Abstract
Background
There has been an increasing need to acquire rigorous scientific data to answer the concerns of physicians, patients, and the FDA regarding the self-reported illness identified as breast implant illness (BII). There are no diagnostic tests or specific laboratory values to explain the reported systemic symptoms described by these patients.
Objectives
The aim of this study was to determine if there are quantifiable laboratory findings that can be identified in blood, capsule tissue pathology, or microbes that differentiate women with systemic symptoms they attribute to their implants from 2 control groups.
Methods
A prospective blinded study enrolled 150 subjects into 3 cohorts: (A) women with systemic symptoms they attribute to implants who requested implant removal; (B) women with breast implants requesting removal or exchange who did not have symptoms attributed to implants; and (C) women undergoing cosmetic mastopexy who have never had any implanted medical device. Capsule tissue underwent detailed analysis and blood was sent from all 3 cohorts to evaluate for markers of inflammation.
Results
No significant histologic differences were identified between the cohorts, except there were more capsules with synovial metaplasia in the non-BII cohort. There was no statistical difference in thyroid-stimulating hormone, vitamin D levels, or complete blood count with differential between the cohorts. Next-generation sequencing revealed no statistically significant difference in positivity between Cohort A and B. Of the 12 cytokines measured, 3 cytokines, interleukin (IL)-17A, IL-13, and IL-22, were found to be significantly more often elevated in sera of subjects in Cohort A than in Cohorts B or C. The enterotoxin data demonstrated an elevation in immunoglobulin G (IgG) anti–Staphylococcus aureus enterotoxin A in Cohort A. There was no correlation between the presence of IgE or IgG anti-Staphylococcal antibody and a positive next-generation sequencing result.
Conclusions
This study adds to the current literature by demonstrating few identifiable biomedical markers to explain the systemic symptoms self-reported by patients with BII.
[Translated to Chinese, below:]摘要
背景
获得严格的科学数据来回答医生、患者和 FDA 对自我报告的乳房假体植入相关疾病 (BII) 关切的需求日益增加。尚无诊断性试验和特定的实验室测定值来解释这些患者所描述的主诉全身症状。
目标
本研究旨在确定是否可以在血液、囊组织病理学或微生物中识别出的可量化实验室结果, 将患有可归因于植入物的全身症状女性患者与 2 个对照组的患者区分开来。
方法
这项前瞻性盲法研究招募 150 名受试者, 并纳入 3 个队列的试验组: (A) 要求取出植入物的女性患者, 其具有自身归因于植入物的全身症状; (B) 要求取出或更换乳房植入物的女性, 其没有归因于植入物的症状; (C) 接受美容乳房固定术的女性患者, 其从未植入任何医疗器械。本研究对囊组织进行了详细分析, 并从所有 3 个队列收集血液, 以评估炎症标志物。
结果
除了在非 BII 队列中有更多滑膜样化生的囊组织外, 各队列之间没有发现显著的组织学差异。各队列在促甲状腺激素、维生素 D 水平或全血细胞分类计数方面没有统计学差异。下一代测序揭示, 队列 A、B 之间的阳性率差异无统计学意义。在测量的 12个细胞因子中, 白细胞介素 (IL) -17A、IL-13 和 IL-22 这 3 个细胞因子在队列 A 受试者血清中升高的频率显著高于队列 B、C。肠毒素数据表明, 队列 A 中免疫球蛋白G (IgG) 抗金黄色葡萄球菌肠毒素 A 升高。IgE 或 IgG 抗葡萄球菌抗体的存在与下一代测序阳性结果之间无相关性。
结论
本研究通过证实几乎没有可识别的生物医学标志物来解释 BII 患者主诉的全身症状, 进一步补充了现有文献。
Level of Evidence: 4

Breast implant illness (BII) describes a variety of symptoms reported by patients that they attribute to their implants. Over 100 symptoms have been reported in no specific configuration. A variety of potential causes have been postulated, including heavy metals in the implants and the potential for biofilms. The previously published Part 2 of the Systemic Symptoms Biospecimen Analysis Study showed that heavy metals contained within implant capsules are well below what are considered safe levels of exposure, and those that were higher in the symptomatic cohort could be attributed to lifestyle differences and potential environmental exposures and are unlikely to be a cause of symptoms.1
Subclinical infections, specifically biofilms, have been reported with medical devices and have been suggested as a potential contributing factor in capsular contracture and in the development of breast implant–associated anaplastic large cell lymphoma.2–4 One of the few published prospective studies on BII cultured the capsules from patients undergoing explantation. In this study, cultures were positive for Cutibacterium acnes in 39% of the BII subjects compared with less than 10% of historical control patients.5 We report here the results of next-generation sequencing (NGS) for bacterial and fungal DNA analysis to see if the capsule and/or surface of the implant indicated the presence of bacteria or fungus to determine if subclinical infection or biofilms play a role in the development of systemic symptoms. To further explore the potential role of bacteria and inflammation in patients with self-described breast implant illness, we also examined peripheral blood for the presence 12 cytokines and immunoglobulin G (IgG) and IgE antibodies to Staphylococcal enterotoxins.
The patients in Cohort A (see below) self-reported a wide variety of nonspecific symptoms with many potential causes. A previous prospective study showed a higher incidence of synovial metaplasia in the capsules of patients undergoing explantation for systemic symptoms compared to a historic control group.5 Peripheral blood was collected from all 3 cohorts to analyze for any statistical differences in complete blood count (CBC), vitamin D, C-reactive protein (CRP), and thyroid levels. Vitamin D deficiency affects up to 40% of women in the United States and can cause many of the symptoms reported in women with self-described BII. Thyroid disease is commonly reported by women with BII and could also produce many of the symptoms reported, so this was included in the evaluation. This study was funded entirely by the Aesthetic Surgery Education and Research Foundation (ASERF).
METHODS
Eligible patients were sequentially enrolled into 1 of 3 cohorts: (A) women with systemic symptoms they attribute to their implants who requested implant removal; (B) women with breast implants requesting removal or exchange who do not have symptoms they attribute to their implants; and (C) women undergoing cosmetic mastopexy who have never had any implanted medical device. This study was approved by the Rhode Island Hospital IRB, performed under the guidance of the Declaration of Helsinki, and registered at clinicaltrials.gov. (NCT04255810). Patients were consecutively enrolled from November 2019 to May 2022. Specimens were sent to the respective laboratories blinded and deidentified, with no indication of their cohort. On the day of surgery all patients in Cohorts A and B underwent at least a partial capsulectomy as biospecimens were collected as per the study protocol. Capsulectomies ranged from partial to intact total (en bloc). Capsule tissue removed from the patients was photographed in the operating room by the operating surgeon and sent within 24 hours to Brown University for routine histologic analysis. Data collection by the operating surgeon included details about the implant shell (ie, textured or smooth), and whether the implant was silicone or saline. The integrity of the device was also noted. The capsule was described as thin and translucent, thin and opaque, thick without calcifications, or thick with calcifications.
The capsule and implant microbiome were analyzed by NGS in preference to routine cultures. The aggregate of swabs of the implant and capsule surfaces and a piece of capsule tissue together were sent for NGS (16SrRNA gene sequencing at MicroGen DX, Lubbock, TX). Prior to formal analysis, we investigated whether technical variance and/or sampling strategy regarding multiple sites would impact relevant endpoints and developed a uniform protocol. Samples were aggregated per patient and a patient was considered NGS positive if any sample was found to be NGS positive for an individual. Additional β-diversity analysis was performed to investigate whether the overall incidence profiles in each cohort were distinct. To consider the relative importance of cohort and collection facility to the number of species recovered per patient, 2-way analysis of variance was used, incorporating both factors. Additional factors were screened for significance in association with richness including implant manufacturer, implant fill, surface, capsule grade, rupture status (Y/N), and observation of gel bleeding. Finally, bacterial species incidence was considered between each cohort.
Three tubes of systemic blood were collected from all cohorts on the day of surgery and sent to Brown University within 24 hours for CBC, CRP, thyroid-stimulating hormone (TSH), and vitamin D levels. The subject’s sera were transported to Rhode Island Hospital, and stored in 2-mL aliquots at –80C until use. One aliquot was analyzed with a BD Biosciences LSRII Analyzer/Flow Cytometer for cytokines interleukin (IL)-2, IL-4, IL-5, IL-6, IL-9, IL-10, IL-13, IL-17A, IL17F, IL-21, IL-22, interferon γ (IFNγ), and tumor necrosis factor α (TNFα) with the LEGENDplex Human T helper (Th) Cytokine Panel (13-plex) (catalog number 741001; BioLegend, San Diego, CA). In 2021, BioLegend discontinued IL-21 in their kits. Each analysis was controlled by an internal standard provided by the manufacturer. Prior to analysis, all serum samples were diluted 1:10 to reduce background interference and analyzed in duplicate, while a subset of samples was also analyzed undiluted (neat). In 10 instances, one value was normal but the other elevated; these sera were reanalyzed in different dilutions and the most reproducible results were used. The remaining aliquots of sera were sent in batches to Johns Hopkins University Department of Dermatology, Allergy and Clinical Immunology Reference Laboratory, and analyzed with an ImmunoCAP autoanalyzer (Thermo Fisher Scientific/Phadia, Uppsala, Sweden) for IgE and IgG anti–Staphylococcus aureus enterotoxin A (anti-SEA), anti–S. aureus enterotoxin B (anti-SEB), and anti–toxic shock syndrome toxin (anti-TSST).6 The analytical sensitivities of the assays were <0.1 kUa/L of IgE anti-SEA, anti-SEB, and anti-TSST and <2 mgA/L of IgG anti-SEA, anti-SEB, and anti-TSST.7
RESULTS
CBC, White Blood Cells, Histology, TSH, Vitamin D, and CRP
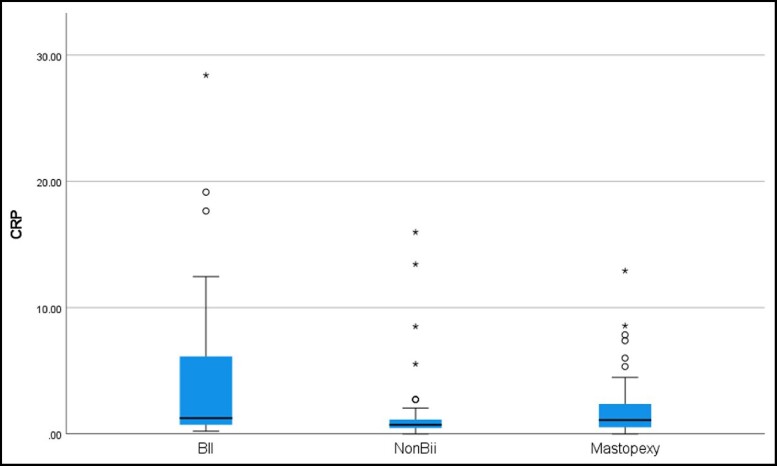
Subjects ranged in age from 30 to 65 years as per enrollment criteria. The average for each cohort was similar: Cohort A, 44.5 years; Cohort B, 46.9 years; and Cohort C, 46.5 years. The evaluation of peripheral blood for vitamin D levels and TSH showed no statistical difference between the cohorts. There were no abnormalities found in any cohort in the CBC, white blood cells, and differential. The CRP levels were statistically higher in Cohort A than in Cohorts B or C, but there was no difference between Cohorts B and C (Figure 1). The normal CRP range is below 8.0 mg/L; levels of 8 to 100 mg/L are mild to moderately elevated, and levels of 100 to 500 mg/L are elevated and signify inflammation of blood vessels, or major trauma. Cohort A CRP levels ranged from 0.20 to 28.41 mg/L, Cohort B from 0.35 to 15.98 mg/L, and Cohort C from 0.20 to 12.40 mg/L. Of the 9 patients in Cohort A with mild to moderately elevated CRP, 8 subjects had smooth implants and 1 had textured; 7 patients had saline implants and 2 had gel implants. In Cohort B, 3 subjects had mild to moderate elevations. Of these subjects, 1 had textured implants and 2 were smooth; 2 were gel implants and 1 saline. Seven of the 9 subjects in Cohort A self-reported connective tissue disease or autoimmune disease such as irritable bowel syndrome (IBS), hypothyroidism, Lyme’s disease, Sjogren’s syndrome, or lichen sclerosis. The subjects in this cohort also had documented significant confounding lifestyle choices and previous medical histories that can contribute to an elevated CRP such as smoking and obesity.8
Figure 1.
CRP: Cohort A (BII) showed a significantly higher expression of CRP than both Cohort B (non-BII) (P = 0.006) and Cohort C (control) (P = 0.014). BII, breast implant illness; CRP, C-reactive protein.
Histology
Microscopic evaluation was performed on the capsules in the Department of Pathology at Brown University. Initial pathologic examination of the capsule tissue from Cohorts A and B did not reveal any cases of malignancy. There was a statistically significant difference in capsule thickness between the cohorts, with thinner capsules identified in Cohort A than in Cohort B.
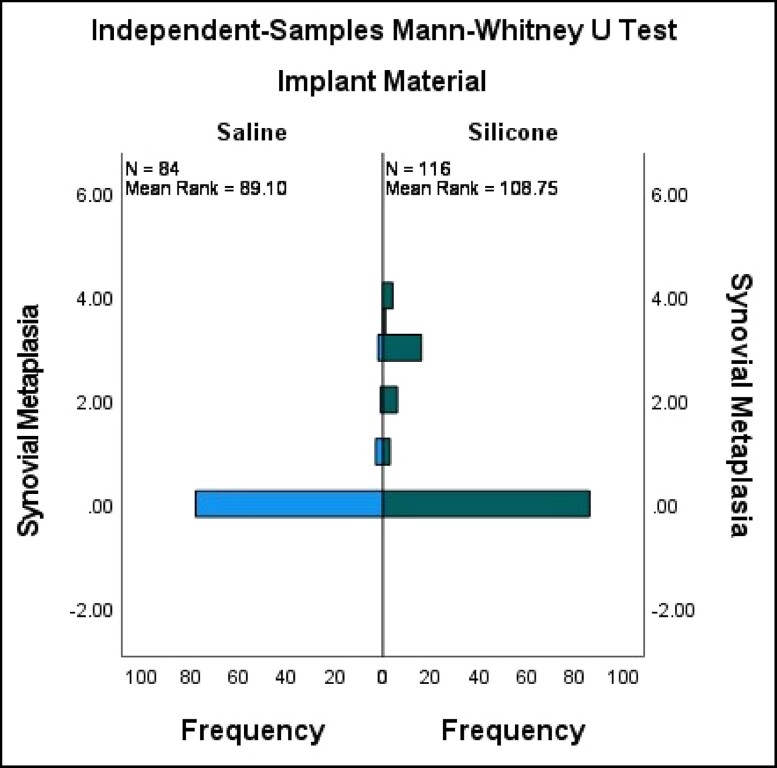
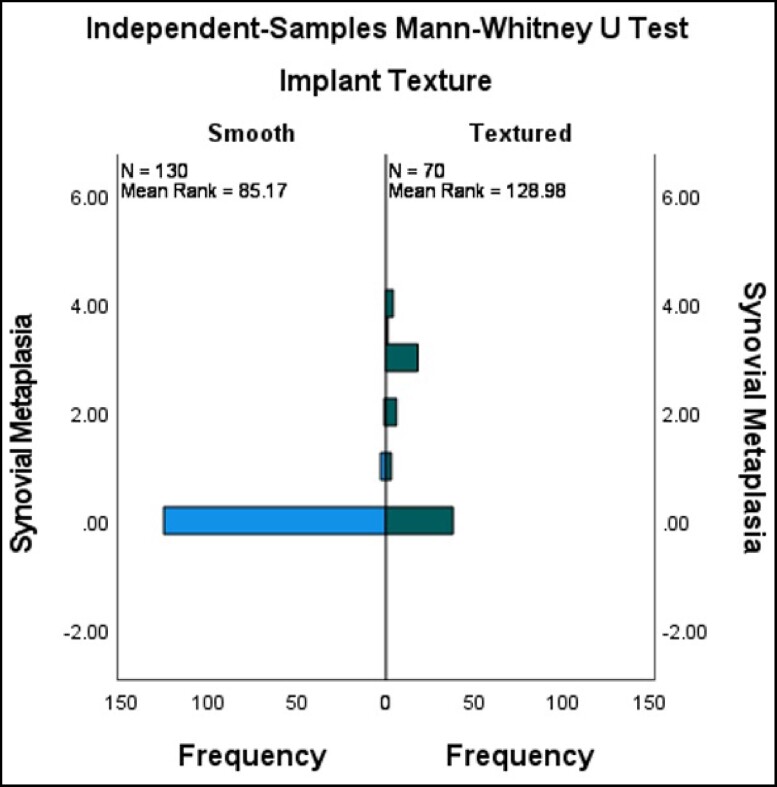
Capsule tissue in both cohorts revealed characteristic fibrosis, lymphoplasmacytic infiltration, calcifications, giant cells, foam cells, and epithelioid histiocytic capsules. We specifically evaluated the capsules for synovial metaplasia as this has previously been reported to be more prevalent in the capsules of patients with self-described BII vs historical control.5 The pathology revealed a higher incidence of synovial metaplasia in Cohort B, which is most likely related to the higher number of textured devices and silicone devices (Figures 2, 3). Synovial metaplasia was present in 9% of capsules in Cohort A and in 27% of capsules in Cohort B. In addition, the capsules were evaluated for the presence of eosinophils which can be present in allergic or other inflammatory conditions. Of the 96 capsules evaluated in Cohort A, 2 capsules, from the same subject, were positive for eosinophils. In Cohort B, eosinophils were identified in 2 capsules. There was no statistically significant difference in the presence of eosinophils between the 2 implant cohorts. There are no specific histologic methods to test for silicone in implant capsules, either morphologically or by special stains. On histologic examination, amorphous refractile material was observed in some of the study capsules. This is thought to represent fragments of the implant shell as this is consistent with published pathology reports of silicone in tissues. This could be confirmed with techniques such as dispersive X-ray analysis which was not available for this study. Amorphous materials were seen in 12 out of 50 capsules in the BII cohort and in 33 out of 50 capsules of the non-BII implant cohort. Amorphous refractile material was observed most often in capsules that surrounded textured surface implants.
Figure 2.
Synovial metaplasia saline vs silicone fill. Mann-Whitney U-test for synovial metaplasia vs implant material (silicone vs saline). Silicone has a significantly higher level of synovial metaplasia vs saline (P = 0.000).
Figure 3.
Textured vs smooth implants: synovial metaplasia. Mann-Whitney U-test for synovial metaplasia vs implant texture (textured vs smooth). Textured implants have a significantly higher correlation with synovial metaplasia vs smooth (P = 0.000).
Microbes
Following data aggregation and filtering, 94 subjects were represented equally among Cohorts A and B (n = 47 each). The mean number of bacterial species detected (ie, richness) study-wide was 2, compared to 2.45 (95% CI, 1.25, 3.64) in Cohort A and 1.55 (95% CI, 0.86, 2.25) in Cohort B. There was no statistically significant difference in NGS positivity between the cohorts (Table 1). A positive NGS demonstrating the presence of bacterial DNA was found in 48% of the subjects in Cohort A and in 46% of Cohort B (P = 1.000) (Table 2). Specifically, there was no statistical difference between the presence of C. acnes (Cohort A, 44%; Cohort B, 32%; P = 0.3) and Staphylococcus epidermidis (Cohort A, 18%; Cohort B, 18%; P = 1.0). A statistical analysis was performed to determine if there was any correlation between the number of systemic symptoms reported at baseline and the presence of bacteria. NGS positivity was not associated with an increased number of self-reported systemic symptoms in both Cohorts A and B, and symptom improvement after explantation was the same regardless of NGS positivity. Cutibacterium has been implicated as a potential cause of symptoms in women with self-described BII.5 There was no statistical difference between the percentage reduction of symptoms at either 3 to 6 weeks or 6 months in Cohorts A or B and the presence of C. acnes (Table 3).
Table 1.
Next-Generation Sequencing Positivity by Cohort
| Cohort | Negative | Positive |
|---|---|---|
| A (n = 50) | 24 (48%) | 26 (52%) |
| B (n = 50) | 23 (46%) | 27 (54%) |
There was no statistical significance between the next-generation sequencing positivity in Cohort A vs Cohort B (P = 1.0)
Table 2.
Bacteria Identified at Baseline: Cohort A vs Cohort B
| Presence of bacteria | Cohort A (n = 50) | Cohort B (n = 50) | P-valuea |
|---|---|---|---|
| Any bacterial growth? | 24 (48.0) | 23 (46.0) | 1.0000 |
| Staphylococcus hominus | 1 (2.0) | 3 (6.0) | 0.6173 |
| Corynebacterium tuberculosteratum | 4 (8.0) | 5 (10.0) | 1.0000 |
| Aerococcus | 0 | 2 (4.0) | 0.4949 |
| Staphylococcus epidermidis | 9 (18.0) | 9 (18.0) | 1.0000 |
| Cutibacterium acnes | 22 (44.0) | 16 (32.0) | 0.3030 |
| Enterobacter | 1 (2.0) | 6 (12.0) | 0.1117 |
| Lactobacillus | 0 | 3 (6.0) | 0.2424 |
| Oryzomicrobium | 1 (2.0) | 1 (2.0) | 1.0000 |
| Phylobacter | 0 | 1 (2.0) | 1.0000 |
| Pseudomonas | 0 | 2 (4.0) | 0.4949 |
| Agrobacterium | 0 | 1 (2.0) | 1.0000 |
| Azoperillus | 0 | 1 (2.0) | 1.0000 |
| Haemophilus influenza | 0 | 1 (2.0) | 1.0000 |
| Staphylococcus pastueri | 1 (2.0) | 1 (2.0) | 1.0000 |
| Massilia | 0 | 1 (2.0) | 1.0000 |
| Mycobacterium marinum | 0 | 1 (2.0) | 1.0000 |
| Escherichia coli | 2 (4.0) | 2 (4.0) | 1.0000 |
| Sneathia | 0 | 1 (2.0) | 1.0000 |
| Serratia marcescens | 0 | 1 (2.0) | 1.0000 |
| Staphylococcus lundungensis | 1 (2.0) | 0 | 1.0000 |
| Staphylococcus saccrolyticus | 3 (6.0) | 0 | 0.2424 |
| Streptococcus mitis | 1 (2.0) | 0 | 1.0000 |
| Acinobacter | 3 (6.0) | 1 (2.0) | 0.6173 |
| Bacillus | 1 (2.0) | 0 | 1.0000 |
| Gordonia otitis | 1 (2.0) | 0 | 1.0000 |
| Klebsiella | 2 (4.0) | 0 | 0.4949 |
| Burkholdia | 3 (6.0) | 0 | 0.2424 |
| Staphylococcus capitus | 1 (2.0) | 0 | 1.0000 |
Values are n (%). aP-value from Fisher’s exact test, testing the null hypothesis that the true percentages of patients with bacterial growth are equal for the 2 groups vs the alternative hypothesis that these percentages are not equal.
Table 3.
Reduction in Symptom Number by Cohort and Visit and Presence of Cutibacterium acnes at Baseline
| Cohort | Parameter | Visit | Cutibacterium acnes | P-valuea | |
|---|---|---|---|---|---|
| Yes (n = 22) | No (n = 28) | ||||
| Cohort A | 50% or more reduction in symptom number | 3-6 weeks | 14 (66.7) | 17 (63.0) | 1.0000 |
| 6 months | 18 (81.8) | 18 (66.7) | 0.3328 | ||
| 80% or more reduction in symptom number | 3-6 weeks | 6 (28.6) | 11 (40.7) | 0.5442 | |
| 6 months | 13 (59.1) | 10 (37.0) | 0.1567 | ||
| Cohort B | 50% or more reduction in symptom number | 3-6 weeks | 6 (85.7) | 13 (46.4) | 0.0964 |
| 6 months | 3 (60.0) | 9 (37.5) | 0.6221 | ||
| 80% or more reduction in symptom number | 3-6 weeks | 2 (28.6) | 10 (35.7) | 1.0000 | |
| 6 months | 1 (20.0) | 5 (20.8) | 1.0000 | ||
Values are n (%). Results are limited to patients with at least 1 reported symptom at baseline based on solicited symptoms listed on the Case Report Form. aP-value from Fisher’s exact test, testing the null hypothesis that the true percentages of patients with reduction in symptom number are equal for the 2 groups vs the alternative hypothesis that these percentages are not equal.
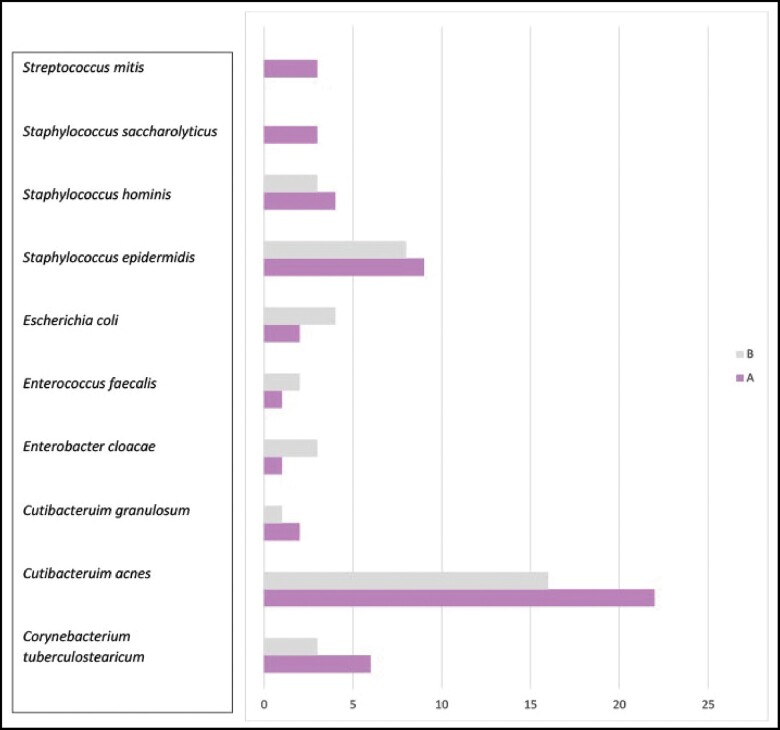
To further identify if one species of bacteria was distinctly responsible for the systemic symptoms reported in Cohort A, β-diversity analysis was calculated in both Cohorts A and B. Although 92 unique species were detected at least once, 66 species were detected only once and the most prevalently detected species included C. acnes, S. epidermidis, and Corynebacterium tuberculostearicum. The top 10 most prevalent species were screened for association to cohort by log regression and none were found to significantly discriminate between the 2 groups (Figure 4).
Figure 4.
Most common organisms (by next-generation sequencing) per cohort: occurrences estimated per species in each cohort. Only species in the top 10 most prevalent are shown here. None were found to be significantly associated with cohort by log-regression–based screening.
Data collected, including implant manufacturer, implant fill, surface, rupture status (Y/N), and observation of gel bleeding, were documented, and none reached the threshold for significance. Data were also analyzed for the presence of capsular contracture and the presence of bacteria. There was a statistically lower level of capsular contracture in Cohort A than in Cohort B. There was a statistically significant relationship between the presence of S. epidermidis and capsular contracture in cohort B. Fifty percent of the subjects in Cohort B with Grade III or IV capsules were positive for S. epidermidis vs only 7% of those with Grade I or II capsules. Most capsules in Cohort A were Grade I or II, and only 14% had Grade III or IV capsule, with no statistical difference in the presence of any bacterial species (Table 4). Interestingly, there was no statistical difference in NGS positivity or bacterial species in either cohort between the subjects with textured or smooth implants (Table 5). There was a statistical significance to NGS positivity and implant fill in Cohort A, with a P value of 0.002 for S. epidermidis between gel and saline. There was no statistical difference identified in Cohort B and no statistical difference between Cohorts A and B and the presence of bacteria as compared to implant fill (Table 6). There was no statistical significance to the presence of gel rupture and NGS positivity.
Table 4.
Comparison by Cohort and Capsular Contracture Category for Presence of Bacteria
| Cohort | Presence of bacteria | Grade III or IV capsular contracture | P-valuea | |
|---|---|---|---|---|
| Yes (n = 7) | No (n = 43) | |||
| Cohort A | Any bacterial growth? | 3 (42.9) | 21 (48.8) | 1.0000 |
| Staphylococcus hominus | 0 | 1 (2.3) | 1.0000 | |
| Corynebacterium tuberculosteratum | 1 (14.3) | 3 (7.0) | 0.4641 | |
| Aerococcus | 0 | 0 | — | |
| Staphylococcus epidermidis | 1 (14.3) | 8 (18.6) | 1.0000 | |
| Cutibacterium acnes | 3 (42.9) | 19 (44.2) | 1.0000 | |
| Enterobacter | 0 | 1 (2.3) | 1.0000 | |
| Lactobacillus | 0 | 0 | — | |
| Oryzomicrobium | 0 | 1 (2.3) | 1.0000 | |
| Phylobacter | 0 | 0 | — | |
| Pseudomonas | 0 | 0 | — | |
| Agrobacterium | 0 | 0 | — | |
| Azoperillus | 0 | 0 | — | |
| Haemophilus influenza | 0 | 0 | — | |
| Staphylococcus pastueri | 0 | 1 (2.3) | 1.0000 | |
| Massilia | 0 | 0 | — | |
| Mycobacterium marinum | 0 | 0 | — | |
| Escherichia coli | 0 | 2 (4.7) | 1.0000 | |
| Sneathia | 0 | 0 | — | |
| Serratia marcessens | 0 | 0 | — | |
| Staphylococcus lundungensis | 0 | 1 (2.3) | 1.0000 | |
| Staphylococcus saccrolyticus | 1 (14.3) | 2 (4.7) | 0.3704 | |
| Streptococcus mitis | 0 | 1 (2.3) | 1.0000 | |
| Acinobacter | 0 | 3 (7.0) | 1.0000 | |
| Bacillus | 0 | 1 (2.3) | 1.0000 | |
| Gordonia otitis | 0 | 1 (2.3) | 1.0000 | |
| Klebsiella | 0 | 2 (4.7) | 1.0000 | |
| Burkholdia | 0 | 3 (7.0) | 1.0000 | |
| Staphylococcus capitus | 0 | 1 (2.3) | 1.0000 | |
| Cohort B | Any bacterial growth? | 9 (75.0) | 14 (36.8) | 0.0435 |
| Staphylococcus hominus | 1 (8.3) | 2 (5.3) | 1.0000 | |
| Corynebacterium tuberculosteratum | 2 (16.7) | 3 (7.9) | 0.5819 | |
| Aerococcus | 0 | 2 (5.3) | 1.0000 | |
| Staphylococcus epidermidis | 6 (50.0) | 3 (7.9) | 0.0033 | |
| Cutibacterium acnes | 5 (41.7) | 11 (28.9) | 0.4859 | |
| Enterobacter | 2 (16.7) | 4 (10.5) | 0.6210 | |
| Lactobacillus | 1 (8.3) | 2 (5.3) | 1.0000 | |
| Oryzomicrobium | 1 (8.3) | 0 | 0.2400 | |
| Phylobacter | 1 (8.3) | 0 | 0.2400 | |
| Pseudomonas | 1 (8.3) | 1 (2.6) | 0.4261 | |
| Agrobacterium | 0 | 1 (2.6) | 1.0000 | |
| Azoperillus | 0 | 1 (2.6) | 1.0000 | |
| Haemophilus influenza | 1 (8.3) | 0 | 0.2400 | |
| Staphylococcus pastueri | 0 | 1 (2.6) | 1.0000 | |
| Massilia | 1 (8.3) | 0 | 0.2400 | |
| Mycobacterium marinum | 1 (8.3) | 0 | 0.2400 | |
| Escherichia coli | 0 | 2 (5.3) | 1.0000 | |
| Sneathia | 0 | 1 (2.6) | 1.0000 | |
| Serratia marcessens | 0 | 1 (2.6) | 1.0000 | |
| Staphylococcus lundungensis | 0 | 0 | — | |
| Staphylococcus saccrolyticus | 0 | 0 | — | |
| Streptococcus mitis | 0 | 0 | — | |
| Acinobacter | 1 (8.3) | 0 | 0.2400 | |
| Bacillus | 0 | 0 | — | |
| Gordonia otitis | 0 | 0 | — | |
| Klebsiella | 0 | 0 | — | |
| Burkholdia | 0 | 0 | — | |
| Staphylococcus capitus | 0 | 0 | — | |
Values are n (%). aP-value from Fisher’s exact test, testing the null hypothesis that the true percentages of patients with bacterial growth are equal for the 2 groups vs the alternative hypothesis that these percentages are not equal.
Table 5.
Comparison by Cohort and Type of Implant for Presence of Bacteria—Next Generation Sequencing
| Cohort | Presence of bacteria | Type of implant | P-valuea | |
|---|---|---|---|---|
| Textured (n = 5) | Smooth (n = 45) | |||
| Cohort A | Any bacterial growth? | 3 (60.0) | 21 (46.7) | 0.6613 |
| Staphylococcus hominus | 0 | 1 (2.2) | 1.0000 | |
| Corynebacterium tuberculosteratum | 0 | 4 (8.9) | 1.0000 | |
| Aerococcus | 0 | 0 | — | |
| Staphylococcus epidermidis | 2 (40.0) | 7 (15.6) | 0.2161 | |
| Cutibacterium acnes | 3 (60.0) | 19 (42.2) | 0.6428 | |
| Enterobacter | 1 (20.0) | 0 | 0.1000 | |
| Lactobacillus | 0 | 0 | — | |
| Oryzomicrobium | 0 | 1 (2.2) | 1.0000 | |
| Phylobacter | 0 | 0 | — | |
| Pseudomonas | 0 | 0 | — | |
| Agrobacterium | 0 | 0 | — | |
| Azoperillus | 0 | 0 | — | |
| Haemophilus influenza | 0 | 0 | — | |
| Staphylococcus pastueri | 0 | 1 (2.2) | 1.0000 | |
| Massilia | 0 | 0 | — | |
| Mycobacterium marinum | 0 | 0 | — | |
| Escherichia coli | 1 (20.0) | 1 (2.2) | 0.1918 | |
| Sneathia | 0 | 0 | — | |
| Serratia marcessens | 0 | 0 | — | |
| Staphylococcus lundungensis | 0 | 1 (2.2) | 1.0000 | |
| Staphylococcus saccrolyticus | 0 | 3 (6.7) | 1.0000 | |
| Streptococcus mitis | 0 | 1 (2.2) | 1.0000 | |
| Acinobacter | 1 (20.0) | 2 (4.4) | 0.2760 | |
| Bacillus | 0 | 1 (2.2) | 1.0000 | |
| Gordonia otitis | 0 | 1 (2.2) | 1.0000 | |
| Klebsiella | 0 | 2 (4.4) | 1.0000 | |
| Burkholdia | 1 (20.0) | 2 (4.4) | 0.2760 | |
| Staphylococcus capitus | 1 (20.0) | 0 | 0.1000 | |
| Cohort B | Any bacterial growth? | 11 (40.7) | 12 (52.2) | 0.5701 |
| Staphylococcus hominus | 1 (3.7) | 2 (8.7) | 0.5881 | |
| Corynebacterium tuberculosteratum | 4 (14.8) | 1 (4.3) | 0.3573 | |
| Aerococcus | 1 (3.7) | 1 (4.3) | 1.0000 | |
| Staphylococcus epidermidis | 4 (14.8) | 5 (21.7) | 0.7147 | |
| Cutibacterium acnes | 7 (25.9) | 9 (39.1) | 0.3726 | |
| Enterobacter | 2 (7.4) | 4 (17.4) | 0.3946 | |
| Lactobacillus | 2 (7.4) | 1 (4.3) | 1.0000 | |
| Oryzomicrobium | 1 (3.7) | 0 | 1.0000 | |
| Phylobacter | 1 (3.7) | 0 | 1.0000 | |
| Pseudomonas | 1 (3.7) | 1 (4.3) | 1.0000 | |
| Agrobacterium | 1 (3.7) | 0 | 1.0000 | |
| Azoperillus | 1 (3.7) | 0 | 1.0000 | |
| Haemophilus influenza | 0 | 1 (4.3) | 0.4600 | |
| Staphylococcus pastueri | 1 (3.7) | 0 | 1.0000 | |
| Massilia | 0 | 1 (4.3) | 0.4600 | |
| Mycobacterium marinum | 0 | 1 (4.3) | 0.4600 | |
| Escherichia coli | 0 | 2 (8.7) | 0.2065 | |
| Sneathia | 0 | 1 (4.3) | 0.4600 | |
| Serratia marcessens | 0 | 1 (4.3) | 0.4600 | |
| Staphylococcus lundungensis | 0 | 0 | — | |
| Staphylococcus saccrolyticus | 0 | 0 | — | |
| Streptococcus mitis | 0 | 0 | — | |
| Acinobacter | 0 | 1 (4.3) | 0.4600 | |
| Bacillus | 0 | 0 | — | |
| Gordonia otitis | 0 | 0 | — | |
| Klebsiella | 0 | 0 | — | |
| Burkholdia | 0 | 0 | — | |
| Staphylococcus capitus | 0 | 0 | — | |
Values are n (%). aP-value from Fisher’s exact test, testing the null hypothesis that the true percentages of patients with bacterial growth are equal for the two groups vs the alternative hypothesis that these percentages are not equal.
Table 6.
Comparison by Cohort and Implant Fill for Presence of Bacteria—Next-Generation Sequencing
| Cohort | Presence of bacteria | Implant fill | P-valuea | |
|---|---|---|---|---|
| Gel (n = 16) | Saline (n = 34) | |||
| Cohort A | Any bacterial growth? | 9 (56.3) | 15 (44.1) | 0.5470 |
| Staphylococcus hominus | 0 | 1 (2.9) | 1.0000 | |
| Corynebacterium tuberculosteratum | 1 (6.3) | 3 (8.8) | 1.0000 | |
| Aerococcus | 0 | 0 | — | |
| Staphylococcus epidermidis | 7 (43.8) | 2 (5.9) | 0.0027 | |
| Cutibacterium acnes | 9 (56.3) | 13 (38.2) | 0.3600 | |
| Enterobacter | 1 (6.3) | 0 | 0.3200 | |
| Lactobacillus | 0 | 0 | — | |
| Oryzomicrobium | 0 | 1 (2.9) | 1.0000 | |
| Phylobacter | 0 | 0 | — | |
| Pseudomonas | 0 | 0 | — | |
| Agrobacterium | 0 | 0 | — | |
| Azoperillus | 0 | 0 | — | |
| Haemophilus influenza | 0 | 0 | — | |
| Staphylococcus pastueri | 0 | 1 (2.9) | 1.0000 | |
| Massilia | 0 | 0 | — | |
| Mycobacterium marinum | 0 | 0 | — | |
| Escherichia coli | 1 (6.3) | 1 (2.9) | 0.5420 | |
| Sneathia | 0 | 0 | — | |
| Serratia marcessens | 0 | 0 | — | |
| Staph lundungensis | 0 | 1 (2.9) | 1.0000 | |
| Staphylococcus saccrolyticus | 0 | 3 (8.8) | 0.5420 | |
| Streptococcus mitis | 1 (6.3) | 0 | 0.3200 | |
| Acinobacter | 1 (6.3) | 2 (5.9) | 1.0000 | |
| Bacillus | 0 | 1 (2.9) | 1.0000 | |
| Gordonia otitis | 1 (6.3) | 0 | 0.3200 | |
| Klebsiella | 1 (6.3) | 1 (2.9) | 0.5420 | |
| Burkholdia | 2 (12.5) | 1 (2.9) | 0.2367 | |
| Staphylococcus capitus | 1 (6.3) | 0 | 0.3200 | |
| Cohort B | Any bacterial growth? | 18 (46.2) | 5 (45.5) | 1.0000 |
| Staphylococcus hominus | 2 (5.1) | 1 (9.1) | 0.5337 | |
| Corynebacterium tuberculosteratum | 4 (10.3) | 1 (9.1) | 1.0000 | |
| Aerococcus | 1 (2.6) | 1 (9.1) | 0.3951 | |
| Staphylococcus epidermidis | 7 (17.9) | 2 (18.2) | 1.0000 | |
| Cutibacterium acnes | 11 (28.2) | 5 (45.5) | 0.2972 | |
| Enterobacter | 4 (10.3) | 2 (18.2) | 0.6014 | |
| Lactobacillus | 3 (7.7) | 0 | 1.0000 | |
| Oryzomicrobium | 1 (2.6) | 0 | 1.0000 | |
| Phylobacter | 1 (2.6) | 0 | 1.0000 | |
| Pseudomonas | 2 (5.1) | 0 | 1.0000 | |
| Agrobacterium | 1 (2.6) | 0 | 1.0000 | |
| Azoperillus | 1 (2.6) | 0 | 1.0000 | |
| Haemophilus influenza | 1 (2.6) | 0 | 1.0000 | |
| Staphylococcus pastueri | 1 (2.6) | 0 | 1.0000 | |
| Massilia | 1 (2.6) | 0 | 1.0000 | |
| Mycobacterium marinum | 1 (2.6) | 0 | 1.0000 | |
| Escherichia coli | 1 (2.6) | 1 (9.1) | 0.3951 | |
| Sneathia | 1 (2.6) | 0 | 1.0000 | |
| Serratia marcessens | 1 (2.6) | 0 | 1.0000 | |
Values are n (%). aP-value from Fisher’s exact test, testing the null hypothesis that the true percentages of patients with bacterial growth are equal for the 2 groups vs the alternative hypothesis that these percentages are not equal.
Cytokines
Twelve cytokines were evaluated in the peripheral blood of all 3 study cohorts. Of the serum cytokines measured in this study, only 3 cytokines, IL-17A, IL-13. and IL-22, were found to be significantly higher in sera of Cohort A than in sera of Cohorts B and C (Tables 7, 8). It should be noted that a limitation of this study from the start is that it is difficult to determine which individuals had serum cytokine levels above those of healthy or normal subjects due to the wide variation of healthy/normal serum values for these cytokines in the literature.9 However, we were able to find statistically significant differences of cytokine serum levels among the 3 cohorts. An elevated IL-22 level was found in 12% of Cohort A, 2% of Cohort B, but in 0% of Cohort C. There was no relationship between elevated cytokines and either the implant surface or fill. Of the 7 subjects in Cohort A with elevations, all reported additional medical diagnoses, such as Hashimoto’s thyroiditis, IBS, and allergies at baseline. IL-17A levels were elevated in 6% of Cohort A, 0% of Cohort B, and 2% of Cohort C. Of the 3 subjects who had elevated IL-17A, all had a coexisting medical illness. An elevated IL-13 was found in 16% of Cohort A, 0% of Cohort B, and 2% of Cohort C. Of the 8 subjects in Cohort A with an elevated IL-13, all but 1 patient had coexisting medical illnesses.
Table 7.
Cytokine Comparison Between Cohorts
| Cytokine | Cohort A (n = 50) | Cohort B (n = 50) | Cohort C (n = 48) |
|---|---|---|---|
| IL-5 | 5 | 3 | 4 |
| IL-13 | 8 | 0 | 1 |
| IL-2 | 2 | 0 | 0 |
| IL-6 | 9 | 8 | 3 |
| IL-9 | 4 | 1 | 0 |
| IL-10 | 2 | 0 | 0 |
| IFNγ | 9 | 4 | 1 |
| TNFα | 8 | 9 | 4 |
| IL-17A | 3 | 0 | 1 |
| IL-17F | 5 | 1 | 2 |
| IL-4 | 6 | 2 | 2 |
| IL-22 | 6 | 1 | 0 |
The table shows the number of subjects within each cohort who had above-normal levels of the respective cytokine. The number of subjects with higher levels for IL-13, IL17A, and IL-22 were significantly higher in Cohort A. TNF and IL-6 proinflammatory cytokines were higher in Cohorts A and B than in Cohort C. IL-2, IFNγ, IL-10, and IL-17F were not statistically different between cohorts. IL-4 was higher in Cohorts A and B than in Cohort C. IFN, interferon; IL, interleukin; TNF, tumor necrosis factor.
Table 8.
Analysis to Find Baseline Characteristics That Are Predictive of Patients Self-reporting BII: Enterotoxins and Cytokines
| Baseline characteristic | Reference category | Cohort A vs Cohort B | Cohort A vs Cohort C | ||
|---|---|---|---|---|---|
| Odds ratio | P-value | Odds ratio | P-value | ||
| Enterotoxins | |||||
| ȃIgE anti-SEA (continuous) | — | 1.196 | 0.9600 | 126.899 | 0.4272 |
| ȃIgE anti-SEB (continuous) | — | 0.041 | 0.4948 | 0.167 | 0.6403 |
| ȃIgE anti-TSST (continuous) | — | 2.349 | 0.5323 | 0.618 | 0.6689 |
| ȃIgG anti-SEA (continuous) | — | 1.023 | 0.0973 | 1.052 | 0.0068 |
| ȃIgG anti-SEB (continuous) | — | 1.022 | 0.0551 | 1.017 | 0.1315 |
| ȃIgG anti-TSST (continuous) | — | 1.005 | 0.4630 | 1.007 | 0.3125 |
| Cytokines | |||||
| ȃElevated IL-5 (yes/no) | No | 1.363 | 0.6958 | 1.022 | 0.9762 |
| ȃElevated IL-13 (yes/no) | No | >999.999 | 0.0168 | 4.355 | 0.1956 |
| ȃElevated IL-2 (yes/no) | No | >999.999 | 0.0934 | >999.999 | 0.0911 |
| ȃElevated IL-6 (yes/no) | No | 1.000 | 1.0000 | 4.000 | 0.0945 |
| ȃElevated IL-9 (yes/no) | No | 2.042 | 0.5653 | >999.999 | 0.0911 |
| ȃElevated IL-10 (yes/no) | No | >999.999 | 0.0934 | >999.999 | 0.0911 |
| ȃElevated IFNγ (yes/no) | No | 1.000 | 1.0000 | 4.355 | 0.1956 |
| ȃElevated TNFα (yes/no) | No | 0.334 | 0.1228 | 1.022 | 0.9796 |
| ȃElevated IL-17A (yes/no) | No | >999.999 | 0.0392 | 3.196 | 0.3219 |
| ȃElevated IL-17F (yes/no) | No | 4.267 | 0.2020 | 4.355 | 0.1956 |
| ȃElevated IL-22 (yes/no) | No | >999.999 | 0.0392 | >999.999 | 0.0378 |
| ȃElevated IL-4 (yes/no) | No | 2.042 | 0.5653 | 1.021 | 0.9835 |
The odds ratios and P-values are from a logistic regression analysis with group as the dependent variable and the baseline characteristic as the explanatory variable. The P-value is for a 2-sided test of the null hypothesis that the true odds ratio equals 1. For enterotoxins, values of ‘<0.1’ (IgE) and ‘<2.0’ (IgG) have been converted to 0 for analysis. For cytokine values, when there was a discrepancy between readings of fluorescent intensity of separate samples in the initial analysis, the analysis was repeated, and most consistent values were used. BII, breast implant illness; IFN, interferon; Ig, immunoglobulin; IL, interleukin; TNF, tumor necrosis factor; SEA(B), Staphylococcus aureus enterotoxin A(B); TSST, toxic shock syndrome toxin.
Enterotoxins
To investigate the possible role of Staphylococcal infection causing a potential systemic inflammatory response, we analyzed blood of subjects for the presence of a humoral (antibody) immune response to bacterial antigens. Sera from all 3 cohorts were analyzed for the presence of IgG and IgE anti-SEA, anti-SEB, and anti-TSST. There were no significant differences in the frequency or levels of IgE anti-SEA, anti-SEB, and anti-TSST between Cohorts A and B, and this was consistent with the overall absence of reported Type 1 hypersensitivity adverse reactions in either group (Table 9). Regarding IgG antibody responses, there is no universally accepted reference range for the concentration of IgG anti-SEA, anti-SEB, and anti-TSST in a healthy adult population. There were no significant differences in IgG anti-SEB or IgG anti-TSST between Cohorts A and B. There was a statistical difference between Cohorts A and B in detected level of IgG anti-SEA (P = 0.0068) (Table 10).
Table 9.
Prevalence of IgE Anti-Staphylococcus aureus Enterotoxin (Superantigen) in the 3 Study Cohorts
| Cohort | Number | IgE anti-SEA >0.1 kUa/L | IgE anti-SEB >0.1 kUa/L | IgE anti-TSST >0.1 kUa/L | IgE anti-SEA, SEB, or TSST >0.1 kUa/L | IgE >0.35 kUa/L |
|---|---|---|---|---|---|---|
| Cohort A | 49 | 2 (4.0%) | 2 (4.0%) | 7 (14.2%) | 9 (18.3%) | 3 (6.1%) |
| Cohort B | 48 | 3 (6.2%) | 2 (4.1%) | 8 (16%) | 1 (20.8%) | 2 (4.1%) |
| Cohort C | 46 | 1 (2.1%) | 3 (6.5%) | 11 (24%) | 11 (23.9%) | 5 (10.8%) |
No significant difference in the levels of IgE antibodies between the cohorts (Mantel-Haenszel chi square). Ig, immunoglobulin; SEA(B), Staphylococcus aureus enterotoxin A(B); TSST, toxic shock syndrome toxin.
Table 10.
Prevalence of IgG Anti-Staphylococcus aureus Enterotoxin (Superantigen) in the 3 Study Cohorts
| Cohort | Number | IgG anti-SEA >32.2 mgA/L | IgG anti-SEB >53.5 mgA/L | IgG anti-TSST 70.5 mgA/L | IgG anti-SEA, SEB or TSST positive |
|---|---|---|---|---|---|
| Cohort A | 49 | 13 (26.5%) | 5 (10.2%) | 7 (14.3%) | 18 (36.7%) |
| Cohort B | 48 | 7 (14.6%) | 4 (8.2%) | 4 (8.2%) | 9 (18.8%) |
| Cohort C | 46 | 3 (6.5%) | 5 (10.8%) | 5 (10.8%) | 7 (15.2%) |
The 95% CI limits for age-adjusted IgG anti-Staphylococcus aureus superantigen reference ranges for healthy female adults derived from Cohort C are 32.3 mgA/L (SEA), 53.6 mgA/L (SEB) and 70.5 mgA/L (TSST). Ig, immunoglobulin; SEA(B), Staphylococcus aureus enterotoxin A(B); mgA/L, milligrams of antigen-specific antibody per liter; TSST, toxic shock syndrome toxin.
DISCUSSION
Forty years ago, Burkhart first described subacute periprosthetic infections and identified the responsible organism as S. epidermidis.10 Research by Ahn et al in 1996 looked at the frequency, type, and clinical significance of microbial colonization on implant surfaces removed from symptomatic patients. C. acnes, formerly known as Propionibacterium acnes, was found most often (57.5%), followed by S. epidermidis (47%).11 Research by Adams et al in 2006 demonstrated a reduction in Grade III and IV capsular contracture when triple antibiotics were added as an irrigant during breast augmentation and reconstruction. Their landmark data demonstrated a wide variety of organisms, rather than only S. epidermidis, cultured from periprosthetic capsules.2 Previous published reports have presented a biofilm hypothesis as a possible etiology for the systemic symptoms reported by women with breast implants. Lee et al specifically cultured capsular tissue removed from women who self-reported systemic symptoms that they associated with their implants.5 Biofilms are generally polymicrobial and under certain environments may be overrepresented by a particular species. Previous efforts to analyze breast implant microbial contamination by culture-based methods are limited as not all pathogens are suitable for culturing. Originally described in 1982, NGS (ie, 16S rRNA gene sequencing) is a microbial diagnostic method that can detect all the nucleic acids present in a specimen. Many of the human pathogens routinely identified on the surface of an implant and within the capsular tissue can enter a nonculturable but still viable state.12 During this state, pathogens retain their cellular structure and gene expression, allowing for polymerase chain reaction testing while remaining nonculturable. In this ASERF study, the pathogens on the surface of the implant and capsules were identified by NGS and the optimal technique for procuring samples was determined by first validating methods that would detect the highest yield of pathogen DNA. Swabs of the entire surface of the breast implant and inside of the capsule as well as 5 grams of capsule tissue were sent for NGS. This aggregate of swabs and tissue provided the most accurate mapping of pathogenic bacterial DNA often missed with routine cultures. The conclusions from the NGS reveal no statistical difference in bacterial positivity on the surface of the implant or within the capsule between Cohorts A and B. The only statistical difference in Cohort A for <10 systemic symptoms or >10 systemic symptoms was the presence of S. epidermidis in subjects who reported fewer than 10 symptoms, not more. In Cohort B, there was no difference. The differences in microbial detection between the 2 cohorts in this study are minimal.
Synovial metaplasia and epithelioid histiocytic reaction are indicators of host reaction to something, especially to foreign bodies.12,13 Synovial metaplasia was more frequently seen and was more severe in Cohort B than in Cohort A, and present surrounding both saline- and silicone-filled implants. It was also much more likely to be associated with textured silicone implants. Low-molecular-weight silicones and gel bleed have been implicated as potential causes of systemic symptoms, with the suggestion that the presence of silicone in capsules is an indication of proof of an association between silicone and systemic symptoms.14,15 This study showed that the presence of silicone particles in the capsule was significantly more common in the non-BII cohort and was most associated with textured implants. There is also no current scientific evidence to support an association between the presence of silicone particles in the capsules and systemic symptoms. In this paper the presence of silicone particles was not associated with symptoms as there was significantly fewer amorphous particles in the capsules of Cohort A.
A limitation of this study was the measurement of IgG antibody to only 1 microbe, S. aureus, for reasons of the well-stablished superantigen responses that are known to produce IgG antibody.16S. epidermidis does not produce the toxic enterotoxins produced by S. aureus. We chose to study immune responses to S. aureus because an estimated 20% to 30% of the human population are long-term carriers of S. aureus in their upper respiratory tract, on normal skin, and in the lower reproductive organs of women.17,18S. aureus also produces α-toxin which can produce pores in cellular membranes, causing localized inflammation and cell death. The presence of superantigen-specific antibody would support a possible Staphylococcal infection–induced mechanism of an inflammatory response to a current or past localized infectious process. Of the toxic proteins S. aureus produces, Staphylococcal enterotoxin superantigens are known to be among the most potent and involved in inducing toxic shock syndrome.19 The presence and levels of SEA-, SEB-, and TSST-specific IgG and IgE antibody were measured using state-of-the-art ImmunoCAP Technology and did find 26% of subjects in Cohort A with elevated IgG anti-SEA levels.20 Of the 18 subjects identified in Cohort A with elevated IgG anti-SEA/SEB/TSST, only 5 subjects demonstrated positive NGS. Cutibacterium was the predominant species detected on their implant surface and in their capsules. Further study may be indicated to measure IgG antibody responses to Cutibacterium. Although the findings of elevated IgG anti-SEA may suggest a possible role for IgG antibody responses or a possible indication of a bacterial infection involved in inducing implant-related symptoms in the Cohort A subjects, it is important to note that due to immunological memory, the IgG response needs to be viewed as a “historical” or “integrated” indicator which reflects both past and current infections. Moreover, the presence of elevated quantitative IgG antibody levels specific for the Staphylococcal superantigens did not consistently match with the presence of detectable S. aureus DNA on the surface of removed implants or capsules from subjects in Cohorts A and B. In addition, the symptoms reported most frequently by the subjects in Cohort A (fatigue, brain fog, muscle pain and weakness, memory issues, and anxiety) are not commonly reported by individuals who are experiencing infectious or allergy/immune response–related adverse reactions. The DNA and antibody data generated fail to support an “infectious” theory as a primary cause for the systemic symptoms reported in BII. This information may aid surgeons and patients when making decisions concerning implant or capsule removal.
CRP is an acute-phase reactant protein that has both proinflammatory and anti-inflammatory properties. Unlike the erythrocyte sedimentation rate, which is an indirect test for the presence of inflammation, the CRP can rise and fall quickly in response to the onset or removal of an inflammatory stimulus. Cohort A demonstrated an elevated CRP compared with Cohorts B and C. An elevated CRP is most often related to an infectious etiology; however, there was no statistical difference in NGS positivity between the cohorts or a higher grade of capsular contracture in Cohort A compared with Cohort B. CRP elevations are reported in patients with IBS, sleep disturbances, autoimmune disease, and mixed connective tissue disease. There are also studies which show an association between anxiety and stress-related disorders and higher levels of inflammatory markers, as measured by CRP.21,22 The baseline symptom surveys and PROMIS data showed a significantly higher percentage of subjects with a high severity level of anxiety in Cohort A than in Cohort B or the control cohort. Subjects in Cohort A also reported statistically higher rates of chronic fatigue syndrome, fibromyalgia, Sjogren’s syndrome, IBS, and general autoimmune illness than subjects in Cohorts B or C.
Cytokines are proteins produced by cells that regulate the body’s response to disease and infection and mediate normal cellular functions. Because many symptoms attributed to BII are thought to be due to unique responses to implants, we hypothesized that specific cytokines might be increased in serum of subjects of Cohort A relative to Cohorts B or C. To test our hypothesis, we analyzed serum levels of 12 cytokines representing different T helper subsets, Th1, Th2, and Th17. This panel previously was able to distinguish cytokine levels in seromas of women with breast implant–associated anaplastic large cell lymphoma from those with benign seromas.23 Significantly, in the current study group of 150 subjects, most subjects in all 3 cohorts had normal levels of all cytokines. However, 3 serum cytokines (IL-22, IL-13, IL-17A) were statistically higher in the sera of subjects in Cohort A than in subjects in Cohorts B and C. There were also statistically higher levels of IL-13 and IL-17 in Cohort A vs Cohort B, but not between Cohort A and Cohort C, for which we have no explanation. There was a statistically higher level of IL-22 in Cohort A than in both control cohorts. The subjects with elevated IL-17 also had elevated IL-6 and TNFα which has also been associated with anxiety disorders.24 It is important to note that although there were statistical differences between the cohorts, cytokine elevations occurred in a minority of subjects in Cohort A.
IL-22 has been assigned to the Th17 group but also has been delegated its own Th22 subset by immunologists.25 Increased Th22 cells and high serum levels of IL-22 have been associated with autoimmune thyroiditis including early phases of Hashimoto’s thyroiditis.26 Interestingly, 5 of 6 patients with elevated serum IL-22 levels had hypothyroidism and/or Hashimoto’s thyroiditis. IL-22 is associated with tissue repair and is predominantly active at interfaces with the environment such as skin, respiratory tract, and gastrointestinal tract.27 IL-22 is regulated by the aryl hydrocarbon receptor, which is activated by environmental toxins.28,29 Of particular significance in this group of women with BII is that the aryl hydrocarbon receptor is activated by arsenic, which is associated with tobacco and smoking.30 Among the 7 women with higher serum IL-22 levels, 4 had a history of smoking. IL-22 has also been found to increase synovial hyperplasia in joints of psoriatic arthritis; however, there was no correlation with synovial metaplasia in capsules of subjects in Cohort A.
IL-13 is a Th2 cytokine that is commonly associated with allergic or atopic disease, eg, atopic dermatitis (eczema), asthma, and food or airborne allergies.31,32 Among the 8 women in Cohort A with increased serum levels of IL-13, 4 reported airborne allergies. IL-13 upregulates eotaxins, a subfamily of cytokines which recruit eosinophils that are associated with atopic diseases.33 We did not find any remarkable infiltration of capsular tissues with eosinophils in these patients. IL-13 is active in promoting fibrosis and could play a role in formation of fibrous capsules and diseases with prominent fibrosis, eg, scleroderma.34,35 However, most capsules in Cohort A did not show remarkable fibrosis and no patient with elevated serum IL-13 reported scleroderma.
IL-17A is a Th17 cytokine that is involved in autoimmune diseases, eg, psoriasis, systemic lupus, and rheumatoid arthritis.36,37 IL-17 inhibitors have been used to treat these diseases.38 Among the 3 subjects with higher serum IL-17, 1 had fibromyalgia, mixed connective tissue disease, Raynaud’s syndrome, and scleroderma. Antinuclear antibodies (ANAs) are commonly increased in autoimmune diseases; however, about 26% of the normal population are ANA positive, with 17% having moderate levels and 9% having high ANA antibody levels.39 Cohort A self-reported a higher ANA positivity relative to the other 2 cohorts.
One of the limitations of this study was the sample size, which included 50 subjects in each cohort. The cohorts, however, provided a large enough sample size to determine significant relationships from the data. Systemic symptoms data relied on the use of self-reported systemic symptom surveys, which may be limited by the fact that they cannot be independently verified. These data were therefore supplemented with the National Institutes of Health PROMIS questionnaire, a validated instrument. Several additional analyses were considered during this research. Extensive validation was undertaken prior to the collection of specimens for NGS analysis as there is reason to believe that the distribution of microbes may not be uniform on the surface of an implant or capsule. Prior to study specimen collection, multiple methods were tested to validate the collection method that yielded the highest positivity. It would have been ideal to submit the entire capsule for NGS; however, part of the capsule was also required for heavy-metal analysis and tissue pathology. Further investigation, including whole genomic sequencing of microbes identified by NGS, was not pursued as the microbial data did not demonstrate specific bacterial pathogens that were statistically related to specific systemic symptoms. Finally, although rigorous histopathology was performed on capsule tissue, techniques such as energy-dispersive X-ray analysis may prove useful in the future for detection of heavy metals and silicone in fresh tissues as well as specimens stored on fixed slides.40
CONCLUSIONS
This study sought to determine if there were quantitative differences in biospecimens in patients with self-described breast implant illness compared to 2 control groups. Advanced metagenomics revealed no statistical difference in bacterial positivity on the surface of the implant or within the capsule between Cohorts A and B, and the presence of bacteria did not influence the number of symptoms reported or symptom relief postoperatively. Although there were statistically significant differences in some of the biospecimen analysis performed, these findings did not occur in all, or even a majority, of subjects in the cohort of women with systemic symptoms they attribute to implants. Had any specific finding been identified in most subjects, it could potentially have been used to make a diagnosis of BII as a specific pathologic entity and potentially point to a possible cause for the systemic symptoms that patients experience. This study adds to the literature by demonstrating a lack of consistent, identifiable biomedical markers that differentiate subjects with self-described BII from control groups.
Contributor Information
Patricia McGuire, Washington University, St Louis, MO, USA and a clinical editor for Aesthetic Surgery Journal.
Caroline Glicksman, Hackensack Meridian School of Medicine, Nutley, NJ, USA and a clinical editor for Aesthetic Surgery Journal.
Roger Wixtrom, LSCI, Springfield, VA, USA.
C James Sung, Warren Alpert School of Medicine of Brown University, Providence, RI, USA.
Robert Hamilton, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Marisa Lawrence, plastic surgeon in private practice, Atlanta, GA, USA.
Melinda Haws, Vanderbilt University, Nashville, TN, USA.
Sarah Ferenz, Chicago Medical School, North Chicago, IL, USA.
Marshall Kadin, Brown University Alpert School of Medicine, Providence, RI, USA.
Disclosures
Dr McGuire is a clinical investigator and consultant for Establishment Labs (Alejuela, Costa Rica). Dr Glicksman is the medical director for Motiva US Breast Implant Clinical Trial (Establishment Labs). Dr Wixtrom is a consultant, Mentor (Mentor Worldwide LLC, Irvine, CA) and PhaseOne Health (Nashville, TN). Dr Haws is an advisory board member for Sientra Strategic (Santa Barbara, CA) and RealSelf Business (Seattle, WA) and an investor in Strathspey Crown (Newport Beach, CA). Dr Kadin receives grants from the Aesthetic Surgery Education and Research Foundation (ASERF; The Aesthetic Society, Garden Grove, CA), Allergan (Irvine, CA), and Abbvie (North Chicago, IL) for his laboratory research. The remaining authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding
This study was funded entirely by the Aesthetic Surgery Education and Research Foundation (ASERF; The Aesthetic Society, Garden Grove, CA).
REFERENCES
- 1. Wixtrom R, Glicksman C, Kadin M, et al. Heavy metals in breast implant capsules and breast tissue: findings from the systemic symptoms in women—biospecimen analysis study: Part 2. Aesthet Surg J. 2022;42(9):1067–1076. doi: 10.1093/asj/sjac106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Adams WPRios JL Jr, Smith SJ. Enhancing patient outcomes in aesthetic and reconstructive breast surgery using triple antibiotic breast irrigation: six-year prospective clinical study. Plast Reconstr Surg. 2006;117(1):30–36. doi: 10.1097/01.prs.0000185671.51993.7e [DOI] [PubMed] [Google Scholar]
- 3. Wixtrom RN, Stutman RL, Burke RM, Mahoney AK, Codner MA. Risk of breast implant bacterial contamination from endogenous breast flora, prevention with nipple shields, and implications for biofilm formation. Aesthet Surg J. 2012;32(8):956–963. doi: 10.1177/1090820X12456841 [DOI] [PubMed] [Google Scholar]
- 4. Tamboto H, Vickery K, Deva AK. Subclinical (biofilm) infection causes capsular contracture in a porcine model following augmentation mammaplasty. Plast Reconstr Surg. 2010;126(3):835–842. doi: 10.1097/prs.0b013e3181e3b456 [DOI] [PubMed] [Google Scholar]
- 5. Lee M, Ponraia G, McLeod K, et al. Breast implant illness: a biofilm hypothesis. Plastic Reconstr Surg Global Open. 2020; 8(4):e2755. doi: 10.1097/GOX.0000000000002755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hamilton RG, Hemmer W, Nopp A, Kleine-Tebbe J. Advances in IgE testing for diagnosis of allergic disease. J Allergy Clin Immunol Pract. 2020;8(8):2495–2504. doi: 10.1016/j.jaip.2020.07.02 [DOI] [PubMed] [Google Scholar]
- 7. Hamilton RG, Matsson PNJ, Adkinson NF Jr, et al. ILA20 Analytical Performance Characteristics, Quality assurance, and Clinical Utility of Immunological Assays for Human Immunoglobulin E Antibodies of Defined Allergen Specificities, 3rd ed. Clinical and Laboratory Standards Institute; 2017. [Google Scholar]
- 8. National Library of Medicine . Medline Plus. C-Reactive protein test. https://medlineplus.gov/heartattack.html. Updated December 3, 2020. Accessed April 20, 2021
- 9. Monastero RN, Pentyala S. Cytokines as biomarkers and their respective clinical cutoff levels. Int J Inflam. 2017;2017:4309485. doi: 10.1155/2017/4309485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Burkhardt BR, Fried M, Schnur PL, Tofield JJ. Capsules, infection, and intraluminal antibiotics. Plast Reconstr Surg. 1981;68(1):43–49. doi: 10.1097/00006534-198107000-00010 [DOI] [PubMed] [Google Scholar]
- 11. Ahn CY, Ko CY, Wagar EA, Wong, RS, Shaw WW. Microbial evaluation: 139 implants removed from symptomatic patients. Plast Reconstr Surg. 1996;98(7):1225–1229. doi: 10.1097/00006534-199612000-00016 [DOI] [PubMed] [Google Scholar]
- 12. Wagley S, Morcrette H, Kovacs-Simon A, et al. Bacterial dormancy: a subpopulation of viable but non-culturable cells demonstrates better fitness for revival. PLoS Pathog. 2021;17(1):e1009194. doi: 10.1371/journal.ppat.1009194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stone J, Boost T. Cytological features of breast peri-implant synovial metaplasia. Acta Cytologica. 2014;58:511–513. doi: 10.1159/000369054 [DOI] [PubMed] [Google Scholar]
- 14. Dijkman HBPM, Slaats I, Bult P. Assessment of silicone particles migration among women undergoing removal or revision of silicone breast implants in the Netherlands. JAMA Network Open. 2021;4(9):e2125381. 10.1001/jamanetworkopen.2021.25381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Spoor J, de Jong D, Leeuwens F. Silicone particle migration: a misleading report. Aesthetic Surg J. 2022;42(4):NP261–NP262. doi: 10.1093%2Fasj%2Fsjab377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Johnson HM, Russell JK, Pontzer CH. Staphylococcal enterotoxin microbial superantigens. FASEB J. 1991;5(12):2706–2712. doi: 10.1096/fasebj.5.12.1916093 [DOI] [PubMed] [Google Scholar]
- 17. Senok AC, Verstraelen H, Temmerman M, Botta GA. Probiotics for the treatment of bacterial vaginosis. Cochrane Database Syst Rev. 2009;4:CD006289. doi: 10.1002/14651858.cd006289.pub2. [DOI] [PubMed] [Google Scholar]
- 18. Tong SY, Davis JS, Eichenberger E, Holland TL, Fowler VG. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev. 2015;28(3):603–661. doi: 10.1128/cmr.00134-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jarraud S, Peyrat MA, Lim A, et al. A highly prevalent operon of enterotoxin gene forms a putative nursery of superantigens in Staphylococcus aureus. J Immunol. 2001;166(1):669–677. doi: 10.4049/jimmunol.166.1.669 [DOI] [PubMed] [Google Scholar]
- 20. Orfali RL, Sato MN, Santos VG, et al. Staphylococcal enterotoxin B induces specific IgG4 and IgE antibody serum levels in atopic dermatitis. Int J Dermatol. 2014;54:898–904. doi: 10.1111/ijd.12533 [DOI] [PubMed] [Google Scholar]
- 21. Kennedy E, Niedzwiedz C. The association of anxiety and stress related disorders with C-reactive protein (CRP) within the UK Biobank. Brain Behav Immun Health. 2021;19:100410. doi: 10.1016/j.bbih.2021.100410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Costello H, Gould RL, Abrol E, Howard R. Systematic review and meta-analysis of the association between peripheral inflammatory cytokines and generalized anxiety disorder. BMJ Open. 2019;9(7):e027925. doi: 10.1136/bmjopen-2018-027925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kadin ME, Morgan J, Kouttab N, et al. Comparative analysis of cytokines of tumor cell lines, malignant and benign effusions around breast implants. Aesthet Surg J. 2020;40(6):630–637. doi: 10.1093/asj/sjz243 [DOI] [PubMed] [Google Scholar]
- 24. Quagliato LA, Nardi AE. Cytokine alterations in panic disorder: a systematic review. J Affect Disord. 2018;228:91–96. doi: 10.1016/j.jad.2017.11.094 [DOI] [PubMed] [Google Scholar]
- 25. Eyerich S, Eyerich K, Pennino D, et al. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest. 2009;119(12):3573–3585. doi: 10.1172/jci40202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ruggeri RM, Minciullo P, Saitta S, et al. Serum interleukin-22 (IL-22) is increased in the early stage of Hashimoto’s thyroiditis compared to non-autoimmune thyroid disease and healthy controls. Hormones. 2014;13(3):338–44. doi: 10.14310/horm.2002.1483. [DOI] [PubMed] [Google Scholar]
- 27. Avitabile S, Odorisio T, Madonna S, et al. Interleukin-22 promotes wound repair in diabetes by improving keratinocyte pro-healing functions. J Invest Dermatol. 2015;135(11):2862–2870. doi: 10.1038/jid.2015.278. [DOI] [PubMed] [Google Scholar]
- 28. Alam MS, Maekawa Y, Kitamura A, et al. Notch signaling drives IL-22 secretion in CD4+ T cells by stimulating the aryl hydrocarbon receptor. Proc Natl Acad Sci USA. 2010;107(13):5943–5948. doi: 10.1073/pnas.0911755107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rogers S, de Souza AR, Zago M, et al. Aryl hydrocarbon receptor (AhR)-dependent regulation of pulmonary miRNA by chronic cigarette smoke exposure. Sci Rep. 2017;7:40539. doi: 10.1038%2Fsrep40539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mohammadi-Bardbori A, Vikstrom Bergander L, Rannug U, Rannug A. NADPH oxidase-dependent mechanism explains how arsenic and other oxidants can activate aryl hydrocarbon receptor signaling. Chem Res Toxicol. 2015;28(12):2278–2286. doi: 10.1021/acs.chemrestox.5b00415 [DOI] [PubMed] [Google Scholar]
- 31. Buzney CD, Gottlieb AB, Rosmarin D. Asthma and atopic dermatitis: a review of targeted inhibition of interleukin-4 and interleukin-13 as therapy for atopic disease. J Drugs Dermatol. 2016;15(2):165–171. [PubMed] [Google Scholar]
- 32. Corren J. Role of interleukin-13 in asthma. Curr Allergy Asthma Rep. 2013;13(5):415–420. doi: 10.1007/s11882-013-0373-9 [DOI] [PubMed] [Google Scholar]
- 33. Matsukura S, Stellato C, Georas SN, et al. Interleukin-13 upregulates eotaxin expression in airway epithelial cells by a STAT6-dependent mechanism. Am J Respir Cell Mol Biol. 2001;24(6):755–761. doi: 10.1165/ajrcmb.24.6.4351 [DOI] [PubMed] [Google Scholar]
- 34. Lee CG, Homer RJ, Zhu Z, et al. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor β1. J Exp Med. 2001;194(6):809–821. doi: 10.1084/jem.194.6.809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fuschiotti P. Role of IL-13 in systemic sclerosis. Cytokine. 2011;56(3):544–549. doi: 10.1016/j.cyto.2011.08.030 [DOI] [PubMed] [Google Scholar]
- 36. Brembilla NC, Montanari E, Truchetet ME, Raschi E, Meroni P, Chizzolini C. Th17 cells favor inflammatory responses while inhibiting type I collagen deposition by dermal fibroblasts: differential effects in healthy and systemic sclerosis fibroblasts. Arthritis Res Ther. 2013;15(5):R151. doi: 10.1186/ar4334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen K, Kolls JK. Interluekin-17A (IL17A). Gene. 2017;614:8–14. doi: 10.1016/j.gene.2017.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kim BS, Park YJ, Chung Y. Targeting IL-17 in autoimmunity and inflammation. Arch Pharm Res. 2016;39(11):1537–1547. doi: 10.1007/s12272-016-0823-8 [DOI] [PubMed] [Google Scholar]
- 39. Prithvi R, Li QZ, Karp D, et al. Antinuclear antibodies in general population: what does that mean? J Immun. 2013;190(1 Supplement)197.2:0022–1767. [Google Scholar]
- 40. Scimeca M, Bischetti S, Lamsira HK, Bonfiglio R, Bonanno E. Energy dispersive X-ray (EDX) microanalysis: a powerful tool in biomedical research and diagnosis. Eur J Histochem. 2018;62(1):2841. doi: 10.4081/ejh.2018.2841 [DOI] [PMC free article] [PubMed] [Google Scholar]