Abstract
Background:
Although electrical stimulation (ES) can improve nerve regeneration, the impact of nerve block, such as lidocaine (Lido), on the therapeutic benefits of ES remains unclear. We used a rat tibial nerve transection-and-repair model to explore how either preoperative (PreOp) or postoperative (PostOp) nerve block affects ES-related improvement in regeneration.
Methods:
Lewis rats were used in 1 of 2 studies. The first evaluated the effects of extraneural Lido on both healthy and injured nerves. In the second study, rats were randomized to 5 experimental groups: No ES (negative control), PreOp Lido, ES + PreOp Lido, PostOp + ES, and ES (positive control). All groups underwent tibial nerve transection and repair. In both studies, nerves were harvested for histological analysis of regeneration distal to the injury site.
Results:
Application of extraneural Lido did not damage healthy or injured nerve based on qualitative histological observations. In the context of nerve transection and repair, the ES group exhibited improved axon regeneration at 21 days measured by the total number of myelinated fibers compared with No ES. Fiber density and percentage of neural tissue in the ES group were greater than those in both No ES and PreOp Lido + ES groups. ES + PostOp Lido was not different from No ES or ES group.
Conclusions:
Extraneural application of Lido did not damage nerves. Electrical stimulation augmented nerve regeneration, but Lido diminished the ES-related improvement in nerve regeneration. Clinical studies on the effects of ES to nerve regeneration may need to consider nerve block as a variable affecting ES outcome.
Keywords: electrical stimulation, peripheral nerve, rat, nerve regeneration, nerve, diagnosis, lidocaine
Introduction
There are an estimated 410 000 extremity trauma injuries presenting to emergency departments in the United States each year. 1 Of these encounters, 2.6% of upper extremity trauma patients and 1.2% of lower extremity trauma patients are diagnosed with a nerve injury within 2 years, 1 more than previously estimated. 2 Furthermore, an estimated 17% of nerve injuries are iatrogenic in nature, and compression neuropathy is common. 3 Despite their regenerative ability, peripheral nerve injuries in humans often result in inadequate functional recovery and debilitating morbidity. Therefore, new therapeutics are needed to improve functional outcomes after nerve repair.
Electrical stimulation (ES) is an emerging therapeutic that, when applied intraoperatively to repaired nerve, shows promise in overcoming biological obstacles to nerve regeneration. These obstacles include anatomical distance between the repair site and target, slow axonal regeneration (3 mm/day in animal models 4 and 1 mm/day in humans 5 ), staggered axonal growth resulting in ineffective regeneration, 6 and transient expression of growth factors to accompany this slow growth following nerve injury. 7
The current proposed mechanism of ES is as follows: When a nerve injury is repaired and ES is applied proximally, voltage-gated ion channels are activated, resulting in a retrograde depolarization to the nerve soma. In the nerve soma, this results in upregulation of neurotrophic factors, including brain-derived neurotrophic factor and its receptor tropomyosin receptor kinase B, Tα1-tubulin, and GAP-43.8,9 Tetrodotoxin (TTX), a potent toxin that inhibits nerve conduction by binding to voltage-gated sodium channels on the axon membrane, has been shown to diminish the regenerative effects of ES by preventing its ability to sufficiently depolarize the axonal membrane. 6 Based on this study, a proposed mechanism for therapeutic ES to improve nerve regeneration relies entirely on “backward depolarization” signaling to the cell body.
Importantly, the effects of clinically relevant nerve block, such as lidocaine, remain understudied in the setting of ES. Lidocaine binds to voltage-gated sodium channels, inhibiting the production of action potentials and, therefore, the sensation of pain. For ES to be used successfully in a clinical setting, interactions with common surgical therapeutics such as nerve blocks must be closely examined. We hypothesized that the addition of a preoperative lidocaine block would diminish the effects of ES to promote nerve regeneration. Conversely, we hypothesized that postoperative nerve block would enable ES to maintain efficacy in improving nerve regeneration.
Materials and Methods
Animals
Inbred adult male Lewis rats (Charles River Laboratories; Wilmington, Massachusetts) were used. All rats were held in a central animal care facility and were given rat chow (PicoLab rodent diet 20; Purina Animal Nutrition LLC, St. Louis, Missouri) and water ad libitum. All institutional and national guidelines for the care and use of laboratory animals were followed. Surgical procedures and perioperative care measures were conducted in compliance with the Association for Assessment and Accreditation of Laboratory Animal Care–accredited Washington University Institutional Animal Care and Use Committee.
Experimental Design
The study was carried out in 2 arms. In arm 1, the effects of lidocaine alone were studied on an uninjured nerve as well as on injured nerve left unrepaired. Previous work has highlighted the neurodegenerative effects of intraneural injection of local anesthetics. 10 To observe the baseline effects of local lidocaine on the nerve, 9 rats were randomized to 3 groups: lidocaine block with no transection, tibial nerve transection without repair, and tibial nerve transection without repair followed by lidocaine block. For groups receiving a block, blocks were achieved by applying lidocaine (2%) to the exposed nerve (including after transection for the applicable group). Histologic analysis of harvested nerves was qualitatively evaluated 7 days after each procedure for signs of axon loss or pathology.
Arm 2 explored: (1) how pre-operative nerve block affects nerve regeneration using ES; and (2) whether post-ES nerve block disrupts ES-related improvement in nerve regeneration. Sixty rats were randomized to 5 experimental groups: ES + Pre-Op Block, ES + Post-Op Block, No ES + Pre-Op Block, ES (positive control), and No ES (negative control). Each group underwent a tibial nerve transection and immediate direct repair, as described in the “Surgical Procedures” section. For groups receiving a block, blocks were achieved by applying lidocaine (2%) to the exposed nerve. Preoperative nerve block was applied prior to nerve transection and repair. Postoperative nerve block was applied following nerve repair and ES. In the groups receiving ES, an ES device (Checkpoint Stimulator/Locator; Checkpoint Surgical, Inc, Cleveland, Ohio) was used to deliver intraoperative ES. Nerves were harvested, and histologic analysis of axon regeneration and myelination was performed 3 weeks after tibial nerve transection and repair in each group.
Surgical Procedures
Surgeries were performed using aseptic technique and with the aid of an operating microscope. Anesthesia was delivered using a cocktail of ketamine (75 mg/kg; Zoetis Inc, Kalamazoo, Michigan) and dexmedetomidine (0.5 mg/kg; Zoetis Inc). During surgery, animals were placed on a warming pad for body temperature maintenance and given 1 mL of normal saline subcutaneously for hydration. The tibial nerve was exposed using a gluteal-muscle-splitting approach. In applicable groups based on the study arm, the tibial nerve was transected 5 mm distal to the sciatic trifurcation and either unrepaired or repaired immediately with 9-0 nylon suture (Sharpoint, Wyomissing, Pennsylvania). For groups receiving a block, blocks were achieved by applying 3 mL of lidocaine (2%) to the exposed nerve for 5 minutes. Preoperative nerve block was applied prior to nerve transection and repair. Postoperative nerve block was applied following nerve repair and ES. After the block, the operative area was copiously irrigated with bacteriostatic saline. For groups receiving ES, a stainless steel 304 wire electrode (Component Supply Company, Sparta, Tennessee) was fashioned into a half-circle and hooked around the tibial nerve 2 mm proximal to the site of repair, providing gentle tension to secure it to the nerve. A return current electrode was inserted securely in musculocutaneous fascia in the surgical field. Electrical stimulation was delivered for 10 minutes at 0.5 mA of current using a monopolar stimulation paradigm. The device provides feedback using a flashing light-emitting diode to indicate whether the circuit is complete and request stimulation intensity is being provided. Contractions of the leg, even after tibial nerve transection and repair, was also an indicator that ES was successfully applied to the nerve, while preoperative block completely abolished this effect. Stimulation in blocked animals was provided to the region of the “blocked” nerve. After completion of ES, electrodes were gently removed from the nerve and musculocutaneous fascia. Wounds were closed in a layered fashion with 6-0 vicryl suture for muscle and 4-0 nylon suture for skin (Ethicon, Somerville, New Jersey).
Animals were recovered with a subcutaneous injection of atipamezole HCl (Antisedan 1 mg/kg; Orion Corporation, Espoo, Finland) and placed on a warming pad postoperatively. Postoperative pain was managed intraoperatively with a single dose of buprenorphine (Buprenorphine SR 1 mg/kg; ZooPharm, Windsor, Colorado). Animals were returned to the central housing facility and closely monitored for infection, distress, and other morbidities.
Nerve Histology and Histomorphometry
In arms 1 and 2, animals were reanesthetized and prepared for exposure of the right tibial nerve at, respectively, 7 and 21 days postoperatively. Postoperative day 21 was chosen as an endpoint for histologic analysis of nerve based on previous work, indicating that 21 days is a sensitive endpoint to detect the increase in early axonal regeneration provoked by ES.11,12 En bloc specimens of the tibial nerve 5 mm distal to the transection or repair site underwent histomorphometric analysis as previously described.13,14 Nerves were harvested and stored in 3% glutaraldehyde (Polysciences, Warrington, Pennsylvania). Nerves were postfixed in 1% osmium tetroxide, serially dehydrated in ethanol and toluene, embedded in epoxy (Polysciences), and sectioned on an ultramicrotome into 1-μm cross sections. Slides were counterstained with 1% toluidine blue dye. The slides were then analyzed at ×1000 on a Leitz Laborlux S microscope. Custom histomorphometry software (Clemex Vision Professional; Clemex Technologies, Longueuil, Québec, Canada) was used to quantify nerve fiber counts, percentage of neural tissue, fiber sizes, and myelin thickness. Animals were humanely killed following tissue harvest using >200 mg/kg sodium pentobarbital (Vortech Pharmaceutical Ltd., Dearborn, Michigan). All analyses were performed by an observer blinded to the experimental groups.
Statistical Analysis
All statistical analyses were performed in Prism 9 (GraphPad Software, La Jolla, California). Data are presented as mean ± SD. Nerve histomorphometry data were analyzed using 1-way analysis of variance, followed by the Tukey multiple comparison test for post hoc analysis between groups for each variable. The study was adequately powered (β = 0.80), and significance (α) was set at .05.
Results
Extraneural Lidocaine Does Not Damage Nerve
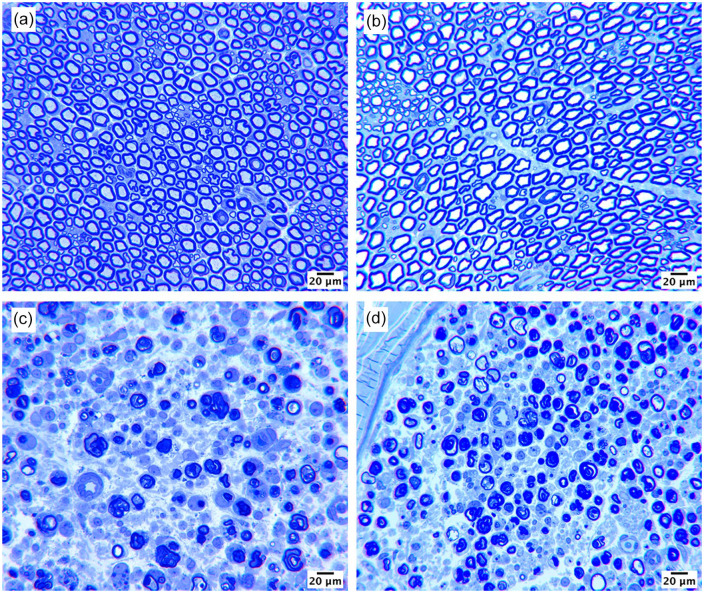
Arm 1 served to investigate the effect of local lidocaine on injured and uninjured nerves. Representative sections of nerves from each group harvested at 7 days are presented in Figure 1. The addition of extraneural lidocaine did not result in additional damage to either group. Healthy myelinated fibers with mature architecture were found in the lidocaine block with no transection group (Figure 1b). Injured nerves, regardless of the presence of lidocaine, specifically showed healthy Wallerian degeneration (Figure 1c and 1d). No major differences in axon loss, cellular infiltrate, or nerve environment were observed between groups receiving tibial transection alone and tibial transection followed by lidocaine block.
Figure 1.
Representative histologic sections of effects of lidocaine on tibial nerve. Nerves were harvested 7 days after lidocaine extraneural administration.
Note. (a) Uninjured nerve, (b) uninjured nerve after lidocaine, (c) transected distal nerve, and (d) transected distal nerve after lidocaine. Scale bars represent 20 μm.
Lidocaine Block May Diminish Some Benefits of ES
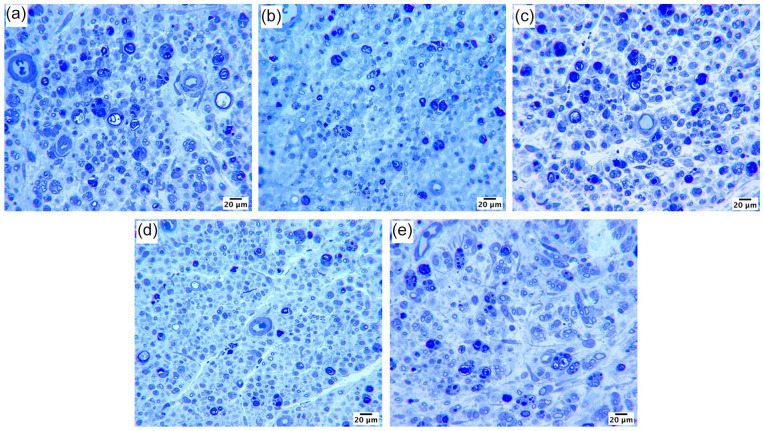
All treatment groups in arm 2 exhibited histological evidence of axon regeneration at 21 days after tibial nerve transection and repair, indicated by the presence of small and large myelinated fibers (Figure 2). The ES group (positive control) exhibited improved axon regeneration measured by the total number of fibers compared with the No ES group (negative control) (Figure 3a; P < .05). Fiber density in the ES group was greater than that in both No ES and PreOp Lido + ES groups (Figure 3b; P < .05 vs No ES, P < 0.05 vs PreOp Lido + ES). The percentage of neural tissue in the ES group was also greater than that in No ES and PreOp Lido + ES groups (Figure 3c; P < .01 vs No ES; P < .05 vs PreOp Lido + ES). ES + PostOp Lido was not different from No ES or ES group. There were no significant differences observed in fiber width, myelin width, or percentage of debris among all groups (Table 1).
Figure 2.
Representative histological sections of distal tibial nerve at 21 days after repair: (a) electrical stimulation (ES), (b) No ES, (c) PreOp Lido + ES, (d) PreOp Lido, and (e) ES + PostOp Lido. Scale bars represent 20 μm. PreOp = preoperative; PostOp = postoperative; Lido = lidocaine.
Figure 3.
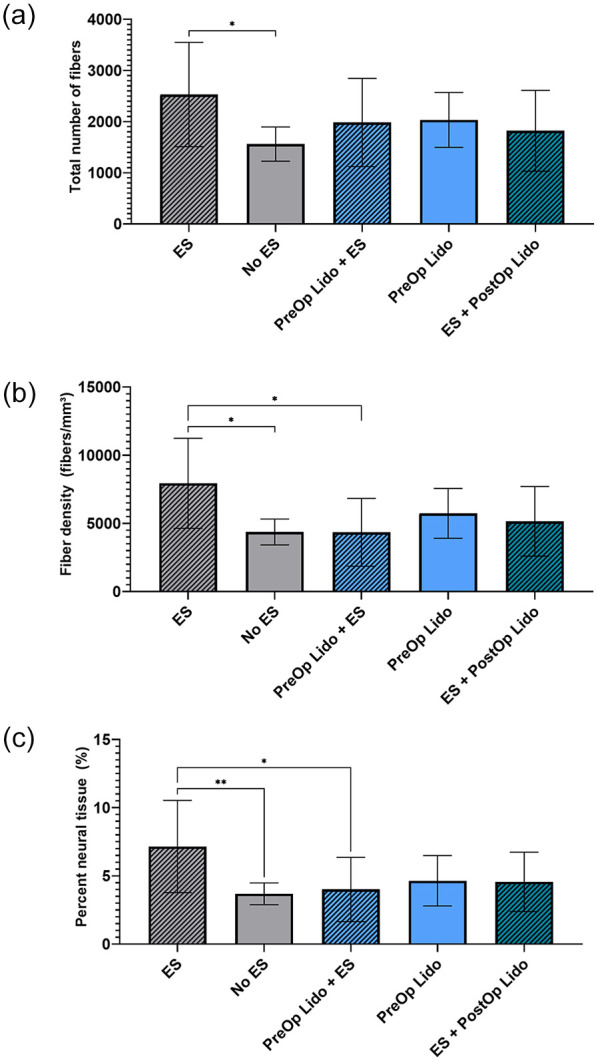
Histomorphometric parameters of nerve regeneration 21 days after tibial nerve transection and repair.
Note. Quantitative evaluation of (a) myelinated axon counts, (b) myelinated fiber density, and (c) percentage of neural tissue. All data are expressed as mean ± SD (n = 12/group). **P < .01, *P < .05. ES = electrical stimulation; PreOp = preoperative; PostOp = postoperative; Lido = lidocaine.
Table 1.
Histomorphometric Data 21 Days After Tibial Nerve Transection and Immediate Direct Repair.
| Outcome metric | ES | PreOp Lido + ES | PreOp Lido | ES + PostOp Lido | No ES |
|---|---|---|---|---|---|
| Total number of fibers | 2530 ± 1020 a | 1983 ± 862 | 2034 ± 537 | 1821 ± 826 | 1561 ± 334 |
| Nerve density (fibers/mm³) | 7941 ± 3303a,b | 4346 ± 2487 | 5734 ± 1828 | 5151 ± 2553 | 4372 ± 952 |
| Percent neural tissue | 7.15 ± 3.38b,c | 4.00 ± 2.36 | 4.64 ± 1.85 | 4.57 ± 2.18 | 3.69 ± 0.80 |
| Fiber width (μm) | 2.85 ± 0.20 | 2.91 ± 0.27 | 2.71 ± 0.17 | 2.87 ± 0.21 | 2.76 ± 0.18 |
| Percent debris | 10.22 ± 3.12 | 10.92 ± 1.80 | 9.04 ± 4.46 | 8.90 ± 4.01 | 8.84 ± 2.40 |
Note. ES = electrical stimulation; PreOp = preoperative; PostOp = postoperative; Lido = lidocaine.
P < .05 vs No ES.
P < .05 vs PreOp Lido + ES.
P < .01 vs No ES.
Discussion
To effectively translate ES to a clinical setting, its effects on axon regeneration must be evaluated in the setting of clinically relevant nerve block, such as lidocaine. This study demonstrated that ES applied intraoperatively for 10 minutes accelerated axon regeneration. However, a lidocaine nerve block appeared to diminish the efficacy of ES to accelerate axon regeneration.
Previous work has shown a detrimental effect of intrafascicular injection of nerve block, which decreased both the total number of fibers and fiber area. 10 We determined that local extraneural lidocaine in the setting of normal nerve or transected nerve does not promote damage. Furthermore, in the setting of transection and repair of the nerve, no histological differences were observed in the PreOp Lido and No ES (control) groups. These results suggest that nerve lidocaine block does not negatively impact nerve regeneration.
In the setting of transection and repair of the nerve, our results showed significant increases in total fiber number between ES and No ES groups. It has also been shown that 10 minutes of ES and 60 minutes of ES provide similar therapeutic potential measured by functional outcomes and histomorphometry in a rat model. 15 Our results corroborate the efficacy of 10 minutes of intraoperative ES to promote greater axonal regeneration in a rat tibial nerve transection-and-repair model 21 days after surgery. However, we found that there was no difference in the total fiber number distal to the injury site between groups receiving a lidocaine block at any point versus either control group (no ES or ES). But fiber density and percentage of neural tissue were significantly decreased in animals receiving a preoperative lidocaine block and ES compared with those receiving ES alone. This evidence suggests that administering lidocaine block at any point, but more prominently block applied prior to ES, may confound the application of ES as a therapy to accelerate axon regeneration after nerve repair.
Prior literature suggests that the introduction of a lidocaine block to a nerve in the setting of ES could confound the therapeutic benefit of ES. However, the mechanisms of therapeutic ES are still not entirely understood. Therapeutic ES effects have been shown to be offset by inhibitors of voltage-gated sodium channels such as TTX, 6 supporting the idea that retrograde depolarization causes changes in gene expression in the neuron soma to promote axonal outgrowth and regeneration. However, TTX has also been shown to have long-term damaging effects on nerve regeneration, including slow optic nerve regeneration and long-term impaired transport of glycolipids.16,17 Local anesthetics, including lidocaine, which also block voltage-gated ion channels, have also been shown to have a damaging effect on nerve. Several studies have shown that local anesthetics can lead to fragmentation of DNA and disruption of the mitochondrial membrane, resulting in an increase in proapoptotic enzymes such as caspases, p38 mitogen-activated protein kinase, and Jun N-terminal kinase, eventually leading to apoptosis.18-20 We show here that application of a nerve block by bathing the nerve in lidocaine for 5 minutes does not cause damage based on histological evaluation at 7 days. However, it is possible that molecular changes from lidocaine could disrupt regeneration kinetics, possibly explaining our findings that demonstrate lidocaine diminished the proregenerative effects of ES. These proposed molecular changes from lidocaine could explain why postoperative lidocaine applied after ES did not promote increased axon regeneration compared with no ES, despite the generation of action potentials from ES prior to block.
This study provides evidence that application of a lidocaine nerve block diminishes the therapeutic benefit of ES on nerve regeneration, but the exact mechanism behind the diminished therapeutic benefit remains unknown. A major limitation of our study is an investigation as to how lidocaine may affect nerve regeneration at a molecular level, which could help better explain our findings. This molecular evaluation would also provide additional insight into possible mechanisms for the therapeutic benefit of ES on nerve regeneration. It is also important to note that differences between the PreOp and PostOp Lido ES groups could be attributed to how the lidocaine was delivered in our study. Bathing the nerve in lidocaine is clinically similar to a local anesthetic block, but this general delivery may have also prevented the ES from activating the nerve in the lidocaine groups. Given the constraints of the rodent model and method of lidocaine delivery, it is possible that the area of nerve stimulated was affected by the lidocaine block. Clinically, the area of ES and location of lidocaine block would likely differ. Therefore, while a limitation, this method of lidocaine delivery as a local nerve block is clinically relevant and does provide key information for the clinical translation of therapeutic ES. Additional study is needed to further examine temporal differences in nerve block delivery relative to ES delivery, as well as whether similar effects are observed when a more targeted nerve block, located proximal to the site of ES delivery, is used.
Conclusion
In conclusion, addition of a nerve block may hinder the positive therapeutic effects of intraoperative ES. More investigation, including close monitoring of functional outcomes, is necessary to potentially elucidate the interaction between nerve block and ES. These findings are important to consider for clinical trials to evaluate the efficacy of ES. These findings also further support preclinical evidence of the efficacy of 10 minutes of intraoperative ES to promote early axon regeneration.
Footnotes
Ethical Approval: This study was approved by our institutional review board.
Statement of Human and Animal Rights: All institutional and national guidelines for the care and use of laboratory animals were followed.
Statement of Informed Consent: This study does not contain any studies with human subjects.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: M.D.W has been the recipient of sponsored research agreements from Checkpoint Surgical, Inc. and has consulted for AxoGen, Foundry Therapeutics, LLC, The Foundry, LLC, and Renerva, LLC. E.R.W. is an employee of Checkpoint Surgical, Inc. None of the other authors have any conflicts of interest to disclose.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded in part by an industry sponsored research agreement from Checkpoint Surgical, Inc. to Washington University through M.D.W.
ORCID iDs: Grace C. Keane  https://orcid.org/0000-0002-9601-0531
https://orcid.org/0000-0002-9601-0531
Evan B. Marsh  https://orcid.org/0000-0001-8031-308X
https://orcid.org/0000-0001-8031-308X
Matthew D. Wood  https://orcid.org/0000-0001-8132-6827
https://orcid.org/0000-0001-8132-6827
References
- 1. Padovano WM, Dengler J, Patterson MM, et al. Incidence of nerve injury after extremity trauma in the United States [published online ahead of print October 21, 2020]. HAND. doi: 10.1177/1558944720963895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Taylor CA, Braza D, Rice JB, et al. The incidence of peripheral nerve injury in extremity trauma. Am J Phys Med Rehabil. 2008;87(5):381-385. doi: 10.1097/PHM.0b013e31815e6370. [DOI] [PubMed] [Google Scholar]
- 3. Kretschmer T, Antoniadis G, Braun V, et al. Evaluation of iatrogenic lesions in 722 surgically treated cases of peripheral nerve trauma. J Neurosurg. 2001;94(6):905-912. doi: 10.3171/jns.2001.94.6.0905. [DOI] [PubMed] [Google Scholar]
- 4. Gutmann E, Guttmann L, Medawar PB, et al. The Rate of Regeneration of Nerve. J Exp Biol. 1942;1(7):83-83. doi: 10.1093/oxfordjournals.bmb.a070228. [DOI] [Google Scholar]
- 5. Fu SY, Gordon T. The cellular and molecular basis of peripheral nerve regeneration. Mol Neurobiol. 1997;14(1-2):67-116. doi: 10.1007/BF02740621. [DOI] [PubMed] [Google Scholar]
- 6. Al-Majed AA, Neumann CM, Brushart TM, et al. Brief electrical stimulation promotes the speed and accuracy of motor axonal regeneration. J Neurosci. 2000;20(7):2602-2608. doi: 10.1523/jneurosci.20-07-02602.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gordon T. The physiology of neural injury and regeneration: the role of neurotrophic factors. J Commun Disord. 2010;43(4):265-273. doi: 10.1016/j.jcomdis.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 8. Gordon T. Electrical stimulation to enhance axon regeneration after peripheral nerve injuries in animal models and humans. Neurotherapeutics. 2016;13(2):295-310. doi: 10.1007/s13311-015-0415-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gordon T, Amirjani N, Edwards DC, et al. Brief post-surgical electrical stimulation accelerates axon regeneration and muscle reinnervation without affecting the functional measures in carpal tunnel syndrome patients. Exp Neurol. 2010;223(1):192-202. doi: 10.1016/j.expneurol.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 10. Farber SJ, Saheb-Al-Zamani M, Zieske L, et al. Peripheral nerve injury after local anesthetic injection. Anesth Analg. 2013;117(3):731-739. doi: 10.1213/ANE.0b013e3182a00767. [DOI] [PubMed] [Google Scholar]
- 11. Jo S, Pan D, Halevi AE, et al. Comparing electrical stimulation and tacrolimus (FK506) to enhance treating nerve injuries. Muscle Nerve. 2019;60(5):629-636. doi: 10.1002/mus.26659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Keane GC, Pan D, Roh J, et al. The effects of intraoperative electrical stimulation on regeneration and recovery after nerve isograft repair in a rat model [published online ahead of print July 15, 2020]. HAND. doi: 10.1177/1558944720939200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hunter DA, Moradzadeh A, Whitlock EL, et al. Binary imaging analysis for comprehensive quantitative histomorphometry of peripheral nerve. J Neurosci Methods. 2007;166(1):116-124. doi: 10.1016/j.jneumeth.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hunter DA, Pan D, Wood MD, et al. Design-based stereology and binary image histomorphometry in nerve assessment. J Neurosci Methods. 2020;336:108635. doi: 10.1016/j.jneumeth.2020.108635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Calvey C, Zhou W, Stakleff KS, et al. Short-term electrical stimulation to promote nerve repair and functional recovery in a rat model. J Hand Surg Am. 2015;40(2):314-322. doi: 10.1016/j.jhsa.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 16. Louise Edwards D, Grafstein B. Intraocular tetrodotoxin in goldfish hinders optic nerve regeneration. Brain Res. 1983;269(1):1-14. doi: 10.1016/0006-8993(83)90957-5. [DOI] [PubMed] [Google Scholar]
- 17. Edwards DL, Grafstein B. Intraocular injection of tetrodotoxin in goldfish decreases fast axonal transport of [3H]glucosamine-labeled materials in optic axons. Brain Res. 1984;299(1):190-194. doi: 10.1016/0006-8993(84)90807-2. [DOI] [PubMed] [Google Scholar]
- 18. Lirk P, Haller I, Myers RR, et al. Mitigation of direct neurotoxic effects of lidocaine and amitriptyline by inhibition of p38 mitogen-activated protein kinase in vitro and in vivo. Anesthesiology. 2006;104(6):1266-1273. doi: 10.1097/00000542-200606000-00023. [DOI] [PubMed] [Google Scholar]
- 19. Perez-Castro R, Patel S, Garavito-Aguilar ZV, et al. Cytotoxicity of local anesthetics in human neuronal cells. Anesth Analg. 2009;108(3):997-1007. doi: 10.1213/ane.0b013e31819385e1. [DOI] [PubMed] [Google Scholar]
- 20. Lirk P, Haller I, Colvin HP, et al. In vitro, inhibition of mitogen-activated protein kinase pathways protects against bupivacaine- and ropivacaine-induced neurotoxicity. Anesth Analg. 2008;106(5):1456-1464. doi: 10.1213/ane.0b013e318168514b. [DOI] [PubMed] [Google Scholar]