Patients with low renal function have an increased risk of critical COVID-19. While chronic kidney disease is associated with impairment of the immune system, it is still not known whether their worse COVID-19 outcome can be explained by a weaker antiviral response or by systemic inflammation. Importantly, there is still no literature on the immunological characteristics of COVID-19 patients with low renal function, which could potentially explain their increased fatality rate. Here, we performed an observational cohort study on 173 consecutively enrolled hospitalized COVID-19 patients, a non-vaccinated cohort recruited in 2020, which was classified according to their estimated glomerular filtration rate (eGFR) at admission. We analysed the immunological differences between patients with low and normal renal function, including circulating T and B cell subsets, SARS-CoV-2 reactive T cells and serum cytokines in follow-up. For more details on the methods, see the Supplementary Methods.
The patients were recruited (initial visit) at a median of 3 [IQR 1–6] days after the first positive PCR test. One hundred forty-three (82.7%) patients showed normal renal function at admission (hereafter Normal-eGFR; eGFR > 60 ml/min/1.73m2), while 30 patients (17.3%) suffered from low renal function (Low-eGFR; < 60 ml/min/1.73m2). Low-eGFR patients had significantly higher age (P < 0.001; see Table S1) and Charlson comorbidity index (P = 0.002). Therefore, we controlled for these factors in our analyses employing multivariate regression, as explained in the Supplementary Methods. Twelve patients (7.3%) died during follow-up; the Normal-eGFR sub-cohort had 5 fatal cases (3.5%), while for Low-eGFR there were 7 (23.3%). The association between Low-eGFR and patient death was significant (P = 0.022), independently from confounders.
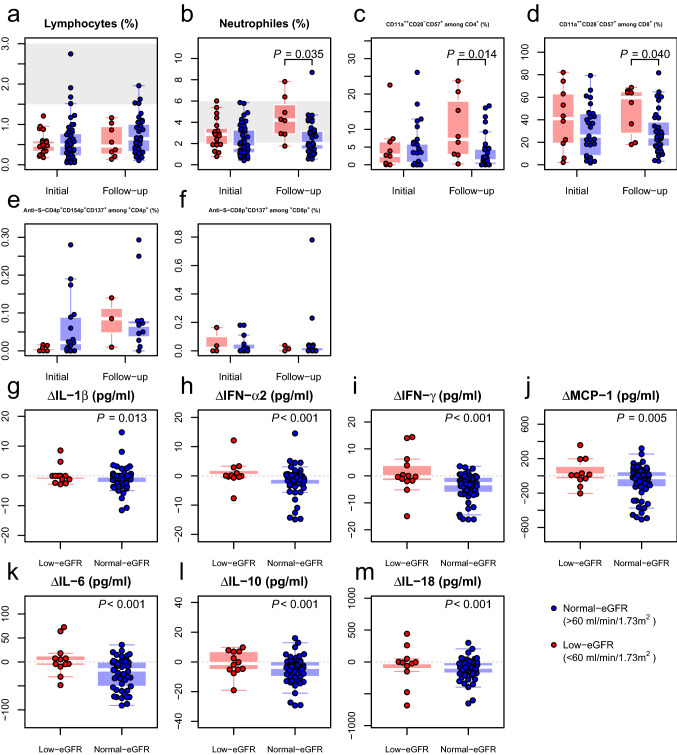
We first analysed general immunological parameters: Almost all patients were lymphopenic (Fig. 1a). We observed significantly higher neutrophil counts among patients in the Low-eGFR group (Fig. 1b). Higher neutrophil counts have been associated with increased COVID-19 severity [1]. Regarding the T cell subset, patients with low eGFR had higher levels of in vivo activated HLA-DR+ CD4+ and CD8+ T cells, albeit without reaching statistical significance (Figure S1). On the other hand, we found significantly increased levels for terminally differentiated CD11a++CD28−CD57+ T cells among Low-eGFR at follow-up (Fig. 1c, d). These cells express markers of tissue migration (CD11a++) and do not require costimulatory molecules for activation (CD28−). This is in line with previous studies that identified such alterations in COVID-19 critical versus less severe disease manifestations [2]. Furthermore, an increase in CD28−CD57+ T cells has been observed repeatedly in a context of severe CKD [3]. These cells also expressed high levels of CD11a, a marker highly expressed in memory T cells and associated with activation and tissue migration [2]. CD11a++CD28−CD57+CD8+ T cells are associated with bystander activation in inflamed tissue, significantly contributing to tissue damage [4]. Higher frequencies of this cell subset might therefore contribute to the worse clinical outcome of patients with lower eGFR. Since these terminally differentiated cells are likely associated with ageing and chronic antigen exposure, we analysed the T cell memory composition in the patient cohorts but did not find any significant differences between the sub-cohorts (Figure S2). Finally, we did not find any significant differences between the study sub-cohorts for the SARS-CoV-2-specific T cell response (Fig. 1e, f) nor in the B cell compartment (Figure S3).
Fig. 1.
Patients in the Low-eGFR sub-cohort demonstrated an altered immune system. The figure depicts the levels of circulating immune subsets at the initial and follow-up visit (a–f), as well as the changes in serum cytokines between the two visits (g–m). For the immune subsets, peripheral blood from 57 patients, 41 from the Normal-eGFR sub-cohort (blue) and 16 from the Low-eGFR sub-cohort (red) was characterized. The figure depicts the frequencies of circulating lymphocytes (a), neutrophils (b), terminally differentiated T cells (c, d) and T cells specific against the viral spike antigen (e–f) using multiparametric flow cytometry. The P values were calculated controlling for differences in age and Charlson comorbidity index (see Statistical methods). In all cases, the left boxplots show the data for the initial visit, while the right boxplots depict the data at follow-up. The area shaded in grey represents the reference range for each parameter, if applicable. For the cytokine kinetics (g–m), 74 patients were analysed, 61 Low-eGFR sub-cohort (red) and 13 Normal-eGFR sub-cohort (blue). The figure shows the change in cytokine levels between initial and follow-up for each patient, where a negative value means a decrease in concentration and vice versa. A dashed grey line marks the threshold of a null change between the visits. Importantly, the P value does not refer to a comparison between the two sub-cohorts, but to the significance of the change in cytokine concentrations within each sub-cohort. eGFR estimated glomerular filtration rate; IFN interferon; IL interleukin; MCP monocyte chemoattractant protein
We further examined the differences in the cytokine profiles of the two sub-cohorts. While there were no significant differences in cytokine concentrations at either study visit (Figure S4), there was a significant decrease in the concentration of IL-1β, IFN-α2, IFN-γ, MCP-1, IL-6, IL-10 and IL-18 in the Normal-eGFR sub-cohort during follow-up, while the cytokine level remained unchanged in the Low-eGFR sub-cohort (Fig. 1g–m). We hypothesize that the latter dynamics are the result of chronic systemic inflammation. Previous studies on patients with senescent immunity demonstrate that chronic inflammation is associated with a higher mortality through infection [5]. Therefore, cytokine data suggest that patients with impaired kidney function might suffer from chronic inflammation, which could cause higher immunopathology and overall mortality.
In summary, we present a characterization of the immune system of COVID-19 patients with reduced renal function. Here, low eGFR emerges as a factor associated with T cell immunosenescence and an altered inflammatory response. These immunological alterations could potentially explain the worse disease outcomes of patients with reduced renal function. Main limitations of our work include the low number of patients within the Low-eGFR group, availability of sufficient samples, the differences in age and co-morbidities between the sub-cohorts and the fact that the cohort was recruited between spring and autumn 2020. Further, prospective studies with larger patient cohorts and long-term follow-up data are needed to confirm our observations.
COVID-19, chronic kidney disease, renal function, immune system, immunosenescence.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We want to thank the patients who donated their blood samples and clinical data for this project.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported by grants from the German Federal Ministry for Education and Research (BMBF) e:KID (01ZX1612A) and NoChro (FKZ 13GW0338B), the Mercator Foundation, Germany (St-2018–0014) and SepsisDataNet (EFRE-0800984).
Availability of data and material
Limited data access requests can be sent to Nina Babel (nina.babel@charite.de).
Code availability
Not applicable.
Declarations
Conflict of interest
The authors have no conflicts of interest.
Ethical approval
The study was approved by the ethical committee of the University Hospitals Bochum (20–6886) and Essen (20-9214-BO).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Arturo Blazquez-Navarro and Lisa Mittmann contributed equally.
References
- 1.Kuri-Cervantes L, Pampena MB, Meng W, Rosenfeld AM, Ittner CAG, Weisman AR, Agyekum RS, Mathew D, Baxter AE, Vella LA, Kuthuru O, Apostolidis SA, Bershaw L, Dougherty J, Greenplate AR, Pattekar A, Kim J, Han N, Gouma S, Weirick ME, Arevalo CP, Bolton MJ, Goodwin EC, Anderson EM, Hensley SE, Jones TK, Mangalmurti NS, Luning Prak ET, Wherry EJ, Meyer NJ, Betts MR. Comprehensive mapping of immune perturbations associated with severe COVID-19. Sci Immunol. 2020 doi: 10.1126/sciimmunol.abd7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anft M, Paniskaki K, Blazquez-Navarro A, Doevelaar A, Seibert FS, Hölzer B, Skrzypczyk S, Kohut E, Kurek J, Zapka J, Wehler P, Kaliszczyk S, Bajda S, Thieme CJ, Roch T, Konik MJ, Berger MM, Brenner T, Kölsch U, Meister TL, Pfaender S, Steinmann E, Tempfer C, Watzl C, Dolff S, Dittmer U, Abou-El-Enein M, Westhoff TH, Witzke O, Stervbo U, Babel N. COVID-19-induced ARDS is associated with decreased frequency of activated memory/effector T cells expressing CD11a++ Mol Ther. 2020;28(12):2691–2702. doi: 10.1016/j.ymthe.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crépin T, Legendre M, Carron C, Vachey C, Courivaud C, Rebibou JM, Ferrand C, Laheurte C, Vauchy C, Gaiffe E, Saas P, Ducloux D, Bamoulid J. Uraemia-induced immune senescence and clinical outcomes in chronic kidney disease patients. Nephrol Dial Transpl. 2020;35(4):624–632. doi: 10.1093/ndt/gfy276. [DOI] [PubMed] [Google Scholar]
- 4.Reinke S, Geissler S, Taylor WR, Schmidt-Bleek K, Juelke K, Schwachmeyer V, Dahne M, Hartwig T, Akyüz L, Meisel C, Unterwalder N, Singh NB, Reinke P, Haas NP, Volk HD, Duda GN. Terminally differentiated CD8+ T cells negatively affect bone regeneration in humans (Science Translational Medicine) Sci Transl Med. 2013;5(187):1–11. doi: 10.1126/scitranslmed.3006523. [DOI] [PubMed] [Google Scholar]
- 5.Zoccali C, Vanholder R, Massy ZA, Ortiz A, Sarafidis P, Dekker FW, Fliser D, Fouque D, Heine GH, Jager KJ, Kanbay M, Mallamaci F, Parati G, Rossignol P, Wiecek A, London G. The systemic nature of CKD. Nat Rev Nephrol. 2017;13(6):344–358. doi: 10.1038/nrneph.2017.52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Limited data access requests can be sent to Nina Babel (nina.babel@charite.de).
Not applicable.