Abstract
In response to starvation, bacilli and clostridia undergo a specialized program of development that results in the production of a highly resistant dormant cell type known as the spore. A proteinacious shell, called the coat, encases the spore and plays a major role in spore survival. The coat is composed of over 25 polypeptide species, organized into several morphologically distinct layers. The mechanisms that guide coat assembly have been largely unknown until recently. We now know that proper formation of the coat relies on the genetic program that guides the synthesis of spore components during development as well as on morphogenetic proteins dedicated to coat assembly. Over 20 structural and morphogenetic genes have been cloned. In this review, we consider the contributions of the known coat and morphogenetic proteins to coat function and assembly. We present a model that describes how morphogenetic proteins direct coat assembly to the specific subcellular site of the nascent spore surface and how they establish the coat layers. We also discuss the importance of posttranslational processing of coat proteins in coat morphogenesis. Finally, we review some of the major outstanding questions in the field.
When challenged by stresses such as starvation, a variety of bacilli and clostridia produce a dormant cell type, called a spore, that can withstand a wide range of assaults that would destroy a vegetative cell. It was the observation by Koch over 100 years ago that Bacillus anthracis spores could survive boiling which prompted researchers to begin studies of spores with the goal of discovering how this special dormant cell type could withstand heat and other stresses. In the century that followed, it was learned that the dehydrated state of the spore interior endows that spore with the capacity to survive heating (119) and that it is the spore coat, a multilayered structure surrounding the spore and composed of upward of 25 often highly cross-linked polypeptide species (Fig. 1A to C), which grants the spore resistance to, for example, treatment with chloroform or challenge by lysozyme. While providing this high level of resistance, the coat nonetheless allows the spore to respond to the renewed presence of nutrients in the environment, the condition under which the spore can convert to a growing cell through a process called germination. These remarkable capabilities have focused attention on the coat and stimulated research into the developmental and morphological processes required to correctly assemble such a complex macromolecular structure.
FIG. 1.
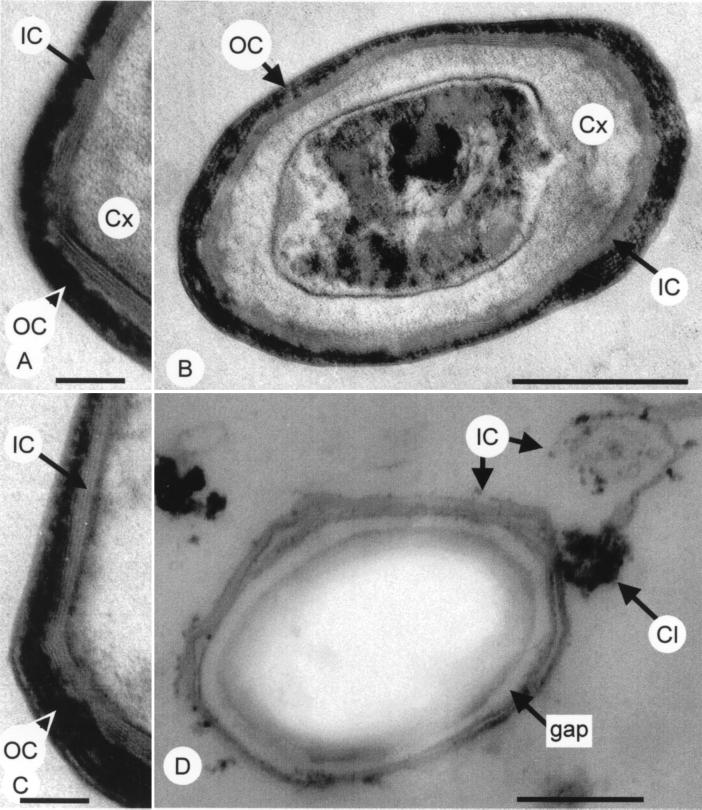
B. subtilis spore. (A to C) Wild-type spore (B) or arcs of spore coats (A and C). (D) cotE spore. IC, inner coat; OC, outer coat; Cx, cortex; gap, the space between the inner coat and the cortex in a cotE spore; Cl, clump of darkly staining material of unknown composition. Bars, 500 nm (B and D) and 300 nm (A and C).
The study of spore morphogenesis, and in particular the assembly of the coat, is part of the broad effort to learn how bacteria build subcellular components, direct them to their correct locations, and alter the organization of components in response to cell cycle events or environmental stimuli (77). The ornate structures assembled by prokaryotes include complex envelope layers and a variety of specialized surface appendages such as pili (51) and flagella (79). In Caulobacter crescentus, for example, the appearance of polar structures offers an example of changes in organelle assembly in a cycle-dependent manner (27), while the crossbands of the C. crescentus stalk serve as an illustration of intriguing internal components of striking design but unknown function (103). The attractiveness of such examples and the sophistication of genetic, biochemical, and structural techniques have made bacteria important systems for the study of the dynamic aspects of morphogenesis during development (121, 122). The particular ease with which these methods can be applied to Bacillus subtilis makes it a premier system for such analysis, and the study of its spore coat especially appealing.
The study of the coat is not relevant solely to spore formation and function. It also contributes to, and is informed by, our understanding of the broader cell-biological problem of how cells build their organelles. It is still largely mysterious how, in general, cells are able to build complex macromolecular structures at the correct subcellular locations and at the proper times in development and how they achieve such remarkable precision in assembly. Nonetheless, studies of assembly in a number of other systems have given us a very deep view into the logic of cellular design and a basic framework with which to attack the problem of the spore coat. It is readily apparent that the control of gene expression will play an important role. Gene regulatory mechanisms ensure that the correct protein products are available at appropriate times and in sufficient quantities. However, an understanding of gene regulation does not solve the question of how an organelle is actually built. The ability of the cell to accurately guide the components of a complex into exactly the correct position requires that structural information be encoded in the proteins of the macromolecular complexes themselves or in accessory proteins that may transiently associate with the organelle during its construction (these concepts are elegantly discussed in reference 62). Understanding this level of morphogenetic control is a major challenge in describing the assembly of the spore coat.
This discussion of coat formation begins with a review of the morphological stages of spore formation and of the control of gene expression during this developmental process. Next, I consider several classical issues in spore coat biology including studies of the morphology and biochemical composition of the coat. I then examine a series of more recent experiments which gave us methods with which to identify coat proteins and their genes and which have enabled us to determine the roles of these proteins in coat morphogenesis. I focus on proteins that direct the coat to assemble at the proper location, that guide the formation of the major coat layers, and that coordinate coat assembly with other cellular events: the so-called morphogenetic proteins. With this description of the known players in hand, I describe a model for coat assembly. Finally, I will consider some of the findings from the completion of the sequencing of the B. subtilis genome and outline some of the remaining questions in the field.
SPORULATION PROGRAM: MORPHOLOGICAL STAGES AND GENETIC CONTROL
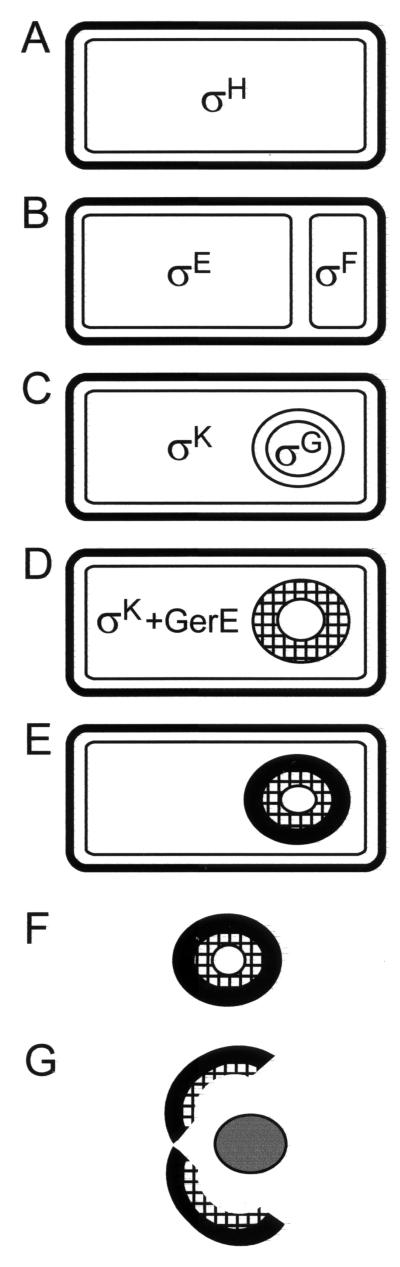
Spore formation, referred to as sporulation, occurs in a series of stages that can be monitored by light and electron microscopy and, once initiated, requires approximately 8 h to complete. The morphological process of sporulation is driven by a temporally and spatially controlled program of gene expression. Commencement of this program requires that the cell has reached a certain stage in the cell cycle (37, 98), that the tricarboxylic acid cycle is intact (52), that at least one extracellular pheromone is present in the appropriate amount, and that an unknown environmental stimulus has activated a complex phosphorylation cascade (45). When these conditions are met (as they are during starvation), sporulation ensues and the pattern of vegetative gene expression is largely replaced with the specialized program of sporulation gene expression. The rapid series of morphological changes that ensue during sporulation is due, in large part, to the sequential appearance of a series of transcription factors, called sigma factors, which bind to core polymerase and direct it to transcribe only from specific promoters (76). An early and dramatic morphological event is the formation of the asymmetrically placed sporulation septum, which divides the cell into the forespore and mother cell compartments, at about the second hour of sporulation (Fig. 2B). The smaller compartment (the forespore) will go on to become the spore. The larger compartment (the mother cell) will serve to nurture the spore until its development is complete. After the sporulation septum is laid down, the sporulation gene expression program splits and two distinct programs become active, one in each of the resulting cellular compartments. These two divergent programs of gene expression, in the mother cell and in the forespore, result in the spore being built from the outside (as a result of protein synthesis in the mother cell) and from the inside (as a consequence of the proteins produced in the forespore). At about the third hour, the edge of the septum migrates in the direction of the forespore pole of the cell, pinching the forespore compartment off to become a protoplast which sits free in the mother-cell cytoplasm and is surrounded by a double layer of membrane (Fig. 2C). After engulfment, a cell wall-like material is deposited between the membrane layers that surround the forespore (Fig. 2D). This cell wall has two layers: an inner layer, called the germ cell wall, which will become the peptidoglycan layer of the nascent cell after germination, and an outer layer, called the cortex, which participates in maintenance of the dehydrated state of the spore. This is followed by formation of the coat from proteins synthesized in the mother cell, which then assemble around the forespore (Fig. 2E). The coat is evident by about the fifth hour of sporulation. Two major coat layers can be discerned in the electron microscope: a darkly staining outer coat and a more lightly staining lamellar inner coat (Fig. 1A to C). The final step in sporulation is lysis of the mother cell and release of the fully formed spore (Fig. 2F). If conditions are suitable, the spore can germinate and thereby convert back into a growing cell. When this occurs, first the spore core rehydrates and swells and then the coat cracks, releasing the nascent cell (Fig. 2G).
FIG. 2.
Stages of sporulation. (A) Once the cell commits to sporulation, ςH activity increases. (B) In the next stage, an asymmetrically positioned septum divides the cell into the forespore and mother cell compartments. ςF becomes active in the forespore, and ςE becomes active in the mother cell. (C) The forespore engulfs into a membrane-bound protoplast. ςG becomes active in the forespore, and ςK directs gene expression in the mother cell. (D) The cortex (the hatched area) forms between the forespore membranes. GerE works in conjunction with ςK to direct a final phase of gene expression. (E) The coat (the dark ring surrounding the hatched cortex) becomes visible by electron microscopy. (F) In the final stage of sporulation, the mother cell lyses and releases the mature spore into the environment. (G) When nutrient returns to the medium, the spore can germinate and the cell can resume vegetative growth. This involves rehydration of the interior of the spore and cracking open of the coat.
A strict program of sigma factor activation directs sporulation gene expression to occur at the right time and place. The first sporulation-specific sigma factor to direct sporulation-specific gene expression is ςH (which acts before the sporulation septum forms [Fig. 2A]). ςH, in combination with the major housekeeping factor ςA, directs the expression of a large set of genes, some of which play early roles in sporulation and in the appearance of the sporulation septum. ςA activates the expression of sporulation genes in conjunction with the transcription factor Spo0A, which becomes active immediately after sporulation is initiated. The ςH-controlled genes include spoIIB (81), spoVG (89), and spoVS (107), which may be involved in the formation and maturation of the septum. Once the sporulation septum has established the nascent forespore and mother cell chambers, ςF becomes active in the forespore compartment (Fig. 2B) (80). ςF is, in fact, synthesized prior to septation and therefore is present in both cell compartments, but it is held inactive until the septum appears. After the septum is formed, it becomes active in only the forespore. This restriction of activity to only one cell type is a consequence of an anti-sigma factor called SpoIIAB and an additional set of regulatory proteins that ensure that ςF remains inactive in the mother cell (3, 4, 85, 118). The ςF regulon includes genes involved in engulfment, such as spoIIQ (75), and genes whose products act later, such as katX (12), which encodes a catalase that protects germinating spores from hydrogen peroxide. It also includes the gene (spoIIIG) that encodes the next sigma factor to be active in the forespore (135). Once ςF is active, ςE becomes active in the mother cell (Fig. 2B). The activity of ςE is a consequence of ςF, but the confinement of this activity to the mother cell is not (see below). Like ςF, ςE is synthesized prior to septation. Unlike ςF, however, ςE is synthesized as a proprotein, requiring the removal of its amino terminus before becoming active. This processing, which is restricted to the mother cell (58), converts the membrane-associated proprotein into a cytoplasmic factor capable of directing transcription (46). An additional forespore-specific proteolytic event removes pro-ςE from the forespore (102). These mechanisms ensure that ςE activity is confined to the mother cell (28). The restriction of functional ςF to the forespore raises the tempting possibility that its asymmetric activation is responsible for the presence of mature ςE exclusively in the mother cell. In fact, the confinement of ςE activation to the mother cell does not depend on ςF or any of the genes it controls (150). The feature of the mother cell that marks it as the compartment in which ςE activity will emerge remains unknown.
ςE activity results in the engulfment of the forespore compartment, creating a free protoplast encircled by a double membrane layer. It also directs the appearance of an important transcription factor, SpoIIID (70, 71), as well as an inactive proform of ςK, which will subsequently become active in the mother cell after proteolytic removal of the propeptide (72, 113). SpoIIID works with ςE to activate a second phase of mother cell gene expression. After engulfment, the next sigma factor, ςG, becomes active in the forespore (Fig. 2C). The gene encoding ςG is under the control of ςF and is expressed just after the appearance of the sporulation septum. However, the gene product remains inactive until forespore engulfment is complete (132). ςG activates a variety of forespore genes, including those that encode the major forespore-specific DNA-binding proteins, the small acid-soluble proteins (119). The core of the forespore dehydrates soon after ςG becomes active. Dehydration presumably renders the spore metabolically inactive, thereby terminating forespore gene expression. Before the forespore becomes dormant, it sends a signal to the mother cell to activate the next stage of sporulation gene expression. This occurs via the ςG-controlled gene spoIVB. SpoIVB sends a signal across the double membranes of the forespore into the mother cell to activate ςK. This sigma factor has been sitting dormant in the mother cell since its previous synthesis under the control of ςE (20). ςK is activated by a proteolysis event that appears to occur on the outer forespore membrane, which is in contact with the mother cell cytoplasm (Fig. 2C) (78, 106, 108, 146). ςK is required for a variety of events that occur late in sporulation, including the synthesis of most of the known coat proteins and (in collaboration with ςG) the synthesis of the cortex (135). The last known phase of mother cell gene expression is directed by ςK along with the small DNA-binding protein GerE (153) (Fig. 2D). GerE controls coat protein genes and genes that may be involved in the glycosylation of the coat (50a, 110).
ROLE OF GENE EXPRESSION IN COAT ASSEMBLY
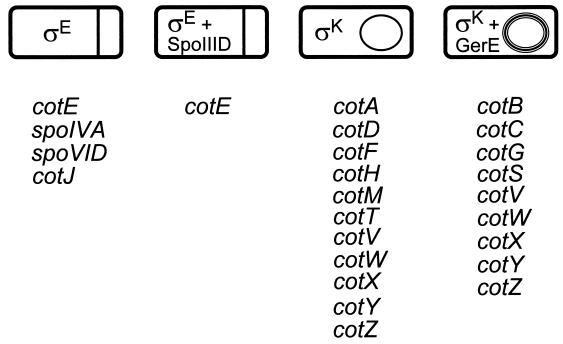
To understand the role of gene expression in the assembly of the coat, it is necessary to focus primarily on events in the mother cell, since all of the known coat protein genes and coat morphogenetic genes are expressed in this compartment (Fig. 3; Table 1). Therefore, I will review the roles of the transcription factors that control mother cell gene expression: ςE, SpoIIID, ςK and GerE.
FIG. 3.
Program of mother cell gene expression. The stages of sporulation are shown at the top of the figure, and the transcription factors that direct mother cell gene expression at each stage are shown within the mother cell compartment. Below each cell are the coat protein genes that are active at that time. The repressive functions of SpoIIID and GerE are not indicated.
TABLE 1.
The known coat and morphogenetic genes
| Gene | Size of product (kDa) | Genetic control | Map position (degrees) | Coat layerb | Morphogenetic rolec | Commentsc |
|---|---|---|---|---|---|---|
| cotA | 65 | ςK | 52 | OC | None known | Controls brown pigment |
| cotB | 59 | ςK + GerE | 314 | OC | None known | |
| cotC | 12 | ςK + GerE | 168 | OC | None known | |
| cotD | 11 | ςK | 198 | OC | None known | |
| cotE | 24 | ςE, ςE + SpoIIID | 150 | OC | Required for outer coat assembly | |
| cotF | 5, 8 | ςK | 356 | ? | None known | Proteolytically processed |
| cotG | 24 | ςK + GerE | 314 | OC | Controls CotB assembly | Nine tandem copies of 13-aa repeat |
| cotH | 42.8 | ςK | 314 | IC | Controls OC protein assembly | Near cotB and cotG |
| cotJA | 9.7 | ςE | 62 | Matrix | None known | Interacts with CotJC |
| cotJC | 21.7 | ςE | 62 | Matrix | None known | Interacts with CotJA |
| cotK | 6 | ? | 164 | ? | None known | Putative coat protein |
| cotL | 5.4 | ? | 164 | ? | None known | Putative coat protein |
| cotM | 14 | ςK | 164 | OC | Controls outer coat assembly | α-Crystallin-like |
| cotS | 41 | ςK + GerE | 270 | IC | None known | CotE dependent |
| cotT | 7.8 | ςK | 108 | IC | Controls inner coat assembly | Proteolytically processed |
| cotV | 14 | ςK ςK + GerE | 107 | ? | None known | Putative coat protein |
| cotW | 12 | ςK, ςK + GerE | 107 | ? | None known | Putative coat protein |
| cotX | 18.6 | ςK, ςK + GerE | 107 | OC | Controls outer coat assembly | Insoluble fraction |
| cotY | 17.9 | ςK, ςK + GerE | 107 | OC | May control outer coat assembly | Insoluble fraction |
| cotZ | 16.5 | ςK, ςK + GerE | 107 | OC | May control outer coat assembly | Insoluble fraction |
| spoIVA | 55 | ςE | 204 | NCP | Attaches the precoat to the forespore | |
| spoVID | 65 | ςE | 244 | NCP | Involved in coat attachment | |
| sodA | 25 | ? | 221 | NCP | Involved in coat morphogenesis |
Only the sigma factors and major positive regulators are listed. See the text for a more complete description of the genetic control.
Assignments of coat proteins to coat layers have been directly demonstrated only for CotE and CotS. Otherwise, the assignments are inferences. IC, inner coat; OC, outer coat; NCP, not considered a coat protein.
These comments must be read in conjunction with the caveats discussed in the text. aa, amino acid.
ςE directs the expression of at least two coat protein gene loci, cotE and cotJ, as well as the coat morphogenetic genes spoIVA and spoVID (13, 42, 109, 120, 128). A significant amount of cotE expression is also under the control of SpoIIID and ςE (153). SpoIIID can repress gene expression as well as activate it, and it represses both the cortex biosynthetic gene spoVD (145) and the coat protein gene cotD (40, 70). After engulfment, ςK directs the expression of a large group of coat protein genes. The first ςK-controlled regulon is composed of cotA, cotD, cotF, cotH, cotM, cotT, cotV, cotW, cotY, and cotZ (17, 23, 41, 114, 149, 153). The transcription factor gene gerE is also part of this regulon (22). SpoIIID controls ςK-directed genes also, since it represses cotD, as noted above. GerE works in conjunction with ςK to activate a final regulon, encompassing cotB, cotC, cotG, cotS, cotV, cotW, cotX, cotY, and cotZ (the genes cotV, cotW, cotY, and cotZ are under the control of ςK as well as ςK and GerE together) (113, 136, 149, 152). GerE can also modulate the expression of genes in the ςK regulon. It down regulates cotA (114) and cotM (41) and activates cotD (152), cotV, cotW, cotX, cotY, and cotZ. A further level of complexity in the control of mother cell gene expression comes from a type of feedback regulation in which late regulatory events modulate ones that were initiated earlier (147). For example, ςK down regulates transcription of the gene encoding ςE, thereby helping to terminate expression of ςE-directed genes. Furthermore, GerE is also able to down regulate the activity of ςK, by inhibiting the expression of the gene encoding this factor (51a, 152). These regulatory pathways appear to fine-tune the timing of the production of sporulation proteins. They may permit certain adjustments to the level of coat protein gene expression as a function of the availability of energy or nutrients (51a).
The elucidation of this gene-regulatory hierarchy has been one of the major triumphs of modern sporulation research. Clearly, the production of the spore coat proteins in the correct cellular compartment and at the proper time is critical to the formation of the coat. Beyond this, however, the precise way in which gene expression participates in coat assembly remains unclear. As far as is known, there is no dedicated regulatory program for the coat protein genes alone; the transcription factors that direct coat protein genes direct other sporulation genes as well. It is not known whether the fine levels of control built into the mother cell program are essential for a functioning coat or even whether the control of gene expression per se contributes significantly to the fine structure of the coat. We do not know, for example, what would happen if cotA expression was forced to occur earlier than normal (for example, by placing the gene under the control of ςE). We do know that when ςK is activated about 1 h earlier than its normal time, some kind of subtle defect in spore assembly occurs (although it is unclear whether this is a consequence of altered coat protein gene expression [21]). It is plausible that the importance of gene-regulatory controls is largely to ensure that the coat components are available in the correct cell type at roughly the proper time and that the job of coat construction is directed, for the most part, by proteins dedicated to assembly.
SPORE MORPHOLOGY
The B. subtilis spore coat has a striking appearance in the electron microscope (Fig. 1A to C). In thin sections, the coat appears as a series of concentric layers. The number and fine structure of the layers differ for different species, ranging from the simple coat of B. cereus (composed largely of a single protein species [6]) to the relatively complex coat of B. sphaericus (see, for example, the micrographs in reference 47). It is possible that these species-specific differences in structure reflect functional differences in the coat that help spore-forming bacteria thrive in their diverse habitats (which range from the soil to the intestinal tracts of insects [105]). However, structural differences between spore coats of different species have yet to be correlated with differences in coat function. It is noteworthy that B. subtilis, like B. cereus, is found in the soil but has a much more complex coat. As discussed below, deletion of many of the B. subtilis coat protein genes has little or no detectable consequence for spore survival, as measured in the laboratory. Possibly, they are not required for survival in the soil either. Therefore, it remains possible that many of the coat proteins do not serve an important function and would be poorly conserved in nature. This issue might be clarified by an examination of the coat proteins present in spores recovered from the wild.
In B. subtilis, there are two major coat layers: an inner coat and an outer coat (Fig. 1A to C) (8, 60, 141). The inner coat has a fine lamellar appearance and stains lightly, as shown by electron microscopy. It is composed of several (between two and five, usually about four) layers and is about 75 nm wide. The lamellar appearance of the inner coat is suggestive of multiple membrane layers, but biochemical analysis indicates that the coat consists largely of protein (66). The inner coat is surrounded by the outer coat, which is often thicker than the inner coat, ranging from about 70 to 200 nm wide. In thin sections, the outer coat stains more darkly than the inner coat and has a more coarsely layered appearance. The electron density of the coat layers can be enhanced by chemical fixatives such as osmium tetroxide, which allows the inner coat to be more readily visualized. The basic protocol for the chemical fixation of spores for electron microscopy (61) has remained essentially unchanged for many years, but there is likely to be room for improvement (see, for example, references 63 and 69).
In some cases, the surface of the outer coat is covered by a somewhat less electron-dense layer. This thin layer of material may simply be a morphologically distinct outermost layer of the outer coat, as argued by Holt and Leadbetter (48). Alternatively, it could be an additional component that is distinct from the outer coat (125, 127). Such a structure, called an exosporium, is found in a variety of species including B. cereus, B. thuringienesis, and B. anthracis (8, 14, 39, 68). Typically, the exosporium is a multilayered shell that surrounds the entire spore, including the coat, but is not connected to the spore or the coat. In contrast to the usual exosporium, in B. subtilis this structure is tightly associated with the outer coat. The application of solubilizing agents such as urea, mercaptoethanol, or sodium hydroxide causes this layer to pull away from the outer coat (127). This may indicate that although this structure closely abuts the outer coat, it is a separate layer. It should be noted that in addition to exosporia, filamentous or pilus-like structures are present on the spores of a variety of Bacillus species but have not been observed in B. subtilis (38, 39, 68, 86).
In addition to the two predominant coat layers, a third layer of density is often visible between the inner coat and the cortex on electron micrographs; it is thinner than either of the coat layers but appears thicker than a membrane. This could be a layer of the coat, or it could be the outer forespore membrane. Aronson and colleagues propose that this layer is part of the coat and designate it the undercoat (7). There is evidence for the outer forespore membrane remaining intact in spores of B. megaterium (67), and this issue must be resolved in B. subtilis. Fujita et al. (32) demonstrated the presence of some vegetative cell membrane proteins in the coat, possibly indicating that at least some of the outer forespore membrane is still intact in the dormant spore.
A number of investigators have applied highly sophisticated electron microscopic methods, including cryofixation and freeze-substitution, freeze-etch techniques, and microprobe analysis, to the coats of a variety of species. The inner and outer coat layers can be readily distinguished when cryofixation and freeze-substitution, in the absence of chemical fixatives, is used to prepare spores for electron microscopy, but no new layers are revealed (27a). However, certain previously observed structural details may become clearer (see, for example, reference 30). As anticipated from thin-section studies, freeze-etch examination of spores of a number of species demonstrated that the coat has several morphologically distinct layers. When freeze-etching was used to uncover the underlying layers, fibrous, cross-hatched, and pitted surfaces were revealed (8, 48, 142). In B. subtilis, layers with a fibrous morphology can be detected. Intriguingly, the fibers in the two layers run at right angles to each other. The identities of the proteins that make up these fibers should be revealed as the locations of the known coat proteins are determined (see below).
A promising technique that could be very informative is electron probe X-ray microanalysis. In this approach, the elements present in a thin section of a sample can be identified and their precise location in the plane of the section can be determined. The resolution and sensitivity are adequate to identify elements that are present in the coat in significant amounts. For example, Stewart et al. found that iron in B. coagulans spores was largely in the coat (131) but was not detected in the coat of B. cereus (130). It would be interesting to apply this method to B. subtilis, as the roles of metals and other elements that might function as cofactors for coat proteins are beginning to be studied (see the discussion of CotA, below). Another underutilized technique is scanning electron microscopy. Images of Bacillus spores obtained by this method have appeared in the literature from time to time and often show a series of ridges parallel to the long axis of the spore (see, for example, reference 91), but this technique does not appear to have been applied in a systematic way to B. subtilis. Clearly, such a study is needed. In particular, it would be useful to observe the changes in the spore surface upon germination (see reference 97 for an example of such an experiment in clostridia).
Several unexplained electron microscopic observations deserve further examination. For example, images of sporangia at early and intermediate times of sporulation suggest that coat assembly initiates at the poles of the spore (that is, along the long axis of the spore [27a]). The resulting caps of coat material contain both inner and outer coat layers and gradually encircle the spore as assembly proceeds. Commonly, the coat at the poles of a mature spore is thicker than the coat that surrounds the midpoint of the spore, perhaps reflecting this polar assembly. An additional unexplained observation is that the thickness of the coat can vary depending on the culture medium (27a). This is intriguing in light of the finding that the composition of the sporulation medium can have a significant effect on spore coat gene expression (153). The mechanism behind this relationship is not known. However, this effect may be evidence of how changes in coat protein gene expression can occur on the fly, so to speak. Possibly, the cell can sense its surroundings after sporulation has begun and can adjust the precise levels of coat protein gene expression depending on the environment. Such a mechanism would permit the spore to make last-minute alterations to the coat in response to a rapidly changing environment (88a).
BIOCHEMICAL ANALYSIS OF THE COAT
Classical studies, carried out during the 1960’s and 1970’s, established the basic biochemical features of the coat (see, for example, references 44, 66, 92, 138, and 141). These biochemical experiments demonstrated that the coat is composed largely of protein with minor amounts of carbohydrate and lipid and that the coat proteins are especially rich in the amino acids tyrosine and cysteine. An important next step in spore coat biochemistry was the analysis of partially purified coat fractions, prepared by chemical extraction procedures (5, 8, 65). This approach has been modified by a variety of researchers but generally makes use of alkaline pH, heat, reducing agents such as dithiothreitol, detergents such as sodium dodecyl sulfate (SDS), denaturants such as urea, or, most commonly, some combination of these conditions (24). The results of chemical extraction vary considerably depending on the precise method used and can be difficult to reproduce. Nonetheless, this technique permitted the isolation of individual coat proteins (by fractionation on polyacrylamide gels) and the first identification of coat protein genes, and it is still a major tool in coat protein analysis. It soon became clear that spore coats of different bacilli contain different numbers of polypeptide species, ranging from one major protein in B. cereus (10) to more than 25 in B. subtilis. These studies also found that at least some coat proteins were highly cross-linked, resulting in large amounts of insoluble material in spore coat extracts.
A variety of studies used biochemical analysis to characterize the B. subtilis spore coat (34, 35, 57, 90, 144) and coats of other Bacillus species (36, 129). A classic example of such a study is the work of Pandey and Aronson (96) (see also reference 6), in which the authors carried out an extensive characterization of the spore coat proteins of B. subtilis after extraction with dithioerythritol, SDS, urea, and 2-(N-cyclohexylamino)ethanesulfonic acid (CHES) buffer at pH 9.8. This procedure solubilized about 70% of the coat proteins. They determined that about 6% of the dry weight of this solubilized fraction was polysaccharide and that the remainder was largely protein. Six or seven major proteins (including a glycoprotein) could be reproducibly detected by SDS-polyacrylamide gel electrophoresis (PAGE), and several less reproducible minor bands were often evident as well. Synthesis of these proteins started at about the second hour of sporulation and continued until about the sixth hour. They also found evidence for proteolytic processing of coat proteins. In agreement with earlier studies, their amino acid analysis indicated that the coat proteins are tyrosine rich, and this has been borne out by subsequent sequencing of coat protein genes. This study also identified dityrosine cross-links in the coat, but this finding was not supported by a later study (35). Recently, studies using high-pressure liquid chromatography analysis have indicated that dityrosine species are present in the coat (25a).
Another important study, from the Mandelstam laboratory (57), characterized the timing of coat protein synthesis and assembly. This work confirmed earlier studies suggesting that coat protein synthesis begins several hours prior to the appearance of a functional coat (96) and indicated that coat formation involves control at the level of assembly as well as at the level of coat protein synthesis. These results anticipated the more recent findings identifying the complex roles that morphogenetic proteins play in the formation of the coat (discussed below). In further studies (56), Mandelstam and colleagues demonstrated that a protease is built into the coat in a manner that depends on the transcription factor GerE. Given the finding that some coat proteins are cleaved to a mature form, a coat-associated protease could be an important regulator of coat assembly. Attempts have also been made to reconstruct the coat in vitro, by applying coat proteins to a spore that has been chemically stripped of its coat. Reconstitution of the B. subtilis coat in a semi-in vitro system has not yet been achieved, but a similar experiment had been at least partially successful for the simpler coat of B. cereus (9).
Once individual coat proteins were identified, it became important to determine where they were located within coat layers. Mandelstam and colleagues addressed this question biochemically (57). They isolated spores at various times after coat assembly had begun and then applied an iodinating reagent that would most readily label the coat proteins that were at an outermost position. From their analysis of the labeled proteins, these authors proposed that the coat consisted of at least three layers and that the predominant proteins in the outermost layer were a 12-kDa species synthesized early and a 36-kDa protein synthesized late.
Several important lessons emerged from these classical studies. First, the complex structure of the coat is the result of a multistep assembly process that involves the ordered synthesis of a large number of coat components. Second, at least some of these proteins undergo modification of several types: proteolytic processing, cross-linking via disulfide residues and other cross-links (8), and glycosylation. Third, the proteins of the coat take up specific positions within the coat layers. It became clear that the study of the coat was an excellent model system for studies of both the control of protein synthesis during development and the mechanisms that guide the assembly of complex structures. These studies also revealed a close connection between the integrity of the coat and the ability to germinate correctly, since defects in germination were found to be characteristic of coat mutants (8). This point is discussed in more detail below, but for the moment it is sufficient to note that this connection is puzzling since there has been no evidence that any part of the germination machinery is in the coat or interacts with the coat.
INTRODUCTION OF MOLECULAR GENETICS TO THE PROBLEM OF SPORE COAT FORMATION
In 1987, Losick and colleagues cloned the first coat protein genes by using a reverse genetics approach (26). They extracted coat proteins from spores and microsequenced several of the resulting species. They then generated oligonucleotide probes based on the sequence information they obtained and used these to clone the coat protein genes cotA, cotB, cotC, and cotD from a recombinant DNA library. As a result of these experiments, it became possible to determine the consequences of deleting coat protein genes and to describe the regulatory mechanisms that govern their expression. With knowledge of the deduced protein sequences, it became feasible to explore the mechanism of coat assembly and the basis of protection from the environment through structure-function and biochemical experiments. Several findings resulted from the initial characterization of these genes. First, the sequences of CotA, CotB, CotC, and CotD were not obviously similar to those of other proteins or to each other. The finding that coat proteins were not significantly similar to proteins of other species has, for the most part, held true as more have been identified and studied. However, this does not necessarily imply that the coat proteins do not share secondary, tertiary, or quaternary features. An exception to the lack of similarity is the resemblance between CotA and copper oxidases that has been noticed by a number of researchers (31a; see below). More recently, several similarities between the coat proteins themselves have been detected. CotB and CotC show homology to CotG, a coat protein that was identified in a later study and is described below. Second, although previous biochemical studies suggested that some coat proteins would be derived from larger precursors, CotA, CotB, CotC, and CotD were not produced by cleavage of larger proteins and did not appear to be coat protein precursors. More recent studies have identified coat proteins that are derived from larger precursors (see below). Third, the viability, measurable resistance properties, and electron microscopic appearance of spores bearing deletions in any of these genes were the same as those of wild-type spores. This indicates either that these genes were responsible for functions that the researchers did not know how to measure (but which were presumably useful to the spore nonetheless) or that they were functionally redundant with respect to other intact coat proteins. These authors showed that germination of spores missing these coat proteins was indistinguishable from that of wild-type spores, with the exception of a slight alteration in germination in response to l-alanine in a cotD spore (26) (although Bourne et al. [17] reported that cotD mutant spores germinated normally). Uniquely among these mutants, cotA spores showed a significant phenotype: the loss of the characteristic brown color of normal spores. Sandman et al. (114) showed that cotA expression began after 4 to 5 h of sporulation. They further determined that expression depended on all the genes known to be required for events in sporulation up to the stage of engulfment and on a subset of the genes that acted later in sporulation. This finding demonstrated that coat protein gene expression is temporally controlled and is a part of a cascade of developmentally regulated events in which later events are dependent upon the completion of earlier ones.
The next coat gene to be identified was cotE, and, in contrast to cotA, cotB, cotC, and cotD, deletion of this gene had a striking consequence for coat assembly (151). Electron microscopy revealed that these spores possessed only an inner coat (Fig. 1D). Consistent with this morphological defect, the mutant spores were deficient in lysozyme resistance and in the ability to germinate. Despite the absence of the outer coat, at least two (and probably all) the outer coat protein genes were still expressed, suggesting that CotE was not significantly involved in outer coat protein gene expression. Therefore, CotE was judged to be involved primarily in assembly and thus was designated a morphogenetic protein to reflect its special role. These authors also realized that they could identify a coat protein as likely to be an outer coat component if it was missing from an extract of a cotE spore. Using this approach, Zheng et al. (151) showed that cotA, cotB, and cotC were probably outer coat proteins and cotD was most probably an inner coat protein. The discovery of the cotE phenotype indicated that coat proteins fall into one of two classes. Either they are not apparently required for the assembly of any coat proteins other than themselves (such as CotA, CotB, CotC or CotD), or they are required for correct assembly of other coat proteins (as is the case for CotE). Proteins that are not morphogenetic could be expected to play roles in other coat functions, such as resistance.
The cloning of cotA, cotB, cotC, cotD, and cotE was followed by a series of experiments, in several laboratories, in which coat protein genes were identified after microsequencing of proteins extracted from polyacrylamide gels. This led to the identification of the coat protein genes cotF, cotG, cotH, cotS, cotT, cotV, cotW, cotY, cotX, and cotZ (1, 17, 23, 93, 113, 148), as well as the identification of fragments of coat proteins (2). Three additional coat protein genes, cotJA, cotJC, and cotM, were identified in a search of genes activated by ςE or ςK (41, 42). Oddly, only one of these 18 genes, cotA (originally called pig to indicate that it is associated with spore pigment [111, 114]), had been identified in extensive previous screens for sporulation genes (see, for example, reference 99).
Although the number of cloned coat protein genes was now 18, it was clear that some genes remained undiscovered. This was evident for at least two reasons: there were still protein bands on SDS-PAGE analyses of coat extracts for which no gene had been described, and the complexity of the coat suggested that more proteins with clear morphogenetic roles would probably be needed to build such an ornate structure. Nonetheless, enough genes were identified for a clear outline of the transcriptional control of the coat protein genes to emerge. This program of control is reviewed, and then the mechanisms by which the gene products are built into the coat are discussed, focusing on those proteins with morphogenetic activities.
REGULATION OF COAT PROTEIN GENES
The organization of the coat protein genes into four regulons of mother cell-expressed genes (Fig. 3; Table 1) ensures that the coat proteins appear in a particular sequence and in the correct compartment during sporulation. The first regulon, activated immediately after the formation of the sporulation septum, includes cotE (153) and the cotJ operon (42). The second regulon contains a second promoter of cotE (153) and the gene encoding ςK (70, 72). The third regulon contains a large set of coat protein genes including cotA, cotD, cotF, cotH, cotM, cotT, cotV, cotW, cotX, cotY, and cotZ (23, 41, 93, 149, 153). The fourth and final regulon consists of cotB, cotC, cotG, and cotS, as well as genes that are also part of the third regulon, such as cotV, cotW, cotX, cotY, and cotZ (1, 113, 136, 149, 153). GerE, which activates the fourth regulon, also down regulates cotA and cotM (41, 152). In general, the coat protein genes are dispersed around the chromosome, but cotB, cotG, and cotH are in close proximity. Although they are transcribed by separate promoters, the possibility that their expression is coupled in some subtle way has not been excluded (93, 113, 153). Layered over this regulatory program are the effects of the feedback regulatory cascade in mother cell gene expression (discussed above).
The order of coat protein gene expression appears to be entirely a function of the gene expression program described above and is largely the same regardless of the specific protocol for inducing sporulation (153) (but see the discussion above regarding how gene expression might be quantitatively altered). However, the expression of cotC was found to be substantially higher when sporulation was induced by allowing growing cells to deplete the medium of nutrient instead of by resuspending the cells in a minimal medium. Strangely, this difference in the levels of cotC expression was largely eradicated in a spoIVA mutant (which is not known to contribute directly to gene expression). A role for spoIVA has been noted in the expression of other coat protein genes as well (78). Mutations in spoIVA impair the processing of pro-ςK to active ςK. The reason for this effect is unknown, but both SpoIVA and the pro-ςK-processing machinery are located on or close to the forespore surface (29, 106, 108). Although the localization of the pro-ςK-processing proteins is not under the control of SpoIVA, it is possible that pro-ςK processing is influenced by other proteins whose cellular position is dependent on SpoIVA.
The existence of a hierarchical cascade of coat protein gene regulation raises the question of whether coat assembly is a consequence of the order of coat protein gene expression. Such a hypothesis would be tenable if the more interior layers of the coat were composed of proteins synthesized early in sporulation and the more exterior layers were made up of proteins that appear later. In fact, an analysis of the locations of the inner and outer coat proteins and of the timing of the appearance of the coat proteins indicates that it is unlikely that transcriptional control alone guides the formation of the coat (discussed in references 29 and 134). It is evident that the inner coat protein genes are not necessarily expressed before the outer coat protein genes and that the genes encoding any one layer of the coat are not necessarily members of a single sporulation gene regulon. For example, cotE is among the first known coat protein genes to be expressed, but the gene product appears in the outermost layer of the coat as opposed to an inner layer, as might have been anticipated from the early expression of cotE. Furthermore, cotD, which encodes an inner coat protein, and cotA, which encodes an outer coat protein, are both expressed in the middle phase of mother cell gene expression. Most strikingly, spores which lack GerE and which are therefore unable to activate the final phase of mother cell gene expression, still produce some outer coat but are entirely missing the inner coat. These observations are not consistent with a sequential stepwise assembly of the layers of the coat from inside to outside (i.e., from inner coat to outer coat), and so the order of the layers of the coat cannot be simply a consequence of the temporal control of mother cell gene expression. Therefore, other mechanisms must guide coat assembly. Nonetheless, gene-regulatory controls may play an important role in coat assembly (they are simply not the only means of control), and the effects of altering the timing of coat protein gene regulation remain to be determined.
ROLES OF THE COAT AND MORPHOGENETIC PROTEINS
The formation of any complex macromolecular structure is likely to require specific morphogenetic proteins dedicated to the control of assembly. These proteins may or may not be present after assembly is complete. They might exert their control only after the relevant structural proteins have been synthesized and therefore after regulation at the level of gene expression has already occurred (as in the case of the control of phage tail length [59]), or they might enable the assembly process to feed back into gene-regulatory mechanisms (as seen for the expression of the class III flagellar genes [50]). These proteins can control assembly in several ways. They can initiate the assembly of a multimeric structure (by acting as a nucleator), they can control the length of such a structure (by acting as a ruler), and they can direct assembly to occur at specific subcellular locations. For spore coat assembly, there is compelling evidence for the existence of several morphogenetic factors. First I discuss CotE and SpoIVA, since examination of these proteins has revealed important early events in coat assembly. Then I discuss morphogenetic proteins with intermediate roles in coat assembly. Finally I examine the coat proteins that appear not to play a measurable morphogenetic role. Much of this information is summarized in Table 1.
Major Morphogenetic Proteins
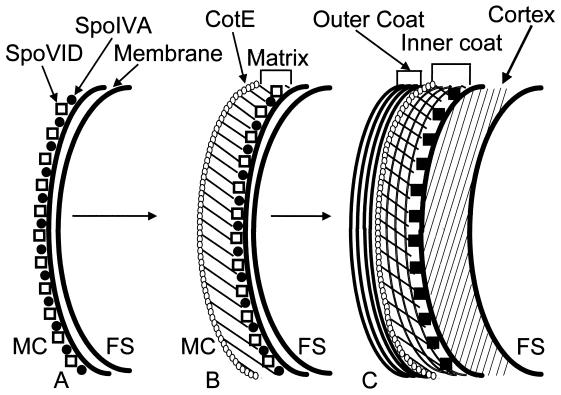
The question of how CotE participates in outer coat assembly was initially addressed by ultrastructural methods. Immunoelectron microscopy experiments indicated that CotE probably sits at the juncture of the inner and outer coat layers, consistent with its role as a coat protein required for outer coat formation (29). CotE could be reasonably assigned to the outer layer, because CotE is still present in a gerE mutant spore, in which the inner coat is missing but the outer coat is still partially intact. Zheng and Losick (153) demonstrated that CotE is synthesized early in sporulation, well before the electron-dense coat structure appears, and immunoelectron microscopy studies showed that CotE takes up a discrete subcellular location just after the formation of the sporulation septum (Fig. 4B) (29). At this time, CotE forms a layer, standing a short distance off the septum, on its mother cell side. After the forespore is engulfed to form a protoplast, the layer of CotE appears as a ring that encircles the forespore and is separated from it by a gap of ∼75 nm. Presumably, some material resides in the gap that would connect the ring of CotE to the forespore surface. Originally, this hypothetical material was referred to as the scaffold, but I propose using the term “matrix” instead to avoid the suggestion that the material in the gap is necessarily a temporary component of the coat, as would be suggested by the usage of the term “scaffold” in the virus assembly literature. An intriguing and unexplained feature of the data from the localization experiment was the tendency of CotE to cluster at the forespore poles, along the long axis of the prespore (which is coincident with the long axis of the sporangium). This is reminiscent of the way that formation of the inner and outer coat layers initiates at the poles. It is not known whether this observation has any biological significance. If it does, it might indicate that there is something unique about the poles of the forespore, perhaps as a consequence of the prior events of fusion of the septal disk (to form the septum) and then fusion after engulfment of the forespore.
FIG. 4.
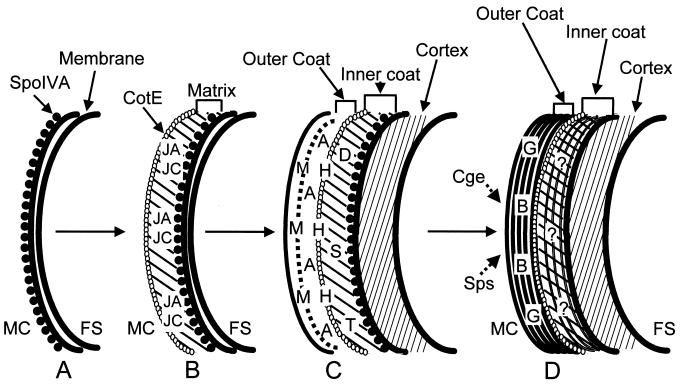
Model for the assembly of the B. subtilis spore coat. The diagrams represent arcs of the forespore surface. The letters within the diagrams indicate the possible times of assembly and locations of some of the coat proteins. For example, CotJA assembles early, in panel B, within the matrix. Once a coat protein is indicated, it is presumed to remain in the coat for the duration of sporulation; i.e., CotJA is still present although not explicitly shown in panels C and D. (A) After septation and as a result of ςE activity, SpoIVA localizes to the mother cell side of the forespore membranes. (B) The precoat, consisting of the matrix and the layer of CotE, is assembled over SpoIVA. CotJA and CotJC may be matrix components. (C) After ςK becomes active, the cortex appears and a large set of coat protein genes are expressed, of which only a subset are represented. CotD, CotH, CotS, and CotT assemble into the inner coat, and CotA and CotM are built into the outer coat. CotH could sit near CotE at the interface of the inner and outer coat layers. CotM may form a substructure within the outer coat. (D) In a final stage, under the control of GerE, an unknown morphogen (represented by ?) directs the completion of the inner coat. CotB and CotG are synthesized and incorporated into the outer coat. Further modifications to the coat, including glycosylation (due to the Sps and Cge proteins), proteolysis, and cross-linking, bring the coat to its final form. Several especially speculative aspects of this model should be noted. The products of coat protein genes that are expressed under the control of ςK are illustrated as being incorporated into the coat soon after synthesis but may in fact be assembled later, after GerE is active. For example, we do not know that CotD is assembled into the coat before CotB. FS, forespore; MC, mother cell.
Although no outer coat is apparent in a cotE null mutant spore, there are unusual clumps of darkly staining material associated with the inner coat (Fig. 1D). These clumps are present in old cultures, well after the spore is released, but are not detected during the course of sporulation (27a). The identity of this material and whether it is composed of coat proteins is unknown. It is possible that this material is composed of outer coat proteins that have formed an aggregate. These structures could represent an alternate dead-end assembly product formed by the outer-coat proteins in the absence of CotE. A very curious feature of cotE null mutant spores is that the remaining inner coat, which is often missassembled and appears to be falling off the spore, is not tightly appossed to the cortex surface, as is the inner coat of a wild-type spore (Fig. 1B). This gives the impression that the inner coat is not in direct contact with the cortex surface in this mutant. Given that CotE resides between the inner and outer coat layers, it is unclear how a cotE mutation causes this apparent disconnection between the inner coat and the cortex. Aronson and Fitz-James (9a) have noted that the undercoat layer, which they have identified as a coat layer between the inner coat and the cortex, is not found in cotE mutant spores. Possibly, the connection occurs via the undercoat layer. Alternatively, it is also possible that in a wild-type spore, the outer coat acts something like a drawstring, squeezing the inner coat into a compressed state, thus forcing the cortex and inner coat into close contact and giving the appearance that the two coat layers are linked. The absence of the outer coat in a cotE mutant would then allow the inner coat to expand away from the cortex surface. This implies that the inner coat and the cortex are not connected, at least by the time the spore is mature. This view is entirely speculative, but the possibility that the coat can go from a compressed to an uncompressed state and thereby allow the spore to achieve a larger diameter might help to explain the increase in spore size as it swells during germination (115).
The ring of CotE and the matrix together constitute the precoat (Fig. 4B). This precoat assembles at the forespore surface early in spore formation, preceding the appearance of the electron-dense coat that is seen in the mature spore (29, 101). The next step in coat formation is the assembly of the inner and outer coat proteins (Fig. 4C). Presumably, the inner coat proteins infiltrate into the gap between the ring of CotE and the forespore membrane surface to form the lamellar inner coat. The width of the gap between the ring of CotE and the forespore membranes is roughly equal to the width of the inner coat, suggesting that the dimensions of the ring of CotE are relatively stable once the precoat is assembled. The outer coat proteins would simultaneously assemble around the ring of CotE to form the darkly staining outer coat. The primary role of the precoat, therefore, would be to establish the two major layers of the coat.
The existence of a precoat located at the spore surface leads to the question of what guides the precoat to that location. The answer turns out to be a protein called SpoIVA. It was known from classic studies of sporulation mutants that this protein was required for correct coat assembly (99). In a spoIVA mutant, the coat is assembled but does not encircle the forespore as in a wild-type cell. Instead, the coat appears as swirls in the mother cell cytoplasm, unattached to the forespore. The finding that coat assembly is not entirely prevented implies that SpoIVA does not nucleate coat assembly, distinguishing it from CotE. In addition to the coat assembly defect, there is no cortex (the thick cell wall of the spore) between the double membrane layers of the forespore in spoIVA cells. spoIVA expression is ςE directed, implying that SpoIVA synthesis occurs in the mother cell compartment (109, 128). It would seem plausible, therefore, that SpoIVA somehow attaches the coat to the forespore surface. Immunoelectron and immunofluorescence microscopy studies suggested that this view was likely to be correct in that SpoIVA forms a ring around the forespore, very close to or directly on the forespore membranes (Fig. 4A) (29). In light of the partially polar localization of CotE mentioned above, it should be noted that SpoIVA did not show polar localization but, rather, formed a relatively contiguous ring around the forespore. When the precoats of spoIVA sporangia were examined by immunoelectron microscopy, they were found to be detached from the forespore and to form clumps in the mother cell cytoplasm, reminiscent of the swirls of coat seen at a later time in sporulation in this mutant (29). Taken together with the fact that spoIVA is transcribed by the mother cell-active factor ςE, it appears that SpoIVA sits on the mother cell side of the forespore membranes and attaches the matrix to the forespore. Therefore, the classic observation that a spoIVA mutation causes the coat to form as a swirl in the mother cell cytoplasm can be explained by a model in which SpoIVA attaches the precoat to forespore at an early time in sporulation, before the electron dense coat is apparent. Experiments with immunofluorescence microscopy (101) confirmed the locations of CotE and SpoIVA and further demonstrated that SpoIVA localizes to the forespore before CotE does, an important prediction of the model proposed from the immunoelectron microscopy studies.
An intriguing feature of SpoIVA is its participation in both cortex synthesis and coat assembly. It is tempting to speculate that the formation of these two structures is coupled via SpoIVA. Such a role is plausible given that SpoIVA sits between the cortex and coat layers (29). The reliance of coat and cortex synthesis on a common protein could be useful to the cell in at least two ways. It could ensure that the two structures are localized to the two sides of a common membrane (the outer of the two forespore membranes), and it might help to coordinate the formation of the cortex and the coat whose periods of assembly largely overlap (25, 126).
SpoIVA is not the only protein with roles in the formation of both the cortex and the coat. SpoVM and SpoVS also play important roles in cortex and coat formation (19, 74, 107). Unlike SpoIVA, they are less likely to be directly involved in these processes, since they affect several other sporulation processes as well, apparently at relatively early times in development. It is likely that the roles of SpoVM and SpoVS in cortex and coat formation reflect pleiotropic effects of these proteins rather than specific couplings between the biosynthesis of the coat and the cortex.
An additional protein, SpoVID, is also involved in the attachment of the coat to the forespore. Beall et al. (13) identified spoVID in the course of searching for ςE-directed promoters. spoVID cells displayed a previously unseen phenotype: the coat was detached from the spore, in much the same way as for spoIVA mutant spores, but the cortex was intact. As a result of the presence of the cortex, the spore was fairly robust, and coatless spores were released when the mother cell lysed at the end of sporulation. Because the spoVID mutant appeared to be similar in phenotype to a spoIVA mutant, at least as far as the defect in coat assembly was concerned, it was a surprise to learn that the precoat is not detached in a spoVID mutant (29). This suggested that, whatever the role of SpoVID in coat attachment, it functioned after SpoIVA had established a connection between the precoat and the forespore. This notion was supported by the finding that in a spoVID mutant, SpoIVA was still correctly located on the forespore. It appears that SpoVID is involved in the attachment of the coat to the forespore at late times, as evidenced by the swirls of coat, but that it is not essential for the initial attachment of the precoat. SpoVID was not detected in mature spores (29) and so might not be a coat protein. The precise role of SpoVID in coat assembly remains unclear, in part because it is not obvious what requirement there would be for an attachment protein in addition to SpoIVA. A possible clue to its role might be the presence of a cortex in spoVID cells. Perhaps, because SpoIVA participates in coat assembly as well as cortex synthesis, it must pass off the function of coat attachment to SpoVID when the matrix is complete and cortex assembly commences.
Morphogenetic Proteins with Intermediate Roles
Not all proteins involved in the coat have either severe effects when missing (such as CotE) or virtually no detectable consequence (such as CotC). Several proteins that have important but less absolutely required roles in guiding coat construction have been identified. One of these is CotT. The absence of CotT has only a subtle effect on coat function: spores respond more slowly to germination with glucose, fructose, and l-asparagine (although normally with l-alanine plus inosine), but the spore is otherwise normal in resistance properties (17). The inner coat, while not entirely missing, is reduced in thickness in cotT spores. Spores from a strain that overproduces CotT have thicker inner coats and are slow to germinate not only in response to glucose, fructose, and l-asparagine but also in response to l-alanine, indicating a more severe defect. CotT is notable for its skewed amino acid composition: 13 common amino acids are not present, and there is a preponderance of tyrosine, proline, and glycine residues (11, 17). The carboxy-terminal 17 amino acids are exclusively tyrosine and glycine. CotT is a 7.8-kDa protein that is generated by the cleavage of a 10.1-kDa precursor form. In a strain bearing both a wild-type copy of cotT and a mutant copy whose product cannot be cleaved to the mature form, the precursor is associated with the spore coat. It is plausible that processing occurs after CotT has assembled into the coat. Processing appears to be under the control of GerE, based on the presence of the 10.1-kDa but not the 7.8-kDa species in a gerE mutant. Possibly, after a CotT precursor is built into the coat, a late event under the control of GerE results in maturation. The significance of these observations is unclear, but it seems plausible that CotT is a structural component of the inner coat and acts either at the level of matrix assembly or later as the inner coat is assembled. cotT transcription is probably under the control of ςK, since it is coincident with that of cotD (149). There is unpublished data (17) indicating that CotT is an inner coat protein, like CotD.
CotG is another coat protein with an intermediate effect on assembly when deleted (113). CotG is a 24-kDa protein, but the protein isolated for microsequencing by SDS-PAGE migrated as a 36-kDa protein (43). It is not known whether this larger species is the result of aberrant mobility of CotG or the formation of a dimer that resists denaturation during electrophoresis. CotG has an unusual primary sequence. It is predicted to be highly hydrophilic, and almost half of its amino acids are positively charged. Even more strikingly, about 60% of CotG is composed of nine tandem copies of a 13-amino-acid lysine-, serine-, arginine-, and tyrosine-rich sequence that is similar among the tandem copies. Both the lysine and tyrosine residues could participate in the formation of cross-links within CotG and with other coat proteins. cotG is transcribed by ςK in conjunction with GerE. CotG is not incorporated into the coat in a cotE mutant and is therefore regarded as an outer coat protein. SDS-PAGE analysis of cotG spores detected the loss of an additional coat protein besides CotG: the outer coat protein CotB. Intriguingly, CotB and CotG appear to be have significant homology (see below), and both are especially cysteine rich. A region within the carboxy terminus of CotB is composed almost entirely of cysteine residues. The other predominant residues in this region are lysine, arginine, aspartate, glutamine, and tyrosine. Like cotG, cotB is also under the control of ςK and GerE. There is no strong effect of a cotG mutation on spore resistance or germination properties, implying that CotG and CotB are not required for these functions. Surprisingly, spores missing CotG have a significant defect by electron microscopy (43). Although the inner coat has a normal appearance, the outer coat is highly abnormal. Instead of the usual set of dark layers, the outer coat is largely diffuse in appearance, with a single, thin, darkly staining band remaining. Since the only significant change in the overall polypeptide composition of the coat is that CotG and CotB were missing (as far as could be detected), it is puzzling that there would be such a dramatic change in the electron-dense features of the coat.
A possible explanation for the apparent importance of CotG in coat structure comes from a remarkable observation from the Moran laboratory. They found that in the absence of SodA, a manganese-dependent superoxide dismutase (18), the morphology of the coat was grossly altered (43). The number of inner coat layers was decreased, and the outer coat was also reduced in thickness. A relatively electron-translucent gap separated the remnants of the two layers. In spite of the significant change in the structure of the coat, the polypeptide composition was altered primarily only in that greater amounts of CotG could be extracted from the coats of mutant spores than from those of wild-type spores. Consistent with this minor change in the extractable coat proteins, the resistance properties of sodA spores were essentially unchanged. These researchers propose that SodA activity is ultimately responsible for the cross-linking of CotG into the coat. This role would be in addition to its involvement in the protection of growing cells (18). In a cotG spore, these cross-links would not be made and this would be reflected in the coat structure. Since the structural defect in a cotG spore coat is different from and apparently more severe than that of a sodA spore coat, other proteins may also participate in CotG cross-linking. They also found that the coat of a sodA cotG spore was similar in structure to the coat of cotG spore. This suggests that SodA acts largely through CotG. A notable potential implication of this model is that the modification of the coat by postassembly cross-linking is directly responsible for much of the striated appearance of the coat.
The ability of superoxide dismutases to produce hydrogen peroxide suggests that SodA could provide hydrogen peroxide to a coat-located peroxidase, which could then cross-link the tyrosine-rich CotG either into a multimeric form or to other coat proteins. A possible candidate peroxidase would be CotE (see below). Previously, it was shown that SodA is dispensable for protection against oxidizing agents or heat (18), and it is possible that the primary role of SodA is in coat assembly. The catalase activity of CotJC (120) (see below) could be involved as well, by helping to control the levels of available hydrogen peroxide. Although the details are unclear, these results provide a compelling case for the further investigation of oxidative mechanisms in coat assembly. It would be interesting to generate a sodA mutation which permits synthesis of the full-length protein but which does not generate the superoxide dismutase activity and to characterize the resulting phenotype. Furthermore, it would be worthwhile to learn if there is an appropriate oxidase activity in the coat to provide superoxide for SodA.
Another example of a mutation that has a clear morphological consequence but no measurable effect on spore resistance or germination is cotM (41). cotM is transcribed under the control of ςK and is negatively regulated by GerE, similarly to cotA. CotM has not been found in spore coat extracts, but cotM spores have reduced amounts of several coat proteins, including the outer coat protein CotC (but not the inner coat protein CotD). In this regard, it differs from other morphogenetic proteins such as CotE and CotG in that when cotM is deleted, the levels of several coat proteins are reduced but they are not absent. In the electron microscope, the outer coat of a cotM mutant appears diffuse and its peripheral structure is altered in two ways. First, several thin lamellar layers, reminiscent of the inner coat, are visible in the outermost layer of the outer coat. Second, instead of the usual smooth appearance of the outer surface of the coat, the mutant spore surfaces often appear to have a series of fine ridges. It is possible that the cotM mutation results in the loss of some outer coat material and exposes structures that are occluded when the full structure is present. Based on the changes in coat protein composition and the alterations in outer coat morphology in a cotM mutant, it appears that CotM is involved mostly in outer coat formation and might be an outer coat component. The 40 amino-terminal-most amino acids are highly acidic, and the middle portion of the protein is more hydrophobic. CotM shows similarity to α-crystallin low-molecular-weight heat shock proteins, particularly in its carboxy-terminal 44 residues. Members of this family have a number of activities that might be expected of a morphogenetic coat protein, including chaperone functions and the capacity to act as substrates for cross-linking by transglutaminases (49, 54, 83). It is reasonable to imagine that CotM is cross-linked by transglutaminases as well, particularly in light of the presence of such activities in the coat (64; see below). Henriques et al. (41) propose that at an intermediate time in coat assembly, CotM helps to build an insoluble structure (perhaps around the shell of CotE) that is required for the assembly of a robust outer coat. In its absence, an outer coat is assembled but its outermost layers are not properly formed and it is has less overall structural integrity, as evidenced by the loss of outer coat proteins in a nonspecific manner. Two genes adjacent to cotM, cotK and cotL, have been tentatively designated coat protein genes, based on their proximity to cotM and the presence in their deduced amino acid sequences of clusters of lysines, suggesting the possibility that their protein products are cross-linked to CotM by a transglutaminase. They potentially encode 6.1- and 5.4-kDa proteins, respectively.
CotH plays a role in outer coat assembly, but in a somewhat different way from CotG and CotM (93). cotH lies between cotB and cotG on the chromosome and is expressed under the control of ςK; therefore, it will be transcribed in the mother cell at an intermediate to late time in sporulation. cotH spores have a small but detectable germination defect and normal resistance properties. Although the effect of a cotE mutation on the presence of CotH in the coat has not been reported, a cotE cotH strain has a more severe germination deficiency and is more lysozyme sensitive than a cotE mutant. This suggests that CotH is present in a cotE spore and therefore that CotH is an inner coat protein. Oddly for an inner coat protein gene mutant, cotH spores are missing the outer coat proteins CotB and CotG as well as CotH and have a reduced amount of CotC. It may be noteworthy that CotC and CotG are relatively homologous and both these proteins have some identity to CotB. Even more intriguingly, the difference in germination between a cotE mutant and a cotE cotH mutant argues that the role of CotH is likely to be more complex than simply assembling these other coat proteins, since these other coat proteins are not known to play any role in germination. Whatever this second role may be, it would seem that CotH works in conjunction with CotE in guiding outer coat assembly and that both proteins are required. CotH might control a set of contacts with outer coat proteins that are independent of the contacts mediated by CotE, in which case both sets of contacts would be critical. Alternatively, CotH could work through CotE, with CotE controlling the interactions with the outer coat proteins. In this case, a cotH mutation would prevent CotE from making the appropriate contacts.
Coat Proteins with No Known Morphogenetic Roles
The absence of CotA has no detectable effect on spore properties, but it does prevent the appearance of the usual brown color of the spore coat (26, 114). Although this pigmentation is of uncertain utility to the spore, it is a useful marker of outer coat assembly and a convenient way to confirm that a colony has entered a late stage of sporulation. CotA shows strong similarity to multicopper oxidases (31a, 140). It is not known if CotA has such an activity in B. subtilis, but the possibility that CotA is an oxidase is intriguing in light of the involvement of the superoxide dismutase SodA and the possible catalase CotJC in the coat (see below). As discussed above, cotA is under the control of ςK and is repressed by GerE.
Also within the ςK-directed regulon is cotF (23). There is no detectable consequence to the coat or to sporulation when this gene is deleted. cotF specifies a 19-kDa protein, but the product of the gene is processed to give 5- and 8-kDa coat proteins. The 5-kDa protein is encoded within the 5′ portion of cotF. In addition to the cleavage site that generates the 5- and 8-kDa species, there appears to be a second cleavage site that allows the removal of first five amino-terminal amino acids, since this sequence is not present in the 5-kDa protein. It is not known whether this cleavage occurs before or after CotF is assembled into the coat.
cotJA and cotJC are, along with cotE, the only known coat protein genes under the control of ςE (42, 120). CotJA and CotJC are encoded by an operon that comprises cotJA, cotJB, and cotJC. CotJA and CotJC are both coat components that are present in spores of a cotE gerE mutant strain. Therefore, they are unlikely to be components of either the inner or outer coat. Most probably, they reside in an early-assembled layer of the coat, either the under-coat layer or the matrix. Studies of the interactions made by the CotJ proteins indicate that the two proteins form a complex in the coat. CotJA and CotJC have been shown to interact with each other in vivo (by using immunoprecipitation experiments) and in vitro (by using the two-hybrid system). Furthermore, the incorporation of CotJA into the coat requires CotJC and the assembly of CotJC requires the presence of CotJA. A species of the correct size to be a CotJA-CotJC heterodimer is present in coat protein extracts and reacts with anti-CotJC antibodies. The function of cotJB is unknown, but there is no reason to exclude the possibility that it encodes a coat protein as well. In addition, there is a second, cotJ-dependent species of about 75 kDa that also depends on GerE. Although CotJC (and possibly CotJA) is an abundant coat protein, deletion of the cotJ operon has no effect on measurable spore properties. A notable sequence feature of CotJC is the significant number of acidic residues in the carboxy-terminal one-third of the protein (13 of 50 residues [42]). CotJC shows significant homology to a catalase from Lactobacillus plantarum (120). Although CotJC has not yet been shown to have catalase activity, it is a candidate participant in oxidative cross-linking of coat proteins.
Several coat protein genes that are transcribed by ςK both alone and in conjunction with GerE have relatively subtle or intermediate effects on the coat when deleted. CotX, a protein with a predicted size of 18.6 kDa, falls into this class (148). It is encoded by an operon that also contains cotV and cotW. CotX was identified by microsequencing of a peptide purified from a hydrolysate of the insoluble fraction of the coat. cotX expression is complex (149) and initiates from two promoters, one which directs a transcript with cotX alone and which is absolutely dependent on both ςK and GerE, and another which directs a multicistronic transcript containing cotVWX and which is transcribed both by ςK and GerE and by ςK alone. cotX spores are largely normal, except that they germinate slightly faster than wild-type spores and their surface characteristics are altered, as evidenced by a tendency for the outer coat to stain abnormally in electron microscopy and for the spores to clump. Also, the relative amounts of insoluble and soluble coat fractions are abnormal in cotX mutant spores, with less coat protein appearing in the insoluble fraction. CotX appears to be present primarily in the insoluble protein of the coat. cotV and cotW were identified by their proximity to cotX, and they have not yet been deleted; therefore, their roles in the coat are unknown. CotV and CotX have significant homology.
Two other coat proteins, CotY and CotZ, are encoded immediately downstream of the cotVWX operon (149). They are cysteine rich, have significant identity, and are transcribed from a single promoter, which is activated by ςK and also by ςK and GerE. This promoter produces both cotY and cotYZ transcripts. CotY is a 17.9-kDa coat protein. High-molecular-weight species that react with an anti-CotY antibody are also present in coat protein extracts, and these may represent disulfide-linked multimers of CotY. CotY does not appear to be required for normal spore properties, but a cotY mutation does result in a subtle germination defect that is similar to the cotX phenotype. cotZ encodes a 16.5-kDa protein that has not yet been found in the coat. CotZ is unlikely to make a significant contribution to the usual spore resistance properties, since spores missing cotX, cotY, and cotZ simultaneously are phenotypically similar to those harboring deletions of only cotX and cotY. However, the outer coat of the triple mutant is reduced in thickness. This implies that CotY and/or CotZ contributes to proper assembly of the coat, possibly in combination with CotX. A band corresponding to CotX was not detected by SDS-PAGE in coat extracts from wild-type spores, and extracts of spore coats from cotX spores were similar to those of coats of wild-type spores, except that two bands, at 18 and 26 kDa (the larger of which reacted with an anti-CotY antiserum) were more intense. These two bands were missing in cotYZ and cotXYZ mutants. The 18-kDa protein is likely to be CotZ. Therefore, CotY and CotZ may be more easily extracted from a cotX spore and coat cross-linking may be somewhat reduced in this strain. An anti-CotX antibody identified two bands, of 24 and 48 kDa, in a Western blot of coat extracts. It is not known whether these species represent monomer and dimer forms of CotX or heteromeric complexes.
CotS is a 41-kDa coat protein, whose absence has no detectable effect on germination or resistance (1, 136). cotS expression requires both ςK and GerE, and the assembly of the gene product into the spore is under the control of CotE. Immunoelectron microscopy experiments indicate that CotS is an inner coat protein. This is noteworthy in light of the previously described role for CotE in outer coat assembly (151), and it suggests that CotE plays a role in inner coat formation as well. Alternatively, the failure to retain CotS in the inner coat could be an indirect consequence of the apparently misassembled inner coat of a cotE mutant spore. This result also serves as a caution regarding the practice of identifying coat proteins as outer coat proteins if they are missing from a cotE mutant spore or as inner coat proteins if they remain spore associated. Such judgments should be regarded as preliminary pending localization of the coat protein by direct methods.
Posttranslational Modification of the Coat Proteins
In addition to structural and morphogenetic coat proteins, it is reasonable to suspect that there will be coat proteins as well as non-coat-associated proteins that are involved in postassembly modification of the coat. One important set of modifications is the cross-linking that contributes to the strength of the coat. Several types of cross-linked species have been implicated in coat assembly, including disulfide cross-links (8) and dityrosine cross-links (96). The potential importance of dityrosine species to the integrity of the coat is reinforced by the preponderance of tyrosine residues in the sequences of the known coat proteins and the recent detection of dityrosine or dityrosine-related species in the coat (25a). Initial studies have found that CotE overproduced in Escherichia coli has a modest peroxidase activity (25a). This activity could generate dityrosine cross-links and thereby contribute to the formation of the outer coat by CotE. Aronson and Fitz-James raised the possibility that a ɛ-(γ-glutamyl)lysine cross-link, typically generated by a transglutaminase activity, is important in coat assembly (8). Subsequently, Kobayashi and colleagues identified a transglutaminase activity in the coat, encoded by tgl (64, 73). It will be informative to determine the controls on the activity of this transglutaminase and its precise location within the coat.
In addition to modification by cross-linking, there is evidence for glycosylation of coat proteins late in development. Roels et al. (109) identified a cluster of genes, called cge genes, that are activated under the control of GerE. One of these, cgeD, is similar to glycosyl transferases that participate in polysaccharide biosynthesis. Mutations that deleted genes in this cluster resulted in spores that appeared normal by the standard assays and by electron microscopy. However, these spores tended to aggregate and had abnormal adsorption properties, suggesting a surface alteration. It is possible that the cge locus encodes one or more coat proteins, but it is perhaps more likely that it is responsible for a terminal step in coat formation that involves the glycosylation of the coat. If so, the cge genes probably do not function alone. Hullo and colleagues at the Institut Pasteur (50a) have identified a cluster of 10 genes which also show homology to polysaccharide biosynthetic genes and whose expression is repressed by GerE. One of these, spsA, is very similar to cgeD. Spores from a strain missing the entire sps operon were similar to those missing both cgeA and cgeB and were very hydrophobic. The deletion of the sps locus does not result in a gross morphological defect in spore structure (27b), but the effects of these genes on the coat have not yet been fully investigated. Glycosylation of the spore surface could determine the hydrophobicity of the spore and therefore its adherence properties (143). It is unknown whether genes similar to cge or sps could be responsible for the significant carbohydrate content of the exosporia of species such as B. cereus (92). The cge and sps genes have, so far, been identified only in B. subtilis, which does not have a clearly defined exosporium. A final modification that deserves mention is the proteolytic processing of certain coat proteins, such as CotF and CotT, already discussed above. Little is known about how or precisely when coat protein processing occurs. The enzymes responsible for these cleavage events have not been identified, but a GerE-dependent coat-associated protease activity has been found (56). It is unclear whether these coat-localized proteases are involved in coat maturation, and no genes that might encode these activities have been identified.
Certainly, the genes and activities listed here do not constitute all the players in coat morphogenesis. In particular, it seems likely that there is at least one undiscovered morphogenetic factor under the control of GerE. gerE spores appear to be missing any discernible inner coat (27a, 87). Therefore, it seems likely that some gene in the GerE regulon plays a significant role in inner coat assembly. Alternatively, it is possible that no single inner coat protein plays a significant morphogenetic role in inner coat formation but, rather, that a number of GerE-controlled genes are required together for the inner coat to be built. Studies of the known GerE-controlled genes have not yet clarified this issue. Deletion of these genes either individually or, in some cases, in various combinations has not resulted in significant morphological phenotypes.
MODEL FOR COAT ASSEMBLY
From the results discussed above, it is possible to propose a tentative model to explain how the layered structure of the coat is established and how coat formation is directed to occur at the location of the spore surface (Fig. 4). In this view, there are four major steps in coat assembly: binding of SpoIVA to the forespore surface (Fig. 4A), formation of the precoat (Fig. 4B) (both of these steps occur under the control of ςE), a ςK-dependent phase of inner and outer coat layer assembly (Fig. 4C), and a ςK-plus-GerE-dependent phase of inner and outer coat layer assembly that includes postassembly modification of the coat (Fig. 4D).
The assembly of the coat begins when ςE becomes active in the mother cell, immediately after the appearance of the sporulation septum. As a result of the action of ςE, several proteins with crucial roles in coat assembly are synthesized. One of these, SpoIVA, appears to act first by positioning itself at the mother cell side of the septum (Fig. 4A) (29, 101). The nature of the interaction between SpoIVA and the membrane is unknown. The amino acid sequence of SpoIVA does not suggest the presence of transmembrane regions, possibly indicating that SpoIVA localization requires an as yet unidentified protein. At this time, SpoVID probably also binds at this location (29) (Fig. 5A). (The function of SpoVID is given in a separate figure because of the complexity of its role and the especially speculative nature of this aspect of the model.) Since SpoIVA remains attached in a spoVID mutant, SpoIVA is not dependent on SpoVID for attachment to the forespore (29). It is not known whether SpoVID attachment depends on SpoIVA.
FIG. 5.
Roles of SpoIVA and SpoVID in attachment of the coat to the forespore membrane. The last two stages represented in Fig. 4 are compressed into a single diagram in panel C. The open boxes represent the early form of SpoVID, when it is not required for matrix attachment. The filled boxes represent the late state of SpoVID, when it becomes required for matrix attachment. FS, forespore; MC, mother cell.
The localization of SpoIVA at the mother cell-facing surface of the septum allows the second step in coat assembly, i.e., the formation of the precoat on top of the layer of SpoIVA (Fig. 4B). The precoat consists of a layer of CotE that is parallel to the plane of the septum and is on its mother cell side. It is separated from the septum by a gap, which is filled by the matrix. In the next step, the inner coat proteins infiltrate into the matrix and form the inner coat. Therefore, the matrix should be a loose porous network into which the inner coat proteins can infiltrate. cotE is expressed under the control of ςE, and it is reasonable to propose that matrix synthesis requires ςE as well (29). The components of the matrix are unknown, but CotJA and CotJC are possible candidates (see above). After engulfment, the forespore is encircled by what appears to be a closed shell of precoat, which may impart some stability to the spore. It is not, however, sufficient for resistance properties, such as resistance to lysozyme, or for germination, since spores missing one or both layers of the coat but retaining the precoat are deficient in resistance and in germination (27a, 87, 151). The precoat does not require SpoVID for attachment to the forespore (29).
The precoat sets the stage for the third step in coat assembly: the appearance of the inner and outer layers of the coat (Fig. 4C). As discussed above, gene expression during this final stage is under complex temporal control. The result is the production of a large number of coat proteins in at least two phases of synthesis (153). Likely inner coat proteins such as CotD, CotH, CotS and CotT presumably assemble into the loose skeleton of the matrix, between the shells of SpoIVA and CotE, while outer coat proteins such as CotA and CotF assemble around the shell of CotE in a CotE-dependent manner. CotT may participate in defining the precise width of the final inner coat. CotE is responsible for the assembly of a significant number of outer coat proteins (151) and one likely inner coat protein (136). Since it is unlikely that CotE directly contacts so many proteins, it probably interacts with at least one, as yet undiscovered, subsidiary morphogen that aids coat protein nucleation. At least some proteases that cleave coat protein precursors to result in their mature forms could become active at this time. This may also be the time when CotM forms a cross-linked superstructure that helps to keep the outer coat intact. CotH would work in conjunction with CotE to guide outer coat assembly. Possibly, CotH sits alongside CotE in the shell that surrounds the forespore (Fig. 4C and D). From this location, it could direct the assembly of a subset of the outer coat and still remain attached to the inner coat in a CotE-independent manner.
Around the time when the coat is visible by electron microscopy, SpoVID becomes required for the continued attachment of the coat; in its absence, the coat forms swirls in the mother cell (13). Because SpoVID is dispensable for precoat attachment, we speculate that when SpoVID is initially placed at the mother cell side of the forespore membranes, it is not required for binding to the matrix (Fig. 5A and B). After some point (perhaps the synthesis of the cortex), it becomes required for coat attachment by switching to an active form (Fig. 5C), explaining why a spoVID strain produces detached swirls of coat. Although the reason for an additional protein of this sort is not known, it is plausible that the requirement for SpoVID is a consequence of the role of SpoIVA in cortex synthesis as well as in coat attachment.
Several coat protein genes are expressed late under the control of GerE and ςK (Fig. 4D). As far as is known, the gene products reside in the outer coat. One of these, CotG, directs the assembly of CotB and also contributes a large degree of the layered appearance of the coat, possibly by directing cross-linking events (113). Therefore, a set of events important for outer coat morphogenesis occurs only after GerE acts. The appearance of the inner coat also depends on GerE, raising the possibility that an unknown inner coat morphogen becomes active at this time as well. The observation that the inner and outer coat layers form together and that there are no obvious morphological intermediates prior to the simultaneous appearance of these two layers may indicate that most or all of the electron-dense features of the coat usually appear only after GerE becomes active. However, a gerE spore has a thin remnant of outer coat. Therefore, it is alternatively possible that there is a brief ςK-dependent period of morphogenesis that builds a small part of the outer coat, which is rapidly followed by the assembly of the full inner and outer coats.
In the final stage of coat assembly, the correctly positioned coat proteins are further cross-linked and modified. This is likely to include, at the very least, GerE-directed events controlled by the cge, tgl, and sps genes. Additionally, there is at least one GerE-dependent coat-associated protease that could play a role in coat protein cleavage (56). These final events remain largely unexplored, in part because of the considerable technical challenges in performing the relevant biochemistry. Nonetheless, characterizing these modifications is critical to understanding coat function. Progress in this area, in particular in understanding glycosylation, will be accelerated now that the B. subtilis Genome-Sequencing Project has been completed (73).
FUNCTIONS OF THE COAT
The major known function of the coat is to protect the spore. Clearly, both the inner and outer coat layers play critical roles in protection. The contributions of the inner and outer layers to this function can be measured by analyzing mutant spores that have not assembled one or the other layer. Traditionally, the ability of the coat to act as a barrier has been studied by measuring its ability to protect the cortex from lysozyme. Studies of cotE spores indicate that the outer coat is important for lysozyme resistance (151). Spores from a gerE mutant strain, in which the inner coat is missing and only a remnant of the outer coat remains, are severely lysozyme sensitive (more so than cotE spores) (87) and are about as sensitive as spores missing both the inner and outer coat layers (as a result of combining the gerE and cotE mutations) (27a). The ability of the coat to withstand lysozyme appears to be due, at least to some degree, to a sieving function. The size of molecules that can pass through the coat has been estimated to be approximately 8 and 2 kDa for B. cereus and B. megaterium, respectively (95, 117). Although spores from these organisms have coats with structures that are distinct from B. subtilis spore coats, they are both resistant to lysozyme, and it seems likely that the coats in all three species play a similar protective role. This degree of porosity is important, because the coat must allow small-molecule germinants to pass through to the spore interior (see below). This sieving capacity is apparently a property of the whole coat, not of any one coat protein, since B. subtilis spores are largely resistant to lysozyme when of any one of the coat protein genes are deleted with the exception of cotE. These deletions may, however, alter as yet untested activities of the coat. These other activities may rely on the biochemical properties of certain coat proteins, apart from the manner in which these proteins contribute to the integrity of the coat as a whole. It is therefore still unclear whether the complete defensive function of the coat absolutely depends on any individual coat protein or whether it is a collective property of an intact coat.
To avoid confusion, it is worth mentioning several resistance properties that do not depend on the coat. The most thoroughly described example is heat resistance. This property depends on the dehydrated state of the spore core, which, in turn, depends for its maintenance on the cortex (104). The cortex also provides resistance against organic solvents (84). The spore chromosome is protected against UV radiation and free radical damage by the small acid-soluble proteins to which it is bound (119). Although these types of protection do not rely principally on the coat, it is possible that the coat enhances them (94a).
The coat is also involved in the ability of the spore to convert from the dormant state back to vegetative growth. This process is called germination, but the term “germination” refers more strictly to an initial phase of the process, during which the spore senses a nutrient in the environment (usually a sugar molecule, an amino acid, or some other small molecule) and then begins the process of rehydration that precedes the resumption of metabolism (31, 88). In the next phase, called outgrowth, the cortex diminishes in width and the coat cracks open to liberate the revived cell (115). The peptidoglycan layer that will become the cell wall of the outgrowing cell is actually visible in the dormant spore as a thin, dark layer between the inner forespore membrane and the thick whitish cortex. This germ cell wall does not break open along with the coat.
The influence of the coat on germination was noted in early studies and has remained unexplained (8). Spores with severe defects in coat assembly (due to mutations in cotE or gerE) are highly deficient in germination as determined by the tetrazolium overlay assay (55, 151), an indirect test that measures the resumption of growth after germination (53). Spores missing CotH or CotT are also deficient in germination (17, 93). These mutant spores are not entirely incapable of germinating, and a significant (but lower than wild-type) proportion of spores do break dormancy. The coat is unlikely to play an active role in germination, since none of the known germination machinery is coat associated. Therefore, the role for the coat in germination is usually regarded as a passive one which merely permits or hinders the passage of germinants to the interior of the spore, where the germinant receptors are likely to be located. This is somewhat paradoxical, however, in that if the coat is merely a sieve, removal of part of the coat might be expected to accelerate germination, not inhibit it. In our own studies, we found that a spore with a particular mutant version of CotE, which assembled an outer coat of altered structure, seemed to germinate either more quickly or more completely than the wild type did (12a). Mutants with other versions of CotE, which were more severely defective in coat assembly, were highly deficient in germination. These results may not be consistent with the role of the coat in germination being that of a simple sieve. In addition to whatever passive role the coat may play in controlling the entry of germinants into the spore, it could also play an active role if, for example, coat proteases initiate cracking. In any event, the germination deficiencies of cotH and cotT spores are likely to be due largely to early events in germination that occur prior to cracking (17, 93).
It has been pointed out that proteins critical to germination, such as germinant receptors, could conceivably be sensitive to some of the endoproteases produced during sporulation (36a). The coat could protect these sensitive germination components during spore assembly. If this is the case, the absence of certain coat proteins would result in limited proteolysis of some of the germination machinery. The importance of the coat to germination would then be more an accident of the physiology of sporulation than a reflection of a fundamental role in the process.
Insights into the functions of the B. subtilis coat may well come from studies of other species. An example is the study of the marine Bacillus sp. strain SG-1. This bacterium encases itself in a crust of manganese oxide that is clearly visible by electron microscopy as an electron-dense halo (112). A wide variety of microorganisms are able to catalyze the oxidation of manganese, and this makes a significant contribution to the geochemical cycling of this element (94). The shell of manganese oxide around SG-1 spores is in direct contact with the outer surface of the spore, and its appearance is under the control of the mnx operon (139, 140). One gene within this operon, mnxG, shows homology to several genes that encode multicopper oxidases, a diverse group of proteins that utilize multiple copper ions to oxidize a variety of substrates (124). CotA is similar to this family of proteins as well. Recently, MnxG has been localized to the ridged outermost layer of the SG-1 spores, which is now believed to be an exosporium (31a, 137). The formation of the metal shell clearly involves this layer, and future studies should reveal whether the other mnx gene products are also present at this location. The surprising fact that both mnxG of SG-1 and cotA of B. subtilis both show similarity to multicopper oxidases makes it tempting to speculate that the CotA-dependent brown pigment is due to a multicopper oxidase activity (although it should be noted that the pigment of B. subtilis does not contain significant manganese [139]). It will be informative to use site-directed mutagenesis to delete regions of CotA that are similar to multicopper oxidases and to determine the effects on both coat pigmentation and the localization of CotA.
It is likely that we do not know the full range of roles played by the coat. In particular, the observation that deletion of any one of a large number of coat protein genes has little or no effect on the usually studied resistance characteristics suggests that some coat functions have yet to be identified. This makes it important to identify the various stresses that spores encounter in nature and to determine whether these are accurately measured by the tests normally applied in the laboratory. In soil, an important habitat of B. subtilis (123), there are likely to be a variety of significant challenges. The coat may act to prevent antibacterial compounds produced by competing organisms or by plants from entering the spore. An intriguing speculation is that the coat enables the spore to survive a sojourn through the digestive tract of an organism that has ingested it. Organisms such as nematodes might consume spores in the course of extracting nutrients from the soil or feeding on other soil-borne organisms, and it may be important for spores to be able to pass safely through the nematode gut. The possibility that B. subtilis is able to survive such a transit appears to be unexplored. This is not to suggest that B. subtilis might be a nematode pathogen. Indeed, some sporulating bacteria, including members of the genus Pasteuria (15), have made elaborate adaptations to the environment of the nematode gut and are effective pathogens (116). Recently, it has been demonstrated that B. cereus can live as a symbiont of a large number of species of arthropods (82). Additionally, spores have been observed in the food vacuoles of amoebae and plasmodia and survive to be incorporated, apparently intact, in the food vacuole remnants of the sporangial stalk of a slime mold, Protophysarum phloiogneum (16a). These observations indicate that the spores are able to resist the degradative enzymes that usually destroy the contents of the food vacuoles (16). It will be interesting to test whether the coat proteins that appear dispensable in normal resistance assays contribute to the survival of spores inside soil-dwelling organisms. The role of the coat need not be limited to protection or survival. Spores in the soil exist in complex and largely uncharacterized relationships with plants, other bacteria, and a variety of other organisms (123). For example, proximity to plant roots can stimulate sporulation in Bacillus species isolated from the soil (33). The coat could conceivably play an active role in these interactions.
Understanding how the coat provides resistance would lead not only to the resolution of the questions posed above but to practical applications as well. For example, the coats of the spore-forming pathogens Clostridium botulinum and B. anthracis provide a degree of protection that makes decontamination extremely difficult. With sufficient knowledge of spore coat assembly, we may be able to at least partially disassemble the coats of pathogenic spores and render them vulnerable to relatively mild chemical sterilization. Spore coats could also be important in materials science applications, where an extremely tough but porous shell is required. Any practical application of the coat in industry will rely on a basic understanding of coat assembly mechanisms.
INSIGHTS FROM THE B. SUBTILIS GENOME-SEQUENCING PROJECT
The completion of the sequencing of the B. subtilis genome challenges us to identify all the genes involved in coat assembly and function. This is not easy to do, since most of the already identified spore coat proteins are largely unlike each other in sequence and do not resemble proteins from other species in the databases. Nonetheless, several open reading frames in B. subtilis that could encode proteins very similar to certain known coat protein genes have been identified. We have studied one, ynzH, which could encode a protein with significant identity to CotC and CotG (18a). We did not detect a phenotype when ynzH was deleted, nor did we see a band of the predicted size for YnzH by SDS-PAGE analysis of coat protein extracts. Currently, we are generating an antibody directed against overproduced YnzH to determine the location of this protein within the sporangium.
Several putative genes encode products with significant similarity to CotF. These include yraD, yraE, yraF, yraG, and yhcQ. An in-depth analysis of the significance of these similarities is outside the scope of this review, but we note that the predicted products of yraE, yraG, and cotF have identity at their amino termini. The predicted products of yraD and yraF have identity to each other at their amino termini and to amino acids toward the middle of CotF. There is also significant identity between cotS and the putative gene ytaA. Intriguingly, these two genes are separated from each other on the chromosome by only a single potential open reading frame, ytxN, which appears to encode a lipopolysaccharide N-acetylglucosaminyltransferase. An additional homology has become apparent with the determination of the complete sequence of cotB. The cysteine-rich carboxy terminus of CotB shows homology to CotG, primarily at cysteine, tyrosine, and arginine residues. It is too early to speculate on whether these similarities might define functionally related groups of coat proteins or whether they could be used to identify functionally important regions within the coat proteins.
FUTURE DIRECTIONS FOR THE STUDY OF THE COAT
Now that we understand the general framework of gene expression that leads to the synthesis of the spore coat proteins and we know something about the steps in the assembly of these proteins into the coat, we can address some of the most interesting questions concerning coat assembly. An important goal will be identifying all the coat proteins and in particular the components of the matrix. Most probably, progress in this endeavor will come both from continued application of the reverse genetics approach and from further analysis of genes of unknown function identified by the sequencing project. These studies may also reveal new morphogenetic proteins. The application of classical genetic techniques to identify mutants with altered coat morphology should also continue. This could identify not only previously undiscovered morphogenetic genes but also new alleles of already studied coat genes, possibly uncovering new information about their roles. The relatively large number of characterized proteins involved in the coat makes their further analysis very timely. We need to determine, for example, how CotE organizes the outer coat and how SpoIVA links the matrix to the forespore. It should be possible to study some of the interactions between the coat proteins. In particular, the molecular details of the interaction between CotJA and CotJC are probably ready to be elucidated. Furthermore, we should determine which regions of the coat proteins direct them to either the inner or outer layers. An especially important area of study will be the biochemical modification of the coat. Examination of proteins such as SodA, Tgl, and the Sps proteins, for which there is good evidence for roles in coat modification, is likely to be productive. An unexplored and very interesting aspect of assembly is the control of the cleavage of coat proteins such as CotF and CotT. These studies may lead to the identification of the genes encoding the coat-associated proteases and the mechanisms that control coat protein cleavage. It may even be possible to study the capacity of proteins in coat extracts to self-assemble. An intriguing set of experiments, initiated by Aronson et al., discovered fibrillar structures in coat extracts (7). These studies must be continued and extended.
The goal of these genetic and biochemical studies will be a clear view of the mechanisms that control the assembly of this complex structure and an explanation of its functions. As noted above, the function of the coat in germination is somewhat paradoxical, and it would be satisfying if the molecular characterization of the coat yielded an explanation for this role. A basic molecular understanding of the coat could also shed light on any other functions of the coat apart from its activity as a shield. However, to grasp the full purpose of the coat, we will have to determine the various tasks performed by the coat in the very different niches that coat-encased sporulating organisms occupy. We will have to continue to characterize the coats of different species and to learn the purposes the coats serve in these organisms. This will require an ecological approach in which we analyze spore coat function from the perspective of the actual challenges faced in the wild.
ACKNOWLEDGMENTS
Thanks to Chris Francis for directing my attention to the similarity of CotA to metal oxidases; to Arthur Aronson, Keith Chater, Tom Deits, Jean Greenberg, and Taha Taha for helpful discussions; and to Elizabeth Wellington for raising the idea that the spore coat might protect against nematodes. I also thank Francesca Catalano, Tom Deits, Chris Francis, Thomas Gallagher, Adriano Henriques, Marie-Francoise Hullo, Lee Kroos, Charles Moran, Ezio Ricca, Bradley Tebo, Alan Wolfe, and especially Shawn Little for comments on the manuscript. Finally, thanks to everyone who participated in the Bacillus subtilis Genome-Sequencing Project for creating such an important resource.
Work in my laboratory is funded by Public Health Service grant GM539898 from the National Institutes of Health and a grant from the Schweppe Foundation.
REFERENCES
- 1.Abe A, Koide H, Kohno T, Watabe K. A Bacillus subtilis spore coat polypeptide gene, cotS. Microbiology. 1995;141:1433–1442. doi: 10.1099/13500872-141-6-1433. [DOI] [PubMed] [Google Scholar]
- 2.Abe A, Ogawa S, Kohno T, Watabe K. Purification of Bacillus subtilis spore coat protein by electrophoretic elution procedure and determination of NH2-terminal amino acid sequences. Microbiol Immunol. 1993;37:809–812. doi: 10.1111/j.1348-0421.1993.tb01709.x. [DOI] [PubMed] [Google Scholar]
- 3.Alper S, Duncan L, Losick R. An adenosine nucleotide switch controlling the activity of a cell type-specific transcription factor in B. subtilis. Cell. 1994;77:195–206. doi: 10.1016/0092-8674(94)90312-3. [DOI] [PubMed] [Google Scholar]
- 4.Arigoni F, Pogliano K, Webb C D, Stragier P, Losick R. Localization of protein implicated in establishment of cell type to sites of asymmetric division. Science. 1995;270:637–640. doi: 10.1126/science.270.5236.637. [DOI] [PubMed] [Google Scholar]
- 5.Aronson A, Fitz-James P C. Biosynthesis of bacterial spore coats. J Mol Biol. 1968;33:199–212. doi: 10.1016/0022-2836(68)90288-x. [DOI] [PubMed] [Google Scholar]
- 6.Aronson A, Pandey N K. Comparative structural and functional aspects of spore coats. In: Chambliss G, Vary J C, editors. Spores—VII. Washington, D.C: American Society for Microbiology; 1978. pp. 54–61. [Google Scholar]
- 7.Aronson A I, Ekanayake L, Fitz-James P C. Protein filaments may initiate the assembly of the Bacillus subtilis spore coat. Biochimie. 1992;74:661–667. doi: 10.1016/0300-9084(92)90138-5. [DOI] [PubMed] [Google Scholar]
- 8.Aronson A I, Fitz-James P. Structure and morphogenesis of the bacterial spore coat. Bacteriol Rev. 1976;40:360–402. doi: 10.1128/br.40.2.360-402.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aronson A I, Fitz-James P C. Reconstitution of bacterial spore coat layers in vitro. J Bacteriol. 1971;108:571–578. doi: 10.1128/jb.108.1.571-578.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9a.Aronson, A. I., and P. C. Fitz-James. Personal communication.
- 10.Aronson A I, Horn D. Characterization of the spore coat protein of Bacillus cereus T. In: Halvorson H O, Hansen R, Campbell L L, editors. Spores—V. Washington, D.C: American Society for Microbiology; 1972. pp. 19–27. [Google Scholar]
- 11.Aronson A I, Song H-Y, Bourne N. Gene structure and precursor processing of a novel Bacillus subtilis spore coat protein. Mol Microbiol. 1988;3:437–444. doi: 10.1111/j.1365-2958.1989.tb00189.x. [DOI] [PubMed] [Google Scholar]
- 12.Bagyan I, Casillas-Martinez L, Setlow P. The katX gene, which codes for the catalase in spores of Bacillus subtilis, is a forespore-specific gene controlled by sigmaF, and KatX is essential for hydrogen peroxide resistance of the germinating spore. J Bacteriol. 1998;180:2057–2062. doi: 10.1128/jb.180.8.2057-2062.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12a.Bauer, T., A. G. Stover, and A. Driks. Unpublished data.
- 13.Beall B, Driks A, Losick R, Moran C P., Jr Cloning and characterization of a gene required for assembly of the Bacillus subtilis spore coat. J Bacteriol. 1993;175:1705–1716. doi: 10.1128/jb.175.6.1705-1716.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beaman T C, Pankratz H S, Gerhardt P. Ultrastructure of the exosporium and underlying inclusions in spores of Bacillus megaterium strains. J Bacteriol. 1972;109:1198–1209. doi: 10.1128/jb.109.3.1198-1209.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berkeley R C, Ali N. Classification and identification of endospore-forming bacteria. Soc Appl Bacteriol Symp Ser. 1994;23:1S–8S. doi: 10.1111/j.1365-2672.1994.tb04352.x. [DOI] [PubMed] [Google Scholar]
- 16.Blackwell M. An ultrastructural study of stalk development in a myxomycete, Protophysarum phoiogenum. Arch Microbiol. 1974;99:331–334. doi: 10.1007/BF00696247. [DOI] [PubMed] [Google Scholar]
- 16a.Blackwell, M. Personal communication.
- 17.Bourne N, Fitz-James P C, Aronson A I. Structural and germination defects of Bacillus subtilis spores with altered contents of a spore coat protein. J Bacteriol. 1991;173:6618–6625. doi: 10.1128/jb.173.20.6618-6625.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Casillas-Martinez L, Setlow P. Alkyl hydroperoxide reductase, catalase, MrgA, and superoxide dismutase are not involved in resistance of Bacillus subtilis spores to heat or oxidizing agents. J Bacteriol. 1997;179:7420–7425. doi: 10.1128/jb.179.23.7420-7425.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18a.Correa, M., and A. Driks. Unpublished data.
- 19.Cutting S, Anderson M, Lysenko E, Page A, Tomoyasu T, Tatematsu K, Tatsuta T, Kroos L, Ogura T. SpoVM, a small protein essential to development in Bacillus subtilis, interacts with the ATP-dependent protease FtsH. J Bacteriol. 1997;179:5534–5542. doi: 10.1128/jb.179.17.5534-5542.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cutting S, Driks A, Schmidt R, Kunkel B, Losick R. Forespore-specific transcription of a gene in the signal transduction pathway that governs pro-ςK processing in Bacillus subtilis. Genes Dev. 1991;5:456–466. doi: 10.1101/gad.5.3.456. [DOI] [PubMed] [Google Scholar]
- 21.Cutting S, Oke V, Driks A, Losick R, Lu S, Kroos L. A forespore checkpoint for mother cell gene expression during development in B. subtilis. Cell. 1990;62:239–250. doi: 10.1016/0092-8674(90)90362-i. [DOI] [PubMed] [Google Scholar]
- 22.Cutting S, Panzer S, Losick R. Regulatory studies on the promoter for a gene governing synthesis and assembly of the spore coat in Bacillus subtilis. J Mol Biol. 1989;207:393–404. doi: 10.1016/0022-2836(89)90262-3. [DOI] [PubMed] [Google Scholar]
- 23.Cutting S, Zheng L, Losick R. Gene encoding two alkali-soluble components of the spore coat from Bacillus subtilis. J Bacteriol. 1991;173:2915–2919. doi: 10.1128/jb.173.9.2915-2919.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cutting S M, Vander Horn P B. Molecular biological methods for Bacillus. Chichester, United Kingdom: John Wiley & Sons Ltd.; 1990. [Google Scholar]
- 25.Dawes I W, Kay D, Mandelstam J. Sporulation in Bacillus subtilis. Establishment of a time scale for the morphological events. J Gen Microbiol. 1969;56:171–179. doi: 10.1099/00221287-56-2-171. [DOI] [PubMed] [Google Scholar]
- 25a.Deits, T. Personal communication.
- 26.Donovan W, Zheng L, Sandman K, Losick R. Genes encoding spore coat polypeptides from Bacillus subtilis. J Mol Biol. 1987;196:1–10. doi: 10.1016/0022-2836(87)90506-7. [DOI] [PubMed] [Google Scholar]
- 27.Doumian I J, Quon K C, Shapiro L. The control of temporal and spatial organization during the Caulobacter cell cycle. Curr Opin Genet Dev. 1996;6:538–544. doi: 10.1016/s0959-437x(96)80081-5. [DOI] [PubMed] [Google Scholar]
- 27a.Driks, A. Unpublished data.
- 27b.Driks, A., and M.-F. Hullo. Unpublished data.
- 28.Driks A, Losick R. Compartmentalized expression of a gene under the control of sporulation transcription factor ςE in Bacillus subtilis. Proc Natl Acad Sci USA. 1991;88:9934–9938. doi: 10.1073/pnas.88.22.9934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Driks A, Roels S, Beall B, Moran C P, Jr, Losick R. Subcellular localization of proteins involved in the assembly of the spore coat of Bacillus subtilis. Genes Dev. 1994;8:234–244. doi: 10.1101/gad.8.2.234. [DOI] [PubMed] [Google Scholar]
- 30.Ebersold H R, Luthy P, Corier J L, Muller M. A freeze-substitution and freeze-fracture study of bacterial spore structures. J Ultrastruct Res. 1981;76:17–81. doi: 10.1016/s0022-5320(81)80051-2. [DOI] [PubMed] [Google Scholar]
- 31.Foster S J, Johnstone K. The trigger mechanism of bacterial spore germination. In: Smith I, Slepecky R, Setlow P, editors. Regulation of procaryotic development. Washington, D.C: American Society for Microbiology; 1989. pp. 223–241. [Google Scholar]
- 31a.Francis, C., and B. Tebo. Personal communication.
- 32.Fujita Y, Yasuda Y, Kozuka S, Tochikubo K. Presence of proteins derived from the vegetative cell membrane in the dormant spore coat of Bacillus subtilis. Microbiol Immunol. 1989;33:391–401. doi: 10.1111/j.1348-0421.1989.tb01987.x. [DOI] [PubMed] [Google Scholar]
- 33.Gochnauer M B, Sealey L J, McCully M E. Do detached root-cap cells influence bacteria associated with maize roots? Plant Cell Environ. 1990;13:793–801. [Google Scholar]
- 34.Goldman R C, Tipper D J. Bacillus subtilis spore coats: complexity and purification of a unique polypeptide component. J Bacteriol. 1978;135:1091–1106. doi: 10.1128/jb.135.3.1091-1106.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldman R C, Tipper D J. Coat protein synthesis during sporulation of Bacillus subtilis: immunological detection of soluble precursors to the 12,200-dalton spore coat protein. J Bacteriol. 1981;147:1040–1048. doi: 10.1128/jb.147.3.1040-1048.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gould G W, Stubbs J M, King W L. Structure and composition of resistant layers in bacterial spore coats. J Gen Microbiol. 1970;60:347–355. doi: 10.1099/00221287-60-3-347. [DOI] [PubMed] [Google Scholar]
- 36a.Greenberg, J. Personal communication.
- 37.Grossman A D. Genetic networks controlling the initiation of sporulation and the development of genetic competence in Bacillus subtilis. Annu Rev Genet. 1995;29:477–508. doi: 10.1146/annurev.ge.29.120195.002401. [DOI] [PubMed] [Google Scholar]
- 38.Hachisuka Y, Kozuka S. A new test of differentiation of Bacillus cereus and Bacillus anthracis based on the existence of spore appendages. Microbiol Immunol. 1981;25:1201–1207. doi: 10.1111/j.1348-0421.1981.tb00128.x. [DOI] [PubMed] [Google Scholar]
- 39.Hachisuka Y, Kozuka S, Tsujikawa M. Exosporia and appendages of spores of Bacillus species. Microbiol Immunol. 1984;28:619–624. doi: 10.1111/j.1348-0421.1984.tb00714.x. [DOI] [PubMed] [Google Scholar]
- 40.Halberg R, Oke V, Kroos L. Effects of Bacillus subtilis sporulation regulatory protein SpoIIID on transcription by sigma K RNA polymerase in vivo and in vitro. J Bacteriol. 1995;177:1888–1891. doi: 10.1128/jb.177.7.1888-1891.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Henriques A O, Beall B W, Moran C P J. CotM of Bacillus subtilis, a member of the alpha-crystallin family of stress proteins, is induced during development and participates in spore outer coat formation. J Bacteriol. 1997;179:1887–1897. doi: 10.1128/jb.179.6.1887-1897.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Henriques A O, Beall B W, Roland K, Moran C P J. Characterization of cotJ, a sigma E-controlled operon affecting the polypeptide composition of the coat of Bacillus subtilis spores. J Bacteriol. 1995;177:3394–3406. doi: 10.1128/jb.177.12.3394-3406.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Henriques A O, Melsen L R, Moran C P. Involvement of superoxide dismutase in spore coat assembly in Bacillus subtilis. J Bacteriol. 1998;180:2285–2291. doi: 10.1128/jb.180.9.2285-2291.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hiragi Y. Physical, chemical and morphological studies of spore coat of Bacillus subtilis. J Gen Microbiol. 1972;72:87–99. doi: 10.1099/00221287-72-1-87. [DOI] [PubMed] [Google Scholar]
- 45.Hoch J A. Regulation of the phosphorelay and the initiation of sporulation in Bacillus subtilis. Annu Rev Microbiol. 1993;47:441–465. doi: 10.1146/annurev.mi.47.100193.002301. [DOI] [PubMed] [Google Scholar]
- 46.Hofmeister A E. Activation of the proprotein transcription factor pro-ςE is associated with its progression through three patterns of subcellular localization during sporulation in Bacillus subtilis. J Bacteriol. 1998;1980:2426–2433. doi: 10.1128/jb.180.9.2426-2433.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Holt S C, Gauther J J, Tipper D J. Ultrastructural studies of sporulation in Bacillus sphaericus. J Bacteriol. 1975;122:1322–1338. doi: 10.1128/jb.122.3.1322-1338.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Holt S C, Leadbetter E R. Comparative ultrastructure of selected aerobic spore-forming bacteria: a freeze-etching study. Bacteriol Rev. 1969;33:346–378. doi: 10.1128/br.33.2.346-378.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Horwitz J. Alpha-crystallin can function as a molecular chaperone. Proc Natl Acad Sci USA. 1992;89:10449–10453. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hughes K T, Gillen K L, Semon M J, Karlinsey J E. Sensing structural intermediates in bacterial flagellar assembly by export of a negative regulator. Science. 1993;262:1277–1280. doi: 10.1126/science.8235660. [DOI] [PubMed] [Google Scholar]
- 50a.Hullo, M.-F. Personal communication.
- 51.Hultgren S J, Abraham S, Caparon M, Falk P, St Geme J W D, Normark S. Pilus and nonpilus bacterial adhesins: assembly and function in cell recognition. Cell. 1993;73:887–901. doi: 10.1016/0092-8674(93)90269-v. [DOI] [PubMed] [Google Scholar]
- 51a.Ichikawa, H., and L. Kroos. Personal communication.
- 52.Ireton K, Jin S, Grossman A D, Sonenshein A L. Krebs cycle function is required for activation of the Spo0A transcription factor in Bacillus subtilis. Proc Natl Acad Sci USA. 1995;92:2845–2849. doi: 10.1073/pnas.92.7.2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Irie R, Okamoto T, Fujita Y. A germination mutant of Bacillus subtilis deficient in response to glucose. J Gen Appl Microbiol. 1982;28:345–354. [Google Scholar]
- 54.Jakob U, Gaestel M, Engel K, Buchner J. Small heat shock proteins are molecular chaperones. J Biol Chem. 1993;268:1517–1520. [PubMed] [Google Scholar]
- 55.James W, Mandelstam J. Protease production during sporulation of germination mutants of Bacillus subtilis and the cloning of a functional gerE gene. J Gen Microbiol. 1985;131:2421–2430. doi: 10.1099/00221287-131-9-2421. [DOI] [PubMed] [Google Scholar]
- 56.Jenkinson H F, Lord H. Protease deficiency and its association with defects in spore coat structure, germination and resistance properties in a mutant of Bacillus subtilis. J Gen Microbiol. 1983;129:2727–2737. [Google Scholar]
- 57.Jenkinson H F, Sawyer W D, Mandelstam J. Synthesis and order of assembly of spore coat proteins in Bacillus subtilis. J Gen Microbiol. 1981;123:1–16. [Google Scholar]
- 58.Ju J, Luo T, Haldenwang W. Bacillus subtilis pro-sigma E fusion protein localizes to the forespore septum and fails to be processed when synthesized in the forespore. J Bacteriol. 1997;179:4888–4893. doi: 10.1128/jb.179.15.4888-4893.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Katsura I, Hendrix R W. Length determination in bacteriophage lambda tails. Cell. 1984;39:691–698. doi: 10.1016/0092-8674(84)90476-8. [DOI] [PubMed] [Google Scholar]
- 60.Kay D, Warren S C. Sporulation in Bacillus subtilis. Biochem J. 1968;109:819–824. doi: 10.1042/bj1090819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kellenberger E, Ryter A, Sechaud J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958;4:671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.King J. Regulation of structural protein interactions as revealed in phage morphogenesis. In: Goldberger R F, editor. Biological regulation and development. Vol. 2. New York, N.Y: Plenum Press; 1980. pp. 101–132. [Google Scholar]
- 63.Knott A G, Hann A C, Russell A D. A note on the development of a non-aldehyde fixation technique for examining spores of Bacillus subtilis under the electron microscope. J Appl Bacteriol. 1995;79:470–474. doi: 10.1111/j.1365-2672.1995.tb03163.x. [DOI] [PubMed] [Google Scholar]
- 64.Kobayashi K, Kumazawa Y, Miwa K, Yamanaka S. ɛ-(γ-Glutamyl)lysine cross-links of spore coat proteins and transglutaminase activity in Bacillus subtilis. FEMS Microbiol Lett. 1996;144:157–160. [Google Scholar]
- 65.Kondo M, Foster J W. Chemical and electron microscope studies on fractions prepared from coats of Bacillus spores. J Gen Microbiol. 1967;47:257–271. doi: 10.1099/00221287-47-2-257. [DOI] [PubMed] [Google Scholar]
- 66.Kornberg A, Spudich J A, Nelson D L, Deutscher M. Origin of proteins in sporulation. Annu Rev Biochem. 1968;37:51–78. doi: 10.1146/annurev.bi.37.070168.000411. [DOI] [PubMed] [Google Scholar]
- 67.Koshikawa T, Beaman T C, Pankratz H S, Nakashio S, Corner T R, Gerhardt P. Resistance, germination and permeability correlates of Bacillus megaterium spores successively divested of integument layers. J Bacteriol. 1984;159:624–632. doi: 10.1128/jb.159.2.624-632.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kozuka S, Tochikubo K. Properties and origin of filamentous appendages on spores of Bacillus cereus. Microbiol Immunol. 1985;29:21–37. doi: 10.1111/j.1348-0421.1985.tb00799.x. [DOI] [PubMed] [Google Scholar]
- 69.Kozuka S, Tochikubo K. Triple fixation of Bacillus subtilis dormant spores. J Bacteriol. 1983;156:409–413. doi: 10.1128/jb.156.1.409-413.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kroos L, Kunkel B, Losick R. Switch protein alters specificity of RNA polymerase containing a compartment-specific sigma factor. Science. 1989;243:526–529. doi: 10.1126/science.2492118. [DOI] [PubMed] [Google Scholar]
- 71.Kunkel B, Kroos L, Poth H, Youngman P, Losick R. Temporal and spatial control of the mother-cell regulatory gene spoIIID of Bacillus subtilis. Genes Dev. 1989;3:1735–1744. doi: 10.1101/gad.3.11.1735. [DOI] [PubMed] [Google Scholar]
- 72.Kunkel B, Sandman K, Panzer S, Youngman P, Losick R. The promoter for a sporulation gene in the spoIVC locus of Bacillus subtilis and its use in studies of temporal and spatial control of gene expression. J Bacteriol. 1988;170:3513–3522. doi: 10.1128/jb.170.8.3513-3522.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kunst F, Ogasawara N, Moszer I, Albertini A M, Alloni G, Azevedo V, Bertero M G, Bessieres P, Bolotin A, Borchert S, Borriss R, Boursier L, Brans A, Braun M, Brignell S C, Bron S, Brouillet S, Bruschi C V, Caldwell B, Capuano V, Carter N M, Choi S K, Codani J J, Connerton I F, Danchin A, et al. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature. 1997;390:249–256. doi: 10.1038/36786. [DOI] [PubMed] [Google Scholar]
- 74.Levin P, A, Fan N, Ricca E, Driks A, Losick R, Cutting S. An unusually small gene required for sporulation by Bacillus subtilis. Mol Microbiol. 1993;9:761–771. doi: 10.1111/j.1365-2958.1993.tb01736.x. [DOI] [PubMed] [Google Scholar]
- 75.Londono-Vallejo J A, Frehel C, Stragier P. spoIIQ, a forespore-expressed gene required for engulfment in Bacillus subtilis. Mol Microbiol. 1997;24:29–39. doi: 10.1046/j.1365-2958.1997.3181680.x. [DOI] [PubMed] [Google Scholar]
- 76.Losick R, Stragier P. Crisscross regulation of cell-type-specific gene expression during development in Bacillus subtilis. Nature. 1992;355:601–604. doi: 10.1038/355601a0. [DOI] [PubMed] [Google Scholar]
- 77.Losick R, Youngman P. Microbial development. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1984. [Google Scholar]
- 78.Lu S, Halberg R, Kroos L. Processing of the mother-cell ς factor, ςK, may depend on events occurring in the forespore during Bacillus subtilis development. Proc Natl Acad Sci USA. 1990;87:9722–9726. doi: 10.1073/pnas.87.24.9722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Macnab R M. Genetics and biogenesis of bacterial flagella. Annu Rev Genet. 1992;26:131–158. doi: 10.1146/annurev.ge.26.120192.001023. [DOI] [PubMed] [Google Scholar]
- 80.Margolis P, Driks A, Losick R. Establishment of cell type by compartmentalized activation of a transcription factor. Science. 1991;254:562–565. doi: 10.1126/science.1948031. [DOI] [PubMed] [Google Scholar]
- 81.Margolis P S, Driks A, Losick R. Sporulation gene spoIIB from Bacillus subtilis. J Bacteriol. 1993;175:528–540. doi: 10.1128/jb.175.2.528-540.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Margulis L, Jorgensen J Z, Dolan S, Kolchinsky R, Rainey F A, Lo S C. The Arthromitus stage of Bacillus cereus: intestinal symbionts of animals. Proc Natl Acad Sci USA. 1998;95:1236–1241. doi: 10.1073/pnas.95.3.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Merck K B, Groenen P J, Voorter C E, de Haard-Hoekman W A, Horwitz J, Bloemendal H, de Jong W W. Structural and functional similarities of bovine alpha-crystallin and mouse small heat-shock protein. A family of chaperones. J Biol Chem. 1993;268:1046–1052. [PubMed] [Google Scholar]
- 84.Milhaud P, Balassa G. Biochemical genetics of bacterial sporulation. IV. Sequential development of resistance to chemical and physical agents during sporulation of Bacillus subtilis. Mol Gen Genet. 1973;125:241–250. doi: 10.1007/BF00270746. [DOI] [PubMed] [Google Scholar]
- 85.Min K-T, Hilditch C M, Diederich B, Errington J, Yudkin M D. ςF, the first compartment-specific transcription factor of B. subtilis, is regulated by an anti-sigma factor that is also a protein kinase. Cell. 1993;74:735–742. doi: 10.1016/0092-8674(93)90520-z. [DOI] [PubMed] [Google Scholar]
- 86.Mizuki E, Ohba M, Ichimatsu T, Hwang S-H, Higuchi K, Saitoh H, Akao T. Unique appendages associated with spores of Bacillus cereus isolates. J Basic Microbiol. 1998;38:33–39. doi: 10.1002/(sici)1521-4028(199803)38:1<33::aid-jobm33>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 87.Moir A. Germination properties of a spore coat-defective mutant of Bacillus subtilis. J Bacteriol. 1981;146:1106–1116. doi: 10.1128/jb.146.3.1106-1116.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Moir A, Kemp E H, Robinson C, Corfe B M. The genetic analysis of bacterial spore germination. J Appl Bacteriol. 1994;77:9S–16S. [PubMed] [Google Scholar]
- 88a.Moran, C. P., Jr. Personal communication.
- 89.Moran C P, Jr, Lang N, Banner C D, Haldenwang W G, Losick R. Promoter for a developmentally regulated gene in Bacillus subtilis. Cell. 1981;25:783–791. doi: 10.1016/0092-8674(81)90186-0. [DOI] [PubMed] [Google Scholar]
- 90.Munoz L, Sadaie Y, Doi R H. Spore coat protein of Bacillus subtilis. J Biol Chem. 1978;253:6694–6701. [PubMed] [Google Scholar]
- 91.Murphy J A, Campbell L L. Surface features of Bacillus polymyxa spores as revealed by scanning electron microscopy. J Bacteriol. 1969;98:737–743. doi: 10.1128/jb.98.2.737-743.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Murrell W G. Chemical composition of spores and spore structures. In: Gould G W, Hurst A, editors. The bacterial spore. New York, N.Y: Academic Press, Inc.; 1969. pp. 215–273. [Google Scholar]
- 93.Naclerio G, Baccigalupi L, Zilhao R, De Felice M, Ricca E. Bacillus subtilis spore coat assembly requires cotH gene expression. J Bacteriol. 1996;178:4375–4380. doi: 10.1128/jb.178.15.4375-4380.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nealson K H, Tebo B M, Rosson R A. Occurrence and mechanisms of microbial oxidation of manganese. Adv Appl Microbiol. 1988;33:279–318. [Google Scholar]
- 94a.Nicholson, W. Personal communication.
- 95.Nishihara T Y, Takubo E, Kawamata T, Koshikawa J, Ogaki J, Kondo M. Role of outer coat in resistance of Bacillus megaterium spore. J Biochem. 1989;106:270–273. doi: 10.1093/oxfordjournals.jbchem.a122843. [DOI] [PubMed] [Google Scholar]
- 96.Pandey N K, Aronson A I. Properties of the Bacillus subtilis spore coat. J Bacteriol. 1979;137:1208–1218. doi: 10.1128/jb.137.3.1208-1218.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Panessa-Warren B J, Tortora G T, Warren J B. Exosporial membrane plasticity of Clostridium sporogenes and Clostridium difficile. Tissue Cell. 1997;29:449–461. doi: 10.1016/s0040-8166(97)80031-6. [DOI] [PubMed] [Google Scholar]
- 98.Parker G F, Daniel R A, Errington J. Timing and genetic regulation of commitment to sporulation in Bacillus subtilis. Microbiology. 1996;142:3445–3452. doi: 10.1099/13500872-142-12-3445. [DOI] [PubMed] [Google Scholar]
- 99.Piggot P J, Coote J G. Genetic aspects of bacterial endospore formation. Bacteriol Rev. 1976;40:908–962. doi: 10.1128/br.40.4.908-962.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Reference deleted.
- 101.Pogliano K, Harry L, Losick R. Visualization of the subcellular localization of sporulation proteins in Bacillus subtilis using immunofluorescence microscopy. Mol Microbiol. 1995;18:459–470. doi: 10.1111/j.1365-2958.1995.mmi_18030459.x. [DOI] [PubMed] [Google Scholar]
- 102.Pogliano K, Hofmeister A E M, Losick R. Disappearance of the ςE transcription factor from the forespore and the SpoIIE phosphatase from the mother cell contributes to establishment of cell-specific gene expression during sporulation in Bacillus subtilis. J Bacteriol. 1997;179:3331–3341. doi: 10.1128/jb.179.10.3331-3341.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Poindexter J S, Staley J T. Caulobacter and Asticcacaulis stalk bands as indicators of stalk age. J Bacteriol. 1996;178:3939–3948. doi: 10.1128/jb.178.13.3939-3948.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Popham D L, Helin J, Costello C E, Setlow P. Muramic lactam in peptidoglycan of Bacillus subtilis spores is required for spore outgrowth but not for spore dehydration or heat resistance. Proc Natl Acad Sci USA. 1996;93:15405–15410. doi: 10.1073/pnas.93.26.15405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Priest F G. Systematics and ecology of Bacillus. In: Sonenshein A L, Hoch J, Losick R, editors. Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology, and molecular genetics. Washington, D.C: American Society for Microbiology; 1993. pp. 3–16. [Google Scholar]
- 106.Resnekov O, Alper S, Losick R. Subcellular localization of proteins governing the proteolytic activation of a developmental transcription factor in Bacillus subtilis. Genes Cells. 1996;1:529–542. doi: 10.1046/j.1365-2443.1996.d01-262.x. [DOI] [PubMed] [Google Scholar]
- 107.Resnekov O, Driks A, Losick R. Identification and characterization of sporulation gene spoVS from Bacillus subtilis. J Bacteriol. 1995;177:5628–5635. doi: 10.1128/jb.177.19.5628-5635.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Resnekov O, Losick R. Negative regulation of the proteolytic activation of a developmental transcription factor in Bacillus subtilis. Proc Natl Acad Sci USA. 1998;95:3162–3167. doi: 10.1073/pnas.95.6.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Roels S, Driks A, Losick R. Characterization of spoIVA, a sporulation gene involved in coat morphogenesis in Bacillus subtilis. J Bacteriol. 1992;174:575–585. doi: 10.1128/jb.174.2.575-585.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Roels S, Losick R. Adjacent and divergently oriented operons under the control of the sporulation regulatory protein GerE in Bacillus subtilis. J Bacteriol. 1995;177:6263–6275. doi: 10.1128/jb.177.21.6263-6275.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rogolsky M. Genetic mapping of a locus which regulates the production of pigment associated with spores of Bacillus subtilis. J Bacteriol. 1968;95:2426–2427. doi: 10.1128/jb.95.6.2426-2427.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rosson R A, Nealson K H. Manganese binding and oxidation by spores of a marine Bacillus. J Bacteriol. 1982;151:1027–1034. doi: 10.1128/jb.151.2.1027-1034.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sacco M, Ricca E, Losick R, Cutting S. An additional GerE-controlled gene encoding an abundant spore coat protein from Bacillus subtilis. J Bacteriol. 1995;177:372–377. doi: 10.1128/jb.177.2.372-377.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sandman K, Kroos L, Cutting S, Youngman P, Losick R. Identification of the promoter for a spore coat protein gene in Bacillus subtilis and studies on the regulation of its induction at a late stage of sporulation. J Mol Biol. 1988;200:461–473. doi: 10.1016/0022-2836(88)90536-0. [DOI] [PubMed] [Google Scholar]
- 115.Santo L Y, Doi R H. Ultrastructural analysis during germination and outgrowth of Bacillus subtilis spores. J Bacteriol. 1974;120:475–481. doi: 10.1128/jb.120.1.475-481.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sayre R M, Wergin W P. Bacterial parasite of a plant nematode: morphology and ultrastructure. J Bacteriol. 1977;129:1091–1101. doi: 10.1128/jb.129.2.1091-1101.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Scherrer R, Beaman T C, Gerhardt P. Macromolecular sieving by the dormant spore of Bacillus cereus. J Bacteriol. 1971;108:868–873. doi: 10.1128/jb.108.2.868-873.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Schmidt R, Margolis P, Duncan L, Coppolecchia R, Moran C P, Jr, Losick R. Control of developmental transcription factor ςF by sporulation regulatory proteins SpoIIAA and SpoIIAB in Bacillus subtilis. Proc Natl Acad Sci USA. 1990;87:9221–9225. doi: 10.1073/pnas.87.23.9221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Setlow P. Mechanisms for the prevention of damage to DNA in spores of Bacillus species. Annu Rev Microbiol. 1995;49:29–54. doi: 10.1146/annurev.mi.49.100195.000333. [DOI] [PubMed] [Google Scholar]
- 120.Seyler R W J, Henriques A O, Ozin A J, Moran C P J. Assembly and interactions of cotJ-encoded proteins, constituents of the inner layers of the Bacillus subtilis spore coat. Mol Microbiol. 1997;25:955–966. doi: 10.1111/j.1365-2958.1997.mmi532.x. [DOI] [PubMed] [Google Scholar]
- 121.Shapiro L. Protein localization and asymmetry in the bacterial cell. Cell. 1993;73:841–855. doi: 10.1016/0092-8674(93)90266-s. [DOI] [PubMed] [Google Scholar]
- 122.Shapiro L, Losick R. Protein localization and cell fate in bacteria. Science. 1997;276:712–718. doi: 10.1126/science.276.5313.712. [DOI] [PubMed] [Google Scholar]
- 123.Slepecky R A. Ecology of bacterial sporeformers. In: Halvorson H O, Hansen R, Campbell L L, editors. Spores—V. Washington, D.C: American Society for Microbiology; 1971. pp. 297–313. [Google Scholar]
- 124.Solomon E I, Sundaram U M, Machonkin T E. Multicopper oxidases and oxygenases. Chem Rev. 1996;96:2563–2605. doi: 10.1021/cr950046o. [DOI] [PubMed] [Google Scholar]
- 125.Sousa J C, Silva M T, Balassa G. An exosporium-like outer layer in Bacillus subtilis spores. Nature. 1976;263:53–54. doi: 10.1038/263053a0. [DOI] [PubMed] [Google Scholar]
- 126.Sousa J C, Silva M T, Balassa G. Spore control (sco) mutations in Bacillus subtilis. Mol Gen Genet. 1978;163:285–291. [Google Scholar]
- 127.Sousa J C, Silva M T, Balassa G. Ultrastructure and development of an exosporium-like outer spore envelope in Bacillus subtilis. Ann Microbiol (Paris) 1978;129:339–362. [PubMed] [Google Scholar]
- 128.Stevens C M, Daniel R, Illing N, Errington J. Characterization of a sporulation gene spoIVA involved in spore coat morphogenesis in Bacillus subtilis. J Bacteriol. 1992;174:586–594. doi: 10.1128/jb.174.2.586-594.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Stewart G S A B, Ellar D J. Characterization, purification and synthesis of spore-coat protein in Bacillus megaterium KM. Biochem J. 1982;202:231–241. doi: 10.1042/bj2020231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Stewart M, Somlyo A P, Somlyo A V, Shuman H, Lindsay J A, Murrell W G. Distribution of calcium and other elements in cryosectioned Bacillus cereus T spores, determined by high-resolution scanning electron probe X-ray microanalysis. J Bacteriol. 1980;143:481–491. doi: 10.1128/jb.143.1.481-491.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Stewart M, Somlyo A P, Somlyo A V, Shuman H, Lindsay J A, Murrell W G. Scanning electron probe X-ray microanalysis of elemental distributions in freeze-dried cryosections of Bacillus coagulans spores. J Bacteriol. 1981;147:670–674. doi: 10.1128/jb.147.2.670-674.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Stragier P. Establishment of forespore-specific gene expression during sporulation of Bacillus subtilis. In: Cole J A, Mohan F, Dow C, editors. Procaryotic structure and function. London, United Kingdom: Society for General Microbiology; 1992. pp. 297–310. [Google Scholar]
- 133.Stragier P, Kunkel B, Kroos L, Losick R. Chromosomal rearrangement generating a composite gene for a developmental transcription factor. Science. 1989;243:507–512. doi: 10.1126/science.2536191. [DOI] [PubMed] [Google Scholar]
- 134.Stragier P, Losick R. Molecular genetics of sporulation in Bacillus subtilis. Annu Rev Genet. 1996;30:297–341. doi: 10.1146/annurev.genet.30.1.297. [DOI] [PubMed] [Google Scholar]
- 135.Sun D, Stragier P, Setlow P. Identification of a new ς-factor involved in compartmentalized gene expression during sporulation of Bacillus subtilis. Genes Dev. 1989;3:141–149. doi: 10.1101/gad.3.2.141. [DOI] [PubMed] [Google Scholar]
- 136.Takamatsu H, Chikahiro Y, Kodama T, Koide H, Kozuka S, Tochikubo K, Watabe K. A spore coat protein, CotS, of Bacillus subtilis is synthesized under the regulation of sigma K and GerE during development and is located in the inner coat layer of spores. J Bacteriol. 1998;180:2968–2974. doi: 10.1128/jb.180.11.2968-2974.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tebo B M, Ghiorse W C, van Waasbergen L G, Siering P L, Caspi R. Bacterially mediated mineral formation: insights into manganese(II) oxidation from molecular genetic and biochemical studies. In: Nealson K, editor. Reviews in minerology. Washington, D.C: The Minerological Society of America; 1997. pp. 225–266. [Google Scholar]
- 138.Tipper D J, Gauthier J J. Structure of the bacterial endospore. In: Halvorson H O, Hanson R, Cambell L L, editors. Spores—V. Washington, D.C: American Society for Microbiology; 1972. pp. 3–12. [Google Scholar]
- 139.van Waasbergen L G, Hildebrand M, Tebo B M. Identification and characterization of a gene cluster involved in manganese oxidation by spores of the marine Bacillus sp. strain SG-1. J Bacteriol. 1996;178:3517–3530. doi: 10.1128/jb.178.12.3517-3530.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.van Waasbergen L G, Hoch J A, Tebo B M. Genetic analysis of the marine manganese-oxidizing Bacillus sp. strain SG-1: protoplast transformation, Tn917 mutagenesis, and identification of chromosomal loci involved in manganese oxidation. J Bacteriol. 1993;175:7594–7603. doi: 10.1128/jb.175.23.7594-7603.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Warth A D, Ohye D F, Murrell W G. The composition and structure of bacterial spores. J Cell Biol. 1963;16:579–592. doi: 10.1083/jcb.16.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wehrli E, Scherrer P, Kubler O. The crystalline layers in spores of Bacillus cereus and Bacillus thuringiensis studied by freeze-etching and high resolution electron microscopy. Eur J Cell Biol. 1980;20:283–289. [PubMed] [Google Scholar]
- 143.Wiencek K M, Klapes N A, Foegeding P M. Hydrophobicity of Bacillus and Clostridium spores. Appl Environ Microbiol. 1990;56:2600–2605. doi: 10.1128/aem.56.9.2600-2605.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wood D A. Sporulation in Bacillus subtilis. Properties and time of synthesis of alkali-soluble protein of the spore coat. Biochem J. 1972;130:505–514. doi: 10.1042/bj1300505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhang B, Daniel R A, Errington J, Kroos L. Bacillus subtilis SpoIIID protein binds to two sites in the spoVD promoter and represses transcription by sigma E RNA polymerase. J Bacteriol. 1997;179:972–975. doi: 10.1128/jb.179.3.972-975.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhang B, Hofmeister A, Kroos L. The prosequence of pro-sigma K promotes membrane association and inhibits RNA polymerase core binding. J Bacteriol. 1998;180:2434–2441. doi: 10.1128/jb.180.9.2434-2441.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhang B, Kroos L. A feedback loop regulates the switch from one sigma factor to the next in the cascade controlling Bacillus subtilis mother cell gene expression. J Bacteriol. 1997;179:6138–6144. doi: 10.1128/jb.179.19.6138-6144.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhang J, Fitz-James P C, Aronson A I. Cloning and characterization of a cluster of genes encoding polypeptides present in the insoluble fraction of the spore coat of Bacillus subtilis. J Bacteriol. 1993;175:3757–3766. doi: 10.1128/jb.175.12.3757-3766.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhang J, Ichikawa H, Halberg R, Kroos L, Aronson A I. Regulation of the transcription of a cluster of Bacillus subtilis spore coat genes. J Mol Biol. 1994;240:405–415. doi: 10.1006/jmbi.1994.1456. [DOI] [PubMed] [Google Scholar]
- 150.Zhang L, Higgins M L, Piggot P J, Karow M L. Analysis of the role of prespore gene expression in the compartmentalization of mother cell-specific gene expression during sporulation of Bacillus subtilis. J Bacteriol. 1996;178:2813–2817. doi: 10.1128/jb.178.10.2813-2817.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zheng L, Donovan W P, Fitz-James P C, Losick R. Gene encoding a morphogenic protein required in the assembly of the outer coat of the Bacillus subtilis endospore. Genes Dev. 1988;2:1047–1054. doi: 10.1101/gad.2.8.1047. [DOI] [PubMed] [Google Scholar]
- 152.Zheng L, Halberg R, Roels S, Ichikawa H, Kroos L, Losick R. Sporulation regulatory protein GerE from Bacillus subtilis binds to and can activate or repress transcription from promoters for mother-cell-specific genes. J Mol Biol. 1992;226:1037–1050. doi: 10.1016/0022-2836(92)91051-p. [DOI] [PubMed] [Google Scholar]
- 153.Zheng L, Losick R. Cascade regulation of spore coat gene expression in Bacillus subtilis. J Mol Biol. 1990;212:645–660. doi: 10.1016/0022-2836(90)90227-d. [DOI] [PubMed] [Google Scholar]