Abstract
Otitis externa is a common condition encountered in the ENT outdoors. For long, it was thought to be of fungal etiology but after World War II, bacterial pathogens were found to be most commonly involved. Pseudomonas has been described as the most common causative organism in the literature. This prospective study aimed to study the microbiological profile and antibiotic sensitivity of 100 patients of otitis externa. 100 diagnosed cases of otitis externa were included in the study after informed consent. Swabs were taken from the external acoustic canal maintaining asepsis. The swabs were analysed using microscopy, culture and sensitivity testing. The samples were cultured on blood agar, MacConkey agar and Sabourad’s dextrose agar with antibiotics. Antimicrobial susceptibility testing was done by Kirby–Bauer disc diffusion method. The most common bacteria identified was Pseudomonas aeruginosa (36.36%), followed by Staphylococcus aureus (15.45%), Escherichia coli (2.73%), Klebsiella (1.82%), and Proteus sp (1.82%). Aspergillus sp (19.09%) and Candida albicans (8.18%) were the fungal species identified. Pseudomonas showed excellent sensitivity to imipenem, piperacillin and ofloxacin, while Staphylococcus showed good sensitivity to vancomycin, ofloxacin and netilmycin. Topical fluoroquinolones can be used as empirical treatment in most cases of bacterial otitis externa. In resistant cases, culture and antibiotic sensitivity should be done to manage the infection.
Keywords: Otitis externa, Pseudomonas, Antibiotic sensitivity, Ofloxacin
Introduction
Since its description by Mayer in 1844 till World War II, otitis externa was considered to be of fungal origin. Due to high percentage of cases during World War II, its etiology was re-examined and established to be bacterial in nature. Otitis externa is a common condition encountered in ENT outdoors. In warm and humid environment, the incidence of this disease increases further more. It is defined as the inflammation of the external ear. It is also commonly known as “Swimmer’s Ear” or “Tropical Ear”. It can take the form of an acute or a chronic condition with multiple etiologies including infections, trauma and allergic reactions [1].
The most common bacteria isolated include Pseudomonas species (50–65%), other gram-negative organisms (25–35%), Staph aureus (15–30%) and Streptococci (9–15%). The most common fungi isolated includes Aspergillus species (80–90%) followed by Candida (10–20%) [2]. Most of the cases reporting to tertiary care centers have received some form of topical or systemic antibiotic treatment, hence microbiological examination and antibiotic sensitivity is vital to effectively manage the condition. Very few recent studies have examined the causative agents in otitis externa, hence this prospective study aims to evaluate the microbiological profile and antibiotic sensitivity in 100 cases of otitis externa.
Materials and Methods
A total of 100 patients attending the OPD of a tertiary care center with symptoms of otitis externa were enrolled in the study after taking written informed consent. The protocol of the study was approved from the Institutional Review Board for Ethical Clearance and all procedures were performed in accordance with the Code of Ethics of the World Medical Association according to the Declaration of Helsinki of 1975, as revised in 2000. Patients between age groups of 15 to 60 years with confirmed diagnosis of otitis externa were included in the study.
Swabs were collected from each affected ear to identify the causative organism. Cavum conchae and external meatus was cleaned with 70% isopropyl alcohol. Two specimens from each affected external canal were obtained with two separate sterile cotton tipped swabs with precautions taken to avoid contact with cavum conchae and external meatus. These swabs were immediately transferred to microbiology lab in sterile container and processed within 60 min. Swabs were taken during first appointment in the outdoor department. Subsequent swabs were not included in the study. Patients with otitis media, malignant otitis externa, perichondritis and other ear pathologies were excluded from the study.
The swabs were analyzed using microscopy, culture and sensitivity testing in the same laboratory. The samples were cultured on blood agar and MacConkey agar for bacterial agents and were aerobically incubated for 18–24 h. Gram Staining, motility, biochemical reactions like Coagulase test for Staphylococci and Oxidase test for Pseudomonas were carried out. For fungal agents, swabs were inoculated on Sabourad’s Dextrose Agar with antibiotics for 7 days. Antimicrobial susceptibility testing was done by Kirby–Bauer disc diffusion method.
Results
A total of 110 ears of 100 patients were included in the study with maximum patients in the age group of 21–30 years. The commonest predisposing factor observed was self-induced trauma (cotton bud, match stick injury) in 50% of cases, followed by water entry in the affected ear during bathing or swimming in 32.72% of patients. Other predisposing factors observed were hearing aid use (12.72%) and co-morbid conditions like diabetes mellitus (4.54%).
10% of cases (11 ears) showed no growth on culture. 58.18% and 27.27% cases had bacterial and fungal growths in their culture respectively (Fig. 1).
Fig. 1.

Culture results of 110 ears of 100 cases
The most common bacteria identified was Pseudomonas aeruginosa (36.36%), followed by Staphylococcus aureus (15.45%), Escherichia coli (2.73%), Klebsiella (1.82%), and Proteus sp (1.82%). Aspergillus sp (19.09%) and Candida albicans (8.18%) were the fungal species identified (Fig. 2).
Fig. 2.
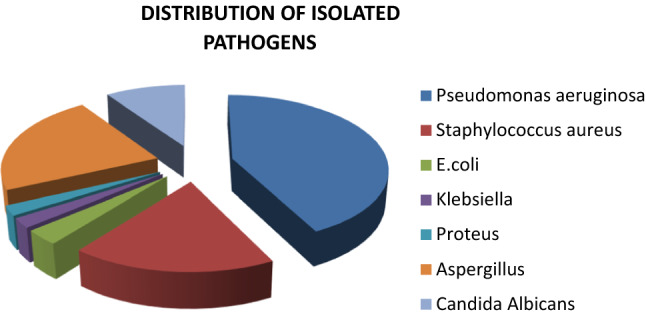
Distribution of isolated pathogens
Polymicrobial infection was seen in 5 cases out of which Staphylococcus with Candida was seen in 2 cases while Staphylococcus with Pseudomonas, Pseudomonas with Candida and Proteus with Candida was seen in 1 patient each.
40 isolates of Pseudomonas were tested for antibiotic sensitivity to cefotaxime, ceftazidime, ceftriaxone, ciprofloxacin, ofloxacin, cefpodoxime, gentamycin, netilimycin, amikacin, piperacillin/tazobactum and imipenem. The maximum sensitivity was with imipenem (100%) while piperacillin/tazobactum and ofloxacin showed 97.5% sensitivity. Least sensitivity was seen with ceftriaxone (30%) and ceftazidime (37.5%). Table 1 summarizes results.
Table 1.
Antibiogram of Staphylococcus isolates. N = 17
| Antibiotics | Sensitivity | Resistant | % Sensitivity |
|---|---|---|---|
| Penicillin | 3 | 14 | 17.64 |
| Amoxyclav | 14 | 3 | 82.35 |
| Gentamycin | 11 | 6 | 64.70 |
| Netilmycin | 15 | 2 | 88.2 |
| Erythromycin | 10 | 7 | 58.82 |
| Ofloxacin | 16 | 1 | 94.1 |
| Clindamycin | 12 | 5 | 70.58 |
| Vancomycin | 17 | 0 | 100 |
| Cefoxitin | 14 | 3 | 82.35 |
Staphylococcus was isolated in 17 swabs and was tested for sensitivity to penicillin, amoxyclav, gentamycin, netilmycin, erythromycin, ofloxacin, clindamycin, vancomycin and cefoxitin. The most effective antibiotics were vancomycin (100%), ofloxacin (94.1%), netilmycin (88.2%) while the least effective antibiotic was penicillin (17.64%) (Table 2).
Table 2.
Antibiogram of Pseudomonas aeruginosa. N = 40
| Antibiotics | Sensitivity | Resistant | % Sensitivity |
|---|---|---|---|
| Cefotaxime | 15 | 25 | 37.5 |
| Ceftazidime | 14 | 26 | 35 |
| Ceftriaxone | 12 | 28 | 30 |
| Ciprofloxacin | 38 | 2 | 95 |
| Ofloxacin | 39 | 1 | 97.5 |
| Cefpodoxime | 21 | 19 | 52.5 |
| Gentamycin | 27 | 13 | 67.5 |
| Netilimycin | 23 | 17 | 57.5 |
| Amikacin | 34 | 6 | 85 |
| Piperacillin/tazobactum | 39 | 1 | 97.5 |
| Imipenem | 40 | 0 | 100 |
All anaerobic organisms were sensitive to metronidazole
Discussion
Otitis externa is a generalized condition of the skin of the external auditory canal. It is characterized by oedema and erythema associated with itch, pain and discharge. It affects between 5 and 20% of patients attending ENT clinics. Since World War II, microbiologic studies of otitis externa have usually identified P. aeruginosa as the most common causative pathogen and S. aureus as the species second most common, but the relative incidences have varied substantially [3].
110 ears of 100 patients were studied with maximum number of cases in the third decade of life. Self-induced trauma and water entry were the most common triggering factors in our study. Bell et al. in his study reported that otitis externa could be caused by trauma to the ear canal, skin disorders and viral and bacterial infections. Qader and Yaseen found that the commonest predisposing factor was water entrance to the affected ear during bathing and swimming in 51% of patients, self-induced trauma (by cotton bud, match stick, hair clips…etc.) in 30% of patients and in 15% of patients there was history of chronic suppurative otitis media [4].
Pseudomonas has been identified as the most common bacterial causative pathogen of otitis externa in world literature. Since Pseudomonas rarely affects the epithelium of healthy individuals Sundstorm studied characteristics of the strain of P. aeruginosa isolated from ear canals and found that the isolates produced less pyocyanin, urase and mucous than Pseudomonas isolated from other sites. He concluded that the strains causing otitis externa have water as their natural habitat [5]. Roland and Stroman conducted a multicenter trial collecting specimens from 2039 participants across United States between 1998 and 2002. The most frequent isolates were P. aeruginosa (38%), Staphylococcus epidermidis (9.1%), S. aureus (7.8%), Microbacterium otitidis (6.6%), Microbacterium alconae (2.9%), Staphylocpccus caprae (2.6%), Staphylococcus auricularis (2.0%), Enterococcus faecalis (1.9%), Enterobacter cloacae (1.6%), Staphylococcus capitis subspecies ureolyticus (1.4%) and Staphylococcus haemolyticus (1.3%) [6]. Pseudomonas was also the most common pathogen found in studies conducted by Amigot et al. [7] and Nincovic et al. [8]. On the contrary, S. aureus was found to be the most common isolate in Taiwan (43.5%) by Juen-Haur et al. [9] and in Norway (34.1%) by Dibbs [10]. Increasing incidence of methicillin resistant S. aureus in otitis externa reported in Taiwan [9] (13.7%) and Ireland [11] (6%) do not seem to correspond to our findings as we did not find any case of MRSA in our study.
Antibiotic sensitivity in otitis externa patients is of increasing importance as most of the patients have received some form of antibiotic treatment before reporting to higher centers. This leads to increasing incidence of antibiotic resistance and superinfections. This reflected in the incidence of fungal growths in our study (27.7%) which was significantly higher than 17.4% reported by Nincovic and 9.8% by Dibbs in Norway. Factors like humidity, temperature and other weather conditions could also have contributed to higher fungal growths in our study.
In our study, Pseudomonas showed excellent sensitivity to imipenam, piperacillin/tazobactam and ofloxacin while least sensitivity was seen with cephalosporins. We did not demonstrate total resistance to any of the antibiotics tested. One of the limitations of our study was the inability to test sensitivity to common topical agents like neomycin and polymyxin B. Nincovic reported 100% resistance of Pseudomonas species to neomycin, chloramphenicol, trimethoprim and amoxicillin, while no resistance was found to polymyxin B and ciprofloxacin. Declining susceptibility of pathogens to neomycin and polymixin B in patients suffering from otitis externa was reported in 2000 by Cantrell [12]. Staphylococcus isolates in our study showed maximum sensitivity to vancomycin and ofloxacin. Battikhi and Ammari demonstrated good sensitivity to ciprofloxacin by both Pseudomonas (93.4%) and Staph (96.5%) isolates [13].
Other limitations of our study were low sample size and limited geographic distribution of cases. A nationwide multicentric study including patients from varying geographical locations needs to be conducted to understand the effect of climatic conditions on etiological agents of otitis externa and also to evaluate antibiotic sensitivity in different regions. Information about antibiotic therapy received at primary care will also help understand the microbiological findings obtained.
Conclusion
Microbial culture and antibiotic sensitivity in otitis externa can help give targeted treatment to obtain better results. Pseudomonas and Staphylococcus were the most common pathogens identified in our study. We observed excellent sensitivity of both pathogens to ofloxacin which can be used as the first line topical therapy in most cases. In resistance cases, oral and parenteral antibiotics can also be used. We did not find absolute resistance to any of the antibiotics tested.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hawke M, Wong J, Krajden S. Clinical and microbiological features of otitis externa. J Otorhinolaryngol. 1984;13:289–295. [PubMed] [Google Scholar]
- 2.Rutka J. Acute otitis externa: treatment perspectives. Ear Nose Throat J. 2004;83:20–22. doi: 10.1177/01455613040839s408. [DOI] [PubMed] [Google Scholar]
- 3.Syverton J, Hess W. Otitis externa: clinical observation and microbiologic flora. Arch Otolaryngol. 1946;43:213–225. doi: 10.1001/archotol.1946.00680050228002. [DOI] [PubMed] [Google Scholar]
- 4.Qader SN, Yaseen MA. Management of acute otitis externa using aural wick versus local drops. Zanco J Med Sci. 2012;16(3):187–193. doi: 10.15218/zjms.2012.0033. [DOI] [Google Scholar]
- 5.Sundstrom J, Jacobson K, Munck-Wikland E, Ringertz S. Pseudomonas aeruginosa in otitis externa. A particular variety of the bacteria? Arch Otolaryngol Head Neck Surg. 1996;122(August (8)):833–836. doi: 10.1001/archotol.1996.01890200023004. [DOI] [PubMed] [Google Scholar]
- 6.Roland PS, Stroman DW. Microbiology of acute otitis externa. Laryngoscope. 2002;112(July (7)):1166–1177. doi: 10.1097/00005537-200207000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Amigot SL, Gomez CR, Luque AG, Ebner G. Microbiological study of external otitis in Rosario City. Argent Mycoses. 2003;46(8):312–315. doi: 10.1046/j.1439-0507.2003.00902.x. [DOI] [PubMed] [Google Scholar]
- 8.Ninkovic G, Dullo V, Saunders NC. Microbiology of otitis externa in the secondary care in United Kingdom and antimicrobial sensitivity. Auris Nasus Larynx. 2008;35:480–484. doi: 10.1016/j.anl.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 9.Juen-Haur H, Chu CK, Liu TC. Changes in bacteriology of discharging ears. JLO. 2002;116(September (9)):686–690. doi: 10.1258/002221502760237957. [DOI] [PubMed] [Google Scholar]
- 10.Dibbs WL. Microbial aetiology of otitis externa. J Infect. 1991;22(3):233–239. doi: 10.1016/S0163-4453(05)80004-0. [DOI] [PubMed] [Google Scholar]
- 11.Walshe P, Rowley H, Timon C. A worrying development in the microbiology of otitis externa. Clin Otolaryngol Allied Sci. 2001;26(June (3)):218–220. doi: 10.1046/j.1365-2273.2001.00455.x. [DOI] [PubMed] [Google Scholar]
- 12.Cantrell FH. Declining susceptibility to neomycin and polymixin B of pathogens recovered in otitis externa clinical trials. South Med J. 2004;97(May (5)):465–471. doi: 10.1097/00007611-200405000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Battikhi MN, Ammar SI. Otitis externa infection in Jordan. Saudi Med J. 2004;25(9):1199–1203. [PubMed] [Google Scholar]


