Abstract
Antiplatelet therapy is key to reducing local thrombotic complications and systemic ischaemic events among patients undergoing percutaneous coronary interventions (PCI), but it is inevitably associated with increased bleeding. The continuous refinement in stent technologies, together with the high incidence of ischaemic recurrences after PCI and the understanding of prognostic implications associated with bleeding, have led to a substantial evolution in antiplatelet treatment regimens over the past decades. Numerous investigations have been conducted to better stratify patients undergoing PCI according to their ischaemic and bleeding risks and to implement antithrombotic regimens accordingly. Evidence from these investigations have resulted in a number of antithrombotic treatment options as recommended by recent guidelines. In this State-of-the-Art review we provide the rationale, summarise the evidence, and discuss current and future directions of antiplatelet treatment regimens after PCI.
Introduction
In patients with coronary artery disease (CAD), percutaneous coronary interventions (PCI) are the cornerstone of treatment for those presenting with an acute coronary syndrome (ACS); PCI has also been largely adopted in patients with chronic coronary syndromes (CCS)1. Adjunctive pharmacotherapy, in particular antithrombotic therapy, has a pivotal role in optimising outcomes in patients undergoing PCI2,3. In fact, patients undergoing PCI may develop both acute and long-term ischaemic events4. Therefore, antithrombotic drugs, in particular antiplatelet agents, are key to the treatment and prevention of both local and systemic thrombotic complications2,3. Over the past four decades there has been an evolution in antiplatelet treatment regimens being used in patients undergoing PCI2. This is attributed to a number of factors including refinement in stent technologies, leading to safer (i.e., less thrombogenic) stent platforms, the development of new antiplatelet drugs, as well as an understanding of the prognostic implications associated with bleeding, the most feared trade-off associated with the use of antiplatelet therapies2,5. Moreover, numerous investigations have also helped to develop a profile of patients at increased risk of ischaemic and bleeding complications2. These profiles, paralleled with an understanding of how individuals may have variable reactions to specific antiplatelet agents, have also laid the foundation for personalised treatment regimens with the goal of optimising efficacy and safety outcomes6. In this State-of-the-Art review, we provide the rationale, discuss the evidence and summarise the current and future directions of antiplatelet treatment regimens after PCI, with a specific focus on oral antiplatelet agents.
Rationale for the use of antiplatelet therapy in patients undergoing percutaneous coronary intervention
Arterial thrombus formation is a complex and dynamic pathological process that is initiated within an injured blood vessel wall or by contact activation on a foreign surface7. Platelets play a key role in thrombus formation and their initial tethering is mediated by the interaction between the complex glycoprotein (GP) Ib-IX-V and Von Willebrand factor and by collagen receptors present on the platelet surface such as GPVI (Figure 1)7,8,9. The crosstalk between platelets, the haemostatic system and inflammatory pathways are key to atherosclerotic development and thrombotic complications occurring after plaque destabilisation10,11,12,13,14,15,16. In ACS patients, when plaque rupture or erosion occurs, the activation of coagulation cascade and platelets can lead to either subocclusive or occlusive thrombosis, causing a symptomatic event. Alternatively, a plaque healing phenomenon occurs when thrombus formation is contained; repeated cycles of this process promote disease progression among patients with CCS (Figure 1)14,17,18. Therefore, the rationale for using antiplatelet agents in patients with CAD is not only for the treatment of acute thrombotic events, but also to modulate the progression of atherosclerotic disease14,17,18.
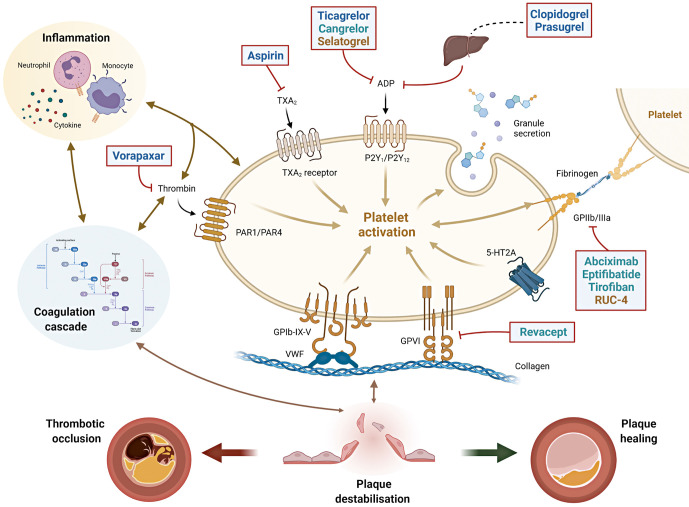
Figure 1. Interplay between platelets, coagulation and inflammation in atherothrombosis and sites of action of antiplatelet agents.
The interplay between platelets, coagulation and inflammation is a key modulator of atherosclerosis and its thrombotic complications. When plaque destabilisation occurs, due to plaque rupture or erosion, platelets and coagulation factors, as well as subsequent inflammatory processes, it may lead to either a thrombotic occlusion of the coronary artery (leading to acute coronary syndromes) or plaque healing (favouring plaque progression with stable coronary artery disease). Initial platelet tethering is mediated by the interaction between the complex glycoprotein (GP) Ib-IX-V and Von Willebrand factor (vWF) and by other collagen receptors present on the platelet surface such as GPVI. Thrombin is a key linking factor between platelet and coagulation cascade. Sites of action of common antiplatelet agents are reported: aspirin inhibits thromboxane A2 (TXA2); clopidogrel, prasugrel, ticagrelor and cangrelor are inhibitors of the ADP P2Y12 receptor. Clopidogrel and prasugrel require hepatic activation. Vorapaxar is a thrombin receptor inhibitor (protease-activated receptor, PAR-1); abciximab, eptifibatide, tirofiban and RUC-4 are GPIIb/IIIa receptor inhibitors. Revacept is a competitive antagonist of the collagen-GPVI signalling. Routes of administration: Blue=oral; Green=intravenous; Yellow=subcutaneous/intramuscular. ADP: adenosine diphosphate; GP: glycoprotein; PAR-1: platelet protease-activated receptor-1; TXA2: thromboxane A2; vWF: von Willebrand factor; 5HT2A: serotonine receptor.
Antiplatelet therapy reduces thrombotic-related periprocedural myocardial damage associated with blood vessel wall trauma such as dissections or plaque ruptures, embolisation or side branch occlusions. In addition, it decreases the risk of stent thrombosis (ST), which is more frequent in the acute or subacute phase of PCI (Figure 2)19,20,21. Importantly, peri-PCI thrombotic complications impact long-term prognosis, to the extent that their occurrence has challenged the long-term clinical benefit of PCI as compared to medical therapy among patients with CCS22,23. Intravenous antiplatelet agents, including glycoprotein IIb/IIIa inhibitors (GPIs) and cangrelor, reduce the risk of peri-PCI thrombotic complications24. A detailed description of intravenous antiplatelet agents goes beyond the scope of this manuscript and is summarised elsewhere24.
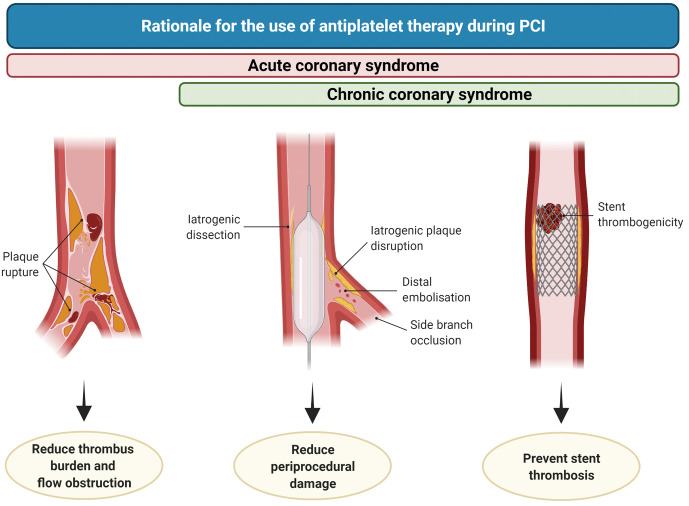
Figure 2. Rationale for the use of antiplatelet therapy during PCI.
In patients undergoing PCI in the setting of chronic coronary syndrome (CCS), antiplatelet therapy reduces the occurrence of intraprocedural or very early stent thrombosis (right), periprocedural damage caused by iatrogenic dissections, plaque disruption, distal embolisation or side branch occlusion (middle). During acute coronary syndrome, antiplatelet therapy also plays a role in reducing thrombus burden and flow obstruction (left). PCI: percutaneous coronary intervention.
Oral antiplatelet agents are essential to both short- and long-term management after PCI2,3. Aspirin is an irreversible inhibitor of platelet cyclooxygenase (COX)-1 and traditionally has been a backbone treatment for patients with atherosclerotic disease manifestations25. In patients undergoing PCI, the association of aspirin plus a P2Y12 inhibitor, a strategy known as dual antiplatelet therapy (DAPT), has represented the cornerstone of treatment for patients undergoing PCI2,3. The clinical development of DAPT is based on investigations showing that the adjunctive use of a P2Y12 inhibitor with aspirin is associated with synergistic platelet inhibitory effects, resulting in improved antithrombotic efficacy in the setting of ACS and patients undergoing PCI26,27,28,29,30,31. Ticlopidine, a first-generation thienopyridine, was characterised by several drawbacks (i.e., bone marrow suppression) and replaced by clopidogrel, a second-generation thienopyridine, due to its more favourable safety profile30. Clopidogrel remains the most widely studied P2Y12 inhibitor. The key role of platelet P2Y12 signalling in platelet activation and amplification processes explains why a blockade of this pathway in patients undergoing PCI results not only in a reduction of acute, local, thrombotic events (i.e., ST) but also prevents long-term ischaemic recurrences due to atherosclerotic plaque progression and destabilisation both in the coronary and extra-coronary vasculature9.
Despite its undisputed benefits, several investigations have revealed heterogeneity in individual response profiles to clopidogrel, with a considerable number of patients yielding inadequate platelet inhibitory effects, resulting in increased risk of thrombotic events post-PCI6,32,33. These observations have prompted the development of newer-generation oral P2Y12 inhibitors including prasugrel, a third-generation thienopyridine, and ticagrelor, a first-in-class cyclopentyltriazolopyrimidine, characterised by potent and predictable antiplatelet effects resulting in greater antithrombotic efficacy compared to clopidogrel in the setting of ACS, albeit at the expense of increased bleeding34. The time course of benefit and risk associated with DAPT in patients undergoing PCI are summarised in Figure 3.
Figure 3. Time course of benefit and risk of antiplatelet therapy after PCI.
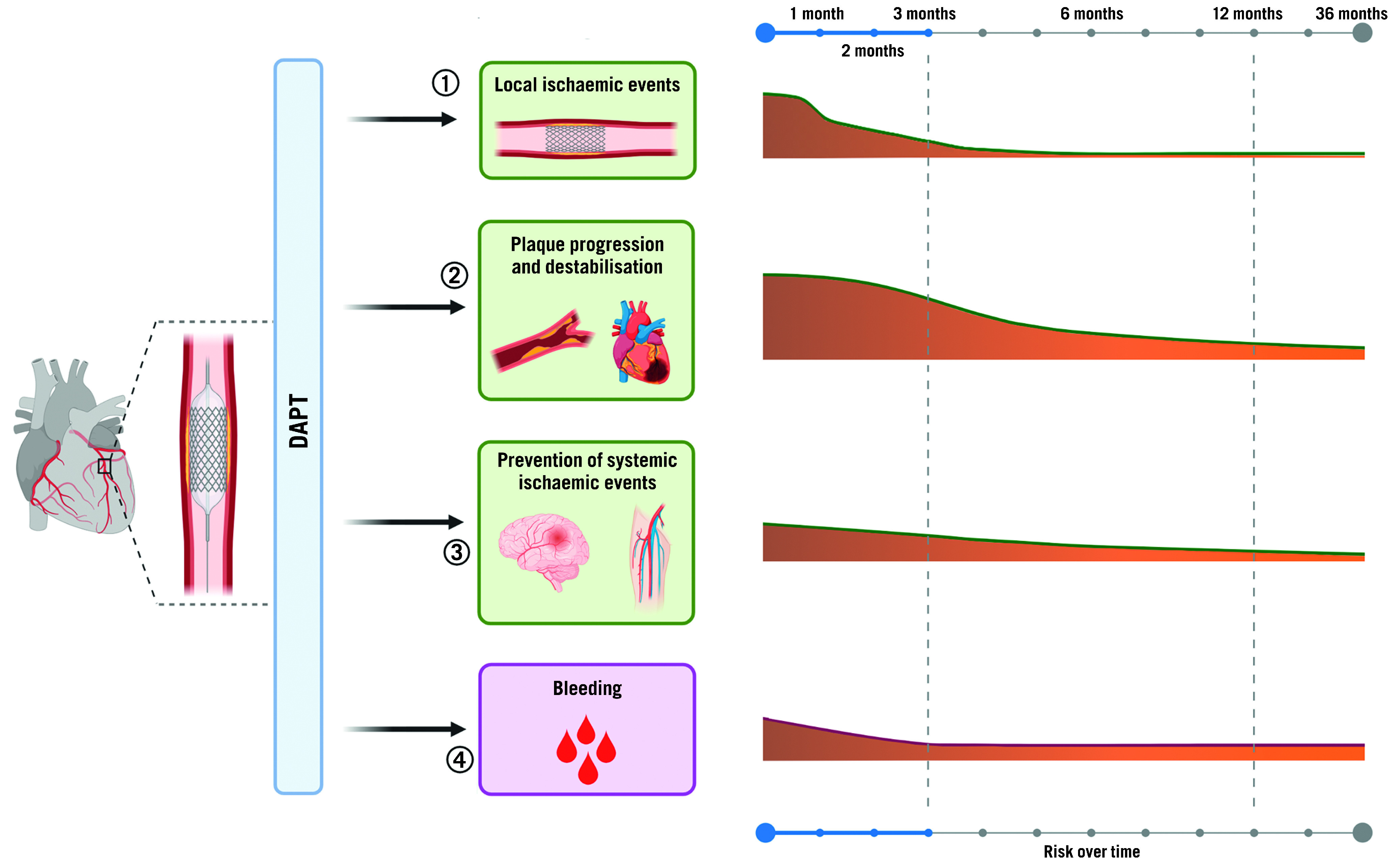
Antiplatelet agents administered after PCI may 1) reduce the incidence of stent-related ischaemic events such as stent thrombosis or target vessel revascularisation; 2) reduce the incidence of cardiovascular ischaemic recurrence and their consequences, such as myocardial infarction and cardiovascular death; 3) prevent cerebrovascular events in other areas affected by atherosclerotic disease, such as peripheral and carotid arteries; 4) be inevitably associated with increased risk of bleeding. Importantly, the potential benefit of antiplatelet agents varies over time, with the greatest benefit in terms of less ischaemic events maximised during the first months after PCI and decreasing over time, while bleeding events remain stable over time. DAPT: dual antiplatelet therapy; PCI: percutaneous coronary intervention.
Indeed, bleeding is the main drawback of antithrombotic therapies, particularly given its association with adverse prognosis35. Multiple mechanisms can explain the adverse outcomes, including ischaemic events and mortality, associated with bleeding. These include, but are not limited to: interruption of antiplatelet treatment as a reaction to the bleeding event, activation of coagulation and inflammation in case of bleeding, and depletion of 2,3-diphosphoglyceric acid and nitric oxide triggered by blood transfusion which modulates oxygen exchange at the tissue level and favours vasoconstriction and platelet aggregation36,37. The risk of bleeding is proportional to the intensity of antithrombotic treatment38. This explains why bleeding complications are highest in the early phase post-PCI, given that patients warrant vascular access and are exposed to adjuvant intraprocedural antithrombotic therapy. Whilst both ischaemic and bleeding risks are highest in the periprocedural phase, the risk of bleeding tends to be stable over time while ischaemic risk decreases after 1 to 3 months post-PCI, albeit with variability according to the clinical presentation of the patients and complexity of the PCI39. These considerations have stimulated interest for tailoring antiplatelet regimens according to the ischaemic and bleeding risk of the individual patient.
Current recommendations on the use of oral antiplatelet therapy after PCI
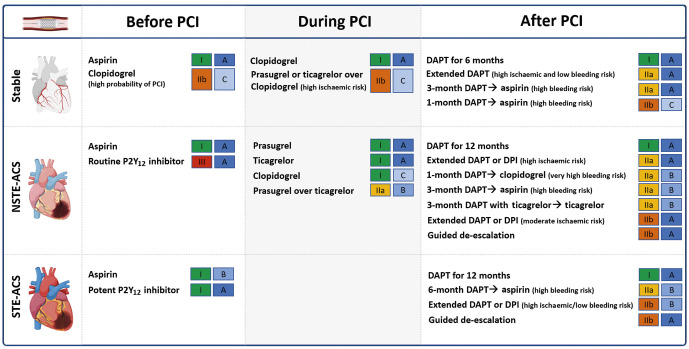
DAPT is the standard of care for patients undergoing PCI1,40,41,42,43. Aspirin (loading dose of 160-325 mg orally or 250-500 mg intravenously, followed by an oral maintenance dose of 75-100 mg once daily [od]) should be administered in all patients. The DAPT regimen, including the choice of P2Y12 inhibitor, varies according to the clinical setting (i.e., ACS or CCS) as well as the thrombotic and bleeding risk of the individual patient. Guideline recommendations from the European Society of Cardiology (ESC) are provided in Figure 4.
Figure 4. Current Guidelines of the European Society of Cardiology recommendations for oral antiplatelet agents among patients undergoing PCI.
DAPT: dual antiplatelet therapy; DPI: dual pathway inhibition; NSTE-ACS: non-ST-elevation acute coronary syndrome; PCI: percutaneous coronary intervention; STE-ACS: ST segment-elevation acute coronary syndrome
Chronic coronary syndrome
In patients with CCS, initiation of oral P2Y12 inhibitors is usually delayed until the coronary anatomy is defined40,43. Clopidogrel, administered as a 600 mg loading dose followed by a 75 mg maintenance dose, is the P2Y12 inhibitor of choice in CCS patients undergoing PCI40,43. After PCI, 6-month DAPT, followed by long-life aspirin monotherapy is the default recommendation with the option of shortening DAPT duration to either 1 or 3 months according to the bleeding risk40,43.
Acute coronary syndromes
In patients with ACS, while pre-treatment with an oral P2Y12 inhibitor has the theoretical advantage of providing greater antiplatelet protection by the time of PCI, not all patients undergoing coronary angiography undergo PCI. This inevitably may expose patients unnecessarily to adjunctive antiplatelet treatment and increase their risk of bleeding44. This also represents a major drawback for patients requiring surgical revascularisation who then need to wait a washout period before undergoing their procedure, prolonging hospital stay and costs44. Ultimately, most recent randomised controlled trials (RCTs) have failed to show any benefit of pre-treatment versus in-lab use of P2Y12 inhibitors45,46,47. More specifically, pre-treatment versus in-lab treatment with prasugrel in patients with a non-ST-elevation ACS (NSTE-ACS) did not reduce ischaemic events but increased major bleeding complications47. In turn, pre-treatment with prasugrel is contraindicated in NSTE-ACS41,43. Moreover, pre-treatment with ticagrelor compared with in-lab treatment with prasugrel favoured the latter45. Accordingly, the latest NSTE-ACS guidelines do not recommend routine pre-treatment with a P2Y12 inhibitor, stating that this strategy can only be considered (using ticagrelor 180 mg followed by 90 mg twice daily [bid] or clopidogrel 600 mg followed by 75 mg/od) for patients at low bleeding risk who are not scheduled to undergo an early invasive strategy41. On the contrary, among STE-ACS patients, the use of a potent P2Y12 inhibitor at the time of diagnosis is encouraged, as primary-PCI is the treatment of choice in these patients42,43. It is however important to note that, particularly in the setting of ST-segment elevation myocardial infarction (STEMI), the onset of action of oral P2Y12 inhibitors is significantly delayed, requiring up to 4-6 hours to achieve full antiplatelet effects, underscoring the need for strategies to bridge this gap in platelet inhibition, such as the use of intravenous antiplatelet therapies48.
In ACS patients undergoing PCI, the standard recommendation is 12 months of DAPT including a potent P2Y12 inhibitor41,42,43. Prasugrel and ticagrelor are preferred over clopidogrel in patients with NSTE-ACS in the absence of contraindications, with prasugrel (60 mg followed by 10 mg/od) being recently recommended over ticagrelor41,49. NSTE-ACS guidelines also introduce the use of ticagrelor monotherapy 3 months after PCI among patients at low ischaemic risk41. Among patients with moderate or high bleeding risk, clopidogrel should be preferred over more potent P2Y12 inhibitors and DAPT can be shortened to either 1 month, when followed by clopidogrel monotherapy, or to 3 months in NSTE-ACS and to 6 months in STE-ACS when followed by aspirin monotherapy41,42. Moreover, guided or unguided de-escalation of P2Y12 inhibitors can also be considered41. Conversely, among patients with high or moderate ischaemic risk and low bleeding risk, prolonged antithrombotic regimens can be considered 12 months after PCI for ACS41. Treatment options are the prolongation of DAPT with either clopidogrel (75 mg/od), prasugrel (10 mg/od), or ticagrelor (60 mg/bid) in addition to aspirin, or alternatively, to consider a strategy of dual pathway inhibition (DPI) with the use of a vascular dose of rivaroxaban (2.5 mg/bid) in addition to aspirin41,42.
Special scenarios
Among patients who undergo PCI but who also have an indication to be treated with oral anticoagulants (OAC), such as those with atrial fibrillation (AF), recommendations have varied over the years50. The latest recommendations now indicate that the default strategy to be used is triple therapy (TT), consisting of aspirin, a P2Y12 inhibitor (preferably clopidogrel) and a novel oral anticoagulant (NOAC) up to 1 week post-PCI, followed by dual antithrombotic therapy (DAT) with a P2Y12 inhibitor and an NOAC (i.e., discontinue aspirin)41,51. The duration of TT could be prolonged up to 1 month, but not beyond, among patients with high ischaemic risk but at low risk for bleeding41,51. DAT should be maintained for up to 12 months after which antiplatelet therapy should be discontinued, except in those patients deemed to be at increased risk of long-term ischaemic recurrences41,51. Discontinuation of antiplatelet therapy and maintaining OAC treatment alone may be considered sooner (i.e., at 6 months) if patients are at increased risk of bleeding or at low ischaemic risk41,51. Although clopidogrel is the P2Y12 inhibitor of choice, potent P2Y12 inhibitors may be considered in patients at high thrombotic risk and low bleeding risk. However, the use of aspirin should not go beyond one week41,51.
Qualitative and quantitative approaches for risk stratification
A careful stratification at a single patient level represents the foundation of a personalised selection of antiplatelet strategies among patients undergoing PCI52. This can be achieved by an integrated assessment of 3 key factors: bleeding risk, ischaemic risk and responsiveness to an antiplatelet agent (Figure 5).
Figure 5. Risk assessment to guide antiplatelet therapy among percutaneous coronary disease patients.
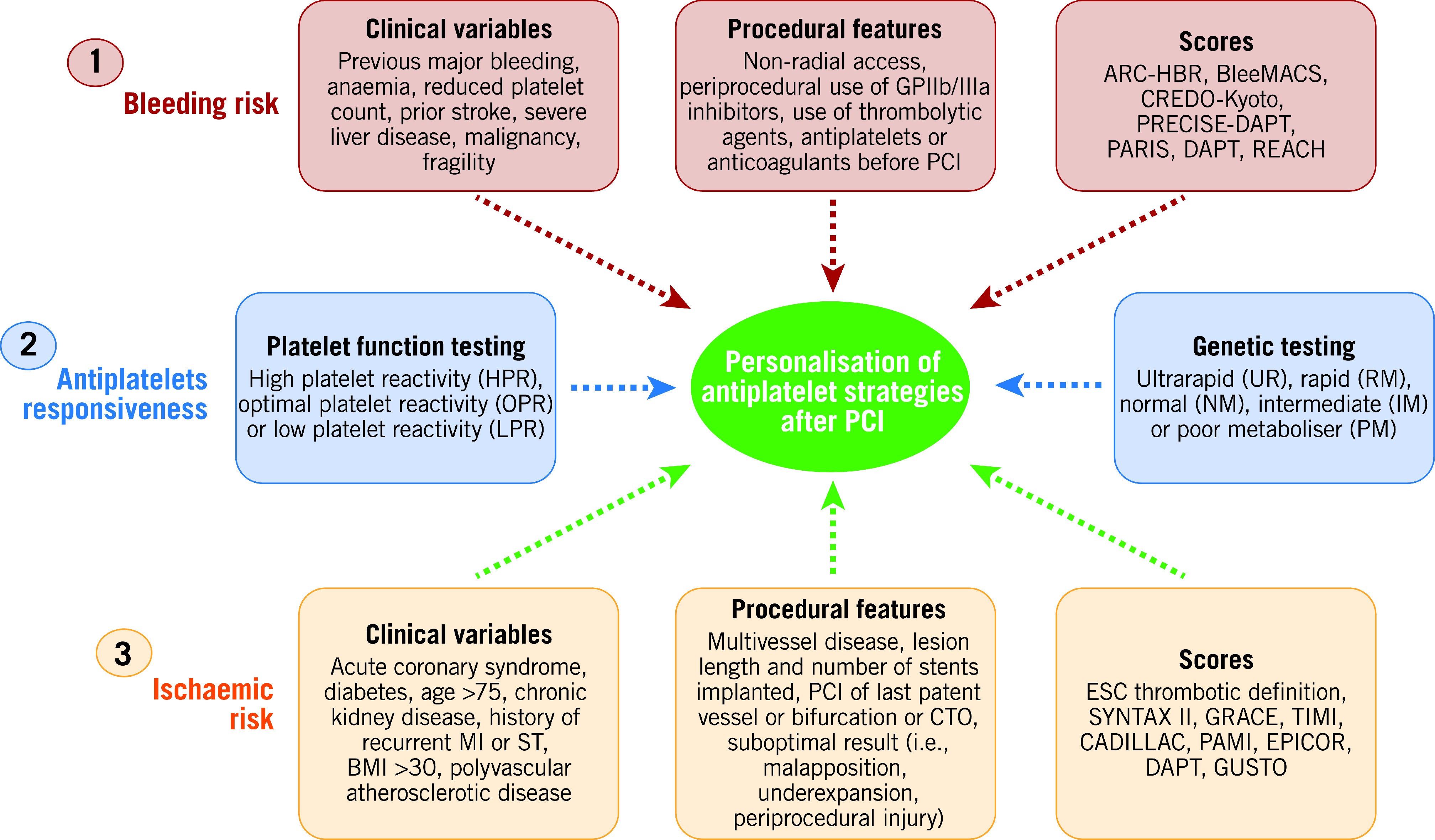
Bleeding and ischaemic risk assessments are based on clinical variables, procedural features and the use of scores/definitions. Antiplatelet responsiveness can be assessed by either platelet function or genetic testing. BMI: body mass index; CTO: chronic total occlusion; GP: glycoprotein; MI: myocardial infarction; PCI: percutaneous coronary intervention; ST: stent thrombosis.
Bleeding and ischaemic risk assessment
The assessment of bleeding and ischaemic risks is achieved by the evaluation of clinical and procedural features which can be defined qualitatively as well as quantified by means of a scoring system (Figure 5). Clinical variables, including patient history, fragility and general status as well as comorbidities and laboratory exams represent the cornerstone for risk assessment.
Nevertheless, in the setting of patients undergoing PCI, procedural and technical features play an important role in determining ischaemic or bleeding risks and should be taken into consideration53,54,55. Several clinical, procedural and laboratory factors that have been found to be associated with increased bleeding or ischaemic risk in retrospective studies have been included in the scores and definitions for risk assessment, in the hope of providing a prognostic stratification and predicting bleeding and/or ischaemic events to help guide the choice of antiplatelet therapy after PCI, as well as to improve the standardisation of the design of clinical trials and ease their interpretation52,55,56. The most commonly adopted ischaemic scores (i.e., GRACE or TIMI) are mainly used for prognostic stratification. Concerning the use of scores or definitions for ischaemic risk stratification to guide the selection of antiplatelet therapy, the recent ESC guidelines provide a thrombotic risk stratification for intensified antithrombotic treatment after the standard DAPT duration by defining patients at high or moderate thrombotic risk (Table 1)40,41.
Table 1. Latest recommendations to guide antiplatelet duration according to ischaemic (European Society of Cardiology Guidelines) and bleeding (Academic Research Consortium for high bleeding risk definition) risks.
| Academic Research Consortium high bleeding risk definition | European Society of Cardiology ischaemic risk definition | ||
|---|---|---|---|
| Major criteria | Minor criteria | High thrombotic risk | Moderate thrombotic risk |
| At least 1 criterion | At least 2 criteria |
Complex CAD and at least 1 criterion |
Non-complex CAD and at least 1 criterion |
| Risk enhancers | |||
| Anticipated use of long-term oral anticoagulation | Age ≥75 years | Diabetes mellitus requiring medication | Diabetes mellitus requiring medication |
| Severe or end-stage CKD (eGFR <30 mL/min/1.73 m2) | Moderate CKD (eGFR 30 to 59 mL/min/1.73 m2) | History of recurrent MI | History of recurrent MI |
| Haemoglobin <11 g/dL | Haemoglobin 11 to 12.9 g/dL for men and 11 to 11.9 g/dL for women | Any multivessel CAD | Polyvascular disease (CAD plus PAD) |
| Spontaneous bleeding requiring hospitalisation or transfusion in the past 6 months or at any time, if recurrent | Spontaneous bleeding requiring hospitalisation or transfusion within the past 12 months not meeting the major criterion | Polyvascular disease (CAD plus PAD) | CKD with eGFR 15-59 mL/min/1.73 m2 |
| Moderate or severe baseline thrombocytopaenia (platelet count <100×109/L) | Long-term use of oral NSAIDs or steroids | Premature (<45 years) or accelerated (new lesion within a 2-year time frame) CAD | – |
| Chronic bleeding diathesis | Any ischaemic stroke at any time not meeting the major criterion | Concomitant systemic inflammatory disease (e.g., human immunodeficiency virus, systemic lupus erythematosus, chronic arthritis) | – |
| Liver cirrhosis with portal hypertension | – | CKD with eGFR 15-59 mL/min/1.73 m2 | – |
| Active malignancy (excluding non-melanoma skin cancer) within the past 12 months | – | Technical aspects | |
| Previous spontaneous ICH (at any time) | – | At least 3 stents implanted | – |
| Previous traumatic ICH within the past 12 months | – | At least 3 lesions treated | – |
| Presence of a bAVM | – | Total stent length >60 mm | – |
| Moderate or severe ischaemic stroke within the past 6 months | – | History of complex revascularisation (left main, bifurcation stenting with >2 stents implanted, chronic total occlusion, stenting of last patent vessel) | – |
| Non-deferrable major surgery on DAPT | – | History of stent thrombosis on antiplatelet treatment | – |
| Recent major surgery or major trauma within 30 days before PCI | – | – | – |
|
ESC guidelines thrombotic risk definition: in line with guideline recommendations, CAD patients are stratified into 2 different risk groups (high vs moderately increased thrombotic or ischaemic risk). Stratification of patients towards complex vs non-complex CAD is based on individual clinical judgement with knowledge of patients’ cardiovascular history and/or coronary anatomy. ARC-HBR: Academic Research Consortium for high bleeding risk; bAVM: brain arteriovenous malformation; CAD: coronary artery disease; CKD: chronic kidney disease; DAPT: dual antiplatelet therapy; eGFR: estimated glomerular filtration rate; ESC: European Society of Cardiology; ICH: intracranial haemorrhage; MI: myocardial infarction; NSAID: non-steroidal anti-inflammatory drug; PAD: peripheral artery disease; PCI: percutaneous coronary intervention | |||
Specific risk scores have been developed aimed at managing antithrombotic duration after PCI according to both bleeding and ischaemic risks. Among these, the DAPT and the PRECISE-DAPT scores are calculated at patient discharge and at one year from the index event, respectively57,58. The DAPT score includes clinical and procedural features and supports DAPT extension up to 30 months when the score is ≥257. Similarly, the PRECISE-DAPT score, which includes clinical and laboratory features, suggests the shortening of DAPT when the score is ≥25, as in these patients a longer DAPT duration was associated with increased bleeding without reducing ischaemic events58. Of note, both scores were developed and validated in patients without an indication to oral anticoagulation, although limited evidence is available in this setting59,60. It is important to note that many patients are at risk of both increased bleeding and ischaemic events; when these are concordant, observational data suggest that bleeding, more than ischaemic risk, should inform the decision-making on the duration of DAPT61. Guidelines also recommend that the bleeding risk of an individual patient be the key determinant in defining DAPT duration1,3,41. To date however, there are no studies that have provided definitive evidence of the advantage of bleeding risk stratification as compared to ischaemic risk stratification as a guide for the intensity and duration of DAPT.
Prompt identification of high bleeding risk patients by a standardised score or definition could play an important role in defining patients for whom a reduction in intensity of antiplatelet therapy could be advantageous. Over the years, a number of scores have been proposed to address this. Recently, the Academic Research Consortium for High Bleeding Risk (ARC-HBR) proposed a consensus for the definition of high bleeding risk, including 14 major and 6 minor criteria (Table 1)62. High bleeding risk is defined by at least 1 major or 2 minor criteria. Although retrospective studies have validated this definition, prospective studies using ARC-HBR criteria to stratify patients to specific antiplatelet regimens are warranted63. Although risk scores and definitions are useful for standardisation purposes, their use should always be integrated with other factors such as clinical and procedural characteristics as well as antiplatelet drug response52.
Antiplatelet drug response
Compared to prasugrel and ticagrelor, clopidogrel is characterised by less potent platelet inhibition, slower onset of action and wide interindividual variability in response profiles. This leads to high on-treatment platelet reactivity (HPR) in approximately 30% of patients, although this prevalence may vary based on distribution of risk factors and patient ethnic background6,32. Importantly, HPR status is associated with an increased risk of thrombotic complications6,32,64. Of note, while HPR is a modifiable marker of thrombotic risk, low platelet reactivity (LPR), which is more common with prasugrel and ticagrelor, is associated with increased risk of bleeding without any reduction of ischaemic events among patients responding to clopidogrel32. Individual responsiveness to P2Y12 inhibitors can be assessed through platelet function tests6,65. Although platelet function testing has the advantage of defining the platelet phenotype which is associated with ischaemic outcomes, its implementation in clinical practice has inherent challenges65,66. Indeed, although commercially available “point-of-care” or “near-patient-based assays”, which are more user-friendly than some more complex laboratory-based methods, have the advantage of providing results in a timely fashion, they are still limited by the variability in the results obtained and by the fact that patients need to be on clopidogrel to define responsiveness6. Genetic testing represents an alternative tool to assist with the guidance of antiplatelet therapy6,67. Indeed, genetic polymorphisms of the hepatic cytochrome P450 (CYP) 2C19 enzyme required to transform clopidogrel into its active metabolite, has an important role, with carriers of loss-of-function (LoF) alleles being characterised by reduced active metabolite generation, increased HPR rates and enhanced thrombotic risk, including ST6,68,69. CYP2C19*2 and *3 are the most common LoF alleles6,67. It is important to note that CYP2C19 genotypes are not the sole contributors to clopidogrel response and thus may not always identify HPR status. For this reason, integrating clinical variables with genotypes to predict HPR status has been suggested to identify clopidogrel-HPR status more accurately70.
Strategies focused on reducing ischaemic events
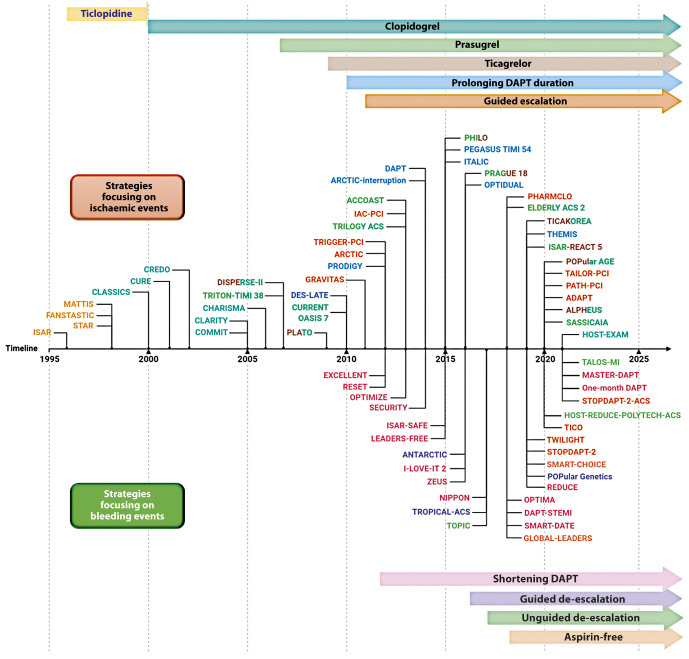
Several strategies aimed at reducing the residual burden of ischaemic events among patients undergoing PCI, especially among high-risk cohorts of patients, have been tested over the years and are discussed below. In the Central illustration we illustrate a detailed timeline of the RCTs that focused on antiplatelet agents over the past 40 years. Table 2 summarises the strategies focused on the reduction of ischaemic events. The major drawback of these strategies is their trade-off in bleeding risk. Strategies that are no longer recommended by guidelines, such as use of ticlopidine, cilostazol or double-dose clopidogrel will not be discussed. RCTs and meta-analyses, as opposed to observational or registry studies, will be discussed as they represent the highest level of evidence. Moreover, it is important to acknowledge that amongst the strategies aimed at reducing ischaemic events in patients with ACS, that adding a NOAC to standard antiplatelet treatment regimens, including DAPT, has also been tested71,72. Although a detailed description of this topic goes beyond the scope of this manuscript which is focused on antiplatelet therapies, it is important to acknowledge that a number of NOACs have been tested in this context, but only the low-dose rivaroxaban was shown to meet the objectives of this strategy in the Anti-Xa Therapy to Lower Cardiovascular Events in Addition to Standard Therapy in Subjects With Acute Coronary Syndrome ACS 2-Thrombolysis In Myocardial Infarction 51 (ATLAS ACS 2-TIMI 51) trial73. In particular, for patients with a recent ACS (n=15,526), rivaroxaban (twice-daily doses of either 2.5 mg or 5 mg) on top of standard of care antiplatelet therapy, most commonly aspirin and clopidogrel, reduced the risk of the composite endpoint of death from cardiovascular (CV) causes, myocardial infarction (MI), or stroke, at the expense of an increased risk of major bleeding and intracranial haemorrhage, but not fatal bleeding. The twice-daily 2.5 mg dose of rivaroxaban was associated with less bleeding. Despite the ischaemic benefit, this strategy has had limited uptake in clinical practice due to concerns surrounding the increased bleeding and the fact that the trial was not reflective of current recommendations that prefer P2Y12 inhibition with prasugrel and ticagrelor in patients with ACS. In contrast, a strategy of DPI with the use of rivaroxaban 2.5 mg/bid, in addition to aspirin, for long-term secondary prevention in patients with stable atherosclerotic disease, has received a stronger endorsement from practice guidelines and has been more broadly adopted41.
Central illustration. Timeline of randomised controlled trials on antiplatelet therapy focusing on strategies aiming at reducing ischaemic (upper) or bleeding (lower) events.
DAPT: dual antiplatelet therapy
Table 2. Randomised controlled trials testing antiplatelet strategies aiming at reducing ischaemic events among patients undergoing percutaneous coronary intervention.
| Study name | Year of publication | Number of patients enrolled | Clinical presentation (%) | Treatment arms and population | Primary endpoint definition | Primary endpoint met? | Follow-up duration | |
|---|---|---|---|---|---|---|---|---|
| ACS | CCS | |||||||
| Potent P2Y12 inhibiting therapy | ||||||||
| TRITON TIMI 38 | 2007 | 13,608 | 100 | 0 | Prasugrel versus clopidogrel among ACS | CV death, MI or stroke | Yes | 14 months |
| PLATO | 2009 | 18,624 | 100 | 0 | Ticagrelor versus clopidogrel among ACS | CV death, MI or stroke | Yes | 12 months |
| PHILO | 2015 | 801 | 100 | 0 | Ticagrelor versus clopidogrel among ACS | Any major bleeding CV death, MI or stroke | No | 12 months |
| PRAGUE-18 | 2016 | 1,230 | 100 | 0 | Ticagrelor versus prasugrel among STEMI patients | All-death, reinfarction, urgent target vessel revascularisation, stroke and serious bleeding requiring transfusion or prolonging hospitalisation at 7 days | No | 12 months |
|
ELDERLY ACS 2 |
2018 | 1,443 | 100 | 0 |
Reduced dose of prasugrel (5 mg die) versus clopidogrel among ACS |
All death, MI, disabling stroke and rehospitalisation for CV causes or bleeding | No | 12 months |
| TICAKOREA | 2019 | 800 | 100 | 0 | Ticagrelor versus clopidogrel among ACS | Major or minor PLATO bleeding | Yes | 12 months |
| ISAR-REACT 5 | 2019 | 4,018 | 100 | 0 | Ticagrelor versus prasugrel among ACS | CV death, MI or stroke | Yes | 12 months |
| POPular AGE | 2020 | 1,002 | 100 | 0 | Ticagrelor versus clopidogrel among elderly (>70 years) ACS | Major or minor PLATO bleeding All-cause death, myocardial infarction, stroke, major and minor PLATO bleeding | No | 12 months |
| SASSICAIA | 2020 | 781 | 0 | 100 | Prasugrel versus clopidogrel among patients undergoing elective PCI | All death, any MI, definite/probable ST, stroke and urgent vessel revascularisation | No | 30 days |
| ALPHEUS | 2020 | 1,910 | 0 | 100 | Ticagrelor versus clopidogrel among patients undergoing high-risk PCI |
PCI-related type 4 (a or b) MI or major myocardial injury Major bleeding |
No |
48 hours 30 days |
| Prolonging DAPT duration | ||||||||
| DES-LATE | 2010 | 2,701 | 63 | 37 | 12 months versus 24 months DAPT | CV death or MI | No | 2 years |
| PRODIGY | 2012 | 2,013 | 74 | 26 | 6 months versus 24 months DAPT 30 days after PCI | All death, myocardial infarction or cerebrovascular accident | No | 2 years |
| DAPT | 2014 | 9,960 | 46 | 54 | 12 months versus 30 months DAPT | Stent thrombosis All death, MI, or stroke Moderate and severe bleeding | Yes | 33 months |
| ARCTIC-interruption | 2014 | 1,259 | 0 | 100 | 12 months versus 18 months DAPT | All death, myocardial infarction, stent thrombosis, stroke and urgent revascularisation | No | 17 months |
| ITALIC | 2015 | 2,301 | 23 | 76 | 6 months versus 24 months DAPT | All death, MI, urgent target vessel revascularisation, stroke and major bleeding | No | 12 months |
| PEGASUS-TIMI 54 | 2015 | 21,162 | 0 | 100 | Ticagrelor 90 mg or ticagrelor 60 mg versus placebo 1 to 3 years after MI | CV death, MI or stroke and TIMI major bleeding | Yes | 33 months |
| OPTIDUAL | 2016 | 1,799 | 35 | 65 | 12 months versus 48 months DAPT | All death, MI, stroke or major bleeding | No | 33 months |
| THEMIS | 2019 | 19,220 | 0 | 100 | Ticagrelor plus aspirin versus placebo plus aspirin among stable patients with DM | CV death, MI or stroke | No | 40 months |
| Guided escalation of P2Y12 inhibitors | ||||||||
| GRAVITAS | 2011 | 2,214 | 45 | 55 | High-dose (150 mg) versus standard-dose clopidogrel (75 mg daily) among clopidogrel non-responders defined by PFT | CV death, MI or ST | No | 6 months |
| ARCTIC | 2012 | 2,440 | 0 | 100 | High-dose (150 mg) prasugrel versus standard-dose clopidogrel (75 mg daily) among clopidogrel non-responders defined by PFT | All death, MI, ST, stroke and urgent revascularisation | No | 12 months |
| TRIGGER-PCI | 2012 | 423 | 0 | 100 | Prasugrel versus clopidogrel among clopidogrel non-responders defined by PFT | CV death or MI | No | 6 months |
| PHARMCLO | 2018 | 888 | 97 | 3 | Prasugrel or ticagrelor among clopidogrel non-responders defined by genetic testing versus standard therapy | CV death, MI, stroke and BARC bleeding 3-5 | Yes | 12 months |
| PATH-PCI | 2019 | 2,285 | 0 | 100 | Ticagrelor among clopidogrel non-responders defined by PFT versus standard therapy | CV death, MI, stroke, ST, urgent revascularisation and BARC bleeding 3-5 | Yes | 6 months |
| TAILOR-PCI | 2020 | 5,302 | 69 | 31 | Prasugrel or ticagrelor among clopidogrel non-responders defined by genetic testing versus standard therapy | CV death, MI, stroke, ST and severe recurrent ischaemia | No | 12 months |
| ADAPT | 2020 | 504 | 50 | 50 | Prasugrel or ticagrelor among clopidogrel non-responders defined by genetic testing versus standard therapy | CV death, MI, stroke, urgent need for revascularisation and ST | No | 16 months |
| ACS: acute coronary syndrome; BARC: Bleeding Academic Research Consortium; CCS: chronic coronary syndrome; CRNM: clinically relevant non-major; CV: cardiovascular; DAPT: dual antiplatelet therapy; ISTH: International Society on Thrombosis and Haemostasis; MI: myocardial infarction; PFT: platelet function testing; PLATO: Platelet Inhibition and Patient Outcomes; ST: stent thrombosis; TIMI: Thrombolysis in Myocardial Infarction; VKA: vitamin K antagonists | ||||||||
Potent P2Y<sub>12</sub> inhibiting therapy
The first strategy proposed to reduce the occurrence of ischaemic events among patients undergoing PCI, was to use more potent P2Y12 inhibitors as compared to clopidogrel34.
Prasugrel
In 2007, the pioneering Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel-Thrombolysis in Myocardial Infarction 38 (TRITON-TIMI 38) trial showed prasugrel reduced the incidence of the primary endpoint of major adverse cardiovascular events (MACE) by 19% at 15 months, compared to clopidogrel, among 13,608 patients with ACS undergoing PCI74. These differences were driven by a reduction in MI; prasugrel also reduced rates of stent thrombosis74. However, prasugrel was also associated with a significant 32% increase in non-coronary artery bypass graft (CABG)-related Thrombolysis In Myocardial Infarction (TIMI) major bleeding, as compared to clopidogrel. Such an increased risk of bleeding offsets the benefits of prasugrel in certain patient cohorts, resulting in neutral effects in the elderly (aged ≥75 years) and low body weight (<60 kg) patients, and net harm to those with a history of stroke or transient ischaemic attack74. Although data from pharmacokinetic/pharmacodynamic (PK/PD) modelling suggested the use of a lower maintenance dose regimen (i.e., 5 mg/od) in the elderly and low-weight patients75,76, to date there are no studies supporting superior efficacy of this regimen over clopidogrel77. The ischaemic benefits of prasugrel in ACS patients undergoing PCI has been confirmed in meta-analyses showing reduced MI and ST but yielding a 25-30% increase in major bleeding compared to clopidogrel78. Studies conducted in patients with medically-managed ACS or high-risk CCS undergoing PCI have failed to show any benefit of prasugrel79,80.
Ticagrelor
In 2009, the Platelet Inhibition and Patient Outcomes (PLATO) trial, showed a 16% reduction of the composite primary endpoint of MACE at 12 months, with ticagrelor compared to clopidogrel among 18,624 patients with ACS managed either invasively or non-invasively81. These differences were driven by a reduction in MI and CV death81. The efficacy of ticagrelor was consistent among patients undergoing revascularisation; ticagrelor also reduced rates of stent thrombosis among patients undergoing PCI82,83. Although the overall incidence of PLATO major bleeding was similar in the 2 groups, ticagrelor treatment was associated with a significant 25% increase in non-CABG-related TIMI major bleeding and an increase of fatal intracranial bleeding81. Moreover, a common non-bleeding adverse effect of ticagrelor was dyspnoea, which occurred in 15-22% of patients and was the most common cause of drug withdrawal81.
Although in PLATO there weren’t any subgroups in which the bleeding risk offset the efficacy of ticagrelor, absolute bleeding rates increased among the elderly treated with ticagrelor81. In the Ticagrelor or Prasugrel Versus Clopidogrel in Elderly Patients With an Acute Coronary Syndrome and a High Bleeding Risk: Optimization of Antiplatelet Treatment in High-risk Elderly (POPular AGE) study conducted in NSTE-ACS patients aged >70 (n=1,002), clopidogrel was associated with fewer bleeding events compared with ticagrelor and without an increase in the combined endpoint of all-cause death, MI, stroke, and bleeding84. Parallel findings were observed in several registry studies of ACS patients85,86. The safety and efficacy of ticagrelor compared with clopidogrel among Asian patients with ACS was tested in 2 relatively small RCTs showing no ischaemic benefit and more bleeding, questioning the effectiveness of ticagrelor in this population or the potential need for dose adjustments, although neither study was sufficiently powered to demonstrate efficacy87,88.
Subsequent meta-analyses in ACS patients showed ticagrelor to be the only P2Y12 inhibitor associated with a reduction in all-cause and CV death, but yielding a 20-30% increase of major bleeding compared to clopidogrel78. This finding can potentially be attributed to the pleiotropic mechanisms of ticagrelor, including increased plasma levels of adenosine, and supports the concept that there may be a mortality benefit independent from a reduction of ischaemic events89. However, such observations have not been observed in other studies45,85,90,91. Ultimately, in patients with CCS undergoing high-risk elective PCI, ticagrelor was not superior to clopidogrel in reducing periprocedural myocardial necrosis92.
Prasugrel versus ticagrelor
Whether 1 of the potent P2Y12 inhibitors is superior to the others has been a topic of debate for years93. Although earlier investigations were inconclusive94, The Intracoronary Stenting and Antithrombotic Regimen: Rapid Early Action for Coronary Treatment (ISAR-REACT 5) trial, conducted in 4,018 ACS patients, showed that prasugrel administered at the time of PCI after defining coronary anatomy, compared with pre-treatment with ticagrelor before defining coronary anatomy, was associated with a significant 36% reduction of the primary endpoint of MACE at 1 year without any increase in bleeding45. These findings were consistent, irrespective of clinical presentation (NSTE-ACS and STEMI)95,96. Mechanisms that can explain these findings include the more potent platelet inhibitory effects of prasugrel over ticagrelor as confirmed in the pharmacodynamic substudy of the trial, as well as better compliance to treatment given that ticagrelor is more commonly associated with treatment discontinuation due to dyspnoea97. A network meta-analysis including both direct and indirect comparisons of oral P2Y12 inhibitors in ACS showed no significant differences between prasugrel and ticagrelor, but when compared to clopidogrel, only prasugrel reduced MI, while only ticagrelor reduced all-cause and CV death78.
Guided escalation of P2Y<sub>12</sub> inhibitors
The use of tools to guide the selection of antiplatelet therapy can lead to an escalation of P2Y12 inhibiting potency (i.e., prasugrel or ticagrelor) among patients identified to have clopidogrel-HPR (i.e., using platelet function tests) or CYP2C19 LoF alleles (i.e., using genetic testing)65,67. From a clinical standpoint, an escalation strategy is aimed at reducing ischaemic events without a trade-off in bleeding6. Two lines of research have investigated the impact of a guided escalation among patients undergoing PCI: in the first, escalation was compared to standard therapy selectively among patients with clopidogrel-HPR or carriers of the CYP2C19 LoF allele, and in the second, this was tested as a strategy (i.e., to assess the clinical benefit of testing versus no-testing in all-comers PCI). In the first line of research, several trials were performed but failed to demonstrate any benefit98,99,100. However, these trials were characterised by design limitations including poor definition of clopidogrel-HPR, implementation of strategies inadequate to overcome clopidogrel-HPR, and inclusion of low-risk patients. These observations have allowed for subsequent studies with ameliorated designs that support the clinical benefit of the use of guided escalation as a strategy (Table 2)101,102,103.
The second line of research tested the use of platelet function or genetic testing as a strategy among the entire population of patients undergoing PCI. These latter studies are of clinical interest since they tested the effectiveness of implementing a guided selection of antiplatelet therapy in clinical practice101,102,103,104. Tailored Antiplatelet Therapy Following PCI (TAILOR-PCI) randomised 5,302 patients undergoing PCI to either a guided or a standard selection of antiplatelet therapy103. The primary analysis was in patients with CYP2C19 LoF variants, and the secondary analysis included all randomised patients. This trial thus provided evidence on both the first and the second line of research. Nevertheless, despite a 34% reduction of MACE at 12 months found in both settings, but favouring the guided selection over the standard selection of antiplatelet therapy, this was not statistically significant given the choice of a very ambitious 85% power to show a 50% reduction of the primary endpoint with guided therapy103. Collectively, the negative results of RCTs in this setting were largely driven by their limited sample sizes. A recent meta-analysis overcoming such limitations found a strategy of a guided escalation of antiplatelet therapy to be associated with a 26% reduction of composite ischaemic events and no difference in bleeding as compared to standard selection of antiplatelet therapy among patients undergoing PCI33.
Prolonging DAPT duration
The observation that patients with CAD, particularly those with prior MI, remain at risk for long-term, even beyond one year, ischaemic recurrences has laid the foundation for investigations evaluating the safety and efficacy of prolonging DAPT (Table 2). Although earlier investigations were designed based on concerns surrounding the long-term safety (i.e., ST) of earlier-generation drug-eluting stents (DES), these concerns have been largely overcome with newer-generation DES platforms2,5. Hence, the focus of studies evaluating prolonged antithrombotic regimens shifted from being centred on “stent” outcomes to “patient” outcomes. Indeed, a number of earlier studies, relatively small in sample size, failed to demonstrate any benefit of prolonging DAPT105,106,107,108,109. This prompted the design of the larger-scale Dual Antiplatelet Therapy (DAPT) study which enrolled 9,961 patients and found that, compared to 12-month DAPT, an additional 18 months of DAPT (with either clopidogrel 75 mg/od or prasugrel 10 mg/od) significantly reduced the primary endpoint of MACE by 29%. ST rates were also significantly reduced. However, this benefit occurred at the expense of a significant increase in moderate and severe bleeding110. Of note, the reduction of major adverse cardiac and cerebrovascular events (MACCE) with prolonged DAPT was enhanced among patients with a prior history of MI compared with those without (p-interaction=0.03)111. Subsequent meta-analyses showed an overall reduction of MI and ST with prolonged, as compared to standard, DAPT but at the cost of increased bleeding112,113,114,115.
The available evidence from post hoc analyses of larger studies that suggested that patients with prior MI potentially benefit from prolongation of intensified antiplatelet regimens, prompted a selective investigation in patients with prior MI111,116. In particular, the Prevention of Cardiovascular Events in Patients with Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin-Thrombolysis in Myocardial Infarction 54 (PEGASUS-TIMI 54) trial tested a P2Y12 inhibiting therapy with either ticagrelor 90 mg/bid or 60 mg/bid on top of aspirin versus a placebo. The trial included 21,162 patients with MI 1 to 3 years earlier who were at least 50 years of age, and had 1 of the following additional high-risk features: age ≥65, diabetes mellitus requiring medication, a second prior MI, multivessel CAD, or chronic renal dysfunction90. At 33 months, both ticagrelor doses significantly reduced the incidence of the primary endpoint of MACE by 15%. However, this occurred at the expense of an increase in major bleeding with both ticagrelor regimens. Results were consistent irrespective of prior PCI, which represented 83% of the study population117. However, the safety profile, both in terms of bleeding and non-bleeding side effects (i.e., dyspnoea) was more favourable with the 60 mg/bid dose, and is the reason for which this dosing regimen is recommended for long-term, beyond 1 year, secondary prevention in practice guidelines41,90. The benefits of intensified antiplatelet therapy among high-risk patients without any prior history of an acute ischaemic event (prior MI or cerebrovascular event) was tested in The Effect of Ticagrelor on Health Outcomes in Diabetes Mellitus Patients Intervention Study (THEMIS) study which was selectively conducted in patients with type 2 diabetes mellitus and stable coronary artery disease (58% of total population had undergone prior PCI)91. The study randomised 19,220 patients and showed that ticagrelor 60 mg/bid plus aspirin 75-150 mg/od, as compared with aspirin plus placebo, reduced MACE by 10%, but significantly increased major bleeding at 40 months91. The net clinical benefit was more favourable among patients with a history of PCI compared to those without118. A relevant aspect in both the PEGASUS-TIMI 54 and THEMIS studies is the consistent efficacy of low-dose ticagrelor over time, as the benefit was present irrespective of time from most recent MI and PCI118,119.
Finally, the Cardiovascular Outcomes for People Using Anticoagulation Strategies (COMPASS) trial was the first study to assess whether adding a vascular dose regimen of rivaroxaban (2.5 mg/bid) to low-dose aspirin, a strategy known as DPI, could reduce ischaemic events in patients with stable atherosclerotic disease at risk for recurrences120. The trial randomised 27,395 patients to receive either rivaroxaban (2.5 mg twice daily) plus aspirin (100 mg once daily), rivaroxaban (5 mg twice daily), or aspirin (100 mg once daily). Rivaroxaban 2.5 mg plus aspirin, but not rivaroxaban 5 mg twice daily, reduced MACE by 24% compared to aspirin alone120. This occurred at the expense of a significant increase in major bleeding.
Overall, these above-mentioned trials led to the most recent changes in guideline recommendations for long-term intensified antithrombotic regimens by means of DAPT or DPI among high and moderate ischaemic risk patients in the absence of high bleeding risk40,41. The guidelines, however, do not provide recommendations for choosing between the 2 strategies (DAPT vs DPI) nor as to which P2Y12 inhibitor to consider if a DAPT strategy is chosen.
Strategies focused on reducing bleeding events
The introduction of stent platforms with low thrombogenicity, together with the fact that thrombotic risk is highest during the first months after PCI and decreases thereafter, while bleeding risk remains stable over time (Figure 3), has prompted investigations focused on the reduction of bleeding events55. Importantly, both major and minor bleeding have important prognostic implications. In particular, major bleeding has shown to have prognostic relevance (i.e., mortality) similar to or greater than that of a major ischaemic event; minor bleeding can result in an abrupt suspension of antiplatelet treatment, potentially resulting in higher ischaemic events35. We next discuss the major strategies tested in RCTs proposed to reduce bleeding, in hopes of providing a more favourable trade-off between bleeding and ischaemic risk over time. These strategies include shortening DAPT, the use of P2Y12 monotherapy and de-escalation of P2Y12 inhibitors (Central illustration). The vast majority of them were not designed to reduce MACE (Table 3). Thus, meta-analyses play an important role in increasing statistical power with respect to defining the impact on hard ischaemic endpoints in this setting.
Table 3. Randomised controlled trials testing antiplatelet strategies aiming at reducing bleeding events among patients undergoing percutaneous coronary intervention.
| Study name | Year of publication | Number of patients enrolled | Clinical presentation (%) | Treatment arms and population | Primary endpoint definition | Primary endpoint met? | Follow-up duration (months) | |
|---|---|---|---|---|---|---|---|---|
| ACS | CCS | |||||||
| Shortening DAPT | ||||||||
| EXCELLENT | 2012 | 1,443 | 51 | 49 | 6 versus 12 months DAPT | CV death, MI and ischaemia-driven target vessel revascularisation | Yes | 12 |
| RESET | 2012 | 2,148 | 54 | 46 | 3 versus 12 months DAPT | CV death, MI, ST, target vessel revascularisation and bleeding | Yes | 12 |
| OPTIMIZE | 2013 | 3,211 | 32 | 68 | 3 versus 12 months DAPT | All death, MI, stroke and major bleeding | Yes | 12 |
| SECURITY | 2014 | 1,404 | 39 | 61 | 6 versus 12 months DAPT | CV death, MI, stroke, definite or probable ST and BARC bleeding 3-5 | Yes | 12 |
| ISAR-SAFE | 2015 | 4,005 | 39 | 61 | 6 versus 12 months DAPT | All death, MI, ST, stroke and TIMI major bleeding | Yes | 9 |
| I-LOVE-IT 2 | 2016 | 1,829 | 85 | 15 | 3 versus 12 months DAPT | CV death, target vessel MI or clinically-indicated target lesion revascularisation | Yes | 18 |
| NIPPON | 2017 | 3,773 | 32 | 68 | 6 versus 12 months DAPT | All death, MI, stroke and major bleeding | Yes | 36 |
| DAPT-STEMI | 2018 | 1,100 | 100 | 0 | 6 versus 12 months DAPT | All death, MI, any revascularisation, stroke, and TIMI major bleeding | Yes | 18 |
| SMART-DATE | 2018 | 2,712 | 100 | 0 | 6 versus 12 months DAPT | All death, MI or stroke | Yes | 18 |
| OPTIMA-C | 2018 | 1,368 | 51 | 49 | 6 versus 12 months DAPT | All death, MI or ischaemia-driven target lesion revascularisation | Yes | 12 |
| REDUCE | 2019 | 1,496 | 100 | 0 | 3 versus 12 months DAPT | All death, MI, ST, stroke, target vessel revascularisation and BARC 2-5 bleeding | Yes | 12 |
| One-Month DAPT | 2021 | 3,020 | 39 | 61 | 1 versus 6-12 months DAPT in non-complex PCI | CV death, MI, target vessel revascularisation, stroke and major bleeding | Yes | 12 |
| MASTER DAPT | 2021 | 4,434 | 49 | 51 | 1 versus 5 months DAPT among HBR patients |
All death, MI, stroke, or major bleeding All death, MI, stroke Major or clinically relevant non-major bleeding |
Yes | 11 |
| P2Y12 monotherapy | ||||||||
| GLOBAL-LEADERS | 2018 | 15,968 | 47 | 53 | Ticagrelor monotherapy for 23 months versus DAPT with ticagrelor for 12 months | All death or MI | Yes | 24 |
| TWILIGHT | 2019 | 7,119 | 64 | 36 | Ticagrelor monotherapy after 3 months of DAPT versus standard DAPT in uneventful patients with high-risk PCI | BARC bleeding type 2, 3, or 5 and all-cause death or MI and stroke | Yes | 15 |
| SMART-CHOICE | 2019 | 2,993 | 58 | 42 | P2Y12 inhibitor monotherapy after 3 months of DAPT versus standard DAPT | All death, MI or stroke | Yes | 12 |
| STOPDAPT-2 | 2019 | 3,045 | 38 | 62 | Clopidogrel monotherapy after 1 month of DAPT versus standard DAPT | CV death, MI, stroke, ST and TIMI major or minor bleeding | Yes | 12 |
| TICO | 2020 | 3,056 | 100 | 0 | Ticagrelor monotherapy after 3 months of DAPT versus standard DAPT | TIMI major bleeding, all-cause death, MI, ST, stroke and target vessel revascularisation | Yes | 12 |
| STOPDAPT-2-ACS | 2021 | 4,169 | 100 | 0 | Clopidogrel monotherapy after 1 month of DAPT versus standard DAPT among ACS | CV death, MI, stroke, ST and TIMI major or minor bleeding | No | 12 |
| Guided de-escalation | ||||||||
| ANTARTIC | 2016 | 877 | 100 | 0 | PFT-guided de-escalation versus standard DAPT | CV death, MI, stroke, ST, urgent revascularisation and BARC 2-5 bleeding | No | 12 |
| TROPICAL-ACS | 2017 | 2,610 | 100 | 0 | PFT-guided de-escalation versus standard DAPT | CV death, MI, stroke and BARC 2-5 bleeding | Yes | 12 |
| POPular Genetics | 2019 | 2,488 | 100 | 0 | Genotype-guided de-escalation versus standard DAPT | All death, MI, definite ST, stroke, or PLATO major bleeding and PLATO major or minor bleeding | Yes | 12 |
| Unguided de-escalation | ||||||||
| TOPIC | 2017 | 646 | 100 | 0 | Clopidogrel-based DAPT versus standard DAPT | CV death, urgent revascularisation, stroke and BARC 2-5 bleeding | Yes | 12 |
| HOST-REDUCE-POLYTHEC-ACS | 2020 | 3,429 | 100 | 0 | Prasugrel 5 mg-based DAPT versus prasugrel 10 mg-based DAPT | All death, MI, ST, repeat revascularisation, stroke and BARC 2-5 bleeding | Yes | 12 |
| TALOS-MI | 2021 | 2,697 | 100 | 0 | Clopidogrel-based DAPT versus ticagrelor-based DAPT | CV death, MI, stroke and BARC 2-5 bleeding | Yes | 12 |
| ACS: acute coronary syndrome; BARC: Bleeding Academic Research Consortium; CCS: chronic coronary syndrome; CRNM: clinically relevant non-major; CV: cardiovascular; DAPT: dual antiplatelet therapy; HBR: high bleeding risk; ISTH: International Society on Thrombosis and Haemostasis; MI: myocardial infarction; PFT: platelet function test; PLATO: Platelet Inhibition and Patient Outcomes; ST: stent thrombosis; TIMI: Thrombolysis in Myocardial Infarction; VKA: vitamin K antagonists | ||||||||
Shortening DAPT
Shortening the duration of DAPT as a strategy to reduce bleeding events has been the most broadly explored approach in 13 published RCTs (Table 3). The shortening of DAPT traditionally consists of the withdrawal of the P2Y12 inhibitor before the recommended standard period of DAPT, usually 3 or 6 months after PCI. Alternatively, shortened DAPT can also occur due to the discontinuation of aspirin while maintaining P2Y12 inhibitor monotherapy, described in greater detail in the aspirin-free approach section below. Seven trials included shortened DAPT, the discontinuation of a P2Y12 inhibitor while maintaining aspirin, and compared 6-month versus 12-month DAPT. Four trials included 3-month versus 12-month DAPT and 2 trials a 1-month versus 6- and 12-month DAPT121,122,123,124,125,126,127,128,129,130,131. Although a number of registries with different stent platforms conducted in high bleeding risk (HBR) patients have evaluated the safety and efficacy of short DAPT, the Management of High Bleeding Risk Patients Post Bioresorbable Polymer Coated Stent Implantation with an Abbreviated versus Standard DAPT Regimen (MASTER-DAPT) was the first RCT to selectively enrol HBR patients130,132,133. Overall, results of the individual studies, as well as pooled analyses of RCTs, showed the early withdrawal of P2Y12 inhibitor reduced bleeding, including major bleeding without any significant increase of thrombotic events112,134. However, it has been argued that many of the studies enrolled patients at low ischaemic risk or that the studies were not powered for hard ischaemic endpoints; whether a trade-off in thrombotic complication can be ruled out in high-risk settings remains to be fully defined. The recent ARC-HBR trade-off model may represent a useful tool to balance bleeding and ischaemic risks135. In fact, patients with ACS had an increase in MI with 6 months compared with 12 months of DAPT127. Overall, these considerations are the reason for which shortened DAPT durations should be reserved for HBR patients for whom the benefits are more likely to outweigh the risks. Further studies are warranted to support shortened DAPT among high-risk populations for both bleeding and thrombotic events.
P2Y<sub>12</sub> monotherapy
For decades, aspirin has represented the backbone therapy for all novel antithrombotic regimens34. Accordingly, the relative benefit of new drugs has remained elusive given that treatment regimens were developed with aspirin as an integral component. Hence, with the development of new therapies, often with enhanced antithrombotic efficacy, the stacking on of treatment consistently showed an increase in bleeding, offsetting any ischaemic benefit134,136. These considerations in conjunction with the established gastrointestinal toxicity associated with aspirin have prompted investigations evaluating the safety and efficacy of P2Y12 monotherapy regimens137.
The first therapeutic area exploring this concept was in patients requiring OAC, such as those with atrial fibrillation, undergoing PCI50. These patients have the theoretical need for triple antithrombotic therapy (TAT, defined as DAPT+OAC), which, however, is associated with prohibitively high rates of bleeding. In light of the pivotal role of P2Y12 mediated signalling on arterial thrombotic complications in patients undergoing PCI, a strategy of dropping aspirin as an attempt to reduce (mostly gastrointestinal) bleeding, and maintaining DAPT with a P2Y12 inhibitor and an OAC, was explored. Importantly, pharmacodynamic studies have shown a synergism in antithrombotic effects with treatment with an OAC and a P2Y12 inhibitor138,139,140. A seminal investigation demonstrating that stented patients who were also treated with a vitamin K antagonist had a significant reduction in bleeding without any increase in thrombotic complications by discontinuing aspirin and maintaining a P2Y12 inhibitor141, prompted subsequent RCTs to test all 4 commercially available agents (dabigatran, rivaroxaban, apixaban, and edoxoban)142,143,144,145. The results of these trials support overall the concept that after a brief period of DAPT, aspirin can be safely discontinued without any trade-off in ischaemic complications in most patients. While the optimal timing of aspirin discontinuation for patients on an OAC is a topic of controversy (between hospital discharge up to 1 week versus 1 month), it is now commonly accepted that this should not be prolonged beyond 30 days41,51. It is, however, important to note that these studies are of limited scope, due to their sample size, to rule out any increase in hard ischaemic events. To this extent, several meta-analyses have been performed146. Some, but not all, showed early discontinuation of aspirin to be associated with a significant increase in risk of thrombotic complications, suggesting that aspirin be maintained for at least 30 days in high-risk subjects147,148,149,150,151. Moreover, given that the recommended antiplatelet agent to be used in these patients is clopidogrel, the potential risk deriving from aspirin withdrawal among patients non-responsive to clopidogrel, as well as the impact of potent P2Y12 inhibitors in this setting, requires further investigations32,33,103.
The favourable safety findings observed with aspirin discontinuation in patients undergoing PCI who also require treatment with an OAC has stimulated interest in investigations evaluating dropping aspirin even among patients not requiring OAC in the presence of effective P2Y12 inhibition. Of note, in vitro and ex vivo PD investigations have suggested that aspirin provides limited antithrombotic effects in addition to potent P2Y12 blockade, and have represented the rationale for the use of P2Y12 monotherapy approaches138,152,153,154,155,156. GLOBAL-LEADERS was the first study to clinically test this strategy, comparing 1-month DAPT followed by 23 months of ticagrelor 90 mg/bid monotherapy versus standard DAPT for 12 months followed by aspirin among 15,968 patients undergoing PCI. Although at a follow-up of 24 months, there was no significant difference in the primary ischaemic endpoint of all-cause death and MI between arms, there were no safety (i.e., bleeding) concerns that emerged from this study157. GLOBAL-LEADERS was however followed by a number of RCTs that consistently showed that discontinuing aspirin after 1-3 months and maintaining P2Y12 inhibitor monotherapy markedly reduced bleeding without any increase in thrombotic events144,158,159,160.
The Ticagrelor With Aspirin or Alone in High-Risk Patients After Coronary Intervention (TWILIGHT) trial was a double-blind, placebo-controlled design trial including 7,119 patients with at least 1 clinical and 1 angiographic feature associated with a high risk of ischaemic or bleeding events, who were randomised after 3 months of uneventful DAPT with ticagrelor plus aspirin to take ticagrelor and receive aspirin or a placebo for 1 year144. There was a significant 44% reduction of the primary endpoint of Bleeding Academic Research Consortium (BARC) 2-5 bleeding, favouring ticagrelor monotherapy and no difference in MACE between groups144. Importantly, MACE results were consistent among subgroups of high-risk patients such as those with diabetes mellitus and ACS and those undergoing complex PCI161,162,163. The use of prasugrel monotherapy in the setting of P2Y12 monotherapy strategies is limited, and thus far has only been tested in a pilot study including 201 patients with CCS undergoing low-risk PCI164. Overall, pooled analyses showed a very short DAPT followed by a P2Y12 inhibitor monotherapy reduced bleeding, including major bleeding, without a trade-off in ischaemic events134,136.
It is important to note that while some of the above-mentioned studies were conducted with clopidogrel, these were in low-risk patients and thus question whether the lack of increase of thrombotic complications would be preserved in ACS patients. The ShorT and OPtimal Duration of Dual AntiPlatelet Therapy-2 ACS study (STOPDAPT-2 ACS), including a total of 4,169 patients, failed to meet non-inferiority for the primary endpoint of net adverse clinical events (NACE) with clopidogrel monotherapy after a 1-month DAPT versus the standard 12-month DAPT, mainly driven by an increase of MI (H W. STOPDAPT-2 ACS: 1-month dual antiplatelet therapy followed by clopidogrel monotherapy in acute coronary syndrome. ESC Congress 2021). These findings call for caution on dropping aspirin too early among high-risk patients, such as those with ACS, when a non-potent P2Y12 inhibitor is used as monotherapy.
De-escalation of P2Y<sub>12</sub> inhibitors
De-escalation of P2Y12 inhibiting therapy consists in switching from more potent (i.e., prasugrel or ticagrelor) to less potent (i.e., clopidogrel) agents, and aims at reducing bleeding without any trade-off in ischaemic events165. Accordingly, the strategy of de-escalation typically applies to the setting of ACS, in which more potent P2Y12 inhibitors are recommended as the standard of care. De-escalation can be guided or un-guided (Table 3). A guided approach implies the use of platelet function or genetic tests that rule out clopidogrel-HPR or the presence of CYP2C19 LoF alleles which are known to be associated with an increased risk of thrombotic complications post-PCI. An unguided approach consists in de-escalation without the aid of platelet function or genetic testing. A guided approach allows for de-escalation early after PCI. On the contrary, an unguided de-escalation early after PCI has been associated with an increase in thrombotic complications166. Accordingly, waiting for the highest risk period of thrombotic complications post-PCI to elapse (e.g., 1 month) prior to de-escalation, represents a safer time frame for considering unguided de-escalation (Figure 3).
Guided de-escalation
A guided de-escalation approach has been tested in 3 RCTs, all in patients with ACS, using either platelet function testing (n=2) or genetic testing (n=1) (Table 3)167,168,169. Testing Responsiveness to Platelet Inhibition on Chronic Antiplatelet Treatment For Acute Coronary Syndromes Trial (TROPICAL-ACS) is the largest of the studies using platelet function testing, the results of which had an impact on guideline recommendations167. In particular, among 2,610 ACS patients undergoing PCI, guided de-escalation was non-inferior for the primary composite endpoint of NACE as compared to standard of care, with a trend towards reduced bleeding at 12 months compared to the standard group167.
The Cost-effectiveness of Genotype Guided Treatment With Antiplatelet Drugs in ST-segment elevation MI (STEMI) Patients: Optimization of Treatment (POPular Genetics) used genetic testing to guide de-escalation and was conducted in 2,488 STEMI patients undergoing primary PCI who were randomised to either a genotype-guided de-escalation or a standard of care selection (mostly ticagrelor, 91%) of oral P2Y12 inhibitors169. The genotype-guided strategy was found to be non-inferior for NACE and superior in terms of PLATO major or minor bleeding, as compared to standard of care at 12-month follow-up169. The observation that platelet function testing-guided de-escalation was not associated with significantly reduced bleeding as compared to standard therapy can be explained by the fact that some patients who de-escalate to clopidogrel are found to have HPR and need to escalate to more potent P2Y12 inhibition, which enhances the risk of bleeding. It is also important to note that 7-14 days of maintenance treatment with clopidogrel is needed after de-escalation before assessing platelet responsiveness, a time frame during which patients who have HPR are at increased risk of thrombotic events66,166. Overall, it may be argued that the individual RCTs were of limited power to assess with granularity the ischaemic and bleeding endpoints. A recent meta-analysis overcoming such limitations found that a strategy of guided de-escalation of antiplatelet therapy is associated with a 19% reduction of any bleeding, driven by a reduction of minor bleeding, without any trade-off in efficacy, as compared to standard selection of antiplatelet therapy among patients undergoing PCI33. Moreover, there were no differences between the use of genetic or platelet tests33. Finally, a network meta-analysis focusing on ACS has shown that, compared with routine selection of potent P2Y12 inhibiting therapy (prasugrel or ticagrelor), a guided selection of P2Y12 inhibiting therapy is associated with the most favourable balance between safety and efficacy170.
Unguided de-escalation
The Timing of Platelet Inhibition After Acute Coronary Syndrome (TOPIC) was a single-centre RCT with 646 patients comparing standard versus unguided de-escalation from a potent platelet inhibitor to clopidogrel 1 month after ACS171. There was a significant reduction of both the primary endpoint of NACE and bleeding171. However, it should be noted that MI was not included in the primary composite endpoint (CV death, urgent revascularisation, stroke and BARC bleeding 2-5) and this population was mostly composed of patients undergoing non-complex PCI. Prasugrel-based de-escalation of DAPT after PCI in patients with ACS (HOST-REDUCE-POLYTECH-ACS), compared standard versus unguided de-escalation of antiplatelet therapy by reducing prasugrel dosage (from 10 mg to 5 mg daily) versus standard prasugrel dosing (10 mg daily) 1 month after ACS among East Asian patients undergoing PCI (n=2,338) and showed that de-escalation was non-inferior to standard therapy for the primary endpoint of MACE172. Finally, the TicAgrelor Versus CLOpidogrel in Stabilized Patients With Acute Myocardial Infarction (TALOS-MI), comparing de-escalation from ticagrelor- to clopidogrel 1 month after PCI versus standard ticagrelor-based DAPT among 2,697 East Asian ACS patients, showed that the primary endpoint of NACE, as well of BARC bleeding 2-5, was significantly reduced in the de-escalation arm173. It is important to note that in these trials the de-escalation of P2Y12 inhibitors was performed 1 month after PCI - the period in which the risk of ischaemic events is highest - while among trials using a guided de-escalation this was performed earlier (0-14 days) after PCI. A recent meta-analysis has shown that both guided and unguided de-escalation reduce bleeding without any trade-off in ischaemic events174.
Future perspectives
Future directions in the field of antiplatelet therapy among PCI patients include the development of new antiplatelet agents, the implementation of new antiplatelet strategies with available agents and the conduct of further studies in support of current antiplatelet strategies (Table 4). Several new antiplatelet agents intended for use among patients undergoing PCI are under clinical development. Agents include selatogrel (a reversible non-thienopyridine P2Y12 receptor antagonist administered subcutaneously), RUC-4 (a GPIIb/IIIa inhibitor with a novel mechanism of action for intramuscular administration) and revacept (an inhibitor of GPVI, the major platelet collagen receptor, administered intravenously) (Figure 1)175,176,177. A detailed description of these agents intended for acute use goes beyond the scope of this manuscript.
Table 4. Ongoing studies on antiplatelet therapy among patients who underwent percutaneous coronary intervention.
| Study name | NCT | Number of patients | Treatment arms and population | Primary endpoint |
|---|---|---|---|---|
| New drugs | ||||
| SOS-AMI | NCT04957719 | 1,400 | Self-administration of selatogrel 16 mg versus placebo subcutaneously with the autoinjector upon occurrence of symptoms suggestive of an acute MI | Status as assessed by a 6-point ordinal scale BARC bleeding 3-5 |
| CELEBRATE | NCT04825743 | 1,668 | Subcutaneous injection or RUC-4 at the dose of 0.110 mg/kg or 0.130 mg/kg versus placebo in the ambulance after diagnosis of STEMI and before hospital arrival | Restoration of the coronary artery blood flow Resolution of ST segment deviation BARC 3-5 and GUSTO severe bleeding |
| New strategies | ||||
| TAILORED-CHIP | NCT03465644 | 2,000 | 6-month DAPT with ticagrelor 60 mg/bid followed by 6-month clopidogrel monotherapy versus 12-month DAPT with clopidogrel among high-risk patients undergoing complex PCI | NACE |
| Optimized-APT | NCT04338919 | 2,020 |
DAPT with ticagrelor 90 mg/bid for the first month, followed by ticagrelor 90 mg/bid monotherapy from the second to the sixth month and ticagrelor 45 mg/bid monotherapy from the seventh to the twelfth month versus DAPT with ticagrelor 90 mg/bid for 12 months among ACS |
MACE NACE BARC 2-5 bleeding |
| OPT-PEACE | NCT03198741 | 593 | Aspirin monotherapy versus clopidogrel monotherapy versus DAPT after 6 months of DAPT among PCI patients | Gastrointestinal mucosal injury |
| E5TION | NCT04734353 | 492 | Prasugrel 5 mg/day for 12 months versus ticagrelor 60 mg/bid for 12 months among high-risk PCI with PCI with the Firehawk stent | BARC 2-5 bleeding |
| ELECTRA-SIRIO | NCT04718025 | 4,500 | DAPT with ticagrelor 90 mg/bid for 1 month followed by DAPT with ticagrelor 60 up to 12 months versus discontinuation of ticagrelor 60 mg/bid at 3 months versus placebo among ACS | MACE BARC 3-5 bleeding |
| CAGEFREEII | NCT04971356 | 1,908 | Aspirin plus ticagrelor for 1 month followed by 5 months ticagrelor monotherapy versus aspirin plus ticagrelor for 12 months in ACS patients with drug-coated balloon | NACE |
| LD-ASPIRIN | NCT04240834 | 1,220 | Aspirin (50 mg od) plus ticagrelor (90 mg bid) for 12 months versus aspirin (75 mg od) plus ticagrelor (90 mg bid) for 12 months among ACS | MACCE |
| STOPDAPT-3 | NCT04609111 | 3,110 | 1-month prasugrel monotherapy followed by clopidogrel monotherapy versus 1-month DAPT with prasugrel followed by aspirin monotherapy after PCI with everolimus-eluting cobalt-chromium | MACE BARC 3-5 bleeding |
| Guided escalation of P2Y12 inhibitors | ||||
| GUARANTEE | NCT03783351 | 3,780 | Genotype-guided versus standard antiplatelet therapy among PCI patients | MACCE |
| Potent P2Y12 inhibiting therapy | ||||
| ATTEMPT | NCT04014803 | 3,500 | 12-month DAPT with prasugrel versus 12-month DAPT with clopidogrel among patients undergoing elective complex PCI | MACE |
| Shortening DAPT | ||||
| DUAL-ACS2 | NCT03252249 | 19,519 | 3-month versus 12-month DAPT among ACS patients | All-death |
| PARTHENOPE | NCT04135989 | 2,106 | Personalised (3-, 6- or 24-month) DAPT versus standard (12-month) DAPT among PCI patients | NACE |
| TARGET SAFE | NCT03287167 | 1,720 | 1-month versus 6-month DAPT among HBR patients undergoing PCI with the Firehawk stent | NACE |
| TARGET DAPT | NCT03008083 | 2,446 | 3-month versus 12-month DAPT among patients undergoing PCI with the Firehawk stent | NACCE |
| Long term P2Y12 inhibiting therapy | ||||
| OPT-BIRISK | NCT03431142 | 7,700 | 9 months of clopidogrel monotherapy versus additional 9 months of DAPT after initial 9-12 months of DAPT among ACS patients with high ischaemic and bleeding risk with prior PCI (≥12 months) | BARC bleeding 2-5 |
| SMART-CHOICE 2 | NCT03119012 | 1,520 | P2Y12 inhibitor monotherapy with clopidogrel or ticagrelor 60 mg/bid from 12 to 36 months versus extended DAPT with ticagrelor 60 mg/bid for 36 months among patients with prior uneventful PCI (≥12 months) | MACCE |
| SMART-CHOICE 3 | NCT04418479 | 5,000 | Clopidogrel monotherapy versus aspirin monotherapy among patients at high ischaemic risk with prior PCI (≥12 months) | MACCE |
| A-CLOSE | NCT03947229 | 3,200 | Clopidogrel monotherapy from 12 to 36 months versus extended DAPT for 36 months among patients at high ischaemic risk and event free for 12 months after DES implantation | NACE |
| Prolonging DAPT duration | ||||
| DAPT-MVD | NCT04624854 | 8,250 | Aspirin alone versus DAPT with clopidogrel among patients with multivessel disease who underwent DES implantation for 12 months | MACCE |
| Aspirin-free | ||||
| NEOMINDSET | NCT04360720 | 3,400 | Prasugrel monotherapy for 12 months versus 12-month DAPT among non-HBR and ticagrelor monotherapy for 12 months versus 6 months DAPT among HBR ACS patients undergoing PCI |
MACCE and BARC bleeding 2-5 |
| ULTIMATE-DAPT | NCT03971500 | 3,486 | Ticagrelor monotherapy versus standard DAPT in ACS patients undergoing PCI |
MACCE and BARC bleeding 2-5 |
| Short-term Dual Antiplatelet Therapy After Deployment of Bioabsorbable Polymer Everolimus-eluting Stent | NCT03447379 | 1,452 | Clopidogrel or ticagrelor monotherapy after 3 months of DAPT versus 12 months of DAPT among patients undergoing PCI with everolimus-eluting stent | MACCE |
| BULK-STEMI | NCT04570345 | 1,002 | 3-month DAPT followed by ticagrelor monotherapy versus 12-month DAPT after second-generation sirolimus stent | NACE |
| ACS: acute coronary syndrome; BARC: Bleeding Academic Research Consortium; CCS: chronic coronary syndrome; CRNM: clinically relevant non-major; CV: cardiovascular; DAPT: dual antiplatelet therapy; DES: drug-eluting stent; HBR: high bleeding risk; ISTH: International Society on Thrombosis and Haemostasis; MACCE: major adverse cardiovascular and cerebrovascular events; MI: myocardial infarction; PFT: platelet function test; PLATO: Platelet Inhibition and Patient Outcomes; NACE: net adverse clinical events; NACCE: Net Adverse Clinical and Cerebral Events; PCI: percutaneous coronary intervention; TIMI: Thrombolysis in Myocardial Infarction; VKA: vitamin K antagonists | ||||
Although prolonging intensified antithrombotic therapy by adding a P2Y12 inhibitor (i.e., DAPT) or rivaroxaban 2.5 mg bid (i.e., DPI) to aspirin reduces the risk of ischaemic recurrences in high-risk patients with CCS compared to aspirin alone, the concerns surrounding the increased risk of bleeding has stimulated research on identified treatments with a better trade-off between ischaemic and bleeding events. Compared with aspirin, rivaroxaban 5 mg/bid did not significantly reduce ischaemic events in patients with stable vascular disease, underscoring the importance of antiplatelet therapy for patients with CAD120. Although the strategy of P2Y12 inhibitor monotherapy and discontinuing aspirin after a brief period of DAPT has shown to reduce bleeding complications without any trade-off in ischaemic events, the available evidence is limited to up to 12 months of therapy after which most patients resumed aspirin monotherapy. Whether P2Y12 inhibitor monotherapy is superior to aspirin monotherapy in patients who have completed required DAPT after DES implantation was tested in the Harmonizing Optimal Strategy for Treatment of Coronary Artery Stenosis- EXtended Antiplatelet Monotherapy (HOST-EXAM) trial178. The trial randomised 5,530 patients from South Korea without clinical events for 6-18 months after PCI to either aspirin 100 mg/od or clopidogrel 75 mg/od monotherapy. At 24 months, the primary endpoint of MACE as well as BARC bleeding type 3-5 were reduced with clopidogrel compared to aspirin178. These results are consistent with those from the CAPRIE trial, published 20 years earlier, which enrolled 19,185 patients with atherosclerotic disease, showing clopidogrel to be associated with a reduction, albeit of marginal statistical significance, of ischaemic events with better gastrointestinal tolerability. It is important to note that in CAPRIE, a 325 mg dose regimen of aspirin was used179.
Ultimately, new formulations of aspirin designed to reduce gastrointestinal toxicity while maintaining adequate absorption and antiplatelet effects are currently being designed. Among these, a liquid formulation of a novel pharmaceutical lipid-aspirin complex (PL-ASA) has been approved. In particular, PL-ASA has pharmacokinetic and pharmacodynamic profiles similar to immediate-release aspirin and provides more reliable drug absorption than enteric-coated aspirin formulations known to be more delayed and erratic180,181. Moreover, its formulation and release properties mitigate disruption of the protective phospholipid bilayer of the gastrointestinal mucosa, resulting in less acute gastric injury (erosions and/or ulcers)182. How PL-ASA compares with standard enteric-coated aspirin and its long-term gastrointestinal safety remain unknown.
Conclusions
Antiplatelet therapy represents the cornerstone of treatment for the prevention of local and systemic ischaemic complications in patients with CAD undergoing PCI. Over the past decades different antiplatelet regimens using aspirin and P2Y12 inhibitors have been developed and implemented in clinical practice. A better understanding of the ischaemic and bleeding risk profile, as well as individual responsiveness to antiplatelet agents, has been instrumental in defining the optimal regimen for the individual patient. In particular, the intensity and the duration of aspirin and P2Y12 inhibiting therapy should be adjusted to reduce the risk of ischaemic complications while minimising the risk of bleeding. Strategies developed to mitigate the risk of bleeding include shortening DAPT duration, P2Y12 inhibitor monotherapy and de-escalation. In the absence of high bleeding risk, patients at increased ischaemic risk may consider prolonging intensified antithrombotic therapy either by means of DAPT or DPI. The use of platelet function and genetic testing can indeed be of aid in the selection of P2Y12 inhibitor therapy. An integrated approach, including scores/definitions to define ischaemic and bleeding risk, procedural characteristics, and tools to help assess drug response, represents the most promising approach for a personalised selection of antiplatelet agents among patients undergoing PCI.
Acknowledgments
Acknowledgements
BioRender platform and templates were used for creating figures.
Conflict of interest statement
D.J. Angiolillo declares that he has received consulting fees or honoraria from Abbott, Amgen, Aralez, AstraZeneca, Bayer, Biosensors, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, Daiichi-Sankyo, Eli Lilly, Haemonetics, Janssen, Merck, PhaseBio, PLx Pharma, Pfizer, Sanofi, and The Medicines Company and has received payments for participation in review activities from CeloNova and St Jude Medical, outside the present work. D.J. Angiolillo also declares that his institution has received research grants from Amgen, AstraZeneca, Bayer, Biosensors, CeloNova, CSL Behring, Daiichi-Sankyo, Eisai, Eli Lilly, Gilead, Janssen, Matsutani Chemical Industry Co., Merck, Novartis, Osprey Medical, Renal Guard Solutions and the Scott R. MacKenzie Foundation. J.P Collet has received financial support/sponsorship for research support, consultation or speaker fees from the following companies: AstraZeneca, Boston Scientific, Bristol-Myers Squibb, Lead-Up, Medtronic, WebMD and Sanofi Aventis. M.L. O'Donoghue declares that she has received grants via Brigham and Women’s Hospital from Amgen, Novartis, AstraZeneca, Janssen, Intarcia, Merck, and Pfizer and honararia from Novartis, AstraZeneca, Amgen, and Janssen. The other authors have no conflicts of interest to declare.
Contributor Information
Dominick J. Angiolillo, Division of Cardiology, University of Florida College of Medicine, Jacksonville, FL, USA.
Mattia Galli, Department of Cardiovascular and Thoracic Sciences, Fondazione Policlinico Universitario A. Gemelli IRCCS, Catholic University of the Sacred Heart, Rome, Italy.
Jean-Philippe Collet, ACTION Study Group, Institut de Cardiologie, Hôpital Pitié-Salpêtrière, Sorbonne Université, Paris, France.
Adnan Kastrati, Deutsches Herzzentrum München, Technische Universität München, Munich, Germany.
Michelle L. O'Donoghue, TIMI Study Group, Cardiovascular Division, Brigham and Women’s Hospital, Boston, MA, USA.
References
- Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, Jüni P, Kastrati A, Koller A, Kristensen SD, Niebauer J, Richter DJ, Seferović PM, Sibbing D, Stefanini GG, Windecker S, Yadav R, Zembala MO. 2018 ESC/EACTS Guidelines on myocardial revascularization. EuroIntervention. 2019;14(14):1435–534. doi: 10.4244/EIJY19M01_01. [DOI] [PubMed] [Google Scholar]
- Cao D, Chandiramani R, Chiarito M, Claessen BE, Mehran R. Evolution of antithrombotic therapy in patients undergoing percutaneous coronary intervention: a 40-year journey. Eur Heart J. 2020;42:339–51. doi: 10.1093/eurheartj/ehaa824. [DOI] [PubMed] [Google Scholar]
- Capodanno D, Alfonso F, Levine GN, Valgimigli M, Angiolillo DJ. ACC/AHA Versus ESC Guidelines on Dual Antiplatelet Therapy: JACC Guideline Comparison. J Am Coll Cardiol. 2018;72:2915–31. doi: 10.1016/j.jacc.2018.09.057. [DOI] [PubMed] [Google Scholar]
- Prasad A, Herrmann J. Myocardial infarction due to percutaneous coronary intervention. N Engl J Med. 2011;364:453–64. doi: 10.1056/NEJMra0912134. [DOI] [PubMed] [Google Scholar]
- Moon JY, Franchi F, Rollini F, Angiolillo DJ. Evolution of Coronary Stent Technology and Implications for Duration of Dual Antiplatelet Therapy. Prog Cardiovasc Dis. 2018;60:478–90. doi: 10.1016/j.pcad.2017.12.004. [DOI] [PubMed] [Google Scholar]
- Sibbing D, Aradi D, Alexopoulos D, Ten Berg, Bhatt DL, Bonello L, Collet JP, Cuisset T, Franchi F, Gross L, Gurbel P, Jeong YH, Mehran R, Moliterno DJ, Neumann FJ, Pereira NL, Price MJ, Sabatine MS, So DYF, Stone GW, Storey RF, Tantry U, Trenk D, Valgimigli M, Waksman R, Angiolillo DJ. Updated Expert Consensus Statement on Platelet Function and Genetic Testing for Guiding P2Y12 Receptor Inhibitor Treatment in Percutaneous Coronary Intervention. JACC Cardiovasc Interv. 2019;12:1521–37. doi: 10.1016/j.jcin.2019.03.034. [DOI] [PubMed] [Google Scholar]
- Lippi G, Franchini M, Targher G. Arterial thrombus formation in cardiovascular disease. Nat Rev Cardiol. 2011;8:502–12. doi: 10.1038/nrcardio.2011.91. [DOI] [PubMed] [Google Scholar]
- Cosemans JM, Schols SE, Stefanini L, de Witt, Feijge MA, Hamulyák K, Deckmyn H, Bergmeier W, Heemskerk JW. Key role of glycoprotein Ib/V/IX and von Willebrand factor in platelet activation-dependent fibrin formation at low shear flow. Blood. 2011;117:651–60. doi: 10.1182/blood-2010-01-262683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angiolillo DJ, Ueno M, Goto S. Basic principles of platelet biology and clinical implications. Circ J. 2010;74:597–607. doi: 10.1253/circj.cj-09-0982. [DOI] [PubMed] [Google Scholar]
- Borissoff JI, Spronk HMH, ten Cate. The hemostatic system as a modulator of atherosclerosis. N Engl J Med. 2011;364:1746–60. doi: 10.1056/NEJMra1011670. [DOI] [PubMed] [Google Scholar]
- Stark K, Massberg S. Interplay between inflammation and thrombosis in cardiovascular pathology. Nat Rev Cardiol. 2021;18:666–82. doi: 10.1038/s41569-021-00552-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:2045–51. doi: 10.1161/ATVBAHA.108.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biasucci LM, La Rosa, Pedicino D, D'Aiello A, Galli M, Liuzzo G. Where Does Inflammation Fit? Curr Cardiol Rep. 2017;19:84. doi: 10.1007/s11886-017-0896-0. [DOI] [PubMed] [Google Scholar]
- Libby P, Pasterkamp G, Crea F, Jang IK. Reassessing the Mechanisms of Acute Coronary Syndromes. Circ Res. 2019;124:150–60. doi: 10.1161/CIRCRESAHA.118.311098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angiolillo DJ, Capodanno D, Goto S. Platelet thrombin receptor antagonism and atherothrombosis. Eur Heart J. 2010;31:17–28. doi: 10.1093/eurheartj/ehp504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley JH, Conway EM. Cross Talk Pathways Between Coagulation and Inflammation. Circ Res. 2016;118:1392–408. doi: 10.1161/CIRCRESAHA.116.306853. [DOI] [PubMed] [Google Scholar]
- Vergallo R, Crea F. Atherosclerotic Plaque Healing. N Engl J Med. 2020;383:846–57. doi: 10.1056/NEJMra2000317. [DOI] [PubMed] [Google Scholar]
- Vergallo R, Porto I, D’Amario D, Annibali G, Galli M, Benenati S, Bendandi F, Migliaro S, Fracassi F, Aurigemma C, Leone AM, Buffon A, Burzotta F, Trani C, Niccoli G, Liuzzo G, Prati F, Fuster V, Jang IK, Crea F. Coronary Atherosclerotic Phenotype and Plaque Healing in Patients With Recurrent Acute Coronary Syndromes Compared With Patients With Long-term Clinical Stability: An In Vivo Optical Coherence Tomography Study. JAMA Cardiol. 2019;4:321–9. doi: 10.1001/jamacardio.2019.0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavender MA, Bhatt DL, Stone GW, White HD, Steg PG, Gibson CM, Hamm CW, Price MJ, Leonardi S, Prats J, Deliargyris EN, Mahaffey KW, Harrington RA CHAMPION PHOENIX Investigators. Consistent Reduction in Periprocedural Myocardial Infarction With Cangrelor as Assessed by Multiple Definitions: Findings From CHAMPION PHOENIX (Cangrelor Versus Standard Therapy to Achieve Optimal Management of Platelet Inhibition). Circulation. 2016;134:723–33. doi: 10.1161/CIRCULATIONAHA.115.020829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt DL, Stone GW, Mahaffey KW, Gibson CM, Steg PG, Hamm CW, Price MJ, Leonardi S, Gallup D, Bramucci E, Radke PW, Widimský P, Tousek F, Tauth J, Spriggs D, McLaurin BT, Angiolillo DJ, Généreux P, Liu T, Prats J, Todd M, Skerjanec S, White HD, Harrington RA CHAMPION PHOENIX Investigators. Effect of platelet inhibition with cangrelor during PCI on ischemic events. N Engl J Med. 2013;368:1303–13. doi: 10.1056/NEJMoa1300815. [DOI] [PubMed] [Google Scholar]
- Galli M, Migliaro S, Rodolico D, G DIS, Piccinni C, Restivo A, Andreotti F, Vergallo R, Montone RA, Besis G, Buffon A, Romagnoli E, Aurigemma C, Leone AM, Burzotta F, Niccoli G, Trani C, Crea F, D'Amario D. Intracoronary bolus of glycoprotein IIb/IIIa inhibitor as bridging or adjunctive strategy to oral P2Y12 inhibitor load in the modern setting of STEMI. Minerva Cardiol Angiol. 2021 Apr 7. [Epub ahead of print]. doi: 10.23736/S2724-5683.21.05669-6. [DOI] [PubMed] [Google Scholar]
- Galli M, Vescovo GM, Andreotti F, D'Amario D, Leone AM, Benenati S, Vergallo R, Niccoli G, Trani C, Porto I. Impact of coronary stenting on top of medical therapy and of inclusion of periprocedural infarctions on hard composite endpoints in patients with chronic coronary syndromes: a meta-analysis of randomized controlled trials. Minerva Cardiol Angiol. 2021 May 4. [Epub ahead of print]. doi: 10.23736/S2724-5683.21.05645-3. [DOI] [PubMed] [Google Scholar]
- Silvain J, Zeitouni M, Paradies V, Zheng HL, Ndrepepa G, Cavallini C, Feldman DN, Sharma SK, Mehilli J, Gili S, Barbato E, Tarantini G, Ooi SY, von Birgelen, Jaffe AS, Thygesen K, Montalescot G, Bulluck H, Hausenloy DJ. Procedural myocardial injury, infarction and mortality in patients undergoing elective PCI: a pooled analysis of patient-level data. Eur Heart J. 2021;42:323–34. doi: 10.1093/eurheartj/ehaa885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capodanno D, Milluzzo RP, Angiolillo DJ. Intravenous antiplatelet therapies (glycoprotein IIb/IIIa receptor inhibitors and cangrelor) in percutaneous coronary intervention: from pharmacology to indications for clinical use. Ther Adv Cardiovasc Dis. 2019;13:1753944719893274. doi: 10.1177/1753944719893274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrono C, García Rodríguez, Landolfi R, Baigent C. Low-dose aspirin for the prevention of atherothrombosis. N Engl J M. 2005;353:2373–83. doi: 10.1056/NEJMra052717. [DOI] [PubMed] [Google Scholar]
- Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345:494–502. doi: 10.1056/NEJMoa010746. [DOI] [PubMed] [Google Scholar]
- Cadroy Y, Bossavy JP, Thalamas C, Sagnard L, Sakariassen K, Boneu B. Early potent antithrombotic effect with combined aspirin and a loading dose of clopidogrel on experimental arterial thrombogenesis in humans. Circulation. 2000;101:2823–8. doi: 10.1161/01.cir.101.24.2823. [DOI] [PubMed] [Google Scholar]
- Leon MB, Baim DS, Popma JJ, Gordon PC, Cutlip DE, Ho KK, Giambartolomei A, Diver DJ, Lasorda DM, Williams DO, Pocock SJ, Kuntz RE. A clinical trial comparing three antithrombotic-drug regimens after coronary-artery stenting. Stent Anticoagulation Restenosis Study Investigators. N Engl J Med. 1998;339:1665–71. doi: 10.1056/NEJM199812033392303. [DOI] [PubMed] [Google Scholar]
- Schömig A, Neumann FJ, Kastrati A, Schühlen H, Blasini R, Hadamitzky M, Walter H, Zitzmann-Roth EM, Richardt G, Alt E, Schmitt C, Ulm K. A randomized comparison of antiplatelet and anticoagulant therapy after the placement of coronary-artery stents. N Engl J Med. 1996;334:1084–9. doi: 10.1056/NEJM199604253341702. [DOI] [PubMed] [Google Scholar]
- Bertrand ME, Rupprecht HJ, Urban P, Gershlick AH CLASSICS Investigators. Double-blind study of the safety of clopidogrel with and without a loading dose in combination with aspirin compared with ticlopidine in combination with aspirin after coronary stenting : the clopidogrel aspirin stent international cooperative study (CLASSICS). Circulation. 2000;102:624–9. doi: 10.1161/01.cir.102.6.624. [DOI] [PubMed] [Google Scholar]
- Steinhubl SR, Berger PB, Mann JT, Fry ET, DeLago A, Wilmer C, Topol EJ CREDO Investigators. Clopidogrel for the Reduction of Events During Observation. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA. 2002;288:2411–20. doi: 10.1001/jama.288.19.2411. [DOI] [PubMed] [Google Scholar]
- Aradi D, Kirtane A, Bonello L, Gurbel PA, Tantry US, Huber K, Freynhofer MK, ten Berg, Janssen P, Angiolillo DJ, Siller-Matula JM, Marcucci R, Patti G, Mangiacapra F, Valgimigli M, Morel O, Palmerini T, Price MJ, Cuisset T, Kastrati A, Stone GW, Sibbing D. Bleeding and stent thrombosis on P2Y12-inhibitors: collaborative analysis on the role of platelet reactivity for risk stratification after percutaneous coronary intervention. Eur Heart J. 2015;36:1762–71. doi: 10.1093/eurheartj/ehv104. [DOI] [PubMed] [Google Scholar]
- Galli M, Benenati S, Capodanno D, Franchi F, Rollini F, D'Amario D, Porto I, Angiolillo DJ. Guided versus standard antiplatelet therapy in patients undergoing percutaneous coronary intervention: a systematic review and meta-analysis. Lancet. 2021;397:1470–83. doi: 10.1016/S0140-6736(21)00533-X. [DOI] [PubMed] [Google Scholar]
- Franchi F, Angiolillo DJ. Novel antiplatelet agents in acute coronary syndrome. Nat Rev Cardiol. 2015;12:30–47. doi: 10.1038/nrcardio.2014.156. [DOI] [PubMed] [Google Scholar]
- Buccheri S, Capodanno D, James S, Angiolillo DJ. Bleeding after antiplatelet therapy for the treatment of acute coronary syndromes: a review of the evidence and evolving paradigms. Expert Opin Drug Saf. 2019;18:1171–89. doi: 10.1080/14740338.2019.1680637. [DOI] [PubMed] [Google Scholar]
- Bassand JP. Acute Coronary Syndromes and Percutaneous Coronary Interventions: impact of bleeding and blood transfusion. Hamostaseologie. 2009;29:381–7. [PubMed] [Google Scholar]
- Rao SV, Eikelboom JA, Granger CB, Harrington RA, Califf RM, Bassand JP. Bleeding and blood transfusion issues in patients with non-ST-segment elevation acute coronary syndromes. Eur Heart J. 2007;28:1193–204. doi: 10.1093/eurheartj/ehm019. [DOI] [PubMed] [Google Scholar]
- Valgimigli M, Costa F, Lokhnygina Y, Clare RM, Wallentin L, Moliterno DJ, Armstrong PW, White HD, Held C, Aylward PE, Van de, Harrington RA, Mahaffey KW, Tricoci P. Trade-off of myocardial infarction vs. bleeding types on mortality after acute coronary syndrome: lessons from the Thrombin Receptor Antagonist for Clinical Event Reduction in Acute Coronary Syndrome (TRACER) randomized trial. Eur Heart J. 2017;38:804–10. doi: 10.1093/eurheartj/ehw525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez F, Harrington RA. Management of Antithrombotic Therapy after Acute Coronary Syndromes. N Engl J Med. 2021;384:452–60. doi: 10.1056/NEJMra1607714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, Agewall S, Dickstein K, Edvardsen T, Escaned J, Gersh BJ, Svitil P, Gilard M, Hasdai D, Hatala R, Mahfoud F, Masip J, Muneretto C, Valgimigli M, Achenbach S, Bax JJ ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407–77. doi: 10.1093/eurheartj/ehz425. [DOI] [PubMed] [Google Scholar]
- Collet JP, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, Dendale P, Dorobantu M, Edvardsen T, Folliguet T, Gale CP, Gilard M, Jobs A, Jüni P, Lambrinou E, Lewis BS, Mehilli J, Meliga E, Merkely B, Mueller C, Roffi M, Rutten FH, Sibbing D, Siontis GCM ESC Scientific Document Group. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42:1289–367. doi: 10.1093/eurheartj/ehaa575. [DOI] [PubMed] [Google Scholar]
- Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský P ESC Scientific Document Group. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119–77. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, Granger CB, Lange RA, Mack MJ, Mauri L, Mehran R, Mukherjee D, Newby LK, O'Gara PT, Sabatine MS, Smith PK, Smith SC. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: An Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention, 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease, 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction, 2014 AHA/ACC Guideline for the Management of Patients With Non-ST-Elevation Acute Coronary Syndromes, and 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery. Circulation. 2016;134:e123–55. doi: 10.1161/CIR.0000000000000404. [DOI] [PubMed] [Google Scholar]
- Capodanno D, Angiolillo DJ. Pretreatment with antiplatelet drugs in invasively managed patients with coronary artery disease in the contemporary era: review of the evidence and practice guidelines. Circ Cardiovasc Interv. 2015;8:e002301. doi: 10.1161/CIRCINTERVENTIONS.114.002301. [DOI] [PubMed] [Google Scholar]
- Schüpke S, Neumann FJ, Menichelli M, Mayer K, Bernlochner I, Wöhrle J, Richardt G, Liebetrau C, Witzenbichler B, Antoniucci D, Akin I, Bott-Flügel L, Fischer M, Landmesser U, Katus HA, Sibbing D, Seyfarth M, Janisch M, Boncompagni D, Hilz R, Rottbauer W, Okrojek R, Möllmann H, Hochholzer W, Migliorini A, Cassese S, Mollo P, Xhepa E, Kufner S, Strehle A, Leggewie S, Allali A, Ndrepepa G, Schühlen H, Angiolillo DJ, Hamm CW, Hapfelmeier A, Tölg R, Trenk D, Schunkert H, Laugwitz KL, Kastrati A. ISAR-REACT 5 Trial Investigators. Ticagrelor or Prasugrel in Patients with Acute Coronary Syndromes. N Engl J Med. 2019;381:1524–34. doi: 10.1056/NEJMoa1908973. [DOI] [PubMed] [Google Scholar]
- Tarantini G, Mojoli M, Varbella F, Caporale R, Rigattieri S, Andò G, Cirillo P, Pierini S, Santarelli A, Sganzerla P, Cacciavillani L, Babuin L, De Cesare, Limbruno U, Massoni A, Rognoni A, Pavan D, Belloni F, Cernetti C, Favero L, Saia F, Fovino LN, Masiero G, Roncon L, Gasparetto V, Ferlini M, Ronco F, Rossini R, Canova P, Trabattoni D, Russo A, Guiducci V, Penzo C, Tarantino F, Mauro C, Corrada E, Esposito G, Marchese A, Berti S, Martinato M, Azzolina D, Gregori D, Angiolillo DJ, Musumeci G DUBIUS Investigators; Italian Society of Interventional Cardiology. Timing of Oral P2Y12 Inhibitor Administration in Patients With Non-ST-Segment Elevation Acute Coronary Syndrome. J Am Coll Cardiol. 2020;76:2450–9. doi: 10.1016/j.jacc.2020.08.053. [DOI] [PubMed] [Google Scholar]
- Montalescot G, Bolognese L, Dudek D, Goldstein P, Hamm C, Tanguay JF, ten Berg, Miller DL, Costigan TM, Goedicke J, Silvain J, Angioli P, Legutko J, Niethammer M, Motovska Z, Jakubowski JA, Cayla G, Visconti LO, Vicaut E, Widimsky P ACCOAST Investigators. Pretreatment with prasugrel in non–ST-segment elevation acute coronary syndromes. N Engl J Med. 2013;369:999–1010. doi: 10.1056/NEJMoa1308075. [DOI] [PubMed] [Google Scholar]
- Capranzano P, Angiolillo DJ. Tackling the gap in platelet inhibition with oral antiplatelet agents in high-risk patients undergoing percutaneous coronary intervention. Expert Rev Cardiovasc Ther. 2021;19:519–35. doi: 10.1080/14779072.2021.1920925. [DOI] [PubMed] [Google Scholar]
- Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F, Jeppsson A, Jüni P, Kastrati A, Kolh P, Mauri L, Montalescot G, Neumann FJ, Petricevic M, Roffi M, Steg PG, Windecker S, Zamorano JL, Levine GN ESC Scientific Document Group; ESC Committee for Practice Guidelines (CPG); ESC National Cardiac Societies. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2018;39:213–60. doi: 10.1093/eurheartj/ehx419. [DOI] [PubMed] [Google Scholar]
- Capodanno D, Huber K, Mehran R, Lip GYH, Faxon DP, Granger CB, Vranckx P, Lopes RD, Montalescot G, Cannon CP, Ten Berg, Gersh BJ, Bhatt DL, Angiolillo DJ. Management of Antithrombotic Therapy in Atrial Fibrillation Patients Undergoing PCI: JACC State-of-the-Art Review. J Am Coll Cardiol. 2019;74:83–99. doi: 10.1016/j.jacc.2019.05.016. [DOI] [PubMed] [Google Scholar]
- Angiolillo DJ, Bhatt DL, Cannon CP, Eikelboom JW, Gibson CM, Goodman SG, Granger CB, Holmes DR, Lopes RD, Mehran R, Moliterno DJ, Price MJ, Saw J, Tanguay JF, Faxon DP. Antithrombotic Therapy in Patients with Atrial Fibrillation Treated with Oral Anticoagulation Undergoing Percutaneous Coronary Intervention: A North American Perspective: 2021 Update. Circulation. 2021;143:583–96. doi: 10.1161/CIRCULATIONAHA.120.050438. [DOI] [PubMed] [Google Scholar]
- Galli M, Gargiulo G. Towards a personalized selection of antithrombotic agents in patients undergoing PCI: the role of clinical presentation in tools for risk assessment. J Thromb Thrombolysis. 2022;53:495–8. doi: 10.1007/s11239-021-02553-w. [DOI] [PubMed] [Google Scholar]
- Giustino G, Chieffo A, Palmerini T, Valgimigli M, Feres F, Abizaid A, Costa RA, Hong MK, Kim BK, Jang Y, Kim HS, Park KW, Gilard M, Morice MC, Sawaya F, Sardella G, Genereux P, Redfors B, Leon MB, Bhatt DL, Stone GW, Colombo A. Efficacy and Safety of Dual Antiplatelet Therapy After Complex PCI. J Am Coll Cardiol. 2016;68:1851–64. doi: 10.1016/j.jacc.2016.07.760. [DOI] [PubMed] [Google Scholar]
- Galli M, Andreotti F, D'Amario D, Vergallo R, Vescovo GM, Giraldi L, Migliaro S, Ameri P, Porto I, Crea F. Antithrombotic therapy in the early phase of non-ST-elevation acute coronary syndromes: a systematic review and meta-analysis. Eur Heart J Cardiovasc Pharmacother. 2020;6:43–56. doi: 10.1093/ehjcvp/pvz031. [DOI] [PubMed] [Google Scholar]
- Capodanno D, Bhatt DL, Gibson CM, James S, Kimura T, Mehran R, Rao SV, Steg PG, Urban P, Valgimigli M, Windecker S, Angiolillo DJ. Bleeding avoidance strategies in percutaneous coronary intervention. Nat Rev Cardiol. 2022;19:117–32. doi: 10.1038/s41569-021-00598-1. [DOI] [PubMed] [Google Scholar]
- Capodanno D, Morice MC, Angiolillo DJ, Bhatt DL, Byrne RA, Colleran R, Cuisset T, Cutlip D, Eerdmans P, Eikelboom J, Farb A, Gibson CM, Gregson J, Haude M, James SK, Kim HS, Kimura T, Konishi A, Leon MB, Magee PFA, Mitsutake Y, Mylotte D, Pocock SJ, Rao SV, Spitzer E, Stockbridge N, Valgimigli M, Varenne O, Windhovel U, Krucoff MW, Urban P, Mehran R. Trial Design Principles for Patients at High Bleeding Risk Undergoing PCI: JACC Scientific Expert Panel. J Am Coll Cardiol. 2020;76:1468–83. doi: 10.1016/j.jacc.2020.06.085. [DOI] [PubMed] [Google Scholar]
- Yeh RW, Secemsky EA, Kereiakes DJ, Normand SL, Gershlick AH, Cohen DJ, Spertus JA, Steg PG, Cutlip DE, Rinaldi MJ, Camenzind E, Wijns W, Apruzzese PK, Song Y, Massaro JM, Mauri L DAPT Study Investigators. Development and Validation of a Prediction Rule for Benefit and Harm of Dual Antiplatelet Therapy Beyond 1 Year After Percutaneous Coronary Intervention. JAMA. 2016;315:1735–49. doi: 10.1001/jama.2016.3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa F, van Klaveren, James S, Heg D, Räber L, Feres F, Pilgrim T, Hong MK, Kim HS, Colombo A, Steg PG, Zanchin T, Palmerini T, Wallentin L, Bhatt DL, Stone GW, Windecker S, Steyerberg EW, Valgimigli M PRECISE-DAPT Study Investigators. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: a pooled analysis of individual-patient datasets from clinical trials. Lancet. 2017;389:1025–34. doi: 10.1016/S0140-6736(17)30397-5. [DOI] [PubMed] [Google Scholar]
- Nammas W, Kiviniemi T, Schlitt A, Rubboli A, Valencia J, Lip GYH, Karjalainen PP, Biancari F, Juhani Airaksinen. Value of DAPT score to predict adverse outcome in patients with atrial fibrillation undergoing percutaneous coronary intervention: A post-hoc analysis from the AFCAS registry. Int J Cardiol. 2018;253:35–9. doi: 10.1016/j.ijcard.2017.07.074. [DOI] [PubMed] [Google Scholar]
- Costa F, Valgimigli M, Steg PG, Bhatt DL, Hohnloser SH, Ten Berg, Miede C, Nordaby M, Lip GYH, Oldgren J, Cannon CP. Antithrombotic therapy according to baseline bleeding risk in patients with atrial fibrillation undergoing percutaneous coronary intervention: applying the PRECISE-DAPT score in RE-DUAL PCI. Eur Heart J Cardiovasc Pharmacother. 2020 Dec 1. [Epub ahead of print]. doi: 10.1093/ehjcvp/pvaa135. [DOI] [PubMed] [Google Scholar]
- Costa F, Van Klaveren, Feres F, James S, Räber L, Pilgrim T, Hong MK, Kim HS, Colombo A, Steg PG, Bhatt DL, Stone GW, Windecker S, Steyerberg EW, Valgimigli M PRECISE-DAPT Study Investigators. Dual Antiplatelet Therapy Duration Based on Ischemic and Bleeding Risks After Coronary Stenting. J Am Coll Cardiol. 2019;73:741–54. doi: 10.1016/j.jacc.2018.11.048. [DOI] [PubMed] [Google Scholar]
- Urban P, Mehran R, Colleran R, Angiolillo DJ, Byrne RA, Capodanno D, Cuisset T, Cutlip D, Eerdmans P, Eikelboom J, Farb A, Gibson CM, Gregson J, Haude M, James SK, Kim HS, Kimura T, Konishi A, Laschinger J, Leon MB, Magee PFA, Mitsutake Y, Mylotte D, Pocock S, Price MJ, Rao SV, Spitzer E, Stockbridge N, Valgimigli M, Varenne O, Windhoevel U, Yeh RW, Krucoff MW, Morice MC. Defining High Bleeding Risk in Patients Undergoing Percutaneous Coronary Intervention. Circulation. 2019;140:240–61. doi: 10.1161/CIRCULATIONAHA.119.040167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargiulo G, Esposito G. Consolidating the value of the standardised ARC-HBR definition. EuroIntervention. 2021;16:1126–8. doi: 10.4244/EIJV16I14A202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parodi G, Marcucci R, Valenti R, Gori AM, Migliorini A, Giusti B, Buonamici P, Gensini GF, Abbate R, Antoniucci D. High residual platelet reactivity after clopidogrel loading and long-term cardiovascular events among patients with acute coronary syndromes undergoing PCI. JAMA. 2011;306:1215–23. doi: 10.1001/jama.2011.1332. [DOI] [PubMed] [Google Scholar]
- Franchi F, Rollini F, Cho JR, Ferrante E, Angiolillo DJ. Platelet function testing in contemporary clinical and interventional practice. Curr Treat Options Cardiovasc Med. 2014;16:300. doi: 10.1007/s11936-014-0300-y. [DOI] [PubMed] [Google Scholar]
- Angiolillo DJ. Dual antiplatelet therapy guided by platelet function testing. Lancet. 2017;390:1718–20. doi: 10.1016/S0140-6736(17)32279-1. [DOI] [PubMed] [Google Scholar]
- Galli M, Franchi F, Rollini F, Cavallari LH, Capodanno D, Crea F, Angiolillo DJ. Genetic testing in patients undergoing percutaneous coronary intervention: rationale, evidence and practical recommendations. Expert Rev Clin Pharmacol. 2021;14:963–78. doi: 10.1080/17512433.2021.1927709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuldiner AR, O’Connell JR, Bliden KP, Gandhi A, Ryan K, Horenstein RB, Damcott CM, Pakyz R, Tantry US, Gibson Q, Pollin TI, Post W, Parsa A, Mitchell BD, Faraday N, Herzog W, Gurbel PA. Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA. 2009;302:849–57. doi: 10.1001/jama.2009.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mega JL, Simon T, Collet JP, Anderson JL, Antman EM, Bliden K, Cannon CP, Danchin N, Giusti B, Gurbel P, Horne BD, Hulot JS, Kastrati A, Montalescot G, Neumann FJ, Shen L, Sibbing D, Steg PG, Trenk D, Wiviott SD, Sabatine MS. Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: a meta-analysis. JAMA. 2010;304:1821–30. doi: 10.1001/jama.2010.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angiolillo DJ, Capodanno D, Danchin N, Simon T, Bergmeijer TO, Ten Berg, Sibbing D, Price MJ. Derivation, Validation, and Prognostic Utility of a Prediction Rule for Nonresponse to Clopidogrel: The ABCD-GENE Score. JACC Cardiovasc Interv. 2020;13:606–17. doi: 10.1016/j.jcin.2020.01.226. [DOI] [PubMed] [Google Scholar]
- Moon JY, Nagaraju D, Franchi F, Rollini F, Angiolillo DJ. The role of oral anticoagulant therapy in patients with acute coronary syndrome. Ther Adv Hematol. 2017;8:353–66. doi: 10.1177/2040620717733691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli M, Capodanno D, Benenati S, D'Amario D, Crea F, Andreotti F, Angiolillo DJ. Efficacy and safety of dual pathway inhibition in patients with cardiovascular disease: a systematic review and Meta-analysis. Eur Heart J Cardiovasc Pharmacother. 2021 Jun 19. [Epub ahead of print]. doi: 10.1093/ehjcvp/pvab043. [DOI] [PubMed] [Google Scholar]
- Mega JL, Braunwald E, Wiviott SD, Bassand JP, Bhatt DL, Bode C, Burton P, Cohen M, Cook-Bruns N, Fox KA, Goto S, Murphy SA, Plotnikov AN, Schneider D, Sun X, Verheugt FW, Gibson CM ATLAS ACS 2–TIMI 51 Investigators. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med. 2012;366:9–19. doi: 10.1056/NEJMoa1112277. [DOI] [PubMed] [Google Scholar]
- Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann FJ, Ardissino D, De Servi, Murphy SA, Riesmeyer J, Weerakkody G, Gibson CM, Antman EM TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–15. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- Erlinge D, Ten Berg, Foley D, Angiolillo DJ, Wagner H, Brown PB, Zhou C, Luo J, Jakubowski JA, Moser B, Small DS, Bergmeijer T, James S, Winters KJ. Reduction in platelet reactivity with prasugrel 5 mg in low-body-weight patients is noninferior to prasugrel 10 mg in higher-body-weight patients: results from the FEATHER trial. J Am Coll Cardiol. 2012;60:2032–40. doi: 10.1016/j.jacc.2012.08.964. [DOI] [PubMed] [Google Scholar]
- Erlinge D, Gurbel PA, James S, Lindahl TL, Svensson P, Ten Berg, Foley DP, Wagner H, Brown PB, Luo J, Zhou C, Moser BA, Jakubowski JA, Small DS, Winters KJ, Angiolillo DJ. Prasugrel 5 mg in the very elderly attenuates platelet inhibition but maintains noninferiority to prasugrel 10 mg in nonelderly patients: the GENERATIONS trial, a pharmacodynamic and pharmacokinetic study in stable coronary artery disease patients. J Am Coll Cardiol. 2013;62:577–83. doi: 10.1016/j.jacc.2013.05.023. [DOI] [PubMed] [Google Scholar]
- Savonitto S, Ferri LA, Piatti L, Grosseto D, Piovaccari G, Morici N, Bossi I, Sganzerla P, Tortorella G, Cacucci M, Ferrario M, Murena E, Sibilio G, Tondi S, Toso A, Bongioanni S, Ravera A, Corrada E, Mariani M, Di Ascenzo, Petronio AS, Cavallini C, Vitrella G, Rogacka R, Antonicelli R, Cesana BM, De Luca, Ottani F, De Luca, Piscione F, Moffa N, De Servi Elderly ACS 2 Investigators. Comparison of Reduced-Dose Prasugrel and Standard-Dose Clopidogrel in Elderly Patients With Acute Coronary Syndromes Undergoing Early Percutaneous Revascularization. Circulation. 2018;137:2435–45. doi: 10.1161/CIRCULATIONAHA.117.032180. [DOI] [PubMed] [Google Scholar]
- Navarese EP, Khan SU, Kołodziejczak M, Kubica J, Buccheri S, Cannon CP, Gurbel PA, De Servi, Budaj A, Bartorelli A, Trabattoni D, Ohman EM, Wallentin L, Roe MT, James S. Comparative Efficacy and Safety of Oral P2Y12 Inhibitors in Acute Coronary Syndrome: Network Meta-Analysis of 52 816 Patients From 12 Randomized Trials. Circulation. 2020;142:150–60. doi: 10.1161/CIRCULATIONAHA.120.046786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehilli J, Baquet M, Hochholzer W, Mayer K, Tesche C, Aradi D, Xu Y, Thienel M, Gschwendtner S, Zadrozny M, Jochheim D, Sibbing D, Schüpke S, Mansmann U, Hoffmann E, Kastrati A, Neumann FJ, Massberg S. Randomized Comparison of Intensified and Standard P2Y12-Receptor-Inhibition Before Elective Percutaneous Coronary Intervention: The SASSICAIA Trial. Circ Cardiovasc Interv. 2020;13:e008649. doi: 10.1161/CIRCINTERVENTIONS.119.008649. [DOI] [PubMed] [Google Scholar]
- Roe MT, Armstrong PW, Fox KA, White HD, Prabhakaran D, Goodman SG, Cornel JH, Bhatt DL, Clemmensen P, Martinez F, Ardissino D, Nicolau JC, Boden WE, Gurbel PA, Ruzyllo W, Dalby AJ, McGuire DK, Leiva-Pons JL, Parkhomenko A, Gottlieb S, Topacio GO, Hamm C, Pavlides G, Goudev AR, Oto A, Tseng CD, Merkely B, Gasparovic V, Corbalan R, Cinteză M, McLendon RC, Winters KJ, Brown EB, Lokhnygina Y, Aylward PE, Huber K, Hochman JS, Ohman EM. TRILOGY ACS Investigators. Prasugrel versus clopidogrel for acute coronary syndromes without revascularization. N Engl J Med. 2012;367:1297–309. doi: 10.1056/NEJMoa1205512. [DOI] [PubMed] [Google Scholar]
- Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, Mahaffey KW, Scirica BM, Skene A, Steg PG, Storey RF, Harrington RA PLATO Investigators, Freij A, Thorsén M. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–57. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- Steg PG, James S, Harrington RA, Ardissino D, Becker RC, Cannon CP, Emanuelsson H, Finkelstein A, Husted S, Katus H, Kilhamn J, Olofsson S, Storey RF, Weaver WD, Wallentin L PLATO Study Group. Ticagrelor versus clopidogrel in patients with ST-elevation acute coronary syndromes intended for reperfusion with primary percutaneous coronary intervention: A Platelet Inhibition and Patient Outcomes (PLATO) trial subgroup analysis. Circulation. 2010;122:2131–41. doi: 10.1161/CIRCULATIONAHA.109.927582. [DOI] [PubMed] [Google Scholar]
- Lindholm D, Varenhorst C, Cannon CP, Harrington RA, Himmelmann A, Maya J, Husted S, Steg PG, Cornel JH, Storey RF, Stevens SR, Wallentin L, James SK. Ticagrelor vs. clopidogrel in patients with non-ST-elevation acute coronary syndrome with or without revascularization: results from the PLATO trial. Eur Heart J. 2014;35:2083–93. doi: 10.1093/eurheartj/ehu160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimbel M, Qaderdan K, Willemsen L, Hermanides R, Bergmeijer T, de Vrey, Heestermans T, Tjon Joe, Waalewijn R, Hofma S, den Hartog, Jukema W, von Birgelen, Voskuil M, Kelder J, Deneer V, Ten Berg. Clopidogrel versus ticagrelor or prasugrel in patients aged 70 years or older with non-ST-elevation acute coronary syndrome (POPular AGE): the randomised, open-label, non-inferiority trial. Lancet. 2020;395:1374–81. doi: 10.1016/S0140-6736(20)30325-1. [DOI] [PubMed] [Google Scholar]
- You SC, Rho Y, Bikdeli B, Kim J, Siapos A, Weaver J, Londhe A, Cho J, Park J, Schuemie M, Suchard MA, Madigan D, Hripcsak G, Gupta A, Reich CG, Ryan PB, Park RW, Krumholz HM. Association of Ticagrelor vs Clopidogrel With Net Adverse Clinical Events in Patients With Acute Coronary Syndrome Undergoing Percutaneous Coronary Intervention. JAMA. 2020;324:1640–50. doi: 10.1001/jama.2020.16167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capranzano P, Angiolillo DJ. Tailoring P2Y12 Inhibiting Therapy in Elderly Patients With Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention. J Am Heart Assoc. 2019;8:e014000. doi: 10.1161/JAHA.119.014000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto S, Huang CH, Park SJ, Emanuelsson H, Kimura T. Ticagrelor vs. clopidogrel in Japanese, Korean and Taiwanese patients with acute coronary syndrome -- randomized, double-blind, phase III PHILO study. Circ J. 2015;79:2452–60. doi: 10.1253/circj.CJ-15-0112. [DOI] [PubMed] [Google Scholar]
- Park DW, Kwon O, Jang JS, Yun SC, Park H, Kang DY, Ahn JM, Lee PH, Lee SW, Park SW, Choi SW, Lee SG, Yoon HJ, Ahn T, Kim MH, Nah DY, Lee SY, Chae JK, Park SJ TICAKOREA Investigators. Clinically Significant Bleeding With Ticagrelor Versus Clopidogrel in Korean Patients With Acute Coronary Syndromes Intended for Invasive Management: A Randomized Clinical Trial. Circulation. 2019;140:1865–77. doi: 10.1161/CIRCULATIONAHA.119.041766. [DOI] [PubMed] [Google Scholar]
- Cattaneo M, Schulz R, Nylander S. Adenosine-mediated effects of ticagrelor: evidence and potential clinical relevance. J Am Coll Cardiol. 2014;63:2503–9. doi: 10.1016/j.jacc.2014.03.031. [DOI] [PubMed] [Google Scholar]
- Bonaca MP, Bhatt DL, Cohen M, Steg PG, Storey RF, Jensen EC, Magnani G, Bansilal S, Fish MP, Im K, Bengtsson O, Ophuis TO, Budaj A, Theroux P, Ruda M, Hamm C, Goto S, Spinar J, Nicolau JC, Kiss RG, Murphy SA, Wiviott SD, Held P, Braunwald E, Sabatine MS PEGASUS-TIMI 54 Steering Committee and Investigators. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372:1791–800. doi: 10.1056/NEJMoa1500857. [DOI] [PubMed] [Google Scholar]
- Steg PG, Bhatt DL, Simon T, Fox K, Mehta SR, Harrington RA, Held C, Andersson M, Himmelmann A, Ridderstråle W, Leonsson-Zachrisson M, Liu Y, Opolski G, Zateyshchikov D, Ge J, Nicolau JC, Corbalán R, Cornel JH, Widimský P, Leiter LA THEMIS Steering Committee and Investigators. Ticagrelor in Patients with Stable Coronary Disease and Diabetes. N Engl J Med. 2019;381:1309–20. doi: 10.1056/NEJMoa1908077. [DOI] [PubMed] [Google Scholar]
- Silvain J, Lattuca B, Beygui F, Rangé G, Motovska Z, Dillinger JG, Boueri Z, Brunel P, Lhermusier T, Pouillot C, Larrieu-Ardilouze E, Boccara F, Labeque JN, Guedeney P, El Kasty, Laredo M, Dumaine R, Ducrocq G, Collet JP, Cayla G, Blanchart K, Kala P, Vicaut E, Montalescot G ALPHEUS investigators. Ticagrelor versus clopidogrel in elective percutaneous coronary intervention (ALPHEUS): a randomised, open-label, phase 3b trial. Lancet. 2020;396:1737–44. doi: 10.1016/S0140-6736(20)32236-4. [DOI] [PubMed] [Google Scholar]
- Crea F, Thiele H, Sibbing D, Barthélémy O, Bauersachs J, Bhatt DL, Dendale P, Dorobantu M, Edvardsen T, Folliguet T, Gale CP, Gilard M, Jobs A, Jüni P, Lambrinou E, Lewis BS, Mehilli J, Meliga E, Merkely B, Mueller C, Roffi M, Rutten FH, Siontis GCM, Barbato E, Collet JP, Giannitsis E, Hamm CW, Böhm M, Cornel JH, Ferreiro JL, Frey N, Huber K, Kubica J, Navarese EP, Mehran R, Morais J, Storey RF, Valgimigli M, Vranckx P, James S. Debate: Prasugrel rather than ticagrelor is the preferred treatment for NSTE-ACS patients who proceed to PCI and pretreatment should not be performed in patients planned for an early invasive strategy. Eur Heart J. 2021;42:2973–85. doi: 10.1093/eurheartj/ehab277. [DOI] [PubMed] [Google Scholar]
- Motovska Z, Hlinomaz O, Miklik R, Hromadka M, Varvarovsky I, Dusek J, Knot J, Jarkovsky J, Kala P, Rokyta R, Tousek F, Kramarikova P, Majtan B, Simek S, Branny M, Mrozek J, Cervinka P, Ostransky J, Widimsky P PRAGUE-18 Study Group. Prasugrel Versus Ticagrelor in Patients With Acute Myocardial Infarction Treated With Primary Percutaneous Coronary Intervention: Multicenter Randomized PRAGUE-18 Study. Circulation. 2016;134:1603–12. doi: 10.1161/CIRCULATIONAHA.116.024823. [DOI] [PubMed] [Google Scholar]
- Valina C, Neumann FJ, Menichelli M, Mayer K, Wöhrle J, Bernlochner I, Aytekin A, Richardt G, Witzenbichler B, Sibbing D, Cassese S, Angiolillo DJ, Kufner S, Liebetrau C, Hamm CW, Xhepa E, Hapfelmeier A, Sager HB, Wustrow I, Joner M, Trenk D, Laugwitz KL, Schunkert H, Schüpke S, Kastrati A. Ticagrelor or Prasugrel in Patients With Non-ST-Segment Elevation Acute Coronary Syndromes. J Am Coll Cardiol. 2020;76:2436–46. doi: 10.1016/j.jacc.2020.09.584. [DOI] [PubMed] [Google Scholar]
- Aytekin A, Ndrepepa G, Neumann FJ, Menichelli M, Mayer K, Wöhrle J, Bernlochner I, Lahu S, Richardt G, Witzenbichler B, Sibbing D, Cassese S, Angiolillo DJ, Valina C, Kufner S, Liebetrau C, Hamm CW, Xhepa E, Hapfelmeier A, Sager HB, Wustrow I, Joner M, Trenk D, Fusaro M, Laugwitz KL, Schunkert H, Schüpke S, Kastrati A. Ticagrelor or Prasugrel in Patients With ST-Segment-Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention. Circulation. 2020;142:2329–37. doi: 10.1161/CIRCULATIONAHA.120.050244. [DOI] [PubMed] [Google Scholar]
- Mayer K, Bongiovanni D, Karschin V, Sibbing D, Angiolillo DJ, Schunkert H, Laugwitz KL, Schüpke S, Kastrati A, Bernlochner I. Ticagrelor or Prasugrel for Platelet Inhibition in Acute Coronary Syndrome Patients: The ISAR-REACT 5 Trial. J Am Coll Cardiol. 2020;76:2569–71. doi: 10.1016/j.jacc.2020.09.586. [DOI] [PubMed] [Google Scholar]
- Price MJ, Berger PB, Teirstein PS, Tanguay JF, Angiolillo DJ, Spriggs D, Puri S, Robbins M, Garratt KN, Bertrand OF, Stillabower ME, Aragon JR, Kandzari DE, Stinis CT, Lee MS, Manoukian SV, Cannon CP, Schork NJ, Topol EJ GRAVITAS Investigators. Standard- vs high-dose clopidogrel based on platelet function testing after percutaneous coronary intervention: the GRAVITAS randomized trial. JAMA. 2011;305:1097–105. doi: 10.1001/jama.2011.290. [DOI] [PubMed] [Google Scholar]
- Trenk D, Stone GW, Gawaz M, Kastrati A, Angiolillo DJ, Müller U, Richardt G, Jakubowski JA, Neumann FJ. A randomized trial of prasugrel versus clopidogrel in patients with high platelet reactivity on clopidogrel after elective percutaneous coronary intervention with implantation of drug-eluting stents: results of the TRIGGER-PCI (Testing Platelet Reactivity In Patients Undergoing Elective Stent Placement on Clopidogrel to Guide Alternative Therapy With Prasugrel) study. J Am Coll Cardiol. 2012;59:2159–64. doi: 10.1016/j.jacc.2012.02.026. [DOI] [PubMed] [Google Scholar]
- Collet JP, Cuisset T, Rangé G, Cayla G, Elhadad S, Pouillot C, Henry P, Motreff P, Carrié D, Boueri Z, Belle L, Van Belle, Rousseau H, Aubry P, Monségu J, Sabouret P, O'Connor SA, Abtan J, Kerneis M, Saint-Etienne C, Barthélémy O, Beygui F, Silvain J, Vicaut E, Montalescot G ARCTIC Investigators. Bedside monitoring to adjust antiplatelet therapy for coronary stenting. N Engl J Med. 2012;367:2100–9. doi: 10.1056/NEJMoa1209979. [DOI] [PubMed] [Google Scholar]
- Notarangelo FM, Maglietta G, Bevilacqua P, Cereda M, Merlini PA, Villani GQ, Moruzzi P, Patrizi G, Malagoli Tagliazucchi, Crocamo A, Guidorossi A, Pigazzani F, Nicosia E, Paoli G, Bianchessi M, Comelli MA, Caminiti C, Ardissino D. Pharmacogenomic Approach to Selecting Antiplatelet Therapy in Patients With Acute Coronary Syndromes: The PHARMCLO Trial. J Am Coll Cardiol. 2018;71:1869–77. doi: 10.1016/j.jacc.2018.02.029. [DOI] [PubMed] [Google Scholar]
- Zheng YY, Wu TT, Yang Y, Hou XG, Gao Y, Chen Y, Yang YN, Li XM, Ma X, Ma YT, Xie X. Personalized antiplatelet therapy guided by a novel detection of platelet aggregation function in stable coronary artery disease patients undergoing percutaneous coronary intervention: a randomized controlled clinical trial. Eur Heart J Cardiovasc Pharmacother. 2019;6:211–21. doi: 10.1093/ehjcvp/pvz059. [DOI] [PubMed] [Google Scholar]
- Pereira NL, Farkouh ME, So D, Lennon R, Geller N, Mathew V, Bell M, Bae JH, Jeong MH, Chavez I, Gordon P, Abbott JD, Cagin C, Baudhuin L, Fu YP, Goodman SG, Hasan A, Iturriaga E, Lerman A, Sidhu M, Tanguay JF, Wang L, Weinshilboum R, Welsh R, Rosenberg Y, Bailey K, Rihal C. Effect of Genotype-Guided Oral P2Y12 Inhibitor Selection vs Conventional Clopidogrel Therapy on Ischemic Outcomes After Percutaneous Coronary Intervention: The TAILOR-PCI Randomized Clinical Trial. JAMA. 2020;324:761–71. doi: 10.1001/jama.2020.12443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuteja S, Glick H, Matthai W, Nachamkin I, Nathan A, Monono K, Carcuffe C, Maslowski K, Chang G, Kobayashi T, Anwaruddin S, Hirshfeld J, Wilensky RL, Herrmann HC, Kolansky DM, Rader DJ, Giri J. Prospective CYP2C19 Genotyping to Guide Antiplatelet Therapy Following Percutaneous Coronary Intervention: A Pragmatic Randomized Clinical Trial. Circ Genom Precis Med. 2020;13:e002640. doi: 10.1161/CIRCGEN.119.002640. [DOI] [PubMed] [Google Scholar]
- Valgimigli M, Campo G, Monti M, Vranckx P, Percoco G, Tumscitz C, Castriota F, Colombo F, Tebaldi M, Fucà G, Kubbajeh M, Cangiano E, Minarelli M, Scalone A, Cavazza C, Frangione A, Borghesi M, Marchesini J, Parrinello G, Ferrari R Prolonging Dual Antiplatelet Treatment After Grading Stent-Induced Intimal Hyperplasia Study (PRODIGY) Investigators. Short- versus long-term duration of dual-antiplatelet therapy after coronary stenting: a randomized multicenter trial. Circulation. 2012;125:2015–26. doi: 10.1161/CIRCULATIONAHA.111.071589. [DOI] [PubMed] [Google Scholar]
- Collet JP, Silvain J, Barthélémy O, Rangé G, Cayla G, Van Belle, Cuisset T, Elhadad S, Schiele F, Lhoest N, Ohlmann P, Carrié D, Rousseau H, Aubry P, Monségu J, Sabouret P, O'Connor SA, Abtan J, Kerneis M, Saint-Etienne C, Beygui F, Vicaut E, Montalescot G ARCTIC investigators. Dual-antiplatelet treatment beyond 1 year after drug-eluting stent implantation (ARCTIC-Interruption): a randomised trial. Lancet. 2014;384:1577–85. doi: 10.1016/S0140-6736(14)60612-7. [DOI] [PubMed] [Google Scholar]
- Gilard M, Barragan P, Noryani AAL, Noor HA, Majwal T, Hovasse T, Castellant P, Schneeberger M, Maillard L, Bressolette E, Wojcik J, Delarche N, Blanchard D, Jouve B, Ormezzano O, Paganelli F, Levy G, Sainsous J, Carrie D, Furber A, Berland J, Darremont O, Le Breton, Lyuycx-Bore A, Gommeaux A, Cassat C, Kermarrec A, Cazaux P, Druelles P, Dauphin R, Armengaud J, Dupouy P, Champagnac D, Ohlmann P, Endresen K, Benamer H, Kiss RG, Ungi I, Boschat J, Morice MC. 6- versus 24-month dual antiplatelet therapy after implantation of drug-eluting stents in patients nonresistant to aspirin: the randomized, multicenter ITALIC trial. J Am Coll Cardiol. 2015;65:777–86. doi: 10.1016/j.jacc.2014.11.008. [DOI] [PubMed] [Google Scholar]
- Helft G, Steg PG, Le Feuvre, Georges JL, Carrie D, Dreyfus X, Furber A, Leclercq F, Eltchaninoff H, Falquier JF, Henry P, Cattan S, Sebagh L, Michel PL, Tuambilangana A, Hammoudi N, Boccara F, Cayla G, Douard H, Diallo A, Berman E, Komajda M, Metzger JP, Vicaut E OPTImal DUAL Antiplatelet Therapy Trial Investigators. Stopping or continuing clopidogrel 12 months after drug-eluting stent placement: the OPTIDUAL randomized trial. Eur Heart J. 2016;37:365–74. doi: 10.1093/eurheartj/ehv481. [DOI] [PubMed] [Google Scholar]
- Park SJ, Park DW, Kim YH, Kang SJ, Lee SW, Lee CW, Han KH, Park SW, Yun SC, Lee SG, Rha SW, Seong IW, Jeong MH, Hur SH, Lee NH, Yoon J, Yang JY, Lee BK, Choi YJ, Chung WS, Lim DS, Cheong SS, Kim KS, Chae JK, Nah DY, Jeon DS, Seung KB, Jang JS, Park HS, Lee K. Duration of dual antiplatelet therapy after implantation of drug-eluting stents. Ne Engl J Med. 2010;362:1374–82. doi: 10.1056/NEJMoa1001266. [DOI] [PubMed] [Google Scholar]
- Mauri L, Kereiakes DJ, Yeh RW, Driscoll-Shempp P, Cutlip DE, Steg PG, Normand SL, Braunwald E, Wiviott SD, Cohen DJ, Holmes DR, Krucoff MW, Hermiller J, Dauerman HL, Simon DI, Kandzari DE, Garratt KN, Lee DP, Pow TK, Ver Lee, Rinaldi MJ, Massaro JM DAPT Study Investigators. Twelve or 30 Months of Dual Antiplatelet Therapy after Drug-Eluting Stents. N Engl J Med. 2014;371:2155–66. doi: 10.1056/NEJMoa1409312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh RW, Kereiakes DJ, Steg PG, Windecker S, Rinaldi MJ, Gershlick AH, Cutlip DE, Cohen DJ, Tanguay JF, Jacobs A, Wiviott SD, Massaro JM, Iancu AC, Mauri L DAPT Study Investigators. Benefits and Risks of Extended Duration Dual Antiplatelet Therapy After PCI in Patients With and Without Acute Myocardial Infarction. J Am Coll Cardiol. 2015;65:2211–21. doi: 10.1016/j.jacc.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarese EP, Andreotti F, Schulze V, Kołodziejczak M, Buffon A, Brouwer M, Costa F, Kowalewski M, Parati G, Lip GY, Kelm M, Valgimigli M. Optimal duration of dual antiplatelet therapy after percutaneous coronary intervention with drug eluting stents: meta-analysis of randomised controlled trials. BMJ. 2015;350:h1618. doi: 10.1136/bmj.h1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmerini T, Bruno AG, Gilard M, Morice MC, Valgimigli M, Montalescot G, Collet JP, Della Riva, Bacchi-Reggiani ML, Steg PG, Diallo A, Vicaut E, Helft G, Nakamura M, Généreux P, Vahl TP, Stone GW. Risk-Benefit Profile of Longer-Than-1-Year Dual-Antiplatelet Therapy Duration After Drug-Eluting Stent Implantation in Relation to Clinical Presentation. Circ Cardiovasc Interv. 2019;12:e007541. doi: 10.1161/CIRCINTERVENTIONS.118.007541. [DOI] [PubMed] [Google Scholar]
- Benenati S, Crimi G, Canale C, Pescetelli F, De Marzo, Vergallo R, Galli M, Della Bona, Canepa M, Ameri P, Crea F, Porto I. Duration of dual antiplatelet therapy and subsequent monotherapy type in patients undergoing drug eluting stent implantation: a network Meta-analysis. Eur Heart J Cardiovasc Pharmacother. 2022;8:56–64. doi: 10.1093/ehjcvp/pvaa127. [DOI] [PubMed] [Google Scholar]
- Bittl JA, Baber U, Bradley SM, Wijeysundera DN. Duration of Dual Antiplatelet Therapy: A Systematic Review for the 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2016;68:1116–39. doi: 10.1016/j.jacc.2016.03.512. [DOI] [PubMed] [Google Scholar]
- Bhatt DL, Flather MD, Hacke W, Berger PB, Black HR, Boden WE, Cacoub P, Cohen EA, Creager MA, Easton JD, Hamm CW, Hankey GJ, Johnston SC, Mak KH, Mas JL, Montalescot G, Pearson TA, Steg PG, Steinhubl SR, Weber MA, Fabry-Ribaudo L, Hu T, Topol EJ, Fox KA CHARISMA Investigators. Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial. J Am Coll Cardiol. 2007;49:1982–8. doi: 10.1016/j.jacc.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Bergmark BA, Bhatt DL, Steg PG, Budaj A, Storey RF, Gurmu Y, Kuder JF, Im K, Magnani G, Oude Ophuis, Hamm C, Špinar J, Kiss RG, Van de, Montalescot G, Johanson P, Braunwald E, Sabatine MS, Bonaca MP. Long-Term Ticagrelor in Patients With Prior Coronary Stenting in the PEGASUS-TIMI 54 Trial. J Am Heart Assoc. 2021;10:e020446. doi: 10.1161/JAHA.120.020446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt DL, Steg PG, Mehta SR, Leiter LA, Simon T, Fox K, Held C, Andersson M, Himmelmann A, Ridderstråle W, Chen J, Song Y, Diaz R, Goto S, James SK, Ray KK, Parkhomenko AN, Kosiborod MN, McGuire DK, Harrington RA THEMIS Steering Committee and Investigators. Ticagrelor in patients with diabetes and stable coronary artery disease with a history of previous percutaneous coronary intervention (THEMIS-PCI): a phase 3, placebo-controlled, randomised trial. Lancet. 2019;394:1169–80. doi: 10.1016/S0140-6736(19)31887-2. [DOI] [PubMed] [Google Scholar]
- Furtado RHM, Nicolau JC, Magnani G, Im K, Bhatt DL, Storey RF, Steg PG, Spinar J, Budaj A, Kontny F, Corbalan R, Kiss RG, Abola MT, Johanson P, Jensen EC, Braunwald E, Sabatine MS, Bonaca MP. Long-term ticagrelor for secondary prevention in patients with prior myocardial infarction and no history of coronary stenting: insights from PEGASUS-TIMI 54. Eur Heart J. 2020;41:1625–32. doi: 10.1093/eurheartj/ehz821. [DOI] [PubMed] [Google Scholar]
- Eikelboom JW, Connolly SJ, Bosch J, Dagenais GR, Hart RG, Shestakovska O, Diaz R, Alings M, Lonn EM, Anand SS, Widimsky P, Hori M, Avezum A, Piegas LS, Branch KRH, Probstfield J, Bhatt DL, Zhu J, Liang Y, Maggioni AP, Lopez-Jaramillo P, O’Donnell M, Kakkar A, Fox KAA, Parkhomenko AN, Ertl G, Störk S, Keltai M, Ryden L, Pogosova N, Dans AL, Lanas F, Commerford PJ, Torp-Pedersen C, Guzik TJ, Verhamme PB, Vinereanu D, Kim JH, Tonkin AM, Lewis BS, Felix C, Yusoff K, Steg PG, Metsarinne KP, Cook Bruns, Misselwitz F, Chen E, Leong D, Yusuf S COMPASS Investigators. Rivaroxaban with or without Aspirin in Stable Cardiovascular Disease. N Engl J Med. 2017;377:1319–30. doi: 10.1056/NEJMoa1709118. [DOI] [PubMed] [Google Scholar]
- Gwon HC, Hahn JY, Park KW, Song YB, Chae IH, Lim DS, Han KR, Choi JH, Choi SH, Kang HJ, Koo BK, Ahn T, Yoon JH, Jeong MH, Hong TJ, Chung WY, Choi YJ, Hur SH, Kwon HM, Jeon DW, Kim BO, Park SH, Lee NH, Jeon HK, Jang Y, Kim HS. Six-month versus 12-month dual antiplatelet therapy after implantation of drug-eluting stents: the Efficacy of Xience/Promus Versus Cypher to Reduce Late Loss After Stenting (EXCELLENT) randomized, multicenter study. Circulation. 2012;125:505–13. doi: 10.1161/CIRCULATIONAHA.111.059022. [DOI] [PubMed] [Google Scholar]
- Kim BK, Hong MK, Shin DH, Nam CM, Kim JS, Ko YG, Choi D, Kang TS, Park BE, Kang WC, Lee SH, Yoon JH, Hong BK, Kwon HM, Jang Y RESET Investigators. A new strategy for discontinuation of dual antiplatelet therapy: the RESET Trial (REal Safety and Efficacy of 3-month dual antiplatelet Therapy following Endeavor zotarolimus-eluting stent implantation). J Am Coll Cardiol. 2012;60:1340–8. doi: 10.1016/j.jacc.2012.06.043. [DOI] [PubMed] [Google Scholar]
- Schulz-Schüpke S, Byrne RA, Ten Berg, Neumann FJ, Han Y, Adriaenssens T, Tölg R, Seyfarth M, Maeng M, Zrenner B, Jacobshagen C, Mudra H, von Hodenberg, Wöhrle J, Angiolillo DJ, von Merzljak, Rifatov N, Kufner S, Morath T, Feuchtenberger A, Ibrahim T, Janssen PW, Valina C, Li Y, Desmet W, Abdel-Wahab M, Tiroch K, Hengstenberg C, Bernlochner I, Fischer M, Schunkert H, Laugwitz KL, Schömig A, Mehilli J, Kastrati A Intracoronary Stenting and Antithrombotic Regimen: Safety And EFficacy of 6 Months Dual Antiplatelet Therapy After Drug-Eluting Stenting (ISAR-SAFE) Trial Investigators. ISAR-SAFE: a randomized, double-blind, placebo-controlled trial of 6 vs. 12 months of clopidogrel therapy after drug-eluting stenting. Eur Heart J. 2015;36:1252–63. doi: 10.1093/eurheartj/ehu523. [DOI] [PubMed] [Google Scholar]
- Han Y, Xu B, Xu K, Guan C, Jing Q, Zheng Q, Li X, Zhao X, Wang H, Zhao X, Li X, Yu P, Zang H, Wang Z, Cao X, Zhang J, Pang W, Li J, Yang Y, Dangas GD. Six Versus 12 Months of Dual Antiplatelet Therapy After Implantation of Biodegradable Polymer Sirolimus-Eluting Stent: Randomized Substudy of the I-LOVE-IT 2 Trial. Circ Cardiovasc Interv. 2016;9:e003145. doi: 10.1161/CIRCINTERVENTIONS.115.003145. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Iijima R, Ako J, Shinke T, Okada H, Ito Y, Ando K, Anzai H, Tanaka H, Ueda Y, Takiuchi S, Nishida Y, Ohira H, Kawaguchi K, Kadotani M, Niinuma H, Omiya K, Morita T, Zen K, Yasaka Y, Inoue K, Ishiwata S, Ochiai M, Hamasaki T, Yokoi H NIPPON Investigators. Dual Antiplatelet Therapy for 6 Versus 18 Months After Biodegradable Polymer Drug-Eluting Stent Implantation. JACC Cardiovasc Interv. 2017;10:1189–98. doi: 10.1016/j.jcin.2017.04.019. [DOI] [PubMed] [Google Scholar]
- Kedhi E, Fabris E, van der, Buszman P, von Birgelen, Roolvink V, Zurakowski A, Schotborgh CE, Hoorntje JCA, Eek CH, Cook S, Togni M, Meuwissen M, van Royen, van Vliet, Wedel H, Delewi R, Zijlstra F. Six months versus 12 months dual antiplatelet therapy after drug-eluting stent implantation in ST-elevation myocardial infarction (DAPT-STEMI): randomised, multicentre, non-inferiority trial. BMJ. 2018;363:k3793. doi: 10.1136/bmj.k3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn JY, Song YB, Oh JH, Cho DK, Lee JB, Doh JH, Kim SH, Jeong JO, Bae JH, Kim BO, Cho JH, Suh IW, Kim DI, Park HK, Park JS, Choi WG, Lee WS, Kim J, Choi KH, Park TK, Lee JM, Yang JH, Choi JH, Choi SH, Gwon HC SMART-DATE investigators. 6-month versus 12-month or longer dual antiplatelet therapy after percutaneous coronary intervention in patients with acute coronary syndrome (SMART-DATE): a randomised, open-label, non-inferiority trial. Lancet. 2018;391:1274–84. doi: 10.1016/S0140-6736(18)30493-8. [DOI] [PubMed] [Google Scholar]
- Lee BK, Kim JS, Lee OH, Min PK, Yoon YW, Hong BK, Shin DH, Kang TS, Kim BO, Cho DK, Jeon DW, Woo SI, Choi S, Kim YH, Kang WC, Kim S, Kim BK, Hong MK, Jang Y, Kwon HM. Safety of six-month dual antiplatelet therapy after second-generation drug-eluting stent implantation: OPTIMA-C Randomised Clinical Trial and OCT Substudy. EuroIntervention. 2018;13:1923–30. doi: 10.4244/EIJ-D-17-00792. [DOI] [PubMed] [Google Scholar]
- De Luca, Damen SA, Camaro C, Benit E, Verdoia M, Rasoul S, Liew HB, Polad J, Ahmad WA, Zambahari R, Postma S, Kedhi E, Suryapranata H Collaborators. Final results of the randomised evaluation of short-term dual antiplatelet therapy in patients with acute coronary syndrome treated with a new-generation stent (REDUCE trial). EuroIntervention. 2019;15:e990–8. doi: 10.4244/EIJ-D-19-00539. [DOI] [PubMed] [Google Scholar]
- Valgimigli M, Frigoli E, Heg D, Tijssen J, Jüni P, Vranckx P, Ozaki Y, Morice MC, Chevalier B, Onuma Y, Windecker S, Tonino PAL, Roffi M, Lesiak M, Mahfoud F, Bartunek J, Hildick-Smith D, Colombo A, Stanković G, Iñiguez A, Schultz C, Kornowski R, Ong PJL, Alasnag M, Rodriguez AE, Moschovitis A, Laanmets P, Donahue M, Leonardi S, Smits PC MASTER DAPT Investigators. Dual Antiplatelet Therapy after PCI in Patients at High Bleeding Risk. N Engl J Med. 2021;385:1643–55. doi: 10.1056/NEJMoa2108749. [DOI] [PubMed] [Google Scholar]
- Feres F, Costa RA, Abizaid A, Leon MB, Marin-Neto JA, Botelho RV, King SB, Negoita M, Liu M, de Paula, Mangione JA, Meireles GX, Castello HJ, Nicolela EL, Perin MA, Devito FS, Labrunie A, Salvadori D, Gusmão M, Staico R, Costa JR, de Castro, Abizaid AS, Bhatt DL OPTIMIZE Trial Investigators. Three vs twelve months of dual antiplatelet therapy after zotarolimus-eluting stents: the OPTIMIZE randomized trial. JAMA. 2013;310:2510–22. doi: 10.1001/jama.2013.282183. [DOI] [PubMed] [Google Scholar]
- Kirtane AJ, Stoler R, Feldman R, Neumann FJ, Boutis L, Tahirkheli N, Toelg R, Othman I, Stein B, Choi JW, Windecker S, Yeh RW, Dauerman HL, Price MJ, Underwood P, Allocco D, Meredith I, Kereiakes DJ. Primary Results of the EVOLVE Short DAPT Study: Evaluation of 3-Month Dual Antiplatelet Therapy in High Bleeding Risk Patients Treated With a Bioabsorbable Polymer-Coated Everolimus-Eluting Stent. Circ Cardiovasc Interv. 2021;14:e010144. doi: 10.1161/CIRCINTERVENTIONS.120.010144. [DOI] [PubMed] [Google Scholar]
- Mehran R, Cao D, Angiolillo DJ, Bangalore S, Bhatt DL, Ge J, Hermiller J, Makkar RR, Neumann FJ, Saito S, Picon H, Toelg R, Maksoud A, Chehab BM, De la, Kunadian V, Sardella G, Thiele H, Varenne O, Vranckx P, Windecker S, Zhou Y, Krucoff MW, Ruster K, Wang J, Valgimigli M XIENCE 90 and XIENCE 28 Investigators. 3- or 1-Month DAPT in Patients at High Bleeding Risk Undergoing Everolimus-Eluting Stent Implantation. JACC Cardiovasc Interv. 2021;14:1870–83. doi: 10.1016/j.jcin.2021.07.016. [DOI] [PubMed] [Google Scholar]
- Benenati S, Galli M, De Marzo, Pescetelli F, Toma M, Andreotti F, Della Bona, Canepa M, Ameri P, Crea F, Porto I. Very short vs. long dual antiplatelet therapy after second generation drug-eluting stents in 35 785 patients undergoing percutaneous coronary interventions: a meta-analysis of randomized controlled trials. Eur Heart J Cardiovasc Pharmacother. 2021;7:86–93. doi: 10.1093/ehjcvp/pvaa001. [DOI] [PubMed] [Google Scholar]
- Urban P, Gregson J, Owen R, Mehran R, Windecker S, Valgimigli M, Varenne O, Krucoff M, Saito S, Baber U, Chevalier B, Capodanno D, Morice MC, Pocock S. Assessing the Risks of Bleeding vs Thrombotic Events in Patients at High Bleeding Risk After Coronary Stent Implantation: The ARC-High Bleeding Risk Trade-off Model. JAMA Cardiol. 2021;6:410–9. doi: 10.1001/jamacardio.2020.6814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donoghue ML, Murphy SA, Sabatine MS. The Safety and Efficacy of Aspirin Discontinuation on a Background of a P2Y12 Inhibitor in Patients After Percutaneous Coronary Intervention: A Systematic Review and Meta-Analysis. Circulation. 2020;142:538–45. doi: 10.1161/CIRCULATIONAHA.120.046251. [DOI] [PubMed] [Google Scholar]
- Capodanno D, Mehran R, Valgimigli M, Baber U, Windecker S, Vranckx P, Dangas G, Rollini F, Kimura T, Collet JP, Gibson CM, Steg PG, Lopes RD, Gwon HC, Storey RF, Franchi F, Bhatt DL, Serruys PW, Angiolillo DJ. Aspirin-free strategies in cardiovascular disease and cardioembolic stroke prevention. Nat Rev Cardiol. 2018;15:480–96. doi: 10.1038/s41569-018-0049-1. [DOI] [PubMed] [Google Scholar]
- Franchi F, Rollini F, Garcia E, Rivas Rios, Rivas A, Agarwal M, Kureti M, Nagaraju D, Wali M, Briceno M, Moon JY, Kairouz V, Yaranov D, Been L, Suryadevara S, Soffer D, Zenni MM, Bass TA, Angiolillo DJ. Effects of Edoxaban on the Cellular and Protein Phase of Coagulation in Patients with Coronary Artery Disease on Dual Antiplatelet Therapy with Aspirin and Clopidogrel: Results of the EDOX-APT Study. Thromb Haemost. 2020;120:83–93. doi: 10.1055/s-0039-1695772. [DOI] [PubMed] [Google Scholar]
- Perzborn E, Heitmeier S, Laux V. Effects of Rivaroxaban on Platelet Activation and Platelet-Coagulation Pathway Interaction: In Vitro and In Vivo Studies. J Cardiovasc Pharmacol Ther. 2015;20:554–62. doi: 10.1177/1074248415578172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker E, Perzborn E, Klipp A, Luecker C, Butehorn U, Kast R, Laux V. Effects of rivaroxaban, ASA and clopidogrel alone and in combination in a porcine model of stent thrombosis. J Thromb Haemost. 2012;10:2470–80. doi: 10.1111/jth.12033. [DOI] [PubMed] [Google Scholar]
- Dewilde WJ, Oirbans T, Verheugt FW, Kelder JC, De Smet, Herrman JP, Adriaenssens T, Vrolix M, Heestermans AA, Vis MM, Tijsen JG, van ’t, ten Berg WOEST study investigators. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet. 2013;381:1107–15. doi: 10.1016/S0140-6736(12)62177-1. [DOI] [PubMed] [Google Scholar]
- Gibson CM, Mehran R, Bode C, Halperin J, Verheugt FW, Wildgoose P, Birmingham M, Ianus J, Burton P, van Eickels, Korjian S, Daaboul Y, Lip GY, Cohen M, Husted S, Peterson ED, Fox KA. Prevention of Bleeding in Patients with Atrial Fibrillation Undergoing PCI. N Engl J Med. 2016;375:2423–34. doi: 10.1056/NEJMoa1611594. [DOI] [PubMed] [Google Scholar]
- Cannon CP, Bhatt DL, Oldgren J, Lip GYH, Ellis SG, Kimura T, Maeng M, Merkely B, Zeymer U, Gropper S, Nordaby M, Kleine E, Harper R, Manassie J, Januzzi JL, Ten Berg, Steg PG, Hohnloser SH RE-DUAL PCI Steering Committee and Investigators. Dual Antithrombotic Therapy with Dabigatran after PCI in Atrial Fibrillation. N Engl J Med. 2017;377:1513–24. doi: 10.1056/NEJMoa1708454. [DOI] [PubMed] [Google Scholar]
- Lopes RD, Heizer G, Aronson R, Vora AN, Massaro T, Mehran R, Goodman SG, Windecker S, Darius H, Li J, Averkov O, Bahit MC, Berwanger O, Budaj A, Hijazi Z, Parkhomenko A, Sinnaeve P, Storey RF, Thiele H, Vinereanu D, Granger CB, Alexander JH AUGUSTUS Investigators. Antithrombotic Therapy after Acute Coronary Syndrome or PCI in Atrial Fibrillation. N Engl J Med. 2019;380:1509–24. doi: 10.1056/NEJMoa1817083. [DOI] [PubMed] [Google Scholar]
- Vranckx P, Valgimigli M, Eckardt L, Tijssen J, Lewalter T, Gargiulo G, Batushkin V, Campo G, Lysak Z, Vakaliuk I, Milewski K, Laeis P, Reimitz PE, Smolnik R, Zierhut W, Goette A. Edoxaban-based versus vitamin K antagonist-based antithrombotic regimen after successful coronary stenting in patients with atrial fibrillation (ENTRUST-AF PCI): a randomised, open-label, phase 3b trial. Lancet. 2019;394:1335–43. doi: 10.1016/S0140-6736(19)31872-0. [DOI] [PubMed] [Google Scholar]
- Galli M, Andreotti F, D'Amario D, Vergallo R, Montone RA, Niccoli G, Crea F. Randomised trials and meta-analyses of double vs triple antithrombotic therapy for atrial fibrillation-ACS/PCI: A critical appraisal. Int J Cardiol Heart Vasc. 2020;28:100524. doi: 10.1016/j.ijcha.2020.100524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capodanno D, Di Maio, Greco A, Bhatt DL, Gibson CM, Goette A, Lopes RD, Mehran R, Vranckx P, Angiolillo DJ. Safety and Efficacy of Double Antithrombotic Therapy With Non-Vitamin K Antagonist Oral Anticoagulants in Patients With Atrial Fibrillation Undergoing Percutaneous Coronary Intervention: A Systematic Review and Meta-Analysis. J Am Heart Assoc. 2020;9:e017212. doi: 10.1161/JAHA.120.017212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargiulo G, Goette A, Tijssen J, Eckardt L, Lewalter T, Vranckx P, Valgimigli M. Safety and efficacy outcomes of double vs. triple antithrombotic therapy in patients with atrial fibrillation following percutaneous coronary intervention: a systematic review and meta-analysis of non-vitamin K antagonist oral anticoagulant-based randomized clinical trials. Eur Heart J. 2019;40:3757–67. doi: 10.1093/eurheartj/ehz732. [DOI] [PubMed] [Google Scholar]
- Galli M, Andreotti F, D'Amario D, Vergallo R, Montone RA, Porto I, Crea F. Dual therapy with direct oral anticoagulants significantly increases the risk of stent thrombosis compared to triple therapy. Eur Heart J Cardiovasc Pharmacother. 2020;6:128–9. doi: 10.1093/ehjcvp/pvz030. [DOI] [PubMed] [Google Scholar]
- Galli M, Andreotti F, Porto I, Crea F. Intracranial haemorrhages vs. stent thromboses with direct oral anticoagulant plus single antiplatelet agent or triple antithrombotic therapy: a meta-analysis of randomized trials in atrial fibrillation and percutaneous coronary intervention/acute coronary syndrome patients. Europace. 2020;22:538–46. doi: 10.1093/europace/euz345. [DOI] [PubMed] [Google Scholar]
- Galli M, Andreotti F, D'Amario D, Porto I, Crea F. Stent Thrombosis With Dual Antithrombotic Therapy in Atrial Fibrillation-ACS/PCI Trials. J Am Coll Cardiol. 2020;75:1727–8. doi: 10.1016/j.jacc.2020.01.054. [DOI] [PubMed] [Google Scholar]
- Baber U, Zafar MU, Dangas G, Escolar G, Angiolillo DJ, Sharma SK, Kini AS, Sartori S, Joyce L, Vogel B, Farhan S, Gurbel P, Gibson CM, Fuster V, Mehran R, Badimon JJ. Ticagrelor With or Without Aspirin After PCI: The TWILIGHT Platelet Substudy. J Am Coll Cardiol. 2020;75:578–86. doi: 10.1016/j.jacc.2019.11.056. [DOI] [PubMed] [Google Scholar]
- Armstrong PC, Leadbeater PD, Chan MV, Kirkby NS, Jakubowski JA, Mitchell JA, Warner TD. In the presence of strong P2Y12 receptor blockade, aspirin provides little additional inhibition of platelet aggregation. J Thromb Haemost. 2011;9:552–61. doi: 10.1111/j.1538-7836.2010.04160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchi F, Rollini F, Faz G, Rivas JR, Rivas A, Agarwal M, Briceno M, Wali M, Nawaz A, Silva G, Shaikh Z, Maaliki N, Fahmi K, Been L, Pineda AM, Suryadevara S, Soffer D, Zenni MM, Baber U, Mehran R, Jennings LK, Bass TA, Angiolillo DJ. Pharmacodynamic Effects of Vorapaxar in Prior Myocardial Infarction Patients Treated With Potent Oral P2Y12 Receptor Inhibitors With and Without Aspirin: Results of the VORA-PRATIC Study. J Am Heart Assoc. 2020;9:e015865. doi: 10.1161/JAHA.120.015865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchi F, Rollini F, Kairouz V, Rivas Rios, Rivas A, Agarwal M, Briceno M, Wali M, Nawaz A, Silva G, Shaikh Z, Maaliki N, Been L, Piraino J, Pineda AM, Suryadevara S, Soffer D, Zenni MM, Jennings LK, Bass TA. Pharmacodynamic Effects of Vorapaxar in Patients With and Without Diabetes Mellitus: Results of the OPTIMUS-5 Study. JACC Basic Transl Sci. 2019;4:763–75. doi: 10.1016/j.jacbts.2019.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli M, Capodanno D, Andreotti F, Crea F, Angiolillo DJ. Safety and efficacy of P2Y12 inhibitor monotherapy in patients undergoing percutaneous coronary interventions. Expert Opin Drug Saf. 2021;20:9–21. doi: 10.1080/14740338.2021.1850691. [DOI] [PubMed] [Google Scholar]
- Vranckx P, Valgimigli M, Jüni P, Hamm C, Steg PG, Heg D, van Es, McFadden EP, Onuma Y, van Meijeren, Chichareon P, Benit E, Möllmann H, Janssens L, Ferrario M, Moschovitis A, Zurakowski A, Dominici M, Van Geuns, Huber K, Slagboom T, Serruys PW, Windecker S GLOBAL LEADERS Investigators. Ticagrelor plus aspirin for 1 month, followed by ticagrelor monotherapy for 23 months vs aspirin plus clopidogrel or ticagrelor for 12 months, followed by aspirin monotherapy for 12 months after implantation of a drug-eluting stent: a multicentre, open-label, randomised superiority trial. Lancet. 2018;392:940–9. doi: 10.1016/S0140-6736(18)31858-0. [DOI] [PubMed] [Google Scholar]
- Kim BK, Hong SJ, Cho YH, Yun KH, Kim YH, Suh Y, Cho JY, Her AY, Cho S, Jeon DW, Yoo SY, Cho DK, Hong BK, Kwon H, Ahn CM, Shin DH, Nam CM, Kim JS, Ko YG, Choi D, Hong MK, Jang Y TICO Investigators. Effect of Ticagrelor Monotherapy vs Ticagrelor With Aspirin on Major Bleeding and Cardiovascular Events in Patients With Acute Coronary Syndrome: The TICO Randomized Clinical Trial. JAMA. 2020;323:2407–16. doi: 10.1001/jama.2020.7580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H, Domei T, Morimoto T, Natsuaki M, Shiomi H, Toyota T, Ohya M, Suwa S, Takagi K, Nanasato M, Hata Y, Yagi M, Suematsu N, Yokomatsu T, Takamisawa I, Doi M, Noda T, Okayama H, Seino Y, Tada T, Sakamoto H, Hibi K, Abe M, Kawai K, Nakao K, Ando K, Tanabe K, Ikari Y, Hanaoka KI, Morino Y, Kozuma K, Kadota K, Furukawa Y, Nakagawa Y, Kimura T STOPDAPT-2 Investigators. Effect of 1-Month Dual Antiplatelet Therapy Followed by Clopidogrel vs 12-Month Dual Antiplatelet Therapy on Cardiovascular and Bleeding Events in Patients Receiving PCI: The STOPDAPT-2 Randomized Clinical Trial. JAMA. 2019;321:2414–27. doi: 10.1001/jama.2019.8145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn JY, Song YB, Oh JH, Chun WJ, Park YH, Jang WJ, Im ES, Jeong JO, Cho BR, Oh SK, Yun KH, Cho DK, Lee JY, Koh YY, Bae JW, Choi JW, Lee WS, Yoon HJ, Lee SU, Cho JH, Choi WG, Rha SW, Lee JM, Park TK, Yang JH, Choi JH, Choi SH, Lee SH, Gwon HC SMART-CHOICE Investigators. Effect of P2Y12 Inhibitor Monotherapy vs Dual Antiplatelet Therapy on Cardiovascular Events in Patients Undergoing Percutaneous Coronary Intervention: The SMART-CHOICE Randomized Clinical Trial. JAMA. 2019;32:2428–37. doi: 10.1001/jama.2019.8146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angiolillo DJ, Baber U, Sartori S, Briguori C, Dangas G, Cohen DJ, Mehta SR, Gibson CM, Chandiramani R, Huber K, Kornowski R, Weisz G, Kunadian V, Oldroyd KG, Ya-Ling H, Kaul U, Witzenbichler B, Dudek D, Sardella G, Escaned J, Sharma S, Shlofmitz RA, Collier T, Pocock S, Mehran R. Ticagrelor With or Without Aspirin in High-Risk Patients With Diabetes Mellitus Undergoing Percutaneous Coronary Intervention. J Am Coll Cardiol. 2020;75:2403–13. doi: 10.1016/j.jacc.2020.03.008. [DOI] [PubMed] [Google Scholar]
- Baber U, Dangas G, Angiolillo DJ, Cohen DJ, Sharma SK, Nicolas J, Briguori C, Cha JY, Collier T, Dudek D, Džavik V, Escaned J, Gil R, Gurbel P, Hamm CW, Henry T, Huber K, Kastrati A, Kaul U, Kornowski R, Krucoff M, Kunadian V, Marx SO, Mehta S, Moliterno D, Ohman EM, Oldroyd K, Sardella G, Sartori S, Shlofmitz R, Steg PG, Weisz G, Witzenbichler B, Han YL, Pocock S, Gibson CM, Mehran R. Ticagrelor alone vs. ticagrelor plus aspirin following percutaneous coronary intervention in patients with non-ST-segment elevation acute coronary syndromes: TWILIGHT-ACS. Eur Heart J. 2020;41:3533–45. doi: 10.1093/eurheartj/ehaa670. [DOI] [PubMed] [Google Scholar]
- Dangas G, Baber U, Sharma S, Giustino G, Mehta S, Cohen DJ, Angiolillo DJ, Sartori S, Chandiramani R, Briguori C, Dudek D, Escaned J, Huber K, Collier T, Kornowski R, Kunadian V, Kaul U, Oldroyd K, Sardella G, Shlofmitz R, Witzenbichler B, Ya-Ling H, Pocock S, Gibson CM, Mehran R. Ticagrelor With or Without Aspirin After Complex PCI. J Am Coll Cardiol. 2020;75:2414–24. doi: 10.1016/j.jacc.2020.03.011. [DOI] [PubMed] [Google Scholar]
- Kogame N, Guimaraes PO, Modolo R, De Martino, Tinoco J, Ribeiro EE, Kawashima H, Ono M, Hara H, Wang R, Cavalcante R, Moulin B, Falcao BAA, Leite RS, de Almeida, Morais GR, Meireles GC, Campos CM, Onuma Y, Serruys PW, Lemos PA. Aspirin-Free Prasugrel Monotherapy Following Coronary Artery Stenting in Patients With Stable CAD: The ASET Pilot Study. JACC Cardiovasc Interv. 2020;13:2251–62. doi: 10.1016/j.jcin.2020.06.023. [DOI] [PubMed] [Google Scholar]
- Angiolillo DJ, Rollini F, Storey RF, Bhatt DL, James S, Schneider DJ, Sibbing D, So DYF, Trenk D, Alexopoulos D, Gurbel PA, Hochholzer W, De Luca, Bonello L, Aradi D, Cuisset T, Tantry US, Wang TY, Valgimigli M, Waksman R, Mehran R, Montalescot G, Franchi F, Price MJ. International Expert Consensus on Switching Platelet P2Y12 Receptor-Inhibiting Therapies. Circulation. 2017;136:1955–75. doi: 10.1161/CIRCULATIONAHA.117.031164. [DOI] [PubMed] [Google Scholar]
- De Luca, D'Ascenzo F, Musumeci G, Saia F, Parodi G, Varbella F, Marchese A, De Servi, Berti S, Bolognese L. Incidence and outcome of switching of oral platelet P2Y12 receptor inhibitors in patients with acute coronary syndromes undergoing percutaneous coronary intervention: the SCOPE registry. EuroIntervention. 2017;13:459–66. doi: 10.4244/EIJ-D-17-00092. [DOI] [PubMed] [Google Scholar]
- Sibbing D, Aradi D, Jacobshagen C, Gross L, Trenk D, Geisler T, Orban M, Hadamitzky M, Merkely B, Kiss RG, Komócsi A, Dézsi CA, Holdt L, Felix SB, Parma R, Klopotowski M, Schwinger RHG, Rieber J, Huber K, Neumann FJ, Koltowski L, Mehilli J, Huczek Z, Massberg S TROPICAL-ACS Investigators. Guided de-escalation of antiplatelet treatment in patients with acute coronary syndrome undergoing percutaneous coronary intervention (TROPICAL-ACS): a randomised, open-label, multicentre trial. Lancet. 2017;390:1747–57. doi: 10.1016/S0140-6736(17)32155-4. [DOI] [PubMed] [Google Scholar]
- Cayla G, Cuisset T, Silvain J, Leclercq F, Manzo-Silberman S, Saint-Etienne C, Delarche N, Bellemain-Appaix A, Range G, El Mahmoud, Carrié D, Belle L, Souteyrand G, Aubry P, Sabouret P, du Fretay, Beygui F, Bonnet JL, Lattuca B, Pouillot C, Varenne O, Boueri Z, Van Belle, Henry P, Motreff P, Elhadad S, Salem JE, Abtan J, Rousseau H, Collet JP, Vicaut E, Montalescot G ANTARCTIC investigators. Platelet function monitoring to adjust antiplatelet therapy in elderly patients stented for an acute coronary syndrome (ANTARCTIC): an open-label, blinded-endpoint, randomised controlled superiority trial. Lancet. 2016;388:2015–22. doi: 10.1016/S0140-6736(16)31323-X. [DOI] [PubMed] [Google Scholar]
- Claassens DMF, Vos GJA, Bergmeijer TO, Hermanides RS, van ’t, van der, Barbato E, Morisco C, Tjon Joe, Asselbergs FW, Mosterd A, Herrman JR, Dewilde WJM, Janssen PWA, Kelder JC, Postma MJ, de Boer, Boersma C, Deneer VHM, ten Berg. A Genotype-Guided Strategy for Oral P2Y12 Inhibitors in Primary PCI. N Engl J Med. 2019;381:1621–31. doi: 10.1056/NEJMoa1907096. [DOI] [PubMed] [Google Scholar]
- Galli M, Benenati S, Franchi F, Rollini F, Capodanno D, Biondi-Zoccai G, Vescovo GM, Cavallari LH, Bikdeli B, ten Berg, Mehran R, Gibson CM, Crea F, Pereira NL, Sibbing D, Angiolillo DJ. Comparative Effects of Guided versus Potent P2Y12 Inhibitor Therapy in Acute Coronary Syndrome: A Network Meta-Analysis of 61 898 Patients from 15 Randomized Trials. Eur Heart J. 2021 Dec 16. [Epub ahead of print]. doi: 10.1093/eurheartj/ehab836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuisset T, Deharo P, Quilici J, Johnson TW, Deffarges S, Bassez C, Bonnet G, Fourcade L, Mouret JP, Lambert M, Verdier V, Morange PE, Alessi MC, Bonnet JL. Benefit of switching dual antiplatelet therapy after acute coronary syndrome: the TOPIC (timing of platelet inhibition after acute coronary syndrome) randomized study. Eur Heart J. 2017;38:3070–8. doi: 10.1093/eurheartj/ehx175. [DOI] [PubMed] [Google Scholar]
- Kim HS, Kang J, Hwang D, Han JK, Yang HM, Kang HJ, Koo BK, Rhew JY, Chun KJ, Lim YH, Bong JM, Bae JW, Lee BK, Park KW HOST-REDUCE-POLYTECH-ACS investigators. Prasugrel-based de-escalation of dual antiplatelet therapy after percutaneous coronary intervention in patients with acute coronary syndrome (HOST-REDUCE-POLYTECH-ACS): an open-label, multicentre, non-inferiority randomised trial. Lancet. 2020;396:1079–89. doi: 10.1016/S0140-6736(20)31791-8. [DOI] [PubMed] [Google Scholar]
- Kim CJ, Park MW, Kim MC, Choo EH, Hwang BH, Lee KY, Choi YS, Kim HY, Yoo KD, Jeon DS, Shin ES, Jeong YH, Seung KB, Jeong MH, Yim HW, Ahn Y, Chang K TALOS-AMI investigators. Unguided de-escalation from ticagrelor to clopidogrel in stabilised patients with acute myocardial infarction undergoing percutaneous coronary intervention (TALOS-AMI): an investigator-initiated, open-label, multicentre, non-inferiority, randomised trial. Lancet. 2021;398:1305–16. doi: 10.1016/S0140-6736(21)01445-8. [DOI] [PubMed] [Google Scholar]
- Tavenier AH, Mehran R, Chiarito M, Cao D, Pivato CA, Nicolas J, Beerkens F, Nardin M, Sartori S, Baber U, Angiolillo DJ, Capodanno D, Valgimigli M, Hermanides RS, van 't, Ten Berg, Chang K, Kini AS, Sharma SK, Dangas G. Guided and unguided de-escalation from potent P2Y12 inhibitors among patients with ACS: a meta-analysis. Eur Heart J Cardiovasc Pharmacother. 2021 Aug 30. [Epub ahead of print]. doi: 10.1093/ehjcvp/pvab068. [DOI] [PubMed] [Google Scholar]
- Li J, Vootukuri S, Shang Y, Negri A, Jiang JK, Nedelman M, Diacovo TG, Filizola M, Thomas CJ, Coller BS. RUC-4: a novel αIIbβ3 antagonist for prehospital therapy of myocardial infarction. Arterioscler Thromb Vasc Biol. 2014;34:2321–9. doi: 10.1161/ATVBAHA.114.303724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milluzzo RP, Franchina GA, Capodanno D, Angiolillo DJ. Selatogrel, a novel P2Y12 inhibitor: a review of the pharmacology and clinical development. Expert Opin Investig Drugs. 2020;29:537–46. doi: 10.1080/13543784.2020.1764533. [DOI] [PubMed] [Google Scholar]
- Mayer K, Hein-Rothweiler R, Schüpke S, Janisch M, Bernlochner I, Ndrepepa G, Sibbing D, Gori T, Borst O, Holdenrieder S, Kupka D, Petzold T, Bradaric C, Okrojek R, Leistner DM, Trippel TD, Münzel T, Landmesser U, Pieske B, Zeiher AM, Gawaz MP, Hapfelmeier A, Laugwitz KL, Schunkert H, Kastrati A, Massberg S. Efficacy and Safety of Revacept, a Novel Lesion-Directed Competitive Antagonist to Platelet Glycoprotein VI, in Patients Undergoing Elective Percutaneous Coronary Intervention for Stable Ischemic Heart Disease: The Randomized, Double-blind, Placebo-Controlled ISAR-PLASTER Phase 2 Trial. JAMA Cardiol. 2021;6:753–61. doi: 10.1001/jamacardio.2021.0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo BK, Kang J, Park KW, Rhee TM, Yang HM, Won KB, Rha SW, Bae JW, Lee NH, Hur SH, Yoon J, Park TH, Kim BS, Lim SW, Cho YH, Jeon DW, Kim SH, Han JK, Shin ES, Kim HS HOST-EXAM investigators. Aspirin versus clopidogrel for chronic maintenance monotherapy after percutaneous coronary intervention (HOST-EXAM): an investigator-initiated, prospective, randomised, open-label, multicentre trial. Lancet. 2021;397:2487–96. doi: 10.1016/S0140-6736(21)01063-1. [DOI] [PubMed] [Google Scholar]
- CAPRIE Steering. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet. 1996;348:1329–39. doi: 10.1016/s0140-6736(96)09457-3. [DOI] [PubMed] [Google Scholar]
- Bhatt DL, Grosser T, Dong JF, Logan D, Jeske W, Angiolillo DJ, Frelinger AL, Lei L, Liang J, Moore JE, Cryer B, Marathi U. Enteric Coating and Aspirin Nonresponsiveness in Patients With Type 2 Diabetes Mellitus. J Am Coll Cardiol. 2017;69:603–12. doi: 10.1016/j.jacc.2016.11.050. [DOI] [PubMed] [Google Scholar]
- Angiolillo DJ, Bhatt DL, Lanza F, Cryer B, Dong JF, Jeske W, Zimmerman RR, von Chong, Prats J, Deliargyris EN, Marathi U. Pharmacokinetic/pharmacodynamic assessment of a novel, pharmaceutical lipid-aspirin complex: results of a randomized, crossover, bioequivalence study. J Thromb Thrombolysis. 2019;48:554–62. doi: 10.1007/s11239-019-01933-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryer B, Bhatt DL, Lanza FL, Dong JF, Lichtenberger LM, Marathi UK. Low-dose aspirin-induced ulceration is attenuated by aspirin-phosphatidylcholine: a randomized clinical trial. Am J Gastroenterol. 2011;106:272–7. doi: 10.1038/ajg.2010.436. [DOI] [PubMed] [Google Scholar]