Abstract
Objective
This study investigated the association between obesity, assessed using body mass index (BMI) and waist circumference (WC), and pre-frailty/frailty among older adults over 21 years of follow-up.
Design
Prospective cohort study.
Setting
Population-based study among community-dwelling adults in Tromsø municipality, Norway.
Participants
2340 women and 2169 men aged ≥45 years attending the Tromsø study in 1994–1995 (Tromsø4) and 2015–2016 (Tromsø7), with additional BMI and WC measurements in 2001 (Tromsø5) and 2007–2008 (Tromsø6).
Primary outcome measure
Physical frailty was defined as the presence of three or more and pre-frailty as the presence of one to two of the five frailty components suggested by Fried et al: low grip strength, slow walking speed, exhaustion, unintentional weight loss and low physical activity.
Results
Participants with baseline obesity (adjusted OR 2.41, 95% CI 1.93 to 3.02), assessed by BMI, were more likely to be pre-frail/frail than those with normal BMI. Participants with high (OR 2.14, 95% CI 1.59 to 2.87) or moderately high (OR 1.57, 95% CI 1.21 to 2.03) baseline WC were more likely to be pre-frail/frail than those with normal WC. Those at baseline with normal BMI but moderately high/high WC or overweight with normal WC had no significantly increased odds for pre-frailty/frailty. However, those with both obesity and moderately high/high WC had increased odds of pre-frailty/frailty. Higher odds of pre-frailty/frailty were observed among those in ‘overweight to obesity’ or ‘increasing obesity’ trajectories than those with stable normal BMI. Compared with participants in a stable normal WC trajectory, those with high WC throughout follow-up were more likely to be pre-frail/frail.
Conclusion
Both general and abdominal obesity, especially over time during adulthood, is associated with an increased risk of pre-frailty/frailty in later years. Thus maintaining normal BMI and WC throughout adult life is important.
Keywords: NUTRITION & DIETETICS, PUBLIC HEALTH, EPIDEMIOLOGY
Strengths and limitations of this study.
This study has a long follow-up period of 21 years.
This study takes into account changes in body mass index and waist circumference occurring through the follow-up period.
Frailty status was defined using a slightly modified version of Fried’s physical frailty criteria.
Frailty and pre-frailty were combined as one outcome.
Information on frailty was only available at follow-up.
Background
Frailty is a dynamic multifactorial geriatric syndrome characterised by physiological deterioration, increased vulnerability and decreased resilience towards external stressors.1 2 Frailty is associated with an increased risk of adverse events such as falls, disability, hospitalisation, reduced quality of life and mortality.1 2 It is preceded by pre-frailty, a multidimensional, transitional risk state.3 4 Fried’s frailty phenotype identifies pre-frailty as the presence of one or two and frailty as three or more of the five criteria: unintentional weight loss, self-reported exhaustion, weakness, slow walking speed and low physical activity.5 The prevalence of frailty and pre-frailty, defined using Fried’s physical frailty measure,5 among community-dwelling people aged ≥50 years across 62 countries, has been estimated to be 12% and 46%, respectively.6
Rapid population ageing has become a global phenomenon.7 Ageing is typically associated with changes in body composition, such as decreased muscle mass and redistribution of total and regional fat.8–10 Underweight older adults with minimal reserve capacity are at risk of adverse health outcomes,5 11 and unintentional weight loss is commonly acknowledged as a significant frailty indicator.5 However, a growing body of evidence also suggests a positive association between obesity among older adults and the risk of frailty.10 12–16 Obesity aggravates the age-related decline in muscle strength, aerobic capacity and physical functionality, thus worsening health and well-being.10 11 14 17 18 It is also closely associated with metabolic disorders, inflammageing and oxidative stress, all of which have been suggested to contribute to the risk of frailty.14 19
Anthropometric measures, including body mass index (BMI) and waist circumference (WC), are simple, cost-effective tools that reflect an individual’s body composition and nutritional status. They are one of the widely used nutritional items for detecting frailty.20 BMI indicates general obesity, while WC indicates abdominal obesity. When used together, they effectively assess obesity-related risks at the population level.21–23 Some studies have detected a U-shaped association between BMI and frailty.13 15 24 Midlife overweight and obesity, assessed by BMI, have been associated with the risk of pre-frailty and frailty in older age.25 26 Similarly, a positive association between high WC and frailty among older adults has been observed in some studies.9 16 27–29 These findings are even more relevant in the present context, where obesity prevalence is increasing across all age groups, posing a global public health challenge.30
Though the evidence is expanding, there have been limited longitudinal studies exploring and comparing the relationship of both BMI and WC with the risk of developing pre-frailty and frailty over a long follow-up period.29 Few have explored changes in BMI31 32 and its association with frailty, while studies that consider changes in WC in association with the development of frailty seem to be lacking. Therefore, the present study aimed to investigate the association of BMI and WC, separately and concurrently, with the risk of pre-frailty/frailty after 21 years of follow-up. Additionally, this study assessed changes in BMI and WC through the follow-up period and their association with pre-frailty/frailty.
Methods
The Tromsø study
This study uses data from the Tromsø study, an ongoing population-based study in the Tromsø municipality, Norway, consisting of seven surveys: Tromsø1 (1974), Tromsø2 (1979–1980), Tromsø3 (1986–1987), Tromsø4 (1994–1995), Tromsø5 (2001), Tromsø6 (2007–2008) and Tromsø7 (2015–2016). More than 45 000 women and men have participated in at least one of the surveys.33 The earlier surveys (Tromsø1–Tromsø3) did not include WC measurements. Therefore, the present study uses data from Tromsø4 (baseline) to Tromsø7 (follow-up). Tromsø4 included 27 158 participants aged 25–97 years, Tromsø5 included 8130 participants aged 30–89 years, Tromsø6 included 12 984 participants aged 30–87 years and Tromsø7 included 21 083 participants aged 40–99 years. The detailed information on the recruitment and the attendance of the participants has been described in the Tromsø study's website.33
Study sample
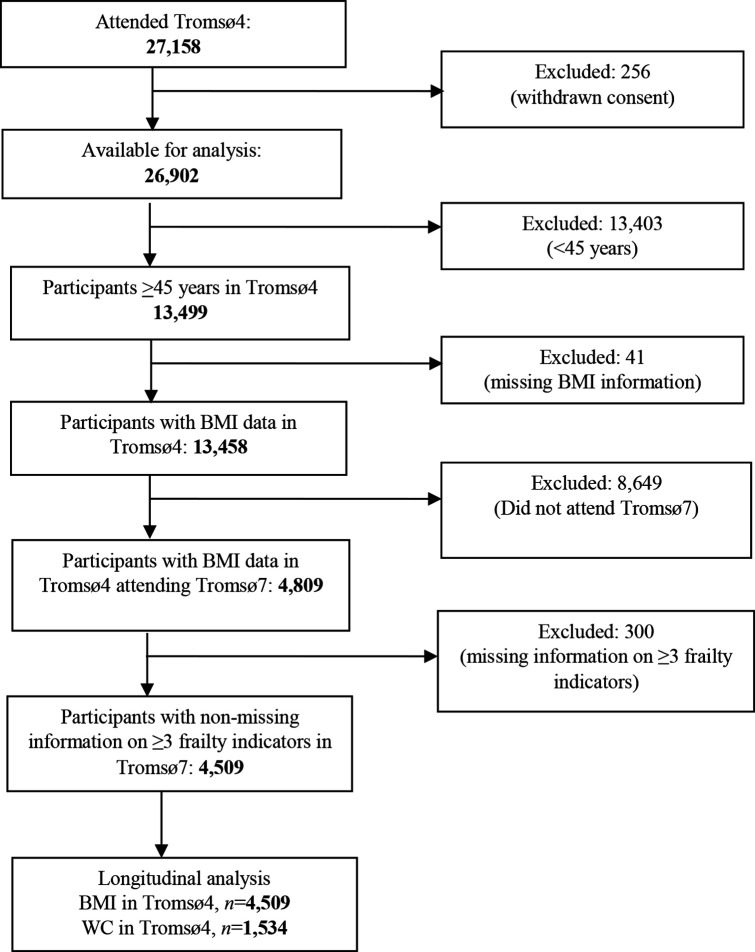
The present study included Tromsø4 participants aged ≥45 years with valid information on BMI who also attended Tromsø7, that is, 21 years of follow-up (n=4809). Participants with missing information on three or more frailty indicators in Tromsø7 were excluded (figure 1). Our primary analytical sample had 4509 participants. Out of these, 1534 participants had information on WC at Tromsø4, and 1391 had repeated measurements on both BMI and WC between Tromsø4 and Tromsø7.
Figure 1.
Flowchart displaying participants’ inclusion and exclusion. BMI, body mass index; WC, waist circumference.
Exposure
Bodyweight in kilograms and height in metres were measured wearing light clothes and no footwear. WC was measured using tape to the nearest centimetre at the umbilical level. All measurements were performed by trained personnel. BMI was calculated as the weight divided by the square of the height (kg/m2) and categorised as underweight (<18.5 kg/m2), normal (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2) and obesity (≥30.0 kg/m2) according to the WHO criteria.34 WC was categorised as normal (men ≤94 cm and women ≤80 cm), moderately high (men 95–102 cm and women 81–88 cm) and high (men >102 cm and women >88 cm) according to WHO.35
Frailty assessment
A modified version of Fried et al’s frailty phenotype5 was used to operationalise frailty in Tromsø7. Frailty was not operationalised at baseline as complete information on frailty indicators was unavailable.
Five indicators were assessed at follow-up (online supplemental table 1):
bmjopen-2022-065707supp001.pdf (325.8KB, pdf)
Unintentional weight loss: Self-reported involuntary weight loss during the last 6 months.36
Exhaustion: Response ‘pretty much’ or ‘very much’ to the question: ‘During the last week, have you experienced that everything is a struggle?’ from the Hopkins’ Symptom Checklist-10.37
Walking speed: Short Physical Performance Battery test,38 39 where the fastest time out of two walks was selected and converted to seconds per 15 feet from seconds per 4 metres. Sex-adjusted and height-adjusted cut-offs, according to Fried et al,5 were used to identify participants with a low walking speed.
Weakness: Grip strength was measured using a newly calibrated Jamar+ Digital Dynamometer (Patterson Medical, Warrenville, Illinois, USA) following the Southampton protocol procedures.40 Sex-specific and BMI-specific cut-offs suggested by Fried et al 5 were used to identify participants with low grip strength.
Low physical activity: Response ‘Reading, watching TV/screen or other sedentary activity’ to the question: ‘Describe your exercise and physical exertion in leisure time over the last year’ from the Saltin-Grimby Physical Activity Level Scale for leisure-time physical activity.41
Participants were categorised as robust (0), pre-frail (1–2) and frail (≥3) based on the number of frailty indicators present.
Covariates
The potential covariates in this study were selected based on the existing knowledge and literature on frailty status. Sociodemographic characteristics included age, sex, educational level (primary/partly secondary education (up to 10 years of schooling), upper secondary education (minimum of 3 years), college/university short (<4 years) and college/university long (≥4 years)) and marital/cohabitation status (married/cohabiting or single/not cohabiting with a partner). Self-reported smoking status was categorised as current, former or never smoker. Self-reported alcohol intake level was categorised as never-drinker, infrequent drinker (<2–4 times/month) and frequent drinker (>2–3 times/week). Comorbidity was defined using Charlson’s comorbidity index42 without weighting the diseases. It was categorised as ‘no comorbidity’ and ‘comorbidity’ based on the self-reported presence of coronary heart disease (angina pectoris/myocardial infarction), stroke, diabetes, cancer, pulmonary disease (asthma/chronic bronchitis/emphysema) and peptic ulcer. Social support was categorised as self-reported ‘not enough good friends’ or ‘enough good friends’. Self-perceived health status was categorised as ‘poor’ or ‘good’. Baseline physical activity level was categorised as no/low physical activity (0 hours/week spent in hard physical activity or ≤2 hours/week spent in light physical activity) and high physical activity (≥1 hour/week in hard physical activity or ≥3 hours/week in light physical activity).
Statistical analysis
The sociodemographic and lifestyle factors at baseline across robust and pre-frail/frail groups were described using mean and SD for continuous variables and proportion and count for categorical variables. The differences between the two groups were tested using the student’s t-test for continuous variables and the χ2 test for categorical variables.
Multivariable logistic regression analysis was used to assess the effect of BMI and WC on pre-frailty/frailty at follow-up. Five different longitudinal associations were assessed: baseline BMI and pre-frailty/frailty; baseline WC and pre-frailty/frailty; joint BMI and WC profile at baseline and pre-frailty/frailty; BMI trajectories and pre-frailty/frailty; and WC trajectories and pre-frailty/frailty. The models were minimally adjusted for age and sex (Model 1) and further adjusted for educational level, marital/cohabitation status, smoking status, alcohol intake, social support, self-perceived health and physical activity level at baseline (Model 2). The adjustment variables were selected using a stepwise backward regression procedure. No significant collinearity or interaction was detected between covariates in the model.
Group-based trajectory modelling (GBTM) was conducted among 1391 participants to assess changes in the BMI and WC throughout the 21-year follow-up period, with measurements on both BMI and WC available at Tromsø4, Tromsø5, Tromsø6 and Tromsø7. GBTM, also known as latent class growth analysis, is a semiparametric technique that identifies distinct subgroups of individuals following a similar pattern of change over time on a given variable, using finite mixtures of defined probability distributions.43 Different models with varying numbers of trajectory groups, varying functional forms and orders were compared. The most appropriate model was selected based on the Bayesian Information Criterion44 and then introduced into longitudinal multivariable logistic regression models. The distinct BMI and WC trajectories were named based on their observed pattern. The WC trajectories were sex-stratified due to varying cut-off levels for men and women.
A new variable with five distinct strata (normal BMI and normal WC; normal BMI and moderately high/high WC; overweight and low WC; overweight and moderately high/high WC; and obesity and moderately high/high WC) was formed by combining different categories of BMI and WC. They were then introduced into the multivariable models to assess the concurrent effects of BMI and WC on frailty status. While forming the new joint variable, the underweight group was removed because of low prevalence (<1%), and moderately high and high WC groups were combined because of their low sample size when stratified.
Additional supplementary analyses were carried out. The cross-sectional association between BMI and WC level and frailty status at Tromsø7 was assessed. Since pre-frailty/frailty could not be assessed at baseline, the primary longitudinal analyses were repeated in a subpopulation (n=4050), excluding participants aged 60 years and older at Tromsø4 who might have had an increased probability of being pre-frail/frail at that time point. The majority of the participants in the pre-frail/frail group had a frailty score of 1. In order to account for potential misclassification, analyses were performed on a further restricted subsample with a frailty score ≥2 at Tromsø7 (n=3124). The primary longitudinal analyses were also repeated among the subgroup of participants with non-missing information on all five frailty components (n=2864), and the association of obesity with each frailty component were assessed.
All the statistical analyses were conducted using Stata V.16.45 Statistical significance was set at p<0.05. The results are expressed as adjusted ORs with 95% CIs.
Patient and public involvement
Patients and the public were not involved in this research’s design, conduct, reporting or dissemination plans.
Results
Study population
The mean age at baseline was 51.6 years, and the participants were followed up for 21 years. Among the participants 28.4% were pre-frail, 1.1% were frail and 70.5% were robust at follow-up (table 1). In total, 50.6% of the robust group and 55.0% of the pre-frail/frail group were women. Most robust and pre-frail/frail participants were either married or cohabiting (84.3% and 80.3%) and reported having enough good friends (83.1% and 80.5%) at baseline. All the baseline characteristics, except comorbidity, were significantly different in the robust and the pre-frail/frail groups (table 1).
Table 1.
Baseline characteristics of participants by frailty status at follow-up: the Tromsø study 1994–2016
| Frailty status | P value | ||
| Robust (% (n)) 70.5 (3179) |
Pre-frail/frail (% (n)) 29.5 (1330) |
||
| Age in years, mean (SD) | 51.1 (5.1) | 52.8 (5.9) | 0.000* |
| Women | 50.6 (1608) | 55.0 (732) | 0.006 |
| Smoking status | |||
| Current smokers | 27.0 (858) | 33.7 (448) | |
| Former smokers | 36.1 (1149) | 34.0 (452) | 0.001 |
| Never | 36.9 (1172) | 32.3 (430) | |
| High physical activity level | 69.5 (2210) | 56.9 (756) | 0.001 |
| Married or cohabiting | 84.3 (2679) | 80.3 (1068) | 0.001 |
| Self-perceived health—good | 75.4 (2394) | 61.5 (818) | <0.001 |
| Social support—enough good friends | 83.1 (2404) | 80.5 (976) | 0.041 |
| Educational level | |||
| Primary/partly secondary | 32.8 (1041) | 42.4 (562) | |
| Upper secondary | 34.3 (1085) | 34.2 (453) | <0.001 |
| College/university short | 16.5 (524) | 12.8 (169) | |
| College/university long | 16.4 (520) | 10.6 (141) | |
| Alcohol intake | |||
| Never/abstaining | 9.0 (286) | 11.9 (158) | |
| Infrequent drinker | 76.2 (2419) | 76.6 (1015) | <0.001 |
| Frequent drinker | 14.8 (468) | 11.5 (152) | |
| Prevalent diseases | |||
| Pulmonary disease† | 8.6 (272) | 9.5 (126) | 0.323 |
| Coronary heart disease‡ | 2.3 (73) | 4.5 (59) | <0.001 |
| Diabetes | 0.4 (12) | 0.6 (8) | 0.300 |
| Cancer | 2.8 (79) | 3.5 (42) | 0.210 |
| Stroke | 0.6 (19) | 0.8 (11) | 0.386 |
| Peptic ulcer | 7.0 (197) | 8.9 (105) | 0.033 |
| Comorbidity | 1.9 (59) | 2.7 (36) | 0.070 |
Values are percentages (numbers); p value: χ2 test for categorical variables.
*Student’s t-test.
†including asthma/chronic bronchitis/emphysema.
‡including angina pectoris/myocardial infarction.
When assessed at follow-up, all the sociodemographic, lifestyle and disease-related factors were significantly associated with pre-frailty/frailty (online supplemental table 2). When the eligible participants lost to follow-up (n=8649) were compared with the attendees, they were found to be older (mean age 63.2 years) with a less healthy lifestyle and higher comorbidities (online supplemental table 3).
BMI and WC
At baseline, the proportion of individuals with underweight was low (<1%) (table 2). The proportion of individuals with normal BMI was higher among the robust group than the pre-frail/frail group (47.6% vs 39.3%), whereas the proportion of individuals with obesity was higher among the pre-frail/frail group (17.1% vs 8.4%). The robust group had a higher proportion of individuals with normal WC than the pre-frail/frail group (51.5% vs 37.3%), whereas the pre-frail/frail group had a higher proportion of individuals with high WC (27.7% vs 17.4%). A similar distribution of different BMI and WC categories across robust and pre-frail/frail groups was observed at follow-up (online supplemental table 2). Both robust and pre-frail/frail groups at follow-up had an increased proportion of individuals with obesity and high WC compared with baseline (table 2; online supplemental table 2).
Table 2.
Longitudinal association between BMI and WC, and pre-frailty/frailty: the Tromsø study 1994–2016
| Frailty status | ||||
| Robust (% (n)) |
Pre-frail/frail (% (n)) |
Model 1 OR (95% CI) |
Model 2 OR (95% CI) |
|
| BMI, kg/m2 | 70.5 (3179) | 29.5 (1330) | ||
| Underweight | 0.3 (11) | 0.7 (9) | 2.15 (0.88 to 5.29) | 1.32 (0.49 to 3.54) |
| Normal | 47.6 (1513) | 39.3 (522) | Ref. | Ref. |
| Overweight | 43.7 (1388) | 43.0 (572) | 1.18 (1.02 to 1.36) | 1.19 (1.02 to 1.39) |
| Obesity | 8.4 (267) | 17.0 (227) | 2.42 (1.98 to 2.98) | 2.41 (1.93 to 3.02) |
| WC, cm | n=952 | n=582 | ||
| Normal | 51.5 (490) | 37.3 (217) | Ref. | Ref. |
| Moderately high | 31.1 (296) | 35.0 (204) | 1.54 (1.21 to 1.96)* | 1.57 (1.21 to 2.03)* |
| High | 17.4 (166) | 27.7 (161) | 2.16 (1.65 to 2.83)* | 2.14 (1.59 to 2.87)* |
Model 1: adjusted for age and sex (*excluding sex) at baseline. Model 2: adjusted for age, sex, educational level, marital/cohabitation status, smoking status, alcohol intake, social support, self-perceived health and physical activity level (*excluding sex) at baseline.
BMI categories: Underweight: <18.5 kg/m2. Normal: 18.5–24.9 kg/m2. Overweight: 25.0–29.9 kg/m2. Obesity: ≥30 kg/m2.
WC categories: Normal: men ≤94 cm; women ≤80 cm. Moderately high: men 95–102 cm; women 81–88 cm. High: men >102 cm; women >88 cm.
BMI, body mass index; WC, waist circumference.
When BMI and WC level was assessed jointly at baseline (table 3), the robust group had a higher proportion of individuals with both BMI and WC in the normal range than the pre-frail/frail group (36.1% vs 29.1%). The proportion of individuals with both obesity and moderately high/high WC was higher among the pre-frail/frail group (16.9% vs 7.4%).
Table 3.
Association between combined BMI and WC profiles, and pre-frailty/frailty: the Tromsø study 1994–2016
| Longitudinal | Frailty status | |||
| Robust | Pre-frail/frail | Model 1 | Model 2 | |
| (% (n)) | (% (n)) | OR (95% CI) | OR (95% CI) | |
| BMI and WC profile, baseline | 62.8 (870) | 37.2 (515) | ||
| Normal BMI and normal WC | 36.1 (314) | 29.1 (150) | Ref. | Ref. |
| Normal BMI and moderately high/high WC | 8.4 (73) | 8.0 (41) | 1.13 (0.73 to 1.74) | 1.01 (0.63 to 1.61) |
| Overweight and normal WC | 15.9 (139) | 9.5 (49) | 0.74 (0.50 to 1.08) | 0.79 (0.53 to 1.19) |
| Overweight and moderately high/high WC | 32.2 (280) | 36.5 (188) | 1.40 (1.07 to 1.84) | 1.48 (1.11 to 1.98) |
| Obesity and moderately high/high WC | 7.4 (64) | 16.9 (87) | 2.86 (1.96 to 4.18) | 3.11 (2.07 to 4.70) |
Model 1: adjusted for age at baseline. Model 2: adjusted for age, educational level, marital/cohabitation status, smoking status, alcohol intake, social support, self-perceived health and physical activity level at baseline.
BMI, body mass index; WC, waist circumference.
The GBTM resulted in four distinct trajectories of BMI (n=1391): stable normal BMI (25.8%), stable overweight (44.8%), overweight to obesity (23.9%) and increasing obesity (5.5%) (online supplemental figure 1). The increasing obesity trajectory included individuals with BMI ≥30 kg/m2 at baseline, which kept increasing to a higher obesity level, that is, BMI ≥35 kg/m2. Four distinct WC trajectories were identified for both women (n=660) and men (n=731) (online supplemental figure 2). The WC trajectories for women were: stable normal WC (23.3%), moderately high to high WC (45.8%), gradually increasing high WC (26.6%) and steeply increasing high WC (4.3%). The WC trajectories for men were: stable normal WC (21.0%), stable moderately high WC (39.9%), moderately high to high WC (30.6%) and increasing high WC (8.5%).
BMI, WC and pre-frailty/frailty
Individuals who had obesity (OR 2.41, 95% CI 1.93 to 3.02) or overweight (OR 1.19, 95% CI 1.02 to 1.39) at baseline had significantly higher odds of becoming pre-frail/frail at follow-up compared with individuals with normal BMI (Model 2, table 2). No statistically significant association was detected between the underweight group and the odds of pre-frailty/frailty; however, the number of underweight individuals was insufficient to reach any conclusion. Participants with moderately high WC (OR 1.57, 95% CI 1.21 to 2.03) or high WC (OR 2.14, 95% CI 1.59 to 2.87) at baseline had higher odds of becoming pre-frail/frail at follow-up compared with individuals with a normal WC (Model 2, table 2).
The supplementary cross-sectional analysis (online supplemental table 4) indicated a significant association between obesity and pre-frailty/frailty among older adults (OR 1.88, 95% CI 1.54 to 2.30), whereas no association was detected between overweight and pre-frailty/frailty. As for WC, only high WC was associated with increased odds of pre-frailty/frailty (OR 1.45, 95% CI 1.20 to 1.76) in the cross-sectional analysis.
The longitudinal model that included joint BMI and WC profile at baseline showed that participants who had overweight with moderately high/high WC (OR 1.48, 95% CI 1.11 to 1.98) or participants who had obesity with moderately high/high WC (OR 3.11, 95% CI 2.07 to 4.70) had higher odds of being pre-frail/frail compared with participants with normal BMI and normal WC (Model 2, table 3). No significant association with pre-frailty/frailty was detected among participants who had normal BMI with moderately high/high WC or overweight with normal WC at baseline.
The sensitivity analyses restricted to participants with baseline age <60 years (online supplemental table 5) and further restricted to those with a frailty score ≥2 at follow-up (online supplemental table 6) confirmed the higher odds of pre-frailty/frailty among participants with baseline obesity and/or moderately high/high WC. However, no significant association was detected between participants in the overweight category and pre-frailty/frailty. The sensitivity analysis among participants with complete information on all five frailty components (online supplemental table 7) also generated similar results.
The model with BMI trajectories (Model 2, table 4) indicated higher odds of pre-frailty/frailty among participants in the overweight to obesity trajectory (OR 1.67, 95% CI 1.19 to 2.35) or those in the constantly increasing obesity trajectory (OR 3.12, 95% CI 1.80 to 5.41), compared with those in the stable normal BMI trajectory. Contrarily, there was no significant association in the stable overweight category. The model with WC trajectories (Model 2, table 4) showed that women in the gradually increasing high WC trajectory (OR 2.17, 95% CI 1.32 to 3.59) or the steeply increasing high WC trajectory (OR 4.09, 95% CI 1.54 to 10.90) had higher odds of being pre-frail/frail compared with women in the normal WC trajectory. Similarly, men in the increasing high WC trajectory (OR 3.36, 95% CI 1.71 to 6.59) had higher odds of pre-frailty/frailty compared with men in the normal WC trajectory. The same trend in the association between different BMI and WC trajectories and pre-frailty/frailty was observed in sensitivity analyses restricted to participants with baseline age <60 years (online supplemental table 5).
Table 4.
Association between BMI and WC trajectories and pre-frailty/frailty: the Tromsø study 1994–2016
| Frailty status | Model 1 | Model 2 | ||
| Robust (% (n)) |
Pre-frail/frail (% (n)) |
OR (95% CI) | OR (95% CI) | |
| BMI trajectories | 62.8 (874) | 37.2 (517) | ||
| Stable normal BMI | 27.8 (243) | 22.4 (116) | Ref. | Ref. |
| Stable overweight | 46.6 (407) | 42.4 (219) | 1.20 (0.91 to 1.59) | 1.21 (0.90 to 1.62) |
| Overweight to obese | 21.8 (191) | 26.5 (137) | 1.62 (1.18 to 2.22) | 1.67 (1.19 to 2.35) |
| Increasing obesity | 3.8 (33) | 8.7 (45) | 3.07 (1.85 to 5.09) | 3.12 (1.80 to 5.41) |
| WC trajectories (women) | 59.4 (392) | 40.6 (268) | ||
| Stable normal WC | 26.3 (103) | 17.5 (47) | Ref. | Ref. |
| Moderately high to high WC | 49.7 (195) | 42.5 (114) | 1.27 (0.84 to 1.94)* | 1.30 (0.83 to 2.05)* |
| Gradually increasing high WC | 20.9 (82) | 33.6 (90) | 2.34 (1.47 to 3.70)* | 2.17 (1.32 to 3.59)* |
| Steeply increasing high WC | 3.1 (13) | 6.3 (17) | 3.04 (1.34 to 6.90)* | 4.09 (1.54 to 10.90)* |
| WC trajectories (men) | 65.9 (482) | 34.1 (249) | ||
| Stable normal WC | 22.4 (108) | 18.1 (45) | Ref. | Ref. |
| Stable moderately high WC | 41.1 (198) | 38.5 (96) | 1.18 (0.77 to 1.80)* | 1.12 (0.72 to 1.76)* |
| Moderately high to high WC | 31.5 (152) | 28.9 (72) | 1.18 (0.75 to 1.85)* | 1.12 (0.69 to 1.79)* |
| Increasing high WC | 5.0 (24) | 14.5 (36) | 3.73 (1.99 to 6.97)* | 3.36 (1.71 to 6.59)* |
Model 1: adjusted for age and sex at baseline (*adjusted for age only). Model 2: adjusted for age, sex, educational level, marital/cohabitation status, smoking status, alcohol intake, social support, self-perceived health and physical activity level (*excluding sex) at baseline.
BMI, body mass index; WC, waist circumference.
When the association was assessed separately for each frailty component (online supplemental table 8), overweight or obesity at baseline was associated with higher odds of slow walking speed, low physical activity and low grip strength at follow-up. However, the association between BMI and grip strength was no longer significant in the fully adjusted model. Moderately high or high WC at baseline was associated with higher odds of slow walking speed and low physical activity.
Discussion
The present study followed 4509 community-dwelling participants from the population-based Tromsø study from 1994 to 2016 to examine the association between general and abdominal obesity and the risk of frailty. This study suggests an increased likelihood of pre-frailty/frailty among those with baseline obesity. Increased likelihood of pre-frailty/frailty was also observed among those with high or moderately high WC at baseline. When assessed jointly, participants with both obesity and moderately high/high WC at baseline had increased odds of being pre-frail/frail compared with those with BMI and WC in the normal range. Participants in the ‘overweight to obesity’ or the ‘increasing obesity’ trajectories had increased odds of pre-frailty/frailty compared with those in the stable normal BMI trajectory. Additionally, participants with a high WC at baseline, whose WC gradually or steeply increased throughout the follow-up period, had increased odds of being pre-frail/frail compared with those in a stable normal WC trajectory.
Our conclusions align with the findings from two previous longitudinal studies with a similar follow-up period (26 and 22 years) that reported a significant positive association between midlife overweight or obesity and the development of pre-frailty and frailty in later life.25 26 However, we should be cautious while interpreting the association between baseline overweight BMI and pre-frailty/frailty. In our study, this association was not significant in the sensitivity analyses where we excluded participants aged 60 years and older at baseline. A prospective study with a follow-up period of 3.5 years observed a significantly increased risk of frailty among underweight women and women with overweight and obesity.24 No significant association between baseline underweight status and risk of pre-frailty/frailty was detected in our study. However, the number of underweight individuals in our study was too low, resulting in a low statistical power to reach any conclusion. In terms of WC and frailty status, similar to our results, a positive association between higher WC and frailty among older adults was reported by a 3.5-year follow-up study from two prospective Spanish cohorts.29 A positive association between high WC and frailty was observed in a few other studies9 16 27; however, they were cross-sectional and used slightly different cut-offs to categorise WC. We identified BMI and WC trajectories to account for the dynamic change in the adiposity level that might occur during adulthood. In line with our findings regarding BMI trajectories, comparable trajectories and observations about a higher risk of pre-frailty and frailty among those with increasing BMI were observed in a 26-year follow-up study.32 A large study that followed adults aged ≥51 years for 10 years reported a higher incidence of frailty among weight gain class, weight loss class and consistent obesity class.31 Literature on long-term changes in WC and its association with frailty seems lacking. Few epidemiological studies have explored the combined effect of BMI and WC on frailty among older adults. Two studies conducted among adults aged ≥65 years in Portugal46 and ≥60 years in Spain29 observed a positive association between frailty and adiposity only when the individuals had both a high WC and a high BMI. It aligns with our results to a certain extent, as we observed an increased likelihood of pre-frailty/frailty among individuals with both obesity and moderately high/high WC at baseline. We also observed higher odds of pre-frailty/frailty among those who had overweight with a moderately high/high WC at baseline. However, this association was not significant in the sensitivity analyses where we excluded participants aged 60 years and older at baseline. On the contrary, high WC was reported to be associated with frailty regardless of their BMI categories by two cross-sectional studies conducted among community-dwelling adults aged ≥65 years in China27 and England,15 indicating WC to be better linked with frailty. Notably, participants who had normal BMI with moderately high/high WC or those who were overweight with normal WC did not have significantly increased odds of pre-frailty/frailty in our study. This finding indicates the importance of considering both BMI and WC to identify the risk of frailty.
There are different mechanisms through which obesity might contribute to pre-frailty/frailty. Increased adiposity leads to increased secretion of pro-inflammatory adipokines, thus contributing to inflammation,14 19 which is also associated with frailty among older adults.47 Obesity leads to increased fat mass and increased lipid infiltration in muscle fibres resulting in reduced muscle strength and function.14 48 When coupled with an age-related decline in muscle mass and strength, it causes ‘sarcopenic obesity’, which is linked to an increased risk of frailty and disability.19 49 50 Grip strength, often used as a proxy for muscle strength in older adults, was found to be associated with baseline overweight and obesity assessed using BMI in our study. However, the association was no longer significant when further adjusted for potential covariates. Slow walking speed and low physical activity, which often represent lower physical functioning at an older age, were significantly associated with baseline BMI and WC. The primary strength of this study is its prospective design with a long follow-up period of two decades. However, several changes in participant’s lifestyle, diet, habits and physical and psycho-social environments might have occurred during this period. We could not account for these factors, which potentially impacted the development of pre-frailty/frailty. So, the result of this study should be cautiously interpreted in light of these contextual issues. We used BMI and WC to define general and abdominal obesity. BMI is often criticised for its inability to provide information on fat distribution,22 while WC is criticised for its limitation in distinguishing between visceral and subcutaneous fat.51 However, they are effective in assessing obesity-related risks at the population level.21 22 A study among Tromsø7 participants aged ≥40 years found a strong correlation between BMI and visceral adipose tissue (VAT) mass and WC and VAT mass. It also concluded them to be a satisfactory substitute to identify cardiometabolic risk.23 Further, they are simple to measure, easy to replicate and widely used in routine health assessments, thus, helping identify individuals at risk of frailty to provide timely interventions. The repeated measures on BMI and WC allowed us to account for changes in participants’ obesity status through the follow-up period and gain a comprehensive understanding of the long-term effects of these exposures on the risk of frailty in later life. However, we could not account for the development and change in frailty status that might have occurred over time as repeated measures on frailty were unavailable. Our outcome was physical frailty, assessed using Fried et al’s frailty phenotype definition.5 Though widely used,52 it defines frailty from the unidimensional perspective of reduced physical functioning and declining physiological reserves. In the context where frailty is being recognised as a multidimensional construct encompassing not just physical but also cognitive, social and psychological dimensions,53 the scope of our results focusing just on physical aspects of frailty might be limited. This study’s objectively measured physical frailty components (low grip strength and low walking speed) aligned with Fried’s definition; however, the questionnaires for self-reported components (exhaustion, low physical activity and unintentional weight loss) varied slightly. Each frailty indicator we used has been validated in different research contexts.36–38 41 The self-reported frailty components are nevertheless prone to information bias. A systematic review that investigated 262 physical frailty phenotypes acknowledged that modifications in the definition of frailty phenotype are common and have an important impact on the classification and predictive ability of the definition.54 A fair agreement has been reported between Fried’s definition and the completely questionnaire-based physical frailty definition.55 56
The main limitation of our study is the selection bias resulting from differential loss to follow-up. Those lost to follow-up were comparatively older and had a higher proportion of general and abdominal obesity and other potential risk factors for frailty. This might have led to a lower prevalence of frailty in Tromsø7. In total, 1.1% of the participants aged ≥66 years at Tromsø7 were frail, and 28.4% were pre-frail which is much lower than the pooled prevalence estimates provided by O’Caoimh et al.6 It aligns with the findings from a study where the grip strengths of Tromsø7 participants and Russian Know Your Heart study participants aged 40–69 years were compared. The average Norwegian participant had a mean grip strength comparable to a 7-year younger Russian counterpart.57 This indicates that the nordic population might be comparatively healthier,58 thus limiting the generalisability of our findings to other populations across the globe. Only a subsample of our study population had information on both BMI and WC, and an even lower number had repeated measurements available for both exposures. Therefore, the models including both BMI and WC might have low statistical power, particularly when considering the repeated measures. Information on frailty measures was not available at baseline. However, most participants were in their mid-life (median age 50) at baseline, lowering their likelihood of having frailty components. The sensitivity analyses, where we excluded participants aged ≥60 years from baseline as a proxy for exclusion of pre-frail/frail individuals, showed a similar trend in the association between baseline obesity, assessed using BMI and WC, and pre-frailty/frailty at an older age. We adjusted for several confounding factors; however, the potential for residual confounding remains. Most covariates in our study, including comorbidity, were self-reported.
We combined pre-frailty and frailty as a single outcome because of the low frailty prevalence in this study. The pre-frail/frail population in this study is primarily pre-frail with a frailty score of 1, half of which were the ones with low physical activity. So, misclassification of comparatively healthier but less active participants with severely pre-frail/frail participants might have occurred. The sensitivity analyses on participants with ≥2 frailty score, which mostly supported results from the primary analysis, addressed this issue to some extent. It would have been informative to assess the association with pre-frailty and frailty separately. Nevertheless, understanding factors associated with pre-frailty is highly relevant because pre-frailty is gaining broader interest as an ideal opportunity for administering timely intervention to delay or reverse frailty and the associated adverse outcomes.59 Of note, as our outcome pre-frailty/frailty is common, the OR estimates obtained might slightly overestimate the relative risk, and caution should be applied while interpreting it as a risk.
In the context where the population is rapidly ageing and the obesity epidemic is rising, growing evidence recognises the subgroup of ‘fat and frail’ older individuals in contrast to viewing frailty only as a wasting disorder.12 15 26 In this study, participants with both high BMI and high WC, that is, general and abdominal obesity, especially for a long duration throughout their adulthood, were observed to have an increased likelihood of pre-frailty/frailty. It highlights the importance of routinely assessing and maintaining optimal BMI and WC throughout adulthood to lower the risk of frailty in older age.
Supplementary Material
Acknowledgments
We thank the NutriFrail team for their support and the Tromsø study team for their cooperation in data acquisition.
Footnotes
Twitter: @uchaishreeshti
Contributors: SU was responsible for conceptualisation, data acquisition, analysis, interpretation, writing original draft, review and editing. LFA was responsible for conceptualisation, funding acquisition, supervision, writing critical review and editing. LAH was responsible for data acquisition for the Tromsø study, constant coordination, writing critical review and editing. AH was responsible for conceptualisation, funding acquisition, data acquisition, supervision, writing critical review and editing. AH is responsible for the overall content as the guarantor.
Funding: This work was supported by the Throne Holst Foundation (Grant number N/A) and the Institute of Basic Medical Sciences, University of Oslo (Grant number N/A). The project also received funding from Aktieselskabet Freia Chocolade Fabriks Medisinske fond (Grant number N/A). The funders had no role in the research manuscript's design, conduct, analysis, interpretation or drafting.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data may be obtained from a third party and are not publicly available. The legal restriction on data availability is set by the Tromsø Study Data and Publication Committee in order to control data sharing, including publication of data sets with the potential of reverse identification of de-identified sensitive participant information. The data can be made available from the Tromsø study upon application to the Tromsø Study Data and Publication Committee. Contact information: The Tromsø Study, Department of Community Medicine, Faculty of Health Sciences, UiT The Arctic University of Norway; e-mail: tromsous@uit.no <mailto:tromsous@uit.no>.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by Regional Committee of Medical and Health Research Ethics (REK) (ref. 2021/234146) and the Norwegian Centre for Research Data (NSD) (ref. 364331). Participants gave informed consent to participate in the study before taking part.
References
- 1. Kojima G, Iliffe S, Jivraj S, et al. Association between frailty and quality of life among community-dwelling older people: a systematic review and meta-analysis. J Epidemiol Community Health 2016;70:716–21. 10.1136/jech-2015-206717 [DOI] [PubMed] [Google Scholar]
- 2. Conroy S, Elliott A. The frailty syndrome. Medicine 2017;45:15–18. 10.1016/j.mpmed.2016.10.010 [DOI] [Google Scholar]
- 3. Sezgin D, O'Donovan M, Woo J, et al. Early identification of frailty: developing an international Delphi consensus on pre-frailty. Arch Gerontol Geriatr 2022;99:104586. 10.1016/j.archger.2021.104586 [DOI] [PubMed] [Google Scholar]
- 4. Sezgin D, Liew A, O'Donovan MR, et al. Pre-frailty as a multi-dimensional construct: a systematic review of definitions in the scientific literature. Geriatr Nurs 2020;41:139–46. 10.1016/j.gerinurse.2019.08.004 [DOI] [PubMed] [Google Scholar]
- 5. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146–57. 10.1093/gerona/56.3.M146 [DOI] [PubMed] [Google Scholar]
- 6. O'Caoimh R, Sezgin D, O'Donovan MR, et al. Prevalence of frailty in 62 countries across the world: a systematic review and meta-analysis of population-level studies. Age Ageing 2021;50:96–104. 10.1093/ageing/afaa219 [DOI] [PubMed] [Google Scholar]
- 7. United Nations Department of Economic and Social Affairs . World population ageing 2019, 2020.
- 8. Reinders I, Visser M, Schaap L. Body weight and body composition in old age and their relationship with frailty. Curr Opin Clin Nutr Metab Care 2017;20:11–15. 10.1097/MCO.0000000000000332 [DOI] [PubMed] [Google Scholar]
- 9. Xu L, Zhang J, Shen S, et al. Association between body composition and frailty in elder inpatients. Clin Interv Aging 2020;15:313–20. 10.2147/CIA.S243211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Villareal DT, Apovian CM, Kushner RF, et al. Obesity in older adults: technical review and position statement of the American Society for nutrition and NAASO, the obesity Society. Obes Res 2005;13:1849–63. 10.1038/oby.2005.228 [DOI] [PubMed] [Google Scholar]
- 11. Bowen ME. The relationship between body weight, frailty, and the disablement process. J Gerontol B Psychol Sci Soc Sci 2012;67:618–26. 10.1093/geronb/gbs067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Blaum CS, Xue QL, Michelon E, et al. The association between obesity and the frailty syndrome in older women: the women's health and aging studies. J Am Geriatr Soc 2005;53:927–34. 10.1111/j.1532-5415.2005.53300.x [DOI] [PubMed] [Google Scholar]
- 13. Rietman ML, van der A DL, van Oostrom SH, et al. The association between BMI and different frailty domains: a U-shaped curve? J Nutr Health Aging 2018;22:8–15. 10.1007/s12603-016-0854-3 [DOI] [PubMed] [Google Scholar]
- 14. Porter Starr KN, McDonald SR, Bales CW. Obesity and physical frailty in older adults: a scoping review of lifestyle intervention trials. J Am Med Dir Assoc 2014;15:240–50. 10.1016/j.jamda.2013.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hubbard RE, Lang IA, Llewellyn DJ, et al. Frailty, body mass index, and abdominal obesity in older people. J Gerontol A Biol Sci Med Sci 2010;65:377–81. 10.1093/gerona/glp186 [DOI] [PubMed] [Google Scholar]
- 16. Crow RS, Lohman MC, Titus AJ, et al. Association of obesity and frailty in older adults: NHANES 1999-2004. J Nutr Health Aging 2019;23:138–44. 10.1007/s12603-018-1138-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Himes CL, Obesity HCL. Obesity, disease, and functional limitation in later life. Demography 2000;37:73–82. 10.2307/2648097 [DOI] [PubMed] [Google Scholar]
- 18. Bales CW, Buhr G. Is obesity bad for older persons? A systematic review of the pros and cons of weight reduction in later life. J Am Med Dir Assoc 2008;9:302–12. 10.1016/j.jamda.2008.01.006 [DOI] [PubMed] [Google Scholar]
- 19. Jarosz PA, Bellar A. Sarcopenic obesity: an emerging cause of frailty in older adults. Geriatr Nurs 2009;30:64–70. 10.1016/j.gerinurse.2008.02.010 [DOI] [PubMed] [Google Scholar]
- 20. Zupo R, Castellana F, Bortone I, et al. Nutritional domains in frailty tools: working towards an operational definition of nutritional frailty. Ageing Res Rev 2020;64:101148. 10.1016/j.arr.2020.101148 [DOI] [PubMed] [Google Scholar]
- 21. Liu X-C, Huang Y, Lo K, et al. Quotient of waist circumference and body mass index: a valuable indicator for the high-risk phenotype of obesity. Front Endocrinol 2021;12:1–10. 10.3389/fendo.2021.697437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cornier M-A, Després J-P, Davis N, et al. Assessing adiposity: a scientific statement from the American heart association. Circulation 2011;124:1996–2019. 10.1161/CIR.0b013e318233bc6a [DOI] [PubMed] [Google Scholar]
- 23. Lundblad MW, Jacobsen BK, Johansson J. Anthropometric measures are satisfactory substitutes for the DXA-derived visceral adipose tissue in the association with cardiometabolic risk - The Tromsø Study. Obes Sci Pract:2015–6. 10.1002/osp4.517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Woods NF, LaCroix AZ, Gray SL, et al. Frailty: emergence and consequences in women aged 65 and older in the women's health Initiative observational study. J Am Geriatr Soc 2005;53:1321–30. 10.1111/j.1532-5415.2005.53405.x [DOI] [PubMed] [Google Scholar]
- 25. Stenholm S, Strandberg TE, Pitkälä K, et al. Midlife obesity and risk of frailty in old age during a 22-year follow-up in men and women: the Mini-Finland follow-up survey. J Gerontol A Biol Sci Med Sci 2014;69:73–8. 10.1093/gerona/glt052 [DOI] [PubMed] [Google Scholar]
- 26. Strandberg TE, Sirola J, Pitkälä KH, et al. Association of midlife obesity and cardiovascular risk with old age frailty: a 26-year follow-up of initially healthy men. Int J Obes 2012;36:1153–7. 10.1038/ijo.2012.83 [DOI] [PubMed] [Google Scholar]
- 27. Liao Q, Zheng Z, Xiu S, et al. Waist circumference is a better predictor of risk for frailty than BMI in the community-dwelling elderly in Beijing. Aging Clin Exp Res 2018;30:1319–25. 10.1007/s40520-018-0933-x [DOI] [PubMed] [Google Scholar]
- 28. Falsarella GR, Gasparotto LPR, Barcelos CC, et al. Body composition as a frailty marker for the elderly community. Clin Interv Aging 2015;10:1661. 10.2147/CIA.S84632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. García-Esquinas E, José García-García F, León-Muñoz LM, et al. Obesity, fat distribution, and risk of frailty in two population-based cohorts of older adults in Spain. Obesity 2015;23:847–55. 10.1002/oby.21013 [DOI] [PubMed] [Google Scholar]
- 30. Malenfant JH, Batsis JA. Obesity in the geriatric population - a global health perspective. J Glob Health Rep 2019;3. 10.29392/joghr.3.e2019045. [Epub ahead of print: 01 08 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mezuk B, Lohman MC, Rock AK, et al. Trajectories of body mass indices and development of frailty: evidence from the health and retirement study. Obesity 2016;24:1643–7. 10.1002/oby.21572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Landré B, Czernichow S, Goldberg M, et al. Association between life-course obesity and frailty in older adults: findings in the GAZEL cohort. Obesity 2020;28:388–96. 10.1002/oby.22682 [DOI] [PubMed] [Google Scholar]
- 33. UiT . The Arctic University of Norway. The Tromsø study | uit. Available: https://uit.no/research/tromsostudy [Accessed 29 Jan 2022].
- 34. World Health Organisation (WHO). . Obesity : preventing and managing the global epidemic : report of a WHO consultation (WHO technical report series ; 894)., 2000. Available: https://apps.who.int/iris/handle/10665/42330 [PubMed]
- 35. World Health Organisation (WHO) . Waist circumference and Waist–Hip ratio. Report of a who expert consultation. Geneva, 8-11 December 2008. Geneva, 2008. Available: http://apps.who.int/iris/bitstream/handle/10665/44583/9789241501491_eng.pdf?sequence=1
- 36. Elia M. The ‘MUST’report. Nutritional screening of adults: a multidisciplinary responsibility. Development and use of the ‘Malnutrition Universal Screening Tool’ (MUST) for adults. BAPEN, 2003. [Google Scholar]
- 37. Derogatis LR, Lipman RS, Rickels K, et al. The Hopkins symptom checklist (HSCL): a self-report symptom inventory. Behav Sci 1974;19:1–15. 10.1002/bs.3830190102 [DOI] [PubMed] [Google Scholar]
- 38. Bergland A, Strand BH. Norwegian reference values for the short physical performance battery (SPPB): the Tromsø study. BMC Geriatr 2019;19:216. 10.1186/s12877-019-1234-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Freiberger E, de Vreede P, Schoene D, et al. Performance-Based physical function in older community-dwelling persons: a systematic review of instruments. Age Ageing 2012;41:712–21. 10.1093/ageing/afs099 [DOI] [PubMed] [Google Scholar]
- 40. Roberts HC, Denison HJ, Martin HJ, et al. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing 2011;40:423–9. 10.1093/ageing/afr051 [DOI] [PubMed] [Google Scholar]
- 41. Grimby G, Börjesson M, Jonsdottir IH, et al. The "Saltin-Grimby Physical Activity Level Scale" and its application to health research. Scand J Med Sci Sports 2015;25 Suppl 4:119–25. 10.1111/sms.12611 [DOI] [PubMed] [Google Scholar]
- 42. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 43. De Rubeis V, Andreacchi AT, Sharpe I, et al. Group‐based trajectory modeling of body mass index and body size over the life course: a scoping review. Obes Sci Pract 2021;7:100–28. 10.1002/osp4.456 [DOI] [Google Scholar]
- 44. Jones BL, Nagin DS. A note on a Stata plugin for estimating group-based trajectory models. Sociol Methods Res 2013;42:608–13. 10.1177/0049124113503141 [DOI] [Google Scholar]
- 45. StataCorp. Stata statistical software: release 16. College Station, TX: Stata Corp LLC, 2019. [Google Scholar]
- 46. Afonso C, Sousa-Santos AR, Santos A, et al. Frailty status is related to general and abdominal obesity in older adults. Nutr Res 2021;85:21–30. 10.1016/j.nutres.2020.10.009 [DOI] [PubMed] [Google Scholar]
- 47. Soysal P, Stubbs B, Lucato P, et al. Inflammation and frailty in the elderly: a systematic review and meta-analysis. Ageing Res Rev 2016;31:1–8. 10.1016/j.arr.2016.08.006 [DOI] [PubMed] [Google Scholar]
- 48. Goodpaster BH, Theriault R, Watkins SC, et al. Intramuscular lipid content is increased in obesity and decreased by weight loss. Metabolism 2000;49:467–72. 10.1016/S0026-0495(00)80010-4 [DOI] [PubMed] [Google Scholar]
- 49. Villareal DT, Banks M, Siener C, et al. Physical frailty and body composition in obese elderly men and women. Obes Res 2004;12:913–20. 10.1038/oby.2004.111 [DOI] [PubMed] [Google Scholar]
- 50. Baumgartner RN, Wayne SJ, Waters DL, et al. Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obes Res 2004;12:1995–2004. 10.1038/oby.2004.250 [DOI] [PubMed] [Google Scholar]
- 51. Grundy SM, Neeland IJ, Turer AT, et al. Waist circumference as measure of abdominal fat compartments. J Obes 2013;2013:1–9. 10.1155/2013/454285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Buta BJ, Walston JD, Godino JG, et al. Frailty assessment instruments: systematic characterization of the uses and contexts of highly-cited instruments. Ageing Res Rev 2016;26:53–61. 10.1016/j.arr.2015.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Panza F, Lozupone M, Solfrizzi V, et al. Different cognitive frailty models and health- and Cognitive-related outcomes in older age: from epidemiology to prevention. J Alzheimers Dis 2018;62:993–1012. 10.3233/JAD-170963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Theou O, Cann L, Blodgett J, et al. Modifications to the frailty phenotype criteria: systematic review of the current literature and investigation of 262 frailty phenotypes in the survey of health, ageing, and retirement in Europe. Ageing Res Rev 2015;21:78–94. 10.1016/j.arr.2015.04.001 [DOI] [PubMed] [Google Scholar]
- 55. Kim S, Kim M, Jung H-W, et al. Development of a frailty phenotype questionnaire for use in screening community-dwelling older adults. J Am Med Dir Assoc 2020;21:660–4. 10.1016/j.jamda.2019.08.028 [DOI] [PubMed] [Google Scholar]
- 56. Van der Elst MCJ, Schoenmakers B, Op Het Veld LPM, et al. Validation of replacement questions for slowness and weakness to assess the fried phenotype: a cross-sectional study. Eur Geriatr Med 2020;11:793–801. 10.1007/s41999-020-00337-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cooper R, Shkolnikov VM, Kudryavtsev AV, et al. Between-study differences in grip strength: a comparison of Norwegian and Russian adults aged 40-69 years. J Cachexia Sarcopenia Muscle 2021;12:2091–100. 10.1002/jcsm.12816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. OECD . European Observatory on health systems and policies (2021). Norway: country health profile 2021, state of health in the EU. OECD Publishing 2021. 10.1787/25227041 [DOI] [Google Scholar]
- 59. Gordon SJ, Baker N, Kidd M, et al. Pre-frailty factors in community-dwelling 40-75 year olds: opportunities for successful ageing. BMC Geriatr 2020;20:96. 10.1186/s12877-020-1490-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-065707supp001.pdf (325.8KB, pdf)
Data Availability Statement
Data may be obtained from a third party and are not publicly available. The legal restriction on data availability is set by the Tromsø Study Data and Publication Committee in order to control data sharing, including publication of data sets with the potential of reverse identification of de-identified sensitive participant information. The data can be made available from the Tromsø study upon application to the Tromsø Study Data and Publication Committee. Contact information: The Tromsø Study, Department of Community Medicine, Faculty of Health Sciences, UiT The Arctic University of Norway; e-mail: tromsous@uit.no <mailto:tromsous@uit.no>.