Abstract
A majority of the plant-infecting viruses and many of the animal-infecting viruses are dependent upon arthropod vectors for transmission between hosts and/or as alternative hosts. The viruses have evolved specific associations with their vectors, and we are beginning to understand the underlying mechanisms that regulate the virus transmission process. A majority of plant viruses are carried on the cuticle lining of a vector’s mouthparts or foregut. This initially appeared to be simple mechanical contamination, but it is now known to be a biologically complex interaction between specific virus proteins and as yet unidentified vector cuticle-associated compounds. Numerous other plant viruses and the majority of animal viruses are carried within the body of the vector. These viruses have evolved specific mechanisms to enable them to be transported through multiple tissues and to evade vector defenses. In response, vector species have evolved so that not all individuals within a species are susceptible to virus infection or can serve as a competent vector. Not only are the virus components of the transmission process being identified, but also the genetic and physiological components of the vectors which determine their ability to be used successfully by the virus are being elucidated. The mechanisms of arthropod-virus associations are many and complex, but common themes are beginning to emerge which may allow the development of novel strategies to ultimately control epidemics caused by arthropod-borne viruses.
Nearly 100 years ago yellow fever virus was confirmed to be transmitted by mosquitoes (128). Shortly thereafter, leafhoppers were established as the vector of rice dwarf virus (145), although leafhopper transmission had been reported as early as 1895 (see reference 44 for details). In the following decades, numerous arthropod vectors of plant and animal viruses were identified (33). Today over 500 animal viruses are classified as arboviruses, i.e., viruses able to replicate in a blood-feeding arthropod and to infect a vertebrate host whenever the arthropod feeds on that host (109). Additionally, numerous vertebrate-infecting viruses are transmitted by arthropod vectors but do not replicate in the vector (23). Finally, there are many hundreds of plant viruses (18), most of which are dependent upon a vector for transmission between and inoculation into plant hosts. Plant-infecting viruses have evolved many interesting and biologically complex associations with their vectors, which include arthropods, nematodes, and fungi. The arthropod-plant virus associations are the focus of this review, but analogies and comparisons with animal-infecting viruses are discussed where possible.
Fifty years ago, relatively few arboviruses were known (33). Most did not infect humans, and many had evolved a stable, unobtrusive relationship with both their arthropod and animal hosts. As humans intruded into previously undisturbed ecosystems during their efforts to domesticate the land, they also intruded into virus-vector relationships that were quick to take advantage of the new animal (human) host. Hence, the number of arboviruses has increased exponentially and “new” viruses continue to emerge or reemerge into the headlines (see references 66 and 104 for discussions of emerging viruses), the most notable being yellow fever, equine encephalomyelitis, dengue, and other related hemorrhagic fever viruses.
The expansion of humans into new ecosystems has been fueled primarily by a need to develop and expand agricultural land. More often than not, the main agricultural practice has been monoculture, i.e., the planting of large acreages with a single monogenic crop. Modern agricultural techniques and practices have, in part, contributed to an explosion of newly discovered and emerging plant viruses, the most notable being the geminiviruses, closteroviruses, and tospoviruses; the last group is a plant-infecting group within the otherwise animal-infecting Bunyaviridae. Similar to the emerging animal virus diseases, many of the emerging plant virus disease problems may be the result of humans disturbing a rather stable, unobtrusive relationship between viruses, insects, and their natural plant host. The development of new agroecosystems provides opportunities for viruses and vectors to exploit the newly and widely available cultivated plant host.
Despite the notable arboviruses that are responsible for devastating and horrific human suffering and death, as well as the arthropod-vectored plant viruses that are responsible for billions of dollars in annual crop losses, the mechanisms of virus transmission by arthropods are only now beginning to be understood. There is an enormous literature describing various virus-arthropod associations. However, little is known about the molecular and cellular mechanisms that regulate the transmission processes and determine the efficiency of transmission. The advent of molecular biology and the ability to genetically manipulate viruses, plants, and now insects has fueled a resurgence in studies on the mechanisms of insect transmission of viruses. Animal virologists and medical entomologists have focused the bulk of their efforts on understanding the vector, including the genetic and physiological parameters that influence the replication, survival, and transmission of the virus. This is an appropriate focus since most the animal-infecting viruses transmitted by arthropods also infect and replicate in the arthropod vector. In contrast, most the arthropod-transmitted plant viruses do not replicate in their vectors. Therefore, research has focused more on the viral genes and gene products required for the interactions of virus and vector. Although the interactions between medical entomologists, animal virologists, and plant virologists studying virus transmission have been limited in the past, a number of common themes are emerging that may facilitate a more interactive approach to understanding all virus-vector interactions in the future. The purpose of this review is to describe the current state of plant virus-insect vector research and to relate findings in the plant virus world to similar findings or reports from the animal virus world. It is not meant to be a comprehensive treatment of arbovirus transmission or even plant virus transmission.
GENERAL MECHANISMS OF VIRUS TRANSMISSION
In the early years, viruses were said to be either mechanically transmitted or biologically transmitted by their arthropod vectors (33). Mechanical transmission referred to the nonspecific transmission of viruses by single or multiple vector taxa, usually on contaminated mouthparts. The viruses were unable to replicate in the vector. Although it became clear early on that a simple “flying-pin” or “flying-needle” explanation often did not fully characterize the mechanical transmission process, the process was not considered to be a complex biological association. The mechanisms of mechanical transmission of animal viruses have not received much attention, since all of these viruses can spread between their hosts without an arthropod intermediary in nature or at least in the laboratory. Biological transmission referred to the specific association of a virus with a particular arthropod species or genus and, more important, to the fact that the virus was able to propagate within the vector. These definitions of mechanical and biological transmission came primarily from the animal virology community and are still in use today (23, 164). Most of the animal viruses that are associated with an arthropod vector would fall into the biological-transmission category. In fact, the definition of an arbovirus would specifically preclude mechanical transmission.
Plant virologists have long recognized the “mechanical” and “biological” terms to be an inadequate representation of the mechanisms of insect transmission of plant-infecting viruses and have struggled to produce terminology that accurately reflects the many general mechanisms that apply to plant virus-insect vector associations. Much of the early work on plant virus-insect vector associations was related to timing events, e.g., acquisition and inoculation periods, retention periods, and latent periods (the time between ingestion of the virus and the ability of the insect to inoculate a host). Therefore, the terminology evolved to describe time events (for reviews, see references 64, 65, 94, 141, 163).
Viruses were said to be nonpersistent if they were not retained by the vector for more than a few hours. Semipersistent viruses were retained for days or possibly weeks. Viruses in both these categories were acquired and inoculated within seconds or minutes, and did not require a latent period, and did not replicate in the vector. Persistent viruses, once acquired, were associated with the vector for the remainder of its life. These viruses required longer acquisition and inoculation times (hours to days) and latent periods of 1 day to several weeks.
As additional data on the mechanisms of transmission were generated, other variations of the terminology evolved. The nonpersistent and semipersistent viruses were found to specifically associate with the epicuticle that lines the stylets (mouthparts) or foreguts of their vectors, respectively, and were often referred to as stylet-borne or foregut-borne viruses. The cuticle (including the lining of the mouthparts and foregut) is shed during each molt, and therefore any acquired virus is also lost. Collectively, all of these viruses have been referred to as noncirculative. The viruses are not internalized by the vector in the sense that they do not enter the hemocoel of the vector or cross any vector cell membrane.
In contrast, successful transmission of persistent viruses requires that the ingested virus be internalized. Virus is actively transported across multiple cell membranes, is found in the hemocoel (vector body cavity), and ultimately must associate with the vector salivary system to be inoculated into a host. These viruses are now referred to as circulative viruses and can be further divided into propagative viruses, which replicate in their arthropod vector in addition to their plant hosts, and nonpropagative viruses, which replicate only in their plant hosts. The insect vector is only a conduit for the nonpropagative viruses to move between plant hosts, although very specific virus-vector interactions are required. All of the circulative viruses are retained by the vector following a molt.
All the above terms were developed for use with aphid and leafhopper vectors and are applicable to many plant viruses. Terminology problems arose, however, as additional arthropod, nematode, and especially fungal vectors (21) were discovered and virus-vector associations were studied. Watson (162) and later Hull (72) proposed a terminology including internally borne and externally borne viruses. The former would include persistent viruses, and the latter would include nonpersistent and semipersistent viruses. We suggested (58) that the use of “circulative” and “noncirculative” be retained, where “circulative” refers to viruses that are transmitted only if the virus is transported across cell membranes and carried internally within the vector body cavity or fungal cells. Noncirculative viruses do not cross vector cell membranes and are carried externally either on the vector surface (as for some fungi) or on the cuticle lining of the vector’s mouthparts or foregut (as for some arthropods or nematodes). The noncirculative and circulative classification is simple and could be used for animal- and plant-infecting viruses that require a vector for optimal existence in nature. There is some loss of definition and categorization, but subgroupings such as nonpersistent and semipersistent could be added if they pertain to a particular vector taxon. There would, of course, be the paradoxical virus-vector associations that do not fit easily into the proposed scheme. For example, beetle-transmitted viruses and the myirid-bug-transmitted velvet tobacco mottle virus may use both circulative and noncirculative transmission mechanisms (46, 48).
VECTOR FEEDING: MECHANICS AND BEHAVIOR
A majority of arthropod and nematode vectors of plant viruses have a common feature: the mechanics of feeding. Their mouthparts are best described as piercing-sucking (5, 73, 125) (Fig. 1 and 2). The hollow, needle-like mouthparts can penetrate the plant cell wall, either by mechanical force and/or with the help of salivary and gut enzymes. The cell membrane is easily breached by mechanical force, making the cell contents available as food. The most significant feature of this type of feeding is that it does not always irreparably damage the plant cell. This nonlethal cell feeding is critical for survival of the virus, since it must be able to replicate in the cell to which it is delivered. Plant virus genomes encode movement proteins that enable them to move to neighboring cells (24). The fungal vectors do not have piercing-sucking mouthparts. Instead, the virus-carrying motile zoospores attach to the plant root surface, enzymatically and mechanically penetrate the cell wall and membrane, and then establish an infection within the plant cell cytoplasm (22). At some point after gaining entrance to the host cytoplasm, virus is released by the fungus. The mechanisms of virus release by fungi are unknown, but again virus is inoculated into a viable cell.
FIG. 1.
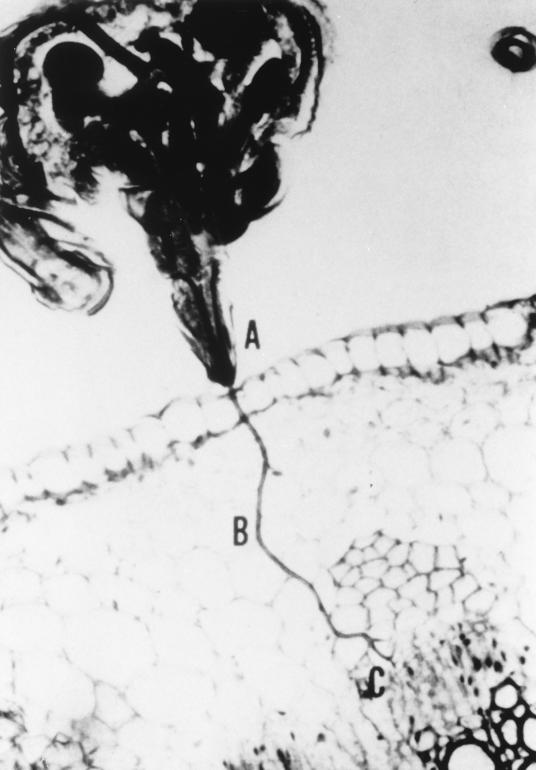
Light micrograph of a longitudinal section through an aphid head and leaf as the aphid is feeding on the plant. The aphid stylet protrudes from the proboscis (A) and penetrates intracellularly through the mesophyll cells (B) and into the vascular bundle (C).
FIG. 2.
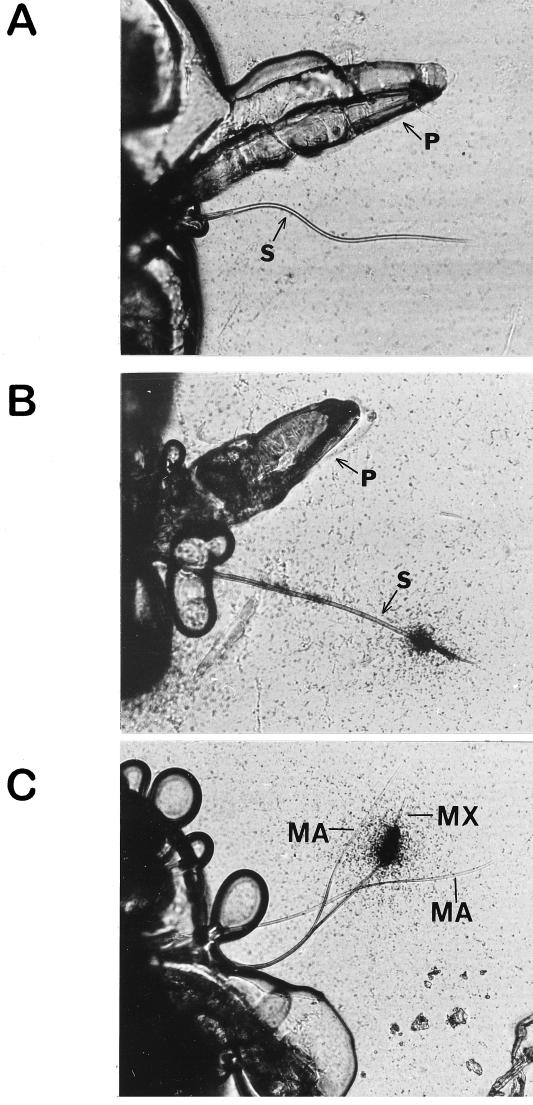
Autoradiographs of stylets of Myzus persicae given acquisition access to 125I-labeled tobacco etch potyvirus virions. (A) Stylets of an aphid that has not fed on an infected plant. (B) Stylets of an aphid that acquired labeled virus through a plastic membrane. (C) Distribution of label in stylets that have separated, showing label associated only with the food canal formed by the maxillary stylets. MA, mandibular stylets; MX, maxillary stylets; P, proboscis; S, stylet. Magnification, ×420. Reproduced from reference 161a with permission of the publisher.
The general feeding behavior of many arthropod and nematode vectors also aids in virus transmission to plants (5, 125, 148). The acceptance or rejection of a plant host by a vector with piercing-sucking mouthparts is performed by a series of brief probes into multiple plant epidermal cells. These brief probes are sufficient to inoculate the noncirculative nonpersistent viruses. Another benefit of this type of transmission mechanism is that the plant need not be a host of the vector for the virus to establish an infection. That is, the virus and the vector do not require overlapping host ranges for the virus to be efficiently transmitted to a wide variety of plant hosts. In general, the noncirculative, nonpersistent plant viruses are not vector species specific but are vector taxon specific. For example, individual potyviruses are transmitted by numerous aphid species but are not transmitted by whiteflies or leafhoppers. These viruses have evolved a transmission strategy based on a numbers game: quantity rather than quality. A virus will associate with many vectors in the hope that a few vectors will rapidly move to and probe another plant that can serve as a host for the virus.
If brief feeding probes indicate the plant is an acceptable host or food source, the vector is likely to initiate prolonged feeding. This may occur in numerous epidermal or mesophyll cells, or, more often, the insect will seek out its preferred feeding site, the carbohydrate-rich phloem sap (Fig. 1). Prolonged feeding allows for inoculation not only of the semipersistent, noncirculative viruses but also of the circulative viruses. These viruses have evolved a very different transmission strategy from the noncirculative, nonpersistent viruses. In general, they are transmitted by a single or a few vector species. The mechanisms of transmission are such that the virus associates with the vector for longer periods. This ensures that the virus survives until the vector finds a suitable host. However, since the same plant must serve as a host for both the vector and the virus, the host range of the virus is determined by the vector.
Direct inoculation of plant viruses into the plant vascular tissue is somewhat analogous to inoculation of arboviruses into the bloodstream of an animal host. Most of the arbovirus vectors feed and transmit viruses by piercing or cutting minor blood vessels and sucking up blood while injecting salivary secretions into the feeding site to prevent the blood from coagulating (37). At the same time, viruliferous vectors are releasing saliva-associated or mouthpart-associated viruses. The question arises of why arboviruses are often efficiently mechanically transmitted by a simple mechanism of mouthpart contamination and without any appreciable vector specificity (23, 164) whereas even high-titer plant viruses are rarely or inefficiently transmitted on the contaminated mouthparts of insects (112, 120).
This differential ability of plant- and animal-infecting viruses to be mechanically transmitted by several insect taxa may be explained by a few fundamental properties. (i) Most plant viruses do not occur in the extremely high titers required for the nonspecific mechanical transmission of animal viruses. (ii) Inoculation into the plant vasculature or “bloodstream” takes time. Plant-feeding insects with piercing-sucking mouthparts require approximately 15 to 30 min to wind their stylets between cells to reach vascular bundles (Fig. 1). The enzymatic action of breaking down the intracellular material is likely to dislodge or inactivate any contaminating virus before it can be injected into the plant vascular system. In contrast, blood-feeding insects locate their feeding sites very quickly. (iii) Plant viruses must be inoculated directly into a viable cell. They are unable to independently cross the cell wall or cell membrane. In contrast, arboviruses are inoculated into the bloodstream, not cells. Virus can then attach to and infect vertebrate cells independent of the vector.
There are insect vectors (mainly beetles) of plant viruses that have chewing mouthparts and a more indiscriminate feeding behavior than the piercing-sucking insects and nematodes. Inoculation of plant viruses by beetles was once considered to be a mechanical process in which either virus contaminating the mouthparts was deposited into the wound or virus in the gut was regurgitated as the beetle fed (137). Recent work has shown this process to be extremely specific and biologically complex. The reader is referred to reference 46 for in-depth coverage of this process. Briefly, the beetle-transmitted viruses can be inoculated into a chewing wound because the virus can rapidly translocate in xylem elements away from the site of inoculation and infect cells at a distance from the feeding site. Several viruses that are not transmitted by beetles were found to be acquired by the beetles and to be present in the hemolymph as well as the gut regurgitant that is deposited into and around the wound. The nontransmissible virus was apparently inactivated at the wound site or was unable to gain entrance to a functional plant cell that was capable of sustaining a virus infection. The mechanism by which beetle-transmitted viruses infect cells at a distance from the wound is unknown.
The transmission of plant viruses is now known to be biologically complex even in situations where initially it appeared to be a simple, nonspecific mechanical inoculation. The details of many of these molecular and cellular mechanisms regulating the transmission of plant viruses are described in subsequent sections.
NONCIRCULATIVE TRANSMISSION
The noncirculative method of transmission is not widely associated with animal virus transmission, but it is the method of choice for a majority of plant viruses (Table 1). All of the major insect vector taxa, including aphids, whiteflies, and leafhoppers, as well as the nematode vectors, transmit plant viruses in a noncirculative manner. The necroviruses and some other members within the Tombusviridae are carried on the external surface of soil-borne fungal vectors (22). The fungus-transmitted viruses are not considered in this review, although the classification of circulative and noncirculative would hold true in terms of membrane transport and internalization.
TABLE 1.
Mechanisms of transmission and principal vector species of plant virus families
| Virus taxon | No. of members | Principal vectora | Helper required |
|---|---|---|---|
| Noncirculative, nonpersistent | |||
| Caulimovirus | 17 | Aphids | Yes |
| Fabavirus | 2 | Aphids | No |
| Potyvirus | 186 | Aphids | Yes |
| Carlavirus | 55 | Aphids | No |
| Cucumovirus | 3 | Aphids | No |
| Alfamovirus | 1 | Aphids | No |
| Machlomovirus | 1 | Thrips, beetles | No |
| Macluravirus | 2 | Aphids | No |
| Potexvirus | 55 | Aphids (7/10), mites (2/10), mechanical | No |
| Noncirculative, semipersistent | |||
| Badnavirus | 16 | Mealybugs (3/6), leafhoppers (1/6) | No |
| Closterovirus | 25 | Aphids (10/19), whiteflies (6/19), mealybugs (2/19) | —b |
| Nepovirus | 39 | Nematodes | — |
| Sequivirus | 2 | Aphids | No |
| Tobravirus | 4 | Nematodes | No |
| Trichovirus | 6 | Aphids (1/3), mealybugs (1/3), mites (1/3) | No |
| Waikavirus | 3 | Aphids (1/3), leafhoppers (2/3) | Yes |
| Noncirculative, (Other) | |||
| Necrovirus | 3 | Fungi | No |
| Tombusvirus | 12 | Fungi (1/12), mechanical | No |
| Varicosavirus | 4 | Fungi | No |
| Circulative, nonpropagative | |||
| Enamovirus | 1 | Aphids | No |
| Geminivirus | |||
| Bigeminivirus | 41 | Whiteflies | — |
| Hybrigeminivirus | 2 | Treehoppers | No |
| Monogeminivirus | 11 | Leafhoppers | No |
| Luteovirus | 27 | Aphids | No |
| Nanavirus | 5 | Aphids | No |
| Umbravirus | 10 | Aphids | Yes |
| Bromovirus | 6 | Beetles | No |
| Carmovirus | 22 | Beetles (3/10) | No |
| Comovirus | 14 | Beetles | No |
| Sobemovirus | 17 | Beetles (6/8) | No |
| Tymovirus | 21 | Beetles | No |
| Bymovirus | 6 | Fungi | No |
| Furovirus | 12 | Fungi | No |
| Rymovirus | 7 | Mites | No |
| Circulative, propagative | |||
| Bunyaviridae | |||
| Tospovirus | 5 | Thrips | No |
| Marafivirus | 3 | Leafhoppers | No |
| Reoviridae | |||
| Phytoveovirus | 5 | Leafhoppers | No |
| Fijivirus | 6 | Planthoppers | No |
| Oryzavirus | 2 | Planthoppers | No |
| Rhabdoviridae | |||
| Phytorhabdovirus | 32 | Aphids (1/3), leafhoppers (1/3), planthoppers (1/3) | No |
| Cytorhabdovirus | 17 | Aphids (3/7), planthoppers (4/7) | No |
| Nucleorhabdovirus | 38 | Aphids (7/17), leafhoppers (4/17), planthoppers (6/17) | No |
| Tenuivirus | 10 | Planthoppers | No |
Numbers in parentheses indicate the number of viruses within the group that were reported to be transmitted by that vector divided by the total number of viruses within the group that were tested. Information was compiled from reference 18.
—, there is information to indicate that a helper factor may be required for the transmission of some members of the group.
A Biological, Not a Mechanical Process
The noncirculative viruses transmitted by arthropod and nematode vectors can be further subdivided into semipersistent and nonpersistent viruses (17, 65, 93). These two groups share some characteristics, but in general, semipersistent viruses tend to be associated with the foregut of the vector and are retained for several days or weeks (months or years in some cases). Transmission efficiency increases as the acquisition feeding time increases, which suggests that the virus is stably bound and accumulates until binding sites are saturated. In contrast, the nonpersistent viruses are associated with the stylets of the vector (Fig. 2), are retained for only a few hours, and are easily lost during feeding probes. Furthermore, transmission efficiency rapidly decreases as the acquisition feeding time increases. This suggests that bound virus is easily dislodged during prolonged feeding and that subsequently ingested virus cannot be reacquired by the formerly occupied sites along the stylets.
The site of virus attachment for nonpersistent viruses was recently identified to be near the distal tip of the maxillary stylets (95, 161). Virus could also be found in the proximal regions of the stylet and foregut (3, 161); however, there was no correlation between the amount of virus accumulated at these regions and transmission (124). In contrast, virus retention in the distal region of the stylets was highly correlated with virus transmission (161).
Three theories have been proposed for the mechanics of noncirculative transmission. (i) The stylet-borne theory, adapted from the mechanical-transmission theory of the animal virus literature, suggests that virus nonspecifically associates with or contaminates the distal tip of the stylet and is simply inoculated into the next plant as the vector begins to feed (79). In this mechanism, the vector is essentially a “needle.” (ii) Harris (64) proposed an ingestion-egestion mechanism in which transmissible virus adheres to multiple sites along the anterior alimentary canal during ingestion of plant material and is subsequently released during periods of regurgitation and salivation. In this mechanism the vector acts as a “syringe” rather than a “needle.” The ingestion-egestion hypothesis offered a potential mechanism of noncirculative transmission but did not distinguish between virus transported on the stylet tips or further inside the mouthparts or foregut. (iii) Recently developed technologies have led to a third hypothesis: ingestion-salivation (95). Virus can associate with multiple sites along the anterior alimentary canal, but the only virus to be transmitted is the virus attached to the proximal tip of the maxillary stylets, where the food and salivary canals are fused. Virus is released by the act of salivation rather than by regurgitation (Fig. 3). This could be viewed as a return to the “needle” analogy but not to the mechanical-transmission theory.
FIG. 3.
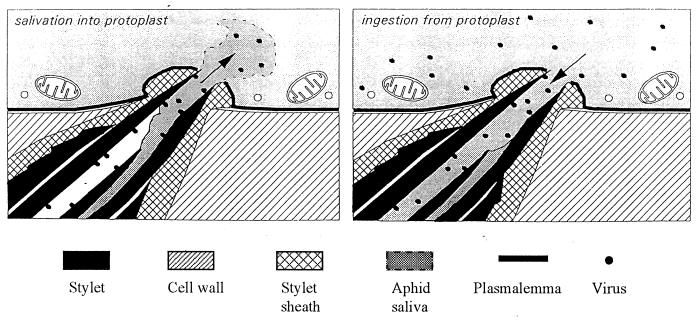
Model of the ingestion-salivation mechanism of noncirculative, nonpersistent transmission. Virus is ingested into the food canal (right), along with the cytoplasm. Virus adheres to the epicuticular lining of the food canal and the common duct at the very distal tip of the stylet, which is shared with the salivary canal. When the aphid first probes a cell after acquiring virus (left), saliva is injected into the cell. The watery salivary secretions will release virus from the cuticle lining the common duct, but virus farther inside the food canal would not be released by this mechanism. Reproduced from reference 95 with permission of the publisher.
Whether the insect or nematode acts as a needle or a syringe is perhaps not as important as the (now irrefutable) fact that the process is not a nonspecific mechanical transfer via contaminated mouthparts but, rather, a complex and very specific biological process (123). The most complete understanding of the mechanisms of noncirculative virus transmission comes from work on the aphid-transmitted potyviruses and caulimoviruses, both of which are nonpersistent (123). The reader is also directed to numerous other reviews on the subject of noncirculative transmission of plant viruses by arthropods and nematodes (17, 58, 65, 72, 79, 93, 94, 141).
Several groups of viruses, including the potyviruses and caulimoviruses, require a nonstructural, virus-encoded protein referred to as a helper component, a helper factor, or a helper (Table 1). Purified virus fed to aphids through a Parafilm membrane sachet was not transmissible, but if aphids were given access to a solution of plant sap from an infected plant (virus removed) before or along with purified virus, transmission was possible (55). Sap from a healthy plant did not mediate the transmission of purified virus. Some viruses require another virus, referred to as a helper virus, to be transmitted (Table 1), but it is not known for all cases if the helper virus particle itself is required or if the helper virus simply provides a helper factor. In addition to functioning in vector transmission, helper proteins have other functions in the virus life cycle. The potyvirus helper functions in polyprotein processing, movement of the virus in its plant host, and viral genome amplification (92, 135). The caulimovirus helper can bind microtubules and was proposed to be involved in movement of the virus in its host (13). Although there are several hypotheses for the role of helper in virus-vector interactions (58, 123), one is emerging as the most plausible and will be the focus of discussion here. The “bridge” hypothesis, i.e., that the helper acts to mediate the attachment of virus to the vector, was first proposed by Govier and Kassanis (55), but only recently has direct evidence been established.
Ammar et al. (3) provided ultrastructural evidence that potyvirus fed to an aphid in the presence of purified helper was embedded in a matrix material associated with the epicuticle. Virus was not retained and the matrix material was absent in aphids fed on the potyvirus alone. Immunolabeling demonstrated that helper protein was associated with virus retained in the matrix. Direct evidence of virus-helper-aphid interactions came from the identification of specific domains in the potyvirus coat protein and helper component that are required for aphid transmission. The potyvirus coat protein contains a DAG amino acid motif located near the N terminus. Mutations within or adjacent to this domain prevented the binding of virus to helper in vitro (11) and were shown to render numerous potyviruses nontransmissible (11, 74, 123, 161). Recently, Wang et al. (161) used radiolabeled virus to observe the effects of mutations in the coat protein DAG motif on the retention of virus in the stylet food canal and found that mutations in this region prevented the accumulation of virus in the stylets and prevented transmission.
The potyvirus helper factor has two characteristic amino acid motifs, a KITC box and a PTK box (123). Natural or engineered mutations in or adjacent to these motifs rendered the virus nontransmissible by the natural vector (4, 71). A specific mutation of the KITC sequence to EITC abolished transmission but did not affect the in vitro binding of virus to the helper (122a). Furthermore, virus was not observed in the stylets when acquired with the EITC mutant helper but was observed when acquired along with wild-type (KITC) helper (161). These data indicate that the KITC box functions in aphid-helper interactions, specifically in retention of the virus in the stylet. Mutations in the PTK box abolished helper-virus interactions in vitro (116). Therefore, this domain may play a role in attachment of the virus to the helper. Alternatively, mutations in this domain may prevent dimerization of the helper to the active configuration (150). All of these results strongly support the hypothesis that the potyvirus helper acts as a bridge to bind virus to the aphid stylet.
Further evidence that indirectly supports the bridging function of helper proteins was provided by analysis of the cauliflower mosaic caulimovirus (CaMV) helper. The CaMV helper accumulated in paracrystals in the cytoplasm of infected plant cells from which active helper was solubilized (12). The predicted structure of the CaMV helper indicates an N-terminal β-sheet domain and a C-terminal α-helix. Random structure separates the two terminal domains. The C-terminal domain mediates binding of the helper to virions in vitro. Mutations in this region abolished helper-virus binding in vitro and aphid transmission. Mutations in the N terminus also abolished aphid transmission but did not abolish the ability of the helper to bind to virions in vitro (134). The current working model is that the C terminus of the helper protein binds to virus particles whereas the N terminus is free to bind to sites in the aphid alimentary canal and mediate or bridge the indirect association of the virus particles to the insect cuticle.
The requirement for helper has also been demonstrated or suggested for a number of other insect- and nematode-transmitted viruses carried in both a nonpersistent and semipersistent manner (Table 1). It is unknown if the helpers for these other viruses function in a manner similar to the potyvirus and caulimovirus helpers or if the “bridge” hypothesis will apply. A common feature of all helper-mediated viruses that have been observed in their vector is that the virus particles are embedded in a semiopaque matrix material associated with the epicuticle lining of the anterior alimentary canal (3, 17, 27). The origin and composition of the matrix material are unknown.
Not all viruses transmitted in a noncirculative manner require a helper protein or helper virus (Table 1). Purified virions of members of the alfamoviruses, carlaviruses, and cucumoviruses can be transmitted by aphids without helpers. Studies with cucumber mosaic cucumovirus have shown that transmission is regulated solely by the capsid protein (26, 118). It is not known if these viruses are retained in similar locations in the vector to those that contain the helper-dependent viruses.
Why, if viruses can evolve a seemingly simpler capsid-mediated transmission strategy, have a majority of the noncirculative viruses evolved a helper-dependent transmission strategy? Pirone and Blanc (123) suggest that helpers may offer a method to widen the evolutionary bottleneck imposed by vector-dependent transmission. They make the point that a majority of the helper-dependent plant viruses are RNA viruses or DNA pararetroviruses. The low fidelity of RNA polymerases and the reverse transcriptase replication strategy used by the pararetroviruses are primarily responsible for the development of quasispecies, i.e., populations of viruses with a continuum of genome variants and invariably a continuum of biological properties (1, 87). Interestingly, a majority of the arboviruses are RNA viruses or DNA retroviruses, and a similar concept of populations existing as a collection of variants has been applied to their evolution (109).
Pirone and Blanc (123) argue that helpers can mediate the transmission of not only the homologous virus particle but also those of a number of related species (86, 90, 121); therefore, helpers should mediate the transmission of a number of coat protein variants within a quasispecies. Similarly, a single coat protein species should be able to interact with several helper variants within a quasispecies. Therefore, mutations in either the coat protein or the helper genes that reduce the overall transmission fitness of a specific virus-helper pair may not be detrimental to the overall transmission of a quasispecies. An additional benefit is that the mutations in helper proteins may allow the variant access to a new species of vector, thereby increasing the chances of transmission out of a host and possibly into a different set of recipient hosts. The helper strategy may actually preserve the genomic diversity within a quasispecies rather than limiting the number of viable genomes. Helpers may also provide an efficient means of expanding the number of vector species that can efficiently transmit a noncirculative virus.
Clearly, we have much to learn about the mechanisms of noncirculative transmission. Semipersistent and nonpersistent viruses may share some attributes, but differences in the sites of virus retention in the vector and in the times of retention indicate major differences in release of virus, in addition to mechanisms of binding. There is little experimental data to explain how virus particles bound to the epicuticle substrate are released. The N terminus of the potyvirus coat protein which binds to the helper protein is often proteolytically cleaved in vitro without having any deleterious effect on viral infectivity (133). Similarly, the C terminus of the nematode-transmitted tobacco rattle tobravirus (98) can be cleaved from the particle without adversely affecting viral infectivity. Interestingly, the coat protein structure changes in response to pH and the C-terminal region does not appear to be part of the structural framework of the virus (98). It is possible that proteinases in the vector saliva or regurgitated gut secretions can act as the scissors that cut the virus particle loose.
Viruses retained at different sites in the vector are likely to be exposed to different enzymes and ionic conditions that may affect the surface structure of virions or the conformation of structural proteins. Similarly, the chemical makeup of salivary and/or digestive secretions is likely to differ among vector species or even among biotypes within a species. These differences could contribute to differences between nonpersistent and semipersistent viruses and to the differences in the specificity or transmission efficiency of vectors for the same virus.
Potential Connection to Animal Viruses
The mechanisms of noncirculative transmission described for plant viruses may also apply to animal viruses. As mentioned above, noncirculative transmission is not widely associated with animal viruses; however there are numerous reports of mechanically transmitted animal viruses (23, 151). Equine infectious anemia virus represents an extreme for animal viruses. This virus does not infect its tabanid fly vector, but it is apparently dependent upon the fly for transmission between equine hosts (23). Members of the Poxviridae, Herpesviridae, Papovaviridae, and Retroviridae are also mechanically transmitted by arthropods but are not totally dependent upon the vector to spread between hosts (23). There is general agreement that mechanical transmission is important in the epidemiology of numerous animal viruses (23, 151, 164), but there also seems to be a widespread belief that mechanical transmission is a nonspecific, short-term incidental association of a virus with a blood- or wound-feeding arthropod (62, 164). Is the mechanical transmission of animal viruses by arthropods just the result of contamination of mouthparts, or is the transmission process of some of these viruses more specifically mediated?
Myxoviruses are perhaps the best-studied “mechanically” transmitted animal viruses (reference 40 and references within). Laboratory studies indicated that there was no vector specificity, indeed not even taxonomic specificity for the myxoma virus. In addition to being transmitted by multiple arthropod species, the virus could be experimentally transmitted via pins or thorns. It is likely that the association of the myxoma virus with many vectors is a laboratory phenomenon and is not relevant to natural transmission and epidemic development. However, the virus-vector relationship may not involve strictly mechanical contamination for all the vectors of myxoma virus. The early successes of the myxomatosis epidemics in Australia, which were deliberately begun to control the rabbit host, were dependent upon several species of mosquito. Success was partly due to the behavior and ecology of the vector that placed it in close proximity to the rabbits. However, other data would argue for a more complex interaction between the virus and mosquito than the “flying-pin” model would suggest. For example, the virus was retained by several mosquito species for extended periods and multiple inoculations from a single insect were documented. Furthermore, there were differences in the efficiency of transmission by different species that were not correlated to the titer of virus imbibed. Virus was associated with the proboscis and head region but not with the body of the insect (40). All of this is similar to the noncirculative mode of transmission of plant viruses and suggests that the association of myxoma virus with its mosquito vectors may be more than just mouthpart contamination.
It is possible, perhaps even likely, that many animal-infecting viruses have evolved vector relationships that are biological but do not include virus replication in the vector. However, the commonly used methods of testing and classifying arboviruses would not identify these viruses. Generally, viruses that are isolated from arthropods are evaluated by being injected into insects to determine if they replicate in that host and thus can be classified as arboviruses. A similar strategy for plant viruses would have identified few “arthropod-transmitted viruses.” Clearly, some animal viruses are mouthpart borne, and this type of transmission is important in the epidemiology of some viruses (23). Furthermore, animal viruses can be foregut borne, similar to the semipersistent, noncirculative plant viruses. For example, retroviruses can associate with and remain infectious in the foreguts of insects and subsequently can be transmitted to a new host after being regurgitated into the feeding site by the insect (82). This is not to say that insect transmission of retroviruses is important or common, only that noncirculative mechanisms of transmission similar to those described for insect-transmitted plant viruses, e.g., ingestion-egestion or ingestion-salivation, have been described for animal viruses. The noncirculative transmission of plant viruses is a very specific and complex biological process; perhaps the same is true for certain animal viruses. The noncirculative transmission of poxviruses may become even more important in light of recent findings that retroviruses (not normally efficiently transmitted by insects) can integrate into the poxvirus genome and can be efficiently transmitted by insects in a noncirculative manner (67).
CIRCULATIVE TRANSMISSION
Viruses transmitted in a circulative manner must be internalized by their vector to be successfully transmitted. That is, the virus must be transported across cell membranes. Members of the furoviruses and bymoviruses are transmitted internally by the motile zoospores of soil-borne fungi (36, 76, 132). These viruses are not discussed in this review, but by the definitions used here, they would be considered circulative viruses. All the remaining circulative plant viruses are transmitted by arthropods; there are no known circulative nematode-transmitted plant viruses (Table 1). As mentioned above, the circulative viruses are further divided into two subgroups, propagative viruses, i.e., those which replicate in their arthropod vectors (similar to the arboviruses) and nonpropagative viruses. The propagative viruses include members of five groups of viruses, three of which, the reoviruses, rhabdoviruses, and bunyaviruses, also have members that infect animals. There are no animal-infecting members of the tenuiviruses or marafiviruses. The nonpropagative viruses include the luteoviruses and the single-member enamovirus group. Geminiviruses are currently considered to be nonpropagative viruses, but the mechanism of transmission is undefined and they are discussed separately.
The general circulative pathway of virus movement through arthropod vectors (Fig. 4) is similar for both subgroups (and for arboviruses) and involves ingestion into the gut followed by association with and uptake by midgut or hindgut epithelial cells. Virus is then released into the hemocoel or secondarily infects other tissues. Eventually, all circulative viruses must associate with the salivary glands and be released into the salivary ducts. Once in the salivary duct, virus is free to be inoculated into plant (or animal) hosts as the insect salivates during feeding. Currently there is no evidence that saliva components contribute, negatively or positively, to the transmission of circulative plant viruses akin to the phenomenon of saliva-activated transmission (SAT) of arboviruses (110). SAT potentiates the transmission of some arboviruses through the release of pharmacologically active substances in saliva into the bloodstream of the vertebrate host. These substances have vasodilatory (117), antihemostatic (155), and host defense suppression (83) properties. SAT is also believed to be the underlying mechanism of “nonviremic transmission” of arboviruses between infected and uninfected vectors cofeeding in close proximity on a host that is not necessarily infected (84, 110). We are unaware of any studies that have investigated the role of insect saliva in reducing a plant defense response and facilitating virus infection. Interestingly, Mowry (105) reported that aggregates of aphids placed on noninoculated leaves of plants previously inoculated with the circulative, nonpropagative potato leafroll luteovirus caused a significant increase in the amount of virus accumulating at the feeding site relative to the amount accumulating in aphid-free leaves on the same plant. As stated above, insect saliva is probably involved in the release of noncirculative viruses from vector mouthparts, but it would be interesting to determine if insect saliva can potentiate the transmission of circulative plant viruses by influencing the infection site in the plant.
FIG. 4.
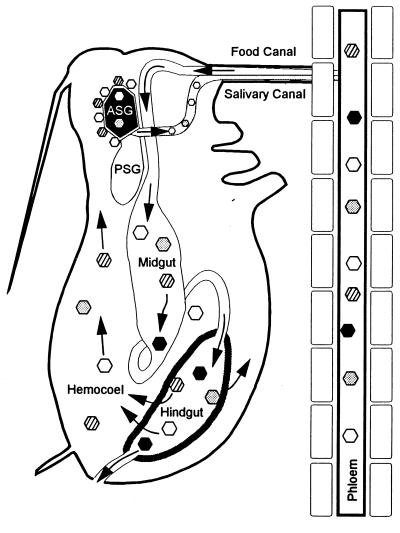
Circulative route of barley yellow dwarf luteoviruses (BYDVs) through aphids. All BYDV strains can be ingested from phloem into the aphid’s alimentary canal and arrive in the hindgut intact. The hindgut epithelium is the first transmission barrier; most BYDVs can bind to hindgut epithelial cells and be transported into the hemocoel, but some are excluded (solid hexagons). BYDVs acquired in the hemocoel must migrate to the ASG. The basal lamina of the ASG may selectively filter BYDVs or may concentrate virions, thereby increasing the efficiency of transport into the ASG. BYDVs (gray hexagons) not concentrated at the ASG may be transported into the ASG if they encounter it, but the efficiency of transmission is low. BYDVs may be concentrated at the ASG but be prevented from entering the ASG by an inability to bind to the ASG plasmalemma and initiate endocytosis (striped hexagons). Efficiently transmitted BYDVs are concentrated at the ASG and efficiently transported in the ASG and the salivary canal (open hexagons). Reprinted from reference 127 with permission of the publisher.
Circulative, Nonpropagative Transmission
The luteoviruses and pea enation enamovirus (PEMV) have a common circulative pathway and biology within their aphid vectors. The members of the luteovirus group and PEMV are each efficiently transmitted by one or, at most, a few aphid species. The transmission pathway through the aphid and the biological factors contributing to the vector specificity were recently reviewed (34, 52) and are only briefly described here.
Ultrastructural studies have shown that virus is not degraded or inactivated in the gut and that entry of virus into the hemocoel occurs through either the midgut (45) or hindgut (50) epithelial cells by endocytosis (Fig. 5). Virus is transported through the cytoplasm in vesicles that ultimately fuse with the basal plasmalemma, and particles are released into the space between the membrane and the basal lamina. Virus apparently moves rapidly across the basal lamina and into the hemocoel. In most virus isolate-aphid species combinations studied, virus was acquired in the hemocoel regardless of whether the aphid was a vector of that particular virus isolate (51). The gut does not appear to be a major barrier to luteovirus acquisition, although the process is specific for luteoviruses. Other morphologically similar viruses were observed in high concentrations in the gut lumen but were not acquired in the hemocoel (51).
FIG. 5.
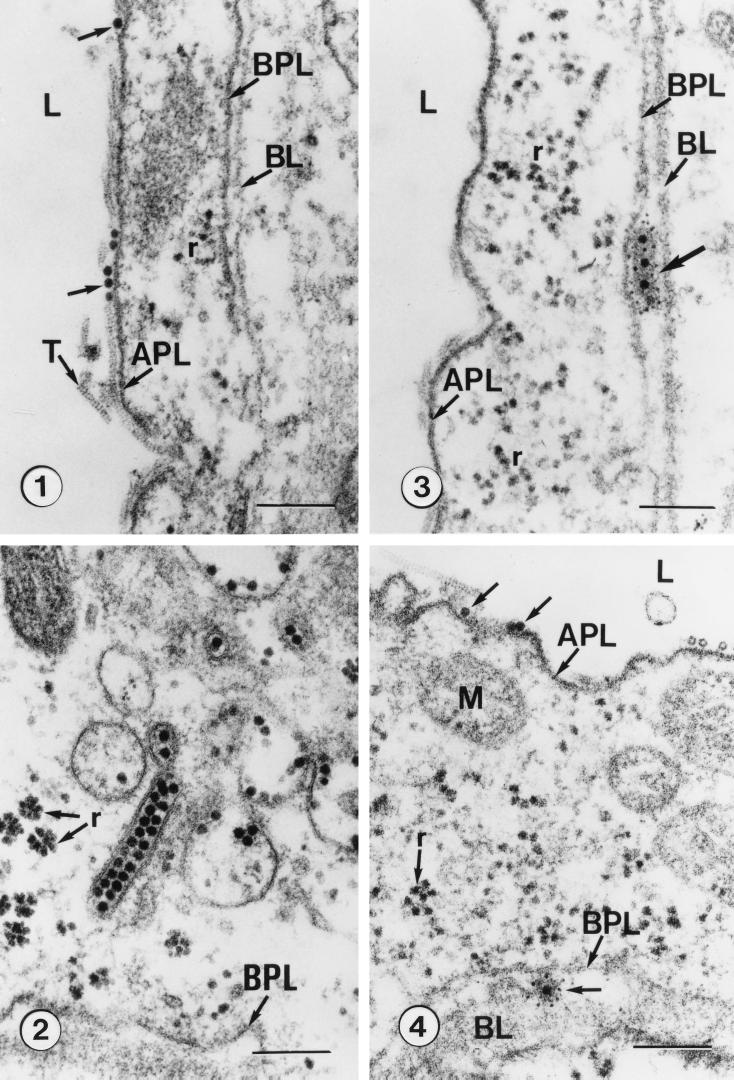
Electron micrographs of the hindgut of Rhopalosiphum padi microinjected with anti-barley yellow dwarf luteovirus (BYDV) antibodies for immunolabeling following acquisition feedings on Parafilm membranes containing purified BYDV or on oats infected with BYDV. (Panel 1) Ingested virions (arrows) in the hindgut lumen (L) adsorbed to the apical plasmalemma (APL). Note the longitudinal views of extracellular tubules (T), ribosomes (r), basal plasmalemma (BPL), and basal lamina (BL). (Panel 2) Unlabeled virions concentrated in receptosome-like vesicles and in a tubular vesicle adjacent to the basal plasmalemma (BPL) and basal lamina. Ribosomes (r) are also shown. (Panel 3) Ferritin-labeled virions (arrow) captured between the basal plasmalemma (BPL) and basal lamina (BL) upon release from the hindgut cell into the hemocoel. Apical plasmalemma (APL), hindgut lumen (L), and ribosomes (r) are also shown. (Panel 4) Unlabeled virions (arrows) in the hindgut lumen (L) adjacent to the apical plasmalemma (APL) and an anti-BYDV-labeled virion adjacent to the basal plasmalemma (BPL) following transport to the hemocoel. The basal lamina (BL), mitochondria (M), and ribosomes (r) are also shown. Bars, 200 nm. Reproduced from reference 51 with permission of the publisher.
Luteoviruses are able to survive in the aphid hemolymph despite potential insect immune responses that may be capable of neutralizing the invading virus. Potential mechanisms for this evasion are discussed below, but similar to the gut barrier, these mechanisms appear general to all luteoviruses and do not contribute to vector-specific transmission (156).
The salivary glands in aphids consist of two principal glands and two accessory salivary glands (ASG) (126). Luteoviruses and PEMV associate exclusively with the ASG and more specifically with the anterior portion of these four-celled glands (52). The ASG produce a watery secretion, containing few or no enzymes, that is thought to be involved in deposition of the stylet sheath during feeding (126). The highly invaginated apical plasmalemma of ASG cells suggests a rapid transport of water and ions, which would be consistent with the suggestions that the ASG function as an excretory organ and play a role in the removal of waxy material originating from degenerating fat cells in the hemolymph (126). The salivary glands of ticks have also been found to play a role in removal of foreign substances from hemolymph and may be part of the tick self-defense system (159). It is possible that luteoviruses have evolved to take advantage of specific excretory pathways to access the salivary ducts. Ultrastructural evidence indicates that the pathway of luteovirus through the ASG (Fig. 6 and 7) is similar to mechanisms used to cross the hindgut. An inability of luteovirus isolates to penetrate the ASG of nonvector aphids has long been known to contribute to vector specificity (130). Recently it was shown that the basal lamina and the basal plasmalemma function as independent barriers to transmission in different luteovirus isolate-aphid species combinations (Fig. 7) (51, 54a, 115). The two ASG-associated barriers and the hindgut barrier can function as the primary barrier of transmission for the same virus in different aphid species or in the same aphid for different virus isolates. This indicates that different membrane attachment sites (receptors) and different virus attachment protein domains are used at each transmission barrier by different virus isolate-aphid species combinations.
FIG. 6.
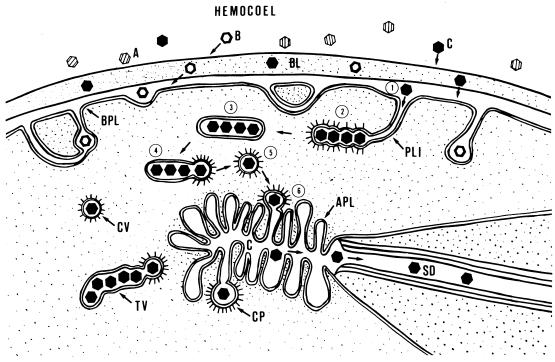
Model of the interactions of luteoviruses with the ASG of aphid vector species. Three types of interactions were observed when virions of the MAV isolate of barley yellow dwarf virus were acquired by aphids that fed on infected plants or were injected with purified virions into the hemocole. In the first type of interaction, MAV virions had no affinity for the salivary basal lamina (BL) of specific aphid species and did not attach to or penetrate the basal lamina (A, nonpenetrating, nontransmitted virions). In other species, MAV virions did exhibit affinity for the salivary basal lamina and were able to attach to and, in some cases, penetrate the basal lamina. However, these virions were unable to initiate endocytosis at the basal plasmalemma (BPL) and were not transmitted (B, penetrating, nontransmitted virions). In the third type of interaction, virions consistently penetrated the basal lamina (step 1), were aggregated in plasmalemma invaginations (PLI), and were endocytosed into the cell by coated-pit formation (step 2). Virions acquired in the cytoplasm accumulated at the apical end of the cell in tubular vesicles (step 3). Individual virions budded from the tubular vesicles by coated-pit formation (step 4) and were transported to the salivary canal (Cn) in coated vesicles (step 5) that fused to the apical plasmalemma (APL), releasing the virion into the canal lumen (step 6). Transcytosed virions were then able to move into the salivary duct (SD) (C, penetrating-transmitted virions; TV, tubular vesicle; CP, coated pit; CV, coated vesicle). Reproduced from reference 54a with permission of the publisher.
FIG. 7.
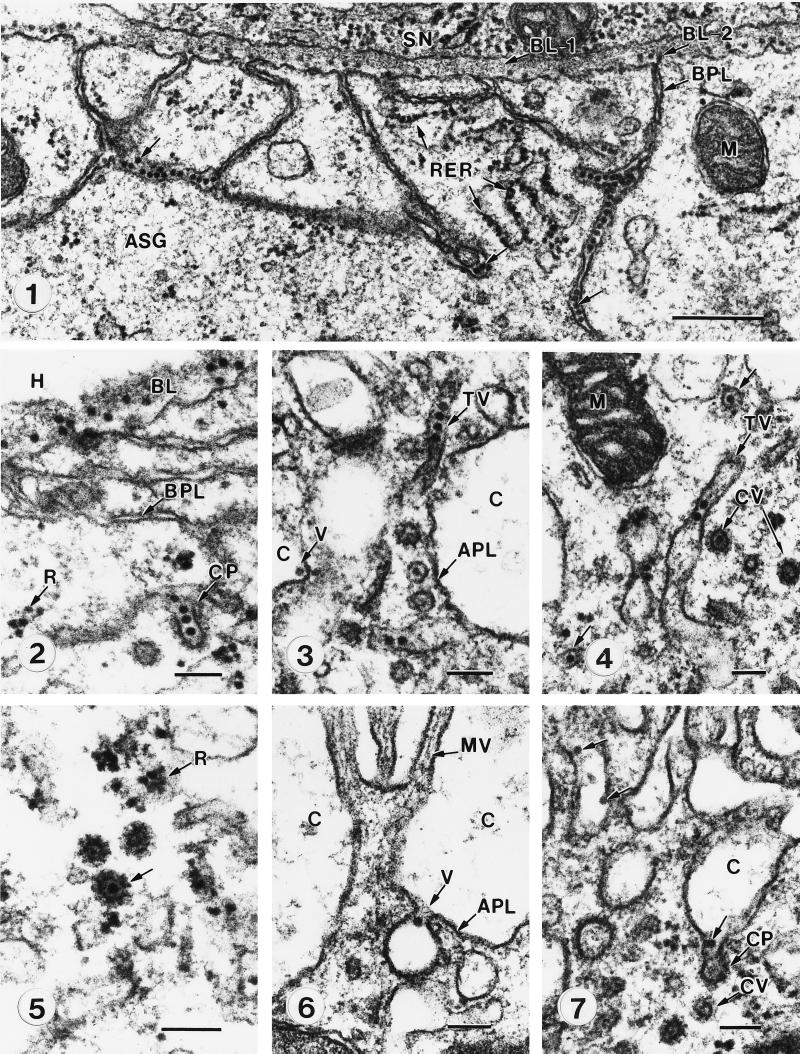
Ultrastructure of membranes associated with transcellular transport of microinjected virions of the MAV isolate of barley yellow dwarf virus through the ASG of Sitobion avenae. (Panel 1) Virions of MAV embedded in the ASG basal lamina (BL-2) and concentrated in basal plasmalemma (BPL) invaginations (arrows). Note the absence of virions in the basal lamina (BL-1) of the adjacent subesophageal nerve ganglion (SN). Particles in the cytoplasm are ribosomes associated with the rough endoplasmic reticulum (RER). M, mitochondria. Bar, 500 nm. (Panel 2) Virions penetrating the basal lamina (BL) from the hemocole (H) and in a coated pit (CP) during endocytosis through the basal plasmalemma (BPL). The small, irregularly shaped particles observed free in the cytoplasm are ribosomes (r), as determined by RNase digestion (3) and observation of ultrastructure under higher magnification. (Panel 3) Virions packaged in tubular vesicles (TV) adjacent to the apical plasmalemma (APL) lining a microvillus-lined canal (C). V, virion in canal. (Panel 4) Virions in tubular vesicles (TV) and associated coated vesicles (CV). M, mitochondria. (Panel 5) Comparison of a virion in a coated vesicle (arrow) to cytoplasmic ribosomes (R). (Panel 6) A virion (V) being released from the accessory salivary gland cell into the canal lumen (c) by exocytosis through the apical plasmalemma (APL). MV, microvilli. (Panel 7) Virions (arrows) in the canal lumen (C) released from coated pits (CP) following fusion of coated vesicles (CV) with the cell membrane. Bars, 100 nm. Reproduced from reference 54a with permission of the publisher.
The luteovirus- and PEMV-encoded proteins involved in aphid transmission have been studied, and the two virus groups share some features at the molecular level. The luteoviruses are currently divided into three taxonomic subgroups based on differences in genome organization, and all three are different from the bipartite PEMV. However, the luteoviruses all share a conserved arrangement of three open reading frames, two of which encode the structural proteins (102). The virus capsid contains a predominant coat protein (ca. 22 to 24 kDa) and a minor amount of a larger protein translated via a readthrough of the coat protein stop codon. The full-length luteovirus readthrough protein is ca. 72 to 74 kDa, but the carboxyl-terminal portion of the readthrough domain is proteolytically processed to yield a 55- to 58-kDa readthrough protein commonly associated with purified virus preparations (14, 42, 77, 160). It is not known if this type of processing actually occurs in vivo. Purified virus and virus produced from cloned cDNA copies of the virus that do not translate the carboxyl terminus of the readthrough domain are transmissible (19, 58a, 160). These findings indicated that the carboxyl-terminal portion of the readthrough domain was not required for aphid transmission and also that there is no requirement for a nonstructural helper in the transmission process. The PEMV readthrough protein is inherently smaller than its luteovirus counterpart and does not undergo further processing (35).
The readthrough protein was not required for particle assembly or plant infection (35, 42, 129), but particles containing only the 22- to 24-kDa coat protein were no longer transmissible by aphids to plants (14, 25, 35, 156). This led to the widespread assumption that readthrough protein was responsible for the aphid transmission phenotype. However, virions without readthrough protein ingested by aphids were detected in the hemolymph, indicating that the coat protein contained all the determinants for uptake of the virus through the hindgut (25). There are a number of highly conserved domains in the coat proteins of all luteoviruses that are likely candidates for mediating virus attachment and transport through the aphid gut. This theory of a common virus sequence mediating gut uptake is consistent with the above-mentioned biological data that the hindgut does not contribute significantly to vector-specific transmission of luteoviruses. A detailed mutational analysis of the coat protein is needed to validate this hypothesis.
Luteovirus coat protein genes without the corresponding readthrough sequences have been expressed in insect cells by using a baculovirus vector, and virus-like particles (VLP) were observed (85). The readthrough-minus VLP were purified and either fed to aphids through a Parafilm membrane sachet or injected directly into the hemocoel. Ultrastructural examination of the aphids revealed the ingested particles were acquired through the gut into the hemocoel, and, surprisingly, VLP were observed in the accessory salivary gland cells and in the salivary ducts (54). These results are consistent with earlier studies showing that readthrough was not required for acquisition through the gut but contrasted with the hypothesis that readthrough determined vector specificity by regulating the transport of virus through the accessory salivary gland. What, then, is the function, if any, of the readthrough domain in the aphid transmission process?
It has long been assumed that the only barriers to luteovirus transmission were the tissue-associated membranes, e.g., the hindgut and accessory salivary gland. However, one potentially important aspect of the vector has been neglected in the search for aphid-virus interactions: the insect immune system. The effect of insect immune systems on parasite infection and transmission has received widespread attention (61, 97), but the effect on virus infection has not been as widely studied (106). The immune system of aphids has received little attention. Aphid hemolymph was reported to be void of hemocytes (126), but it is unknown if this is a general phenomenon for all aphids. Other types of defenses, such as humeral encapsulation (158) or the production of defense-related proteins such as interferon (29, 106), may exist. These types of defense systems are active in many insects (60), including Homopterans. How, then, are luteoviruses or PEMV able to survive for extended periods in the aphid hemolymph, an environment shown to be hostile to insect pathogens and parasites? The answer may be related to the readthrough protein.
Aphids harbor endosymbiotic bacteria of the genus Buchnera in specialized cells located in the abdomen, called mycetocytes (126). Neither the aphids nor the bacteria are able to survive and reproduce without the other. Not all the benefits that the bacteria provide for the aphid are known, but one function is to provide essential amino acids that the aphid is unable to synthesize (7). In addition, the bacteria produce copious amounts of a chaperonin protein named symbionin, a homologue of the Escherichia coli GroEL protein (7, 41). The role of this protein in aphid metabolism is unknown, but the chaperonin class of proteins generally functions in protein folding, translocation across membranes, and recovery from stress. Symbionin is produced and stored exclusively in the mycetocytes and is unlikely to be exported into the aphid hemolymph (43). The reported detection of symbionin in hemolymph (157) is most probably due to the degradation of the endosymbionts and mycetocytes as the aphid reaches maturity (7, 126).
Interestingly, symbionin has been shown to bind to purified luteoviruses in vitro or to a recombinant luteovirus readthrough polypeptide (41, 68, 156, 157). When aphids were cured of endosymbionts by treatment with antibiotic, their ability to transmit virus was significantly reduced and the amount of coat protein detected in the aphid was diminished. Strangely, the amount of readthrough was not affected (68, 157). The results of these experiments must be interpreted carefully. The destruction of the endosymbionts is likely to have dramatic effects on the metabolism and physiology of the aphids, and these changes may be directly or indirectly responsible for the effects on luteovirus protein detection and virus transmission.
Recently, six luteoviruses and the related PEMV were all shown to bind specifically but differentially to E. coli GroEL and symbionin homologues from vector and nonvector aphids (156). The binding capacity was not correlated with transmission ability or efficiency, suggesting that if symbionin plays a role in transmission, it does not play a role in vector specificity. Furthermore, an analysis of the ability of a series of readthrough deletion mutants to bind symbionin in vitro indicated that the amino-terminal portion of the readthrough domain contained the determinants for symbionin binding. Finally, virions that did not contain readthrough protein and did not bind symbionin in vitro were less persistent in the aphid hemolymph than was wild-type virus. These studies provide convincing data that symbionin can interact specifically with luteoviruses and PEMV in the aphid hemolymph and can slow the degradation of virus. The mechanisms of degradation of virus in the hemolymph are unknown, and it is unknown if the attachment of symbionin to the virus protects the virus from targeting by the aphid immune system or, alternatively, if it facilitates virus movement into the accessory salivary gland.
The circulative, nonpropagative plant viruses have evolved several mechanisms to utilize insect cell membrane functions and to interact with the products of the aphid endosymbionts. Why develop such a complex and nonproductive association with the vector? The virus must maintain a number of defenses and tricks to move through various membranes and survive in several hostile environments. The virus does prolong its association with a vector, but it does not replicate and is constantly fighting a losing battle to remain viable in a hostile vehicle of transport. The luteoviruses and geminiviruses are phloem restricted and must be inoculated directly into phloem tissues to cause an infection. Aphids, whiteflies, leafhoppers, and planthoppers, the vectors of these viruses, are all phloem feeders. It takes time for the insects to reach the phloem, and they will feed on the phloem only if the plant is a host of the insect. Therefore, to ensure their long-term survival and maximize their chances of moving between host plants, these viruses have evolved a transmission strategy that requires that they have a long term relationship with the vector. Furthermore, this type of transmission means that the vector will determine the host range of the virus, since the virus will be inoculated into phloem tissues only if the plant is a host of the vector. The ability of the virus to survive for extended periods without replicating in the vector and being pathogenic to the vector is good for the vector, although long-term survival of a nonreplicating virus in the potentially hostile environment of a vector does not appear to be advantageous to the virus.
Luteoviruses, PEMV, and geminiviruses appear to have used reassortment as a driving force in their evolution (15, 49, 96). The various luteovirus subgroups have ties to different virus supergroups relative to their replication, but they all have acquired and retained a conserved arrangement of structural and movement proteins.
Perhaps these viruses have not yet been able to acquire the genes that would allow them to replicate in their insect vector. Alternatively, they may be evolutionarily undecided whether to ultimately pursue a noncirculative mode of transmission or a circulative propagative mode. For example, PEMV is a bipartite virus; RNA 1 is luteovirus-like and, as discussed above, contains the genes encoding the transmission-associated structural proteins. This virus has acquired a second RNA which, in addition to other functions, has allowed the virus to escape its phloem limitation. It can be acquired from and inoculated into epidermal cells, a feature that allows it to be inoculated, perhaps even acquired, by aphids during brief feeding probes. This could lead to a broader host range of the virus, since it is not completely dependent upon the vector to determine its host range. Perhaps the virus will ultimately acquire a helper or a new capsid that will allow it to associate with the mouthparts of its aphid vectors and become noncirculative. One member of the geminiviruses may be moving in the opposite direction, toward becoming propagative in its vector (see below).
Geminivirus transmission.
Geminiviruses are single-stranded circular DNA viruses that have been divided into three taxonomic groups or genera (Table 1). The viruses within the monogeminivirus and hybrigeminivirus genera are each transmitted by a different species of leafhopper or treehopper. Viruses within the bigeminivirus genera are all transmitted by whiteflies, although there is likely to be more than one mechanism of transmission. The coat protein has been shown to be the sole determinant of transmission of some whitefly-borne viruses (113), a property that was recently mapped to the N terminus of the coat protein of abutilon mosaic bigeminivirus (166). The coat protein was also shown to be the sole determinant of whether a geminivirus is transmitted by a whitefly or a leafhopper (16). However, the coat protein does not solely determine the transmission phenotype of all geminiviruses. Recently the genomic analysis of another whitefly-transmitted bigeminivirus, tomato golden mosaic virus, indicated that although the coat protein was required for acquisition of the virus, both genomic components (DNA A and DNA B) were required for transmission. DNA B was essential for the accumulation of virus in the whitefly, while DNA A was required for the successful inoculation of plants by viruliferous insects (88).
The transmission of all geminiviruses has been classified as circulative and nonpropagative. Virus has been observed in the gut epithelial cells and associated with salivary glands of whitefly vectors, and it is assumed to follow a similar circulative strategy as the aphid-transmitted luteoviruses, although no detailed ultrastructural studies have been published (30). Whiteflies also possess endosymbionts (31), but it is not known if they produce a symbionin homologue, nor has the ability of geminiviruses to bind any of the characterized symbionin homologues been reported.
Several lines of evidence, in addition to the nonstructural gene requirement for transmission (88), suggest that some whitefly-transmitted geminiviruses have evolved interesting twists that may indicate a more complex transmission pathway than that of luteoviruses. Studies to determine virus titers over time in the insects have not conclusively shown an increase that would suggest virus replication, but the viral DNA does persist in the insect longer than its infectivity would suggest (20, 30, 131). No replicative forms of the viral DNA have been detected within the insect, which argues against the replication of virus in the insect. However, squash leaf curl virus was observed in several whitefly tissues, and the presence of virus was associated with cytopathological abnormalities in some tissues (119). Furthermore, the presence of the virus in the insect can have detrimental effects on the biology and reproduction of the vector (131). Both of these observations would suggest virus replication. No cytopathological or deleterious reproductive effects have been documented for aphids fed on luteovirus-infected plants.
Another interesting twist is the recent finding that a monopartite isolate of the tomato yellow leaf curl virus is transmitted transovarily in its whitefly vector (47). Although this is in contrast to previously published reports (30), the data are convincing that this tomato yellow leaf curl virus isolate can be transovarily passaged. One criterion for the classification as a nonpropagative, circulative plant virus is an inability to be transovarily transmitted (65), since this type of vertical transmission usually indicates that the virus is replicating in the vector. Geminiviruses may have evolved a mechanism to cross the transovarial transmission barriers without replicating in that tissue, or perhaps there is some low level of infection of reproductive tissues. A potential paradox (if the geminiviruses are found to replicate in their vectors) is that the complete genome organizations of the various groups of geminiviruses are known and specific and required functions in the plant infection process have been assigned to all genes and gene products. Therefore, the same genes would have to function in virus replication within both plant and insect hosts, although presumably any host components required by the virus would be different.
Circulative, Propagative Transmission
The plant-infecting viruses within the circulative, propagative classification (Table 1) are those most closely related to the arboviruses; indeed three of the five taxonomic groups considered here have animal-infecting members: rhabdoviruses, reoviruses, and bunyaviruses. The plant viruses within these groups could be considered plant-infecting arboviruses or phytoarboviruses (165). It is odd, however, that the arboviruses and phytoarboviruses are classified as vertebrate-infecting and plant-infecting viruses, respectively, rather than both being classified as invertebrate viruses. The arthropod host is more important in the evolution and survival of the virus because it will exert a greater selection pressure (109). The virus must evolve and maintain the ability to infect and survive in the arthropod, an accomplishment that requires the virus to surmount a number of barriers not present in the vertebrate host (see “Vector competence”). Furthermore, most of these viruses do not have a pronounced deleterious effect on their arthropod host and they depend on the arthropod host for long-term survival. In contrast, the animal or plant host is often a temporary host that serves only as a high-titer source to allow the efficient infection of more arthropod hosts (109, 164). There are, of course, exceptions to this general trend. Several arboviruses and phytoarboviruses adversely affect the longevity and fecundity of their vectors, while others are avirulent in their plant or vertebrate host (references 2, 109, and 164 and references within).
Until recently, the phytoarboviruses had not received a great deal of attention. With few notable exceptions, such as the tomato spotted wilt tospovirus, many of these viruses are not economically important. Their genomes tend to be relatively large and complex, and most have remained recalcitrant to analyses by many of the modern molecular biology techniques. In addition, it has been difficult to generate sufficient numbers of stable mutants with phenotypes related to vector transmission. Although these problems have also plagued arbovirus research, it has benefited tremendously from the establishment of cultured vector cell lines (101) and the ability to conduct detailed genetic studies on vector populations (144, 164). Both of these research strategies have been difficult to develop and apply to the insect vectors of the phytoarboviruses.
Vector competence.
The individual arboviruses and phytoarboviruses tend to be transmitted by only one or a limited number of closely related vector species. Furthermore, intraspecific variation in susceptibility and the ability to transmit virus has been reported for populations of vectors of numerous viruses (69, 70, 109, 146, 149). Consequently, much of the research on transmission has investigated the vector specificity of these viruses. The general pathway through the arthropod is similar for all these viruses. Virus is imbibed along with the plant sap or the bloodmeal. It then attaches to and infects midgut cells, usually reaching high titers in these tissues. It is released into the hemocoel and secondarily infects other tissues, including reproductive tissues, from which it can spread vertically to offspring. Horizontal transmission to other plant or animal hosts occurs following infection of salivary tissues and subsequent release of infectious virus in the salivary secretions that are injected into the host during feeding.
Vector competence (ability to transmit) is determined not only by the ability of the virus to infect the tissues of the vector but also by the ability of the virus to successfully enter and exit the critical tissues. Extensive studies have been done to identify the cellular barriers to transmission, and Ammar (2) has provided an excellent and comprehensive review of this topic for both phytoarboviruses and arboviruses. The barriers include the midgut infection barrier, which was first demonstrated for eastern equine encephalomyelitis alphavirus (99) and has subsequently been demonstrated for other animal- and plant-infecting viruses. An active midgut infection barrier will effectively render the arthropod immune to the virus. This barrier was once considered the reason why many potential vectors were not capable of virus transmission. However, there were several viruses that were able to infect a potential vector but were not transmitted. Hardy et al. (63) first reported the existence of two other barriers that could explain this phenomenon, a midgut escape barrier and a salivary gland infection barrier. A midgut escape barrier has been demonstrated for tomato spotted wilt tospovirus (Bunyaviradae) in the adult stage of the thrips vector. The virus must be acquired by the larval thrips to be transmitted. Virus can infect and replicate in midgut cells of both larval and adult thrips but can disseminate only from larval midgut cells into other thrips tissues (153). The wound tumor reovirus (57) and sowthistle yellow vein rhabdovirus (9), both phytoarboviruses, were able to invade and replicate in several tissues of their leafhopper or aphid vectors, respectively. However, in nontransmitting individuals, the viruses were not associated with the salivary glands. This suggests the existence of a salivary gland infection barrier but does not rule out the possibility that the virus is not able to survive in the hemolymph or hemolymph-associated cells that would come in contact with the salivary glands. There is also a salivary gland escape barrier, which has been demonstrated for some arboviruses in their mosquito vectors (59), but this has not been demonstrated for any phytoarbovirus. The phenomenon of saliva-activated transmission (110) appears to be yet another potential barrier to the successful transmission of some arboviruses, but it has not been investigated for phytoarboviruses. The successful transmission of any of these viruses requires the virus to run the gauntlet of potential barriers, each of which have been shown to be active in some virus-vector combination. Similar to the situation described above for the circulative, nonpropagative luteoviruses, the specific barrier may differ for any combination of virus and vector and no generalities seem to be applicable.
The molecular and physiological basis for virus-vector interactions that regulate transmission are not well understood, but it is clear that genetic elements within both the virus and the vector ultimately decide if a particular species or individual within a species of arthropod is able to be a vector for a particular virus strain. Environmental or abiotic factors also play a role in determining virus-vector interactions, but in general these factors seem to influence the efficiency of the interaction rather than to determine the ability of the interaction to take place (164).
The genetics of vector competence are receiving widespread attention, and results have begun to change the central dogma that all individuals within a vector species are potential vectors. A more enlightened concept states that populations within a species will differ in their ability to be efficient vectors for certain viruses (144). Intraspecific variation in vector capacity is not unique to arboviruses; it has long been known for vectors that transmit plant viruses by all of the mechanisms described thus far (138). Understanding why a vector is a vector and developing the tools to rapidly and accurately identify potential vectors is important for understanding the epidemiology of a virus and for developing control measures. Viruses are extremely difficult, if not impossible, to control once they have infected a susceptible animal or plant host. Current strategies for virus disease control are usually aimed at protecting the host, e.g., vaccines in animal hosts or pathogen-derived resistance strategies in plant hosts (89, 114), but a more direct strategy would be to prevent the infection of the arthropod host and/or transmission of the virus.
Arbovirologists and medical entomologists have begun to develop systems to investigate the genetics of vector populations and have begun to develop the tools to allow the molecular mapping of elements that differ between individuals that are able to efficiently transmit viruses and individuals that are refractive to virus infection or transmission (144). In contrast to the simple monogenic control of vector susceptibility to malaria parasites or filarial infection, the competence of viral vectors appears to be a complex multigenic phenomenon in several instances (144, 147). However, a single locus that controls the susceptibility of Culicoides variipennis to bluetongue virus was identified (143). The inability of a strain of Aedes aegypti to transmit several flaviviruses was also found to be regulated by a single gene or set of closely linked genes (100). Similarly, the ability of rice hoja blanca tenuivirus to replicate in its planthopper host, Sagosodes oryzicola, is controlled by a single recessive gene (168).
Genetic maps are being constructed for several insect species that are the principal vectors of several important human and animal viruses (144). In addition, techniques are advancing so that the stable transformation of insects with foreign genes is now possible (75, 80). Analogous systems are beginning to be developed for the insect vectors of the phytoarboviruses. The routine generation of sexual forms and the sexual crossing of many important aphid vector species have been accomplished in several laboratories, and genetic polymorphisms are being identified (10, 136, 152). One of the problems in the analysis and mapping of genetic traits in insects such as mosquitoes has been in the relatively small amount of DNA that can be extracted from an insect for analysis. Aphids may offer a solution to this problem, since they reproduce parthenogenetically unless environmental factors induce a switch to sexual reproduction. The clonal propagation of aphids provides a limitless supply of identical individuals for genetic and biological study. Furthermore, the long-term stability of virus transmission phenotypes of clonal populations has been established (127). Although the genetic mechanisms regulating vector competence for arboviruses and phytoarboviruses remains largely unknown, modern molecular technologies are likely to allow for the identification of these genetic elements in the future. Similar technologies have been well developed and are very successful in the identification of genes and assignment of gene function in plants and animals.
Virus competence.
The genetics of virus transmission by vectors is not controlled solely by the vector; the virus also contributes to the overall process (58, 122). The current knowledge of virus genetics in relation to noncirculative transmission and circulative, nonpropagative transmission was described earlier in this review. Limited progress has been made in understanding the phytoarbovirus genes and gene products that influence vector transmission. A lack of stable cell lines from insect vector species and a difficulty in developing stable mutants with a transmission-deficient phenotype have contributed to the slow progress.
The establishment of continuous mosquito cell lines (56) has greatly facilitated our knowledge of arbovirus-vector interactions. The cell lines have allowed the study of virus entry into and release from insect cells and of virus replication and gene expression in insect cells relative to their animal host counterpart (101). It has been difficult to establish stable cell lines for many of the important phytoarbovirus vectors (32). Chiu and Black (28) established cell lines from the leafhopper vector of wound tumor reovirus, and, more recently, additional cell lines have been established for additional leafhopper vectors of other reoviruses (154) as well as for thrips vectors of tomato spotted wilt tospovirus (107). These successes, coupled with recent progress on the molecular biology of several reoviruses and the tospoviruses, is likely to allow significant progress to be made in the future in understanding how these viruses can infect their insect hosts.
Despite an absence of vector cell lines, progress on the identification of virus genes whose function is related to transmission has been made by studying virus associations with whole insects. Total-protein extracts from whole thrips or from thrips gut tissue were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and subsequently probed with tomato spotted wilt bunyavirus or its glycoproteins in a gel overlay assay. Two independent laboratories identified two different thrips proteins, one of which was associated with midgut tissues (6). The tissue association of the other was not determined (81). The animal-infecting LaCrosse bunyavirus glycoproteins have long been implicated in vector transmission (8), and recently it was shown that the GP1 interacts with both mosquito and vertebrate cells in culture but not with mosquito gut cells in vivo. GP2 binds only to mosquito cells. Both glycoproteins are most probably involved in cell attachment and virus entry (91).
Studies on whole, virus-infected insects have also identified differences in viral RNA and protein accumulation between the plant and insect hosts. Falk et al. (39) identified a nonstructural protein of maize stripe tenuivirus that accumulated in maize but not in the leafhopper vector. Similar results were recently reported for the related rice grassy stunt tenuivirus in rice and its leafhopper host (103). The function of the nonstructural protein is unknown. Perhaps it serves as a plant virus movement protein or aids in the initial uptake of virus by the vector but does not subsequently need to be produced by the vector. In contrast to the aforementioned studies, the RNAs encoding the two maize stripe tenuivirus glycoproteins are abundant in both insect and plant host cells (38). It is not known if the proteins accumulate to similar levels or are required for infection of both hosts, but they are integral membrane proteins and, by analogy to other viruses (91), are assumed to be involved in insect cell membrane attachment. All serologically detectable rice dwarf reovirus proteins are also present in both insect and plant hosts (140). Similar findings of no qualitative differences in viral RNAs or proteins have been reported for the alphaviruses (101, 139). However, posttranslational processing of viral proteins differed in mosquito and vertebrate hosts. Differences in glycosylation pathways in mosquito cells and vertebrate cells resulted in differences in attached oligosaccharides and subsequent differences in proteolytic cleavage. These differences do not appear to alter transport of the protein but apparently affect budding of the virions through cell membranes (101, 139). In addition, differences in methylation and RNA-capping pathways in invertebrate and vertebrate cells apparently affect alphavirus replication and accumulation rates (139). These fundamental differences are likely to contribute to the distinct differences in pathologic findings induced by alphaviruses in mosquito and vertebrate cell cultures (78).
Another major problem in developing an understanding of phytoarbovirus-vector interactions has been in the development or identification of stable mutants that have altered vector transmission phenotypes. The viruses have all remained recalcitrant to manipulation by many of the modern molecular biology techniques. Infectious DNA clones have not been produced, and therefore neither a directed mutational strategy nor a reverse genetic approach to identifying gene function is possible. A limited number of transmission mutants have been obtained by repeated mechanical inoculation of plant hosts without going through the insect host. A strain of the rice dwarf reovirus maintained for 12 years in vegetatively propagated rice plants lost the P2 outer capsid protein. The genomic sequence encoding P2 was present in the rice dwarf reovirus-infected plants, but a point mutation that introduced a termination codon in the open reading frame prevented translation of the protein. The P2 protein was not required for infection of the plant host, but the lack of P2 protein did prevent infection of the insect host, perhaps due to an inability of the virus to attach to the vector cells (111, 167). Consequently the virus was not transmitted by the insects to plants.
Vegetative propagation of wound tumor reovirus-infected plants also resulted in leafhopper transmission-defective virus isolates. This was later found to result from the generation of defective RNAs of 4 of the 12 genomic segments. These defective viruses were not able to infect leafhopper vector cells but were able to maintain a near-wild-type infection of the plant host (108). Presumably all of the virus functions necessary to infect the plant host were contained on the eight remaining genomic segments whereas one or more of the four defective segments provided as yet undefined functions specific for infection of the insect host. The data generated thus far points to the conclusion that outer capsid proteins of the phytoarboviruses are, as would be predicted, involved in the infection of insect cells, i.e., attachment proteins. These would not be necessary for plant hosts due to the cell wall, which, along with the cell membrane, must be breached by the insect vector stylet.
There are very few references to the continued serial passage of phytoarboviruses in their insect vector. Sonchus yellow vein rhabdovirus was mechanically passaged by injecting virus into the hemocoel of aphid vectors. Continuous serial passage did give rise to virus isolates that were difficult to transmit to plants and also were more pathogenic to the vector (142). The reasons for the low probability of transmission were not determined and might have been associated with a deficiency in systemic plant infection or an inability to move through all the transmission barriers in the aphid.
The technique of mechanically passaging the virus in either the insect or plant host is apparently useful in the generation of mutants. With the advances in technologies that now allow further characterization of such mutants, there should be advances in our understanding of virus-vector interactions despite the difficulties with vector cell cultures and a lack of infectious clones of the viruses.
FUTURE DIRECTIONS AND CHALLENGES
Except for the phytoarboviruses and a few other notable examples, genetic analysis of viral gene expression and function is currently possible and is progressing rapidly. We will soon know which viral genes and gene products are required for vector transmission, but for the most part we will not understand their exact role. The challenges ahead lie in our ability to understand the interactions of the viruses inside the arthropod (or other) vector and to determine what genetic factors the vector contributes to the overall transmission process. The obstacle for most researchers studying virus transmission involves the present inability to get inside the vector and observe what is going on. New technologies must be and are being developed (e.g., transformation of insects and genetic manipulation of aphids). The sensitivity and detection limits of existing technologies are being improved (e.g., detection and visualization of potyvirus particles on aphid stylets, or the association of symbionin with luteovirus readthrough proteins). However, roadblocks are ever present. For example, it is likely that virus particles undergo conformational changes in their vectors as they are exposed to various environments within the gut, hemocole, or salivary system. These changes are likely to be critical for certain aspects of virus-vector interactions and the virus transmission process. The presence of relatively few virus particles in relatively few vector cells or on a small area of vector cuticle presents a problem for analysis of biological interactions. The production of continuous vector cell lines may provide the tools to answer some of these questions for researchers studying phytoarboviruses, but it will not provide definitive experimental systems for those studying viruses which do not replicate in their vectors.
Insect vectors cannot always be controlled by chemical means, and with the increasing sensitivity to the environment and the loss of many chemical control measures, vector control is not at present a long-term option. Protective measures of vector control are not really even an option for many of the nonpersistent plant viruses or perhaps even the mechanically transmitted animal viruses, since the infection process is too quick. Understanding the mechanisms of virus transmission is the key to developing effective strategies to block virus-vector interactions. If the virus can no longer be transmitted or even if the efficiency of transmission can be reduced to manageable levels, the disease will be less of a problem. The answer surely lies in our ability to render the host immune to infection or in our ability to control the transmission process. Genetic engineering and plant breeding have produced many virus-resistant plants, but immunity is the exception rather than the rule, and therefore genetically engineered resistance may not be the silver bullet that will solve all the problems. Our ability to control virus transmission is dependent upon our ability to first understand the transmission process and to effectively predict steps in the process that are vulnerable to intervention. Arthropod-borne viruses are likely to continue to emerge or reemerge and become more, rather than less, problematic. Success will depend on the continued integration of the expertise of animal and plant virologists along with entomologists, molecular biologists and geneticists.
REFERENCES
- 1.Al K N, Covey S N. Variation in biological properties of cauliflower mosaic virus clones. J Gen Virol. 1994;75:3137–3145. doi: 10.1099/0022-1317-75-11-3137. [DOI] [PubMed] [Google Scholar]
- 2.Ammar E D. Propagative transmission of plant and animal viruses by insects: factors affecting vector specificity and competence. Adv Dis Vector Res. 1994;10:289–331. [Google Scholar]
- 3.Ammar E D, Jarlfors U, Pirone T P. Association of potyvirus helper component protein with virions and the cuticle lining the maxillary food canal and foregut of an aphid vector. Phytopathology. 1994;84:1054–1060. [Google Scholar]
- 4.Atreya C D, Pirone T P. Mutational analysis of the helper component proteinase gene of a potyvirus: effects of amino acid substitutions, deletions, and gene replacement on virulence and aphid transmissibility. Proc Natl Acad Sci USA. 1993;90:11919–11923. doi: 10.1073/pnas.90.24.11919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Backus E A. Anatomical and sensory mechanisms of planthopper and leafhopper feeding behavior. In: Nault L R, Rodriguez J G, editors. The leafhoppers and planthoppers. New York, N.Y: John Wiley & Sons, Inc.; 1985. pp. 163–194. [Google Scholar]
- 6.Bandla M D, Campbell L, Ullman D E, Sherwood J L. Interaction of tomato spotted wilt tospovirus glycoproteins with a thrips midgut protein, a potential cellular receptor for TSWV. Phytopathology. 1998;88:98–104. doi: 10.1094/PHYTO.1998.88.2.98. [DOI] [PubMed] [Google Scholar]
- 7.Baumann P, Baumann L, Lai C, Rouhbakhsh D, Moran N A, Clark M A. Genetics, physiology and evolutionary relationships of the genus Buchnera: intracellular symbionts of aphids. Annu Rev Microbiol. 1995;49:55–94. doi: 10.1146/annurev.mi.49.100195.000415. [DOI] [PubMed] [Google Scholar]
- 8.Beaty B J, Holterman M, Tabachnick W, Shope R E, Rozhon E J, Bishop D H L. Molecular basis of bunyavirus transmission by mosquitoes: role of the middle-sized RNA segment. Science. 1981;211:1433–1435. doi: 10.1126/science.6781068. [DOI] [PubMed] [Google Scholar]
- 9.Behncken G M. Evidence of multiplication of sowthistle yellow vein virus in an inefficient aphid vector, Macrosiphum euphorbiae. Virology. 1973;53:405–412. doi: 10.1016/0042-6822(73)90220-1. [DOI] [PubMed] [Google Scholar]
- 10.Birch A N E, Fenton B, Malloch G, Jones A T, Phillips M S, Harrower B E, Woodford J A T, Catley M A. Ribosomal spacer length variability in the large raspberry aphid, Amphorophora idaei (Aphidinae: Macrosiphini) Insect Mol Biol. 1994;3:239–245. doi: 10.1111/j.1365-2583.1994.tb00172.x. [DOI] [PubMed] [Google Scholar]
- 11.Blanc S, Lopez Moya J J, Wang R, Garcia Lampasona S, Thornbury D W, Pirone T P. A specific interaction between coat protein and helper component correlates with aphid transmission of a potyvirus. Virology. 1997;231:141–147. doi: 10.1006/viro.1997.8521. [DOI] [PubMed] [Google Scholar]
- 12.Blanc S, Schmidt I, Kuhl G, Esperandieu P, Lebeurier G, Hull R, Cerutti M, Louis C. Paracrystalline structure of cauliflower mosaic virus aphid transmission factor produced both in plants and in a heterologous system and relationship with a solubilized active form. Virology. 1993;197:283–292. doi: 10.1006/viro.1993.1589. [DOI] [PubMed] [Google Scholar]
- 13.Blanc S, Schmidt I, Vantard M, Scholthof H B, Kuhl G, Esperanddieu P, Cerutti M, Louis C. The aphid transmission factor of cauliflower mosaic virus forms a stable complex with microtubules in both insect and plant cells. Proc Natl Acad Sci USA. 1996;93:15158–15163. doi: 10.1073/pnas.93.26.15158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brault V, et al. Aphid transmission of beet western yellows luteovirus requires the minor capsid read-through protein P74. EMBO J. 1995;14:650–659. doi: 10.1002/j.1460-2075.1995.tb07043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Briddon R W, Bedford I D, Tsai J H, Markham P G. Analysis of the nucleotide sequence of the treehopper-transmitted geminivirus, tomato pseudo-curly top virus, suggests a recombinant origin. Virology. 1996;219:387–394. doi: 10.1006/viro.1996.0264. [DOI] [PubMed] [Google Scholar]
- 16.Briddon R W, Pinner M S, Stanley J, Markham P G. Geminivirus coat protein replacement alters insect specificity. Virology. 1990;177:85–94. doi: 10.1016/0042-6822(90)90462-z. [DOI] [PubMed] [Google Scholar]
- 17.Brown D J F, Robertson W M, Trudgill D L. Transmission of viruses by plant nematodes. Annu Rev Phytopathol. 1995;33:223–249. doi: 10.1146/annurev.py.33.090195.001255. [DOI] [PubMed] [Google Scholar]
- 18.Brunt A A, Crabtree K, Dallwitz M J, Gibbs A J, Watson L. Viruses of plants. Cambridge, United Kingdom: CAB International; 1996. [Google Scholar]
- 19.Bruyere A, Brault V, Ziegler Graff V, Simonis M T, Van Den Heuvel J F J M, Richards K, Guilley H, Jonard G, Herrbach E. Effects of mutations in the beet western yellows virus readthrough protein on its expression and packaging and on virus accumulation, symptoms, and aphid transmission. Virology. 1997;230:323–334. doi: 10.1006/viro.1997.8476. [DOI] [PubMed] [Google Scholar]
- 20.Caciagli P, Bosco D. Quantitation over time of tomato yellow leaf curl geminivirus DNA in its whitefly vector. Phytopathology. 1997;87:610–613. doi: 10.1094/PHYTO.1997.87.6.610. [DOI] [PubMed] [Google Scholar]
- 21.Campbell R N. Persistence: A vector relationship not applicable to fungal vectors. Phytopathology. 1993;83:363. [Google Scholar]
- 22.Campbell R N. Fungal transmission of plant viruses. Annu Rev Phytopathol. 1996;34:87–108. doi: 10.1146/annurev.phyto.34.1.87. [DOI] [PubMed] [Google Scholar]
- 23.Carn V M. The role of dipterous insects in the mechanical transmission of animal viruses. Br Vet J. 1996;152:377–393. doi: 10.1016/s0007-1935(96)80033-9. [DOI] [PubMed] [Google Scholar]
- 24.Carrington J C, Kasschau K D, Mahajan S K, Schaad M C. Cell-to-cell and long-distance transport of viruses in plants. Plant Cell. 1996;8:1669–1681. doi: 10.1105/tpc.8.10.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chay C A, Gunasinge U B, Dinesh K S P, Miller W A, Gray S M. Aphid transmission and systemic plant infection determinants of barley yellow dwarf luteovirus-PAV are contained in the coat protein readthrough domain and 17-kDa protein, respectively. Virology. 1996;219:57–65. doi: 10.1006/viro.1996.0222. [DOI] [PubMed] [Google Scholar]
- 26.Chen B, Francki R I B. Cucumovirus transmission by the aphid Myzus-persicae is determined solely by the viral coat protein. J Gen Virol. 1990;71:939–944. [Google Scholar]
- 27.Childress S A, Harris K F. Localization of virus-like particles in the foreguts of viruliferous Graminella nigrifrons leafhoppers carrying the semi-persistent maize chlorotic dwarf virus. J Gen Virol. 1989;70:247–251. [Google Scholar]
- 28.Chiu R J, Black L M. Monolayer cultures of insect cell lines and their inoculation with a plant virus. Nature. 1967;215:1076–1078. doi: 10.1038/2151076a0. [DOI] [PubMed] [Google Scholar]
- 29.Cohen S. In vivo effects in whiteflies of a possible antiviral factor. Virology. 1967;37:448–454. doi: 10.1016/0042-6822(69)90228-1. [DOI] [PubMed] [Google Scholar]
- 30.Cohen S, Antignus Y. Tomato yellow leaf curl virus, a whitefly-borne geminivirus of tomatoes. Adv Dis Vector Res. 1994;10:259–288. [Google Scholar]
- 31.Costa H S, Westcot D M, Ullman D E, Rosell R, Brown J K, Johnson M W. Morphological variation in Bemisia endosymbionts. Protoplasma. 1995;189:194–202. [Google Scholar]
- 32.Creamer R. Invertebrate tissue culture as a tool to study insect transmission of plant viruses. In Vitro Cell Dev Biol. 1993;29A:284–288. doi: 10.1007/BF02633954. [DOI] [PubMed] [Google Scholar]
- 33.Day M F, Bennetts M J. A review of problems of specificity in arthropod vectors of plant and animal viruses. Canberra, Australia: Division of Entomology, CSIRO; 1954. [Google Scholar]
- 34.Demler S A, de Zoeten G A, Adam G, Harris K F. Pea enation mosaic enamovirus: properties and aphid transmission. In: Harrison B D, Murant A F, editors. The plant viruses. 5. Polyhedral virions and bipartite RNA genomes. New York, N.Y: Plenum Press; 1996. pp. 303–344. [Google Scholar]
- 35.Demler S A, Rucker Feeney D G, Skaf J S, De Zoeten G A. Expression and suppression of circulative aphid transmission in pea enation mosaic virus. J Gen Virol. 1997;78:511–523. doi: 10.1099/0022-1317-78-3-511. [DOI] [PubMed] [Google Scholar]
- 36.Dubois F, Sangwan R S, Sangwan N B S. Immunogold labeling and electron microscopic screening of beet necrotic yellow vein virus in the fungus Polymyxa betae infecting Beta vulgaris root cortical parenchyma cells. Int J Plant Sci. 1994;155:545–552. [Google Scholar]
- 37.Edman J D, Spielman A. Blood feeding by vectors: physiology, ecology, behavior, and vertebrate defense. In: Monath T P, editor. The arboviruses: epidemiology and ecology. Boca Raton, Fla: CRC Press, Inc.; 1988. pp. 153–190. [Google Scholar]
- 38.Estabrook E M, Suyenaga K, Tsai J H, Falk B W. Maize stripe tenuivirus RNA2 transcripts in plant and insect hosts and analysis of pvc2, a protein similar to the Phlebovirus virion membrane glycoproteins. Virus Genes. 1996;12:239–247. doi: 10.1007/BF00284644. [DOI] [PubMed] [Google Scholar]
- 39.Falk B W, Tsai J H, Lommel A. Differences in levels of detection for the maize stripe virus capsid and major non-capsid proteins in plant and insect hosts. J Gen Virol. 1987;68:1801–1812. [Google Scholar]
- 40.Fenner F, Ratcliffe F N. Myxomatosis. Cambridge, United Kingdom: Cambridge University Press; 1965. [Google Scholar]
- 41.Filichkin S A, Brumfield S, Filichkin T P, Young M J. In vitro interactions of the aphid endosymbiotic symL chaperonin with barley yellow dwarf virus. J Virol. 1997;71:569–577. doi: 10.1128/jvi.71.1.569-577.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Filichkin S A, Lister R M, McGrath P F, Young M J. In vivo expression and mutational analysis of the barley yellow dwarf virus readthrough gene. Virology. 1994;205:290–299. doi: 10.1006/viro.1994.1645. [DOI] [PubMed] [Google Scholar]
- 43.Fukatsu T, Ishikawa H. Synthesis and localization of symbionin an aphid endosymbiont protein. Insect Biochem Mol Biol. 1992;22:167–174. [Google Scholar]
- 44.Fukushi T. Relationship between propagative rice viruses and their vectors. In: Maramorosch K, editor. Viruses, vectors and vegetation. New York, N.Y: John Wiley & Sons, Inc.; 1969. pp. 279–301. [Google Scholar]
- 45.Garret A, Kerlan C, Thomas D. The intestine is a site of passage for potato leafroll virus from the gut lumen into the haemocoel in the aphid vector Myzus-persicae. Arch Virol. 1993;131:377–392. doi: 10.1007/BF01378639. [DOI] [PubMed] [Google Scholar]
- 46.Gergerich R C, Scott H A. Determinants in the specificity of virus transmission by leaf-feeding beetles. Adv Dis Vector Res. 1991;8:1–14. [Google Scholar]
- 47.Ghanim M, Morin S, Zeidan M, Czosnek H. Evidence for transovarial transmission of tomato yellow leaf curl virus by its vector, the whitefly Bemesia tabaci. Virology. 1998;240:295–303. doi: 10.1006/viro.1997.8937. [DOI] [PubMed] [Google Scholar]
- 48.Gibb K S, Randles J W. Transmission of velvet tobacco mottle virus and related viruses by the Mirid Cyrtopeltis nicotianae. Adv Dis Vector Res. 1991;7:1–18. [Google Scholar]
- 49.Gibbs M J, Cooper J I. A recombinational event in the history of luteoviruses probably induced by base-pairing between the genomes of two distinct viruses. Virology. 1995;206:1129–1132. doi: 10.1006/viro.1995.1037. [DOI] [PubMed] [Google Scholar]
- 50.Gildow F E. Virus-membrane interactions involved in circulative transmission of luteoviruses by aphids. Adv Dis Vector Res. 1987;4:93–120. [Google Scholar]
- 51.Gildow F E. Evidence for receptor-mediated endocytosis regulating luteovirus acquisition by aphids. Phytopathology. 1993;83:270–277. [Google Scholar]
- 52.Gildow, F. E. Luteovirus transmission and mechanisms regulating vector-specificity. In Luteoviridea, in press. CAB International, Wallingford, United Kingdom.
- 53.Gildow F E, Damsteegt V D, Smith O P, Gray S M. Cellular mechanisms regulating circulative transmission and aphid vector specificity of soybean dwarf luteoviruses. Phytopathology. 1994;84:1155–1156. [Google Scholar]
- 54.Gildow F E, Reavy B, Mayo M A, Woodford T, Duncan G H. Potato leafroll virus-like particles lacking readthrough protein are transmitted by Myzus persicae. Phytopathology. 1997;87:S33. doi: 10.1094/PHYTO.2000.90.10.1153. [DOI] [PubMed] [Google Scholar]
- 54a.Gildow F E, Gray S M. The aphid salivary gland basal lamina as a selective barrier associated with vector-specific transmission of barley yellow dwarf luteovirus. Phytopathology. 1993;83:1293–1302. [Google Scholar]
- 55.Govier D A, Kassanis B. A virus induced component of plant sap needed when aphids acquire potato virus Y from purified preparations. Virology. 1974;78:420–426. doi: 10.1016/0042-6822(74)90278-5. [DOI] [PubMed] [Google Scholar]
- 56.Grace T D C. Establishment of a line of mosquito cells grown in vitro. Nature. 1966;211:366–367. doi: 10.1038/211366a0. [DOI] [PubMed] [Google Scholar]
- 57.Granados R R, Hirumi H, Maramorosch K. Electron microscopic evidence for wound tumor virus accumulation in various organs of an inefficient leafhopper vector, Agalliopsis novella. J Invertebr Pathol. 1967;9:147–159. [Google Scholar]
- 58.Gray S M. Plant virus proteins involved in natural vector transmission. Trends Microbiol. 1996;4:253–294. doi: 10.1016/0966-842X(96)10040-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58a.Gray, S. M., et al. Unpublished data.
- 59.Grimstad P R, Paulson S L, Craig G B. Vector competence of Aedes hendersoni for La Crosse virus and evidence of a salivary-gland escape barrier. J Med Entomol. 1985;22:447–453. doi: 10.1093/jmedent/22.4.447. [DOI] [PubMed] [Google Scholar]
- 60.Gupta A P. Immunology of insects and other arthropods. Boca Raton, Fla: CRC Press, Inc.; 1991. [Google Scholar]
- 61.Ham P J. Immunity in haematophagous insect vectors of parasitic infection. Adv Dis Vector Res. 1992;9:41–66. [Google Scholar]
- 62.Hardy J L. Susceptibility and resistance of vector mosquitoes. In: Monath T P, editor. The arboviruses: epidemiology and ecology. I. Boca Raton, Fla: CRC Press, Inc.; 1988. pp. 127–152. [Google Scholar]
- 63.Hardy J L, Houk E J, Kramer L D, Reeves W C. Intrinsic factors affecting vector competence of mosquitoes for arboviruses. Annu Rev Entomol. 1983;28:229–262. doi: 10.1146/annurev.en.28.010183.001305. [DOI] [PubMed] [Google Scholar]
- 64.Harris K F. An ingestion-egestion hypothesis of noncirculative virus transmission. In: Harris K F, Maramorosch K, editors. Aphids as virus vectors. New York, N.Y: Academic Press, Inc.; 1977. pp. 165–220. [Google Scholar]
- 65.Harris K F. Aphid transmission of plant viruses. In: Mandahar C L, editor. Plant viruses. II. Pathology. Boca Raton, Fla: CRC Press, Inc.; 1990. pp. 177–204. [Google Scholar]
- 66.Henig R M. A dancing matrix: voyages along the viral frontier. New York, N.Y: Alfred Knopf; 1993. [Google Scholar]
- 67.Hertig C, Coupar B E H, Gould A R, Boyle D B. Field and vaccine strains of fowlpox virus carry integrated sequences from the avian retrovirus, reticuloendotheliosis virus. Virology. 1997;235:367–376. doi: 10.1006/viro.1997.8691. [DOI] [PubMed] [Google Scholar]
- 68.Hogenhout S A, Verbeek M, Hans F, Houterman P M, Fortass M, Van Der Wilk F, Huttinga H, Van Den Heuvel J F J M. Molecular bases of the interactions between luteoviruses and aphids. Agronomie. 1996;16:167–173. [Google Scholar]
- 69.Holbrook F R, Tabachnick W J. Culicoides variipennis (Diptera: Ceratopogonidae) complex in California. J Med Entomol. 1995;32:413–419. doi: 10.1093/jmedent/32.4.413. [DOI] [PubMed] [Google Scholar]
- 70.Holbrook F R, Tabachnick W J, Brady R. Genetic variation in populations of Culicoides variipennis complex in the six New England states, USA. Med Vet Entomol. 1996;10:173–180. doi: 10.1111/j.1365-2915.1996.tb00725.x. [DOI] [PubMed] [Google Scholar]
- 71.Huet H, Gal O A, Meir E, Lecoq H, Raccah B. Mutations in the helper component protease gene of zucchini yellow mosaic virus affect its ability to mediate aphid transmissibility. J Gen Virol. 1994;75:1407–1414. doi: 10.1099/0022-1317-75-6-1407. [DOI] [PubMed] [Google Scholar]
- 72.Hull R. Molecular biology of plant virus-vector interactions. Adv Dis Vector Res. 1994;10:361–386. [Google Scholar]
- 73.Hunter W B, Ullman D E. Anatomy and ultrastructure of the piercing-sucking mouthparts and paraglossal sensilla of Frankliniella-Occidentalis Pergande Thysanoptera Thripidae. Int J Insect Morphol Embryol. 1992;21:17–35. [Google Scholar]
- 74.Husted K. Reason for non-aphid transmissibility in a strain of Kalanchoe mosaic potyvirus. Virus Genes. 1995;11:59–61. doi: 10.1007/BF01701663. [DOI] [PubMed] [Google Scholar]
- 75.Jasinskiene N, Coates C J, Benedict M Q, Cornel A J, Rafferty C S, James A A, Collins F H. Stable transformation of the yellow fever mosquito, Aedes aegypti, with the Hermes element from the housefly. Proc Natl Acad Sci USA. 1998;95:3743–3747. doi: 10.1073/pnas.95.7.3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jianping C, Swaby A G, Adams M J, Yili R. Barley mild mosaic virus inside its fungal vector polymyxa-graminis. Ann Appl Biol. 1991;118:615–622. [Google Scholar]
- 77.Jolly C A, Mayo M A. Changes in the amino acid sequence of the coat protein readthrough domain of potato leafroll luteovirus affect the formation of an epitope and aphid transmission. Virology. 1994;201:182–185. doi: 10.1006/viro.1994.1283. [DOI] [PubMed] [Google Scholar]
- 78.Karpf A R, Brown D T. Comparison of Sindbis virus-induced pathology in mosquito and vertebrate cell cultures. Virology. 1998;240:193–201. doi: 10.1006/viro.1997.8914. [DOI] [PubMed] [Google Scholar]
- 79.Kennedy J S, Day M F, Eastop V F. A conspectus of aphids as vectors of plant viruses. London, United Kingdom: Commonwealth Institute of Entomology; 1962. [Google Scholar]
- 80.Kidwell M G, Wattam A R. An important step forward in the genetic manipulation of mosquito vectors of human disease. Proc Natl Acad Sci USA. 1998;95:3349–3350. doi: 10.1073/pnas.95.7.3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kikkert M, Meurs C, van de Wetering F, Dorfmuller S, Peters D, Kormelink R, Goldbach R. Binding of tomato spotted wilt virus to a 94-kDa thrips protein. Phytopathology. 1998;88:63–69. doi: 10.1094/PHYTO.1998.88.1.63. [DOI] [PubMed] [Google Scholar]
- 82.Kloft W J. Radioisotopes in vector research. Adv Dis Vector Res. 1992;9:41–66. [Google Scholar]
- 83.Kubes M, Fuchsberger N, Labuda M, Zuffova E, Nuttall P A. Salivary gland extracts of partially fed Dermacentor reticulatus ticks decrease natural killer cell activity in vitro. Immunology. 1994;82:113–116. [PMC free article] [PubMed] [Google Scholar]
- 84.Labuda M, Austyn J M, Zuffova E, Kozuch O, Fuchsberger N, Lysy J, Nuttall P A. Importance of localized skin infection in tick-borne encephalitis virus transmission. Virology. 1996;219:357–366. doi: 10.1006/viro.1996.0261. [DOI] [PubMed] [Google Scholar]
- 85.Lamb J W, Duncan G H, Reavy B, Gildow F E, Mayo M A, Thay R T. Assembly of virus-like particles in insect cells infected with a baculovirus containing a modified coat protein gene of potato leafroll luteovirus. J Gen Virol. 1996;77:1349–1358. doi: 10.1099/0022-1317-77-7-1349. [DOI] [PubMed] [Google Scholar]
- 86.Lecoq H, Pitrat M. Specificity of the helper component-mediated aphid transmission of three potyviruses infection muskmelon. Phytopathology. 1985;75:890–893. [Google Scholar]
- 87.Legavre T, Maia I G, Casse Delbart F, Bernardi F, Robaglia C. Switches in the mode of transmission select for or against a poorly aphid-transmissible strain of potato virus Y with reduced helper component and virus accumulation. J Gen Virol. 1996;77:1343–1347. doi: 10.1099/0022-1317-77-7-1343. [DOI] [PubMed] [Google Scholar]
- 88.Liu S, Bedford I D, Briddon R W, Markham P G. Efficient whitefly transmission of African cassava mosaic geminivirus requires sequences from both genomic components. J Gen Virol. 1997;78:1791–1794. doi: 10.1099/0022-1317-78-7-1791. [DOI] [PubMed] [Google Scholar]
- 89.Lomonossoff G P. Pathogen-derived resistance to plant viruses. Annu Rev Phytopathol. 1995;33:323–343. doi: 10.1146/annurev.py.33.090195.001543. [DOI] [PubMed] [Google Scholar]
- 90.Lopez Moya J J, Canto T, Diaz Ruiz J R, Lopez Abella D. Transmission by aphids of a naturally non-transmissible plum pox virus isolate with the aid of potato virus Y helper component. J Gen Virol. 1995;76:2293–2297. doi: 10.1099/0022-1317-76-9-2293. [DOI] [PubMed] [Google Scholar]
- 91.Ludwig G V, Israel B A, Christensen B M, Yull T M, Schultz K T. Role of La Crosse virus glycoproteins in attachment of virus to host cells. Virology. 1991;181:564–571. doi: 10.1016/0042-6822(91)90889-j. [DOI] [PubMed] [Google Scholar]
- 92.Maia I G, Haenni A, Bernardi F. Potyviral HC-Pro: a multifunctional protein. J Gen Virol. 1996;77:1335–1341. doi: 10.1099/0022-1317-77-7-1335. [DOI] [PubMed] [Google Scholar]
- 93.Mandahar C L. Virus transmission. In: Mandahar C L, editor. Plant viruses. II. Pathology. Boca Raton, Fla: CRC Press; 1990. pp. 205–254. [Google Scholar]
- 94.Maramorosch K. Arthropod transmission of plant viruses. Annu Rev Entomol. 1963;8:369–414. doi: 10.1146/annurev.en.08.010163.002101. [DOI] [PubMed] [Google Scholar]
- 95.Martin B, Collar J L, Tjallingii W F, Fereres A. Intracellular ingestion and salivation by aphids may cause the acquisition and inoculation of non-persistently transmitted plant viruses. J Gen Virol. 1997;78:2701–2705. doi: 10.1099/0022-1317-78-10-2701. [DOI] [PubMed] [Google Scholar]
- 96.Martin R R, Keese P K, Young M J, Waterhouse P M, Gerlach W L. Evolution and molecular biology of luteoviruses. Annu Rev Phytopathol. 1990;28:341–364. [Google Scholar]
- 97.Maudlin I. Transmission of African trypanosomiasis: interactions among Tsetse immune system, symbionts, and parasites. Adv Dis Vector Res. 1991;7:117–148. [Google Scholar]
- 98.Mayo M A, Brierley K M, Goodman B A. Developments in the understanding of the particle structure of tobraviruses. Biochimie (Paris) 1993;75:639–644. doi: 10.1016/0300-9084(93)90093-8. [DOI] [PubMed] [Google Scholar]
- 99.Merrill M H, TenBroeck C. The transmission of equine encephalomyelitis virus by Aedes aegypti. J Exp Med. 1935;62:687–695. doi: 10.1084/jem.62.5.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Miller B R, Mitchell C J. Selection of a flavivirus-refractory strain of the yellow fever mosquito Aedes aegypti. Am J Trop Med Hyg. 1991;45:399–407. doi: 10.4269/ajtmh.1991.45.399. [DOI] [PubMed] [Google Scholar]
- 101.Miller M L, Brown D T. Alphavirus infection in cultured tissue cells. Adv Dis Vector Res. 1991;8:107–142. [Google Scholar]
- 102.Miller W A, Dinesh K S P, Paul C P. Luteovirus gene expression. Crit Rev Plant Sci. 1995;14:179–211. [Google Scholar]
- 103.Miranda G J, Koganezawa H. Identification, purification, and serological detection of the major noncapsid protein of rice grassy stunt virus. Phytopathology. 1995;85:1530–1533. [Google Scholar]
- 104.Morse S S. Examining the origins of emerging viruses. In: Morse S S, editor. Emerging viruses. New York, N.Y: Oxford University Press; 1993. pp. 10–28. [Google Scholar]
- 105.Mowry T M. Within-plant accumulation of potato leafroll virus by aggregated green peach aphid feeding. Phytopathology. 1995;85:859–863. [Google Scholar]
- 106.Murphy F A, Whitfield S G, Sudia W D, Chamberlain R W. Interaction of vector with vertebrate pathogenic viruses. In: Maramorosch K, Shope R E, editors. Invertebrate immunity. New York, N.Y: Academic Press, Inc.; 1975. pp. 25–53. [Google Scholar]
- 107.Nagata T, Storms M M H, Goldbach R, Peters D. Multiplication of tomato spotted wilt virus in primary cell cultures derived from two thrips species. Virus Res. 1997;49:59–66. doi: 10.1016/s0168-1702(97)01453-6. [DOI] [PubMed] [Google Scholar]
- 108.Nuss D L, Summers D. Variant dsRNAs associated with transmission-defective isolates of wound tumor virus represent terminally conserved remnants of genome sequences. Virology. 1984;133:276–288. doi: 10.1016/0042-6822(84)90395-7. [DOI] [PubMed] [Google Scholar]
- 109.Nuttall P A, Jones L D, Davies C R. The role of arthropod vectors in arbovirus evolution. Adv Dis Vector Res. 1991;8:15–45. [Google Scholar]
- 110.Nuttall P A, Jones L D, Labuda M, Kaufman W R. Adaptations of arboviruses to ticks. J Med Entomol. 1994;31:1–9. doi: 10.1093/jmedent/31.1.1. [DOI] [PubMed] [Google Scholar]
- 111.Omura T, Maruyama W, Ichimi K, Fukui Y, Yan J, Zhu Y, Kamiunten H. Involvement in virus infection to insect vector cells of the P2 outer capsid proteins of rice gall dwarf and rice dwarf phytoreoviruses. Phytopathology. 1997;87:S72. doi: 10.1007/s007050050218. [DOI] [PubMed] [Google Scholar]
- 112.Orlob G B. Reappraisal of transmission of tobacco mosaic virus by insects. Phytopathology. 1963;53:822–830. [Google Scholar]
- 113.Ozzam O, Frazer J, De La Rosa D, Beaver J S, Ahlquist P, Maxwell D P. Whitefly transmission and efficient ssDNA accumulation of bean golden mosaic geminivirus require functional coat protein. Virology. 1994;204:289–296. doi: 10.1006/viro.1994.1533. [DOI] [PubMed] [Google Scholar]
- 114.Pappu H R, Niblett C L, Lee R F. Application of recombinant DNA technology to plant protection: molecular approaches to engineering virus resistance in crop plants. World J Microbiol Biotechnol. 1995;11:426–437. doi: 10.1007/BF00364618. [DOI] [PubMed] [Google Scholar]
- 115.Peiffer M L, Gildow F E, Gray S M. Two distinct mechanisms regulate luteovirus transmission efficiency and specificity at the aphid salivary gland. J Gen Virol. 1997;78:495–503. doi: 10.1099/0022-1317-78-3-495. [DOI] [PubMed] [Google Scholar]
- 116.Peng Y H, Kadoury D, Gal On A, Huet H, Wang Y, Raccah B. Mutations in the HC-Pro gene of zucchini yellow mosaic potyvirus: effects on aphid transmission and binding to purified virions. J Gen Virol. 1998;79:897–904. doi: 10.1099/0022-1317-79-4-897. [DOI] [PubMed] [Google Scholar]
- 117.Perez de Leon A A, Ribeiro J M C, Tabachnick W J, Valenzuela J G. Identification of a salivary vasodilator in the primary North American vector of bluetongue viruses, Culicoides variipennis. Am J Trop Med Hyg. 1997;57:375–381. doi: 10.4269/ajtmh.1997.57.375. [DOI] [PubMed] [Google Scholar]
- 118.Perry K L, Zhang L, Shintaku M H, Palukaitis P. Mapping determinants in cucumber mosaic virus for transmission by Aphis gossypii. Virology. 1994;205:591–595. doi: 10.1006/viro.1994.1686. [DOI] [PubMed] [Google Scholar]
- 119.Pesic-van Esbroeck Z, Harris K F, Duffus J E. Immunocytochemical localization of squash leaf curl virus in squash and the sweet potato whitefly. Phytopathology. 1995;85:1180. [Google Scholar]
- 120.Pirone T P. Does tobacco mosaic virus infect plant through wounds produced by aphid stylets? Virology. 1972;49:801–803. doi: 10.1016/0042-6822(72)90536-3. [DOI] [PubMed] [Google Scholar]
- 121.Pirone T P. Efficiency and selectivity of the helper component mediated aphid transmission of purified potyviruses. Phytopathology. 1981;71:922–924. [Google Scholar]
- 122.Pirone T P. Viral genes and gene products that determine insect transmissibility. Semin Virol. 1991;2:81–87. [Google Scholar]
- 122a.Pirone, T. P. Personal communication.
- 123.Pirone T P, Blanc S. Helper-dependent vector transmission of plant viruses. Annu Rev Phytopathol. 1996;34:227–247. doi: 10.1146/annurev.phyto.34.1.227. [DOI] [PubMed] [Google Scholar]
- 124.Pirone T P, Thornbury D W. Quantity of virus required for aphid transmission of a potyvirus. Phytopathology. 1988;78:104–107. [Google Scholar]
- 125.Pollard D G. Aphid penetration of plant tissues. In: Harris K F, Maramorosch K, editors. Aphids as virus vectors. New York, N.Y: Academic Press, Inc.; 1977. pp. 165–220. [Google Scholar]
- 126.Ponsen M B. The site of potato leafroll virus multiplication in its vector, Myzus persicae: an anatomical study. Meded Landbouwhogesch Wageningen. 1972;72-16:1–147. [Google Scholar]
- 127.Power A G, Gray S M. Aphid transmission of barley yellow dwarf viruses: interactions between viruses, vectors, and host plants. In: D’Arcy C J, Burnett P A, editors. Barley yellow dwarf: 40 years of progress. St. Paul, Minn: APS Press; 1995. pp. 259–291. [Google Scholar]
- 128.Reed W. Recent researches concerning the etiology, propagation and prevention of yellow fever by the United States Army Commission. J Hyg. 1902;2:101–119. doi: 10.1017/s0022172400001856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Reutenauer A, Ziegler G V, Lot H, Scheidecker D, Guilley H, Richards K, Jonard G. Identification of beet western yellows luteovirus genes implicated in viral replication and particle morphogenesis. Virology. 1993;195:692–699. doi: 10.1006/viro.1993.1420. [DOI] [PubMed] [Google Scholar]
- 130.Rochow W F, Foxe M J, Muller I. A mechanism of vector specificity for circulative aphid-transmitted plant viruses. Ann New York Acad Sci. 1975;266:293–301. [Google Scholar]
- 131.Rubinstein G, Czosnek H. Long-term association of tomato yellow leaf curl virus with its whitefly vector Bemisia tabaci: effect on the insect transmission capacity, longevity and fecundity. J Gen Virol. 1997;78:2683–2689. doi: 10.1099/0022-1317-78-10-2683. [DOI] [PubMed] [Google Scholar]
- 132.Rysanek P, Stocky G, Haeberle A M, Putz C. Immunogold labelling of beet necrotic yellow vein virus particles inside its fungal vector Polymyxa betae. Agronomie (Paris) 1992;12:651–659. [Google Scholar]
- 133.Salomon R. Proteolytic cleavage of the N-terminal region of potyvirus coat protein and its relation to host recovery and vector transmission. Arch Virol Suppl. 1992;5:75–76. doi: 10.1007/978-3-7091-6920-9_8. [DOI] [PubMed] [Google Scholar]
- 134.Schmidt I, Blanc S, Esperandieu P, Kul G, Devauchelle G, Louis C, Cerutti M. Interaction between the aphid transmission factor and virus particles is a part of the molecular mechanism of cauliflower mosaic virus aphid transmission. Proc Natl Acad Sci USA. 1994;91:8885–8889. doi: 10.1073/pnas.91.19.8885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shi X M, Miller H, Verchot J, Carrington J C, Vance V B. Mutations in the region encoding the central domain of helper component-proteinase (HC-Pro) eliminate potato virus X-potyviral synergism. Virology. 1997;231:35–42. doi: 10.1006/viro.1997.8488. [DOI] [PubMed] [Google Scholar]
- 136.Shufran K A, Peters D C, Webster J A. Generation of clonal diversity by sexual reproduction in the greenbug, Schizaphis graminum. Insect Mol Biol. 1997;6:203–209. doi: 10.1046/j.1365-2583.1997.00174.x. [DOI] [PubMed] [Google Scholar]
- 137.Smith C E. Transmission of cowpea mosaic by the bean leaf beetle. Science. 1924;60:268. doi: 10.1126/science.60.1551.268. [DOI] [PubMed] [Google Scholar]
- 138.Smith K M. Plant virus-vector relationships. Adv Virus Res. 1965;11:61–96. doi: 10.1016/s0065-3527(08)60543-6. [DOI] [PubMed] [Google Scholar]
- 139.Stollar V. Insect-transmitted vertebrate viruses: alphatogaviruses. In Vitro Cell Dev Biol. 1993;29A:289–295. doi: 10.1007/BF02633957. [DOI] [PubMed] [Google Scholar]
- 140.Suzuki N, Sugawara M, Kusano T, Mori H, Matsuura Y. Immunodetection of rice dwarf phytoreoviral proteins in both insect and plant hosts. Virology. 1994;202:41–48. doi: 10.1006/viro.1994.1320. [DOI] [PubMed] [Google Scholar]
- 141.Swenson K G. Plant virus transmission by insects. Methods Virol. 1967;1:267–307. [Google Scholar]
- 142.Sylvester E S, Richardson J. Decreased survival of Hypermyzus lactucae inoculated with serially passed sowthistle yellow vein virus. Virology. 1971;46:310–317. doi: 10.1016/0042-6822(71)90032-8. [DOI] [PubMed] [Google Scholar]
- 143.Tabachnick W J. Genetic control of oral susceptibility to infection of Culicoides variipennis for bluetongue virus. Am J Trop Med Hyg. 1991;45:666–671. doi: 10.4269/ajtmh.1991.45.666. [DOI] [PubMed] [Google Scholar]
- 144.Tabachnick W J. Genetics of insect vector competence for arboviruses. Adv Dis Vector Res. 1994;10:93–108. [Google Scholar]
- 145.Takami N. On dwarf disease of rice plant and “tsumaguro-yokabai.”. J Jpn Agric Soc. 1901;241:22–30. [Google Scholar]
- 146.Tardieux I, Poupel O, Lapchin L, Rodhain F. Variation among strains of Aedes aegypti in susceptibility to oral infection with dengue type 2. Am J Trop Med Hyg. 1990;43:308–313. doi: 10.4269/ajtmh.1990.43.308. [DOI] [PubMed] [Google Scholar]
- 147.Tardieux I, Poupel O, Lapchin L, Rodhain F. Analysis of inheritance of oral susceptibility of Aedes aegypti to dengue-2 virus using isofemale lines. J Med Entomol. 1991;28:518–521. doi: 10.1093/jmedent/28.4.518. [DOI] [PubMed] [Google Scholar]
- 148.Taylor C E. Nematodes. In: Harris K F, Maramorosch K, editors. Vectors of plant pathogens. New York, N.Y: Academic Press, Inc.; 1980. pp. 375–416. [Google Scholar]
- 149.Tesh R B, Gubler D J, Rosen L. Variation among geographic strains of Aedes albopictus in susceptibility to infection with chikungunya virus. Am J Trop Med Hyg. 1976;25:326–333. doi: 10.4269/ajtmh.1976.25.326. [DOI] [PubMed] [Google Scholar]
- 150.Thornbury D W, Hellman G M, Rhodes R E, Pirone T P. Purification and characterization of potyvirus helper component. Virology. 1985;144:260–267. doi: 10.1016/0042-6822(85)90322-8. [DOI] [PubMed] [Google Scholar]
- 151.Turell M J. Horizontal and vertical transmission of viruses by insect and tick vectors. In: Monath T P, editor. The arboviruses: epidemiology and ecology. I. Boca Raton, Fla: CRC Press, Inc.; 1988. pp. 127–152. [Google Scholar]
- 152.Ullah F, Peters D C. Sexual reproduction capabilities of greenbugs (Homoptera: Aphididae) J Kans Entomol Soc. 1996;69:153–159. [Google Scholar]
- 153.Ullman D E, Cho J J, Mau R F L, Westcot D M, Custer D M. A midgut barrier to tomato spotted wilt virus acquisition by adult western flower thrips. Phytopathology. 1992;82:1333–1342. [Google Scholar]
- 154.Uyeda I, Kimura I, Shikata E. Characterization of genome structure and establishment of vector cell lines for plant reoviruses. Adv Virus Res. 1995;45:249–279. doi: 10.1016/s0065-3527(08)60063-9. [DOI] [PubMed] [Google Scholar]
- 155.Valenzuela J G, Chuffe O M, Ribeiro J M C. Apyrase and anti-platelet activities from the salivary glands of the bed bug Cimex lectularius. Insect Biochem Mol Biol. 1996;26:557–562. [Google Scholar]
- 156.Van Den Heuvel J F J M, Bruyere A, Hogenhout S A, Ziegler Graff V, Brault V, Verbeek M, Van Der Wilk F, Richards K. The N-terminal region of the luteovirus readthrough domain determines virus binding to Buchnera GroEL and is essential for virus persistence in the aphid. J Virol. 1997;71:7258–7265. doi: 10.1128/jvi.71.10.7258-7265.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Van Den Heuvel J F J M, Verbeek M, Van Der Wilk F. Endosymbiotic bacteria associated with circulative transmission of potato leafroll virus by Myzus persicae. J Gen Virol. 1994;75:2559–2565. doi: 10.1099/0022-1317-75-10-2559. [DOI] [PubMed] [Google Scholar]
- 158.Vey A. Humeral encapsulation. In: Pathak J P N, editor. Insect immunity. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1993. pp. 59–68. [Google Scholar]
- 159.Wang H, Nuttall P A. Excretion of host immunoglobulin in tick saliva and detection of IgG-binding proteins in tick haemolymph and salivary glands. Parasitology. 1994;109:525–530. doi: 10.1017/s0031182000080781. [DOI] [PubMed] [Google Scholar]
- 160.Wang J Y, Chay C, Gildow F E, Gray S M. Readthrough protein associated with virions of barley yellow dwarf luteovirus and its potential role in regulating the efficiency of aphid transmission. Virology. 1995;206:954–962. doi: 10.1006/viro.1995.1018. [DOI] [PubMed] [Google Scholar]
- 161.Wang R Y, Ammar E D, Thornbury D W, Lopez Moya J J, Pirone T P. Loss of potyvirus transmissibility and helper-component activity correlate with non-retention of virions in aphid stylets. J Gen Virol. 1996;77:861–867. doi: 10.1099/0022-1317-77-5-861. [DOI] [PubMed] [Google Scholar]
- 161a.Wang R Y, Pirone T P. Mineral oil interferes with retention of tobacco etch potyvirus in the stylets of Myzus persicae. Phytopathology. 1996;86:820–823. [Google Scholar]
- 162.Watson M A. Seventh Commonwealth Entomological Conference. London, United Kingdom: Eastern Press, Ltd.; 1960. The ways in which plant viruses are transmitted by vectors. [Google Scholar]
- 163.Watson M A, Plumb R T. Transmission of plant-pathogenic viruses by aphids. Annu Rev Entomol. 1972;17:425–452. [Google Scholar]
- 164.Weaver S C. Vector biology in arboviral pathogenesis. In: Nathanson N, editor. Viral pathogenesis. Philadelphia, Pa: Lippincott-Raven Publishers; 1997. pp. 329–352. [Google Scholar]
- 165.Whitcomb R F, Davis R E. Mycoplasma and phytoarboviruses as plant pathogens persistently transmitted by insects. Annu Rev Entomol. 1970;15:405–464. [Google Scholar]
- 166.Wu Z C, Hu J S, Polston J E, Ullman D E, Hiebert E. Complete nucleotide sequence of a nonvector-transmissible strain of abutilon mosaic geminivirus in Hawaii. Phytopathology. 1996;86:608–613. [Google Scholar]
- 167.Yan J, Tomaru M, Takahashi A, Kimura I, Hibino H, Omura T. P2 protein encoded by genome segment S2 of rice dwarf phytoreovirus is essential for virus infection. Virology. 1996;224:539–541. doi: 10.1006/viro.1996.0560. [DOI] [PubMed] [Google Scholar]
- 168.Zeigler R S, Morales F J. Genetic determination of replication of rice hoja blanca virus within its planthopper vector, Sogatodes oryzicola. Phytopathology. 1990;80:559–566. [Google Scholar]