Abstract
Objectives:
Prevalence of synovitis, tenosynovitis, erosions, acro-osteolysis and bone marrow edema in systemic sclerosis is not extensively reported. We aimed to estimate the prevalence of changes in individual joints of hands in systemic sclerosis patients.
Method:
A cross-sectional analytical study consisting of 34 adults (females, n = 32) with systemic sclerosis. Patients with clinical synovitis were excluded. All patients underwent ultrasound (US) and magnetic resonance imaging of bilateral hands.
Results:
On US, synovitis, tenosynovitis, erosions, and acro-osteolysis were detected in 97%, 94%, 97%, and 29% patients. Grade I synovitis observed in 67% joints—first carpometacarpal joint (55%), first metacarpophalangeal joint (54%), distal radioulnar joint (50%), and intercarpal joints (47%) were commonly affected. Erosions were common in distal phalanges (first DP72% to fifth DP39%). On magnetic resonance imaging, synovitis, tenosynovitis, erosions, and bone edema were observed in 91%, 85%, 97%, and 85% patients. Grade I synovitis was seen in 70% joints, affecting intercarpal joint (70.6%) and third metacarpophalangeal joint (52.9%) commonly. Grade I erosions were seen in 61%, affecting distal phalanges (55.8%), capitate (60.3%), and lunate (55.8%). Grade I edema was commonly affecting lunate (39%) and capitate (26%). On magnetic resonance imaging, acro-osteolysis was present in 28% (97/340) distal phalanges. Fair agreement (0.21–0.40) was noted between US and magnetic resonance imaging for synovitis and erosions.
Conclusion:
High prevalence of low-grade inflammation is found in systemic sclerosis patients on US and magnetic resonance imaging. Distal joint assessment in addition to proximal joints improves accurate estimation of prevalence of early arthropathy.
Keywords: Systemic sclerosis, ultrasound, magnetic resonance imaging, arthropathy, erosion, synovitis, edema, RAMRIS, OMERACT, US-7
Introduction
Ultrasound (US) and magnetic resonance imaging (MRI) are sensitive modalities to detect inflammatory and destructive changes in inflammatory arthritis. Inflammatory changes have been found on MRI in 59%–100% of systemic sclerosis (SSc) patients with arthralgia,1,2 but limited number of studies have compared and graded individual joints of hand and wrist in SSc. Validated US and MRI scores exist for patients with rheumatoid arthritis (RA). US-7 score 3 is a sonographic grading of synovitis in seven joints, and RAMRIS is used for grading of synovitis, erosions, and bone marrow edema on MRI in RA. 4 Both RAMRIS and US-7 focus on the proximal joints of hands—for RAMRIS this includes distal radioulnar, intercarpal, and carpometacarpal joints and for US-7 score this inclues the wrist, second and third MCPJ (MCPJ), second and fifth metatarsophalangeal, and PIPJ of the clinically dominant hand and foot. There is scarce data about commonly involved joints, severity of inflammatory changes, and comparison between US and MRI in SSc. We conducted a cross-sectional imaging study comparing US and MRI inflammatory changes affecting wrist and hands without clinically active synovitis in SSc. RAMRIS and US-7 scores were utilized to evaluate all joints of hands and wrist in contrast to the proximal joints in RA. The primary aim of our study was to determine the prevalence of inflammatory and erosive changes in individual joints of wrist and hand in SSc patients on US and MRI and to evaluate the agreement between US with MRI findings of inflammatory arthropathy (synovitis, tenosynovitis, erosions, and acro-osteolysis) of bilateral hands.
Material and methods
We conducted a prospective cross-sectional study on adult subjects diagnosed with SSc with arthralgia without clinical synovitis during the 2-year study period. Informed written consent was obtained from all subjects. Institutional ethical committee approval was taken.
Patients
Inclusion criteria
Consecutive adults (>18 years) (n = 34) meeting American College of Rheumatology (ACR) 2013 classification criteria for SSc, presenting with unilateral or bilateral wrist and/or hand arthralgia to the rheumatology clinic were included.
Exclusion criteria
Patients with SSc/RA overlap (defined as patients who had positive RA factor on serology) or mixed connective tissue disorder (MCTD), and patients with clinical evidence of synovitis (redness, swelling, or palpable synovial effusion) were excluded. Those patients who had equivocal findings of synovitis on clinical examination (due to extensive skin thickening, fibrosis, and contractures) were also excluded.
All patients received multiple medications including low-dose steroids (5 mg/day), nifedipine, cyclophosphamide, and angiotensin converting enzyme (ACE) inhibitors. All patients underwent US and MRI of bilateral hands. Anti-topoisomerase (Anti-Scl-70), Anti-centro-mere, Anti-U1-RNP, and Anti-CCP assays were performed on a fully automated immunoassay analyzer (Phadia100, ThermoFisher Scientific, Uppsala, Sweden).
Technique: high-resolution ultrasound of wrist and hand joints
Two radiologists (AV and AS) performed US of both hands—including distal radioulnar joint (DRUJ), radiocarpal joint, intercarpal joint (ICJ), carpometacarpal joint (CMC), MCPJ, PIPJ, and DIPJ for synovitis and erosions—and tendons of the wrist for tenosynovitis using a Philips iU-22 (Philips Healthcare, Eindhoven, Netherlands) machine and a 5-17 MHz phased-array linear transducer, according to EULAR working-group guidelines. 5
Technique: MRI acquisition
MRI was performed in a 3-Tesla MRI (Siemens Magnetom Verio, Siemens Healthcare, Erlangen, Germany). T1-weighted (TR/TE-700–800/10–13 ms), fat-saturated T2-Weighted (TR/TE-4000–8000/70 ms), Short-Tau Inversion Recovery/STIR (TR/TE-4200/57 ms), 3D-MEDIC (TR/TE-40/14 ms), and post-contrast (TR/TE-700/10 ms) sequences were acquired.
Interpretation of US images
Synovitis, tenosynovitis, and erosions were defined according to OMERACT (Outcome Measures in Rheumatoid Arthritis Clinical Trial) criteria. 5 Power Doppler was used for grading the synovial vascularity. Synovial hypertrophy and synovial vascularity were graded semi-quantitatively on a scale of 0 to 3 according to the OMERACT-EULAR task force.5–7 Tenosynovitis and erosions were evaluated. Acro-osteolysis was defined as an abrupt ending of the hyperechoic cortex or complete disappearance of the cortical hyperechoic line of distal phalanx. 5
Interpretation of MRI
RAMRIS (Rheumatoid Arthritis Magnetic Resonance Imaging Score) was used for analysis of MRI. 4
Image analysis
Two radiologists (AV and AS, 2.5 and 15 years of experience) acquired and stored the US and MRI images of the study participants in DICOM format. Both radiologists at first independently and then by consensus interpreted and scored the anonymized US and MRI data. Both radiologists were blinded to the findings of the MRI at the time of US interpretation, and interpretation of MRI and US of patients was done randomly. MRI was reviewed after a period of 1 month after the US images were reviewed. Images were sent for tie-break read to third reader (MP, 17 years of experience) in case of lack of consensus. RAMRIS scores for RA were adapted for grading of synovitis, tenosynovitis, erosions, and edema. 4 We included MCPJ, PIPJ, and DIPJ in addition to joints evaluated under RAMRIS. For bone edema, we included all phalanges. Tenosynovitis and acro-osteolysis were assessed for presence/absence.
Statistical analysis
Statistical analysis done using R-system, two-tailed tests (chi-square) were used, and p-value < 0.05 was considered statistically significant. Agreement between US and MRI findings was evaluated using Cohen’s kappa test. Prevalence of antibodies and their association with synovitis was performed using chi-square test.
Results
Demographic data
Thirty-four patients (68 hands), 32 (94.1%) females, with a median age of 39 years (range: 20–63 years) were included (Table 1).
Table 1.
Demographic and clinical characteristics of patients with systemic sclerosis.
| Characteristics | Metrics |
|---|---|
| Age, median (range) | 39 years (20–63 years) |
| Gender, number (percentage) | |
| Female | 32 (94.1%) |
| Male | 02 (5.9%) |
| Duration of illness, median duration (range) a | 4.35 years (1–15 years) |
| Sub-type, number (percentage) | |
| Diffuse scleroderma (dcSSc) | 25 (73.5%) |
| Limited scleroderma (lcSSc) | 9 (26.5%) |
| Antibody positivity status, number (%) | |
| Anti-topoisomerase (Anti-Scl-70) | 26 (76.47%) |
| Anti-centromere | 4 (11.76%) |
| Anti-U1-RNP | 2 (5.9%) |
| Anti-CCP | 2 (5.9%) |
Disease duration from the onset of Raynaud’s phenomenon or other symptoms of SSc (skin tightening, heartburn, dysphagia, polyarthralgia, etc.).
Prevalence of arthropathic changes on US
On US, we found synovitis, tenosynovitis, and erosions in 97% (33/34), 94% (32/34), and 97% (33/34), respectively, and acro-osteolysis in 29% (10/34) of subjects (Table 2).
Table 2.
Prevalence of arthropathy changes on US and MRI.
| Parameter | Ultrasound findings, n (%) | MRI findings, n (%) |
|---|---|---|
| Synovitis | ||
| Number of subjects (n = 34) | 33 (97%) | 31 (91%) |
| Number of hands (n = 68) | 65 (95.58%) | 59 (86.76%) |
| Number of joints (n = 1132) | 282 (24.91%) | 312 (26.99%) |
| Grade I | 190 (16.78%) | 220 (19.4%) |
| Grade II | 83 (7.3%) | 67 (5.9%) |
| Grade III | 9 (0.79%) | 25 (2.2%) |
| Tenosynovitis | ||
| Number of subjects affected (n = 34) | 32 (94%) | 29 (85%) |
| Number of hands affected (n = 68) | 52 (76.47%) | 53 (77.94%) |
| Flexor | 16 (23.5%) | 29 (54.7%) |
| Extensor | 21 (30.8%) | 8 (15%) |
| Both (flexor and extensor) | 15 (22%) | 16 (30.1%) |
| Erosions | ||
| Number of subjects (n = 34) | 33 (97%) | 33 (97%) |
| Number of hands | 66 (97%) | 66 (97%) |
| Number of joints (n = 1094) | 291 (26.59%) | 505 (46.16%) |
| Grade I | Not relevant (NR) | 309 (28.24%) |
| Grade II | NR | 103 (9.41%) |
| Grade III | NR | |
| Acro-osteolysis | ||
| Number of subjects | 10 (29%) | 15 (44.11%) |
| Number of hands | 20 (29.41%) | 42 (52.50%) |
| Distal phalanges (n = 340) | 55 (16.17%) | 97 (28.53%) |
| Marrow edema | ||
| Number of subjects | NR | 29 (85%) |
| Number of hands | NR | 51 (75%) |
| Number of bones (n = 1972) | NR | 155 |
| Grade I | NR | 121 |
| Grade II | NR | 27 |
| Grade III | NR | 7 |
MRI: magnetic resonance imaging.
Synovitis was observed in 65/68 (95.8%) hands. Due to contracture, 24/1156 joints could not be evaluated; 282/1132 (24%) joints evaluated had synovitis. First CMC joint (55%), first MCP (54%), DRUJ (50%), and intercarpal joints (47%) were commonly involved. Erosions were seen 66/68 (97%) hands. The most common bones with erosions were distal phalanges (DP)—first DP to fifth DP (72%–39%) (Table 2, Supplemental Table 1). Tenosynovitis was seen in 52/68 (76%) hands and acro-osteolysis in 20 (29%) hands. Of 340 distal phalanges, 55 (16%) showed acro-osteolysis (Figure 1(a)–(c)).
Figure 1.
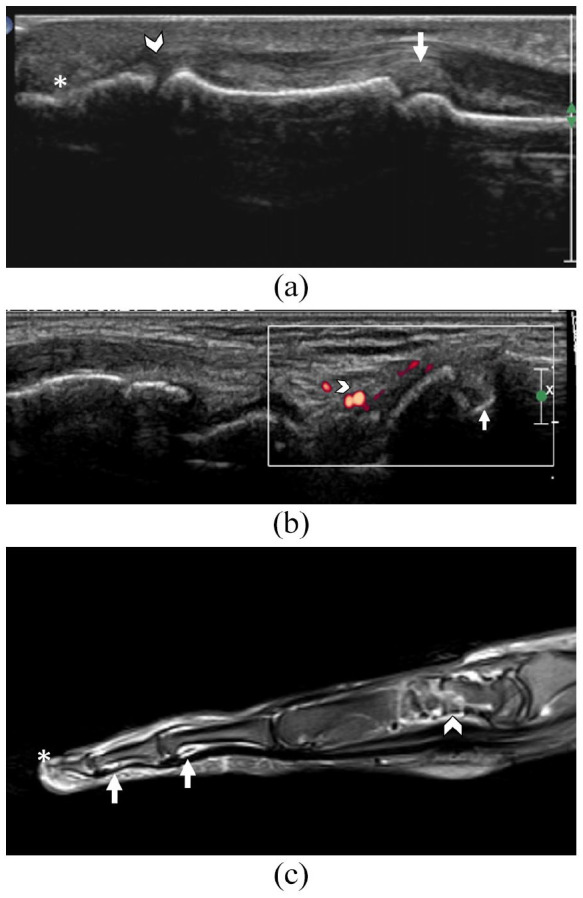
45-year-old female with diffuse systemic sclerosis and bilateral arthralgia.Fig.1A. US shows convex hyperechoic bulging synovium (Grade III) at the PIPJ (small arrow), hypoechoic (Grade III) synovitis at the distal interphalangeal joint(arrowhead) and irregular erosion of distal phalanx (*) of the middle finger.Fig.1B.US of the 1st carpometacarpal joint of the patient shows erosion of the base of 1st metacarpal (arrow) and synovitis with few confluent vessel signals on Doppler involving less than half of the synovium (Grade II) (arrowhead). Fig.1C. T2-weighted fat saturated sagittal image of the middle finger of the same patient shows erosions of the carpal bone with synovitis of intercarpal joints(arrowhead). Synovitis (small arrows) is also seen in the proximal and distal interphalangeal joints. Acro osteolysis with abrupt termination and narrowing of distal phalanx tip is seen (*).
Prevalence of arthropathic changes on MRI
On MRI, synovitis, tenosynovitis, erosions, and edema were seen in 91% (31/34), 85% (29/34), 97% (33/34), and 85% (29/34) patients, respectively.
Synovitis was seen in 59/68 hands (86.76%) and in 312/1156 joints (26.9%). 70% had grade-I synovitis, 67(21%) had Grade II synovitis. Intercarpal (70.6%), third MCP (52.9%), first MCPJ (45.6%), DRUJ (45.6%), CMC (44.1%), and second MCPJ (41.1%) were commonly involved. DIP joints were least commonly involved (Supplemental Table 2).
Erosions were seen in 66/68 (97%) hands, and among 505 (26.52%) of 1904 bones. Grade I (309 bones, 61%) erosions were most common. Erosions were frequent in distal phalanges (55.8%) and carpals (capitates (60.3%), lunate (55.8%), triquetrum (54.4%), and scaphoid (30.8%)). Less frequently involved were fifth metacarpal (2.94%) and third middle phalanx (8.82%) (Supplemental Table 1).
Bone marrow edema was seen in 51/68 hands (75%), with Grade I edema in 78% of the affected hands. Edema was seen in 155 (7.86%) of the 1972 evaluated bones, affecting lunate (n = 21, 39%) and capitates (n = 17, 26%) bones commonly. The most frequently encountered grade of edema was Grade I (78.06%) followed by Grade II (17.4%) and Grade III edema (4.5%). The fourth metacarpal and first proximal phalanx did not have any edema (Supplemental Table 1).
Tenosynovitis was seen in 53/68 hands (77.9%). On MRI, 18 patients (31 hands) showed acro-osteolysis (Table 2). Of 340 distal phalanges, acro-osteolysis was present in 97 (28%) (Figure 1(a)–(c)).
Agreement
There was fair agreement between US and MRI for synovitis (kappa = 0.234), and erosions (kappa = 0.346) in individual joints. Agreement was seen in 771/1132 joints (68.1%) for synovitis. MRI detected more erosions than US (Table 3).
Table 3.
Agreement between US and MRI of both hands.
| Observations | Kappa | p-value | Percentage of agreement |
|---|---|---|---|
| Synovitis | 0.234 (95% CI, 0.162–0.306) | <0.05 | 68.1% |
| Erosions | 0.346 (95% CI, 0.274–0.418) | <0.05 | 50.4% |
| Tenosynovitis | 0.123 (95% CI, 0.051–0.195) | 0.311 | 78.52% |
Prevalence of arthropathic manifestations in disease subtypes and association
There was no significant association between disease sub-type (lcSSc and dcSSc) and synovitis (US/MRI) or acro-osteolysis (p = 0.855), though prevalence of synovitis in dcSSc (96.0% vs 77%) was higher. There was significant association between disease sub-type and bone edema (p = 0.03) on MRI.
Association between synovitis on MRI and antibodies positivity
There was no significant association between synovitis and Anti-topoisomerase (Anti-Scl-70) and Anti-centromere-antibody positivity.
Discussion
In this study, we report high prevalence of synovitis, erosions, tenosynovitis, and bone edema of the in SSc patients. Low-grade synovitis was most common as reported in previous studies. 8 There was fair agreement between US and MRI in detection and grading of tenosynovitis, synovitis, and erosions (78.62%, 68.1%, and 50.4%). Our findings are in concordance with studies by other authors like Abdel-Magied et al. 9 (agreement of 62.5%, 43.8%, and 43.8%, for tenosynovitis, synovitis, and erosion, respectively) and Chitale et al. (62%, 38%, and 25%).
The utility of MRI and US in RA has been extensively evaluated and validated in multiple trials.4,5,10 In patients with clinical features of early RA such as joint stiffness and pain, there is good agreement between US and MRI, with MRI showing marginally higher sensitivity and accuracy for detecting subclinical synovitis. 11 Diagnostic accuracy of US has been validated for synovitis in the wrist and hand joints in a systematic review by Takase-Minegishi et al. 12 and it is considered competitive to MRI in routine evaluation of RA. Another study by Navalho et al. 13 in patients with new onset polyarthritis demonstrated that MRI outperformed US in detecting early synovitis and tenosynovitis in patients, although both modalities had good diagnostic yield. Ohrndorf et al. 14 have found that US is less sensitive than MRI for both synovitis and tenosynovitis, but has lesser non-specific findings when compared to MRI, with specificity for tenosynovitis approaching 100%. Another study by Wang et al. 15 has shown that in early RA, there is no significant difference in bone erosions detection on US or MRI, although there is significant difference between both the modalities in synovial proliferation, tenosynovitis, and bone marrow edema.
In comparison to RA, there is limited experience of US and MRI in SSc-related arthropathy. The US and MRI findings in the small joints of SSc have not been standardized in previous studies. Ultrasound is operator- and machine-dependent, and the probe and machine settings have great impact on the interpretation of findings. Skin thickening, fibrosis, and contractures can also make US technically difficult to perform. This also makes MRI evaluation difficult, especially in areas like the finger where subcutaneous fat is very little and thickened and inflamed skin close to the joint capsule can be mistaken for synovitis, leading to false-positives. MRI of the hands includes simultaneous evaluation of all the small joints of both hands; therefore, the field of view (FOV) is kept large enough to include the area of interest. The increased FOV to afford evaluation of more number of joints can compromise the resolution of the study, and reduce the chances of picking up milder synovitis and erosions. Similar to Navalho et al, in our study, MRI has detected involvement of more number of joints than US in the form of synovitis, erosions, and bone marrow edema.
We found synovitis in 97% patients on US, which is higher than 39% in a cross-sectional study, 15 or a reported rate of 25% and 31.1% patients at baseline and follow-up US, respectively. 9
On MRI, 31 patients had synovitis, similar to that reported by Abdel-Magied et al. 9 We report higher prevalence of inflammatory changes on MRI than Low et al., 2 who reported synovitis in 47% patients with clinically inflamed joints. However, Low et al did not perform a contrast enhanced MRI, which may be the cause of low prevalence of synovitis.
Chitale et al. 1 reported erosions in 8 (75%) subjects on MRI. Abdel-Magied et al. 9 reported erosions in 6.3% on US, whereas prevalence of erosions on MRI in the same study was 62.5%. We used a high-frequency 17-MHz probe as compared to Chitale et al., which could have resulted in better detection of erosions. We evaluated both hands, whereas other studies either excluded distal joints 2 or performed MRI of the more symptomatic hand, 1 with possible underestimation of the actual prevalence.
The prevalence of tenosynovitis observed in our study was similar to previous studies which have reported tenosynovitis in 43% to 50% of subjects on US, and 47% to 88% on MRI.1,2 None of our patients had a layered pattern of tenosynovitis as reported by Freire et al. 16
Bone marrow edema was present in 75% hands and in 7.86% of bones. Previous studies have reported bone marrow edema in 53%–75% of patients.1,2,9 Individual bone involvement has not been specified in the previous studies. Edema of the lunate was most common, affecting 47% patients.
On US, synovitis was most common in first CMC and first MCPJ, similar to a previous study where synovitis was most commonly detected in the MCPJ.17,18 Grade I synovitis was most common, similar to predominant low grade synovitis in 57% of SSc patients in a cross-sectional study. 8
Previous studies have found MRI to be more sensitive than US in diagnosing synovitis, whereas we found US to be more sensitive.1,9 Use of high-frequency probes (17 MHz) could lead to increased sensitivity.
About 80% of scleroderma patients have limited SSc; 19 however, in our study, we had a significantly higher proportion of dsSSc patients. Previous imaging studies have reported higher rates of arthropathic changes with dcSSc. 20 This could explain the high prevalence of arthropathic changes in our study, although we did not find a significant difference in the presence of synovitis, tenosynovitis or erosions between both SSc subtypes. Another factor that could have contributed to the high prevalence of arthropathy in our study was that most patients had long standing disease and reported in more advanced stages of SSC. We also found no significant association between SSc subtypes and US or MRI findings, again the possible cause could be due to late presentation of disease.
The high prevalence of synovitis and erosions in our study highlights the role of imaging for early detection of inflammatory changes.
Limitation
Grading systems like DAS28 score, US-7, and GLOESS (Global OMERACT-EULAR Synovitis Score) are well established in assessment of RA.3,4,6,21 Joint changes in SSc are less studied and no scoring system is available to quantify the changes. In the absence of a comprehensive grading system for SSc, we performed a detailed evaluation of all bones and joints of wrist and hands. The process was time-consuming on both US and MRI. A scoring system, like those established for RA, is essential for a focused assessment of few of the most involved joints. It is also important to standardize the acquisition protocols for both MRI and US for evaluation of SSc arthropathy.
Referral bias with disproportionate number of dcSSc patients and advanced disease of long duration is a prime concern. We did not correlate the findings with modified Rodnan skin score or hand mobility scores to assess functional limitation. Patients were not treatment naïve. In the absence of a standardized scoring system in SSc, comprehensive joint imaging was time-consuming. We did not evaluate interobserver variability.
In conclusion, US and MRI can detect inflammatory changes in SSc without clinical involvement. The relatively high prevalence of joint involvement and lack of robust agreement between US and MRI imply the need for standardization of techniques. A scoring system similar to that in RA of the commonly involved joints would improve reporting standards in a more reproducible and time-efficient manner.
Supplemental Material
Supplemental material, sj-docx-1-jso-10.1177_23971983221140673 for Ultrasound and magnetic resonance imaging of hands in systemic sclerosis: A cross-sectional analytical study of prevalence of inflammatory changes in patients with subclinical arthropathy by Akash Babulal Vadher, Anindita Sinha, Shayeri Roy Choudhury, Mahesh Prakash, Muniraju Maralakunte, Tanveer Rehman, Shefali Sharma and Yashwant Kumar in Journal of Scleroderma and Related Disorders
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Anindita Sinha  https://orcid.org/0000-0002-5034-3488
https://orcid.org/0000-0002-5034-3488
Supplemental material: Supplemental material for this article is available online.
References
- 1. Chitale S, Ciapetti A, Hodgson R, et al. Magnetic resonance imaging and musculoskeletal ultrasonography detect and characterize covert inflammatory arthropathy in systemic sclerosis patients with arthralgia. Rheumatology 2010; 49(12): 2357–2361. [DOI] [PubMed] [Google Scholar]
- 2. Low AH, Lax M, Johnson SR, et al. Magnetic resonance imaging of the hand in systemic sclerosis. J Rheumatol 2009; 36(5): 961–964. [DOI] [PubMed] [Google Scholar]
- 3. Backhaus M, Ohrndorf S, Kellner H, et al. Evaluation of a novel 7-joint ultrasound score in daily rheumatologic practice: a pilot project. Arthritis Rheum 2009; 61(9): 1194–1201. [DOI] [PubMed] [Google Scholar]
- 4. Østergaard M, Peterfy C, Conaghan P, et al. OMERACT rheumatoid arthritis magnetic resonance imaging studies. Core set of MRI acquisitions, joint pathology definitions, and the OMERACT RA-MRI scoring system. J Rheumatol 2003; 30(6): 1385–1386. [PubMed] [Google Scholar]
- 5. Brown AK, Machold KP, Conaghan PG; OMERACT 7 Special Interest Group. Musculoskeletal ultrasound including definitions for ultrasonographic pathology. J Rheumatol 2005; 32: 2485–2487. [PubMed] [Google Scholar]
- 6. Ventura-Ríos L, Hernández-Díaz C, Ferrusquia-Toríz D, et al. Reliability of ultrasound grading traditional score and new global OMERACT-EULAR score system (GLOESS): results from an inter- and intra-reading exercise by rheumatologists. Clin Rheumatol 2017; 36(12): 2799–2804. [DOI] [PubMed] [Google Scholar]
- 7. D’Agostino MA, Wakefield R, Backhaus M, et al. Evaluation of the intra- and inter-reader reliability of power Doppler ultrasonography (PDUS) for detecting and scoring synovitis of several joints in rheumatoid arthritis (RA) by using a validated scoring system: a report of the EULAR/OMERACT US group. Ann Rheum Dis 2008; 67(Suppl. 2): 422.17878211 [Google Scholar]
- 8. Elhai M, Guerini H, Bazeli R, et al. Ultrasonographic hand features in systemic sclerosis and correlates with clinical, biologic, and radiographic findings. Arthritis Care Res 2012; 64(8): 1244–1249. [DOI] [PubMed] [Google Scholar]
- 9. Abdel-Magied RA, Lotfi A, AbdelGawad EA. Magnetic resonance imaging versus musculoskeletal ultrasonography in detecting inflammatory arthropathy in systemic sclerosis patients with hand arthralgia. Rheumatol Int 2013; 33(8): 1961–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. D’Agostino M, Terslev L, Aegerter P, et al. Scoring ultrasound synovitis in rheumatoid arthritis: a EULAR-OMERACT ultrasound taskforce – part 1: definition and development of a standardised, consensus-based scoring system. RMD Open 2017; 3: e000428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bao Z, Zhao Y, Chen S, et al. Ultrasound versus contrast-enhanced magnetic resonance imaging for subclinical synovitis and tenosynovitis: a diagnostic performance study. Clinics 2020; 75: e1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Takase-Minegishi K, Horita N, Kobayashi K, et al. Diagnostic test accuracy of ultrasound for synovitis in rheumatoid arthritis: systematic review and meta-analysis. Rheumatology 2018; 57(1): 49–58. [DOI] [PubMed] [Google Scholar]
- 13. Navalho M, Resende C, Rodrigues AM, et al. Bilateral evaluation of the hand and wrist in untreated early inflammatory arthritis: a comparative study of ultrasonography and magnetic resonance imaging. J Rheumatol 2013; 40(8): 1282–1292. [DOI] [PubMed] [Google Scholar]
- 14. Ohrndorf S, Boer AC, Boeters DM, et al. Do musculoskeletal ultrasound and magnetic resonance imaging identify synovitis and tenosynovitis at the same joints and tendons? A comparative study in early inflammatory arthritis and clinically suspect arthralgia. Arthritis Res Ther 2019; 21(1): 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang MY, Wang XB, Sun XH, et al. Diagnostic value of high-frequency ultrasound and magnetic resonance imaging in early rheumatoid arthritis. Exp Ther Med 2016; 12(5): 3035–3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Freire V, Bazeli R, Elhai M, et al. Hand and wrist involvement in systemic sclerosis: US features. Radiology 2013; 269(3): 824–830. [DOI] [PubMed] [Google Scholar]
- 17. Baron M, Lee P, Keystone EC. The articular manifestations of progressive systemic sclerosis (scleroderma). Ann Rheum Dis 1982; 41(2): 147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chapin R, Hant FN. Imaging of scleroderma. Rheum Dis Clin 2013; 39(3): 515–546. [DOI] [PubMed] [Google Scholar]
- 19. Erre GL, Marongiu A, Fenu P, et al. The ‘sclerodermic hand’: a radiological and clinical study. Joint Bone Spine 2008; 75(4): 426–431. [DOI] [PubMed] [Google Scholar]
- 20. Ostojić P, Damjanov N. Different clinical features in patients with limited and diffuse cutaneous systemic sclerosis. Clin Rheumatol 2006; 25(4): 453–457. [DOI] [PubMed] [Google Scholar]
- 21. D’Agostino MA, Boers M, Wakefield RJ, et al. Exploring a new ultrasound score as a clinical predictive tool in patients with rheumatoid arthritis starting abatacept: results from the APPRAISE study. RMD Open 2016; 2(1): e000237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-jso-10.1177_23971983221140673 for Ultrasound and magnetic resonance imaging of hands in systemic sclerosis: A cross-sectional analytical study of prevalence of inflammatory changes in patients with subclinical arthropathy by Akash Babulal Vadher, Anindita Sinha, Shayeri Roy Choudhury, Mahesh Prakash, Muniraju Maralakunte, Tanveer Rehman, Shefali Sharma and Yashwant Kumar in Journal of Scleroderma and Related Disorders


