Abstract
Since the start of the coronavirus disease 2019 (COVID-19) pandemic, several biomarkers have been proposed to assess the diagnosis and prognosis of this disease. The present systematic review evaluated endocan (a marker of endothelial cell damage) as a potential diagnostic and prognostic biomarker for COVID-19. PubMed, Scopus, Web of Science, and Embase were searched for studies comparing circulating endocan levels between COVID-19 cases and controls, and/or different severities/complications of COVID-19. Eight studies (686 individuals) were included, from which four reported significantly higher levels of endocan in COVID-19 cases compared with healthy controls. More severe disease was also associated with higher endocan levels in some of the studies. Studies reported higher endocan levels in patients who died from COVID-19, were admitted to an intensive care unit, and had COVID-19-related complications. Endocan also acted as a diagnostic and prognostic biomarker with different cut-offs. In conclusion, endocan could be a novel diagnostic and prognostic biomarker for COVID-19. Further studies with larger sample sizes are warranted to evaluate this role of endocan.
Keywords: endocan, COVID-19, endothelial cell-specific molecule-1, ESM-1, systematic review
Introduction
Coronavirus disease 2019 (COVID-19) is a multisystem inflammatory disease caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) that mainly affects the respiratory system and most commonly presents with fever, dry cough, and dyspnea.1 Reverse transcriptase polymerase chain reaction (RT-PCR) is currently the most commonly used test to diagnose COVID-19 due to its high speed and accuracy. However, analysis of its false-negative rate has suggested that it should not be used in isolation to rule out COVID-19 infection.2 Additional reliable blood biomarkers associated with SARS-CoV-2 infection could be helpful in aiding clinical decision-making based on the patient’s most likely outcome.
Endocan, also known as endothelial cell-specific molecule 1 (ESM-1), is a dermatan sulfate proteoglycan secreted upon cytokine stimulation, mainly by pulmonary and renal endothelial cells.3 Hence, it is considered a marker of endothelial cell damage and it plays a role in endothelial-dependent pathologic diseases, including inflammatory diseases, angiogenesis, and adhesion.4,5 Evidence also suggests that endocan levels in peripheral blood are elevated in pneumonia, acute respiratory distress syndrome (ARDS), and pulmonary thromboembolism implicating endocan in the molecular mechanisms of vascular injury in the respiratory system.6
A large body of evidence supports the involvement of endothelial dysfunction in COVID-19.7 This can occur either directly (virus infection via angiotensin-converting enzyme-2 (ACE2) receptor, C-type lectin receptor L-SIGN, and other receptors) or indirectly (through cytokine storm).8 All of these findings make endocan an attractive target for investigation as a potential diagnostic and prognostic marker for COVID-19.
The present review systematically summarizes and highlights the findings relating endocan levels with the presence, severity, and complications of COVID-19 infection and discusses its possible roles in the management of this disease. This evidence could help determine the role of endocan and endothelial dysfunction in COVID-19 disease. Furthermore, clinicians may benefit if this biomarker predicts outcomes.
Methods
Search Strategy and Screening
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) 2020 guidelines.9Supplementary Table 1 provides the PRISMA checklist for this review. An initial systematic search was performed in international online databases, including PubMed, Scopus, Web of Science, and Embase, through November 2022 without any publication type or language restriction. Terms related to “COVID-19” and “endocan” were searched. We also manually reviewed the references list of included studies and websites in order to find any possible additional studies. Although we did not register a protocol for this study, details of the search strategy are available in Supplementary Table 2.
Study Selection
We included original published articles if they included ≥1 of the following: (1) reported serum and/or plasma levels of endocan in COVID-19 cases and controls, (2) reported serum and/or plasma levels of endocan in different COVID-19 severities, (3) Assessed relation between endocan levels and outcomes or hospitalization characteristics of COVID-19 patients, or (4) provided diagnostic and/or prognostic value of endocan levels in COVID-19. The exclusion criteria were: (1) not reporting serum endocan levels, (2) non-English full texts, and (3) case reports, conference abstracts, and reviews.
The PICO (Population, Intervention, Control, and Outcome) for study selection was defined as P: COVID-19, I: Endocan levels as a diagnostic or prognostic marker, C: RT-PCR testing or symptom-based testing for diagnosis, and endocan’s ability to distinguish different COVID-19 severities or outcomes (mortality, intensive care unit (ICU) admission), O: could the test significantly differentiate COVID-19 patients from controls or the cases with poorer outcomes from the ones with better outcomes. Two reviewers (SS and NA) searched titles and abstracts for studies relating endocan to COVID-19. Any disagreement was resolved through discussion with a third author (AHB). The full texts were then reviewed to assess inclusion criteria.
Data Extraction and Quality Assessment
Two authors (AHB and AK) performed the data extraction independently. The following data were retrieved from all studies: (1) First author’s name; (2) publication year; (3) location; (4) population in each group; (5) endocan specimen (plasma and/or serum); (6) the number of individuals in each study group; (7) mean age; (8) male percentage; (9) reported diagnostic and prognostic values of endocan, and (10) main findings, in addition to all endocan levels in study groups. The Newcastle Ottawa Scale (NOS) of non-randomized studies was used to assess the risk of bias in included studies.10
Data Synthesis and Statistical Analysis
Results of each included study regarding the comparison of endocan levels in COVID-19 patients and controls or within the COVID-19 group, with different stages, comorbidities, or complications, were used to synthesize data. Data are represented as mean and standard deviation, median and interquartile ranges, or median and range. A 2-sided P < .05 was considered statistically significant.
Results
Literature Search and Characteristics of Included Studies
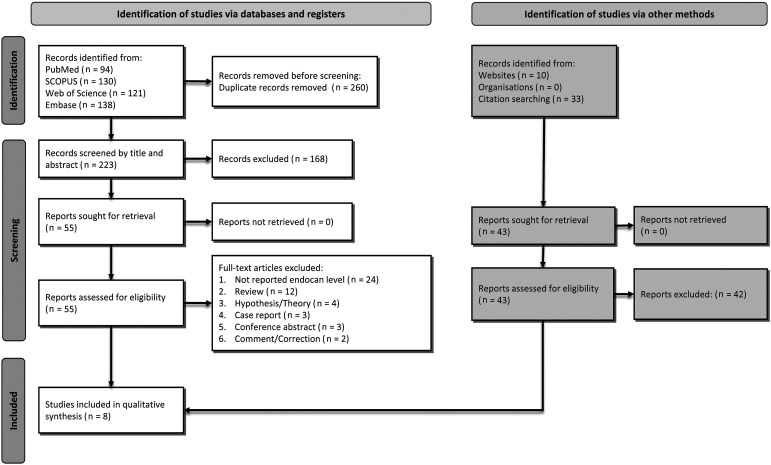
The initial search identified 483 records, 94 from PubMed, 130 from SCOPUS, 121 from Web of Science, and 138 from Embase. After the removal of 260 duplicate articles, 223 remained. After the exclusion of another 168 articles following title/abstract screening, the remaining 55 were screened as full text. Subsequently, 48 articles were excluded due to various reasons, while one was added by reference searching, as described in Figure 1. Finally, eight studies,11–18 including 686 cases, were included in the systematic review. A detailed flowchart of the search and selection process is shown in Figure 1. Characteristics of included studies are shown in Table 1. The mean age of participants was 60.1 ± 14.0 years, and 62.9% were male. Four studies11,12,15,18 measured endocan in plasma, and the other four13,14,16,17 measured serum endocan levels. Half of the studies were conducted in Turkey13,14,16,17 and three in France.11,12,18 Qualities of the included studies, assessed by NOS criteria, are shown in Table 2. All the studies had high quality, despite the fact that none of them except one15 fulfilled the comparability item.
Figure 1.
Flow diagram summarizing the selection of eligible studies based on the PRISMA guidelines.
Table 1.
Characteristics of included studies.
| Author | Year | Location | Specimen | Population | N Total | Age (years) | Male (%) | Findings |
|---|---|---|---|---|---|---|---|---|
| Chenevier-Gobeaux et al | 2022 | France | Plasma | COVID-19 patients with and without thrombotic events | 79 | 59.8 ± 18.6 | 55.7 | Endocan was significantly higher in COVID-19 patients with thrombotic events (16.2 [IQR: 5.53–26.7] vs 1.81 ng/mL [IQR: .71–10.5], P < .001). Endocan level of 2.83 ng/mL had 93.8% sensitivity, 54.7% specificity, 97.2% NPV, and 34.1% PPV with an AUC of .78 [95% CI: .67–.86] for distinguishing COVID-19 patients with and without a thrombotic event |
| Gaudet et al | 2022 | France | Plasma | COVID-19 patients admitted to ICU | 151 | 64.9 ± 11.7 | 76.2 | Patients with late ARF worsening had higher levels of endocan (9.13±15.34 vs 3.39±3.08 ng/mL, P < .01). Endocan was one of the top three predictive variables for late ARF worsening (per additional ng/mL, OR: 1.13 [95% CI: 1.04–1.31]) |
| Gorgun et al | 2021 | Turkey | Serum | Hospitalized COVID-19 patients and HCs | 88 | 54.1 ± 14.1 | 54.5 | Median endocan level was significantly higher in COVID-19 patients compared to HCs (243.5 vs 201.5 ng/mL, P = .002). Also, the endocan level of 202 ng/mL had 86.7% sensitivity and 50% specificity for COVID-19 diagnosis |
| Guzel et al | 2022 | Turkey | Serum | Hospitalized mild/moderate and severe COVID-19 patients | 80 | 57.8 ± 14.3 | 43.8 | There was no significant relationship between serum endocan and the degree of pneumonia (P = .22) and prognosis (P = .761) |
| Kim et al | 2021 | South Korea | Plasma | Severe COVID-19 patients and HCs | 44 | NR | NR | Patients with severe COVID-19 had higher levels of endocan, compared with HCs (878 [IQR: 616–1618] vs 477 pg/mL [IQR: 368-548], P = .005) with an adjusted OR of 293.42 (P = .005) at a threshold 632.25 pg/mL |
| Laloglu et al | 2022 | Turkey | Serum | PCR + suspected COVID-19, PCR- suspected COVID-19, and HCs | 90 | 56.1 ± 16.0 | 56.7 | Endocan was significantly higher both in PCR+ and PCR- cases, compared with HCs with P < .05. Endocan level of 444.2 pg/mL had 92% sensitivity, 80% specificity, 82% PPV, and 91% NPV with an AUC of .94 [95% CI: .89–.98] |
| Medetalibeyoglu et al | 2021 | Turkey | Serum | COVID-19 patients with and without ICD admission or death | 80 | 62 ± 16 | 65 | Serum endocan was significantly higher in patients with composite endpoint (mortality/ICU admission) compared with those without (852.2±522.7 vs 550.2±440.8 ng/L, P < .01). In addition, serum endocan of 276.4 ng/L had 97% sensitivity and 85% specificity for the prediction of the composite endpoint |
| Pascreau et al | 2021 | France | Plasma | HCs, mild-to-moderate COVID-19 patients, and severe ARDS | 74 | 63.3 ± 12.1 | 79.7 | COVID-19 patients had significantly higher endocan levels compared with controls (P = .0031). Also, there was no difference between patients who developed ARDS and those who did not (P = .223) |
NR: not reported, CI: confidence interval, HC: healthy control, ARDS: acute respiratory distress syndrome, ARF: acute respiratory failure, AUC: area under the curve, NPV: negative predictive value, PPV: positive predictive value, ICU: intensive care unit, PCR: polymerase chain reaction.
Table 2.
Quality Assessment of Included Studies Based on Newcastle–Ottawa Scale (NOS).
| Study | Selection | Comparability | Outcome | Overall Score | ||||
|---|---|---|---|---|---|---|---|---|
| Representation | Sample size | Non-respondents | Exposure | Outcome | Statistical test | |||
| Chenevier-Gobeaux et al (2022) | * | * | * | ** | - | ** | * | 8 |
| Gaudet et al (2022) | * | * | * | ** | - | ** | * | 8 |
| Gorgun et al (2021) | * | * | * | ** | - | ** | * | 8 |
| Guzel et al (2022) | * | * | * | ** | - | ** | * | 8 |
| Kim et al (2021) | * | * | * | ** | ** | ** | * | 10 |
| Laloglu et al (2022) | * | * | * | ** | - | ** | * | 8 |
| Medetalibeyoglu et al (2021) | * | * | * | ** | - | ** | * | 8 |
| Pascreau et al (2021) | * | * | * | ** | - | ** | * | 8 |
Endocan Levels in COVID-19 Patients Compared With Healthy Controls
Comparison of endocan levels in COVID-19 patients and healthy controls was carried out in four studies.13,15,16,18 Gorgun et al13 conducted a study on hospitalized COVID-19 patients (n = 60, of which 6.7% died), and 28 healthy subjects as control. Endocan levels were significantly higher (P = .002) in the patient group, with a median level of 243.5 ng/mL, compared with healthy controls (median = 201.5 ng/mL). They suggested endocan as a useful biomarker for the diagnosis of COVID-19. A pilot study by Kim et al15 investigated 31 hospitalized patients with severe COVID-19 and compared endocan levels with 13 controls. Plasma endocan levels were significantly higher in COVID-19 groups (878 [616-1618] vs 477 [368-548] pg/mL, P = .005), suggesting endocan as a biomarker for COVID-19 diagnosis. Laloglu et al16 assessed endocan levels in three groups: (1) 30 patients with positive RT-PCR, (2) 30 patients with negative RT-PCR but history, physical examination, and radiological findings compatible with COVID-19, and (3) 30 healthy controls. The main purpose of their study was to assess the role of endocan in diagnosing COVID-19 in patients with negative RT-PCR tests and clinical symptoms of COVID-19. They found that endocan levels were significantly higher in both RT-PCR positive and RT-PCR negative groups, compared with controls (821.9 ± 99.4, 803.9 ± 97.0, and 382.9 ± 37.6 pg/mL, respectively) (P < .05 for both comparisons). Finally, Pascreau et al18 reported plasma endocan levels in 59 COVID-19 cases and 10 healthy controls. The median endocan level at admission was significantly higher in patients compared with healthy controls (3.4 [1.8–7.5] vs 1.6 [1.0–2.1] ng/mL, P = .003).
Endocan Levels and Severity of COVID-19
Endocan levels were assessed based on COVID-19 severity in four studies.11,13,14,16 Chenevier-Gobeaux et al11 investigated endocan in four stages of the disease (stage 0: mild, stage 1: moderate, stage 2: severe, and stage 3: critical) in 79 patients and found that admission peripheral endocan level significantly increases with COVID-19 severity (P < .001). Moreover, in all pairwise comparisons of endocan levels between different stages of the disease, the higher stage of COVID-19 was associated with a significantly higher level of endocan (P < .05 for all comparisons). Gorgun et al13 compared endocan levels in different severities of COVID-19 based on a chest computed tomography scan. They could not demonstrate any difference in endocan values within severities (P = .399). Serum endocan was reported to be 297.6 pg/mL in the study by Guzel et al,14 with no statistical difference between the mild-moderate group (n = 40) and severe COVID-19 (n = 40), of whom 3 (7.5%) died (P = .22). Finally, Laloglu et al16 used endocan as a biomarker to distinguish between different severities of COVID-19 (uncomplicated, mild-to-moderate, severe, and critical) and reported a trend toward higher endocan levels from uncomplicated COVID-19 to critical-level disease (P < .001).
Endocan Levels and the Presence of Comorbidities
Blood endocan was assessed based on the presence of comorbidities in COVID-19 in two studies.11,13 In the Gorgun et al study,13 the most prevalent comorbidity in hospitalized COVID-19 patients was hypertension (23.3%), followed by diabetes mellitus (16.7%). The presence and absence of comorbidities were not associated with a significant difference in median endocan levels (237.5 vs 248 ng/mL, respectively). Chenevier-Gobeaux et al11 came to the same conclusion that arterial hypertension or cardiovascular disease was not associated with higher endocan levels.
Endocan Levels and ICU Admission And/or Death
The Medetalibeyoglu et al17 study defined a composite endpoint of mortality and ICU admission and compared endocan levels in COVID-19 cases with and without a composite endpoint. The primary composite endpoint was associated with higher endocan serum levels (852.2 ± 522.7 vs 550.2 ± 440.8 ng/L, P < .01). In addition, Gorgun et al13 investigated endocan levels in patients who died (n = 4) compared with the ones discharged with recovery (n = 56). It was concluded that patients who died from COVID-19 had significantly higher median serum levels of endocan compared with the other group (558 [400-989] vs 240.5 [158-920] ng/mL, P = .001). Likewise, Chenevier-Gobeaux et al11 compared patients admitted to ICU and the ones admitted to a conventional ward. ICU patients had significantly higher endocan levels (14.30 [4.54–25.48] vs 1.41 [.68–10.28] ng/mL, P < .001).
Endocan Levels and COVID-19–Related Complications
Chenevier-Gobeaux et al11 compared mild to critical COVID-19 patients with thrombotic events (n = 16) with the ones with no thrombotic events (n = 63). Among patients with thrombotic events, 11 (69%) experienced pulmonary embolism while 5 (31%) had venous thromboembolism. Thrombotic events were associated with higher endocan levels compared with patients without thrombotic events (16.2 [5.5–26.7] vs 1.81 [.71–10.5] ng/mL, P < .001). The same study compared endocan levels in patients with and without the need for oxygen. The need for oxygen was associated with statistically higher endocan, in comparison with those without its need (8.40 [4.25–22.20] vs 1.00 [.36–7.25] ng/mL, P < .001).
In another study, Pascreau et al18 conducted an analysis based on the presence of ARDS in COVID-19 patients. They found no significant difference between endocan levels of ARDS and non-ARDS groups of COVID-19 cases (P = .2231). Gaudet et al,12 on the other hand, investigated COVID-19 patients admitted to ICU and categorized them based on acute respiratory failure (ARF) and the need for subsequent ventilation with 15 day follow-up. The ARF cases had significantly higher endocan levels compared with the non-ARF group (3.39 ± 3.08 vs 9.13 ± 15.34 ng/mL, P < .001).
Endocan as a Diagnostic or Prognostic Biomarker
Several studies assessed blood endocan as a potential biomarker for the detection of COVID-19, compared with RT-PCR as a gold standard, or for the prediction of complications. Gorgun et al13 defined a cutoff of 202 ng/mL for the diagnosis of COVID-19 with sensitivity and specificity of 86.7% and 50%, respectively. Laloglu et al16 also determined 444.2 pg/mL serum endocan with 92% sensitivity and 80% specificity (area under the receiver operating characteristic curve (AUC) = .94, P < .001, positive likelihood ratio = 4.6) for distinguishing COVID-19 cases from controls.
For the prediction of thrombotic events in COVID-19 patients, Chenevier-Gobeaux et al11 found a threshold of 2.83 ng/mL with 93.8% sensitivity, 54.7% specificity, and an AUC of .776 (P < .001). Moreover, Medetalibeyoglu et al17 reported 276.4 ng/mL as a cut-off for the detection of the primary composite endpoint (mortality and ICU admission).
Discussion
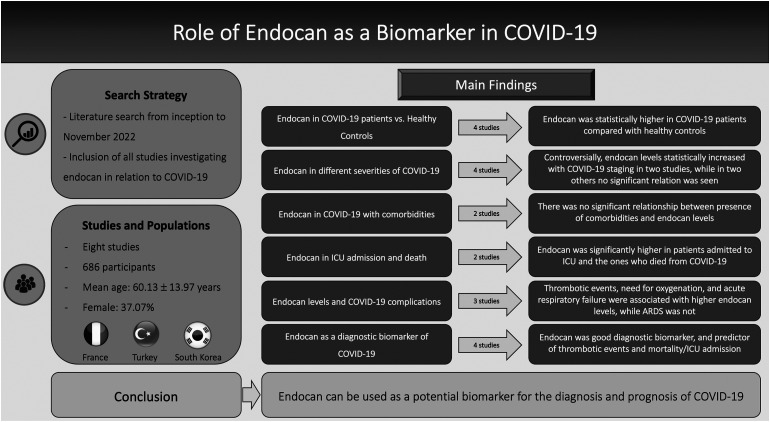
To our knowledge, this is the first comprehensive systematic review investigating the association between endocan and COVID-19. The main findings from this study include: (1) Endocan levels were significantly higher in COVID-19 patients compared with healthy individuals and can have added diagnostic benefits in assessing COVID-19 patients, (2) Higher endocan levels can be a prognostic factor in terms of adverse outcomes such as thrombotic events, ICU admission, and mortality, and, (3) Increasing endocan levels could signify worsening of disease course. The summary of all the findings of the present study is depicted in Figure 2.
Figure 2.
Summary of findings of systematic review regarding the role of endocan in COVID-19.
The main target of SARS-CoV-2 is the pulmonary system; however, extrapulmonary manifestations such as micro- and macro-vascular involvements were also reported in several studies.19 Endothelial dysfunction is one of the reported extrapulmonary manifestations and a pathological characteristic of COVID-19.20 Endothelial dysfunction in COVID-19 includes endothelial cell degradation and apoptosis.21 In addition to endothelial damage, disruption of the glycocalyx, which is essential for vascular homeostasis, may increase susceptibility to SARS-CoV-2 infection by increasing oxidative stress and hyper-inflammatory response.8,22
Many biomarkers have been investigated for detecting glycocalyx damage such as endocan, syndecan-1, heparan sulfates, heparinase, and angiopoietins.23 Of these, endocan, a soluble endothelial proteoglycan, is a novel biomarker of endothelial and glycocalyx damage which is released from the endothelium during inflammation.24 Endocan is expressed in actively proliferative tissues such as bronchi and lung submucosal glands' endothelium.25 In addition to the role of endocan in inflammation, it plays a role in endothelial cell rearrangement, cell adhesion, migration, proliferation, and angiogenesis.26 Finally, endocan also plays a role in the transfer of leukocytes through the endothelium.27
One of the main pathways by which endocan may have an association with COVID-19 severity, complications, and death is cardiovascular events.28,29 The role of endocan in association with cardiovascular events such as hypertension,30 coronary artery disease,31 atherosclerosis,32 lipid metabolism disorder,33 and diabetes,34 or even rare ones such as aortic dissection35 have been reported. Due to this and the presence of cardiovascular complications in COVID-19,36,37 endocan may be a useful biomarker of disease severity.
Endocan is an inflammatory marker and also increases with endothelial damage.5 COVID-19 can elevate endocan levels through the inflammatory process, which affects all organs, including the endothelium, in addition to endothelial damage directly caused by SARS-CoV-2 infection. In line with our results, it seems that the main pathophysiology behind the increased levels of endocan in COVID-19 patients compared with healthy controls and also higher levels of endocan in severe forms of COVID-19 compared with mild-to-moderate ones is the extrapulmonary inflammatory process which includes the endothelium and its damage causing an increase in endothelial markers, including endocan.
Endocan’s efficacy as a diagnostic or prognostic factor in diseases with endothelial damage is under investigation. Han et al38 investigated endocan as a prognostic factor in a cohort of 227 patients presenting with an acute ischemic stroke which followed up for three months. They found endocan acceptable for prediction of death (AUC = .61 [.55–.67]) and major disability defined by modified Rankin Scale score ≥3 (AUC = .68 [.59–.76]). In another study, Laloğlu et al39 found that endocan levels can differentiate between rheumatic aortic regurgitation and non-rheumatic aortic regurgitation.
Endocan levels could be used as a prognosis predictor for COVID-19 patients. The potential ability of endocan as a prognostic biomarker was also shown in patients with inflammatory conditions, in addition to its relation with inflammatory indices, such as C-reactive protein.40 Serum endocan levels were higher in patients with more severe COVID-19. Furthermore, serum endocan levels were higher in patients who experienced adverse effects of the disease such as thrombotic events and ARF.11,12 A point of contention regarding the prognostic value of endocan levels is that the associated comorbidities that increase the severity index of the disease could be an independent cause of higher endocan levels. However, two of the eight studies addressed this issue and questioned it.11,13 They found no difference between levels of endocan in COVID-19 patients presenting with comorbidities compared with those without comorbidities. Accordingly, it could be concluded that higher endocan levels are due to the pathophysiology behind COVID-19 rather than associated comorbidities.41,42 This can add value to its application as a novel diagnostic and prognostic biomarker.
We found promising results for endocan, showing added benefits in terms of being a diagnostic biomarker for patients. Serum or plasma endocan levels showed promise as a diagnostic tool in the detection of COVID-19. Four of our selected studies11,13,14,16 assessed the diagnostic value of endocan for COVID-19. This notion was cemented by Laloglu et al,16 establishing a 92% sensitivity and 80% specificity for serum endocan levels for diagnosing, while the gold standard diagnostic tool in COVID-19, RT-PCR test, had a sensitivity of 87.8% [81.5–92.2%] in a meta-analysis.43 The diagnostic ability of endocan in COVID-19, however, should be questioned due to its low specificity. Therefore, its use may be more of interest where the RT-PCR test is negative, but there is high clinical suspicion, as investigated in one of our included studies.16 Large controlled studies are required for better evaluation of endocan’s additional diagnostic value over RT-PCR.
As with all novel biomarkers, the clinical use and cost-effectiveness of that biomarker should always be considered. Endocan measurement was described as feasible, relatively fast (3 h by enzyme-linked immunosorbent assay (ELISA) method), and low-cost in the study by Seo et al.44 This cost-effectiveness of biomarkers is of higher importance in developing nations. Although our results showed higher endocan levels in COVID-19 cases compared with controls, the cost-effectiveness of its utility compared with current common methods should be investigated. Perhaps, as endocan is an endothelium-dependent biomarker, its measurement in serum may be more rational for the prediction of outcomes related to endothelial injury. All these possibilities highlight the need for future studies with large sample sizes.
The present study has some limitations. First, some studies reported endocan levels with median and range which made it impossible for us to convert them to mean and SD, due to high skewness and unavailability of methods suggested by Luo et al and Wan et al.45,46 Accordingly, we were unable to perform meta-analyses comparing endocan levels between patients and controls and also between different severities of COVID-19. Second, the low number of included studies, the low sample size in these studies, and the high heterogeneity among them may lead to a bias. This was also true for each individual outcome assessed which was supported by a relatively low number of studies. Furthermore, due to the chronological limitations facing the selected studies, a dynamic assessment of endocan levels throughout the course of the disease was not possible. Fourth, the geographical distribution of included studies may be influenced by local factors and patterns; hence, not generalizable to all patients around the world. Finally, the inherent biases in each of the included studies which could not be modified should be taken into consideration.
Conclusion
Endocan showed encouraging prospects as a prognostic laboratory test in addition to its potential added value in the diagnosis of patients presenting with symptoms of COVID-19. However, further studies with a larger study population are warranted to determine its clinical use.
Supplemental Material
Supplemental Material for Systematic Review of Endocan as a Potential Biomarker of COVID-19 by Amirmohammad Khalaji, Nikan Amirkhani, Sourena Sharifkashani, Soheil Peiman, and Amir Hossein Behnoush in Angiology
Authors’ Contributions: All authors contributed to: (1) substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data, (2) drafting the article or revising it critically for important intellectual content, and, (3) final approval of the version to be published.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material: Supplemental material for this article is available online.
ORCID iD
Amir Hossein Behnoush https://orcid.org/0000-0002-9955-4227
References
- 1.Yuki K, Fujiogi M, Koutsogiannaki S. COVID-19 pathophysiology: a review. Clin Immunol. 2020;215:108427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kanji JN, Zelyas N, MacDonald C, et al. False negative rate of COVID-19 PCR testing: a discordant testing analysis. Virol J. 2021;18:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kali A, Shetty KS. Endocan: a novel circulating proteoglycan. Indian J Pharmacol. 2014;46:579-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yücel M, Kotan D, Gurol Çiftçi G, Çiftçi IH, Cikriklar HI. Serum levels of endocan, claudin-5 and cytokines in migraine. Eur Rev Med Pharmacol Sci. 2016;20:930-936. [PubMed] [Google Scholar]
- 5.Balta S, Mikhailidis DP, Demirkol S, Ozturk C, Celik T, Endocan IA. A novel inflammatory indicator in cardiovascular disease? Atherosclerosis. 2015;243:339-343. [DOI] [PubMed] [Google Scholar]
- 6.Kechagia M, Papassotiriou I, Gourgoulianis KI. Endocan and the respiratory system: a review. Int J Chronic Obstr Pulm Dis. 2016;11:3179-3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qian Y, Lei T, Patel PS, et al. Direct activation of endothelial cells by sars-cov-2 nucleocapsid protein is blocked by simvastatin. J Virol. 2021;95:e0139621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu S-W, Ilyas I, Weng J-P. Endothelial dysfunction in COVID-19: an overview of evidence, biomarkers, mechanisms and potential therapies. Acta Pharmacol Sin. 2022:1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wells G, Shea B, O’Connell D, et al. The newcastle–ottawa Scale (NOS) for assessing the quality of non-randomized studies in meta-analysis. 2000. [Google Scholar]
- 11.Chenevier-Gobeaux C, Ducastel M, Meritet JF, et al. Plasma endocan as a biomarker of thrombotic events in COVID-19 patients. J Clin Med. 2022;11:5560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaudet A, Ghozlan B, Dupont A, et al. Derivation and validation of a predictive score for respiratory failure worsening leading to secondary intubation in COVID-19: the ceres score. J Clin Med. 2022;11:2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorgun S, Cindoruk S, Ozgen E, et al. Diagnostic and prognostic value of serum endocan levels in patients with COVID-19. Angiology. 2021;72:942-946. [DOI] [PubMed] [Google Scholar]
- 14.Guzel D, Kalkan EA, Eren F, et al. Can serum endocan levels be used as an early prognostic marker for endothelial dysfunction in COVID-19? Angiology. 2022;73:438-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim WY, Kweon OJ, Cha MJ, Baek MS, Choi SH. Dexamethasone may improve severe COVID-19 via ameliorating endothelial injury and inflammation: a preliminary pilot study. PLoS One. 2021;16:e0254167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laloglu E, Alay H. Endocan as a potential marker in diagnosis and predicting disease severity in COVID-19 patients: a promising biomarker for patients with false-negative RT-PCR. Ups J Med Sci. 2022;127:e8211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Medetalibeyoglu A, Emet S, Kose M, et al. Serum endocan levels on admission are associated with worse clinical outcomes in COVID-19 patients: a pilot study. Angiology. 2021;72:187-193. [DOI] [PubMed] [Google Scholar]
- 18.Pascreau T, Tcherakian C, Zuber B, Farfour E, Vasse M, Lassalle P. A high blood endocan profile during COVID-19 distinguishes moderate from severe acute respiratory distress syndrome. Crit Care. 2021;25:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fodor A, Tiperciuc B, Login C, et al. Endothelial dysfunction, inflammation, and oxidative stress in COVID-19-mechanisms and therapeutic targets. Oxid Med Cell Longev. 2021;2021:8671713-8671715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gu SX, Tyagi T, Jain K, et al. Thrombocytopathy and endotheliopathy: crucial contributors to COVID-19 thromboinflammation. Nat Rev Cardiol. 2021;18:194-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383:120-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.du Preez HN, Aldous C, Hayden MR, Kruger HG, Lin J. Pathogenesis of COVID-19 described through the lens of an undersulfated and degraded epithelial and endothelial glycocalyx. Faseb J. 2022;36:e22052. [DOI] [PubMed] [Google Scholar]
- 23.Martin L, Koczera P, Zechendorf E, Schuerholz T. The endothelial glycocalyx: new diagnostic and therapeutic approaches in sepsis. BioMed Res Int. 2016;2016:3758278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sarrazin S, Lyon M, Deakin JA, et al. Characterization and binding activity of the chondroitin/dermatan sulfate chain from endocan, a soluble endothelial proteoglycan. Glycobiology. 2010;20:1380-1388. [DOI] [PubMed] [Google Scholar]
- 25.Zhang SM, Zuo L, Zhou Q, et al. Expression and distribution of endocan in human tissues. Biotech Histochem. 2012;87:172-178. [DOI] [PubMed] [Google Scholar]
- 26.Afsar B, Takir M, Kostek O, Covic A, Kanbay M. Endocan: a new molecule playing a role in the development of hypertension and chronic kidney disease? J Clin Hypertens. 2014;16:914-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leite AR, Borges-Canha M, Cardoso R, Neves JS, Castro-Ferreira R, Leite-Moreira A. Novel biomarkers for evaluation of endothelial dysfunction. Angiology. 2020;71:397-410. [DOI] [PubMed] [Google Scholar]
- 28.Zhao T, Kecheng Y, Zhao X, et al. The higher serum endocan levels may be a risk factor for the onset of cardiovascular disease: a meta-analysis. Medicine (Baltim). 2018;97:e13407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen J, Jiang L, Yu XH, et al. Endocan: a key player of cardiovascular disease. Front Cardiovasc Med. 2021;8:798699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klisic A, Kavaric N, Vujcic S, Spasojevic-Kalimanovska V, Ninic A, Kotur-Stevuljevic J, et al. Endocan and advanced oxidation protein products in adult population with hypertension. Eur Rev Med Pharmacol Sci. 2020;24:7131-7137. [DOI] [PubMed] [Google Scholar]
- 31.Ziaee M, Mashayekhi S, Ghaffari S, Mahmoudi J, Sarbakhsh P, Garjani A. Predictive value of endocan based on timi risk score on major adverse cardiovascular events after acute coronary syndrome. Angiology. 2019;70:952-959. [DOI] [PubMed] [Google Scholar]
- 32.Icli A, Cure E, Cure MC, et al. Endocan levels and subclinical atherosclerosis in patients with systemic lupus erythematosus. Angiology. 2016;67:749-755. [DOI] [PubMed] [Google Scholar]
- 33.Nalbantoğlu A, Kızılca Ö, Güzel S, Emeksiz HC, Nalbantoğlu B. Increased carotid intima-media thickness and endothelial cell-specific molecule-1 (endocan) levels in obese children. Angiology. 2021;72:633-639. [DOI] [PubMed] [Google Scholar]
- 34.Arman Y, Akpinar TS, Kose M, et al. Effect of glycemic regulation on endocan levels in patients with diabetes: a preliminary study. Angiology. 2016;67:239-244. [DOI] [PubMed] [Google Scholar]
- 35.Metschl S, Bruder L, Paloschi V, et al. Changes in endocan and dermatan sulfate are associated with biomechanical properties of abdominal aortic wall during aneurysm expansion and rupture. Thromb Haemost. 2022;122:1513-1523. [DOI] [PubMed] [Google Scholar]
- 36.Long B, Brady WJ, Koyfman A, Gottlieb M. Cardiovascular complications in COVID-19. Am J Emerg Med. 2020;38:1504-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramandi A, Akbarzadeh MA, Khaheshi I, Khalilian MR. Aortic dissection and Covid-19; a comprehensive systematic review. Curr Probl Cardiol. 2022:101129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han F, Liao W, Duan X, Shi Y, Hu Z. The association between serum endocan level and short-term prognosis of patients with acute ischemic stroke. Angiology. 2022;73:344-349. [DOI] [PubMed] [Google Scholar]
- 39.Laloğlu F, Laloğlu E, Ceviz N, Güler MA. Serum endocan levels in children with rheumatic aortic insufficiency: can it differentiate bicuspid aortic valve disease from rheumatic heart disease? Cardiol Young. 2022:1-5. [DOI] [PubMed] [Google Scholar]
- 40.Yilmaz MI, Siriopol D, Saglam M, et al. Plasma endocan levels associate with inflammation, vascular abnormalities, cardiovascular events, and survival in chronic kidney disease. Kidney Int. 2014;86:1213-1220. [DOI] [PubMed] [Google Scholar]
- 41.Landstra CP, de Koning EJP. COVID-19 and diabetes: understanding the interrelationship and risks for a severe course. Front Endocrinol. 2021;12:649525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sheppard JP, Nicholson BD, Lee J, et al. Association between blood pressure control and coronavirus disease 2019 outcomes in 45 418 symptomatic patients with hypertension: an observational cohort study. Hypertension. 2021;77:846-855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jarrom D, Elston L, Washington J, et al. Effectiveness of tests to detect the presence of SARS-CoV-2 virus, and antibodies to SARS-CoV-2, to inform COVID-19 diagnosis: a rapid systematic review. BMJ Evidence-Based Medicine. 2022;27:33-45. [DOI] [PubMed] [Google Scholar]
- 44.Seo K, Kitazawa T, Yoshino Y, Koga I, Ota Y. Characteristics of serum endocan levels in infection. PLoS One. 2015;10:e0123358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range and/or mid-quartile range. Stat Methods Med Res. 2018;27:1785-1805. [DOI] [PubMed] [Google Scholar]
- 46.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Systematic Review of Endocan as a Potential Biomarker of COVID-19 by Amirmohammad Khalaji, Nikan Amirkhani, Sourena Sharifkashani, Soheil Peiman, and Amir Hossein Behnoush in Angiology