Abstract
INTRODUCTION:
Alzheimer’s disease (AD) biomarkers are increasingly more reliable in predicting neuropathology. To facilitate interpretation of phosphorylated tau sites as an early fluid biomarker, we sought to characterize which neurofibrillary tangle maturity levels (pretangle, intermediary 1, mature tangle, intermediary 2, and ghost tangle) are recognized.
METHODS:
We queried the Florida Autopsied Multi-Ethnic (FLAME) cohort for cases ranging from Braak stages I-VI, excluding non-AD neuropathologies and tauopathies. Thioflavin-S staining was compared to immunohistochemical measures of phosphorylated threonine (pT) 181, pT205, pT217, and pT231 in two hippocampal subsectors across n=24 cases.
RESULTS:
Each phosphorylated tau site immunohistochemically labeled early neurofibrillary tangle maturity levels compared to advanced levels recognized by thioflavin-S. Hippocampal burden generally increased with each Braak stage.
DISCUSSION:
These results provide neurobiologic evidence that these phosphorylated tau fluid biomarker sites are present during early neurofibrillary tangle maturity levels and may explain why these fluid biomarker measures are observed before symptom onset.
Keywords: Neuropathology, tau, neurofibrillary tangle, biomarker, Alzheimer’s disease, postmortem
1. INTRODUCTION:
Alzheimer’s disease (AD) is a neurodegenerative disorder characterized by two hallmark neuropathologies: plaques composed of amyloid-β and neurofibrillary tangles composed of hyperphosphorylated tau [1–3]. Amyloid-β plaques reside outside the neuron whereas neurofibrillary tangles accumulate inside the neuron. Although current biomarkers lack the microscopic granularity of neuropathologic examination, they are important tools critical to reliably measure AD neuropathology in vivo [4]. One such biomarker is positron emission tomography (PET), which can identify patients with increased tau or amyloid-β levels. PET imaging can lead to a more informed antemortem diagnosis and provide spatial information of which regions have a high neuropathologic burden [5–7]. However, disadvantages to PET imaging include cost, accessibility, and patient exposure to radioactivity, albeit minimal. Alternatively, fluid biomarkers are useful in identifying changes in amyloid-β and tau in patients without the use of radioactivity [8]; however, these biomarkers lack spatial information. Over 20 years ago, total tau [9–11] and phosphorylated tau [12] were found increased in cerebrospinal fluid of AD patients compared to controls. Multiple phosphorylated (p) tau sites were elevated in cerebrospinal fluid, including at threonine (T) 181 [12, 13], pT205 [14], pT217 [15, 16], and pT231 [12, 17]. More recently, the AD biomarker field has witnessed the successful translation of cerebrospinal fluid assays to minimally invasive plasma-based assays [18–21]. As of now, there are few studies investigating the neuropathology that these phosphorylated tau fluid biomarkers may be representing.
Neurofibrillary tangles have a lifespan that progresses through three major levels: pretangles, mature tangles, and ghost tangles [22]. Pretangles contain diffuse or granular hyperphosphorylated tau in otherwise morphologically normal neurons with perinuclear tau accumulation often observed [23]. Mature tangles are intensely stained bundles of fibers that take the shape of the neuron they occupy. The nucleus may appear shrunken and misplaced towards the cell membrane [1–3]. Ghost tangles are the remnants of mature tangles once the neurons have died and are recognized by faintly stained bundles of fibers with no associated nucleus [1–3].We and others have previously provided support that flortaucipir, a tau-PET ligand, recognizes a more middling to advanced neurofibrillary tangle maturity levels (mature tangles and ghost tangles) [24–26].
There is increasing evidence that phosphorylated tau fluid biomarkers are elevated in AD prior to tau-PET positivity [27–29]. Therefore, we hypothesized that these phosphorylated tau sites recognize early neurofibrillary tangle maturity levels. To test our hypothesis, the hippocampus was specifically chosen to evaluate the large pyramidal neurons that undergo morphologic changes as the neurofibrillary tangle matures. Thus, our first goal was to histologically characterize neurofibrillary tangle maturity levels recognized by four fluid biomarker phosphorylated tau sites: pT181, pT205, pT217, and pT231. Secondly, as studies have shown that the subiculum has twice the number of thioflavin-S positive neurofibrillary tangles as the cornu ammonis 1 (CA1), [30, 31] we sought to examine regional differences of the phosphorylated tau sites leveraging the vulnerability of subiculum compared to CA1 using digital pathology to quantify tau burden. Lastly, we sought to identify changes in the phosphorylated tau sites based on global severity measured by Braak stage [32, 33] to evaluate shifting recognition of neurofibrillary tangle maturity levels.
2. METHODS:
2.1. Cohort selection
The Florida Autopsied Multi-Ethnic (FLAME) cohort [34, 35], as of May 27, 2020, was queried to identify cases with a spectrum of neuropathologically diagnosed AD. Cases with significant non-AD neurodegenerative pathology (frontotemporal lobar degeneration, amyotrophic lateral sclerosis, hippocampal sclerosis, Lewy body disease, amygdala predominant Lewy bodies, multiple system atrophy, Creutzfeldt-Jakob disease) and non-AD tauopathies (progressive supranuclear palsy, corticobasal degeneration, Pick’s disease, globular glial tauopathies, MAPT mutation carriers) were excluded. As age related tau astrogliopathy (ARTAG) is not routinely screened in our brain bank and not observed in the hippocampus, these cases were not excluded. Additionally, primary age related tauopathy (PART) was not excluded as these cases fall below the Braak stage requirements for AD. Cases with exhausted paraffin embedded blocks of the posterior hippocampus were also excluded. To examine the entire lifespan of neurofibrillary tangles, we selected 2 males and 2 females for each Braak stage I, II, III, IV, V, and VI [32, 33]. For Braak stages IV-VI, only AD cases with a typical AD subtype were selected [31]. The final cohort included 24 cases summarized in Supplementary Table 1. Tissue sampling and histology is further discussed in Supplementary Methods.
2.2. Immunohistochemistry and Fluorescence staining
Sections were deparaffinized and rehydrated using standard methods. Immunohistochemistry using AT270 (pT181), pT205, pT217, and AT180 (pT231) antibodies was performed on serial sections on the Thermo Scientific Lab Vision Autostainer 480S. Specifications and vendor information for each antibody is reported in Table 1. While AT270, AT180, and the pT217 antibodies were previously used in biomarker assays [7, 12, 13, 16, 21, 36–40], the pT205 antibody used in this study has not been previously used in biomarker assays (Supplementary Table 2). All epitopes fall within the proline rich region (Fig. 1), sometimes described as mid-domain, which lies N-terminal to the microtubule binding region [41]. Sections were developed with the developing kit (Biocare Medical, catalog # M3M530L [mouse] or M3R531L [rabbit]).
Table 1.
Primary antibody information
| Antibody | Epitope | Vendor | Catalog # | Species | Clonality | Isotype | RRID | IHC Dilution | IF Dilution | Antigen Retrieval | Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|
| AT270 | pT181 | Thermo Fisher Scientific | MN1050 | Mouse | Monoclonal | IgG1κ | AB_223651 | 1:10,00 | 1:500 | H2O steam | [74] |
| pT205 | pT205 | Thermo Fisher Scientific | 44–738G | Rabbit | Polyclonal | IgG | AB_2533738 | 1:2,500 | 1:500 | H2O steam | |
| pT217 | pT217 | Thermo Fisher Scientific | 44–744 | Rabbit | Polyclonal | IgG | AB_2533741 | 1:500 | 1:500 | H2O steam | |
| AT180 | pT231 | Thermo Fisher Scientific | MN1040 | Mouse | Monoclonal | IgG1κ | AB_223649 | 1:5,000 | 1:1,000 | H2O steam | [74] |
Abbreviations: IHC, immunohistochemistry; IF, immunofluorescence; Ref, reference
Figure 1.
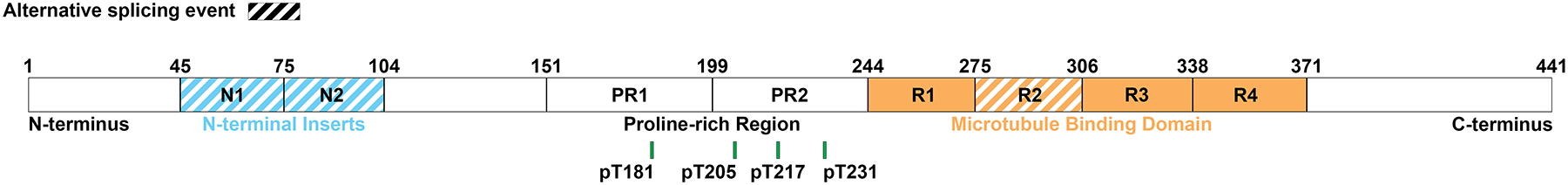
All phosphorylated tau antibodies used in this study fall within the proline-rich region. Amino acid position is illustrated by a green line for phosphorylated threonine (pT) at 181, 205, 217, and 231. Figure adapted from [22].
Posterior hippocampus sections were stained with thioflavin-S and TO-PRO-3. Anterior hippocampus sections were stained with thioflavin-S and pT181, pT205, pT217, or pT231. Fluorescence staining is further elaborated in Supplementary Methods.
To compare thioflavin-S to another stain recognizing advanced tangles, sections adjacent to thioflavin-S/pT231 fluorescent staining of anterior hippocampi were stained with Congo Red following standard procedures.
2.3. Digital pathology
A summary of slides stained/scanned, annotations, and experimental analysis is found in Supplementary Table 3. H&E-, immunohistochemically-, and Congo red-stained slides were digitally scanned at 20x using the Aperio AT2 scanner. The CA1, subiculum, and whole hippocampus were annotated using the pen tool in ImageScope (Leica Biosystems, version 12.4.3.5008). Fluorescently stained thioflavin-S/TO-PRO-3 posterior hippocampi and thioflavin-S/phosphorylated tau anterior hippocampi were digitally scanned at 20X (for scanning parameters, see Supplementary Table 4) using the Pannoramic 250 Flash III scanner (3DHistech). Using CaseViewer (3DHistech, version 2.3.0.99276) annotations were traced with the closed polygon tool for the thioflavin-S/TO-PRO-3-stained sections. The CA1 and subiculum were annotated on both immunohistochemically and thioflavin-S/TO-PRO-3-stained tissue as previously described [31] (Supplementary Methods). Hippocampal area (per mm2) was calculated by averaging the annotated whole hippocampus on each of the immunostained sections for each case. H&E-stained sections facilitated recognition of neuroanatomic boundaries and are thus traced first as a template for subsequent tracing on immunohistochemical slides. Artifacts were excluded using the negative pen tool.
Batch analysis of the brightfield scans was completed using eSlideManager (Leica Biosystems). Digitized slides were analyzed with a positive pixel count (v9) (Supplementary Table 5) macro custom designed for each antibody to recognize the 3, 3’-diaminobenzidine staining on the tissue and to exclude background to obtain burden, as previously described [42–46]. Batch analysis of the fluorescent scans was completed using QuantCenter’s HistoQuant module in CaseViewer (3DHistech), which was designed specifically to recognize thioflavin-S (FITC) positive staining (Supplementary Table 6). The macro was custom designed to exclude dystrophic neurites associated with the amyloid-β plaques across all cases. To negate autofluorescence, FITC and TRITC positive colocalization was not included. Any artifacts were annotated with the closed polygon tool, analyzed, and manually subtracted from the data.
2.4. Neurofibrillary tangle maturity level classifications
Neurofibrillary tangles were classified into the following maturity levels: pretangle, intermediary 1 tangle, mature tangle, intermediary 2 tangle, and ghost tangle [22]. Pretangles were identified by diffuse or granular staining of phosphorylated tau and could have perinuclear accumulation of tau. Pretangles were less intensely stained than mature tangles. Intermediary 1 tangles were identified with intensely stained aggregates that did not fill the entire neuron. These aggregates may be round or fibrillar in appearance. Mature tangles were identified by intense staining throughout most, if not all, of the neuron, with an associated nucleus that was often displaced or shrunken in appearance. Intermediary 2 tangles were identified with intense staining throughout the entire neuron but lack a nucleus. Ghost tangles were identified with weaker staining compared to mature tangles in structures that appear to be loosely arranged bundles of fibers with no associated nucleus.
2.5. Neurofibrillary tangle maturity semi-quantification
Neurofibrillary tangle maturity levels were semi-quantified in the annotated regions in the CA1 and subiculum from phosphorylated tau and thioflavin-S-stained sections using a grading system: absence, rare, and presence. Absence referred to 0 neurofibrillary tangles of a certain maturity level. Rare referred to 1–5 individual tangles of a certain maturity level. Presence referred to greater than 5 tangles of a certain maturity level. For quantification, we assigned each frequency a value: absence=0, rare=0.5, presence=1. For each phosphorylated tau site or thioflavin-S section, the semi-quantitative score of each neurofibrillary tangle maturity level were summed together to obtain a cumulative tangle maturity score per stain.
2.6. Nomenclature
The current study will use “pT” when describing immunohistochemical evidence from the phosphorylated threonine site and “p-tau” when discussing phosphorylated tau fluid biomarkers.
2.7. Statistical analysis
A permutation test with Kendall’s Tau was used to test differences of continuous values between Braak stages and for the whole hippocampal area measures with tau burden. A Pearson’s chi-squared test was used to test differences of categorical values between Braak stages. To calculate the subiculum:CA1 ratios, a small delta=0.1 added to the burden was used to stabilize the variance. Burden measures for subiculum were divided by CA1 and then the natural log was calculated. The sample mean of these logged ratios was calculated and a one-group t-test with a 95% confidence interval was performed. The values were then exponentiated to give fold difference in the CA1 to subiculum. A Kruskal-Wallis rank sum test was used to compare the phosphorylated tau burden and thioflavin-S burden and tangle counts of APOE ε4 carriers to non-carriers. Continuous values are reported as median (range [minimum-maximum]) unless otherwise noted. P-values <0.05 were considered statistically significant. All statistical analyses were performed using R (version 4.0.3). Figure development is elaborated in Supplementary Methods.
3. RESULTS:
3.1. Morphologic characteristics of neurofibrillary tangle and neuritic pathology
To evaluate the neurofibrillary tangle maturity level for each phosphorylated tau site, we immunohistochemically evaluated serial sections of the hippocampus for each antibody. At least one instance of each neurofibrillary tangle maturity level was visualized for all phosphorylated tau sites (Fig. 2). In addition, we found neuritic pathology (neuropil threads, neuritic plaques, and tangle associated neuritic clusters) was recognized by all phosphorylated tau antibodies (Supplementary Fig. 1 a–l). Neuropathologic features of ARTAG (thorn shaped astrocytes) and argyrophilic grains disease (coiled bodies) were also observed (Supplementary Fig. 1 m–t).
Figure 2.
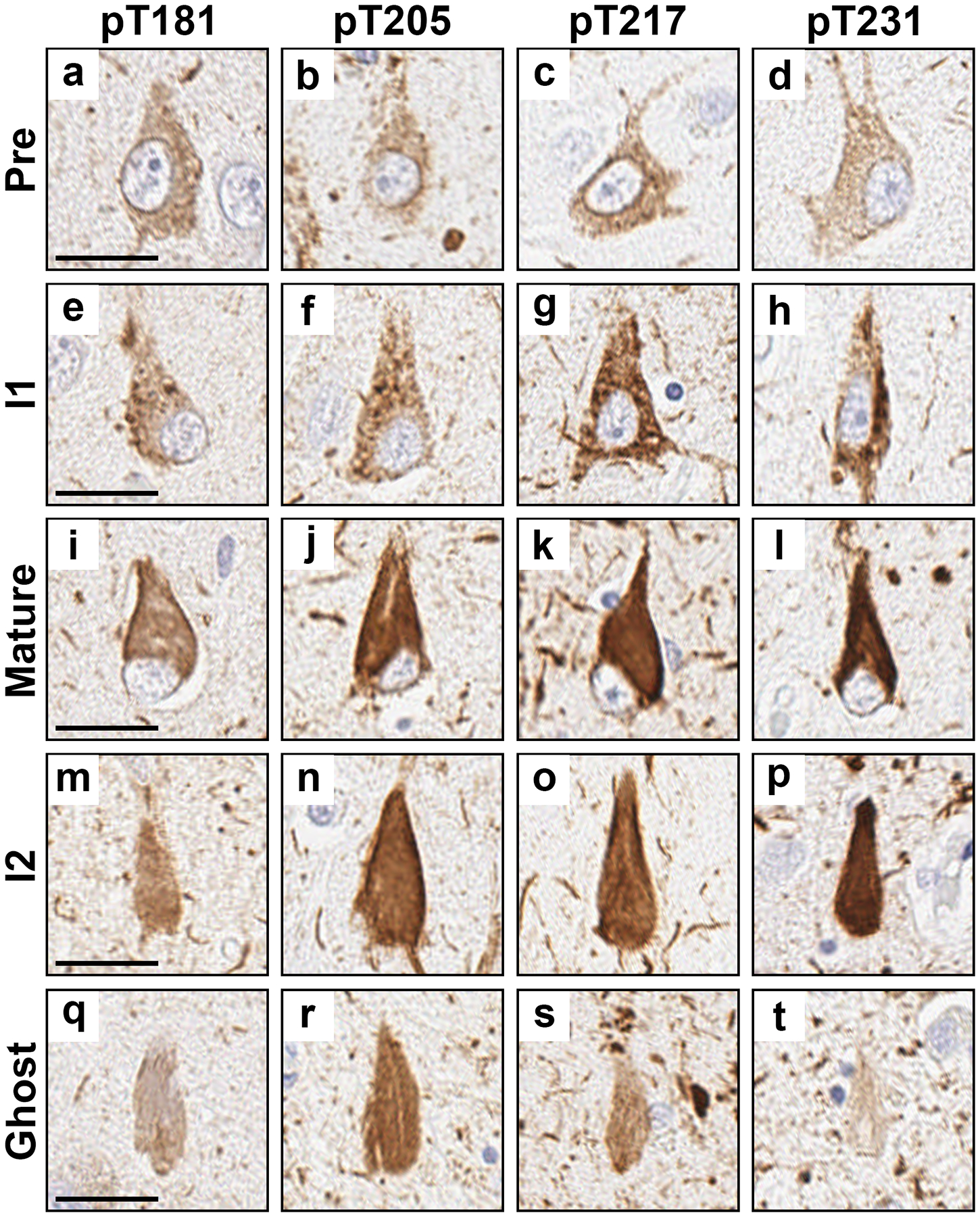
Examples of neurofibrillary tangle maturity levels and neuritic pathology observed with each phosphorylated tau site. a-d Pretangles, e-h intermediary 1 (I1), i-l mature tangles, m-p intermediary 2 (I2), and q-t ghost tangles. a, e, i, m, and q were stained with the pT181 antibody. b, f, j, n, and r were stained with the pT205 antibody. c, g, k, o, and s were stained with the pT217 antibody. d, h, l, p, and t were stained with the pT231 antibody. All images were taken in the CA1 subsector of the hippocampus except for q, r, and t which were taken in the subiculum. a, e, i, l, and t are from case 18. b is from case 9. c is from case 14. d, h, and n are from case 17. f and j are from case 16. g, q, and r are from case 13. k and s are from case 15. m and p are from case 23. o is from case 22. Scale bar measures 25 μm.
To compare the maturity levels recognized by these phosphorylated tau sites to a marker recognizing advanced neurofibrillary tangle maturity levels (mature tangles and ghost tangles), anterior hippocampi of Braak III and VI cases were co-stained with thioflavin-S (Supplementary Fig. 2). As expected, while pretangles stained with the phosphorylated tau antibodies, there was no co-staining of thioflavin-S. Intermediary 1s appeared to be composed of aggregated phosphorylated tau prior to β-pleated sheet structure formation as recognized by thioflavin-S. Mature tangles showed costaining of phosphorylated tau and thioflavin-S. Intermediary 2s showed a predominance of thioflavin-S with limited phosphorylated tau. Ghost tangles were only stained by thioflavin-S. These maturity levels were also compared to neurofibrillary tangles stained by Congo red, another dye that recognizes advanced neurofibrillary tangle maturity levels. Like thioflavin-S, Congo red stained mature tangle and ghost tangles, but did not stain pretangles, in contrast to the early tangles recognized by the phosphorylated tau antibodies (Supplementary Fig. 3).
3.2. Regional tau burden in CA1 and subiculum
We sought to determine the regional differences of tau pathology recognized by these phosphorylated tau antibodies between the CA1 and subiculum (Fig. 3). In the past, we observed nearly twice the number of neurofibrillary tangles in the subiculum compared to CA1 in the typical AD subtype, as visualized by thioflavin-S [31]. The burden of pT181 was 1.45-fold higher in the subiculum compared to the CA1 (95% confidence interval [CI] 1.32–1.59, p<0.001). pT205 burden was 1.25-fold higher in the subiculum compared to the CA1 (CI 1.11–1.40, p<0.001). The burden of pT217 was 1.36-fold higher in the subiculum compared to the CA1 (CI 1.23–1.50, p<0.001). pT231 burden was 1.30-fold higher in the subiculum compared to the CA1 (CI 1.32–1.59, p<0.001). While thioflavin-S burden was not significantly elevated in the subiculum compared to the CA1, thioflavin-S positive tangle counts were 1.36-fold higher in the subiculum compared to the CA1 (CI 1.09–1.70, p=0.009).
Figure 3.
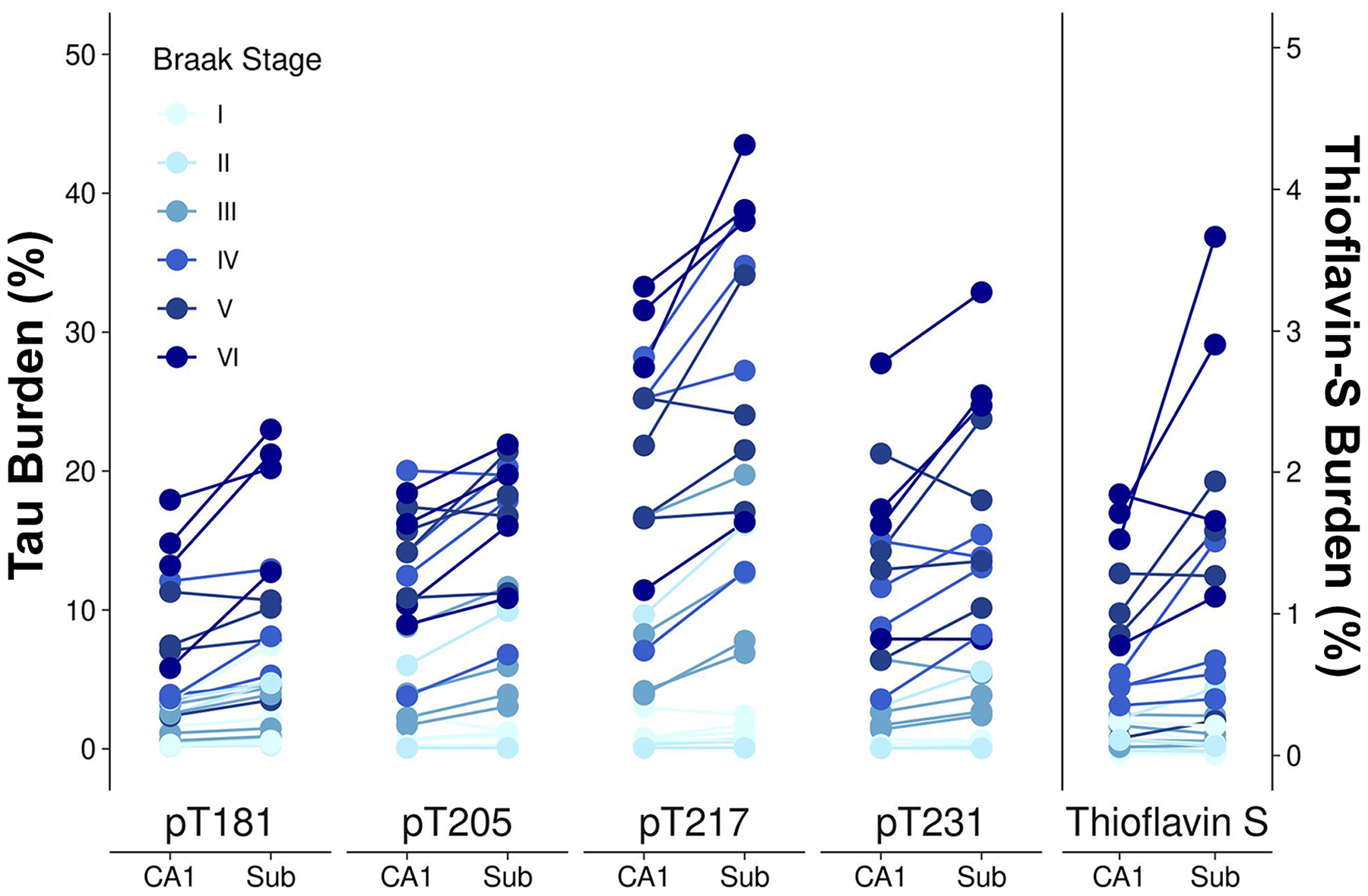
Neurofibrillary tangle burden in the CA1 and subiculum. Burden measures were plotted for the CA1 and subiculum. Data points were vertically jittered ±0.15 for display purposes. Images are from case 18. Scale bar measures 100 μm.
3.3. Global hippocampal evaluation of tau burden
We next sought to determine differences between the phosphorylated tau sites when using the global tau measure, Braak stage [32, 33], to stratify cases. The hippocampus was specifically chosen to allow for cross-sectional interpretation before (i.e. Braak I-II) and after overt limbic involvement (i.e. Braak > III) [33]. In Braak stages I and II, there were primarily rare pretangles and intermediary 1s (Supplementary Fig. 4). By Braak stage III, pretangles, intermediary 1s, and mature tangles were frequently present, with rare intermediary 2s observed. From Braak stages IV to VI, pretangles, intermediary 1s, mature tangles were frequently present, and an increasing amount of intermediary 2s were observed to be present. However, there were relatively fewer pretangles present in Braak stages V and VI. Ghost tangles were absent to rare across all Braak stages.
As expected, tau burden measurements increased through each Braak stage (Table 2, Supplementary Fig. 5–6). pT181 burden increased steadily between Braak stage III and Braak stage VI. pT205 burden increased from Braak stage II to VI, where burden appeared to level off between Braak stages IV and VI. pT217 burden increased from Braak stage II to III, sharply increased from Braak stages III to IV, leveled off from Braak stage IV to V, before increasing again to stage VI. Finally, pT231 burden increased more steadily between Braak stage III to VI.
Table 2.
Characteristics and digital pathology findings by Braak stage.
| Characteristic | Braak I | Braak II | Braak III | Braak IV | Braak V | Braak VI | p-value |
|---|---|---|---|---|---|---|---|
| Number | 4 | 4 | 4 | 4 | 4 | 4 | |
| Age at death, year | 87 (79, 93) | 80 (71, 84) | 89 (87, 93) | 84 (82, 93) | 86 (82, 90) | 79 (71, 84) | 0.041 |
| Females (%) | 2/4 (50%) | 2/4 (50%) | 2/4 (50%) | 2/4 (50%) | 2/4 (50%) | 2/4 (50%) | 1.000 |
| APOE ε4+ (%) | 0/4 (0%) | 0/4 (0%) | 0/4 (0%) | 2/4 (50%) | 3/4 (75%) | 2/4 (50%) | 0.058 |
| Brain weight, g | 1305 (960, 1440) | 1090 (1060, 1220) | 1140 (1000, 1320) | 1050 (1020, 1340) | 1030 (960, 1160) | 890 (760, 1100) | 0.002 |
| Digital pathology findings | |||||||
| Whole hippocampus measures | |||||||
| Area (mm2) | 44 (43, 49) | 45 (39, 60) | 50 (35, 67) | 48 (35, 67) | 31 (28, 54) | 34 (31, 40) | 0.034 |
| pT181 burden, % | 1.6 (0.50, 7.9) | 2.9 (0.47, 7.7) | 1.7 (0.64, 4.5) | 4.1 (2.8, 8.6) | 7.2 (1.8, 8.9) | 13 (10, 14) | <0.001 |
| pT205 burden, % | 0.52 (0.15, 0.82) | 0.19 (0.065, 4.7) | 2.3 (1.2, 5.8) | 10 (2.7, 13) | 9.4 (6.7, 12) | 9.5 (6.8, 12) | <0.001 |
| pT217 burden, % | 0.78 (0.38, 2.0) | 0.70 (0.090, 7.4) | 4.7 (2.9, 10) | 16 (5.6, 18) | 13 (12, 17) | 20 (8.3, 23) | <0.001 |
| pT231 burden, % | 0.25 (0.17, 0.36) | 0.11 (0.048, 2.3) | 1.5 (0.93, 3.5) | 6.6 (2.9, 8.1) | 8.3 (4.9, 13) | 12 (6.4, 17) | <0.001 |
| CA1 measures | |||||||
| Thioflavin-S counts | 0 (0, 1) | 1 (0, 1) | 2 (1, 4) | 5 (2, 10) | 10 (4, 25) | 22 (6, 30) | <0.001 |
| pT181 burden, % | 0.95 (0.24, 3.5) | 1.4 (0.19, 3.6) | 1.8 (0.58, 3.2) | 3.8 (3.5, 12) | 7.3 (2.4, 11) | 14 (5.8, 18) | <0.001 |
| pT205 burden, % | 0.70 (0.25, 2.5) | 0.21 (0.066, 6.0) | 3.1 (1.7, 8.8) | 13 (3.8, 20) | 15 (11, 17) | 13 (8.9, 18) | <0.001 |
| pT217 burden, % | 0.78 (0.60, 3.0) | 0.37 (0.073, 9.6) | 6.2 (3.9, 17) | 25 (7.1, 28) | 19 (17, 25) | 30 (11, 33) | <0.001 |
| pT231 burden, % | 0.27 (0.24, 0.70) | 0.11 (0.046, 3.1) | 2.2 (1.4, 6.5) | 10 (3.6, 15) | 14 (6.4, 21) | 17 (7.9, 28) | <0.001 |
| Thioflavin-S, % | 0.16 (0.014, 0.23) | 0.085 (0.030, 0.26) | 0.16 (0.057, 0.29) | 0.49 (0.35, 0.57) | 0.93 (0.12, 1.3) | 1.6 (0.78, 1.8) | <0.001 |
| Subiculum measures | |||||||
| Thioflavin S counts | 0 (0, 1) | 0 (0, 1) | 2 (1, 4) | 8 (6, 17) | 22 (8, 40) | 40 (25, 55) | <0.001 |
| pT181 burden, % | 1.4 (0.37, 7.4) | 2.8 (0.26, 4.7) | 2.7 (0.91, 4.4) | 6.7 (4.6, 13) | 9.0 (3.5, 11) | 21 (13, 23) | <0.001 |
| pT205 burden, % | 1.1 (0.24, 1.2) | 0.24 (0.064, 9.9) | 4.9 (3.1, 12) | 19 (6.8, 20) | 18 (11, 21) | 18 (11, 22) | <0.001 |
| pT217 burden, % | 1.5 (0.64, 2.4) | 0.64 (0.082, 16) | 10 (6.9, 20) | 31 (13, 39) | 23 (17, 34) | 38 (16, 43) | <0.001 |
| pT231 burden, % | 0.46 (0.34, 0.65) | 0.14 (0.045, 5.6) | 3.3 (2.4, 5.4) | 13 (8.2, 15) | 16 (10, 24) | 25 (7.9, 33) | <0.001 |
| Thioflavin-S, % | 0.072 (0.005, 0.209) | 0.045 (0.013, 0.47) | 0.12 (0.070, 0.28) | 0.62 (0.40, 1.5) | 1.4 (0.24, 1.9) | 2.3 (1.1, 3.7) | <0.001 |
When phosphorylated tau and thioflavin-S measures were stratified by APOE ε4 genotype across all individuals studied, higher values were consistently observed in the APOE carriers (Supplementary Table 7). The CA1 and subiculum phosphorylated tau burden and thioflavin-S burden were combined to visualize differences (Supplementary Figure 7). Data plotted for pT205 and pT217 visually demonstrate the most striking differences.
3.4. Cumulative neurofibrillary tangle maturity score
To determine the predominance of neurofibrillary tangle maturity level recognized by each phosphorylated tau site, we semi-quantified the presence or absence of pretangles, intermediary 1s, mature tangles, intermediary 2s, and ghost tangles (Fig. 4). Overall, the phosphorylated tau fluid biomarker sites immunohistochemically recognized similar tangle maturity levels, as demonstrated by the cumulative tangle maturity score. There was a predilection for each of the phosphorylated tau antibodies towards early neurofibrillary tangle pathology, especially in earlier Braak stages. Visual inspection of the graph suggests pT181 may recognize pretangles to a slightly higher extent and subsequent neurofibrillary tangle maturity levels to a lesser extent. Ghost tangles were extremely rare for all phosphorylated tau sites. In contrast, thioflavin-S, a dye which binds to the β-pleated sheets, predominately recognized mature tangles through ghost tangles (Fig. 4a). The overall score of each tangle maturity level of the phosphorylated tau sties combined in each case revealed a predilection of the early neurofibrillary tangle maturity levels for each case (Fig. 4b).
Figure 4.
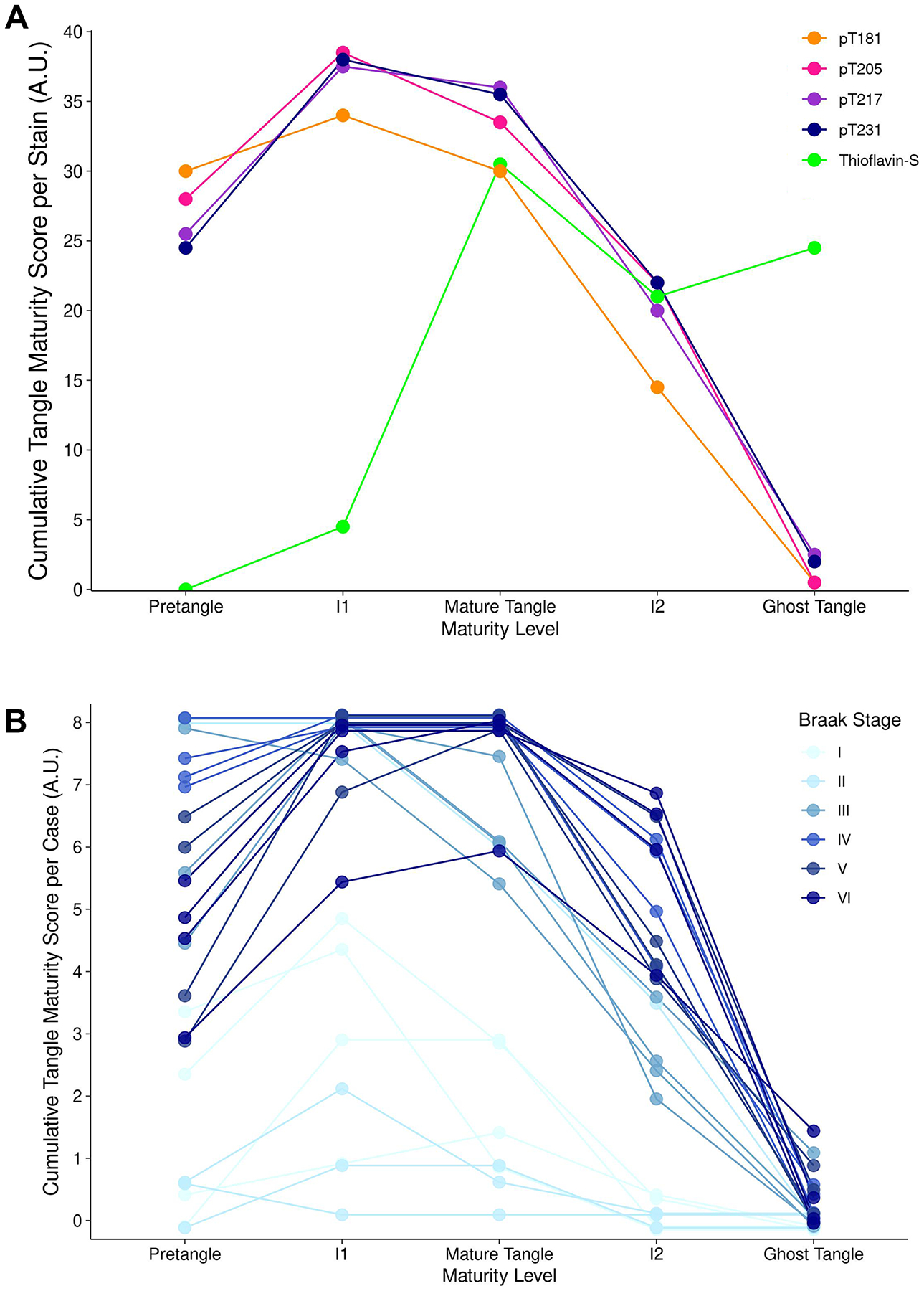
Semi-quantification frequency neurofibrillary tangle maturity levels for each phosphorylated tau site. a For each phosphorylated tau site, semiquantitative values for each neurofibrillary tangle maturity level were added together for each case. Data points were vertically jittered ±0.15 for display purposes. Acronyms: I1, intermediary 1; I2, intermediary 2. b For each case, semiquantitative values for each neurofibrillary tangle maturity level were added together for each phosphorylated tau site. Data points were vertically jittered ±0.15 for display purposes. Acronyms: I1, intermediary 1; I2, intermediary 2.
4. DISCUSSION:
Our findings provide neurobiologic evidence that fluid biomarker-based phosphorylated tau sites in the proline-rich region are present during early neurofibrillary tangle maturity levels. While all neurofibrillary tangle maturity levels were observed in pT181, pT205, pT217, and pT231, there was a predilection toward pretangles, intermediary 1s, and mature tangles. The striking difference in vulnerability between CA1 and subiculum tangle counts observed on thioflavin-S was not as readily observed in tau burden measurements of the phosphorylated tau sites suggesting diminished antibody recognition once tangle pathology matured to advanced levels. Phosphorylated tau burden generally increased as Braak stage increased with some plateauing observed in later Braak stages, even though the phosphorylated tau antibodies primarily recognize early neurofibrillary tangle maturity levels.
While the temporal sequence of detectable phosphorylated tau levels in fluids is still under investigation [15, 16, 19], evidence suggests that fluid tau levels may elevate earlier than observed uptake on tau positron emission tomography (PET) [37]. Previous tau-PET autoradiographic and fluorescence studies suggested that flortaucipir (AV-1451) may recognize middling to advanced neurofibrillary tangle maturity levels [24–26]. There are some hypotheses as to why the phosphorylated tau fluid biomarkers are elevated prior to tau-PET positivity. One hypothesis is that the phosphorylated tau fluid biomarkers may recognize early neurofibrillary tangle maturity levels. Another hypothesis is that a higher burden of neurofibrillary tangle pathology is needed to show positivity on PET scans, whereas the fluid biomarkers may be more sensitive. For this study, we tested the hypothesis that the phosphorylated tau sites in the fluid biomarkers recognize early neurofibrillary tangle maturity levels. Currently, there is a lack of in-depth characterization of the neurofibrillary tangle maturity levels [22] recognized by the phosphorylated tau fluid biomarker sites. To fill this knowledge gap, we performed deep phenotyping of neurofibrillary tangle morphology recognized by pT181, pT205, pT217, and pT231 and employed digital pathology to quantify tau burden. We found that all phosphorylated tau sites studied are present primarily in early neurofibrillary tangle maturity levels. Their recognition of pretangles, intermediary 1s, and mature tangles may help explain why these biomarker sites are observed earlier during the AD dementia course [19, 29, 36, 47].
To put in context of the current study and our morphologic characterization across neurofibrillary tangle maturity, we retrospectively examined photomicrographs from previous studies utilizing antibodies that recognized these phosphorylated tau sites. Evaluation of these photomicrographs support our results that pT181 [48–50], pT205 [51–54], pT217 [50, 55], and pT231 [49, 50, 56–60] recognize early neurofibrillary tangle maturity levels as we observed labeling of pretangles and mature tangles but not ghost tangles. Previously, some phosphorylated tau fluid biomarkers were temporally sequenced based on anticipated projected course in dominantly inherited AD cases, which suggested cerebrospinal fluid levels of p-tau217 may elevate earlier, followed by p-tau181, then p-tau205 [29]. pT231 was recently suggested to be one of the initial phosphorylated sites found in neurons that may develop earlier than pretangles [61], which supports evidence from a plasma study in an autopsy cohort showing plasma p-tau231 levels may distinguish Braak 0 from Braak I-II [19]. While our study was not designed to disentangle the sequence of events, we provide histopathologic evidence that pT181, pT205, pT217, and pT231 are expressed early in the neurofibrillary tangle lifespan. Stratification by APOE ε4 status provides novel histopathologic insight into sensitivity of phospho-tau sites in AD, as we observed a striking difference between APOE ε4 non-carriers and carriers in pT205 and pT217. T205 was suggested to be phosphorylated last based on quantitative mass spectrometry [62] and biomarker pathology staging [29], which taken together with our data may support later phosphorylation event of T205. It is important to consider that these phosphorylated tau fluid biomarkers may not be accurately reflecting the tau species in neurofibrillary tangles as these fluid biomarkers detect tau fragments [63]. Upon co-staining with thioflavin-S, we observed the accumulation of phosphorylated tau prior to thioflavin-S positivity in intermediary 1s. We hypothesize these intermediary 1s, or nucleation sites [64], are the nidus of tau fibrillization, leading to mature tangles.
To further investigate differences in regional vulnerability, we elected to examine the CA1 and subiculum, two hippocampal subsectors known to be vulnerable in AD. Based upon observations of nearly twice the number of thioflavin-S positive tangles in the subiculum compared to the CA1 in typical AD [30, 31], our goal was to investigate differences in phosphorylated tau burden in these two regions. All phosphorylated tau sites had a significant increase in the burden in subiculum compared to the CA1. Interestingly, thioflavin-S burden was not significantly different in the CA1 and subiculum, whereas the thioflavin-S positive tangle counts were elevated in the subiculum compared to the CA1. This may be due to the neurofibrillary tangle counts being obtained in the highest density regions of the CA1 and subiculum. Additionally, the subiculum:CA1 tangle count ratio was less in this study compared to previous studies. This difference to previous findings may be due to advanced AD (Braak >IV) being used to calculate differences in CA1 and subiculum, whereas the current study examined a wider range from Braak I-VI. It is unclear why the subiculum has greater neurofibrillary tangle burden compared to the CA1 [30, 31], especially as the CA1 has more neurons compared to the subiculum as determined by a stereology study [65]. Moreover, initial findings from Braak & Braak detail tangles appearing in the CA1 first [33]. As the subiculum is the major outflow pathway of the hippocampus that receives multiple connections from CA1 and entorhinal cortex [66], these anatomical differences may confer heightened vulnerability to advanced neurofibrillary tangle maturity. Evaluation in the context of reciprocal connections [67] or perhaps populations of excitatory neurons [68] may warrant future investigation.
It is well-established that tau pathology increases across Braak stages [32, 33, 69]. While conformational and truncation events have been widely studied [70, 71], there is a current gap in the literature regarding the systematic evaluation of these four phosphorylated tau sites across a global measure of tau distribution (Braak stage) in the human brain. As expected in Braak stages I-II, we found minimal neurofibrillary tangle pathology primarily consisting of pretangles, intermediary 1s, and rarely mature tangles. However, by Braak stage III and onwards, there was an increase in the neurofibrillary tangle pathology with an increase in mature tangle and rare observations of intermediary 2. By Braak stage V, there was a notable decrease in pretangle population. This is not unexpected as the number of unaffected neurons with potential to accumulate pathology decreases as pathology burden increases [72]. Ghost tangles were not commonly observed, which may indicate the loss of the phosphate group at the advanced maturity levels. We hypothesize this is because as ghost tangles are the remnants of mature tangles once the neuron has died [1, 3] they are no longer contained by a membrane and as such are exposed to the surrounding environment including phosphatases. In a neuropathologically diagnosed cohort, plasma p-tau181 and p-tau231 levels were found to increase with Braak stage [19] and suggested to plateau in very advanced stages of disease [7]. Our findings provide support of the observed plateauing, suggesting that as the shift from early to advanced neurofibrillary tangle maturity occurs recognition by these phosphorylated tau sites in the proline-rich domain may be diminished.
Our study is not without limitations. One such is sample size; while n=4 cases per Braak stage is adequate for the morphologic characterization and semiquantitative methods of neurofibrillary tangle maturity; however, larger sample sizes are necessary to evaluate and interpret tau burden differences between Braak stages. Another limitation concerns the thickness of tissue sections. It is difficult to be certain of intermediary 2 tangles, as it is possible that the nucleus is out of plane of section. To overcome this, thicker sections can be used to include the entire neurofibrillary tangle. In the current study, we observed lighter staining by the pT181 antibody compared to the other phosphorylated tau sites. As antibody affinity may affect immunohistochemical burden analyses, caution is warranted in interpreting which phosphorylated tau site is increased the earliest in the current study. Additionally, we chose to focus on the hippocampus as this region is well characterized in terms of neurofibrillary tangle maturity [22, 23, 73]. As neurofibrillary tangle maturity is not well characterized in cortical regions, future studies should consider characterizing neurofibrillary tangle maturity levels in these regions to facilitate investigation of these phosphorylated tau fluid biomarker sites.
In conclusion, we performed a deep postmortem characterization of four phosphorylated tau fluid biomarker sites (pT181, pT205, pT217, and pT231) that are reliably elevated in AD dementia. To our knowledge, this is the first time these four phosphorylated tau sites were characterized together in the postmortem brain to evaluate recognition of neurofibrillary tangle maturity. While all neurofibrillary tangle maturity levels were visualized by each of the four phosphorylated tau sites, we observed a predilection towards early neurofibrillary tangle maturity with extremely rare observations of ghost tangles. Although fluid biomarkers do not provide regional involvement captured by tau-PET imaging, our study demonstrates these phosphorylated tau sites in the proline-rich domain readily recognize early aspects of neurofibrillary tangle maturity and may provide keen insight into the initial phase of neurofibrillary tangle changes.
Supplementary Material
Highlights:
Immunohistochemical evaluation of four phosphorylated tau fluid biomarker sites
Earlier neurofibrillary tangle maturity levels recognized by phosphorylated tau in proline-rich region
Advanced tangle pathology is elevated in the subiculum compared to the CA1 of the hippocampus
Novel semi-quantitative frequency to calculate tangle maturity frequency
Research in Context:
Systematic review: The authors searched PubMed and Google Scholar for literature on Alzheimer’s disease, tau, neurofibrillary tangles, and tau biomarkers in Alzheimer’s disease.
Interpretation: Phosphorylated tau fluid biomarker measures are elevated in Alzheimer’s disease earlier than tau positron emission tomography measures. As postmortem studies show that tau PET recognizes more advanced neurofibrillary tangle maturity levels, we hypothesize phosphorylated tau fluid biomarkers recognize earlier maturity levels. Our study provides supportive evidence that in the postmortem brain, the phosphorylated tau fluid biomarkers primarily recognize earlier neurofibrillar tangle maturity levels.
Future directions: We recommend comparisons of phosphorylated tau fluid biomarker measures with postmortem digital pathology measures. Additionally, it will be important to determine the source of soluble tau in fluids and if the fluid tau levels accurately reflect insoluble tau in the postmortem brain.
Acknowledgements
We are grateful to Virginia Phillips, Ariston Librero, Jo Landino, Jessica Tranovich, Ashley Wood, Janisse Cabrera, and the Cytometry and Imaging Lab for histologic and imaging support.
Funding sources
The investigators are supported by grants from National Institute on Aging (R01 AG054449, R01 AG075802, R01 AG073282, P30 AG062677, RF1 AG069052, U01 AG057195, P50 AG047266, U19 AG069701) and the Florida Department of Health, Ed and Ethel Moore Alzheimer’s Disease Research Program (8AZ06, 20A22).
Conflicts of interest
CMM, SAL, JEC, HS, CL, MC-C, RD, and DWD have no conflicts of interest to declare. RCP is a consultant for Biogen, Inc., Roche, Inc., Merck, Inc., Genentech Inc. (DSMB) and Eisai, Inc., receives publishing royalties from Mild Cognitive Impairment (Oxford University Press, 2003), UpToDate. NRG-R takes part in multicentre trials supported by AbbVie, Eli Lilly, and Biogen, outside the submitted work. MMM has consulted for Biogen and Brain Protection Company and receives funding from the NIH/NIA and DOD. MEM served as a consultant for AVID Radiopharmaceuticals.
Abbreviations
- A.U.
Arbitrary unit
- AD
Alzheimer’s disease
- ARTAG
Aging-related tau astrogliopathy
- CA
Cornu ammonis
- CB
Coiled body
- CVA
Cerebrovascular accident
- DG
Digital gain
- Exp
Exposure
- F
Female
- F/S
Focus/stitching channel
- FLAME
Florida Autopsied Multi-Ethnic
- FTD/PPA
Frontotemporal dementia/primary progressive aphasia
- H&E
Hematoxylin and eosin
- I1
Intermediary 1
- I2
Intermediary 2
- Int
Intensity
- M
Male
- MCI
Mild cognitive impairment
- NP
Neuritic plaque
- NPH
Normal pressure hydrocephalus
- NT
Neuropil thread
- p
Phosphorylation
- PA
Pathological aging
- PART
Primary age related tauopathy
- PET
Positron emission tomography
- SC
Senile change
- Sub
Subiculum
- T
Threonine
- TANC
Tangle associated neuritic cluster
- Thio-S
Thioflavin-S
- TSA
Thorn shaped astrocytes
- TSA
Thorn shaped astrocyte
- VaD
Vascular disease
- Ref
Reference
- IF
Immunofluorescence
- IHC
Immunohistochemistry
Footnotes
All brains were acquired with appropriate ethical approval, and the research performed on postmortem samples was approved by the Mayo Clinic Research Executive Committee.
References
- [1].Alzheimer A. Über eine eigenartige Erkankung der Hirnrinde. Allg Z Psychiatr Ps. 1907;18:177–9. [Google Scholar]
- [2].Alzheimer A. Über eigenartige Krankheitsfälle des späteren Alters. Z Gesamte Neurol Psy. 1911;4:356–85. [Google Scholar]
- [3].Stelzmann RA, Schnitzlein HN, Murtagh FR. An English translation of Alzheimer’s 1907 paper, “Uber eine eigenartige Erkankung der Hirnrinde”. Clin Anat. 1995;8:429–31. [DOI] [PubMed] [Google Scholar]
- [4].Jack CR Jr., Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14:535–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lowe VJ, Lundt ES, Albertson SM, Min HK, Fang P, Przybelski SA, et al. Tau-positron emission tomography correlates with neuropathology findings. Alzheimers Dement. 2020;16:561–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ossenkoppele R, Schonhaut DR, Schöll M, Lockhart SN, Ayakta N, Baker SL, et al. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer’s disease. Brain. 2016;139:1551–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lantero Rodriguez J, Karikari TK, Suárez-Calvet M, Troakes C, King A, Emersic A, et al. Plasma p-tau181 accurately predicts Alzheimer’s disease pathology at least 8 years prior to post-mortem and improves the clinical characterisation of cognitive decline. Acta Neuropathol. 2020;140:267–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mielke MM, Frank RD, Dage JL, Jeromin A, Ashton NJ, Blennow K, et al. Comparison of Plasma Phosphorylated Tau Species With Amyloid and Tau Positron Emission Tomography, Neurodegeneration, Vascular Pathology, and Cognitive Outcomes. JAMA Neurology. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Mehta PD, Thal L, Wisniewski HM, Grundke-Iqbal I, Iqbal K. Paired Helical Filament Antigen in CSF. The Lancet. 1985;326. [DOI] [PubMed] [Google Scholar]
- [10].Vandermeeren M, Mercken M, Vanmechelen E, Six J, van de Voorde A, Martin JJ, et al. Detection of tau proteins in normal and Alzheimer’s disease cerebrospinal fluid with a sensitive sandwich enzyme-linked immunosorbent assay. J Neurochem. 1993;61:1828–34. [DOI] [PubMed] [Google Scholar]
- [11].Wolozin B, Davies P. Alzheimer-related neuronal protein A68: specificity and distribution. Ann Neurol. 1987;22:521–6. [DOI] [PubMed] [Google Scholar]
- [12].Blennow K, Wallin A, Ågren H, Spenger C, Siegfried J, Vanmechelen E. Tau protein in cerebrospinal fluid: a biochemical marker for axonal degeneration in Alzheimer disease? Mol Chem Neuropathol. 1995;26:231–45. [DOI] [PubMed] [Google Scholar]
- [13].Vanmechelen E, Vanderstichele H, Davidsson P, Van Kerschaver E, Van Der Perre B, Sjögren M, et al. Quantification of tau phosphorylated at threonine 181 in human cerebrospinal fluid: a sandwich ELISA with a synthetic phosphopeptide for standardization. Neurosci Lett. 2000;285:49–52. [DOI] [PubMed] [Google Scholar]
- [14].Barthélemy NR, Mallipeddi N, Moiseyev P, Sato C, Bateman RJ. Tau Phosphorylation Rates Measured by Mass Spectrometry Differ in the Intracellular Brain vs. Extracellular Cerebrospinal Fluid Compartments and Are Differentially Affected by Alzheimer’s Disease. Front Aging Neurosci. 2019;11:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Barthélemy NR, Bateman RJ, Marin P, Becher F, Sato C, Lehmann S, et al. Tau hyperphosphorylation on T217 in cerebrospinal fluid is specifically associated to amyloid-β pathology. bioRxiv. 2017. [Google Scholar]
- [16].Mielke MM, Aakre JA, Algeciras-Schimnich A, Proctor NK, Machulda MM, Eichenlaub U, et al. Comparison of CSF phosphorylated tau 181 and 217 for cognitive decline. Alzheimers Dement. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kohnken R, Buerger K, Zinkowski R, Miller C, Kerkman D, DeBernardis J, et al. Detection of tau phosphorylated at threonine 231 in cerebrospinal fluid of Alzheimer’s disease patients. Neurosci Lett. 2000;287:187–90. [DOI] [PubMed] [Google Scholar]
- [18].Palmqvist S, Janelidze S, Quiroz YT, Zetterberg H, Lopera F, Stomrud E, et al. Discriminative Accuracy of Plasma Phospho-tau217 for Alzheimer Disease vs Other Neurodegenerative Disorders. JAMA. 2020;324:772–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ashton NJ, Pascoal TA, Karikari TK, Benedet AL, Lantero-Rodriguez J, Brinkmalm G, et al. Plasma p-tau231: a new biomarker for incipient Alzheimer’s disease pathology. Acta Neuropathol. 2021;141:709–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Tatebe H, Kasai T, Ohmichi T, Kishi Y, Kakeya T, Waragai M, et al. Quantification of plasma phosphorylated tau to use as a biomarker for brain Alzheimer pathology: pilot case-control studies including patients with Alzheimer’s disease and down syndrome. Mol Neurodegener. 2017;12:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mielke MM, Hagen CE, Xu J, Chai X, Vemuri P, Lowe VJ, et al. Plasma phospho-tau181 increases with Alzheimer’s disease clinical severity and is associated with tau- and amyloid-positron emission tomography. Alzheimers Dement. 2018;14:989–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Moloney CM, Lowe VJ, Murray ME. Visualization of neurofibrillary tangle maturity in Alzheimer’s disease: A clinicopathologic perspective for biomarker research. Alzheimers Dement. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bancher C, Brunner C, Lassmann H, Budka H, Jellinger K, Wiche G, et al. Accumulation of abnormally phosphorylated tau precedes the formation of neurofibrillary tangles in Alzheimer’s disease. Brain Res. 1989;477:90–9. [DOI] [PubMed] [Google Scholar]
- [24].Lowe VJ, Curran G, Fang P, Liesinger AM, Josephs KA, Parisi JE, et al. An autoradiographic evaluation of AV-1451 Tau PET in dementia. Acta Neuropathol Commun. 2016;4:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Marquié M, Normandin MD, Vanderburg CR, Costantino IM, Bien EA, Rycyna LG, et al. Validating novel tau positron emission tomography tracer [F-18]-AV-1451 (T807) on postmortem brain tissue. Ann Neurol. 2015;78:787–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ono M, Sahara N, Kumata K, Ji B, Ni R, Koga S, et al. Distinct binding of PET ligands PBB3 and AV-1451 to tau fibril strains in neurodegenerative tauopathies. Brain. 2017;140:764–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Mattsson-Carlgren N, Andersson E, Janelidze S, Ossenkoppele R, Insel P, Strandberg O, et al. Abeta deposition is associated with increases in soluble and phosphorylated tau that precede a positive Tau PET in Alzheimer’s disease. Sci Adv. 2020;6:eaaz2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Janelidze S, Berron D, Smith R, Strandberg O, Proctor NK, Dage JL, et al. Associations of Plasma Phospho-Tau217 Levels With Tau Positron Emission Tomography in Early Alzheimer Disease. JAMA Neurology. 2021;78:149–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Barthélemy NR, Li Y, Joseph-Mathurin N, Gordon BA, Hassenstab J, Benzinger TLS, et al. A soluble phosphorylated tau signature links tau, amyloid and the evolution of stages of dominantly inherited Alzheimer’s disease. Nat Med. 2020;26:398–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Petersen C, Nolan AL, de Paula França Resende E, Miller Z, Ehrenberg AJ, Gorno-Tempini ML, et al. Alzheimer’s disease clinical variants show distinct regional patterns of neurofibrillary tangle accumulation. Acta Neuropathol. 2019;138:597–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Murray ME, Graff-Radford NR, Ross OA, Petersen RC, Duara R, Dickson DW. Neuropathologically defined subtypes of Alzheimer’s disease with distinct clinical characteristics: a retrospective study. Lancet Neurol. 2011;10:785–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112:389–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–59. [DOI] [PubMed] [Google Scholar]
- [34].Santos OA, Pedraza O, Lucas JA, Duara R, Greig-Custo MT, Hanna Al-Shaikh FS, et al. Ethnoracial differences in Alzheimer’s disease from the FLorida Autopsied Multi-Ethnic (FLAME) cohort. Alzheimers Dement. 2019;15:635–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Liesinger AM, Graff-Radford NR, Duara R, Carter RE, Hanna Al-Shaikh FS, Koga S, et al. Sex and age interact to determine clinicopathologic differences in Alzheimer’s disease. Acta Neuropathol. 2018;136:873–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Janelidze S, Mattsson N, Palmqvist S, Smith R, Beach TG, Serrano GE, et al. Plasma P-tau181 in Alzheimer’s disease: relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer’s dementia. Nat Med. 2020;26:379–86. [DOI] [PubMed] [Google Scholar]
- [37].Karikari TK, Pascoal TA, Ashton NJ, Janelidze S, Benedet AL, Rodriguez JL, et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer’s disease: a diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol. 2020;19:422–33. [DOI] [PubMed] [Google Scholar]
- [38].Karikari TK, Emersic A, Vrillon A, Lantero-Rodriguez J, Ashton NJ, Kramberger MG, et al. Head-to-head comparison of clinical performance of CSF phospho-tau T181 and T217 biomarkers for Alzheimer’s disease diagnosis. Alzheimers Dement. 2021;17:755–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ashton NJ, Benedet AL, Pascoal TA, Karikari TK, Lantero-Rodriguez J, Brum WS, et al. Cerebrospinal fluid p-tau231 as an early indicator of emerging pathology in Alzheimer’s disease. EBioMedicine. 2022;76:103836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Suarez-Calvet M, Karikari TK, Ashton NJ, Lantero Rodriguez J, Mila-Aloma M, Gispert JD, et al. Novel tau biomarkers phosphorylated at T181, T217 or T231 rise in the initial stages of the preclinical Alzheimer’s continuum when only subtle changes in Abeta pathology are detected. EMBO Mol Med. 2020;12:e12921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Horie K, Barthélemy NR, Sato C, Bateman RJ. CSF tau microtubule binding region identifies tau tangle and clinical stages of Alzheimer’s disease. Brain. 2021;144:515–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Crist AM, Hinkle KM, Wang X, Moloney CM, Matchett BJ, Labuzan SA, et al. Transcriptomic analysis to identify genes associated with selective hippocampal vulnerability in Alzheimer’s disease. Nat Commun. 2021;12:2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Hanna Al-Shaikh FS, Duara R, Crook JE, Lesser ER, Schaeverbeke J, Hinkle KM, et al. Selective Vulnerability of the Nucleus Basalis of Meynert Among Neuropathologic Subtypes of Alzheimer Disease. JAMA Neurol. 2020;77:225–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Murray ME, Ferman TJ, Boeve BF, Przybelski SA, Lesnick TG, Liesinger AM, et al. MRI and pathology of REM sleep behavior disorder in dementia with Lewy bodies. Neurology. 2013;81:1681–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Murray ME, Przybelski SA, Lesnick TG, Liesinger AM, Spychalla A, Zhang B, et al. Early Alzheimer’s disease neuropathology detected by proton MR spectroscopy. J Neurosci. 2014;34:16247–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Murray ME, Vemuri P, Preboske GM, Murphy MC, Schweitzer KJ, Parisi JE, et al. A quantitative postmortem MRI design sensitive to white matter hyperintensity differences and their relationship with underlying pathology. J Neuropathol Exp Neurol. 2012;71:1113–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Mattsson N, Zetterberg H, Janelidze S, Insel PS, Andreasson U, Stomrud E, et al. Plasma tau in Alzheimer disease. Neurology. 2016;87:1827–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Scheper W, Hoozemans JJ. The unfolded protein response in neurodegenerative diseases: a neuropathological perspective. Acta Neuropathol. 2015;130:315–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Augustinack JC, Schneider A, Mandelkow EM, Hyman BT. Specific tau phosphorylation sites correlate with severity of neuronal cytopathology in Alzheimer’s disease. Acta Neuropathol. 2002;103:26–35. [DOI] [PubMed] [Google Scholar]
- [50].Wennstrom M, Janelidze S, Nilsson KPR, Netherlands Brain B, Serrano GE, Beach TG, et al. Cellular localization of p-tau217 in brain and its association with p-tau217 plasma levels. Acta Neuropathol Commun. 2022;10:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Strang KH, Goodwin MS, Riffe C, Moore BD, Chakrabarty P, Levites Y, et al. Generation and characterization of new monoclonal antibodies targeting the PHF1 and AT8 epitopes on human tau. Acta Neuropathol Commun. 2017;5:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Trejo-Lopez JA, Sorrentino ZA, Riffe CJ, Prokop S, Dickson DW, Yachnis AT, et al. Generation and Characterization of Novel Monoclonal Antibodies Targeting p62/sequestosome-1 Across Human Neurodegenerative Diseases. J Neuropathol Exp Neurol. 2020;79:407–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Strang KH, Sorrentino ZA, Riffe CJ, Gorion KM, Vijayaraghavan N, Golde TE, et al. Phosphorylation of serine 305 in tau inhibits aggregation. Neurosci Lett. 2019;692:187–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Xia Y, Prokop S, Gorion KM, Kim JD, Sorrentino ZA, Bell BM, et al. Tau Ser208 phosphorylation promotes aggregation and reveals neuropathologic diversity in Alzheimer’s disease and other tauopathies. Acta Neuropathol Commun. 2020;8:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Hanes J, Kovac A, Kvartsberg H, Kontsekova E, Fialova L, Katina S, et al. Evaluation of a novel immunoassay to detect p-tau Thr217 in the CSF to distinguish Alzheimer disease from other dementias. Neurology. 2020;95:e3026–e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Bengoa-Vergniory N, Velentza-Almpani E, Silva AM, Scott C, Vargas-Caballero M, Sastre M, et al. Tau-proximity ligation assay reveals extensive previously undetected pathology prior to neurofibrillary tangles in preclinical Alzheimer’s disease. Acta Neuropathol Commun. 2021;9:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Jicha GA, Lane E, Vincent I, Otvos L Jr., Hoffmann R, Davies P. A conformation- and phosphorylation-dependent antibody recognizing the paired helical filaments of Alzheimer’s disease. J Neurochem. 1997;69:2087–95. [DOI] [PubMed] [Google Scholar]
- [58].Luna-Muñoz J, Chávez-Macías L, García-Sierra F, Mena R. Earliest stages of tau conformational changes are related to the appearance of a sequence of specific phospho-dependent tau epitopes in Alzheimer’s disease. J Alzheimers Dis. 2007;12:365–75. [DOI] [PubMed] [Google Scholar]
- [59].Luna-Muñoz J, Peralta-Ramirez J, Chávez-Macías L, Harrington CR, Wischik CM, Mena R. Thiazin red as a neuropathological tool for the rapid diagnosis of Alzheimer’s disease in tissue imprints. Acta Neuropathol. 2008;116:507–15. [DOI] [PubMed] [Google Scholar]
- [60].Vincent I, Rosado M, Davies P. Mitotic mechanisms in Alzheimer’s disease? J Cell Biol. 1996;132:413–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Aragão Gomes L, Uytterhoeven V, Lopez-Sanmartin D, Tomé SO, Tousseyn T, Vandenberghe R, et al. Maturation of neuronal AD-tau pathology involves site-specific phosphorylation of cytoplasmic and synaptic tau preceding conformational change and fibril formation. Acta Neuropathol. 2021;141:173–92. [DOI] [PubMed] [Google Scholar]
- [62].Wesseling H, Mair W, Kumar M, Schlaffner CN, Tang S, Beerepoot P, et al. Tau PTM Profiles Identify Patient Heterogeneity and Stages of Alzheimer’s Disease. Cell. 2020;183:1699–713 e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Karikari TK, Ashton NJ, Brinkmalm G, Brum WS, Benedet AL, Montoliu-Gaya L, et al. Blood phospho-tau in Alzheimer disease: analysis, interpretation, and clinical utility. Nat Rev Neurol. 2022. [DOI] [PubMed] [Google Scholar]
- [64].Luna-Muñoz J, García-Sierra F, Falcón V, Menéndez I, Chavez-Macias L, Mena R. Regional conformational change involving phosphorylation of tau protein at the Thr231, precedes the structural change detected by Alz-50 antibody in Alzheimer’s disease. J Alzheimers Dis. 2005;8:29–41. [DOI] [PubMed] [Google Scholar]
- [65].Walker MA, Highley JR, Esiri MM, McDonald B, Roberts HC, Evans SP, et al. Estimated neuronal populations and volumes of the hippocampus and its subfields in schizophrenia. Am J Psychiatry. 2002;159:821–8. [DOI] [PubMed] [Google Scholar]
- [66].Matsumoto N, Kitanishi T, Mizuseki K. The subiculum: Unique hippocampal hub and more. Neurosci Res. 2019;143:1–12. [DOI] [PubMed] [Google Scholar]
- [67].Llorens-Martín M, Blazquez-Llorca L, Benavides-Piccione R, Rabano A, Hernandez F, Avila J, et al. Selective alterations of neurons and circuits related to early memory loss in Alzheimer’s disease. Front Neuroanat. 2014;8:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Leng K, Li E, Eser R, Piergies A, Sit R, Tan M, et al. Molecular characterization of selectively vulnerable neurons in Alzheimer’s disease. Nat Neurosci. 2021;24:276–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Braak E, Braak H, Mandelkow EM. A sequence of cytoskeleton changes related to the formation of neurofibrillary tangles and neuropil threads. Acta Neuropathol. 1994;87:554–67. [DOI] [PubMed] [Google Scholar]
- [70].Basurto-Islas G, Luna-Munoz J, Guillozet-Bongaarts AL, Binder LI, Mena R, Garcia-Sierra F. Accumulation of aspartic acid421- and glutamic acid391-cleaved tau in neurofibrillary tangles correlates with progression in Alzheimer disease. J Neuropathol Exp Neurol. 2008;67:470–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Ghoshal N, Garcia-Sierra F, Wuu J, Leurgans S, Bennett DA, Berry RW, et al. Tau conformational changes correspond to impairments of episodic memory in mild cognitive impairment and Alzheimer’s disease. Exp Neurol. 2002;177:475–93. [DOI] [PubMed] [Google Scholar]
- [72].Fukutani Y, Kobayashi K, Nakamura I, Watanabe K, Isaki K, Cairns NJ. Neurons, intracellular and extracellular neurofibrillary tangles in subdivisions of the hippocampal cortex in normal ageing and Alzheimer’s disease. Neurosci Lett. 1995;200:57–60. [DOI] [PubMed] [Google Scholar]
- [73].Pretangles Uchihara T. and neurofibrillary changes: similarities and differences between AD and CBD based on molecular and morphological evolution. Neuropathology. 2014;34:571–7. [DOI] [PubMed] [Google Scholar]
- [74].Goedert M, Jakes R, Crowther RA, Cohen P, Vanmechelen E, Vandermeeren M, et al. Epitope mapping of monoclonal antibodies to the paired helical filaments of Alzheimer’s disease: identification of phosphorylation sites in tau protein. Biochem J. 1994;301:871–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


