Abstract
To find if an association could be established between Human Papilloma Virus (HPV) infection and oropharyngeal cancers (OPCs) in a group of patients known to be regular users of tobacco, and to determine the impact of HPV status on clinical outcomes.Case records of 212 patients with AJCC-7 (The American Joint Committee on Cancer 7th edition) stages II–IVB non metastatic squamous cell carcinoma of the oropharynx treated using radical radiotherapy with or without chemotherapy during the years 2015–2018 were retrieved. Formalin-fixed, paraffin-embedded blocks from oropharyngeal biopsies were available for 177 patients and were evaluated for p16 expression by immunohistochemical (IHC) staining. More than 50% nuclear staining with or without cytoplasmic staining was considered HPV+ . The association between tobacco use and HPV, as well as the influence of HPV status on survival outcomes were assessed. p16 expression was found to be positive in 23(13%) patients. Significant association was found between chewable tobacco usage and HPV positivity (p = 0.051). The median follow up was 20.5 months (range: 3–80). 5-year Overall Survival was 43.4% and 29.8% (p = 0.044) in HPV+ and HPV− patients, respectively. Local control was significantly better in HPV+ patients (38.6% vs. 25.3%, p = 0.049). There was also a trend towards improved Disease-free Survival in HPV+ patients (31 months vs. 15 months, p = 0.078). Though less in prevalence among the Indian population, improved outcomes in HPV+ OPC patients and widely available IHC HPV assays signifies the routine implementation of p16 testing in day-to-day clinical practice.
Keywords: Human papilloma virus, Oropharyngeal cancer, p16 immunohistochemistry, Radiotherapy, Tobacco
Introduction
Oropharyngeal carcinomas (OPC) are among the most common cancers in the globe. As of the year 2018, they accounted for 92,887 new cases and 51,005 cancer related deaths worldwide [1]. Squamous cell cancers (SCC) of the oropharynx are found in older age men, commonly in the sixth and seventh decades [2, 3]. However, in the past three decades, prevalence of oropharyngeal cancers have been on the rise in both sexes in their younger ages. This trend has been attributed to an increased incidence of Human Papilloma Virus (HPV) infection across the same time period [4]. HPV is sexually transmitted and is a known causative factor for SCC involving the cervix and the head and neck, although association with the latter have most strongly been implicated in cancers of the oropharynx [5].
HPV associated OPC are found to have distinct pathological and clinical features, apart from improved survival outcomes [6]. They tend to be poorly differentiated tumours with basaloid features [6]. They commonly occur at an earlier age [7] and are associated with high risk sexual behaviour, including a higher number of oral/vaginal sex partners [8]. Moreover, patients typically are found to be light users or non-users of alcohol and tobacco [9, 10], though the lack of connection has not been consistently established [11].
Studies have found that close to 80% of head and neck cancer patients in India are frequent users of tobacco [12, 13]. Smokeless tobacco is more commonly used than smoked tobacco, and is one of the important risk factors for OPC [14]. We conducted a retrospective review to know if an association could be established between HPV infection and OPC in a group of patients known to be regular users of tobacco, and to determine the influence of HPV status on therapeutic outcomes.
Patients and Methods
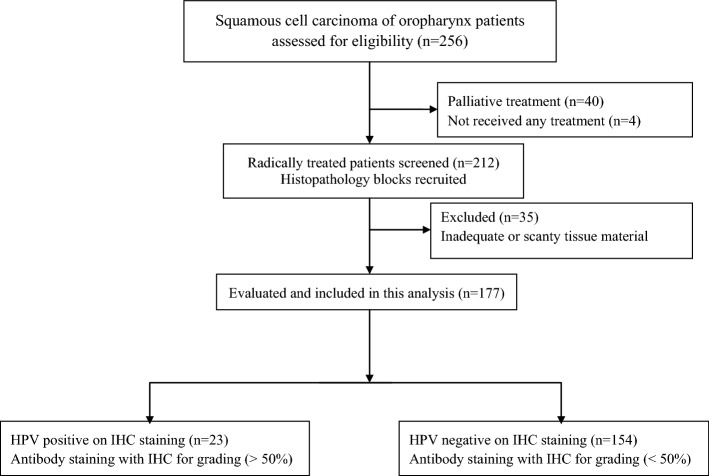
Two hundred and twelve patients of AJCC-7 (The American Joint Committee on Cancer 7th edition) stages II–IVB non metastatic squamous cell carcinoma of the oropharynx treated using radical radiotherapy with or without chemotherapy during the years 2015–2018 were selected, as shown in the study flowchart (Fig. 1). Patients with malignancies at other sites and second primary cancers were excluded. Ethical clearance was obtained from the institutional review board.
Fig. 1.
Study flowchart
All patients receiving treatment at our centre are routinely reviewed by a multidisciplinary team of radiation oncologists, otorhinolaryngologists, radiologists, and pathologists. Biopsies were taken from the oropharyngeal lesions to confirm the diagnosis and fine needle aspiration cytology (FNAC) done from palpable lymph nodes to prove nodal metastasis. Contrast enhanced computed tomography of the head and neck region was performed for staging purpose. Baseline investigations including haemogram, renal function tests, liver function tests, random blood sugars, chest x-ray, and soft tissue x-ray neck were done before initiation of treatments. Pre-treatment dental evaluation is mandated in all patients receiving a radical treatment.
Treatment Details
External radiation was delivered by conventional 2-Dimensional technique to a dose of 66 Gy in 33 fractions, or by IMRT technique to a dose of 66 Gy in 30 fractions. Concomitant boost radiotherapy was given to a total dose of 67.5 Gy/40 fractions/5 weeks in eligible patients, as per the institutional protocol [15]. Treatments were delivered either using Cobalt-60 gamma rays or 6 MV photons. Concurrent chemotherapy was delivered either as a weekly regime (Inj. Cisplatin 40 mg/m2) or as 3-weekly regime (Inj. Cisplatin 100 mg/m2 on days 1, 22 and 43 of the radiation schedule).
A retrospective assessment of case records was done to retrieve demographic data, addiction history, pre-treatment clinical findings, details of treatment, and follow-up status. Formalin-fixed, paraffin-embedded (FFPE) blocks from oropharyngeal biopsies were retrieved and evaluated for p16 expression by immunohistochemical (IHC) staining.
p16 Detection by Immunohistochemistry
IHC for p16 was done on formalin fixed tissue sections using anti-p16 antibody (Roche/Ventana) on a Ventana Benchmark LT automated immunostainer (Ventana Medical Systems, Tucson AZ, USA) according to standard protocols. Briefly, 4 μm sections were cut, deparaffinised, rehydrated and processed with standard methods. Antigen retrieval was done as standard on the machine, by Ventana EDTA-Tris, pH 8.0 solution. Sections were then incubated with the primary antibody for 40 min. Detection involved Ventana's UltraView Universal DAB Detection Kit that utilizes a cocktail of enzyme-labelled secondary antibodies to locate the bound primary antibody. The complex was then visualized with hydrogen peroxide substrate and a 3,3′-diaminobenzidine tetrahydrochloride (DAB) chromogen. Follicular dendritic cells of normal tonsil showing moderate nuclear and cytoplasmic staining with negative germinal centre B cells were used as positive controls.
Interpretation of Staining
Only nuclear staining, with or without cytoplasmic staining was considered positive. Staining distribution was read in a semi-quantitative fashion independent of intensity as follows: 0: no positive cells; 1: 1–25% of cells positive; 2: 26–50% of cells positive; 3: 51–75% of cells positive; 4: 76–100% of cells positive. More than 50% staining was considered positive (Table 1) [13].
Table 1.
HPV immunohistochemistry grading and positivity
| Characteristics | Percentage of staining | No. of patients (%) |
|---|---|---|
| Antibody staining with IHC for HPV grading | < 10% | 153 (72.2) |
| 10–49% | 1 (0.5) | |
| 50–69% | 5 (2.4) | |
| ≥ 70% | 18 (8.5) | |
| HPV status | Positive (≥ 50%) | 23 (13) |
| Negative (< 50%) | 154 (87) |
HPV, Human Papilloma Virus; IHC, Immunochemistry
Statistical Analysis
Descriptive statistics were used to assess the prevalence of HPV and frequency rates in the study cohort. Clinical and demographic parameters between HPV-positive (HPV+) and HPV-negative (HPV−) subgroups were compared with Chi-square and Fisher’s exact test (categorical variables), and Independent t-test (continuous variables). Multivariate analysis was applied by using Cox-regression model to determine the association between various prognostic factors (HPV status) and clinical outcomes. Survival analysis was computed with Kaplan–Meier method using Log-Rank test and survival rates were compared between the HPV+ and HPV− subgroups. Overall survival (OS) and disease free survival (DFS) outcomes were calculated from the date of treatment initiation. Results were considered significant at p value < 0.05. Statistical Package for Social Services version 23 (SPSS v.23) was used for statistical analysis.
Results
Among the 212 patients for whom case records were retrieved, 35 (16.5%) were found ineligible owing to scanty or inadequate pathological material available for diagnostic processing. 177 (83.5%) patients were thus evaluable. The median age of the study group was 59 years (25–79 years). Most of them were males (94.4%).
p16 expression was found to be positive in 23(13%) patients and none of them were females. Tonsil was the most common site positive for p16, accounting for 47.3% of the cases, followed by base of tongue, soft palate and vallecula. The median age of HPV+ patients was five years younger than HPV− patients (53 vs. 58 years, p = 0.092). Based on the median age of both the groups, patients were stratified as being younger (≤ 55 years) or older (> 55 years). HPV+ disease was more common in younger group patients (60.9% vs. 39.1%, p = 0.150).
Median pack-years of smoking in the entire group was 30 (Range: 4–80). Patients were categorised into ≤ 30 pack-years and > 30 pack-years, and there was no correlation with HPV-status in this regard. 87% of HPV+ and 92.2% of HPV− patients were current/former tobacco users (p = 0.399). The clinico-demographic variables, including age of the patients, their smoking status, subsite involvement, stage of the disease and the type of treatment were not significantly different between the HPV+ and HPV− subgroups. Consumption of oral chewable tobacco was, however, significantly higher in the HPV+ group (76.2% vs. 66%, p = 0.051) (Table 2).
Table 2.
Baseline characteristics
| Characteristics | p16 positive | p16 negative | Total patients | p-value | |||
|---|---|---|---|---|---|---|---|
| No | % | No | % | No | % | ||
| Sex | |||||||
| Males | 23 | 100 | 144 | 93.5 | 167 | 94.4 | 0.364 |
| Females | 0 | 0 | 10 | 6.5 | 10 | 5.6 | |
| Age | |||||||
| ≤ 55 years | 14 | 60.6 | 69 | 44.8 | 83 | 46.9 | 0.150 |
| > 55 years | 9 | 39.1 | 85 | 55.2 | 94 | 53.1 | |
| Smoking | |||||||
| Yes | 20 | 87 | 142 | 92.2 | 162 | 91.5 | 0.399 |
| No | 3 | 13 | 12 | 7.8 | 15 | 8.5 | |
| Smokeless tobacco (N = 168) | |||||||
| Yes | 16 | 76.2 | 97 | 66 | 113 | 67.3 | 0.051 |
| No | 5 | 23.8 | 50 | 34 | 55 | 32.7 | |
| Subsite | |||||||
| Tonsil | 3 | 13 | 31 | 20.3 | 34 | 19.3 | 0.376 |
| Base of tongue | 11 | 47.3 | 73 | 47.7 | 84 | 47.7 | |
| Vallecula | 5 | 21.7 | 27 | 17.6 | 32 | 18.2 | |
| Soft palate | 4 | 17.4 | 12 | 7.8 | 16 | 9.1 | |
| PPW | 0 | 0 | 10 | 6.5 | 10 | 5.7 | |
| Tumourstage | |||||||
| T1-2 | 7 | 30.4 | 44 | 28.6 | 51 | 28.8 | 0.443 |
| T3 | 10 | 43.5 | 50 | 32.5 | 60 | 33.9 | |
| T4 | 6 | 26.1 | 60 | 39 | 66 | 37.3 | |
| Nodal stage | |||||||
| N0 | 8 | 34.8 | 39 | 25.3 | 47 | 26.6 | 0.338 |
| N + | 15 | 65.2 | 115 | 74.7 | 130 | 73.4 | |
| Composite stage | |||||||
| I-II | 4 | 17.4 | 14 | 9.1 | 18 | 10.2 | 0.469 |
| III | 6 | 26.1 | 43 | 27.9 | 49 | 27.7 | |
| IV | 13 | 56.5 | 97 | 63 | 110 | 62.1 | |
| EBRT type | |||||||
| Conventional fractionation | 19 | 82.6 | 120 | 120 | 139 | 79.4 | 0.686 |
| Altered fractionation | 4 | 17.4 | 32 | 32 | 36 | 20.6 | |
PPW, Posterior pharyngeal wall; EBRT, External Beam Radiotherapy
Clinical Outcomes
The median follow up for the entire cohort was 20.5 months (range: 3–80 months). Patients who were HPV+ , non-users of tobacco and belonged to earlier clinical stages had significantly better OS than their counterparts. DFS outcomes were significantly better for patients belonging to earlier tumour stages (Table 3).
Table 3.
Survival comparison between HPV positive and HPV negative groups (Log-Rank test)
| Characteristics | p16 positive | p16 negative | p-value |
|---|---|---|---|
| Overall survival | |||
| Median (95% CI) | 43 months (27.8–58.1) | 18 months (12.9–23.0) | |
| No of events/No of patients | 10/23 | 89/154 | |
| 2-year | 65.1% | 44.8% | 0.044 |
| 3-year | 57.9% | 36.7% | |
| 5-year | 43.4% | 29.8% | |
| Disease free survival | |||
| Median (95% CI) | 31 months (9.3–52.6) | 15 months (11.6–18.3) | |
| No of events/No of patients | 11/23 | 90/154 | |
| 2-year | 56.1% | 41.4% | 0.078 |
| 3-year | 49.8% | 32% | |
| 5-year | 42.7% | 27.6% | |
| Local control | |||
| Median (95% CI) | 37 months (29.8–50.6) | 18 months (12.7–30.4) | |
| No of events/No of patients | 9/23 | 79/154 | |
| 2-year | 63.8% | 44.7% | 0.049 |
| 3-year | 47.8% | 33.6% | |
| 5-year | 38.6% | 25.3% | |
HPV, Human Papilloma Virus; CI, Confidence Interval
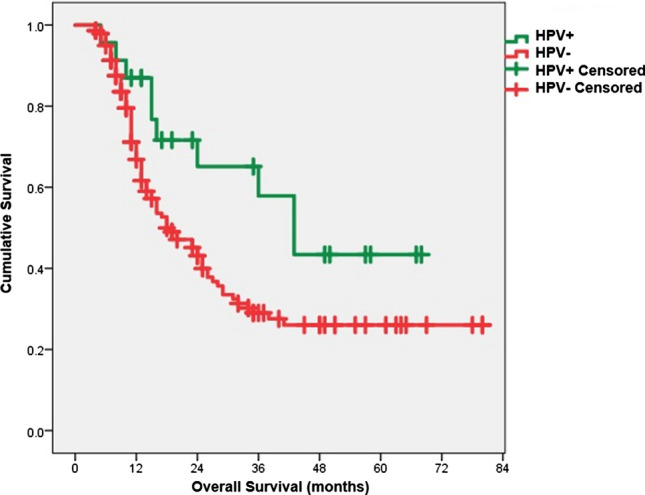
The clinical outcomes of HPV+ patients were better than in HPV− patients. The 5 year OS was 43.4% versus 29.8% (p = 0.044) in HPV+ and HPV− patients, respectively [Fig. 2]. The median OS was 43 months [(95% confidence interval (CI): 27.8–58.1] compared to 18 months (95% CI: 12.9–23). Similarly, local control (LC) was significantly higher in HPV+ patients than their counterparts (5-year LC 38.6% vs 25.3%, p = 0.049). There was also a trend towards improved DFS in HPV+ patients (31 months vs. 15 months, p = 0.078). On multivariate analysis, HPV status [hazard ratio (HR) 1.8, 95% CI: 0.9–3.6, p = 0.043] and composite stage grouping (HR 2.4, 95% CI: 1.1–3.5, p = 0.031) were found to be significant determinants of OS (Table 4).
Fig. 2.

Kaplan-Meier survival curve showing significantly better overall survival in HPV positive patients (5 year OS: 43.4% vs. 29.8%, p = 0.044)
Table 4.
Predictive factors for survival (Cox-regression analysis)
| Characteristics | No of events/No of patients | HR | 95% CI | p-value |
|---|---|---|---|---|
| Overall survival | ||||
| HPV | ||||
| Positive | 10/23 | 1.884 | 0.979–3.627 | 0.043 |
| Negative | 89/154 | |||
| Smoking | ||||
| Yes | 103/193 | 1.479 | 0.628–3.484 | 0.421 |
| No | 9/19 | |||
| Pack-years of smoking | ||||
| ≤ 30 | 68/121 | 1.062 | 0.706–1.598 | 0.772 |
| > 30 | 35/69 | |||
| Chewable tobacco | ||||
| Yes | 71/139 | 0.952 | 0.639–1.417 | 0.807 |
| No | 37/64 | |||
| Composite stage | ||||
| I–III | 27/78 | 2.410 | 1.198–3.565 | 0.031 |
| IV | 85/134 | |||
| EBRT type | ||||
| Conventional fractionation | 88/160 | 1.761 | 0.911–3.425 | 0.092 |
| Altered fractionation | 23/52 | |||
| Disease free survival | ||||
| HPV status | ||||
| Positive | 11/23 | 1.576 | 0.836–2.973 | 0.057 |
| Negative | 90/154 | |||
| Smoking status | ||||
| Yes | 113/193 | 1.345 | 0.615–2.941 | 0.495 |
| No | 9/19 | |||
| Pack-year status | ||||
| ≤ 30 | 72/121 | 1.146 | 0.781–1.682 | 0.487 |
| > 30 | 41/69 | |||
| Chewable tobacco | ||||
| Yes | 80/139 | 0.970 | 0.661–1.424 | 0.876 |
| No | 39/64 | |||
| Composite stage | ||||
| I–III | 30/78 | 1.671 | 1.105–3.053 | 0.033 |
| IV | 92/134 | |||
| EBRT type | ||||
| Conventional fractionation | 96/160 | 0.832 | 0.458–1.511 | 0.397 |
| Altered fractionation | 24/52 | |||
HR, Hazard ratio; CI, Confidence Interval; HPV, Human Papilloma Virus; EBRT, External Beam Radiotherapy
Eighty seven percent of p16 positive and 92.2% of p16 negative patients were current/former tobacco users in any form (p = 0.4). The effects of smoking and chewable tobacco on clinical outcomes of p16 positive patients were independently evaluated in a subset analysis. Among all smokers of tobacco (n = 162), HPV+ (20/162) patients had better survival than HPV− (142/162) patients (5-year OS: 45% vs. 26%, p = 0.039) (Fig. 3). Only 3 patients were HPV+ in the non-smokers sub-group (N = 15) and therefore, a statistical evaluation was not done to compare outcomes. Among the 162 smokers, 113 had a history of chewing smokeless tobacco. Even in this sub-group, HPV+ (16/113) patients had better survival than HPV− (97/113) patients (5-year OS: 54.5% vs. 28.6%, p = 0.053). Similar findings were observed for DFS between HPV+ and HPV- smokers with a trend towards statistical significance (5-year DFS: 44.5% vs. 26.1%, p = 0.083), and also in users of chewable tobacco (5-year DFS: 43% vs. 28.2%, p = 0.080).
Fig. 3.
Kaplan Meier survival curve showing significantly better overall survival in HPV positive smokers compared to HPV negative smokers (5-year OS: 45% vs. 26%, p = 0.039)
Discussion
The association between HPV and OPC has been one of the significant discoveries owing to differences in pathogenesis, clinical profile and treatment outcomes of individuals who test positive for HPV infection compared to the HPV- patients. Many evidences have confirmed this link, as proven in cervical cancers. The virus itself has been directly identified in head and neck cancer specimens [16]. Also, HPV+ head and neck SCC express specific oncoproteins besides showing a significant viral load [7]. HPV associated carcinogenesis due to these oncoproteins have been linked to unique molecular alterations which are not routinely found in the HPV- tumours [16]. Additional grounds for an HPV association have been established in case control studies which prove increased odds of oral HPV infection (with any type) and seropositivity for the HPV-16 L1 capsid protein in oropharyngeal cancer patients [5].
Testing for p16 has long been utilized as a surrogate for transcriptionally active HPV infection due to high sensitivity and ease of clinical utility [17]. In our cohort, 13% of the patients tested positive on p16 testing. The western literature describe a fairly high range of HPV infection, with approximately 50% and 65% prevalence rates reported in the Europe and the United States, respectively [18]. Similar reports from India show a relatively low prevalence averaged around 22% [19]. This difference is also seen in the rest of the world and is most likely due to the differences in ethnic, cultural and geographic characteristics and also due to the variations in high risk sexual behaviour. The global prevalence varies hugely with values ranging 13 to 60% [20]. The low prevalence reported in our study can partly be explained due to the above parameters. Another reason could be due to the increased prevalence of smoking in our cohort. 91% of the entire cohort were smokers of tobacco in one or the other forms. Tobacco induced hypomethylation of p16 gene has been shown to prevent the overexpression of p16 [21].
Evidences have shown that tobacco use is less common in patients with HPV+ OPC(9). This is not always true and similar to the studies reporting otherwise [11], our analysis did not show such an inverse association. This is due to the already high prevalence of smoking in both the subgroups. Of note, 87% of p16 positive and 92.2% of p16 negative patients were current/former tobacco users (p = 0.399). Prevalence of oral chewable tobacco was found to be higher in the HPV+ group (76.2% vs. 66%, p = 0.051). This should be cautiously interpreted because the majority of HPV negative non-users of chewable tobacco were addicted to smoked tobacco.
We were able to find that even among tobacco users, HPV+ patients had significantly better OS compared to HPV- patients (p = 0.039). Using recursive partition analysis, Ang et al. [22] showed similar findings with increasing odds of death in high-risk patients (HPV− smokers) compared to intermediate-risk patients (HPV+ smokers). On the flip side, smoking has proven to adversely affect the outcomes of HPV+ patients [23, 24]. However with majority of our HPV+ patients being significant users of tobacco, (20 smokers vs. 3 non-smokers), an independent analysis could not be performed to analyse such an association.
The median age of HPV+ group was 53 years which is comparable to both national [12, 19] and western data (around 55 years) [6, 22]. Similarly, tonsil was the most common site of HPV positivity, with 47.3% of HPV+ cancers originating from this site. Though the percentage of involvement is reported to vary between studies, most report tonsil to be the primary site of involvement [19, 25, 26], usually followed by base of tongue, as observed in our study group. There were no female patients who tested positive for HPV in the entire cohort. This could be because of the small number of females in the entire group (only 12 patients). Also, 8 of them were consumers of at least 10 pack years of tobacco.
HPV+ cancers are generally reported to have a small primary tumour with large and cystic nodal metastatic disease [27], though the association has not been consistent. In our study, we could not find a significant link between HPV+ cases and their tumour and nodal status (T and N stages), likely due to the small number of patients testing positive.
Our results showed that HPV positivity conferred a survival advantage compared to the HPV− patients. Unsurprisingly, many published literature support the evidence, with improvements in OS and DFS identified specifically within the oropharyngeal subsite [28]. Favourable outcomes with HPV positivity were confirmed in the Eastern Cooperative Oncology Group (ECOG) phase II multicenter trial with significantly improved OS and a lower risk of disease progression [29]. A meta-analysis of the prognostic impact of HPV in head and neck SCC demonstrated that HPV+ patients were likely to have improvements OS and DFS [30]. In our study, we found HPV+ patients to have significantly better OS and LC outcomes at 5 years compared to the HPV− cohort (p = 0.40 and p = 0.049, respectively).
Our study is one among the very few studies from developing countries to assess the prevalence of HPV infection in OPC and to provide a prognostic significance in terms of meaningful clinical outcomes in a large cohort of patients. Molecular biology plays a key role in OPC management as indicated by the use of p16 analysis in these patients. The p16 immunostains are easier for interpretation, affordable, and widely available. In developing countries and resource limited settings where gene sequencing, polymerase chain reaction and other molecular profiling techniques are not regularly accessible and hampered by high cost, careful use of p16 stains greatly help in therapeutics and prognostication. Unlike many other studies which included other sub sites of head and neck cancers within the analytical cohort, we exclusively studied patients with OPCs. The improved outcomes observed in HPV+ group encourage the use of HPV screening in all newly diagnosed OPC patients, as has been recommended for routine use by the American Joint Committee on Cancer and the Union for International Cancer Control eighth edition TNM staging guidelines. This will lead to appropriate patient stratification and treatment. The limitations of our study are its retrospective nature and lack of data on high risk sexual behaviour. Nevertheless, this study undermines the significance of routine HPV testing by IHC in OPC patients.
Author Contributions
All authors contributed to the study conception and design. Methodology: SG, AE, CBD, AS, Amanjot Bal, AD, Amit Bahl, and RKV; Material preparation, investigation, data collection, formal analysis, and original draft preparation: AE, CBD, AS, and Rajesh Kumar; Review, editing and supervision: SG, Amanjot Bal, AD, Amit Bahl, and RKV. All authors read and approved the final manuscript.
Funding
This study was performed as an intramural research project, funded by the Research Grant Cell, Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh, India. Grant Number: 71/2-Edu-16/234. The funding department had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data Availability
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
Code Availability
None.
Compliance with Ethical Standards
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical Approval
Approved from Institutional Ethics Committee (IEC, PGIMER, Chandigarh, India). All authors have followed the principles outlined in the Declaration of Helsinki for all human experimental investigations.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sushmita Ghoshal, Email: rtsushmita@gmail.com.
Chinna Babu Dracham, Email: chinnababudraksham@gmail.com.
Archana Sundaram, Email: archdoc89@gmail.com.
Rajesh Kumar, Email: drrajesh1996@gmail.com.
Amanjit Bal, Email: docaman@hotmail.com.
Ashim Das, Email: ashim126@gmail.com.
Amit Bahl, Email: dramitbahl@yahoo.com.
Roshan Kumar Verma, Email: roshanverma@hotmail.com.
Arun Elangovan, Email: arune195@gmail.com.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Chaturvedi AK, Anderson WF, Lortet-Tieulent J, et al. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J Clin Oncol. 2013;31(36):4550–4559. doi: 10.1200/JCO.2013.50.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shiboski CH, Schmidt BL, Jordan RC. Tongue and tonsil carcinoma: increasing trends in the U.S. population ages 20–44 years. Cancer. 2005;103(9):1843–1849. doi: 10.1002/cncr.20998. [DOI] [PubMed] [Google Scholar]
- 4.Ramqvist T, Dalianis T. Oropharyngeal cancer epidemic and human papillomavirus. Emerg Infect Dis. 2010;16(11):1671–1677. doi: 10.3201/eid1611.100452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D'Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356(19):1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 6.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100(4):261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 7.Vidal L, Gillison ML. Human papillomavirus in HNSCC: recognition of a distinct disease type. Hematol Oncol Clin North Am. 2008;22(6):1125–vii. doi: 10.1016/j.hoc.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 8.Kreimer AR, Alberg AJ, Daniel R, et al. Oral human papillomavirus infection in adults is associated with sexual behavior and HIV serostatus. J Infect Dis. 2004;189(4):686–698. doi: 10.1086/381504. [DOI] [PubMed] [Google Scholar]
- 9.Applebaum KM, Furniss CS, Zeka A, et al. Lack of association of alcohol and tobacco with HPV16-associated head and neck cancer. J Natl Cancer Inst. 2007;99(23):1801–1810. doi: 10.1093/jnci/djm233. [DOI] [PubMed] [Google Scholar]
- 10.Gillison ML, D'Souza G, Westra W, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008;100(6):407–420. doi: 10.1093/jnci/djn025. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz SM, Daling JR, Doody DR, et al. Oral cancer risk in relation to sexual history and evidence of human papillomavirus infection. J Natl Cancer Inst. 1998;90(21):1626–1636. doi: 10.1093/jnci/90.21.1626. [DOI] [PubMed] [Google Scholar]
- 12.Agarwal JP, Mallick I, Bhutani R, et al. Prognostic factors in oropharyngeal cancer–analysis of 627 cases receiving definitive radiotherapy. Acta Oncol. 2009;48(7):1026–1033. doi: 10.1080/02841860902845839. [DOI] [PubMed] [Google Scholar]
- 13.Murthy V, Calcuttawala A, Chadha K, et al. Human papillomavirus in head and neck cancer in India: current status and consensus recommendations. South Asian J Cancer. 2017;6(3):93–98. doi: 10.4103/sajc.sajc_96_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhawna G. Burden of smoked and smokeless tobacco consumption in India - results from the Global adult Tobacco Survey India (GATS-India)- 2009–201. Asian Pac J Cancer Prev. 2013;14(5):3323–3329. doi: 10.7314/apjcp.2013.14.5.3323. [DOI] [PubMed] [Google Scholar]
- 15.Rishi A, Ghoshal S, Verma R, et al. Comparison of concomitant boost radiotherapy against concurrent chemoradiation in locally advanced oropharyngeal cancers: a phase III randomised trial. Radiother Oncol. 2013;107(3):317–324. doi: 10.1016/j.radonc.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 16.Gillison ML, Koch WM, Capone RB, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92(9):709–720. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 17.Craig SG, Anderson LA, Schache AG, et al. Recommendations for determining HPV status in patients with oropharyngeal cancers under TNM8 guidelines: a two-tier approach. Br J Cancer. 2019;120(8):827–833. doi: 10.1038/s41416-019-0414-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stein AP, Saha S, Kraninger JL, et al. Prevalence of human papillomavirus in oropharyngeal cancer: a systematic review. Cancer J. 2015;21(3):138–146. doi: 10.1097/PPO.0000000000000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bahl A, Kumar P, Dar L, et al. Prevalence and trends of human papillomavirus in oropharyngeal cancer in a predominantly north Indian population. Head Neck. 2014;36(4):505–510. doi: 10.1002/hed.23317. [DOI] [PubMed] [Google Scholar]
- 20.Plummer M, de Martel C, Vignat J, et al. Global burden of cancers attributable to infections in 2012: a synthetic analysis. Lancet Glob Health. 2016;4(9):e609–e616. doi: 10.1016/S2214-109X(16)30143-7. [DOI] [PubMed] [Google Scholar]
- 21.Al-Kaabi A, van Bockel LW, Pothen AJ, et al. p16INK4A and p14ARF gene promoter hypermethylation as prognostic biomarker in oral and oropharyngeal squamous cell carcinoma: a review. Dis Markers. 2014;2014:260549. doi: 10.1155/2014/260549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vawda N, Banerjee RN, Debenham BJ. Impact of smoking on outcomes of HPV-related oropharyngeal cancer treated with primary radiation or surgery. Int J Radiat Oncol Biol Phys. 2019;103(5):1125–1131. doi: 10.1016/j.ijrobp.2018.11.046. [DOI] [PubMed] [Google Scholar]
- 24.Mirghani H, Leroy C, Chekourry Y, et al. Smoking impact on HPV driven head and neck cancer's oncological outcomes? Oral Oncol. 2018;82:131–137. doi: 10.1016/j.oraloncology.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 25.Murthy V, Swain M, Teni T, et al. Human papillomavirus/p16 positive head and neck cancer in India: prevalence, clinical impact, and influence of tobacco use. Indian J Cancer. 2016;53(3):387–393. doi: 10.4103/0019-509X.200668. [DOI] [PubMed] [Google Scholar]
- 26.Myers JN, Elkins T, Roberts D, et al. Squamous cell carcinoma of the tongue in young adults: increasing incidence and factors that predict treatment outcomes. Otolaryngol Head Neck Surg. 2000;122(1):44–51. doi: 10.1016/S0194-5998(00)70142-2. [DOI] [PubMed] [Google Scholar]
- 27.Goldenberg D, Begum S, Westra WH, et al. Cystic lymph node metastasis in patients with head and neck cancer: an HPV-associated phenomenon. Head Neck. 2008;30(7):898–903. doi: 10.1002/hed.20796. [DOI] [PubMed] [Google Scholar]
- 28.Yuanyuan X, Suling H, Quan Z, et al. The relationship between human papillomavirus and prognosis of oropharyngeal squamous cell carcinoma: a meta analysis. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2015;50(3):236–243. [PubMed] [Google Scholar]
- 29.Marur S, Li S, Cmelak AJ, et al. E1308: phase II trial of induction chemotherapy followed by reduced-dose radiation and weekly cetuximab in patients with HPV-associated resectable squamous cell carcinoma of the oropharynx- ECOG-ACRIN cancer research group. J Clin Oncol. 2017;35(5):490–497. doi: 10.1200/JCO.2016.68.3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Rorke MA, Ellison MV, Murray LJ, et al. Human papillomavirus related head and neck cancer survival: a systematic review and meta-analysis. Oral Oncol. 2012;48(12):1191–1201. doi: 10.1016/j.oraloncology.2012.06.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
None.