Abstract
Objectives: The purpose of this study was to assess the value of the diffusion MRI with the non-echoplanar imaging (Non-EPI) technique for follow-up the post-operative patients to detect residual cholesteatomas. Study design: This prospective study was performed on 40 patients. All patients were at least one year after Canal Wall Up mastoidectomy surgery for cholesteatoma and scheduled for a second-look surgery. Patients and Methods: This prospective study was performed on 40 patients. All patients were subjected to Canal Wall Up surgery and planned for the second-look operation. After one year as removal of choleasteatoma is uncertain in first surgery. The study done at Tertiary referral centers (Ain shams, Mansoura, and Minia university hospitals), non-echoplanar diffusion MRI (NEP-DWI) technique for follow-up the post-operative patients to detect residual cholesteatomas, then second look surgery done 2 weeks after MRI. Results: Forty patients underwent MRI with Non-echoplanar diffusion-weighted imaging (NEP-DWI). Twenty-six patients had positive MRI results with the remaining 14 patients had negative results. These results were compared to operative findings. All positive MRI cases showed positive intra-operative findings. Ten of negative MRI cases showed negative intra-operative findings. Four of DWI-negative cases showed small cholesteatomas. Conclusion: The use of NEP-DWI is a valuable tool in detecting residual cholesteatoma that could replace the second look surgery in many cases.
Keywords: Cholesteatoma, Diffusion-weighted image, Magnetic resonance imaging, Non-echoplanar DWI, Second-look surgery
Background
Cholesteatomas is a destructive erosive growth of keratinizing squamous epithelium in the temporal bone [1]. It has erosive potential, mostly due to the inflammatory response, which activates osteoclastic activity [2, 3].
Cholesteatomas usually erode middle ear structures like scutum and middle ear ossicles. With progression, the cholesteatoma can invade other important structures such as the labyrinth and tegmen tympani [4, 5]. This can result in serious intracranial life-threatening complications. Surgery is the only definitive therapeutic option, aiming primarily to eradicate the disease and secondarily attempting to maintain the anatomy and preserve the function as possible [6].
There are two basic types of surgery for cholesteatoma, canal wall up (CWU) and canal wall down (CWD) surgeries. Most surgeons tend to favor the less invasive Canal Wall Up surgery for treating cholesteatomas; however, it carries higher rates of recurrence and residual disease [7]. In the postoperative follow-up, the recurring or residual disease is very difficult to detect clinically. Computed tomography (CT) is also not specific in this situation. Second-look surgery is, therefore, usually needed [8].
Many studies reported that magnetic resonance imaging (MRI), especially diffusion-weighted imaging (DWI) can play a crucial role in differentiation between residual cholesteatoma and post mastoidectomy granulation tissue, and thus obviates the need for unnecessary surgery [6, 7, 9].
Several types of DWI techniques have been applied, which can be broadly divided into non-EPI and echoplanar imaging techniques [6].
The purpose of this study was to assess the value of the non-echoplanar diffusion MRI (NEP-DWI) technique for follow-up the post-operative patients to detect residual cholesteatomas.
Patients and Methods
Study Design
This prospective study was performed on 43 patients. All patients were subjected to Canal Wall Up surgery and planned for the second-look operation. After one year as removal of choleasteatoma is uncertain in first surgery.
This study was approved by the local institutional review board. Written consent was obtained from each patient.
Sample size: 40 patients were examined over two years, from August 2017 to September 2019.
Inclusion criteria: Patients underwent CWU surgery and were planned for second-look surgery after one year.
Exclusion criteria: Any patient with contraindication to MRI (e.g. pacemaker) was excluded.
Protocol: After describing the aim, protocol, and risk–benefit ratio of the study, informed consent was obtained. Cleaning the wax and secretions of the external ear was done before the exam to avoid false-positive results. In each case, history has been taken with checking for contraindication to MRI imaging. Previous CT (if available) is obtained. Patients lie supine and were asked not to move during the exam.
MRI was performed using a Philips scanner (Intera, 1.5Tesla, Philips Healthcare, Best, The netherlands). An 8-channel sensitivity encoding head coil was used. The following sequences were done:
Axial non-echo-planar multi-shot turbo spin echo diffusion-weighted imaging: 3 mm thick axial slices, TE: 75 ms, TR: 2460 ms, matrix: 128 × 128, B factors: 0 and 800 s/mm2, FOV: 200 × 150 mm.
Coronal non-echo-planar turbo spin echo diffusion-weighted imaging: 3 mm slice thickness, TE: 75 ms, TR: 2460 ms, matrix: 128 × 128, B factors: 0 and 800 s/mm2, FOV: 200 × 150 mm.
ADC maps were reconstructed after the scan using the diffusion scan raw data. ADC values were not routinely calculated.
Axial T2-weighted Turbo spin-echo: TR/TE, 5000/120 ms; matrix, 320 × 320; section thickness 3 mm; field of view, 180 × 180 mm.
Coronal T2-weighted turbo spin echo: TR/TE, 5000/120 ms; matrix, 320 × 320; section thickness 3 mm; field of view, 180 × 180 mm.
Axial T1-weighted coronal spin-echo (TSE): TR/TE, 550/10 ms; matrix, 256 × 256; section thickness 3 mm; field of view, 180 × 180 mm.
Image Analysis
MRI images were reviewed by two independent expert neuroradiologists (about 7 and 10 years of experience in neuroimaging). A final decision was made based on mutual consultation. The study was considered positive for cholesteatoma if there is high signal intensity on T2WI, with high signal intensity on DWI on b value = 0 s/mm2 images, with persistence or increases of signal on high b value (800 s/mm2) images and low signal in ADC maps. Calculation of the ADC value was not performed.
All patients underwent subsequent surgical exploration within 2 weeks following the MRI.
Statistical Analysis
The data collected was analyzed and findings were obtained using the statistical package for social science (SPSS) windows package version 26.0 (IBM, Armonk, New York). A comparison of intra-operative data with data generated by MRI was made. Sensitivity, specificity, negative and positive predictive values were calculated by using operative findings as standard of reference.
Results
Forty-three patients were sent for DW-MRI examination. Three patients were removed from the study, one patient because of having a metallic implant that was not MR compatible (cardiac pacemaker) and 2 patients were claustrophobic. So, the total number of patients included in our study was 40 patients, who were exposed to MRI.
Of the 40 patients, 27 (67.5%) were male patients and 13 (32.5%) were females. The mean age of the patients was 26.6 years (range, 12–60 years).
Twenty-five (62.5%) of the patients had a right-sided lesion while 15 (37.5%) patients had the lesion on the left side.
Among the 40 patients, 26 patients (65%) had positive DW-MRI results with the remaining 14 patients (35%) had negative results. These results were compared to intra-operative findings. Positive operative findings were found in 30 (75%) patients. Negative intra-operative findings were found in 10 (25%) patients, where only granulation tissue was found, with no evidence of cholesteatoma. Four cases were negative by DWI study but revealed positive results intra-operatively (false-negative cases). Three of these four cases show cholesteatoma measuring 3 mm or less in size intra-operatively and one case show retraction pocket without keratin content.
The sensitivity of the DW-MRI in our study was 86.7%, the specificity 100%, the positive predictive value 100%, and the negative predictive value 71.4%.
Figures from 1, 2, 3, 4, 5 and 6 show MRI- DWI of some cases.
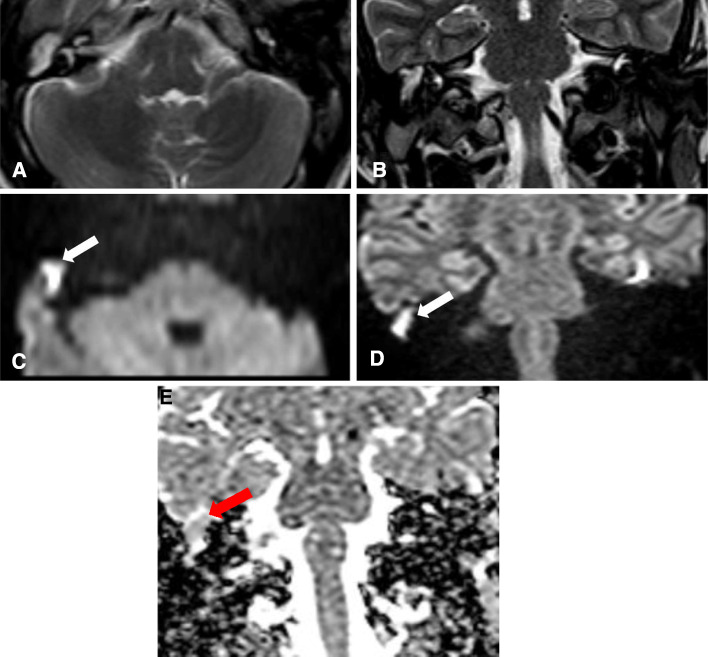
Fig. 1.
a Axial & b Coronal T2WI show an area of abnormal high signal within the right middle ear cavity measuring 11 mm in largest dimension. Axial (c) and Coronal (d) DWIs: show a large area of diffusion restriction within the right middle ear cavity (white arrow). Coronal ADC map (e): shows a corresponding area of low signal within the right middle ear cavity (red arrow)
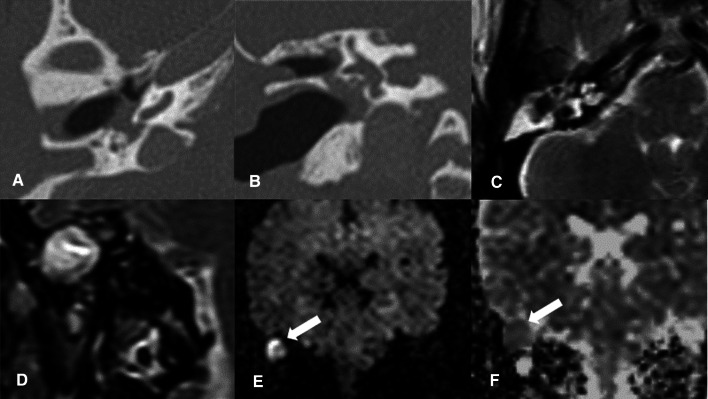
Fig. 2.
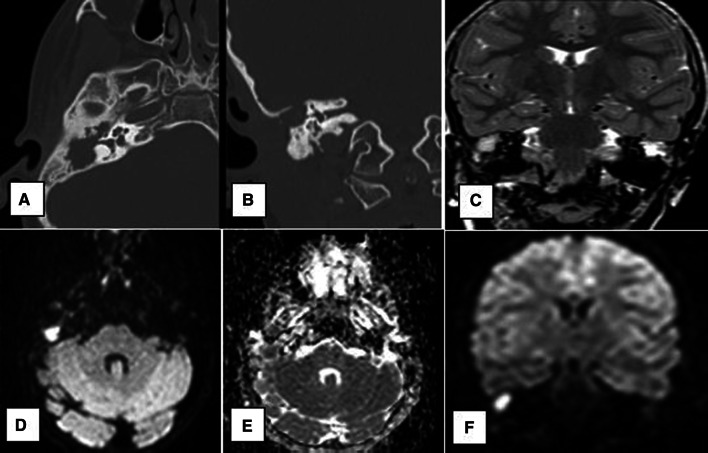
a Axial & b Coronal CT images: show non-specific soft tissue opacification of the right mastoid region as well as the epitympanum and mesotympanum with total ossicular resorption and extensive osseous erosions. c Axial & d Coronal T2 images: show diffuse T2 hyperintensity within the right mastoid and middle ear regions. e Coronal DWIs and f coronal ADC map: show a large area of bright DWI signal within the right middle ear cavity with corresponding low signal on ADC map, confirming true diffusion restriction (white arrow)
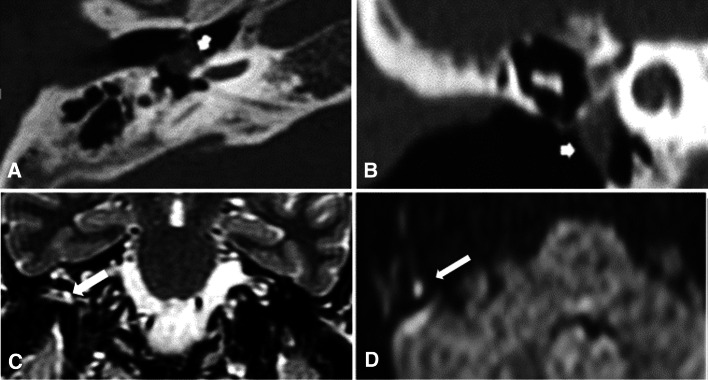
Fig. 3.
a Axial and b coronal CT images: show non-specific small mesotympanic soft tissue density (short arrow). c Coronal T2 image: shows a small area of T2 hyperintensity within the right middle ear region. d Axial DWIs: shows a small area of bright DWI signal within the right middle ear cavity
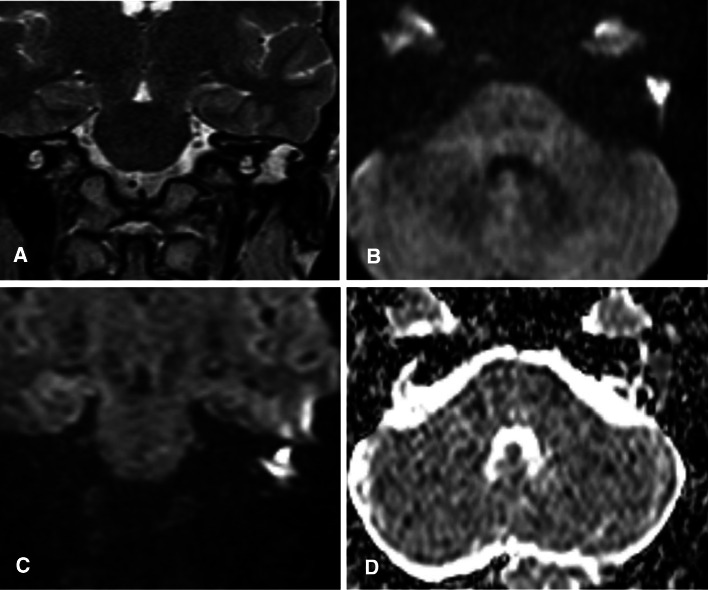
Fig. 4.
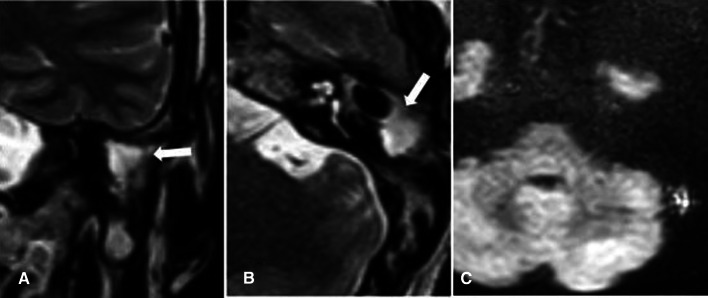
a Coronal T2 images: show diffuse T2 hyperintensity within the left middle ear cavity. b Axial and c coronal DWIs: show area of diffusion restriction within the left middle ear cavity measuring 9 mm. d Axial ADC map revealed a corresponding area of low signal within the left middle ear cavity, confirming diffusion restriction
Fig. 5.
a Axial and b coronal CT images: show non-specific soft tissue density within right middle ear. c Coronal T2 image: shows an area of T2 hyperintensity within the right middle ear region. d Axial DWIs, e Axial ADC map and f Coronal DWIs: show a small area of bright DWI signal within the right middle ear cavity with corresponding low signal in ADC map
Fig. 6.
a Coronal and b Axial T2 WI: show diffuse T2 hyperintensity within the left middle ear cavity. c Axial DWI: show no evidence of diffusion restriction. However, a small cholesteatoma was discovered intra-operatively within the granulation tissue measuring 2 mm
Discussion
Cholesteatoma is an aggressive epithelial lesion that may result in hearing loss, temporal bone destruction, and serious intracranial complications [10]. Surgery is the definitive treatment for cholesteatoma. However, there is a high incidence of residual cholesteatoma. Second-look surgery is usually done between 9 and 18 months after the primary surgery to eradicate any residual or recurrent lesion [11, 12].
The diagnostic accuracy of several imaging modalities was evaluated in many published literatures, trying to find a non-invasive radiological alternative to the elective "second-look" operation. High-resolution Computed tomography (HRCT) has a high negative predictive value (NPV) only when the post-operative cavity is completely clear without any abnormal soft tissue inside [13]. CT has poor specificity as it cannot differentiate between cholesteatoma tissue and other post-operative soft tissue pathologies such as granulation tissue, inflammatory process, or fibrotic tissue [14].
Various MRI techniques were also evaluated for this purpose. Delayed post-contrast MRI was evaluated as a tool to differentiate granulation tissue from residual cholesteatoma. This technique detect larger cholesteatomas but often failed to detect small lesions [15].
Many data have been published during the last decade supporting the use of DWI for the evaluation of postoperative residual/recurrent cholesteatoma. Cholesteatomas appear of high signal on DWI images. The reason is not fully understood, but may be explained by a combination of T2 shine-through and diffusion restriction effects [16].
DWI is more practical than delayed contrast-enhanced MRI; with a shorter examination time lack of IV contrast injection [6]. Single-shot (SS) echoplanar diffusion-weighted imaging (EPI-DWI) is the widely available standard diffusion technique, yet it is prone to susceptibility artifacts, chemical shift, and geometric distortion [17]. These artifacts may mask small cholesteatoma [18]. Moreover, EPI-DWI is of low spatial resolution and requires thick sections, limiting its ability to detect cholesteatoma below 5 mm in size [19, 20].
More recently, different non-echoplanar DWI (NEP-DWI) techniques have been developed by different MR imaging vendors, such as single-shot turbo spin-echo DWI, multi-shot turbo-spin echo, half-Fourier acquisition single-shot turbo spin-echo (HASTE) (Siemens Medical Solutions, Erlangen, Germany), PROPELLER DWI (GE medical systems, USA), and BLADE DWI (Siemens Medical Solutions). Such sequences reduce most susceptibility artifacts and permit thinner sections, resulting in improved sensitivity to 90–100% for lesions as small as 2 mm [6, 14].
These techniques allow for a better selection of cases requiring surgical revision and could help to avoid unnecessary surgical exploration [3].
The current prospective study supports many previous studies that NEP-DWI can confirm or rule out the presence of residual or recurrent cholesteatoma with high sensitivity and specificity.
The sensitivity of the current study was 86.7%, which correlated with other previous studies such as De Foer et al. (90%) [21], Profant et al. (96.15%) [22], Hudgins et al. (93%) [23]. Few published studies documented lower sensitivity, which could be attributed to a larger proportion of small cholesteatoma (less than 3 mm) in their study sample [24, 25].
Lehman et al. and Pizzini et al. found slightly higher sensitivity than our study (96.5%, 100% respectively). This could be attributed to using higher magnetic field strength (3Tesla magnet) [26, 27].
The most common cause for missing residual cholesteatoma on a NEP-DWI is size. The sizes of cholesteatomas detected intra-operatively in three of the false-negative cases in our study were 2 mm, 2.5 mm, and 3 mm. The smallest size detected in our study was 4 mm. The threshold for detection is reported to be 2–3 mm in most studies using NEP-DWI [23, 28–30]. It is better than previous studies using single-shot echoplanar imaging (SS-EPI) such as [19] and [31] (5 mm in both studies).
Authors such as Migirov et al. [29] stated that it may be considered safe to leave such small cholesteatomas, and some researchers suggest a follow-up DWI study to detect such lesions once they increased in size. The need for such follow-up studies and the time interval in-between are still not accurately established.
The last false-negative case in our study measured 4.5 mm in size, yet this was composed of epithelial lining without keratin matrix contents. This could be explained by that the keratin material is responsible for the bright signal in DWI, so lack of this material (empty retraction pocket) reduced the visibility of the lesion [32].
According to our study, the specificity of NEP-DWI for cholesteatoma was 100%, compatible with many earlier studies using NEP-DWI as well as EPI techniques. [19, 21, 26, 33, 34]
Delayed contrast-enhanced MRI improved the sensitivity of EPI to detect postoperative cholesteatoma. However, when NEP-DWI techniques are available, performing this time-consuming technique is not considered necessary anymore. Preoperative CT may not be needed also except for cases with positive DWI examination [35–37]
While the use of the NEP-DWI technique in post-operative follow-up implies additional costs in patient management, the overall cost reduction and the decrease in patient morbidity outweigh these costs because the number of second-look surgery could be significantly reduced [1, 3].
A suggested workup protocol for possible residual cholesteatoma is to use NEP-DWI in low-risk patients with small cholesteatoma or those lacking clinical complaints. Second look surgery can be postponed but those patients should be monitored closely by serial DWI to confirm the absence of cholesteatoma or growth to a size that requires surgery.
Conclusion
This study confirmed that the NEP-DWI technique is a valuable modality in detecting residual cholesteatoma with high accuracy that could significantly reduce the number of elective revision surgery.
Abbreviations
- CT
Computed tomography
- CWD
Canal wall down surgery
- CWU
Canal wall up surgery
- DWI
Diffusion-weighted imaging
- EPI
Echoplanar imaging
- MRI
Magnetic resonance imaging
- NEP-DWI
Nonechoplanar Diffusion-weighted imaging
- SPSS
Statistical package for social science
Authors' Contributions
AMI: radiological evaluation of patients and share in drafting of the paper; AME: radiological evaluation of patients and revise the final draft of the paper; MGE: radiological evaluation of patients, share in study design and statistical evaluation of results; MAG: study design, share in the writing of the article, clinical evaluation of patients, and share in evaluation of results; AAR: share in writing of the article, evaluation, and surgery of the patients; TE: share in editing of the final draft of the article; KH: share in writing of the article, evaluation, and surgery of the patients.
Funding
None.
Data Availability
Yes.
Declarations
Conflicts of interest
Author declares that there is no conflict of interest.
Consent to Participate
Signed informed consent was obtained from each patient.
Consent for Publication
After acceptance, I authorize Indian Journal of Otolaryngology and Head & Neck Surgery to publish the article.
Ethics Approval
The study was approved by the Research Ethics Committee.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Choi DL, Gupta MK, Rebello R, Archibald JD. cost-comparison analysis of diffusion-weighted magnetic resonance imaging (DW-MRI) versus second-look surgery for the detection of residual cholesteatoma. J Otolaryngol - Head Neck Surg. 2019;48:1–7. doi: 10.1186/s40463-019-0384-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lingam RK, Connor SEJ, Casselman JW, Beale T. MRI in otology: applications in cholesteatoma and Ménière’s disease. Clin. Radiol. 2018;73:35–44. doi: 10.1016/j.crad.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Más-Estellés F, Mateos-Fernández M, Carrascosa-Bisquert B, Facal de Castro F, Puchades-Román I, Morera-Pérez C. Contemporary non–echo-planar diffusion-weighted imaging of middle ear cholesteatomas. Radiographics. 2012;32:1197–1213. doi: 10.1148/rg.324115109. [DOI] [PubMed] [Google Scholar]
- 4.V. P. Afzal. (2010) “Hearing Results following Canal Wall Down Mastoidectomy in Attico-Antral Disease.” http://localhost:8080/xmlui/handle/123456789/5774
- 5.Sarkar S. Endoscopic Ear Surgery: A New Horizon. JP Medical Ltd.; 2016. [Google Scholar]
- 6.Henninger B, Kremser C. Diffusion-weighted imaging for the detection and evaluation of cholesteatoma. World J Radiol. 2017;9:217. doi: 10.4329/jr.v9.i5.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.SA Aly, SA Abohedibah ( 2016) The value of half-Fourier-acquisition single-shot turbo spin-echo (HASTE) diffusion-weighted magnetic resonance imaging combined with non-contrast conventional MRI imaging in the evaluation of recurrent cholesteatoma following canal wall up mastoid surgery. J. home page http//arnmsmb. Com /RJMMS 11: 18–22
- 8.Horn RJ, Gratama JWC, van der Zaag-Loonen HJ, Droogh-de Greve KE, van Benthem PG. Negative predictive value of non-echo-planar diffusion-weighted MR imaging for the detection of residual cholesteatoma done at 9 months after primary surgery is not high enough to omit second look surgery. Otol Neurotol. 2019;40(7):911–919. doi: 10.1097/MAO.0000000000002270. [DOI] [PubMed] [Google Scholar]
- 9.Khemani S, Lingam RK, Kalan A, Singh A. The value of non-echo planar HASTE diffusion-weighted MR imaging in the detection, localization, and prediction of extent of postoperative cholesteatoma. Clin Otolaryngol. 2011;3:306–312. doi: 10.1111/j.1749-4486.2011.02332.x. [DOI] [PubMed] [Google Scholar]
- 10.Castle JT. Cholesteatoma pearls: practical points and update. Head Neck Pathol. 2018;12:419–429. doi: 10.1007/s12105-018-0915-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tomlin J, Chang D, McCutcheon B, Harris J. Surgical technique in cholesteatoma: a meta-analysis. Audiol Neurotol. 2013;18:135–142. doi: 10.1159/000346140. [DOI] [PubMed] [Google Scholar]
- 12.Neudert M, Lailach S, Lasurashvili N, Kemper M, Beleites T, Zahnert T. Cholesteatoma recidivism: comparison of three different surgical techniques. Otol Neurotol. 2014;35:1801–1808. doi: 10.1097/MAO.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 13.De Foer B, Nicolay S, Vercruysse J-P, Officers E, Casselman JW, Pouillon M (2014) Imaging of cholesteatoma, In: Temporal Bone Imaging, Springer, 69–87
- 14.Gouda M, Nasr WF, Abd Albany ME-S, Razek MMA. MRI as an alternative to second look mastoid surgery. Indian J Otolaryngol Head Neck Surg. 2018;70:410–414. doi: 10.1007/s12070-018-1407-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dündar Y, Akcan FA, Dilli A, Tatar E, Korkmaz H, Özdek A. Does diffusion-weighted MR imaging change the follow-up strategy in cases with residual cholesteatoma? J Int Adv Otol. 2015;11:58–62. doi: 10.5152/iao.2014.216. [DOI] [PubMed] [Google Scholar]
- 16.Cavaliere M, et al. Cholesteatoma vs granulation tissue: a differential diagnosis by DWI-MRI apparent diffusion coefficient. Eur Arch Oto-Rhino-Laryngol. 2018;275:2237–2243. doi: 10.1007/s00405-018-5082-5. [DOI] [PubMed] [Google Scholar]
- 17.Bammer R. Basic principles of diffusion-weighted imaging. Eur J Radiol. 2003;45:169–184. doi: 10.1016/S0720-048X(02)00303-0. [DOI] [PubMed] [Google Scholar]
- 18.Attenberger UI, et al. Diffusion-weighted imaging: a comprehensive evaluation of a fast spin-echo DWI sequence with BLADE (PROPELLER) k-space sampling at 3 T, using a 32-channel head coil in acute brain ischemia. Invest Radiol. 2009;44:656–661. doi: 10.1097/RLI.0b013e3181af3f0e. [DOI] [PubMed] [Google Scholar]
- 19.Vercruysse J-P, De Foer B, Pouillon M, Somers T, Casselman J, Officers E. The value of diffusion-weighted MR imaging in the diagnosis of primary acquired and residual cholesteatoma: a surgical verified study of 100 patients. Eur Radiol. 2006;16:1461–1467. doi: 10.1007/s00330-006-0160-2. [DOI] [PubMed] [Google Scholar]
- 20.Aikele P, Kittner T, Offergeld C, Kaftan H, Huttenbrink K-B, Laniado M. Diffusion-weighted MR imaging of cholesteatoma in pediatric and adult patients who have undergone middle ear surgery. Am J Roentgenol. 2003;181:261–265. doi: 10.2214/ajr.181.1.1810261. [DOI] [PubMed] [Google Scholar]
- 21.De Foer B, et al. Detection of postoperative residual cholesteatoma with non-echo-planar diffusion-weighted magnetic resonance imaging. Otol Neurotol. 2008;29:513–517. doi: 10.1097/MAO.0b013e31816c7c3b. [DOI] [PubMed] [Google Scholar]
- 22.Profant M, Sláviková K, Kabátová Z, Slezák P, Waczulíková I. Predictive validity of MRI in detecting and following cholesteatoma. Eur Arch Oto-Rhino-Laryngology. 2012;269:757–765. doi: 10.1007/s00405-011-1706-8. [DOI] [PubMed] [Google Scholar]
- 23.Hudgins CT, Singh A, Lingam RK, Kalan A. Detecting cholesteatoma with non-echo planar (HASTE) diffusion-weighted magnetic resonance imaging. Otolaryngol Neck Surg. 2010;143:141–146. doi: 10.1016/j.otohns.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 24.Plouin-Gaudon I, Bossard D, Fuchsmann C, Ayari-Khalfallah S, Froehlich P. Diffusion-weighted MR imaging for evaluation of pediatric recurrent cholesteatomas. Int J Pediatr Otorhinolaryngol. 2010;74:22–26. doi: 10.1016/j.ijporl.2009.09.035. [DOI] [PubMed] [Google Scholar]
- 25.Lecler A, et al. Magnetic resonance imaging at one year for detection of postoperative residual cholesteatoma in children: is it too early? Int J Pediatr Otorhinolaryngol. 2015;79:1268–1274. doi: 10.1016/j.ijporl.2015.05.028. [DOI] [PubMed] [Google Scholar]
- 26.Lehmann P, et al. 3T MR imaging of postoperative recurrent middle ear cholesteatomas: value of periodically rotated overlapping parallel lines with enhanced reconstruction diffusion-weighted MR imaging. Am J Neuroradiol. 2009;30:423–427. doi: 10.3174/ajar.A1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pizzini FB, Barbieri F, Beltramello A, Alessandrini F, Fiorino F. HASTE diffusion-weighted 3-Tesla magnetic resonance imaging in the diagnosis of primary and relapsing cholesteatoma. Otol Neurotol. 2010;31:596–602. doi: 10.1097/MAO.0b013e3181dbb7c2. [DOI] [PubMed] [Google Scholar]
- 28.El Mogy SA, Mazroa JA, Abd El Ghaffar M, El Mogy MS, El Mogy IS. Evaluation of acquired cholesteatoma with PROPELLER diffusion imaging. Egypt J Radiol Nucl Med. 2011;42:9–17. doi: 10.1016/j.ejrnm.2011.02.003. [DOI] [Google Scholar]
- 29.Migirov L, Wolf M, Greenberg G, Eyal A. Non-EPI DW MRI in planning the surgical approach to primary and recurrent cholesteatoma. Otol Neurotol. 2014;35:121–125. doi: 10.1097/MAO.0000000000000234. [DOI] [PubMed] [Google Scholar]
- 30.Lingam RK, Nash R, Majithia A, Kalan A, Singh A. Non-echoplanar diffusion-weighted imaging in the detection of postoperative middle ear cholesteatoma: navigating beyond the pitfalls to find the pearl. Insights Imaging. 2016;7:669–678. doi: 10.1007/s13244-016-0516-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dubrulle F, Couillard R, Chechen D, Vaneecloo FM, Desaulty A, Vincent C. Diffusion-weighted MR imaging sequence in the detection of postoperative recurrent cholesteatoma. Radiology. 2006;238:604–610. doi: 10.1148/radiol.2381041649. [DOI] [PubMed] [Google Scholar]
- 32.Paddle P, Hurtado G, Goergen S, Hong S, Baxter M, Copson B. Turbo spin non-echo-planar diffusion-weighted MRI for cholesteatoma in revision mastoidectomy: a prospective study of diagnostic accuracy and clinical impact. Aust J Otolaryngol. 2018;1:12–12. doi: 10.21037/ajo.2018.02.02. [DOI] [Google Scholar]
- 33.Ilıca AT, et al. HASTE diffusion-weighted MRI for the reliable detection of cholesteatoma. Diagn Interv Radiol. 2012;18:153–158. doi: 10.4261/1305-3825.DIR.4246-11.3. [DOI] [PubMed] [Google Scholar]
- 34.Velthuis S, Van Everdingen KJ, Quak JJ, Colnot DR. The value of non-echo planar, diffusion-weighted magnetic resonance imaging for the detection of residual middle-ear cholesteatoma. J Laryngol Otol. 2014;128:599–603. doi: 10.1017/S0022215114001418. [DOI] [PubMed] [Google Scholar]
- 35.Jindal M, Riskalla A, Jiang D, Connor S, O’Connor AF. A systematic review of diffusion-weighted magnetic resonance imaging in the assessment of postoperative cholesteatoma. Otol Neurotol. 2011;32:1243–1249. doi: 10.1097/MAO.0b013e31822e938d. [DOI] [PubMed] [Google Scholar]
- 36.De Foer B, et al. Middle ear cholesteatoma: non–echo-planar diffusion-weighted MR imaging versus delayed gadolinium-enhanced T1-weighted MR imaging—value in detection. Radiology. 2010;255(3):866–872. doi: 10.1148/radiol.10091140. [DOI] [PubMed] [Google Scholar]
- 37.De Foer B, et al. Diffusion-weighted magnetic resonance imaging of the temporal bone. Neuroradiology. 2010;52:785–807. doi: 10.1007/s00234-010-0742-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Yes.