arranged tubuli of the splitting system. If the splitting system is removed, the cross wall layers are moved aside, exposing the container-like slit of the cross wall. Reference figure, Fig. 4g.
FIG. 13.
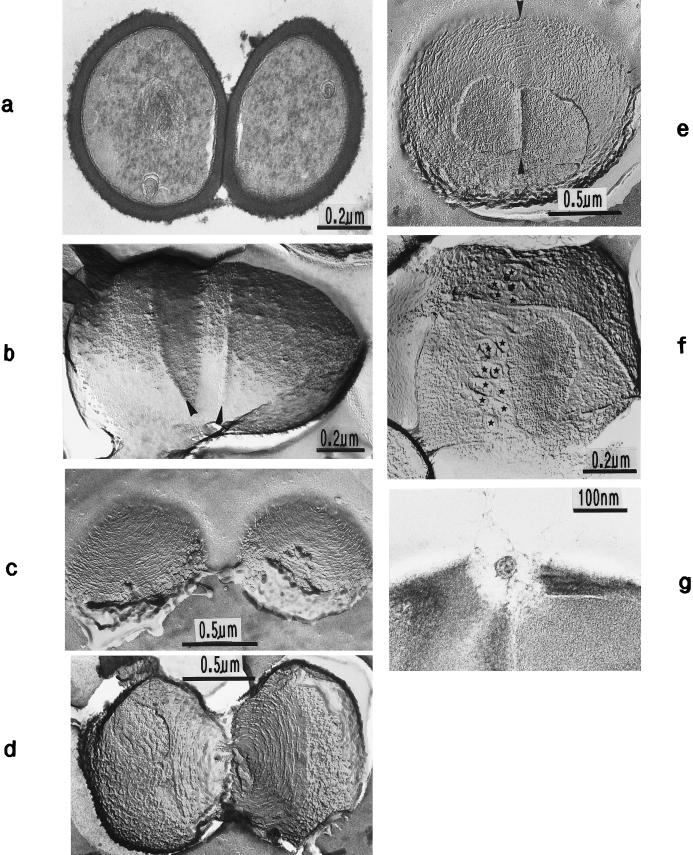
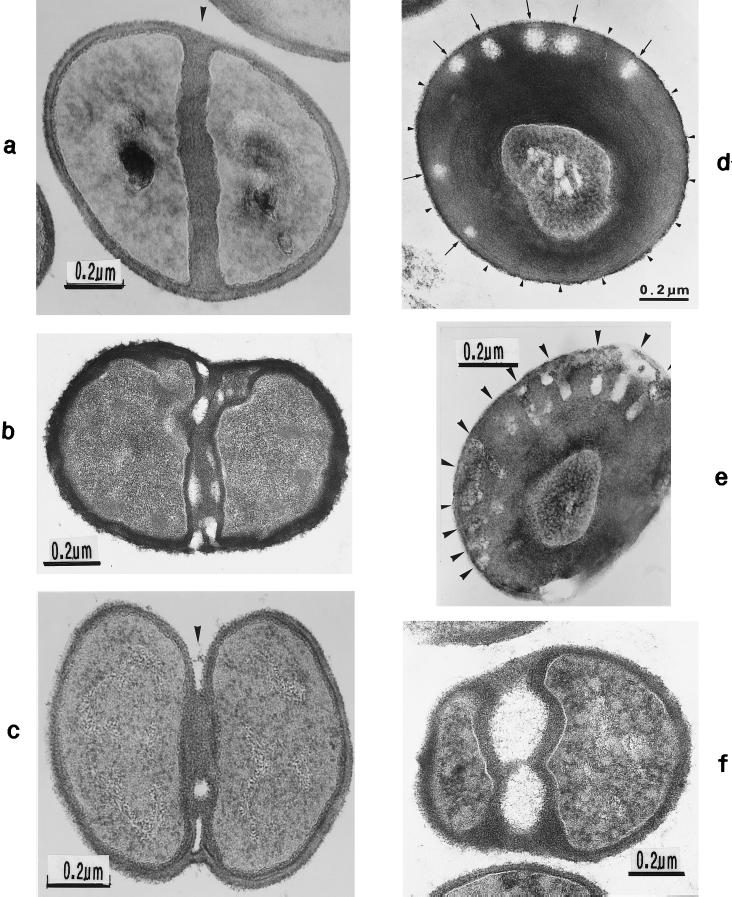
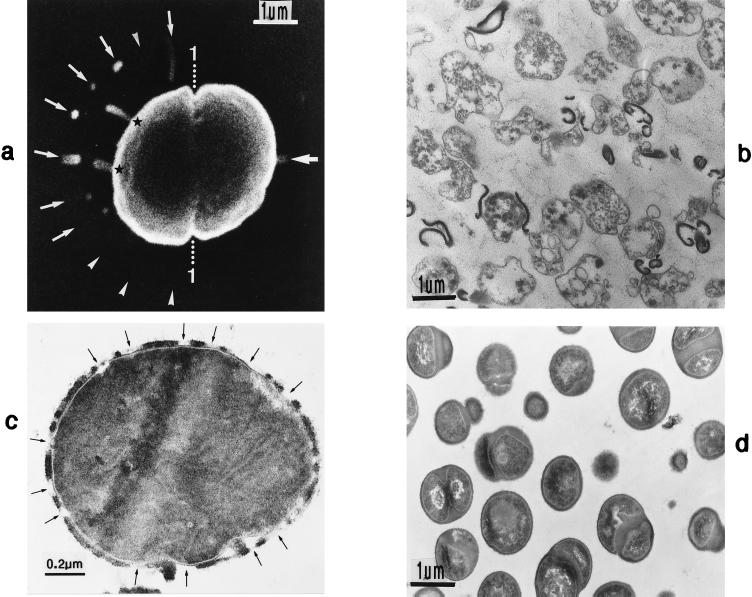
Thin sections (a and g) and freeze fractures (b to f) of staphylococcal cells. (a) Cell separation in untreated cells along the small layer of the splitting system without detectable loss of cross wall material. (b) During the separation of untreated cells not even remnants of the concentrically arranged tubuli of the splitting system can be detected on the just-exposed cross wall surfaces of the daughter cells. Only the clefts (arrowheads) are preserved which mark the site of the initial cutting through of the peripheral wall (reproduced with permission from reference 54). (c) After growth in the presence of chloramphenicol (20 μg/ml) and subsequent regeneration in drug-free medium, the concentrically arranged tubuli of the splitting system are preserved on the just-exposed surfaces of the daughter cells (reproduced with permission from reference 44). (d) This untreated cell of S. aureus SA 113 reveals the concentrically arranged tubuli of the splitting system on the cross wall surfaces of both daughter cells during cell separation. (e) After growth in the presence of chloramphenicol (20 μg/ml) and subsequent regeneration in drug-free medium, the next division plane is already initiated beneath the center of the still-preserved concentrically arranged rings of the splitting system (arrowheads) (reproduced with permission from reference 44). (f) In the presence of penicillin (0.1 μg/ml), the murosome-mediated punching of holes into the peripheral wall for cell separation starts in a zigzag-like manner (stars), resulting in the formation of two parallel rows of circumferential pores (reproduced with permission from reference 38). (g) In spite of growth in the presence of penicillin, this murosome just released from the cell appears to be rather well preserved.
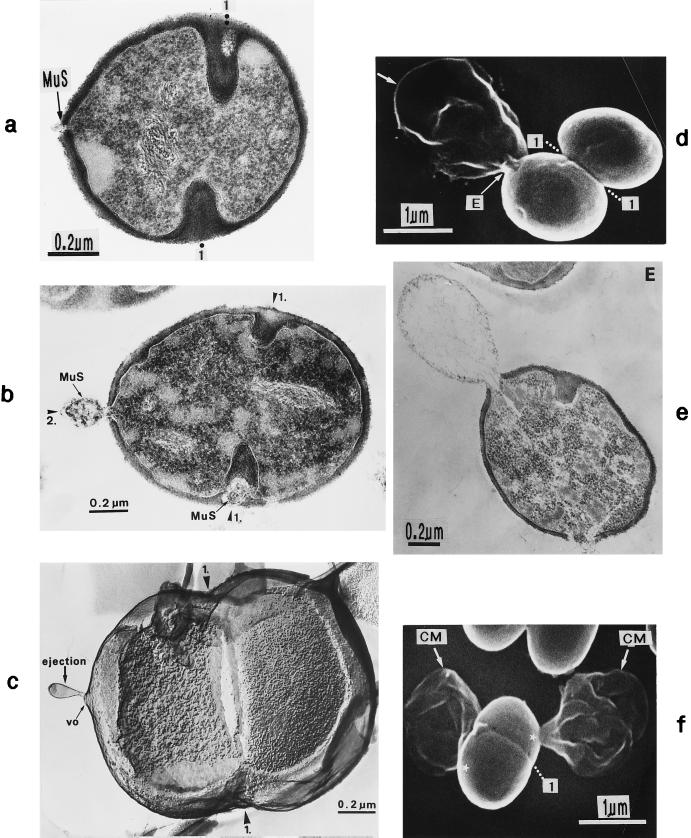
In the presence of penicillin (0.1 μg/ml), characteristic variations were sometimes found in the “staining” of the wall material that had been formed. The various parts of the wall structures reacted differently to the uranium and lead salts applied to raise the contrast for electron microscopy. The central region of the cross wall known to be lysed during cell separation (54) (see “The lytic type of cell separation”) and the primary, peripheral wall both showed a strikingly low electron density, while the newly underlayered secondary wall and the future cross wall of the daughter cells produced under the influence of penicillin were intensely stained (Fig. 4a). Such different staining effects of the two parts of the cross wall not only indicated the existence of different wall qualities in this region of the cell wall but also proved to be helpful for analyzing the sequence of some morphogenetic events (see “Murosomes and their role in cross wall morphogenesis”).
FIG. 4.
Thin sections of staphylococcal cells. (a) After treatment with penicillin (0.1 μg/ml) the secondary wall (scW) is intensely stained while the primary wall and the central parts of the cross wall (the so-called transitory cross wall material) are hardly stained. A murosome (MuS) is detectable in the secondary wall. (b) This section, running exactly through the middle of the cross wall, reveals the concentrically arranged tubuli of the splitting system. The hexagonally shaped inner edge of the closing cross wall is marked with arrows (reproduced with permission from reference 41). (c) The diameter of the tubuli of the splitting system enlarges continuously during growth in the presence of spermine (arrowheads). (d) Treatment with Triton X-100 likewise resulted in an enlargement of the tubular structures of the staphylococcal splitting system (Sp). (e) By extraction of the LTA, the splitting system disappeared. (f) Isolated cell wall of a staphylococcus after removal of the cytoplasm. The splitting system is still detectable (arrows) (reproduced with permission from reference 78). (g) Extraction of LTA from the isolated cell wall leads not only to the disappearance of the splitting system but also to a premature separation of the central cross wall region (arrows) (reproduced with permission from reference 78).
The most distinctive feature in the center of the nascent cross wall was a thin electron-dense layer (Fig. 2d and e); since this layer proved to be involved in cell separation, we have called it the splitting system of the cross wall (41). This splitting system (Fig. 4b) is 7 to 10 nm wide (103) and has been shown to consist of a concentrically arranged system of about 14 to 18 ring-shaped tubuli, each 7 to 10 nm in diameter (41). A similar concentric ring system has been found to be located in the cross wall of the cyanobacterium Phormidium uncinatum (33). Early data concerning the chemical nature of this cyanobacterial system indicated that it does not consist of peptidoglycan (33).
In staphylococci, the splitting system can be influenced by growth conditions; in the presence of spermine, the diameter of the tubuli was continuously inflated (up to 20 nm) (Fig. 4c). Treatment with Triton X-100 enlarged the width of the splitting system slightly, but it caused a considerable increase of its electron density (Fig. 4d), thus indicating its composition of tubular structures. At the same time, the layer directly beneath the cell wall, the so-called membrane-wall interlayer (see Fig. 6h and i), was affected.
FIG. 6.
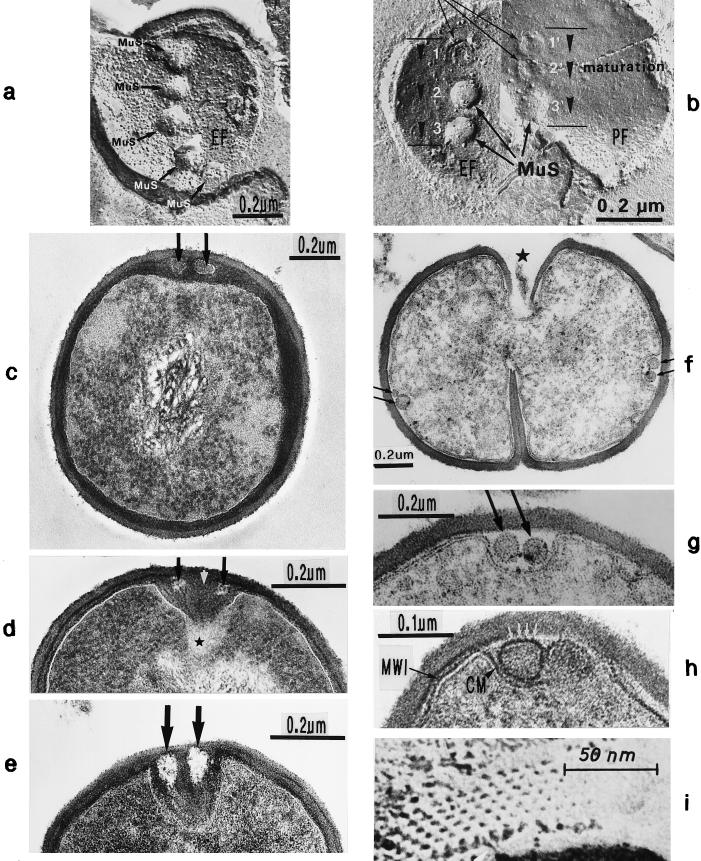
Freeze fractures of staphylococci in the presence of sodium chloride (a and b) or normal freeze fracture (i) and thin sections (c to h) of staphylococcal cells. (a) A row of linearly arranged vesicular murosomes (MuS) on the concave fracture plane (EF). (b) Murosomes (MuS) on the concave (EF) and convex (PF) fracture planes of the cytoplasmic membrane are in different stages of maturation (arrowheads); a ring-like structure is visible in the upper murosomes. (c) In the presence of penicillin (0.1 μg/ml) the underlayered wall material of the secondary wall reveals a higher contrast than the primary wall. Two murosomes (arrows) embedded in the highly contrasted layer are located beneath the primary wall. (d) In the presence of penicillin (0.1 μg/ml) the two murosomes differentiate the nascent cross wall into three parts, a central sector (white arrow) and two lateral ones (black arrows) ★, transparent “lytic” region at the tip of the nascent cross wall. (e) In the presence of penicillin (0.1 μg/ml) the nascent cross wall is divided in three parts by the lytic activity of murosomes (arrows). (f) Even in the presence of trimethoprim (3.13 μg/ml) the murosomes for the second division plane (arrows) are located at a 90° angle to the first division plane. (g) Higher magnification of panel f. The murosomes (arrows) are located outside the protoplast, between the cell wall and an invagination of the cytoplasmic membrane. (h) In the presence of trimethoprim (3.13 μg/ml) the murosomes located between the primary wall and the cytoplasmic membrane (CM) are covered with particles (white arrows) probably derived from the membrane-wall interlayer (MWI) of the cytoplasmic membrane. (i) Hexagonally arranged particles of an isolated cytoplasmic membrane with its membrane-wall interlayer of a control cell (reproduced with permission from reference 43).
No convincing data are so far available about the chemical composition of the staphylococcal splitting system; teichoic acid-like material, the lipoteichoic acid (LTA), has been assumed to be associated with this system (96, 125, 132). In fact, LTA extraction of staphylococci via the phenol method (28) led to the disappearance of the splitting system (Fig. 4e). It is highly interesting to note that extraction of teichoic acid from isolated cell walls of S. aureus resulted not only in the disappearance of the splitting system (cf. Fig. 4f and g) but also in premature separation of the cross walls of the presumed daughter cells exclusively within the region of the concentrically arranged rings of the splitting system (78).
This setting apart of cross wall layers after extraction of teichoic acid was always restricted to the region of the splitting system without affecting the peripheral cross wall. These data suggest that the splitting system can no longer be regarded simply as a special chemical entity which is located in the compact cross wall; it should be seen as a distinct layer fitted into a preformed longitudinal container-like slit of the cross wall (Fig. 3D). We have to consider, therefore, that the completed cross wall consists of different parts, including a central slit-like container in which the concentrically arranged tubuli of the splitting system are located and the rather homogeneous-looking layers of the neighboring cross wall. These findings are important for all considerations concerning the involvement of the splitting system during mechanical cell separation (see “An alternative, mechanical type of cell separation using the splitting system of the cross wall”).
Concerning the origin of the splitting system, no convincing data are presently available. The possibility that we are dealing with a direct extension of the cytoplasmic membrane can, however, be excluded since the characteristic appearance of this membrane (see Fig. 6g and h) has never been demonstrated. Several indications led us to presume that the so-called membrane-wall interlayer (43), located between the cytoplasmic membrane and the cell wall proper (see Fig. 18, MWI), is involved in the formation of the splitting system. This membrane-wall interlayer, in which minute hexagonally arranged particles are embedded (see Fig. 6h and i), regularly covers the staphylococcal cytoplasmic membrane (43). As discussed below the membrane-wall interlayer is, probably, also involved in the formation of the murosomes (see “Murosomes and their role in cross wall morphogenesis”).
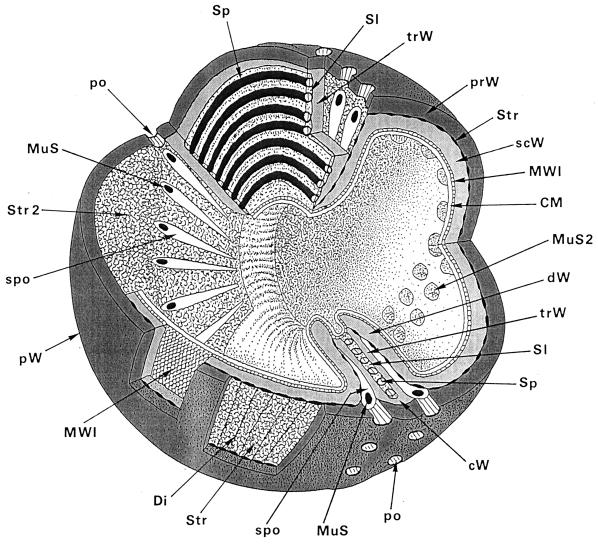
FIG. 18.
Three-dimensional reconstruction of the staphylococcal wall architecture. This sketch depicts all of the essential structural parts of a staphylococcal cell wall which are presently known. CM, cytoplasmic membrane. This membrane, which covers the protoplast, appears in thin sections as a characteristic three-layered structure about 10 nm in width. Reference figure, Fig. 6h. cW, cross wall. The cross wall is differentiated into the central layers comprising about 30% of the cross wall volume including the splitting system (Sp) and the outer layers. The central layers are only destined to be disintegrated during cell separation (transitory cross wall parts [trW]), while the outer layers (dW) represent the presumptive cell walls of the future daughter cells (permanent cross wall parts). Reference figures, Fig. 2d and 11e. Di, disintegrating system. This lytic, wall-disintegrating tool is periodically arranged on the surface of the stripping system (Str) and engaged in cutting through superfluous peripheral cell walls into rather large pieces. Reference figure, Fig. 16f. dW, Presumptive cell wall of the future daughter cell. The outer, permanent layer of the cross wall represents, after completion of the cross wall and subsequent lytic cell separation, the peripheral wall of future daughter cells (see trW below). Reference figure, Fig. 11a, b, and e. MuS, murosome. These minute extraplasmatic wall organelles are found located in two circumferential rows in the peripheral wall above the cross walls, close to the stripping system (Str). They are more or less spherical (diameter about 30 nm) and sometimes equipped with a “tail.” Murosomes contain special autolytic wall enzymes and are involved in three types of lytic disintegration of wall material during wall morphogenesis: (i) initiation of cross wall neoformation, (ii) punching of pores (po) into the peripheral wall, and (iii) attacking transitory cross wall material during cell separation. Reference figures, Fig. 5f and 10e. MuS2, murosomes of the second division plane. They are arranged at a right angle to the first one. Reference figures, Fig. 6a and b. MWI, membrane-wall interlayer. This thin layer sandwiched between the cytoplasmic membrane and the peripheral wall covers the outer surface of the cytoplasmic membrane and, probably, also the murosomes. This layer contains hexagonally arranged particles with center-to-center spacing of about 7 nm. The function of the MWI is unknown. Reference figure, Fig. 6i. po, pore. Pores in the peripheral cell wall are the result of centrifugally directed lytic murosome activities and represent the first step in cell separation. Pore diameters hardly surpass the size of the murosomes. At later stages of cell separation the pores are enlarged and fuse with each other; cutting of circumferential pores marks the beginning of cell separation. Reference figure, Fig. 10a to g. prW, primary wall. Outer layer of the peripheral cell wall. Reference figure, Fig. 2f. pW, peripheral cell wall. The highly elastic peripheral wall determines the bacterial shape and protects the protoplast, having an internal turgor of about 25 atm, against bursting. It functions as an “exoskeleton.” The peripheral wall, about 40 nm wide, is capable of enormous thickening. The seemingly homogeneous peripheral cell wall is, in fact, differentiated into an outer primary wall and an inner secondary wall. Reference figure, Fig. 2f. scW, secondary wall. Inner layer of the peripheral wall which is continued into the cross wall. It is deposited beneath the primary wall in connection with the formation of a new cross wall. The murosomes are positioned in those parts of the secondary wall that are located above the cross wall. Reference figures, Fig. 2f and 6c. Sl, slit. A longitudinal slit in the center of the cross wall which contains the concentrically arranged tubuli of the splitting system. Reference figure, Fig. 4g. Sp, splitting system. The splitting system is located in a container-like longitudinal slit in the center of the cross wall and consists of 14 to 18 concentrically arranged ring-shaped tubuli, each about 7 to 10 nm in diameter. It is involved in mechanical cell separation. Reference figures, Fig. 4b and 13c. spo, spoke-shaped canal. These canals are the result of centripetally directed lytic actions of the murosomes and are formed during lytic cell separation within the transitory cross wall material. Reference figures, Fig. 11c-d and 14e. Str, stripping system. This lytically active system is found sandwiched between the primary wall and the secondary wall and is involved in wall turnover processes. Reference figure, Fig. 16b-c. Str 2, stripping system after removing the cytoplasmic membrane, the membrane-wall interlayer, and the secondary wall, revealing the vast surface of this lytically active system. trW, transitory parts of the cross wall. The transitory parts of the cross wall are only destined to be disintegrated during lytic cell separation (see dW above). Reference figure, Fig. 11a, b, and e.
Murosomes and their role in cross wall morphogenesis.
Minute vesicular structures, 30 to 40 nm in diameter, can be observed in the peripheral cell wall above closed cross walls (Fig. 2e). Often, these structures appear in pairs located in the peripheral wall directly above a closed cross wall (54). We named such transparent, extraplasmatic wall organelles “murosomes” (38, 48, 50); they are capable of performing divers lytic activities in the cell wall material. Murosomes above closed cross walls were demonstrated most clearly in very thin sections of slowly growing and dividing control cells, especially during the so-called stationary phase of growth in which staphylococci exhibit relatively thick cell walls.
Pairs of such minute wall organelles were also traceable in the peripheral cell wall at sites where a new cross wall was initiated (Fig. 5a). Freeze-etching in the presence of sucrose or sodium chloride revealed that the murosomes are enclosed by a definite envelope (Fig. 5b).
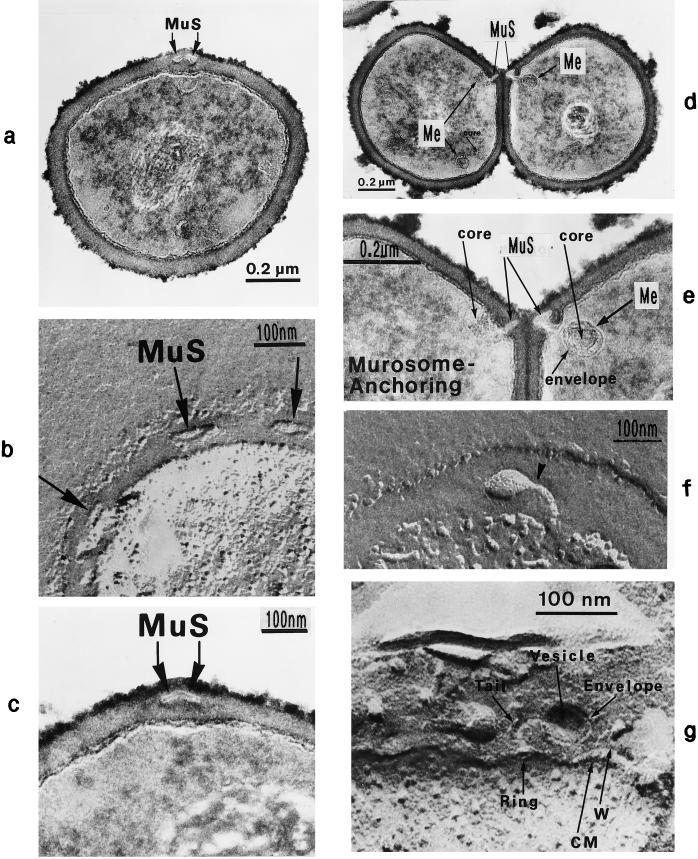
FIG. 5.
Staphylococcal cells after thin sectioning (a and c to e) or freeze fracturing in the presence of sucrose (b and f) or sodium chloride (g). (a) A pair of murosomes (MuS) is located at the site of a new cross wall initiation. (b) The murosomes (MuS [arrows]) appear to be enclosed by a definite envelope (reproduced with permission from reference 50). (c) Flattened or collapsed vesicular murosomes (arrows) in the peripheral cell wall. (d) For initiation of cross wall formation, the murosomes in both the just-separating daughter cells have been anchored in the secondary wall where they induced centripetal lytic processes which cut the secondary wall. Me, membranous body. (e) Higher magnification of panel d showing details of the new cross wall initiation. At the free ends of the secondary wall created by the lytic activity of the murosomes the first assembling of wall material for the formation of the new cross wall can be seen. A local invagination of the cytoplasmic membrane, the membranous body (Me), consisting of an envelope and a core, is associated with the site of cross wall initiation. (f) A vesicular murosome exhibiting a tubular tail-like extension (arrow) is detectable; this extension connects the extraplasmatic murosome with the surface of the cytoplasm (reproduced with permission from reference 49). (g) The fracturing has exposed an envelope and a vesicular part of the murosome and its tail. At the end of the tail-like extension of the murosome, a ring-like structure is revealed which marks the connection site between the murosome and the cytoplasmic membrane (CM). W, wall.
In other control cells, however, instead of vesicular murosomes rather flat structures only 10 to 15 nm wide were found, resembling more or less collapsed vesicular murosomes (Fig. 5c).
Since the initiation of a new cross wall is a very rapid process in staphylococci, taking place within a few minutes from the logarithmic phase of growth, only slowly growing cells from the stationary-growth phase were suitable for analyzing cross wall morphogenesis. Furthermore, advantage was taken of the fact that staphylococci regularly alter their division plane and can therefore start cross wall formation in the second division plane while cell separation is still going on along the first division plane (Fig. 2b). In this way, initiation of cross wall formation could be followed at the same time in both the just-separating daughter cells (Fig. 5d and e). The murosomes of both daughter cells were always found to be symmetrically arranged to each other, indicating the possible existence of a synchronized starting process for the cross wall initiation in both daughter cells.
For initiation of a new cross wall, the murosomes always induced a centripetally directed cutting of some inner layers of the peripheral wall, probably the entire secondary wall (Fig. 5d and e and cf. Fig. 19d and f). It is speculated that after the cutting through of the secondary wall its “free ends” function as assembling sites of newly synthesized wall material for the formation of the new cross wall (see Fig. 7).
FIG. 19.
Thin sections of staphylococcal cells grown in the presence of 0.05 (a) or 0.1 (b to f) μg of penicillin/ml. (a) Huge amounts of fibrillar wall material deposited at the cross wall tips have prevented a fatal tearing apart of the nascent cross wall (arrowheads). The premature initiation of cell separation has only resulted in a limited increase of cell size (arc-shaped arrows). (b) A murosome (MuS) is deposited at the initiation site of the second division plane between the cytoplasmic membrane and the peripheral wall. 1, first division plane (reproduced with permission from reference 50). (c) A murosome (MuS) is found within the peripheral wall of the second division plane (2). 1, first division plane (reproduced with permission from reference 50). (d) By attack from the inside, one murosome has disintegrated a sector of some inner layers of the peripheral wall, the so-called secondary wall (arrow), at the initiation site of the second cell division. The outer layers of the peripheral wall, the so-called primary wall, are not yet affected. (e) A pair of murosomes (black stars at the left side) is found deposited within the peripheral wall at the initiation site of the second division plane. The white stars mark the initiation site of the second division plane of the other daughter cell (reproduced with permission from reference 48). (f) By attack from the inside, a pair of murosomes has disintegrated a sector of the secondary wall at the initiation site of the second division plane, leaving behind a rather extended gap in the inner layer of the peripheral wall (arrow) without, however, affecting its outer layers.
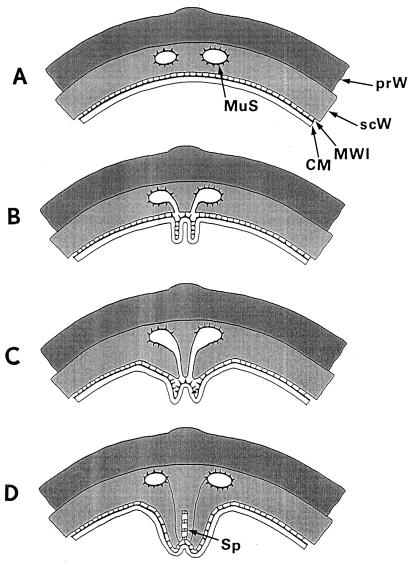
FIG. 7.
Cross-wall morphogenesis. This schematic illustration is intended to tentatively represent the involvement of murosomes in morphogenetic processes during neoformation of a staphylococcal cross wall. Becoming acquainted with these morphogenetic steps is an essential prerequisite for understanding the last minutes in the life of a staphylococcus under penicillin (see Fig. 21 and 22). (A) Site for cross-wall neoformation. The murosomes (MuS) are anchored within the secondary wall (scW) directly above the site of the future cross wall initiation. CM, cytoplasmic membrane; MWI, membrane-wall interlayer; prW, primary wall. Reference figures, Fig. 5a and 6c. (B) Initiation of cross wall morphogenesis. For initiation of a new cross wall, the murosomes induce centripetally directed lytic cutting processes considered to separate the secondary wall into three parts: a central sector and two lateral ones. Sometimes the vesicular murosomes show tail-like tubular extensions which, probably, are involved in this cutting process. Reference figure, Fig. 5d to g. (C) Onset of cross wall morphogenesis. The reason for the cutting processes within the secondary wall seems to become evident: the central sector of the sectioned secondary wall starts to form the so-called transitory part of the cross wall, while the two lateral sectors initiate the formation of the so-called permanent parts of the cross wall (see Fig. 12B). Reference figure, Fig. 6d to e. (D) Initiation of the splitting system. No reliable data are available about the genesis of the splitting system (Sp). It is speculated that the splitting system stems from the membrane-wall interlayer of the cytoplasmic membrane. Reference figure, Fig. 2c and d.
High-resolution freeze-etching in the presence of sucrose or sodium chloride (49) revealed the existence of vesicular murosomes with a tubular “tail-like” extension (Fig. 5f and g) which, probably, are involved in this cutting process. The characteristic result of these cutting processes was a direct connection between the extraplasmatic murosomes and the cytoplasmic surface; a local invagination of the cytoplasmic membrane was often observed beneath these connection sites (Fig. 5e). Sometimes a more-or-less ring-like structure was detected at the connection site of the murosomal tail with the cytoplasmic surface (Fig. 5g). It is, however, still an open question whether the tail of the murosome is in fact a structural peculiarity of these organelles, engaged in cross-wall initiation, or whether it is nothing but a sort of canal within the compact wall material created by the lytic activity of the murosome. Furthermore, no explanation has been found so far as to why at this stage of the cell cycle the lytic activities of the murosomes are always directed centripetally; one can only speculate that at this stage the outer layers of the peripheral wall are more resistant to lytic processes of this type than the inner layers.
Linearly arranged vesicular murosomes, during the onset of cross wall formation on the inner surface of the peripheral cell wall (Fig. 6a), were also demonstrated by such freeze-etching; probably, they were located in the region between the cell wall and the cytoplasmic membrane. Interestingly, the centripetally directed side of some of these murosomes proved to be open (Fig. 6b). It is, however, still unknown whether these openings in the “bottom” of the murosomes can be ascribed to the tail-like extensions of those murosomes involved in the cutting of lower layers of the peripheral wall (Fig. 5f and g).
However, neither thin sections nor freeze-etchings of control cells could answer questions concerning the exact localization of the murosomes within the primary or secondary peripheral walls before the cutting processes for cross wall initiation started. This was only possible after the application of penicillin, by means of which we became able to differentiate between primary and secondary wall material (Fig. 4a). After treatment with this antibiotic the murosomes appeared to be somewhat inflated (Fig. 6c), but for the first time they could be nicely localized within the peripheral wall: they were found closely beneath the primary peripheral wall and clearly within the dark lower “secondary” layer of the peripheral wall formed during the action of penicillin.
Consequently, in nondividing staphylococci, murosomes can hardly be considered to exist a priori in every peripheral cell wall; rather, these wall organelles must always be formed de novo for every new cross wall formation. For this, murosomes are apparently placed beneath the primary cell wall together with a rather thick layer of newly formed secondary wall material. The possibility cannot be excluded, however, that the murosomes are placed beneath both these layers of the peripheral cell wall and are capable of penetrating, in a separate step, into the secondary layer (see Fig. 19b and c).
The location of the murosomes was also monitored during the subsequent stages of early cross wall formation (Fig. 6d and e), indicating that the two murosomes are in some way also involved in the process of cross wall differentiation into three parallel layers (Fig. 4a; see also Fig. 3 and 7).
The stringent positioning of the murosomes certainly requires a highly sophisticated “anchoring” procedure at the peripheral wall. However, it is far from clear whether the apposition of secondary wall material is the intrinsic mechanism by which the staphylococci are capable of placing the murosomes at the necessary 90° angle to the preceding division plane (Fig. 2b). Since the ftsZ-ftsA genes are part of the cell division gene cluster of staphylococci (109) one could seek to determine if they are involved in this alteration of division planes (7). Recent findings have shown that FtsZ can form tubular structures (83); in this regard one should consider whether there exists any relation between these tubular structures and the concentrically arranged tubuli of the splitting systems of the cross wall (Fig. 4b).
In this connection, the reactions of staphylococci to treatment with the bacteriostatic agent trimethoprim are especially interesting (100). This drug is known to inhibit the growth and synthesis of secondary wall material which, after treatment with other bacteriostatic agents such as chloramphenicol, is normally deposited as very thick layers beneath the primary peripheral wall (see Fig. 16a). Since during trimethoprim-mediated growth inhibition there was no such secondary wall material beneath the primary cell wall, the murosomes were found to be deposited between the peripheral wall and an invagination of the cytoplasmic membrane without any detectable association with secondary wall material; this fact was shown especially in cells at the onset of cellular disintegration when the region of the cytoplasmic membrane was better exposed (Fig. 6f and g). This was the first demonstration of the formation of “free murosomes,” i.e., of murosomes not embedded in wall material (see also Fig. 19b). These findings suggest that the apposition of new secondary wall material below the primary peripheral wall cannot be made responsible for the localization of the murosomes at the correct site for inducing new cross wall initiations in the next division plane.
FIG. 16.
Thin sections of staphylococcal cells treated with 20-μg/ml chloramphenicol (a to f) and subsequently transferred into drug-free medium for regeneration (b to f). (a) Considerable wall thickening has taken place (reproduced with permission from reference 40). (b) Wall restoration has started with the formation of a so-called stripping layer (arrowheads) beneath the old thick cell wall; the subsequent synthesis of new wall material has resulted in the formation of a thin peripheral wall underneath the stripping layer (reproduced with permission from reference 44). (c) Removal of superfluous thick wall material starts with the formation of a cutting rim (C1) above the initiation site of the new cross wall. Furthermore, the sequential detachment of the old thick wall material along the stripping layer (St [small arrowheads]) above the underlayered new wall (U [large arrowhead]) is initiated (reproduced with permission from reference 45). (d) The stripping of superfluous thick wall material has proceeded, and the removed wall material is already partially disintegrated above the cutting rim (C1) by a so-called disintegrating system (D). The gap along the stripping layer (St) is enlarged (small arrowheads) (reproduced with permission from reference 45). (e) The continuous disintegration of the thick peripheral wall has already liberated parts of the new peripheral wall from superfluous wall material via sequential activating of the stripping system (St) along the stripping layer (small arrowheads), C2, former cutting rim (reproduced with permission from reference 45). (f) Detachment and disintegration (Di) of thick wall material is sometimes initiated by the formation of periodically arranged holes (arrowheads) (reproduced with permission from reference 44).
It was quite remarkable that in the presence of trimethoprim the staphylococci and their murosomes were always grossly inflated but the murosomes were located rather correctly at an angle of 90° to the first division plane (Fig. 6f). Furthermore, a peculiar surface of the murosomal envelope was revealed with trimethoprim, since its surface was, for the first time, free from masking wall material. These murosomes were shown to be covered with characteristically arranged dark particles (Fig. 6h), the array of which could not be differentiated from that of the typical surface of the so-called membrane-wall interlayer (43). This membrane-wall interlayer is regularly located directly beneath the peripheral cell wall (Fig. 6h and i); it is known to cover the outer surface of the cytoplasmic membrane of staphylococci. Earlier high-resolution freeze-etching of this membrane-wall interlayer revealed the very peculiar, hexagonal array of such particles, exhibiting center-to-center spacings of approximately 7 nm (Fig. 6i).
Tentative identification of some material from the membrane-wall interlayer located on the murosomal envelope suggests that the possibility cannot be excluded that the cytoplasmic membrane and its membrane-wall interlayer are in some way involved in manipulating the position of the murosomes to the correct site for successful initiation of subsequent cross wall formation. Questions concerning the possible origin of the murosomes from the cytoplasmic membrane can now be discussed as well. The surface layer of the murosomes, covered with particles, indicates that the murosomes cannot be created simply by invagination of the cytoplasmic membrane, since in such case the membrane-wall interlayer would always be found inside the vesicles. These murosomes must be formed in an evagination process, either by evagination of the cytoplasmic membrane together with its membrane-wall interlayer or by evagination of the membrane-wall interlayer alone. A highly speculative schematic proposal has been sketched (Fig. 8) to elucidate the possible formation of the murosomes via local invagination of the cytoplasmic membrane, followed by murosome morphogenesis via evagination of the membrane-wall interlayer; it remains a matter of speculation whether, during an additional step, the murosomes are capable of penetrating into the secondary wall (Fig. 8, B-2 and C) or whether they evaginate synchronously with the synthesis of the secondary wall (Fig. 8, B and C).
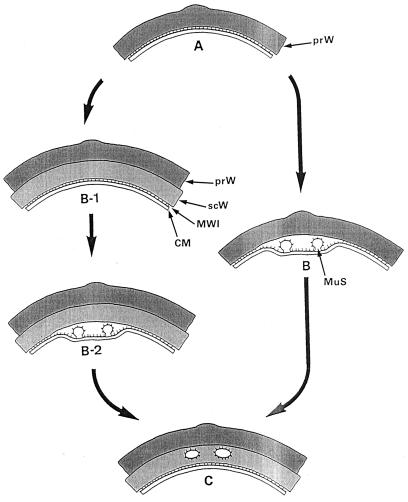
FIG. 8.
Formation and positioning of the murosomes. A highly speculative attempt to reconstruct the formation and localization of staphylococcal murosomes, which are derivatives of the cytoplasmic membrane. (A) The primary wall. A part of the primary wall (prW) is depicted. (B-1) Formation of the secondary wall. By apposition growth the secondary wall (scW) is placed beneath the primary wall. The cytoplasmic membrane (CM) is indicated beneath the primary wall. Sandwiched between the wall and the membrane is the so-called membrane-wall interlayer (MWI). (B and B-2) Murosome morphogenesis. The murosomes (MuS) seem to originate from a local invagination of the cytoplasmic membrane followed by evagination of the membrane-wall interlayer (B and B-2). It is not clear, however, whether the secondary wall is formed before genesis of the murosomes (B-1 to B-2) or whether it is synthesized only after the murosomes are formed (B to C). Moreover, it cannot be excluded that murosomes and secondary wall originate synchronously. The surface of the murosomes is covered with particles which seem to originate from the membrane-wall interlayer. Reference figure, Fig. 6g and h. (C) Positioning of the murosomes. At this stage the murosomes are found to be anchored beneath the primary wall within the secondary wall material. The murosomes are always considered to be placed directly above the site where the next cross wall formation will be initiated. If murosome positioning takes place only after formation of the secondary wall (B-1 to B-2) the murosomes must be assumed to be capable of penetrating into the secondary wall (B-2 to C). Reference figure, Fig. 6c.
However, judging from the contrast evident by electron microscopy, in some cases the envelope of the transparent murosomes of control cells was covered not only with particles but also with some other material which looked like a rather thin but compact layer of wall material and could be clearly differentiated from the wall material in which the murosomes were embedded (Fig. 9a). Furthermore, some antibiotic-induced reactions of this surface layer of the murosomes were also typical of newly formed wall material. It was regularly thickened under chloramphenicol (Fig. 9b); such wall thickening is highly characteristic of the action of this drug (see Fig. 16a). In the presence of penicillin this compact layer changed to a rather fibrillar appearance (Fig. 9c), which is well known for wall material formed in the presence of this antibiotic (see Fig. 14a).
FIG. 9.
Thin sections of staphylococcal cells. (a) In the presence of sucrose a ring-like structure surrounds the murosome (MuS). (b) In the presence of chloramphenicol (3 μg/ml) the murosome (MuS) appears to be enveloped by a rather thick layer of wall material. (c) In the presence of penicillin (0.1 μg/ml) the murosome is enlarged and its surrounding layer is of rather fibrillar appearance (reproduced with permission from reference 38). (d) During treatment with penicillin (0.05 μg/ml) the compact cross wall has been converted into fibrillar wall material seemingly arranged in an arc-shaped configuration (reproduced with permission from reference 42). (e) Simultaneous treatment of staphylococci with chloramphenicol (20 μg/ml) and penicillin (0.1 μg/ml) has resulted in the formation of an extremely thick cross wall exhibiting a layered architecture. (f) Under penicillin (0.1 μg/ml) cross wall material may be assembled even at an extension of the cytoplasmic membrane (CM), seemingly without any contact with preexisting wall material. (g) Staphylococci having lost their splitting system during penicillin treatment restore this system during growth in drug-free medium (arrows) (reproduced with permission from reference 38).
FIG. 14.
Thin sections of penicillin-treated staphylococcal cells. (a) Already during growth in the presence of low doses of penicillin (0.01 μg/ml) the formation of the splitting system is blocked and only fibrillar wall material is synthesized instead of a compact cross wall (arrowhead). (b) Pairs of lytic sites are involved in the process of cross wall degradation during cell separation. (c) Under penicillin (0.01 μg/ml), central parts of the cross wall (arrowhead) are lysed without participation of the splitting system (reproduced with permission from reference 54). (d) This section of a penicillin-treated cell (0.1 μg/ml), running parallel to the middle of the cross wall through the intensely stained cross wall fibrils, reveals several murosome-mediated lytic sites located at the cell periphery (arrows). Further presumable lytic sites are indicated by arrowheads (reproduced with permission from reference 50). (e) Section of a penicillin-treated cell (0.1 μg/ml) running parallel to the middle of the cross wall; the lytic sites from the cell periphery have been extended centripetally and form spoke-shaped canals (arrowheads) (reproduced with permission from reference 42). (f) After simultaneous treatment with penicillin (0.1 μg/ml) and liquoid (2 mg/ml) transitory parts of the cross wall disintegrated without, however, affecting the peripheral cell wall above the cross wall, thus more or less inhibiting cell separation.
These data indicate that the murosomes, besides their lytic capacity, can also serve, at least to a limited extent, as a special site for the assembly of new cell wall material. The significance of such restricted wall assembly effect is still unknown.
At any effect, the morphogenetic importance of the extraplasmatic murosomes is indicated (i) by their sophisticated anchoring process (Fig. 6c and f to h), (ii) by their ability to perform cutting procedures for the initiation of cell division (Fig. 5d to g), (iii) by their involvement in the differentiation of the three-layered cross wall (Fig. 6d and e), and (iv) by their capability of assembling at their surfaces at least some new wall material (Fig. 9a to c). The subsequent induction of these processes, taking place within the secondary wall material, is an important prerequisite for the successful initiation and neoformation of the next cross wall at the correct site and for the accomplishment of the subsequent cell division which has to implement the regular alternation of the division planes. All data available so far indicate that the murosome-induced morphogenesis of the staphylococcal cross wall takes place as tentatively suggested in the schematic drawing shown in Fig. 7.
These findings on cross wall morphogenesis, as summarized in the schematic drawings in Fig. 7 and 8, are important prerequisites for understanding the events which can be observed only minutes before penicillin-induced death (cf. Fig. 21, B3 and Fig. 22A to D).
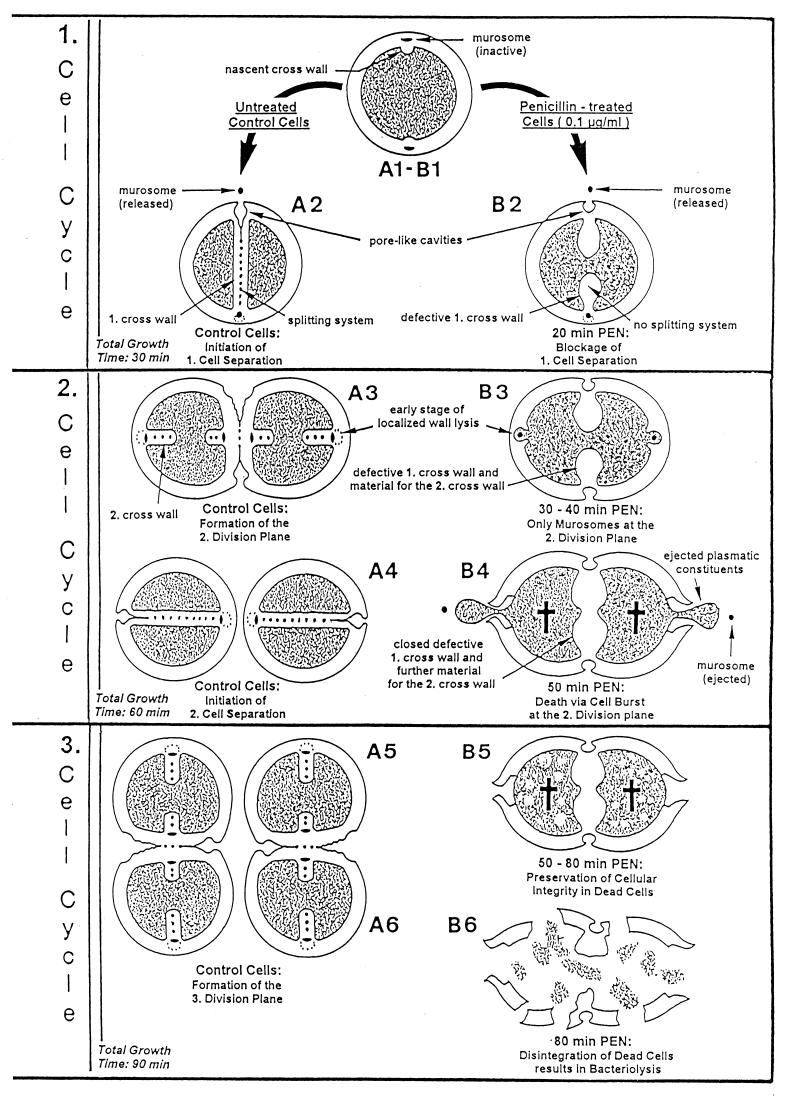
FIG. 21.
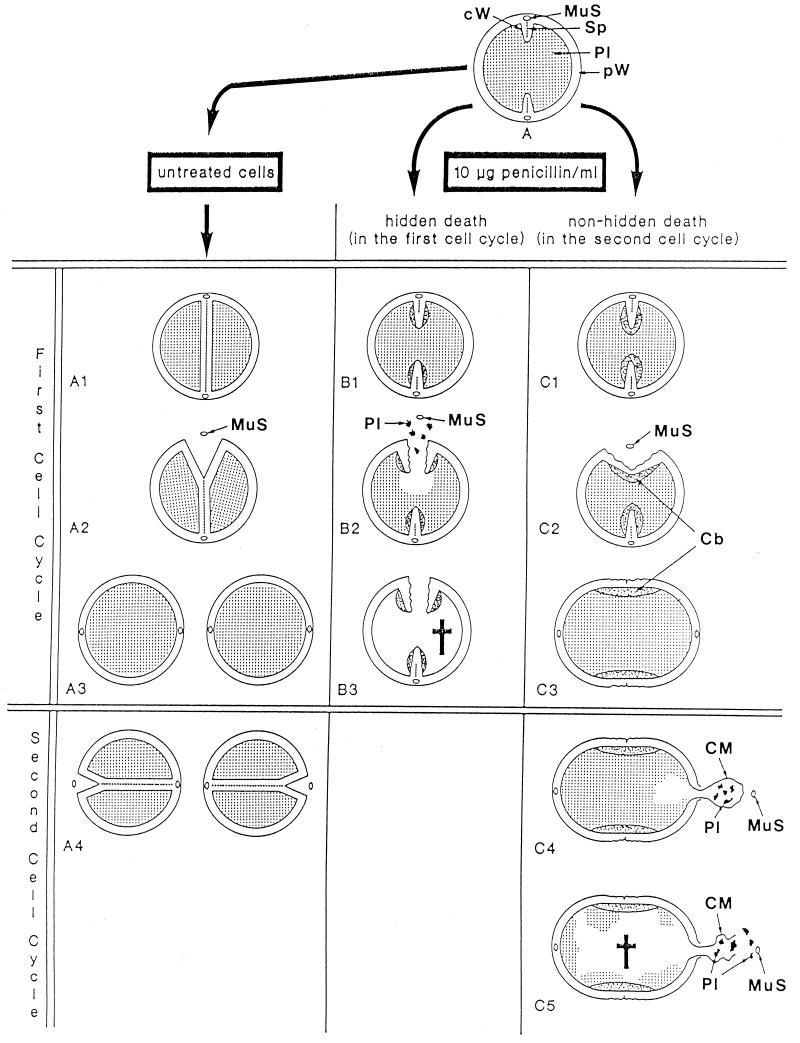
Time course of penicillin-induced death and bacteriolysis. A simplified schematic survey is shown of the events which take place in growing control cells (A) and in staphylococci after the addition of 0.1-μg/ml penicillin (B). The critical first 90 min of treatment with this antibiotic, which include three cell cycles (generation time of staphylococci, 30 min), are illustrated; these events lead to penicillin-induced death and finally to bacteriolysis. First cell cycle. (A1 to A2) In untreated cells a normal, thin, highly organized and complete cross wall will be formed in the first division plane which contains the intact splitting system. Only after this cross wall has been completed do the murosomes of the first cross wall initiate cell separation via perforation of periodically arranged minute pores into the peripheral wall in the first division plane. After such initial step, the subsequent tearing apart of these pores initiates the separation of the two daughter cells. Reference figure, Fig. 10a. (B1 to B2) After the addition of a lethal concentration of penicillin, the staphylococci almost immediately lose the capacity to form a splitting system. Reference figure, Fig. 19a. Furthermore, the cells are now no longer capable of assembling normal, compact cross walls, but they synthesize mainly a network of fine fibrils arranged in rather thick, deformed and often incomplete defective cross walls. Nevertheless, the staphylococci try to start cell separation via murosome-induced perforation of the peripheral wall as if the first cross wall is intact and complete. However, instead of cell separation only rather large, murosome-induced cavities appear in the peripheral wall of the first division plane. Reference figure, Fig. 13f. Second cell cycle. (A3) During separation of the untreated daughter cells the formation of a new cross wall in the second division plane is initiated at a 90° angle with respect to the previous one. Reference figure, Fig. 2b. Cross wall initiation starts with a very localized, murosome-mediated wall lysis which attacks only some inner layers of the peripheral wall; cross wall assembly takes place and proceeds in this small lytic region. Reference figure, Fig. 5e. (B3) In the presence of penicillin, the second division plane is likewise initiated via a very localized, murosome-mediated wall lysis of some inner layers of the peripheral wall. Reference figure, Fig. 19c to f. However, no cross wall assembly takes place here; the cross wall material bound for the second division plane is detoured and deposited further on in the first division plane, so that the deformed, defective first cross wall becomes even thicker. Reference figure, Fig. 19e. (A4) In control cells, only after completion of the cross wall, the next cell separation is initiated via murosome-mediated perforation of the peripheral wall in the second division plane. Reference figure, Fig. 10a. (B4) Like in normal staphylococci, cell separation in the second division plane starts with murosome-mediated punching of pores into the peripheral wall in spite of the fact that in the presence of penicillin no cross wall material has been deposited at this site. Hence, due to their high internal turgor, the cells will burst and eject the murosome and a limited amount of their plasmatic constituents (aneurysm principle). This murosome-induced morphogenetic death takes place about 50 min after addition of the drug. Reference figure, Fig. 20b to f. For further details see Fig. 22. Third cell cycle. (A5) In control cells, already during the course of cell separation in the second division plane, the cross walls for the third division plane are initiated, again at a 90° angle to the previous one. Reference figure, Fig. 2a. (B5) The dead staphylococci, killed in the presence of penicillin during late stages of the second cell cycle, have lost part of their cytoplasm. This loss reduces considerably the tension of the elastic cell wall and results, therefore, in some shrinkage of the cells. The dead staphylococci preserve, however, their seemingly intact cellular integrity and, therefore, hardly differ from bacteria that are still alive. Reference figure, Fig. 23d. (A6) In control cells the cross walls for the third division planes are completed and the next cell separation is initiated (not depicted). (B6) Disintegration of the cell wall and decomposition of the cytoplasm (bacteriolysis) start only about 30 min after penicillin-induced death, leaving behind mainly large pieces of ruptured cell walls, membrane fragments, and plasmatic debris. Reference figure, Fig. 23b. Schematic drawing modified from reference 48 and supplemented and varied. For the sake of simplicity, only one murosome has been depicted at all the murosomal sites.
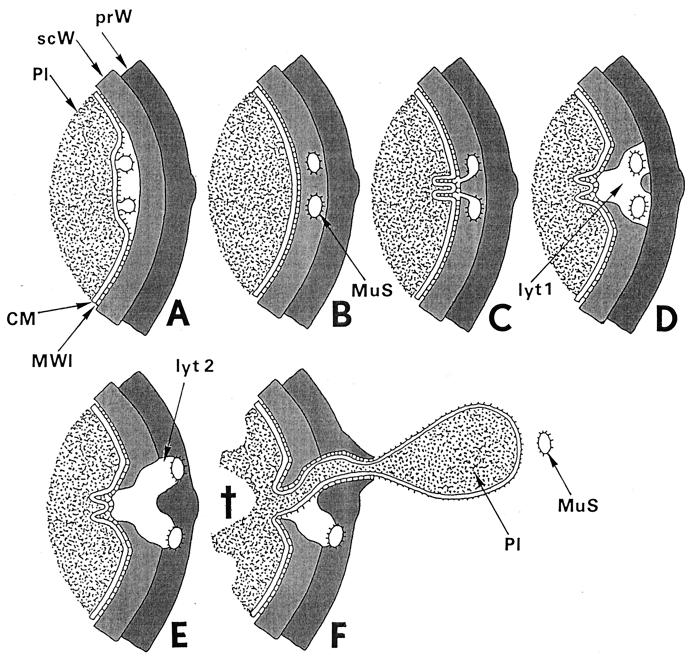
FIG. 22.
Fatal effects of “normal” lytic wall processes. Two consecutive lytic processes of wall morphogenesis in control cells, induced by the same murosomes in an interval of 10 min, are the key events for understanding penicillin-induced death: (i) initiation of cross wall formation and (ii) initiation of cell separation. In penicillin-treated staphylococci both processes take place during the same phase of wall morphogenesis, at the same site, and in the same way as in control cells. Only some penicillin-induced variations in the distribution of wall material proved to be fatal (for an overview, see Fig. 21). The schematic drawing seeks to provide a visual aid for a better understanding of these crucial processes. The depicted area is the site where both these lytic processes take place during the second cell cycle after the addition of penicillin. (A to D) Initiation of cross wall formation. (A) At the site of the second division plane, murosomes are formed by the cytoplasmic membrane (CM) or its membrane-wall interlayer (MWI) via an evagination process. Pl, cytoplasm; prW, primary wall; scW, secondary wall. Reference figures, Fig. 19b and 6f to h. (B) Immediately before cross wall formation starts, the murosomes (MuS) are found to be located in the lower layer of the peripheral cell wall, the so-called secondary wall. Probably, they have penetrated into this secondary wall or they are formed together with this wall layer. Reference figures, Fig. 19b and c and 6c (see also Fig. 8). (C) Lytic processes of the murosomes, directed to the center of the cell, separate the secondary wall into three parts. Folds of the cytoplasmic membrane indicate the first steps for cross wall formation. Reference figure, Fig. 6d and e. (D) The central part of the secondary cross wall starts the formation of the central, “transitory” layer of the future cross wall while the other parts initiate the “permanent” layers (see Fig. 7). However, while in control cells cross wall formation goes on until it is completed, in the presence of penicillin, cross wall formation at this site ceased because the necessary wall material is deposited at another site; furthermore, lytic processes (lyt 1) within the secondary wall proceed, leaving behind a disintegrated sector in the secondary wall. Reference figure, Fig. 6d and 19c to f. (E and F) Fatal initiation of cell separation. (E) In spite of the fact that in the presence of penicillin there is no cross wall material deposited in the second division plane, staphylococci start normal cell separation with murosome-mediated punching of pores into the primary layer of the peripheral wall via outward directed lytic processes (lyt 2). Reference figure, Fig. 20a. (F) As soon as one of the murosomes (MuS) has succeeded in perforating the outer layer of the peripheral wall and is released into the growth medium, the cell will burst and eject limited amounts of its cytoplasm (Pl), due to its extremely high internal turgor. This death (cross) happens only because a protecting cross wall is missing beneath the single wall perforation. Reference figure, Fig. 20 b to f.
The lytic capacity of the murosomes is not restricted to cross-wall morphogenesis but is also involved in two steps of lytic cell separation: in the punching of pores into the peripheral wall (Fig. 10a and b) and in the disintegration of transitory cross wall material (see “The lytic type of cell separation”).
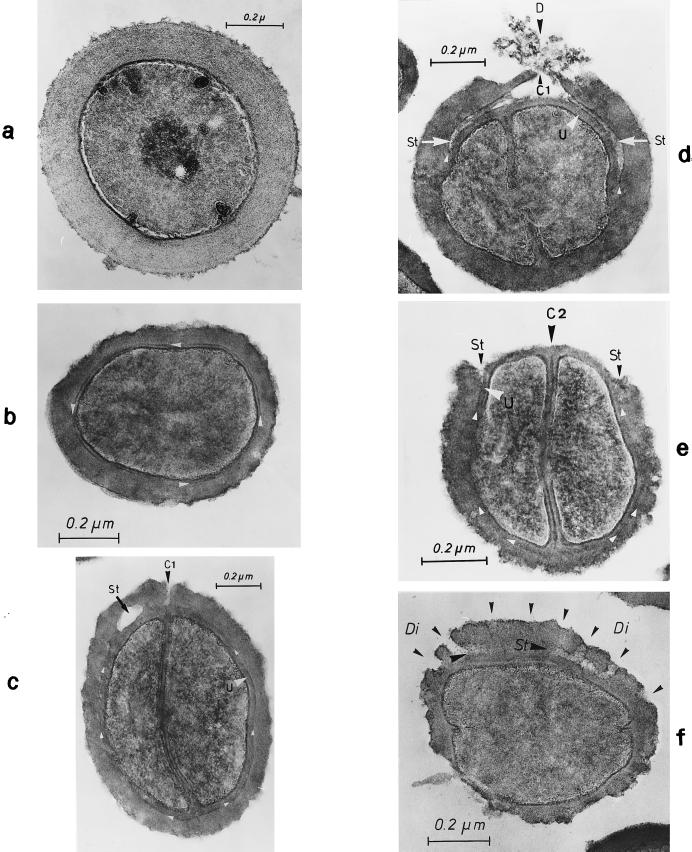
FIG. 10.
Staphylococcal cells after freeze fracturing (a to c) and thin sectioning (d to g). (a) Cell separation starts with a row of minute wall perforations (pores [arrows]) on the surface of the peripheral wall above the completed cross wall (reproduced with permission from reference 41). (b) After deactivation of autolytic wall hydrolases by chloramphenicol (20 μg/ml) and subsequent reactivation of these enzymes by treatment with lysozyme (10 μg/ml), two parallel rows of pores can be detected on the surface of the peripheral wall (arrowheads) (reproduced with permission from reference 50). (c) After deactivation and subsequent reactivation of autolytic wall enzymes a row of blebs (arrows) is located on the cell surface, indicating the release of murosomes into the medium (reproduced with permission from reference 50). (d) After chloramphenicol-mediated deactivation and lysozyme-induced reactivation of autolytic wall enzymes the release of murosomes (MuS) leaves behind pore-like cavities in the peripheral cell wall (stars) (reproduced with permission from reference 50). (e to g) The peripheral area of the completed cross wall is shown after deactivation and subsequent reactivation of autolytic wall enzymes. An attempt to reconstruct the subsequent steps of murosome-mediated lytic perforation of the peripheral cell wall during which the murosomes seem to disintegrate is shown. (e) A murosome (arrowhead), still consisting of an envelope and a core, appears to be rather well preserved (reproduced with permission from reference 54). (f) The murosome (arrowhead) shows the first signs of swelling and core disintegration (reproduced with permission from reference 54). (g) The murosome (arrowhead), having just perforated the peripheral wall, appears only as an undifferentiated vesicular structure (reproduced with permission from reference 54).
However, no reliable experimental data are so far available concerning the question of why murosomes perform certain vectorial lytic wall processes only at defined stages of the cell cycle while at others they are inactive (“resting”).
Speculations about other lytically active vesicles of the staphylococci, the so-called mesosomes, could be helpful in elucidating this problem. For mesosomes, which are also derived from the membrane-wall interlayer, it was assumed (39) that the autolytic wall enzymes of their vesicles (27) are regulated by the charge of their neighboring LTAs (28). Any transformation of their flat, “collapsed” vesicles to ball-shaped structures would, during bulging, result in an enlargement of the outer surface layer of the vesicle, which, in turn, would inevitably reduce the number of LTA molecules per square nanometer and hence enlarge the distance between the regulating LTA molecules and the regulated autolytic wall enzymes. The resulting reduction of surface charge density via the “blowing up” of flat vesicles could be sufficient to transform lytically inactive vesicles into lytically active ones, capable of attacking the staphylococcal cell wall. If such considerations are applied to murosomes, the observation of flat, collapsed murosomes (Fig. 5c) and ball-shaped vesicular ones (Fig. 5a) could likewise reflect the existence of different stages of murosome activation.
The close similarities in structure, function, and genesis between murosomes and mesosomes have led to speculations that mesosomes must be considered as being nothing but enlarged and multiplied murosomes induced by external factors like sucrose-mediated compression (39, 51).
Perturbations of cross wall formation.
After treatment with penicillin, which always affects the PBPs located at the outer surface of the cytoplasmic membrane and in the splitting system (103), the first detectable morphological effect was the cessation of the formation of the splitting system (47). This observation supported the conclusion that penicillin is only capable of inducing structural variations in growing parts of the staphylococcal cell wall, without affecting nongrowing cell wall regions or any part of the cell wall formed before the action of the drug (see “ ‘Hidden death’ at high penicillin concentrations”). Therefore, perturbation of cross wall formation by penicillin proved to be an effective tool for analyzing the structure and function of its different components. Especially after application of very low, nonfatal concentrations of penicillin the cross wall always revealed its composition of fibrillar components (Fig. 9d), which was never detected in untreated staphylococci (42). The penicillin-induced impairment of the transpeptidation reactions necessary to cross-link the peptidoglycan strands for building up a compact cross wall (for an overview, see reference 78) is assumed to have led to the exposure of these fibrillar cross wall components. Remarkably, these fibrillar components often were not randomly distributed but occurred in a seemingly arc-shaped arrangement (Fig. 9d). Similar arrangements of fibrillar components have been observed in several eukaryotic organisms, and they were all interpreted as being the result of a plywood-like superposition of layers containing more or less linearly arranged fibrils, which only optically gives the impression of arc-shaped structures (14, 82). Such plywood-like arrangement of stacked macromolecular layers has been postulated to also represent the macromolecular architecture of the staphylococcal cell wall (75, 76).
When penicillin and chloramphenicol acted simultaneously, the formation of extremely thick cross walls was induced in which, apparently, one layer after another of wall material was laid down, resulting in a pile of such wall material (Fig. 9e). Such observations indicated that layered arrangements of wall material are in fact feasible. However, the characteristic chloramphenicol-induced thickening of the peripheral wall (see Fig. 16a) was prevented by this combination of penicillin and chloramphenicol (73).
Prolonged treatment with penicillin resulted in rather gross defects of the nascent cross walls (85, 98). Sometimes, assembly of cross wall material took place at an extension of the cytoplasmic membrane seemingly without any contact with the preexisting wall material (Fig. 9f). These data suggest that the assembly of cross wall material will take place not only via apposition of new strands of peptidoglycan at preexistent wall material but may start independently at the surface of certain sites of the cytoplasmic membrane (38).
It is interesting that penicillin-induced distortions of the cross wall as well as the disappearance of the splitting system did not persist when staphylococci recovered after penicillin treatment in drug-free medium (38). Under these conditions the reappearance of the splitting system in the newly built wall material always started at the tips of the distorted cross walls (Fig. 9g), and cross walls of normal width were formed concomitantly.
These findings indicate that the splitting system should not be considered exclusively as a tool for cell separation (see “An alternative, mechanical type of cell separation using the splitting system of the cross wall”) as it may also have a function in maintaining the highly sophisticated compact architecture of the cross wall.
Cell Separation in Staphylococci
Cell separation of fully divided bacteria is an important step in the dissemination of daughter cells in an infection. The process of cell separation in staphylococci seems to be unique within the bacterial kingdom. Staphylococci developed a system of highly sophisticated mechanisms to guarantee successful cell separation even under adverse conditions. Depending on the growth conditions, they can use either of two different mechanisms to separate the daughter cells after completion of the cross wall, i.e., a lytic or a mechanical mechanism (54).
A schematic drawing of the components involved in these mechanisms of cell separation contributes to a better understanding of these processes which are essential for growth and multiplication (see Fig. 12).
FIG. 12.
Components of staphylococcal cross walls. These sketches (modified from reference 54) give preliminary information about the cross wall components involved in cell division and in the different types of cell separation of staphylococci. (A) Situation during initiation of cell separation. Illustrated is a divided staphylococcus with a newly completed cross wall before cell separation liberates the two daughter cells; however, in order to look inside the cross wall with its divers components, the right daughter cell is depicted separately, at some distance from its normal location. Cell separation is just being initiated by the centrifugally directed lytic activity of the murosomes (MuS) which punch two rows of pores (po) into the peripheral cell wall. In slowly growing staphylococci cell separation takes place along the concentrically arranged rings of the splitting system (Sp) which is synthesized during cross wall formation; in rapidly growing staphylococci cell separation takes place along the spoke-shaped canals (spo) which originate only after completion of the cross wall by the centripetally directed lytic activity of the murosomes. The cross wall material located between the two rows of pores including the splitting system is only destined for cell separation and will be disintegrated; this material can only be considered as being transitory parts of the staphylococcal cross wall. Reference figures, Fig. 4b and 11d. (B) Situation after the onset of cell separation. Illustrated are those parts of a completed cross wall which are located directly beneath the peripheral cell wall (pW). In a first lytic step the murosomes (MuS) have punched, via their centrifugal lytic activity, two circumferential rows of pores (po) into the peripheral wall (upward arrow, left side). These pores in the peripheral wall are then torn apart along the perforation line (right row). In a second lytic step the murosomes attack central parts of the cross wall via centripetally directed lytic actions (downward arrow, left side), resulting in the formation of spoke-shaped canals (spo). Between the presumptive cell walls of the future daughter cells (dW) and the peripheral wall of the mother cell (pW) is the location of the so-called stripping system (Str) which is involved in cell wall turnover. Reference figures, Fig. 10a and b and e to g and Fig. 11c to e.
Initiation of cell separation via murosomes.
Earlier observations (41) have shown that cell separation in staphylococci always starts with the punching of a row of 18 to 24 minute wall perforations (pores) into the peripheral cell wall (Fig. 10a) above the completed cross wall. This is, apparently, the result of the lytic activity of the murosomes of the cell wall (54). Since calculations have suggested that cell separation in rapidly growing staphylococci takes place within a few minutes (54), the punching of pores will last, probably, less than 1 min. This is why for a more detailed analysis of the mechanism of pore formation this rapidly passing initial step of cell separation had to be slowed down. This was accomplished by applying chloramphenicol, which is capable of reversibly deactivating autolytic wall processes without killing the cells (45, 50, 137). After a subsequent very slow reactivation of the wall autolysins by cationic proteins, the process of cell separation could be extended up to several hours, which made it easy to follow the sequence of pore formation.
Under such specific growth conditions the peripheral pores proved, at the very beginning of cell separation, not to be arranged in a single row but in two parallel ones (Fig. 10b). The single row (Fig. 10a) must be considered to be already the result of so-called pore fusion processes (54) in which two rather small neighboring pores are torn apart and form one larger pore. After formation of the peripheral pores these wall perforations were extended and eventually fused with each other, giving rise to rather large openings in the peripheral wall. The proceeding of such opening processes, which may be compared with the separation of two stamps along their perforation line (see Fig. 12B), is apparently the very beginning of cell separation (54).
A pair of circumferential sets of pores is found not only in staphylococci but also in some oscillatoriacean cyanobacteria (33, 56, 79).
During pore formation, regularly arranged rows of blebs on the cell surface were found to indicate a process of murosome protrusion into the growth medium after pore punching had taken place (Fig. 10c). Such release of murosomes after pore formation leaves behind characteristic spherical cavities in the peripheral cell wall, as seen in thin sections (Fig. 10d); since we are dealing here with two parallel rows of pores, the resulting cavities are not located directly above the cross walls but are laterally displaced.
Interestingly, the inhibition of autolytic wall processes by chloramphenicol and their subsequent reactivation resulted in the best preserved murosomes hitherto demonstrated by thin sectioning; often the murosomes, 30 to 40 nm in diameter, appeared to be covered with a sort of envelope and had a distinct core (Fig. 10e).
During perforation of the peripheral wall the murosomes often showed signs of swelling and disintegration of their inner core (Fig. 10f and g), indicating that we are dealing here with rather short-lived organelles. Probably, this progressive disintegration is the very reason why it has not yet been possible to isolate these minute wall organelles. This tendency of self-disintegration is, probably, also the reason why in thin sections of untreated staphylococci murosomes often appear only as empty holes within the peripheral cell wall (Fig. 2e and 5a, d and e). This effect was even more distinct in staphylococci growing under the influence of lytic concentrations of penicillin (48, 50) where murosomes frequently could be detected only as transparent lytic sites within the wall material (see Fig. 14d and e).
Wall autolysins, apparently associated with such pores, were recently found to form a circumferential double ring on the cell surface above the cross wall (146). These murosome-associated wall autolysins have since been identified as endo-β-N-acetylglucosaminidase and N-acetylmuramyl-l-alanine amidase (123).
The lytic type of cell separation.
During rapid growth staphylococci use the method of lytic cell separation in which they sacrifice specific central parts of their own cross wall (Fig. 11a and b). In this case the murosomes have been shown not only to perforate the peripheral cell wall via centrifugal lytic processes but also to attack some central parts of their cross wall proper via centripetally directed lytic actions; these different steps of lytic cell separation have been described in more detail recently (54). It has been concluded that the lytic processes always start from the murosomal sites and proceed radially to the center of the cell (54). Such bifunctional action of the murosomes during this type of cell separation revealed that, in staphylococci, there are both transitory and permanent parts of the cross wall. To quantify the amount of cross wall material lost during cell separation, the tension of the elastic cell wall had to be reduced. This was achieved by compensating the cell turgor via treatment of unfixed cells with 3 M sucrose. In this way it was shown that during lytic cell separation central cross wall parts, 30 to 40 nm thick, were lost (54), constituting about one third of the total cross wall material (Fig. 11a and b), and which also included the 7- to 10-nm thick concentrically arranged rings of the splitting system (Fig. 12A). These transitory cross wall parts must be considered as being auxiliary structures exclusively designated to be digested during cell separation (54). A schematic drawing (Fig. 12B) depicts the onset of lytic cell separation.
FIG. 11.
Freeze fracturing (a to d) and thin sectioning of staphylococcal cells (e) and B. subtilis (f). (a) After compensating the tension of the elastic cell wall by suspending unfixed cells in 3 M sucrose (a and b), a tripartite architecture of the cross wall was revealed (arrowheads). In the middle of the cross wall the transitory part is located (reproduced with permission from reference 54). (b) During lytic cell separation the central, transitory part of the cross wall is disintegrated (arrowhead) (reproduced with permission from reference 54). (c) After deactivation and subsequent reactivation of autolytic wall enzymes, spoke-like intruding canals or separation scars (stars) appear on the exposed cross wall surface after cell separation. (cW, cross wall, pW, peripheral wall, MuS, murosome) (reproduced with permission from reference 54). (d) A cell recovering from chloramphenicol treatment reveals spoke-like structures (arrows) on the just-exposed surface of the daughter cell during cell separation. (e) After deactivation and subsequent reactivation of autolytic wall enzymes, centripetally directed lytic wall processes (arrowheads) have left behind a lysis-resistant central part of the cross wall (reproduced with permission from reference 54). (f) After deactivation and subsequent reactivation of autolytic wall enzymes, central parts of the cross wall in a B. subtilis cell are disintegrated during cell separation.
The muralytic enzymes involved in this separation process are, apparently, the same enzymes that are capable of punching the pores into the peripheral cell wall for initiating cell separation, i.e., two amidases, the endo-β-N-acetylglucosaminidase and the N-acetylmuramyl-l-alanine amidase (122), which were previously shown to be capable of dispersing cluster-forming mutants of staphylococci (20) (see “Inhibition of cell separation results in the formation of pseudomulticellular staphylococci”).
Experimentally induced lytic cell separation aside from the splitting system.
For a more detailed analysis, the rapid process of lytic cell separation also had to be slowed down via reversal deactivation of wall autolysins by limited chloramphenicol treatment, followed by a slow reactivation of lytic wall processes by cationic proteins (“see Initiation of cell separation via murosomes”). The utilization of this technique is considered to be capable of demonstrating the original pattern of distribution of the bulk of staphylococcal wall autolysins (50). Furthermore, the chloramphenicol-induced thickening of the staphylococcal wall (40, 42) (see “Wall thickening via underlayering processes”) has proven to be of great advantage for ultrastructural analysis.
After application of this slowing-down technique, centripetally intruding spoke-like canals (Fig. 11c) were demonstrated on the surface of the daughter cells (54), which canals to a certain extent resemble the so-called separation scars on the cell surface of yeasts (133).
Apparently, the spoke-like canals of the staphylococci are the result of the reactivated lytic activity of wall autolysins starting from the site of the peripheral murosomes. Even more important was the observation that very differentiated spoke-like structures, resembling chrysanthemum flowers (Fig. 12A), were also found in some staphylococci recovering from chloramphenicol treatment (Fig. 11d). Below it will be seen that the formation of spoke-like canals in the cross wall could also be induced by treatment with penicillin (see Fig. 14e). Demonstration of these radially orientated canals in the cross wall under different experimental conditions is of considerable interest since it confirms the conclusion that in control cells the muralytic processes during lytic cell separation, as mentioned in the preceding section, also start from the murosomal sites and proceed radially into the center of the cell. Furthermore, these findings suggest that the canals cannot be considered as being artificial conformations created by the experimental conditions.
So far there has been no convincing explanation for the preservation of the spoke-shaped tracks under some experimental conditions. They have only been found in staphylococci treated with chloramphenicol or penicillin, and it is suggested that they are rapidly dissolved after the centripetally directed muralytic actions of the murosomes, probably by the activity of the so-called stripping system (see “Wall thickening via underlayering processes” and Fig. 12B). Since after treatment with chloramphenicol all autolytic processes are in some way retarded, one could also speculate that the final cleaning of the surface of the daughter cells, i.e., the complete disintegration of all parts of the cross wall which are to be dissolved, takes more time than in control cells.
At least the induced lytic processes in the cross wall, proceeding centripetally from the peripheral pairs of murosomal sites, at first hardly attack the central region of the cross wall which contains the splitting system; sometimes these processes spared even the entire central part of the cross wall (Fig. 11e).
It should be pointed out that the spared part of the cross wall holds about 30% of the entire cross wall material and also comprises the splitting system; it is, therefore, much thicker than the splitting system proper (only 7 to 10 nm in width) (103). Furthermore, this central part of the cross wall reaches up to the cell periphery, while the splitting system itself is known to be restricted to the slit-like container of the cross wall which extends only to the inner surface of the peripheral wall (Fig. 3). A schematic drawing shows the limited disintegration of transitory parts of the staphylococcal cross wall during this type of cell separation (Fig. 12B).
The fact that the procedure of reactivating autolytic wall enzymes was not capable of inducing lytic processes within the splitting system argues for the assumption that the splitting system contains hardly any autolytic wall enzymes and is normally not involved in this type of lytic cell separation. Hence, one could presume that induced lytic cell separation is mainly the result of the two vectorial lytic activities of the murosomes directed centrifugally and centripetally, supplemented by the autolysins of the stripping system.
A similar process of cell separation, in which certain parts of the cross wall are sacrificed, was also observed in cells of Bacillus subtilis (Fig. 11f), indicating that disintegration of substantial parts of the cross wall during cell separation also takes place in another gram-positive bacterium.
An alternative, mechanical type of cell separation using the splitting system of the cross wall.
During slow growth, for instance in the stationary phase of growth, cell separation again starts with the activation of the centrifugally directed lytic capacity of the murosomes, resulting in the punching of pores into the peripheral cell wall as described above (see “Initiation of cell separation via murosomes”). However, no centripetal lytic processes start out from the peripheral pores, since under these conditions staphylococci utilize mainly the splitting system for cell separation. This method is considered as an alternative, more or less nonlytic, technique for completing the fission process without sacrificing greater parts of the cross wall.
For most of the investigated staphylococcal strains, during slow growth the thin (7 to 10 nm) splitting system of the cross wall disappears in the course of cell separation. One normally observes that after this type of cell separation, running along the splitting system (Fig. 13a), not even traces of their concentric tubuli can be detected on the just-exposed surface of the nascent daughter cells (Fig. 13b) (3). Since no lytic activity could be demonstrated within the splitting system, the immediate disappearance of its tubuli at the onset of cell separation is not yet completely understood. One could speculate that in this case staphylococci utilize the preformed longitudinal slit in the cross wall which contains the tubuli of the splitting system (Fig. 3D) for cell separation and that the tiny tubuli are destroyed or lost during this event.
However, after blocking the muralytic activity with chloramphenicol, which leads to a drastically reduced wall turnover without killing the cells, and after subsequent regeneration of the cells during growth in any normal medium, remnants of the concentrically arranged tubular rings were preserved and nicely exposed on the surface of the daughter cells (42, 44) (Fig. 13c). During all stages of this fission process, when the exposed cross walls started bulging, more or less complementary images of the concentric rings were on the surface of the cross wall of both daughter cells detected (Fig. 13c). These findings have proved that in this case the process of cell separation actually takes place exactly within the middle of the splitting system, i.e., the concentrically arranged tubular rings only 7 to 10 nm wide must be considered to represent the very splitting plane of this type of cell separation. Furthermore, unlike in S. aureus, in S. epidermidis such concentric circular structures are already visible on the surface of control cells (4). The splitting system of a certain mutant strain of S. aureus, characterized by a low turnover rate, was also shown to persist during cell separation (Fig. 13d, strain SA 113) (54, 65). All these observations have shown that cell separation per se will not disintegrate the tubuli of the splitting system.
In discussing the question of why during slow growth the splitting system is sometimes disintegrated but is preserved in other cases, a look at the fate of preserved splitting systems should prove to be helpful. After the first cell separation the next division plane in the more or less ball-shaped daughter cells is initiated directly beneath the center of the concentric ring system (Fig. 13e), and the concentrically arranged rings are still preserved even at this late division stage; only much later were they disintegrated via turnover processes during the next generation time. Such turnover processes, being the result of a so-called stripping system, were capable of disintegrating even rather thick layers of wall material (see “Wall thickening via underlayering processes”). It is conceivable, therefore, that the wall enzymes of this stripping system that are normally competent for wall turnover could be responsible for the disintegration of the tubular structures of the splitting system during cell separation of slowly growing staphylococci. However, experimental data which might support such speculations are not yet available.
But in all cases in which the concentric rings of the splitting system were excellently preserved (Fig. 13c to e) there was no indication that any lytic enzymes were involved in this type of cell separation. However, one could assume that instead of lytic events some physical forces are involved in cell separation within the lipid-containing splitting system. Confirmatory support for such an assumption came from the observation that other lipid-containing layers can be separated rather easily with low mechanical energy. Studies of fracture faces of frozen membranes suggested that in most cases freeze-fracturing splits the membrane along some inner hydrophobic regions (15).
If such considerations are correct, any attempt to enlarge the volume of just-dividing cells via an increase of internal pressure should result in mechanical separation processes. Such increase of cell volume could be realized simply because the staphylococcal cell wall is known for its impressive elasticity (68, 78, 105) and its capability of swelling after exposure to distilled water (93, 94).
The result of such volume increase via uptake of distilled water was impressive. In many staphylococci premature cell separation took place and the concentrically arranged rings of the preserved splitting system could be seen on the exposed surfaces of their cross walls (54). In view of the very rapid cell separation achieved by increasing cell volume via water uptake it is highly probable that this type of mechanical cell separation is mainly caused by physical forces and almost without involvement of autolytic wall enzymes (54). The importance of these findings is discussed below (see “Penicillin-induced death without involvement of autolytic wall enzymes”).
Consequently, it can be concluded that in the case of a presumed deficit of autolytic wall enzymes the staphylococci seem to be capable of applying a two-step procedure of cell separation based on two entirely different principles. The centrifugal muralytic activity of the murosomes, which results in perforation and subsequent cutting-through of the peripheral wall, apparently represents the initial step. However, the second step, the centripetally directed step of cell division, concerns the large central part of the division plane and could well be performed more or less mechanically, i.e., without the involvement of a detectable quantity of autolytic wall processes. The physical forces for performing mechanical cell separation in this case would be the result of growth processes of the cytoplasm, which would increase the cell volume and therefore the tension of the elastic cell wall. The possibility that physical forces are also capable of performing the murosome-mediated initial step of cell separation, resulting in a cell partition without involvement of autolytic wall enzymes, is discussed below (see “Penicillin-induced death without involvement of autolytic wall enzymes”).
If such interpretation was correct, cell separation of this type might utilize the concentric rings of the splitting system as a built-in “emergency tool for mechanical fission”; this would occur in all cases in which the efficacy of autolytic wall enzymes involved in lytic cell separation was affected by internal or external factors.
It must be emphasized, however, that the actual reason why in slowly growing staphylococci no or hardly any murein hydrolases are employed for performing cell separation is still obscure. No experimental data are presently available concerning inactivation of these enzymes in staphylococci during the stationary phase of growth, although the number of bacteriolytic enzymes decreases at this growth phase (69).
Finally, it should be mentioned that during mechanical cell separation hardly any or only small amounts of the transitory wall material are disintegrated. Those large parts of the transitory wall material which are not attacked during mechanical cell separation are not preserved but after termination of cell separation are later turned over. Therefore, in comparison to lytic cell separation, mechanical cell separation must be considered to be insufficient in itself since it transposes the necessary disintegration of the transitory cross wall material, which apparently is only designated to be digested during cell separation, to later stages of the cell cycle.
Cell separation in the absence of the splitting system.
If the splitting system is indeed acting mainly as a mechanical emergency tool only, lytic cell separation should at least also take place in the absence of a splitting system. Such assumption can be examined easily since it is well known that at low concentrations of penicillin the splitting system is no longer synthesized. Under these conditions the newly formed cross walls no longer consist of compact structures but rather are fibrillar structures considered to represent less cross-linked wall material, which does not affect the viability of the cells (42). The concentric ring system of the cyanobacterium Phormidium uncinatum is likewise affected by penicillin (33). Meanwhile the very reason for this lack of the splitting system in S. aureus was found to lie in the inhibition of the key target for penicillin, the high-molecular-weight penicillin-binding protein PBP 1 (8, 112), which is considered to be located in the splitting system itself (103).
Also in the presence of penicillin, cell separation starts with the punching of murosome-induced pores into the peripheral cell wall (Fig. 13f), which pores are sometimes arranged in pairs (38). As already mentioned, most of the murosomes in penicillin-treated cells appear only as more or less empty vesicles (Fig. 6c) or even as lytic sites (Fig. 6d). Only in some cases (Fig. 13g) were murosomes preserved in the presence of penicillin as well as after treatment with chloramphenicol, which is capable of inhibiting their autolytic activities (Fig. 10e).
Thin sections have revealed (54) that, in spite of the lack of the splitting system, cell separation in the presence of nonfatal concentrations of penicillin takes place in a manner similar to that in untreated staphylococci, accompanied by a sacrifice of considerable parts of their fibrillar wall material from the central layer of their cross walls (Fig. 14a to c). Often, pairs of lytic sites are apparently involved in this degradation process (Fig. 14b). In these cells thin sections through the middle of the cross wall, which in control cells show the characteristic concentric ring system (Fig. 4b), reveal only the intensely stained fibrillar cross wall material (Fig. 14d and e) without even traces of the rings of the splitting system. This dark cross wall material formed in the presence of penicillin (Fig. 14d) is, at the periphery, perforated by about as many lytic sites as there are murosome-induced pores on the surface of untreated or chloramphenicol-treated staphylococci (Fig. 10a and b). Some of the lytic murosomal sites appear centripetally extended (Fig. 14e), resembling the spoke-shaped canals (separation scars) after reactivation of inhibited wall autolysins (Fig. 11c and d).
Apparently, this lytic cell separation without involvement of a splitting system is also the result of two differently directed lytic activities of the murosomes, starting with their centrifugal lytic vector of pore formation and followed by their centripetal lytic vector of the disintegration of some transitory cross wall material. These considerations prompted intense studies to find out what would happen if one of these lytic activities was inhibited or even blocked. Such inhibitory effect on the activity of wall autolysins is well documented for some polyanionic substances, especially for negatively charged anticoagulants of the heparinoid type and other negatively charged drugs such as liquoid or suramin (139, 140, 142), which resulted more or less in an inhibition of cell separation. If penicillin was applied simultaneously with liquoid, the polyanionic drug exclusively affected lytic wall processes at the cell periphery, and by this, cell separation was considerably inhibited; however, the centripetally directed lytic actions continued and were, for the first time, analyzed separately and during a considerable period of time. These isolated lytic events within the cross wall eventually resulted in the disintegration of the transitory parts of the cross wall, sparing only its peripheral parts (Fig. 14f).
The fact that, with penicillin, murosome-induced lytic cell separation can be performed even without the existence of the splitting system supports once again the conclusions drawn in the preceding sections, namely, that this staphylococcal tool is not necessary for normal, lytic cell separation and that the equipment most important for cell separation is the murosome system.
Finally, it should be mentioned that murosomes ejected during pore formation in the presence of penicillin may indicate the onset of the penicillin-induced killing process; the role of the murosomes in these fatal events is discussed below (see “Morphogenetic death during the second cell cycle in the presence of 0.1-μg/ml penicillin”).
Inhibition of Cell Separation Results in the Formation of Pseudomulticellular Staphylococci
Since during subsequent cell divisions staphylococci regularly alter their division planes at right angles to each other (Fig. 2b), any delay or inhibition of cell separation must result in the formation of seemingly multicellular bacterial units, forming symmetric cell tetrads (Fig. 15a) or cuboidal packets of eight cells (Fig. 2a). Although there is no indication that such interconnected cells represent more than the sum of several singular staphylococci, we have called such cellular systems “pseudomulticellular bacteria” (37, 42) in order to underline the possibility that differentiated cellular developments might be connected with these clusters.
FIG. 15.
Thin sections (a to c) and a scanning electron micrograph (d) of pseudomulticellular staphylococci. (a) Evans blue-mediated (200 μg/ml) suppression of wall autolysins resulted in the formation of symmetrically arranged pseudomulticellular staphylococci; the two division planes are marked by arrowheads. (b) After simultaneous treatment with penicillin (0.1 μg/ml) and magnesium chloride (0.5%) asymmetrically arranged pseudomulticellular staphylococci were formed due to the inhibition of autolytic wall enzymes. (c) After simultaneous treatment with penicillin (0.05 μg/ml) and trypsin (1%) asymmetrically arranged pseudomulticellular staphylococci were formed via inactivation of autolytic wall enzymes. (d) A femA mutation in S. aureus has induced the formation of pseudomulticellular staphylococci via alteration of the target structure (peptidoglycan) for wall autolysins.
Divers experimental conditions are known to be capable of inducing the formation of pseudomulticellular staphylococci, all of which conditions interfere directly or indirectly with the activity of wall autolysins involved in cell separation (see “The lytic type of cell separation”). These conditions include (i) application of antibiotics, (ii) application of polyelectrolytes, (iii) application of divalent cations, (iv) inactivation of wall autolysins, and (v) genetic manipulations. The data available so far on the induction of pseudomulticellular staphylococci is briefly discussed.
(i) The formation of antibiotic-induced pseudomulticellular staphylococci was first observed during growth of these bacteria in the presence of low concentrations of chloramphenicol (37, 42). The capacity of this antibiotic to inhibit cell separation is probably based on its ability to reversibly deactivate all autolytic wall enzymes, not only those involved in cell separation (50, 54, 137); no explanation is available to account for how chloramphenicol is capable of interfering with the action of autolytic wall enzymes. Furthermore, subinhibitory concentrations of penicillin (84) and other beta-lactam antibiotics (35) are also capable of inducing the formation of pseudomulticellular staphylococci.
(ii) Inhibition of cell separation and subsequent formation of pseudomulticellular staphylococci was especially observed during growth in the presence of negatively charged anticoagulants of the heparinoid type and other negatively charged drugs, such as liquoid, suramin, and Evans blue (Fig. 15a) (138–141). The common chemical characteristic of these inhibiting substances is the existence of sulfate or sulfonate groups in the molecule which are responsible for their negative charge. Another prerequisite for their capability of inhibiting cell separation seems to be their molecular size: while dextran sulfate with a high molecular weight was capable of inhibiting cell separation, the corresponding low-molecular-weight dextran sulfate did not (142). Concerning the mechanism of cell separation inhibition by anionic polyelectrolytes, different possibilities might be discussed. There are no indications of a direct interaction of these substances with autolytic wall enzymes or with their target, i.e., peptidoglycan. Rather, at present, an indirect action of anionic polyelectrolytes is assumed, such as an interaction with LTA as regulator of wall autolysins or a reaction with the peripheral wall teichoic acid, which would change the net charge of the cell surface and thereby inhibit the autolysin activity. Since similar polyanionic substances, such as chondroitin sulfate and dermatan sulfate, are widespread in the human body, the formation of pseudomulticellular staphylococci in vivo also has to be discussed.
In all these experiments with anionic polyelectrolytes it is important to strictly maintain the physiological pH. When staphylococci were cultivated on Trypticase soy agar at pH 5.5 or 7.8, they were still able to multiply, but not to separate; this effect was explained as a pH-dependent inhibition of wall autolysins responsible for cell separation (92).
(iii) Divalent cations, such as Mg2+ ions, proved to act as potent inhibitors of staphylococcal cell separation, since growth in the presence of 0.525 mM MgCl2 resulted already in the formation of pseudomulticellular bacteria (Fig. 15b) (64). Concerning the mode of action of divalent cations on cell separation, their binding to LTA and other anionic wall polymers seems to play an important role in the regulation of staphylococcal wall autolysins (28). It has been suggested that alanyl substitution in teichoic acid and LTA may interfere with the binding of Mg2+ ions which regulate the activity of wall autolysins (143). However, no experimental evidence for such speculations is yet available, so further studies are desirable.
(iv) Since autolytic wall enzymes are involved in the lytic separation of divided daughter cells (see “Cell separation in staphylococci”), the degradation of these enzymes should result in delayed or suppressed cell separation. In fact, treatment of bacteria with agents known to inactivate such wall enzymes (sodium dodecyl sulfate, Triton X-100) induced the formation of pseudomulticellular staphylococci (71). However, staphylococci growing in the presence of trypsin or pancreatin did not show any reaction to these proteases. But if these staphylococci were pretreated with low, nonfatal concentrations of penicillin, these proteases induced the formation of typical pseudomulticellular staphylococci (Fig. 15c). It was discussed, therefore, that proteolytic enzymes are only capable of attacking autolytic wall enzymes after a preceding “loosening” of the staphylococcal cell wall by penicillin (42). Since proteolytic enzymes are widespread in the human body, the formation of pseudomulticellular staphylococci could also be induced in vivo during penicillin treatment.
(v) After chemical mutagenesis, cell packets or large clumps of pseudomulticellular staphylococci have also been observed (19, 63, 71). The formation of such bacterial systems seems to result from a lack of polymeric teichoic acid, which would suppress the activity of wall autolysins involved in cell separation. Other examples of genetically induced formations of pseudomulticellular staphylococci are based on the stepwise inactivation of the femAB operon, which is involved in the formation of the pentaglycine interpeptide bridges of the staphylococcal peptidoglycan (9, 58). The induction of cells without FemB which were only capable of synthesizing the rather short triglycine cross bridges resulted in retarded cell separation (58) which, in turn, led to the formation of rather regularly arranged pseudomulticellular staphylococci, as was the case with liquoid. The inactivation of femA, induced by chemical mutagenesis, likewise yielded the formation of pseudomulticellular staphylococci (70); in this case, however, there arose rather irregularly arranged cell systems (Fig. 15d), since often one daughter cell had lost the capability of forming a new cross wall. Finally, complete inactivation of the femAB operon by allele replacement resulted in a mutant which contained only monoglycine bridges and in which cell separation was intensely inhibited (121). These remarkably shortened glycine interpeptide bridges are obviously the reason for the inhibited separation of the divided daughter cells. It has been discussed that, in this case, steric hindrance could suppress the activity of autolytic wall enzymes which are responsible for cell separation. Recently a mutation in the scdA gene of S. aureus, which gene obviously plays an important role in cell division, was described that led to an aberrant cellular morphology which was comparable to those of femA-femB mutants (7).
All these data for agents that interfere with cell separation are of great importance for therapeutic efforts with beta-lactam antibiotics, since any inhibition of cell separation has been shown to also result in a suppression of penicillin-induced killing and bacteriolysis (142, 143). On the other hand, any acceleration of cell separation seems to correspond with an enhancement of penicillin-induced death and bacteriolysis. Furthermore, autolysin-defective staphylococci capable of forming pseudomulticellular clusters are unable to disseminate daughter cells for spreading an infection; they are, therefore, considered to have reduced virulence (91), a fact that indicates the role of these clusters and their autolysins in pathogenesis.
On the other hand, the question remains why pseudomulticellular staphylococci after blockage of their lytic cell separation system do not utilize their capability of separating via their alternative tool for cell separation, the splitting system, which mainly uses the mechanical forces of cell turgor for this task (see “An alternative, mechanical type of cell separation using the splitting system of the cross wall”). Since it is well-known that pseudomulticellular staphylococci normally contain, as judged from electron micrographs (Fig. 15a), a seemingly intact splitting system, one reason for the missing cell separation might be a defect of the splitting system. But at least for cells of the femA mutant strain 145, containing an increased amount of monoglycine bridges (121), the function of the splitting system was not impaired, since the pseudomulticellular staphylococci could be easily separated by a swelling procedure via suspending these cells in distilled water, thus imitating a rising internal turgor (see “An alternative, mechanical type of cell separation using the splitting system of the cross wall”). Consequently, one explanation for why mechanical forces along the splitting system for cell separation are not used could be the presumption that not only are the autolytic wall enzymes involved in cell separation affected in pseudomulticellular staphylococci but so too is the internal turgor which is a necessary prerequisite for mechanical cell separation. However, no experimental evidence for such speculation has been available so far.
It should be emphasized that this type of mechanical cell separation via artificial volume increase has led to the assumption that in pseudomulticellular staphylococci mechanical cell separation can also occur nearly without involvement of autolytic wall enzymes (see “Penicillin-induced death without involvement of autolytic wall enzymes”). Furthermore, lytic cell separation was also achieved artificially in pseudomulticellular staphylococci (i.e., without involvement of endogenous autolytic wall enzymes) simply by adding isolated wall enzymes (20, 122). The importance of the turgor for cell separation (67) and of inducing cell separation via external factors in pseudomulticellular staphylococci is discussed in some detail below.
Finally, some presently known methods of restoration and reactivation of cell separation in pseudomulticellular staphylococci have to be mentioned. In many cases cell separation in pseudomulticellular staphylococci can be restored simply by transferring the cells into a new growth medium without the inducing agent (i.e., without chloramphenicol or anionic polyelectrolytes). This fact indicates that the inhibition of cell separation is a reversible process. An even more direct reactivation of chloramphenicol-inactivated wall autolysins was possible by the treatment of such cells with cationic proteins, such as lysozyme which acts mainly as an activator of autolytic wall enzymes rather than as an enzyme itself (137, 145). This method of reactivation has previously been used to slow down the rapidly passing steps of cell separation (see “Experimentally induced lytic cell separation aside from the splitting system”).
Wall Thickening via Underlayering Processes
Wall regeneration after chloramphenicol treatment.
Besides linear growth of wall material, as is characteristic of the neoformation of cross walls (see “Neoformation of the cross wall”), staphylococci are also capable of considerably increasing the thickness of their walls (40). This takes place via successive underlayering of the peripheral wall with newly synthesized wall material (inside-to-outside growth mechanism) as is the case in other gram-positive bacteria (5, 106–108). In contrast to linear wall growth this method of wall growth is referred to as apposition growth (42).
For many years it has been well known that the thickness of the staphylococcal cell wall may vary considerably, mostly depending on the strain and especially on the growth conditions. Rapidly multiplying staphylococci often exhibit cell walls about 30 to 40 nm thick, while in many slowly growing cells a characteristic thickening of the cell wall has been observed from the stationary phase of growth (22, 124), resulting in a wall thickness of up to 60 nm. Since wall synthesis is independent of protein synthesis (57, 88, 89), further wall thickening can be induced by inhibiting protein synthesis by means of chloramphenicol, puromycin, or actinomycin, leading sometimes to a wall thickness of more than 100 nm (Fig. 16a). Such cells are still alive and can easily regenerate if transferred to a medium free of protein synthesis inhibitors. After prolonged treatment with chloramphenicol the underlayering of more and more wall material beneath the peripheral wall may even result in the compression of the bacterial protoplast and in its subsequent disintegration, leaving behind only extremely thick, empty cell walls (40).
Consequently, for survival of the staphylococci it is necessary to get rid of these huge amounts of wall material, which can only take place after cessation of chloramphenicol treatment, i.e., after transfer into normal growth medium. For this purpose, the staphylococci have developed a characteristic sequence of wall-disintegrating steps. Surprisingly, to reconstitute normal wall thickness they not only remove the superfluous parts of the peripheral wall but also reestablish a complete new peripheral wall beneath the wall material formed under the influence of chloramphenicol. This fact suggests that the wall material formed in the presence of this antibiotic is superfluous and in some way even obstructive.
Wall regeneration is initiated by placing a so-called stripping layer, which, apparently, is formed by the cytoplasmic membrane or its membrane-wall interlayer, beneath the thickened cell wall. This stripping layer is considered to be involved in centrifugally directed disintegration processes of the peripheral wall material. Under this stripping layer, a new peripheral wall is laid down (Fig. 16b and c); furthermore, a new cross wall is formed (Fig. 16b). Removal of the old, thick superfluous wall material always starts asymmetrically by the formation of a single cutting rim above the initiation site of the new cross wall which cuts through, at this site, all the peripheral wall material located outside the stripping layer (Fig. 16c). This cutting process, which is not associated with the initiation of cell separation, seems to take place without any involvement of murosomes. A sequential stripping of the superfluous wall material along the stripping system (Fig. 16c and d) then begins which is followed by disintegration of stripped wall material (Fig. 16d and e). It is well documented (42, 44) that in the course of detaching superfluous wall material more or less periodically arranged holes are observed on the surface of the stripping system (Fig. 16f), which indicate the involvement of lytic events in the disintegration of superfluous wall material (disintegration system) (45). It is far from being clear, however, which wall hydrolases might be involved in these wall disintegration processes.
If such liberation of rather large fragments of the superfluous wall material (Fig. 16d) was to take place in an infected tissue, these fragments could probably act as antigens capable of catching numerous antibodies. In this case, wall restoration after chloramphenicol treatment could function as an effective method of defense against the attacking capacity of human antibodies (13).
In any event, normal wall thickness is restored, after cessation of chloramphenicol treatment, by a regenerative process comparable to that of snakes when they peel off their skin.
After detachment of the old, excessive wall material the staphylococci always start normal growth via cell separations, resulting in cells with normal wall thickness. In this way, the regeneration cycle of chloramphenicol-treated bacteria uses the entire lytic arsenal which is available to these bacteria for cutting and separating tasks within the staphylococcal cell wall. The consecutive application of all these tools is illustrated in a schematic drawing (Fig. 17).
FIG. 17.
Wall regeneration sequences after chloramphenicol treatment. Growth-inhibiting bacteriostatic antibiotics, such as chloramphenicol, do not interfere with the synthesis of wall material. Consequently, under such bacteriostatic conditions the slowly growing staphylococci are capable of forming huge masses of wall material, resulting in extremely thick cell walls. After being transferred to normal growth medium, a comprehensive sequence of cutting and separation processes in the thick wall material is initiated to restore the normal width of the cell wall. This simplified schematic drawing gives a survey of these processes. (A to B) Wall thickening. By chloramphenicol-mediated, successive underlayerings of the peripheral cell wall with newly synthesized wall material, the staphylococcal cell wall becomes considerably wider. Reference figure, Fig. 16a. (C) Formation of a stripping layer. After transfer into normal growth medium, staphylococci initiate wall regeneration by placing a so-called stripping layer (Str) beneath the thick peripheral wall. Reference figure, Fig. 16b. (D) Synthesis of a new peripheral wall. Under the stripping layer a completely new peripheral cell wall (pW) is synthesized via inside-to-outside processes of wall assembly. Reference figure, Fig. 16c. (E) Cutting through of the old peripheral wall. Removal of the old and apparently obstructive peripheral wall is initiated by an asymmetrical cutting through of this wall via forming a so-called cutting rim (ri) above the cross wall (cW) which is now synthesized. Reference figure, Fig. 16c. (F) Onset of sequential wall-stripping processes. By activation of autolytic wall hydrolases within the stripping layer (Str), the old superfluous peripheral wall is sequentially peeled off from the underlayered new peripheral wall. Reference figure, Fig. 16d and e. (G) Disintegration of stripped cell walls. The detached old cell wall is disintegrated either already during the stripping of the old peripheral cell wall or after this process. The disintegrating processes start from periodically arranged holes (disintegration system [Di]) located between the stripping layer and the old peripheral wall and result in the liberation of rather large pieces of the old, peripheral cell wall. Reference figure, Fig. 16f. MuS, murosomes. (H and I) Normal growth after detachment of the old cell wall. As soon as the old peripheral wall formed under chloramphenicol is removed, normal growth takes place, starting in staphylococci with completed cross walls, by murosome-mediated punching of pores into the new peripheral wall and subsequent cell separation. Reference figures, Fig. 10a and 13a. Sp, splitting system.
Wall regeneration after penicillin treatment.
The mechanism of penicillin-induced death is discussed below (see “Morphogenetic death during the second cell cycle in the presence of 0.1-μg/ml penicillin” and “ ‘Hidden death’ at high penicillin concentrations”). At present it is not well understood why a considerable number of staphylococci always survive treatment with penicillin, especially after the application of high concentrations of the drug (see “ ‘Hidden death’ at high penicillin concentrations”). Among the staphylococci that may survive in the presence of penicillin are those cells which after removal of the drug are capable of restoring penicillin-induced cellular damages, thereby surviving as “persisters” (47).
In those staphylococci that had survived treatment with 10-μg/ml penicillin, complicated steps of wall restoration were observed after transfer into fresh, drug-free medium. A first indication of survival was the fact that some of the seemingly dead cells started to produce wall material which resulted in the formation of extremely thick but rather “loose” peripheral cell walls, the thickness being reminiscent of walls produced under chloramphenicol treatment (see “Wall regeneration after chloramphenicol treatment”). Such formation of extremely thick wall material without protoplasmic growth processes is characteristic of many bacteriostatic antibiotics but is hitherto unknown for beta-lactams. Furthermore, as was the case in staphylococci regenerating after chloramphenicol treatment (Fig. 16b to f), a so-called stripping layer was placed beneath the old peripheral wall, which in thin sections appeared as a rather thin, dark layer; an entire new, but rather thin peripheral wall was placed underneath this stripping layer. After this initial step the newly formed peripheral wall became thicker and thicker, while the lytic capacity of the stripping layer started to attack all wall material that had been formed before or during the penicillin action so that the primary peripheral wall became thinner and thinner. This lytic attack eventually resulted in a nearly complete disintegration of preexistent wall material.
Restoration processes via neoformation of the entire cell wall system presume that the staphylococci have to get rid of wall material formed in the presence of antibiotics either independently, if bacteriostatic drugs such as chloramphenicol have induced extremely thick cell walls, or if potential bactericidal beta-lactams have induced the formation of loose, fibrillar wall material. Probably, treatment with antibiotics results in more wall defects than known so far, leaving behind walls which cannot be repaired but have to be exchanged by completely new wall structures.
The similarity of wall regeneration after treatment with chloramphenicol or penicillin deserves considerable interest, since these restoration processes indicate a hardly known mechanism of reactivating an infection after therapy with antibiotics. Resistance to penicillin in particular might not in all cases be based solely on penicillin-insensitive bacteria or on the activity of beta-lactamases in those cells capable of inactivating this antibiotic but might also be based on restoration processes. However, the extent to which restoration processes contribute to the survival of pathogenic germs after treatment with antibiotics is still not known.
Summation of Structural Parts and Functional Features of Staphylococcal Cell Walls
The data on staphylococcal wall morphogenesis presented in the preceding pages enable us for the first time to sketch a schematic drawing which includes the essential parts of the staphylococcal cell wall architecture (Fig. 18). This drawing also includes the highly sophisticated arsenal of lytic tools which these bacteria use for all types of cutting, separating, or manipulating actions on their cell wall for the sake of morphogenetic differentiation and for survival after antibiotic attack. These data reveal the fascinating beauty of one of the most differentiated members of the eubacterial section of the prokaryotic kingdom; detailed knowledge of these structural components is an essential prerequisite for understanding the complex events which lead to penicillin-induced morphogenetic death. Furthermore, such schematic drawing should, hopefully, enable us to recognize new targets for improved therapeutic efforts which are so badly needed.
PENICILLIN-INDUCED DEATH
Since the discovery of penicillin by Alexander Fleming in 1929 (30), and especially since the first clinical application of penicillin in 1941 (for reviews, see references 1, 2, and 66), scientists have tried to explain the mechanism by which bacteria die in the presence of this drug. No one could imagine that it would take more than 50 years to solve this problem. Considering the enormous amount of data on penicillin-treated bacteria which have been accumulated in all these years, this failure is, at first glance, hardly understandable.
It has been generally accepted for many years that penicillin affects only the peptidoglycan of the bacterial cell wall; since peptidoglycan has only been detected in prokaryotic organisms, penicillin will not attack any eukaryotic cell. The first molecular targets of penicillin are some special bacterial proteins located on the outer surface of the cytoplasmic membrane (or within the splitting system); they are called PBPs (12, 120, 134). The interaction of beta-lactams with the PBPs is known to result in an inhibition of peptidoglycan cross-linking (for a review, see reference 74). In staphylococci cross-linking was reduced by penicillin from 85 to about 60% (119). It should be emphasized that the penicillin reaction at the PBPs takes place outside the bacterial protoplast.
Four PBPs have been identified in staphylococci (32, 72, 126). The degree of cross-linking in staphylococcal cell walls after treatment with low concentrations of penicillin is reduced (119); this result corresponds with the electron microscopic observation that instead of compact, newly formed cross walls, an organized network of wall fibrils is detectable, revealing to a certain extent some details of the macromolecular architecture of this wall. Such a loosening of the staphylococcal cell wall, however, does not result in a mechanically unstable, fragile cell wall which can no longer withstand the internal turgor, as has been postulated (12, 127, 128); growth and cell separation are going on more or less as in untreated staphylococci (Fig. 14a to c). Furthermore, the wall fibrils, formed in the presence of penicillin, have proved to consist of a tough, interconnected network capable of withstanding the internal turgor (see Fig. 25).
FIG. 25.
Hidden death at high penicillin concentrations. Schematic drawing illustrating the fate of staphylococcal control cells (A1 to A4) and of cells in the presence of high penicillin concentrations (B1 to B3 and C1 to C5) during the first and the second cell cycles. (A1 to A3) Control cells during the first cell cycle. (A1) Untreated cells divide by completion of their cross wall; (A2) they initiate cell separation via murosome-induced punching of pores into the peripheral wall above the completed cross wall; (A3) two daughter cells have been generated by this first cell separation. MuS, Murosome. (B1 to B3) Hidden death during the first cell cycle. (B1) In the presence of high concentrations of penicillin the formation of the splitting system stops and cell wall synthesis is largely inhibited. Instead of compact, highly organized cross walls, only some loose fibrillar cross wall material is synthesized which is mainly deposited laterally on the ingrowing cross walls, leaving the tips of the cross wall unprotected. Reference figure, Fig. 24a. (B2). Like in untreated cells (A2), cell separation is then initiated in spite of the fact that cross wall formation has not yet been completed. After liberation of the murosomes (MuS), cell separation proceeds along the splitting system synthesized before the action of the drug. Because of the high internal pressure and since the tips of the cross wall are not sufficiently protected by fibrillar cross wall material, the affected cells erupt granular cytoplasm (Pl) through slit-like openings in the peripheral wall. Reference figure, Fig. 24d. (B3) The incomplete cross wall of the first division plane is ripped up along the splitting system, the cell loses considerable parts of its cytoplasm and dies, leaving behind only empty cell walls which still preserve the shape of the primary staphylococcus. Reference figure, Fig. 24c. (C1 to C5) Nonhidden death during the second cell cycle. (C1) For still unknown reasons, a certain percentage of staphylococci treated with high doses of penicillin is still capable of synthesizing considerable amounts of fibrillar cross wall material after blocking the formation of the splitting system. In those cases not only the lateral parts but also the tips of the ingrowing cross walls of the first cell cycle are covered with these fibrils. Reference figure, Fig. 24e. (C2) Since the cross wall fibrils at the tips are capable of welding with each other, initiation of cell separation via murosome-mediated perforations of the peripheral wall (MuS) and ripping up of the cross wall along the primary splitting system will not result in a loss of cytoplasm and in death, and this in spite of the fact that their cross walls are not completed either: a connecting bridge (Cb) formed by the welding of fibrillar cross wall material proves to be tough enough to resist the high internal pressure. Reference figure, Fig. 24e. (C3) Consequently, those cells capable of welding together the individual primary cross walls of the prospective daughter cells can survive at least the first division cycle in the form of more or less elongated cells. Reference figure, Fig. 24f. (C4) Like in normal cells (A4), cell separation during the second cell cycle may be initiated via murosome-mediated formation of peripheral pores in the second division plane (MuS). Since hardly any cross wall material is deposited at this site, the extremely high internal pressure widens one of these pores, and part of the cytoplasmic membrane (CM) together with cytoplasmic constituents (Pl) is thrown out via an explosion-like ejection. Reference figure, Fig. 20d. (C5) Rupture of the thrown-out part of the cytoplasmic membrane (CM) results in the release of much of the granular cytoplasm (Pl), and the cell dies. Reference figure, Fig. 20f. (Modified from reference 53.)
A second effect of penicillin treatment is the disappearance of the splitting system of the cross wall (Fig. 14a to c) in which PBPs are thought to be located (103); this disappearance is the result of the effects of penicillin on two of the four PBPs, including PBP 1 (8, 112). But staphylococci can grow and perform cell separations without this splitting system (52, 54). Furthermore, at least in the presence of relatively high concentrations of penicillin (10 μg/ml) the absence of the splitting system can sometimes even protect staphylococci from the special case of “hidden” penicillin-induced death during the first cell cycle (see “ ‘Hidden death’ at high penicillin concentrations”).
Besides speculations about penicillin-induced fragile cell walls, many other hypotheses have been put forward during the last 50 years to elucidate the mechanism by which penicillin is capable of killing bacteria. Biochemical data on the inhibition of peptidoglycan synthesis by certain concentrations of penicillin and on an assumed uncontrolled activation of autolytic wall hydrolases have mainly served as the basis for several hypotheses about the very reason of penicillin-induced death and about bacteriolysis. For instance, it was postulated that a balanced system of wall synthetases and wall hydrolases might be essential for wall growth; if a penicillin-induced inhibition of wall synthetases would occur, the ongoing action of wall hydrolases would inevitably destroy penicillin-treated bacteria (144). The same fatal effect would take place when cell wall hydrolases were directly activated by penicillin (31) or when penicillin was capable of inducing the liberation of inhibitors of wall hydrolases (“triggering” model) (129–131).
However, such presumptions of a central role of the uncontrolled action of wall hydrolases in penicillin-induced death are not compatible with findings that in certain bacteria with suppressed activity of wall hydrolases penicillin only caused growth inhibition without bacteriolysis (117, 129). Furthermore, more recent studies on peptidoglycan synthesis and on autolytic wall degradation in the presence of penicillin have revealed that, surprisingly, neither an inhibition of the incorporation of labeled wall material nor an enhancement of autolytic wall degradation were prerequisites for penicillin-induced killing and lysis in staphylococci (77, 111, 119). Finally, penicillin even proved to be capable of inhibiting wall enzymes involved in cell separation (84) and in autolytic wall degradation (47, 51, 111).
Consequently, none of the two generally postulated processes, namely, either uncontrolled, overall activation of autolytic wall processes or the overall inhibition of wall synthesis, can be considered causative for penicillin-induced death. Therefore, all these hypotheses have had to be reevaluated.
Penicillin Kills Only Actively Growing Bacteria
A somewhat deeper understanding of the biological role which this antibiotic plays in the mortal combat between penicillin-producing fungi and their surrounding bacteria would be helpful for analyzing the cause of penicillin-induced death of staphylococci; such death reflects the endless battle between man with his most important antibiotic weapon and bacteria.
As already mentioned, the specific target of penicillin is the peptidoglycan of the bacterial cell wall. However, penicillin is only capable of killing susceptible bacteria which grow and divide, while resting cells are hardly endangered (53, 60). Furthermore, in a growing bacterium only those parts of the wall which are just enlarging and not all regions of the entire cell wall are likely to be affected by penicillin. Therefore, penicillin-induced wall variations take place only at two sites of the wall: (i) in the small longitudinal growth area of the peripheral wall of rod-shaped bacteria, and (ii) on the nascent cross walls as the main centers of linear wall growth of all bacteria (33, 80, 81, 98, 116); the other “resting” parts of the cell wall and the protoplast itself are scarcely affected.
Consequently, if bacteria do not grow, penicillin can hardly attack them, and if bacteria have lost their cell wall, they can grow and replicate in the presence of penicillin (L forms), which then is no longer toxic. It is not surprising, therefore, that penicillin does not impair (i) bacterial protoplasts (staphylococci after removal of their cell wall by lysostaphin; see reference 55 for methods), (ii) bacteria which do not contain a cell wall (genus Mycoplasma), and (iii) microorganisms which live without a peptidoglycan-containing cell wall (Archaea).
Therefore, penicillin can be considered neither simply as a fungi-derived poisonous agent active against bacteria nor as a drug with selective toxicity to the overall bacterial cell wall. The effect of the application of such a simple drug would result in continuously increasing, random wall damages, while studies on synchronously growing staphylococci in the presence of penicillin have revealed that penicillin-induced death is dependent on the moment of drug addition during the cell cycle (86). These studies have further shown that the deadly incident takes place only during the onset of the second cell separation after addition of the drug. Independent of the stage of the division cycle at which penicillin was added, the staphylococci were still capable of surviving only one generation time after addition of this antibiotic; at the end of the next cell cycle, killing took place during intended cell separation, followed by lysis.
Consequently, for analyzing the fatal effect of penicillin on staphylococci research must focus mainly on the first three cell cycles after addition of the drug, i.e., on a total period of only 90 min.
To get a reliable impression of the events which take place during this rather short period, all available data gathered by common analytical techniques have been combined with our findings obtained by electron microscopic methods. For a quick overview and summary, a scheme representing the course of events associated with penicillin-induced death and bacteriolysis in the presence of low doses of penicillin is shown in Fig. 21.
Nonfatal Morphogenetic Wall Variations during the First Cell Cycle in the Presence of 0.1-μg/ml Penicillin
For a better understanding of the structural variations that occur during the first cell cycle, cell division of untreated staphylococci, as has been described above in some detail (see “Cell division in staphylococci”), must be considered once more. Untreated staphylococci having completed cell division via formation of a cross wall (Fig. 2c to e) start cell separation by the lytic activity of murosomes which punch a row of circumferentially arranged minute pores into the peripheral cell wall (Fig. 10a). After this initial step, the subsequent tearing apart of these pores separates the two daughter cells (Fig. 12B and see Fig. 21, A1 and A2).
When a lethal concentration of penicillin (0.1 μg/ml) is added during the first 10 min of the first cell cycle (generation time of staphylococci, 30 min), the drug has only to diffuse through the 30-nm thick peripheral wall to reach the target molecules, the PBPs located on the outer surface of the cytoplasmic membrane and within the splitting system (103). It is assumed, therefore, that the blockage of the PBPs occurs almost instantaneously after addition of the drug, resulting in an immediate inhibition of the capacity of later forming a splitting system. But this loss of the splitting system per se did not affect the viability of the cells (52) (Fig. 14a to c).
Due to the effect of penicillin on the PBPs, the degree of cross-linking of newly synthesized peptidoglycan is normally lowered (119). Consequently, staphylococci are no longer able to form normal, compact cross walls but mainly synthesize a network of fine fibrils. This fibrillar material is always arranged in rather thick, deformed, and often incomplete cross walls (cf. Fig. 19a and 21, B2).
In spite of the synthesis of such deformed cross walls, the penicillin-treated cells tried to start, at the same time as in control cells, normal cell separation via punching of two rows of pores into the peripheral wall (Fig. 13f). Instead of cell separation, however, in most cases only rather large, murosome-induced cavities resulted in the peripheral parts of the cross walls of this first division plane (see Fig. 21, B2). In spite of the high turgor inside the cell, such wall cavities could not endanger the cells, since sufficient wall material had always formed beneath these wall cavities. If, in rare cases, the formation of wall cavities in fact resulted in a local cutting through of the peripheral wall, the underlayered wall material very effectively ensured that this event would not result in a fatal loss of cytoplasm; only a very limited tearing apart of the nascent daughter cells was observed, which increased the size of the cell (Fig. 19a) but never resulted in cell separation.
The incapability of performing cell separation at the end of the first cell cycle after addition of penicillin was also indicated by the observation that no further increase in cell numbers was detected 20 min after addition of penicillin (77).
Morphogenetic Death during the Second Cell Cycle in the Presence of 0.1-μg/ml Penicillin
While in control cells during the second cell cycle the formation of cross walls in the second division plane was initiated at an angle of 90° with respect to the previous one (see Fig. 21, A3), the penicillin-treated staphylococci continued to assemble newly synthesized wall material at the already thickened cross wall in the first division plane instead of depositing it at the site of the presumed cross wall in the second division plane (see Fig. 21, B3). This mistake eventually resulted in the formation of thicker and thicker cross walls in the first division plane, by far exceeding those of control cells (cf. Fig. 19e and 2e). In the second division plane, however, the necessary deposition of sufficient material for cross wall formation did not take place (Fig. 19b and c). This morphogenetic detouring of cross wall material needed for the second division plane towards the first one has to be considered to be the crucial event and the essential prerequisite which enables the occurrence of penicillin-induced death.
It has been speculated that such failure in the correct distribution of newly formed wall material might be due to the activity of “drifting” hydrolytic wall enzymes capable of cutting numerous free ends into the fibrillar cross wall material, which in turn could serve as additional acceptors of newly formed strings of cross wall material (50). Furthermore, one could discuss the possible involvement of one of the PBPs in the wrong distribution of newly synthesized wall material.
The sequence of events which, eventually, lead to penicillin-induced death was nicely followed in thin sections and by freeze etching. Before going into details it should be mentioned that this death proved to be the result of two consecutive murosome-induced lytic effects on the peripheral cell wall, which surprisingly took place during the same phase of wall morphogenesis, at the same site, and in the same way as in control cells. Only minimal penicillin-induced morphogenetic variations in the distribution of wall material proved to be the reason for the killing effect.
Since in the presence of penicillin the murosomes of the second division plane were deposited at their correct place (cf. Fig. 19 b to f and Fig. 21, B3), the first lytic effect on the protecting peripheral wall started at the same time as the initiation of the formation of the second cross wall in control cells; the murosomes, by nonfatal localized wall lysis, attacked only the inner layer of the peripheral wall, the so-called secondary wall, by their wall hydrolases. In the course of this attack the murosomes were first evident in thin sections at the site of the second division plane beneath the cell wall (Fig. 19b). Possibly, they penetrated into the peripheral wall via their lytic enzymes (Fig. 19c) and attacked the peripheral wall from inside until a small region of the inner layer of this wall was disintegrated (Fig. 19d). When two adjacent murosomes attacked this inner layer of the peripheral wall (Fig. 19e), a larger region of this layer was disintegrated (Fig. 19f). We presume that this first, nonfatal step of attacking the peripheral wall corresponds to the initiation of the second cross wall formation in control cells during which the murosomes always induced a corresponding, centripetally directed cutting of some inner layers of the peripheral wall (Fig. 5d and e) before cross wall formation started. Apparently, at this stage the penicillin-treated staphylococci are still alive.
During their lytic action murosomes may show different sizes (cf. the murosome shown in Fig. 19c with those shown in Fig. 19e); possibly, they may swell, an effect which is known to also take place during experimentally induced cell separation (Fig. 10e to g).
Only the second lytic step in the murosome-induced attack of the peripheral wall is considered to result in death. Apparently at the same time that the second cell separation is initiated in control cells (Fig. 10e to g and 21, A4), the penicillin-treated staphylococci at this site initiate their normal second cell division via murosome-mediated, centrifugally directed punching of pores into the outer layer of the peripheral cell wall, the so-called primary wall (Fig. 20a). They start this wall perforation in spite of the fact that hardly any cross wall material has been deposited in the second division plane. Consequently, the murosome is ejected, together with a limited amount of cytoplasm through this unprotected wall perforation (Fig. 20b). The same type of ejection was observed during murosome-mediated killing in the presence of high concentrations of penicillin (Fig. 20c).
FIG. 20.
Thin sectioning (a, b, e), freeze fracturing (c) and scanning electron micrographs (d, f) of staphylococcal cells grown in the presence of 0.1 (a, b, e) or 10 (c, d, f) μg of penicillin/ml. Some cultures were grown in medium supplemented with 3% NaCl and were subsequently transferred to medium with low osmolarity (d and f). (a) The murosome (MuS) has, finally, perforated the outer layers of the peripheral cell wall, the so-called primary wall, at the initiation site of the second division plane. 1, first division plane (reproduced with permission from reference 50). (b) After perforation of the outer, primary wall at the site of the second division plane (2), the murosome (MuS) is ejected together with some plasmatic material (arrowhead). 1, first division plane (reproduced with permission from reference 48). (c) Even in the presence of high concentrations of penicillin the ejection of the murosome can be detected at the initiation site of the second division plane. This ejection starts from a local bulge of the peripheral wall (vo). 1, first division plane. (d) At high concentrations of penicillin the explosion-like liberation of the cytoplasmic membrane (short arrow) has caused a considerable enlargement of the small, murosome-induced wall perforation (E) at the second division plane. 1, first division plane (reproduced with permission from reference 53). (e) A similar explosion-like liberation of the cytoplasmic membrane occurs at low penicillin doses (reproduced with permission from reference 48). (f) At high concentrations of penicillin (10 μg/ml) murosome-induced explosions (stars) can take place simultaneously in the second division planes of both daughter cells, liberating at the same time two cytoplasmic membranes (CM). 1, first division plane.
This perforation of the peripheral wall in the absence of a completed cross wall or of sufficient protecting wall material beneath the murosome-induced pores must result in burst and in the death of such cells (Fig. 21, B4) due to their high internal osmotic pressure of about 25 atm (93, 94). This burst of the staphylococci and the subsequent liberation of bacterial cytoplasm through a perforation of the protective cell wall has been compared with another pressure-stabilized system: the formation of an aneurysm in humans (48, 50, 53).
In some cases, the extremely high internal osmotic pressure may even result in an explosion-like liberation of the murosome and of some cytoplasm together with parts of the cytoplasmic membrane; the number of such explosions could be enhanced if the staphylococci were growing in a medium containing penicillin and 3% NaCl and were subsequently transferred into medium with low osmolarity (Fig. 20d and f). However, parts of the cytoplasmic membrane can be thrown out irrespective of whether the cells are killed by low (Fig. 20e) or high concentrations of penicillin (Fig. 20d); this explosion may also enlarge the fatal peripheral wall opening (Fig. 20d). Sometimes, explosions were even observed in both daughter cells (Fig. 20f).
This sudden penicillin-induced death at the end of the second cell cycle could also be monitored by common analytical tools such as (i) the sudden breakdown of calorimetrically measurable heat production of plasmatic growth processes, (ii) the drastic decline in the number of CFUs, and (iii) the considerable loss of labeled plasmatic components (77). Recently, these observations were also supported by time-lapse photography: it was possible to show very clearly, for the first time in living bacteria, that staphylococci, in the presence of penicillin, “burst away at the second cell division” (101).
Under the applied experimental conditions, the time of death was even predictable for staphylococci: it took place 50 min after application of the drug (Fig. 21, B4), as shown by measurements of the heat production of the culture (77) combined with the determination of CFUs (number of viable cells).
Finally, the question arose whether more than one murosome capable of killing the staphylococcal cell was located in the second division plane. In fact, the number of murosomes involved in penicillin-induced killing could be increased considerably by varying the osmolarity of the growth medium (50), which resulted in the ejection of a fan-shaped row of murosomes at the second division plane (see Fig. 23a). This observation has indicated that the individual murosomes in a murosomal row might have reached different degrees of activation, thus being capable of perforating the peripheral cell wall at slightly different times.
FIG. 23.
Scanning electron micrograph (a) and thin sections (b to d) of staphylococcal cells grown in the presence of penicillin. (a) By varying the osmolarity of the growth medium, several murosomes of the second division plane are ejected simultaneously (arrows); arrowheads mark supposed ejections of murosomes. 1, first division plane (reproduced with permission from reference 50). (b) After a 4-h treatment with 0.1-μg/ml penicillin most cells undergo bacteriolysis and show different degrees of cellular disintegration. (c) Simultaneous treatment with penicillin (0.1 μg/ml) and lysozyme (1 mg/ml) prevents bacteriolysis; the protoplast even remains stabilized in spite of multiple breakages in the peripheral cell wall (arrows) (reproduced with permission from reference 51). (d) After 4 h of simultaneous treatment with 0.1-μg/ml penicillin and 100-μg/ml Evans blue, the staphylococci seem to be intact, although about 99% are already dead.
For a better understanding of these very complex and highly sophisticated events during the second cell cycle in the presence of penicillin, a more detailed schematic drawing is depicted in Fig. 22; it shows only the area where the two consecutive lytic events on the peripheral cell wall take place, both induced by the same murosomes with a time lag of a few minutes: the centripetally directed lytic attack during initiation of the second cross wall formation and the subsequent centrifugally directed lytic attack during intended cell separation. This schematic drawing shows the nonfatal steps during initiation and intended formation of the second cross wall at the beginning and in the middle of the second cell cycle (cf. Fig. 22, A to D, and Fig. 21, B3), taking place about 30 to 40 min after penicillin addition, and the fatal steps during intended cell separation at the end of the second cell cycle (cf. Fig. 22, E and F, with Fig. 21, B4) about 50 min after application of the drug (see also the legend for Fig. 22).
Even when briefly summarizing these highly complex data it becomes evident that, in the presence of a lethal concentration of penicillin (0.1 μg/ml), neither a shortage of wall material nor the activation of autolytic wall hydrolases are causative for penicillin-mediated death of staphylococci. Even both lytic processes of the murosomes on the peripheral cell wall take place like in control cells. The killing process depending on the cell cycle is rather based on a characteristic mistake in the local distribution of newly synthesized wall material which is detoured to an incorrect place in the presence of the drug, i.e., we are dealing here with a penicillin-impaired regulation of local murein synthesis. Because of this mistake in murein distribution the cells are no longer capable of depositing sufficient cross wall material beneath murosome-induced perforations of the peripheral wall, which normally protects the cell against extremely high internal turgor. This unprotected wall perforation by one single “killing murosome” takes place in the second division plane when, in control cells, cell separation is initiated. The deadly incident itself is the result of a throwing out of some cytoplasm through the murosome-mediated hole in the peripheral wall and takes place already at the end of the second cell cycle. Consequently the very reason for penicillin-induced death does not lie in a mistake in the biosynthesis of sufficient staphylococcal wall material but rather in a seemingly minute morphogenetic mistake in its distribution. Therefore, we have named this penicillin-mediated fatal effect of staphylococci, “morphogenetic death.” It is evident that this death can be considered as an induced suicide via interfering with normal growth processes. One cannot but admire the fungi for the ingenious invention of such a sophisticated and effective biological weapon for their daily combat against bacteria.
Bacteriolysis during the Third Cell Cycle in the Presence of 0.1-μg/ml Penicillin
While in control cells, during the third cell cycle, the formation of cross walls in the third division plane was initiated (Fig. 21, A5 and A6), the dead cells killed in the presence of penicillin at the end of the second cell cycle (Fig. 21, B4) shrank due to the loss of cytoplasm and, hence of their internal turgor (Fig. 21, B5). Surprisingly, however, they preserved their outward shape and their seemingly intact cellular integrity for about 30 min, i.e., throughout the entire third cell cycle, before disintegration of the cell wall and subsequent decomposition of the cytoplasm (bacteriolysis) took place. This long-lasting preservation of cellular integrity of dead cells is hardly understood; one can only speculate that wall hydrolases, which are known to attack bacterial cell walls much more rapidly as long as the walls are stressed by the internal turgor (67), can work only very slowly after penicillin-induced killing (Fig. 20d and e) and subsequent cell shrinkage, i.e., when the cell walls have lost their tension.
Bacteriolysis took place only at the end of the third cell cycle (Fig. 21, B6). The onset of bacteriolysis was monitored as a sudden decline in cell numbers about 70 to 90 min after penicillin treatment, accompanied by an increasing release of cytoplasm and a continuously decreasing optical density, till the nutrient broth had been nearly cleared up (77, 140).
In thin sections, bacteriolysis of staphylococci appeared as a rather spectacular event: the disintegration of cellular integrity, presumed to be the result of an uncontrolled activity of endogenous autolytic enzymes, left behind only large pieces of ruptured cell walls, membrane fragments, and plasmatic debris (Fig. 23b).
Such autolytic events, leading to bacteriolysis, could easily be inhibited or even prevented (i) by agents which induce the suppression of autolytic wall degradation and (ii) by agents which are capable of stabilizing the bacterial protoplast.
In the first case, applying combinations of penicillin and anionic polyelectrolytes, bacteriolysis was prevented because the wall appeared in some way to be protected against the autolytic wall system (64, 138, 139, 141); consequently, in electron micrographs, only seemingly intact bacteria could be observed after such simultaneous action of penicillin and an anionic polyelectrolyte (Fig. 23d) in spite of the fact that more than 99% of these cells were already dead (CFU test). This observation has demonstrated that it was hardly possible to differentiate, in thin sections, dead staphylococci from still living ones. This was because staphylococci, after penicillin-induced death, differed from living cells only by rather inconspicuous morphological variations, such as one single, tiny pore-like opening in the peripheral wall at the site of the second cross wall initiation (Fig. 20b) and the loss of some cytoplasm. But any penicillin-induced fatal loss of cytoplasm under conditions which block bacteriolysis is known to result in shrinkage of the staphylococci (Fig. 21, B4 and B5), due to the high elasticity of their cell walls (64, 78, 105). By this shrinkage the remaining part of the cytoplasm in the dead cells is compressed so that the initial, fatal loss of some cytoplasm cannot be detected. That is the reason why, in thin sections, the dead cells always appear rather small but seemingly intact (53). Penicillin-induced fatal loss of cytoplasm and subsequent shrinkage can only be prevented by establishing an external osmotic pressure equaling the pressure inside the staphylococci (53).
In the second case, applying combinations of penicillin and high concentrations of lysozyme, bacteriolysis was prevented because the bacterial protoplast was “stabilized” (46, 51); consequently, in electron micrographs rather intact bacteria were found in spite of the fact that multiple fractionations of the peripheral cell wall had taken place (Fig. 23c).
On the other hand, penicillin-induced bacteriolysis could also be enhanced, for instance by cationic proteins (137), by d-alanine (142), and by lantibiotics (10, 11).
Unfortunately, the very reason why bacteriolysis takes place after bacterial death is still unknown. Only speculations have been put forward about a presumed uncontrolled action of endogenous autolytic enzymes, considered to be the result of an inhibition of peptidoglycan synthesis (for a review, see reference 118). More recently, however, inverse results have been found in staphylococci: application of low concentrations of penicillin which did not interfere with peptidoglycan synthesis (111) resulted in optimal bacteriolysis, while under the influence of higher penicillin concentrations, which progressively reduced the incorporation of wall material, bacteriolysis was inhibited almost completely (119, 136) (see also “On the identification of penicillin-induced bacteriolysis as a postmortem event”). Consequently, an inhibition of peptidoglycan synthesis cannot be causative for bacteriolysis, but it rather prevents bacteriolysis. However, the cell shrinkage which takes place after penicillin-induced killing (Fig. 20a to f and 21, B4 and B5) may contribute to overcoming our present lack of knowledge in this field. When cell shrinkage was effected simply by sucrose-induced osmotic compression of living staphylococci, these bacteria lysed. Such bacteriolysis without any involvement of an antibiotic has been shown to be the result of a perturbation of the cytoplasmic membrane and the membrane-wall interlayer which then generate lytically active mesosomal vesicles capable of disintegrating the staphylococcal cell wall (39, 51, 59).
On the Identification of Penicillin-Induced Bacteriolysis as a Postmortem Event
Since the first application of penicillin, this antibiotic has been well known for its capacity of inducing bacteriolysis which is always easily detected by monitoring the optical density of a bacterial culture growing in nutrient broth, which broth clears up rapidly upon addition of penicillin. Consequently, the most widely accepted idea concerning the typical effect of penicillin was that this antibiotic is killing bacteria via bacteriolysis (lytic death). However, the data presented in the preceding sections have shown that penicillin-induced killing and bacteriolysis are two completely different, consecutive events, proving that bacteriolysis does not cause the penicillin-induced killing process but that it is simply its consequence. Therefore, bacteriolysis must be considered to be nothing but a postmortem process (50–52), and for the future, misleading terms like lytic death should be avoided in connection with penicillin treatment of staphylococci, since such an event does not exist. Consequently, it is concluded that penicillin induces an always nonlytic killing event which is caused, normally, by a rather limited liberation of some cytoplasm; this penicillin-induced death may be followed by bacteriolysis of the dead bacteria (Fig. 21, B4 to B6). Such conclusion is in line with observations that staphylococci treated with penicillin concentrations above 1 μg/ml are killed without bacteriolysis resulting subsequently (77, 119, 136). In the mutant strain SA 113 (see “An alternative, mechanical type of cell separation using the splitting system of the cross wall”), exhibiting a very low wall turnover (54), even low doses of penicillin (0.1 μg/ml) proved to be sufficient to cause growth inhibition and death without subsequent bacteriolysis, resembling certain bacteria with suppressed activity of wall hydrolases (117, 129).
In this regard it should be emphasized that those autolytic wall enzymes which are considered to be responsible for bacteriolysis of staphylococci killed in the presence of penicillin are, apparently, not capable of killing living, intact staphylococci: when these enzymes were externally added to pseudomulticellular staphylococci, only lytic cell separation took place (20, 122). We presume that externally added autolytic wall enzymes of this type would only be capable of killing those staphylococci which had not been able to complete their cross walls before the onset of cell separation (Fig. 21).
After all, the identification of bacteriolysis as a postmortem process is not at all surprising; apparently, bacteria pass only through the same postmortal processes as do other living beings, including humans. They all undergo lysis after having died and never does lysis reveal the manner of their death (53).
“Hidden Death” at High Penicillin Concentrations
For several years it was generally accepted that penicillin is capable of killing staphylococci by one single, unique mechanism (see “Morphogenetic death during the second cell cycle in the presence of 0.1-μg/ml penicillin”); in staphylococci, this killing was induced by 0.1-μg/ml penicillin and occurred about 50 min after addition of the drug, during the second generation time. However, earlier findings on the so-called “paradoxical effect of penicillin” on staphylococci (26, 136) had indicated that, in the presence of high concentrations of penicillin, “bacteriostatic” effects of this antibiotic were also observed and bacteriolysis was missing. These data have indicated that at least certain effects of penicillin on susceptible bacteria were not independent of the drug concentration, and possibly, penicillin-induced killing was even more complicated than expected. Since under therapeutic conditions penicillin concentrations of up to 50-μg/ml have been determined in tissues (see reference 36 for a review), the effect of high concentrations of penicillin on staphylococci had to be examined in some detail.
In fact, hidden behind seemingly bacteriostatic effects a second, very early killing mechanism has been observed at high concentrations of penicillin. Since common analytical tools like monitoring CFUs or optical density failed to detect this early killing effect we have named it hidden death (53). This fatal event takes place as early as 10 to 15 min after drug exposure, i.e., already during the first generation time. This early killing effect differs considerably from the murosome-induced punching of pores into the peripheral wall at sites not protected by sufficient cross wall material.
Electron microscopic studies have shown that such an early killing process in the presence of 10-μg/ml penicillin did not affect cells which had already completed their cross walls. Only those staphylococci were endangered which, at the very moment when the drug was added, were at a rather early stage of the cell cycle in which they had formed only a still incomplete cross wall including the splitting system (Fig. 24a to c). Such high penicillin concentration immediately blocked the formation of the splitting system and the further synthesis of an intact cross wall; only small amounts of fibrillar wall material were then synthesized, which were deposited mainly laterally on the nascent cross walls (Fig. 24a). At the same time that cell separation started in control cells (Fig. 10a), the same murosome-induced perforation of the peripheral wall took place in the presence of high concentrations of the drug. After that, normal cell separation started via ripping up of the cross wall along the splitting system (Fig. 24b), similar to the process of mechanical cell separation of control cells (see “An alternative, mechanical type of cell separation using the splitting system of the cross wall”). Since, however, cross wall formation had prematurely ceased, this ripping up of incomplete cross walls eventually resulted in an opening of the pressure-stabilized cell wall and in the eruption of considerable parts of the cytoplasmic constituents, leading to death (Fig. 24c). Further details of this killing process were detected by scanning electron microscopy. Dividing cells lost cytoplasm (Fig. 24d, upper cells) apparently through slit-like openings in the peripheral wall of the first division plane (53) and subsequently showed some shrinkage, while staphylococci with already completed cross walls were not affected (Fig. 24d, lower cells). Via determination of the number of empty cells (Fig. 24c) we calculated that about 20% of the staphylococci were killed by this hidden death during the first division cycle in the presence of 10-μg/ml penicillin; however, other staphylococci showed only different stages of shrinkage without detectable effects on their cell walls. It cannot be ruled out that such cells stayed alive; one could speculate that such staphylococci are suitable candidates for restoration processes (see “Wall regeneration after penicillin treatment”).
FIG. 24.
Thin sections (a to c and e to f) and a scanning electron micrograph (d) of staphylococci treated with high doses of penicillin (10 μg/ml). (a) Small amounts of fibrillar wall material are deposited mainly laterally on the nascent cross wall (reproduced with permission from reference 53). (b) In spite of the fact that the cross wall is not yet complete, cell separation has started in the first division plane (1) via ripping up of the cross wall along its splitting system (reproduced with permission from reference 53). (c) Opening of the cell wall via ripping up of the incomplete cross wall along the splitting system has resulted in the eruption of considerable parts of the cytoplasm (Pl) and in cell death (reproduced with permission from reference 53). (d) The upper two dividing staphylococci reveal a certain loss of cytoplasm (Pl) along slit-like openings in the peripheral wall along the first division plane while staphylococci having already completed their cross wall (lower cells) are not affected by the drug. (1, first division plane) (reproduced with permission from reference 53). (e) Deposition of sufficient amounts of newly formed wall fibrils at the tips of the ingrowing cross wall has prevented complete ripping up of the cross wall in spite of the initiation of cell separation (arrows and arrowhead), thus protecting the cells from lysis. (f) The incomplete cross wall of this cell is almost completely ripped up, but the cross wall tips are welded together (asterisks), thus preventing fatal consequences; the paired arrows indicate the region of the peripheral wall which is suggested to be derived from the tearing apart of the cross wall; the elongated cell already shows an initiation site for the formation of the next cross wall (arrow) (reproduced with permission from reference 53).
Furthermore, there were other cells which were not killed via hidden death in spite of the fact that they had already formed an incomplete cross wall at the moment of adding high concentrations of this drug. Thin sections have revealed the very reason for this surprising observation: for still unknown reasons these surviving cells had not only laterally covered the nascent cross walls with some fibrillar wall material but they had formed sufficient wall fibrils which were also deposited at the tips of the ingrowing cross wall (Fig. 24e), as was the case in the presence of 0.1-μg/ml penicillin (Fig. 19a). This covering of the cross wall tips with fibrillar wall material prevented a complete ripping up of the unfinished cross walls, since these fibrils were, apparently, welded together. The limited ripping up of the cross wall eventually resulted in an elongation of the cell (Fig. 24f), as was already observed to a certain extent for staphylococci in the presence of low concentrations of penicillin (Fig. 19a). Such welding processes after the formation of sufficient fibrillar wall material, which produced a connecting bridge between the two presumptive daughter cells, must be considered the very reason why hidden death was never observed in the presence of 0.1-μg/ml penicillin. It is important to note that such tough connecting bridges of welded fibrillar wall material between two presumptive individual daughter cells are capable of protecting these staphylococci from penicillin-induced death for at least one additional generation time.
The resulting enlargement of staphylococci partly ripped up at incomplete cross walls led to considerably elongated staphylococci, some of them already showing initiations of the second cross wall, but without sufficient cross-wall material being deposited at this site (Fig. 24f). At these sites, as already mentioned (see “Morphogenetic death during the second cell cycle in the presence of 0.1-μg/ml penicillin”), the same characteristic, murosome-induced fatal events (cell explosions) (Fig. 20c, d, and f) took place as was the case in the presence of low penicillin concentrations (Fig. 20a, b, and e; cf. Fig. 21 and 22). Apparently, the two fatal events in the presence of penicillin are closely connected: early hidden death is followed by the common murosome-induced death. A schematic drawing demonstrates the connection between these two lethal processes (Fig. 25).
Finally, for therapeutic considerations it should be emphasized that hidden death by which staphylococci lose cytoplasm via slit-like openings in incomplete cross walls is only possible if such incomplete cross walls with their intact splitting systems had been formed before penicillin became active. If the splitting system is missing, hidden death is impossible. This fact indicates the danger of any pretreatment of staphylococci with low doses of penicillin; this would prevent the formation of a splitting system and, by this, also prevent hidden death. The apparent advantage of applying high doses of penicillin, the very fast killing of a considerable percentage of staphylococci already in the first division cycle, would be lost, and these cells would thus be preserved for at least one additional cell cycle.
Autolytic Wall Enzymes: Are They Indispensable for Penicillin-Induced Death of Staphylococci?
In the preceding sections, autolytic wall enzymes have been considered to be of some importance to the penicillin-induced death of staphylococci because autolytic wall enzymes are involved in lytic cell separations in control cells as well as in cells with the addition of penicillin (see “Cell separation in staphylococci”). However, the occurrence of mechanical processes during the ripping up of incomplete cross walls at high concentrations of penicillin in the first division plane (hidden death) (cf. Fig. 24b and 25, B1 to B3) indicates that the role of autolytic wall enzymes in penicillin-induced death needs to be discussed in greater detail. On principle, one should also raise the question whether penicillin is even capable of killing staphylococci without any involvement of autolytic wall enzymes. When discussing these questions, we have to consider once again the essentials which lead to the different types of killing, depending on the concentration of penicillin.
Penicillin-induced death in the presence of autolytic wall enzymes.
It has been shown that low doses of penicillin (0.1 μg/ml) cannot prevent the synthesis of the cross wall in the first division plane, although cross wall formation is affected to a considerable extent (synthesis of deformed cross walls without a splitting system; see “Cell separation in the absence of the splitting system”). Normally, at low concentrations of penicillin, death occurs only during the second cell cycle after addition of the drug, since after such a long period of time in the presence of penicillin a complete cross wall can no longer be formed in the second division plane due to a local penicillin-impaired distribution of newly synthesized murein. This penicillin-induced death takes place in such cells because the high internal pressure of the protoplast abruptly kills the cell during the next attempted cell separation (see “Morphogenetic death during the second cell cycle in the presence of 0.1-μg/ml penicillin”). No indications were found that penicillin-induced death resulted from attacking staphylococcal cell walls by murein hydrolases, triggered by this drug (see, however, references 129 and 131).
Since bacteriolysis of the killed cells starts about 30 min later (see “Bacteriolysis during the third cell cycle in the presence of 0.1-μg/ml penicillin”), we presume that low concentrations of penicillin are not capable of inhibiting either autolytic wall enzymes involved in cell separation or those involved in bacteriolysis.
Penicillin-induced death in spite of inhibition of autolytic wall enzymes.
High concentrations of penicillin (10 μg/ml) have been shown to already inhibit or even prevent the completion of cross walls in the first division plane (see “ ‘Hidden death’ at high penicillin concentrations”). Penicillin-induced death can, therefore, occur early in this first division plane in those staphylococci that had already started cross wall formation before the drug was added. Concerning the mechanism of this death it was presumed that after murosome-mediated initiation of cell separation in the first division plane the killing would occur via ripping up of incomplete cross walls along their splitting system (see “ ‘Hidden death’ at high penicillin concentrations” and Fig. 25). It should be emphasized that this ripping up of the incomplete cross walls has already been considered to be a mechanical event (54) for which autolytic wall enzymes are not essential (see “An alternative, mechanical type of cell separation using the splitting system of the cross wall”).
Since, under such conditions, no bacteriolysis of the killed cells takes place (77, 119, 136) we have presumed that high doses of penicillin are capable of inhibiting autolytic wall enzymes involved in bacteriolysis. But one might speculate that high concentrations of this drug would also inhibit autolytic wall enzymes involved in cell separation.
Penicillin-induced death without involvement of autolytic wall enzymes.
We tend to presume that in the presence of high concentrations of penicillin only remnants of the primary activity of autolytic wall enzymes would be available for the initiation of cell separation in the first division plane via murosomes, which finally will result in morphogenetic death via mechanical cell separation (Fig. 25).
Furthermore, in control cells it has been shown that autolytic wall enzymes are not always essential prerequisites for cell separation: very rapid cell separation was achieved by artificially increasing cell volumes via suspending staphylococci in distilled water (54). Such mechanical cell separation, apparently without involvement of autolytic wall enzymes, was induced artificially even in pseudomulticellular staphylococci (see “Inhibition of cell separation results in the formation of pseudomulticellular staphylococci”). Such mechanical cell separation of staphylococci due to water-induced swelling has led to the assumption that mechanical cell separation of staphylococci during normal growth (54) also basically abides by the same rules, with the volume increase being established by the growth of cytoplasm.
Such considerations should apply not only to the separation of control cells but also to cell separation in the presence of 10-μg/ml penicillin. If, under such experimental conditions, the internal turgor is sufficiently increased via cytoplasm growth, cell separation will start even without involvement of a detectable quantity of autolytic wall enzymes. If the onset of such mechanical cell separation occurs in staphylococci with penicillin-mediated incomplete cross walls (Fig. 24a and b), the type of hidden death will be the same as if autolytic wall enzymes are involved in the initiation of cell separation (Fig. 25). Consequently, one might argue that, basically, autolytic wall enzymes are more or less dispensable for inducing the killing of staphylococci by high concentrations of penicillin as long as growth of cytoplasm continues.
Even penicillin-induced death in the presence of 0.1-μg/ml penicillin could occur almost without an involvement of autolytic wall enzymes, if mechanical cell separation via growth-mediated increase of turgor was initiated in the second division plane of staphylococci lacking an intact cross wall (see Fig. 22).
We have to emphasize, therefore, that it is not the activity of autolytic wall enzymes (129, 131) that is essential for penicillin-induced death of staphylococci; this killing effect is rather due to defined morphogenetic defects of their cross walls, induced by this antibiotic. Either the inability of completing the cross wall of the first division plane (in the presence of 10-μg/ml penicillin) or the inability of synthesizing the cross wall of the second division plane (in the presence of 0.1-μg/ml penicillin) will inevitably result in penicillin-induced death at the very moment when mechanical cell separation is initiated and for this type of death autolytic wall enzymes are not essential.
These considerations indicate that in penicillin-induced death the role of autolytic wall enzymes has been overestimated so far, while the importance of a raising turgor due to continual growth of cytoplasm has been neglected. But discussions about the importance of autolytic wall enzymes can, possibly, help to explain the penicillin-induced death without subsequent bacteriolysis of some mutants that use mainly mechanical cell separation, considered to occur without involvement of autolytic wall enzymes (low-wall-turnover strain SA 113 of S. aureus) (cf. reference 54 and Fig. 13d). Since, in staphylococci, penicillin-mediated morphogenetic defects of the nascent cross walls are the very reason for penicillin-induced death, it would make no difference if cell separation in the presence of this drug would be achieved without involvement of a detectable quantity of autolytic wall enzymes (mechanical cell separation) or with these enzymes being involved (lytic cell separation). No indications are available so far that in the absence of autolytic wall enzymes there occurs a more complex mechanism of penicillin-induced killing. Possibly our data about the killing of staphylococci in the presence of penicillin being the result of morphogenetic mistakes during cross wall completion would be sufficient to also explain penicillin-induced death in certain streptococci killed by a mechanism independent of autolytic wall enzymes (the so-called cid mutants), without any speculation about a hypothetical injury of the cytoplasmic membrane (95).
However, we emphasize that autolytic wall enzymes in the region of the murosomes are of great importance to rapid staphylococcal cell separation during the logarithmic-growth phase of untreated staphylococci. Activation and inactivation of these enzymes will influence growth, mainly via enhancement or inhibition of lytic cell separation, respectively. But it should be stressed that staphylococci are not helpless if the activity of autolytic wall enzymes is inhibited or even lost because they can always apply mechanical cell separation. On the other hand, staphylococci lacking autolytic wall enzymes are not protected from penicillin-induced death.
On the basis of these considerations we have to repeat that penicillin-mediated morphogenetic mistakes at nascent cross walls are the most important prerequisites for the fatal action of penicillin, not only for staphylococci but possibly also for other bacteria. Furthermore, we presume that the specific action of penicillin may not be unique. Any other drug capable of preventing the completion of nascent cross walls should be able to induce the same type of morphogenetic death as if penicillin were involved in this killing process.
Why Has Penicillin-Induced Death Escaped Elucidation for Several Decades?
Retrospectively, several decades after Alexander Fleming’s fundamental observations in 1929 regarding the effect of penicillin on staphylococci (30) and after the first clinical application of this drug in 1941 (1, 2, 66), the question can now be answered why penicillin-induced death has escaped elucidation for so long. First, at the very beginning of research when bacteriolysis was still considered as an indication for the onset of penicillin-induced killing (lytic death), most experiments started much too late, i.e., about 2 to 3 h after addition of the drug. Only later on, when bacteriolysis had been identified as a postmortem effect (50–52), was it possible to identify the fatal morphogenetic processes taking place much earlier (Fig. 21 and 25). But obviously, not only some methodological aspects but also a series of structural and functional peculiarities of the bacterium under review proved to be important prerequisites for a successful analysis of the rather complex sequences which eventually resulted in the fatal event. Some of the essentials for solving this problem should be emphasized once more.
(i) Considering that penicillin-induced morphogenetic death depends on very localized minute changes in the structure of the cross wall during a short critical step of cell separation, any of the common biochemical analyses of staphylococcal cultures (or their mutant strains) growing in an unsynchronized manner had virtually no chance of contributing significantly to the solution of this problem.
(ii) Favoring electron microscopic examinations as the method of choice, it was possible to analyze characteristic morphogenetic defects occurring in the presence of penicillin within one single bacterium. Furthermore, it was, in comparison to gram-negative bacteria, the extremely thick cell wall of the gram-positive staphylococci which proved to be especially suitable for elucidating the fatal wall variations, being in the range of only a few nanometers.
(iii) The typical staphylococcal feature exhibiting three division planes arranged rectangularly to each other offered the unique chance of analyzing variations in wall morphogenesis during two consecutive cell separations. Furthermore, since staphylococci lack a definite longitudinal growth zone of their peripheral wall, penicillin almost exclusively affected the very restricted growth area of the nascent cross wall which facilitated considerably the analysis of fatal wall variations.
(iv) Since even in the presence of penicillin the minute murosomes of staphylococci remained sufficiently preserved, their involvement in penicillin-induced death could be elucidated.
(v) The importance of the high internal pressure of the staphylococcal protoplast for penicillin-induced death, for normal cell separation, and for dispersing pseudomulticellular clusters of staphylococci had to be considered.
(vi) The findings that staphylococci are capable of using not only a lytic but also a mechanical cell separation has enabled us to call in question the importance of autolytic wall enzymes in penicillin-induced death.
Therefore, hardly any bacterium other than a staphylococcus would have offered such a chance for the successful investigation of the mechanism by which penicillin is capable of killing bacteria.
CONCLUSION AND FUTURE DIRECTIONS
This review has attempted to give a detailed description of the staphylococcal wall architecture, as far as it can be analyzed with the electron microscope. Unfortunately, progress in determining the basic structural wall elements has been rather limited compared to the knowledge which had already been gained some 20 years ago (see reference 42 for a review). Interesting results have been obtained, however, regarding the wide range of staphylococcal wall reactivities to different antibiotics, the capacity to restore walls via sophisticated tricks after induced wall variations, and the ability to very slowly reactivate inhibited wall autolysins which has enabled us to follow even extremely rapid events in the cell wall involving murosomes. But this review has also shown that, concerning wall morphogenesis, in some cases there are still gaps in our knowledge. These gaps become especially evident when one tries to reconstruct schematically morphogenetic processes such as neoformation of the cross wall (Fig. 7) or murosome morphogenesis (Fig. 8). Consequently, at least some of the schematic drawings of this review are not free from alternative possibilities or even speculative elements, as mentioned in the relevant sections; they are considered to be working models and are mainly depicted to induce new experiments in order to check their accuracy.
Considerable gaps in our knowledge exist concerning autolytic wall enzymes. The localization of certain staphylococcal wall autolysins in a circumferential double ring structure on the cell surface above closed cross walls (146), where the pairs of murosomes are located, indicates that the vesicular murosomes are associated with these wall autolysins, but a real proof is still missing because it has not yet been possible to isolate the easily disintegrating murosomes in sufficient quantities (54). Furthermore, virtually nothing seems to be known so far about the regulatory capability of staphylococci in activating the lytic capacity of the murosomes, which must be stringently controlled. Any mistake in the lytic direction, the lytic target, or the lytic timetable of the regulatory cascade could result in an injury to the protective peripheral wall and in an explosion of the cell, due to the high internal turgor. Unfortunately, at present there is hardly any chance of getting reliable information about the coordinated regulation of lytic murosomal actions. However, since the genome of S. aureus has been fully sequenced and the data, hopefully, will soon be available to all scientists, comprehensive studies of well-characterized mutants are possible which could make significant contributions to all of the relevant questions.
The relations between the murosomes and the stripping system, which is involved in wall turnover, are also hardly understood. The stripping system is sandwiched between the primary and the secondary walls (Fig. 3); it is formed de novo when chloramphenicol-induced masses of wall material have to be disintegrated by centrifugal lytic actions (Fig. 17). The murosomes are normally found in close connection with the stripping system (Fig. 3B). Since both lytically active structures, i.e., the murosomes and the stripping system, are extracellular derivatives of the cytoplasmic membrane or of its membrane-wall interlayer, the possibility cannot be excluded that their functions are more or less closely related or that the murosomes are even part of the stripping system. It has already been mentioned that, for performing lytic cell separation, the actions of the murosome-associated autolysins are, apparently, supplemented by the autolysins of the stripping system (54) and that addition of isolated wall autolysins to nondivided cell clusters (pseudomulticellular staphylococci) resulted in autolysin-mediated lytic cell separation (20, 122). Confirmatory support for placing murosomes into the stripping system also comes from the observation that not only murosomes but also the stripping system is capable of punching periodically arranged holes into peripheral wall material via its disintegrating system (Fig. 17G). The concept of a more or less uniform assembly of lytic wall systems constituting the stripping system with the murosomes and the disintegrating system could prompt a series of new experimental approaches to explore presently unknown mutual relations during wall morphogenesis.
The second part of this review presents a detailed elucidation of the mechanism of penicillin-induced death as being the result of a minute morphogenetic mistake (Fig. 21 and 22), which elucidation allows specific predictions concerning the manner and time of the bacterial death to be made. This may help to end long-lasting disputes in this field. Knowledge of this mechanism will, hopefully, contribute to improvements in penicillin therapy, for instance via manipulating its efficiency with certain additives. Any improvement in beta-lactam therapy is badly needed, especially in the case of multiresistant staphylococcal variants such as MRSA. A prerequisite for attacking such MRSA strains is exact knowledge of a novel structural or morphogenetic weak point within the staphylococcal cell wall which could serve as a new target. For this reason, further and much more detailed knowledge of the staphylococcal wall and its morphogenesis is urgently needed. New experiments should also include our considerations about the possibility that, under certain conditions, even a mechanical cell separation without an involvement of a detectable quantity of autolytic wall enzymes could be sufficient to result in fatal effects after penicillin-induced morphogenetic defects at the staphylococcal cross walls (see “Penicillin-induced death without involvement of autolytic wall enzymes”). It is the hope of all scientists in this field to identify or develop an ingenious drug capable of interfering with a specific step in bacterial wall morphogenesis in a manner similar to that of the drug which the fungi have produced for their daily mortal combat against surrounding bacteria.
REFERENCES
- 1.Abraham E P. From penicillins to cephalosporins. In: Kleinkauf H, von Döhren H, editors. 50 years of penicillin application—history and trends. Berlin, Germany: Technische Universität Berlin; 1993. pp. 7–23. [Google Scholar]
- 2.Abraham E P, Fletcher C M, Florey H W, Gardner A D, Heatley N G, Jennings M A. Further observations on penicillin. Lancet. 1941;ii:177–189. [PubMed] [Google Scholar]
- 3.Amako K, Umeda A. Scanning electron microscopy of Staphylococcus. J Ultrastruct Res. 1977;58:34–40. doi: 10.1016/s0022-5320(77)80005-1. [DOI] [PubMed] [Google Scholar]
- 4.Amako K, Umeda A. Concentric circular structure of the cell wall of Staphylococcus epidermidis revealed by scanning electron microscope. J Electron Microsc. 1978;27:147–148. [PubMed] [Google Scholar]
- 5.Archibald A R, Coapes H E. Bacteriophage SP50 as a marker for cell wall growth in Bacillus subtilis. J Bacteriol. 1976;125:1195–1206. doi: 10.1128/jb.125.3.1195-1206.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.ASM Task Force on Antibiotic Resistance. Report of the ASM Task Force on Antibiotic Resistance. Washington, D.C: American Society for Microbiology; 1995. [Google Scholar]
- 7.Begg K J, Donachie W D. Division planes alternate in spherical cells of Escherichia coli. J Bacteriol. 1998;180:2564–2567. doi: 10.1128/jb.180.9.2564-2567.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beise F, Labischinski H, Giesbrecht P. Selective inhibition of penicillin-binding proteins and its effects on growth and architecture of Staphylococcus aureus. FEMS Microbiol Lett. 1988;55:195–201. [Google Scholar]
- 9.Berger-Bächi B, Barberis-Maino L, Strässle A, Kayser F H. FemA, a host-mediated factor essential for methicillin resistance in Staphylococcus aureus: molecular cloning and characterization. Mol Gen Genet. 1989;219:263–269. doi: 10.1007/BF00261186. [DOI] [PubMed] [Google Scholar]
- 10.Bierbaum G, Sahl H-G. Induction of autolysis of staphylococci by the basic peptide antibiotics Pep 5 and nisin and their influence on the activity of autolytic enzymes. Arch Microbiol. 1985;141:249–254. doi: 10.1007/BF00408067. [DOI] [PubMed] [Google Scholar]
- 11.Bierbaum G, Sahl H-G. Induction of autolysis of Staphylococcus simulans 22 by Pep5 and nisin and influence of the cationic peptides on the activity of the autolytic enzymes. In: Jung G, Sahl H-G, editors. Nisin and novel lantibiotics. Leiden, The Netherlands: ESCOM; 1991. pp. 386–396. [Google Scholar]
- 12.Blumberg P M, Strominger J L. Interaction of penicillin with the bacterial cell: penicillin-binding proteins and penicillin-sensitive enzymes. Bacteriol Rev. 1974;38:291–335. doi: 10.1128/br.38.3.291-335.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blümel P, Uecker W, Giesbrecht P. Zentbl. Bakteriol. Mikrobiol. Hyg. Abt. 1 Suppl. 10:435–439. 1981. In vitro studies on the possible role of cell wall turnover in Staphylococcus aureus during infection. [Google Scholar]
- 14.Bouligand Y. Twisted fibrous arrangements in biological materials and cholesteric mesophases. Tissue Cell. 1972;4:189–217. doi: 10.1016/s0040-8166(72)80042-9. [DOI] [PubMed] [Google Scholar]
- 15.Branton D. Fracture faces of frozen membranes. Proc Natl Acad Sci USA. 1966;55:1048–1056. doi: 10.1073/pnas.55.5.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brunskill E W, Bayles K W. Identification and molecular characterization of a putative regulatory locus that affects autolysis in Staphylococcus aureus. J Bacteriol. 1996;178:611–618. doi: 10.1128/jb.178.3.611-618.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brunskill E W, de Jonge B L M, Bayles K W. The Staphylococcus aureus scdA gene: a novel locus that affects cell division and morphogenesis. Microbiology. 1997;143:2877–2882. doi: 10.1099/00221287-143-9-2877. [DOI] [PubMed] [Google Scholar]
- 18.Canale-Parola E. Genus Sarcina Goodsir 1842, 434AL. In: Sneath P H A, Mair N S, Sharpe M E, Holt J G, editors. Bergey’s manual of systematic bacteriology. Vol. 2. Baltimore, Md: Williams & Wilkins; 1986. pp. 1100–1103. [Google Scholar]
- 19.Chatterjee A N, Mirelman D, Singer H J, Park J T. Properties of a novel pleiotropic bacteriophage-resistant mutant of Staphylococcus aureus H. J Bacteriol. 1969;100:846–853. doi: 10.1128/jb.100.2.846-853.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chatterjee A N, Wong W, Young F E, Gilpin R W. Isolation and characterization of a mutant of Staphylococcus aureus deficient in autolytic activity. J Bacteriol. 1976;125:961–967. doi: 10.1128/jb.125.3.961-967.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen M L. Epidemiology of drug resistance: implications for a post-antimicrobial era. Science. 1992;257:1050–1055. doi: 10.1126/science.257.5073.1050. [DOI] [PubMed] [Google Scholar]
- 22.Cole R M, Chatterjee A N, Gilpin R W, Young F E. Ultrastructure of teichoic acid-deficient and other mutants of staphylococci. Ann NY Acad Sci. 1974;236:22–52. doi: 10.1111/j.1749-6632.1974.tb41480.x. [DOI] [PubMed] [Google Scholar]
- 23.deJonge B L M, Chang Y-S, Gage D, Tomasz A. Peptidoglycan composition of a highly methicillin-resistant Staphylococcus aureus strain. J Biol Chem. 1992;267:11248–11254. [PubMed] [Google Scholar]
- 24.deJonge B L M, Chang Y-S, Gage D, Tomasz A. Peptidoglycan composition in heterogeneous Tn551 mutants of a methicillin-resistant Staphylococcus aureus strain. J Biol Chem. 1992;267:11255–11259. [PubMed] [Google Scholar]
- 25.de Jonge B L M, Sidow T, Chang Y-S, Labischinski H, Berger-Bachi B, Gage D A, Tomasz A. Altered muropeptide composition in Staphylococcus aureus strains with an inactivated femA locus. J Bacteriol. 1993;175:2779–2782. doi: 10.1128/jb.175.9.2779-2782.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eagle H, Musselman A D. The role of bactericidal action of penicillin in vitro as a function of its concentration, and its paradoxically reduced activity at high concentrations against certain organisms. J Exp Med. 1948;88:99–131. doi: 10.1084/jem.88.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ellar, D. J., and J. A. Posgate. 1972. Peptidoglycan lytic system associated with mesosomal membranes from Micrococcus lysodeikticus. Biochem. J. 130. [DOI] [PMC free article] [PubMed]
- 28.Fischer W. Physiology of lipoteichoic acids in bacteria. Adv Microb Physiol. 1988;29:233–302. doi: 10.1016/s0065-2911(08)60349-5. [DOI] [PubMed] [Google Scholar]
- 29.Fischer W, Rösel P, Koch H U. Effect of alanine ester substitution and other structural features of lipoteichoic acids on their inhibitory activity against autolysins of Staphylococcus aureus. J Bacteriol. 1981;146:467–475. doi: 10.1128/jb.146.2.467-475.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fleming A. On the antibacterial action of cultures of a penicillium, with special reference to their use in the isolation of B. influenzae. Br J Exp Pathol. 1929;10:226–236. [Google Scholar]
- 31.Fontana R, Satta G, Romanzi C A. Penicillins activate autolysins extracted from both Escherichia coli and Klebsiella pneumoniae envelopes. Antimicrob Agents Chemother. 1977;12:745–747. doi: 10.1128/aac.12.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fontana R, Varaldo P E, Canepari P, Satta G. Analysis of the penicillin binding proteins in the membranes of staphylococci of different lyogroups (or species). Zentbl. Bakteriol. Mikrobiol. Hyg. Abt. 1. Suppl. 10:67–70. Stuttgart, New York: Gustav Fischer Verlag; 1981. [Google Scholar]
- 33.Frank H, Lefort M, Martin H H. Elektronenoptische und chemische Untersuchungen an Zellwänden der Blaualge Phormidium uncinatum. Z Naturforsch. 1962;17b:262–268. [Google Scholar]
- 34.Garvie E I. Genus Pediococcus Claussen 1903, 68AL. In: Sneath P H A, Mair N S, Sharpe M E, Holt J G, editors. Bergey’s manual of systematic bacteriology. Vol. 2. Baltimore, Md: Williams & Wilkins; 1986. pp. 1075–1079. [Google Scholar]
- 35.Gemmell C G, Lorian V. Effects of low concentrations of antibiotics on bacterial ultrastructure, virulence, and susceptibility to immunodefenses: clinical significance. In: Lorian V, editor. Antibiotics in laboratory medicine. 4th ed. Baltimore, Md: Williams & Wilkins; 1996. pp. 397–452. [Google Scholar]
- 36.Gerding D, Peterson L R, Hughes C E, Bamberger D M, Larson T A. Extravascular antimicrobial distribution and the respective blood concentrations in humans. In: Lorian V, editor. Antibiotics in laboratory medicine. 3rd ed. Baltimore, Md: Williams & Wilkins; 1991. pp. 880–961. [Google Scholar]
- 37.Giesbrecht P. Zur Morphogenese der Zellwand von Staphylokokken. Mikroskopie. 1972;28:323–342. [PubMed] [Google Scholar]
- 38.Giesbrecht P. Novel bacterial wall organelles (“murosomes”) in staphylococci: their involvement in wall assembly. In: Nombela C, editor. Microbial cell wall synthesis and autolysis. Amsterdam, The Netherlands: Elsevier Publishers B.V.; 1984. pp. 177–186. [Google Scholar]
- 39.Giesbrecht P. Zentbl. Bakteriol. Suppl. 21:173–177. 1991. Perturbation of the membrane-wall interlayer by volume reductions of staphylococci results in wall disintegration and bacteriolysis after formation of mesosomal vesicles, being no fixation artifacts but rather multiplied “exosomes.”. [Google Scholar]
- 40.Giesbrecht P, Ruska H. Über Veränderungen der Feinstruktur von Bakterien unter der Einwirkung von Chloramphenicol. Klin Wochenschr. 1968;46:575–582. doi: 10.1007/BF01747836. [DOI] [PubMed] [Google Scholar]
- 41.Giesbrecht P, Wecke J. Zur Morphogenese der Zellwand von Staphylokokken I. Querwandbildung und Zelltrennung 1. Cytobiologie. 1971;4:349–368. [Google Scholar]
- 42.Giesbrecht P, Wecke J, Reinicke B. On the morphogenesis of the cell wall of staphylococci. Int Rev Cytol. 1976;44:225–318. doi: 10.1016/s0074-7696(08)61651-4. [DOI] [PubMed] [Google Scholar]
- 43.Giesbrecht P, Wecke J, Reinicke B, Tesche B. The demonstration of the existence of an interlayer between the cytoplasmic membrane and the cell wall proper of staphylococci. Arch Microbiol. 1977;115:25–35. doi: 10.1007/BF00427841. [DOI] [PubMed] [Google Scholar]
- 44.Giesbrecht P, Wecke J. On the structure and function of autolytic wall systems in gram-positive bacteria. Electron Microsc. 1980;2:446–453. [Google Scholar]
- 45.Giesbrecht P, Wecke J. Electron microscopic studies on the regeneration of staphylococci after treatment with antibiotics. Zentbl Bakteriol Mikrobiol Hyg Abt 1 Suppl. 1981;10:455–459. [Google Scholar]
- 46.Giesbrecht P, Wecke J, Blümel P, Reinicke B, Labischinski H. The role of autolytic wall systems in wall disintegration during the phagocytosis of staphylococci treated with antibiotics. In: Eickenberg H-U, Hahn H, Opferkuch W, editors. The influence of antibiotics on the host-parasite relationship. Berlin, Germany: Springer-Verlag; 1982. pp. 228–241. [Google Scholar]
- 47.Giesbrecht P, Morioka H, Krüger D, Kersten T, Wecke J. Restoration of penicillin-damaged cell walls by de novo murein synthesis and successive murein degradation in staphylococci, revealing a hitherto unknown mechanism of penicillin action: blockage of autolytic wall processes by penicillin. In: Hakenbeck R, Höltje J V, Labischinski H, editors. The target of penicillin: the murein sacculus of bacterial cell walls, architecture and growth. Berlin, Germany: Walter de Gruyter; 1983. pp. 243–248. [Google Scholar]
- 48.Giesbrecht P, Labischinski H, Wecke J. A special morphogenetic wall defect and the subsequent activity of “murosomes” as the very reason for penicillin-induced bacteriolysis in staphylococci. Arch Microbiol. 1985;141:315–324. doi: 10.1007/BF00428843. [DOI] [PubMed] [Google Scholar]
- 49.Giesbrecht, P., and J. Wecke. 1986. A new method in bacterial cell wall research: controlled transition from freeze-fractioning to freeze sectioning in latent living staphylococci. J. Electron Microsc. 35(Suppl.):2057–2060.
- 50.Giesbrecht P, Kersten T, Wecke J. Fan-shaped ejections of regularly arranged murosomes involved in penicillin-induced death of staphylococci. J Bacteriol. 1992;174:2241–2252. doi: 10.1128/jb.174.7.2241-2252.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Giesbrecht P, Kersten T, Madela K, Grob H, Blümel P, Wecke J. Penicillin-induced bacteriolysis of staphylococci as a post-mortem consequence of murosome-mediated killing via wall perforation and attempts to imitate this perforation process without applying antibiotics. In: de Pedro M A, Höltje J-V, Löffelhardt W, editors. Bacterial growth and lysis—metabolism and structure of the bacterial sacculus. New York, N.Y: Plenum Press; 1993. pp. 393–407. [Google Scholar]
- 52.Giesbrecht P, Franz M, Krüeger D, Labischinski H, Wecke J. Bacteriolysis of staphylococci is only a side-effect of penicillin-induced death. In: Kleinkauf H, von Döhren H, editors. 50 years of penicillin application—history and trends. Berlin, Germany: Technische Universität Berlin; 1993. pp. 353–363. [Google Scholar]
- 53.Giesbrecht P, Kersten T, Maidhof H, Krüger D, Blümel P, Grob H, Wecke J. A novel, “hidden” penicillin-induced death of staphylococci at high concentration, occurring earlier than murosome-mediated killing processes. Arch Microbiol. 1994;161:370–383. doi: 10.1007/BF00288946. [DOI] [PubMed] [Google Scholar]
- 54.Giesbrecht P, Kersten T, Maidhof H, Wecke J. Two alternative mechanisms of cell separation in staphylococci: one lytic and one mechanical. Arch Microbiol. 1997;167:239–250. doi: 10.1007/s002030050439. [DOI] [PubMed] [Google Scholar]
- 55.Götz F, Ahrné S, Lindberg M. Plasmid transfer and genetic recombination by protoplast fusion in staphylococci. J Bacteriol. 1981;145:74–81. doi: 10.1128/jb.145.1.74-81.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Halfen L N, Castenholz R W. Gliding motility in the blue-green alga, Oscillatoria princeps. J Phycol. 1971;7:133–145. [Google Scholar]
- 57.Hancock R, Park J T. Cell-wall synthesis by Staphylococcus aureus in the presence of chloramphenicol. Nature. 1958;181:1050–1052. doi: 10.1038/1811050a0. [DOI] [PubMed] [Google Scholar]
- 58.Henze U, Sidow T, Wecke J, Labischinski H, Berger-Bächi B. Influence of femB on methicillin resistance and peptidoglycan metabolism in Staphylococcus aureus. J Bacteriol. 1993;175:1612–1620. doi: 10.1128/jb.175.6.1612-1620.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hirai Y. The development of mesosomes of Staphylococcus aureus during autolysis. Acta Med Okayama. 1979;33:219–238. [PubMed] [Google Scholar]
- 60.Hobby G L, Meyer K, Chaffee E. Observations on the mechanism of action of penicillin. Proc Soc Exp Biol Med. 1942;50:281–288. [Google Scholar]
- 61.Jayaswal R K, Lee Y-I, Wilkinson B J. Cloning and expression of a Staphylococcus aureus gene encoding a peptidoglycan hydrolase activity. J Bacteriol. 1990;172:5783–5788. doi: 10.1128/jb.172.10.5783-5788.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Johannsen Lars. Über den Einfluß der O-Acetylierung des Mureins von Staphylococcus aureus auf die Abbaubarkeit bakterieller Zellwände durch zellwandlytische Enzymsysteme. Ph.D. thesis. Berlin, Germany: Free University; 1986. [Google Scholar]
- 63.Kaneda S. Isolation and characterization of autolysin-defective mutants of Staphylococcus aureus that form cell packets. Curr Microbiol. 1997;34:354–359. doi: 10.1007/s002849900195. [DOI] [PubMed] [Google Scholar]
- 64.Kersten T, Wecke J. Delayed penicillin-induced bacteriolysis of staphylococci by some cations. In: Kleinkauf H, von Döhren H, editors. 50 years of penicillin application—history and trends. Berlin, Germany: Technische Universität Berlin; 1993. pp. 364–372. [Google Scholar]
- 65.Kinsman O, Jonsson P, Haraldsson I, Lindberg M, Arbuthnott J P, Wadström T. Decreased virulence of α-haemolysin negative and coagulase-negative mutants of Staphylococcus aureus in experimental infections in mice. Zentbl Bakteriol Mikrobiol Hyg Abt 1 Suppl. 1981;10:651–659. [Google Scholar]
- 66.Kleinkauf H, von Döhren H, editors. 50 years of penicillin application—history and trends. Berlin, Germany: Technische Universität Berlin-Public Ltd.; 1993. [Google Scholar]
- 67.Koch A. Biophysics of bacterial walls viewed as stress-bearing fabric. Microbiol Rev. 1988;52:337–353. doi: 10.1128/mr.52.3.337-353.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Koch A L, Pinette M F S. Nephelometric determination of turgor pressure in growing gram-negative bacteria. J Bacteriol. 1987;169:3654–3663. doi: 10.1128/jb.169.8.3654-3663.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Komatsuzawa H, Sugai M, Nakashima S, Suginaka H. Alteration of bacteriolytic enzyme profile of Staphylococcus aureus during growth. Microbiol Immunol. 1995;39:629–633. doi: 10.1111/j.1348-0421.1995.tb02253.x. [DOI] [PubMed] [Google Scholar]
- 70.Kopp U, Roos M, Wecke J, Labischinski H. Staphylococcal peptidoglycan interpeptide bridge biosynthesis: a novel antistaphylococcal target? Microb Drug Resist. 1996;2:29–41. doi: 10.1089/mdr.1996.2.29. [DOI] [PubMed] [Google Scholar]
- 71.Koyama T, Yamada M, Matsuhashi M. Formation of regular packets of Staphylococcus aureus cells. J Bacteriol. 1977;129:1518–1523. doi: 10.1128/jb.129.3.1518-1523.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kozarich J W, Strominger J L. A membrane enzyme from Staphylococcus aureus which catalyzes transpeptidase, carboxypeptidase, and penicillinase activities. J Biol Chem. 1978;253:1272–1278. [PubMed] [Google Scholar]
- 73.Krüger D, Giesbrecht P. Microcalorimetric and electron microscopic investigation on staphylococci before and after treatment with penicillin and chloramphenicol and their combinations. In: Hakenbeck R, Höltje J-V, Labischinski H, editors. The target of penicillin: the murein sacculus of bacterial cell walls, architecture and growth. Berlin, Germany: Walter de Gruyter; 1983. pp. 273–278. [Google Scholar]
- 74.Labischinski H. Consequences of the interaction of β-lactam antibiotics with penicillin binding proteins from sensitive and resistant Staphylococcus aureus strains. Med Microbiol Immunol. 1992;181:241–265. doi: 10.1007/BF00198846. [DOI] [PubMed] [Google Scholar]
- 75.Labischinski H, Barnickel G, Bradaczek H, Giesbrecht P. On the secondary and tertiary structure of murein. Low and medium-angle X-ray evidence against chitin-based conformations of bacterial peptidoglycan. Eur J Biochem. 1979;95:147–155. doi: 10.1111/j.1432-1033.1979.tb12949.x. [DOI] [PubMed] [Google Scholar]
- 76.Labischinski H, Barnickel G, Rönspeck W, Roth K, Giesbrecht P. New insights into the three dimensional arrangement of the cell walls of staphylococci and other gram-positive bacteria. Zentbl Bakteriol Mikrobiol Hyg Abt 1 Suppl. 1981;10:427–433. [Google Scholar]
- 77.Labischinski H, Maidhof H, Franz M, Krüger D, Sidow T, Giesbrecht P. Biochemical and biophysical investigations into the cause of penicillin-induced lytic death of staphylococci: checking predictions of the murosome model. In: Actor P, Daneo-Moore L, Higgins M L, Salton M R J, Shockman G D, editors. Antibiotic inhibition of bacterial cell surface assembly and function. Washington, D.C: American Society for Microbiology; 1988. pp. 242–257. [Google Scholar]
- 78.Labischinski H, Maidhof H. Bacterial peptidoglycan: overview and evolving concepts. In: Ghuysen J-M, Hakenbeck R, editors. Bacterial cell wall. Amsterdam, The Netherlands: Elsevier Science; 1994. pp. 23–38. [Google Scholar]
- 79.Lamont H C. Sacrificial cell death and trichome breakage in an oscillatoriacean blue-green alga: the role of murein. Archiv Mikrobiol. 1969;69:237–259. [Google Scholar]
- 80.Lederberg J. Mechanism of action of penicillin. J Bacteriol. 1957;73:144. doi: 10.1128/jb.73.1.144-144.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liebermeister K, Kellenberger E. Studien zur L-Form der Bakterien I. Die Umwandlung der bazillären in die globuläre Zellform bei Proteus unter dem Einfluß von Penicillin. Z Naturforsch Teil b. 1956;11:200–206. [Google Scholar]
- 82.Livolant F, Bouligand Y. New observations on the twisted arrangement of dinoflagellate chromosomes. Chromosoma. 1978;68:21–44. [Google Scholar]
- 83.Löwe J, Amos L A. Crystal structure of the bacterial cell-division protein FtsZ. Nature. 1998;391:203–206. doi: 10.1038/34472. [DOI] [PubMed] [Google Scholar]
- 84.Lorian V. Some effects of subinhibitory concentrations of penicillin on the structure and division of staphylococci. Antimicrob Agents Chemother. 1975;7:864–870. doi: 10.1128/aac.7.6.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lorian V, Atkinson B. Effects of subinhibitory concentrations of antibiotics on cross walls of cocci. Antimicrob Agents Chemother. 1976;9:1043–1055. doi: 10.1128/aac.9.6.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Maidhof H, Johannsen L, Labischinski H, Giesbrecht P. Onset of penicillin-induced bacteriolysis in staphylococci is cell cycle dependent. J Bacteriol. 1989;171:2252–2257. doi: 10.1128/jb.171.4.2252-2257.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Maidhof H, Reinicke B, Blümel P, Berger-Bächi B, Labischinski H. femA, which encodes a factor essential for expression of methicillin resistance, affects glycine content of peptidoglycan in methicillin-resistant and methicillin-susceptible Staphylococcus aureus strains. J Bacteriol. 1991;173:3507–3513. doi: 10.1128/jb.173.11.3507-3513.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mandelstam J, Rogers H J. Chloramphenicol-resistant incorporation of amino acids into staphylococci and cell-wall synthesis. Nature. 1958;181:956–957. doi: 10.1038/181956a0. [DOI] [PubMed] [Google Scholar]
- 89.Mandelstam J, Rogers H J. The incorporation of amino acids into the cell-wall mucopeptide of staphylococci and the effect of antibiotics on the process. Biochem J. 1959;72:654–662. doi: 10.1042/bj0720654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mani N, Tobin P, Jayaswal R K. Isolation and characterization of autolysis-defective mutants of Staphylococcus aureus created by Tn917-lacZ mutagenesis. J Bacteriol. 1993;175:1493–1499. doi: 10.1128/jb.175.5.1493-1499.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mani N, Baddour L M, Offutt D Q, Vijaranakul U, Nadakavukaren M J, Jayaswal R K. Autolysis-defective mutant of Staphylococcus aureus: pathological considerations, genetic mapping, and electron microscopic studies. Infect Immun. 1994;62:1406–1409. doi: 10.1128/iai.62.4.1406-1409.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mirza J, Kim Y, Lorian V. Effect of pH on the morphology of Staphylococcus aureus. Drugs Exp Clin Res. 1985;11:149–153. [PubMed] [Google Scholar]
- 93.Mitchell P, Moyle J. Osmotic function and structure in bacteria. In: Spooner E T C, Stocker B A D, editors. Bacterial anatomy. Cambridge, United Kingdom: University Press; 1956. pp. 150–180. [Google Scholar]
- 94.Mitchell P, Moyle J. Autolytic release and osmotic properties of “protoplasts” from Staphylococcus aureus. J Gen Microbiol. 1957;16:184–194. doi: 10.1099/00221287-16-1-184. [DOI] [PubMed] [Google Scholar]
- 95.Moreillon P, Markiewicz Z, Nachman S, Tomasz A. Two bactericidal targets for penicillin in pneumococci: autolysis-dependent and autolysis-independent killing mechanisms. Antimicrob Agents Chemother. 1990;34:33–39. doi: 10.1128/aac.34.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Morioka H, Tachibana M, Suganuma A. Ultrastructural localization of carbohydrates on thin sections of Staphylococcus aureus with silver methenamine and wheat germ agglutinin-gold complex. J Bacteriol. 1987;169:1358–1362. doi: 10.1128/jb.169.3.1358-1362.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Murray R G E. Family II. Deinococcaceae Brooks and Murray 1981, 356VP. In: Sneath P H A, Mair N S, Sharpe M E, Holt J G, editors. Bergey’s manual of systematic bacteriology. Vol. 2. Baltimore, Md: Williams & Wilkins; 1986. p. 1035. [Google Scholar]
- 98.Murray R G E, Francombe W H, Mayall B H. The effect of penicillin on the structure of staphylococcal cell walls. Can J Microbiol. 1959;5:641–648. doi: 10.1139/m59-078. [DOI] [PubMed] [Google Scholar]
- 99.Murray R G E, Brooks B W. Genus I. Deinococcus Brooks and Murray 1981, 354VP. In: Sneath P H A, Mair N S, Sharpe M E, Holt J G, editors. Bergey’s manual of systematic bacteriology. Vol. 2. Baltimore, Md: Williams & Wilkins; 1986. pp. 1035–1043. [Google Scholar]
- 100.Nishino T, Wecke J, Krüger D, Giesbrecht P. Trimethoprim-induced structural alterations in Staphylococcus aureus and the recovery of bacteria in drug-free medium. J Antimicrob Chemother. 1987;19:147–159. doi: 10.1093/jac/19.2.147. [DOI] [PubMed] [Google Scholar]
- 101.Ohnuma T. Staphylococcus administered by antibiotics. Tokyo, Japan: Yone-Production Ltd.; 1996. . (Scientific film.) [Google Scholar]
- 102.Oshida T, Sugai M, Komatsuzawa H, Hong Y-M, Suginaka H, Tomasz A. A Staphylococcus aureus autolysin that has an N-acetylmuramoyl-l-alanine amidase domain and an endo-β-N-acetylglucosaminidase domain: cloning, sequence analysis, and characterization. Proc Natl Acad Sci USA. 1995;92:285–289. doi: 10.1073/pnas.92.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Paul T R, Venter A, Blaszczak L C, Parr T R, Jr, Labischinski H, Beveridge T J. Localization of penicillin-binding proteins to the splitting system of Staphylococcus aureus septa by using a mercury-penicillin V derivative. J Bacteriol. 1995;177:3631–3640. doi: 10.1128/jb.177.13.3631-3640.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pfennig N. Genus IX. Thiopedia Winogradsky 1888, 85AL. In: Staley J T, Bryant M P, Pfennig N, Holt J G, editors. Bergey’s manual of systematic bacteriology. Vol. 3. Baltimore, Md: Williams & Wilkins; 1989. pp. 1652–1653. [Google Scholar]
- 105.Pinette M F S, Koch A L. Variability of the turgor pressure of individual cells of the gram-negative heterotroph Ancylobacter aquaticus. J Bacteriol. 1987;169:4737–4742. doi: 10.1128/jb.169.10.4737-4742.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pooley H M. Turnover and spreading of old wall during surface growth of Bacillus subtilis. J Bacteriol. 1976;125:1127–1138. doi: 10.1128/jb.125.3.1127-1138.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pooley H M. Layered distribution, according to age, within the cell wall of Bacillus subtilis. J Bacteriol. 1976;125:1139–1147. doi: 10.1128/jb.125.3.1139-1147.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pooley H M, Shockman G D. Relationship between the location of autolysin, cell wall synthesis, and the development of resistance to cellular autolysis in Streptococcus faecalis after inhibition of protein synthesis. J Bacteriol. 1970;103:457–466. doi: 10.1128/jb.103.2.457-466.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pucci M J, Thanassi J A, Discotto L F, Kessler R E, Dougherty T J. Identification and characterization of cell wall-cell division gene clusters in pathogenic gram-positive cocci. J Bacteriol. 1997;179:5632–5635. doi: 10.1128/jb.179.17.5632-5635.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ramadurai L, Jayasval R K. Molecular cloning, sequencing, and expression of lytM, a unique autolytic gene of Staphylococcus aureus. J Bacteriol. 1997;179:3625–3631. doi: 10.1128/jb.179.11.3625-3631.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Reinicke B, Blümel P, Labischinski H, Giesbrecht P. Neither an enhancement of autolytic wall degradation nor an inhibition of the incorporation of cell wall material are pre-requisites for penicillin-induced bacteriolysis in staphylococci. Arch Microbiol. 1985;141:309–314. doi: 10.1007/BF00428842. [DOI] [PubMed] [Google Scholar]
- 112.Reynolds P E. The essential nature of staphylococcal penicillin-binding proteins. In: Actor P, Daneo-Moore L, Higgins M L, Salton M R J, Shockman G D, editors. Antibiotic inhibition of bacterial cell surface assembly and function. Washington, D.C: American Society for Microbiology; 1988. pp. 343–351. [Google Scholar]
- 113.Rippka R, Herdman M. Division patterns and cellular differentiation in cyanobacteria. Ann Inst Pasteur/Microbiol. 1985;136A:33–39. doi: 10.1016/s0769-2609(85)80018-1. [DOI] [PubMed] [Google Scholar]
- 114.Rogers H J, Perkins H R, Ward J B. Microbial cell walls and membranes. London, United Kingdom: Chapman and Hall; 1980. [Google Scholar]
- 115.Salton M R J. The bacterial cell envelope—a historical perspective. In: Ghuysen J-M, Hakenbeck R, editors. Bacterial cell wall. Amsterdam, The Netherlands: Elsevier Science; 1994. pp. 1–22. [Google Scholar]
- 116.Schwarz U, Asmus A, Frank H. Autolytic enzymes and cell division of Escherichia coli. J Mol Biol. 1969;41:419–429. doi: 10.1016/0022-2836(69)90285-x. [DOI] [PubMed] [Google Scholar]
- 117.Shockman G D, Daneo-Moore L, McDowell T D, Wong W. Function and structure of the cell wall—its importance in the life and death of bacteria. In: Salton M R J, Shockman G D, editors. β-Lactam antibiotics. Mode of action, new developments, and future prospects. New York, N.Y: Academic Press; 1981. pp. 31–65. [Google Scholar]
- 118.Shockman G D, Höltje J-V. Microbial peptidoglycan (murein) hydrolases. In: Ghuysen J-M, Hakenbeck R, editors. Bacterial cell wall. Amsterdam, The Netherlands: Elsevier Science; 1994. pp. 131–166. [Google Scholar]
- 119.Sidow T, Johannsen L, Labischinski H. Penicillin-induced changes in the cell wall composition of Staphylococcus aureus before the onset of bacteriolysis. Arch Microbiol. 1990;154:73–81. doi: 10.1007/BF00249181. [DOI] [PubMed] [Google Scholar]
- 120.Spratt B G. Biochemical and genetical approaches to the mechanism of action of penicillin. Philos Trans R Soc Lond Ser B Biol Sci. 1980;289:273–283. doi: 10.1098/rstb.1980.0045. [DOI] [PubMed] [Google Scholar]
- 121.Strandén A M, Ehlert K, Labischinski H, Berger-Bächi B. Cell wall monoglycine cross-bridges and methicillin hypersusceptibility in a femAB null mutant of methicillin-resistant Staphylococcus aureus. J Bacteriol. 1997;179:9–16. doi: 10.1128/jb.179.1.9-16.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sugai M, Komatsuzawa H, Akiyama T, Hong Y-M, Oshida T, Miyake Y, Yamaguchi T, Suginaka H. Identification of endo-β-N-acetylglucosaminidase and N-acetylmuramyl-l-alanine amidase as cluster-dispersing enzymes in Staphylococcus aureus. J Bacteriol. 1995;177:1491–1496. doi: 10.1128/jb.177.6.1491-1496.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sugai M, Yamada S, Nakashima S, Komatsuzawa H, Matsumoto A, Oshida T, Suginaka H. Localized perforation of the cell wall by a major autolysin: atl gene products and the onset of penicillin-induced lysis of Staphylococcus aureus. J Bacteriol. 1997;179:2958–2962. doi: 10.1128/jb.179.9.2958-2962.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Suganuma A. Fine structure of staphylococci: electron microscopy. In: Cohen J O, editor. The staphylococci. New York, N.Y: John Wiley & Sons; 1972. pp. 21–40. [Google Scholar]
- 125.Suganuma A. Electron microscopic observations of staphylococci. Zentbl Bakteriol Suppl. 1991;21:27–35. [Google Scholar]
- 126.Suginaka H, Blumberg P M, Strominger J L. Multiple penicillin-binding components in Bacillus subtilis, Bacillus cereus, Staphylococcus aureus, and Escherichia coli. J Biol Chem. 1972;247:5279–5288. [PubMed] [Google Scholar]
- 127.Tipper D J, Strominger J L. Mechanism of action of penicillins: a proposal based on their structural similarities to acyl-d-alanyl-d-alanine. Proc Natl Acad Sci USA. 1965;54:1133–1141. doi: 10.1073/pnas.54.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tipper D J, Strominger J L. Biosynthesis of the peptidoglycan of bacterial cell walls. XII. Inhibition of cross-linking by penicillins and cephalosporins: studies in Staphylococcus aureus in vivo. J Biol Chem. 1968;243:3169–3179. [PubMed] [Google Scholar]
- 129.Tomasz A. The mechanism of the irreversible antimicrobial effects of penicillins: how the beta-lactam antibiotics kill and lyse bacteria. Annu Rev Microbiol. 1979;33:113–137. doi: 10.1146/annurev.mi.33.100179.000553. [DOI] [PubMed] [Google Scholar]
- 130.Tomasz A. Penicillin tolerance and the control of murein hydrolases. In: Salton M R J, Shockman G D, editors. β-Lactam antibiotics: mode of action, new developments and future projects. New York, N.Y: Academic Press; 1981. pp. 227–247. [Google Scholar]
- 131.Tomasz A, Höltje J-V. Murein hydrolases and the lytic and killing action of penicillin. In: Schlessinger D, editor. Microbiology. Washington, D.C: American Society for Microbiology; 1977. pp. 209–215. [Google Scholar]
- 132.Umeda A, Ikebuchi T, Amako K. Localization of bacteriophage receptor, clumping factor, and protein A on the cell surface of Staphylococcus aureus. J Bacteriol. 1980;141:838–844. doi: 10.1128/jb.141.2.838-844.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Watson K, Arthur H. Cell surface topography of Candida and Leucosporidium yeasts as revealed by scanning electron microscopy. J Bacteriol. 1977;130:312–317. doi: 10.1128/jb.130.1.312-317.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Waxman D J, Strominger J L. Penicillin-binding proteins and the mechanism of action of β-lactam antibiotics. Annu Rev Biochem. 1983;52:825–869. doi: 10.1146/annurev.bi.52.070183.004141. [DOI] [PubMed] [Google Scholar]
- 135.Wecke J, Giesbrecht P. Zur Morphogenese der Zellwand von Staphylokokken. II. Untersuchungen über das Wachstum der Zellwand mit Hilfe von Penicillin-Markern. Cytobiologie. 1973;7:272–288. [Google Scholar]
- 136.Wecke J, Giesbrecht P. Electron microscopic studies on the “paradoxical” reaction of staphylococci during treatment with antibiotics. Zentbl Bakteriol Mikrobiol Hyg Abt 1 Suppl. 1981;10:461–467. [Google Scholar]
- 137.Wecke J, Lahav M, Ginsburg I, Giesbrecht P. Cell wall degradation of Staphylococcus aureus by lysozyme. Arch Microbiol. 1982;131:116–123. doi: 10.1007/BF01053992. [DOI] [PubMed] [Google Scholar]
- 138.Wecke J, Lahav M, Ginsburg I, Kwa E, Giesbrecht P. Cell wall degradation of antibiotic-treated staphylococci under phagocyte-specific conditions: distinction between penicillin-induced effects and wall alterations caused by anticoagulants. In: Hakenbeck R, Höltje J-V, Labischinski H, editors. The target of penicillin: the murein sacculus of bacterial cell walls, architecture and growth. Berlin, Germany: Walter de Gruyter; 1983. pp. 329–334. [Google Scholar]
- 139.Wecke J, Lahav M, Ginsburg I, Kwa E, Giesbrecht P. Inhibition of wall autolysis of staphylococci by sodium polyanethole sulfonate “liquoid.”. Arch Microbiol. 1986;144:110–115. doi: 10.1007/BF00414719. [DOI] [PubMed] [Google Scholar]
- 140.Wecke J, Kwa E, Lahav M, Ginsburg I, Giesbrecht P. Suppression of penicillin-induced bacteriolysis of staphylococci by some anticoagulants. J Antimicrob Chemother. 1987;20:47–55. doi: 10.1093/jac/20.1.47. [DOI] [PubMed] [Google Scholar]
- 141.Wecke J, Franz M, Giesbrecht P. Inhibition of the bacteriolytic effect of β-lactam antibiotics on Staphylococcus aureus by the polyanionic drugs suramin and Evans Blue. Acta Pathol Microbiol Immunol Scand. 1990;98:71–81. [PubMed] [Google Scholar]
- 142.Wecke, J., T. Kersten, and P. Giesbrecht. 1992. The modulation of the bacteriolytic effect of β-lactam antibiotics by non-antibiotics. Acta Pathol. Microbiol. Immunol. Scand. 100(Suppl.):32–39. [PubMed]
- 143.Wecke J, Madela K, Fischer W. The absence of d-alanine from lipoteichoic acid and wall teichoic acid alters surface charge, enhances autolysis and increases susceptibility to methicillin in Bacillus subtilis. Microbiology. 1997;143:2953–2960. doi: 10.1099/00221287-143-9-2953. [DOI] [PubMed] [Google Scholar]
- 144.Weidel W, Pelzer H. Bag-shaped macromolecules—a new outlook on bacterial cell walls. Adv Enzymol Relat Areas Biochem. 1964;26:193–232. doi: 10.1002/9780470122716.ch5. [DOI] [PubMed] [Google Scholar]
- 145.Westmacott D, Perkins H R. Effects of lysozyme on Bacillus cereus 569: rupture of chains of bacteria and enhancement of sensitivity to autolysins. J Gen Microbiol. 1979;115:1–11. doi: 10.1099/00221287-115-1-1. [DOI] [PubMed] [Google Scholar]
- 146.Yamada S, Sugai M, Komatsuzawa H, Nakashima S, Oshida T, Matsumoto A, Suginaka H. An autolysin ring associated with cell separation of Staphylococcus aureus. J Bacteriol. 1996;178:1565–1571. doi: 10.1128/jb.178.6.1565-1571.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]