Abstract
Background:
Chest computed tomography (CT) is increasingly used for phenotyping and monitoring of patients with COPD. The aim of this work was to evaluate the association of Pi10 as a measure of standardized airway wall thickness on CT with exacerbations, mortality, and response to triple therapy.
Methods:
Patients of GOLD grades 1–4 of the COSYCONET cohort with prospective CT scans were included. Pi10 was automatically computed and analyzed for its relationship to COPD severity, comorbidities, lung function, respiratory therapy, and mortality over a 6-year period, using univariate and multivariate comparisons.
Results:
We included n = 433 patients (61%male). Pi10 was dependent on both GOLD grades 1–4 (p = 0.009) and GOLD groups A–D (p = 0.008); it was particularly elevated in group D, and ROC analysis yielded a cut-off of 0.26 cm. Higher Pi10 was associated to lower FEV1 % predicted and higher RV/TLC, moreover the annual changes of lung function parameters (p < 0.05), as well as to an airway-dominated phenotype and a history of myocardial infarction (p = 0.001). These associations were confirmed in multivariate analyses. Pi10 was lower in patients receiving triple therapy, in particular in patients of GOLD groups C and D. Pi10 was also a significant predictor for mortality (p = 0.006), even after including multiple other predictors.
Conclusion:
In summary, Pi10 was found to be predictive for the course of the disease in COPD, in particular mortality. The fact that Pi10 was lower in patients with severe COPD receiving triple therapy might hint toward additional effects of this functional therapy on airway remodeling.
Registration:
ClinicalTrials.gov, Identifier: NCT01245933
Keywords: COPD, CT based phenotyping, mortality, Pi10, triple therapy
Introduction
According to the current GOLD guidelines, chronic obstructive pulmonary disease (COPD) is treated using a stepwise approach, whereby inhaled treatment should be escalated to triple therapy but only after exacerbations have occurred.1 It is known that each exacerbation leads to a deterioration of lung function and increases rehospitalization rate and mortality risk.2–4 Therefore, identification of patients at increased risk for exacerbation or mortality is essential to initiate early, protective treatment escalation. There has been a long search for easy-to-assess biomarkers for these risks, but none of these has yet found its way into widespread clinical use.
For the assessment of COPD severity in terms of symptoms, exacerbations, lung function decline, and phenotyping, established tools are available. Phenotyping is increasingly performed using computed tomography (CT) scans, which is particularly useful for the assessment of emphysema, but can also be used to determine alterations of the airways, including airway lumen and wall thickness.5 Previous investigations found an association between increased airway wall thickness and more severe airflow obstruction,6–8 as well as higher respiratory mortality in patients additional showing a higher degree of emphysema.9 Pi10 represents the average airway wall thickness normalized to a ‘theoretical’ airway lumen of 10-mm inner perimeter and can be derived by established algorithms from standard clinical CT scans used for COPD phenotyping.10 Pi10 has already been shown to be of value in the evaluation of patients without known lung disease, especially population-based cohorts without history of prior respiratory disease, as well as in lung cancer screening cohorts. In these groups, an association between higher Pi10 and increased COPD risk or hospitalization rate and mortality due to respiratory disease has been demonstrated.11,12 Previous studies using cross-sectional data in patients with the established diagnosis of COPD showed a link between higher Pi10, respiratory symptoms, and higher airway obstruction.13–15
These findings raise the question of whether routine CT scans that are becoming part of routine assessment in COPD, can be utilized for the additional evaluation of clinical state and its deteriorations by analysis of Pi10. In the present study, we therefore aimed to reveal whether routine evaluation of CT scans for Pi10 can support the identification of patients who are at increased risk in terms of exacerbations and mortality, and thus possibly need escalation of inhaled therapy. For this purpose, we used data of the German COPD cohort COSYCONET (COPD and Systemic Consequences Comorbidies Network), in which extensive clinical characterization as well as CT scans were available.
Materials and methods
Study population
The German COPD cohort study ‘COPD and SYstemic consequences-COmorbidities NETwork’ (COSYCONET) started in 2010 in 31 study centers all over Germany. The aim of the investigation was the assessment of the interaction of lung disease, comorbidities, and systemic inflammation. After baseline visit (termed as visit 1), follow-up visits were performed 6, 18, 36, 54, 72, and 90 months after baseline. The broad inclusion criteria comprised (1) aged 40 years and older, (2) diagnosis of COPD (according to GOLD criteria) or chronic bronchitis, and (3) availability for repeated study visits over at least 18 months. The exclusion criteria were based on (1) having undergone major lung surgery, (2) moderate or severe exacerbation within the past 4 weeks, (3) having a lung tumor, and (4) physical or cognitive impairment resulting in an inability to walk or to understand the intention of the project.16 Among the patients of the German COPD cohort COSYCONET17 recruited at visit 1, n = 1427 patients participated in the third follow-up visit (V4), of whom 1176 were of GOLD grades 1–4. Of the 1427 patients, a subgroup of 602 patients was studied via prospective CT scans in separate study visits at the time of V4.18 Of these, 433 patients belonged to GOLD grades 1–4 and were evaluated regarding Pi10 (see Figure S1). Functional and clinical data of these patients were obtained at visit 4.
Assessments
Standard procedures were used to determine the anthropometric characteristics and lung function,17 including the assessment of forced expiratory volume in 1 s (FEV1), forced vital capacity, and their ratio FEV1/FVC in spirometry, of the ratio RV/TLC of residual volume (RV) to total lung capacity (TLC) in body plethysmography, and of lung diffusing capacity for carbon monoxide (CO) in terms of total diffusing capacity (TLCO) and diffusing capacity per alveolar volume (KCO). Predicted vales for spirometry19 and diffusing capacity20 were chosen from GLI resources.
The presence of comorbidities was assessed in a structured interview on the basis of physician-based diagnoses reported by patients.17 The present analysis included the comorbidities heart failure, myocardial infarction, coronary artery disease, chronic bronchitis, asthma, sleep apnea, diabetes, hyperlipidemia, hyperuricemia, and osteoporosis. The COPD Assessment Test (CAT), St George’s Respiratory Questionnaire (SGRQ), and modified Medical Research Council (mMRC) questionnaire were used as recommended.17 Following GOLD recommendations, patients were categorized into grades 1–4,1 or into groups A–D according to the frequency/severity of exacerbations and symptom burden quantified via the mMRC.1 According to the GOLD recommendations we used a value of mMRC of 0–1 for GOLD A and C patients, and of mMRC ⩾ 2 for GOLD B and D patients. COPD exacerbations were defined according to the GOLD recommendations as acute worsening of respiratory symptoms that resulted in additional therapy.20 Exacerbation history was recorded repeatedly during the follow-up visits of COSYCONET by structured interviews, whereby exacerbation risk of GOLD groups A–D was based on the 12-month history of exacerbations of all severities, including hospitalization; high risk was indicated by a history of two non-hospitalized exacerbations, or one exacerbation leading to hospital admission as described by GOLD.20
The decline in lung function parameters corresponded to the decline measured between visit 1 and visit 5.21 To assess the intake of inhaled medication, that is, long-acting beta-agonists (LABA), long-acting muscarinic antagonists (LAMA), and inhaled corticosteroids (ICS), patients were asked to bring all their medication for the study visit.17
Using the data from follow-up visits, we additionally investigated all-cause mortality up to 6 years after inclusion at visit 1. Survival status was assessed by contacting partners, relatives, primary care practitioners, and hospitals as done before.22
CT assessments
Overall, 602 patients were enrolled in the radiologic sub-study SP7 of COSYCONET. These patients underwent additional CT thorax examinations in inspiration and expiration18,23 on clinical CT scanners with at least 40-row detector arrays, taking their regular medication. Assessments were based on a non-enhanced low-dose chest CT protocol according to clinical standards and comprised end-inspiratory and end-expiratory acquisitions of the entire lung. The protocol followed the recommendations of the thoracic imaging workshop (AG Thorax) of the German Radiological Society (DRG). It was realized using a thin slice collimation of 0.6 mm, a pitch of 0.6–1.0, a tube potential of 120 kVp, and a tube current of 35 effective mAs for most scanner types.18,24 Owing to the use of different CT scanners, each site-specific study protocol was adapted to the local technical scanner specifications. Details of the CT acquisition protocol are given in Supplemental Table 1. The maximum effective radiation dose of both inspiratory and expiratory CT scans was below 3.5 mSv.
COPD phenotype
All CT scans were subjected to a standardized visual assessment process by an experienced chest radiologist. Based on the modified guidelines of the COPD Gene CT Workshop Group,25 an algorithm for further evaluation was applied. The extent of emphysema was determined semi-quantitatively using a five-point scale (<5%, 5–25%, 26–50%, 51–75%, and >75%) along with the leading type of emphysema (centrilobular versus panlobular) in a lobe-based approach.25,26 Moreover, the additional presence of bronchiectases, bronchial wall thickening, mosaic attenuation, and centrilobular nodules was listed. Furthermore, the presence of paraseptal emphysema and bullae (each for right and left side) was documented, in addition to abnormalities (e.g. collapse or stenosis) of the trachea or the two main stem bronchi.26 Based on these evaluations, the observer set the predominant COPD phenotype (either emphysema-type or not) in a binary score. The final score used in this analysis as well as in previous COSYCONET investigations18,23,26,27 thus comprised emphysema versus non-emphysema type, that is, airway-predominance.
Pi10
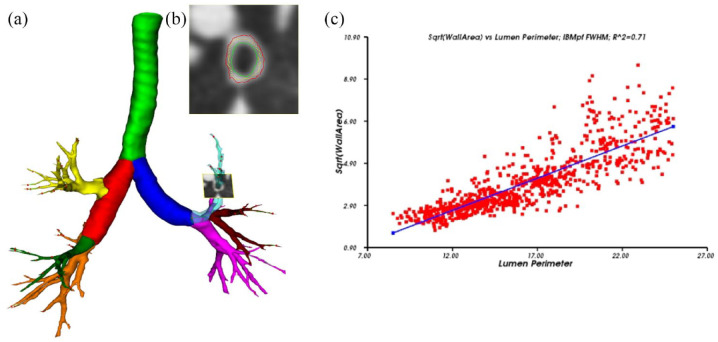
The non-commercial, fully automatic airway analysis software YACTA ( ‘Yet another CT analyzer’, Version 2.8.0.14, programming by O.W.) was used to evaluate CT data as previously described.28–33 The software was used to segment the entire tracheobronchial tree and the results were used to calculate different metrics of airway geometry. According to a previous clinical study by Jobst et al.,32 the airway parameters total diameter (TD), luminal area (LA), wall area (WA), and the ratio of wall area to the sum of wall and lumen area (wall percentage, WP) were investigated for the first to eighth generations – but Pi10 calculation was done based on all segmented airways. A standardized measure of airway wall thickness was determined by plotting the square root of airway wall areas against the inner perimeter of the airway for each airway location.32 Through the use of the corresponding regression line, the square root of the wall area for a hypothetical airway with 10-mm internal perimeter was derived and defined as Pi10.10 Thus, Pi10 characterizes airway morphology, not the number of airways. For further illustration, Figure 1(a)–(c) shows the segmented airway tree including the labeling of different airway structures, as well as the magnification of orthogonal airway slices and the calculation of Pi10.
Figure 1.
(a) Segmented airway tree including labeling of the pulmonary lobes, trachea highlighted in green, right main in red, left main in dark blue, right upper lobe in yellow, middle lobe in green and right lower lobe in orange, left upper lobe in light blue (transparent), lingula in dark red, and left lower lobe in pink. In the left upper lobe an orthogonal slice through an airway is shown, on such slices, all airways of the whole segmented airway tree are measured. (b) Magnification of orthogonal slice shown in (a), inner (green) and outer (red) wall borders are displayed. (c) The calculation of Pi10. The square root of wall area is plotted against the internal perimeter for each measured airway. Regression line delivers the Pi10 which square root of wall area for an airway with an internal perimeter of 10 mm.
Statistical analysis
For data description, mean values and standard deviations, or numbers and percentages were chosen. Univariate analyses were performed as analysis of variance (ANOVA) or contingency tables with chi-square statistics, depending on the type of data. Post hoc comparisons of ANOVA results were performed according to Duncan. In addition, receiver operator characteristics (ROC) for Pi10 were computed. The associations between variables were assessed by linear and logistic regression analyses comprising one dependent and multiple independent variables. In these analyses, age, gender, body mass index (BMI), and decline in FVC % predicted were always included as confounders. Proportional hazard Cox regression analysis was used for the assessment of mortality; models were adjusted for age, BMI, GOLD group D, active smoking, the change in FVC % predicted, a history of myocardial infarction, and the presence of an airway dominated phenotype. All analyses were performed using the software package SPSS (Version 26, IBM Corp., Armonk, NY, USA). The p values below 0.05 were considered as statistically significant, and 95% confidence intervals (CIs) were given where appropriate. Significance levels are given explicitly, as far as possible, without adjustments for the multiplicity of tests.
Results
Baseline characteristics
CT scans with technically valid values of Pi10 were available in 433 patients of GOLD grades 1–4 (Table 1). Table 1 also shows the prevalence of major comorbidities. The number of patients deceased within 6 years was n = 29.
Table 1.
Baseline characteristics of the study cohort. For categorical data absolute numbers and percentages are given, for continuous variables mean values and standard deviations (SD).
| Parameter | Mean value ± SD or numbers (%) |
|---|---|
| Sex (male/female) | 268/165 (61.9%/38.1%) |
| Age (years) | 66.2 ± 8.2 |
| BMI (kg/m²) | 26.6 ± 4.9 |
| FEV1 % predicted (GLI) | 55.4 ± 18.7 |
| FVC % predicted (GLI) | 82.6 ± 20.3 |
| FEV1/FVC (ratio) | 0.51 ± 0.11 |
| RV/TLC (ratio) | 0.53 ± 0.10 |
| Annual change in FEV1 (% predicted) | −1.07 ± 2.9 |
| Annual change in FVC (% predicted) | −0.42 ± 3.8 |
| Annual change in RV/TLC | 0.004 ± 0.03 |
| Annual decline TLCO (% predicted) | −1.6 ± 4.04 |
| SGRQ total | 39.3 ± 19.2 |
| CAT total | 16.9 ± 7.0 |
| Asthma | 105 (24.2%) |
| Hyperuricemia | 97 (22.4%) |
| Hyperlipidemia | 205 (47.3%) |
| Airway predominance (CT) | 249 (57.5%) |
| Triple therapy | 206 (47.6.0%) |
| LABA + LAMA | 99 (22.9%) |
| Deaths | 29 (6.7%) |
BMI, body mass index; CAT, COPD Assessment Test; CT, computed tomography; FEV 1, forced expiratory volume in 1 second; FVC, forced vital capacity; GLI, Global lung initiative; LABA, long-acting beta-agonists; LAMA, long-acting muscarinic antagonists; RV/TLC, residual volume/total lung capacity; SGRQ, St George’s Respiratory Questionnaire; TLCO diffusing capacity.
Association of Pi10 with COPD characteristics
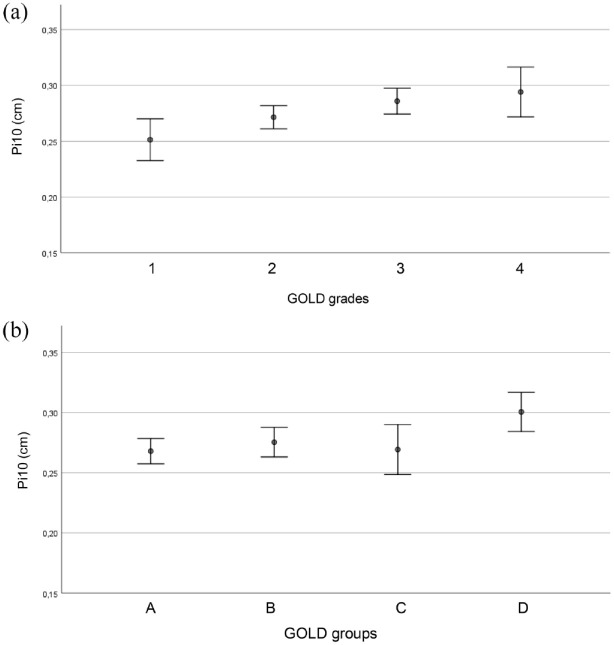
Pi10 was dependent on both GOLD grades 1–4 [p = 0.009, ANOVA, see Figure 2(a)] and GOLD groups A–D [p = 0.008, ANOVA, see Figure 2(b)]. Specifically, grade 1 showed lower values than grades 3–4 (p < 0.05, Duncan), and group D showed higher values of Pi10 than groups A–C (p < 0.05, Duncan). Active smokers had higher values for Pi10 (p = 0.022), while there was no dependence on gender (p = 0.377). For a better description of the dependence on GOLD groups, we performed an ROC analysis of Pi10 regarding the association with GOLD group D. This yielded an optimal cut-off value of 0.26 with an area under the ROC curve (AUC) of 0.628 (95% CI: 0.563–0.694) (p < 0.001).
Figure 2.
(a) Pi10 (vertical axis) for the different GOLD grades (horizontal axis). Mean values and 95% CI intervals are given. (b) Pi10 (vertical axis) for the different GOLD groups (horizontal axis). Mean values and 95% CI intervals are given.
According to linear regression analysis, Pi10 was associated with BMI (p < 0.001) but not age (p = 0.624) Pi10. Higher Pi10 values were associated with higher values of the CAT score (p = 0.006), total SGRQ (p = 0.027), and SGRQ impact sub-score (p = 0.020) but not with the activity and symptom sub-scores. Lower values of FEV1 % predicted (p = 0.002) and FVC % predicted (p < 0.001) were associated with higher Pi10 values, as well as a higher RV/TLC ratio (p = 0.036) and higher KCO % predicted (p < 0.001), but not TLCO % predicted (p = 0.078). The annual decline rates in FEV1 (p = 0.753), TLCO (p = 0.418), and KCO (p = 0.256), each in % predicted, were not significantly associated with Pi10. In contrast, a lower annual decline rate of FVC % predicted (p = 0.015) and a lower annual increase in RV/TLC (p = 0.025) were linked to lower Pi10.
According to the CT evaluation for emphysema, Pi10 values were higher in patients in whom the airway type dominated over the emphysema type (p = 0.002, ANOVA). Correspondingly, Pi10 was higher when mean lung density, the 15th percentile of mean lung density and the inspiratory emphysema index were higher (p < 0.001 each, regression analysis).
According to univariate analyses, Pi10 values were higher in patients with coronary artery disease (p = 0.012) and myocardial infarction (p = 0.001), while there was no dependence on the diagnosis of asthma, chronic bronchitis, gastrointestinal disorders, diabetes, arterial hypertension, heart failure sleep apnoea, hyperlipidemia, hyperuricemia, and osteoporosis.
To identify statistically independent determinants of Pi10, we performed regression analyses with multiple predictors.
The differences regarding GOLD grades and groups remained significant when introducing the lung function decline over time as additional covariate. However, in the multivariate analyses, only the decline in FVC % predicted (p = 0.015) and the increase in RV/TLC (p = 0.012) were significantly associated with Pi10, whereas the decline in FEV1 (p = 0.764), TLCO (p = 0.679), and KCO (p = 0.382), all of them as % predicted, was not. The same was true for changes in SGRQ (p = 0.587).
The association with the airway dominated type (present in 57.5% of patients, see Table 1) or mean lung density remained significant (p < 0.001 each) when GOLD grades and groups, active smoking and the decline in FVC % predicted were introduced as additional predictors. Similarly, the dependence on myocardial infarction remained significant (p = 0.002) when in a multivariate analysis additionally introducing GOLD grades and groups, active smoking status as well as the decline of FVC % predicted. The same was true for coronary artery disease (p = 0.012) but not if coronary artery disease was reported without any previous infarction (p = 0.778).
Association of Pi10 with respiratory COPD medication
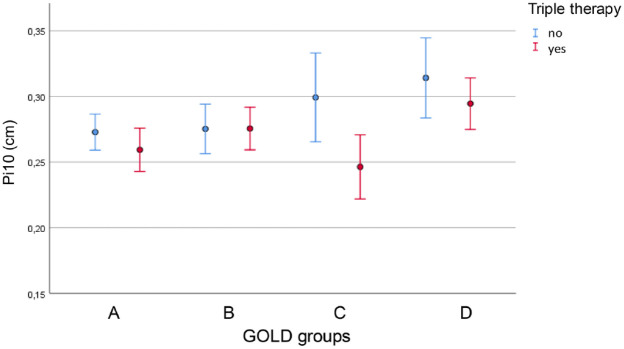
When keeping GOLD grades and GOLD groups as well as the decline in FVC % predicted as predictors of Pi10, there was a significant association with the presence of triple therapy (p = 0.003); remarkably, patients with triple therapy showed lower Pi10 (see Table 2, Figure 3). This result was independent on the inclusion of the anamnestic information on sputum production. There were no significant interaction terms between triple therapy and GOLD groups or grades (p > 0.05). The association of Pi10 with triple therapy was dependent on GOLD groups. When comparing Pi10 in GOLD CD patients with triple therapy versus without, it still showed a significant (p = 0.030) reduction in patients with triple therapy (0.277 cm versus 0.307 cm). This was not the case in GOLD AB patients (0.266 cm versus 0.274 cm).
Table 2.
Association of Pi10 with respiratory COPD medication.
| Predictor | Regression coefficient B | Standard error of B |
p value (compared with ref.) |
95% CI lower | 95% CI upper |
|---|---|---|---|---|---|
| GOLD 1 | ref | ||||
| GOLD 2 | 0.023 | 0.011 | 0.043 | 0.001 | 0.045 |
| GOLD 3 | 0.038 | 0.012 | 0.002 | 0.014 | 0.062 |
| GOLD 4 | 0.043 | 0.016 | 0.009 | 0.011 | 0.076 |
| GOLD A | ref | ||||
| GOLD B | 0.004 | 0.009 | 0.664 | −0.013 | 0.021 |
| GOLD C | 0.002 | 0.011 | 0.847 | −0.020 | 0.024 |
| GOLD D | 0.028 | 0.010 | 0.008 | 0.007 | 0.048 |
| Triple | −0.022 | 0.007 | 0.003 | −0.036 | −0.007 |
| Annual delta FVC % predicted | 0.002 | 0.001 | 0.009 | 0.001 | 0.004 |
COPD, chronic obstructive pulmonary disease; FVC, forced vital capacity.
The table shows the results of multivariate linear regression analysis in terms of non-standardized regression coefficients (B), their standard errors, p values and 95% confidence intervals. All GOLD grades and groups refer to visit 4, the change of FVC % predicted to that between baseline and each patient’s last visit, expressed as % predicted at baseline. Significant differences (p < 0.05) are marked in bold.
Figure 3.
Pi10 (vertical axis) for the different GOLD groups (horizontal axis) and patients with or without triple therapy. Mean values and 95% CI intervals are given.
Similar results were obtained for the combination of LAMA and LABA (irrespective of ICS, p = 0.003) but not LABA + ICS (irrespective of LAMA, p = 0.122).
Associations of Pi10 with mortality
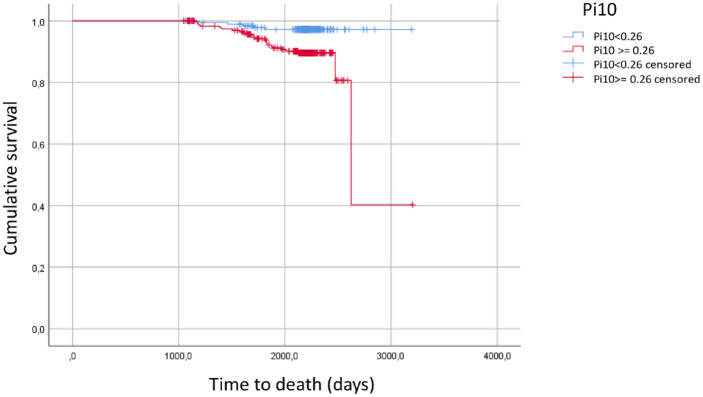
ROC analysis of Pi10 with regard to mortality also revealed an association (p = 0.004), yielding an optimal cut-off value of 0.26 with an AUC of 0.658 (95% CI: 0.571–0.745). Using BMI, active smoking, the change in FVC % predicted, myocardial infarction, the airway dominated phenotype, GOLD group D and additionally age as further predictors, the Pi10 value in terms of the cut-off value 0.26 was still significantly linked to mortality (p = 0.006), with an odds ratio of 4.56 (95% CI: 1.55–13.42) (Table 3 and Figure 4).
Table 3.
Associations of Pi10 with mortality.
| B | SE | p value | Exp(B) | 95% CI for Exp(B) | ||
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Pi10 ⩾ 0.26 cm | 1.518 | 0.550 | 0.006 | 4.562 | 1.551 | 13.418 |
| BMI (kg/m2) | 0.020 | 0.046 | 0.662 | 1.020 | 0.933 | 1.116 |
| Age (years) | 0.102 | 0.030 | 0.001 | 1.107 | 1.043 | 1.175 |
| Current smoking | 0.619 | 0.470 | 0.188 | 1.856 | 0.739 | 4.663 |
| History of myocardial infarction | −1.026 | 0.801 | 0.200 | 0.358 | 0.074 | 1.723 |
| Emphysema versus airway type | 1.082 | 0.421 | 0.010 | 2.951 | 1.292 | 6.740 |
| Annual delta in FVC (% predicted) | −0.089 | 0.042 | 0.033 | 0.915 | 0.843 | 0.993 |
| GOLD D versus ABC | 0.490 | 0.437 | 0.263 | 1.632 | 0.693 | 3.842 |
CT, computed tomography; BMI, body mass index; CI, confidence interval; FVC, forced vital capacity.
The table shows the results of Cox proportional hazards regression analysis for Pi10 ⩾ 0.26 cm as predictor. Further potential predictors are shown for comparison: BMI, age, active smoking, a history of myocardial infarction, the CT-derived emphysema versus airway dominated phenotype and GOLD group D versus ABC. Significant differences (p < 0.05) are marked in bold. For all predictors, the regression coefficients (B), their standard errors (SE), p values, the derived odds ratios [exp(B)] and their 95% confidence intervals are shown.
Figure 4.
Kaplan–Meier estimates of the study cohort regarding mortality and depicting survival for patients having values of Pi10 of at least 0.26 cm versus patients with values below 0.26 cm.
Discussion
The present analysis aimed to elucidate the role of Pi10, an easily derived morphological parameter from routine chest CT scans, in patients with COPD. We found that for practical purposes a cut-off value of 0.26 cm was suitable to provide additional information on exacerbation risk and mortality, even when other, known predictors were taken into account. Interestingly, a reduced Pi10 value was linked to triple therapy even after adjusting for clinical correlates of this therapy, suggesting a genuine morphological effect of triple therapy. Although these conclusions are limited by the cross-sectional nature of the analysis, they indicate a potential clinical value of Pi10 as an easily available biomarker, which can be automatically extracted from clinical chest CT scans for COPD phenotyping.
Pi10 measurements may depend on the algorithms and definitions used in the CT evaluation.34 We used the YACTA algorithm for the investigation of airway parameters (total diameter, luminal area, wall area and the ratio of wall area to the sum of wall and lumen area) for the first to eighth generations – but Pi10 calculation in our study was done based on all segmented airways, according to previously published data of our group.32 As shown previously, the average highest airway generation showed low variability between the different patient groups and time points.32 It is important to note that the definition of Pi10 does not depend on the specific attribution of airway generation number but only describes the relationship between airway wall thickness against internal diameter from a regression analysis of both parameters. In the regression analysis, generation number does not explicitly enter and the computation is valid as far as the interpolated value corresponding to 10-mm internal perimeter is within the range of airway generations measured. The fact that different numbers of generations can be determined in COPD patients of different severity should therefore only affect the signal-to-noise ratio, as from a broader range of perimeters a more reliable regression analysis results.
Higher Pi10 were associated with a higher symptom burden and lower disease-specific quality of life, in line with the results of previous investigations.34,35 Pi10 was also associated with the CT-morphological, functional, and clinical picture of an airway-dominated phenotype.36 This concordance with the literature suggests that the CT scans used by us were of comparable quality, and that thus the additional information on the clinical value of Pi10 was not specific for COSYCONET.
Previous studies addressing the association between Pi10 and lung function decline were limited to patients without pre-existing chronic obstructive pulmonary disease.12 Pi10 was associated with airway obstruction in terms of the annual fall of FEV1.11,12 In the present study, we also evaluated the lung function decline in association with Pi10 but included patients with COPD. We found the annual decline of FVC and the annual increase of the ratio RV/TLC to be linked to Pi10; this was not the case for the changes in FEV1 or diffusing capacity (TLCO). The change was computed between visit 1 and visit 5 to cover the CT study visit which occurred between visits 4 and 5. FVC and RV/TLC both reflect the lung volume available for breathing, which is critically dependent on the function of the small airways, while FEV1 more reflects the central airways and diffusing capacity the alveolar space. FEV1 also depends stronger than FVC on the occurrence of acute bronchoconstriction. From this perspective, it seems not unplausible that we observed the changes of FVC, and not of FEV1, as correlate of morphological changes assessed via Pi10.
We also addressed the potential role of inhaled triple therapy for Pi10. Triple therapy was more prevalent in patients with increased symptoms and exacerbations (GOLD groups C and D), particularly in group D. Group D also showed an elevated value of Pi10. On the contrary, when including all GOLD groups, triple therapy was linked to a lower value of Pi10. The association with triple therapy depended on GOLD groups and was present in groups CD but not AB, suggesting that triple therapy was particularly effective in patients with increased rate and/or severity of exacerbations.
A recent study found that even patients with no or minor previous history of exacerbations had a significant risk for future exacerbations.37 Owing to the importance of exacerbations, all easy-to-obtain parameters comprising information on this risk are of interest. In the present study, we identified Pi10 as a potentially useful parameter for the quantification of exacerbation risk, with a cut-off value of 0.26 cm. Our findings regarding triple therapy also suggest that Pi10 might be helpful in the decision for early treatment escalation, especially in patients of GOLD group D.
Pi10, as a measure of small airway alterations, was associated with the presence of coronary artery disease and a previous history of myocardial infarction. Data from the SPIROMICS cohort showed an association between Pi10 and higher coronary calcium load,38 although these associations were dependent on the use of multivariable adjustment. The underlying pathomechanisms for the association between COPD and coronary artery disease probably include oxidative stress, systemic inflammation, increased thrombogenicity, impaired vascular function, and hypoxemia.39,40 An interesting pathway could be the reduction of CXCL5 affecting cholesterol efflux,41 thereby increasing atherogenesis41 and potentially explaining the association between airway obstruction and coronary artery disease. If Pi10 was higher than the cut-off value of 0.26 cm, mortality risk increased by a factor of 4.5. As this result was obtained accounting for additional predictors such as age, previous myocardial infarction, FVC decline and GOLD group D, it indicates an independent effect of changes in airway morphology on mortality risk.
Considering the pattern of associations found in this observational study, the results appear compatible with those of randomized controlled trials, such as the ETHOS study, which found a reduction of overall mortality, particularly due to cardiovascular causes, in patients with triple therapy.42 Our findings suggest that beneficial effects on airway morphology may play a role in this, either as direct factor regarding the lung or as marker of indirect positive effects on the heart.
Limitations
While the cross-sectional study design showed a correlation between Pi10, risk of exacerbation and triple therapy, direct causal relationships cannot be derived, although we also used data on mortality. In the follow-up visits, overall mortality was not very high, but due to the large study cohort and a long observation period of 6 years, a mortality analysis could still be performed. Another limitation of this study was the missing information on the cause of death, thus mortality had to be analyzed as all-cause mortality. The information on the presence of comorbidities was based on the physician-based diagnoses that were recorded in a structured interview and not verified by independent assessments; however, in previous studies this did not appear to affect the validity of the analyses.21,43,44 It is clear that the results regarding triple therapy merely represent an association, in particular as the presence of this therapy could depend on symptoms of bronchitis. However, when introducing symptoms such as sputum production as additional predictors, the association with triple was not affected.
Conclusion
These observational data suggest that Pi10, as determined from routine CT chest imaging using an automated processing software, provided information on the future clinical course of COPD patients. Pi10 was independently associated with both, exacerbations and mortality, in addition to other, established predictors. For this purpose, a cut-off value of 0.26 cm could be used. The additional result on the lower value of Pi10 in patients with triple therapy suggests a potential, unrecognized benefit of this therapy, suggesting that Pi10 might be implemented in randomized trials addressing appropriateness and escalation of COPD therapy.
Supplemental Material
Supplemental material, sj-docx-2-tar-10.1177_17534666221148663 for Standardized airway wall thickness Pi10 from routine CT scans of COPD patients as imaging biomarker for disease severity, lung function decline, and mortality by Kathrin Kahnert, Rudolf A. Jörres, Hans-Ulrich Kauczor, Peter Alter, Franziska C. Trudzinski, Felix Herth, Bertram Jobst, Oliver Weinheimer, Sebastian Nauck, Pontus Mertsch, Diego Kauffmann-Guerrero, Jürgen Behr, Robert Bals, Henrik Watz, Klaus F. Rabe, Tobias Welte, Claus F. Vogelmeier and Jürgen Biederer in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-1-tar-10.1177_17534666221148663 for Standardized airway wall thickness Pi10 from routine CT scans of COPD patients as imaging biomarker for disease severity, lung function decline, and mortality by Kathrin Kahnert, Rudolf A. Jörres, Hans-Ulrich Kauczor, Peter Alter, Franziska C. Trudzinski, Felix Herth, Bertram Jobst, Oliver Weinheimer, Sebastian Nauck, Pontus Mertsch, Diego Kauffmann-Guerrero, Jürgen Behr, Robert Bals, Henrik Watz, Klaus F. Rabe, Tobias Welte, Claus F. Vogelmeier and Jürgen Biederer in Therapeutic Advances in Respiratory Disease
Acknowledgments
We are grateful to the COSYCONET study group and study centers who contributed in patient recruitment and data collection, as well as to all patients participating in this study.
COSYCONET Study Group: Andreas, Stefan (Lungenfachklinik, Immenhausen); Bals, Robert (Universitätsklinikum des Saarlandes); Behr, Jürgen and Kahnert, Kathrin (Klinikum der Ludwig-Maximilians-Universität München); Bewig, Burkhard (Universitätsklinikum Schleswig Holstein); Buhl, Roland (Universitätsmedizin der Johannes-Gutenberg-Universität Mainz); Ewert, Ralf and Stubbe, Beate (Universitätsmedizin Greifswald); Ficker, Joachim H. (Klinikum Nürnberg, Paracelsus Medizinische Privatuniversität Nürnberg); Gogol, Manfred (Institut für Gerontologie, Universität Heidelberg); Grohé, Christian (Ev. Lungenklinik Berlin); Hauck, Rainer (Kliniken Südostbayern AG, Kreisklinik Bad Reichenhall); Held, Matthias and Jany, Berthold (Klinikum Würzburg Mitte gGmbH, Standort Missioklinik); Henke, Markus (Asklepios Fachkliniken München-Gauting); Herth, Felix (Thoraxklinik Heidelberg gGmbH); Höffken, Gerd (Fachkrankenhaus Coswig GmbH); Katus, Hugo A. (Universitätsklinikum Heidelberg); Kirsten, Anne-Marie and Watz, Henrik (Pneumologisches Forschungsinstitut an der Lungenclinic Grosshansdorf GmbH); Koczulla, Rembert and Kenn, Klaus (Schön Klinik Berchtesgadener Land); Kronsbein, Juliane (Berufsgenossenschaftliches Universitätsklinikum Bergmannsheil, Bochum); Kropf-Sanchen, Cornelia (Universitätsklinikum Ulm); Lange, Christoph and Zabel, Peter (Forschungszentrum Borstel); Pfeifer, Michael (Klinik Donaustauf); Randerath, Winfried J. (Wissenschaftliches Institut Bethanien e. V., Solingen); Seeger, Werner (Justus-Liebig-Universität Gießen); Studnicka, Michael (Uniklinikum Salzburg); Taube, Christian and Teschler, Helmut (Ruhrlandklinik gGmbH Essen); Timmermann, Hartmut (Hamburger Institut für Therapieforschung GmbH); Virchow, J. Christian (Universitätsklinikum Rostock); Vogelmeier, Claus (Universitätsklinikum Gießen und Marburg GmbH, Standort Marburg); Wagner, Ulrich (Klinik Löwenstein gGmbH); Welte, Tobias (Medizinische Hochschule Hannover); Wirtz, Hubert (Universitätsklinikum Leipzig).
Footnotes
ORCID iDs: Kathrin Kahnert  https://orcid.org/0000-0001-9633-3368
https://orcid.org/0000-0001-9633-3368
Klaus F. Rabe  https://orcid.org/0000-0002-7020-1401
https://orcid.org/0000-0002-7020-1401
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Kathrin Kahnert, Department of Medicine V, Comprehensive Pneumology Center, Member of the German Center for Lung Research (DZL), University Hospital, LMU Munich, Ziemssenstr. 5, Munich 80336, Germany.
Rudolf A. Jörres, Institute and Outpatient Clinic for Occupational, Social and Environmental Medicine, Comprehensive Pneumology Center Munich (CPC-M), Member of the German Center for Lung Research (DZL), Ludwig-Maximilians-Universität München, Munich, Germany
Hans-Ulrich Kauczor, Department of Diagnostic and Interventional Radiology, University Hospital of Heidelberg, Heidelberg, Germany; Translational Lung Research Centre Heidelberg (TLRC), Member of the German Center for Lung Research, Heidelberg, Germany.
Peter Alter, Department of Medicine, Pulmonary and Critical Care Medicine, University Medical Center Giessen and Marburg, Philipps-University Marburg, Member of the German Center for Lung Research (DZL), Marburg, Germany.
Franziska C. Trudzinski, Thoraxklinik-Heidelberg gGmbH, Translational Lung Research Centre Heidelberg (TLRC), Member of the German Center for Lung Research (DZL), Heidelberg, Germany.
Felix Herth, Thoraxklinik-Heidelberg gGmbH, Translational Lung Research Centre; Heidelberg (TLRC), Member of the German Center for Lung Research (DZL), Heidelberg, Germany.
Bertram Jobst, Department of Diagnostic and Interventional Radiology, University Hospital of Heidelberg, Heidelberg, Germany; Translational Lung Research Centre Heidelberg (TLRC), Member of the German Center for Lung Research, Heidelberg, Germany.
Oliver Weinheimer, Department of Diagnostic and Interventional Radiology, University Hospital of Heidelberg, Heidelberg, Germany; Translational Lung Research Centre Heidelberg (TLRC), Member of the German Center for Lung Research, Heidelberg, Germany.
Sebastian Nauck, Department of Diagnostic and Interventional Radiology, University Hospital of Heidelberg, Heidelberg, Germany; Translational Lung Research Centre Heidelberg (TLRC), Member of the German Center for Lung Research, Heidelberg, Germany.
Pontus Mertsch, Department of Medicine V, Comprehensive Pneumology Center, Member of the German Center for Lung Research (DZL), University Hospital, LMU Munich, Munich, Germany.
Diego Kauffmann-Guerrero, Department of Medicine V, Comprehensive Pneumology Center, Member of the German Center for Lung Research (DZL), University Hospital, LMU Munich, Munich, Germany.
Jürgen Behr, Department of Medicine V, Comprehensive Pneumology Center, Member of the German Center for Lung Research (DZL), University Hospital, LMU Munich, Munich, Germany.
Robert Bals, Department of Internal Medicine V – Pulmonology, Allergology, Respiratory Intensive Care Medicine, Saarland University Hospital, Homburg, Germany; Helmholtz Institute for Pharmaceutical Research Saarland (HIPS), Helmholtz Centre for Infection Research (HZI), Saarland University Campus, Saarbrücken, Germany.
Henrik Watz, Pulmonary Research Institute at LungenClinic Grosshansdorf, Airway Research Center North (ARCN), Member of the German Center for Lung Research (DZL), Grosshansdorf, Germany.
Klaus F. Rabe, Lung Clinic Grosshansdorf, Airway Research Center (ARCN), Grosshansdorf, German Faculty of Medicine, Christian-Albrechts-Universität zu Kiel, Kiel, Germany.
Tobias Welte, Department of Pneumology, Hannover Medical School, Hannover, Germany.
Claus F. Vogelmeier, Department of Medicine, Pulmonary and Critical Care Medicine, University Medical Center Giessen and Marburg, Philipps-University Marburg, Member of the German Center for Lung Research (DZL), Marburg, Germany
Jürgen Biederer, Department of Diagnostic and Interventional Radiology, University Hospital of Heidelberg, Heidelberg, Germany; Translational Lung Research Centre Heidelberg (TLRC), Member of the German Center for Lung Research, Heidelberg, Germany; Faculty of Medicine, Christian-Albrechts-Universität zu Kiel, Kiel, Germany; University of Latvia, Faculty of Medicine, Raina bulvaris 19, Riga, LV-1586 Latvia.
Declarations
Ethics approval: The study was based on 2741 patients recruited within the COSYCONET framework (ClinicalTrials.gov, Identifier: NCT01245933).
For further information see:
Karch A, Vogelmeier C, Welte T, Bals R, Kauczor HU, Biederer J, Heinrich J, Schulz H, Glaser S, Holle R et al.: The German COPD cohort COSYCONET: Aims, methods and descriptive analysis of the study population at baseline. Respir Med 2016, 114:27–37.
All patients gave their written informed consent to participation in the study including the assessment of CT scans. Within the ethical approval, the participants of the study gave their informed consent to publish the data collected during the study period.
Consent for publication: Within the ethical approval, the participants of the study gave their informed consent to publish the data collected during the study period.
Authors contributions: Kathrin Kahnert: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Visualization; Writing – original draft
Rudolf A. Jörres: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Writing – original draft
Hans-Ulrich Kauczor: Methodology; Resources; Software; Validation; Writing – review & editing
Peter Alter: Conceptualization; Formal analysis; Writing – review & editing
Franziska C. Trudzinski: Formal analysis; Investigation; Writing – review & editing
Felix Herth: Investigation; Visualization; Writing – review & editing
Bertram Jobst: Data curation; Software; Writing – review & editing
Oliver Weinheimer: Software; Validation; Visualization; Writing – review & editing
Sebastian Nauck: Software; Visualization; Writing – review & editing
Pontus Mertsch: Visualization; Writing – review & editing
Diego Kauffmann-Guerrero: Formal analysis; Writing – review & editing
Jürgen Behr: Conceptualization; Writing – review & editing
Robert Bals: Methodology; Visualization; Writing – review & editing
Henrik Watz: Data curation; Writing – review & editing
Klaus F. Rabe: Methodology; Validation; Writing – review & editing
Tobias Welte: Funding acquisition; Methodology; Writing – review & editing
Claus F. Vogelmeier: Conceptualization; Methodology; Supervision; Writing – review & editing
Jürgen Biederer: Conceptualization; Validation; Writing – review & editing
Third-party submissions: Not applicable.
Writing assistance: Not applicable.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work is supported by the German Centre for Lung Research (DZL), grant number 82DZLI05A2 (COSYCONET), the BMBF, grant number FKZ 01GI0881, and is furthermore supported by unrestricted grants from AstraZeneca GmbH, Boehringer Ingelheim Pharma GmbH & Co. KG, GlaxoSmithKline, Grifols Deutschland GmbH, Novartis Deutschland GmbH. The funding body was not involved in the design of the study, or the collection, analysis or interpretation of the data.
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: KK reports personal fees from Astra Zeneca and GSK outside the submitted work. RJ has nothing to disclose. HUK grants and personal fees from Bayer, Siemens, Boehringer Ingelheim, Philips, MSD, Sanofi and Median outside the submitted work. PA has nothing to disclose with regard to this manuscript. FCT reports personal fees from Boehringer Ingelheim, CSL Behring, Chiesi, GSK, Grifols, Novartis outside the submitted work. FH has nothing to disclose with regard to this manuscript. BJ has nothing to disclose with regard to this manuscript. OW reports that parts of the airways analysis algorithm have been licensed to the company Imbio, LLC. SN has nothing to disclose with regard to this manuscript. PM has nothing to disclose with regard to this manuscript. DK has nothing to disclose with regard to this manuscript. JB has nothing to disclose with regard to this manuscript. RB reports grants and personal fees from AstraZeneca, grants and personal fees from Boehringer Ingelheim, personal fees from GlaxoSmithKline, personal fees from Grifols, grants and personal fees from Novartis, personal fees from CSL Behring, grants from German Federal Ministry of Education and Research (BMBF) Competence Network Asthma and COPD (ASCONET), grants from Sander Stiftung, grants from Schwiete Stiftung, grants from Krebshilfe, grants from Mukoviszidose eV, outside the submitted work. HW has nothing to disclose with regard to this manuscript. JB has nothing to disclose with regard to this manuscript. KFR reports personal fees from Astra Zeneca, Boehringer Ingelheim, Chiesi Pharmaceuticals, Novartis, Sanofi & Regeneron, GlaxoSmithKline, Berlin Chemie, Roche Pharma, as well as leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid for German Center for Lung Research (DZL), German Chest Society (DGP), American Thoracic Society (ATS) outside the submitted work. TW reports grants from German Federal Ministry of Education and Research (BMBF). CFV reports personal fees from Aerogen, AstraZeneca, Boehringer Ingelheim, CSL Behring, Chiesi, GlaxoSmithKline, Insmed, Menarini, Novartis, Nuvaira, Novartis outside the submitted work, as well as grants for the institution from German Federal Ministry of Education and Research (BMBF) and AstraZeneca, Boehringer Ingelheim, Chiesi, CSL Behring, GlaxoSmithKline, Grifols and Novartis. JBi reports leadership of the ESTI (European Society of Thoracic Imaging) president 2022/2023 (unpaid) and outside the submitted work.
Availability of data and materials: Data may be obtained from a third party and are not publicly available. The full dataset supporting the conclusions of this article is available upon request and application from the Competence Network Asthma and COPD (ASCONET, http://www.asconet.net/html/cosyconet/projects).
References
- 1. Global initiative for chronic obstructive lung disease, 2022. [Google Scholar]
- 2. Lindberg A, Sawalha S, Hedman L, et al. Subjects with COPD and productive cough have an increased risk for exacerbations and death. Respir Med 2015; 109: 88–95. [DOI] [PubMed] [Google Scholar]
- 3. Kunisaki KM, Dransfield MT, Anderson JA, et al. Exacerbations of chronic obstructive pulmonary disease and cardiac events. A post hoc cohort analysis from the SUMMIT randomized clinical trial. Am J Respir Crit Care Med 2018; 198: 51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rothnie KJ, Mullerova H, Smeeth L, et al. Natural history of chronic obstructive pulmonary disease exacerbations in a general practice-based population with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2018; 198: 464–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Telenga ED, Oudkerk M, van Ooijen PM, et al. Airway wall thickness on HRCT scans decreases with age and increases with smoking. BMC Pulm Med 2017; 17: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arakawa H, Fujimoto K, Fukushima Y, et al. Thin-section CT imaging that correlates with pulmonary function tests in obstructive airway disease. Eur J Radiol 2011; 80: e157–e163. [DOI] [PubMed] [Google Scholar]
- 7. Nakano Y, Muro S, Sakai H, et al. Computed tomographic measurements of airway dimensions and emphysema in smokers. Correlation with lung function. Am J Respir Crit Care Med 2000; 162: 1102–1108. [DOI] [PubMed] [Google Scholar]
- 8. Hasegawa M, Nasuhara Y, Onodera Y, et al. Airflow limitation and airway dimensions in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2006; 173: 1309–1315. [DOI] [PubMed] [Google Scholar]
- 9. Johannessen A, Skorge TD, Bottai M, et al. Mortality by level of emphysema and airway wall thickness. Am J Respir Crit Care Med 2013; 187: 602–608. [DOI] [PubMed] [Google Scholar]
- 10. Grydeland TB, Dirksen A, Coxson HO, et al. Quantitative computed tomography: emphysema and airway wall thickness by sex, age and smoking. Eur Respir J 2009; 34: 858–865. [DOI] [PubMed] [Google Scholar]
- 11. Mohamed Hoesein FA, de Jong PA, Lammers JW, et al. Airway wall thickness associated with forced expiratory volume in 1 second decline and development of airflow limitation. Eur Respir J 2015; 45: 644–651. [DOI] [PubMed] [Google Scholar]
- 12. Oelsner EC, Smith BM, Hoffman EA, et al. Prognostic significance of large airway dimensions on computed tomography in the general population. The multi-ethnic study of atherosclerosis (MESA) lung study. Ann Am Thorac Soc 2018; 15: 718–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim V, Han MK, Vance GB, et al. The chronic bronchitic phenotype of COPD: an analysis of the COPDGene study. Chest 2011; 140: 626–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Martinez CH, Chen YH, Westgate PM, et al. Relationship between quantitative CT metrics and health status and BODE in chronic obstructive pulmonary disease. Thorax 2012; 67: 399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xie X, Dijkstra AE, Vonk JM, et al. Chronic respiratory symptoms associated with airway wall thickening measured by thin-slice low-dose CT. AJR Am J Roentgenol 2014; 203: W383–W390. [DOI] [PubMed] [Google Scholar]
- 16. Karch A, Vogelmeier C, Welte T, et al. The German COPD cohort COSYCONET: aims, methods and descriptive analysis of the study population at baseline. Respir Med 2016; 114: 27–37. [DOI] [PubMed] [Google Scholar]
- 17. Karch A, Vogelmeier C, Welte T, et al. The German COPD cohort COSYCONET: aims, methods and descriptive analysis of the study population at baseline. Respir Med 2016; 114: 27–37. [DOI] [PubMed] [Google Scholar]
- 18. Kahnert K, Jorres RA, Kauczor HU, et al. Relationship between clinical and radiological signs of bronchiectasis in COPD patients: results from COSYCONET. Respir Med 2020; 172: 106117. [DOI] [PubMed] [Google Scholar]
- 19. Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J 2012; 40: 1324–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stanojevic S, Graham BL, Cooper BG, et al. Official ERS technical standards: global lung function initiative reference values for the carbon monoxide transfer factor for Caucasians. Eur Respir J 2017; 50: 1700010. [DOI] [PubMed] [Google Scholar]
- 21. Kahnert K, Andreas S, Kellerer C, et al. Reduced decline of lung diffusing capacity in COPD patients with diabetes and metformin treatment. Sci Rep 2022; 12: 1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lutter JI, Jorres RA, Welte T, et al. Impact of education on COPD severity and all-cause mortality in lifetime never-smokers and longtime ex-smokers: results of the COSYCONET cohort. Int J Chron Obstruct Pulmon Dis 2020; 15: 2787–2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Marietta von Siemens S, Alter P, Lutter JI, et al. CAT score single item analysis in patients with COPD: results from COSYCONET. Respir Med 2019; 159: 105810. [DOI] [PubMed] [Google Scholar]
- 24. Biederer JFS, Tuengerthal S, Rehbock B. Protocol recommendations for computed tomography of the lung: consensus of the chest imaging workshop of the German radiologic society. Röfo 2008; 5: 1–24. [Google Scholar]
- 25. COPDGene CT Workshop Group, Barr RG, Berkowitz EA, et al. A combined pulmonary-radiology workshop for visual evaluation of COPD: study design, chest CT findings and concordance with quantitative evaluation. COPD 2012; 9: 151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kahnert K, Jobst B, Biertz F, et al. Relationship of spirometric, body plethysmographic, and diffusing capacity parameters to emphysema scores derived from CT scans. Chron Respir Dis 2019; 16: 1479972318775423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kellerer C, Jorres RA, Schneider A, et al. Prediction of lung emphysema in COPD by spirometry and clinical symptoms: results from COSYCONET. Respir Res 2021; 22: 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wielputz MO, Eichinger M, Weinheimer O, et al. Automatic airway analysis on multidetector computed tomography in cystic fibrosis: correlation with pulmonary function testing. J Thorac Imaging 2013; 28: 104–113. [DOI] [PubMed] [Google Scholar]
- 29. Achenbach T, Weinheimer O, Brochhausen C, et al. Accuracy of automatic airway morphometry in computed tomography-correlation of radiological-pathological findings. Eur J Radiol 2012; 81: 183–188. [DOI] [PubMed] [Google Scholar]
- 30. Weinheimer O, Achenbach T, Bletz C, et al. About objective 3-d analysis of airway geometry in computerized tomography. IEEE Trans Med Imaging 2008; 27: 64–74. [DOI] [PubMed] [Google Scholar]
- 31. Leutz-Schmidt P, Weinheimer O, Jobst BJ, et al. Influence of exposure parameters and iterative reconstruction on automatic airway segmentation and analysis on MDCT – an ex vivo phantom study. PLoS ONE 2017; 12: e0182268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jobst BJ, Weinheimer O, Buschulte T, et al. Longitudinal airway remodeling in active and past smokers in a lung cancer screening population. Eur Radiol 2019; 29: 2968–2980. [DOI] [PubMed] [Google Scholar]
- 33. Weinheimer O, Wielpütz MO, Konietzke P, et al. Fully automated lobe-based airway taper index calculation in a low dose MDCT CF study over 4 time-points. In: Proceedings of the SPIE 10133, Medical Imaging 2017: Image Processing, Orlando, FL, USA, 2017. [Google Scholar]
- 34. Smith BM, Hoffman EA, Rabinowitz D, et al. Comparison of spatially matched airways reveals thinner airway walls in COPD. The Multi-Ethnic Study of Atherosclerosis (MESA) COPD study and the subpopulations and intermediate outcomes in COPD study (SPIROMICS). Thorax 2014; 69: 987–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Charbonnier JP, Pompe E, Moore C, et al. Airway wall thickening on CT: relation to smoking status and severity of COPD. Respir Med 2019; 146: 36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Han MK, Kazerooni EA, Lynch DA, et al. Chronic obstructive pulmonary disease exacerbations in the COPDGene study: associated radiologic phenotypes. Radiology 2011; 261: 274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kellerer C, Kahnert K, Trudzinski FC, et al. COPD maintenance medication is linked to left atrial size: results from the COSYCONET cohort. Respir Med 2021; 185: 106461. [DOI] [PubMed] [Google Scholar]
- 38. Bhatt SP, Nath HP, Kim YI, et al. Centrilobular emphysema and coronary artery calcification: mediation analysis in the SPIROMICS cohort. Respir Res 2018; 19: 257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Boschetto P, Beghé B, Fabbri LM, et al. Link between chronic obstructive pulmonary disease and coronary artery disease: implication for clinical practice. Respirology 2012; 17: 422–431. [DOI] [PubMed] [Google Scholar]
- 40. Macnee W, Maclay J, McAllister D. Cardiovascular injury and repair in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2008; 5: 824–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rousselle A, Qadri F, Leukel L, et al. CXCL5 limits macrophage foam cell formation in atherosclerosis. J Clin Invest 2013; 123: 1343–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Martinez FJ, Rabe KF, Ferguson GT, et al. Reduced all-cause mortality in the ETHOS trial of budesonide/glycopyrrolate/formoterol for chronic obstructive pulmonary disease. A randomized, double-blind, multicenter, parallel-group study. Am J Respir Crit Care Med 2021; 203: 553–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kahnert K, Lucke T, Biertz F, et al. Transfer factor for carbon monoxide in patients with COPD and diabetes: results from the German COSYCONET cohort. Respir Res 2017; 18: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Trudzinski FC, Jorres RA, Alter P, et al. Sex-specific associations of comorbidome and pulmorbidome with mortality in chronic obstructive pulmonary disease: results from COSYCONET. Sci Rep 2022; 12: 8790. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-2-tar-10.1177_17534666221148663 for Standardized airway wall thickness Pi10 from routine CT scans of COPD patients as imaging biomarker for disease severity, lung function decline, and mortality by Kathrin Kahnert, Rudolf A. Jörres, Hans-Ulrich Kauczor, Peter Alter, Franziska C. Trudzinski, Felix Herth, Bertram Jobst, Oliver Weinheimer, Sebastian Nauck, Pontus Mertsch, Diego Kauffmann-Guerrero, Jürgen Behr, Robert Bals, Henrik Watz, Klaus F. Rabe, Tobias Welte, Claus F. Vogelmeier and Jürgen Biederer in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-1-tar-10.1177_17534666221148663 for Standardized airway wall thickness Pi10 from routine CT scans of COPD patients as imaging biomarker for disease severity, lung function decline, and mortality by Kathrin Kahnert, Rudolf A. Jörres, Hans-Ulrich Kauczor, Peter Alter, Franziska C. Trudzinski, Felix Herth, Bertram Jobst, Oliver Weinheimer, Sebastian Nauck, Pontus Mertsch, Diego Kauffmann-Guerrero, Jürgen Behr, Robert Bals, Henrik Watz, Klaus F. Rabe, Tobias Welte, Claus F. Vogelmeier and Jürgen Biederer in Therapeutic Advances in Respiratory Disease