Abstract
Objective:
To investigate the prevalence of and independent predictors for digital ischemic complications in patients with systemic sclerosis.
Method:
Patients enrolled in the Siriraj Systemic Sclerosis Cohort registry during 2013–2019 were classified as having or not having digital ischemic complications at the baseline and 1-year timepoints.
Results:
A total of 171 patients with systemic sclerosis were included. The prevalence of digital pulp loss, digital pitting scar, digital ulcer, and digital amputation at baseline and 1 year was 41.5%, 39.8%, 3.5%, 7.6% and 37.4%, 43.9%, 14.1%, 6.4%, respectively. Over half (58.5%) of overall systemic sclerosis had developed new digital ischemic complications during the 1-year follow-up. Those with digital ischemic complications at baseline were at high risk for developing new digital ischemic complications (odds ratio: 15.9). Diffuse cutaneous systemic sclerosis is associated with digital ischemic complications (odds ratio: 6.0), digital pitting scar (odds ratio: 4.9), and digital pulp loss (odds ratio: 6.4). Tendon friction rub is associated with digital pitting scar (odds ratio: 5.0). Salt-and-pepper skin appearance is associated with digital pulp loss (odds ratio: 3.0) and digital ulcer (odds ratio: 6.9). Disease duration > 3 years is associated with digital ulcer (odds ratio: 4.4). Male gender is associated with digital ulcer (odds ratio: 5.4).
Conclusion:
Digital pulp loss, digital pitting scar, digital ulcer, and digital amputation were common manifestations of digital ischemic complications, and diffuse cutaneous systemic sclerosis was the strongest of the six independent predictors.
Keywords: Prevalence, factors, digital ischemic complications, patients, systemic sclerosis
Introduction
Microvascular endothelial cell injury via autoimmunity and specific environmental triggers is a primary event in the pathogenesis of systemic sclerosis (SSc). 1 Endothelial cell damage results in both structural and functional aberrations. 2 Structural alterations include two pathological features. The first feature is impaired vasculogenesis and angiogenesis that are combined with the destruction of capillary arterioles via destructive vasculopathy that is caused by chronic progressive endothelial cell damage and apoptosis. The second feature is occlusion of small vessels that is caused by intimal fibroproliferation that results in proliferative obliterative vasculopathy, which is characterized by luminal narrowing and occlusion. Functional abnormalities cause low nitric oxide availability and increased expression of cell adhesion molecules that induce inflammatory cell infiltration, which leads to fibroproliferative change and fibrin deposits. 2 Loss of capillary arterioles leads to necrotic lesions, most notably digital ischemic complications (DIC).
The spectrum of ischemia severity in this clinical setting can include Raynaud phenomenon (RP), digital pitting scar (DPS), digital pulp loss (DPL) or tuft resorption, digital tip ulcer (DU), digital gangrene (DG), and eventually digital (auto) amputation (DA). A systematic review of the reported prevalence of active or any history of DU varied from 17% to 58% in cross-sectional studies; however, different disease subsets and organ complications were studied and reported. 3 A meta-analysis of the prevalence of active DU reported a rate of 15% (95% confidence interval: 10%–20%), which was lower than the proportion of patients with a history of prior DU. 4 Diffuse cutaneous SSc (dcSSc), early-onset RP, early onset of non-RP symptom, high modified Rodnan skin score (mRSS), anti-topoisomerase-I antibody (ATA), and male gender were reported to be independent risk factors for the development of DU. 3 DU occurs within 5 years of onset of the first non-RP symptom in approximately 70% of SSc patients. 5 Geographical location also affects the risk of DU development. People living in the subtropical region had a five times higher risk of developing DU than those living in the tropical zone. 6 In 2018, Wangkaew et al. 7 conducted a cross-sectional study of DIC in SSc. The prevalence of RP, DPS, DPL, DU, and DA was 89.1%, 44.5%, 36.4%, 12.7%, and 3.6%, respectively. Few studies have longitudinally examined the prevalence of and risk factors for the various digital ischemic complications in SSc. Accordingly, the aim of this study was to investigate the prevalence of and factors independently associated with DIC in patients diagnosed with SSc who were enrolled in an SSc registry during a recent 5-year period in Thailand.
Methods
Patients and study design
This study was conducted at the Division of Rheumatology of the Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University. This post hoc analysis included patients aged ⩾18 years who were enrolled in the Siriraj Systemic Sclerosis Cohort (SiSSC) registry during 21 November 2013 to 21 July 2019. The SiSSC registry is an ongoing single-center observational prospective adult SSc registry at Siriraj Hospital, which is a super tertiary care center that is located in Bangkok, Thailand. Adult SSc patients who met the American College of Rheumatology (ACR) 1980 8 or the ACR/European League Against Rheumatism (EULAR) 2013 9 criteria were included. Patients with clinical features of other connective tissue diseases (CTD), including rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), dermatomyositis (DM), polymyositis (PM), and Sjögren syndrome (SjS), who also fulfilled the aforementioned standard classification criteria10–13 were then classified as overlapping scleroderma syndrome. SiSSC registry participants with mixed connective tissue disease (MCTD) or SLE with anti-U1RNP positivity were excluded because they have several clinical features similar to those found in early undifferentiated SSc (eSSc) patients. Included patients had to have been followed as routine clinical visits for at least 1 year. Patients with history and clinical evidence that other conditions could be the cause of DU, DG, or DA, such as traumatic ulcer, embolism, vasculitis, antiphospholipid antibody syndrome (APS)-related thrombosis, or surgical/traumatic amputation, were excluded.
Main outcome variable
Patients enrolled in the SiSSC registry were followed up prospectively in an observational fashion. Patients attended follow-up visits as needed or from every 2 weeks to every 6 months depending on disease condition, comorbidity, living area, and financial status. Every patient underwent clinical and laboratory assessments as part of routine follow-up. Types of DIC were recorded by the same assessor (C.M.) throughout the study period using symbolic codes drawn on paper-based pictures of the palmar and dorsal surfaces of both hands. Each kind of DIC at each consecutive visit would be evaluated to determine whether they were the old lesions, newly developed lesions, or progressive/worsening preexisting lesions. The digital ischemic complications of interest in this study were RP; DPS—pitted scars of digital tips, excluding posttraumatic scars; DPL—loss of digital (soft tissue) pulp, excluding distal bony phalanges; DU—a denuded area with defined border and loss of epithelialization; DG—necrosis of the tip of a finger; and, DA—autoamputation of the tip of the finger, including distal bony phalanges, finger (non-tip) ulcer (FU), and a non-hand ulcer (NHU).
Study factors
Variables/outcomes of interest in this study included patient demographics; disease duration from non-RP symptom to the first visit; the disease subsets eSSc, lcSSc, dcSSc, and SSc overlap syndrome; serological status; organ involvement, and laboratory studies.
Procedures
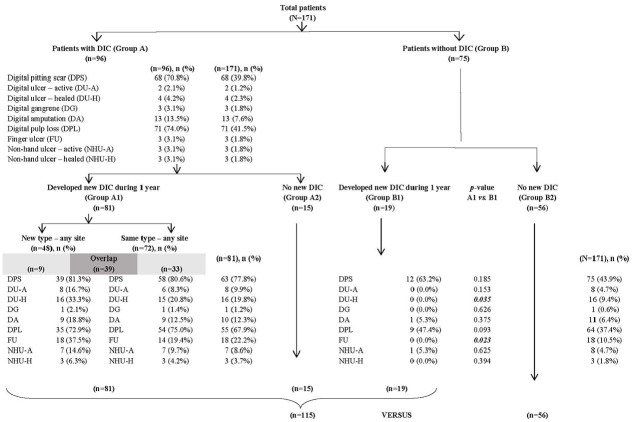
Included patients were classified at baseline as having DIC (Group A) or not having DIC (Group B). Each of those two groups was further categorized into those who did and who did not develop new DIC. Patients with no DIC at baseline who did not develop DIC during 1 year of follow-up were defined as patients with no DIC (Group B2). Group B2 patients were then compared with patients with DIC, including patients with DIC at baseline (Group A) and patients with no DIC at baseline, but who developed DIC during the 1-year follow-up (Group B1) (Figure 1).
Figure 1.
Flow diagram of the patient enrollment and group allocation scheme.
Ethical approval
The protocol for this study (protocol ID: 677/2561-EC2) was approved by the Siriraj Institutional Review Board (SIRB) (certificate of approval no. Si658/2018). In this post hoc analysis, included patients were from the Siriraj Systemic Sclerosis Cohort registry (SiSSC registry) (protocol ID 284/2558-EC3; certificate of approval no. Si417/2015). Written informed consent to participate in present and future SSc study was obtained from all the subjects when they agreed to participate in the SiSSC registry.
Sample size calculation and statistical analysis
Sunderkötter et al. 14 found DU to be significantly associated with mRSS. They reported the mean ± standard deviation (SD) of mRSS in patients who had and did not have DU to be 13.56 ± 10.05 and 8.84 ± 8.58, respectively. Using that data, a sample size of 58 patients per group was calculated to ensure a 95% confidential interval (95% CI) (Z = 1.96), a level of significance of 0.05, and the power of the test of 80%.
Chi-square test was used to compare categorical variables, and the results of those comparisons are given as frequency and percentage. Independent samples t-test and Mann–Whitney U test were used to compare normally and non-normally distributed data, respectively. The results of those analyses are shown as mean ± SD and median and interquartile range (IQR), respectively. Variables with a p-value less than 0.05 in univariate analysis and any other variables of special interest were entered into multivariate analysis. The results of multivariate analysis are shown as odds ratio (OR) and 95% CI. PASW Statistics v.18.0 (SPSS, Inc. Chicago, IL, USA) was used to perform all statistical analyses, and a p-value < 0.05 was considered statistically significant for all tests.
Results
During the enrollment period, there were 208 potentially eligible patients. Of those, 2 patients did not satisfy the ACR 1980 or ACR/EULAR 2013 criteria for SSc, 3 were referred to another center, 4 died, and 28 were lost to follow-up before completing 1 year of follow-up. The remaining 171 patients were included in this study. The average age of patients was 50.0 ± 11.6 years, 86% were women, 76% were dcSSc subset, 98% were ANA positive, 75.3% were anti-Scl-70 positive, 11% were anti-centromere positive, and the median disease duration from the onset of non-RP symptom was 2.9 years (IQR: 1.0, 6.7) years. A flow diagram of the patient enrollment and group allocation scheme is shown in Figure 1.
Among the entire cohort, 96 of 171 (56.1%) patients had DIC at baseline (Group A), and the other 75 (43.9%) patients did not have DIC at baseline (Group B). The three most common digital ischemic complications at baseline were DPL (41.5%), DPS (39.8%), and DA (7.6%). The prevalence of history of prior DU was 3.5%, of which 1.2% and 2.3% were active and healed DU, respectively. Among all study patients, 100 of 171 patients had developed new DIC during the 1-year follow-up for an incidence rate of 58.5/100 patient-years (95% CI, 50.7–66.0). The incidence rate of patients who developed new DIC (Group A1) in Group A (84.4/100 patient-years, 95% CI: 75.5–91.0) was significantly higher (OR: 15.9, 95% CI: 7.5–34.0; p < 0.0001) than the incidence rate of patients who developed new DIC (Group B1) in Group B patients (25.3/100 patient-years, 95% CI: 16.0–36.7).
Among Group A1 patients, the development of new DIC included both new type and same type at any site among digital tips, fingers, and the body. By default, Group B1 patients developed only new types of DIC. DPL and DPS were still the top two most common DIC in both groups. There were higher rates of most newly developed DIC in Group A1 relative to Group A, except for DG, DA, and DPL. Among the patients who developed DIC during the 1-year follow-up, Group A1 patients had significantly more healed DU and FU compared to Group B1 patients (p < 0.05), but the other types of DIC did not differ significantly between groups.
At the 1-year timepoint, the majority of patients in this study had DIC (n = 115; 67.3%), and about one-third of SSc (n = 56; 32.7%) did not develop DIC. Comparisons of demographic, clinical, laboratory, and medication data between groups are shown in Tables 1–3. Patients with DIC were significantly more likely to be dcSSc subset (87.8% vs 51.8%; p < 0.0001), to have significantly longer disease duration (3.3 years vs 1.7 years; p = 0.039), to have significantly higher baseline mRSS (8.0 vs 3.5; p = 0.001), to have significantly more baseline tendon friction rub (TFR; 19.1% vs 3.6%; p = 0.006) and salt-and-pepper skin presentation (60.9% vs 41.1%; p = 0.015), to have significantly less interincisor distance (3 cm vs 4 cm; p < 0.0001) and lower baseline forced vital capacity (FVC) % predicted (70.2% vs 80.1%; p = 0.018), to have significantly more symptomatic usual interstitial pneumonia (UIP) (16.5% vs 5.4%; p = 0.041), and to have received a significantly higher median dosage of nifedipine (12.5 mg/day vs 0.0 mg/day; p < 0.0001)—all compared to those without DIC. The early undifferentiated SSc (eSSc) (4.3% vs 25.0%; p < 0.0001) and limited cutaneous SSc (lcSSc) (7.8% vs 23.2%; p = 0.005) subsets were significantly less prevalent among patients with DIC. Anti-centromere (ACA) positivity (7.1% vs 18.6%; p = 0.051) was very close to being statistically significantly less prevalent in patients with DIC. Regarding baseline laboratory variables, there were no significantly different variables between groups (Table 2). However, white blood cell count (7950 vs 8085 cells/mm3; p = 0.056) demonstrated a trend toward being significantly lower among patients with DIC, whereas erythrocyte sedimentation rate (ESR; 41.0 vs 37.5 mm/h; p = 0.054) and serum globulin levels (3.8 vs 3.5 g/dL; p = 0.058) showed a trend toward being significantly higher in patients with DIC—all compared to patients without DIC.
Table 1.
Baseline demographic and clinical characteristics of all included patients, and compared between those with and without DIC.
| Characteristics | All patients (N = 171) | Patients with DIC (n = 115) | Patients without DIC (n = 56) | p |
|---|---|---|---|---|
| Age (years) | 50.0 ± 11.6 | 49.7 ± 12.1 | 50.6 ± 10.6 | 0.640 |
| Female gender | 147/171 (86.0%) | 97/115 (84.3%) | 50/56 (89.3%) | 0.383 |
| Disease duration with non-RP symptom to the first visit (years) | 2.9 (1, 6.7) | 3.3 (1.4, 7.6) | 1.7 (0.6, 5.2) | 0.039 |
| Disease duration > 3 years | 85/171 (49.7%) | 64/115 (55.7%) | 21/56 (37.5%) | 0.026 |
| Early undifferentiated SSc (eSSc) | 19/171 (11.1%) | 5/115 (4.3%) | 14/56 (25.0%) | <0.0001 |
| Limited cutaneous SSc (lcSSc) | 22/171 (12.9%) | 9/115 (7.8%) | 13/56 (23.2%) | 0.005 |
| Diffuse cutaneous SSc (dcSSc) | 130/171 (76.0%) | 101/115 (87.8%) | 29/56 (51.8%) | <0.0001 |
| SSc overlap syndrome | 32/171 (18.7%) | 22/115 (19.1%) | 10/56 (17.9%) | 0.841 |
| SSc with RA | 22/171 (12.9%) | 15/115 (13.0%) | 7/56 (12.5%) | 0.921 |
| SSc with SLE | 4/171 (2.3%) | 3/115 (2.6%) | 1/56 (1.8%) | 0.738 |
| SSc with PM | 2/171 (1.2%) | 1/115 (0.9%) | 1/56 (1.8%) | 0.601 |
| SSc with RA and SLE | 3/171 (1.8%) | 2/115 (1.7%) | 1/56 (1.8%) | 0.983 |
| SSc with SLE and PM | 1/171 (0.6%) | 1/115 (0.9%) | 0/56 (0.0%) | 0.484 |
| mRSS (range: 0–51) | 6.0 (0, 13) | 8.0 (3.0, 12.0) | 3.5 (2.0, 5.75) | 0.001 |
| mRSS > 20 | 17/171 (9.9%) | 16/115 (13.9%) | 1/56 (1.8%) | 0.013 |
| Tendon friction rub | 24/171 (14.0%) | 22/115 (19.1%) | 2/56 (3.6%) | 0.006 |
| Disseminated telangiectasia | 52/171 (30.4%) | 38/115 (33.0%) | 14/56 (25.0%) | 0.283 |
| Salt-and-pepper skin appearance | 93/171 (54.4%) | 70/115 (60.9%) | 23/56 (41.1%) | 0.015 |
| Interincisor distance (cm) | 3.0 (3.0, 4.0) | 3.0 (2.5, 4.0) | 4.0 (3.3, 4.4) | <0.0001 |
| %FVC predicted | 75.4 (63.7, 88.1) | 70.2 (58.3, 85.1) | 80.1 (73.7, 86.9) | 0.018 |
| %TLC predicted | 70.0 (59.5, 79.0) | 63.0 (53.0, 81.0) | 77.0 (66.5, 82.3) | 0.066 |
| %DLCO/VA predicted | 68.0 (55.8, 76.3) | 53.0 (49.0, 69.0) | 72.5 (58.5, 76.8) | 0.429 |
| 6MWD (m) | 414.5 (360.0, 449.5) | 378.0 (360.0, 443.0) | 396.5 (280.3, 484.3) | 0.935 |
| %FVC < 80% predicted | 51/86 (59.3%) | 40/61 (65.6%) | 11/25 (44.0%) | 0.064 |
| %TLC < 80% predicted | 60/78 (76.9%) | 46/56 (82.1%) | 14/22 (63.6%) | 0.081 |
| %DLCO/VA < 70% predicted | 46/78 (59.0%) | 35/55 (63.6%) | 11/23 (47.8%) | 0.196 |
| Symptomatic NSIP | 76/171 (44.4%) | 54/115 (47.0%) | 22/56 (39.3%) | 0.343 |
| Symptomatic UIP | 22/171 (12.9%) | 19/115 (16.5%) | 3/56 (5.4%) | 0.041 |
| Symptomatic NSIP and UIP | 3/171 (1.8%) | 1/115 (0.9%) | 2/56 (3.6%) | 0.207 |
| LVEF (%) | 67.0 (64.2, 71.3) | 69.0 (63.0, 73.0) | 67.5 (64.2, 69.0) | 0.882 |
| RVSP (mmHg) | 33.7 (28.0, 40.3) | 33.8 (30.1, 52.9) | 35.2 (27.9, 43.0) | 0.329 |
| mPAP (mmHg) | 20.1 (17.7, 24.8) | 20.1 (17.1, 23.4) | 19.5 (17.7, 28.1) | 0.783 |
| RVSP > 50 mmHg | 5/56 (8.9%) | 5/44 (11.4%) | 0/12 (0.0%) | 0.221 |
| mPAP > 20 mmHg | 21/41 (51.2%) | 17/31 (54.8%) | 4/10 (40.0%) | 0.414 |
| Scleroderma renal crisis ever | 5/171 (2.9%) | 3/115 (2.6%) | 2/56 (3.6%) | 0.726 |
| Antinuclear antibody positivity | 159/162 (98.1%) | 107/108 (99.1%) | 52/54 (96.3%) | 0.216 |
| Anti-Scl-70 positivity | 113/150 (75.3%) | 78/98 (79.6%) | 35/52 (67.3%) | 0.097 |
| Anti-centromere positivity | 14/127 (11.0%) | 6/84 (7.1%) | 8/43 (18.6%) | 0.051 |
| Anti-ribonucleoproteins | 11/76 (14.5%) | 10/52 (19.2%) | 1/24 (4.2%) | 0.083 |
| Anti-dsDNA | 4/63 (6.3%) | 2/44 (4.5%) | 2/19 (10.5%) | 0.372 |
| Anti-Sm | 5/68 (7.4%) | 4/49 (8.2%) | 1/19 (5.3%) | 0.681 |
| Lupus anticoagulant | 3/34 (8.8%) | 2/29 (6.9%) | 1/5 (20.0%) | 0.340 |
| Anti-cardiolipin IgM or IgG | 3/33 (9.1%) | 2/27 (7.4%) | 1/6 (16.7%) | 0.475 |
| Anti-β2GP1 IgM or IgG | 4/28 (14.3%) | 3/24 (12.5%) | 1/4 (25.0%) | 0.508 |
| Rheumatoid factor | 14/44 (31.8%) | 7/28 (25.0%) | 7/16 (43.8%) | 0.199 |
| Anti-citrullinated peptide | 6/27 (22.2%) | 4/17 (23.5%) | 2/10 (20.0%) | 0.831 |
DIC: digital ischemic complications; RP: Raynaud phenomenon; SSc: systemic sclerosis; eSSc: early undifferentiated SSc; lcSSc: limited cutaneous SSc; dcSSc: diffuse cutaneous SSc; RA: rheumatoid arthritis; SLE: systemic lupus erythematosus; PM: polymyositis; mRSS: modified Rodnan skin score; FVC: forced vital capacity; TLC: total lung capacity; DLCO/VA: diffusion capacity of carbon monoxide/ventilatory area; 6MWD: 6 meter walking distance; NSIP: nonspecific interstitial pneumonia; UIP: usual interstitial pneumonia; LVEF: left ventricular ejection fraction; RVSP: right ventricular systolic pressure; mPAP: mean pulmonary arterial pressure; Anti-dsDNA: anti-double stranded deoxyribonucleic acid; Anti-Sm: anti-Smith antibody; IgM: immunoglobulin M; IgG: immunoglobulin G.
Data presented as mean ± standard deviation, number and percentage, or median and interquartile range.
A p-value < 0.05 (italicized) indicates statistical significance.
Table 2.
Baseline laboratory data for all included patients, and compared between those with and without DIC.
| Laboratory data | All patients (N = 171) | Patients with DIC (n = 115) | Patients without DIC (n = 56) | p |
|---|---|---|---|---|
| Hemoglobin (g/dL) | 12.2 (11.2, 13.4) | 11.4 (9.9, 12.6) | 12.8 (11.9, 14.9) | 0.552 |
| Red cell distribution width | 14.5 (13.6, 15.7) | 15.5 (13.2, 17.2) | 14.3 (13.0, 15.0) | 0.185 |
| White blood cell count (cells/mm3) | 7310.0 (5630, 8440) | 7950.0 (6010.0, 8750.0) | 8085.0 (6380.0, 8372.5) | 0.056 |
| Eosinophils (percent count) | 2.4 (1.4, 4.2) | 2.1 (1.0, 4.2) | 2.1 (1.4, 9.5) | 0.543 |
| Eosinophils (absolute count) | 142.7 (80.8, 287.8) | 156.6 (78.1, 319.3) | 141.0 (112.1, 785.0) | 0.139 |
| Erythrocyte sedimentation rate (mm/h) | 36.5 (18.0, 54.3) | 41.0 (17.0, 63.0) | 37.5 (12.5, 53.5) | 0.054 |
| C-reactive protein (mg/L) | 3.2 (1.2, 9.2) | 5.5 (0.9, 11.0) | 1.6 (0.8, 2.4) | 0.105 |
| Creatinine (mg/dL) | 0.7 (0.6, 0.9) | 0.7 (0.6, 0.9) | 0.7 (0.7, 1.2) | 0.776 |
| Albumin (g/dL) | 4.0 (3.8, 4.3) | 4.0 (3.5, 4.1) | 3.9 (3.7, 4.1) | 0.548 |
| Globulin (g/dL) | 3.7 (3.2, 4.1) | 3.8 (3.2, 4.3) | 3.5 (3.2, 4.3) | 0.058 |
| Creatine phosphokinase (IU/L) | 101.0 (73.5, 160.0) | 153.0 (78.0, 269.0) | 94.0 (52.5, 138.5) | 0.399 |
| Urine-protein-creatinine ratio (g/mg) | 0.1 (0.1, 0.2) | 0.1 (0.1, 0.3) | 0.1 (0.1, 0.2) | 0.868 |
DIC: digital ischemic complications.
Data presented as median and interquartile range.
A p-value < 0.05 indicates statistical significance.
Table 3.
Baseline medication data for all included patients, and compared between those with and without DIC.
| Medication data | Total cohort (N = 171) | Patients with DIC (n = 115) | Patients with no DIC (n = 56) | p |
|---|---|---|---|---|
| Hydroxychloroquine | 16/171 (9.4%) | 12/115 (10.4%) | 4/56 (7.1%) | 0.488 |
| Hydroxychloroquine dose (mg/day) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.957 |
| Chloroquine | 139/171 (81.3%) | 94/115 (81.7%) | 45/56 (80.4%) | 0.828 |
| Chloroquine dose (mg/day) | 78.2 (35.7, 125.0) | 86.0 (35.7, 125.0) | 72.0 (32.6, 107.1) | 0.512 |
| Methotrexate | 23/171 (13.5%) | 15/115 (13.0%) | 8/56 (14.3%) | 0.823 |
| Methotrexate dose (mg/week) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.916 |
| Cyclophosphamide | 20/171 (11.7%) | 17/115 (14.8%) | 3/56 (5.4%) | 0.072 |
| Cyclophosphamide dose (mg/day) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.189 |
| Mycophenolate mofetil | 23/171 (13.5%) | 16/115 (13.9%) | 7/56 (12.5%) | 0.799 |
| Mycophenolate mofetil dose (mg/day) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.7) | 0.0 (0.0, 0.0) | 0.621 |
| Baby ASA | 148/171 (86.5%) | 99/115 (86.1%) | 49/56 (87.5%) | 0.799 |
| Nifedipine | 68/171 (39.8%) | 51/115 (44.3%) | 17/56 (30.4%) | 0.079 |
| Nifedipine dose (mg/day) | 1.9 (0.0, 20.0) | 12.5 (0.0, 20.0) | 0.0 (0.0, 20.0) | < 0.0001 |
| Sildenafil | 5/171 (2.9%) | 4/115 (3.5%) | 1/56 (1.8%) | 0.538 |
| Sildenafil dose (mg/day) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.681 |
| Amlodipine | 41/171 (24.0%) | 30/115 (26.1%) | 11/56 (19.6%) | 0.354 |
| Amlodipine dose (mg/day) | 0.0 (0.0, 2.5) | 0.0 (0.0, 3.2) | 0.0 (0.0, 0.0) | 0.165 |
| Manidipine | 3/171 (1.8%) | 1/115 (0.9%) | 2/56 (3.6%) | 0.207 |
| Manidipine dose (mg/day) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.814 |
| Verapamil | 1/171 (0.6%) | 1/115 (0.9%) | 0/56 (0.0%) | 0.484 |
| Verapamil dose (mg/day) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.814 |
| Enalapril | 9/171 (5.3%) | 6/115 (5.2%) | 3/56 (5.4%) | 0.969 |
| Enalapril dose (mg/day) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.946 |
| Captopril | 3/171 (1.8%) | 2/115 (1.7%) | 1/56 (1.8%) | 0.983 |
| Captopril dose (mg/day) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.695 |
| Losartan | 4/171 (2.3%) | 4/115 (3.5%) | 0/56 (0.0%) | 0.158 |
| Losartan dose (mg/day) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.549 |
| Doxazosin | 2/171 (1.2%) | 2/115 (1.7%) | 0/56 (0.0%) | 0.321 |
| Doxazosin dose (mg/day) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.271 |
| Prazosin | 14/171 (8.2%) | 10/115 (8.7%) | 4/56 (7.1%) | 0.728 |
| Prazosin dose (mg/day) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.115 |
| Prednisolone | 36/171 (21.1%) | 28/115 (24.3%) | 8/56 (14.3%) | 0.130 |
| Prednisolone dose (mg/day) | 0.0 (0.0, 0.3) | 0.0 (0.0, 0.7) | 0.0 (0.0, 0.0) | 0.067 |
DIC: digital ischemic complications; ASA: acetylsalicylic acid.
Data presented as number and percentage or median and interquartile range.
A p-value < 0.05 (italicized) indicates statistical significance.
Multivariate regression analysis adjusted for continuous (age and interincisor distance) and categorical (gender, disease duration > 3 years, dcSSc subset, mRSS > 20, TFR, salt-and-pepper skin appearance, anti-Scl-70, and anti-centromere) variables revealed the following independent associations with DIC. The dcSSc subgroup was associated with DIC (OR: 6.0, 95% CI: 2.6–14.0; p < 0.0001), DPS (OR: 4.9, 95% CI: 1.8–13.2; p = 0.002), and DPL (OR: 6.4, 95% CI: 1.7–23.5; p = 0.005). TFR was associated with DPS (OR: 5.0, 95% CI: 1.5–16.4; p = 0.008). Salt-and-pepper skin appearance was associated with DPL (OR: 3.0, 95% CI: 1.2–7.4; p = 0.014) and DU (OR: 6.9, 95% CI: 1.6–30.5; p = 0.011). Longer disease duration of more than 3 years was associated with DU (OR: 4.4, 95% CI: 1.2–16.4; p = 0.026). Finally, male gender was associated with DU (OR: 5.4, 95% CI: 1.2–24.6; p = 0.028) (Table 4).
Table 4.
Variables found to be independently associated with DIC in multivariable regression analysis.
| Variables | B | SE | Wald test result | p | Exp (B) | 95% CI for Exp (B) | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Digital ischemic complications | |||||||
| Diffuse cutaneous SSc | 1.793 | 0.432 | 17.242 | 0.000 | 6.008 | 2.577 | 14.004 |
| Digital pitting scars | |||||||
| Diffuse cutaneous SSc | 1.587 | 0.507 | 9.798 | 0.002 | 4.887 | 1.810 | 13.196 |
| Tendon friction rub | 1.610 | 0.607 | 7.043 | 0.008 | 5.004 | 1.524 | 16.436 |
| Digital pulp loss | |||||||
| Diffuse cutaneous SSc | 1.856 | 0.665 | 7.803 | 0.005 | 6.400 | 1.740 | 23.540 |
| Salt-and-pepper skin appearance | 1.110 | 0.454 | 5.985 | 0.014 | 3.030 | 1.247 | 7.379 |
| Age | −0.039 | 0.019 | 4.076 | 0.043 | 0.962 | 0.927 | 0.999 |
| Digital ulcer | |||||||
| Disease duration > 3 years | 1.488 | 0.669 | 4.941 | 0.026 | 4.429 | 1.192 | 16.449 |
| Salt-and-pepper skin appearance | 1.928 | 0.760 | 6.438 | 0.011 | 6.875 | 1.551 | 30.482 |
| Male gender | 1.692 | 0.770 | 4.830 | 0.028 | 5.432 | 1.201 | 24.575 |
DIC: digital ischemic complications; B: unstandardized beta; SE: standard error; Exp (B): odds ratio; CI: confidence interval; SSc: systemic sclerosis; dcSSc: disseminated cutaneous SSc; mRSS: modified Rodnan skin score; TFR: tendon friction rub.
Continuous variables adjusted for age and interincisor distance.
Categorical variables adjusted for gender, disease duration > 3 years, dcSSc, mRSS > 20, TFR, salt-and-pepper skin appearance, anti-Scl70, and anti-centromere.
A p-value < 0.05 indicates statistical significance.
Discussion
This post hoc study investigated the incidence of various types of DIC and their independently associated factors among a survival cohort of SSc patients (dcSSc majority subset) who lived in a tropical zone and who had an overall median disease duration of approximately 2.9 years. At baseline, 56% of SSc patients had DIC. The prevalence rates of DPS, DPL, DU, and DA of 39.8%, 41.5%, 3.5%, and 7.6%, respectively, in our study are in good agreement with the rates reported by Wangkaew et al., 7 who had similar patient demographics and geographical area, and are comparable to the rates reported by Poormoghim et al. 15 The present study encountered substantially less DU (3.5%) compared to that encountered by Wangkaew et al. 7 (12.7%) and Poormoghim et al. 15 (39.0%). Wangkaew et al. 7 conducted a cross-sectional analysis of a prospective registry in a tertiary care center in Northern Thailand among SSc patients with a median disease duration of approximately 4.9 years. Poormoghim et al. 15 conducted a retrospective analysis of a registry in a university-affiliated hospital in Iran among patients with a median disease duration of approximately 5.6 years, and they included patients with DG. It was nonetheless the case that 14% of the entire cohort developed DU during the 1-year follow-up. The prevalence was increased in both active DU (1.2% increased to 4.7%) and healed DU (2.3% increased to 9.4%). Therefore, the prevalence of any history of DU gradually accumulated over time. This indicates that the vasculopathy progressed over time, resulting in various types of DIC.
The severity and disease duration of SSc may affect the prevalence of DIC, as was the case for DU in the present study. The incidence of both DU and DPS had two waves—during the early stage followed by a gradual increase during the later stage of SSc disease evolution. Utsunomiya et al. 16 conducted a prospective multicenter study in 207 patients for 7 years. They found rates of prevalence of DU and DPS at baseline and at 7 years of 18.0% versus 28.6%, and 40.3% versus 49.0%, respectively. The first wave of disease development may be influenced by the severity of vasculopathy or SSc disease severity. The second wave of disease development may be influenced by the progression of vasculopathy over time combined with the effects age and other atherosclerotic risk factors. Hachulla et al. 5 conducted a retrospective study of 101 patients with long disease duration (12.3 ± 6.3 years) for 7 years and found that 43% and 73% of the first DU occurred within 1 and 5 years, respectively—the first wave. Many studies reported that factors associated with SSc severity, such as dcSSc subset,17–20 anti-Scl-70 positivity,14,17,19–23 early onset of RP or non-RP symptoms,5,14,18,21,22,24,25 male gender,14,24 and mRSS,5,7,24 were independently correlated with the occurrence of DU. In addition, Tiev et al. 24 and Morrisroe et al. 20 reported that patients with DU had a longer disease duration since the first onset of non-RP symptom(s)—the second wave.
The incidence rates of active DU (8%–24%)14,24,25 and in patients with a history of prior DU (17%–58%)17,20,24–26 that were reported from studies conducted in the subtropical region were higher than the DU incidence rates found in this study. There is, therefore, a strong relationship between climate and the rates of developing DU in SSc patients. 6 The incidence rate of DIC in this study was 58.5%. Of these patients, the incidence rate of DU was only 14%. Our reported incidence rates of DU are lower than those previously reported. Matucci-Cerinic et al., 27 Hachulla et al., 5 and Brand et al. 19 reported incidence rates of DU of 46.2%, 50.0%, and 66% within 1 year. However, the SSc patients included in the immediately aforementioned studies had a longer disease duration (>6 years) and they lived in a subtropical area. Foocharoen et al. 28 and Wangkaew et al. 29 (overlapped population with Wangkaew et al. 7 ) from another two Thailand SSc referral centers reported prevalence rates of DU of 19% and 8.7%. These series had a longer disease duration (>8 years). Janardana et al. 30 from India reported a prevalence rate of DU of 23%. This study had a short disease duration (2.5 years). It was noted that even the prevalence of DU from the Asian equatorial cohorts was lower than those studies conducted in the subtropical region. The geo-ethnic difference may also influence the occurrence of DIC in SSc.
The relationship between preexisting vasculopathy and the recurrence of DU has also been reported. Matucci-Cerinic et al. 27 and Hachulla et al. 5 reported a recurrence rate of DU of 46.2% and 66%, respectively, and 50% of cases occurred within 1 year. 5 Mecoli et al. 31 studied a prospective Johns Hopkins University cohort of 300 long disease duration (10 ± 8.4 years) patients for 5 years, and they found that patients were significantly more likely to develop DIC if they had a history of prior DIC (hazard ratio (HR): 7.0, p < 0.001). Our study also showed that patients with a history of prior DIC developed new DIC at a higher incidence rate than those who had no history of prior DIC (OR: 15.9, p < 0.0001).
DIC is a surrogate marker for the peripheral vascular domain of the SSc disease severity scale proposed by Medsger et al. 32 Disease severity reflects the combined effect of damage to internal organs that was caused by SSc. SSc vasculopathy results from both structural and functional aberrations 2 and is correlated with other markers of SSc severity. Consistent with the results of the present study, patients with DIC were usually dcSSc subset, had longer disease duration, and had higher baseline mRSS in univariate analysis.5,7,17–20,24 Other factors found to be significantly associated with DIC were TFR, salt-and-pepper skin presentation, less interincisor distance, lower FVC %predicted, and symptomatic UIP. Inflammatory process as indicated by ESR and serum globulin levels was nonsignificant in our study, but increased in patients with DIC. Sunderkötter et al. 14 reported ESR to be a factor that independently influences the appearance of DU.
Our multivariate analysis identified dcSSc as an independent risk factor for developing DIC, DPS, or DPL during the following year. TFR was found to be an independent predictor of DPS, and salt-and-pepper skin appearance was found to independently predict both DPL and DU. Moreover, male gender was independently associated with DU, and age had a negative independent association with the development of DPL. Previous studies reported dcSSc17–20 and anti-Scl-70 positivity14,17,19–23 to be independently associated with the development of DU since they were at risk for major SSc internal organ complications and survival.33–35 However, the fact that our study did not find significant association between anti-Scl-70 positivity and any DIC may be due to the high prevalence of anti-Scl-70 positivity in the overall population or the small sample size. A referral bias may cause a high frequency of anti-Scl-70 antibodies. However, it was similar to that described in the Northern (79%) 29 and the North-Eastern (81%) 28 Thai series. Anyway, all of these centers were the SSc referral centers. These figures were higher than those in the Chinese 22 and Malaysian36–38 series. TFR was prevalent among those with dcSSc subset; however, TFR was independently associated with DU and was not confounded by dcSSc. 39 Hughes et al. 40 studied the EUSTAR database of 9671 patients. They found DPS was associated with severe internal organ involvement, for example, interstitial lung disease (ILD), pulmonary arterial hypertension (PAH), conduction blocks, calcinosis cutis, arthritis, capillaroscopic abnormality, DU, and death. Likewise, DPS did not reflect only severe vasculopathy but also severe skin, poor disease course, and mortality.
Multivariate logistic regression analysis revealed salt-and-pepper skin appearance to be independently associated with DU (Table 4), which has not been reported from any previous study. The strong positive correlation between salt-and-pepper skin appearance and baseline mRSS > 20 units might explain the non-significance of baseline mRSS > 20 units by our statistical model. When baseline mRSS as a continuous variable instead of baseline mRSS > 20 units was included in the regression analysis, the presence of mRSS (OR: 1.075, 95% CI: 1.011–1.142; p = 0.02) showed a significant independent association with DU, which is concordant with previous studies.5,7,24 Leroy et al. 41 found that diffuse skin hyper/hypo-pigmentation was associated not only with the dcSSc subset but also with a peak mRSS. In addition, diffuse skin hyperpigmentation was correlated with the presence of vascular involvement, DU, and ILD. Endothelin-1 (ET-1) was among keratinocyte-derived factors that regulate melanocytic skin pigmentation. 42 Increased levels of ET-1 productivity in keratinocytes of dcSSc patients were correlated with skin pigmentation. 43 Therefore, the elevation of ET-1 could connect skin pigmentation and vascular complication in SSc. Treatment with Bosentan—an endothelin receptor antagonist—was effective for PAH, peripheral vascular disease, and mRSS. 44 However, there was no evidence for the improvement of skin pigmentation. Similar to previous studies,14,24 we found male gender to be an independent predictor of DU. We also found that age negatively correlated to DPL since early onset of SSc was associated with DIC, especially DU.5,14,18,21,22,24,25 Contrarily—over the longer term, the development of DPL might be decreased via vasodilator treatment or progressed to DA due to the development of acro-osteolysis.
Strengths and limitations
The strength of this study is the epidemiological reporting of various types of DIC other than DU using a prospective cohort of short disease duration SSc patients who lived in a tropical zone. The results are in good agreement with those reported from previous studies in spite of our small incidence and prevalence of DU. We also described and compared various baseline characteristics between DIC and non-DIC SSc (Tables 1–3) and then described and compared the progression of certain variables at 1 year (Figure 1).
This study also has some mentionable limitations. First, the retrospective nature of this post hoc study suggests its potential vulnerability to certain biases, as well as to missing and/or incomplete data. Second, the data included in this study was taken from a registry from one center, which also happens to be a national tertiary referral center. Since our center is routinely referred cases thought to be complex, our results may not be immediately generalizable to other care settings. Third, our results reflect the study of SSc patients with short disease duration, dcSSc as the major SSc subset, and living in a tropical area, so the generalizability of our results to study populations with other characteristics should be performed with caution. Fourth, less than half of our patients underwent pulmonary function testing and echocardiography, so this could have influenced the inability of our study to identify existing, but unfound significant associations between those parameters and the various types of DIC. Fifth, the relatively short 1-year follow-up period could influence the epidemiography of particular types of DIC, such as DPL and DA. Sixth, and finally, this study had/included no data specific to the severity of micro and macro vasculopathy, such as nail fold capillaroscopy and Doppler ultrasonography of digit and hand arteries.
Conclusion
Among the various SSc-associated digital ischemic complications, DPL, DPS, DU, and DA were the most common accounting for more than half of incidents per year, especially among those who had a previous history of those complications. Of the six identified independent risk factors for DIC among the evaluated digital ischemic complication, including dcSSc, TFR, salt-and-pepper skin appearance, age, disease duration > 3 years, and male gender, dcSSc was the strongest predictor of DIC. Close monitoring and vasodilator therapy are essential for improving favorable patient outcomes.
Acknowledgments
The authors gratefully acknowledge Miss Khemajira Karaketklang of the Research and Academic Services Unit, Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand, for her assistance with statistical analysis. And Asst. Prof. Kevin P. Jones of the Siriraj Medical Research Center, Faculty of Medicine, Siriraj Hospital, Mahidol University, Bangkok, Thailand, for his assistance with medical research manuscript editing.
Footnotes
Author contributions: T.G. and C.M. contributed to study conception; data acquisition, analysis, and interpretation; drafting and critical revision of the manuscript; final approval of the manuscript; and agreement to be responsible for the accuracy and integrity of the data and findings.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Chayawee Muangchan  https://orcid.org/0000-0002-5412-4055
https://orcid.org/0000-0002-5412-4055
References
- 1. Furue M, Mitoma C, Mitoma H, et al. Pathogenesis of systemic sclerosis—current concept and emerging treatments. Immunol Res 2017; 65(4): 790–797. [DOI] [PubMed] [Google Scholar]
- 2. Asano Y, Sato S. Vasculopathy in scleroderma. Semin Immunopathol 2015; 37: 489–500. [DOI] [PubMed] [Google Scholar]
- 3. Silva I, Almeida J, Vasconcelos C. A PRISMA-driven systematic review for predictive risk factors of digital ulcers in systemic sclerosis patients. Autoimmun Rev 2015; 14(2): 140–152. [DOI] [PubMed] [Google Scholar]
- 4. Muangchan C, Canadian Scleroderma Research Group, Baron M, et al. The 15% rule in scleroderma: the frequency of severe organ complications in systemic sclerosis. A systematic review. J Rheumatol 2013; 40(9): 1545–1556. [DOI] [PubMed] [Google Scholar]
- 5. Hachulla E, Clerson P, Launay D, et al. Natural history of ischemic digital ulcers in systemic sclerosis: single-center retrospective longitudinal study. J Rheumatol 2007; 34(12): 2423–2430. [PubMed] [Google Scholar]
- 6. Souza E, Muller CS, Horimoto A, et al. Geographic variation as a risk factor for digital ulcers in systemic sclerosis patients: a multicentre registry. Scand J Rheumatol 2017; 46(4): 288–295. [DOI] [PubMed] [Google Scholar]
- 7. Wangkaew S, Sivasomboon C, Leungwatthananon W, et al. Prevalence and predictors of hand involvement in Thai patients with systemic sclerosis. Int J Rheum Dis 2018; 21(1): 240–248. [DOI] [PubMed] [Google Scholar]
- 8. Preliminary criteria for the classification of systemic sclerosis (scleroderma). Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis Rheum 1980; 23(5): 581–590. [DOI] [PubMed] [Google Scholar]
- 9. van den Hoogen F, Khanna D, Fransen J, et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League Against Rheumatism Collaborative Initiative. Ann Rheum Dis 2013; 72: 1747–1755. [DOI] [PubMed] [Google Scholar]
- 10. Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism Collaborative Initiative. Ann Rheum Dis 2010; 69: 1580–1588. [DOI] [PubMed] [Google Scholar]
- 11. Petri M, Orbai AM, Alarcón GS, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 2012; 64(8): 2677–2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lundberg IE, Tjärnlund A, Bottai M, et al. 2017 European League Against Rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Ann Rheum Dis 2017; 76: 1955–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shiboski CH, Shiboski SC, Seror R, et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjögren’s syndrome: a consensus and data-driven methodology involving three international patient cohorts. Ann Rheum Dis 2017; 76: 9–16. [DOI] [PubMed] [Google Scholar]
- 14. Sunderkötter C, Herrgott I, Brückner C, et al. Comparison of patients with and without digital ulcers in systemic sclerosis: detection of possible risk factors. Br J Dermatol 2009; 160(4): 835–843. [DOI] [PubMed] [Google Scholar]
- 15. Poormoghim H, Moghadam AS, Moradi-Lakeh M, et al. Systemic sclerosis: demographic, clinical and serological features in 100 Iranian patients. Rheumatol Int 2013; 33(8): 1943–1950. [DOI] [PubMed] [Google Scholar]
- 16. Utsunomiya A, Hasegawa M, Oyama N, et al. Clinical course of Japanese patients with early systemic sclerosis: a multicenter, prospective, observational study. Mod Rheumatol 2021; 31(1): 162–170. [DOI] [PubMed] [Google Scholar]
- 17. Simeón-Aznar CP, Fonollosa-Plá V, Tolosa-Vilella C, et al. Registry of the Spanish network for systemic sclerosis: clinical pattern according to cutaneous subsets and immunological status. Semin Arthritis Rheum 2012; 41(6): 789–800. [DOI] [PubMed] [Google Scholar]
- 18. Denton CP, Krieg T, Guillevin L, et al. Demographic, clinical and antibody characteristics of patients with digital ulcers in systemic sclerosis: data from the DUO Registry. Ann Rheum Dis 2012; 71(5): 718–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brand M, Hollaender R, Rosenberg D, et al. An observational cohort study of patients with newly diagnosed digital ulcer disease secondary to systemic sclerosis registered in the EUSTAR database. Clin Exp Rheumatol 2015; 33(4 Suppl. 91): S47–S54. [PubMed] [Google Scholar]
- 20. Morrisroe K, Stevens W, Sahhar J, et al. Digital ulcers in systemic sclerosis: their epidemiology, clinical characteristics, and associated clinical and economic burden. Arthritis Res Ther 2019; 21: 299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Walker UA, Tyndall A, Czirják L, et al. Clinical risk assessment of organ manifestations in systemic sclerosis: a report from the EULAR Scleroderma Trials and Research Group database. Ann Rheum Dis 2007; 66(6): 754–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xu D, Li MT, Hou Y, et al. Clinical characteristics of systemic sclerosis patients with digital ulcers in China. Clin Exp Rheumatol 2013; 31(2 Suppl. 76): 46–49. [PubMed] [Google Scholar]
- 23. Hunzelmann N, Riemekasten G, Becker MO, et al. The predict study: low risk for digital ulcer development in patients with systemic sclerosis with increasing disease duration and lack of topoisomerase-1 antibodies. Br J Dermatol 2016; 174(6): 1384–1387. [DOI] [PubMed] [Google Scholar]
- 24. Tiev KP, Diot E, Clerson P, et al. Clinical features of scleroderma patients with or without prior or current ischemic digital ulcers: post-hoc analysis of a nationwide multicenter cohort (ItinérAIR-Sclérodermie). J Rheumatol 2009; 36(7): 1470–1476. [DOI] [PubMed] [Google Scholar]
- 25. Khimdas S, Harding S, Bonner A, et al. Associations with digital ulcers in a large cohort of systemic sclerosis: results from the Canadian Scleroderma Research Group registry. Arthritis Care Res 2011; 63(1): 142–149. [DOI] [PubMed] [Google Scholar]
- 26. Steen V, Denton CP, Pope JE, et al. Digital ulcers: overt vascular disease in systemic sclerosis. Rheumatology 2009; 48(Suppl. 3): iii19–iii24. [DOI] [PubMed] [Google Scholar]
- 27. Matucci-Cerinic M, Krieg T, Guillevin L, et al. Elucidating the burden of recurrent and chronic digital ulcers in systemic sclerosis: long-term results from the DUO registry. Ann Rheum Dis 2016; 75(10): 1770–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Foocharoen C, Peansukwech U, Mahakkanukrauh A, et al. Clinical characteristics and outcomes of 566 Thais with systemic sclerosis: a cohort study. Int J Rheum Dis 2020; 23(7): 945–957. [DOI] [PubMed] [Google Scholar]
- 29. Wangkaew S, Tungteerabunditkul S, Prasertwittayakij N, et al. Comparison of clinical presentation and incidence of cardiopulmonary complications between male and female Thai patients with early systemic sclerosis: inception cohort study. Clin Rheumatol 2020; 39(1): 103–112. [DOI] [PubMed] [Google Scholar]
- 30. Janardana R, Nair AM, Surin AK, et al. Unique clinical and autoantibody profile of a large Asian Indian cohort of scleroderma-do South Asians have a more aggressive disease? Clin Rheumatol 2019; 38(11): 3179–3187. [DOI] [PubMed] [Google Scholar]
- 31. Mecoli CA, Shah AA, Boin F, et al. Vascular complications in systemic sclerosis: a prospective cohort study. Clin Rheumatol 2018; 37(9): 2429–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Medsger TA, Jr, Bombardieri S, Czirjak L, et al. Assessment of disease severity and prognosis. Clin Exp Rheumatol 2003; 21: S42–S46. [PubMed] [Google Scholar]
- 33. Simeón-Aznar CP, Fonollosa-Plá V, Tolosa-Vilella C, et al. Registry of the Spanish network for systemic sclerosis: survival, prognostic factors, and causes of death. Medicine 2015; 94(43): e1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kranenburg P, van den Hombergh WM, Knaapen-Hans HK, et al. Survival and organ involvement in patients with limited cutaneous systemic sclerosis and anti-topoisomerase-I antibodies: determined by skin subtype or auto-antibody subtype? A long-term follow-up study. Rheumatology 2016; 55(11):2001–2008. [DOI] [PubMed] [Google Scholar]
- 35. Foocharoen C, Suwannachat P, Netwijitpan S, et al. Clinical differences between Thai systemic sclerosis patients with positive versus negative anti-topoisomerase I. Int J Rheum Dis 2016; 19(3): 312–320. [DOI] [PubMed] [Google Scholar]
- 36. Pagalavan L, Ong SG. Demography, clinical and laboratory features of systemic sclerosis in a Malaysian rheumatology centre. Med J Malaysia 2007; 62(2): 117–121. [PubMed] [Google Scholar]
- 37. Teh CL, Kuan YC, Wong JS. Systemic sclerosis in Sarawak: a profile of patients treated in the Sarawak General Hospital. Rheumatol Int 2009; 29(10):1243–1245. [DOI] [PubMed] [Google Scholar]
- 38. Sujau I, Ng CT, Sthaneshwar P, et al. Clinical and autoantibody profile in systemic sclerosis: baseline characteristics from a West Malaysian cohort. Int J Rheum Dis 2015; 18(4): 459–465. [DOI] [PubMed] [Google Scholar]
- 39. Avouac J, Walker U, Tyndall A, et al. Characteristics of joint involvement and relationships with systemic inflammation in systemic sclerosis: results from the EULAR Scleroderma Trial and Research Group (EUSTAR) database. J Rheumatol 2010; 37(7):1488–1501. [DOI] [PubMed] [Google Scholar]
- 40. Hughes M, Heal C, Henes J, et al. Digital pitting scars are associated with a severe disease course and death in systemic sclerosis: a study from the EUSTAR cohort. Rheumatology 2022; 61: 1141–1147. [DOI] [PubMed] [Google Scholar]
- 41. Leroy V, Henrot P, Barnetche T, et al. Association of skin hyperpigmentation disorders with digital ulcers in systemic sclerosis: analysis of a cohort of 239 patients. J Am Acad Dermatol 2019; 80(2): 478–484. [DOI] [PubMed] [Google Scholar]
- 42. Yamaguchi Y, Hearing VJ. Physiological factors that regulate skin pigmentation. Biofactors 2009; 35(2): 193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tabata H, Hara N, Otsuka S, et al. Correlation between diffuse pigmentation and keratinocyte-derived endothelin-1 in systemic sclerosis. Int J Dermatol 2000; 39(12): 899–902. [DOI] [PubMed] [Google Scholar]
- 44. Funauchi M, Kishimoto K, Shimazu H, et al. Effects of bosentan on the skin lesions: an observational study from a single center in Japan. Rheumatol Int 2009; 29(7): 769–775. [DOI] [PubMed] [Google Scholar]