Abstract
Myometrial contraction is stringently controlled throughout pregnancy and parturition. Progesterone signaling, effecting through the progesterone receptor (PR), is pivotal in modulating uterine activity. Evidence has shown that two major PR isoforms, PR-A and PR-B, have distinct activities on gene regulation, and the ratio between these isoforms determines the contractility of the myometrium at different gestational stages. Herein, we focus on the regulation of PR activity in the myometrium, especially the differential actions of the two PR isoforms, which maintain uterine quiescence during pregnancy and regulate the switch to a contractile state at the onset of labor. To demonstrate the PR regulatory network and its mechanisms of actions on myometrial activity, we summarized the findings into three parts: Regulation of PR Expression and Isoform Levels, Progesterone Receptor Interacting Factors, and Biological Processes Regulated by Myometrial Progesterone Receptor Isoforms. Recent genomic and epigenomic data, from human specimens and mouse models, are recruited to support the existing knowledge and offer new insights and future directions in myometrial biology.
Keywords: progesterone signaling, progesterone receptor isoform, myometrium, pregnancy, parturition, epigenetic regulation
1. Introduction
The myometrium is the muscular compartment in uterus that supports structural integrity of the organ and plays a dual role in regulating the switch of uterine contraction states during pregnancy and parturition. The myometrium lies between the inner endometrium and the outer perimetrium, forming the thickest part of the uterine wall. Smooth muscle cells (myocytes or myometrial cells) are the most abundant cell component constructing the circular and longitudinal muscle bundles in this layer, which is capable of both tonic and phasic contractions without nerve control. Uterine myocytes have their own intrinsic rhythmicity and pacemaker to control contractile activities depending on different situations, and they are finely modulated by hormones, mechanical stretch, and other intracellular signals such as calcium (Ca2+) flux (1). Proper exertion of the contractile forces coordinated by myometrial cells is critical for the myometrium throughout all gestational stages and at term parturition.
Uterine contraction is physiologically relevant for both nonpregnant and pregnant uteri. Adequate uterine contractility assists transportation of sperm toward the fallopian tubes, helps fertilized eggs implant, and sheds menstrual debris (2,3) (4). Emerging evidence suggests that excessive contractions could lead to implantation failure (2,5). The myometrium becomes relaxed once the embryo implants and starts to develop (6). Reduced muscle movement during this gestational period keeps the myometrium quiescent, preventing the fetus from being expelled by preterm labor. Early in the third trimester of pregnancy, intermittent but mild Braxton Hicks contractions begin (7), which serve to provide more oxygen-rich blood flow to the placenta and prepare for true labor. Once parturition is initiated, the myometrium begins frequent and intense contractions until the completion of fetus and placental expulsion, which concludes the labor process. The arrest of labor caused by insufficient contractile force during parturition could consequently develop into dystocia (8), increasing the risk of both maternal and neonatal hemorrhage, infections, and even birth asphyxia (9).
Progesterone, a key steroid hormone in female reproduction, plays a vital role in modulating the phenotypic transition between different gestational stages in the uterus. In mice, progesterone is supplied by the ovarian corpus luteum. In humans, luteal progesterone production is subsequently taken over by the developing placenta around the 8th-12th week of gestation after successful implantation (10). Progesterone levels increase after ovulation to prepare the uterus for pregnancy. At early pregnancy, progesterone signaling is important for embryo reception and decidual differentiation in the endometrium (11) (12). Subsequently, a sustained level of functional progesterone throughout pregnancy maintains low myometrial activities and stabilizes the maternal environment, allowing the fetus to mature. Animal models, including mice, rabbits, pigs, and goats, show a measurable drop of serum progesterone around the end of gestation (13–16), releasing the myometrium from contraction inhibition. However, in humans, the systemic level of progesterone is not decreased preceding parturition. Instead, functional suppression of progesterone responsiveness is employed in the human myometrium to work with other stimulating factors, such as estrogen, prostaglandins, and oxytocin, to transform the muscle into a contractile phenotype for subsequent parturition (17). On the other hand, progesterone also serves as an anti-inflammatory factor that allows for the tolerance of the fetus in the maternal body. During the onset of labor, the loss of progesterone’s effects facilitates the effects of pro-inflammatory cytokines, chemokines, oxidative stress, and infiltrated leukocytes to stimulate the vigorous rhythmic contractions in the myometrium for parturition (18).
Progesterone signaling is transduced by genomic and nongenomic mechanisms (19,20). In the female reproductive tract, progesterone genomic actions are primarily driven by the progesterone receptor (PR). Upon ligand induction, PR translocate to the nucleus and acts as a transcription factor to regulate target gene expression. PR-A and PR-B isoforms are made from the same PR gene locus via alternative promoters (21,22). While both variants bear DNA binding domains, PR-A is a truncated form of PR-B, lacking the first 164 amino acids of the N-terminal site where one of the three activation function (AF) domains resides (12). PR isoforms can act as transcription activators or repressors depending on the context (23–26). Additionally, global genomic actions of PR-A and PR-B can be similar or distinct depending on the tissue type (27–30). Moreover, subcellular localization of PR isoforms in response to local ligand availability also determines the activity of target genes (26). Physiologically, female mice lacking the PR-A isoform are infertile due to the compromised ovulation and decidualization capability, similar to the PR null phenotypes (31,32). No myometrial abnormality was reported in these two mouse models (31,32). In contrast, PR-B knockout females can carry out pregnancy and produce live pups (33). These observations indicate that PR-A is required and sufficient to exert myometrial functions for mouse pregnancy. On the other hand, female mice of smooth muscle PR-B overexpression manifest a labor dystocia phenotype (28), showing the function of the PR-B isoform in maintaining myometrial quiescence during pregnancy. These findings collectively demonstrate the versatility of progesterone receptors that permit progesterone dependent control on a wide spectrum of biological processes in various contexts.
The human myometrium expresses both PR-A and PR-B isoforms and their relative protein ratio changes along gestation (25,34). The changing PR isoform ratio has been proposed to mediate the “functional progesterone withdrawal” that determines the switch to the contractile state in the pregnant human myometrium (25,35). Microarray analyses using immortalized human myometrial cells engineered to express these two isoforms have demonstrated that these two isoforms regulate separate target genes. PR-A modulates gene signatures corresponding to inflammation and the promotion of parturition, while PR-B modulates genes inhibiting inflammation that are necessary for the maintenance of pregnancy (29). This in vitro observation was validated in vivo by engineering mice with deregulated expression of PR-A or PR-B in smooth muscle cells. In vivo, more than 80% of uterine genes under the control of either myometrial PR-A or PR-B are distinct between these two isoforms in mice (28). Uteri of myometrial PR-A overexpression manifest a pro-inflammatory gene signature, in contrast with the anti-inflammation, anti-contraction, and pro-muscle building transcriptomic profile of the PR-B overexpressor (28). Such an agreement between human and mouse studies reveals an evolutionary conservation of PR isoform functions in the myometrium via conferring overlapping and independent transcriptomes (28,29). In this review, we will discuss the regulation, the interacting partners, and the downstream actions of the progesterone receptor isoforms PR-A and PR-B with a focus on the myometrial compartment.
2. Regulation of PR Expression and Isoform Levels
In 1965, Csapo and colleagues mentioned that even though there is a distinct dynamic pattern of systemic progesterone level in humans, especially during the onset of labor, progesterone has a similar effect on myometrial contractility in all mammals, implying that progesterone signaling is not only a prerequisite for pregnancy termination, but built with refined and complicated networks in response to endocrine milieu diversity (35). The flip-flop of progesterone responsiveness in the myometrium is predominantly determined by how intracellular PR signaling works, which factors in the protein level of PR, particularly the two major isoforms PR-A and PR-B, their locations in subcellular compartments, and the regulation of their downstream genes that are functionally related to muscle contraction.
2.1. Transcriptional Control
Human myometrial PR-A protein abundance exhibits a gradual increase from preterm nonlabor to term labor stages while PR-B maintains its levels (25). This differential expression pattern results in a major increase of the PR-A-to-PR-B ratio with a PR-A dominance status in the term labor myometrium (25). Such an increase of the myometrial PR-A-to-PR-B ratio is also seen in the transcript level (36,37), suggesting that transcription regulation is one of the mechanisms that determines the PR isoform ratio.
The genetic program that modulates expression of myometrial PR and its isoforms remains elusive. In term pregnant human myometrial tissues, the PR-encoding PGR locus manifests several open chromatin regions at the promoter, introns, and untranslated regions (Figure 1) (38) that permit transcription regulators’ access to the vicinity for gene modulation. Among which, the open chromatin regions at the PGR promoter and untranslated regions are potential hotspots of regulator occupancy, as suggested by the meta-analysis of 338 transcription factors’ genome binding patterns across 130 cell types from the ENCODE project (Figure 1) (39). In human breast cancer cells, previous work has found that PR gene expression is stimulated by estrogen and cyclic AMP (40). cAMP response elements (CRE)-like sequences were identified in the 5′-flanking region of the human PR gene (41), although their direct regulation of PR transcription has not been shown. Estrogen-induced increases in PR expression are dependent upon estrogen receptor α (ERα). Specifically, ERα-null mice have lower levels of PR and PR transcription cannot be induced by estrogen treatment (42). Interestingly, in the regulatory regions surrounding and within the PR gene, there are no full estrogen response elements (ERE), but there is an ERE half-site (43,44). This ERE half site is upstream of two adjacent specificity protein 1 (Sp1) sites (from +571 to +595), thus it is called the +571 ERE/Sp1 site, and requires the binding of ERα and Sp1 for effective regulation of PR gene transcription (45,46). This +571ERE/Sp1 site is conserved in the mouse and rat (47,48). Additionally, there is another region from −80 to −34 in the PR gene that contains two Sp1 sites (46). Here again, cooperation between Sp1 and ERα regulates PR gene transcription in an E2-dependent manner (46). In the mouse endometrium, NR2F2 promotes PR expression (49,50), while NR2F2 can tether on Sp1 proteins to promote gene expression (51). Since ESR1 (the gene encoding ERα), NR2F2, and SP1 are expressed in the human myometrium (38,52), myometrial cells could potentially adopt similar regulatory effects of these transcription factors on PR expression. Notably, occupancy of CTCF, a protein involved in the tertiary structure of the chromatin, at the PR promoter is observed in MCF-7 cells (39). This finding raises a possibility of PR being regulated by distal enhancers and warrants future investigations on the impact of topological interactions among cis-acting elements on the regulation of PR expression.
Figure 1.
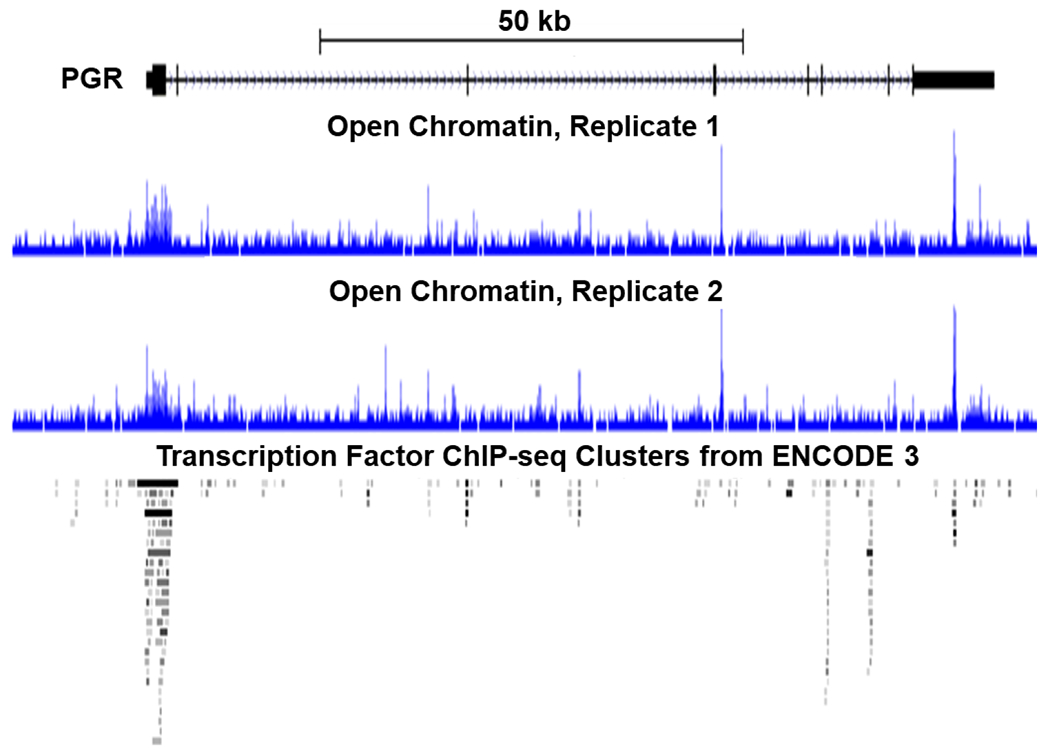
Open chromatin regions in the PGR locus of term pregnant human myometrial tissues. The track view was modified from the UCSC genome browser using the ATAC-seq data from NCBI accession number GSE137549. The scale bar depicts the size of the displayed genomic region in kilobases. The PGR gene body is displayed to the right of the PGR label and oriented with the first exon on the left and the 3’ end on the right. Arrowheads depict the gene orientation from 5’ to 3’. On the gene body, bigger blocks are exons; smaller blocks are untranslated regions; and horizontal lines in between are introns. The ATAC-seq signals are displayed in histogram-like images for each individual specimen. The transcription factor occupancy patterns of 338 factors from 130 cell lines of the ENCODE (ENCyclopedia Of DNA Elements) 3 project (128) are displayed in a two-dimension density plot to show potential hotspots of transcription factor bindings.
2.2. Epigenetic Regulation
Epigenetic mechanisms emerge as a layer of control for myometrial PR isoform expression. PR-A mRNA levels are higher in the laboring human term myometrium than nonlaboring specimens (37). Chai et al. further found that the PR-A promoter exhibits higher enrichment levels of the active histone marks acetyl-histone H3 and acetyl-histone H4 compared with the PR-B promoter, and this difference was established prior to the labor-associated increase of PR-A/PR-B mRNA ratio (37). This observation echoes Shchuka and colleagues’ findings that, at the genome wide scale, promoters of labor-associated genes and the putative enhancers are already epigenetically active four days prior to labor in the mouse myometrium, preceding the labor-associated change of gene expression patterns at the transition to the active laboring stage (53). Moreover, the term pregnant human myometrium also has a distinct epigenomic environment as evidenced by a stage-specific open chromatin pattern (38). These results indicate that the myometrial epigenomic landscape reprograms ahead of parturition, paving the way to adjust the PR isoform ratio and switch transcriptome to the contractile program.
Epigenetic modifiers that alter chromatin and DNA moiety profiles have played a role in promoting PR-A level during parturition (54,55). Histone deacetylase 1 (HDAC1), that works to remove acetylation modifications from histones, has lower protein and mRNA levels in laboring human myometrial tissues compared with nonlaboring specimens (54). Ke et al. showed that HDAC1 occupies the PR-A promoter and suppresses PR-A expression in primary myometrial cells (54). Collectively, these data suggest that the reduction of HDAC1 expression promotes the increase of the PR-A/PR-B ratio for parturition. Unlike HDAC1, the Jumonji AT-rich interactive domain 1A (JARID1A, KDM5A), an enzyme that eliminates methylation at H3K4, has comparable mRNA levels between nonlaboring and laboring term myometrial specimens. Instead, JARID1A protein occupancy on the PR-A promoter is lower in laboring human myometrium compared with nonlaboring tissues (55), coinciding with the increase of H3K4 trimethylation in the PR promoter at labor (37). Notably, JARID1A also occupies the PR locus and attenuates estrogen-induced PR promoter activities in MCF-7 cells (56). These observations implicate that an unknow mechanism in the myometrium removes JARID1A from the PR promoter region to permit the change of the PR isoform expression pattern at parturition.
Alterations of the DNA methylation profile at the PR promoter region of human myometrial tissues have also been observed. Bisulfite sequencing data show a reduction in the number of methylated cytosine-guanine dinucleotide (CpG) sites in between the PR-B and the PR-A transcription start sites in laboring myometrium compared to nonlaboring specimens (55,57). Concomitantly, DNA methyltransferases (DNMT), DNMT1 and DNMT3a, exhibit lower mRNA abundance in laboring specimens compared to the nonlaboring human myometrial tissues (57), which potentially links the DMNT levels to PR promoter methylation status. While functional significance of the promoter DNA methylation status and DMNT levels remain unknown for PR in the myometrium, studies in the endometrium shed light on DNA methylation as a regulatory mechanism of PR expression. Hypermethylation of the PR-B promoter is associated with lower PR-B mRNA abundance in ectopic endometrial tissues of endometriosis patients (58). Emerging evidence shows that the inverse correlation between PR-B promoter methylation and expression levels is present in epithelial and stromal cells, the two major cell types of the endometrium (59,60). Functionally, treating cultured endometrial stromal cells with demethylation agent 5-aza-20-deoxycytidine increases PR-B mRNA abundance and adding the histone deacetylase inhibitor trichostatin A can further boost the induction levels (60). These observations indicate that epigenetic regulators likely work in concert to modulate the activities of PR isoform promoters in a context dependent manner.
2.3. Protein stability and posttranslational modifications
Control of protein stability regulates the abundance of and the ratio between PR isoforms in the myometrium. In cultured myometrial cells, progesterone treatment increases the PR-A/PR-B protein ratio by reducing the rate of protein decay on PR-A and increasing that of PR-B (61). The differential protein turnover rates between PR-A and PR-B are 26S proteosome dependent and can be facilitated by either pro-inflammatory interleukin-1β (IL-1β) or lipopolysaccharide (LPS) in the presence of progesterone (61). Protein levels of both PR isoforms have been reported to decrease in cultured myometrial explants and further in isolated myometrial cells compared to freshly isolated nonpregnant myometrial tissues (62). Treating the myometrial explants with progesterone together with either IL-1β or LPS slows down the PR-A isoform decay rate (61), supporting the in vitro findings. Notably, progestin R-5020, a PR agonist, also facilitates PR-B degradation in a 26S proteosome dependent manner in MDA-MB-231 breast cancer cells and Ishikawa endometrial cancer cells (63). These observations collectively suggest a conserved biological process in regulation of PR-B protein stability across various cell types.
Modifications of PR proteins with phosphorylation, acetylation, ubiquitination, and SUMOylation have been discussed elsewhere (64,65). In human myometrial tissues, phosphorylation on the serine 345 residue (pSer345) of the PR-A isoform increases at the laboring stage (66). In cultured human myometrial cells, mutating this serine residue to alanine abolishes the PR-A’s antagonizing function on PR-B’s repressive effect over the interleukin-8 gene expression (66), which supports the functional significance of PR-A pSer345. Progesterone alone increases pSer345 levels of both PR-A and PR-B in these cells. In contrast, progesterone together with IL-1β elevate pSer345 abundance on only the PR-A isoform in human myometrial explants (66). In summary, these findings indicate that progesterone and the pro-inflammatory IL-1β differentially control the PR isoform protein stability and activities in the myometrium.
3. Progesterone Receptor Interacting Factors
As a transcriptional regulator, PR interacts with major transcription factors and cofactors to control gene expression. Numerous PR interacting proteins have been identified in various systems. Here, the discussion focuses on the ones that have functional roles in the myometrial compartment.
3.1. Activator protein-1 (AP-1)
AP-1 is a regulatory heterodimer composed of Jun and Fos family proteins, whose structure bears conserved DNA-binding domains (DBD) for specific DNA region recognition (67,68). AP-1 is an important regulator for laboring processes, which is evidenced by the histone ChIPseq analysis done in the pregnant and laboring mouse myometrium by Shchuka et al. Both proximal and distal regulatory regions of labor-associated genes show enriched AP-1 recognizing sequence motif in H3K27Ac-positive (active mark) chromatins at labor (53), suggesting that AP-1 may act through these putative enhancers to regulate expression of labor-associated genes. The AP-1 protein is also found to increase during parturition (69), and it is reported that AP-1 serves a central role in mediating PR signaling by guiding the PR complex to designated promoter regions of contractile genes (26). For instance, the association with AP-1 is important for the PR complex to recognize the binding site in the promoter of connexin 43 (CX43, Gja1), a key labor gene for myometrial contraction (70). A study done by Dong et al. showed that, during pregnancy stage, AP-1 is essential for leading a PR multiprotein complex containing p54nrb (official gene symbol NONO), a heterodimer component of polypyrimidine tract-binding protein-associated splicing factor (PSF), which is already known as a PR corepressor (71), to the AP-1 binding site in the Gja1 promoter for transcriptional repression (70). Compared to the nonpregnant stage, protein levels of p54nrb increase and peak at mid-pregnancy, followed by a gradual reduction, resulting in significantly lower levels at term in the rat myometrium (70). In humans, NONO mRNA abundance also trends lower at term pregnant than nonpregnant myometrial tissues (38), in line with the observation in the animal model. The reduction of p54nrb levels at term may lift the repression on associated contractile genes in preparation for labor. Different component proteins that comprise AP-1 could also change the binding affinity of AP-1 to different PR isoforms, and this could pose a large impact on the phenotypic switch of the myometrium between pregnancy and parturition states (26). Nadeem et al. found, constituted by Jun/Jun homodimers, that myometrial AP-1 prefers to associate with PR-B as a transcriptional repressor complex during pregnancy. At the laboring stage, Fos increases to generate more Fos/Jun heterodimers that exhibit less binding affinity with PR-B but higher binding affinity with PR-A (26). As a result, the Fos/Jun complex, including PR-A homodimers, becomes dominant and serves as a strong inducer for Gja1 transcription for the laboring process. Moreover, the AP-1-associated proteins could also regulate progesterone signaling by directly interacting with PR. It has been reported that Jun activation domain-binding protein-1 (JAB1), one of the co-activators in the AP-1 multiprotein complex that potentiates the binding specificity of AP-1, directly interacts with PR to stabilize the DNA-multiprotein complex during transcription (67). Using the two-hybrid screening method, Chauchereau et al. demonstrated that JAB1 could physically associate with both PR and steroid co-activator-1 (SRC-1), a common core protein in nuclear receptor protein complex, which enhances the transactivation of corresponding target gene induced by progesterone-dependent signaling (67).
Assessments of PR functions and genome occupancy in human and mouse myometrial tissues provide physiological relevance for previous in vitro findings. Based on the sequencing analysis from our published PR ChIPseq data from human myometrial tissues (38), PR occupying sites show significant enrichments of binding motifs of the Jun and Fos family, including JUN, FOSL1, and FOSL2. These results indicate a genome-wide permissive environment for Jun and Fos family proteins to interact with PR in the human myometrium. Moreover, predicted FOS molecular activities are lower in pregnant myometrial PR-B overexpression mouse uteri compared with the control, reflecting the PR-B preference of the JUN/JUN AP-1 composition (28). Our in vivo observations are in line with and serve as a physiological support for the in vitro results (26,70). It is noteworthy that the myometrium expresses multiple members of the AP-1 complex (72) that permit versatile regulation of PR functions. Therefore, further investigation of how the AP-1 complex interacts with PR to determine the switch of myometrium contraction in various contexts of pregnancy is warranted.
3.2. Nuclear Factor kappa B
The role of the Nuclear Factor kappa B (NF/κB) complex and its functional interaction with PR in regulation of myometrial contractility have been recently reviewed elsewhere (73–75). Liganded PR suppresses expression of NFκB dependent pro-inflammatory genes by recruiting co-repressors to the loci or by promoting the expression of inhibitory factors IκBα and MKP-1 to maintain myometrial quiescence. In vitro, the p65 subunit of the NFκB complex RELA can physically interact with PR at the region commonly shared between PR-A and PR-B isoforms (76). In the genome, the overrepresentation of the NFκB binding motif in myometrial PR occupying sites formulates opportunities of interactions by permitting PR and NFκB in a proximity (38). Functionally, myometrial PR-A overexpression is associated with a prominent increase in predicted activities of the NFκB complex in mouse uteri (28), which is in line with the model that PR-A works with NFκB in regulating myometrial gene expression (73,74). Notably, RELA physically interacts with AP-1 proteins JUN and FOS in U1 leukemia cells (77). Given that PR isoforms preferentially interact with different combinations of the AP-1 proteins (26), further investigations would shed light on the role of potential interactions among PR, NFκB and AP-1 in determination of isoform specific activities of myometrial PR.
3.3. GATA zinc finger domain-containing 2B
Harnessing the mass spectrometry technique, Chen et al. discovered and characterized a novel PR-associated repressor, GATA zinc finger domain-containing 2B (GATAD2B) (78). GATAD2B directly interacts with both PR-A and PR-B isoforms in hTERT-HM cells. Loss of GATAD2B reduces progesterone dependent repression of proinflammatory gene COX-2 and IL8 expression under IL-1beta stimulation. In this system, PR-A and PR-B mediated the progesterone action in the same direction. In human myometrial tissues, GATAD2B mRNA levels are lower in the laboring compared to the nonlaboring specimens. Notably, Gatad2b mRNA abundance decreases two days prior to parturition in the mouse myometrium, suggesting that the switch to the parturition program may begin days before the onset of laboring at both the genetic and epigenetic levels. Physiologically, GATAD2B is essential for mouse survival (Mouse Genome Informatics, MGI: 2443225) and is also a metastatic driver of lung cancer in the KRAS mutation background (79). The functional significance of myometrial GATAD2B in a physiological context remains to be determined.
3.4. Krüppel-Like Factor 9
Krüppel-like factor 9 (KLF9), encoded by the gene of basic transcription element-binding protein-1 (BTEB1), has been studied as a crucial coregulator for mediating PR activity in various compartments in the uterus (80–82). KLF9 is a transcription factor that belongs to the Sp/Krüppel-like family, which has a conserved triple-C2H2 DBD at the C-terminus for direct gene regulation and carries highly variable transactivation/repression domains at the N-terminus that diversify its transcriptional regulation functions (83). It has been reported that klf9 deficient mice exhibit altered progesterone responsiveness and a subfertility phenotype (80,81,84). KLF9 expression is detected in the term pregnant myometrium in both human and mice (38,82,85). Clinically, women who experienced late term pregnancy (greater or equal to 41 weeks) have lower KLF9 protein levels in the nuclear extracts of myometrial tissues compared to term pregnancy (34). Functionally, Klf9 deficient mice exhibited prolonged pregnancy compared to wildtype mice (82). Myometrial tissues of Klf9 null mice exhibit lower PR-A protein levels, lessened mRNA abundance of contractile genes Oxtr and Gja1, and reduced NF-κB p65/RELA DNA binding activities, compared to wild type animals (82). These findings suggest that KLF9 promotes expression of PR-A and other contractile machinery genes for parturition. Emerging data implicate that PR and KLF9 might regulate myometrial gene expression in concert. The KLF9 binding motif is enriched in PR occupying sites of human myometrial tissues (38) and estimated PR-A activities are increased in term pregnant human myometrial specimens (28). On the other hand, KLF9 and PR-B are found in the same protein complex from pig endometrial nuclear extracts (84). KLF9 also enhances liganded PR-B dependent transactivation activities in the human endometrial carcinoma cell line Flec-1-A (86). These observations collectively support a context-dependent role of KLF9 in modulating progesterone signaling through regulating PR isoform expression and working with PR in gene control. Further experimentations are needed to determine the molecular functionality of KLF9 on myometrial progesterone signaling modification.
3.5. Signal transducer and activator of transcription proteins
The Janus kinase (JAK)- Signal transducer and activator of transcription (STAT) signaling pathway has been associated with the transcriptomic changes between non-laboring and laboring stages in human myometrial tissues (87). mRNA of all seven gene members in the STAT family, including STAT1, STAT2, STAT3, STAT4, STAT5 (a/b), and STAT6, are expressed in human myometrial tissues (38). STAT proteins serve as transcription factors to transduce extracellular signals from cell-surface cytokine and growth factor receptors to nuclei for gene actions. STAT signaling participates in many biological processes, including cell proliferation, survival, differentiation, and inflammatory responses, which are mainly driven by external stimuli acting through JAK phosphorylation for downstream cascades (88,89). In human myometrial tissues, STAT5B mRNA abundance in term pregnant samples is 30% lower than that in the nonpregnant specimens (38). This observation is in line with the decreased Stat5b mRNA and protein abundance in term pregnant mouse myometrial tissues compared to those at mid-pregnancy (90), which suggests a conserved temporal gene expression pattern of myometrial STAT5B between humans and mice. Myometrial STAT5B mediates progesterone signaling to repress expression of the 20-α-hydroxysteroid dehydrogenase (20αHSD) encoding gene AKR1C1 (90). A reduction of the progesterone catabolizing 20αHSD abundance supports the local progesterone ligand availability in the myometrial tissues. At the transition to laboring, the further reduction of STAT5B mRNA and protein levels is inversely correlated with those of 20αHSD in the myometrium (90). Such an increase of myometrial 20αHSD levels may in part contribute to the lower progesterone ligand presence in the laboring myometrial nuclei (26). STAT factors are also involved in PR signaling in endometrium and breast cancer models. It has been shown that STAT proteins, including STAT1, STAT5B, and STAT6, take part in controlling the endometrial decidualization process (91), and knockdown of PR leads to compromised STAT signaling for endometrial cells to differentiate (92).
Binding motifs of STAT proteins are significantly enriched in PR occupying sites of human myometrial specimens (38). Comparing between term pregnant in-active-labor and term pregnant not-in-labor stages, STAT motifs are uniquely enriched in laboring human myometrial tissues at H3K27ac-positive enhancers and at H3K4me3-marked promoter regions (93). STAT5 proteins interact with PR in breast cancer cells (94,95). It has been reported that progesterone stimulates co-binding of PR and STAT5A on the enhancer of the receptor and activator of NF-κB ligand (RANKL) to promote the gene expression in breast cancer cells, showing direct evidence of PR and STAT protein interaction (95). Another piece of evidence from the study done by Hagan et al. shows that, in T47D-Y breast cancer cells, PR-B mediates progesterone signaling to promote WNT1 expression, which can be suppressed by inhibiting JAK/STAT signaling (96). Importantly, PR-B and STAT5 occupancy is seen on the WNT1 locus together with dual specific phosphatase 6 and casein kinase IIα, suggesting a potential interaction between STAT5 and PR-B in a regulatory complex for the control of WNT1 expression (96). The enrichment of STAT binding motifs in myometrial PR occupying sites and the functional significance of PR-STAT interactions in breast cancer cells support the possibility of PR working with STAT proteins for gene regulation in the myometrium.
4. Biological Processes Regulated by Myometrial Progesterone Receptor Isoforms
The two major biological processes in the myometrium under the control of progesterone signaling are the inflammatory pressure and the contractile status. Employing PR isoforms allow context-dependent temporal control of myometrial activities via their versatile utilities in modulation of expression of different sets of genes. The following discussions highlight their regulation of inflammatory and contractile genes.
4.1. Prostaglandins Synthesis
Prostaglandins are a group of lipid molecules that serve as pivotal inflammation mediators in regulating uterine activity during pregnancy. Derived from arachidonic acid, prostaglandins are metabolized by prostaglandin G/H synthase (PTGS), a group of enzymes that is also known as cyclo-oxygenase (COX). These enzymes convert arachidonic acid into prostaglandin G2 (PGG2), and subsequently transform PGG2 into prostaglandin H2 (PGH2), which is a common substrate for producing other active prostaglandins including PGD2, PGE2, PGF2, PGI2, and thromboxane A2. PGE2 and PGF2α, mainly produced by the amnion, are the major effectors that participate in regulating myometrial contraction (97). PGE2 and PGF2α act through their corresponding receptors, EP and FP, to differentially control muscle contractility in different pregnancy stages (98,99). Low levels of PGE2 is detected during pregnancy, and EP2/EP4 are the main receptors bound by PGE2, which pose a relaxation effect on the myometrium via cAMP signaling (99,100). Global level of prostaglandins increase after the onset of labor, which mainly function through EP1/EP2 and FP leading to a highly inflamed state that promotes muscle contraction for parturition (100).
Progesterone signaling regulates prostaglandin effects during pregnancy mainly by affecting its levels. One of the crucial enzymes for prostaglandin synthesis is COX-2 (encoded by the PTGS2 gene), whose local level is mainly produced by the fetal membrane, myometrium, and decidua (101,102). COX-2 protein levels are higher in laboring than nonlaboring term human myometrial tissues (103) and PR dependent regulation of myometrial COX-2 has been comprehensively reviewed (74). In cultured human myometrial cells, PR-B has a more pronounced effect on progesterone dependent repression of IL-1β-induced PTGS2 expression than PR-A does (78). Progesterone signaling promotes the expression of the ZEB1 transcription factor, which upregulates miR-199a and miR-214 levels to reduce COX-2 protein abundance in human myometrial cells (90). Notably, PR-B mediates progesterone dependent induction of ZEB1 promoter activities in HEK293 cells (104). PR-B has been reported as the dominant form of PR prior to term in human myometrial tissues (25), and PR occupancy on the ZEB1 locus is observed in human myometrial tissues (38). These observations together support the view in which myometrial PR-B suppresses COX-2 abundance via the ZEB1-miR-199a/214 pathway to maintain uterine quiescence prior to term.
The activity of 15-hydroxyprostaglandin dehydrogenase (encoded by the HPGD gene), the protein responsible for inactivating prostaglandin synthesis, is also controlled by progesterone signaling (105). PR, as well as other associated factors including AP-1 complex proteins, cAMP, and Ets family proteins, promotes HPGD expression. Both PR-A and PR-B enhance the HPGD promoter activity upon ligand treatment, while only PR-B is responsive to the stimulatory effect of cAMP on HPGD promoter activity in the primary human myometrial cells (105). This suggests that PR-A may have less synergistic effects with other gonadotropins, which may partially explain the phenomenon of “functional progesterone withdrawal” observed during laboring period. Notably, myometrial PR occupancy is found in an intergenic zone, 33 kilobases downstream of the HPGD gene body (38). Further studies may be needed to investigate the potential topological interaction between this PR occupying site and HPGD locus, as well as its functional relevance.
4.2. Gap junctions
Gap junctions are the channel arrays on the plasma membrane that control ion passage for electrical coupling. A collective group of neighboring myocytes coordinate electrical activities via the membrane gap junctions, to generate a systemic, strong, rhythmic contractile force (106). The connexin protein is the basic unit that constitutes gap junctions and has been shown to have a rapid increase of levels in the myometrium of both human and other mammals during the transition period from uterine quiescence to laboring stage (107). There are four different types of connexins that have been characterized in the myometrium, which are Cx43 (GJA1), Cx40 (GJA5), Cx26 (GJB2), and Cx45 (GJC1), with Cx43 being the major protein participating in myometrial contractions during parturition (108,109). Mice lacking functional Cx43 exhibit prolonged gestation and the presence of suffocated fetuses due to weaker contraction forces being generated by the myometrium. Our previous RNA sequencing analysis on myometrium tissues from both nonpregnant and term pregnant human subjects also revealed a significant increase of GJA1 mRNA abundance at term pregnancy compared to the non-laboring stage (38).
PR is the major transcriptional mediator that regulates the expression switch of myometrial Cx43 between pregnancy and laboring stages for muscle contraction. One of the principal mechanisms is the differential transcription mediated by PR isoforms. Before parturition, Cx43 transcription is inhibited by the upstream binding of a multiprotein complex recruited by liganded PR-B, which includes Jun/Jun AP-1 dimers, PSF, and p54nrb/Sin3 homolog A (mSin3A)/histone deacetylase (HDAC), to restrict the production of gap junction proteins (110). The activity of this transcriptional complex is maintained by the high levels of PSF and the dominant expression of Jun/Jun component in the AP-1 complex during pregnancy (110). After unliganded PR-A gradually becomes the major PR at the time of labor, another protein complex, containing PR-A and Jun/Fos AP-1 heterodimers, is formed at the AP-1 binding domain located upstream of Gja1 to regulate its transcription (26,110). Another study done by Renthal et al. showed that PR-B could also work in an indirect manner to regulate the levels of GJA1 mRNA via the effect of transcription factors ZEB1 and ZEB2 (104). As mentioned previously, PR-B stimulates ZEB1 promoter activities in the presence of progesterone (104). Increased ZEB1 expression reduces GJA1 mRNA abundance and impairs oxytocin-induced contraction in cultured human myometrial cells (104). Since ZEB1 occupies the mouse Gja1 locus in the myometrial tissues and this occupancy is downregulated at the laboring stage (104), ZEB1 likely directly repress GJA1 transcription in a conserved manner. Similarly, ZEB2 overexpression decreases GJA1 transcript levels and blocks myometrial cell contraction as well (103). On the other hand, the upregulation of the miR-200 family in a mouse parturition model causes the decrease of ZEB1/ZEB2 expression via post-transcriptional degradation, which in turn derepresses Cx43 level in the myometrium during laboring stage to support muscle contraction (104).
4.3. Oxytocin Signaling
Oxytocin (encoded by OXT) is a 9-amino-acid neuropeptide predominantly produced from the mammalian hypothalamus and exerts its cellular action via the oxytocin receptor (encoded by OXTR). The oxytocin receptor is a kind of Gq-coupled receptor that requires calcium (Ca2+) as a secondary messenger to transmit the cascades for muscle contraction (111). The role of oxytocin signaling in the myometrium that regulate muscle contractions during parturition is controversial, since mice lacking either Oxt or Oxtr exhibit normal parturition (112,113). However, it is observed that both oxytocin and myometrial OXTR are dramatically increased in humans at the onset of labor (38,114–116), and synthetic oxytocin has been widely used to induce labor by stimulating uterine contractions (111). This suggests that the effect of oxytocin signaling could be compensated, and that it must be through the same downstream pathway driven by oxytocin to regulate muscle contraction during pregnancy. Local oxytocin production from decidua and chorion-amnion tissues, which increases during parturition (117) due to the estrogenic and inflammatory environment (118,119), is crucial to determine the onset of labor. For the cognate receptor OXTR, PR also plays a role in modulating OXTR expression in the myometrium at different gestation stages. Peavey and colleagues demonstrate that the PR-B overexpression compromises oxytocin signaling in the pregnant uterus by reducing Oxtr levels, leading to attenuated uterine contractility (28). PR-B may act through ZEB1 to suppress OXTR transcription because PR-B promotes ZEB1 expression, and myometrial ZEB1 occupancy on the mouse Oxtr locus also varies in a stage-specific manner (104). On the contrary, specifically overexpressing PR-A in the muscle induces myometrial contractions (28). This in vivo result provides support to in vitro observations of differential PR isoform functions that PR-B suppresses oxytocin signaling and PR-A acts as a pro-contraction regulator. The fact that Oxtr-null mice has no parturition phenotype indicates the presence of a redundant system to activate the downstream calcium signaling for the mouse parturition process.
Once OXTR has been activated, phospholipase C (PLC) -β hydrolyzes phosphatidylinositol bisphosphate (PIP2) to generate diacylglycerol (DAG) and inositol triphosphate (IP3), and IP3 subsequently promotes more calcium release from sarcoplasmic reticulum into cytosol (120). The elevated cytosol calcium then forms a complex with calmodulin (CaM), calcium-modulated protein, to activate calcium-dependent myosin light chain kinase (MLCK), which further phosphorylates myosin light chain (MLC) to generate force and contraction by interacting with actin filaments in an ATP-dependent cross-bridge cycling (120). Our recent finding shows that PR regulation of myometrial contraction is accomplished via mediating the expression of the proteins involved in these calcium-myosin activation cascades (28,38). In human myometrial tissues, term pregnant myometrium specimens show a significant reduction of phospholipase C like 1 (PLCL1) compared to the non-laboring myometrium (38). At term, PLCL1 mRNA abundance becomes even lower in the laboring myometrium in comparison to nonlaboring tissues (121). PLCL1 has a similar structure to phospholipase C but lacking catalytic activities, which sequester PIP2 and attenuate the overall PLC efficiency in the signaling pathway (122,123). Therefore, decreased levels of PLCL1 in the term myometrium likely reduces the dampening effect on PLC functions and subsequently permits a higher capacity of PIP2 conversion for the generation of contraction force. PLCL1 and its paralog phospholipase C like 2 (PLCL2) are both expressed in human and mouse uterine tissues and have a similar function in calcium sequestration and attenuating muscle contraction (28,38,124,125). Comparing their mRNA abundance, PLCL1 is higher in the human myometrium, while Plcl2 is dominant in the pregnant mouse uterine tissues, implicating a functional conservation on the management of PIP2 metabolism between human and mice amid an evolutionary divergence. PLCL1 expression is induced by progesterone in endometrial stromal cells (126). In the term pregnant human myometrial tissue, PR occupancy is observed at the PLCL1 promoter and 92 kilobases downstream of the PLCL1 gene body in open chromatin regions (Figure 2) (38). Moreover, the PLCL1 promoter region that is rich with transcription factor occupancy has CTCF binding in numerous cell types and the downstream PR occupying regions also manifest CTCF binding in BE2C cells (Figure 2) (39). These findings collectively implicate that progesterone acts through PR in both the promoter and the distal enhancer to promote PLCL1 expression. In mice, myometrial PR-B overexpression increases the level of uterine Plcl2 and attenuates uterine contractility (28). These observations together support a mechanism in which progesterone acts through PR-B to promote both PLCL1 and PLCL2 expression for the management of the calcium cascades on myometrial contraction.
Figure 2.
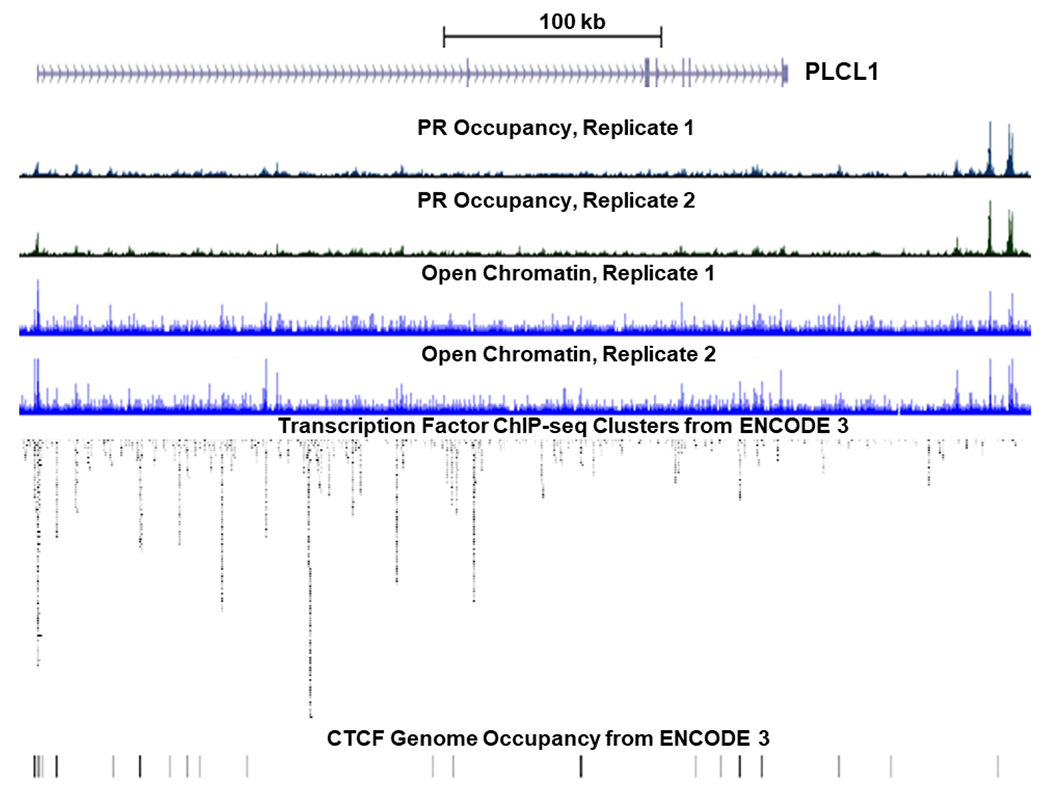
PR genome occupancy and open chromatin regions in the PLCL1 locus of term pregnant human myometrial tissues. The track view was modified from the UCSC genome browser using the ATAC-seq data from NCBI accession numbers GSE137549 and the PR ChIP-seq data from GSE137550. The PLCL1 gene body is displayed to the left of the PLCL1 label and oriented with the first exon on the left and 3’ end on the right. PR ChIP-seq and ATAC-seq signals are displayed in histogram-like images for each individual specimen. The transcription factor occupancy patterns of 338 factors from 130 cell lines of the ENCODE (ENCyclopedia Of DNA Elements) 3 project (128) are displayed in a two-dimension density plot to show potential hotspots of transcription factor bindings. CTCF genome occupancy from ENCODE 3 are displayed in vertical lines.
Additionally, myometrial expression of transient receptor potential channel subfamily c member 3 (TRPC3) is also repressed by PR-B overexpression (28). TRPC are a group of non-selective cation channels that could activate calcium influx from sarcoplasmic reticulum to enhance the calcium-mediated muscle contraction (127). The regulation of Oxtr, Plcl2, and Trpc3 expression indicates that PR-B imposes control on multiple points of the calcium signaling cascade to reduce the cytoplasmic calcium level and prohibit the myometrium from augmenting contractile force before parturition.
5. Conclusion and Perspectives
Smooth muscle provides mechanic force for uterine contraction and structural integrity among a multi-component system for pregnancy. From the myocyte’s perspective, myometrial progesterone signaling directs the cellular adaptation to the demand of pregnancy, which is largely determined by the relative levels and posttranslational modifications of PR isoforms, the interaction between PR and partner regulators, and the downstream pathways that control uterine inflammatory pressure and muscle contractility (Figure 3). Alterations on any member of this PR dependent signaling network could impact the tocolytic effect of progesterone. While investigations on PR interacting partners have gained steam, much is still unknown about the mechanisms that determine PR isoform expression in the myometrium over the course of pregnancy. The mapped myometrial enhancers provide candidate cis-acting elements that may confer the regulation of PR isoform expression (53). Future identification of topologically associated enhancers, followed by CRISPR-mediated manipulation of gene expression, could test whether utilizing alternative cis-acting elements determines the expression of specific PR isoforms. Similar approaches could also be used to identify the crucial cis-acting elements for major PR downstream target genes by integrative analysis of PR isoform specific transcriptome and myometrial enhancer datasets. This information would facilitate the identification and examination of the regulators that control myometrial genes in conjunction with PR isoforms.
Figure 3.
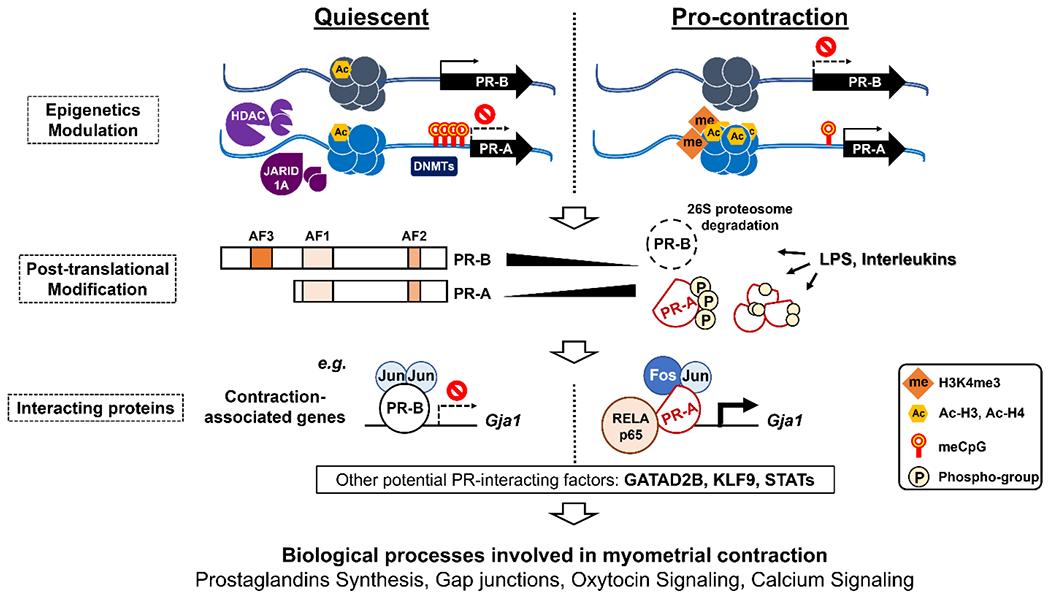
A progesterone signaling model for myometrial activities during pregnancy. PR: progesterone receptor; HDAC: Histone deacetylase 1; JARID1A: Jumonji AT-rich interactive domain 1A; DNMT: DNA methyltransferase; me: methyl group; Ac: Acetyl group; AF: activation function domain; LPS: lipopolysaccharide; Gja1: Gap Junction Protein Alpha 1, Cx43; GATAD2B: GATA zinc finger domain-containing 2B; KLF9:Krüppel-like factor 9; STAT: Signal transducer and activator of transcription; H: histone; CpG: cytosine-guanine dinucleotide.
The significant difference between transcriptome profiles of nonpregnant and term pregnant myometrial tissues reflects major structural and functional changes of the myometrium to support pregnancy and in preparation for parturition (38). During this process, myometrial PR-A and PR-B isoforms orchestrate the expression of common and distinct sets of genes on an epigenetically pre-determined landscape to translate progesterone actions and build a parturition-capable contractile machinery (28,53). This includes, but is not limited to, contraction-associated and inflammatory genes, as well as programs for vascularization, energy metabolism, and extracellular matrix arrangement that are important for operation and expansion of the myometrial compartment in a dynamic environment during pregnancy (28). How the myometrium is expanded and remodeled at the time when the muscle already carries the load remains largely unknown, except that the epigenome and transcriptome appear to be placed in advance (28,53). Meanwhile, how do the activities, in addition to the levels of the PR isoforms, change in response to the functional demand of pregnancy is also unclear. Future investigations on the role of progesterone signaling and PR isoforms in this tightly controlled muscle remodeling process will shed light on this uncharted territory.
Highlights.
Progesterone via the progesterone receptor (PR) controls uterine contractions.
PR isoforms and cofactors together permit a wide spectrum of progesterone actions.
Isoform-specific protein levels and modifications contribute to overall PR activities.
Myometrial PR-A and PR-B confers divergent cellular actions via unique target genes.
Acknowledgement
The authors thank Ms. Sylvia Hewitt and Ms. Skylar Montague for English editing. This work is supported by an Intramural Research Program of the National Institute of Environmental Health Sciences, National Institutes of Health (NIH) Z1AES103311 (FJD) and Z99-ES999999 (SPW) and the Intramural Research Training Award (MJD). WNL is a recipient of the fellowship of Taiwan Ministry of Science and Technology Overseas Project for Post Graduate Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CRediT authorship contribution statement
Wan-Ning Li: Conceptualization, Writing – original draft, Writing – review & editing, Visualization. Mackenzie J. Dickson: Writing – original draft. Francesco J. DeMayo: Writing – review & editing, Supervision, Funding acquisition. San-Pin Wu: Conceptualization, Investigation, Writing – original draft, Writing – review & editing, Visualization, Supervision.
References
- 1.Garfield RE, Maner WL. Physiology and electrical activity of uterine contractions. Semin Cell Dev Biol. 2007;18(3):289–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuijsters NPM, Methorst WG, Kortenhorst MSQ, Rabotti C, Mischi M, Schoot BC. Uterine peristalsis and fertility: current knowledge and future perspectives: a review and meta-analysis. Reprod Biomed Online. 2017;35(1):50–71. [DOI] [PubMed] [Google Scholar]
- 3.Akerlund M Myometrial activity and endometrial blood flow in an ectopic pregnancy. Acta Obstet Gynecol Scand. 1978;57(5):479–481. [DOI] [PubMed] [Google Scholar]
- 4.Flores D, Madhavan M, Wright S, Arora R. Mechanical and signaling mechanisms that guide pre-implantation embryo movement. Development. 2020; 147(24). [DOI] [PubMed] [Google Scholar]
- 5.Lan VT, Khang VN, Nhu GH, Tuong HM. Atosiban improves implantation and pregnancy rates in patients with repeated implantation failure. Reprod Biomed Online. 2012;25(3):254–260. [DOI] [PubMed] [Google Scholar]
- 6.Bulletti C, de Ziegler D. Uterine contractility and embryo implantation. Curr Opin Obstet Gynecol. 2005;17(3):265–276. [DOI] [PubMed] [Google Scholar]
- 7.Raines DA, Cooper DB. Braxton Hicks Contractions. StatPearls. Treasure Island (FL)2021. [PubMed] [Google Scholar]
- 8.Committee on Practice Bulletins-Obstetrics ACoO, Gynecologists. Dystocia and augmentation of labor. Int J Gynaecol Obstet. 2004;85(3):315–324. [DOI] [PubMed] [Google Scholar]
- 9.Moshiro R, Mdoe P, Perlman JM. A Global View of Neonatal Asphyxia and Resuscitation. Front Pediatr. 2019;7:489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar P, Magon N. Hormones in pregnancy. Niger Med J. 2012;53(4):179–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blanks AM, Brosens JJ. Progesterone Action in the Myometrium and Decidua in Preterm Birth. Facts Views Vis Obgy. 2012;4(3):188–194. [PMC free article] [PubMed] [Google Scholar]
- 12.DeMayo FJ, Lydon JP. 90 YEARS OF PROGESTERONE: New insights into progesterone receptor signaling in the endometrium required for embryo implantation. J Mol Endocrinol. 2020;65(1):T1–T14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Csapo A Progesterone Block. American Journal of Anatomy. 1956;98(2):273–291. [DOI] [PubMed] [Google Scholar]
- 14.Csapo AI, Wiest WG. An examination of the quantitative relationship between progesterone and the maintenance of pregnancy. Endocrinology. 1969;85(4):735–746. [DOI] [PubMed] [Google Scholar]
- 15.Ellendorff F, Taverne M, Elsaesser F, Forsling M, Parvizi N, Naaktgeboren C, Smidt D. Endocrinology of Parturition in the Pig. Animal Reproduction Science. 1979;2(1-3):323–334. [Google Scholar]
- 16.Currie WB, Thorburn GD. Parturition in Goats - Studies on Interactions between Fetus, Placenta, Prostaglandin-F and Progesterone before Parturition, at Term or at Parturition Induced Prematurely by Corticotropin Infusion of Fetus. Journal of Endocrinology. 1977;73(2):263–278. [DOI] [PubMed] [Google Scholar]
- 17.Mesiano S, Chen EC, Fitter JT, Kwek K, Yeo G, Smith R. Progesterone withdrawal and estrogen activation in human parturition are coordinated by progesterone receptor A expression in the myometrium. Journal of Clinical Endocrinology & Metabolism. 2002;87(6):2924–2930. [DOI] [PubMed] [Google Scholar]
- 18.Edey LF, Georgiou H, O’Dea KP, Mesiano S, Herbert BR, Lei K, Hua R, Markovic D, Waddington SN, MacIntyre D, Bennett P, Takata M, Johnson MR. Progesterone, the maternal immune system and the onset of parturition in the mouse. Biol Reprod. 2018;98(3):376–395. [DOI] [PubMed] [Google Scholar]
- 19.Medina-Laver Y, Rodriguez-Varela C, Salsano S, Labarta E, Dominguez F. What Do We Know about Classical and Non-Classical Progesterone Receptors in the Human Female Reproductive Tract? A Review. Int J Mol Sci. 2021;22(20). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halasz M, Szekeres-Bartho J. The role of progesterone in implantation and trophoblast invasion. J Reprod Immunol. 2013;97(1):43–50. [DOI] [PubMed] [Google Scholar]
- 21.Conneely OM, Mulac-Jericevic B, Lydon JP. Progesterone-dependent regulation of female reproductive activity by two distinct progesterone receptor isoforms. Steroids. 2003;68(10-13):771–778. [DOI] [PubMed] [Google Scholar]
- 22.Kastner P, Krust A, Turcotte B, Stropp U, Tora L, Gronemeyer H, Chambon P. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J. 1990;9(5):1603–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hovland AR, Powell RL, Takimoto GS, Tung L, Horwitz KB. An N-terminal inhibitory function, IF, suppresses transcription by the A-isoform but not the B-isoform of human progesterone receptors. Journal of Biological Chemistry. 1998;273(10):5455–5460. [DOI] [PubMed] [Google Scholar]
- 24.Vegeto E, Shahbaz MM, Wen DX, Goldman ME, O’Malley BW, McDonnell DP. Human progesterone receptor A form is a cell- and promoter-specific repressor of human progesterone receptor B function. Mol Endocrinol. 1993;7(10):1244–1255. [DOI] [PubMed] [Google Scholar]
- 25.Merlino AA, Welsh TN, Tan HQ, Yi LJ, Cannon V, Mercer BM, Mesiano S. Nuclear progesterone receptors in the human pregnancy myometrium: Evidence that parturition involves functional progesterone withdrawal mediated by increased expression of progesterone receptor-A. J Clin Endocr Metab. 2007;92(5):1927–1933. [DOI] [PubMed] [Google Scholar]
- 26.Nadeem L, Shynlova O, Matysiak-Zablocki E, Mesiano S, Dong X, Lye S. Molecular evidence of functional progesterone withdrawal in human myometrium. Nat Commun. 2016;7:11565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li R, Wang X, Huang Z, Balaji J, Kim TH, Wang T, Zhou L, Deleon A, Cook ME, Marbrey MW, Wu SP, Jeong JW, Arora R, DeMayo FJ. The role of epithelial progesterone receptor isoforms in embryo implantation. iScience. 2021;24(12):103487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peavey MC, Wu SP, Li R, Liu J, Emery OM, Wang T, Zhou L, Wetendorf M, Yallampalli C, Gibbons WE, Lydon JP, DeMayo FJ. Progesterone receptor isoform B regulates the Oxtr-Plcl2-Trpc3 pathway to suppress uterine contractility. Proc Natl Acad Sci U S A. 2021;118(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tan HQ, Yi LJ, Rote NS, Hurd WW, Mesiano S. Progesterone Receptor-A and -B Have Opposite Effects on Proinflammatory Gene Expression in Human Myometrial Cells: Implications for Progesterone Actions in Human Pregnancy and Parturition. Journal of Clinical Endocrinology & Metabolism. 2012;97(5):E719–E730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaya HS, Hantak AM, Stubbs LJ, Taylor RN, Bagchi IC, Bagchi MK. Roles of progesterone receptor A and B isoforms during human endometrial decidualization. Mol Endocrinol. 2015;29(6):882–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA Jr., Shyamala G, Conneely OM, O’Malley BW. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9(18):2266–2278. [DOI] [PubMed] [Google Scholar]
- 32.Mulac-Jericevic B, Mullinax RA, DeMayo FJ, Lydon JP, Conneely OM. Subgroup of reproductive functions of progesterone mediated by progesterone receptor-B isoform. Science. 2000;289(5485):1751–1754. [DOI] [PubMed] [Google Scholar]
- 33.Mulac-Jericevic B, Lydon JP, DeMayo FJ, Conneely OM. Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc Natl Acad Sci U S A. 2003; 100(17):9744–9749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pabona JMP, Zhang D, Ginsburg DS, Simmen FA, Simmen RCM. Prolonged Pregnancy in Women Is Associated With Attenuated Myometrial Expression of Progesterone Receptor Co-Regulator Kruppel-Like Factor 9. Journal of Clinical Endocrinology & Metabolism. 2015;100(1):166–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Csapo AI, Pinto-Dantas CA. The effect of progesterone on the human uterus. Proc Natl Acad Sci U S A. 1965;54(4):1069–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mesiano S, Chan EC, Fitter JT, Kwek K, Yeo G, Smith R. Progesterone withdrawal and estrogen activation in human parturition are coordinated by progesterone receptor A expression in the myometrium. J Clin Endocrinol Metab. 2002;87(6):2924–2930. [DOI] [PubMed] [Google Scholar]
- 37.Chai SY, Smith R, Zakar T, Mitchell C, Madsen G. Term myometrium is characterized by increased activating epigenetic modifications at the progesterone receptor-A promoter. Molecular Human Reproduction. 2012;18(8):401–409. [DOI] [PubMed] [Google Scholar]
- 38.Wu SP, Anderson ML, Wang T, Zhou L, Emery OM, Li X, DeMayo FJ. Dynamic transcriptome, accessible genome, and PGR cistrome profiles in the human myometrium. FASEB J. 2020;34(2):2252–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lou S, Li T, Kong X, Zhang J, Liu J, Lee D, Gerstein M. TopicNet: a framework for measuring transcriptional regulatory network change. Bioinformatics. 2020;36(Suppl_1):i474–i481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Katzenellenbogen BS, Norman MJ. Multihormonal Regulation of the Progesterone Receptor in MCF-7 Human Breast Cancer Cells: Interrelationships among Insulin/Insulin-Like Growth Factor-1, Serum, and Estrogen. Endocrinology. 1990;126(2):891–898. [DOI] [PubMed] [Google Scholar]
- 41.Cho H, Aronica SM, Katzenellenbogen BS. Regulation of Progesterone Receptor Gene Expression in MCF-7 Breast Cancer Cells: A Comparision of the Effects of Cyclic Adenosine 3’,5’-Monophosphate, Estradiol, Insulin-Like Growth Factor-I, and Serum Factors. Endocrinology. 1994; 134(2):658–664. [DOI] [PubMed] [Google Scholar]
- 42.Hewitt SC, Korach KS. Progeterone action and responses in the alpha ERKO mouse. Steroids. 2000(10-11):551–557. [DOI] [PubMed] [Google Scholar]
- 43.Kastner P, Krust A, Turcotte B, Stropp U, Tora L, Gronemeyer H, Chambon P. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. The EMBO Journal. 1990;9(5):1603–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Savouret JF, Bailly A, Misrashi M, Rauch C, Redeuilh G, Chauchereau A, Milgrom E. Characterization of the hormone responsive element involved in the regulation of the progesterone receptor. EMBO Journal. 1991;10(7):1875–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petz LN, Nardulli AM. Sp1 Binding Sites and An Estrogen Response Element Half-Site Are Involved in Regulation of the Human Progesterone Receptor A Promoter. Mol Endocrinol. 2000;14(7):972–985. [DOI] [PubMed] [Google Scholar]
- 46.Petz LN, Ziegler YS, Schultz JR, Kim H, Kemper JK, Nardulli AM. Differential regulation of the human progesterone receptor gene through an estrogen response element half site and Sp1 sites. J Steroid Biochem Mol Biol. 2004;88(2):113–122. [DOI] [PubMed] [Google Scholar]
- 47.Hagihara K, Wu-Peng XS, Funabashi T, Kato J, Pfaff DW. Nucleic Acid Sequence and DNase Hypersensitive Sites of the 5’ Region of the Mouse Progesterone Receptor Gene. Biochemical and Biophysical Research Communications. 1994;205(2): 1093–1101. [DOI] [PubMed] [Google Scholar]
- 48.Kraus WL, Montano MM, Katzenellenbogen BS. Identification of multiple, widely spaced estrogen-responsive regions in the rat progesterone receptor gene. Mol Endocrinol. 1994;8(8):952–969. [DOI] [PubMed] [Google Scholar]
- 49.Kurihara I, Lee DK, Petit FG, Jeong J, Lee K, Lydon JP, DeMayo FJ, Tsai MJ, Tsai SY. COUP-TFII mediates progesterone regulation of uterine implantation by controlling ER activity. PLoS Genet. 2007;3(6):e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rubel CA, Wu SP, Lin L, Wang T, Lanz RB, Li X, Kommagani R, Franco HL, Camper SA, Tong Q, Jeong JW, Lydon JP, DeMayo FJ. A Gata2-Dependent Transcription Network Regulates Uterine Progesterone Responsiveness and Endometrial Function. Cell Rep. 2016;17(5):1414–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yu CT, Tang K, Suh JM, Jiang R, Tsai SY, Tsai MJ. COUP-TFII is essential for metanephric mesenchyme formation and kidney precursor cell survival. Development. 2012;139(13):2330–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Phillips RJ, Tyson-Capper AJ, Bailey J, Robson SC, Europe-Finner GN. Regulation of expression of the chorionic gonadotropin/luteinizing hormone receptor gene in the human myometrium: Involvement of specificity protein-1 (Sp1), Sp3, Sp4, Sp-like proteins, and histone deacetylases. J Clin Endocr Metab. 2005;90(6):3479–3490. [DOI] [PubMed] [Google Scholar]
- 53.Shchuka VM, Abatti LE, Hou H, Khader N, Dorogin A, Wilson MD, Shynlova O, Mitchell JA. The pregnant myometrium is epigenetically activated at contractility-driving gene loci prior to the onset of labor in mice. PLoS Biol. 2020;18(7):e3000710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ke W, Chen C, Luo H, Tang J, Zhang Y, Gao W, Yang X, Tian Z, Chang Q, Liang Z. Histone Deacetylase 1 Regulates the Expression of Progesterone Receptor A During Human Parturition by Occupying the Progesterone Receptor A Promoter. Reprod Sci. 2016;23(7):955–964. [DOI] [PubMed] [Google Scholar]
- 55.Chai SY, Smith R, Fitter JT, Mitchell C, Pan X, Ilicic M, Maiti K, Zakar T, Madsen G. Increased progesterone receptor A expression in labouring human myometrium is associated with decreased promoter occupancy by the histone demethylase JARID1A. Molecular Human Reproduction. 2014;20(5):442–453. [DOI] [PubMed] [Google Scholar]
- 56.Stratmann A, Haendler B. The histone demethylase JARID1A regulates progesterone receptor expression. FEBS J. 2011;278(9):1458–1469. [DOI] [PubMed] [Google Scholar]
- 57.Li X, Chen C, Luo H, van Velkinburgh JC, Ni B, Chang Q. Decreased DNA Methylations at the Progesterone Receptor Promoter A Induce Functional Progesterone Withdrawal in Human Parturition. Reproductive Sciences. 2014;21(7):898–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu Y, Strawn E, Basir Z, Halverson G, Guo SW. Promoter hypermethylation of progesterone receptor isoform B (PR-B) in endometriosis. Epigenetics. 2006;1(2):106–111. [DOI] [PubMed] [Google Scholar]
- 59.Esfandiari F, Heidari Khoei H, Saber M, Favaedi R, Piryaei A, Moini A, Shahhoseini M, Ramezanali F, Ghaffari F, Baharvand H. Disturbed progesterone signalling in an advanced preclinical model of endometriosis. Reprod Biomed Online. 2021;43(1):139–147. [DOI] [PubMed] [Google Scholar]
- 60.Jichan N, Xishi L, Guo SW. Promoter hypermethylation of progesterone receptor isoform B (PR-B) in adenomyosis and its rectification by a histone deacetylase inhibitor and a demethylation agent. Reprod Sci. 2010;17(11):995–1005. [DOI] [PubMed] [Google Scholar]
- 61.Peters GA, Yi L, Skomorovska-Prokvolit Y, Patel B, Amini P, Tan H, Mesiano S. Inflammatory Stimuli Increase Progesterone Receptor-A Stability and Transrepressive Activity in Myometrial Cells. Endocrinology. 2017;158(1):158–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Georgiou EX, Lei K, Lai PF, Yulia A, Herbert BR, Castellanos M, May ST, Sooranna SR, Johnson MR. The study of progesterone action in human myometrial explants. Mol Hum Reprod. 2016;22(8):877–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Khan JA, Amazit L, Bellance C, Guiochon-Mantel A, Lombes M, Loosfelt H. p38 and p42/44 MAPKs differentially regulate progesterone receptor A and B isoform stabilization. Mol Endocrinol. 2011;25(10):1710–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Abdel-Hafiz HA, Horwitz KB. Post-translational modifications of the progesterone receptors. J Steroid Biochem Mol Biol. 2014;140:80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dwyer AR, Truong TH, Ostrander JH, Lange CA. 90 YEARS OF PROGESTERONE: Steroid receptors as MAPK signaling sensors in breast cancer: let the fates decide. J Mol Endocrinol. 2020;65(1):T35–T48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Amini P, Michniuk D, Kuo K, Yi L, Skomorovska-Prokvolit Y, Peters GA, Tan H, Wang J, Malemud CJ, Mesiano S. Human Parturition Involves Phosphorylation of Progesterone Receptor-A at Serine-345 in Myometrial Cells. Endocrinology. 2016;157(11):4434–4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chauchereau A, Georgiakaki M, Perrin-Wolff M, Milgrom E, Loosfelt H. JAB1 interacts with both the progesterone receptor and SRC-1. Journal of Biological Chemistry. 2000;275(12):8540–8548. [DOI] [PubMed] [Google Scholar]
- 68.Claret FX, Hibi M, Dhut S, Toda T, Karin M. A new group of conserved coactivators that increase the specificity of AP-1 transcription factors. Nature. 1996;383(6599):453–457. [DOI] [PubMed] [Google Scholar]
- 69.Mitchell JA, Lye SJ. Differential activation of the connexin 43 promoter by dimers of activator protein-1 transcription factors in myometrial cells. Endocrinology. 2005;146(4):2048–2054. [DOI] [PubMed] [Google Scholar]
- 70.Dong XS, Yu C, Shynlova O, Challis JRG, Rennie PS, Lye SJ. p54nrb Is a Transcriptional Corepressor of the Progesterone Receptor that Modulates Transcription of the Labor-Associated Gene, Connexin 43 (Gja1). Molecular Endocrinology. 2009;23(8):1147–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dong XS, Shylnova O, Challis JRG, Lye SJ. Identification and characterization of the protein-associated splicing factor as a negative co-regulator of the progesterone receptor. Journal of Biological Chemistry. 2005;280(14):13329–13340. [DOI] [PubMed] [Google Scholar]
- 72.Nadeem L, Farine T, Dorogin A, Matysiak-Zablocki E, Shynlova O, Lye S. Differential expression of myometrial AP-1 proteins during gestation and labour. J Cell Mol Med. 2018;22(1):452–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Khader N, Shchuka VM, Shynlova O, Mitchell JA. Transcriptional control of parturition: insights from gene regulation studies in the myometrium. Mol Hum Reprod. 2021;27(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mendelson CR, Gao L, Montalbano AP. Multifactorial Regulation of Myometrial Contractility During Pregnancy and Parturition. Front Endocrinol (Lausanne). 2019;10:714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li JKH, Lai PF, Tribe RM, Johnson MR. Transcription factors regulated by cAMP in smooth muscle of the myometrium at human parturition. Biochemical Society Transactions. 2021;49(2):997–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kalkhoven E, Wissink S, van der Saag PT, van der Burg B. Negative interaction between the RelA(p65) subunit of NF-kappaB and the progesterone receptor. J Biol Chem. 1996;271(11):6217–6224. [DOI] [PubMed] [Google Scholar]
- 77.Yang X, Chen Y, Gabuzda D. ERK MAP kinase links cytokine signals to activation of latent HIV-1 infection by stimulating a cooperative interaction of AP-1 and NF-kappaB. J Biol Chem. 1999;274(39):27981–27988. [DOI] [PubMed] [Google Scholar]
- 78.Chen CC, Montalbano AP, Hussain I, Lee WR, Mendelson CR. The transcriptional repressor GATAD2B mediates progesterone receptor suppression of myometrial contractile gene expression. J Biol Chem. 2017;292(30):12560–12576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Grzeskowiak CL, Kundu ST, Mo X, Ivanov AA, Zagorodna O, Lu H, Chapple RH, Tsang YH, Moreno D, Mosqueda M, Eterovic K, Fradette JJ, Ahmad S, Chen F, Chong Z, Chen K, Creighton CJ, Fu H, Mills GB, Gibbons DL, Scott KL. In vivo screening identifies GATAD2B as a metastasis driver in KRAS-driven lung cancer. Nat Commun. 2018;9(1):2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Simmen RC, Eason RR, McQuown JR, Linz AL, Kang TJ, Chatman L Jr., Till SR, Fujii-Kuriyama Y, Simmen FA, Oh SP. Subfertility, uterine hypoplasia, and partial progesterone resistance in mice lacking the Kruppel-like factor 9/basic transcription element-binding protein-1 (Bteb1) gene. J Biol Chem. 2004;279(28):29286–29294. [DOI] [PubMed] [Google Scholar]
- 81.Velarde MC, Geng Y, Eason RR, Simmen FA, Simmen RC. Null mutation of Kruppel-like factor9/basic transcription element binding protein-1 alters peri-implantation uterine development in mice. Biol Reprod. 2005;73(3):472–481. [DOI] [PubMed] [Google Scholar]
- 82.Zeng Z, Velarde MC, Simmen FA, Simmen RC. Delayed parturition and altered myometrial progesterone receptor isoform A expression in mice null for Kruppel-like factor 9. Biol Reprod. 2008;78(6):1029–1037. [DOI] [PubMed] [Google Scholar]
- 83.Presnell JS, Schnitzler CE, Browne WE. KLF/SP Transcription Factor Family Evolution: Expansion, Diversification, and Innovation in Eukaryotes. Genome Biol Evol. 2015;7(8):2289–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang D, Zhang XL, Michel FJ, Blum JL, Simmen FA, Simmen RC. Direct interaction of the Kruppel-like family (KLF) member, BTEB1, and PR mediates progesterone-responsive gene expression in endometrial epithelial cells. Endocrinology. 2002;143(1):62–73. [DOI] [PubMed] [Google Scholar]
- 85.Pabona JMP, Simmen FA, Nikiforov MA, Zhuang DZ, Shankar K, Velarde MC, Zelenko Z, Giudice LC, Simmen RCM. Kruppel-Like Factor 9 and Progesterone Receptor Coregulation of Decidualizing Endometrial Stromal Cells: Implications for the Pathogenesis of Endometriosis. Journal of Clinical Endocrinology & Metabolism. 2012;97(3):E376–E392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang XL, Zhang DY, Michel FJ, Blum JL, Simmen FA, Simmen RCM. Selective interactions of Kruppel-like factor 9/basic transcription element-binding protein with progesterone receptor isoforms A and B determine transcriptional activity of progesterone-responsive genes in endometrial epithelial cells. Journal of Biological Chemistry. 2003;278(24):21474–21482. [DOI] [PubMed] [Google Scholar]
- 87.Stanfield Z, Lai PF, Lei K, Johnson MR, Blanks AM, Romero R, Chance MR, Mesiano S, Koyuturk M. Myometrial Transcriptional Signatures of Human Parturition. Front Genet. 2019;10:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Morris R, Kershaw NJ, Babon JJ. The molecular details of cytokine signaling via the JAK/STAT pathway. Protein Sci. 2018;27(12):1984–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zeinalzadeh E, Valerievich Yumashev A, Rahman HS, Marofi F, Shomali N, Kafil HS, Solali S, Sajjadi-Dokht M, Vakili-Samiani S, Jarahian M, Hagh MF. The Role of Janus Kinase/STAT3 Pathway in Hematologic Malignancies With an Emphasis on Epigenetics. Front Genet. 2021;12:703883. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 90.Williams KC, Renthal NE, Condon JC, Gerard RD, Mendelson CR. MicroRNA-200a serves a key role in the decline of progesterone receptor function leading to term and preterm labor. Proc Natl Acad Sci U S A. 2012;109(19):7529–7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rytkonen KT, Erkenbrack EM, Poutanen M, Elo LL, Pavlicev M, Wagner GP. Decidualization of Human Endometrial Stromal Fibroblasts is a Multiphasic Process Involving Distinct Transcriptional Programs. Reproductive Sciences. 2019;26(3):323–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cloke B, Huhtinen K, Fusi L, Kajihara T, Yliheikkila M, Ho KK, Teklenburg G, Lavery S, Jones MC, Trew G, Kim JJ, Lam EW, Cartwright JE, Poutanen M, Brosens JJ. The androgen and progesterone receptors regulate distinct gene networks and cellular functions in decidualizing endometrium. Endocrinology. 2008;149(9):4462–4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dotts AJ, Reiman D, Yin P, Kujawa S, Grobman WA, Dai Y, Bulun SE. In Vivo Genome-Wide PGR Binding in Pregnant Human Myometrium Identifies Potential Regulators of Labor. Reprod Sci. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cerliani JP, Guillardoy T, Giulianelli S, Vaque JP, Gutkind JS, Vanzulli SI, Martins R, Zeitlin E, Lamb CA, Lanari C. Interaction between FGFR-2, STAT5, and progesterone receptors in breast cancer. Cancer Res. 2011;71(10):3720–3731. [DOI] [PubMed] [Google Scholar]
- 95.Obr AE, Grimm SL, Bishop KA, Pike JW, Lydon JP, Edwards DP. Progesterone Receptor and Stat5 Signaling Cross Talk Through RANKL in Mammary Epithelial Cells. Molecular Endocrinology. 2013;27(11):1808–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hagan CR, Knutson TP, Lange CA. A Common Docking Domain in Progesterone Receptor-B links DUSP6 and CK2 signaling to proliferative transcriptional programs in breast cancer cells. Nucleic Acids Res. 2013;41(19):8926–8942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Challis JRG, Matthews SG, Gibb W, Lye SJ. Endocrine and paracrine regulation of birth at term and preterm. Endocr Rev. 2000;21(5):514–550. [DOI] [PubMed] [Google Scholar]
- 98.Bhattacharya M, Peri K, Ribeiro-da-Silva A, Almazan G, Shichi H, Hou X, Varma DR, Chemtob S. Localization of functional prostaglandin E2 receptors EP3 and EP4 in the nuclear envelope. J Biol Chem. 1999;274(22):15719–15724. [DOI] [PubMed] [Google Scholar]
- 99.Astle S, Thornton S, Slater DM. Identification and localization of prostaglandin E2 receptors in upper and lower segment human myometrium during pregnancy. Mol Hum Reprod. 2005;11(4):279–287. [DOI] [PubMed] [Google Scholar]
- 100.Erkinheimo TL, Saukkonen K, Narko K, Jalkanen J, Ylikorkala O, Ristimaki A. Expression of cyclooxygenase-2 and prostanoid receptors by human myometrium. J Clin Endocrinol Metab. 2000;85(9):3468–3475. [DOI] [PubMed] [Google Scholar]
- 101.Romero R, Munoz H, Gomez R, Parra M, Polanco M, Valverde V, Hasbun J, Garrido J, Ghezzi F, Mazor M, Tolosa JE, Mitchell MD. Increase in prostaglandin bioavailability precedes the onset of human parturition. Prostaglandins Leukot Essent Fatty Acids. 1996;54(3):187–191. [DOI] [PubMed] [Google Scholar]
- 102.Slater DM, Dennes WJ, Campa JS, Poston L, Bennett PR. Expression of cyclooxygenase types-1 and -2 in human myometrium throughout pregnancy. Mol Hum Reprod. 1999;5(9):880–884. [DOI] [PubMed] [Google Scholar]
- 103.Williams KC, Renthal NE, Gerard RD, Mendelson CR. The microRNA (miR)-199a/214 cluster mediates opposing effects of progesterone and estrogen on uterine contractility during pregnancy and labor. Mol Endocrinol. 2012;26(11):1857–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Renthal NE, Chen CC, Williams KC, Gerard RD, Prange-Kiel J, Mendelson CR. miR-200 family and targets, ZEB1 and ZEB2, modulate uterine quiescence and contractility during pregnancy and labor. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(48):20828–20833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Greenland KJ, Jantke I, Jenatschke S, Bracken KE, Vinson C, Gellersen B. The human NAD(+)-dependent 15-hydroxyprostaglandin dehydrogenase gene promoter is controlled by Ets and activating protein-1 transcription factors and progesterone. Endocrinology. 2000;141(2):581–597. [DOI] [PubMed] [Google Scholar]
- 106.Tong D, Lu X, Wang HX, Plante I, Lui E, Laird DW, Bai D, Kidder GM. A dominant loss-of-function GJA1 (Cx43) mutant impairs parturition in the mouse. Biol Reprod. 2009;80(6):1099–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kidder G, Winterhager E. Connexins: A Guide. 2008. [Google Scholar]
- 108.Orsino A, Taylor CV, Lye SJ. Connexin-26 and connexin-43 are differentially expressed and regulated in the rat myometrium throughout late pregnancy and with the onset of labor. Endocrinology. 1996;137(5):1545–1553. [DOI] [PubMed] [Google Scholar]
- 109.Kilarski WM, Dupont E, Coppen S, Yeh HI, Vozzi C, Gourdie RG, Rezapour M, Ulmsten U, Roomans GM, Severs NJ. Identification of two further gap-junctional proteins, connexin40 and connexin45, in human myometrial smooth muscle cells at term. European Journal of Cell Biology. 1998;75(1):1–8. [DOI] [PubMed] [Google Scholar]
- 110.Xie N, Liu L, Li Y, Yu C, Lam S, Shynlova O, Gleave M, Challis JR, Lye S, Dong X. Expression and function of myometrial PSF suggest a role in progesterone withdrawal and the initiation of labor. Mol Endocrinol. 2012;26(8):1370–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bell AF, Erickson EN, Carter CS. Beyond labor: the role of natural and synthetic oxytocin in the transition to motherhood. J Midwifery Womens Health. 2014;59(1):35–42: quiz 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nishimori K, Young LJ, Guo QX, Wang ZX, Insel TR, Matzuk MM. Oxytocin is required for nursing but is not essential for parturition or reproductive behavior. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(21):11699–11704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Takayanagi Y, Yoshida M, Bielsky IF, Ross HE, Kawamata M, Onaka T, Yanagisawa T, Kimura T, Matzuk MM, Young LJ, Nishimori K. Pervasive social deficits, but normal parturition, in oxytocin receptor-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(44):16096–16101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chard T, Gibbens GL. Spurt release of oxytocin during surgical induction of labor in women. Am J Obstet Gynecol. 1983;147(6):678–680. [DOI] [PubMed] [Google Scholar]
- 115.Fuchs AR, Romero R, Keefe D, Parra M, Oyarzun E, Behnke E. Oxytocin Secretion and Human Parturition - Pulse Frequency and Duration Increase during Spontaneous Labor in Women. American Journal of Obstetrics and Gynecology. 1991;165(5) :1515–1523. [DOI] [PubMed] [Google Scholar]
- 116.Fuchs AR, Fuchs F, Husslein P, Soloff MS. Oxytocin receptors in the human uterus during pregnancy and parturition. Am J Obstet Gynecol. 1984;150(6):734–741. [DOI] [PubMed] [Google Scholar]
- 117.Chibbar R, Miller FD, Mitchell BF. Synthesis of Oxytocin in Amnion, Chorion, and Decidua May Influence the Timing of Human Parturition. Journal of Clinical Investigation. 1993;91(1):185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chibbar R, Wong S, Miller FD, Mitchell BF. Estrogen stimulates oxytocin gene expression in human chorio-decidua. J Clin Endocrinol Metab. 1995;80(2):567–572. [DOI] [PubMed] [Google Scholar]
- 119.Rauk PN, Friebe-Hoffmann U, Winebrenner LD, Chiao JP. Interleukin-6 up-regulates the oxytocin receptor in cultured uterine smooth muscle cells. Am J Reprod Immunol. 2001;45(3):148–153. [DOI] [PubMed] [Google Scholar]
- 120.Arrowsmith S, Wray S. Oxytocin: Its Mechanism of Action and Receptor Signalling in the Myometrium. J Neuroendocrinol. 2014;26(6):356–369. [DOI] [PubMed] [Google Scholar]
- 121.Chan YW, van den Berg HA, Moore JD, Quenby S, Blanks AM. Assessment of myometrial transcriptome changes associated with spontaneous human labour by high-throughput RNA-seq. Exp Physiol. 2014;99(3):510–524. [DOI] [PubMed] [Google Scholar]
- 122.Yoshida M, Kanematsu T, Watanabe Y, Koga T, Ozaki S, Iwanaga S, Hirata M. D-myoinositol 1,4,5-trisphosphate-binding proteins in rat brain membranes. J Biochem. 1994;115(5):973–980. [DOI] [PubMed] [Google Scholar]
- 123.Uji A, Matsuda M, Kukita T, Maeda K, Kanematsu T, Hirata M. Molecules interacting with PRIP-2, a novel Ins(1,4,5)P3 binding protein type 2: Comparison with PRIP-1. Life Sci. 2002;72(4-5):443–453. [DOI] [PubMed] [Google Scholar]
- 124.Kanematsu T, Takeuchi H, Terunuma M, Hirata M. PRIP, a novel Ins(1,4,5)P3 binding protein, functional significance in Ca2+ signaling and extension to neuroscience and beyond. Mol Cells. 2005;20(3):305–314. [PubMed] [Google Scholar]
- 125.Takenaka K, Fukami K, Otsuki M, Nakamura Y, Kataoka Y, Wada M, Tsuji K, Nishikawa S, Yoshida N, Takenawa T. Role of phospholipase C-L2, a novel phospholipase C-like protein that lacks lipase activity, in B-cell receptor signaling. Mol Cell Biol. 2003;23(20):7329–7338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Muter J, Brighton PJ, Lucas ES, Lacey L, Shmygol A, Quenby S, Blanks AM, Brosens JJ. Progesterone-Dependent Induction of Phospholipase C-Related Catalytically Inactive Protein 1 (PRIP-1) in Decidualizing Human Endometrial Stromal Cells. Endocrinology. 2016;157(7):2883–2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kaneko Y, Szallasi A. Transient receptor potential (TRP) channels: a clinical perspective. Br J Pharmacol. 2014;171(10):2474–2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Consortium EP, Moore JE, Purcaro MJ, Pratt HE, Epstein CB, Shoresh N, Adrian J, Kawli T, Davis CA, Dobin A, Kaul R, Halow J, Van Nostrand EL, Freese P, Gorkin DU, Shen Y, He Y, Mackiewicz M, Pauli-Behn F, Williams BA, Mortazavi A, Keller CA, Zhang XO, Elhajjajy SI, Huey J, Dickel DE, Snetkova V, Wei X, Wang X, Rivera-Mulia JC, Rozowsky J, Zhang J, Chhetri SB, Zhang J, Victorsen A, White KP, Visel A, Yeo GW, Burge CB, Lecuyer E, Gilbert DM, Dekker J, Rinn J, Mendenhall EM, Ecker JR, Kellis M, Klein RJ, Noble WS, Kundaje A, Guigo R, Farnham PJ, Cherry JM, Myers RM, Ren B, Graveley BR, Gerstein MB, Pennacchio LA, Snyder MP, Bernstein BE, Wold B, Hardison RC, Gingeras TR, Stamatoyannopoulos JA, Weng Z. Expanded encyclopaedias of DNA elements in the human and mouse genomes. Nature. 2020;583(7818):699–710. [DOI] [PMC free article] [PubMed] [Google Scholar]


