Abstract
Background and Aims
Pre‐eclampsia is a particular type of pregnancy condition. Although the primary etiology of pre‐eclampsia is unclear, it hypothesizes that the alteration of trace elements and macro‐minerals may play a crucial function in the pathogenesis of Pre‐eclampsia. Therefore, our research sought to ascertain the serum level of trace elements (zinc, iron) and macro‐minerals (sodium, calcium, potassium) and their possible association with pre‐eclampsia.
Methods
The present study was conducted with 74 pre‐eclampsia pregnant women (case) and 118 pregnant women having normal blood pressure (controls). Atomic Absorption Spectroscopy determined the serum level of trace components and electrolytes.
Results
The researchers discovered notable differences in maternal age, gestational period, body mass index, systolic and diastolic blood pressure, hemoglobin, and creatinine level. Results of serum analysis revealed that calcium (52.06 ± 3.71 mg/L vs. 65.93 ± 2.57 mg/L, p < 0.05) and potassium (63.44 ± 5.33 mg/L vs. 102.54 ± 4.25 mg/L, p < 0.001) concentrations were substantially lower in the patient group than in control. Serum zinc (0.34 ± 0.02 mg/L vs. 0.52 ± 0.02 mg/L, p < 0.001) and iron (0.38 ± 0.03 mg/L vs. 0.46 ± 0.02 mg/L, p < 0.05) concentration were also considerably decreased in pre‐eclampsia participants compared with a pregnant normotensive group. Pearson's correlation research results in the patient group revealed a connection between trace elements or macro minerals. In addition, the systolic blood pressure was positively correlated with sodium (r = 0.392, p < 0.01) and negatively correlated with potassium (r = −0.257, p < 0.05) in the control group.
Conclusions
This study concludes that calcium, potassium, iron, and zinc levels were lower, whereas sodium levels were higher in Bangladeshi pre‐eclampsia patients compared to controls. These findings with Pearson's correlation and the inter‐element relationship between the patient and a control subject results can act as critical indication factors for patients with pre‐eclampsia in Bangladesh and, as a result, may require a higher intake of calcium, potassium, iron, and zinc for effective therapeutic intervention and reduce the intake of sodium.
Keywords: macro‐minerals, pre‐eclampsia, pregnant women, trace elements
1. INTRODUCTION
Pre‐eclampsia is a pregnancy‐related hypertension disease with substantial proteinuria. 1 , 2 , 3 It occurs in about 2%–8% of pregnancies and is one of the primary indications for premature delivery as there is currently no other cure. 1 It may develop after 20 weeks of gestation. 4 About 12% of pregnant women die only from pre‐eclampsia, and about 1,00,000 have an eclamptic convulsion. 5 It is one of the leading causes of intrauterine hypoxia and growth restriction (IUGR), pregnancy complications, and perinatal mortality. Although experts are not sure about the causes of pre‐eclampsia, and belief is that placental hypoxia and ischemia, high peroxidation, and endothelial malfunction might link numerous factors responsible for the clinical manifestation of pre‐eclampsia. 6 Endothelial dysfunctions are mediated by low amounts of proangiogenic molecules such as placental growth factor (PlGF) and vascular endothelial growth factor (VEGF) and elevated amounts of circulating soluble Fms‐like tyrosine kinase 1 (sFlt1) and soluble endoglin. 7 Reactive oxygen species (ROS.) proceed in a chain reaction with stable molecules and produce another free radical, which reacts with another stable molecule. Lipid peroxidation is a chain reaction that damages endothelial cells and is responsible for endothelial dysfunction. 8 With the help of the antioxidant defense system, cells can protect themselves from oxidative damage. 9
Zinc, an essential trace element, acts as a co‐factor for synthesizing several enzymes, DNA. and RNA., and is necessary for reproductive health. 10 , 11 Previous studies showed that zinc deficiency due to reduced dietary intake increases the risk of pre‐eclampsia and insufficiency are due to decreased nutritional information. 12 Evaluation of serum level of zinc concentration recommends as an index for predicting the severity of pre‐eclampsia. 13 Iron is an essential trace element that, in excess or deficiency, causes DNA damage. 14 Lewandowska et al. 15 conducted a meta‐analysis and reported that pre‐eclamptic individuals have a greater blood iron level compared to healthy pregnant women. A possible source of iron in an ischemic placenta is the breakdown of RBCs. 16 By using the Fenton reaction, these molecules may produce the extremely reactive hydroxyl radical; these species can generate the highly reactive hydroxyl radical by the Fenton reaction, 17 which can start the processes of lipid peroxidation and endothelial cell damage. 18 Calcium, sodium, and potassium are essential electrospray in regulating blood pressure. In healthy people, there is a balance of these electrolyte concentrations. Disturbance of this balance causes many complications in the human body. Both calcium and sodium are essential physiologically since they facilitate muscular contraction and control the cellular water balance, respectively. A low serum calcium level can cause abnormal heart rhythm and elevation in the maintenance of blood pressure in preeclamptic mothers. 19 Sodium is the principal cation related to blood volume, water balance, and cell membrane potential. A high serum sodium level is associated with severe hypertension. 20 Potassium plays a vital function in the activity of vascular smooth muscle, responsible for vasodilation that results from the hyperpolarisation of vascular cells. 21 Pre‐eclampsia in an area depends mainly on the dwellers' lifestyle. The incidence of pre‐eclampsia is less common in developed countries, but in third‐world countries, pre‐eclampsia occurs frequently and is as high as 5% in Bangladesh. 22 In developed countries, the rates of pre‐eclampsia can be as low as 0.4%. 23 In the Millennium Development Goals, a country's level of development is measured by how many mothers die during childbirth. The Bangladesh Government's mission is to minimize maternal deaths. To achieve this, Bangladesh should emphasize the management of pre‐eclampsia and eclampsia. However, there is very little research on the serum level of trace elements and macro minerals of pre‐eclampsia patients in Bangladesh, which encouraged us to conduct this study to assess the serum level of macro‐minerals like sodium, calcium, and potassium trace elements Zinc and Iron.
2. METHODS
2.1. Study design
This case–control research included 74 women with pre‐eclampsia identified at a gestational age ≥20 weeks, while 118 women with normal blood pressure throughout pregnancy served as controls. Study participants were recruited from the Department of Obstetrics and Gynecology, Noakhali Medical College Hospital, from August 2019 to November 2019. An obstetrician and gynecologist carried out the diagnosis. Before information collection, a well‐designed questionnaire was used to get patients' histories, and each patient received a short explanation of the study's goals. Participants with hyperglycemia, kidney, cardiac, hepatic, endocrine, or other chronic ailment were excluded. Ethical approval was taken from the Ethical Committee of Noakhali Science and Technology University (13/2018), and informed consent was obtained from each of the participants.
2.2. Sample collection and processing
After an 8 h overnight fast, 5 mL of venous blood was drawn from each diagnosed patient and placed in the metal‐free tube. The serum was extracted from the blood samples by centrifuging them at 3000 rpm for 10 min after they had been left to clot at room temperature for 30 min. At −80°C, the serum was kept until investigation.
2.3. Analytical procedure
We follow the methods of Haque et al. 24 2016 for determining trace elements and macro‐minerals concentration in blood serum. Using a graphite furnace and flame atomic absorption spectrometry (Shimadzu AA 6800), the content of trace elements and macro‐minerals in the serum sample was precisely measured. 0.1 N HCl was used as a diluent. Different concentrations of standard solutions were prepared to construct the calibration curve. Absorbances were read at 422.7, 589.0, 766.5, 213.9, and 248.3 nm for calcium, sodium, potassium, zinc, and iron. The concentration was calculated using the WizAArd AA application. Our assay precision was calculated by doing standard solution runs for every 10 test samples and excluding carryover; blank solution runs were required for 5 test samples.
2.4. Statistical analysis
All findings were presented as mean ± standard error mean (mean ± SEM). The statistical analysis was carried out with the help of the statistical software program SPSS, version 16.0. (SPSS Inc). Using an independent sample t‐test, trace element levels between the patient and control groups were compared. Pearson's correlation analysis was utilized to determine the relationship between the different test parameters.
3. RESULTS
3.1. Characteristics of participants
Obstetric, anthropometric, clinical, and biochemical features of the research population are presented in Table 1. The average mother age of the cases and controls were 27.51 ± 0.98 and 24.59 ± 0.51 years, p < 0.05 and whereas the average gestational age at diagnosis was 31.02 ± 0.71 and 25.98 ± 0.55 weeks, p < 0.001, respectively. Present data revealed that mothers aged 28–33 had the most frequency of mild pre‐eclampsia (MPE) and severe pre‐eclampsia (SPE). A recent study described the link between hypertension and low blood selenium level in pregnant women from Bangladesh. 24 So, the previously published article may also support this demographic data. 25 Statistically, a significant difference was found in mother age between cases and control groups, which contradicts a prior investigation. 26 A statistical evaluation of these variables revealed that the patients had significantly higher creatinine (1.00 ± 0.03 mg/dL vs. 0.76 ± 0.02 mg/dL, p < 0.001), SBP (146.22 ± 3.31 mmHg vs. 111.53 ± 1.05 mmHg, p < 0.001), and DBP (105.41 ± 2.81 mmHg vs. 72.54 ± 0.98 mmHg, p < 0.001) than the control group. A Hb level below 11 mg/dL was considered a sign of anemia in pregnancy. According to the present investigation, pregnant women with pre‐eclampsia had decreased Hb levels, and there was a significant difference between the patient and control groups (patient 10.64 ± 0.14% vs. control 11.51 ± 0.18%, p < 0.001). We also observed a substantial difference in BMI between the preeclamptic pregnant and healthy pregnant women.
Table 1.
Obstetric clinical and biochemical profile between control and patient group.
| Variables | Patient | Control | p Value |
|---|---|---|---|
| Maternal age (years) | 27.51 ± 0.98 | 24.59 ± 0.51 | <0.05* |
| Gestational Period(weeks) | 31.02 ± 0.71 | 25.98 ± 0.55 | <0.001*** |
| BMI (kg/m2) | 26.47 ± 0.29 | 25.58 ± 0.16 | <0.05* |
| Hb (%) | 10.64 ± 0.14 | 11.51 ± 0.18 | <0.001*** |
| Creatinine (mg/dL) | 1.00 ± 0.03 | 0.76 ± 0.02 | <0.001*** |
| SBP (mmHg) | 146.22 ± 3.31 | 111.53 ± 1.05 | <0.001*** |
| DBP (mmHg) | 105.41 ± 2.81 | 72.54 ± 0.98 | <0.001*** |
p < 0.05 and ***p < 0.001 indicates the significant difference between control and patient groups.
3.2. Concentration of serum macro‐minerals and trace elements
The results of a serum macro‐mineral and trace element analysis suggest that mean values of Calcium, Sodium, Potassium, Zinc, and Iron were 52.06 ± 3.71, 3503.6 ± 272.4, 63.44 ± 5.33, 0.34 ± 0.02 and 0.38 ± 0.03 mg/L for the patient group and 65.93 ± 2.57, 4096.3 ± 236.5, 102.54 ± 4.25, 0.52 ± 0.024, and 0.46 ± 0.02 mg/L for the control group. A lower concentration of all the elements except sodium was found in the cases group compared to the control group. Table 2 represents the serum concentrations of minerals with the corresponding p values. The distribution of serum Sodium, Calcium, Potassium, Zinc, and Iron concentrations in mild pre‐eclampsia, severe pre‐eclampsia, and control participants are shown in Figures 1, 2, 3, 4, and 5, respectively.
Table 2.
Serum level of Ca, Na, K, Zn, Fe, and Se between control and patient groups.
| Elements | Patient | Control | p Value |
|---|---|---|---|
| Ca (mg/L) | 52.06 ± 3.71 | 65.93 ± 2.57 | <0.05* |
| Na (mg/L) | 3503.6 ± 272.4 | 4096.3 ± 236.5 | <0.05* |
| K (mg/L) | 63.44 ± 5.33 | 102.54 ± 4.25 | <0.001*** |
| Zn (mg/L) | 0.34 ± 0.02 | 0.52 ± 0.02 | <0.001*** |
| Fe (mg/L) | 0.38 ± 0.03 | 0.46 ± 0.02 | <0.05* |
p < 0.05 and ***p < 0.001 indicates the level of significant difference between the control and patient groups.
Figure 1.
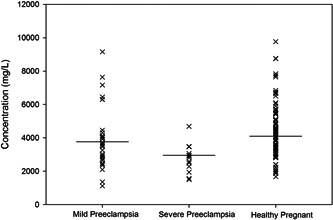
Distribution of serum sodium concentrations in mild pre‐eclampsia, severe pre‐eclampsia and control participants. Horizontal bars represent mean values.
Figure 2.
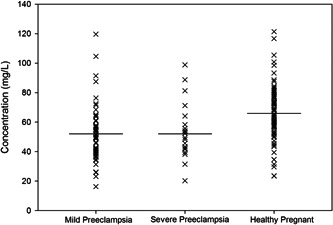
Serum calcium concentrations in mild pre‐eclampsia, severe pre‐eclampsia, and control participants. Horizontal bars represent mean values.
Figure 3.
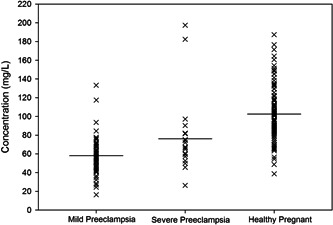
Serum potassium concentrations in mild pre‐eclampsia, severe pre‐eclampsia, and control participants. Horizontal bars represent mean values.
Figure 4.
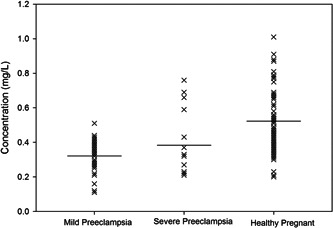
Distribution of serum zinc concentrations in mild pre‐eclampsia, severe pre‐eclampsia and control participants. Horizontal bars represent mean values.
Figure 5.
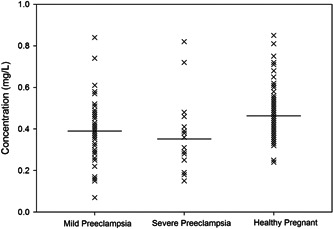
Serum iron concentrations in mild pre‐eclampsia, severe pre‐eclampsia, and control participants. Horizontal bars represent mean values.
3.3. Correlation study
The correlation between gestational age and BMI, creatinine, hemoglobin, and minerals was also studied. In the patient group, Pearson's correlation (Table 3) revealed that there was a significant positive association between hemoglobin and potassium (r = 0.397, p < 0.05), gestational period and calcium (r = 0.448, p < 0.05) and a strong negative correlation between creatinine and potassium (r = −0.490, p < 0.05) creatinine and zinc (r = −0.447, p < 0.05). A significant positive association between SBP and sodium (r = 0.392, p < 0.01) and a negative correlation between hemoglobin and sodium (r = −0.296, p < 0.05), SBP and potassium (r = −0.257, p < 0.05) shown in the control group (Table 4). Data were also analyzed to establish an inter‐element relationship between the patient and control subjects, which exhibited either a positive or negative correlation between selected elements (Table 5). No significant association was not found between sodium and calcium, sodium and potassium, calcium and potassium, and iron and zinc in the patient group; however, sodium, potassium, and calcium were shown to be significantly positively correlated in the control group, Na and Ca (r = 0.329, p < 0.05); Na and K (r = 0.436, p < 0.05); Ca and K (r = 0.332, p < 0.05). Iron and zinc did not show any significant correlation in the control group.
Table 3.
Correlation between systolic blood pressure, diastolic blood pressure, gestational period, hemoglobin, creatinine, and trace elements of pre‐eclampsia patients.
| Na | Ca | K | Zn | Fe | ||
|---|---|---|---|---|---|---|
| SBP | r | 0.014 | 0.303 | 0.222 | 0.010 | 0.010 |
| p | 0.395 | 0.069 | 0.187 | 0.634 | 0.951 | |
| DBP | r | 0.037 | 0.072 | −0.229 | 0.036 | 0.059 |
| p | 0.828 | 0.674 | 0.172 | 0.832 | 0.729 | |
| Gestational period | r | 0.064 | 0.448 | 0.088 | 0.203 | −0.001 |
| p | 0.706 | 0.005* | 0.605 | 0.228 | .994 | |
| Hemoglobin | r | 0.048 | 0.083 | 0.397 | 0.075 | 0.076 |
| p | 0.776 | 0.626 | 0.015* | 0.661 | 0.653 | |
| Creatinine | r | 0.081 | 0.125 | −0.490 | −0.447 | 0.156 |
| p | 0.635 | 0.346 | 0.002* | 0.006* | 0.353 |
p < 0.05 means correlation is significant at a 0.05 level.
Table 4.
Correlation between systolic blood pressure, diastolic blood pressure, gestational period, hemoglobin, creatinine, and trace elements of the control group.
| Na | Ca | K | Zn | Fe | ||
|---|---|---|---|---|---|---|
| SBP | r | 0.392 | −0.204 | −0.257 | −0.239 | −0.089 |
| p | 0.002* | 0.068 | 0.049* | 0.068 | 0.504 | |
| DBP | r | 0.240 | −0.048 | −0.205 | −0.164 | −0.086 |
| p | 0.068 | 0.719 | 0.120 | 0.215 | 0.517 | |
| Gestational period | r | −0.190 | −0.061 | −0.219 | 0.040 | −0.103 |
| p | 0.149 | 0.228 | 0.096 | 0.765 | 0.436 | |
| Hemoglobin | r | −0.296 | −0.160 | −0.188 | 0.038 | 0.018 |
| p | 0.023* | 0.228 | 0.153 | 0.777 | 0.436 | |
| Creatinine | r | −0.033 | 0.125 | 0.244 | −0.050 | −0.137 |
| p | 0.806 | 0.346 | 0.062 | 0.707 | 0.300 |
p < 0.05 means correlation is significant at a 0.05 level.
Table 5.
Correlation among macro‐minerals and trace elements.
| Case | Control | |
|---|---|---|
| Na and Ca | r = 0.016, p = 0.927 | r = 0.392, p < 0.05* |
| Na and K | r = 0.240, p = 0.152 | r = 0.436, p < 0.001* |
| Ca and K | r = −0.166, p = 0.326 | r = 0.332, p < 0.01* |
| Fe and Zn | r = 0.170, p = 0.313 | r = −0.101, p = 0.445 |
p < 0.05 means correlation is significant at a 0.05 level.
4. DISCUSSION
During pregnancy, hypertension becomes a significant public health challenge. Though many research efforts have already been undertaken in this area of medical science, etiologic causes responsible for the condition are yet to unravel. The mean mother's age was 27.51 ± 0.98 in the patient group, whereas it was 24.59 ± 0.51 for the controls. Patients exhibited higher levels of creatinine (1.00 ± 0.03 mg/dL vs. 0.76 ± 0.02 mg/dL; p < 0.001), systolic blood pressure (146.22 ± 3.31 mmHg vs. 111.53 ± 1.05 mmHg; p < 0.001), and diastolic blood pressure (105.41 ± 2.81 mmHg vs. 72.54 ± 0.98 mmHg; p < 0.001) than the control group, whereas patients exhibited significantly lower Hb levels than controls (10.64 ± 0.14%, vs. 11.51 ± 0.18%; p < 0.001). All findings indicate that an imbalance of physiologically essential nutrients may manifest in harmful health effects and much more in pregnancy. 27 , 28 , 29 , 30 , 31 , 32 Pre‐eclampsia occurs in 3% to 5% of all pregnancies, considered a properly‐customary chance marker for heart disease. Within 10 to 20 years after giving birth, women with pre‐eclampsia have a twofold chance of developing a stroke, heart ischemia, or venous thrombosis. They had a threefold increased risk of type 2 diabetes mellitus and a fourfold increased risk of excessive blood pressure, respectively. 33 Generally, there is a physiological balance between antioxidants and free radicals. When the antioxidants are lowered in the marked body level of lipid peroxidation damages the blood vessels leading to the alteration of polyunsaturated fatty acids, which destroy the mechanism and formation of the capillary endothelial cell; thus, endothelial cell damage or harm may be the main element for initiating pathophysiological events of pre‐eclampsia. 34 Macro‐minerals like calcium (Ca), sodium (Na), and potassium (K) manifested in the development of pre‐eclampsia through gambling, an important position within the functioning of the vascular smooth muscle mass. 35 During pregnancy, several physiological adjustments reason manifest in calciuria, and the transfer of calcium retard the growth of the fetus. These mechanisms lower the serum calcium level in the pregnant mother. 36 One finding suggested that from mother to fetus Maximum of 350 mg/day of calcium is transported at 245 days of gestation. 37 Pre‐eclampsia sufferers have notably low concentrations of 1, 25‐dihydroxy vitamin D [1, 25‐(OH)2 D] than nonpreeclamptic pregnant women, which may contribute to the suboptimal intestinal absorption of calcium. In contrast, calcium demand inside the cell is high. 38 This growth in intracellular calcium affects extended vascular contraction and vascular resistance that increases blood stress. 35 Additionally, calcium controls the homeostasis of mobile water and muscular tension. Our research found that serum calcium concentration (patient: 52.06 ± 3.71 mg/L vs. control: 65.93 ± 2.57 mg/L, p < 0.05) was significantly lower in pre‐eclamptic pregnant women than in the control group. Other studies in India and part of the world region find similar findings. 28 , 39 , 40 During Pearson's correlation study, the gestational period and calcium (r = 0.448, p < 0.05) showed positive relation in the patient group. In the inter‐element relationship analysis, a significant association between Ca and K (r = 0.332, p < 0.05) was found in the control group. Although in our study, lowering the frequency of serum Ca levels was similar in both MPE. and SPE. groups. Low blood Ca levels encourage the production of renin and parathyroid hormone, which subsequently promote cytoplasmic Ca ion concentration in vascular smooth muscle. This raises the cytoplasmic calcium content. 41 Because of this vasoconstriction, preeclamptic moms are experiencing an increase in vascular resistance and a subsequent rise in blood pressure. 28 , 42 Surprisingly, a previous study in South Africa found no difference in serum and hair calcium concentration between pre‐eclamptic and normotensive women. 43
Severe hyponatremia is an infrequent, mortal complication of pre‐eclampsia, and this may be due to the human chorionic gonadotropin during pregnancy. 44 In the brain, Oxytocin's natriuresis provides a stimulus to suppress salt appetite. 31 At the same time, relaxin is a peptide hormone that also affects pregnancy's hydration levels and vasopressin production., resulting in hyponatremic hypervolemia in pre‐eclampsia pregnant women. 32 If hyponatremia is mild to severe (>125 mmol/L) and persistent (>48 h), then it may not cause any symptoms, 45 while Severe hyponatremia defines as sodium <125 mmol/L. 46 It is crucial to keep in mind that when blood sodium falls below 120 mmol/L within 24 h, up to 50% of people may die. This is true even if mild hyponatremia may not be noticed during pregnancy. In our present study, serum sodium concentration in pre‐eclampsia patients was depleted compared to the control group (3503.6 ± 272.4 mg/L vs. 4096.3 ± 236.5 mg/L, p < 0.05). In the inter‐element relationship analysis, no significant association was found in the patient group, but the control group Na and Ca (r = 0.329, p < 0.05) and Na and K (r = 0.436, p < 0.05) showed a strong positive correlation. Particularly with twin pregnancies, the risk was increased. The distribution of serum potassium concentrations was lower in severe pre‐eclampsia but found to be comparatively higher in mild pre‐eclampsia. Our present study supports the data from Kahramanoglu et al. 47 An analysis of the current and prior instances demonstrates that pre‐eclampsia may independently result in severe hyponatremia.
Potassium is an electrolyte that prevents hypertension by decreasing vascular responsiveness to vasopressors like norepinephrine. 48 Our present finding showed significant hypokalemia in patients with pre‐eclampsia compared to healthy pregnant women (patient 63.44 ± 5.33 mg/L vs. control 102.54 ± 4.25 mg/L, p < 0.001). In Pearson's correlation analysis, Hemoglobin and potassium (r = 0.397, p < 0.05) showed positive relation in the patient group, and a significant negative correlation was shown between creatinine and potassium (r = −0.490, p < 0.05). Elevated plasma concentrations of aldosterone and other mineralocorticoids may contribute to hypokalemic alterations seen during a healthy pregnancy, an also increase in glomerular filtration rate, 49 and inadequate potassium conservation by kidney & alimentary canal; Potassium lost in feces may be higher than that lost via urine, 50 and a similar hypothesis also suggested by Bera et al. 48 and Yussif et al. 51 Their predictive analysis show that pregnant women with hypertension had lower blood potassium levels than pregnant women with normotension. Their speculations depicted a possible link between hypertensive pregnancies and low potassium levels. The serum potassium concentrations distribution was lower in mild pre‐eclampsia than in severe pre‐eclampsia and control participants. Some clinical studies have shown that a diet low in potassium (10–16 mmol/day) may be associated with blood pressure elevation. 51
Zinc is essential for the metabolic processes of glucose, protein, and nucleic acids; the production of energy; the maintenance of antioxidant defenses; the proliferation and differentiation of cells; and the repair of DNA. Zinc also takes part in embryogenesis. During the third trimester, demand for zinc is approximately twofold increased in nonpregnant women. 52 In the 1980s, a common link between Zn deficiency and pre‐eclampsia observe in adolescent pregnancies. 53 , 54 The study suggests that slow serum Zn concentrations in pre‐eclampsia mothers may be partly due to minimizing secretion of estrogen and depletion of zinc‐binding protein concentrations. 55 Cross‐sectional retrospective studies also showed lower placental Zn concentration in pre‐eclampsia, with placental Zn values effectively correlated with newborn weights. 56 , 57 Numerous diseases are made worse by a lack of zinc, which also leads to oxidative damage in the body. 58 , 59 , 60 Patients with pre‐eclampsia were found to have considerably decreased blood zinc concentrations compared to the control group (0.34 ± 0.02 mg/L vs. 0.52 ± 0.02 mg/L, p < 0.001). Some other studies support this by Atamer et al. 61 A significant negative correlation between creatinine and zinc (r = −0.447, p < 0.05) was shown in the pre‐eclampsia patients after performing the Pearson's correlation study. The serum zinc concentrations distribution was lower in mild pre‐eclampsia than in severe pre‐eclampsia and control participants. However, the mean serum zinc in pre‐eclampsia was nonsignificantly lower than in this study's normal pregnancies.
Numerous studies have evaluated the iron level in pregnancy for decades; all studies suggest a contributory role in oxidative stress in pre‐eclampsia. 62 , 63 Our research reveals that Serum iron concentration was also significantly lower in pre‐eclampsia cases than in the control group (patient 0.38 ± 0.03 mg/L vs. control 0.46 ± 0.02 mg/L, p < 0.05). In the inter‐element relationship analysis, Iron and zinc did not show any significant correlation in both patients and the control group. According to one analysis, normal blood iron and ferritin levels drop for most women in their third trimester of pregnancy. Pregnant women with severe pre‐eclampsia had a more comprehensive range of blood iron values than those with moderate pre‐eclampsia. The mean is shown by the horizontal bar, and it is hypothesized that women need to draw on their reserve of iron during the third trimester of pregnancy to meet the demands of the developing fetus and the rapid growth of the red blood cell mass, 64 thus strongly supporting our present study data. A study carried out by Hubel and colleagues also found a lower serum iron level in pre‐eclampsia patients than in healthy pregnant women. 63 In pregnant women, this may be because the total iron‐binding capacity (TIBC) is the joint mon preeclamptic group compared to the control group. 65 Preeclamptic women, compared to those experiencing a healthy pregnancy, have a decreased unsaturated iron‐binding capacity. 66 However, an elevated serum iron level in pre‐eclamptic compared to average pregnant women has also been reported. 67 Thus, further research on the serum concentration of iron in pre‐eclampsia is required to define its exact role in the pathogenesis of pre‐eclampsia.
Our research was limited as we did not assess the effects of dietary supplements; therefore, more research is needed to see if eating habits influence our study parameters in pre‐eclampsia patients. A high number of study subjects and samples should be used in our investigation to produce a more appreciable conclusion.
5. CONCLUSIONS
Our research found that pre‐eclampsia patients have significantly lower serum calcium, potassium, zinc and iron levels than healthy pregnant women and substantially higher sodium concentrations in pre‐eclampsia patients. Thus from this study, it can be concluded that antioxidant vitamins, trace elements, and macro‐minerals significantly affect the pathogenesis of pre‐eclampsia. Increasing awareness and a dietary supplement may prevent associated complications of the disease.
AUTHOR CONTRIBUTIONS
S. M. Naim Uddin: Data curation; formal analysis; investigation; writing – original draft. Mahmodul Haque: Formal analysis; investigation; resources; writing – original draft. Md Abdul Barek: Data curation; validation; writing – original draft. Mohammad Nizam Uddin Chowdhury: Data curation; project administration; writing – original draft. Abhijit Das: Data curation; investigation; writing – original draft. Md Giash Uddin: Investigation; methodology; project administration; writing – review & editing. Mohammad Safiqul Islam: Conceptualization; methodology; project administration; resources; software; supervision; validation; writing – review & editing.
CONFLICT OF INTEREST STATEMENT
Mohammad Safiqul Islam is an Editorial Board member of Health Science Reports and a corresponding author of this article. To minimize bias, he was excluded from all editorial decision‐making related to the acceptance of this article for publication. The remaining authors declare no other conflict of interest.
TRANSPARENCY STATEMENT
The lead author Mohammad Safiqul Islam affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
ACKNOWLEDGMENTS
The authors are thankful to all the staff, nurses, and physicians of the Department of Obstetrics and Gynaecology, Noakhali Medical College Hospital, Bangladesh, for their technical and administrative support. The authors are also thankful to all the participants of this study. No formal funding was received for this work.
Uddin SMN, Haque M, Barek MA, et al. Analysis of serum calcium, sodium, potassium, zinc, and iron in patients with pre‐eclampsia in Bangladesh: a case–control study. Health Sci Rep. 2023;6:e1097. 10.1002/hsr2.1097
S. M. Naim Uddin and Mahmodul Haque contributed equally to this study.
DATA AVAILABILITY STATEMENT
The authors confirm that the data supporting the findings of this study are available within the article and/or its Supporting Information.
REFERENCES
- 1. Al‐Jameil N, Tabassum H, Al‐Mayouf H, et al. Analysis of serum trace elements‐copper, manganese and zinc in preeclamptic pregnant women by inductively coupled plasma optical emission spectrometry: a prospective case controlled study in Riyadh, Saudi Arabia. Int J Clin Exp Pathol. 2014;7:1900‐1910. [PMC free article] [PubMed] [Google Scholar]
- 2. Ghulmiyyah L, Sibai B. Maternal mortality from preeclampsia/eclampsia. Semin Perinatol. 2012;36:56‐59. [DOI] [PubMed] [Google Scholar]
- 3. Braunthal S, Brateanu A. Hypertension in pregnancy: pathophysiology and treatment. SAGE Open Med. 2019;7:1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Parikh NI, Gonzalez J. Preeclampsia and hypertension: courting a long while: time to make it official. JAMA Intern Med. 2017;177(7):917‐918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mou AD, Barman Z, Hasan M, et al. Prevalence of preeclampsia and the associated risk factors among pregnant women in Bangladesh. Sci Rep. 2021;11(1):21339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shim SS, Lee CH, Jun JK. Midtrimester maternal plasma concentrations of angiopoietin 1, angiopoietin 2, and placental growth factor in pregnant women who subsequently develop preeclampsia. Obstet Gynecol Sci. 2015;58:10‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eiland E, Nzerue C, Faulkner M. Preeclampsia 2012. J Pregnancy. 2012;2012:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sena CM, Pereira AM, Seiça R. Endothelial dysfunction—a major mediator of diabetic vascular disease. Biochim Biophys Acta Mol Basis Dis. 2013;1832:2216‐2231. [DOI] [PubMed] [Google Scholar]
- 9. Lo JO, Mission JF, Caughey AB. Hypertensive disease of pregnancy and maternal mortality. Curr Opin Obstet Gynecol. 2013;25:124‐132. [DOI] [PubMed] [Google Scholar]
- 10. Farzin L, Sajadi F. Comparison of serum trace element levels in patients with or without pre‐eclampsia. J Res Med Sci. 2012;17:938‐941. [PMC free article] [PubMed] [Google Scholar]
- 11. Ma Y, Shen X, Zhang D. The relationship between serum zinc level and preeclampsia: a meta‐analysis. Nutrients. 2015;7:7806‐7820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen CW, Jaffe IZ, Karumanchi SA. Pre‐eclampsia and cardiovascular disease. Cardiovasc Res. 2014;101:579‐586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jin S, Hu C, Zheng Y. Maternal serum zinc level is associated with risk of preeclampsia: a systematic review and meta‐analysis. Front Public Health. 2022;10:968045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Johal T, Lees CC, Everett TR, Wilkinson IB. The nitric oxide pathway and possible therapeutic options in pre‐eclampsia. Br J Clin Pharmacol. 2014;78:244‐257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lewandowska M, Sajdak S, Lubiński J. Can serum iron concentrations in early healthy pregnancy be risk marker of pregnancy‐induced hypertension? Nutrients. 2019;11(5):1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rudra CB, Williams MA, Schiff MA, Koenig JQ, Dills R, Yu J. A prospective study of maternal carboxyhaemoglobin and pre‐eclampsia risk. Paediatr Perinat Epidemiol. 2010;24:35‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gutteridge JMC, Quinlan GJ. Antioxidant protection against organic and inorganic oxygen radicals by normal human plasma: the important primary role for iron‐binding and iron‐oxidising proteins. Biochim Biophys Acta. 1992;1159:248‐254. [DOI] [PubMed] [Google Scholar]
- 18. McCance DR, Holmes VA, Maresh MJA, et al. Vitamins C and E for prevention of preeclampsia in women with type 1 diabetes (DAPIT): a randomized placebo‐controlled trial. Obstet Gynecol Surv. 2010;65:753‐754. [Google Scholar]
- 19. Ephraim RKD, Osakunor DNM, Denkyira SW, Eshun H, Amoah S, Anto EO. Serum calcium and magnesium levels in women presenting with pre‐eclampsia and pregnancy‐induced hypertension: a case–control study in the Cape Coast metropolis, Ghana. BMC Pregnancy Childbirth. 2014;14:390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cole NI, Suckling RJ, Swift PA, et al. The association between serum sodium concentration, hypertension and primary cardiovascular events: a retrospective cohort study. J Hum Hypertens. 2019;33(1):69‐77. [DOI] [PubMed] [Google Scholar]
- 21. Haddy FJ, Vanhoutte PM, Feletou M. Role of potassium in regulating blood flow and blood pressure. Am J Physiol Regul Integr Comp Physiol. 2006;290:R546‐R552. [DOI] [PubMed] [Google Scholar]
- 22. Ullah M, Koch C, Tamanna S, Rouf S, Shamsuddin L. Vitamin D deficiency and the risk of preeclampsia and eclampsia in Bangladesh. Horm Metab Res. 2013;45:682‐687. [DOI] [PubMed] [Google Scholar]
- 23. Machano MM, Joho AA. Prevalence and risk factors associated with severe pre‐eclampsia among postpartum women in Zanzibar: a cross‐sectional study. BMC Public Health. 2020;20(1):1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Haque MM, Moghal MMR, Sarwar MS, et al. Low serum selenium concentration is associated with preeclampsia in pregnant women from Bangladesh. J Trace Elem Med Biol. 2016;33:21‐25. [DOI] [PubMed] [Google Scholar]
- 25. Czupryn M, Falchuk KH, Stankiewicz A, Vallee BL. A Euglena gracilis zinc endonuclease. Biochemistry. 1993;32:1204‐1211. [DOI] [PubMed] [Google Scholar]
- 26. Yanik FF, Amanvermez R, Yanik A, Çelik C, Kökçü A. Pre‐eclampsia and eclampsia associated with increased lipid peroxidation and decreased serum vitamin E levels. Int J Gynaecol Obstet. 1999;64:27‐33. [DOI] [PubMed] [Google Scholar]
- 27. Marshall NE, Abrams B, Barbour LA, et al. The importance of nutrition in pregnancy and lactation: lifelong consequences. Am J Obstet Gynecol. 2022;226(5):607‐632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chaurasia PP, Jadav PA, Jasani JH. Changes in serum calcium and serum magnesium level in preeclamptic vs normal pregnancy. Int J Biomed Adv Res. 2012;3:511‐513. [Google Scholar]
- 29. Onyegbule O, Meludu S, Dioka C, et al. Comparison of serum levels of calcium and magnesium among preeclamptic and normotensive pregnant women at Nnamdi Azikiwe University Teaching Hospital, Nnewi, Nigeria. J Res Med Sci. 2014;2:404‐408. [Google Scholar]
- 30. Enaruna N, Ande A, Okpere E. Clinical significance of low serum magnesium in pregnant women attending the university of Benin teaching hospital. Niger J Clin Pract. 2013;16:448‐453. [DOI] [PubMed] [Google Scholar]
- 31. Nguyen H, Odelola OA, Rangaswami J, Amanullah A. A review of nutritional factors in hypertension management. Int J Hypertens. 2013;2013:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Golmohammad S, Amirabi A, Yazdian M, et al. Evaluation of serum calcium, magnesium, copper, and zinc levels in women with pre‐eclampsia. Iran J Med Sci. 2008;33:231‐234. [Google Scholar]
- 33. Gongora M, Wenger N. Cardiovascular complications of pregnancy. Int J Mol Sci. 2015;16:23905‐23928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Seoud MAF, Nassar AH, Usta IM, Melhem Z, Kazma A, Khalil AM. Impact of advanced maternal age on pregnancy outcome. Am J Perinatol. 2002;19:001‐008. [DOI] [PubMed] [Google Scholar]
- 35. Kashyap MK, Saxena SV, Khullar M, Sawhney H, Vasishta K. Role of anion gap and different electrolytes in hypertension during pregnancy (preeclampsia). Mol Cell Biochem. 2006;282:157‐167. [DOI] [PubMed] [Google Scholar]
- 36. Milman N, Paszkowski T, Cetin I, Castelo‐Branco C. Supplementation during pregnancy: beliefs and science. Gynecol Endocrinol. 2016;32(7):509‐516. [DOI] [PubMed] [Google Scholar]
- 37. Kovacs CS. Maternal mineral and bone metabolism during pregnancy, lactation, and post‐weaning recovery. Physiol Rev. 2016;96(2):449‐547. [DOI] [PubMed] [Google Scholar]
- 38. Seely EW, Seely EW, Wood RJ, Brown EM, Graves SW. Lower serum ionized calcium and abnormal calciotropic hormone levels in preeclampsia. J Clin Endocrinol Metab. 1992;74:1436‐1440. [DOI] [PubMed] [Google Scholar]
- 39. Akhtar S, Begum S, Ferdousi S. Calcium and zinc deficiency in preeclamptic women. J Bangladesh Soc Physiol. 2012;6:94‐99. [Google Scholar]
- 40. Abdelmarouf HM, Asma AD, Yousif HMO, et al. Serum calcium level as a marker of pregnancy‐induced hypertension. Sudan J Med Sci. 2007;2:245‐248. [Google Scholar]
- 41. Geraldo Lopes Ramos J, Brietzke E, Martins‐Costa SH, Vettorazzi‐Stuczynski J, Barros E, Carvalho C. Reported calcium intake is reduced in women with preeclampsia. Hypertens Pregnancy. 2006;25:229‐239. [DOI] [PubMed] [Google Scholar]
- 42. Indumati V, Kodliwadmath M, Sheela M. The role of serum electrolytes in pregnancy induced hypertension. J Clin Diagnostic Res. 2011;5:66‐69. [Google Scholar]
- 43. Richards D, Lindow S, Carrara H, Knight R, Haswell S, Van der Spuy Z. A comparison of maternal calcium and magnesium levels in pre‐eclamptic and normotensive pregnancies: an observational case–control study. BJOG: Int J Obstet Gynaecol. 2014;121:327‐336. [DOI] [PubMed] [Google Scholar]
- 44. Camara‐Lemarroy CR, de Leon‐Cruz A, Rodriguez‐Gutierrez R, Galarza‐Delgado DA. Severe hyponatremia associated with pre‐eclampsia. Gynecol Endocrinol. 2013;29(8):801‐803. [DOI] [PubMed] [Google Scholar]
- 45. Nawathe A, Govind A. Pregnancy with known syndrome of inappropriate antidiuretic hormone. J Obstet Gynaecol (Lahore). 2013;33:9‐13. [DOI] [PubMed] [Google Scholar]
- 46. Wakil A, Ng JM, Atkin SL. Investigating hyponatraemia. BMJ. 2011;342:d1118. [DOI] [PubMed] [Google Scholar]
- 47. Kahramanoglu I, Baktiroglu M, Yucel O, et al. Preeclampsia as a rare cause of hyponatremia. Gynecol Obstet. 2014;4:1‐4. [Google Scholar]
- 48. Bera S, Siuli RA, Gupta S, et al. Study of serum electrolytes in pregnancy induced hypertension. J Indian Med Assoc. 2011;109:546‐548. [PubMed] [Google Scholar]
- 49. Forestiero V, Sconfienza E, Mulatero P, Monticone S. Primary aldosteronism in pregnancy. Rev Endocr Metab Disord. 2023;24:39‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cheung KL, Lafayette RA. Renal physiology of pregnancy. Adv Chronic Kidney Dis. 2013;20:209‐214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yussif MN, Salih R, Sami A, et al. Estimation of serum zinc, sodium and potassium in normotensive and hypertensive primigravide pregnant women. Tikrit Med J. 2009;15:13‐18. [Google Scholar]
- 52. Wibowo N, Irwinda R, Rivai AT. Serum zinc, selenium, iron, and copper levels in pregnant women with fetal growth restriction. Clin Exp Obstet Gynecol. 2019;46(6):892‐896. [Google Scholar]
- 53. Kiilholma P, Paul R, Pakarinen P, Gränroos M. Copper and zinc in pre‐eclampsia. Acta Obstet Gynecol Scand. 1984;63:629‐631. [DOI] [PubMed] [Google Scholar]
- 54. Cherry FF, Bennett EA, Bazzano GS, Johnson LK, Fosmire GJ, Batson HK. Plasma zinc in hypertension/toxemia and other reproductive variables in adolescent pregnancy. Am J Clin Nutr. 1981;34:2367‐2375. [DOI] [PubMed] [Google Scholar]
- 55. Bassiouni BA, Foda AI, Rafei AA. Maternal and fetal plasma zinc in pre‐eclampsia. Eur J Obstet Gynecol Reprod Biol. 1979;9:75‐80. [DOI] [PubMed] [Google Scholar]
- 56. Díaz E, Halhali A, Luna C, Díaz L, Avila E, Larrea F. Newborn birth weight correlates with placental zinc, umbilical insulin‐like growth factor I, and leptin levels in preeclampsia. Arch Med Res. 2002;33:40‐47. [DOI] [PubMed] [Google Scholar]
- 57. Açikgoz S, Harma M, Harma M, Mungan G, Can M, Demirtas S. Comparison of angiotensin‐converting enzyme, malonaldehyde, zinc, and copper levels in preeclampsia. Biol Trace Elem Res. 2006;113:1‐8. [DOI] [PubMed] [Google Scholar]
- 58. Marreiro D, Cruz K, Morais J, Beserra J, Severo J, de Oliveira A. Zinc and oxidative stress: current mechanisms. Antioxidants. 2017;6(2):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hussain A, Jiang W, Wang X, et al. Mechanistic impact of zinc deficiency in human development. Front Nutr. 2022;9:717064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Schuessel K, Schäfer S, Bayer TA, et al. Impaired Cu/Zn‐SOD activity contributes to increased oxidative damage in APP transgenic mice. Neurobiol Dis. 2005;18:89‐99. [DOI] [PubMed] [Google Scholar]
- 61. Atamer Y, Koçyigit Y, Yokus B, Atamer A, Erden AC. Lipid peroxidation, antioxidant defense, status of trace metals and leptin levels in preeclampsia. Eur J Obstet Gynecol Reprod Biol. 2005;119:60‐66. [DOI] [PubMed] [Google Scholar]
- 62. Siddiqui IA, Jaleel A, Kadri HMFA, Saeed WA, Tamimi W. Iron status parameters in preeclamptic women. Arch Gynecol Obstet. 2011;284(3):587‐591. [DOI] [PubMed] [Google Scholar]
- 63. Fisher AL, Nemeth E. Iron homeostasis during pregnancy. Am J Clin Nutr. 2017;106(6):1567S‐1574S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Balla G, Jacob HS, Eaton JW, Belcher JD, Vercellotti GM. Hemin: a possible physiological mediator of low density lipoprotein oxidation and endothelial injury. J Atheroscler Thromb. 1991;11:1700‐1711. [DOI] [PubMed] [Google Scholar]
- 65. Taeubert MJ, Wiertsema CJ, Vermeulen MJ, et al. Maternal iron status in early pregnancy and blood pressure throughout pregnancy, placental hemodynamics, and the risk of gestational hypertensive disorders. J Nutr. 2022;152(2):525‐534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ugwuja EI, Akubugwo EI, Ibiam UA, Obidoa O. Impact of maternal copper and zinc status on pregnancy outcomes in a population of pregnant Nigerians. Pak J Nutr. 2010;9:678‐682. [Google Scholar]
- 67. Basher K, Deb K. Alteration in iron status in pre eclampsia. Mymensingh Med J. 2006;15:22‐24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and/or its Supporting Information.


