Key Points
Question
What are the cost and cost-effectiveness of management strategies for treating eyes with moderate or more severe vision loss from diabetic macular edema (DME) over 2 years?
Findings
In this economic evaluation in 228 participants with DME, while the cost of aflibercept monotherapy was approximately $12 000 more than treating with bevacizumab first and then switching to aflibercept for suboptimal response, aflibercept monotherapy had an incremental cost-effectiveness ratio of $837 077 per quality-adjusted life-year gained compared with bevacizumab first.
Meaning
The findings suggest that for eyes with vision loss from DME, treating with bevacizumab first and then switching to aflibercept for suboptimal response may confer substantial cost savings on a societal level without sacrificing visual acuity gains.
This economic evaluation determines the cost-effectiveness of aflibercept monotherapy vs bevacizumab first, then aflibercept for suboptimal response, in treating patients with diabetic macular edema.
Abstract
Importance
The DRCR Retina Network Protocol AC showed no significant difference in visual acuity outcomes over 2 years between treatment with aflibercept monotherapy and bevacizumab first with switching to aflibercept for suboptimal response in treating diabetic macular edema (DME). Understanding the estimated cost and cost-effectiveness of these approaches is important.
Objective
To evaluate the cost and cost-effectiveness of aflibercept monotherapy vs bevacizumab-first strategies for DME treatment.
Design, Setting, and Participants
This economic evaluation was a preplanned secondary analysis of a US randomized clinical trial of participants aged 18 years or older with center-involved DME and best-corrected visual acuity of 20/50 to 20/320 enrolled from December 15, 2017, through November 25, 2019.
Interventions
Aflibercept monotherapy or bevacizumab first, switching to aflibercept in eyes with protocol-defined suboptimal response.
Main Outcomes and Measures
Between February and July 2022, the incremental cost-effectiveness ratio (ICER) in cost per quality-adjusted life-year (QALY) over 2 years was assessed. Efficacy and resource utilization data from the randomized clinical trial were used with health utility mapping from the literature and Medicare unit costs.
Results
This study included 228 participants (median age, 62 [range, 34-91 years; 116 [51%] female and 112 [49%] male; 44 [19%] Black or African American, 60 [26%] Hispanic or Latino, and 117 [51%] White) with 1 study eye. The aflibercept monotherapy group included 116 participants, and the bevacizumab-first group included 112, of whom 62.5% were eventually switched to aflibercept. Over 2 years, the cost of aflibercept monotherapy was $26 504 (95% CI, $24 796-$28 212) vs $13 929 (95% CI, $11 984-$15 874) for the bevacizumab-first group, a difference of $12 575 (95% CI, $9987-$15 163). The aflibercept monotherapy group gained 0.015 (95% CI, −0.011 to 0.041) QALYs using the better-seeing eye and had an ICER of $837 077 per QALY gained compared with the bevacizumab-first group. Aflibercept could be cost-effective with an ICER of $100 000 per QALY if the price per dose were $305 or less or the price of bevacizumab was $1307 per dose or more.
Conclusions and Relevance
Variability in individual needs will influence clinician and patient decisions about how to treat specific eyes with DME. While the bevacizumab-first group costs still averaged approximately $14 000 over 2 years, this approach, as used in this study, may confer substantial cost savings on a societal level without sacrificing visual acuity gains over 2 years compared with aflibercept monotherapy.
Introduction
Intravitreous anti–vascular endothelial growth factor (VEGF) injections are the standard treatment for eyes with vision loss from diabetic macular edema (DME).1 The most widely used anti-VEGF agents for DME are aflibercept (Regeneron); ranibizumab (Genentech/Roche); and bevacizumab (Genentech/Roche), which is used off label.1 The DRCR Retina Network Protocol T demonstrated that all 3 medications are highly effective in improving visual acuity for DME.2,3 However, in eyes with visual acuity of 20/50 or worse, the aflibercept group had better visual acuity through 2 years than the bevacizumab group, despite many eyes in the bevacizumab group achieving good visual outcomes.2
Based on 2022 Centers for Medicare & Medicaid Services reimbursement rates for dose and injection procedures, bevacizumab costs $182.06 per injection ($67.86 for the drug and $114.20 for the procedure), and aflibercept costs $1945.69 per injection ($1831.49 for the drug and $114.20 for the procedure).4,5 As a result of the cost difference, some insurance companies may require a patient to initiate DME treatment with bevacizumab and switch to another anti-VEGF agent if the clinical response is not adequate. Until recently, it was not known whether this strategy would compromise long-term visual acuity relative to giving aflibercept alone. Results from DRCR Retina Network Protocol AC have now demonstrated that in eyes with center-involved DME and starting visual acuity of 20/50 or worse,6 no clinically meaningful differences in visual acuity outcomes over 2 years exist between eyes treated with aflibercept monotherapy vs bevacizumab first, with eyes receiving aflibercept if needed (mean [SD] improvement in visual acuity over 2 years was 15.0 (8.5) letters in the aflibercept monotherapy group and 14.0 (8.8) letters in the bevacizumab-first group [P = .37]).6 During follow-up, aflibercept treatment was received in approximately 70% of eyes in the bevacizumab-first group. Given the respective costs of the treatments and the results of Protocol AC, it is important to evaluate the relative cost-effectiveness of these different management strategies for treating DME.
Methods
Participants
The DRCR Retina Network Protocol AC included 312 study eyes of 270 participants at 54 clinical sites in the US.7 Participants were enrolled between December 2017 and November 2019. The protocol and written informed consent form adhered to the tenets of the Declaration of Helsinki and were approved by the Jaeb Center for Health Research institutional review board. This economic evaluation study followed the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) reporting guideline.
Participant characteristics, including sex, age, self-reported race and ethnicity, and baseline visual acuity letter score, were collected. Participants received a $25 gift or money card at each nonannual study visit and a $100 gift or money card at annual visits. Participants with 1 study eye were randomly assigned with equal probability to 2.0-mg aflibercept monotherapy or 1.25-mg bevacizumab, with a switch to aflibercept when the eye had a suboptimal response. This report focuses on the 228 participants with only 1 study eye enrolled.
Detailed procedures, protocol, and statistical methods have been reported previously.6 In summary, participants were aged 18 years or older with center-involved DME on optical coherence tomography (OCT) and clinical examination and best-corrected visual acuity (Snellen fraction) of 20/50 to 20/320 (electronic Early Treatment Diabetic Retinopathy Study letter score 69-24) in the study eye. Participants were followed up every 4 weeks through 1 year and every 4 to 16 weeks in year 2. Anti-VEGF injections were given as frequently as monthly based on protocol-specific criteria.6 Eyes in the bevacizumab group were switched to aflibercept treatment starting at the 12-week visit if the eye had a suboptimal visual acuity and OCT thickness response based on predefined protocol criteria. A suboptimal response was defined as persistent center-involved DME for an eye that received bevacizumab at the previous 2 visits, without improvement of visual acuity by at least 5 letters and without a decrease in central subfield thickness of at least 10%, and visual acuity of 20/50 or worse before 24 weeks or 20/32 or worse at 24 weeks or later.
Costs
The cost for resource utilization during the trial included costs for the clinic visit, OCT, injection, and anti-VEGF agent. Participants incurred costs of OCT at each visit. If there was no injection, participants incurred costs for a level 3 visit (Current Procedural Terminology [CPT] code 99213), and if an injection were given, participants instead incurred the costs of injection administration (CPT code 67028) and injection dose. Injection costs were based on Medicare reimbursement of each anti-VEGF agent and Medicare physician fees for administration in an office-based setting (Table 1).8,9 Costs were measured in 2022 dollars, and costs in year 2 were discounted by 3% as is standard in cost-effectiveness analyses to account for the time value of money.10 Costs not expected to vary among the treatment arms were not captured, including systemic and ocular adverse events and other ocular procedures. Thus, this analysis provides an accurate estimate of incremental costs between treatment strategies but not of overall medical costs associated with DME.
Table 1. Cost for Resource Utilization During the Trial and Mapping From Visual Acuity to Utility.
| Parameter | Base value (range) | Distributiona | Notes |
|---|---|---|---|
| Costs, $ | |||
| OCT (for all visits) | 41.18 (30.89-51.48) | Normal | CPT code 92134 |
| Injection procedure (for visits with injection) | 114.20 (85.65-142.75) | Normal | CPT code 67028 |
| Aflibercept, 2 mg | 1831.49 (1373.62-2289.37) | Normal | CPT code J0178 |
| Bevacizumab | 67.86 (50.9-84.83) | Normal | CPT code J9035 |
| Annual discount rate, % | 3 (0-7) | Normal | NA |
| Utilities | |||
| Visual acuity letter score in better-seeing eye | |||
| 100 (20/10) | 0.97 (0.95-1.00) | Correlated normal | Brown et al,82003 |
| 83-99 (20/25-20/10) | 0.92 (0.87-0.97) | Correlated normal | NA |
| 79-82 (20/25) | 0.87 (0.82-0.92) | Correlated normal | NA |
| 74-78 (20/32) | 0.84 (0.79-0.89) | Correlated normal | NA |
| 68-73 (20/50-20/40) | 0.80 (0.74-0.86) | Correlated normal | NA |
| 62-67 (20/63-20/50) | 0.77 (0.7-0.84) | Correlated normal | NA |
| 55-61 (20/80-20/63) | 0.74 (0.67-0.81) | Correlated normal | NA |
| 43-54 (20/160-20/80) | 0.67 (0.57-0.77) | Correlated normal | NA |
| 31-42 (20/250-20/160) | 0.66 (0.55-0.77) | Correlated normal | NA |
| 24-30 (20/320-20/250) | 0.63 (0.54-0.72) | Correlated normal | NA |
| 19-23 (20/400) | 0.54 (0.43-0.65) | Correlated normal | NA |
| 1-18 (<20/800-20/500) | 0.52 (0.36-0.68) | Correlated normal | NA |
| 0 | 0.35 (0.1-0.60) | Correlated normal | NA |
| Visual acuity letter score in treated eye (sensitivity analysis) | NA | ||
| 86-100 (20/20-20/10) | 0.86 (0.793-0.927) | Correlated normal | RESTORE; Mitchell et al,9 2012 |
| 76-85 (20/32-20/20) | 0.86 (0.833-0.887) | Correlated normal | NA |
| 66-75 (20/50-20/32) | 0.813 (0.789-0.837) | Correlated normal | NA |
| 56-65 (20/80-20/50) | 0.802 (0.775-0.829) | Correlated normal | NA |
| 46-55 (20/125-20/80) | 0.770 (0.735-0.805) | Correlated normal | NA |
| 36-45 (20/200-20/125) | 0.760 (0.707-0.813) | Correlated normal | NA |
| 26-35 (20/320-20/200) | 0.681 (0.577-0.785) | Correlated normal | NA |
| 0-25 (<20/800-20/320) | 0.547 (0.384-0.71) | Correlated normal | NA |
Abbreviations: CPT, Current Procedural Terminology; NA, not applicable; OCT, optical coherence tomography.
Correlated normal indicates that the utilities drawn for the Monte Carlo simulation have a correlation of 0.75 between adjacent visual acuity levels.
Health Utility
Participant visual acuity scores at each visit were converted to quality-adjusted life-years (QALYs) using data from Brown et al.8 This approach uses the visual acuity from the participant’s better-seeing eye at each visit and links the acuity to a health-related quality-of-life level. Quality-of-life levels during the first and second year were separately averaged into 2 QALY values, and the sum of the QALYs in year 1 and the discounted QALYs in year 2 provided an aggregate QALY value for the entire study for each participant. Since the visual acuity scores were similar at the end of the 2 years and prior studies have demonstrated that visual acuity remains stable on average beyond 2 years,11,12,13 we did not simulate or project outcomes beyond 2 years.
Cost-effectiveness
The evaluation of costs and health outcomes were from a health system perspective. The incremental cost-effectiveness ratio (ICER) was calculated by dividing the incremental cost of the 2 treatments by the incremental QALYs gained.
Sensitivity Analysis
To assess robustness of the results and explore how different assumptions might affect cost-effectiveness of the therapies, several sensitivity analyses were performed. In a univariable sensitivity analysis, each parameter was varied, 1 at a time, and the overall ICER results assessed. In the base case analysis, quality of life was mapped to visual acuity in the participant’s better-seeing eye; a sensitivity analysis used data from the UK-based RESTORE trial of anti-VEGF therapy for DME to map quality of life to visual acuity in the participant’s treated eye (whether it was the better- or worse-seeing eye).9 The associations of varying the utility values and costs were assessed and are displayed as tornado plots. The costs of aflibercept and bevacizumab were varied to determine the price that would yield an ICER less than $100 000 per QALY, a threshold commonly considered meaningful for determining cost-effectiveness in the US.14,15,16,17,18
Assessment of overall uncertainty in cost-effectiveness was conducted using Monte Carlo simulation in a probabilistic sensitivity analysis. First, synthetic trial treatment groups were created using bootstrapping by randomly drawing participants from each trial arm, with replacement; values for model parameters, including per-unit costs and mapping of visual acuity to quality of life, were drawn at random from distributions reflecting their uncertainty (Table 1). This process was repeated 250 times, with cost-effectiveness results calculated for each iteration to obtain a distribution of probabilities for each treatment strategy to be cost-effective at different societal willingness-to-pay values per QALY.
Statistical Analysis
The protocol prespecified that an economic analysis would be performed. For these analyses, visual acuity assessed every 4 weeks during the first year and every 4 months during the second year were included. Forty-nine percent of participants were missing at least 1 visual acuity measurement at some point during the study, but most of these were brief, with an overall 12% of measurements missing. Missing visual acuity scores were interpolated between adjacent assessments or carried forward from the last observed assessment if no final assessment was available. Between February and July 2022, 2-tailed t tests were used to assess the cost and quality-of-life differences in 2-year data from the trial. Calculated P values and 95% CIs for cost and quality-of-life differences reflect participant-level variation from trial data but do not account for uncertainty in unit cost or in the mapping of visual acuity to quality of life from outside sources. The threshold for significance was set at a 2-sided P < .05. Statistical analyses were performed using Microsoft Excel, version 365 software (Microsoft Corporation).
Results
Between December 15, 2017, and November 25, 2019, 228 participants with 1 study eye were randomly assigned to aflibercept monotherapy (116 eyes) vs bevacizumab first (112 eyes). Median age was 62 years (range, 34-91 years). The sample included 116 women (51%) and 112 men (49%). Forty-four participants were Black or African American (19%), 60 Hispanic or Latino (26%), 117 White (51%), and 7 of other race or ethnicity (including Asian, Native Hawaiian or other Pacific Islander, multiple races, or unknown/not reported). The median baseline visual acuity letter score was 61 (range, 68-25; approximate Snellen equivalent 20/63), and mean baseline OCT central subfield thickness was 504 μm (95% CI, 487-521 μm). Excluding 12 deaths, the 2-year completion rate was 89% (192 of 216).
The mean number of visits completed over 2 years was 19.9 (95% CI, 18.8-21.0 visits) in the aflibercept monotherapy group and 20.4 (95% CI, 19.3-21.6 visits) in the bevacizumab-first group. Over 2 years, the aflibercept monotherapy group received a mean of 13.0 injections (95% CI, 12.1-13.9 injections), while the bevacizumab-first group received 14.7 injections (95% CI, 13.8-15.7 injections) of either bevacizumab or aflibercept. During the 2-year follow-up, 70 eyes (62.5%) in the bevacizumab-first group received aflibercept. Over 2 years, eyes in the bevacizumab-first group received a mean of 9.0 injections (95% CI, 8.0-9.9 injections) and 5.7 injections (95% CI, 4.7-6.8 injections) of aflibercept.
Health-Related Quality of Life
The mean visual acuity letter score in the aflibercept monotherapy group was 58.8 (20/63) (95% CI, 56.8-60.1) at baseline and 72.4 (20/40) (95% CI, 69.7-75.2) at 2 years (mean change, 13.9; 95% CI, 11.3-16.6) in the study eye and 76.0 (20/32) (95% CI, 74.1-77.9) at baseline and 80.5 (20/25) (95% CI, 78.6-82.3) at 2 years (mean change, 4.4; 95% CI, 2.9-6.0) in the better-seeing eye. The mean visual acuity letter score in the bevacizumab-first group was 57.2 (20/80) (95% CI, 55.3-59.1) at baseline and 72.7 (20/40) (95% CI, 70.1-75.2) at 2 years (mean change, 15.4; 95% CI, 13.7-17.7) in the study eye and 74.8 (20/32) (95% CI, 72.8-76.8) at baseline and 79.9 (20/25) (95% CI, 78.1-81.6) at 2 years (mean change, 5.1; 95% CI, 3.2-6.9) in the better-seeing eye. In the aflibercept monotherapy group, the study eye was the better-seeing eye for 14 participants (12.1%) at baseline and 14 participants (36.2%) at 2 years, while in the bevacizumab group, these numbers were 14 (12.5%) and 46 (41.1%), respectively.
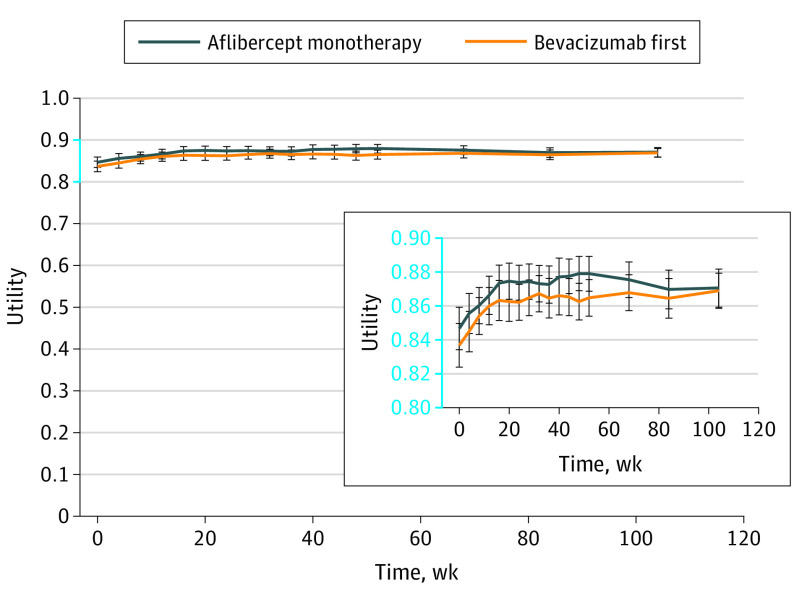
The utility was similar between the 2 groups, where there were no statistically significant differences observed between treatment groups at any point (Figure 1). Results were similar using the RESTORE approach to mapping visual acuity of the study eye to QALYs9 (eFigure 1 in Supplement 1).
Figure 1. Utility Over Time.
Quality of life was mapped to visual acuity letter score in the participant’s better-seeing eye at each visit using data from Brown et al,8 with a more detailed view shown in the inset. Error bars represent the 95% CIs.
Cost-effectiveness Results
Although the bevacizumab-first group had more total injections over the 2-year period than the aflibercept monotherapy group (mean [SD], 14.7 [5.1] vs 13.0 [4.8]; P = .01), it had fewer aflibercept injections (mean [SD], 5.7 [5.7] vs 13.0 [4.8]; P < .001). Accounting for the number and types of anti-VEGF treatments and number of visits, the aflibercept monotherapy group cost per participant over 2 years was $26 504 (95% CI, $24 796-$28 212) compared with the bevacizumab-first group costs of $13 929 (95% CI, $11 984-$15 874), a savings in the bevacizumab-first group of $12 575 (95% CI, $9987-$15 163). Given the higher cost but 0.015 (95% CI, −0.011 to 0.041) increase in QALYs in the aflibercept monotherapy group, the ICER was $837 077 per QALY gained (Table 2). The RESTORE approach using utility based on the treated eye led to a similar result with an even greater ICER of $1 090 496 per QALY gained (Table 2).
Table 2. Cost-effectiveness Results.
| Study group | Mean (95% CI) | Incremental | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Costs, $ | 2-y Results | Mean (95% CI) | ICER, $ | |||||||
| Visit | OCT | Injection procedure | Aflibercept drug | Bevacizumab drug | Total costs, $ | QALYs | Total costs, $ | QALYs | ||
| Brown et al8 utility mapping method based on the better-seeing eye | ||||||||||
| Bevacizumab first | 519 (462 to 577) | 832 (786 to 878) | 1663 (1557 to 1770) | 10 308 (8406 to 12 210) | 606 (543 to 670) | 13 929 (11 984 to 15 874) | 1.704 (1.685 to 1.722) | NA | NA | NA |
| Aflibercept monotherapy | 625 (557 to 693) | 810 (764 to 856) | 1471 (1373 to 1570) | 23 597 (22 023 to 25 172) | 0 (0 to 0) | 26 504 (24 796 to 28 212) | 1.719 (1.7 to 1.737) | 12 575 (9987 to 15 163) | 0.015 (−0.011 to 0.041) | 837 077 |
| RESTORE9 utility mapping method based on the treated eye | ||||||||||
| Bevacizumab first | 519 (462 to 577) | 832 (786 to 878) | 1663 (1557 to 1770) | 10 308 (8406 to 12 210) | 606 (543 to 670) | 13 929 (11 984 to 15 874) | 1.615 (1.602 to 1.628) | NA | NA | NA |
| Aflibercept monotherapy | 625 (557 to 693) | 810 (764 to 856) | 1471 (1373 to 1570) | 23 597 (22 023 to 25 172) | 0 (0 to 0) | 26 504 (24 796 to 28 212) | 1.627 (1.616 to 1.638) | 12 575 (9987 to 15 163) | 0.012 (−0.005 to 0.028) | 1 090 496 |
Abbreviations: ICER, incremental cost-effectiveness ratio; NA, not applicable; OCT, optical coherence tomography; QALY, quality-adjusted life-year.
Sensitivity Analysis
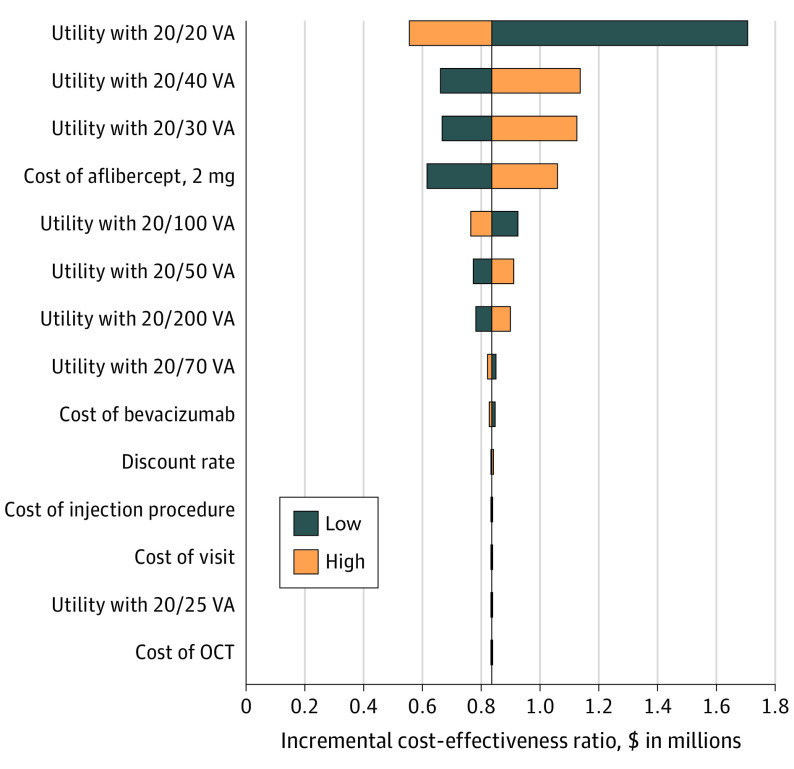
Findings from the 1-way sensitivity analysis suggested that varying assumptions about the utilities and costs from a high range to a low range, as detailed in Table 1, may moderately change the results, but the ICER still would not drop below $100 000 per QALY (Figure 2). Sensitivity results using the RESTORE approach using utilities based on the treated eye similarly showed that the ICER did not drop below $100 000 per QALY when varying assumptions (eFigure 2 in Supplement 1).
Figure 2. Tornado Diagram of 1-Way Sensitivity Analysis.
The diagram shows how the incremental cost-effectiveness ratio on the horizontal axis varies as the individual parameter assumptions (on the vertical axis) vary between the high and low ranges (shown in Table 1). Quality of life was mapped to visual acuity (VA) letter score in the participant’s better-seeing eye at each visit using data from Brown et al.8 OCT indicates optical coherence tomography.
Threshold Cost
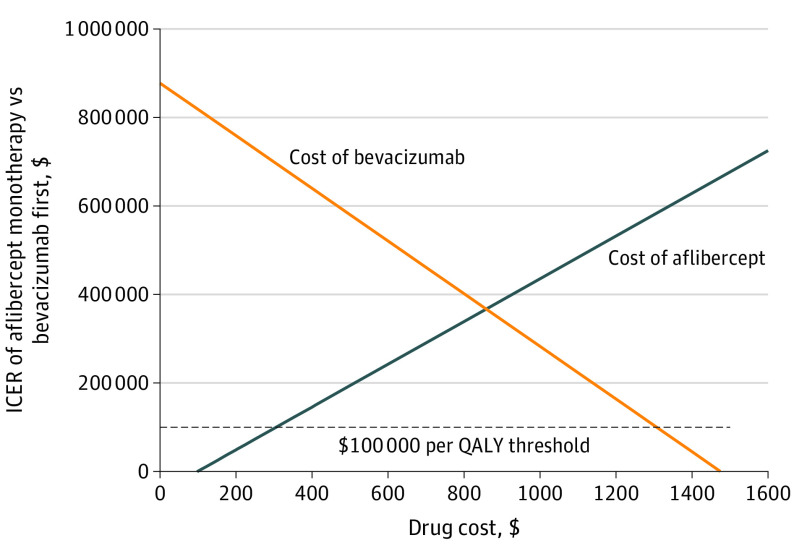
Figure 3 shows the ICER for varying drug costs separately for aflibercept and bevacizumab based on utilities from the Brown et al8 method on the better-seeing eye. The cost per dose of aflibercept would have to drop to $305 (from $1831.49) for aflibercept to have an ICER of $100 000 per QALY (Figure 3). Conversely, if the bevacizumab costs were to increase to $1307 (from $67.86), the aflibercept ICER would be $100 000 per QALY. Thresholds using the RESTORE method for the study eye are presented in eFigure 3 in Supplement 1.
Figure 3. Sensitivity to Cost of Treatment per Dose.
The lines show the incremental cost-effectiveness ratio (ICER) of aflibercept vs bevacizumab first (vertical axis) at the varying costs of aflibercept and bevacizumab (horizontal axis). An ICER less than $100 000 per quality-adjusted life-year (QALY) is commonly considered meaningful for determining cost-effectiveness in the US.14,15,16,17,18 Quality of life was mapped to visual acuity letter score in the participant’s better-seeing eye at each visit using data from Brown et al.8
Probabilistic Sensitivity Analysis
The probabilistic sensitivity analysis results showed that aflibercept monotherapy would not be cost-effective compared with a bevacizumab-first strategy at the 2022 Medicare rate. There is essentially a 0% chance that aflibercept monotherapy would be cost-effective at a willingness to pay below $200 000 per QALY gained (eFigures 4 and 5 in Supplement 1). Willingness to pay per QALY gained has to be more than $850 000 for aflibercept monotherapy to be more than 50% likely to be cost-effective.
Discussion
This economic evaluation using a cost-effectiveness analysis found that an aflibercept monotherapy treatment approach is not cost-effective compared with a bevacizumab-first approach for eyes with moderate to severe vision loss from center-involved DME. The health-related quality-of-life gains that favored aflibercept monotherapy in the first year were small and not statistically significant. Over 2 years, the average cost of aflibercept monotherapy was approximately $26 500 compared with approximately $14 000 for the bevacizumab-first group. This increased cost was substantial at more than $12 500 per participant over 2 years.
Even though eyes in the bevacizumab-first group received, on average, 1.7 more injections than their aflibercept monotherapy counterparts over 2 years and the majority (62.5%) of participants in the bevacizumab-first group eventually transitioned to aflibercept, the findings suggest that the initiation of therapy with bevacizumab with a switch to aflibercept when visual and structural improvement is suboptimal is cost-effective. Aflibercept monotherapy may be cost-effective if the per-dose price drops to $300 or less or if the bevacizumab price increases to $1307 per dose. We estimate that more than 50 000 individuals are treated with anti-VEGF therapy for center-involved DME with visual acuity of 20/50 or worse each year in the US. At 2022 prices and conservatively assuming that each patient requires unilateral rather than bilateral treatment, for every 10 000 new patients starting therapy for DME, we estimate a cost savings of more than $125 million with a bevacizumab-first vs aflibercept monotherapy treatment strategy.19
This analysis is based solely on the treatment numbers and visual acuity results from 2 years of follow-up within DRCR Retina Network Protocol AC. It is possible that the significant difference in cost-effectiveness favoring the bevacizumab-first group might be reduced over time if eyes treated with this approach needed far more injections than eyes treated with aflibercept monotherapy. However, previous studies have demonstrated that in general, there is a rapidly declining need for treatment after the first year of therapy in eyes receiving anti-VEGF for DME.11,12 A reduced need for treatment between years 1 and 2 was present in eyes treated with bevacizumab monotherapy in DRCR Retina Network Protocol T. Thus, eyes in the bevacizumab-first group may not have a substantially increased need for injections after the first 2 years of treatment. Similarly, visual outcomes in eyes with DME are generally stable after the first year of treatment, suggesting that the differences in vision between the aflibercept monotherapy and bevacizumab-first groups would not increase dramatically after the first 2 years of follow-up. In both Protocol T and Protocol AC, the aflibercept-only strategy was not cost-effective compared with a strategy that includes bevacizumab either as monotherapy as in Protocol T or as the first therapy as in Protocol AC.20
Limitations
This study has several limitations. First, the results are based on a single trial. However, the cost differences between the aflibercept monotherapy and bevacizumab-first approaches are large, and the visual acuity outcomes with these 2 approaches were similar. Thus, the findings from this study appear robust. Second, we based costs on Medicare reimbursement values. The costs of individual medications might vary with substantially lower negotiated rates for aflibercept. Our sensitivity analysis findings show what the price would have to be for aflibercept to be cost-effective under different payment systems. Third, this study is based on 2 years of outcomes; therefore, it did not evaluate for potential longer-term outcomes. However, at the end of the 2-year period, visual acuity outcomes were similar between groups. Fourth, in the base case, we converted visual acuity letter scores to QALYs using data from Brown et al,8 who evaluated quality of life in patients with neovascular age-related macular degeneration, which may or may not be a good proxy for patients with DME. However, in sensitivity analysis, we also used a conversion from the RESTORE study in patients with DME.9 In addition, although the study did not obtain detailed treatment information on the nonstudy eye and the primary analyses were conducted using utilities based on visual acuity from the better-seeing eye, the sensitivity analysis based on the study eye resulted in similar findings. Fifth, the switching criteria used are just 1 approach developed by consensus of DRCR Retina Network investigators. It is unknown whether criteria that would have tolerated more intraretinal fluid or reduced vision for a longer period or a different retreatment regimen would have led to different results. These results only apply to the anti-VEGF agents evaluated in this trial.
Conclusions
Variability in individual needs will influence clinician and patient decisions about how to treat specific eyes with center-involved DME. Although the bevacizumab-first group costs still averaged approximately $14 000 over 2 years, this approach, as used in this economic evaluation in which 62.5% of this group eventually switched to aflibercept, may confer substantial cost savings on a societal level without sacrificing visual acuity gains over 2 years compared with aflibercept monotherapy for treatment of center-involved DME.
eFigure 1. Utility Over Time Using Ranibizumab Monotherapy or Combined With Laser Vs Laser Monotherapy for Diabetic Macular Edema (RESTORE) Utility Mapping Based on the Treated Eye
eFigure 2. Tornado Diagram Using Ranibizumab Monotherapy or Combined With Laser Vs Laser Monotherapy for Diabetic Macular Edema Utility Mapping Based on the Treated Eye
eFigure 3. Sensitivity to Cost of Treatment Per Dose Using Ranibizumab Monotherapy or Combined With Laser Vs Laser Monotherapy for Diabetic Macular Edema Utility Mapping Based on the Treated Eye
eFigure 4. Cost-Effectiveness Acceptability Curves
eFigure 5. Cost-Effectiveness Acceptability Curves Using Ranibizumab Monotherapy or Combined With Laser Vs Laser Monotherapy for Diabetic Macular Edema Utility Mapping Based on the Treated Eye
eReferences
Nonauthor Collaborators
Data Sharing Statement
References
- 1.American Society of Retina Specialists. Preferences and Trends (PAT) survey. 2021. Accessed August 15, 2022. https://www.asrs.org/asrs-community/pat-survey/pat-survey
- 2.Wells JA, Glassman AR, Ayala AR, et al. ; Diabetic Retinopathy Clinical Research Network . Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema: two-year results from a comparative effectiveness randomized clinical trial. Ophthalmology. 2016;123(6):1351-1359. doi: 10.1016/j.ophtha.2016.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wells JA, Glassman AR, Ayala AR, et al. ; Diabetic Retinopathy Clinical Research Network . Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372(13):1193-1203. doi: 10.1056/NEJMoa1414264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Medicare & Medicaid Services. 2022 ASP drug pricing files. 2022. Accessed February 24, 2022. https://www.cms.gov/medicare/medicare-part-b-drug-average-sales-price/2022-asp-drug-pricing-files
- 5.Centers for Medicare & Medicaid Services. RVU22A. 2022. Accessed August 15, 2022. https://www.cms.gov/medicaremedicare-fee-service-paymentphysicianfeeschedpfs-relative-value-files/rvu22a
- 6.Jhaveri CD, Glassman AR, Ferris FL III, et al. ; DRCR Retina Network . Aflibercept monotherapy or bevacizumab first for diabetic macular edema. N Engl J Med. 2022;387(8):692-703. doi: 10.1056/NEJMoa2204225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DRCR.Net aflibercept vs. bevacizumab + deferred aflibercept for the treatment of CI-DME (DRCR AC). ClinicalTrials.gov identifier: NCT03321513. Updated December 1, 2022. Accessed August 15, 2022. https://www.clinicaltrials.gov/ct2/show/NCT03321513
- 8.Brown MM, Brown GC, Sharma S, Landy J. Health care economic analyses and value-based medicine. Surv Ophthalmol. 2003;48(2):204-223. doi: 10.1016/S0039-6257(02)00457-5 [DOI] [PubMed] [Google Scholar]
- 9.Mitchell P, Annemans L, Gallagher M, et al. Cost-effectiveness of ranibizumab in treatment of diabetic macular oedema (DME) causing visual impairment: evidence from the RESTORE trial. Br J Ophthalmol. 2012;96(5):688-693. doi: 10.1136/bjophthalmol-2011-300726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gold M. Panel on cost-effectiveness in health and medicine. Med Care. 1996;34(12)(suppl):DS197-DS199. [PubMed] [Google Scholar]
- 11.Elman MJ, Ayala A, Bressler NM, et al. ; Diabetic Retinopathy Clinical Research Network . Intravitreal ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: 5-year randomized trial results. Ophthalmology. 2015;122(2):375-381. doi: 10.1016/j.ophtha.2014.08.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyer DS, Nguyen QD, Brown DM, Basu K, Ehrlich JS; RIDE and RISE Research Group . Outcomes with as-needed ranibizumab after initial monthly therapy: long-term outcomes of the phase III RIDE and RISE trials. Ophthalmology. 2015;122(12):2504-2513.e1. doi: 10.1016/j.ophtha.2015.08.006 [DOI] [PubMed] [Google Scholar]
- 13.Heier JS, Korobelnik JF, Brown DM, et al. Intravitreal aflibercept for diabetic macular edema: 148-week results from the VISTA and VIVID Studies. Ophthalmology. 2016;123(11):2376-2385. doi: 10.1016/j.ophtha.2016.07.032 [DOI] [PubMed] [Google Scholar]
- 14.Grosse SD. Assessing cost-effectiveness in healthcare: history of the $50,000 per QALY threshold. Expert Rev Pharmacoecon Outcomes Res. 2008;8(2):165-178. doi: 10.1586/14737167.8.2.165 [DOI] [PubMed] [Google Scholar]
- 15.Anderson JL, Heidenreich PA, Barnett PG, et al. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(21):2304-2322. doi: 10.1016/j.jacc.2014.03.016 [DOI] [PubMed] [Google Scholar]
- 16.Hutton D, Newman-Casey PA, Tavag M, Zacks D, Stein J. Switching to less expensive blindness drug could save Medicare part B $18 billion over a ten-year period. Health Aff (Millwood). 2014;33(6):931-939. doi: 10.1377/hlthaff.2013.0832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness–the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371(9):796-797. doi: 10.1056/NEJMp1405158 [DOI] [PubMed] [Google Scholar]
- 18.Ubel PA, Hirth RA, Chernew ME, Fendrick AM. What is the price of life and why doesn’t it increase at the rate of inflation? Arch Intern Med. 2003;163(14):1637-1641. doi: 10.1001/archinte.163.14.1637 [DOI] [PubMed] [Google Scholar]
- 19.Varma R, Bressler NM, Doan QV, et al. Diabetic population-based model to estimate impact of ranibizumab on diabetic retinopathy severity in patients with diabetic macular edema. Clin Ophthalmol. 2020;14:1249-1259. doi: 10.2147/OPTH.S236636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ross EL, Hutton DW, Stein JD, Bressler NM, Jampol LM, Glassman AR; Diabetic Retinopathy Clinical Research Network . Cost-effectiveness of aflibercept, bevacizumab, and ranibizumab for diabetic macular edema treatment: analysis from the Diabetic Retinopathy Clinical Research Network Comparative Effectiveness Trial. JAMA Ophthalmol. 2016;134(8):888-896. doi: 10.1001/jamaophthalmol.2016.1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Utility Over Time Using Ranibizumab Monotherapy or Combined With Laser Vs Laser Monotherapy for Diabetic Macular Edema (RESTORE) Utility Mapping Based on the Treated Eye
eFigure 2. Tornado Diagram Using Ranibizumab Monotherapy or Combined With Laser Vs Laser Monotherapy for Diabetic Macular Edema Utility Mapping Based on the Treated Eye
eFigure 3. Sensitivity to Cost of Treatment Per Dose Using Ranibizumab Monotherapy or Combined With Laser Vs Laser Monotherapy for Diabetic Macular Edema Utility Mapping Based on the Treated Eye
eFigure 4. Cost-Effectiveness Acceptability Curves
eFigure 5. Cost-Effectiveness Acceptability Curves Using Ranibizumab Monotherapy or Combined With Laser Vs Laser Monotherapy for Diabetic Macular Edema Utility Mapping Based on the Treated Eye
eReferences
Nonauthor Collaborators
Data Sharing Statement