Abstract
The aim of this study is to eveluate levels of advanced oxidation protein products (AOPP) which are thought to increase in the polyp tissue and superoxide dismutase (SOD), an antioxidant enzyme, with using specthrophotometry in polyp tissue and healthy mucosa. 30 nasal polyp patients without inflammatory disease except for nasal polyposis were included in the study. The control group consisted of 18 patients who did not have allergy, asthma, inflammatory and granulomatous disease and planned surgery due to septum deviation and concha hypertrophy. AOPP and SOD tissue levels were measured by spectrophotometry in polyp tissue specimens taken from patients with nasal polyps and concha samples taken from patients in the control group. The mean AOPP tissue level of patients in the nasal polyposis group was statistically significantly higher than the control group. (p < 0.05). The mean SOD activity level was significantly lower in the nasal polyposis group than the control group. (p < 0.05) As a result of this study, high AOPP levels in polyp tissue and low SOD levels in polyp tissue compared to healthy nasal mucosa, oxidative stress plays an important role in nasal polyp development.
Keywords: Advanced oxidation protein products, Nasal polyposis, Oxidative stress, Superoxide, Dismutase
Introduction
Nasal polyposis (NP), which incidence varies among different societies between 1–4% and causes a decrease in quality of life (QoL) and economical burden due to its relatively high incidence, is a disease characterized by the inflammation of nasal cavity and paranasal sinus mucosa[1, 2]. The etiopathogenesis of nasal polyposis has not been fully clarified, because genetic background, immunologic factors and anatomic differences are involved in its development, and the etiology of nasal polyposis is multifactorial. The main factors in the formation of polyps are edema and inflammation. Mucosal damage resulting from different factors that cause inflammation sets the ground for the development of polyps [3].
Unpaired single electron parts in molecular structures are called free radicals or reactive oxygen particles (ROP). Although free radicals are needed for the functioning of especially neutrophils and macrophages that are among the immune system cells, excess production of free radicals leads to tissue damage [4]. The increase of reactive oxygen molecules up to a certain level is balanced with antioxidant systems in the organism [4]. If this balance is impaired, the resulting oxidative stress disrupts the organelles in cells and the structure of the cell membrane, organic respiration in mitochondria and electrolyte balance, beginning to damage the organism [5].
Although the main source of reactive oxygen particles (ROPs) is aerobic respiration, polymorphonuclear leukocytes can also produce ROP as part of inflammatory response. Molecules such as nitric oxide (NO), hydroxyl radical (OH−), superoxide anion (O2−) and hydrogen peroxide (H2O2) are the most prominent molecules in oxidative stress. The potential role of oxidative stress has been proven in the development of many chronic diseases including asthma, allergy, chronic tonsillitis, and ulcerative colitis [6, 7]. The effects of free oxygen radicals in the body are struggled to be balanced by various enzymatic and non-enzymatic mechanisms [8]. Superoxide dismutase (SOD), catalase and glutathione peroxidase are the most important enzymes that provide this balance [9]. Superoxide dismutase converts superoxide anion (O2−) to hydrogen peroxide (H2O2) and has an important role in balancing oxidative stress [10].
Advanced oxidation protein products (AOPP) are sensitive markers of oxidative stress, involved in cellular functions and act like inflammatory mediators [11]. AOPPs are dityrosine containing cross-linked protein products and were described for the first time by Witko-Sarsat et al. in the plasma of chronic uremic patients in 1996 [12]. AOPPs have been shown to activate mononuclear phagocytes, acting as a mediator providing the connection between monocytes and neutrophils [11]. Protein oxidation products are more stable compared to lipid oxidation products, making them AOPP a better marker in determining oxidative stress [13, 14].
Oxidative stress, which physiopathology has not yet been fully understood and which has been demonstrated in the different studies to increase in the development of nasal polyposis [10, 15], is of importance in order to understand the development of the disease. The objective of this study is to evaluate tissue levels of SOD, which is an important enzyme in balancing oxidative stress, and AOPP which to our knowledge has not been studied in polyps tissue so far, in polyps and healthy nasal mucosa.
Methods
A total of 30 patients who presented to the otolaryngology clinic of XXX University Faculty of Medicine between December 2016 and May 2017, and diagnosed with chronic rhinosinusitis with nasal polyps (CRSwNP) through anamnesis, anterior rhinoscopy, endoscopic examination and paranasal sinus computed tomography (CT) based on the “European Position Paper on Rhinosinusitis and Nasal Polyps” [1] criteria included in the study. The study included patients with bilateral nasal polyposis who did not receive systemic or local treatment for at least 4 weeks and were scheduled for surgery. Samples were taken from the patients with nasal polyposis during the surgery. The control group consisted of 18 patients who had no additional disease, chronic sinusitis and allergy, and who underwent partial turbinectomy due to concha hypertrophy concurrent with septoplasty operation. Turbinate samples were collected from these patients during the operation. The study was conducted according to the Declaration of Helsinki, and approved by the ethics committee of our faculty. All patients were informed in advance and consent forms were obtained.
Patients were questioned for allergy, inflammatory and granulomatous diseases, diabetes mellitus, malignancy, and cystic fibrosis, and those with the specified diseases were excluded from the study. Patients with unilateral nasal polyps, those who used topical or systemic steroids within the last month and patient upper respiratory tract infection within the last two weeks were also excluded. There were no smokers in the patient and control groups. Patients underwent endoscopic examination at the time of the diagnosis, the staging of polyps was made as described by Rasp et al. [16, 17]. Endoscopic staging of the nasal polyps used in the study is shown in Table 1. Whereas, Lund-Mackay staging system was used for paranasal sinus CT staging in the patients with nasal polyposis. Score evaluation of the severity of NP related symptoms was made with Visual Analog Scale (VAS).
Table 1.
Polyp grading system
| Grade | |
|---|---|
| 0 | No visible NPs; |
| 1 | Small amount of polypoid disease confined within the middle meatus; |
| 2 | Multiple polyps occupying the middle meatus: |
| 3 | Polyps extending beyond the middle meatus, within the sphenoethmoid recess but not totally obstructing, or both; |
| 4 | Polyps completely obstructing the nasal cavity; |
NP Nasal polyp
Nasal polyp tissues and samples in the control group were stored at -85 °C until biochemical analyzes. Biochemical measurements were performed in the research laboratories of our hospital, Department of Medical Biochemistry. All reagents and chemicals used in the analyzes were of analytical grade. Spectrophotometric measurements were made using Libra S70 instrument (Biochrom Ltd, Cambridge, England). Tissue protein levels were estimated by Lowry method [18].
The levels of superoxide dismutase (SOD) activity in tissues were estimated with the method described by Sun et al. [19]. According to the method, in the reaction catalyzed by the xanthine oxidase enzyme (XO), xanthine produces superoxide radicals (O2˙−). Then, the formed superoxide radicals react with nitroblue tetrazolium (NBT) to form a colored complex. On the other hand, SOD inhibits the latter reaction by catalyzing the destruction of superoxide radicals. The more SOD activity in the reaction medium, the less colored complex will be formed, resulting in lower absorbance values measured by spectrophotometer. The SOD activity in nasal polyp tissues were estimated by the degree of inhibition of this reaction, at 560 nm wavelength. One unit (1U) of SOD activity was defined as the amount of enzyme causing 50% inhibition of the NBT reduction rate. The results obtained by measuring SOD activity were divided into the amount of tissue protein and expressed as unit per mg protein (U/mg protein).
AOPP levels in tissues were measured by spectrophotometric method determined by Witko-Sarsat et al. and Çakatay et al. [12, 20]. In order to determine AOPP levels, triiodide ion formation through the oxidation of potassium iodide (KI) with chloramine-T was used. The results were expressed as nmol/mg protein.
Data obtained in this study were statistically evaluated using IBM SPSS Statistics 23.0 (Statistical Package for Social Sciences, SPSS Inc. Chicago, IL, United States) software. In addition to descriptive statistics (mean, median, percentage distribution), Mann–Whitney U test was used in the evaluation of the numeric variables, while Spearman’s correlation analysis was utilized to evaluate the correlations. p < 0.05 values were considered statistically significant.
Results
Nineteen (63.3%) patients were male and 11 (36.7%) were female in the nasal polyposis group, while 10 (55.6%) patients were male and 8 (44.4%) patients were female in the control group. The mean age was found as 44.6 ± 12.57 years in the patient group and 39.29 ± 6.87 years in the control group. When both groups were evaluated in terms of age and gender, there was no statistically significant difference between them.
The mean VAS score was found as 8.6. When complaints of the patients were questioned; the most common complaint was nasal congestion (96.6%) followed by smell disorder (80%). In the NP group, the mean Lund – Mackay staging score was found as 18.67 ± 2.32. When endoscopy scores of the patients with nasal polyposis were evaluated; the mean score was 2.70 ± 0.82.
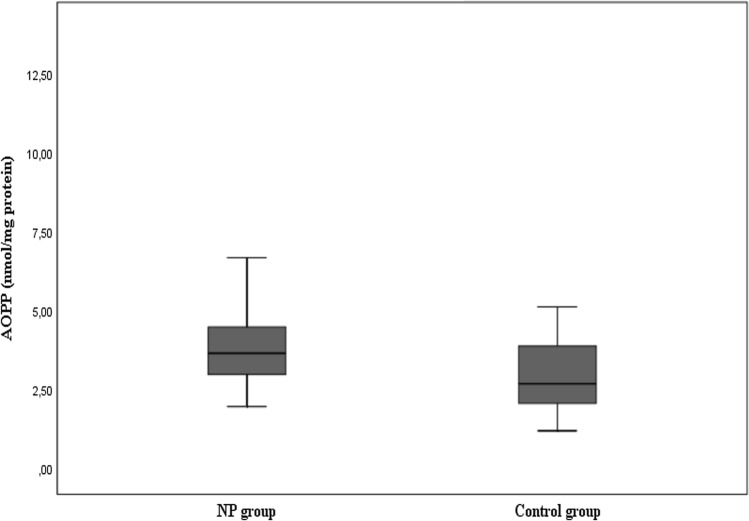
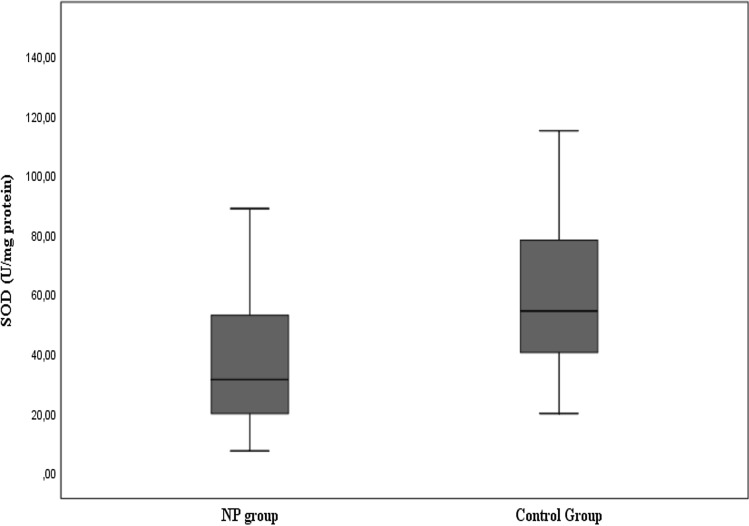
The mean AOPP tissue level was found as 4.22 ± 2.19 nmol/mg protein and the median value as 3.68 nmol/mg protein in the patients with nasal polyposis, while these values were respectively found as 3.11 ± 1.41 nmol/mg protein and 2.72 nmol/mg protein as seen in Fig. 1 and Table 2. The difference between the two groups was statistically significant (p < 0.05). The mean SOD tissue level was found as 41.6 ± 31.12 U/mg protein and the median value as 31.6 U/mg protein in the patients with nasal polyposis, while these values were respectively found as 62.75 ± 32.31 U/mg and 54.63 U/mg in the control group as shown in Fig. 2 and Table 2. The difference between the two groups was statistically significant (p < 0.05).
Fig. 1.
Distribution of AOPP tissue levels of the groups
Table 2.
AOPP and SOD values between the nasal polyp and control groups
| NP Group | Control Group | p value | |||
|---|---|---|---|---|---|
| Mean | Median | Mean | Median | ||
| SOD | 41.6 ± 31.12 | 31.6 | 62.75 ± 32.31 | 54.63 | 0.008 |
| AOPP | 4.22 ± 2.19 | 3.68 | 3.11 ± 1.41 | 2.72 | 0.017 |
p < 0.05 was accepted as significant, AOPP Advanced oxidation protein products, SOD superoxide dismutase
Fig. 2.
Distribution of SOD activity levels of the groups
No correlation was observed between endoscopy scores, and the levels of AOPP and SOD in the patients. Similarly, there was no significant correlation between VAS scores, and the levels of AOPP and SOD (Table 3). Whereas no significant correlation was found between the Lund–Mackay scores and AOPP levels of the patients, a low-to-moderate correlation was observed between the Lund–Mackay scores and SOD levels (p < 0.05).
Table 3.
Correlations between AOPP and SOD levels, and VAS, endoscopic stage and Lund-Mackay scores
| Lund-Mackay | Endoskopic score | VAS | |
|---|---|---|---|
| AOPP | > 0.05 | > 0.05 | > 0.05 |
| SOD | < 0.05 (r:0.389) | > 0.05 | > 0.05 |
p < 0.05 was accepted as significant, r correlation coefficient, VAS visuel analog scale
Discussion
In our study, we observed that the mean AOPP tissue level was statistically significantly higher in the nasal polyposis group compared to the control group. It has been shown that AOPP is the major uremic toxin in chronic renal failure and is associated with systemic diseases such as Type 2 diabetes mellitus and atherosclerosis [11, 21, 22]. In their study, Bochi et al. [23] demonstrated in an experimental in vitro environment that AOPP is formed after hypochlorous acid (HOCI)− released by neutrophils interacts with collagen. In the same study, the authors found that apoptosis increased with the presence of collagens transformed into AOPP in the environment, the number of viable cells decreased, and these effects of AOPP could be prevented by alpha-tocopherol (vitamin E). On the other hand, in their study on patients with metabolic syndrome Venturini et al. [24] showed that AOPP was more associated with metabolic syndrome components such as waist circumference and insulin resistance compared to lipid peroxidation products.
Besides its association with systemic diseases, serum level of AOPP increases also in local diseases, similar to lipid peroxidation products. Aksoy et al. [25], in their study evaluating the serum AOPP levels of patients with allergic rhinitis, found that serum AOPP values were higher in the patient group compared to the controls. Veyseller et al. [26] measured serum levels of AOPP in patients with nasal polyposis and those undergone septoplasty, and found statistically significantly higher serum AOPP levels in the NP group. Although a correlation was observed between free radical levels and polyps stage in different previous studies [27, 28], in our study no correlation was found between AOPP and Lund-Mackay scores and endoscopic stages.
In the present study, SOD activity level was significantly lower in the nasal polyposis group compared to the control group. High levels of superoxide dismutase, which is an antioxidant enzyme converting superoxide anion (O2−) to hydrogen peroxide, in the control tissue indicates that oxidative stress-antioxidant defense balance that is needed for normal physiology was impaired.
Studies have reported lower levels of antioxidants such as SOD, selenium, zinc, retinol and higher levels of oxidants such as malondialdehyde (MDA) and eosinophilic cationic protein (ECP) in the nasal polyps tissue compared to the healthy tissue [9, 10, 27, 29, 30]. Topal et al. [27] have found that NO level was negatively correlated with nasal congestion, total antioxidant status (TAS) was correlated with nasal congestion, and ECP level was correlated with nasal congestion and rhinorrhea in the patients with nasal polyps.
Recurrence rates are still high in nasal polyposis despite maximum surgical and medical treatment combinations. It is important to resolve the relationship between oxidative stress and nasal polyps, which has been shown in different studies to be effective in the development of nasal polyposis, but the physiopathology is not fully understood, in order to overcome the difficulties encountered in the treatment of the disease. Lawrance et al. [15] demonstrated that SOD can be used as local antioxidant therapy in inflammation caused by Aspergillus fumigatus and Alternaria alternata fungi. Sagit et al. [31] investigated the effect of antioxidants in NP patients and showed that antioxidant therapy involving oral vitamins A,C,E and selenium statistically significantly lowered MDA levels.
Although in our study a weak positive correlation was found between SOD activity level and Lund-Mackay score, Ono et al. observed an inverse correlation between Lund Mackay score and SOD activity [32]. This might have resulted from that Ono et al. studied SOD isoforms individually and classified polyps as eosinophilic and non-eosinophilic.
Given the etiology of the disease and many effective pathways, patients are likely to be grouped according to some molecular markers and treatments to differ according to these determined patient groups in the next years. Considering that AOPP is an important marker showing protein oxidation and more stable than other oxidation products, it can be used in identification of patients with prominent oxidative stress. If patient groups where oxidative stress is more effective in the development of the disease can be determined, antioxidant therapies such as vitamins A, C, E, selenium and coenzyme Q10 can be promising treatment methods in this regard.
The study's main limitations include a small number of patients in the control group, and lack of the repeat measurements following medical and surgical treatments, and not distinguishing the nasal polyp endotype through eosinphil count.
Conclusion
In our study, we found that tissue AOPP level increased and SOD activity level decreased in the polyps tissue. These results suggest that AOPP can be used in the evaluation of oxidative stress induced protein damage in patients with nasal polyposis.
Acknowledgements
Not applicable.
Author Contributions
All authors contributed to the study conception and design. MEZ and KU performed the literature search. Material preparation, data collection and analysis were performed by MEZ, YK, SY and ÖÖD. SU and UA performed the statistical analysis. The first draft of the manuscript was written by MEZ and SU. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This study did not receive financial support.
Data Availability
Data used in the study are included in the manuscript.
Declarations
Conflict of interest
The authors declare no conflict of interest to disclose.
Ethics Approval
This study was approved by the local ethics committee of our hospital with the 13/03/2017 dated and 51 numbered decision.
Informed Consent
All patients were informed about the objective of the study and signed written consent forms.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mehmet Ekrem Zorlu, Email: m_ekrem_zorlu@hotmail.com.
K. Kemal Uygur, Email: kkemaluygur@gmail.com.
Niyazi Samet Yılmaz, Email: dr.sametyilmaz.0653@gmail.com.
Özlem Özbaş Demirel, Email: drozbas@gmail.com.
Utku Aydil, Email: utkuaydil@yahoo.com.
Yusuf Kızıl, Email: yusufkizil@yahoo.com.
Sabri Uslu, Email: s_uslu@hotmail.com.
References
- 1.Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology. 2012;50(1):1–12. doi: 10.4193/rhino12.000. [DOI] [PubMed] [Google Scholar]
- 2.Johansson L, Akerlund A, Holmberg K, Melen I, Bende M. Prevalence of nasal polyps in adults: the Skovde population-based study. Ann Otol Rhinol Laryngol. 2003;112(7):625–629. doi: 10.1177/000348940311200709. [DOI] [PubMed] [Google Scholar]
- 3.Orlandi RR, Kingdom TT, Hwang PH, Smith TL, Alt JA, et al. International consensus statement on allergy and rhinology: rhinosinusitis. Int Forum Allergy Rhinol. 2016;6(Suppl 1):S22–209. doi: 10.1002/alr.21695. [DOI] [PubMed] [Google Scholar]
- 4.Folkerts G, Kloek J, Muijsers RB, Nijkamp FP. Reactive nitrogen and oxygen species in airway inflammation. Eur J Pharmacol. 2001;429(1–3):251–262. doi: 10.1016/S0014-2999(01)01324-3. [DOI] [PubMed] [Google Scholar]
- 5.Halliwell B, Gutteridge JM, Cross CE. Free radicals, antioxidants, and human disease: where are we now? J Lab Clin Med. 1992;119(6):598–620. [PubMed] [Google Scholar]
- 6.Kaygusuz I, Ilhan N, Karlidag T, Keles E, Yalcin S, et al. Free radicals and scavenging enzymes in chronic tonsillitis. Otolaryngol Head Neck Surg. 2003;129(3):265–268. doi: 10.1016/S0194-5998(03)00630-2. [DOI] [PubMed] [Google Scholar]
- 7.Roessner A, Kuester D, Malfertheiner P, Schneider-Stock R. Oxidative stress in ulcerative colitis-associated carcinogenesis. Pathol Res Pract. 2008;204(7):511–524. doi: 10.1016/j.prp.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 8.Mrowicka M, Zielinska-Blizniewska H, Milonski J, Olszewski J, Majsterek I. Evaluation of oxidative DNA damage and antioxidant defense in patients with nasal polyps. Redox Rep. 2015;20(4):177–183. doi: 10.1179/1351000215Y.0000000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dagli M, Eryilmaz A, Besler T, Akmansu H, Acar A, et al. Role of free radicals and antioxidants in nasal polyps. Laryngoscope. 2004;114(7):1200–1203. doi: 10.1097/00005537-200407000-00013. [DOI] [PubMed] [Google Scholar]
- 10.Cheng YK, Hwang GY, Lin CD, Tsai MH, Tsai SW, et al. Altered expression profile of superoxide dismutase isoforms in nasal polyps from nonallergic patients. Laryngoscope. 2006;116(3):417–422. doi: 10.1097/01.MLG.0000199738.37455.55. [DOI] [PubMed] [Google Scholar]
- 11.Witko-Sarsat V, Friedlander M, Nguyen Khoa T, Capeillere-Blandin C, Nguyen AT, et al. Advanced oxidation protein products as novel mediators of inflammation and monocyte activation in chronic renal failure. J Immunol. 1998;161(5):2524–2532. doi: 10.4049/jimmunol.161.5.2524. [DOI] [PubMed] [Google Scholar]
- 12.Witko-Sarsat V, Friedlander M, Capeillere-Blandin C, Nguyen-Khoa T, Nguyen AT, et al. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996;49(5):1304–1313. doi: 10.1038/ki.1996.186. [DOI] [PubMed] [Google Scholar]
- 13.Ozbay I, Kucur C, Kocak FE, Savran B, Oghan F. Advanced oxidation protein product levels as a marker of oxidative stress in paediatric patients with chronic tonsillitis. Acta Otorhinolaryngol Ital. 2016;36(5):381–385. doi: 10.14639/0392-100X-897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Massy ZA, Nguyen-Khoa T. Oxidative stress and chronic renal failure: markers and management. J Nephrol. 2002;15(4):336–341. [PubMed] [Google Scholar]
- 15.Lawrence LA, Mulligan JK, Roach C, Pasquini WN, Soler ZM, et al. Superoxide dismutase reduces the inflammatory response to Aspergillus and Alternaria in human sinonasal epithelial cells derived from patients with chronic rhinosinusitis. Am J Rhinol Allergy. 2015;29(2):89–93. doi: 10.2500/ajra.2015.29.4155. [DOI] [PubMed] [Google Scholar]
- 16.Rasp G, Kramer MF, Ostertag P, Kastenbauer E. A new system for the classification of ethmoid polyposis Effect of combined local and systemic steroid therapy. Laryngorhinootologie. 2000;79(5):266–72. doi: 10.1055/s-2000-8806. [DOI] [PubMed] [Google Scholar]
- 17.Meltzer EO, Hamilos DL, Hadley JA, Lanza DC, Marple BF, et al. Rhinosinusitis: developing guidance for clinical trials. Otolaryngol Head Neck Surg. 2006;135(5 Suppl):S31–80. doi: 10.1016/j.otohns.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 18.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193(1):265–275. doi: 10.1016/S0021-9258(19)52451-6. [DOI] [PubMed] [Google Scholar]
- 19.Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988;34(3):497–500. doi: 10.1093/clinchem/34.3.497. [DOI] [PubMed] [Google Scholar]
- 20.Cakatay U, Telci A, Kayali R, Tekeli F, Akcay T, et al. Relation of aging with oxidative protein damage parameters in the rat skeletal muscle. Clin Biochem. 2003;36(1):51–55. doi: 10.1016/S0009-9120(02)00407-1. [DOI] [PubMed] [Google Scholar]
- 21.Kaneda H, Taguchi J, Ogasawara K, Aizawa T, Ohno M. Increased level of advanced oxidation protein products in patients with coronary artery disease. Atherosclerosis. 2002;162(1):221–225. doi: 10.1016/S0021-9150(01)00706-7. [DOI] [PubMed] [Google Scholar]
- 22.Taylor EL, Armstrong KR, Perrett D, Hattersley AT, Winyard PG. Optimisation of an advanced oxidation protein products assay: its application to studies of oxidative stress in diabetes mellitus. Oxid Med Cell Longev. 2015;2015:496271. doi: 10.1155/2015/496271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bochi GV, Torbitz VD, de Campos LP, Sangoi MB, Fernandes NF, et al. In vitro oxidation of collagen promotes the formation of advanced oxidation protein products and the activation of human neutrophils. Inflammation. 2016;39(2):916–927. doi: 10.1007/s10753-016-0325-3. [DOI] [PubMed] [Google Scholar]
- 24.Venturini D, Simao AN, Dichi I. Advanced oxidation protein products are more related to metabolic syndrome components than biomarkers of lipid peroxidation. Nutr Res. 2015;35(9):759–765. doi: 10.1016/j.nutres.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 25.Aksoy F, Demirhan H, Veyseller B, Yildirim YS, Ozturan O, et al. Advanced oxidation protein products as an oxidative stress marker in allergic rhinitis. Kulak Burun Bogaz Ihtis Derg. 2009;19(6):279–284. [PubMed] [Google Scholar]
- 26.Veyseller B, Aksoy E, Ertas B, Keskin M, Ozturan O, et al. A new oxidative stress marker in patients with nasal polyposis: advanced oxidation protein products (AOPP) B-ENT. 2010;6(2):105–109. [PubMed] [Google Scholar]
- 27.Topal O, Kulaksizoglu S, Erbek SS. Oxidative stress and nasal polyposis: does it affect the severity of the disease? Am J Rhinol Allergy. 2014;28(1):e1–4. doi: 10.2500/ajra.2014.28.3963. [DOI] [PubMed] [Google Scholar]
- 28.Cheng YK, Tsai MH, Lin CD, Hwang GY, Hang LW, et al. Oxidative stress in nonallergic nasal polyps associated with bronchial hyperresponsiveness. Allergy. 2006;61(11):1290–1298. doi: 10.1111/j.1398-9995.2006.01228.x. [DOI] [PubMed] [Google Scholar]
- 29.Okur E, Gul A, Kilinc M, Kilic MA, Yildirim I, et al. Trace elements in nasal polyps. Eur Arch Otorhinolaryngol. 2013;270(8):2245–2248. doi: 10.1007/s00405-012-2319-6. [DOI] [PubMed] [Google Scholar]
- 30.Cekin E, Ipcioglu OM, Erkul BE, Kapucu B, Ozcan O, et al. The association of oxidative stress and nasal polyposis. J Int Med Res. 2009;37(2):325–330. doi: 10.1177/147323000903700206. [DOI] [PubMed] [Google Scholar]
- 31.Sagit M, Erdamar H, Saka C, Yalcin S, Akin I. Effect of antioxidants on the clinical outcome of patients with nasal polyposis. J Laryngol Otol. 2011;125(8):811–815. doi: 10.1017/S0022215111001149. [DOI] [PubMed] [Google Scholar]
- 32.Ono N, Kusunoki T, Miwa M, Hirotsu M, Shiozawa A, et al. Reduction in superoxide dismutase expression in the epithelial mucosa of eosinophilic chronic rhinosinusitis with nasal polyps. Int Arch Allergy Immunol. 2013;162(2):173–180. doi: 10.1159/000353122. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data used in the study are included in the manuscript.