Abstract
Malignant tumors of external auditory canal (EAC) constitute less than 0.2% of all head and neck cancers. The incidence of carcinoma of the EAC is estimated to be between one in six per million populations. Majority of cancers of EAC are squamous cell carcinomas and basal cell carcinomas. Some rare and unusual tumors do occur within the ear canal including malignant melanoma, merkel cell carcinoma, angiosarcoma, lymphoma and adnexal carcinomas like ceruminous adenocaricinoma and adenoid cystic carcinoma. Ceruminous glands tumors constitute about 5% of all external auditory canal tumors. Carcinoma of the external auditory canal is a difficult diagnosis unless the tumors presents as a fungating mass protruding from the external auditory canal. Syringocystadenocarcinoma Papilliferum (SCACP) is an extremely rare cutaneous adnexal neoplasm. Syringocystadenoma papilliferum (SCAP) is thought to be precursor of SCACP. About 50 cases of SCACP have been reported in literature all over the body. The diagnosis is difficult and excisional biopsy becomes mandatory for diagnosis and treatment. We present a case of SCACP in the external auditory canal in a middle-aged female. To the best of our knowledge and belief, this is the first case of SCACP in the external auditory canal in the English literature. This prompted us to report this case.
Keywords: Syringocystadenocarcinoma papilliferum, SCACP, Syringocystadenoma papilliferum, SCAP, Sweat gland neoplasm
Introduction
Malignant tumors of the external auditory canal (EAC) are infrequent. The commonest histological type of carcinoma of the EAC is squamous cell carcinoma. The other histological types are basal cell carcinoma, malignant melanoma. Merkel cell carcinoma, angiosarcoma adnexal carcinoma and lymphoma [1]. Ceruminous gland tumors or ceruminoma arising from apocrine glands are classified as adenoma, ceruminous adenocarcinoma and ceruminous adenoid cystic carcinoma and mixed tumors [2]. Syringocystadenoma papilliferum (SCAP), a non-malignant tumor arising from modified apocrine and/or eccrine sweat glands in the outer part of EAC. Syringocystadenocarcinoma papilliferum (SCACP) is a rare adnexal malignant tumor. SCAP is considered as a precursor of SCACP. Since its description by Dissanayake et al. in 1980, less than 50 cases have been reported in the literature [3].
Literature review shows the histologic and immunohistochemical features of SCACP are not well defined outside of their clear morphologic overlap with SCAP. The optimal treatment has not yet been established because of the rarity of the tumor. Wide local excision has been the preferred treatment for localized SCACP. To our knowledge, no case of SCACP in the external auditory canal has been reported yet in the English medical literature. We report the first case of SCACP involving external auditory canal in a middle-aged female.
Case Report
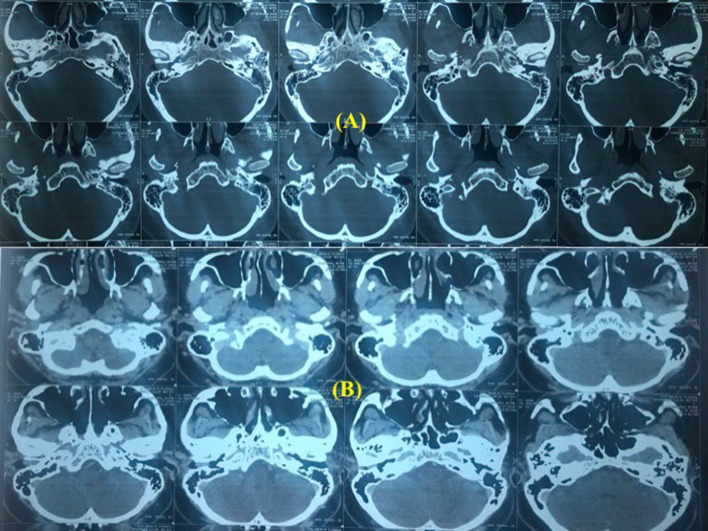
NK, 40 female presented with complaints of pain, mass in the ear and recurrent scanty watery discharge from right ear for last 1.5 years. She had fullness in the right ear and impaired hearing. She was operated upon 8 years back somewhere else on the same ear. The details were not available. Examination revealed a polypoidal firm mass occupying the 7/8 part of the external auditory canal in the posterior, inferior and anterior part with uninvolved superior canal wall and a part of tympanic membrane could be seen while pushing the mass inferiorly (Fig. 1). Lateral surface of the mass was covered with normal looking hair bearing skin but medially the mass was ulcerated. There was scanty foul smelling discharge in the canal. Tuning fork test was positive on left and negative on right side and weber lateralized to right side. Tonal audiogram showed average 25 db loss in all frequencies in right ear. Clinically, no neck nodes were palpable. FNAC showed dysplastic squamous cells. High resolution computed tomography (HRCT) of petro-temporal bone showed a soft tissue density mass with bony destruction and resorption. Contrast CT study petrotemporal complex demonstrated a mildly enhanced mass 16.1 × 9.4 × 7.6 mm in external auditory canal with associated resorption and defects of the bony walls predominantly postero-inferior, inferior and anterior portions of external auditory canal. There was no extension of the mass posteriorly into the mastoid or anteriorly into temporo-mandibular joint, parotid gland and inferiorly into parotid space. No extension is seen to the middle ear cavity. No metastatic lymph nodes were detected on ultrasound scan of the neck (Fig. 2).
Fig. 1.
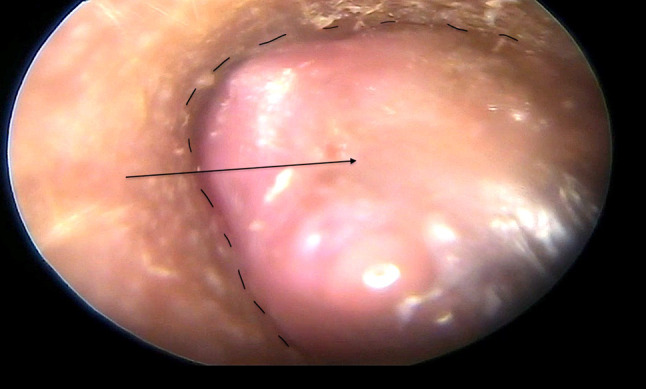
Polypoidal mass in the external auditory canal
Fig. 2.
Mildly enhancing soft tissue density in external auditory canal associated with bony destruction and resorption
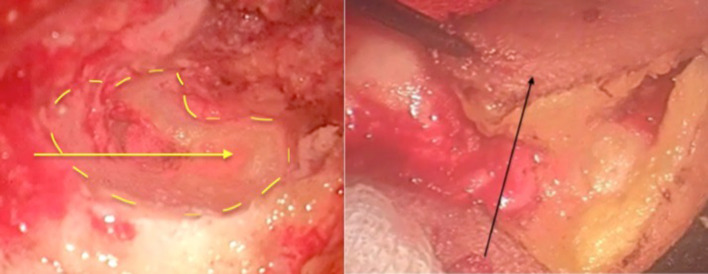
The patient was operated upon via post aural approach. Anterior and posterior canalplasty was done to delineate the extent of tumor. The mass was attached laterally to the cartilage. The mass was dissected with the cartilaginous portion of the canal with the outer perichondrium. There was destruction of the tympanic part of the temporal bone infero-medially. The mass was easily separated out from the muscle with the outer periosteum inferiorly with sufficient safe margins. Anteriorly the mass was dissected of the TM joint area. TM joint was not involved and healthy bone was present. Posteriorly, the tumor was limited and not involving the mastoid beyond periosteum. Medially the tumor was extending up to about 2–3 mm lateral to tympanic membrane and tympanic membrane was intact. The bony defect thus created in the inferior part of tympano-mastoid bone was smoothed off with diamond burr. The inferior bony defect so formed was lined with inferior based musculo-cutaneous transposition flap (Fig. 3). A partial thickness graft taken from the posterior surface of pinna to cover exposed the posterior and anterior bony canal walls. Under TNM classification this was categorized as T2N0M0.
Fig. 3.
Bony defect after removal of mass (yellow line). Musculocutaneous flap used to cover the defect (black arrow)
The histopathology revealed skin-covered tissue with an underlying lesion composed of papillae lined by dual layer of cuboidal to low columnar cells along with basal cells. Nests of cells are also seen infiltrating the stroma with nuclear atypia and mitosis (Fig. 4). Immunohistochemistry showed EMA (E29), P63 (DAK-P63), CK (AE1/AE3) and S-100 (EP-32) were positive in basal cells Ki67 (MIB-1) was 50% and mCEA (2-7(MONO)) was focal positive. D-2-40 (CLONE D2-40), PAXS (ZR-1) and TTF1 (EP229) were negative (Fig. 5). All these findings collectively favoured a final diagnosis of syringocystadenocarcinoma papilliferum.
Fig. 4.
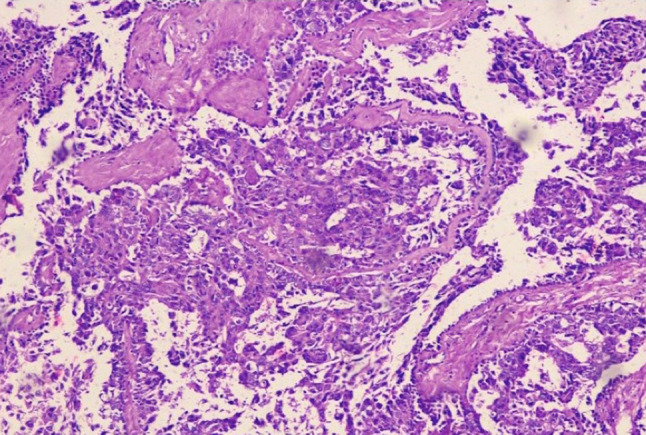
Papillae lined by cuboidal to low columnar cells. Nests of cells infiltrating the stroma with nuclear atypia and mitosis
Fig. 5.
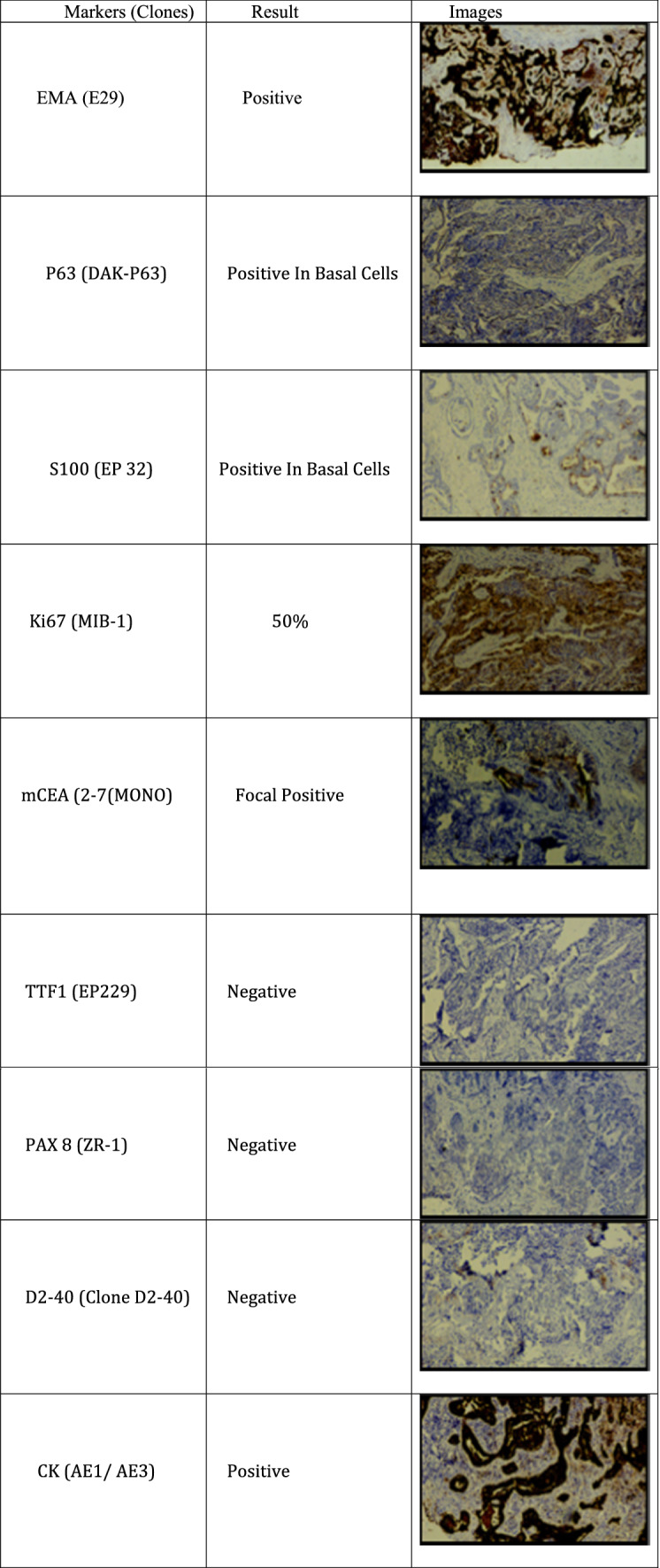
Immunohistochemistry
Discussion
Human body has two types of sweat glands–apocrine and eccrine glands. Eccrine glands are found all over the body except hair bearing areas of armpits, eye lids, areola, nostrils, perianal and ear canal where apocrine glands are present. There are modified apocrine glands as ciliary glands of eyelids, mammary glands and ceruminous glands of external auditory canal. Two types of glands are found in the cartilaginous portion of the ear canal, sebaceous and sudoriferous apocrine glands. Ceruminous gland tumors are classified as pleomorphic adenoma, ceruminous adenoma, ceruminous adenocarcinoma and ceruminous adenoid cystic carcinoma [8]. Additional variants, including syringocystadenoma papilliferum, mucoepidermoid carcinoma, and benign-malignant eccrine cylindroma have been added.
SCAP is a rare benign ceruminous gland tumor, seen in head and neck region and characterized by asymptomatic, skin-colored to pink papules or plaques with a highly variable appearance. In the EAC, these tumors may present with pain, hearing loss, itching, bleeding, headache, tinnitus, facial weakness. Examination may reveal a mass in the canal, ear discharge, periauricular edema and facial palsy.
The incidence of malignant tumors of EAC is very low. The histological types are squamous cell carcinoma, basal cell carcinoma, malignant melanoma, Merkel cell carcinoma, angiosarcoma, adnexal carcinoma including ceruminous adenocarcinoma and adenoid cystic carcinoma, lymphoma and sebaceous carcinoma [1]. The diagnosis of carcinoma of the external auditory canal is difficult unless the tumor presents as a fungating mass protruding from the external auditory canal. Long standing SCAP seems to give rise to SCACP with over all similar configuration except cytological atypia [4].
SCACP is considered by WHO as the malignant form of SCAP [5]. SCACP is believed to arise in a pre existing nevus sebaceous of Jadassohn (NSJ) through a multistep progression process. This hypothetical process involves an NSJ giving rise to SCAP, which then presumably undergoes malignant transformation in rare circumstances to give rise to SCACP in situ, which finally progresses to an invasive SCACP [6].
Controversy exists about the origin of SCACP-apocrine versus eccrine. It grows with variable speed and may even alternate between periods of stability and growth. Time to diagnosis ranges from several weeks to 30 years. The classification, clinical behavior, and management of this rare tumor is a matter of debate. Due to the rarity of this entity, clinical and pathologic diagnosis is challenging. These tumors are small and localized and hence the early diagnosis is difficult. The anatomy of EAC is complex; clinically it is difficult to assess the accurate extent of the tumor. Fine needle aspiration is difficult to perform due to the size and anatomic position. In our case, the pathologists could not perform fine needle aspiration biopsy but the senior author helped the pathologists to procure the FNAC specimen and which yielded dysplastic squamous cells, a provisional diagnosis of malignancy. Biopsy for histopathological examination is difficult and often inadequate to confirm the diagnosis. Local excision and excisional biopsy should be carried out to reach a definite diagnosis.
Mass lesions in the external auditory canal are difficult to diagnose based on clinical and radiological data. High resolution computed tomography (HRCT) and magnetic resonance imaging (MRI) are important modalities for diagnosing and differentiating masses in the EAC. Judging from the CT images, complete surgical excision can be achieved if there has been no spread into the bony canal and no bone destruction is observed. Following the total removal of the neoplasm, periodic follow up is suggested.
SCACP has many structural similarities with SCAP, but it can be differentiated from SCAP in that it has an asymmetric and poorly circumscribed structure of tumor, often extending deep into the subcutaneous fat and atypical cells, many of which are in mitosis [7].
SCACP, despite its rarity, should be kept in mind in the differential diagnosis of mass lesions in the external auditory canal. A rare site of origin of such an uncommon tumor is described in the present article.
Histologically, SCACP can present as (1) SCACP in situ. (2) Invasive SCACP and rarely (3) invasive squamous cell carcinoma (SCC), characterized by the presence of invasive SCC component in the SCAP background [8]. SCACP characteristically presents with squamous cell invaginations extending from the epidermis into the dermis. The invaginations and papillary projections are lined by two-layer epithelium: the luminal layer composed of columnar cells with decapitation secretions and the outer layer composed of small cuboidal cells. SCACP needs to be understood for the elucidation of progression of a lesion from a benign or premalignant lesion to an adenocarcinoma. In situ carcinoma and SCACP may coexist serving as histologic evidence of malignant progression.
There is no specific immunohistochemical marker that defines the diagnosis of SCACP and still under study. Solid and cystic glandular structures with cribriform and tubular architecture along with CK5/6, pankeratin, and p63 immuno-profile set apart SCACP from other cutaneous malignancies [9]. It is suggested that this tumor could derive from pluripotent-like cells and need to reanalyze the SCACP cases in the presence of evidences regarding histogenesis and stem cell-like properties. SCACP is very infrequent skin tumor but could be a good model to verify the existence of the cancer stem cells and their trans differentiable properties in skin neoplasms [10].
Altunel et al. [9] suggested molecular characterization (genomic profiling) of the patient’s tumor. It is a potentially interesting finding and further studies will need to be performed to validate these genomic alterations, which may help targeted therapy. Reporting molecular profile of the rare tumors with no established standard treatment options should be encouraged. It has been recently discovered by some authors that nevus sebaceous of Jadassohn (NSJ) is a mosaic RASopathy, a mosaic mutation of HRAS and KRAS genes with activation of the mitogen-activated proteins kinase (MAPK) and phosphatidylinositol-3-kinase (P13k)-Akt signaling pathways [6]. The tumor may spread to the regional and distant lymph nodes [9]. Of the 50 cases reported, 22% had loco regional and 3 patients had distant metastasis.
Surgical excision is thought to be the standard treatment of choice. Wide local excision has been the mainstay treatment for localized SCACP. Though surgery is nearly always performed in cancers of external auditory canal, no consensus exists as to what type of procedure should be chosen. Some authors advocate en bloc resection (Partial, subtotal, or total temporal bone resection) depending upon the extent of lesion determined by preoperative imaging techniques. The operative procedures are categorized as, (1) Local canal resection with or without mastoidectomy, removal of incus and malleus and/or parotidectomy leaving behind some of the osseous external auditory canal wall. (2) En bloc removal of the entire external auditory canal, lateral to the facial nerve including the tympanic membrane with the malleus and incus. There is no standard systemic therapy for the unresectable tumors especially in cases of loco regional metastases [9].
Conclusion
A case report of SCACP in the external auditory canal is presented. To the best of our knowledge, it is the first case of SCACP being reported in the English literature. Though extremely rare, the differential must be kept in mind. Histopathology and immunohistochemistry with CT scan and MRI are the various modalities to reach the correct diagnosis. In limited disease, wide local excision is the treatment of choice with or without loco-regional node dissection. In advanced cases chemo-radiation may advised but the success rate is still a matter to study. Since there have been reports of loco regional and distant metastasis and recurrence, these patients must be closely followed up. Molecular characterization (genomic profiling) of this type of tumors is suggested. It is a potentially interesting finding and further studies will need to be performed to validate genomic alterations, which may help better understanding about the targeted therapy and prognosis.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Devaney KO, Boschman CR, Willard SC, Ferlito PA, Rinaldo A. Tumours of the external ear and temporal bone. Lancet Oncol. 2005;6:411–420. doi: 10.1016/S1470-2045(05)70208-4. [DOI] [PubMed] [Google Scholar]
- 2.Wetly CV, Pardo V, Millard M, Gerston K. Tumors of ceruminous glands. Cancer. 1972;29:1169–1178. doi: 10.1002/1097-0142(197205)29:5<1169::aid-cncr2820290507>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 3.Dissanayake RV, Salm R. Sweat-gland carcinomas: prognosis related to histological type. Histopathology. 1980;4:445–466. doi: 10.1111/j.1365-2559.1980.tb02939.x. [DOI] [PubMed] [Google Scholar]
- 4.Requena L, Kiryu H, Ackerman AB (1988) In Ackerman’s histologic diagnosis of neoplastic skin disease: a method by pattern analysis. Neoplasms with Apocrine Differentiation. Lippincott-Raven, Philadelphia, pp 665–75
- 5.Abrari A, Mukherjee U. Syringocystadenocarcinoma papilliferum at unusual site: inherent lesional histologic polymorphism is the pathognomon. BMJ Case Rep. 2011 doi: 10.1136/bcr.05.2011.4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parekh V, Guerrero CE, Knapp CF, Elmets CA, Mckay KM. A histological snapshot of hypothetical multistep progression from nevus sebaceous to invasive syringocystadenocarcinoma papilliferum. Am J Dermatopathol. 2016;38:56–62. doi: 10.1097/DAD.0000000000000370. [DOI] [PubMed] [Google Scholar]
- 7.Park SH, Shin YM, Shin DH. Syringocystadenocarcinoma papilliferum: a case report. J Korean Med Sci. 2007;22:762–765. doi: 10.3346/jkms.2007.22.4.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee KG, Choi W, Lim JS, Hahn HJ, Myung KB, Cheong SH. Syringocystadenocarcinoma papilliferum: a case report and review of the literature. Ann Dermatol. 2019;31(5):559–562. doi: 10.5021/ad.2019.31.5.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Altunel E, Perepletchikov A, Kozyreva O. Metastatic syringocystadenocarcinoma papilliferum: a case report, tumor genomic profiling, and literature review. Case Rep Oncol Med. 2020 doi: 10.1155/2020/9056209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paradiso B, Bianchini E, Cifelli P, Cavazzini L, Lanza G. A new case of Syringocystadenocarcinoma papilliferum: a rare pathology for a wide- ranging comprehension. Case Rep Med. 2014;2014:453874. doi: 10.1155/2014/453874. [DOI] [PMC free article] [PubMed] [Google Scholar]