Abstract
This clinical trial discusses the efficacy of premedication with desmopressin in the management of bleeding and clears the surgical field during rhinoplasty surgery. This study is a randomized, double-blinded placebo-control clinical trial. Seventy patients were enrolled in this study and divided into two equal intervention-control groups. Thirty minutes before surgery, the intervention group received 500 ml of normal saline containing 0.1 μg/kg desmopressin and, the control group received 500 ml of normal saline. According to the surgeon’s opinion, the local distribution of bleeding was dramatically different in both groups. While DDAVP receivers had grade 1 or 2 bleeding (according to the FROMME-BOEZAART grading score), the control group had grade 3 or 4 bleeding, and this difference was statistically meaningful. It seems that intravenous DDAVP can reduce bleeding and clear the surgical field during rhinoplasty surgery, but further studies are needed to determine the exact role and dose of the DDAVP.
Keywords: Desmopressin, DDAVP, Intraoperative hemorrhage, Rhinoplasty, Otorhinolaryngology surgery
Introduction
Despite recent efforts and developments in surgery and anesthesiology techniques, bleeding is still an unsolved concern during operation [1]. Massive bleeding is rare but, micro-bleedings can commonly damage surgeon’s sight, lead to longer operation time and stop the operation in severe cases. So, keeping appropriate sight for the surgeon is important [2, 3]. Reduction in bleeding, which can result in stable hemodynamics, is also favorable for an anesthesiologist [4]. Several methods like cauterization, vasoconstrictors local injection and, reducing blood pressure are applicable for this purpose in head and neck surgeries. Cauterizations may cause direct tissue damage and lead to secondary hemorrhage. Local vasoconstrictors may cause instability in hemodynamic status and may have ischemic heart disease consequences. Reducing blood pressure will expose the patient to the surplus of anesthesiology medications and cause unwanted complications. Therefore, all these methods have pitfalls, making them less useful in the clinic [5].
Massive bleedings during operation are rather uncommon, and most of the time, micro-bleedings can hinder surgeons’ sight and be problematic. Vasoconstrictors like desmopressin, aprotinin, tranexamic acid, E-aminocaproic acid and, estrogen are common medications used [6, 7]. Desmopressin (1-deamino-8-d-arginine vasopressin), which is sold as DDAVP, is the synthetic form of the human normal hormone arginine vasopressin. DDAVP is used to treat diabetes insipidus, bedwetting, hemophilia A and von Willebrand disease. DDAVP can reduce bleeding from the surgery site in patients with renal insufficiencies, enhance platelet function, increase factor VIII and VWF factor. In addition to beneficial effects on hemostasis, DDAVP can reduce blood pressure and blood loss in uncontrolled hemorrhages [8, 9].
Rhinoplasty is one of the most prevalent, precise, and problematic plastic surgeries worldwide [10]. Most of the ways of DDAVP effects on blood pressure and hemostasis have been unknown yet, so the consequences and benefits of this medication in Rhinoplasty surgery are discussed in this article.
Materials and methods
This study is a single-center randomized clinical trial on patients were referred to Rhinoplasty surgery department and were conducted and designed by Amiralam Hospital in Tehran, Iran from 21st of March 2018 until 21st of March 2019. The inclusion and exclusion criteria are showed beneath. The demographic and clinical outcomes of all 70 patients who were included in the study and were divided into two groups of interventions and controls (N1 = N2 = 35), then analyzed and discussed.
Inclusion criteria
patients who were candidates for primary rhinoplasty surgery (not revision surgery) based on cosmetic appearance and did not suffer from any platelet dysfunction or coagulopathy of any kind.
Exclusion criteria
Patients who had discontent for being included in study, patients with underlying renal disease, hepatic disease, heart disease of any kind, neurologic problems of any kind, bleeding problems and coagulopathies were excluded. Patients who took medications like OCP, warfarin, and other medications which may alter hemostasis were also debarred from study. Finally, patients requiring rib cartilage for rhinoseptoplasty were also disqualified.
Randomization and random hiding technique
This study is a randomized clinical trial using the permuted block randomization method. Each block used contained 4 samples. The randomized number table was used for study. specialist consultation for cardiac, hepatic, renal, hematology and neurology problems was done before entering samples and if not excluded, the study nature and its purpose was thoroughly explained to the patients. The complications and benefits of the procedure were explained. Confidentiality of information was guaranteed during and after study and patients had utter right to exit study at any point during the study in which they desired. Informed consent of patients was taken.
Basic demographics like sex, age, history and physical exams were taken and registered. The intervention group received 0/1 to 0/3 µg per kilograms of DDAVP infused in 500 cc of normal saline serum, 30 min prior to the surgery. The anesthesiology team was the same for both intervention and control groups and used the same method for both groups. The surgeons were blinded in knowing which group contained interventions -controls and the final results were gathered by the first author of this publication. The amount of bleeding was measured both quantitatively and qualitatively. Quantitatively via measurement of blood in suction minus serum used for irrigation and number of bloody gauzes. Qualitatively by the surgeon using the FROMME-BOEZAART grading score (Grade1: No bleeding, Grade2: Slight bleeding, Grade3: Moderate bleeding, Grade4: Severe bleeding.) and results were compared with each other [11, 12].
The 26th version of SPSS software was used for analyzing the data and qualitative variables were described by tables and diagrams. Central and dispersion indexes were used for describing quantitative variables. Due to having quantitative dependent variables, for comprehending their relation with independent variables the two-sample t test and the Pearson correlation (if normal dispersion) were used. Nonparametric equivalent measures were applied for variables not normally disputed. The Mann–Whitney test was used to investigate whether two independent samples were selected from populations having the same distribution. The p value of less than 5 percent was considered as epidemiologically significant.
Results
The mean age of intervention group was 32.11 years old and consisted 48.6% females and 51.4% males while the average age of control group was 28.2 years old and contained 57.1% females and 42.9% males, the demographic data are available in Table 1.
Table 1.
Demographic and clinical data of patients were categorized in intervention (DDAVP +) and control (DDAVP −) groups
| DDAVP + | DDAVP − | p value | |
|---|---|---|---|
| Age (years) | 32.11 (9.12) | 28.20 (7.79) | 0.43 |
| Gender (male) | 18 (51.4%) | 15 (42.9%) | 0.12 |
| Operation time (min) | 209 | 235.29 | < 0.001 |
| Na serum level preoperations (mEq/L) | 135.02 (1.31) | 135.22 (1.33) | 0.72 |
| Na serum level post operation (mEq/L) | 133.57 | 135.14 | < 0.001 |
| Maximum mean arterial blood pressure | 94.48 | 88.13 | < 0.001 |
| Minimum mean arterial blood pressure | 79.21 | 71.36 | < 0.001 |
| Blood loss (cc) | 108.29 | 189.57 | < 0.001 |
The fluctuations of sodium and hyponatremia before and after operation in case group receiving DDAVP was higher compared to control group, (p value < 0.001). The maximum mean arterial blood pressure was also suggestively different in both groups (p value < 0.001), and the minimum mean arterial blood pressure was higher in the case group (Table 1). The operating time was significantly lower in patients receiving DDAVP compared to the control group (p value < 0.001).
The local distribution of bleeding according to surgeon’s opinion was also dramatically different in both groups, while DDAVP receivers had grade 1 or 2 bleeding (according to the FROMME-BOEZAART grading score) the control group had grade 3 or 4 of bleeding and this difference was statically meaningful (p value < 0.001) (Fig. 1).
Fig. 1.
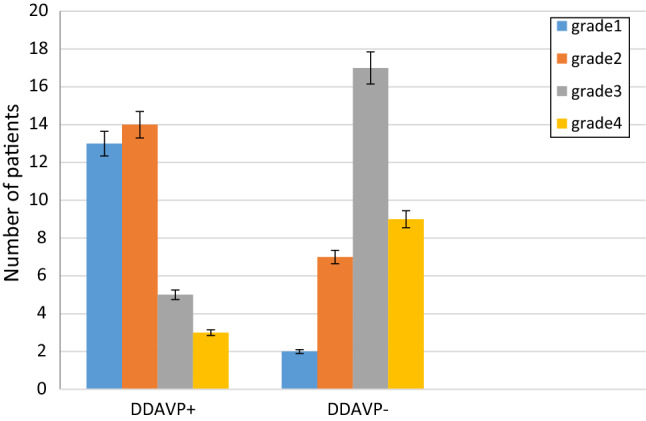
The local distribution of bleeding according to the FROMME-BOEZAART grading score in intervention (DDAVP +) and control (DDAVP-) groups
Discussion
We discussed the efficacy of DDAVP in management of bleeding of surgery site and therefore providing a satisfactory sight for surgeon for rhinoplasty in this clinical trial. We concluded that bleeding was lower in patients receiving DDAVP while the blood pressure was higher during surgery. One of the main factors contributing to desired results in procedures is maintaining normal blood pressure using vasoconstrictors.
DDAVP is one of the main vasoconstrictor medications used for otolaryngology surgeries. According to Nitu-Whalley et al. [13], using DDAVP for hemostasis and preventing bleeding was more effective compared to Tranexamic acid. DDAVP is widely used for hematologic problems in patients who suffer from inherited blood diseases and undergo otolaryngology surgery but there is not adequate data to support using DDAVP for controlling hemorrhage in healthy patients and the mild side effects of this medication and its frequency is yet to be discussed in upcoming studies. There are several studies indicating that DDAVP could be beneficial for treating bleeding during surgery in otherwise healthy patients. In one study by Reusser [14], the amount of bleeding in Adenotonsilectomy was significantly lower in healthy patients receiving DDAVP. Although many studies support using DDAVP can decrease bleeding in patients undergoing head and neck surgery, the distribution and epidemiologic data could not be scientifically proven. Using DDAVP in patients having congenital hematologic disease could decrease bleeding in otolaryngology surgery. Still, they differ in rating how successful this medication is and the quality and quantity are not described well and the dosage of medication is the same throughout studies [15].
In a meta-analysis performed in 2017, regardless of any previous congenital blood disease, mild side effects have been discussed and only one patient had severe hyponatremia leading to seizure [16]. Severe complications of advising desmopressin were more prevalent in studies done before 2000 and could be related to elder medication guidelines, inadequate hydration, and electrolyte management of patients [17]. A meta-analysis by Barinsky et al., reported that mild hyponatremia is fairly common in patients receiving desmopressin (74/6%). This hyponatremia can be a physiologic response to the return of free fluids. Other complications like nausea, vomiting and temporary hypotension due to loss of fluids are also common in patients. Moreover, it was suggested that DDAVP could also be used for healthy patients undergoing surgery due to having mild side effects and being safe if not contraindicated [18, 19]. This also could be very helpful in delicate head and neck surgeries, in which the surgeon pertains to a non-bleeding site for appropriate access to anatomy of region. Providing a better visual site for the surgeon directly and less time for exchanging devices used for hemostasis indirectly could lessen bleeding and reduce the time of surgery [9, 16, 18].
Septoplasty, rhinoplasty and turbinectomy are usually done synchronously and using desmopressin could be challenging. Especially knowing that FDA has not approved using this medication for healthy patients and it has been studied for patients having underlying hematologic problems and the confounding factors have not been well managed [20].
In a study performed by Gurber et al., it was concluded that using desmopressin, in some cases in lower doses than 0/3 µg per kilogram, could be beneficial and obtain a better vision site for surgeon. Several issues have to be discussed regarding this study: first of all, it is a retrospective study and it lacks blinding of case–control groups which lead to misinterpretation and prejudgment of surgeon on grading hemorrhage and hemostasis. The question that why 43 out of 73(59%) of patients in the study received increased dose of 0/3 µg per kilogram in spite of 0/1 while only 9 patients out of 300 retrospective patients got adequate hemostatic results remains unanswered. Also it is not explained why 91% of patients received desmopressin and was it essential for treatment or not. Therefore, it is not recommended to use desmopressin for every patient according to what Gurber said but it could be reasonable to prescribe it for patients in which surgeon believes may have more than usual bleeding like those with prior history of complex cosmetic rhinoplasty manipulating bone structure or hypertensive patients [4].
The possible side effects of desmopressin are flushing, paresthesia, headache, nausea and vomiting, tachycardia and rarely delusional hyponatremia due to excess usage of fluids in the elderly and very young patients [20].
After initial incision for surgery damage to vasculature causes bleeding, as time goes by the coagulation system becomes more efficient. Recent studies prove that an intranasal dose of desmopressin one hour prior to operation could be useful in subsiding hemorrhage during operation. A study conducted by Haddadi et al. suggests that desmopressin could be advantageous in septoplasty surgery; it aids the surgeon in providing a drier field for better vision and has better outcomes like less ecchymosis and less edema for patients [3].
In a meta-analysis done in 2016 by Coroneos et al. it was suggested that intravenous dose of desmopressin is helpful for controlling bleeding and epistaxis without any need for any superseded technique. An intranasal desmopressin is an excellent choice for use in clinic due to great availability and being safe. Water retention, hyponatremia, nausea and vomiting, nasal congestion and epistaxis are possible side effects which need to be followed carefully by the practitioner and if two puffs are used one hour prior to surgery in each nostril, it will help patient feel relieved after surgery [21]. Another finding in the Gurber study was that using lesser doses of desmopressin could be as effective as higher doses in preventing hemorrhage during surgery. So, lesser doses will reduce possible complications made by higher doses [4]. Based on Gurber and Barinsky study [4, 18], desmopressin could be used in a broader spectrum of cosmetic rhinoplastic surgeries which prefer a dryer field of the incision site. Examples are submentoplasty in males, lower blepharoplasty, and facial lifting with a higher hemorrhage rate, especially in hypertensive patients under medication and borderline blood pressures. In a nutshell benefits of desmopressin exceed its defects. The Gurber study alongside Guyuron et al. and Faber et al. recommend using desmopressin and surgeon should be aware of its remunerations in plastic and reconstruction surgeries [4, 12, 22]].
Conclusion
Based on this study and literature review of similar studies, intravenous DDAVP can reduce bleeding and offer an enhanced clear vision for the surgeon during rhinoplasty surgery. But further studies are needed to determine the exact role and dose of the DDAVP. Determining the minimum effective dose in controlling hemorrhage with less side effects and more therapeutic use could be the subject of forthcoming investigations in this field and makes desmopressin a candidate for further use in rhinoplasty surgery.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This study was approved by the ethics committees of the collaborating hospitals. Informed consent was obtained from all participants. The study protocol was approved by the local Ethics Review Committee of our institution (Tehran University of medical sciences). The approval ID is: IR.TUMS.MEDICINE.REC.1397.897.
Footnotes
The clinical trial registration number (IRCT) is IRCT20170120032069N7.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Abolqasem Youssefy, Email: yousefi@sina.tums.ac.ir.
AmirHossein Ghabasiah, Email: ah.ghabasiah@gmail.com.
Farrokh Heidari, Email: farrokh.heidari@yahoo.com.
Sepideh Alvandi, Email: Sepideh_alvandi@yahoo.com.
Shahin Bastaninezhad, Email: shahinbastani@razi.tums.ac.ir.
Jawad Hosseini, Email: Jawadhosseini.md@gmail.com.
Ardavan Tajdini, Email: a-tajdini@sina.tums.ac.ir.
References
- 1.Wormald P-J, Athanasiadis T, Rees G, Robinson S. An evaluation of effect of pterygopalatine fossa injection with local anesthetic and adrenalin in the control of nasal bleeding during endoscopic sinus surgery. Am J Rhinol. 2005;19(3):288–292. doi: 10.1177/194589240501900313. [DOI] [PubMed] [Google Scholar]
- 2.Wormald P (2005) The surgical field in endoscopic sinus surgery. Endoscopic sinus surgery: anatomy, three-dimensional reconstruction and surgical technique. Thieme, New York
- 3.Haddady-Abianeh S, Rajabpour AA, Sanatkarfar M, Farahvash MR, Khorasani G, Molaei H. The hemostatic effect of desmopressin on bleeding as a nasal spray in open septorhinoplasty. Aesthetic Plast Surg. 2019;43(6):1603–1606. doi: 10.1007/s00266-019-01485-4. [DOI] [PubMed] [Google Scholar]
- 4.Gruber RP, Zeidler KR, Berkowitz RL. Desmopressin as a hemostatic agent to provide a dry intraoperative field in rhinoplasty. Plast Reconstr Surg. 2015;135(5):1337–1340. doi: 10.1097/PRS.0000000000001158. [DOI] [PubMed] [Google Scholar]
- 5.Riegle EV, Gunter JB, Lusk RP, Muntz HR, Weiss KL. Comparison of vasoconstrictors for functional endoscopic sinus surgery in children. Laryngoscope. 1992;102(7):820–823. doi: 10.1288/00005537-199207000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Flordal PA, Ljungström K-G, Ekman B, Neander G. Effects of desmopressin on blood loss in hip arthroplasty: controlled study in 50 patients. Acta Orthop Scand. 1992;63(4):381–385. doi: 10.3109/17453679209154749. [DOI] [PubMed] [Google Scholar]
- 7.Testa LD, Tobias JD. Pharmacologic drugs for controlled hypotension. J Clin Anesth. 1995;7(4):326–337. doi: 10.1016/0952-8180(95)00010-F. [DOI] [PubMed] [Google Scholar]
- 8.Shao H, Kuang L-T, Hou W-J, Zhang T. Effect of desmopressin administration on intraoperative blood loss and quality of the surgical field during functional endoscopic sinus surgery: a randomized, clinical trial. BMC Anesthesiol. 2015;15(1):53. doi: 10.1186/s12871-015-0034-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jahanshahi J, Tayebi E, Hashemian F, Bakhshaei MH, Ahmadi MS, Rabiei MAS. Effect of local desmopressin administration on intraoperative blood loss and quality of the surgical field during functional endoscopic sinus surgery in patients with chronic rhinosinusitis: a triple-blinded clinical trial. Eur Arch Otorhinolaryngol. 2019;276(7):1995–1999. doi: 10.1007/s00405-019-05435-3. [DOI] [PubMed] [Google Scholar]
- 10.Mohseni M, Ebneshahidi A. The effect of oral clonidine premedication on blood loss and the quality of the surgical field during endoscopic sinus surgery: a placebo-controlled clinical trial. J Anesth. 2011;25(4):614. doi: 10.1007/s00540-011-1157-9. [DOI] [PubMed] [Google Scholar]
- 11.Boezaart AP, van der Merwe J, Coetzee A. Comparison of sodium nitroprusside-and esmolol-induced controlled hypotension for functional endoscopic sinus surgery. Can J Anaesth. 1995;42(5):373–376. doi: 10.1007/BF03015479. [DOI] [PubMed] [Google Scholar]
- 12.Guyuron B, Vaughan C, Schlecter B. The role of DDAVP (desmopressin) in orthognathic surgery. Ann Plast Surg. 1996;37(5):516–519. doi: 10.1097/00000637-199611000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Nitu-Whalley IC, Griffioen A, Harrington C, Lee CA. Retrospective review of the management of elective surgery with desmopressin and clotting factor concentrates in patients with von Willebrand disease. Am J Hematol. 2001;66(4):280–284. doi: 10.1002/ajh.1058. [DOI] [PubMed] [Google Scholar]
- 14.Reusser NM, Bender RW, Agrawal NA, Albright JT, Duncan NO, Edmonds JL (2017) Post-tonsillectomy hemorrhage rates in children compared by surgical technique. ENT Ear Nose Throat J 96(7) [DOI] [PubMed]
- 15.Dimichele DM, Hathaway WE. Use of DDAVP in inherited and acquired platelet dysfunction. Am J Hematol. 1990;33(1):39–45. doi: 10.1002/ajh.2830330108. [DOI] [PubMed] [Google Scholar]
- 16.Desborough MJ, Oakland K, Brierley C, Bennett S, Doree C, Trivella M et al (2017) Desmopressin use for minimising perioperative blood transfusion. Cochrane Database Syst Rev 2017(7) [DOI] [PMC free article] [PubMed]
- 17.Allen GC, Armfield DR, Bontempo FA, Kingsley LA, Goldstein NA, Post JC. Adenotonsillectomy in children with von Willebrand disease. Arch Otolaryngol Head Neck Surg. 1999;125(5):547–551. doi: 10.1001/archotol.125.5.547. [DOI] [PubMed] [Google Scholar]
- 18.Barinsky GL, Buziashvili D, Svider PF, Carron MA, Folbe AJ, Hsueh WD, et al. Perioperative desmopressin for patients undergoing otolaryngologic procedures: a systematic review. Otolaryngol Head Neck Surg. 2019;161(1):36–45. doi: 10.1177/0194599819831288. [DOI] [PubMed] [Google Scholar]
- 19.Hajimohamadi F, Hosseini J, Heidari F, Alvandi S, Bastaninezhad S, Ghabasiah A et al (2021) Desmopressin effects on bleeding during functional endoscopic sinus surgery on patients with chronic rhinosinusitis. Am J Otolaryngol 42(5):103024 [DOI] [PubMed]
- 20.Cattaneo M, Mannucci PM (2019) Desmopressin (DDAVP). Platelets: Elsevier, pp 1111–1120
- 21.Coroneos CJ, Voineskos SH, Cook DJ, Farrokhyar F, Thoma A. Perioperative corticosteroids reduce short-term edema and ecchymosis in rhinoplasty: a meta-analysis. Aesthetic Surg J. 2016;36(2):136–146. doi: 10.1093/asj/sjv138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Faber C, Larson K, Amirlak B, Guyuron B. Use of desmopressin for unremitting epistaxis following septorhinoplasty and turbinectomy. Plast Reconstr Surg. 2011;128(6):728e–e732. doi: 10.1097/PRS.0b013e318230bf39. [DOI] [PubMed] [Google Scholar]


