ABSTRACT.
Quality is one of the essential components of medicines and needs to be ensured to preserve the population’s health. This can be achieved through post-marketing quality control of medicines and is one of the most important duties of national regulatory authorities. In collaboration with the Cameroonian National Drug Quality Control and Valuation Laboratory, the decision was made to initiate a prevalence study to assess the quality of antiinfective medicines in Cameroon. A total of 150 samples of ciprofloxacin tablets and 142 samples of metronidazole tablets were collected from 76 licensed pharmacies and 75 informal vendors in three cities in Cameroon using a random strategy wherever possible and a mystery shopper approach. Three tests were carried out on each of the samples. Visual inspection allowed to find two falsified samples (0.7%) due to lack of information about the manufacturing company, and five more samples (1.7%) were deemed to be substandard due to flaws in the product. An additional 13 samples (4.5%) failed disintegration testing, and six (2.1%) others failed high-performance liquid chromatography assay testing due to insufficient active pharmaceutical ingredient (API) content. All samples were found to contain some API. A prevalence of 7.9% substandard or falsified (SF) medicines was found. Moreover, the prevalence of outlets selling SF medicines was greater in the informal sector (26.7%) than in the formal sector (2.6%). Although the prevalence of SF medicines found was low, efforts need to be made by national regulatory authorities to monitor the pharmaceutical market more closely.
INTRODUCTION
Access to safe medicines is an important element of the Right to Health.1–3 Despite improvements to the accessibility of medicines in recent years in some developing countries, their availability has been estimated to remain below the target of 80% set by the WHO.4 In addition to this issue of accessibility, substandard and falsified (SF) medicines are of major concern.5 This may partly be explained by the shortcomings of national medicine regulatory systems in these regions of the world.6–8
Falsifiers typically focus on the most needed medicines, exploiting their scarcity to infiltrate the pharmaceutical market. This issue has been highlighted during the coronavirus pandemic with the falsification of chloroquine and COVID-19 vaccines.9–11
In May 2017, the WHO agreed on three new definitions based on a public health focus:
“Substandard medical products,” also called “out of specification,” are authorized medical products that fail to meet either their quality standards, specifications or both.
“Unregistered/unlicensed medical products” are medical products that have not undergone evaluation and/or approval by the National or Regional Regulatory Authority for the market in which they are marketed/distributed or used, subject to permitted conditions under national or regional regulation and legislation.
“Falsified medical products” are medical products that deliberately/fraudulently misrepresent their identity, composition or source.5,12
In a recent study, the WHO has estimated that, on average, 10.5% of medicines in circulation in low- and middle-income countries are either substandard or falsified.5,13 Some authors stated that the prevalence of SF medicines in the African region was 18.7%.14
These estimations are global since very few prevalence studies have been conducted in some regions of the world, particularly in Central Africa, where Cameroon is located.15 A recent review of SF medicines prevalence studies conducted in Cameroon highlighted the low methodological quality of the majority of them, which could significantly compromise the reliability of the data presented. However, these studies are essential to both determine the prevalence and identify strategies to address SF medicines.16 Their quality is therefore of the utmost importance.
The main objective of the present study was to conduct a field survey to estimate the prevalence of poor-quality medicines in Cameroon by implementing the following tests: visual inspection, a simplified disintegration test, and a high-performance liquid chromatography assay (HPLC) test.
This study focused on two antiinfective medicines, ciprofloxacin and metronidazole, both in tablet forms. Ciprofloxacin is an antibiotic substance of the fluoroquinolone family, generally prescribed for urinary tract infections and usually for the treatment of salmonellosis.17,18 Metronidazole is an antibiotic and antiparasitic substance member of the nitroimidazole class, widely used in the treatment of a number of infections caused by anaerobic bacteria and some types of protozoa.19 These two medicines were selected because they are on the national list of essential medicines in Cameroon20 and have been listed among the most readily available on the African pharmaceutical market in two studies conducted in Cameroon, the Democratic Republic of Congo (DRC), and Togo.21,22 The galenic tablet form was chosen because it represents the most often sold formulation and is in turn highly targeted by falsifiers.23
METHODOLOGY
Study period.
The collection of samples took place from December 2019 to December 2020.
Sampling design.
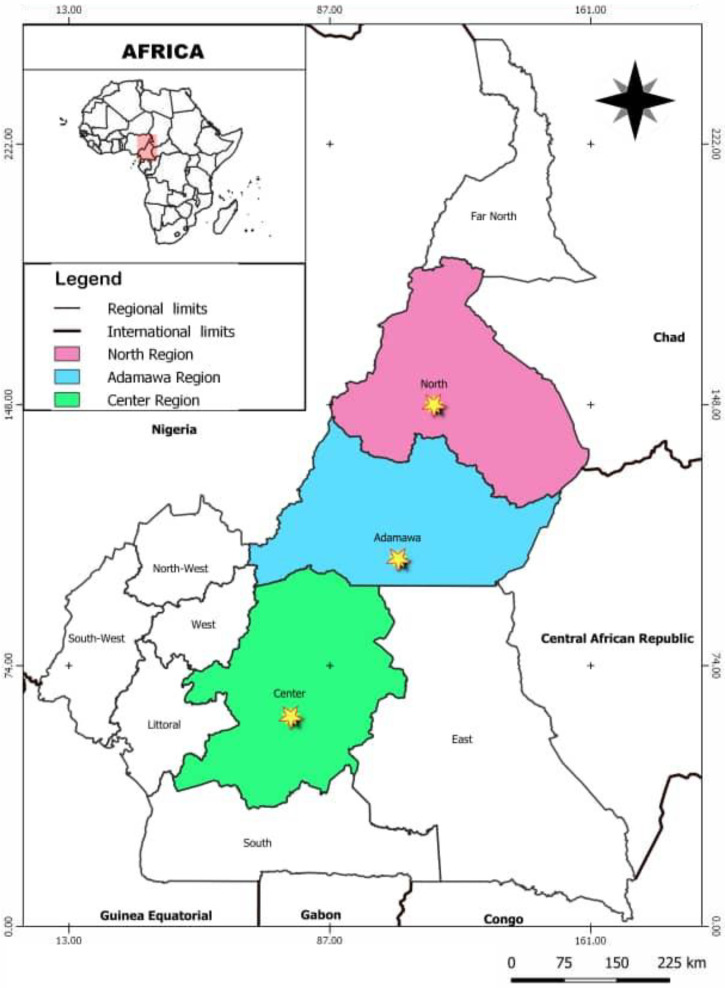
For the sampling design, the WHO recommendations24 and Medicines Quality Assessment Reporting Guidelines (MEDQUARG) from Newton et al.25 were taken into account. The samples were collected in the three main cities of the Center, Adamawa, and Northern regions—Yaoundé, Ngaoundéré, and Garoua respectively (Figure 1). These samples were collected in licensed pharmacies in the formal sector and informal sector outlets respectively classified as levels II and III according to the classification of principal medicines distribution sectors.24 These two levels were selected for their proximity to patients.
Figure 1.
Geographic location of sampling sites in Cameroon.
The city of Yaoundé was chosen because of its high representativeness at a national pharmaceutical level with the highest number of pharmacies in the country.26 Adamawa and North regions were picked to cover the least studied areas regarding medicines quality studies in Cameroon.16
A random selection of sampling sites and a mystery shopper approach for sample collection were used because this has the advantage of inducing less sampling bias.25 Mystery shoppers presented themselves at each outlet as patients or their relatives without any medical prescription, asking: “Could I have ciprofloxacin and metronidazole 500-mg tablets?” in either French or local languages, especially in the informal sector. When the sellers asked for the reason for purchasing these medicines, the shoppers replied: “The medicines are intended for an adult suffering from typhoid fever and amoebiasis.” At each point of purchase, when several products were presented, the cheaper ones were selected following recommendations by Newton et al.25
The formal sector was exclusively represented by licensed pharmacies in the cities of Yaoundé, Ngaoundéré, and Garoua from a list provided by the Cameroonian National Order of Pharmacists. The total number of licensed pharmacies on this list was 168 at the time of collection. The sampled pharmacies were randomly selected in Yaoundé using the rand function in Matlab (R2018a) (Mathworks, Inc., Natick, MA). In the other two cities, all pharmacies were sampled because there were few. There was no database for the informal medicine sector, and thus outlets located close to the randomly selected pharmacies and those found in the most recognized places for illicit medicine trade were arbitrarily selected. Where several collection points were in close proximity to each another, only one was visited to avoid raising suspicion. These informal outlets were either kiosks, stores, roadside stands, or markets. They did not have any authorization from the Ministry of Health in contrast to licensed pharmacies.
Generally, a minimum of 10 tablets was collected per sample at each point of purchase because the smallest package available on the market contained 10 tablets. In this way, the risk of collecting several batches per sample at the same sampling point was avoided. Newton et al.25 also recommend only small samples be collected to avoid raising suspicion. Samples were collected in their original packaging where possible and were given a code for anonymization purposes.
Data collection.
The following information was collected for each sample: date of collection, brand name, international nonproprietary name, galenic form, dosage, batch number, manufacturing and expiry dates, number of tablets, country of origin, price, registration status, stated manufacturer, and address. All information was encoded in an Excel 2016 file (Microsoft Corp., Redmond WA).
Samples storage and transport.
After collection, the samples were stored in a temperature-controlled storage room (20 ± 5°C) until an analysis could be carried out at the National Drug Quality Control and Valuation Laboratory (LANACOME) in Yaoundé. Once collected and analyzed, the samples were then transported to Liège, Belgium, in less than 24 hours and stored in a dedicated room at the Laboratory of Pharmaceutical Analytical Chemistry at a controlled temperature (20 ± 5°C).
Ethical considerations.
This study was approved by the Ministry of Health of Cameroon through the National Committee of Ethics of Research for Human Health (ethical clearance N°2019/07/1173/CE/CNERSH/SP). In addition to this ethical clearance, a data-sharing agreement was drawn up and signed between the two laboratories where the validation of methods and analyses of samples were performed.
Definitions of SF medicines in the study.
The definitions used for SF medicines in this study were those recommended by the WHO.5,12 The samples for which there was no way of identifying the manufacturer and/or the marketing authorization holder were considered to have been falsified.
Testing laboratories and tests performed.
The analyses were initially planned to be performed at LANACOME (Cameroon). However, due to several logistical issues including instrument failure, long delays for the repairs, and power and supplies shortages, the disintegration tests and LC-UV assays were performed on only 96 samples in Cameroon and with 186 remaining samples being tested in Belgium.
Visual inspection test.
Samples were examined visually, and information related to packaging, identity, traceability, and physical appearance were checked using a simplified checklist from the Institute of Tropical Medicine (Antwerp, Belgium).7 Any samples displaying issues with packaging, the physical appearance of tablets, and traceability were considered noncompliant at this stage.
Disintegration test.
The disintegration test was selected because it can be considered a prescreening method for dissolution testing.15 Moreover, Nickerson et al.27 have demonstrated a relationship between dissolution and disintegration testing for immediate-release tablets, which was the case for these samples. The equipment used for disintegration in Cameroon and Belgium were type DT2 SOTAX and DT3 SOTAX (Aesch, Basel, Switzerland), respectively. The disintegration test was performed according to the <701> general chapter for tablets, capsules, or granules in the U.S. Pharmacopeia (USP)28: the apparatus consisted of a basket-rack assembly. This test was performed with distilled water at a temperature of 37 ± 2°C on three tablets per sample. A sample was considered noncompliant if at least one tablet had not disintegrated within 30 minutes.
HPLC assay.
For this study, it was decided to adapt the Ciprofloxacin USP method28 to analyze metronidazole and ciprofloxacin simultaneously within a shorter analysis time frame. Acetonitrile was replaced by methanol, and the dimensions and properties of the stationary phase were modified to obtain a faster and cheaper method (comparing methanol and acetonitrile prices) than the original one.
The chromatography system used in Belgium for the validation and the analyses of samples was a Waters 2695 separation module coupled to a Waters selector valve 7678 and a Waters 996 Photodiode array detector (Waters, Eschborn, Germany). The method was likewise validated in Cameroon on an Agilent 1260 Infinity II HPLC system including a quaternary pump and a DAD 1260 infinity II detector (Agilent Technologies, Santa Clara, CA).
Details of the adaptations made to the pharmacopeial method and the validation are given in the supplementary materials Supplemental Tables 1–4,Supplemental Figure 1).
Where the active pharmaceutical ingredient (API) content was noncompliant with what was claimed on the packaging ± 10%, the sample was considered substandard. These acceptance limits were in line with the USP monographs of ciprofloxacin tablets and metronidazole tablets.
Software.
MATLAB (R2018a) (The Mathworks, Inc.) was used to generate random numbers for the selection of pharmacies for sampling. Validation data processing was made using E-noval 4.0b software (Pharmalex Belgium, Mont-Saint-Guibert, Belgium).
Information of authorities and stakeholders.
The WHO Global Surveillance and Monitoring System and the national pharmaceutical regulatory authority: Direction de la Pharmacie du Médicament et des Laboratoires (DPML) were notified of any SF and unregistered medicines detected during this study. LANACOME was also informed about the results obtained, according to the terms of the data-sharing agreement.
RESULTS
Overview of the samples.
A total of 292 samples were collected from the three target cities (Table 1). In Ngaoundéré and Garoua cities, metronidazole 500 mg was not frequently available in either pharmacies (one time out of 10) or informal sector outlets (one time out of four). In the city of Yaoundé, ciprofloxacin 500 mg could not be found in one pharmacy and was therefore replaced by another arbitrarily selected pharmacy. Samples were taken from a total of 151 selling points as follows: 96 in Yaoundé (48 from both the formal and informal sector), 25 in Ngaoundéré (13 in the formal and 12 from the informal sector), and 30 in Garoua (15 from both the formal and informal sector).
Table 1.
Distribution of samples collected according to API and sampling location
| Location | Formal sector | Informal sector | Total | ||
|---|---|---|---|---|---|
| Ciprofloxacin | Metronidazole | Ciprofloxacin | Metronidazole | ||
| Yaoundé | 48 | 48 | 48 | 48 | 192 |
| Ngaoundéré | 12 | 12 | 12 | 12 | 48 |
| Garoua | 15 | 14 | 15 | 8 | 52 |
| Total | 75 | 74 | 75 | 68 | 292 |
API = active pharmaceutical ingredient.
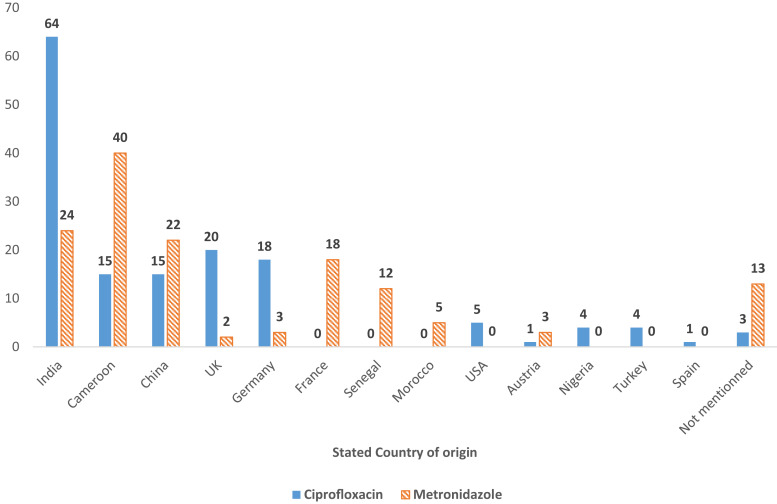
As shown in Figure 2 and Supplemental Table 5, the samples had 13 stated countries of origin. Interestingly, Asia (India and China) was the main point of origin for both ciprofloxacin and metronidazole samples with 52.7% (n = 79) and 32.4% (n = 46), respectively. The second was Europe for ciprofloxacin (26.7%, n = 40) and Africa for metronidazole (40.1%, n = 57).
Figure 2.
Distribution of samples according to stated country of origin.
For the ciprofloxacin samples, a total of 46 brands was recorded, coming from 44 manufacturing laboratories, for a total of 91 batches.
Concerning metronidazole samples, 18 brands were collected, representing 62 batches.
Registration status of samples.
Regarding the registration status at the Cameroon national medicines regulatory authority (DPML), 62% of the samples (n = 181) were registered (Table 2). The unregistered samples included 27.5% (n = 39) of metronidazole samples and 48% (n = 72) of ciprofloxacin samples. Among those that were not registered, 23 had a registration number from Nigerian National Agency for Food and Drug Administration and Control (NAFDAC).
Table 2.
Summary of analyses results by samples characteristics
| Samples characteristics | Visual inspection | Disintegration | HPLC analysis | ||||
|---|---|---|---|---|---|---|---|
| C | NC | C | NC | C | NC | ||
| Sampling cities | Yaoundé | 188 | 4 | 180 | 12 | 190 | 2 |
| Ngaoundéré | 47 | 1 | 47 | 1 | 45 | 3 | |
| Garoua | 51 | 1 | 52 | 0 | 51 | 1 | |
| Stated continent of origin | Africa | 76 | 0 | 76 | 0 | 72 | 4 |
| America | 5 | 0 | 5 | 0 | 5 | 0 | |
| Asia | 126 | 3 | 122 | 7 | 128 | 1 | |
| Europe | 66 | 0 | 60 | 6 | 65 | 1 | |
| Unknown | 13 | 3 | 16 | 0 | 16 | 0 | |
| Production | Local | 55 | 0 | 55 | 0 | 55 | 0 |
| Foreign | 217 | 4 | 208 | 13 | 215 | 6 | |
| Unknown | 13 | 3 | 16 | 0 | 16 | 0 | |
| Registration | Registered | 181 | 0 | 175 | 6 | 181 | 0 |
| Unregistered | 106 | 5 | 104 | 7 | 105 | 6 | |
| Sampling sector | Formal | 147 | 2 | 149 | 0 | 148 | 1 |
| Informal | 138 | 5 | 130 | 13 | 138 | 5 | |
| International nonproprietary name | Ciprofloxacin | 144 | 6 | 144 | 6 | 144 | 6 |
| Metronidazole | 142 | 0 | 135 | 7 | 142 | 0 | |
C = compliant; HPLC = high-performance liquid chromatography; NC = not compliant.
According toSupplemental Figure 2, in the formal sector, 8.1% (n = 12) were unregistered (five ciprofloxacin and seven metronidazole samples). This proportion rose to 69.2% (n = 99) in the informal sector (67 ciprofloxacin and 32 metronidazole samples). The registration status was checked on the DPML website.29
Visual inspection test.
The main issues raised by the visual inspection were related to the integrity of primary packaging, traceability, and physical appearance. Some samples collected in the informal sector (5.5%, N = 16) did not have any stated manufacturing company information: 13 metronidazole and three ciprofloxacin samples (Figure 2). The metronidazole samples were considered suspicious instead of noncompliant because information about the stated manufacturing company or country of origin could have been present on their potential secondary packaging even though the mystery shoppers did not have access to it. In respect of the three ciprofloxacin samples, one had already been repackaged in a plastic bag at the time of sampling and was considered substandard because the integrity of the medicine could have been compromised (Supplemental Figure 3A). The two others did not have any stated manufacturing company or country of origin on either the primary or the secondary packaging (Supplemental Figure 3B); they were therefore considered falsified.
Four ciprofloxacin samples raised concerns due to their physical appearance: one from the formal sector had visible stains on the tablets (Supplemental Figure 3C). These stains were only detected after removing the tablets from the aluminum-aluminum blister pack. The second, from the informal sector, had a half tablet in an intact blister (Supplemental Figure 3D). The primary packaging of a third sample, from the informal sector, had been cut into two separate halves (Figure 3E). In the fourth, collected from the formal sector, a piece of sticky paper had been pasted over the original printed batch number, manufacturing, and expiry dates on the secondary packaging to make it the same as the information on the blister pack (Supplemental Figure 3F). All these samples were therefore considered substandard.
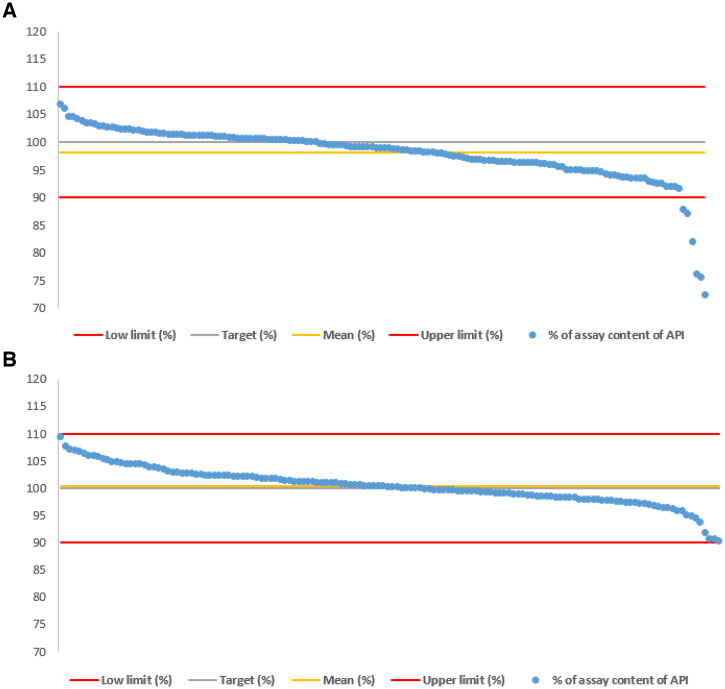
Figure 3.
(A) Assay results of ciprofloxacin samples. (B) Assay results of metronidazole samples.
Among the samples with visual inspection issues, four had the same brand name: “Ciprolif 500.” Two of them claimed to be from China and India, and the two others were those without visible manufacturing company information (Supplemental Figure 3B, C, and F). Other samples with approximately the same brand name, “AD Ciprolif 500,” had alleged to come from different manufacturers in India (Supplemental Figure 4A and B). These samples thought suspicious rather than falsified because there was no way to verify their authenticity. As a result of the visual inspection test, a total of seven samples were deemed noncompliant: five substandard and two falsified (Supplemental Figure 3A–F).
Disintegration test.
A total of 4.9% (n = 7) of the metronidazole samples and 4% (n = 6) of the ciprofloxacin samples did not comply with the specifications of the disintegration test. All these samples came from the informal sector: 12 were collected in Yaoundé and one in Ngaoundéré.
HPLC tests.
Presence of the correct API (metronidazole or ciprofloxacin) was confirmed for all samples by comparing the retention times (1.7 minutes for metronidazole and 3.3 minutes for ciprofloxacin) and ultraviolet spectra to those of the reference substances (Supplemental Figure 5).
In respect of the assay results, 4% (n = 6) of ciprofloxacin samples were found noncompliant, as illustrated in Figure 3A. These samples presented insufficient API contents varying from 72.4% to 88.0%. One of these samples was collected in the formal sector (88.0% of the stated amount of ciprofloxacin) and the remaining five from the informal sector. As can be seen in Figure 3B, the assay confirmed that all metronidazole samples were compliant with API contents between 90.0% and 110.0%.
DISCUSSION
Prevalence of SF medicines and cause of noncompliance.
In terms of the samples collected, 7.2% (N = 21) were found substandard: 4.9% (n = 7) of metronidazole samples that failed the disintegration test and 9.3% of ciprofloxacin samples (n = 14). From the substandard ciprofloxacin samples, three failed the visual inspection test, three the assay, two both visual inspection and assay, five the disintegration test, and one both disintegration test and assay. The prevalence of falsified medicines (0.7%) concerned exclusively the two ciprofloxacin samples with information gaps regarding traceability (Supplemental Figures 3B).
The resulting prevalence of SF medicines (7.9%) was lower than the one obtained by the WHO (37%) in Cameroon and by Hauk et al. (16.5%) in Cameroon, Malawi, and DRC.30,31 However, it was close to that of Schäfermann et al. (10%) for ciprofloxacin and metronidazole exclusively in a study conducted in Cameroon and DRC.32 It was also approaching those found by Petersen et al.33 (7.1%) in Cameroon, Rahman et al.34 in Bangladesh (9.5%) and to the WHO global prevalence for the class of antibiotics and other antiinfective medicines (7.2%).5 In fact, the problem could be more serious than it seemed as the present study only included two antiinfective medicines, a single pharmaceutical form, and a single dosage in three cities in Cameroon. Nevertheless, the present study has provided a more comprehensive overview of the prevalence of SF medicines in Cameroon.
Visual inspection issues revealed problems related to the lack of application of Good Manufacturing Practices.7
Regarding the assay results, underdosed antibiotics could be responsible for therapy failures and antibiotic resistance, which can lead to the reduction or absence of effectiveness of first-line therapies.5,35–38 The WHO has recently reported high resistance levels for Escherichia coli and Salmonella spp. to ciprofloxacin.17 According to Fasugba et al., the highest rate of resistance to this antibiotic was reported in developing countries.39
Concerning disintegration problems, they can lead to therapeutic failures and even resistance in the case of antiinfective medicines.40 Some samples that failed this test claimed to come from the same manufacturer and batch (for each substance) as others that had passed it. Many of them had been collected from the informal sector where storage requirements had not been followed. These observations were consistent with the findings of Lalani et al.,40 which proved that poor storage conditions may result in tablet disintegration problems.
Of all the 151 sampling points, 14.6% (n = 22) were found dispensing SF medicines of which 26.7% (n = 20) were informal outlets and 2.6% (n = 2) were licensed pharmacies. It is important to note that all substandard samples regarding API content were unregistered, which is consistent with the finding from the WHO.30
Prevalence of SF medicines according to the city of sampling, stated country, and the continent of origin.
The highest prevalence of SF medicines (10.4%) was observed in Ngaoundéré (Supplemental Figure 6). Ngaoundéré is the main city in the Adamawa region (see Figure 1), and borders Nigeria to the west. Extensive commercial exchanges are carried out in this region and would logically include drug trafficking. Three of the four samples claiming to be from Nigeria collected from here were classified as substandard. Our data concern only three main cities of Cameroon and the situation could be worse in rural areas with limited accessibility.
Almost half, 47.8% (n = 11), of the SF samples stated to be from Asia: China (n = 2) and India (n = 9). This was similar to the results obtained by Khuluza et al.15 where most of the out-of-specifications samples displayed India as the country of origin. Moreover, India was identified in 2020 by the Organization for Economic Cooperation and Development as one of the top source countries for most SF medicines.41
Prevalence of SF medicines according to sampling sector.
The prevalence of SF medicines in the informal sector was 14.7% (n = 21), which was higher than that of the formal sector (1.3%, n = 2). This situation was similar to that found in South Africa,42 Cameroon, and DRC16,32 for which most SF medicines were found in the informal sector.
In terms of affordability, prices in the formal sector were 2.5 times higher than prices in the informal sector for both ciprofloxacin and metronidazole (Supplemental Figure 7). Comparable results were found by Schäfermann et al.21 in southern Togo. A survey conducted in Cameroon showed that 53.6% of the population were buying their medicines in the informal sector.43 This preference for the unlicensed sector could be motivated on the one hand by the lack of awareness of the population of the risks associated with this sector and, on the other hand, by the lower prices and higher accessibility than the formal sector. Intervention is necessary to improve access to medicines by reducing prices in the formal sector or implementing strategies to reduce out-of-pocket payments in the health system. In addition, pharmaceutical governance, supply chain management, and technical capacity must be strengthened and associated with repressive actions to eradicate the illicit and uncontrolled sector of medicines in Cameroon.
Findings about dispensing practices.
The present study also highlighted irregularities in dispensing medicines. Indeed, medicines were dispensed despite the lack of medical prescription in both formal and informal sectors. In Cameroon, although policies are in place for the regulation of antibiotic dispensing, access to antibiotics among the population remains uncontrolled and could lead to misuse and potential abuse of these types of medications.44 The same observation was recently made in Malawi.45 In addition, there was no secondary packaging in 42.1% of the samples. Therefore, information related to storage conditions and traceability are unavailable to patients.
Limitations.
The present study was intended as an overview of the quality of the most readily available antiinfective medicines in the Cameroonian pharmaceutical market and a snapshot of the situation in only three cities in Cameroon. Therefore, the results obtained cannot be extrapolated to all other pharmaceutical classes, galenic forms, or to the whole country.
Some difficulties concerned the realization of HPLC analyses in Cameroon due to the logistical issues previously mentioned. The shipment of the samples to Belgium within 24 hours took place without a temperature control system and noncompliant samples found in Cameroon were reanalyzed in Belgium, where consistent results were obtained.
Although the sampling size was large, there were limited quantities of tablets per sample. It was therefore not possible to achieve pharmacopeial testing of the samples. Nevertheless, the prevalence of SF medicines obtained in this study was comparable to the results obtained by other authors for ciprofloxacin and metronidazole.32
In samples that had a limited number of tablets, disintegration testing was carried out on three rather than six tablets. Unfortunately, it was not possible to repeat the test on noncompliant samples as recommended by the USP.28 Nevertheless, because most of these samples had the same stated manufacturer and batch number (for each substance), they could have been considered as a single sample (even if it was not the case in the present study), but that would not have met the pharmacopeia requirements.
The stated manufacturing companies of suspicious and noncompliant samples were contacted (when information was available) to confirm the authenticity of the samples. Only one replied and confirmed the authenticity of three substandard samples. For the others, no reply had been received at the time of writing this paper.
One issue concerned the gray area of the WHO definition of SF medicines. Indeed, the status of samples without information about the manufacturing company is unclear. To clarify this situation, the WHO was contacted, and it was confirmed that if it was not possible to identify the manufacturer and/or marketing authorization holder of a product, the samples should be classified as falsified.
CONCLUSION
This field study was conducted in Cameroon following WHO and MEDQUARG recommendations.24,25 The visual inspection, assay, and disintegration test results revealed that the Cameroonian pharmaceutical market is not safe from SF medicines. Most of these SF medicines were gathered in the informal sector and were unregistered. It is therefore risky to buy medicines in the informal sector. The results obtained are encouraging with a relatively low SF medicines prevalence, but more efforts should be made by governments in a multi-stakeholder collaboration to eradicate the illicit trade of medicines. Moreover, regular surveillance of the pharmaceutical market needs to be implemented.
Supplemental files
Note: Supplemental tables, figures, and materials appear at www.ajtmh.org.
REFERENCES
- 1. UN Human Rights, Office of the High Commissioner Access to Medicines and the Right to Health. Available at: https://www.ohchr.org/en/development/access-medicines-fundamental-element-right-health. Accessed May 3, 2022.
- 2. Bassat Q, Tanner M, Guerin PJ, Stricker K, Hamed K, 2016. Combating poor-quality anti-malarial medicines: a call to action. Malar J 15: 302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Green MD, Hostetler DM, Nettey H, Swamidoss I, Ranieri N, Newton PN, 2015. Integration of novel low-cost colorimetric, laser photometric, and visual fluorescent techniques for rapid identification of falsified medicines in resource-poor areas: application to artemether–lumefantrine. Am J Trop Med Hyg 92 (Suppl 6): 8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization , 2013. Global Action Plan for the Prevention and Control of Noncommunicable Diseases 2013–2020. Available at: https://www.who.int/publications/i/item/9789241506236. Accessed April 20, 2022.
- 5. World Health Organization , 2017. A Study on the Public Health and Socioeconomic Impact of Substandard and Falsified Medical Products. Available at: https://www.who.int/publications/i/item/9789241513432. Accessed May 3, 2022.
- 6. Schiavetti B. et al. , 2018. The quality of medicines used in children and supplied by private pharmaceutical wholesalers in Kinshasa, Democratic Republic of Congo: a prospective survey. Am J Trop Med Hyg 98: 894–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schiavetti B, Wynendaele E, Melotte V, Van Der Elst J, De Spiegeleer B, Ravinetto R, 2020. A simplified checklist for the visual inspection of finished pharmaceutical products: a way to empower frontline health workers in the fight against poor-quality medicines. J Pharm Policy Pract 13: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Newton PN, Bond KC; on behalf of the Oxford Statement signatories , 2019. Global access to quality-assured medical products: the Oxford Statement and call to action. Lancet Glob Health 7: e1609–e1611. [DOI] [PubMed] [Google Scholar]
- 9. Schneider M, Ho Tu Nam N, 2020. Africa and counterfeit pharmaceuticals in the times of COVID-19. J Intellect Prop Law Pract. 15: 417–418. [Google Scholar]
- 10. Alerte produit médical N°5/2021 Vaccin COVISHIELD falsifié (mise à jour) Available at: https://www.who.int/fr/news/item/31-08-2021-medical-product-alert-n-5-2021-falsified-covishield-vaccine. Accessed September 20, 2021.
- 11. Waffo Tchounga CA, Sacre PY, Ciza P, Ngono R, Ziemons E, Hubert P, Marini R, 2021. Composition analysis of falsified chloroquine phosphate samples seized during the COVID-19 pandemic. J Pharm Biomed Anal 194: 113761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. World Health Organization , 2017, WHO Member State Mechanism On Substandard/Spurious/Falselylabelled/Falsified/Counterfeit (SSFFC) Medical Products Working Definitions, Appendix 3. Geneva, Switzerland. Available at: https://apps.who.int/gb/ebwha/pdf_files/WHA70/A70_23-en.pdf. Accessed October 8, 2021.
- 13. Organisation Mondiale de la Santé , 2017. Dans les pays en développement, 1 médicament sur 10 est de qualité inférieure ou falsifié. Available at: https://www.who.int/fr/news/item/28-11-2017-1-in-10-medical-products-in-developing-countries-is-substandard-or-falsified. Accessed October 8, 2021.
- 14. Ozawa S, Evans DR, Bessias S, Haynie DG, Yemeke TT, Laing SK, Herrington JE, 2018. Prevalence and estimated economic burden of substandard and falsified medicines in low- and middle-income countries: a systematic review and meta-analysis. JAMA Netw Open 1: e181662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Khuluza F, Kigera S, Heide L, 2017. Low prevalence of substandard and falsified antimalarial and antibiotic medicines in public and faith-based health facilities of southern Malawi. Am J Trop Med Hyg 96: 1124–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Waffo Tchounga C, Sacré P-Y, Hamuli PC, Mballa RN, Nga NE, Hubert P, Marini R, 2021. Poor-quality medicines in Cameroon: a critical review. Am J Trop Med Hyg 105: 284–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. World Health Organization , 2020. Global Antimicrobial Resistance and Use Surveillance System (GLASS). Geneva, Switzerland: WHO. Available at: https://www.who.int/publications/i/item/9789240027336. Accessed May 25, 2021.
- 18. Schaeffer AJ, 2003. The expanding role of fluoroquinolones. Am J Med 49: 129–147. [DOI] [PubMed] [Google Scholar]
- 19. Sakira AK, Mees C, De Braekeleer K, Delporte C, Yameogo J, Yabre M, Some TI, Van Antwerpen P, Mertens D, Kauffmann JM, 2021. Determination of the quality of metronidazole formulations by near-infrared spectrophotometric analysis. Talanta Open 3: 100027. [Google Scholar]
- 20. Ministère de la santé publique, Direction de la pharmacie, du médicament et des laboratoires Liste nationale des médicaments essentiels du Cameroun 2017. Available at: https://dpml.cm/index.php/fr/catalogue/medicaments-essentiels. Accessed August 18, 2021.
- 21. Schäfermann S, Wemakor E, Hauk C, Heide LI, 2018. Quality of medicines in southern Togo: investigation of antibiotics and of medicines for non-communicable diseases from pharmacies and informal vendors. PLoS One 13: e0207911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schäfermann S, Neci R, Ngah Ndze E, Nyaah F, Basolanduma Pondo V, Heide LI, 2020. Availability, prices and affordability of selected antibiotics and medicines against non-communicable diseases in western Cameroon and northeast DR Congo. PLoS One 15: e0227515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Coic L, Sacré P-Y, Dispas A, Karim Sakira A, Fillet M, Marini RD, Hubert P, Ziemons E, 2019. Comparison of hyperspectral imaging techniques for the elucidation of falsified medicines composition. Talanta 198: 457–463. [DOI] [PubMed] [Google Scholar]
- 24. World Health Organization Good Pharmacopoeial Practices , 2016. In: WHO Expert Committee on Specifications for Pharmaceutical Preparations. WHO Technical Fiftieth Report Series 996, Geneva, Switzerland. Available at: https://cdn.who.int/media/docs/default-source/medicines/norms-and-standards/guidelines/distribution/trs966-annex7-who-guidelines-on-the-conduct-of-surveys-of-the-quality-of-medicines.pdf?sfvrsn=4dbb5519_2. Accessed June 17, 2022.
- 25. Newton PN. et al. , 2009. Guidelines and guidance guidelines for field surveys of the quality of medicines: a proposal. PLoS One 6: e1000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ministère de la santé publique, Direction de la pharmacie, du médicament et des laboratoires Politique pharmaceutique nationale du Cameroun, 2013. Available at: https://dpml.cm/index.php/fr/s-informer/actualite/annee-2018/546-politique-pharmaceutique-nationale-du-cameroun-ppn-2013 Accessed October 8, 2021.
- 27. Nickerson B, Kong A, Gerst P, Kao S, 2018. Correlation of dissolution and disintegration results for an immediate-release tablet. J Pharm Biomed Anal 150: 333–340. [DOI] [PubMed] [Google Scholar]
- 28. U.S. Pharmacopeia , 2021. USP NF online, Issue 2. Available at: https://online.uspnf.com/uspnf. Accessed June 2, 2022.
- 29. Ministère de la santé publique, Direction de la pharmacie, du médicament et des laboratoires Repertoire des médicaments homologués. Available at: https://dpml.cm/repertoireDesAmm/index.php. Accessed October 8, 2021.
- 30. World Health Organization , 2011. Survey of the Quality of Selected Antimalarial Medicines Circulating in Six Countries of Sub-Saharan Africa. Available at: https://www.afro.who.int/sites/default/files/2017-06/WHO_QAMSA_report.pdf. Accessed April 25, 2020.
- 31. Hauk C, Hagen N, Heide L, 2021. Identification of substandard and falsified medicines: influence of different tolerance limits and use of authenticity inquiries. Am J Trop Med Hyg 104: 1936–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schäfermann S. et al. , 2020. Substandard and falsified antibiotics and medicines against noncommunicable diseases in Western Cameroon and Northeastern Democratic Republic of Congo. Am J Trop Med Hyg 103: 894–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Petersen A, Held N, Heide L, 2017. Surveillance for falsified and substandard medicines in Africa and Asia by local organizations using the low-cost GPHF Minilab. PLoS One 12: e0184165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rahman MS, Yoshida N, Tsuboi H, Karmoker JR, Kabir N, Schaefermann S, Akimoto Y, Bhuiyan MA, Reza MS, Kimura K, 2022. A comprehensive analysis of select medicines collected from private drug outlets of Dhaka City, Bangladesh in a simple random survey. Sci Rep 12: 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. OMS , 2020, Résistance aux antibiotiques. Available at: https://www.who.int/fr/news-room/fact-sheets/detail/antibiotic-resistance. Accessed September 21, 2021.
- 36. Tulkens PM, Van Bambeke F, Zinner SH, 2019. Profile of a novel anionic fluoroquinolone-delafloxacin. Clin Infect Dis 68: S213–S222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ching C, Zaman MH, 2021. Impact of ciprofloxacin impurities on bacterial growth, antibiotic resistance development and content assays. Lett Appl Microbiol 73: 220–228. [DOI] [PubMed] [Google Scholar]
- 38. Ching C, Zaman MH, 2021. Identification of multiple low-level resistance determinants and coselection of motility impairment upon sub-MIC ceftriaxone exposure in Escherichia coli. MSphere 6: e0077821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fasugba O, Gardner A, Mitchell BG, Mnatzaganian G, 2015. Ciprofloxacin resistance in community-and hospital-acquired Escherichia coli urinary tract infections: a systematic review and meta-analysis of observational studies. BMC Inf Dis 15: 545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lalani M, Kaur H, Mohammed N, Mailk N, Van Wyk A, Jan S, Kakar RM, Mojadidi KM, Tobie L, 2015. Substandard antimalarials available in Afghanistan: a case for assessing the quality of drugs in resource poor settings. Am J Trop Med Hyg 92: 51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Organization for Economic Cooperation and Development/EU Intellectual Property Office , 2020. Trade in Counterfeit Pharmaceutical Products, Illicit Trade. Paris, France: OECD Publ, 94. [Google Scholar]
- 42. Lehmann A, Katerere DR, Dressman J, 2018. Drug quality in South Africa: a field test. J Pharm Sci 107: 2720–2730. [DOI] [PubMed] [Google Scholar]
- 43. Essomba N, Adiogo D, Essome MJ, Lehman L, Coppieters Y, 2014. Habitudes d’approvisionnement en médicaments par les populations d’une ville semi-rurale au Cameroun. Health Sci Dis 15. Available at: https://www.hsd-fmsb.org/index.php/hsd/article/view/438. Accessed October 5, 2021. [Google Scholar]
- 44. Ekambi GAE, Ebongue CO, Penda C, Nga EN, Mpondo EM, Moukokoid CEE, 2019. Knowledge, practices and attitudes on antibiotics use in Cameroon: self-medication and prescription survey among children, adolescents and adults in private pharmacies. PLoS One 14: e0212875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chikowe I, Bliese SL, Lucas S, Lieberman M, 2018. Amoxicillin quality and selling practices in urban pharmacies and drug stores of Blantyre, Malawi. Am J Trop Med Hyg 99: 233–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.