Abstract
Background:
The present, open-labeled study aimed to compare the distal approach (DA) for local corticosteroid injection (LCI) with the conventional proximal approach (PA) in alleviating the symptom and improving the electrodiagnostic parameters of the patients with carpal tunnel syndrome (CTS).
Methods:
A total of 60 participants with nonsevere CTS were included in the present randomized controlled trial (RCT), of which 29 and 31 were assigned to the DA and PA groups, respectively. Each group received a single, landmark-guided injection of local methylprednisolone. The participants were assessed preintervention and 3 months later using the measures of visual analog scale (VAS), Boston Carpal Tunnel Questionnaire (BCTQ), hand grip strength, and nerve conduction study (NCS).
Results:
Following a 3-month follow-up, both groups had significant improvements in VAS, both functional and severity subscales of BCTQ, hand grip strength, and some electrodiagnostic parameters (all P-values < .05). Moreover, the DA group had a significantly lower procedure duration than the PA group (9.80 ± 1.12 vs. 27.61 ± 1.77; P < .001).
Conclusions:
LCI using the DA should be considered a feasible, safe, and effective therapeutic method in patients with mild to moderate CTS. It had a shorter procedure duration than conventional PA, while their clinical and electrophysiological results were similar.
Keywords: carpal tunnel syndrome, median nerve entrapment, local corticosteroid injection, neuropathic pain
Introduction
Carpal tunnel syndrome (CTS) is the most common focal peripheral neuropathy encountered by physicians, accounting for 90% of entrapment neuropathy cases. 1 The condition is caused by the median nerve entrapment within the wrist’s carpal tunnel and subsequent nerve irritation. 2 Clinically, it is characterized by nocturnal pain, burning or tingling sensation, and paresthesia in the cutaneous area on the fingers innervated by the median nerve. 3 Moreover, atrophy and weakness of the thenar muscles are observed in advanced disease. 4 The point prevalence is approximately 3.8% in the general population, with an estimated incidence rate of 276:100,000 per year and a female-to-male ratio of 3-5:1.5,6 The precise underlying mechanism is not fully understood yet; however, it is believed that increased pressure inside the carpal tunnel results in the ischemic injury of the median nerve. 7 The main risk factors of the disorder include some certain activities, such as repetitive wrist movements, prolonged, and inappropriate positions (e.g., driving, typing, hanging the phone, holding objects, opening buttons, or closing buttons), trauma, rheumatoid arthritis, diabetes mellitus, menopause, oral contraceptive pill use, and pregnancy.8-10 The diagnosis is usually clinical-based and is confirmed by the nerve conduction study (NCS). 11
In general, CTS management can be surgical or nonsurgical. 12 Surgical interventions include different carpal tunnel release (CTR) procedures, which are mainly associated with favorable clinical results; however, these approaches are only recommended in severe cases or if the conservative treatment fails. 5 Nonsurgical or conservative alternatives include various therapeutic options, such as activity modifications, night splinting of the wrist, physical therapy, and pharmacologic treatments, such as non-steroidal anti-inflammatory drugs (NSAIDs) and oral or local corticosteroids.4,7
Local corticosteroid injection (LCI) is one of the most recommended options, with a rapid and effective response in patients with mild to moderate CTS. 13 This modality is associated with a significant reduction in tendon swelling and subsequent decompression of the median nerve within the carpal tunnel. 14 For several decades, the injection has been conventionally performed at a site 4 cm proximal to the distal crease in the palmar surface of the wrist, just medial to the palmaris longus (PL) or flexor carpi radialis (FCR) tendons.15,16 The technique has been associated with successful short-term results; however, several complications have been reported, with the median nerve injury being the most serious one. Another side effect is the traumatic injury to the nearby tendons. 16 A needle with an incorrect position can directly traumatize the median nerve. Even with the correct needle insertion and positioning, patients with CTS are more vulnerable to trauma due to the swallowing in the median nerve around the wrist crease.17-19 By the way, there is good evidence that ultrasound-guided injection is associated with more favorable outcomes. However, it seems that landmark-guided injection is still one of the options most widely used due to its acceptable effectiveness, more convenience, and lower costs. 20
For the first time, Habib et al 21 described a new approach for local corticosteroid injection in CTS in 2006. In this technique, the needle is inserted distal to the middle of the distal crease in the wrist palmar surface, between the thenar and hypothenar muscles. 22 Up to now, 5 other studies have compared this new distal approach (DA) with the well-known conventional proximal technique using different methods and have found similar results in symptom relief and electrophysiological parameter improvement.22-26
Therefore, the present study intended to compare the distal and proximal approaches (PAs) used for local corticosteroid injection in CTS symptom management. Despite the 2 approved proximal methods, ultrasound-, and landmark-guided injections, we aimed to focus on the relatively new alternative of DA as a less time-consuming, safe, practical, and effective method for CTS treatment.
Materials and Methods
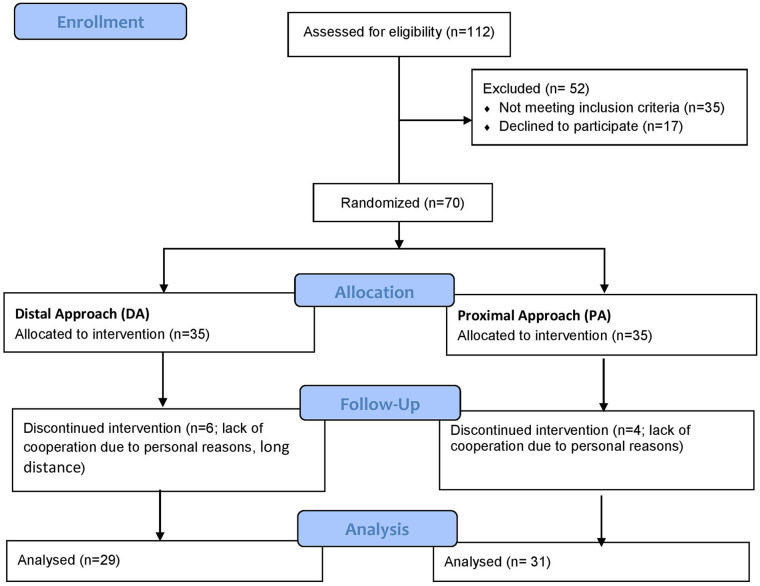
The participants were selected from the patients referred to the Physical Medicine and Rehabilitation clinic and Rheumatology center of Shariati Hospital during 2019 to 2020 with clinical manifestations suggestive of CTS. The inclusion criteria were the involvement with mild or moderate CTS based on the electrodiagnostic studies, 27 an age higher than 18 years, and a symptom severity score of ≥ 4 on the visual analogue scale (VAS). The exclusion criteria included severe CTS or severe and persistent symptoms (paresthesia and pain), a history of any corticosteroid injection within the last 6 months, a history of any CTR procedure, contraindications to corticosteroid administration (such as hypersensitivity), malignancy, cutaneous infection at the injection site, pregnancy, concomitant neurological disorders based on the electrodiagnostic findings, and patients indicated for surgical intervention. If bilateral involvement was present, only the hand with more severe involvement was included, and the other hand was excluded from the study. Eventually, 60 patients were eligible based on the inclusion and exclusion criteria (Figure 1).
Figure 1.
Flow diagram of the study population.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008. Informed consent was obtained from all patients for being included in the study. The randomized controlled trial (RCT) was approved by the ethics committee of the institutional review board of the Tehran University of Medical Sciences (TUMS) with the approval code of (IR.TUMS.MEDICINE.REC.1398.423). Also, it was registered at the Iranian Registry of Clinical Trials with the registration number of (IRCT20180804040685 N1). This study conforms to all CONSORT guidelines and reports the required information accordingly (see Supplementary Checklist).
Intervention
The participants were randomly divided into 2 intervention groups of the DA group and the PA group using block randomization. Patients in each group received a single, landmark-guided injection of local methylprednisolone through either the distal or PAs by an experienced physiatrist with 10 years of experience musculoskeletal interventions. All injections were performed using a 29-gauge, 0.5-inch-long needle containing 40 mg of methylprednisolone while the patients were in the supine position. In the PA group, the needle was inserted at the palmar side of the wrist medial to the PL tendon and 2 cm proximal to the distal wrist crease with an angle of 30° to 40°. 28 In the DA group, the needle was inserted 2 cm distal to the palmar crease of the wrist perpendicular to the palm while the wrist was at a 40° to 50° dorsiflexion. All patients were prescribed a night splint for the wrist for 6 weeks and were instructed how to use them. Moreover, the patients were reminded to use the splints via regular phone calls.
Outcome Measures
The participants were assessed using VAS (primary outcome), Boston Carpal Tunnel Questionnaire (BCTQ), and NCS before the intervention and 3 months later. Pain assessment was performed using a 10-cm VAS ruler ranged from no symptom = 0 to the most severe symptom possible = 10. The participants were asked to mark the score on the VAS ruler that was correlated with the highest pain experienced during the last 2 days.
As a self-reporting questionnaire specific to CTS, BCTQ is one of the most widely-used tools. It consists of 2 following subscales: the Boston Questionnaire—Symptom Severity Scale (BQ-SS) and the Boston Questionnaire—Functional Status Scale (BQ-FS). The former includes 11 questions on the symptom severity based on a 5-point Likert scale (from “none” or “never” = 0 to “very severe” or “persistent” = 5) while the latter includes 8 items on the difficulty in performing typical daily tasks and is also scored on a 5-point Likert scale (from “no difficulty” = 0 to “not able to perform” = 5). For each subscale, the sum of individual item scores is calculated and reported in percentage. Higher scores indicate a more severe disability. 29 The content validity and test-retest reliability of the Persian version of BCTQ have been described in recent years.30,31
To find the motor and sensory electrodiagnostic parameters of the median nerve, NCS was performed using a Nihon Kohden machine (Neuropack® X1 MEB-2300). The related assessments were performed based on the last guideline update by the American Association of Electrodiagnostic Medicine (AANEM). 32 To determine the Sensory Nerve Action Potential (SNAP), the recording surface electrodes were put on the third digit, and an antidromic stimulation was applied to the median nerve at a site 14 cm proximal to the recording electrodes between PL and FCR tendons. The distal sensory latency (DSL, peak latency), baseline-to-peak amplitude, and sensory conduction velocity (NCV-s) were then calculated.
The recording electrode was positioned on the abductor pollicis brevis (APB) motor point on the thenar eminence to determine the compound muscle action potential (CMAP). Then, an orthodromic stimulation was applied to the median nerve at a site 8 cm proximal to the recording electrode. Subsequently, the distal motor latency (DML, initial latency), baseline-to-peak amplitude, and motor conduction velocity (NCV-m) between 2 points of proximal and distal stimulation were calculated. Based on the electrodiagnostic findings and the criteria by Werner and Andary, 27 the CTS severity was classified as no CTS or mild, moderate, and severe diseases.
The hand grip strength was assessed using a hydraulic grip dynamometer (Baseline, Irvington, New York). Patients were seated with a semi-supinated position of the forearm and wrist. The elbow was flexed at 90°, and the shoulder was abducted at 90°. Among the 3 measurements performed with the maximum possible force, the highest value (kg) was recorded. 33 The procedure duration, which was from finding the appropriate injection site to the end of the procedure and needle withdrawal, was recorded using a stopwatch, and the mean injection time in both groups was calculated. Moreover, any related or unrelated adverse effects were also reported.
Statistical Analysis
Considering the earlier experiences, a standard deviation of 1 and a mean difference of 1, the sample size was estimated as 30 per group. Then, it was increased to 35 to take into account a potential dropout rate of 15% (α = 0.01; β = 0.1). The following equation was used for samples size calculation:
The data were analyzed using the SPSS software (SPSS Inc.®, Chicago, Illinois) version 24.0. The Kolmogorov-Smirnov and Shapiro-Wilk tests were used to ensure the normality of data distribution. Continuous variables were described using the mean ± SD, while the categorical variables were described using the frequency and percentage (%). At first, patients’ characteristics and baseline measurements were compared between the 2 groups. The study variable changes in each group were described in effect size or raw mean difference (MD), which was considered as the difference between pre- and postintervention mean values. The independent samples (student’s) t-test and Chi-square test were used to investigate the inter-variable relationships for quantitative and qualitative variables, respectively. The paired t-test (or the Wilcoxon Signed Ranks Test, if necessary) was used to investigate the intragroup changes in study variables. The significance level was considered as 0.05 for all analyses.
Results
A total of 112 patients with CTS were referred to our clinics in the study duration, of which 35 did not fulfill the inclusion criteria, and 17 were not willing to participate. Seventy patients remained and were divided into 2 groups, with 35 patients in each group. During the study duration, 6 and 4 participants of the DA and PA groups were lost to follow-up, respectively. Finally, a total of 60 participants completed the intervention and follow-up. Therefore, their data entered analysis (Figure 1).
A total of 60 participants with mild to moderate CTS were included in the present RCT. 29 participants, including 5 males and 24 females with a mean age of 56.59 ± 8.81 years, were assigned to the DA group, while 31 participants, including 8 males and 24 females with a mean age of 59.74 ± 10.29 years, were assigned to the PA group. The demographic characteristics of the participants and baseline values of clinical findings are presented in Table 1. There was no significant inter-group difference in demographic characteristics and baseline clinical findings. Therefore, the 2 groups were relatively matched and comparable (Table 1). The only exceptions were SNAP amplitude and NCV-s, which had higher preintervention values in the PA group (P = .004 and .015, respectively). Interestingly, these 2 parameters were considerably improved in the DA group postintervention (Table 2).
Table 1.
Comparison of Baseline Participant’s Characteristics Between Distal and Proximal Approach Groups.
| Characteristics | Distal (n = 29) | Proximal (n = 31) | P-value |
|---|---|---|---|
| Age (year) a | 56.6 ± 8.8 | 59.7 ± 10.3 | .75 b |
| BMI a | 31.4 ± 5.5 | 29.6 ± 3.2 | .27 b |
| Duration (days) a | 36.7 ± 33 | 31.5 ± 34.3 | .49 b |
| Sex c | |||
| Male | 5 (17.2%) | 8 (25.8%) | .42 d |
| Female | 24 (82.3%) | 23 (74.2%) | |
| MPH c | |||
| None | 13 (44.8%) | 17 (54.8%) | .44 d |
| Diabetic | 16 (55.2%) | 14 (45.2%) | |
| Hand side c | |||
| Left | 14 (48.2%) | 12 (38.7%) | .46 d |
| Right | 15 (51.8%) | 19 (61.3%) | |
| DSL (ms) | 4.9 ± 1.2 | 4.4 ± 0.7 | .06 b |
| Amp-s (µv) | 15.7 ± 11 | 24.2 ± 10.8 | .001 b |
| NCV-s (m/s) | 37.6 ± 7.2 | 42.1 ± 6.9 | .02 e |
| DML (ms) | 5.4 ± 1 | 5 ± 0.9 | .08 b |
| Amp-m (mv) | 8.7 ± 2.8 | 9 ± 3 | .7 b |
| NCV-m (m/s) | 50.4 ± 5 | 52.3 ± 4 | .13 b |
| Severity | 2 ± 0.2 | 1.8 ± 0.4 | .06 b |
| Boston 1 | 15.8 ± 5.4 | 17.1 ± 7.2 | .66 b |
| Boston 2 | 27.1 ± 7.7 | 28.3 ± 8.4 | .66 b |
| VAS | 6.5 ± 1.7 | 6.8 ± 1.8 | .47 b |
| Power (kg) | 40.9 ± 15.9 | 44.2 ± 20.4 | .6 b |
Note. BMI = body mass index; DSL = distal sensory latency; NCV-s = sensory conduction velocity; DML = distal motor latency; VAS = visual analog scale.
indicated as mean ± standard deviation.
Mann-Whitney U test.
indicated as N (%).
Chi-Square test.
t test.
Table 2.
Comparison of Studied Variables Within Each Group as a Before-After of Injection.
| Variables a | Time | Difference | P value | |||
|---|---|---|---|---|---|---|
| Before | After | Within-group effect b | Between-groups effect | |||
| DSL (ms) |
Distal | 4.9 ± 1.2 | 4.4 ± 0.7 | 0.4 ± 0.8 | .001 | .35 c |
| Proximal | 4.4 ± 0.7 | 4.2 ± 0.7 | 0.2 ± 0.3 | .002 | ||
| Amp-s (µv) |
Distal | 15.7 ± 11 | 18.3 ± 9.6 | −2.7 ± 4.3 | .001 | .08 c |
| Proximal | 24.2 ± 10.8 | 23.5 ± 10.2 | 0.7 ± 8 | .67 | ||
| NCV-s (m/s) |
Distal | 37.6 ± 7.2 | 40.6 ± 5.6 | −3 ± 4.2 | .001 | .27 d |
| Proximal | 42.1 ± 6.9 | 44.1 ± 6.4 | −2 ± 3.4 | .005 | ||
| DML (ms) |
Distal | 5.4 ± 1 | 4.6 ± 0.8 | 0.7 ± 0.7 | < .001 | .12 c |
| Proximal | 5 ± 0.9 | 4.5 ± 0.8 | 0.5 ± 0.6 | < .001 | ||
| Amp-m (mv) |
Distal | 8.7 ± 2.8 | 8.1 ± 2.3 | 0.6 ± 2.3 | .21 | .44 d |
| Proximal | 9 ± 3 | 9 ± 3.7 | 0 ± 3 | .72 | ||
| NCV-m (m/s) |
Distal | 50.4 ± 5 | 50.4 ± 4.6 | 0 ± 3.3 | .94 | .05 c |
| Proximal | 52.3 ± 4 | 50.2 ± 5 | 2.1 ± 5.2 | .008 | ||
| Severity | Distal | 2 ± 0.2 | 1.8 ± 0.6 | 0.2 ± 0.6 | .06 | .34 c |
| Proximal | 1.8 ± 0.4 | 1.5 ± 0.9 | 0.4 ± 0.7 | .01 | ||
| Boston 1 | Distal | 15.8 ± 5.4 | 13.6 ± 6.2 | 2.2 ± 4.7 | .02 | .52 d |
| Proximal | 17.1 ± 7.2 | 13.9 ± 6.1 | 3.2 ± 6.9 | .01 | ||
| Boston 2 | Distal | 27.1 ± 7.7 | 19.1 ± 7.2 | 8 ± 7.7 | < .001 | .79 d |
| Proximal | 28.3 ± 8.4 | 20.9 ± 10.1 | 7.4 ± 10 | < .001 | ||
| VAS | Distal | 6.5 ± 1.7 | 3.7 ± 1.9 | 2.8 ± 2.1 | < .001 | .98 c |
| Proximal | 6.8 ± 1.8 | 4.1 ± 2.5 | 2.7 ± 2.7 | < .001 | ||
| Power (kg) |
Distal | 40.9 ± 15.9 | 46.6 ± 16.1 | −5.7 ± 12.3 | .02 | .47 c |
| Proximal | 44.2 ± 20.4 | 48.4 ± 21.3 | −4.2 ± 6.7 | .002 | ||
Note. DSL = distal sensory latency; NCV-s = sensory conduction velocity; NCV-m = motor conduction velocity; VAS = visual analog scale.
Indicated as mean ± standard deviation.
Wilcoxon Signed Ranks Test.
Mann-Whitney U test.
t test.
The pre- and postintervention values of the study variables for each group are presented in Table 2. The following electrodiagnostic parameters obtained from the NCS were significantly improved in both DA and PA groups: DSL (DA group: MD = -0.43, P = .005; PA group: MD = -0.19, P = .005), NCV-s (DA group: MD = 3.03, P = .001; PA group: MD = 1.95, P = .003), and DML (DA group: MD = -0.74, P < .001; PA group: MD = -0.53, P < .001). However, SNAP amplitude only improved significantly in the DA group (MD = 2.70, P = .002), while it was even insignificantly decreased in the PA group (P = .632). Unexpectedly, the amplitude and NCV of motor fibers were decreased in both groups; however, this decrease was only significant in the PA group (MD = 2.12, P = .031).
Both groups had significantly improved scores in both subscale of the BCTQ, including the BQ-SS (DA group: MD = -2.21, P = .018; PA group: MD = -3.19, P = .015) and BQ-FS (DA group: MD = -7.97, P < .001; PA group: MD = -7.36, P < .001), while the groups had significantly reduced scores in the VAS (DA group: MD = -2.76, P < .001; PA group: MD = -2.71, P < .001). Moreover, there was a significant increase in the hand group strength in both groups (DA group: MD = 5.72, P = .018; PA group: MD = 4.16, P = .002). Also, only the patients in the PA group had a significant improvement in the CTS severity score (P = .009). However, the procedure duration in the DA group was significantly shorter than the PA group (9.80 ± 1.12 s vs. 27.61 ± 1.77 s; P < .001).
In summary, the BQ-SS score of the PA group was slightly more increased than that of the DA group, which was not significant (P-value = .844). However, other variables, including DSL, BQ-FS, VAS, hand grip strength, and procedure duration, showed higher improvements and larger MDs in the DA group, which were not significant as well. It is worth mentioning that although the preintervention values of NCV-s were better in the PA group, this variable showed a larger MD in the DA group (P-value = 0.030). Also, CTS severity changes were only significant in the PA group.
Discussion
According to our findings, corticosteroid injection using the DA could effectively treat the symptoms of mild or moderate CTS and showed even more favorable results compared to the conventional PA. Almost all the study variables, including the objective (DML, DSL, Amp-s, NCV-s, and hand grip strength) and subjective measures (VA and BQ-FS), were significantly more improved in the DA group than in the PA group. These findings are compatible with the previous studies reporting the effectiveness of LCI using the DA.21-26
In terms of electrodiagnostic parameters, both groups had improvements in the DML and all sensory NCS findings, except for SNAP amplitude, which was only improved in the DA group. These observed improvements can be explained by decreased pressure on the median nerve passing through the carpal tunnel. 23 However, NCV and motor fiber amplitude were insignificantly decreased in both groups, which was unexpected and could be accidental. The only exception was the NCV-m changes in the PA group, which had a borderline significance (Table 2).
Contrary to other investigations on the distal and PAs with different needle gauges (needles with smaller gauges for the DA and those with larger gauges for the PA),21,23 in the present RCT, injections in both groups were performed using similar 29-gauge, 0.5-inch-long needles. Although the needle used for the PA group in the present study was smaller than those used by the previous studies, the outcomes were comparable. Furthermore, the present study used an LCI technique without adding local anesthetics, such as lidocaine, whose administration has been associated with immediate pain relief in CTS patients and is usually used as a diagnostic tool. Given our goal of comparing the 2 techniques for LCI, there was no need for further lidocaine addition.
One of the main strengths of the present research was the extensive range of outcome measures evaluated, including subjective and objective measures. Just a study by EL-Badawy used similar measures. 23 Moreover, some studies only used subjective outcomes, such as the patients’ statements of their recovery 21 or BCTQ.25,26 Özdemir et al 24 and Nair et al 22 assessed both objective and subjective outcomes; however, none evaluated the VAS and BCTQ simultaneously. Also, we assessed the hand grip strength and found significant improvements in both groups, with no significant inter-group difference.
The DA was first described by Habib et al 21 in 2005. The technique was associated with clinical and electrophysiological results comparable to the proximal technique. Moreover, it had a significantly shorter procedure duration, which can potentially lead to higher patient satisfaction. Moreover, other studies reported similar results on the time required for the intervention.21,23 It should be noted that identifying the PL tendon is time-consuming, especially in overweight patients. This will probably play a significant role in the prolongation of the PA.
Although the PA is the most common method of LCI, there is still an ongoing debate on the safest injection site. 23 Several techniques with various needle insertion sites have been described in the literature.34-37 It has been found that the conventional proximal technique may be associated with a higher risk of median nerve injury, 34 ulnar nerve injury, 35 transverse carpal ligament rupture, 33 and traumatic injury of the ulnar artery. 38
In the present study, we aimed to confirm the previous research findings to provide more robust evidence supporting the performance of LCI using the new DA. As well demonstrated before, the novel distal technique caused the same level of functional improvement and symptom alleviation as the conventional method. The present study was conducted to introduce the distal technique as a safe, feasible, effective, and less time-consuming method, compared with the proximal technique, for managing mild to moderate CTS. Moreover, based on the secondary results, the conventional proximal technique can be performed using a smaller-gauge needle, leading to less pain experienced during the intervention.
The present RCT also had some limitations, including the lack of ultrasound evaluation of the exact changes in the cross-sectional view of the median nerve, which could have been considered an anatomic outcome. Moreover, the pain experienced during the procedure and overall patient satisfaction could have been considered as primary outcomes.
Conclusion
Local corticosteroid injection using the DA should be considered as a feasible, safe, and effective method for patients suffering from mild to moderate CTS. It was associated with clinical and electrophysiological results comparable to those of the conventional PA without any significant difference.
Acknowledgments
The authors thankfully acknowledge all staff of the Physical Medicine and Rehabilitation ward who participated in this study, especially Niloofar Shirzad, Hamidreza Tajik, and Parinaz Dalili.
Footnotes
Ethical Approval: This study was approved by the ethics committee of the institutional review board of the Tehran University of Medical Sciences (TUMS).
Statement of Human and Animal Rights: All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Statement of Informed Consent: Informed consent was obtained from all patients for being included in the study.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by School of Medicine (grant number 97-02-30-38913). This study was funded by School of Medicine (grant number 97-02-30-38913).
ORCID iDs: Hamid R. Fateh  https://orcid.org/0000-0003-2469-437X
https://orcid.org/0000-0003-2469-437X
Shahram Rahimi-Dehgolan  https://orcid.org/0000-0001-9067-3943
https://orcid.org/0000-0001-9067-3943
References
- 1. O’Connor D, Marshall S, Massy-Westropp N. Non-surgical treatment (other than steroid injection) for carpal tunnel syndrome. Cochrane Database Syst Rev. 2003;2003(1):CD003219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang JC, Liao KK, Lin KP, et al. Efficacy of combined ultrasound-guided steroid injection and splinting in patients with carpal tunnel syndrome: a randomized controlled trial. Arch Phys Med Rehabil. 2017;98(5):947-956. [DOI] [PubMed] [Google Scholar]
- 3. Chen PC, Wang LY, Pong YP, et al. Effectiveness of ultrasound-guided vs direct approach corticosteroid injections for carpal tunnel syndrome: a doubleblind randomized controlled trial. J Rehabil Med. 2018;50(2):200-208. [DOI] [PubMed] [Google Scholar]
- 4. Padua L, Coraci D, Erra C, et al. Carpal tunnel syndrome: clinical features, diagnosis, and management. Lancet Neurol. 2016;15(12):1273-1284. [DOI] [PubMed] [Google Scholar]
- 5. Ghasemi-Rad M, Nosair E, Vegh A, et al. A handy review of carpal tunnel syndrome: from anatomy to diagnosis and treatment. World J Radiol. 2014;6(6):284-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Klauser AS, Buzzegoli T, Taljanovic MS, et al. Nerve entrapment syndromes at the wrist and elbow by sonography. Semin Musculoskelet Radiol. 2018;22(3):344-353. [DOI] [PubMed] [Google Scholar]
- 7. Kim JC, Jung SH, Lee SU, et al. Effect of extracorporeal shockwave therapy on carpal tunnel syndrome: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2019;98(33):e16870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ghiasian M, Khazaei M, Daneshyar S, et al. Epidemiological survey of patients with a carpal tunnel syndrome referred to Sina Hospital in Hamedan during 2014-2016. Feyz J Kashan Univ Med Sci. 2017;21(5):498-505. [Google Scholar]
- 9. Werner RA, Andary M. Carpal tunnel syndrome: pathophysiology and clinical neurophysiology. Clin Neurophysiol. 2002;113(9):1373-1381. [DOI] [PubMed] [Google Scholar]
- 10. Gelberman RH, Hergenroeder PT, Hargens AR, et al. The carpal tunnel syndrome. A study of carpal canal pressures. J Bone Joint Surg Am. 1981;63(3):380-383. [PubMed] [Google Scholar]
- 11. Borire AA, Hughes AR, Lueck CJ, et al. Sonographic differences in carpal tunnel syndrome with normal and abnormal nerve conduction studies. J Clin Neurosci. 2016;34:77-80. [DOI] [PubMed] [Google Scholar]
- 12. Eftekharsadat B, Roomizadeh P, Torabi S, et al. Effectiveness of Lavendula stoechas essential oil in treatment of mild to moderate carpal tunnel syndrome: a randomized controlled trial. J Hand Ther. 2018;31:437-442. [DOI] [PubMed] [Google Scholar]
- 13. Evers S, Bryan AJ, Sanders TL, et al. Effectiveness of ultrasound-guided compared to blind steroid injections in the treatment of carpal tunnel syndrome. Arthritis Care Res (Hoboken). 2017;69(7):1060-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huisstede BM, Fridén J, Coert JH, et al. Carpal tunnel syndrome: hand surgeons, hand therapists, and physical medicine and rehabilitation physicians agree on a multidisciplinary treatment guideline—results from the European HANDGUIDE Study. Arch Phys Med Rehabil. 2014;95(12):2253-2263. [DOI] [PubMed] [Google Scholar]
- 15. Chen PC, Chuang CH, Tu YK, et al. A Bayesian network meta-analysis: comparing the clinical effectiveness of local corticosteroid injections using different treatment strategies for carpal tunnel syndrome. BMC Musculoskelet Disord. 2015;16(1):363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kasten SJ, Louis DS. Carpal tunnel syndrome: a case of median nerve injection injury and a safe and effective method for injecting the carpal tunnel. J Fam Pract. 1996;43(1):79-82. [PubMed] [Google Scholar]
- 17. Propeck T, Quinn TJ, Jacobson JA, et al. Sonography and MR imaging of bifid median nerve with anatomic and histologic correlation. AJR Am J Roentgenol. 2000;175(6):1721-1725. [DOI] [PubMed] [Google Scholar]
- 18. Kim DH, Jang JE, Park BK. Anatomical basis of ulnar approach in carpal tunnel injection. Pain Physician. 2013;16(3):E191-E198. [PubMed] [Google Scholar]
- 19. Smith J, Wisniewski SJ, Finnoff JT, et al. Sonographically guided carpal tunnel injections: the ulnar approach. J Ultrasound Med. 2008;27(10):1485-1490. [DOI] [PubMed] [Google Scholar]
- 20. Babaei-Ghazani A, Roomizadeh P, Forogh B, et al. Ultrasound-guided versus landmark-guided local corticosteroid injection for carpal tunnel syndrome: a systematic review and meta-analysis of randomized controlled trials. Arch Phys Med Rehabil. 2018;99(4):766-775. doi: 10.1016/j.apmr.2017.08.484. [DOI] [PubMed] [Google Scholar]
- 21. Habib GS, Badarny S, Rawashdeh H. A novel approach of local corticosteroid injection for the treatment of carpal tunnel syndrome. Clin Rheumatol. 2006;25(3):338-340. [DOI] [PubMed] [Google Scholar]
- 22. Nair PP, Wadwekar V, Chakkalakkoombil SV, et al. Comparison of proximal and distal corticosteroid injections for carpal tunnel syndrome. Muscle Nerve. 2020;62:89-94. [DOI] [PubMed] [Google Scholar]
- 23. EL-Badawy MA. Electrophysiological and clinical comparison of local steroid injection by means of proximal versus distal approach in patients with mild and moderate carpal tunnel syndrome. Egypt Rheumatol Rehabil. 2015;42(3):120-127. [Google Scholar]
- 24. Özdemir G, Demir R, Özel L, et al. The effect of steroid injection by Novel method in carpal tunnel syndrome on pain severity and electrophysiological findings. Dicle Med J. 2014;41(2):277-281. [Google Scholar]
- 25. Kamanli A, Bezgincan M, Kaya A. Comparison of local steroid injection into carpal tunnel via proximal and distal approach in patients with carpal tunnel syndrome. Bratisl Lek Listy. 2011;112(6):337-341. [PubMed] [Google Scholar]
- 26. Badarny S, Rawashdeh H, Meer J, et al. Repeated electrophysiologic studies in patients with carpal tunnel syndrome following local corticosteroid injection using a novel approach. Isr Med Assoc J. 2011;13:25-28. [PubMed] [Google Scholar]
- 27. Werner RA, Andary M. Electrodiagnostic evaluation of carpal tunnel syndrome. Muscle Nerve. 2011;44(4):597-607. [DOI] [PubMed] [Google Scholar]
- 28. de Carvalho Leite JC, Jerosch-Herold C, Song F. A systematic review of the psychometric properties of the Boston Carpal Tunnel Questionnaire. BMC Musculoskelet Disord. 2006;7(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hassankhani GG, Moradi A, Birjandinejad A, et al. Translation and validation of the Persian version the Boston carpal tunnel syndrome questionnaire. Arch Bone Jt Surg. 2018;6(1):71-77. [PMC free article] [PubMed] [Google Scholar]
- 30. Rezazadeh A, Bakhtiary AH, Samaei A, et al. Validity and reliability of the Persian Boston questionnaire in Iranian patients with carpal tunnel syndrome. Koomesh. 2014;15(2):138-145. [Google Scholar]
- 31. Dumitru D, Amato AA, Zwarts M, eds. Electrodiagnostic Medicine. 2nd ed. Philadelphia, PA: Hanley & Belfus; 2001. [Google Scholar]
- 32. Fernandes CH, Meirelles LM, Raduan Neto J, et al. Carpal tunnel syndrome with thenar atrophy: evaluation of the pinch and grip strength in patients undergoing surgical treatment. Hand. 2013;8(1):60-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wong SM, Hui AC, Tang A, et al. Local vs systemic corticosteroids in the treatment of carpal tunnel syndrome. Neurology. 2001;56(11):1565-1567. [DOI] [PubMed] [Google Scholar]
- 34. Racasan O, Dubert TH. The safest location for steroid injection in the treatment of carpal tunnel syndrome. J Hand Surg. 2005;30(4):412-414. [DOI] [PubMed] [Google Scholar]
- 35. Green DP, MacKay BJ, Seiler SJ, et al. Accuracy of carpal tunnel injection: a prospective evaluation of 756 patients. Hand. 2020;15:54-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ozturk K, Esenyel CZ, Sonmez M, et al. Comparison of carpal tunnel injection techniques: a cadaver study. Scand J Plast Reconstr Surg Hand Surg. 2008;42(6):300-304. [DOI] [PubMed] [Google Scholar]
- 37. Dubert T, Racasan O. A reliable technique for avoiding the median nerve during carpal tunnel injections. Joint Bone Spine. 2006;73(1):77-79. [DOI] [PubMed] [Google Scholar]
- 38. Hussain SS, Taylor C, Van Rooyen R. Ulnar artery ischaemia following corticosteroid injection for carpal tunnel syndrome. N Z Med J. 2011;124(1335):80-83. [PubMed] [Google Scholar]