Abstract
Recurrent aphthous stomatitis (RAS) is a frequently seen oral ulcerative lesion, manifesting as multiple, recurrent, shallow, irregular ulcers encircled by an erythematous halo. The precise etiopathogenesis is obscure, although the recent understanding suggests an underlying immune-mediated etiology. However, there is no defined management protocol and the principal therapy aims to attain symptomatic respite. 150 clinically diagnosed RAS patients of both sexes in the age range of 15–65 years were randomly allocated and divided into two cohorts, namely Group A (75 RAS patients receiving 5% Amlexanox paste + 100 mg Rebamipide tab.) and Group B (75 RAS patients receiving Dologel CT). Effectiveness of 5% Amlexanox oral paste and Rebamipide tablets in diminution of the ulcer size, erythema and pain was assessed and compared with Dologel CT therapy. The study showed an equal gender distribution. Majority of the patients were in the younger age range (25–35 years) and labial and buccal mucosa were the commonest affected sites. Although, both 5% Amlexanox and Rebamipide tablets and Dologel CT showed statistically significant resolution in the ulcer size, erythema, and associated pain. However, 5% oral Amlexanox paste and Rebamipide tablets are more efficacious in the treatment of RAS and tend to heal the ulcer at a more accelerated pace. Our research concluded that 5% oral Amlexanox paste and Rebamipide tablets tend to heal the ulcer at a more accelerated pace as compared to Dologel CT.
Electronic supplementary material
The online version of this article (10.1007/s12070-020-01858-1) contains supplementary material, which is available to authorized users.
Keywords: Amlexanox, Dologel CT, Oral ulcers, Rebamipide, Recurrent aphthous stomatitis
Introduction
RAS is a common oral mucosal ulcerative condition with an incidence rate of 5–25%. The precise etiology of RAS is not known, hence, the condition requires significant clinical observation with numerous therapeutic options and not much of a relief [1]. Some well-known predisposing factors include genetic variations, nutritional and hematinic deficiency states, mucosal trauma, smoking cessation, endocrine and immune dysfunction, allergic reaction to foodstuff and drugs, and psycho-somatic disorders (anxiety, stress, and depression) [2, 3]. A multitude of systemic conditions (Inflammatory Bowel Disease, cyclic neutropenia) and syndromes (Reiter syndrome, Behçet's syndrome, PFAPA syndrome, MAGIC syndrome, and Sweet syndrome) are also associated with RAS [4].
RAS usually appear as multiple, recurrent, small, shallow irregular ulcers, with defined borders, and circumscribed by erythematous haloes [5]. Stanley (1972) categorized RAS into 3 types- minor, major and herpetiform ulcers [6]. The classification criteria include the size and the depth of the lesion, the number of lesions in one episode, their location and duration [7].
Minor aphthous ulcers (known as canker sore or Mikuliz's aphthae) account for up to 80% of the aphthous stomatitis. The size ranges from 8 to 10 mm with a site predilection for buccal mucosa, labial mucosa, and floor of the mouth. Healing occurs without scarring, usually in 10–14 days. The number of eruptions per one flare-up in this type does not normally exceed 10.
Major aphthous ulcers (known as peri-adenitis mucosa necrotica recurrens or Sutton's disease), constitutes 10–15% of ulcers. The size is usually < 1 cm in diameter with a site predilection for the keratinized mucosa (Lips, soft palate, faucial pillars, gingiva, and dorsum of the tongue). Healing with scarring usually occurs in 6–8 weeks.
Herpetiform ulcers occur as recurrent crops of pin-point (2–3 mm) multiple ulcers (up to 100). The ulcers have a later age of onset, show a female preponderance and persist for about 10–14 days [8].
Topical therapy is usually preferred to diminish inflammation and pain [9]. However, systemic pharmacotherapy is reserved for patients with multiple minor aphthae or major RAS [10]. Local anesthetic agents, antihistamines, antibiotics, immunosuppressive and NSAIDs (non- steroidal anti-inflammatory drugs) are the frequently used topical therapy [11]. A few studies have established that 5% Amlexanox oral paste and Rebamipide tablets have a pivotal role in the pharmacotherapy of RAS [12, 13]. Amlexanox (C16H14N2O4), a widely researched topical agent is an anti-inflammatory and anti-allergic drug. The exact mechanism of action is not known, although, it may hinder the formation and release of leukotrienes and histamine from mast cells, neutrophils, and mononuclear cells [11, 14, 15]. 5% amlexanox paste exhibits an excellent effect if applied during the prodromal ulcer stage [11]. Rebamipide 2-(4-chlorobenzoyllamine)-3-[2-(1H)-quinolinon-4-yl) is a recent muco-protective drug that sustains the lively epithelial cells and restores the damaged tissue through multi-modal actions [16]. Rebamipide has successfully been able to ameliorate various mucosal conditions such as gastric ulceration and erosions [17]. Published literature has demonstrated the efficacy of Rebamipide in the pharmacotherapy for Behcet's disease and RAS. Rebamipide promotes accelerated ulcer healing by decreasing the formation of free oxygen radicals, augmenting the vasculature and enhancing the protective prostaglandin formation in ulcerated mucosa [18].
With this background, the study aimed to assess the efficacy of 5% amlexanox oral paste and rebamipide tablets as a treatment regimen for RAS. The study also aimed to evaluate and compare the differences in the ulcer parameters (size, erythema and pain score) in both the study groups (Group A: receiving 5% amlexanox oral paste and rebamipide tablets and Group B: receiving analgesic and antiseptic gel- Dologel CT).
Material and Methods
A Prospective Cross-sectional study was carried out for a period of eight months (July–March) in the Out Patient Department of Oral Medicine and Radiology, Faculty of Dentistry, Jamia Millia Islamia. A total of 800 patients with various complaints were screened for RAS. The study was conducted after obtaining ethical approval from the Institutional Ethics Committee (IEC), Jamia Millia Islamia FTS-279210/5798-5803 [Appendix 1]. The patient's participation was made after the informed consent of the patient (parent's consent in individuals below 18 years of age), and a detailed history was elicited from the patients.
Patients of both genders, between the age group of 15–65 years, and presenting with aphthous ulcerations (minor, major or herpetiform ulcers as per Stanley’s criteria) were enrolled in the study. However, patients presenting with Oral ulcerations due to other obvious causes (Inflammatory Bowel Disease, hematological ailments, Behchet's syndrome, Reiter syndrome, and dietary deficiencies), accompanying oral mucosal disorders, history of previous therapy for ulcers, and lactating and pregnant females were excluded from the study.
Patient demographics and a thorough history were recorded on a pre-designed questionnaire [Appendix 2] A detailed clinical examination was performed following infection control measures and the number, size, and site of the ulcer was assessed. Pain evaluation was carried out using a visual analog scale (VAS) ranging from 1 to 10 (10 being the most painful). The level of ulcer erythema was evaluated on a scale ranging from 0 to 3 (following Greer et al.), where 0, 1, 2 and 3 represented the absence of erythema, light pink erythema, red and dark red color respectively.
Selected patients were randomly divided into two treatment groups: Group A (RAS patients receiving 5% Amlexanox paste + 100 mg rebamipide tab.) and Group B (RAS patients receiving Dologel CT).
Group A patients were prescribed 5% amlexanox paste for topical application 3–4 times daily for 7 days and rebamipide (Rebagen tablet 100 mg) TDS for 7 days at the initial visit. Amlexanox paste (Trade name Lexenox in the form of a plastic tube containing 5 g of oral paste) and rebamipide tablets were supplied by Macleod's pharmaceuticals, India.
Group B patients were prescribed the anti-inflammatory, analgesic and antiseptic gel (Dologel CT) to be topically applied 3–4 times daily for a week. Dologel-CT was supplied by Dr. Reddy`s Laboratories Ltd., India as a 10 g tube and contains choline salicylate, Benzalkonium chloride, and Lignocaine as the primary constituents.
The subjects in both the groups were instructed regarding the usage and application of the medications. The subjects were also advised to discontinue the medication and report back to the physician in case of any adverse effects experienced.
The patients were then reviewed at 5th, 10th, 15th and 30th day, following the onset of treatment. Treatment efficacy was assessed in both the groups based on reduction in the size, erythema and pain scores of ulcers at 15th and 30th day.
Student's t-test was performed for comparing the two groups. Paired t-test was done for comparing the size and erythema of ulcer, and pain (VAS) scores at various time frames. Day 0 served as a baseline value when the tests were done within the group. Statistically significant results were chosen with p-value = < 0.05.
Results
Out of 800 screened patients, 150 patients presented with RAS (Prevalence rate of 19.8%). The study showed an equal sex distribution of aphthous ulcers. Group A had 30 males and 45 affected females whereas in Group B, there were 45 males and 30 females.
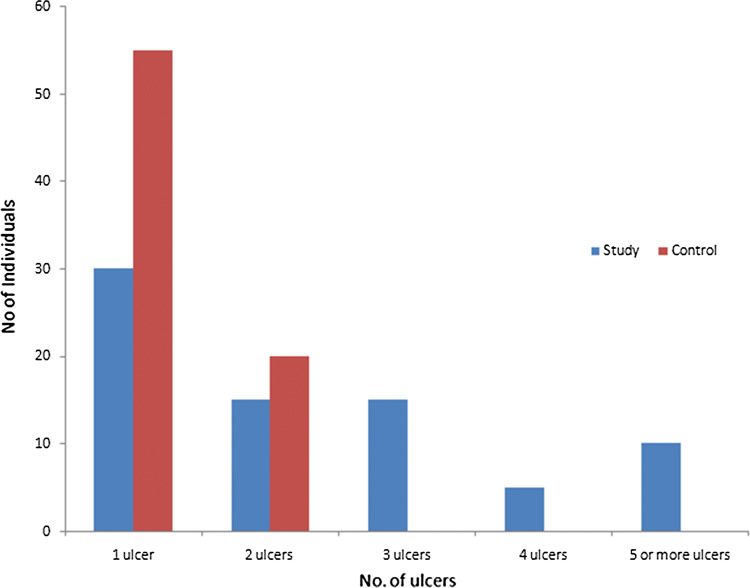
Labial mucosa (45%), dorsum of the tongue (24%), buccal mucosa (10%) and floor of the mouth (7%) were the 4 most common sites that were involved in RAS (Fig. 1). Majority of the subjects enrolled demonstrated the presence of a solitary ulcer (n = 85), followed by subjects in which 2 or more ulcers were found (n = 65) (Fig. 2).
Fig. 1.
Site invlovement in RAS
Fig. 2.
Number of Ulcers in the study and Control Group
The baseline values like the size of the ulcer, erythema in ulcer and VAS scale were documented on day 0. After prescribing 5% oral amlexanox paste and rebamipide tablets, the patients were recalled at 5th, 10th, 15th and 30th day. Pairwise comparison of the 0 days with 5th, 10th, 15th and 30th day of treatment with Amlexanox 5% + Rebapamide regarding the changes in size, erythema, and pain score (VAS) of the ulcers was carried out. Patients in the Group A demonstrated that majority of the ulcers showed complete healing by day 10 or 15 (Table 1). Paired t-test showed statistically significant differences in the size, erythema and pain score of the ulcers (Table 2).
Table 1.
Pair wise comparison of the 0 day with 5th, 10th, 15th and 30th day of treatment with in cases w.r.t the size of ulcers, erythema of ulcers, maximum pain in (VAS) of the ulcers
| Treatment modality | Group A | Day 0 | Day 5 | Day 10 | Day 15 | Day 30 | |||
|---|---|---|---|---|---|---|---|---|---|
| Amlexanox 5% + Rebapamide | Size of ulcers | Mean | 8.056 | 1.193 | 0.1 | 0 | 0 | ||
| SD | 3.679 | 2.4511 | 0.496 | 0 | 0 | ||||
| Erythema of ulcers | Mean | 1.45 | 0.225 | 0 | 0 | 0 | |||
| SD | 0.959 | 0.422 | 0 | 0 | 0 | ||||
| Pain in VAS | Mean | 6.275 | 0.925 | 0.05 | 0 | 0 | |||
| SD | 1.934 | 1.456 | 0.316 | 0 | 0 | ||||
| Dologel-CT | Group B | Day 0 | Day 5 | Day 10 | Day 15 | Day 30 | |||
| Size of ulcers | Mean | 8.7763 | 2.7631 | 0.3421 | 0 | 0 | |||
| SD | 2.7270 | 2.4288 | 0.7081 | 0 | 0 | ||||
| Erythema of ulcers | Mean | 1.210 | 0.4736 | 0.0526 | 0 | 0 | |||
| SD | 0.4188 | 0.6117 | 0.2294 | 0 | 0 | ||||
| Pain in VAS | Mean | 3.157 | 1.0526 | 0.0526 | 0 | 0 | |||
| SD | 1.572 | 1.7471 | 0.2294 | 0 | 0 | ||||
Table 2.
Group A and B: p-value for 5th, 10th, 15th and 30th day compared with 0 day
| Treatment modality | Group A | Day 5 | Day 10 | Day 15 | Day 30 | |
|---|---|---|---|---|---|---|
| 5% Amlexanox paste + 100 mg Rebamipide tab | Size of ulcers | P-value | 2.506E-13 | 2.195E-16 | 1.22E-16 | 1.22E-16 |
| Significance | *S | *S | *S | *S | ||
| Erythema of ulcers | P-value | 1.08068E-09 | 9.05126E-12 | 1.69E-22 | 1.69E-22 | |
| Significance | *S | *S | *S | *S | ||
| Pain in VAS | P-value | 3.63295E-16 | 6.5274E-22 | 9.05E-12 | 9.05E-12 | |
| Significance | *S | *S | *S | *S | ||
| Dologel CT | Group B | Day 5 | Day 10 | Day 15 | Day 30 | |
| Size of ulcers | P-value | 8.93441E-07 | 4.16691E-10 | 3.93293E-11 | 3.93293E-11 | |
| Significance | *S | *S | *S | *S | ||
| Erythema of ulcers | P-value | 0.000362 | 8.08384E-09 | 2.29877E-10 | 2.29877E-10 | |
| Significance | *S | *S | *S | *S | ||
| Pain in VAS | P-value | 0.00115 | 1.04666E-07 | 6.6641E-08 | 6.6641E-08 | |
| Significance | *S | *S | *S | *S | ||
Group B patients (treated with Dologel CT) also showed statistically significant changes in size, erythema, and pain of the ulcers. However, the average period for absolute ulcer healing ranged between day 15th to day 30th (Tables 1, 2).
Further, both the groups were compared for the mean ulcer size. Our study shows that although it is not statistically significant, the mean ulcer size has decreased at a much faster-pace in Group A (Tables 1, 3). Comparison of the VAS scale and level of erythema showed a drastic decrease between the two groups on day 0 and day 5 (Tables 1, 2). Both the groups were compared for statistical significance related to the ulcer size, level of erythema, and pain score. The study concluded that only erythema of the ulcers between the 2 groups was statistically significant for day 5 and VAS score showed statistical significance at day 0 (Table 3).
Table 3.
p-value between group A and group B
| Day 0 | Day 5 | Day 10 | Day 15 | Day 30 | ||
|---|---|---|---|---|---|---|
| Size of ulcers | P-value | 0.451306 | 0.024852 | 0.134026 | 0 | 0 |
| Significance | NS | NS | NS | NS | NS | |
| Erythema of ulcers | P-value | 0.303516 | 0.074045 | 0.148353 | 0 | 0 |
| Significance | NS | *S | NS | NS | NS | |
| Pain in VAS | P-value | 9.15569E-08 | 0.769297 | 0.974276 | 0 | 0 |
| Significance | *S | NS | NS | NS | NS |
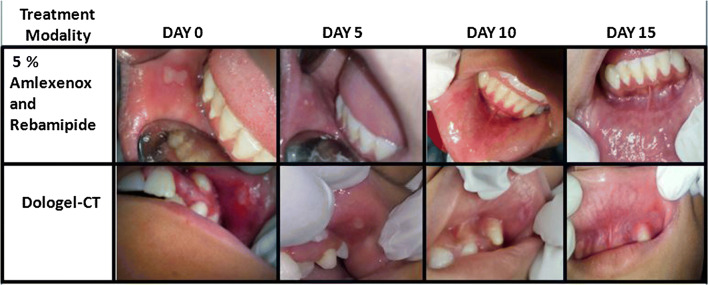
Gradual Resolution of the ulcers for both treatment modalities on follow up visits is depicted in Fig. 3.
Fig. 3.
Gradual Resolution of the ulcers for both treatment modalities
Discussion
There exists no definitive therapeutic regimen for RAS, owing to its multitude of etiologies, uncertain course, and propensity for recurrent episodes. The principal objective of the pharmacotherapy is pain alleviation, accelerated ulcer healing and precludes recurrences. Numerous treatment protocols have been instituted for RAS treatment, encompassing the varied spectrum of topical to systemic agents [19]. Traditional therapy incorporated the topical application of varied anti-inflammatory, analgesic, and antiseptic pastes such as Dologel CT, Dentogel, 0.2% chlorhexidine in rinses or gel, Orasep, etc. [20]. However, topical application of 5% Amlexanox oral paste and systemic ingestion of tablet Rebamipide constitutes the emerging treatment regimen for RAS. At present, 5% Amlexanox oral paste is the only therapeutic product for RAS which has attained acceptance from the Food and Drug Administration (FDA) US [21].
This study aimed at comparing the efficiency of two standard medications- 5% amlexanox oral paste and rebamipide tablets and Dologel CT in Group A and Group B respectively. The diminution in the size, erythema and pain score of the ulcer was noted post-therapy in both the groups.
The present study showed equal sex distribution of aphthous ulcers. Although, 30 males and 45 females were affected in the Group A and 45 males and 30 females had aphthous ulcers in the Gropu B. This was contrary to the studies conducted by Bhatt et al. [22], Darshan et al. [23], and Parvathi et al. [24] where a male predominance was noted. A study by Matsuda et al. [13] demonstrated that RAS has a female preponderance as elderly females usually have an accompanying systemic aspect (haematinic deficiencies are more common in elderly females). In our study, most of the subjects were in the younger age (25–35 years) range with equal gender distribution. Similar findings were reported in the studies conducted by Khandwala et al. [12], and Matsuda et al. [13]. However, Parvathi et al. [24] reported that RAS occurred predominantly in the 21–25 year range. In our study, 30% of the patients were in the 15–25 year age group.
In this study, labial mucosa was the most frequent site of involvement (44%), followed by dorsum of the tongue, buccal mucosa and floor of the mouth, constituting 24%, 12%, and 8% cases respectively. Least common sites of involvement were hard palate, lateral border of the tongue, alveolar mucosa, buccal vestibule, and uvula. These findings are in coherence to the published literature which shows RAS site predilection for the labile mucosa (labial and buccal mucosa) [13, 15, 22, 23, 25, 26].
Among the 150 RAS patients, 85 patients had a solitary ulcer followed by 2, 3, 4 ulcers found in 35, 15, 5 patients respectively. 10 patients had ulcers that accounted for 5 or more. (Fig. 2) Published literature suggests that the majority of RAS patients presents between 2 and 6 ulcers/episode [25].
The baseline values like the size of the ulcer, erythema in ulcer and VAS scale were documented on day 0. After prescribing 5% oral amlexanox paste and rebamipide tablets, the patients were recalled at 5th, 10th and 15th day. Our study concluded statistically significant changes in the size, erythema and pain intensity of the ulcers over 5th, 10th and 15th day.(Tables 1, 2) Studies conducted by Khandwala et al. [12], Matsuda et al. [13], Suraksha Bhatt et al. [22], Parvathi Devi et al. [24], and Meng W et al. [27] also demonstrated similar findings.
Group B also showed statistically significant changes in size, erythema, and pain associated with the ulcers. However, on comparing the mean ulcer size, erythema, and pain scores between the 2 groups, we found that 5% oral amlexanox paste and rebamipide tablets tend to heal the ulcer at a more accelerated pace. This ensures that the topical therapy with 5% oral amlexanox paste and systemic intake of rebamipide tablets would serve as the treatment of choice for RAS.
Usually, 5% Amlexanox paste does not have adverse effects, although, mild transitory tingling sensations and a metallic taste have been reported by a few patients [28]. Uncommon mild gastrointestinal adverse effects (constipation, diarrhea, nausea, and vomiting) are encountered with rebamipide therapy [29]. However, no such adverse effects were noted in our study patients.
Conclusion
The study concluded that both 5% Amlexanox oral paste and Rebapamide tablets and Dologel CT can be used as a treatment modality for aphthous stomatitis. However, the synergistic effect of Amlexanox 5% paste and tablet Rebapamide has a more profound effect on the resolution of pain and erythema, ulcer size and accelerates the ulcer healing. The diminution in the size, erythema and pain intensity are also suggestive of the better efficacy of these drugs in the RAS treatment.
Limitations
This study is a single-center study. Hence, the sample size may not be representative of all the general patients. A multicentre study incorporating a larger sample size should be carried out to eliminate this limitation.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors acknowledge the support of Indian Council of Medical Research for this research under Short Term Studentship 2015 (STS) Programme.
Compliance with ethical standards
Conflict of interest
Authors declare that they have no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shamimul Hasan, Email: shasan1@jmi.ac.in.
Naila Perween, Email: nailap88@outlook.com.
Shazina Saeed, Email: ssaeed@amity.edu.
Mandeep Kaur, Email: mkaur@jmi.ac.in.
Virender Gombra, Email: vgombra@jmi.ac.in.
Arpita Rai, Email: arai@jmi.ac.in.
References
- 1.Hasan S, Saeed S, Rai A, Kumar A, Choudhary P, Panigrahi R, et al. Thalidomide: clinical implications in oral mucosal lesions - an update. Ann Med Health Sci Res. 2018;8:21–28. [Google Scholar]
- 2.Koybasi S, Parlak AH, Serin E, Yilmaz F, Serin D. Recurrent aphthous stomatitis: investigation of possible etiologic factors. Am J Otolaryngol. 2006;27(4):229–232. doi: 10.1016/j.amjoto.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 3.Wray D, Graykowski EA, Notkins AL. Role of mucosal injury in initiating recurrent aphthous stomatitis. Br Med J Clin Res Ed. 1981;283(6306):1569–1570. doi: 10.1136/bmj.283.6306.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Preeti L, Magesh KT, Rajkumar K, Karthik R. Recurrent aphthous stomatitis. J Oral Maxillofac Pathol. 2011;15:252–256. doi: 10.4103/0973-029X.86669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jurge S, Kuffer R, Scully C, Porter SR. Recurrent aphthous stomatitis. Oral Dis. 2006;12(1):21. doi: 10.1111/j.1601-0825.2005.01143.x. [DOI] [PubMed] [Google Scholar]
- 6.Edgar NR, Saleh D, Miller RA. Recurrent aphthous stomatitis: a review. J Clin Aesthet Dermatol. 2017;10(3):26–36. [PMC free article] [PubMed] [Google Scholar]
- 7.Natah SS, Konttinen YT, Enattah NS, Ashammakhi N, Sharkey KA, Häyrinen-Immonen R. Recurrent aphthous ulcers today: a review of the growing knowledge. Int J Oral Maxillofac Surg. 2004;33:221–234. doi: 10.1006/ijom.2002.0446. [DOI] [PubMed] [Google Scholar]
- 8.Sharma D, Garg A. Comprehensive review on aphthous stomatitis, its types, management and treatment available. J Develop Drugs. 2018;7(2):1–8. [Google Scholar]
- 9.Baccaglini L, Lalla RV, Bruce AJ, Sartori-Valinotti JC, Latortue MC, Carrozzo M, et al. Urban legends:recurrent aphthous stomatitis. Oral Dis. 2011;17:755–770. doi: 10.1111/j.1601-0825.2011.01840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Abreu MA, Hirata CH, Pimentel DR, Weckx LL. Treatment of recurrent aphthous stomatitis with clofazimine. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:714–721. doi: 10.1016/j.tripleo.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 11.Murray B, Biagioni PA, Lamey P-J. The efficacy of amelxanox OraDiscTM on the prevention of recurrent minor aphthous ulceration. J Oral Pathol Med. 2006;35:117–122. doi: 10.1111/j.1600-0714.2006.00379.x. [DOI] [PubMed] [Google Scholar]
- 12.Khandwala A, Van Inwegen RG, Alfano MC. 5% amlexanox oral paste, a new treatment for recurrent minor aphthous ulcers: I. clinical demonstration of acceleration of healing and resolution of pain. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83:222–230. doi: 10.1016/S1079-2104(97)90009-3. [DOI] [PubMed] [Google Scholar]
- 13.Matsuda T, Ohno S, Hirohata S, Miyanaga Y, Ujihara H, Inaba G, et al. Efficacy of rebamipide as adjunctive therapy in the treatment of recurrent oral aphthous ulcers in patients with behcet's disease: A randomised, double-blind, placebo-controlled study. Drugs R D. 2003;4:19–28. doi: 10.2165/00126839-200304010-00002. [DOI] [PubMed] [Google Scholar]
- 14.Sharma R, Pallagatti S, Aggarwal A, Sheikh S, Singh R, Gupta D. A randomized, double-blind, placebo-controlled trial on clinical efficacy of topical agents in reducing pain and frequency of recurrent aphthous ulcers. Open Dent J. 2018;12:700–713. doi: 10.2174/1745017901814010700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu J, Zeng X, Chen Q, Cai Y, Chen F, Wang Y, et al. An evaluation on the efficacy and safety of amlexanox oral adhesive tablets in the treatment of recureent minor aphthous ulceration in a chinese cohort: a randomized, double blind, vehicle controlled, unparallelmulticentric clinical trial. Oral Surg Oral Med Oral Pathol Oral RadiolEndod. 2006;102:475–481. doi: 10.1016/j.tripleo.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 16.Kim HK, Kim JI, Kim JK, Han JY, Park SH, Choi KY, et al. Preventive effects of rebamipide on NSAID-induced gastric mucosal injury and reduction of gastric mucosal blood flow in healthy volunteers. Dig Dis Sci. 2007;52:1776–1782. doi: 10.1007/s10620-006-9367-y. [DOI] [PubMed] [Google Scholar]
- 17.Akagi S, Fujiwara T, Nishida M, Okuda A, Nagao Y, Okuda T, et al. The effectiveness of rebamipide mouthwash therapy for radiotherapy and chemoradiotherapy-induced oral mucositis in patients with head and neck cancer: a systematic review and meta-analysis. J Pharm Health Care Sci. 2019;5(16):2–7. doi: 10.1186/s40780-019-0146-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kudur MH, Hulmani M. Rebamipide: A novel agent in the treatment of recurrent aphthous ulcer and Behcet's syndrome. Indian J Dermatol. 2013;58:352–354. doi: 10.4103/0019-5154.117298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tarakji B, Gazal G, Al-Maweri SA, Azzeghaiby SN, Alaizari N. Guideline for the diagnosis and treatment of recurrent aphthous stomatitis for dental practitioners. J Int Oral Health. 2015;7(5):74–80. [PMC free article] [PubMed] [Google Scholar]
- 20.Belenguer-Guallar I, Jiménez-Soriano Y, Claramunt-Lozano A. Treatment of recurrent aphthous stomatitis: a literature review. J Clin Exp Dent. 2014;6(2):168–174. doi: 10.4317/jced.51401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shrivastava K, Naidu G, Deshpande A, Handa H, Raghuvanshi V, Gupta M. Comparative evaluation of the efficacy of topical amlexanox 5% oral paste and triamcinolone acetonide 0.1% oral paste in the treatment of recurrent aphthous stomatitis (RAS) J Indian Acad Oral Med Radiol. 2018;30:235–240. [Google Scholar]
- 22.Bhat S, Sujatha D. A clinical evaluation of 5% amlexanox oral paste in the treatment of minor recurrent aphthous ulcers andcomparison with the placebo paste: a randomized, vehicle controlled, parallel, single center clinical trial. Indian J Dent Res. 2013;24(5):593–598. doi: 10.4103/0970-9290.123382. [DOI] [PubMed] [Google Scholar]
- 23.Darshan DD, Vijay Kumar CN, Manoj Kumar AD, Manikantan NS, Balakrishnan D. Clinical study to know the efficacy of Amlexanox 5% with other topical Antiseptic, Analgesic and Anesthetic agents in treating minor RAS. J Int Oral Health. 2014;6(1):5–11. [PMC free article] [PubMed] [Google Scholar]
- 24.Parvathi Devi MK, Ramesh DNSV, Koppal S, Byatnal AR, Rukmangada T, Byatnal AA. Efficacy of rebamipide and levamisole in the treatment of patients with recurrent aphthous ulcer—a comparative study. J Clin Diagn Res. 2014;8(11):119–122. doi: 10.7860/JCDR/2014/10295.5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greenberg MS, Glick M. In: Burket’s Oral Medicine Diagnosis and Treatment. 11. New York: BC Decker; 2008. [Google Scholar]
- 26.Greer RO, Lindenmuth JE, Juarez T, Khandwala A. A double-blind study of topically applied 5% amlexanox in the treatment of aphthous ulcers. J Oral Maxillofac Surg. 1993;51(3):243–248. doi: 10.1016/S0278-2391(10)80164-8. [DOI] [PubMed] [Google Scholar]
- 27.Meng W, Dong Y, Liu J, Wang Z, Zhong X, Chen R, et al. A clinical evaluation of amlexanox oral adhesive pellicles in the treatment of recurrent aphthous space stomatitis and comparison with amlexanox oral tablets: a randomized, placebo controlled, blinded, multicenter clinical trial. Trials. 2009;10(30):1–7. doi: 10.1186/1745-6215-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bell J. Amlexanox for the treatment of recurrent aphthous ulcers. Clin Drug Investig. 2005;25:555–566. doi: 10.2165/00044011-200525090-00001. [DOI] [PubMed] [Google Scholar]
- 29.Park SH, Cho CS, Lee OY, Jun JB, Lin SR, Zhou LY, et al. Comparison of prevention of NSAID-induced gastrointestinal complications by rebamipide and misoprostol: A randomized, multicenter, controlled trial-storm study. J Clin Biochem Nutr. 2007;40:148–155. doi: 10.3164/jcbn.40.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.