Abstract
Background
Deep vein thrombosis (DVT) and resulting pulmonary embolism (PE) are important complications of stroke. Physical methods to reduce the risk of DVT and PE, such as graduated compression stockings (GCS) or intermittent pneumatic compression (IPC) applied to the legs, do not appear to be associated with any bleeding risk and reduce the risk of DVT in some categories of surgical patients. We sought to assess their effects in stroke patients.
Objectives
To assess the effectiveness and safety of physical methods of reducing the risk of DVT, fatal or non‐fatal PE and death in patients with recent stroke.
Search methods
We searched the Cochrane Stroke Group Trials Register (last searched November 2009), the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library Issue 4, 2009), MEDLINE (1966 to November 2009), EMBASE (1980 to November 2009), CINAHL (1982 to November 2009) and The British Nursing Index (1985 to November 2009). We screened reference lists of all relevant papers, searched ongoing trials registers (November 2009) and contacted experts in the field.
Selection criteria
Unconfounded randomised controlled trials comparing physical methods for reducing the risk of DVT with control and in which prophylaxis was started within seven days of the onset of stroke.
Data collection and analysis
Two review authors searched for trials and extracted data.
Main results
We identified two trials of GCS that included 2615 patients and two small studies of IPC that included 177 patients. Overall, physical methods were not associated with a significant reduction in DVTs during the treatment period (odds ratio (OR) 0.85, 95% confidence interval (CI) 0.70 to 1.04) or deaths (OR 1.12, 95% CI 0.87 to 1.45). Use of GCS was not associated with any significant reduction in risk of DVT (OR 0.88, 95% CI 0.72 to 1.08) or death (OR 1.13, 95% CI 0.87 to 1.47) at the end of follow up. IPC was associated with a non‐significant trend towards a lower risk of DVTs (OR 0.45, 95% CI 0.19 to 1.10) with no evidence of an effect on deaths (OR 1.04, 95% CI 0.37 to 2.89).
Authors' conclusions
Evidence from randomised trials does not support the routine use of GCS to reduce the risk of DVT after acute stroke. There is insufficient evidence to support the routine use of IPC to reduce the risk of DVT in acute stroke and further larger randomised studies of IPC are needed to reliably assess the balance of risks and benefits of this intervention.
Plain language summary
Physical methods for preventing deep vein thrombosis in stroke
After a stroke, blood clots can form in the veins of the legs (deep vein thrombosis, or DVT). These clots can break off and be carried in the blood stream to the heart and lungs (causing pulmonary embolism). This can be life threatening. Although anticoagulant drugs can reduce the risk of DVT they can also cause serious bleeding. A number of physical methods have been developed to prevent DVT forming. These include wearing graduated compression stockings, intermittent pneumatic compression and electrical stimulation of leg muscles. The physical methods are used to increase the blood flow in the leg veins and reduce the risk of clots forming. We aimed to evaluate the effects of these physical methods in patients with a recent stroke. We found two randomised trials of graduated compression stockings, involving 2615 participants, and two small trials of intermittent pneumatic compression involving 177 participants. Graduated compression stockings were no better than 'best medical treatment' in reducing the risk of DVT after stroke. Stockings caused more skin problems (for example ulcers and blisters) on the legs. Intermittent pneumatic compression appeared promising but was not proven to be definitely beneficial. The evidence does not support routine use of graduated compression stockings or intermittent pneumatic compression in patients with a recent stroke. The trials that are ongoing at present should provide reliable evidence on the benefits and harms of intermittent pneumatic compression.
Background
Deep vein thrombosis (DVT) and pulmonary embolism (PE) are important causes of death and morbidity after stroke. The pathophysiological mechanisms underlying DVT include venous stasis and hypercoagulability linked to an increase in thrombin formation and platelet hyperactivity (Virchow 1858). Patients with significant weakness of the leg, and who are immobile appear to be at greatest risk. Recent studies with magnetic resonance imaging appeared to demonstrate DVT in 40% of stroke patients within the first three weeks, and above‐knee DVT in 18% (Kelly 2004). Studies using less sensitive screening techniques, such as compression duplex ultrasound, identify above‐knee DVT in about 10% of patients, although the types of patients included and the duration and timing of follow up influences the frequency (CLOTS 2009). Clinically apparent DVT confirmed on investigation is less common. Unrecognised DVTs may nonetheless cause important complications. Proximal DVT carries a higher risk of PE than distal DVT (Havig 1977). Clinically evident PE has been variably estimated to affect stroke patients, 1% to 16% of patients in prospective randomised trials (Sandercock 2008) and 3% to 30% in observational studies (Davenport 1996).
A Cochrane systematic review of studies of anticoagulants in patients with acute stroke (Sandercock 2008) has shown that treatment with unfractionated heparin, low molecular‐weight heparin, heparinoids or oral anticoagulants was associated with a highly significant 64% reduction in the risk of DVT (95% confidence interval for the relative risk reduction was 54% to 71%, 2P < 0.00001). However, only 3.9% of patients included in this review were systematically screened for asymptomatic DVTs to determine the effect of anticoagulants on the occurrence of symptomatic or asymptomatic DVT. In these small trials, the apparent absolute reduction in DVT with anticoagulation was substantial with 281 DVTs prevented per 1000 patients treated. However, although anticoagulant therapy was associated with about nine fewer recurrent ischaemic strokes per 1000 patients treated, it was also associated with nine additional symptomatic intracranial haemorrhages per 1000 patients treated. Similarly, anticoagulants were associated with about four fewer pulmonary emboli per 1000 but this benefit was offset by an extra nine major extracranial haemorrhages per 1000. As a result, guidelines suggest that use of low‐dose anticoagulants for DVT prophylaxis are generally only applied to patients at high risk of DVT and low risk of bleeding. For example, the UK National Institute for Clinical Excellence guidance states that for stroke patients, "Do not offer pharmacological venous thromboembolism (VTE) prophylaxis to patients with any of the risk factors for bleeding shown .... , unless the risk of VTE outweighs the risk of bleeding" (NICE CG92 2010).
In view of the uncertainties about the net benefit from anticoagulants for DVT prophylaxis after stroke, interest has increased in physical methods of DVT prophylaxis (either alone or in addition to background treatment with low‐dose anticoagulants). Graduated compression stockings (GCS) are thought to reduce the risk of DVT by several mechanisms, compressing the leg and thus reducing the cross‐sectional area of the veins which in turn reduces stasis by increasing the blood flow velocity, increasing flow by providing greater compression around the ankle than more proximally, augmenting the effect of the calf muscle pump, improving the function of venous valves, reduction of venous pooling and altering levels of clotting factors (CLOTS 2009). Intermittent pneumatic compression (IPC) is thought to reduce the risk of DVT by increasing the flow of venous blood through the deep veins of the leg and by stimulating the release of intrinsic fibrinolytic substances (Kohro 2005).
Physical methods of DVT prevention could have harmful effects. Patients at highest risk of harm from this therapy are those with dermatological diseases, severe peripheral arteriopathy or diabetic neuropathy. GCS may cause ischaemic necrosis of the legs in patients with peripheral vascular disease (Kay 1986; Merrett 1993) and ulceration in patients with sensory loss due to neuropathy. IPC is contraindicated in severe congestive heart failure with oedema, it could also disturb patients' sleep and might increase the risk of falling. We aimed to determine whether there is any evidence in the literature that physical methods are effective in reducing the risk of DVT and safe in patients with acute stroke. We therefore updated the previous review (Mazzone 2004) to include the recently completed trials.
Objectives
The aim of the review is to assess the effectiveness and safety of different physical methods to reduce the risk of DVT and fatal or non‐fatal PE in patients with recent stroke in comparison with a control group without such prophylactic treatment.
Methods
Criteria for considering studies for this review
Types of studies
Unconfounded randomised controlled trials comparing physical methods for reducing the risk of DVT with control and in which prophylaxis was started within seven days of the onset of stroke.
Types of participants
Patients of any age and either sex with ischaemic or haemorrhagic stroke who were randomised within seven days of stroke onset. We did not include trials assessing physical methods as a treatment for established DVT or trials restricted to patients with subarachnoid haemorrhage.
Types of interventions
Interventions included any physical methods to reduce the risk of DVT: graduated compression stockings (GCS); intermittent pneumatic compression (IPC); or electrical nerve stimulation (ENS) to induce calf muscle contractions. We only included randomised trials providing an unconfounded comparison of the intervention with control; that is, 'best medical treatment' versus 'best medical treatment plus physical prevention'. For trials of IPC we included studies comparing 'best medical treatment' versus 'best medical treatment plus IPC' and comparing 'best medical treatment plus GCS' versus 'best medical treatment plus GCS plus IPC'.
Types of outcome measures
The Cochrane Stroke Group policy recommends that review authors identify a single primary measure of outcome or, if not specified, justify the use of multiple outcomes (with the attendant risk of data‐dependent emphasis on particular outcomes). The primary purpose in the first version of this review was to assess the three clinically most important outcomes and in this update we have adhered to that protocol.
-
Events during the scheduled treatment period:
deaths from any cause;
DVT;
fatal or non‐fatal PE.
-
Events during the scheduled follow‐up period:
deaths from any cause;
DVT;
fatal or non‐fatal PE.
Adverse effects.
Search methods for identification of studies
See the 'Specialized register' section in the Cochrane Stroke Group module.
Electronic searches
We searched the Cochrane Stroke Group Trials Register (last searched by the Managing Editor in November 2009). In addition, we searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library Issue 4, 2009), MEDLINE (1966 to November 2009) (Appendix 1), EMBASE (1980 to November 2009) (Appendix 2), CINAHL (1982 to November 2009) (Appendix 3) and The British Nursing Index (1985 to November 2009) (Appendix 4) using an intervention‐based strategy to supplement the Cochrane Stroke Group general strategy.
Searching other resources
In an effort to identify further published, unpublished and ongoing trials, we:
screened the reference lists of all relevant papers;
-
searched the following ongoing trials registers (November 2009):
Current Controlled Trials (www.controlled‐trials.com),
ClinicalTrials.gov (http://www.clinicaltrials.gov/),
Stroke Trials Registry (www.strokecenter.org/trials/);
contacted authors and experts in the field.
We searched for relevant trials in all languages and arranged translation of trial reports published in languages other than English.
Data collection and analysis
One author (MN) assessed the studies identified by the electronic searches and reference tracking. Two authors (MN and MD) discussed the potentially relevant trials and extracted information about the characteristics of the included studies (method of randomisation, concealment of treatment allocation, blinding, patients lost to follow up), the characteristics of the patients (age, sex, clinical severity, confinement to bed), the action taken (description of the treatment, duration of treatment, associated administration of anticoagulant, physiotherapy) and the measures of outcome (and duration of follow up). The two data extractors resolved any discrepancies by discussion. We calculated the Peto odds ratio (OR) and 95% confidence interval (CI) for each outcome using the Review Manager software, RevMan 5 (RevMan 2008). We aimed to extract data to permit an intention‐to‐treat analysis, if possible. We also planned to do subgroup analyses according to the type of physical method of prophylaxis (GCS versus control, IPC versus control) and patients (immobile versus mobile), if possible. The review was to include an evaluation of the methodology of the studies, adequacy of randomisation, concealment of treatment allocation and blinding of the study. We had planned to do a sensitivity analysis excluding studies in which we found some ambiguity and excluding studies of poor methodological quality and unpublished studies, but this was not possible.
Results
Description of studies
We reviewed reports of 52 studies and 72 abstracts identified by the searches. We found 12 studies of physical means for preventing DVT or PE, or both, in acute stroke. Only four studies met our inclusion criteria. One study (Prasad 1982) included 26 patients within 72 hours of having had an acute stroke (clinical diagnosis). Patients were randomised to receive below‐knee IPC or not for 10 days. 125I‐Fibrinogen was used for DVT diagnosis. The test was performed within 24 hours of admission and scanning of precordium and legs was carried out for 10 days. The second study (Muir 2000 ) randomly allocated 98 patients (but one patient immediately dropped out) with an acute stroke to one of three treatment groups: routine care, routine care plus thigh‐length Kendall TED stockings (TED group: TED), or routine care plus thigh‐length Brevett TX stockings (TX group: TX). Colour flow duplex ultrasound examination was used at baseline and at day 7 ± 2 for the diagnosis of DVT. The third study (Lacut 2005) included 151 patients (mobile or immobile) within 48 hours of admission for cerebral haemorrhage (spontaneous or traumatic) who were randomly allocated to either thigh‐length 'IPC plus GCS' or 'GCS alone' for 10 ± 2 days. We included this study since the design (IPC + GCS versus GCS) provided an unconfounded comparison of the effects of IPC versus control. At day 10 ± 2 a compression duplex ultrasound was performed for the diagnosis of DVT. Patients were evaluated at 30 and 90 days for symptomatic venous thromboembolism. The fourth study (CLOTS 2009) included 2518 patients enrolled within the first three days after admission for an acute stroke. Patients were randomly allocated either to 'routine care plus thigh‐length GCS' or 'routine clinical care alone' until the patients were mobile, discharged or died. A compression duplex ultrasound was performed between days seven and 10 and, when practical, between days 25 and 30 after randomisation. The study also evaluated complications of GCS use and compliance with the allocated treatment. We did not identify any studies of electrical nerve stimulation.
Risk of bias in included studies
In the Prasad study, the method of randomisation was unclear and it was not clear whether the fibrinogen scans were assessed blind to the treatment allocation (Prasad 1982). In the Muir study, patients were randomised using computer‐generated random numbers in sealed envelopes (Muir 2000). Compression duplex scans were recorded on videotape for independent blind assessment. In the Lacut study, patients were randomised by computer‐generated random numbers in sequentially numbered, sealed, opaque envelopes (Lacut 2005). Compression duplex scans were recorded on videotape for independent blind assessment. This study included patients with traumatic and spontaneous cerebral haemorrhage who were either mobile or immobile at randomisation. In the CLOTS trial 1, patients were randomised by means of central (web or telephone) computer‐generated allocation (CLOTS 2009). Compression stockings were removed before compression duplex ultrasound (CDU) was performed to blind the observer to treatment allocation and hard copies of positive scans were recorded for independent assessment.
Effects of interventions
Death from any cause during the scheduled treatment period
In Prasad 1982 two patients died, both in the IPC group. In Muir 2000 nine of 65 patients (14%) allocated GCS and four of 32 (13%) allocated to avoid GCS died. In Lacut 2005 eight of the 77 (10%) allocated GCS alone and six of the 74 (8%) allocated IPC + GCS died. In CLOTS 2009 122 of 1256 (10%) allocated GCS and 110 of 1262 (9%) allocated to avoid GCS died within 30 days. Overall, there was a non‐significant excess of deaths among patients allocated physical methods of DVT prevention (OR 1.12, 95% CI 0.87 to 1.45) (Analysis 1.1). Subgroup analyses based on the type of physical method used did not show any significant difference from the overall analysis, however the small number of events precluded reliable conclusions. In the IPC subgroup there was moderate heterogeneity (I2 = 57%).
1.1. Analysis.
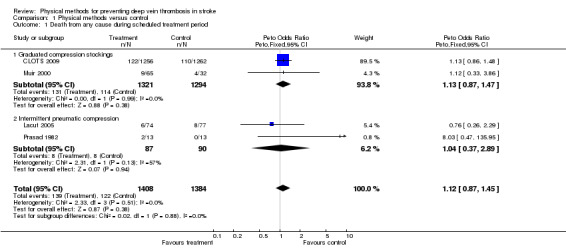
Comparison 1 Physical methods versus control, Outcome 1 Death from any cause during scheduled treatment period.
Deep vein thrombosis (DVT) during the scheduled treatment period
In Prasad 1982 the 125I‐fibrinogen scan was positive in 46% of patients (12 patients out of 26). Clinical signs of DVT were only noted in one patient with a positive scan (treatment allocation not stated). No significant differences were noted in radio‐isotopically detected DVT between the treatment and control groups (six patients versus six patients, P = ns). In Muir 2000, at randomisation nine out of 97 patients (9%) already had detectable DVT on CDU and five additional patients had detectable DVT by days five to nine. Thus 14 of 97 patients (14%) had DVTs within 10 days of stroke. Of the five new DVTs that occurred after randomisation four occurred in the control group. The dropout of patients in the treated group was greater than in the control group but was not statistically significant (dropouts in TX 39% versus TED 24% versus control 19%, P = 0.16). Seven patients withdrew: three for intolerance, the others for unstated reasons in the GCS group. In Muir 2000 there was no significant difference in compliance or tolerability in the two treated groups (TX plus TED) so the two active groups were combined for analyses of efficacy. At the second CDU three of 65 patients allocated GCS and five of 32 allocated to the control group had DVT. Only one patient had a symptomatic DVT. In Lacut 2005 14 patients out of 151 had a positive CDU at day 10 ± 2 (9%). No symptomatic DVT occurred within the treatment period. Three out of the 11 DVTs in the control group involved the proximal veins, while no proximal vein DVT occurred in the IPC group. Ten patients allocated to IPC (14%) and eight patients in the control group (10%) did not have a CDU performed. In CLOTS 2009 205 patients out of 1256 (16%) allocated to GCS had a CDU‐confirmed DVT within the study period; 224 patients out of 1262 (18%) developed DVTs in the control group. However, not all patients had CDU of their calf veins so total DVT rates may have been underestimated. The incidence of proximal DVTs was 10% (126 out of 1256) in the GCS group and 10.5% (133 out of 1262) in the control group. Symptomatic DVTs occurred in 4% of patients allocated to GCS (55 out of 1256) and in 5% of control patients (61 out of 1262) by 30 days. Overall, physical methods were not associated with a significant effect on DVT prevention (OR 0.85, 95% CI 0.70 to 1.04) (Analysis 1.2), with moderate heterogeneity (I2 = 54%). Subgroup analysis based on the type of physical method used showed no evidence of effect of thigh‐length GCS on DVT risk (OR 0.88, 95% CI 0.72 to 1.08) with a trend towards heterogeneity between the two included studies (I2 = 65%). IPC was associated with a non‐significant trend to reduction in the risk of DVT (OR 0.45, 95% CI 0.19 to 1.10; P = 0.08), without heterogeneity (I2 = 38%).
1.2. Analysis.
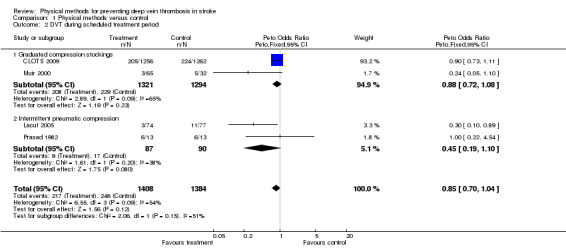
Comparison 1 Physical methods versus control, Outcome 2 DVT during scheduled treatment period.
Death or deep vein thrombosis (DVT) in the scheduled treatment period
The outcome 'DVT in the treatment period' takes no account of any possible adverse effects or death. A more inclusive assessment of effect, that would take account of fatal events, is the outcome 'death or DVT'. This was not a prespecified outcome for this review. In Muir 2000 15 of 65 (23%) patients allocated to thigh‐length GCS and 10 of 32 (31%) allocated to avoiding GCS either died or had CDU‐detected DVTs. In Prasad 1982 the outcome was not reported explicitly but can be derived from the publication. Eight patients in the IPC group and six in the control group died or had a DVT. In Lacut 2005 nine patients out of 74 (12%) allocated to IPC and 19 out of 77 (25%) allocated to avoid IPC either died or developed DVT within the study period. In CLOTS 2009 327 patients out of 1256 (26%) allocated thigh‐length GCS and 334 out of 1262 (26%) allocated to avoiding GCS either died or had CDU‐detected DVTs within 30 days. Overall, physical methods were not associated with a significant reduction in 'death or DVT' (OR 0.94, 95% CI 0.79 to 1.11) (Analysis 1.3). Subgroup analysis based on the type of physical method used showed a non‐significant reduction of 'death or DVT' in the IPC subgroup (OR 0.61, 95% CI 0.29 to 1.24) even though in this subgroup few events occurred during the study period and there was moderate heterogeneity (I2 = 62%).
1.3. Analysis.
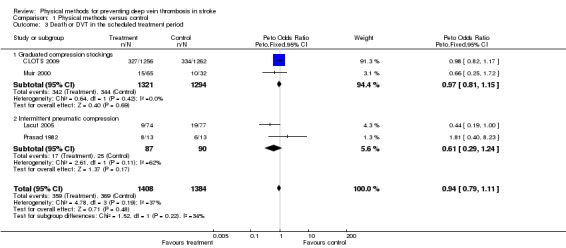
Comparison 1 Physical methods versus control, Outcome 3 Death or DVT in the scheduled treatment period.
Pulmonary embolism (PE), adverse effects and late outcomes
Data on other outcomes in the scheduled treatment period (PE and adverse effects) were not reported by two studies (Muir 2000; Prasad 1982). In Lacut 2005 there were no symptomatic PEs nor any confirmed cases of PE at post‐mortem. During the three‐month follow‐up period only two symptomatic venous thromboembolic events occurred, one in each group. Fourteen patients out of 74 (18.9%) allocated to IPC did not tolerate the device and stopped wearing it within five days following randomisation. In CLOTS 2009 13 patients out of 1256 (1%) allocated to GCS and 20 patients out of 1262 (1.6%) allocated to avoid GCS had imaging or autopsy confirmed PE within the first 30 days (OR 0.65, 95% CI 0.33 to 1.30; P = ns) (Analysis 1.4). Of patients allocated to compression GCS, 73% (916 out of 1256) were compliant with treatment (that is they wore GCS daily until 30 days, discharge or death). Sixty‐four patients out of 1256 (5%) allocated to GCS and 16 patients out of 1262 (1%) allocated to avoiding GCS experienced skin problems on the legs (that is skin breaks, ulcers, blisters, skin necrosis) (OR 3.47, 95 % CI 2.22 to 5.41; P < 0.001).
1.4. Analysis.
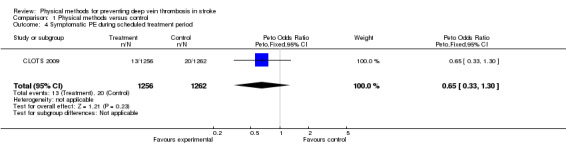
Comparison 1 Physical methods versus control, Outcome 4 Symptomatic PE during scheduled treatment period.
Discussion
Overall, the use of physical methods did not have any clinically or statistically significant effects on any of the outcomes assessed. PEs were rare in the included RCTs of physical methods of prophylaxis. The estimate of the effect of these interventions on the risk of PE is therefore very imprecise.
However, the great majority of data in this review comes from trials of GCS, and CLOTS 2009 yielded 93% of the weight in the estimate of effect on the outcome 'death or DVT'. Use of thigh‐length GCS in stroke patients was not associated with a significant effect on the odds of DVT during the scheduled treatment period (OR 0.88, 95% CI 0.72 to 1.08). Assuming a rate of 16% for any DVT (distal and proximal) this translates into a very modest absolute risk reduction of less than 2% (number needed to treat ≥ 50). This is probably not a clinically worthwhile effect given the cost, difficulties with compliance and frequency of local complications (that is skin breaks, etc).
These data from trials of GCS in stroke are in marked contrast to the effects of GCS in other settings. Apart from a single study carried out in patients with acute myocardial infarction (Kierkegaard 1993) no other randomised clinical trials in high‐risk medical patients, other than those with stroke, have been performed. However, a systematic review of randomised trials of GCS in patients at moderate and high risk of DVT following surgical procedures showed that the use of GCS was associated with a significant reduction in DVT (Amaragiri 2001). In a more recent review that included 18 trials, of which 16 were in perioperative patients, thigh‐length GCS were associated with a significant reduction in the risk of 'any DVT'. When outcomes were restricted to proximal DVT the trend towards reduced risk with GCS was no longer statistically significant (Roderick 2005). Therefore, GCS applied perioperatively seem to reduce the risk of mainly distal DVT in patients undergoing surgery. By contrast, in stroke patients the evidence does not indicate that use of GCS is associated with a clinically worthwhile reduction in risk of either distal or proximal DVT. There is very little information to indicate whether GCS reduce the risk of DVT in other high‐risk groups of medical patients.
There was very limited evidence on the effects of IPC. However, in patients with an acute stroke IPC appears to be a promising method for reducing the risk of DVT, particularly among groups of patients in whom anticoagulants are contraindicated, for example those with intracranial haemorrhage. Therefore, additional larger randomised studies of IPC are needed to reliably assess the balance of risk and benefit after stroke. We did not find any trials of electrical nerve stimulation to induce active calf muscle contractions, but since such devices are being developed it seems likely that trials may be undertaken.
Authors' conclusions
Implications for practice.
The evidence does not support the routine use of graduated compression stockings (GCS) to reduce the risk of DVT in patients with recent stroke. There is currently insufficient evidence to justify the routine use of intermittent pneumatic compression (IPC) in such patients.
Implications for research.
The results of this review suggest that further trials of graduated compression stockings are justified in other categories of high‐risk medical patients. Intermittent pneumatic compression for the prevention of DVT and PE may be effective in stroke patients. The ongoing CLOTS Trial 3 aims to determine whether intermittent pneumatic compression reduces the risk of DVT after acute stroke in the presence or absence of background use of GCS.
What's new
| Date | Event | Description |
|---|---|---|
| 23 April 2010 | New citation required and conclusions have changed | The review conclusions changed from 'no evidence' to 'no evidence of benefit' for graduated compression stockings after stroke. There has been a change of authorship. |
| 23 April 2010 | New search has been performed | We have updated the searches to November 2009, and have added two new trials with 2669 patients. The text of the review has been updated. |
History
Protocol first published: Issue 4, 1999 Review first published: Issue 1, 2002
| Date | Event | Description |
|---|---|---|
| 29 September 2008 | Amended | Converted to new review format. |
| 11 June 2004 | New search has been performed | Two minor corrections have been made: the data from the Prasad trial on deaths were inadvertently omitted from the published review (now included); there was an error in the denominators used for the Muir trial (now corrected). An additional outcome (death or DVT) was added and the results section edited accordingly. The overall conclusions of the review were not altered. |
Acknowledgements
We are grateful to the Editorial Board of the Cochrane Stroke Group; to the Managing Editor, Mrs Hazel Fraser; and to the Trials Search Co‐ordinator, Mrs Brenda Thomas.
Appendices
Appendix 1. MEDLINE (Ovid)
We used the following search strategy for MEDLINE (Ovid) and adapted it to search CENTRAL
cerebrovascular disorders/ or exp basal ganglia cerebrovascular disease/ or exp brain ischemia/ or exp carotid artery diseases/ or exp intracranial arterial diseases/ or exp "intracranial embolism and thrombosis"/ or exp intracranial hemorrhages/ or stroke/ or exp brain infarction/ or vasospasm, intracranial/ or vertebral artery dissection/
(stroke or poststroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw.
((brain$ or cerebr$ or cerebell$ or intracran$ or intracerebral) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$)).tw.
((brain$ or cerebr$ or cerebell$ or intracerebral or intracran$ or subarachnoid) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw.
1 or 2 or 3 or 4
thrombosis/
thromboembolism/
venous thrombosis/ or venous thromboembolism/
thrombophlebitis/
deep venous thrombo$.tw.
deep vein thrombo$.tw.
((venous or vein) adj5 thrombo$).tw.
(DVT or VTE).tw.
thromboprophylaxis.tw.
phlebothrombosis.tw.
exp pulmonary embolism/
pulmonary artery/ and embolism/
((pulmonary or lung) adj5 (embol$ or thrombo$ or infarct$)).tw.
or/6‐18
Intermittent Pneumatic Compression Devices/
bandages/ or stockings, compression/ or gravity suits/
(pneumatic adj5 (compression or device$ or appliance$ or stocking$ or hose or boot$ or suit or suits)).tw.
((pneumatic or electric$ or compression) adj5 pump$).tw.
(intermittent adj5 (compression or impulse device$)).tw.
(compression adj5 (device$ or system$ or stocking$ or hose or boot$)).tw.
((elastic or antiembolic or anti‐embolic) adj5 (stocking$ or hose)).tw.
(mechanical adj5 (prophylaxis or compression)).tw.
(inflatable adj5 (device$ or garment$ or stocking$ or hose or boot$)).tw.
IPC.tw.
(sequential adj5 compression).tw.
exp Electric Stimulation/
exp Electric Stimulation Therapy/
(electric$ adj10 stimulat$).tw.
electrostimulation.tw.
or/20‐34
5 and 19 and 35
Appendix 2. EMBASE (Ovid)
We used the following search strategy to search EMBASE (Ovid)
cerebrovascular disease/ or basal ganglion hemorrhage/ or cerebral artery disease/ or cerebrovascular accident/ or stroke/ or exp carotid artery disease/ or exp brain hematoma/ or exp brain hemorrhage/ or exp brain infarction/ or exp brain ischemia/ or exp intracranial aneurysm/ or exp occlusive cerebrovascular disease/
stroke patient/
(stroke or poststroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw.
((brain$ or cerebr$ or cerebell$ or intracran$ or intracerebral) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$)).tw.
((brain$ or cerebr$ or cerebell$ or intracerebral or intracran$ or subarachnoid) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw.
1 or 2 or 3 or 4 or 5
thromboembolism/ or thrombosis/ or leg thrombosis/ or vein thrombosis/ or deep vein thrombosis/ or leg thrombophlebitis/ or thrombophlebitis/ or venous thromboembolism/
thrombosis prevention/ or postoperative thrombosis/
deep venous thrombo$.tw.
deep vein thrombo$.tw.
((venous or vein) adj5 thrombo$).tw.
(DVT or VTE).tw.
thromboprophylaxis.tw.
phlebothrombosis.tw.
lung embolism/
lung artery/ or pulmonary artery/
embolism/ or artery embolism/ or embolism prevention/
16 and 17
((pulmonary or lung) adj5 (embol$ or thrombo$ or infarct$)).tw.
7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 18 or 19
intermittent pneumatic compression device/
compression/ or compression therapy/ or leg compression/ or pneumatic tool/
clothing/ or protective clothing/ or cuff/ or elastic stockings/
(pneumatic adj5 (compression or device$ or appliance$ or stocking$ or hose or boot$ or suit or suits)).tw.
((pneumatic or electric$ or compression) adj5 pump$).tw.
(intermittent adj5 (compression or impulse device$)).tw.
(compression adj5 (device$ or system$ or stocking$ or hose or boot$)).tw.
((elastic or antiembolic or anti‐embolic) adj5 (stocking$ or hose)).tw.
(mechanical adj5 (prophylaxis or compression)).tw.
(inflatable adj5 (device$ or garment$ or stocking$ or hose or boot$)).tw.
IPC.tw.
electrostimulation/ or electrostimulation therapy/
(electric$ adj10 stimulat$).tw.
electrostimulation.tw.
or/21‐34
6 and 20 and 35
Appendix 3. CINAHL (EBSCO)
We used the following search strategy to search CINAHL (EBSCO) S52 .S9 and S23 and S51 S51 .S24 or S27 or S30 or S33 or S36 or S39 or S42 or S45 or S46 or S47 or S48 or S49 or S50 S50 .TI electrostimulation or AB electrostimulation S49 .TI (electric* N10 stimulat*) or AB (electric* N10 stimulat*) S48 .MH "electrical stimulation" or MH "electrotherapy" S47 .TI (sequential N5 compression) or AB (sequential N5 compression) S46 .TI IPC or AB IPC S45 .S43 and S44 S44 .TI (device* or garment* or stocking* or hose or boot*) or AB (device* or garment* or stocking* or hose or boot*) S43 .TI inflatable or AB inflatable S42 .S40 and S41 S41 .TI (prophylaxis or compression) or AB (prophylaxis or compression) S40 .TI mechanical or AB mechanical S39 .S37 and S38 S38 .TI (stocking* or hose) or AB (stocking* or hose) S37 .TI (elastic or antiembolic or anti‐embolic) or AB (elastic or antiembolic or anti‐embolic) S36 .S34 and S35 S35 .TI (device* or system* or stocking* or hose or boot*) or AB (device* or system* or stocking* or hose or boot*) S34 .TI compression or AB compression S33 .S31 and S32 S32 .TI (compression or impulse device*) or AB (compression or impulse device*) S31 .TI intermittent* or AB intermittent* S30 .S28 and S29 S29 .TI pump* or AB pump* S28 .TI (pneumatic or electric* or compression)or AB (pneumatic or electric* or compression) S27 .S25 and S26 S26 .TI (compression or device* or appliance* or stocking* or hose or boot* or suit or suits) or AB (compression or device* or appliance* or stocking* or hose or boot* or suit or suits) S25 .TI pneumatic or AB pneumatic S24 .(MH "Compression Garments") or (MH "Compression Therapy") S23 .S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S22 S22 .S20 and S21 S21 .TI (embol* or thrombo* or infarct*) or AB (embol* or thrombo* or infarct*) S20 .TI (pulmonary or lung) or AB (pulmonary or lung) S19 .MH "pulmonary artery" and MH "embolism" S18 .MH "pulmonary embolism" S17 .TI phlebothrombosis or AB phlebothrombosis S16 .TI thromboprophylaxis or AB thromboprophylaxis S15 .TI (DVT OR VTE) or AB (DVT OR VTE) S14 .TI (vein N5 thrombo*) or AB (vein N5 thrombo*) S13 .TI (venous N5 thrombo*) or AB (venous N5 thrombo*) S12 .TI deep vein thrombo* or AB deep vein thrombo* S11 .TI deep venous thrombo* or AB deep venous thrombo* S10 .(MH "Thrombosis") or (MH "thromboembolism") or (MH "venous thrombosis") or (MH "thrombophlebitis") S9 .S1 or S2 or S5 or S8 S8 .S6 and S7 S7 .TI ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* ) or AB ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* ) S6 .TI ( brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid ) or AB ( brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid ) S5 .S3 and S4 S4 .TI ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus* ) or AB ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus* ) S3 .TI ( brain* or cerebr* or cerebell* or intracran* or intracerebral ) or AB ( brain* or cerebr* or cerebell* or intracran* or intracerebral ) S2 .TI ( stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc or cva or apoplex or SAH ) or AB ( stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc or cva or apoplex or SAH ) S1 .(MH "Cerebrovascular Disorders+") or (MH "stroke patients") or (MH "stroke units")
Appendix 4. British Nursing Index (Ovid)
We used the following search strategy to search The British Nursing Index (Ovid)
"equipment and supplies"/ and dressings/
(pneumatic adj5 (compression or device$ or appliance$ or stocking$ or hose or boot$ or suit or suits)).tw.
((pneumatic or electric$ or compression) adj5 pump$).tw.
(intermittent adj5 (compression or impulse device$)).tw.
(compression adj5 (device$ or system$ or stocking$ or hose or boot$)).tw.
((elastic or antiembolic or anti‐embolic) adj5 (stocking$ or hose)).tw.
(mechanical adj5 (prophylaxis or compression)).tw.
(inflatable adj5 (device$ or garment$ or stocking$ or hose or boot$)).tw.
ipc.tw.
(sequential adj5 compression).tw.
((electric$ adj10 stimulat$) or electrostimulation).tw.
or/1‐11
thrombosis/
deep venous thrombo$.tw.
deep vein thrombo$.tw.
((venous or vein) adj5 thrombo$).tw.
(DVT or VTE).tw.
thromboprophylaxis.tw.
phlebothrombosis.tw.
((pulmonary or lung) adj5 (embol$ or thrombo$ or infarct$)).tw.
or/13‐20
stroke services/ or stroke/ or stroke rehabilitation/
(stroke or poststroke or post‐stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw.
((brain$ or cerebr$ or cerebell$ or intracran$ or intracerebral) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$)).tw.
((brain$ or cerebr$ or cerebell$ or intracerebral or intracranial or subarachnoid) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw.
or/22‐25
12 and 21 and 26
Data and analyses
Comparison 1. Physical methods versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death from any cause during scheduled treatment period | 4 | 2792 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.12 [0.87, 1.45] |
| 1.1 Graduated compression stockings | 2 | 2615 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.13 [0.87, 1.47] |
| 1.2 Intermittent pneumatic compression | 2 | 177 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.04 [0.37, 2.89] |
| 2 DVT during scheduled treatment period | 4 | 2792 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.85 [0.70, 1.04] |
| 2.1 Graduated compression stockings | 2 | 2615 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.88 [0.72, 1.08] |
| 2.2 Intermittent pneumatic compression | 2 | 177 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.45 [0.19, 1.10] |
| 3 Death or DVT in the scheduled treatment period | 4 | 2792 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.94 [0.79, 1.11] |
| 3.1 Graduated compression stockings | 2 | 2615 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.97 [0.81, 1.15] |
| 3.2 Intermittent pneumatic compression | 2 | 177 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.61 [0.29, 1.24] |
| 4 Symptomatic PE during scheduled treatment period | 1 | 2518 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.65 [0.33, 1.30] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
CLOTS 2009.
| Methods | Study: RCT Exclusion to post‐randomisation: 0 Losses to follow up: 69 missing data (41 in treatment group and 28 in control group) ‐ no CDU prior to death or 30 days DVT diagnosis: CDU (minimum of the popliteal and femoral veins) between day 7 and 10 and between day 25 and 30 Statistical analysis: odds ratio and NNT Scheduled treatment and follow‐up period: 30 days; clinical follow up at 6 months | |
| Participants | Country: UK, Italy and Australia Total number of participants: 2518 Total available for analysis: 2518 Age: 76 years (68 to 83) for both groups Sex: males 49.4% (620/1256) in the treatment group and 49.3% in the control group (622/1262) Immobilisation: yes Inclusion criteria: patients admitted with an acute stroke up to day 3 post‐admission Exclusion criteria: peripheral vascular disease, or with diabetic/sensory neuropathy, if clinicians judged GCS could cause skin damage Full intention‐to‐treat analysis: performed | |
| Interventions | Type: thigh‐length Tyco Healthcare TED GCS
Control: 1262
Treatment: 1256
Duration applied: night and day until death/discharge/mobile/refused Use of anticoagulants post randomisation: group allocated GCS • 117 post‐randomisation prophylactic dose heparin/LMWH prescribed • 78 post‐randomisation treatment dose heparin/LMWH prescribed • 186 post‐randomisation warfarin prescribed Use of anticoagulants post randomisation: group allocated 'avoid GCS' • 129 post‐randomisation prophylactic dose heparin/LMWH prescribed • 97 post‐randomisation treatment dose heparin/LMWH prescribed • 208 post‐randomisation warfarin prescribed |
|
| Outcomes | Any DVT Control: 224 Treatment: 205 P value: ns | |
| Notes | The primary outcome focused on proximal DVTs (popliteal or femoral) rather than any DVT. Randomising clinicians were allowed to elect prior to randomisation whether patients would have a second CDU at 25 to 30 days. The 6‐month outcomes have not yet been reported. The median delay from stroke onset to enrolment was 2 days but there was no trend towards more effect with earlier recruitment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | A ‐ Adequate. Computer‐generated allocation based on within centre minimisation for prognostic factors (stroke onset; stroke severity; leg paresis; use of anticoagulants) plus random allocation |
| Allocation concealment? | Low risk | A ‐ Adequate. Central allocation (web and telephone) |
| Blinding? All outcomes | Low risk | A ‐ Adequate. GCS removed before CDU was performed Hard copies of positive scans were recorded for independent assessment |
| Incomplete outcome data addressed? All outcomes | Low risk | A ‐ Adequate. 69 missing data |
Lacut 2005.
| Methods | Study: RCT Exclusion to post‐randomisation: 0 Losses to follow up: 18 (10 in the treatment group and 8 in the control group) DVT diagnosis: CDU of proximal and distal veins at day 10 ± 2 Statistical analysis: odds ratio and NNT Scheduled treatment and follow‐up period: 10 ± 2 days; follow up at 30 and 90 days | |
| Participants | Country: France Total number of participants: 151 Total available for analysis: 133 Age: mean 59.9 (±14.7) years in the treatment group and 65.7 (±12.7) years in the control group; P < 0.01 Sex: males 62% (46/74) in the treatment group and 55% (42/77) in the control group Immobilisation: no Inclusion criteria: age over 18 years, traumatic or spontaneous ICH with or without SAH Exclusion criteria: extra or subdural haematomas, traumatic ICH due to polytrauma including the lower limbs, haemorrhagic transformation of ischaemic infarct and vasculitis, DVT within the previous 3 months, lower‐limb arteriopathy, venous graft, wound in the lower limb related either to a vascular disease or a trauma, 'do not resuscitate' order, and > 24‐hour delay since hospital admission Full intention‐to‐treat analysis: not performed | |
| Interventions | Type: intermittent pneumatic compression (SC Response Controller) plus GCS (TED) versus GCS (TED) alone. ICP applied sequentially for 11 seconds with pressures of 45, 40 and 30 mmHg at the ankle, calf and thigh. GCS length not stated Control: 77 Treatment: 74 Duration applied: 10 ± 2 days | |
| Outcomes | DVT Control: 11 Treatment: 3 P value: 0.03 | |
| Notes | The study included both traumatic and spontaneous ICH, with (or without) SAH, and the authors did not give any additional information on the number of spontaneous ICH and their outcome. The control group wore GCS. Immobilisation was not an inclusion criteria. Control group patients were significantly younger than the treatment group patients 18.9% patients (14/74) in the treatment group did not tolerate the IPC device | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | A ‐ Adequate. Computer‐generated with random block sizes and stratified according to three variables (age, surgical intervention plan, admission to intensive care unit) |
| Allocation concealment? | Low risk | A ‐ Adequate. Sequentially numbered, sealed, opaque envelopes |
| Blinding? All outcomes | Low risk | A ‐ Adequate. Intermittent pneumatic compression device removed before CDU was performed CDU scans were recorded on videotape for independent blind assessment |
| Incomplete outcome data addressed? All outcomes | Low risk | A ‐ Adequate. 18 patients did not have the scheduled compression duplex ultrasound |
Muir 2000.
| Methods | Study: RCT Exclusion to post‐randomisation: 1 Losses to follow up: 19 DVT diagnosis: CDU at enrolment and at day 7 ± 2 Statistical analysis: odds ratio and NNT Scheduled treatment and follow‐up period 7 ± 2 days | |
| Participants | Country: UK Total number of participants: 98 (1 missing data) Total available for analysis: 71 Age: mean age > 73 years Sex: not specified Immobilisation: yes Inclusion criteria: acute stroke within 24 hours Exclusion criteria: patients with coma, life‐threatening intercurrent illness, critical lower limb ischaemia or severe dermatological conditions Full intention‐to‐treat analysis was not performed | |
| Interventions | Type: GCS thigh‐length Kendall TED or Brevett TX brands Control: 32 Treatment: TED group 37, TX group 28 Duration applied: time of application and duration applied not specified | |
| Outcomes | DVT Control: first exam 3/32, second exam 5/26, either 7/32 Treatment: first exam 6/65, second exam 3/45, either exam 7/65 P value: ns | |
| Notes | TX and TED groups were combined for analysis of efficacy. Only 1 symptomatic DVT occurred during study period, but it is not clear in which group. Some data reported in the results do not correspond to data reported in tables | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | A ‐ Adequate. Computer‐generated random numbers |
| Allocation concealment? | Low risk | A ‐ Adequate. Sealed envelopes |
| Blinding? All outcomes | Low risk | A ‐ Adequate. Examinations videotaped for blinded independent review |
| Incomplete outcome data addressed? All outcomes | Unclear risk | B ‐ Unclear. Not clearly reported. Data in the results do not correspond to data shown in tables |
Prasad 1982.
| Methods | Study: RCT Exclusion to post‐randomisation: 0 Losses to follow up: 0 DVT diagnosis: FUT on first and subsequently every day for 10 days Statistical analysis: Student's T Test Scheduled treatment and follow‐up period 10 days | |
| Participants | Country: UK Total number of participants: 26 Total available for analysis: 26 Age: mean age 78 years in the treatment group and 80 years in the control group Sex: males 53.8% in the treatment group (8/13) and 38.5% (5/13) in the control group Immobilisation: yes Inclusion criteria: all patients admitted for acute stroke within 72 hours Exclusion criteria: patients in coma or not specified other clinically unacceptable conditions Full intention‐to‐treat analysis was not possible | |
| Interventions | Type: intermittent calf pneumatic compression with Flowtron Legging at 40 mmHg to both legs intermittently, each complete cycle lasting 4 minutes Control: 13 Treatment: 13 Duration: 10 days (24 hours per day for first day and then 3 x 3 hours per day for remainder of treatment period | |
| Outcomes | DVT Control: 6 Treatment: 6 P values: ns | |
| Notes | Method of randomisation, allocation concealment and blinding to primary outcome are not clear. 2 patients in the treatment group died within the study period. 1 symptomatic DVT occurred within the study period (FUT confirmed), but it is not clear in which treatment group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | B ‐ Unclear |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Blinding? All outcomes | Unclear risk | B ‐ Unclear |
| Incomplete outcome data addressed? All outcomes | Low risk | A ‐ Adequate. No losses to follow up |
CDU: compression duplex ultrasound DVT: deep vein thrombosis FUT: 125I‐Fibrinogen uptake test (A sustained difference of more than 20% between consecutive or opposite points or a raising count were considered diagnostic of DVT) GCS: graded compression stockings ICH: intracerebral haemorrhage IPC: intermittent pneumatic compression NNT: number needed to treat ns: non‐significant RCT: randomised controlled trial SAH: subarachnoid haemorrhage TED and TX: graded compression stockings brands
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| CLOTS Trial 2 | Comparison of 2 groups of stockings: no 'non‐stocking' control group |
| Desmukh 1991 | This is a controlled randomised study but patients were enrolled between 19 and 21 days after stroke |
| Green 1998 | This study has no control group and patients were randomised 7 days after stroke |
| Kamran 1998 | Not a controlled randomised study |
| Kelly 2004 | This study has no control group |
| Pambianco 1995 | This study enrolled patients 16 days after the stroke The control group is formed of patients with compression stockings |
| Sprigg 2005 | Post‐hoc analysis from a large study on tinzaparin versus aspirin in acute stroke Use of compression stockings not randomised |
| Turpie 1979 | Controlled randomised trial including 199 patients with intracranial disease; among them there were 40 patients with stroke but data for these patients were not separately available |
Characteristics of studies awaiting assessment [ordered by study ID]
Spinelli 2006.
| Methods | RCT DVT diagnosis: CDU in patients with high D‐dimer levels or clinical suspect of DVT Scheduled treatment and follow‐up period: 15 and 60 days |
| Participants | Country: Italy Total number of participants: 93 Immobilisation: yes Inclusion criteria: acute ischaemic stroke within 48 hours |
| Interventions | IPC plus enoxaparin plus normal care versus enoxaparin plus normal care |
| Outcomes | DVT and PE within the study period |
| Notes | Within‐group outcomes, concealment and blinding procedures and total number of compression duplex ultrasound not provided |
CDU: compression duplex ultrasound DVT: deep vein thrombosis IPC: intermittent pneumatic compression PE: pulmonary embolism RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
CLOTS Trial 3.
| Trial name or title | CLOTS Trial 3 |
| Methods | RCT |
| Participants | Country: UK Any patient admitted to hospital within 7 days of a clinical stroke |
| Interventions | IPC in addition to routine care versus routine care alone |
| Outcomes | Primary outcome: presence of definite or probable symptomatic or asymptomatic DVT in the popliteal or femoral veins detected on a screening CDU scan or any symptomatic DVT in the popliteal or femoral veins confirmed on CDU scan or contrast venography or MRI direct thrombus imaging within 30 days of randomisation Secondary outcomes: death within 30 days/6 months; presence of definite or probable DVT in the popliteal or femoral veins detected on a screening CDU scan or contrast venography or MRI direct thrombus imaging within 30 days of randomisation; definite symptomatic or asymptomatic DVT in the popliteal or femoral veins detected on either a CDU scan or contrast venography or MRI direct thrombus imaging within 30 days of randomisation; any definite or probable symptomatic or asymptomatic DVT; confirmed fatal or non‐fatal PE; adherence to allocated treatment; any confirmed symptomatic or asymptomatic DVT or PE occurring between randomisation and 6‐month follow up; any symptomatic DVT or PE occurring between randomisation and 6‐month follow up; place of residence at 6‐month follow up; post‐DVT syndrome; disability (modified Rankin score) at 6‐month follow up; health related quality of life (EuroQol) at 6‐month follow up |
| Starting date | 2009 |
| Contact information | Prof Martin Dennis, Department of Clinical Neurosciences, Western General Hospital, Edinburgh, EH4 2XU, UK |
| Notes |
CDU: compression duplex ultrasound DVT: deep vein thrombosis IPC: intermittent pneumatic compression MRI: magnetic resonance imaging PE: pulmonary embolism RCT: randomised controlled trial
Contributions of authors
Defining review question: Dr Chiodo Grandi Protocol development: Dr Fabio Chiodo Grandi, Prof Martin Dennis, Dr Marcello Naccarato Locating and assessing trials: Dr Marcello Naccarato, Prof Martin Dennis Extracting data: Dr Marcello Naccarato, Dr Fabio Chiodo Grandi, Prof Martin Dennis
In addition, Professor Peter Sandercock contributed to the first version of the review; and commented on the methods, analyses and drafts of the updated review.
Declarations of interest
Martin Dennis is the Principal investigator of the CLOTS trials, Peter Sandercock is a member of the Steering Committee of the trial and Fabio Chiodo Grandi is a co‐investigator. COVIDIEN, a manufacturer of both GCS and IPC devices, supplied the CLOTS trials with their devices and training in their use but had no part in any aspect of this review.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
CLOTS 2009 {published data only}
- CLOTS Trials Collaboration. Effectiveness of thigh‐length graduated compression stockings to reduce the risk of deep vein thrombosis after stroke (CLOTS trial 1): a multicentre, randomised controlled trial. Lancet 2009;373(9679):1958‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lacut 2005 {published data only}
- Lacut K, Bressollette L, Gal G, Etienne E, Tinteniac A, the VICTORIAh (Venous Intermittent Compression and Thrombosis Occurrence Related to Intra‐cerebral Acute hemorrhage) investigators. Prevention of venous thrombosis in patients with acute intracerebral hemorrhage. Neurology 2005;65:865‐69. [DOI] [PubMed] [Google Scholar]
Muir 2000 {published data only}
- Muir KW, Watt A, Baxter G, Grosset DG, Lees KR. Randomized trial of graded compression stockings for prevention of deep‐vein thrombosis after acute stroke. Quarterly Journal of Medicine 2000;93:359‐64. [DOI] [PubMed] [Google Scholar]
Prasad 1982 {published data only}
- Prasad BK, Banerjee AK, Howard H. Incidence of deep vein thrombosis and the effect of pneumatic compression of the calf in elderly hemiplegics. Age and Ageing 1982;11:42‐4. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
CLOTS Trial 2 {published data only}
- CLOTS Trials Collaboration. Effectiveness of thigh‐length graduated compression stockings to reduce the risk of deep vein thrombosis after stroke (CLOTS trial 1): a multicentre, randomised controlled trial. Lancet 2009;373(9679):1958‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Desmukh 1991 {published data only}
- Desmukh M, Bisignani M, Landau P, Orchard TJ. Deep vein thrombosis in rehabilitating stroke patients. Incidence, risk factors and prophylaxis. American Journal Physical Medicine and Rehabilitation 1991;70:313‐6. [PubMed] [Google Scholar]
Green 1998 {published data only}
- Green D, Akuhota V, Eiken M, Feinglass J, Fuller S, Hwang C, et al. Prevention of thromboembolism in stroke rehabilitation patients. Topics in Stroke Rehabilitation 1998;5(2):68‐74. [Google Scholar]
Kamran 1998 {published data only}
- Kamran SI, Downey D, Ruff RL. Pneumatic sequential compressions reduce the risk of deep vein thrombosis in stroke patients. Neurology 1998;50(6):1683‐8. [DOI] [PubMed] [Google Scholar]
Kelly 2004 {published data only}
- Kelly J, Rudd A, Lewis RR, Coshall C, Moody A, Hunt BJ. Venous thromboembolism after acute ischemic stroke: a prospective study using magnetic resonance direct thrombus imaging. Stroke 2004;35(10):2320‐5. [DOI] [PubMed] [Google Scholar]
Pambianco 1995 {published data only}
- Pambianco G, Orchard T, Landau P. Deep vein thrombosis: prevention in stroke patients during rehabilitation. Archives of Physical Medicine and Rehabilitation 1995;76:324‐30. [DOI] [PubMed] [Google Scholar]
Sprigg 2005 {published data only}
- Sprigg N, Gray LJ, Bath PMW, Boysen G, Deyn PP, TAIST Advisory Committee and TAIST Investigators. Compression stockings and the prevention of symptomatic venous thromboembolism: data from the Tinzaparin in Acute Ischemic Stroke Trial. Journal of Stroke and Cerebrovascular Diseases 2005;14(5):203‐9. [DOI] [PubMed] [Google Scholar]
Turpie 1979 {published data only}
- Turpie AG, Delmore T, Hirsh J, Russell H, Genton E, Hiscoe C, et al. Prevention of venous thrombosis by intermittent sequential calf compression in patients with intracranial disease. Thrombosis Research 1979;15:611‐6. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Spinelli 2006 {published data only}
- Spinelli M, Corea F, Bignamini V, Nuzzaco G, Ghidinelli C, Martinelli V, et al. Early vital and functional outcome of acute ischaemic stroke patients: influence of deep vein thrombosis prevention with pneumatic compression devices. Journal of Neurology 2006;253 Suppl 2:135. [Google Scholar]
References to ongoing studies
CLOTS Trial 3 {published data only}
- Dennis M. The CLOTS trials: testing the effect of external compression devices on the risk of deep vein thrombosis (DVT) in acute stroke patients. Proceedings of the 18th European Stroke Conference. 2009.
Additional references
Amaragiri 2001
- Amaragiri SV, Lees TA. Elastic compression stockings for prevention of deep vein thrombosis. Cochrane Database of Systematic Reviews 2001, Issue 1. [Art. No.: CD001484. DOI: 10.1002/14651858.CD001484] [DOI] [PubMed] [Google Scholar]
Davenport 1996
- Davenport RJ, Dennis MS, Wellwood BA, Warlow CP. Complications after acute stroke. Stroke 1996;27:415‐20. [DOI] [PubMed] [Google Scholar]
Havig 1977
- Havig O. Deep vein thrombosis and pulmonary embolism. An autopsy study with multiple regression analysis of possible risk factors. Acta Chirurgica Scandinavica Supplementum 1977;478:1‐120. [PubMed] [Google Scholar]
Kay 1986
- Kay TW, Martin FI. Heel ulcers in patients with long‐standing diabetes who wear antiembolism stockings. Medical Journal of Australia 1986;145:290‐1. [DOI] [PubMed] [Google Scholar]
Kierkegaard 1993
- Kierkegaard A, Norgren L. Graduated compression stockings in the prevention of deep vein thrombosis in patients with acute myocardial infarction. European Heart Journal 1993;14(10):1365‐8. [DOI] [PubMed] [Google Scholar]
Kohro 2005
- Kohro S, Yamakage M, Sato K, Sato JI, Namiki A. Intermittent pneumatic foot compression can activate blood fibrinolysis without changes in blood coagulability and platelet activation. Acta Anaesthesiologica Scandinavica 2005;49(5):660‐4. [DOI] [PubMed] [Google Scholar]
Merrett 1993
- Merrett ND, Harrell KC. Ischemic complications of graduated compression stockings in the treatment of deep venous thrombosis. Postgraduate Medical Journal 1993;69:232‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE CG92 2010
- National Institute for Clinical Excellence. NICE Clinical Guideline 92: Reducing the risk of venous thromboembolism (deep vein thrombosis and pulmonary embolism) in patients admitted to hospital. www.nice.org.uk/guidance/CG92 2010.
RevMan 2008 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
Roderick 2005
- Roderick P, Ferris G, Wilson K, Halls H, Jackson D, Collins R, et al. Towards evidence‐based guidelines for the prevention of venous thromboembolism: systematic reviews of mechanical methods, oral anticoagulation, dextran and regional anaesthesia as thromboprophylaxis. Health Technology Assessment 2005;9(49):1‐94. [DOI] [PubMed] [Google Scholar]
Sandercock 2008
- Sandercock PA, Counsell C, Kamal AK. Anticoagulants for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2008, Issue 4. [Art. No.: CD000024. DOI: 10.1002/14651858.CD000024] [DOI] [PubMed] [Google Scholar]
Virchow 1858
- Virchow R. Die cellularpathologie in ihrer begrundung auf physiologische und pathologische gewebsleher. Berlin: Hirschwald A, 1858. [Google Scholar]
References to other published versions of this review
Mazzone 2002
- Mazzone C, Chiodo Grandi F, Sandercock PAG, Miccio M, Salvi R. Physical methods for preventing deep vein thrombosis in stroke. Cochrane Database of Systematic Reviews 2002, Issue 1. [Art. No.: CD001922. DOI: 10.1002/14651858.CD001922] [DOI] [PubMed] [Google Scholar]
Mazzone 2004
- Mazzone C, Chiodo Grandi F, Sandercock PAG, Miccio M, Salvi R. Physical methods for preventing deep vein thrombosis in stroke. Cochrane Database of Systematic Reviews 2004, Issue 4. [Art. No.: CD001922. DOI: 10.1002/14651858.CD001922.pub2] [DOI] [PubMed] [Google Scholar]


