Abstract
The aim is to retrospectively evaluate the clinical outcomes and prognostic factors in patients of locally advanced nasopharyngeal cancer (NPC) treated with intensity-modulated radiotherapy (IMRT). The northeastern states report relatively more NPC cases in comparison to other states of India. This study is an attempt to assess the treatment outcomes and prognostic factors of locally advanced NPC who had been treated with definitive radiotherapy with or without chemotherapy in our institute from 2012 to 2016 using IMRT. This is a single institutional retrospective study. Thirty-one consecutive patients of locally advanced NPC treated with definitive chemoradiation using the IMRT technique between 2012 to 2016 were evaluated. The survival was analyzed using Kaplan–Meir method and their relations with various clinicopathologic parameters were compared. After a median follow-up time of 36 months, the 5-year overall survival (OS) and disease-free survival (DFS) was 47.3% and 26.1% respectively. The younger patients of < 50 years had improved OS (p = 0.05). Patients of stage IVA had inferior 5-year OS (p = 0.1) and 5-years DFS (0.02) than those of stage III. The patients who received neoadjuvant chemotherapy showed improved DFS at 5 years (p = 0.09). The treatment-related toxicities were within acceptable limit. This retrospective analysis has reported outcomes of locally advanced NPC patients treated with IMRT with concurrent chemotherapy when IMRT was first introduced in our institute. This is the first of its kind from the Northeastern region of India.
Keywords: Nasopharyngeal cancer, IMRT, Chemotherapy, Northeast india
Introduction
Nasopharyngeal Cancer (NPC) is relatively a rare malignancy in most of the world except South East Asia, Southern China, and North Africa [1]. According to GLOBOCAN 2018 data, the number of new cases of nasopharyngeal cancers was found to be 129,079, and the number of deaths related to it is 72,987 [2]. Nasopharyngeal cancers have distinct epidemiology and may be caused by several factors involving genetic variants, environmental factors, and Epstein Barr virus (EBV) infection [3].
The disease is common among the Chinese population with an age-adjusted rate (AAR) of 30/100,000 for males and 13/100,000 for females [4]. In India also, the incidence of the disease is low except in the Northeastern part of the country [5]. Among the nine population-based cancer registries (PBCR) of Northeastern India, eight states have a high age-adjusted ratio (AAR) of NPC with the highest in the state of Nagaland (15.2/100,000 population) [6]. The district-wise distribution (population scattered over various districts within a State) of the AARs of NPC in Kohima district in Nagaland state is 19.4/100 000 as per the PBCR report [7].
Radiotherapy in early-stage lesions and chemoradiotherapy in advanced disease is the standard of treatment in nasopharyngeal carcinomas. In the era of conventional radiotherapy, the two-dimensional conventional radiotherapy (2DRT) for nasopharyngeal cancers had led to severe acute and late toxicities because of its limitation in conformality thus impairing the quality of life. Moreover, 2DRT also led to increased incidences of loco-regional relapses. The 5-year local–regional failure rate of 15.0%–19.1% has been reported in analyses done in the 1990s [8–10].
2DRT has now been replaced by IMRT which is a breakthrough in the treatment of nasopharyngeal carcinomas. IMRT allows a conformal dose distribution to the target volume and thus minimizing the dose to the organs at risk. IMRT has been shown to cause reduced acute and late toxicities in all head and neck cancers including nasopharyngeal cancers. Currently, IMRT is the most widely used technique for the treatment of NPC and it has reduced the 5-year locoregional failure rates to 7·4% [11]. A meta-analysis reviewing more than 3000 participants reported significantly improved 5-year OS [Odd Ratio 1·51 (95% CI 1·23–1·87)] with IMRT compared with 2DRT or three-dimensional conformal radiotherapy (3DCRT), along with a significant reduction of radiation-induced toxicities [12].
IMRT in the North-East region of India was first started in our institute in 2012. From 2012 to 2016, this single linear accelerator with IMRT facility was available to cater the entire region where IMRT treatment of all subsites was performed. This study is an attempt to assess the treatment outcomes, prognostic factors with the assessment of treatment-related toxicities-both acute and late in patients of locally advanced NPC who had been treated with definitive radiotherapy with concurrent chemotherapy in our institute from 2012 to 2016 using IMRT.
Materials and Methods
This hospital-based retrospective study was conducted in compliance with the policy of our institution after approval by the institutional ethical committee.
Patient Selection
Between January 2012 and December 2016, 31 consecutive newly diagnosed, non-metastatic locally advanced NPC patients treated with IMRT at our center with radical intent were included in the study. All patient and treatment-related data were retrieved from the record of hospital files.
Diagnostic Workup
Pretreatment evaluation included a complete medical history, physical examination, hematological and biochemical profiles. Diagnostic and staging investigations included nasopharyngoscopy with biopsy, direct laryngoscopy, upper gastrointestinal endoscopy, contrast-enhanced computed tomography (CECT) scan of the neck, thorax, and abdomen, and magnetic resonance imaging (MRI) of Nasopharynx including the brain. None of the patients underwent a positron emission tomography (PET-CT) scan as the facility was not available in the entire northeastern region during the given period. The pre-radiotherapy dental check-up was carried out for all patients and dental extraction, if deemed necessary, was performed. Patients were staged as per the American Joint Committee on Cancer/Union for International Cancer Control staging system 2010 (AJCC/UICC2010).
Radiotherapy
All the patients were treated with IMRT with radical intent. The patients were immobilized in the supine position using thermoplastic head-neck-shoulder mould. Intravenous contrast-enhanced CT images were acquired using the Siemens Somatom CT simulator with 3-mm slice spacing from the cranial vertex to 2 cm below the clavicle head as the lower limit.
The gross tumor volume (GTV) included the primary nasopharyngeal disease (GTVp) as evident in nasopharyngoscopy and imaging studies and the gross cervical and retropharyngeal nodes (GTVn). In patients receiving neoadjuvant chemotherapy (NACT), the GTV was based on pre-chemotherapy disease volumes. High-risk clinical tumor volume (CTVp1) included GTV with 0.5 cm margins to encompass the high-risk sites of microscopic extension however, the margin was minimized to 2 to 3 mm when CTV abutted the critical structures. The low-risk CTV (CTVp2) was created by adding a 5 mm margin to the CTVp1 and including the parapharyngeal space, the posterior nasal cavity, the pterygoid fossae, the skull base, inferior part of the ethmoid sinus. For T3 and T4 diseases, the ipsilateral cavernous sinus, whole of sphenoid sinus, and clivus were also included in the CTVp2. The CTV of the neck included the gross nodes with 0.5 cm margin and levels Ib, II, III, IV, V, and retropharyngeal nodal regions. The level Ib station was omitted from the neck CTV in case of node-negative cases. An additional 5 mm margin was added around the CTV to create the planning target volume (PTV).
Dose prescriptions were 66-70 Gy in 33–35 fractions to high-risk PTV and 60 Gy in 30 fractions to low-risk PTV. Normal tissue tolerance of radiation dose was respected as per the Quantitative Analyses of Normal Tissue Effects in the Clinic (QUANTEC) data. IMRT planning was performed for all patients using the XiO treatment planning system (TPS). IMRT was delivered with 6-MV X-ray beams modulated by use of a step and shoot technique with Elekta Synergy (Elekta AB, Stockholm, Sweden) linear accelerator. Treatment setup verification was done with image-guided therapy with either daily kV imaging or daily CT verification. Radiotherapy was delivered five fractions in a week.
Chemotherapy
Nineteen patients received neoadjuvant chemotherapy which consisted of two or three cycles. The patients with bulky primary (T3/T4) or nodal disease (N1/N2) received neoadjuvant chemotherapy. The regimens were Docetaxel + Cisplatin and only one patient received three agent chemotherapy with Docetaxel + Cisplatin + 5 Fluorouracil. Out of the 31 patients, 29 had received weekly concurrent chemotherapy ranging from four to seven cycles. The chemotherapy regimens consisted of single-agent Cisplatin or Paclitaxel and Carboplatin.
Follow-up and Assessment
During the radiotherapy treatment, patients were assessed weekly by a radiation oncologist to evaluate and document acute toxicity [using Common Terminology Criteria for Adverse Events (CTCAE) v4.0]. After IMRT completion, the patients were subsequently followed up every 2 monthly for 1 year, every 3 months through the first 3 years, then annually. During every follow-up, a complete physical examination and nasopharyngoscopy, CECT of the neck was carried out. MRI was ordered as clinically indicated.
Statistical Analysis
Baseline variables were depicted as number (percentage) and median. The follow-up period was measured from the day of completion of treatment to the day of the last clinic visit before analysis. Death from any cause was considered an event for the calculation of overall survival (OS) and was measured from the date of histologic diagnosis to the date of last visit or death. The persistence of disease, recurrence, and death due to disease or treatment-related events were the endpoints for disease-free survival (DFS). Kaplan–Meir method was used to evaluate the survival rate, and the log-rank test was used to compare the survival among groups. p < 0.05 was considered as statistically significant at 95% confidence interval (CI). The log-rank test was used for univariate analyses, and the Cox proportional hazard model was used for multivariate prognostic analyses. All the data were analyzed using IBM SPSS Statistics for Windows, version 21 (IBM Corp., Armonk, N.Y., USA).
Results
Baseline Patient Characteristics
Baseline patient and tumor characteristics are shown in Table 1. The median age of the study patients was 44 years (21–68 years). Twenty-two (71%) patients were male and nine (29%) were females. The most common histologic type was non-keratinizing undifferentiated carcinoma, (NKUC), (45.2%) followed by non-keratinizing differentiated carcinoma (NKDC) and keratinizing squamous cell carcinoma (KSCC) contributing 35.40% and 19.40% respectively. The majority of patients (80.6%) had nodal involvement; 16 patients (51.6%) had N2 disease at presentation. The most common T-stage was T3, 18 patients (58.1%). In the study cohort; 20 patients (64.50%) had clinical stage III and 11 patients (35.40%) had clinical stage IVA.
Table 1.
Patient characteristics
| Patient number (n = 31) | Percentage | |
|---|---|---|
| Gender | ||
| Male | 22 | 71% |
| Female | 9 | 29% |
| Age | ||
| < 50 | 22 | 71% |
| ≥ 50 | 9 | 29% |
| Histology | ||
| KSCC | 6 | 19.40% |
| NKDC | 11 | 35.40% |
| NKUC | 14 | 45.20% |
| T-classification | ||
| T2 | 5 | 16.10% |
| T3 | 18 | 58.10% |
| T4 | 8 | 25.80% |
| Stage | ||
| III | 20 | 64.50% |
| IV A | 11 | 35.40% |
| NACT | ||
| Yes | 19 | 61.30% |
| No | 12 | 38.70% |
| RT dose | ||
| ≥ 66 Gy | 22 | 71% |
| < 66 Gy | 9 | 29% |
| RT duration | ||
| ≤ 49 day | 16 | 51.60% |
| > 50 day | 15 | 48.40% |
| Concurrent chemotherapy regimen | ||
| Cisplatin | 25 | 80.60% |
| Carbopltin + paclitaxel | 6 | 19.40% |
Treatment Characteristics
All the study patients were treated with definitive radiotherapy using IMRT with or without concurrent chemotherapy. Nineteen patients (61.30%) received NACT ranging from two to three cycles. The regimens were Docetaxel + Cisplatin or Docetaxel + Cisplatin + 5 Fluorouracil. All the study patients received weekly concurrent chemotherapy ranging from two to seven cycles. The most common regimen for concurrent chemotherapy was single-agent inj Cisplatin 40 mg/m2 weekly. The median radiotherapy dose was 68 Gy (59.4 Gy–70 Gy) and the median duration of radiotherapy was 49 days (43–63 days).
Treatment Outcome
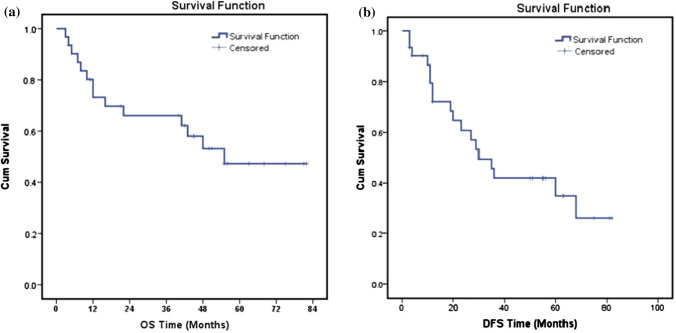
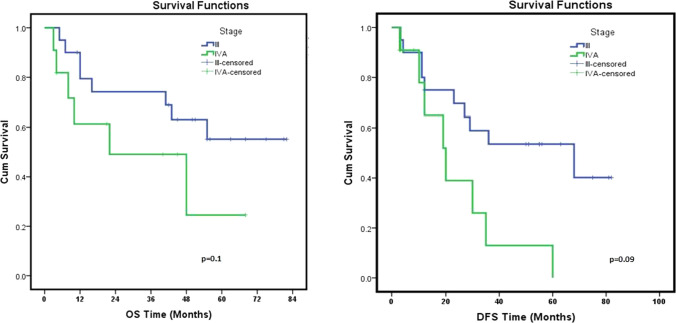
After a median follow-up period of 36.0 months (range 2–72), the three-year and five-year OS of the study patients was 66.1% and 47.3% respectively (Fig. 1a). The three-year and five-year DFS of the patients were 41.3% and 26.1% respectively (Fig. 1b). Potential prognostic factors including gender, age, histological classification, T-classification, clinical staging, radiotherapy dose, duration, use of chemotherapy in neoadjuvant and concurrent settings, chemotherapy drugs were analyzed by the log-rank test for univariate analysis (Table 2) and cox proportional hazards analysis (Table 3). The 22 patients (71%) with age less than 50 years had improved 5-years OS, 64.4% vs 22.2%. However, the statistical difference reached borderline significance only (p = 0.05, Fig. 2). Patients of stage IVA had inferior 5-year OS (p = 0.1) and 5-years DFS (0.02) than those of stage III (Fig. 3). Patients with keratinizing squamous carcinoma had a higher overall survival rate. Although the majority of the patients were diagnosed with non-keratinizing undifferentiated carcinoma, this condition was associated with a relatively poor disease-free survival rate.
Fig. 1.
Kaplan–Meier plot of a overall survival, b disease-free survival
Table 2.
Univariate analysis univariate analysis of potential prognostic factors
| Prognostic factors | N = 31 (percentage) | 5-year OS | p Value | 5 Year-DFS | p Value |
|---|---|---|---|---|---|
| Gender | |||||
| Male | 22 (71%) | 46.3% | 0.8 | 29.1% | 0.354 |
| Female | 9 (29%) | 55.6% | 25.4% | ||
| Age | |||||
| < 50 | 22 (71%) | 61.4% | 0.05 | 24.4% | 0.97 |
| ≥ 50 | 9 (29%) | 22.2% | – | ||
| Histology | |||||
| KSCC | 6 (19.4%) | 80% | 0.08 | – | 0.983 |
| NKDC | 11 (35.4%) | 27% | 40.9% | ||
| NKUC | 14 (45.2%) | 57.1% | 32.1% | ||
| T-classification | |||||
| T2 | 5 (16.1%) | 53.3% | 0.518 | – | 0.078 |
| T3 | 18 (58.1%) | 53.8% | 36.7% | ||
| T4 | 8 (25.8%) | 0% | 0% | ||
| Stage | |||||
| III | 20 (64.5%) | 55.2% | 0.133 | 40.2% | 0.02* |
| IV A | 11(35.4%) | 24.5% | 0% | ||
| NACT | |||||
| Yes | 19 (61.3%) | 56.5% | 0.169 | 33.7% | 0.094 |
| No | 12 (38.7%) | 33.3% | 21.8% | ||
| RT dose | |||||
| ≥ 66 Gy | 22 (71%) | 54.7% | 0.441 | 23.1% | 0.896 |
| < 66 Gy | 9 (29%) | 33.3% | – | ||
| Concurrent chemotherapy drug | |||||
| Cisplatin | 25 (80.6%) | 53% | 0.232 | 29.2% | 0.26 |
| Carboplatin paclitaxel | 6 (19.4%) | 22.2% | 20% | ||
Table 3.
Cox proportional hazards analysis
| Parameters | HR | 95% CI | p Value | |
|---|---|---|---|---|
| Age | 2.737 | 0.952–7.862 | 0.062 | |
| Gender | 1.141 | 0.356–3.661 | 0.825 | |
| Histology | 1.827 | 0.21–15.71 | 0.583 | |
| Stage | III vs IV | 2.23 | 0.76–6.54 | 0.144 |
| NACT | Yes, vs No | 2.628 | 0.342–20.172 | 0.353 |
| RT duration (days) | ≤ 49 vs > 50 | 0.715 | 0.239–2.142 | 0.549 |
| RT Dose (Gy) | ≥ 66 vs < 66 Gy | 1.532 | 0.512–4.582 | 0.446 |
| Chemo regimen | Cisplatin vs other | 2.001 | 0.625–6.411 | 0.243 |
Fig. 2.
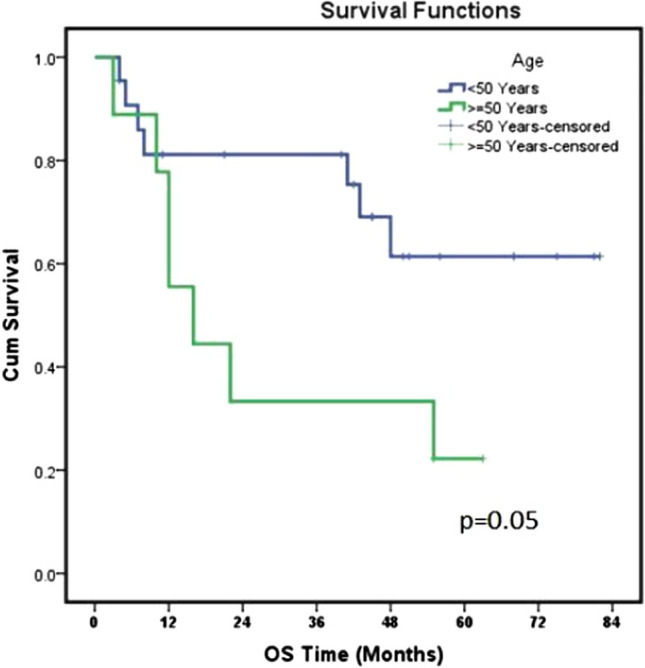
Comparison of OS among two age groups
Fig. 3.
Comparison of OS and DFS among two-stage groups
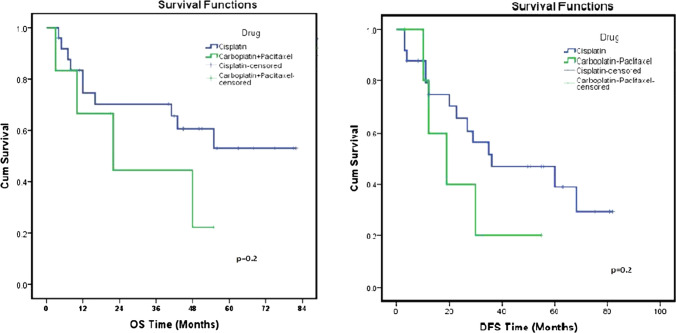
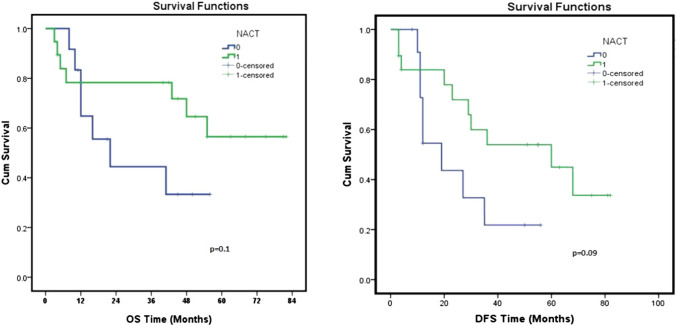
All the patients in the study cohort received concurrent chemotherapy and the most common chemotherapy drug was inj Cisplatin. As compared to the patients receiving concurrent chemotherapy with other drugs, those with Cisplatin had improved 5 years OS (53% vs 22.2%, p = 0.2) and DFS (29.2% vs 20%, p = 0.2), Fig. 4. Similarly, the use of NACT improved the treatment outcome. Nineteen patients (61.30%) in the study cohort receiving NACT had improved OS and DFS at 5 years when compared with those treated without NACT (Fig. 5).
Fig. 4.
Comparison of OS and DFS among the groups receiving concurrent cisplatin vs other drugs
Fig. 5.
Comparison of OS and DFS in terms of use of NACT
Radiotherapy dose and duration are important prognostic factors of NPC. The patients receiving RT dose of 66 Gy or more to the gross primary and nodal disease had improved OS at 5 years (54.7% Vs 33.3%, p = 0.4, Fig. 6).
Fig. 6.
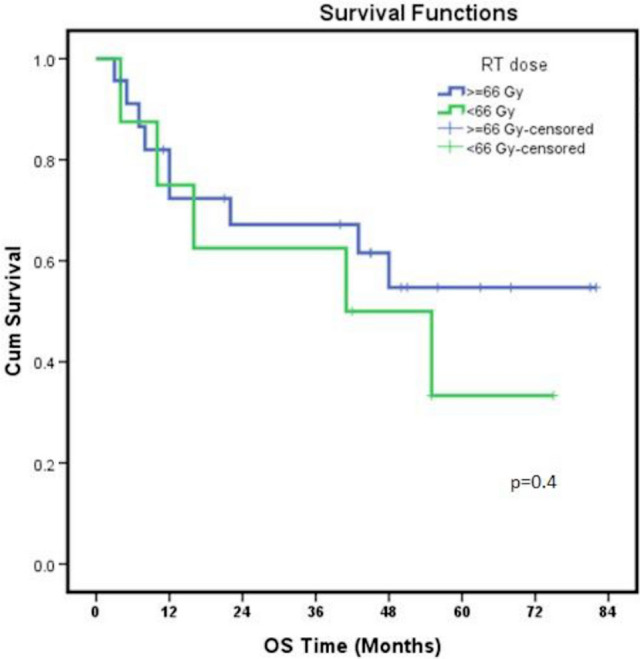
Comparison of OS among two groups in terms of total RT dose
Treatment-Related Toxicity
Acute and late treatment-related toxicities were recorded as per CTCAE version 4.0 and are depicted in Table 4. Eight (25.8%) patients developed grade II and three (9.6%) patients developed grade III oral mucositis during the radiotherapy treatment. Grade II dermatitis was recorded in 12 patients (38.7%). None patients had developed grade IV oral mucositis or dermatitis. Anemia was the most common hematological toxicity recorded during radiotherapy treatment. Twelve patients (38.70%) developed grade I, nine patients (29%) developed grade II and five patients (16.10%) developed grade III anemia. Anemia was managed conservatively with packed cell transfusion. Three patients (9.70%) experienced grade III neutropenia and thrombocytopenia. Three patients (9.70%) developed grade III diarrhea during the treatment period requiring hospital admission.
Table 4.
Treatment related toxicities
| Acute toxicity | Late toxicity | ||
|---|---|---|---|
| Mucositis | Subcutaneous fibrosis | ||
| Grade 1 | 20 (64.5%) | Grade 1 | 19(61.3%) |
| Grade 2 | 8 (25.8%) | Grade 2 | 4(12.9%) |
| Grade3 | 3 (9.6%) | Grade 3 | 0 |
| Dermatitis | Xerostomia | ||
| Grade 1 | 19 (61.3%) | Grade 1 | 15(48.4%) |
| Grade 2 | 12 (38.7%) | Grade 2 | 6(19.4%) |
| Grade 3 | 0 | Grade 3 | 1(3.2%) |
| Odynophagia | Late dysphagia | ||
| Grade 1 | 22 (71%) | Garde 1 | 13(41.9%) |
| Grade 2 | 9 (29%) | Grade 2 | 4(12.9%) |
| Neutropenia | Hearing impairment | ||
| Grade 1 | 10 (32.3%) | Grade 1 | 5(16.1%) |
| Grade 2 | 7 (22.6%) | ||
| Grade 3 | 3 (9.7%) | ||
| Anemia | |||
| Grade 1 | 12 (38.7%) | ||
| Grade 2 | 9 (29%) | ||
| Grade 3 | 5 (16.1%) | ||
| Thrombocytopenia | |||
| Grade 2 | 7 (22.6%) | ||
| Grade 3 | 3 (9.7%) | ||
| Diarrhea | |||
| Grade 2 | 5 (16.1%) | ||
| Grade 3 | 3 (9.6%) | ||
| Vomiting | |||
| Grade 2 | 5 (16.1%) | ||
Subcutaneous fibrosis and xerostomia were the most common late toxicity recorded. Nineteen patients (61.3%) developed grade I and four patients (12.9%) developed grade II subcutaneous fibrosis. Grade I xerostomia was experienced by 15 patients (48.40%), Grade II by six (19.4%) patients and only one patient (3.20%) developed grade III xerostomia. Five patients (16.10%) developed grade I hearing impairment during the follow-up period. Grade I late dysphagia was recorded in 13 patients (41.90%).
Discussion
The current study is an attempt to report the results of treatment of nonmetastatic locally advanced NPC treated with definitive chemoradiation using IMRT during 2012–2016 when IMRT treatment was first introduced in this part of the country. This is the first report of treatment outcomes of locally advanced NPC patients treated with IMRT from the Northeast region of India. The prognostic factors affecting the outcomes have also been analyzed.
The median age of the patients included in the present study was 44 years (21–68 years) with a male: female ratio of 2.4:1. This is comparable with that in other studies carried out in India. Laskar et al. [13] reported treatment outcome of 206 NPC patient treated during 1994–2004 and the median age the patients were 43 years (range: 18–76) with a male: female ratio of 2.6:1. Sharma et al. [14] in a clinicopathological study from Northeastern India reported the mean age of presentation of NPC patients 49.7 ± 10.7 years with a male: female ratio of 2.2:1.
In our study, it was observed that the patients of age < 50 years had improved OS at 5 years than those of age > 50 years 64.4% vs 22.2%. Laskar et al. [13] observed that younger age (< 35 years) patients had significantly better locoregional control rates and DFS. Other reports in the literature showed age as a significant prognostic factor of NPC when treated with RT or Chemo RT [15, 16].
The histology of the primary tumor was also an important prognostic factor in our study. The most common histology was non-keratinizing undifferentiated carcinoma, (45.2%) which is similar to the other study from the Northeast and other parts of India [13, 14]. Worldwide the keratinizing subtype accounts for less than 20% of cases, and is relatively rare in endemic areas; the non-keratinizing subtype constitutes most cases in endemic areas (> 95%) [17]. Although the majority of the patients in our study were diagnosed with non-keratinizing undifferentiated carcinoma, it was associated with relatively poor survival. Similar results are reported by a study from Nigeria by Aliyu et al. [18].
It is a well-established fact that the stage at presentation is an important prognostic factor. The stage IVA patients of our study cohort had statistically significantly improved DFS at 5 years than stage III patients (p = 0.02). The primary tumor size (T) is also a significant prognostic factor. The patients with T4 and T3 tumors had relatively poor survival than those with T2. Various large studies have shown that stage at presentation and T classification is an important prognostic factor of survival in NPC [19, 20].
The addition of NACT to concurrent chemoradiation is a promising approach for locally advanced NPC. NACT reduces the tumor burden which helps in RT planning with adequate sparing of normal tissues. Moreover, it reduces the risk of distance metastases in locally advanced NPC [21]. Two large multicenter phase 3 trials from Guangzhou have reported significantly improved 5-year OS [HR 0·65 (95% CI 0·43–0·98)] in locoregionally advanced nasopharyngeal carcinoma (excluding N0 disease) treated with induction docetaxel, cisplatin, and fluorouracil when added to concurrent chemoradiotherapy [22, 23].
To date, from India, there are no strong published data regarding the outcomes of various commonly used induction chemotherapy regimens before chemoradiation in patients with locally advanced NPC. However, Lokesh et al. [24] reported the results of a retrospective study from southern India that there was no significant difference between taxane-based doublet and triplet chemotherapy regimens, in terms of survival outcomes, although Grade III–IV toxicities were numerically higher with the triplet regimen.
In our study, nineteen patients (61.30%) received NACT with either Docetaxel + Cisplatin or Docetaxel + Cisplatin + 5 Fluorouracil. Although statistically not significant, the patients receiving NACT had improved OS and DFS at 5 years (p = 0.1 and 0.09 respectively).
Radiotherapy dose is an important factor in the treatment outcome of NPC patients. Studies had shown the importance of higher doses (> 66 Gy) for achieving optimal local control [25, 26]. In our study also patients receiving a total radiation dose of ≥ 66 Gy to the primary and gross nodal disease had improved OS at 5 years.
All the patients in our study cohort received concurrent chemotherapy. The most widely used regimen was inj Cisplatin 40 mg/m2 for four to seven cycles. Chan et al. [27] demonstrated good efficacy and tolerability for a regimen consisting of weekly Cisplatin (40 mg/m2). In their study, 78% of patients in the chemoradiotherapy arm received at least four cycles of Cisplatin and was associated with a statistically significant survival benefit after adjusting for age and disease stage. In our study also the majority of the patients (80.6%) received Cisplatin concurrent chemotherapy and had improved 5-year OS and DFS, although the difference is not statistically significant.
Various prognostic factors such as age, stage, radiotherapy dose, T size, the addition of NACT have shown significance in many studies and have become important criteria for the assessment of treatment outcomes, which has also been reflected in the present study.
Patients in our study had an acceptable degree of radiation-related toxicity which is shown in Table 2. Acute grade II mucositis and grade II dermatitis was experirnced by 25.8% and 38.7% of patients in our study population respectively. It is comparable with the Indian study by Kunheri et al. [28] reporting acute grade II mucositis and dermatitis by 35% and 47% of patients respectively. In the current study, 19.4% of patients developed late radiation-induced xerostomia. Wang et al. [19] reported late xerostomia in 23.2% of patients.
After a median follow-up of 36 months, the OS of our study patients at 3 and 5 years were 66.1% and 47.3% respectively. Similarly, the DFS at 3 and 5 years were 41.8% and 26.1% respectively. Our results are slightly inferior as compared to the reported outcomes in endemic regions treated with IMRT. Zhi-Qiang et al. [29] reported a 5-year OS of 78.30% for stage III-IVb patients treated with IMRT and concurrent Cisplatin-based chemotherapy. Wee et al. [30] reported 3-Year OS and DFS of 80% and 53% respectively for the stage III and IV NPC patients treated with concurrent chemoradiotherapy.
The current study has many limitations. Firstly, it is a retrospective study and the study population is heterogeneous. Further, the sample size is very small. However, we have tried to report the first experience of treating locally advanced NPC with the IMRT technique from a geographical area of relatively higher incidence but with limited resources. Therefore, further research in homogenous patients in a prospective design with a larger sample size needs to be done.
Conclusion
Radiotherapy is the primary therapeutic option for patients with NPC. The benefit of using concurrent chemotherapy for the locally advanced disease has been well documented in various published literature. The transition from 2D-RT to three-dimensional RT (3DRT), in particular IMRT, represents a major development forward in the treatment of NPC. This retrospective analysis has reported outcomes in locally advanced NPC patients treated with IMRT with concurrent chemotherapy between 2012 to 2016 when IMRT was first introduced in our institute. This is the first of its kind from the Northeastern region of India, the region which reports the highest incidence of NPC in the country.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
The study was approved by Institutional Ethics Committee; BBCI Medical Ethics Committee (Registration No: ECR/1040/Inst/AS/2018).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zheng YM, Tuppin P, Hubert A, et al. Environmental and dietary risk factors for nasopharyngeal carcinoma: a case-control study in Zangwu County, Guangxi, China. Br J Cancer. 1994;69(3):508–514. doi: 10.1038/bjc.1994.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee AW, Sez WM, Au JS, Leung SF, Leung TW, Chua DT, Zee BC, Law SC, Teo PM, Tung SY, Kwong DL, Lau WH. Treatment results for nasopharyngeal carcinoma in the modern era: the Hongkong experience. Int J Radiat Oncol Biol Phys. 2005;61:1107–1116. doi: 10.1016/j.ijrobp.2004.07.702. [DOI] [PubMed] [Google Scholar]
- 3.Wei WI, Sham JST. Nasopharyngeal carcinoma. Lancet. 2005;365:2041–2054. doi: 10.1016/S0140-6736(05)66698-6. [DOI] [PubMed] [Google Scholar]
- 4.Muir C, Waterhouse J, Mack T, eds (1987) Cancer incidence in five continents. IARC Scientific Publications, Lyon, pp 787–789
- 5.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 6.Three Year Report of PBCR 2012–2014 [Internet]. Ncdirindia.org. 2021 [cited 5 February 2021]. Available from: https://ncdirindia.org/ncrp/ALL_NCRP_REPORTS/PBCR_REPORT_2012_2014/index.htm
- 7.Kataki A, Simons M, Das A, Sharma K, Mehra N. Nasopharyngeal carcinoma in the Northeastern states of India. Chin J Cancer. 2011;30(2):106–113. doi: 10.5732/cjc.010.10607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leung TW, Tung SY, Sze WK, Wong FC, Yuen KK, Lui CM, Lo SH, Ng TY, Saiki O. Treatment results of 1070 patients with nasopharyngeal carcinoma: an analysis of survival and failure patterns. Head Neck. 2005;27:555–565. doi: 10.1002/hed.20189. [DOI] [PubMed] [Google Scholar]
- 9.Yi JL, Gao L, Huan XD, Li SY, Luo JW, Cai WM, Xiao JP, Xu GZ. Nasopharyngeal carcinoma treated by radical radiotherapy alone: ten-year experience of a single institution. Int J Radiat Oncol Biol Phys. 2006;65:161–168. doi: 10.1016/j.ijrobp.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Gregoire V, Coche E, Cosnard G, Hamoir M, Reychler H. Selection and delineation of lymph node target volumes in head and neck conformal radiotherapy. Proposal for standardizing terminology and procedure based on the surgical experience. Radiother Oncol. 2000;56:135–150. doi: 10.1016/S0167-8140(00)00202-4. [DOI] [PubMed] [Google Scholar]
- 11.Mao YP, Tang LL, Chen L, et al. Prognostic factors and failure patterns in non-metastatic nasopharyngeal carcinoma after intensity-modulated radiotherapy. Chin J Cancer. 2016;35:103. doi: 10.1186/s40880-016-0167-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang B, Mo Z, Du W, Wang Y, Liu L, Wei Y. Intensity-modulated radiation therapy versus 2D-RT or 3D-CRT for the treatment of nasopharyngeal carcinoma: a systematic review and meta-analysis. Oral Oncol. 2015;51:1041–1046. doi: 10.1016/j.oraloncology.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Laskar S, Gurram L, Gupta T, Budrukkar A, Murthy V, Agarwal J. Outcomes in nasopharyngeal carcinoma: results from a nonendemic cohort. Indian J Cancer. 2016;53:493–498. doi: 10.4103/0019-509X.204762. [DOI] [PubMed] [Google Scholar]
- 14.Sharma TD, Singh TT, Laishram RS, Sharma LD, Sunita AK, Imchen LT. Nasopharyngeal carcinoma—a clinico-pathological study in a regional cancer centre of Northeastern India. Asian Pac J Cancer Prev. 2011;12:1583–1587. [PubMed] [Google Scholar]
- 15.Shigematsu N, Ito H, Oki Y, Kawada T, Suzuki T, Takeda A, et al (1996) Prognostic factors of nasopharyngeal carcinoma treated by radiotherapy. Nihon Igaku Hoshasen Gakkai Zasshi 56:1050-5.9 [PubMed]
- 16.Cheng SH, Yen KL, Jian JJ, Tsai SY, Chu NM, Leu SY, et al. Examining prognostic factors and patterns of failure innasopharyngeal carcinoma following concomitant radiotherapy and chemotherapy: Impact on future clinical trials. Int J Radiat Oncol Biol Phys. 2001;50:717–726. doi: 10.1016/S0360-3016(01)01509-7. [DOI] [PubMed] [Google Scholar]
- 17.Chen Y, Chan A, Le Q, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. 2021;394:64–80. doi: 10.1016/S0140-6736(19)30956-0. [DOI] [PubMed] [Google Scholar]
- 18.Aliyu U, Folasire A, Ntekim A. Treatment outcome of patients with nasopharyngeal carcinoma in Nigeria: an institutional experience. Precis Radiat Oncol. 2018;2(3):68–75. doi: 10.1002/pro6.44. [DOI] [Google Scholar]
- 19.Wang W, Feng M, Fan Z, Li J, Lang J. Clinical outcomes and prognostic factors of 695 nasopharyngeal carcinoma patients treated with intensity-modulated radiotherapy. Biomed Res Int. 2014;2014:1–10. doi: 10.1155/2014/408514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Au K, Ngan R, Ng A, Poon D, Ng W, Yuen K, et al. Treatment outcomes of nasopharyngeal carcinoma in modern era after intensity modulated radiotherapy (IMRT) in Hong Kong: a report of 3328 patients (HKNPCSG 1301 study) Oral Oncol. 2018;77:16–21. doi: 10.1016/j.oraloncology.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 21.Lee AW, Lau KY, Hung WM, Ng WT, Lee MC, Choi CW, et al. Potential improvement of tumor control probabilityby induction chemotherapy for advanced nasopharyngeal carcinoma. Radiother Oncol. 2008;87:204–210. doi: 10.1016/j.radonc.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 22.Sun Y, Li WF, Chen NY, et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. Lancet Oncol. 2016;17:1509–1520. doi: 10.1016/S1470-2045(16)30410-7. [DOI] [PubMed] [Google Scholar]
- 23.Li WF, Chen NY, Zhang N, et al. Concurrent chemoradiotherapy with/without induction chemotherapy in locoregionally advanced nasopharyngeal carcinoma: long-term results of phase 3 randomized controlled trial. Int J Cancer. 2019;45:295–305. doi: 10.1002/ijc.32099. [DOI] [PubMed] [Google Scholar]
- 24.Lokesh KN, Chaudhuri T, Lakshmaiah KC, Babu KG, Lokanatha D, Jacob LA, Suresh Babu MC, Rudresha AH, Rajeev LK. Induction chemotherapy in locoregionally advanced nasopharyngeal carcinoma in adults: Results from a nonendemic region. Indian J Cancer. 2018;55:257–266. doi: 10.4103/ijc.IJC_115_18. [DOI] [PubMed] [Google Scholar]
- 25.Marks JE, Bedwinek JM, Lee F, Purdy JA, Perez CA. Dose-response analysis for nasopharyngeal carcinoma: anhistorical perspective. Cancer. 1982;50:1042–1050. doi: 10.1002/1097-0142(19820915)50:6<1042::AID-CNCR2820500604>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 26.Vikram B, Strong EW, Manolatos S, Mishra UB. Improved survival in carcinoma of the nasopharynx. Head Neck Surg. 1984;7:123–128. doi: 10.1002/hed.2890070206. [DOI] [PubMed] [Google Scholar]
- 27.Chan AT, Leung SF, Ngan RK, et al. Overall survival after concurrent cisplatin-radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma. J Natl Cancer Inst. 2005;97(7):536–539. doi: 10.1093/jnci/dji084. [DOI] [PubMed] [Google Scholar]
- 28.Kunheri B, Agarwal G, Sunil PS, Anoop R, Pushpaja KU. Nasopharyngeal carcinoma: experience and treatment outcome with radical conformal radiotherapy from a tertiary care centre in India. Indian J Cancer. 2017;54:502–507. doi: 10.4103/ijc.IJC_287_17. [DOI] [PubMed] [Google Scholar]
- 29.Zhi-Qiang W, Qi M, Ji-Bin L, Rui Y, You-Ping L, Rui S et al (2019) The long-term survival of patients with III-IVb stage nasopharyngeal carcinoma treated with IMRT with or without Nimotuzumab: a propensity score-matched analysis. BMC Cancer 19(1) [DOI] [PMC free article] [PubMed]
- 30.Wee J, Tan E, Tai B, Wong H, Leong S, Tan T, et al. Randomized trial of radiotherapy versus concurrent chemoradiotherapy followed by adjuvant chemotherapy in patients with American Joint Committee on cancer/international union against cancer stage III and IV nasopharyngeal cancer of the endemic variety. J Clin Oncol. 2005;23(27):6730–6738. doi: 10.1200/JCO.2005.16.790. [DOI] [PubMed] [Google Scholar]