ABSTRACT.
The WHO recommends handwashing with soap and water for 20–40 seconds. In settings where soap is not available, ash or sand is used for handwashing, yet their efficacy as handwashing materials is underresearched. The purpose of this study was to quantify the removal of viruses using ash and sand as handwashing agents, and compare their efficacy to commonly recommended handwashing methods. We performed a volunteer study to estimate the log reduction value (LRV) of model viruses Phi6 and MS2 on hands after six handwashing conditions: two handwashing agents (ash and water, and sand and water) with two time points (5 and 20 seconds), and two handwashing agents (soap and water, and water only) with one time point (20 seconds). Plaque assays were used to measure infectious virus reduction. Handwashing with any of the handwashing agents for 20 seconds resulted in a greater LRV than the 2-log reduction U.S. Food and Drug Administration criteria for both viruses. Soap and water resulted in a significantly greater LRV (2.7–4.8) than washing with ash and water (2.0–2.8) or sand and water (1.8–2.7) for 5 seconds for both viruses, and water only resulted in a significantly higher LRV (2.8) than all ash (2.0–2.6) and sand (1.8–2.4) conditions for MS2 only. These results suggest that using ash or sand as handwashing agents can be efficacious in reducing viruses but may be less efficacious than soap, especially when used for shorter durations. Further research should investigate the use of ash and sand as handwashing agents in real-world settings.
INTRODUCTION
Handwashing is an important intervention to disrupt disease transmission worldwide, as contaminated hands can serve as a pathway in the spread of diseases such as respiratory and gastrointestinal infections—two of the leading causes of global mortality.1,2 Hands play a crucial role in the transmission of viruses—whether directly to another person or mediated by objects and surfaces. Respiratory viruses such as influenza, respiratory syncytial virus, human parainfluenza virus, severe acute respiratory syndrome coronavirus, and rhinovirus, as well as enteric viruses such as rotavirus, adenovirus, norovirus, and hepatitis A virus, are associated with indirect disease transmission via surfaces and objects.3 It is critical to interrupt the spread of disease via hands and surfaces by taking preventative measures such as handwashing. Interventional studies have found that handwashing results in a 21–24% reduction of respiratory infections4,5 and 31% reduction in gastrointestinal illnesses.4 Handwashing is also one of the main preventative measures recommended against the spread of COVID-19.6,7
The WHO and the U.S. CDC recommend handwashing with soap and water for at least 20–40 seconds as a preferred method to prevent the spread of viruses6–8 but, in settings where soap is not available, the WHO recommends the use of ash and sand as handwashing agents.9 Ash is a product of burning coal, wood, dry leaves, cow dung cakes, and other materials used in a household setting10 and is used as a handwashing agent.11 Ash could potentially inactivate pathogens because it is strongly alkaline, or it may also remove microbes mechanically.12 Sand can be used as an abrasive agent to mechanically remove microbes and other contaminants from hand surfaces.10,12 Studies have quantified bacterial but not viral removal from hands using ash13–15 and, to our knowledge, no study has attempted to quantify the efficacy of sand as a handwashing agent. The structure of the virus and the properties of a handwashing agent both impact the efficacy of handwashing. For instance, enveloped viruses can be inactivated by some agents through chemical or physical disruption of their envelope.16 Therefore, it is important that laboratory handwashing studies evaluate a wide range of materials used for handwashing and evaluate viruses with different morphologies to provide evidence supporting handwashing recommendations.
The purpose of this study is to quantify the efficacy of ash and sand used in conjunction with water as handwashing agents in the removal of enveloped and nonenveloped viruses using nonpathogenic surrogate bacteriophages Phi6 and MS2, and to compare their efficacy against recommended handwashing methods such as soap and water. A commonly accepted threshold criterion for efficacy is the U.S. Food and Drug Administration (FDA) 2-log reduction criterion for removal of microorganisms from hands within 5 minutes after a single wash.17 Efficacy of handwashing methods determined from volunteer studies has been previously reported as the mean log reduction of prewash and postwash bacterial levels,18 and reporting the mean log reduction of organisms,19–21 percent reduction,22 or the number of organisms23 after handwashing in comparison with no handwashing. For this study, we define the efficacy of the handwashing agent as the log reduction of viral titer associated with each handwashing condition in comparison with no handwashing.
MATERIALS AND METHODS
Volunteer enrollment.
Experiments took place at Stanford University between October 12 and December 12, 2021. A total of 19 volunteers (10 self-identifying female and 9 self-identifying male) enrolled in the study. Participation in the experiments was contingent upon volunteers 1) self-reporting as healthy, 2) having no visible sores on hands, and 3) having building access (as deemed appropriate by Stanford’s COVID-19 Research Recovery Plan). Volunteers wore face masks at all times and kept a distance from the technician whenever possible as required by the university’s COVID-19 Research Recovery Plan. Before beginning each experiment, volunteers provided informed consent and reported their age and gender, and their hand measurements (hand length and breadth) were recorded.24 Volunteer enrollment in this study is covered by Stanford Institutional Review Board (IRB) approval (IRB-58904) for human subject research. Based on a power analysis, a sample size of 15 volunteers was determined to provide statistical power greater than 80% for an ANOVA test with six conditions to detect a 40% difference in log reduction values (LRVs).
Materials preparation.
Handwashing materials.
Materials used for handwashing conditions included ash, sand, soap, and water. Water flowing from a tap (tap water) was used for all conditions and decontamination steps. The tap water had a 3.2 mg/L total chlorine residual as of December 21, 2021.25 Antibacterial Softsoap liquid hand soap (Colgate‐Palmolive, New York, NY) was used for the soap handwashing condition and for the decontamination steps of the experiments. Premium Double Sifted Clean Hardwood Ash (Mr. Dirtfarmer, Fleming Island, FL), described as oak and cherry hardwood ash, was used. Ash had an approximate 10- to 102-μm particle size range obtained via microscopy using a microscope (Zeiss, Jena, Germany). For sand, a 1:1 ratio of All-Natural Play Sand (Sakrete, Atlanta, GA) and Ottawa Sand (Fisher Scientific, Waltham, MA) was used. Sand had an approximate 10- to 103-μm particle size range obtained via microscopy. For sterilization, both ash and sand were autoclaved at ∼138 kPa (20 psi) and 121°C for 20 minutes at least 24 hours prior to each experiment to sterilize the materials. In preparation for experiments, 35 cm3 of ash (∼20 g) and 35 cm3 of sand mixture (∼55 g) were individually distributed into a 50-mL Falcon tube to represent the equivalent of a “handful” of material. Prior to each experiment, the pH of ash and sand was measured independently by dipping an MColorpHast pH-indicator strip (Merck Millipore, Burlington, MA) into a mixture containing 6 g of ash or sand and 30 mL of deionized (DI) water.26 The pH of DI water was also measured prior to mixing with each material.
Organisms.
Phi6 and MS2, nonpathogenic biosafety-level 1 bacteriophages, were used for the experiments to protect the health of the volunteers. Phi6 is an enveloped double-stranded RNA nonpathogenic virus (∼80 to 100 nm in diameter) and MS2 is a nonenveloped single-stranded RNA nonpathogenic virus (∼27 nm in diameter) that have been used in laboratory studies investigating handwashing and the interaction between enveloped and nonenveloped viruses and hands.19,27,28 Phi6 (NBRC 105899) and its host, Pseudomonas syringae (ATCC 21781), were donated to the project by K. R. Wigginton at the University of Michigan. P. syringae was propagated by inoculating a loop of P. syringae stock (stored at −80°C) into 25 mL of nutrient broth (media preparation as described by Anderson and Boehm27), and incubating overnight at 30°C on a table shaker operating at 75 revolutions per minute (rpm). The next day, a 100-μL aliquot of the overnight culture was used to inoculate another 25 mL of autoclaved nutrient broth, and incubated overnight at 30°C at 75 rpm. The resultant P. syringae culture was used immediately for enumerating Phi6 in experimental samples (described in the Quantification section below).
Phi6 virus stock (stored at −80°C) was diluted to ∼104 plaque-forming units (PFUs) per milliliter in tryptic soy broth (TSB; Fisher Scientific) and plated as described in the Quantification section below. Phi6 virus stock was created by scraping off the soft agar of a fully lysed plate and suspending it in 5 mL of phosphate-buffered saline (PBS; Fisher Scientific). The solution was kept at 4°C for 3 hours and centrifuged at 3,000 × g for 10 minutes. The supernatant was passed through a 0.22-μm pore size PES syringe filter (Nalgene, Rochester, NY), and concentrated using an Amicon Ultra centrifugal filter (Merck Millipore) at 6,500 rpm for 15 min and used as stock. The resultant solution had a concentration of 109 PFU/mL.
MS2 (DMS 13767) and its host, Escherichia coli (DMS 5695), were obtained from the DMZ German Collection of Microorganism and Cell Cultures (Braunschweig, Germany). Escherichia coli was propagated using a single colony from a 1.5% agar plate streaked with E. coli to inoculate 25 mL of TSB. TSB used for all MS2 procedures contained the antibiotics streptomycin sulfate (Fisher Scientific) and ampicillin sodium salt (Sigma-Aldrich, St. Louis, MO). Escherichia coli culture was incubated overnight at 37°C on a table shaker operating at 75 rpm and then stored at 4°C until the day of the experiments (up to 60 days of storage). On the day of the experiments, a loop of E. coli (stored at 4°C) was used to inoculate 25 mL of the TSB with antibiotics. The inoculated TSB was incubated at 37°C for ∼3 hours until it reached log-phase growth and then used immediately to enumerate MS2 from the experimental samples.
MS2 virus stock (stored at −80°C) was diluted in TSB containing antibiotics to ∼104 PFU/mL in TSB and plated as described in the Quantification section below. MS2 virus stock was created by scraping off the soft agar of a fully lysed plate and suspending it in 5 mL of PBS. The solution was left at 4°C for 3 hours and centrifuged at 3,000 × g for 10 minutes. The supernatant was passed through a 0.22-μm pore size PES syringe filter and used as stock. The resultant concentration was 109 to 1010 PFU/mL.
The virus cocktail for inoculation of volunteers’ hands used in the experiments was prepared on the day of each experiment for immediate use by diluting virus stock in TSB. Virus cocktail preparation (mixture of Phi6 and MS2 into a single solution) was based on Anderson and Boehm.27 For Phi6, 750 μL of Phi6 virus stock (stored at −80°C, ∼109 PFU/mL) and 750 μL of a 1:10 dilution of MS2 virus stock (stored at −80°C, ∼109 to 1010 PFU/mL) was added to 13.5 mL of TSB.
Experimental protocol.
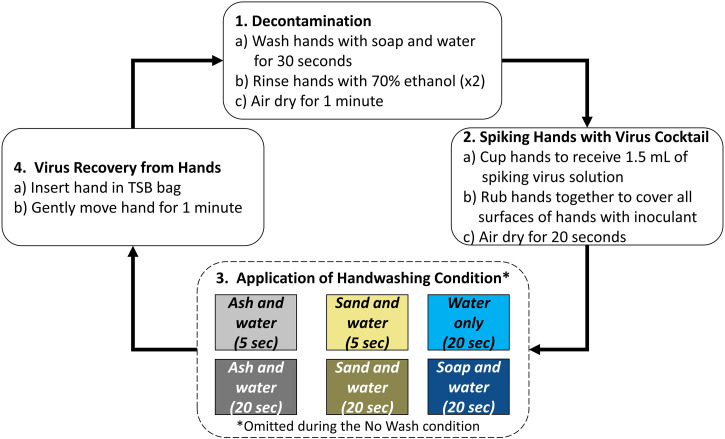
Each volunteer participated in the testing of six handwashing treatments and a control (no handwashing). The six handwashing conditions were as follows: 1) ash and water for 5 seconds, 2) ash and water for 20 seconds, 3) sand and water for 5 seconds, 4) sand and water for 20 seconds, 5) soap and water for 20 seconds, and 6) water only for 20 seconds (Figure 1). Hereafter, ash and water conditions will be referred to as “ash,” sand and water conditions as “sand,” and soap and water conditions as “soap.” For each condition, 1) hands were decontaminated, 2) the virus cocktail of MS2 and Phi6 was applied to the volunteers’ hands, 3) the handwashing condition was applied, and 4) one hand was chosen at random to recover remaining viruses on the hand (Figure 1). Step 1, decontamination, consisted of volunteers washing their hands with soap and water for 30 seconds, hand drying them with a scientific cleaning wipe (Kleenex, Kimberly‐Clark, Irving, TX), and rinsing their hands with a 70% ethanol solution. Volunteers notified the technician when their hands felt dry, and were then instructed to rinse their hands with a 70% ethanol solution a second time. Once the volunteer alerted that their hands were dry a second time, 1 minute elapsed before proceeding with the next step. Step 2, spiking hands with virus cocktail, consisted of pipetting 1.5 mL of virus cocktail solution into volunteers’ cupped hands (750 μL into each hand). Volunteers were instructed to gently rub their hands together to spread the solution across all surfaces of their hands for 20 seconds before proceeding with the next step. Step 3, handwashing, consisted of using a method previously assigned via randomization to wash hands (this step was skipped for the no-wash condition). Volunteers stood in front of the sink and cupped their hands to receive the material used for handwashing (35 cm3 of ash or sand, a single pump of soap, or nothing). The water tap was opened and closed by the technician. Volunteers rubbed their hands with the corresponding material and rinsed their hands under water all within the time specified in the condition. Immediately after handwashing, step 4, virus recovery from hands, consisted of inserting the assigned hand into a 1.6-L Whirl-Pak bag (Nasco, Fort Atkinson, WI) containing 75 mL of sterile TSB. Volunteers were asked to gently swirl their hand in the solution for 1 minute, before finalizing the experiment or restarting at step 1 with the next handwashing condition. Prior to each experiment, 1 mL of the TSB used for the experiment bags was saved in a 1.5-mL microcentrifuge tube to be used as a negative control.
Figure 1.
Experimental design. Note: each experiment included an additional no-wash condition where step 3 was omitted.
Quantification.
After the volunteer finished the experiment, 1 mL from each Whirl-Pak bag sample was transferred into a 1.5-mL microcentrifuge tube to use for plaque assays. Samples were processed via plaque assays immediately after the volunteer experiment was completed, and were stored up to 24 hours at 4°C in the instance that the assay for a sample had to be repeated. Samples and controls were processed via the double agar plaque assay method. Each sample underwent 1:10 serial dilutions in autoclaved TSB. Dilutions up to 1:107 were used; the greatest dilution required to produce countable plaques was identified during pilot stages of the project. A minimum of three dilutions were assayed per sample. Virus stocks used to prepare the virus cocktail solution were used for positive controls. Negative controls included both plating the TSB used to fill the experiment bags and carry out dilutions, and soft agar inoculated only with the host bacteria (no virus or sample). Phi6 plaque assays were completed as described elsewhere19 and MS2 plaque assays were completed using U.S. Environmental Protection Agency method 1602.29 Soft agar was prepared the same day of the experiment, and hard agar plates were prepared up to a week prior to an experiment and stored at 4°C. For the Phi6 plaque assay, soft nutrient agar (0.3% nutrient agar) was inoculated with 100 μL of sample and 100 μL of P. syringae host culture prepared as described in the Organisms section. Soft agar was gently stirred by hand and poured onto a hard nutrient agar plate (2.3% nutrient agar). For the MS2 plaque assay, soft tryptic soy agar (0.7% tryptic soy agar) containing the antibiotics streptomycin sulfate (Fisher Scientific) and ampicillin sodium salt (Sigma-Aldrich) was inoculated with 300 μL of sample and 200 μL of E. coli host culture prepared as described in the Organisms section. Soft agar was gently stirred by hand and poured onto a hard tryptic soy agar plate (1.5% tryptic soy agar). Plates were left to incubate overnight, Phi6 plates at 30°C and MS2 plates at 37°C. PFUs were counted the following day. A result was classified as countable if the number of PFUs was between 1 and 400. The plaques were distinguishable and countable up to 400 due to the small size of the plaques. If the number of plaques observed on the bacterial lawn was greater than 400, the plate was too numerous to count, and when no plaques were observed on the bacterial lawn, a 0 was recorded.
DATA ANALYSIS
Plaque-forming units counts were converted to concentrations of virus in the solution used to rinse hands after each handwashing condition () and divided by concentrations of virus in the solution after no handwashing (). As shown in Equation (1), virus concentration in each rinse solution () was calculated by multiplying PFU counts in each plate by their corresponding dilution, summing them, and then dividing the result by the number of dilutions with countable PFUs. If there was no PFU detected in any dilution plate, 0.5 PFU was substituted in the least diluted plate for the final concentration.
| [Eq. 1] |
The LRV of viral titer associated with each handwashing condition was calculated using the concentration in rinse solution after handwashing compared with the concentration in rinse solution after no handwashing (no-wash condition). The LRV was calculated for each volunteer experiment for each handwashing condition as shown in Equation (2).
| [Eq. 2] |
Log reduction values calculated for each handwashing condition for each volunteer were used for the statistical analysis. Statistical analysis was completed using R version 4.1.2 and RStudio version 1.4.1717 (RStudio, Boston, MA). LRVs for each handwashing condition were tested for normality using the Shapiro-Wilk test, and nonparametric methods were used thereafter because data were not consistently normally distributed. A Kruskal-Wallis test was used to determine whether there were significant differences among the LRVs resulting from the handwashing methods by testing the null hypothesis that the datasets come from the same distribution using a statistical significance of P = 0.05. When significant differences were indicated by the Kruskal-Wallis test, a Conover-Iman post-hoc test was used to determine which conditions were significantly different using a statistical significance of P = 0.025. P values adjusted for the Bonferroni correction are reported Supplemental Tables 2 and 4).
The recovery ratio between virus and the no-wash control (no-wash condition) was calculated for each volunteer using the concentration found in the rinse solution after no handwashing compared with the concentration from the virus stock used to seed volunteers’ hands. The recovery ratios were tested for normality using the Shapiro-Wilk test and were not found to be normally distributed. The nonparametric Kruskal-Wallis test was used to determine whether recovery ratios were significantly different between viruses using a statistical significance of P = 0.05.
RESULTS
The study enrolled a total of 19 volunteers with a median age of 24 years (min = 22, max = 60), median hand length of 17.8 cm (min = 14.6 cm, max = 22.2 cm), and median hand breath of 8.3 cm (min = 7 cm, max = 10.2 cm). Median laboratory conditions during the experiments were 20.5°C (min = 20.2°C, max = 20.7°C) and 42% relative humidity (min = 31%, max = 60%). The median pH of materials (N = 19) used on volunteers’ hands was 12 for ash (min = 11, max = 12) and 6 for the sand mixture (min = 5, max = 6). The Phi6 data from four volunteers were not obtained due to problems with the bacterial host culture, but the study retained sufficient power (greater than 80%) with 15 volunteers.
Quality assurance and control.
Negative controls were negative and positive controls were positive as expected. The negative controls containing only bacterial hosts, and the negative controls containing bacterial host and TSB used in the experiments, had no plaques observed on bacterial lawns. The positive controls containing viral stock had the expected viral titer. The median recovery ratio between the no-wash control and the virus seeded on volunteers’ hands was 0.039 for Phi6 (N = 15, first quantile = 0.023, third quantile = 0.084) and 0.032 for MS2 (N = 19, first quantile = 0.027, third quantile = 0.035). The recovery ratio was not significantly different between viruses (Kruskal-Wallis, P = 0.274). Recoveries less than 1 are expected, as the virus could have become inactivated during application of the seeding cocktail to hands, some of the viral cocktail could have dripped from the volunteers’ hands during application, or some virus could remain adsorbed to the volunteers’ skin.30 By comparing results from the various wash conditions to the no-wash control, we assume that recovery of virus is the same across these conditions, an assumption applied to other handwashing studies.19
Phi6 LRV.
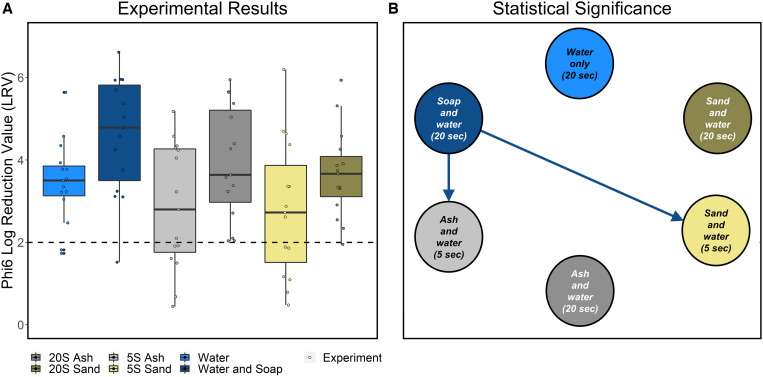
In comparison to the no-wash condition, the median LRV was 3.5 across all conditions for Phi6 (N = 90, min = 0.4, max = 6.6). Overall, all handwashing conditions resulted in greater than 2-log reduction (Table 1, Figure 2A, Supplemental Table 1). Handwashing with soap resulted in the highest LRV (median = 4.8), and handwashing with sand for 5 seconds resulted in the lowest LRV (median = 2.7). There were significant differences in LRV between handwashing conditions (Kruskal-Wallis, P = 0.02). A post-hoc Conover-Iman test indicated that handwashing with soap for 20 seconds had a larger LRV than handwashing with ash for 5 seconds (P adjusted = 0.015) and handwashing with sand for 5 seconds (P adjusted = 0.02). LRVs associated with other handwashing methods were not significantly different from one another (Figure 2B, Supplemental Table 2).
Table 1.
LRVs for Phi6 and MS2
| Water only, 20 seconds | Soap and water, 20 seconds | Ash and water, 5 seconds | Ash and water, 20 seconds | Sand and water, 5 seconds | Sand and water, 20 seconds | |
|---|---|---|---|---|---|---|
| Phi6 LRVs (N = 15) | ||||||
| Median | 3.5 | 4.8 | 2.8 | 3.6 | 2.7 | 3.7 |
| Min | 1.7 | 1.5 | 0.4 | 2.0 | 0.5 | 2.0 |
| Max | 5.6 | 6.6 | 5.2 | 5.9 | 6.2 | 5.9 |
| MS2 LRVs (N = 19) | ||||||
| Median | 2.8 | 2.7 | 2.0 | 2.6 | 1.8 | 2.4 |
| Min | 2.4 | 2.0 | 1.0 | 1.6 | 1.1 | 1.3 |
| Max | 4.2 | 4.0 | 3.0 | 3.5 | 2.6 | 3.5 |
LRV = log reduction value.
Figure 2.
(A) Experimental results: LRV results for Phi6 for all handwashing conditions. The dashed line indicates a 2-log reduction. (B) Statistical significance: dominance-directed graph only including arrows associated with pairs of conditions that were deemed significantly different based on the Conover-Iman test. LRV = log reduction value.
MS2 LRV.
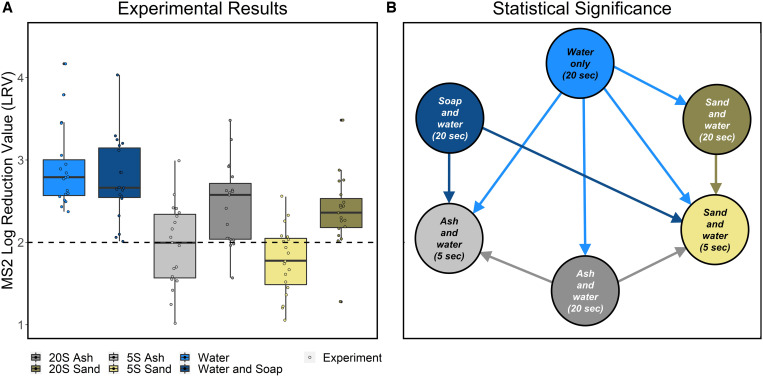
In comparison to the no-wash condition, the median LRV was 2.4 across all conditions for MS2 (N = 114, min = 1.0, max = 4.2). Overall, all 20-second conditions resulted in greater than 2-log reduction, and 5-second conditions resulted in less than 2-log reduction (Table 1, Figure 3A, Supplemental Table 3). Handwashing using water only resulted in the highest LRV (median = 2.8), and handwashing with sand for 5 seconds resulted in the lowest LRV (median = 1.8). There were significant differences in LRV between handwashing conditions (Kruskal-Wallis, P < 0.01). A post-hoc Conover-Iman test indicated that handwashing with water only for 20 seconds had a significantly greater LRV than all handwashing conditions except soap for 20 seconds. Soap and ash for 20 seconds were both associated with a significantly greater LRV than sand and ash for 5 seconds. Sand for 20 seconds had a significantly greater LRV than sand for 5 seconds (P adjusted values can be found in Supplemental Table 4). LRVs associated with other handwashing methods were not significantly different from one another (Figure 3B, Supplemental Table 4).
Figure 3.
(A) Experimental results: LRV results for MS2 for all handwashing conditions. The dashed line indicates a 2-log reduction. (B) Statistical significance: dominance-directed graph only including arrows associated with pairs of conditions that were deemed significantly different based on the Conover-Iman test. LRV = log reduction value.
Phi6 and MS2 LRV comparison.
The median LRV for Phi6 was higher overall than the median LRV for MS2 for all conditions (N = 90, median = 3.5 for Phi6 and N = 114, median = 2.4 for MS2). There were significant differences between Phi6 and MS2 LRVs in each handwashing condition, with the exception of conditions with a duration of 5 seconds, in which no significant difference was found (Kruskal-Wallis, P values can be found in Supplemental Table 5), and in each case the post-hoc Conover-Iman test indicated that LRVs were higher for Phi6 than for MS2 Supplemental Table 5).
DISCUSSION
We carried out a laboratory study with volunteers to quantify the efficacy of handwashing methods common in resource-limited settings (ash and sand with water) for varying amounts of time in comparison to WHO-preferred recommended methods (soap). We compared the removal of both enveloped and nonenveloped surrogate bacteriophages from hands to represent common respiratory and enteric viruses with different morphologies. Overall, handwashing for a full 20 seconds with any method resulted in LRVs greater than a commonly accepted threshold indicating good performance (2-log removal) for both viruses, and increased handwashing time was associated with increased efficacy for ash and sand. There was no significant difference in the performance of handwashing with water and with soap and water, a finding that is consistent with previous studies.19,31 These laboratory results suggest that the tested handwashing agents can perform well when used for a full 20 seconds in the laboratory.
Although handwashing agents such as ash and sand that are primarily used in resource-limited settings are commonly used around the world, only a few studies have attempted to quantify their efficacy.12 To our knowledge, this is the first study to quantify the virus removal efficacy of ash and sand as handwashing agents against enveloped and nonenveloped viruses. Previous studies that focused on the use of ash as a handwashing agent in the field found a similar reduction of fecal coliform bacterial counts on hands after using ash or using soap, and have concluded that using ash and soap have similar efficacy.13,14 Our results suggest that when used for a full 20 seconds, these methods are also similar in efficacy and could meet the U.S. FDA 2-log reduction criteria.17 However, this is only the case when handwashing was conducted for the full 20 seconds.
Although the results between these methods are comparable when used as recommended in a laboratory setting, the use of ash or sand could pose risks in the natural world such as the introduction of new contamination or skin irritation. Several studies have detected pathogens in beach sand including enterovirus, Salmonella, and Campylobacter.32,33 Ash and sand can also be abrasive and/or irritating, which can even increase the risk of transmission of some diseases if the skin is broken. Studies have found that risk of skin irritation increases with frequency of handwashing with soap,34 and irritation potentially caused by other handwashing methods should be investigated. Due to the possible risks associated with ash and sand use, we suggest that follow-up studies in the field are necessary to address these concerns before further recommendations are made based on the performance of the methods in the laboratory.
We found no significant difference in LRVs between soap and water only for removal of either Phi6 or MS2. Our results are consistent with other volunteer experiment studies that have found similar LRVs between handwashing with water with and without soap.19,31 The LRVs found in this study for WHO commonly recommended handwashing methods (soap and water only) are similar to those found by other handwashing studies using enveloped viruses21 and nonenveloped viruses,31,35 and we found no significant difference in the performance of handwashing with and without soap. These results suggest that handwashing with soap and water only may have similar virucidal and/or mechanical removal capacity during handwashing against enveloped and nonenveloped viruses, supporting the theory that mechanical inactivation from hand rubbing and rinsing plays a substantial role in inactivation and removal of viruses during handwashing.
Log reduction values for handwashing with ash and sand were significantly higher for Phi6 (enveloped) than MS2 (nonenveloped), when used for 20 seconds. One possible explanation is that the lipid envelope makes Phi6 more susceptible to inactivation. Enveloped viruses have a lipid envelope that covers the capsid and contains the proteins needed for host infection; nonenveloped viruses lack the lipid layer and contain the proteins needed for host infection directly in the capsid.16 The efficacy of several handwashing agents is attributed to the disruption of the lipid envelope (of enveloped viruses) and the physical removal of viruses from hands.16 All of the handwashing agents tested in this study (ash, sand, and soap) are expected to work in part by physical or chemical disruption of the lipid layer in addition to the mechanical removal of viruses from hands provided by water.
This laboratory study had several limitations. First, only one type each of ash and sand were used for handwashing, and they were sterilized before use. Ash and sand found in the natural world might have varying characteristics and could carry the potential to reintroduce contamination.32,33 An additional limitation of this study was the potential for viruses to carry over into a different handwashing condition if they were not removed in the decontamination step. Piloting stages of the project indicated that the carryover of viruses remaining after a handwashing condition into another condition would be negligible and not affect the final LRV. Handwashing conditions were also randomized for each volunteer experiment to account for this limitation. An additional limitation of this study was that it did not include testing soap and water-only conditions for a duration of 5 seconds. Another limitation present in this study was the chlorine levels already present in the tap water used for each experiment, as chlorine levels can vary in different settings. A study that compared the inactivation efficacy of 0.05% chlorine solutions as handwashing agents against soap and water only found small differences in the efficacy among those conditions.19 The concentration of a 0.05% chlorine solution (500 mg/L)36 is substantially higher than the concentration of residual chlorine found in tap water (∼3.2 mg/L), potentially indicating that the chlorine levels found in tap water are not high enough to chemically inactivate viruses from hands over the timescales of this experiment. Lastly, flowing water from a tap was used for all handwashing conditions; we acknowledge that splashing or scooping water onto hands is common in handwashing, and that ash and sand may also be used without water.37 Those variables were not tested in this study. Future work should further consider the impact of the use of ash and sand under less ideal conditions, including the reduced efficacy at shorter handwashing durations and the potential for contamination of hands from sand and ash obtained from the environment. With the evidence from this study suggesting that ash and sand can be effective for handwashing, further exploring how these factors impact efficacy will be critical to providing effective recommendations for their use in a variety of settings.
Supplemental files
ACKNOWLEDGMENTS
We thank all the volunteers who participated in this study who made the completion of this research possible. We thank Elana Chan for laboratory assistance during the study. We also thank Professor Daniele Lagtangne for her comments on the manuscript.
Note: Supplemental tables appear at www.ajtmh.org.
REFERENCES
- 1. Ansari SA, Springthorpe VS, Sattar SA, Rivard S, Rahman M, 1991. Potential role of hands in the spread of respiratory viral infections: studies with human parainfluenza virus 3 and rhinovirus 14. J Clin Microbiol 29: 2115–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. WHO , 2020. The Top 10 Causes of Death. Available at: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death. Accessed April 26, 2022.
- 3. Boone SA, Gerba CP, 2007. Significance of fomites in the spread of respiratory and enteric viral disease. Appl Environ Microbiol 73: 1687–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aiello AE, Coulborn RM, Perez V, Larson EL, 2008. Effect of hand hygiene on infectious disease risk in the community setting: a meta-analysis. Am J Public Health 98: 1372–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rabie T, Curtis V, 2006. Handwashing and risk of respiratory infections: a quantitative systematic review. Trop Med Int Health 11: 258–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. WHO Handwashing an Effective Tool to Prevent COVID-19, Other Diseases. Available at: https://www.who.int/southeastasia/news/detail/15-10-2020-handwashing-an-effective-tool-to-prevent-covid-19-other-diseases. Accessed February 3, 2022.
- 7. CDC , 2022. How to Protect Yourself and Others. Available at: https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/prevention.html. Accessed February 3, 2022.
- 8. WHO World Hand Hygiene Day. Available at: https://www.who.int/campaigns/world-hand-hygiene-day. Accessed May 11, 2022.
- 9. WHO Handwashing and Handwashing Alternatives. COVID-19 in the Philippines. Available at: https://www.who.int/philippines/emergencies/covid-19-response-in-the-philippines/information/handwashing. Accessed February 16, 2022.
- 10. Nath KJ, Bloomfield SF, 2009. Use of Ash and Mud for Handwashing in Low Income Communities. International Scientific Forum on Home Hygiene. Available at: https://www.ifh-homehygiene.org/review-best-practice/use-ash-and-mud-handwashing-low-income-communities. Accessed February 13, 2022.
- 11. Nizame FA, Nasreen S, Halder AK, Arman S, Winch PJ, Unicomb L, Luby SP, 2015. Observed practices and perceived advantages of different hand cleansing agents in rural Bangladesh: ash, soil, and soap. Am J Trop Med Hyg 92: 1111–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kivuti-Bitok LW, Chepchirchir A, Waithaka P, Ngune I, 2020. Dry taps? A synthesis of alternative “wash” methods in the absence of water and sanitizers in the prevention of coronavirus in low-resource settings. J Prim Care Community Health 11: 2150132720936858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Anuradha P, Devi PY, Prakash MS, 1999. Effect of handwashing agents on bacterial contamination. Indian J Pediatr 66: 7–10. [DOI] [PubMed] [Google Scholar]
- 14. Hoque BA, Mahalanabis D, Alam MJ, Islam MS, 1995. Post-defecation handwashing in Bangladesh: practice and efficiency perspectives. Public Health 109: 15–24. [DOI] [PubMed] [Google Scholar]
- 15. Paludan-Müller AS, Boesen K, Klerings I, Jørgensen KJ, Munkholm K, 2020. Hand cleaning with ash for reducing the spread of viral and bacterial infections: a rapid review. Cochrane Database Syst Rev 2020: CD013597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ijaz MK, Nims RW, de Szalay S, Rubino JR, 2021. Soap, water, and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): an ancient handwashing strategy for preventing dissemination of a novel virus. PeerJ 9: e12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. U.S. FDA , 2015. Safety and Effectiveness of Health Care Antiseptics; Topical Antimicrobial Drug Products for Over-the-Counter Human Use; Proposed Amendment of the Tentative Final Monograph; Reopening of Administrative Record. Federal Register. Available at: https://www.federalregister.gov/documents/2015/05/01/2015-10174/safety-and-effectiveness-of-health-care-antiseptics-topical-antimicrobial-drug-products-for. Accessed April 10, 2022.
- 18. Jensen DA, Macinga DR, Shumaker DJ, Bellino R, Arbogast JW, Schaffner DW, 2017. Quantifying the effects of water temperature, soap volume, lather time, and antimicrobial soap as variables in the removal of Escherichia coli ATCC 11229 from hands. J Food Prot 80: 1022–1031. [DOI] [PubMed] [Google Scholar]
- 19. Wolfe MK, Gallandat K, Daniels K, Desmarais AM, Scheinman P, Lantagne D, 2017. Handwashing and Ebola virus disease outbreaks: a randomized comparison of soap, hand sanitizer, and 0.05% chlorine solutions on the inactivation and removal of model organisms Phi6 and E. coli from hands and persistence in rinse water. PLoS One 12: e0172734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tuladhar E, Hazeleger WC, Koopmans M, Zwietering MH, Duizer E, Beumer RR, 2015. Reducing viral contamination from finger pads: handwashing is more effective than alcohol-based hand disinfectants. J Hosp Infect 90: 226–234. [DOI] [PubMed] [Google Scholar]
- 21. Grayson ML, Melvani S, Druce J, Barr IG, Ballard SA, Johnson PDR, Mastorakos T, Birch C, 2009. Efficacy of soap and water and alcohol‐based hand‐rub preparations against live H1N1 influenza virus on the hands of human volunteers. Clin Infect Dis 48: 285–291. [DOI] [PubMed] [Google Scholar]
- 22. Ansari SA, Sattar SA, Springthorpe VS, Wells GA, Tostowaryk W, 1989. In vivo protocol for testing efficacy of hand-washing agents against viruses and bacteria: experiments with rotavirus and Escherichia coli . Appl Environ Microbiol 55: 3113–3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Burton M, Cobb E, Donachie P, Judah G, Curtis V, Schmidt W-P, 2011. The effect of handwashing with water or soap on bacterial contamination of hands. Int J Environ Res Public Health 8: 97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. NASA Anthropometry and Biomechanics. Man-Systems Integration Standards. Available at: https://msis.jsc.nasa.gov/sections/section03.htm. Accessed February 28, 2022.
- 25. Water Resources City of Palo Alto. Available at: https://www.cityofpaloalto.org/Departments/Utilities/Utilities-Services-Safety/Water-Resources. Accessed January 18, 2022.
- 26. Baker KK. et al. , 2014. Association between moderate-to-severe diarrhea in young children in the Global Enteric Multicenter Study (GEMS) and types of handwashing materials used by caretakers in Mirzapur, Bangladesh. Am J Trop Med Hyg 91: 181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Anderson CE, Boehm AB, 2021. Transfer rate of enveloped and nonenveloped viruses between fingerpads and surfaces. Appl Environ Microbiol 87: e01215-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Julian TR, Leckie JO, Boehm AB, 2010. Virus transfer between fingerpads and fomites. J Appl Microbiol 109: 1868–1874. [DOI] [PubMed] [Google Scholar]
- 29.. U.S. Environmental Protection Agency , 2001. Method 1602: Male-Specific (F+) and Somatic Coliphage in Water by Single Agar Layer (SAL) Procedure EPA 821-R-01-029. Washington, DC: Office of Water, Engineering and Analysis Division. [Google Scholar]
- 30. Pitol AK, Bischel HN, Boehm AB, Kohn T, Julian TR, 2018. Transfer of enteric viruses adenovirus and coxsackievirus and bacteriophage MS2 from liquid to human skin. Appl Environ Microbiol 84: e01809-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sickbertbennett E, Weber D, Gergenteague M, Sobsey M, Samsa G, Rutala W, 2005. Comparative efficacy of hand hygiene agents in the reduction of bacteria and viruses. Am J Infect Control 33: 67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Halliday E, Gast RJ, 2011. Bacteria in beach sands: an emerging challenge in protecting coastal water quality and bather health. Environ Sci Technol 45: 370–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Solo-Gabriele HM. et al. , 2016. Beach sand and the potential for infectious disease transmission: observations and recommendations. J Mar Biol Assoc U K 96: 101–120. [Google Scholar]
- 34. Chamorey E. et al. , 2011. A prospective multicenter study evaluating skin tolerance to standard hand hygiene techniques. Am J Infect Control 39: 6–13. [DOI] [PubMed] [Google Scholar]
- 35. Davies J, 1993. Preliminary study of test methods to assess the virucidal activity of skin disinfectants using poliovirus and bacteriophages. J Hosp Infect 25: 125–131. [DOI] [PubMed] [Google Scholar]
- 36. Iqbal Q, Lubeck-Schricker M, Wells E, Wolfe MK, Lantagne D, 2016. Shelf-life of chlorine solutions recommended in Ebola virus disease response. PLoS One 11: e0156136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Trevett AF, Carter RC, Tyrrel SF, 2004. Water quality deterioration: a study of household drinking water quality in rural Honduras. Int J Environ Health Res 14: 273–283. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.