Abstract
Background
The presence of severe calcific atherosclerosis at the iliofemoral axis may preclude transcatheter aortic valve implantation (TAVI) by the transfemoral (TF) approach. Intravascular lithotripsy (IVL) is a novel technology that fractures intimal/medial calcium and increases vessel compliance allowing TF TAVI in selected patients with peripheral artery disease (PAD).
Aims
The aim of this study was to report on the safety and efficacy of IVL-assisted TF TAVI in an all-comers population.
Methods
Clinical, imaging and procedural data on all consecutive patients treated by IVL-assisted TF TAVI in six high-volume European centres (2018-2020) were collected in this prospective, real-world, multicentre registry.
Results
IVL-assisted TF TAVI was performed in 108 patients, increasing from 2.4% to 6.5% of all TAVI from 2018 to 2020, respectively. The target lesion was most often localised at the common and/or external iliac artery (93.5% of cases; average TL-MLD 4.6±0.9 mm with 318 degrees of calcium arc). Transfemoral aortic valve delivery was successful in 100% of cases; final procedural success in 98.2% (two conversions to cardiac open surgery for annular rupture and valve migration). Complications of the IVL-treated segments consisted of 1 perforation and 3 major dissections requiring stent implantation (2 covered stents and 2 BMS). Access-site-related complications included 3 major bleedings. Three in-hospital deaths were recorded (2.8%, 1 failed surgical conversion after annular rupture, 1 cardiac arrest after initial valvuloplasty, 1 late hyperkalaemia in renal dysfunction).
Conclusions
IVL-assisted TF TAVI proved to be a safe and effective approach, which helps to expand the indications for TF TAVI in patients with severe calcific PAD. However, these patients continue to have a higher-than-average incidence of periprocedural complications.
Introduction
The presence of severe calcific atherosclerosis of the iliac and femoral arteries may preclude transfemoral (TF) delivery for transcatheter aortic valve implantation (TAVI) in 10-15% of cases1,2.
At the start of the TAVI era surgical transapical procedures were used, but only transfemoral TAVI was proved to be equal or superior to surgical aortic valve replacement (SAVR) in randomised trials3. Alternative approaches (i.e., transsubclavian, transaxillary, transcaval, transcarotid, direct aortic) have limited evidence of safety and efficacy and may require a surgical approach and/or general anaesthesia4. Intravascular lithotripsy (IVL) has been used increasingly in coronary and peripheral angioplasty5,6,7. The largest IVL balloons (up to 7 mm) allow its application in iliac and common femoral arteries to facilitate insertion of the TAVI delivery system. Since its first description in a case performed in Florence in December 20178, only a few case reports9,10 and one multicentric registry of less than 50 patients have been published11. No studies have prospectively addressed the advantage conferred by a systematic use of this technique in reducing the need of non-transfemoral approaches.
To confirm the role of IVL in facilitating transfemoral TAVI, consecutive data were collected in a prospective registry including six high-volume European centres. The aims of this study were: first, to report the adoption of IVL in the last five years to pre-treat the iliofemoral axis before TAVI, saving/maintaining TF approach; second, to investigate the safety and efficacy of IVL in facilitating TF TAVI.
Materials and methods
DATA COLLECTION
We prospectively collected data from all consecutive TAVI procedures performed between January 2016 and December 2020 at six high-volume European TAVI centres using a common database focusing on the procedures requiring IVL facilitation.
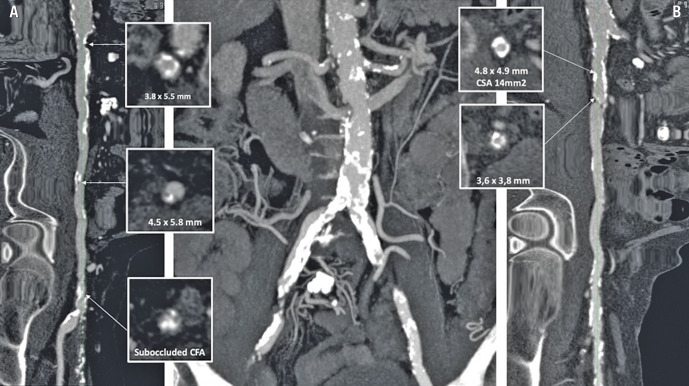
In particular, all consecutive patients with severe symptomatic aortic valve stenosis discussed and approved in the Heart Team and with severe calcific peripheral artery disease (PAD) receiving IVL were enrolled. Complete demographic and clinical data and detailed analysis of the preoperative iliac and femoral computed tomography (CT) angiogram were obtained (Figure 1).
Figure 1. Preprocedural computed tomography (CT) scan to assess iliac and femoral calcification in order to evaluate TAVI access.
Case example (part 1). Central panel: CT image of the iliac bifurcation showing severe tortuosity and calcification of both iliac arteries. CT longitudinal image of the severely calcified right common, external iliac and right common femoral artery (CFA) (panel A) and left common external iliac artery (panel B). In each panel, multiple cross-sections with diameters, area of near circumferential calcification and thick protruding nodules are shown. The right common femoral artery was the access artery selected for this patient, who underwent IVL treatment as shown in Figure 2. CSA: cross-sectional area; IVL: intravascular lithotripsy; TAVI; transcatheter aortic valve implantation
Patient selection required the presence of severe symptomatic aortic valve stenosis with anatomy compatible with safe implantation of available transcatheter valves and discussed and approved in the Heart Team. Complete demographic, clinical data and detailed analysis of the iliac and femoral CT angiogram were obtained in patients with severe calcific PAD deemed unsuitable for standard transfemoral access.
PROCEDURAL TECHNIQUE
The decision to use IVL upfront or as a possible bail-out in case of unsuccessful initial delivery was taken by the individual operators based on the combination of severity of stenosis, circumferential and longitudinal extension of calcification and tortuosity of the iliac vessels. For each target lesion we measured on CT-angiography minimal cross-sectional area, lesion and calcium length, diameter and percentage stenosis. Demographic, clinical and echocardiographic data at baseline were collected through a review of the medical records. Surgical risk was estimated using the Society of Thoracic Surgeons (STS) score12. All patients provided signed informed consent for TAVI, including the possible use of IVL for access preparation.
The femoral puncture for TAVI catheter insertion was performed after administration of local anaesthetics using a combination of ultrasound guidance and contralateral (opposite femoral or left radial) angiography. The type of anaesthesia (local, conscious sedation or general with deep intubation) was left to the best care practices of each participating centre. The combination of local anaesthetics and conscious sedation was preferred, when possible. Protection from the contralateral artery using a 0.018-inch×300 cm long wire was maintained in most cases throughout the procedure. Closure of the puncture site with a percutaneous system was routinely used, normally with two ProGlide clo-sure devices (Abbott Laboratories). In most patients, elective prolonged low-pressure inflation of a balloon was routinely used in some centres to prevent bleeding during tightening of the closure device, with further inflation across the punctured segment after protamine administration. In case of failures not responsive to low-pressure balloon dilatation, covered stents were deployed.
In case of severe calcification or stenosis extending to the common femoral artery, elective surgical vessel exposure and endarterectomy was preferred. Anticoagulation, selection of guidewires and catheters and of the transcatheter heart valves were conducted according to the standard best care practices of each participating institution.
IVL TREATMENT
Predilatation with conventional balloons was left to the operator’s choice.
The decision to use elective IVL upfront was taken by the individual operators based on the combination of severity of stenosis, circumferential and longitudinal extension of calcification and tortuosity of the iliac vessels assessed by CT. For elective cases, after insertion of a 0.014-inch guidewire, a dedicated IVL balloon was delivered, in the majority of cases preferring the largest available IVL balloon (Shockwave M5, 7 mm; Shockwave Medical) (Figure 2). A short 10 Fr sheath was generally used to advance the IVL system from the main access side, after deployment of the pre-implanted closure device system, while a 45 cm, 7 Fr sheath was preferred in case of a contralateral approach. During repeat balloon inflations at low pressure (3-4 atmospheres), multiple pulses were delivered, completing (in the majority of cases) the whole cycle of pulses available (300) for each balloon. Post-dilatation with a conventional non-compliant (NC) balloon was left to the operator’s choice. For bail-out procedures, IVL was used after failure to advance the delivery sheath or the valve deployment system.
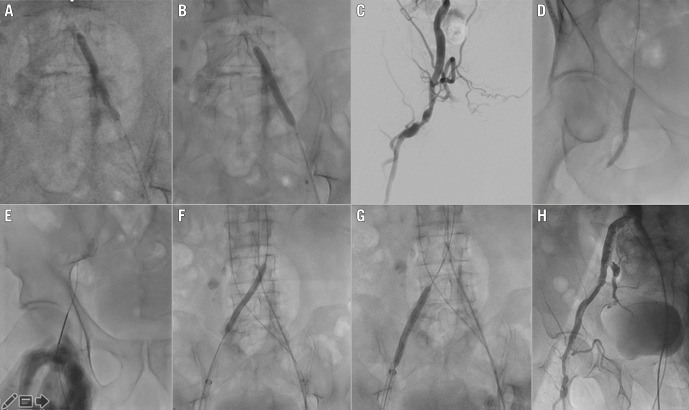
Figure 2. IVL predilatation to allow transfemoral TAVI.
Case example (part 2). A) & B) 7 mm IVL balloon on left common iliac artery (4 atm) with evident dog-boning effect. C) & D) Peripheral angiography showing occluded superficial femoral artery, critical calcific stenosis, and tortuosity of the common femoral artery extending to the deep femoral artery (C) that were treated with a 5 mm IVL balloon (4 atm) (D). E) Right common femoral artery puncture. F) & G) Peripheral lithotripsy with 7 mm Shockwave IVL balloon on right common iliac artery (4 atm), main TAVI access. H) Final aortogram with no dissection or extravasation. IVL: intravascular lithotripsy; TAVI; transcatheter aortic valve implantation
TAVI OUTCOME
We reported the frequency of permanent pacemaker implantation and collected data on post-procedural creatinine and troponin in order to assess kidney and myocardial injury13,14,15.
In-hospital death was reported, distinguished between cardiovascular or non-cardiovascular death.
Successful valve delivery was defined as the ability to advance the TAVI valve delivery system via the femoral route up to the aortic annulus facilitated by IVL. Successful valve implantation was assessed as optimal valve positioning and without greater than moderate aortic valve regurgitation. Valve performance was evaluated by considering the post-procedural angiographic and echocardiographic severity of aortic regurgitation and valve gradient.
IVL-RELATED AND ACCESS-SITE COMPLICATIONS
IVL-related and access-site complications were documented with final angiography in all patients and are reported separately. Vascular complications were registered and reported according to Valve Academic Research Consortium 3 (VARC 3) definitions16. Vascular dissections were graded, based upon angiographic appearance, as types A to F according to the National Heart, Lung and Blood Institute (NHLBI) classification17. Vascular closure device failure was defined as failure to achieve haemostasis at the access site, resulting in alternative treatment (other than manual compression or planned adjunctive endovascular balloon inflation). Bleedings were graded according the Bleeding Academic Research Consortium (BARC) definitions18.
STATISTICAL ANALYSIS
Statistical analysis was performed with SPSS statistical software, Version 27.0 (IBM Corp.). Continuous data were expressed as mean±standard deviation whereas median and interquartile ranges (IQR) were used if a non-normal distribution was present. Fisher’s exact test was performed to examine the relationship between predilation before IVL and the rate of post-procedural dissections.
Results
The baseline patient characteristics of the 108 patients treated with IVL facilitated TAVI are reported in Table 1. Reference vessel diameter was 9.1 mm (IQR 8.3-10.0), target lesion minimum diameter 4.6±0.8 mm with an average diameter stenosis of 50.0±10.7%. Mean calcification length was 50.5 mm (IQR 32.7-74.1), with an average maximum circumferential calcium angle of 317.5 degrees (IQR 282.0-360.0).
Table 1. Baseline patient demographic characteristics and lesion angio-CT assessment.
| Baseline characteristics | n=108 | |
|---|---|---|
| Male, n (%) | 61 (56.5%) | |
| Female, n (%) | 47 (43.5%) | |
| Age at the time of procedure, years | 80.5±6.2 | |
| BMI, kg/m² | 25.5 (23.2-27.6) | |
| Tricuspid aortic valve, n (%) | 103 (95.4%) | |
| Bicuspid aortic valve (type 1 L-R), n (%) | 3 (2.8%) | |
| Valve-in-valve, n (%) | 2 (1.8%) | |
| Baseline valve area, mm2 | 0.7±0.2 | |
| Baseline EF (%) | 54.5 (40.0-60.0) | |
| Baseline mean gradient, mmHg | 42.0 (35.0-50.0) | |
| STS | 3.35 (2.1-5.3) | |
| Lesion characteristics | n=108 | |
| Target lesion location, n (%) | Common iliac artery | 35 (32.4%) |
| External iliac artery | 17 (15.7%) | |
| Common and external iliac arteries | 49 (45.4%) | |
| Common femoral artery | 7 (6.5%) | |
| RVD, mm | 9.1 (8.3-10.0) | |
| Target lesion diameter, mm | 4.6±0.9 | |
| Diameter stenosis % | 50.0+10.7 | |
| Target lesion length*, mm | 20.0 (12.0-30.0) | |
| Calcification max arc, degrees | 317.5 (282.5-360) | |
| Calcification min CSA, mm2 | 18.1 (14.4-25.8) | |
| Calcification length*, mm | 50.5 (32.7-74.1) | |
| Values are % (n) or mean±SD or median + interquartile range. * Calcification length refers to the length of the entire calcified segment, while target lesion length refers to the stenotic calcific segment. BMI: body mass index; CSA: cross-section area; CT: computed tomography; EF: ejection fraction; RVD: reference vessel diameter; STS: surgical risk score | ||
Femoral access was obtained percutaneously in 93.5% of patients, while 7 patients (6.5%) required elective surgical cutdown. Procedural details are summarised in Table 2. Predilatation with 5-6 mm compliant and semi-compliant peripheral balloons (e.g., Armada [Abbott Laboratories]) before IVL was used in 17.6% of lesions. Immediate elective IVL was performed in the most severely calcified and narrowed lesions, while in 33 procedures (30.6%) IVL was kept as a standby and used only after failure to advance the TAVI delivery system. A 7 mm Shockwave M5IVL catheter was employed in 85.7% of cases with the maximum available cycle of impulses (300) delivered. In 4 lesions, 2 IVL balloons were required.
Table 2. Procedural characteristics and IVL-related details.
| Procedural details | n=108 | |
|---|---|---|
| Site access, n (%) | Percutaneous | 101 (93.5%) |
| Cutdown | 7 (6.5%) | |
| Protection crossover wire in place, n (%) | Yes | 71 (65.7%) |
| No | 37 (34.3%) | |
| Conscious sedation, n (%) | 83 (76.9%) | |
| General anaesthesia, n (%) | 25 (23.1%) | |
| Predilatation with conventional balloon, n (%) | Yes | 19 (17.6%) |
| No | 89 (82.4%) | |
| Elective IVL, n (%) | 75 (69.4%) | |
| Bail-out IVL, n (%) | 33 (30.6%) | |
| Number of IVL balloons per lesion, n (%) | 1 IVL balloon per lesion | 104 (96.3%) |
| 2 IVL balloons per lesion | 4 (3.7%) | |
| IVL catheter size, n (%) (112) | 5×60 mm | 1 (0.9%) |
| 5.5×60 mm | 1 (0.9%) | |
| 6×60 mm | 8 (7.1%) | |
| 6.5×60 mm | 6 (5.4%) | |
| 7×60 mm | 96 (85.7%) | |
| Number of IVL pulses per lesion | 300.0 (270.0-300.0) | |
| Residual stenosis post-IVL, %§ | 25.0 (20.0-30.0) | |
| Conventional balloon post-dilatation after IVL | Yes, n (%) | 56 (51.8%) |
| No, n (%) | 52 (48.2%) | |
| Balloon size, n (%) | 6 mm | 3 (5.4%) |
| 7 mm | 14 (25%) | |
| 8 mm | 39 (69.6%) | |
| Balloon pressure, atm | 6.0 (6.0-6.0) | |
| IVL balloon vs NC/high-pressure (HP), n (%) | IVL 7 mm >NC/HP 8 mm | 38 (67.8%) |
| IVL 6 mm >NC/HP 8 mm | 1 (1.8%) | |
| IVL 6 mm >NC/HP 7 mm | 2 (3.6%) | |
| IVL 7 mm >NC/HP 7 mm | 12 (21.4%) | |
| IVL 7 mm >NC 6 mm | 3 (5.4%) | |
| Size of sheet, n (%) | 14 Fr | 55 (50.9%) |
| 16 Fr | 42 (38.9%) | |
| >18 Fr | 11 (10.2%) | |
| Degree of aortic regurgitation post-TAVI, n (%) | None or trace | 54 (50.0%) |
| Mild | 48 (44.4%) | |
| Moderate | 3 (2.8%) | |
| Severe | 1 (0.9%) | |
| Unknown | 2 (1.8%) | |
| Access-site closure method, n (%) | Device | 101 (93.5%) |
| Surgical | 7 (6.5%) | |
| Values are n (%) or mean±SD or median + interquartile range. §Residual stenosis post-IVL was assessed by visual estimation with iliac and femoral angiography after IVL dilatation. IVL: intravascular lithotripsy; TAVI: transcatheter aortic valve implantation | ||
Post-dilatation with 7 and 8 mm high-pressure and non-compliant balloons ((Mustang [Boston Scientific], Z-MED II [B. Braun], and Charger [Boston Scientific]) was performed in 51.8% of cases. Other details are reported in Table 2.
Thirty-four (31.5%) balloon-expandable SAPIEN 3 (S3) and SAPIEN Ultra valves (Edwards Lifesciences) were implanted, while a variety of self-expanding valves were used (most frequently Evolut R or Evolut R PRO [Medtronic] and Portico [Abbott Laboratories]; 38.9% and 22.2%, respectively) (Supplementary Table 1). On the control echocardiogram at the end of the procedure mean gradient decreased to 7.0 mmHg (IQR 4.0-10.0). In 50.0% of aortic valve implantations, aortic regurgitation was absent or minimal with mild regurgitation in 44.4%.
Valve delivery through the femoral route was successfully achieved in all (100%) of the cases, followed by successful valve implantation in 98.2% of procedures. Other post-TAVI outcomes are reported in Table 3.
Table 3. Procedural and in-hospital TAVI outcomes.
| n=108 | |
|---|---|
| Successful delivery of THV, n (%) | 108 (100.0%) |
| Successful valve implantation, n (%) | 106 (98.2%) |
| TAVI performed at the same time as IVL, n (%) | 108 (100.0%) |
| In hospital all-cause death, n (%) | 3 (2.8%) |
| Stroke, n (%) | 0 (0.0%) |
| New permanent pacemaker, n (%) | 8 (7.4%) |
| Acute kidney injury, n (%) | 7 (6.5%) |
| Periprocedural acute MI, n (%) | 0 (0.0%) |
| Values are n (%) or mean±SD or median + interquartile range. MI: myocardial infarction; TAVI: transcatheter aortic valve implantation; THV: transcatheter heart valve | |
Vascular haemostasis was achieved with closure devices (double ProGlide or Prostar XL [Abbott Laboratories] or occasionally MANTA® [Teleflex Medical]) in all of the non-surgical cases with closure device failure (5.9% of procedures). In these patients, haemostasis was achieved with prolonged balloon dilatation and/or covered stent placement (Table 4). Major vascular complications involving the segment undergoing IVL occurred in 3.7% of cases and included one perforation of the external iliac artery and three type F dissections (2 involving the external iliac artery and 1 the common femoral artery). They were detected by angiography immediately after the IVL dilatation and required endovascular treatment with bare metal or covered stents. Overall, IVL-related complications of the treated lesion required 2 covered stents and 3 bare metal stents implanted in the external and common femoral and common iliac arteries. Notably, out of the 7 dissections which occurred with IVL (3 major and 4 minor), 4 (21.1%) dissections were reported in patients who underwent predilatation compared to 3 (3.4%) patients who did not undergo predilatation prior to IVL, with a significative statistical difference (p-value <0.0001).
Conversely, the most frequently reported access-site complications were closure device failure (5.9%), dissection (1.8%), and major bleedings (2.8%), requiring unplanned endovascular interventions in 12% of cases. In particular, 10 covered stent and 1 bare metal stent were implanted (Table 4).
Table 4. IVL-related, access-site- and cardiac structural- and technical valve-related complications.
| IVL-related vascular complications | |
|---|---|
| Perforation, n (%) | 1 (0.9%) |
| Rupture, n (%) | 0 (0.0%) |
| Minor dissection (type A-B-C), n (%) | 4 (3.7%) |
| Major dissection (type D-E-F), n (%) | 3 (2.8%) |
| Covered stent, n (%) | 2 (1.8%) |
| Bare metal stent, n (%) | 3 (2.8%) |
| Access-site-related complications | |
| Vessel perforation, n (%) | 0 (0.0%) |
| Rupture, n (%) | 1 (0.9%) |
| Dissection type, n (%) | 2 (1.8%) |
| Stenosis, n (%) | 3 (2.8%) |
| Ischaemia, n (%) | 0 (0.0%) |
| Arteriovenous thrombosis, n (%) | 0 (0.0%) |
| Arteriovenous fistula, n (%) | 1 (0.9%) |
| Pseudoaneurysm, n (%) | 0 (0.0%) |
| Retroperitoneal haematoma, n (%) | 1 (0.9%) |
| Infection, n (%) | 0 (0.0%) |
| Distal embolisation, n (%) | 0 (0.0%) |
| Closure device failure, n (%) | 6 (5.9%) |
| Bleeding <BARC3a, n (%) | 21 (19.4%) |
| Bleeding >BARC type 3b, n (%) | 3 (2.8%) |
| Unplanned endovascular intervention (balloon dilatation or covered stent implantation), n (%) | 13 (12.0%) |
| Balloon dilatation, n (%) | 4 (3.7%) |
| Covered stent, n (%) | 10 (9.3%) |
| Bare metal stent, n (%) | 1 (0.9%) |
| Acute procedural and technical valve-related complications | |
| Conversion to open-surgery, n (%) | 2 (1.8%) |
| Unplanned use of mechanical circulatory support, n (%) | 1 (0.9%) |
| Valve embolisation, n (%) | 1 (0.9%) |
| Valve migration, n (%) | 0 (0.0%) |
| Ectopic valve deployment, n (%) | 0 (0.0%) |
| Cardiac structural complications | |
| Cardiac structures perforation, injury, n (%) | 1 (0.9%) |
| Pericardial effusion resulting in tamponade, n (%) | 1 (0.9%) |
| Coronary obstruction, n (%) | 0 (0.0%) |
| Values are n (%) or mean±SD or median + interquartile range | |
Three (3%) major bleedings (BARC type ≥ 3B) occurred. Other access-site complications are also reported in Table 4.
We observed one case of annulus rupture after valve implantation with subsequent patient death despite immediate surgery, a second emergency surgical conversion after valve embolisation into the left ventricular cavity, and one new pericardial effusion resulting in cardiac tamponade requiring pericardiocentesis (likely due to right ventricle perforation by a temporary pacemaker electrode).
One patient developed cardiac arrest after sedation at the beginning of the procedure and, after unsuccessful cardiopulmonary resuscitation manoeuvres and emergency aortic balloon valvuloplasty, required mechanical support with arteriovenous extracorporeal membrane oxygenation (ECMO). After IVL preparation of the opposite iliac artery, TAVI was successfully completed with initial patient stabilisation but subsequent death due to sepsis after eight transfusions and prolonged intubation. The other two deaths were caused by a failed surgical repair post annular rupture and a malignant arrhythmia due to hyperkalaemia in a patient under chronic haemodialysis.
Between 2016 and 2020, 3,705 TAVI procedures were performed in the six participating centres that started IVL in 2018: in 3,469 patients (93.6%) TAVI was performed via a transfemoral route, while the remaining 6.4% required alternative approaches, mainly transsubclavian and transapical. IVL-assisted TAVI progressively increased from 16 (2.4%) in 2018 to 33 (3.6%) in 2019 and 59 (6.5%) in 2020 (Central illustration). In parallel to the increase IVL-assisted transfemoral procedures, we observed a decrease in the use of alternative approaches (from 8.7% in January 2018 to 3.0% in 2020).
Central illustration. Cumulative data from Careggi University Hospital (Italy), Rigshospitalet Copenhagen University Hospital (Denmark), CNR Cardiovascular Centre (Italy) and Policlinico San Orsola, University of Bologna (Italy).
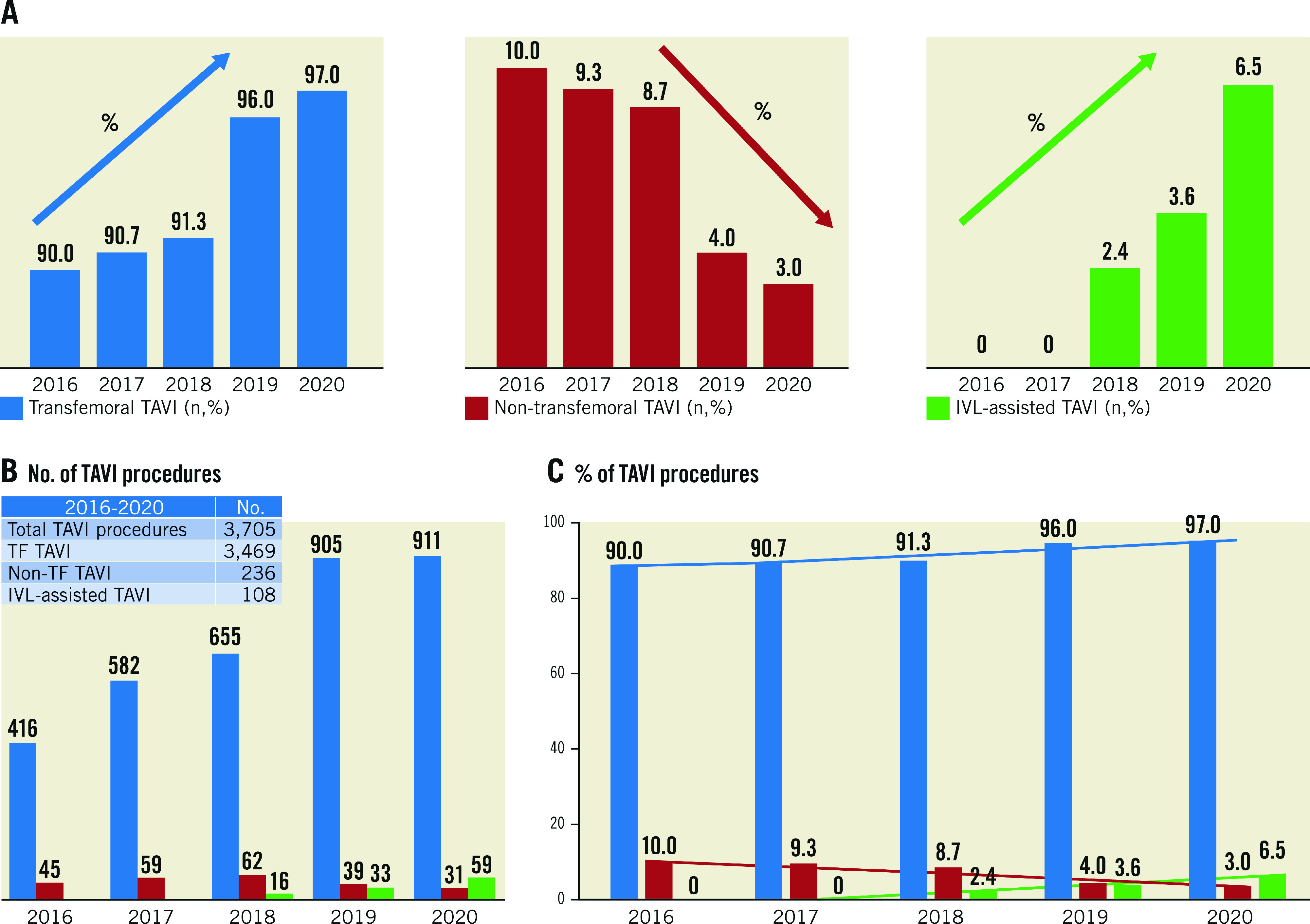
Istituto Clinico Sant'Ambrogio (Italy) and Institut Cœur Poumon, CHU de Lille (France) introduced lithotripsy in their clinical practice in 2020, therefore data from these centres are not included in this figure. Data are reported as percentage (histograms A-C) and as total number of cases (histogram B). Transfemoral TAVI is displayed in blue, non-transfemoral in red and IVL-assisted in green. Between 2016 and 2020, a total of 3,705 TAVI procedures were performed at the four participating centres, with 3,469 (93.6%) performed via a transfemoral route while the remaining 6.4% required alternative approaches mainly represented by trans-subclavian and transapical accesses (B & C). In the period from 2018-2020, a total of 108 cases of IVL-assisted TAVI were performed, increasing from 16 (2.4%) in 2018 to 59 (6.5%) in 2020 (C). The use of alternative approaches progressively decreased from 10% in 2016 to 3% in 2020. IVL: intravascular lithotripsy; TAVI; transcatheter aortic valve implantation; TF: transfemoral
Discussion
This manuscript reports on the largest TAVI series of iliofemoral IVL to facilitate transfemoral valve delivery. In 108 consecutive patients, crossing of the TAVI delivery catheter was always successful despite the presence on CT of severe circumferential iliac of femoral calcifications and narrowings.
The transfemoral approach for TAVI offers multiple advantages4. It is a percutaneous route and is almost painless if generous local anaesthesia is delivered under intravascular ultrasound (IVUS) guidance. It benefits from the availability of multiple percutaneous closure devices with a high success rate, from the preclosure with the suture-based ProGlide and Prostar to the delivery after the procedure of large collagen-based plugs (MANTA). It allows rapid ambulation, with a risk of bleeding and vascular complications progressively decreasing with operator experience, and shorter hospital stays with a lower need for intensive care19. All these aspects are always desirable but especially valuable in the COVID-19 era and in geriatric patients who may require prolonged rehabilitation if kept on bed rest too long. Randomised trials and registries consistently indicate that the best outcome and lowest complication rate is achieved using the transfemoral access20. Guidelines endorse this evidence, recommending TAVI as a superior or equal treatment to SAVR in elderly (>65 years) low-risk patients21, but stress, among the key decision elements, the possibility of treating the patient with a transfemoral approach22.
IVL, a balloon-based system designed to deliver sonic pressure waves to the arterial wall, has been successfully used in coronary arteries with safety and efficacy. This has recently been confirmed in a pooled analysis of 628 patients in four controlled registries23. For peripheral arteries the evidence is equally strong, supported by a recent randomised trial in superficial femoral arteries showing greater lumen gain, fewer flow-limiting dissections and a lesser need for stent implantation when compared with conventional percutaneous transluminal angioplasty (PTA)24. Our experience replicates these results in iliac arteries where IVL obtained sufficient lumen expansion and increased compliance to allow crossing with modern transcatheter heart valve (THV) delivery systems. Consequently, IVL allowed a further expansion of the group of patients suitable for transfemoral TAVI. Our most recent large multicentre experience suggests that with IVL a transfemoral approach is feasible in up to 95 to 97% of patients selected for TAVI (Central illustration).
From our multicentre experience, IVL was effective in treating vascular stenosis with large circumferential and longitudinal extension of calcification. The concomitant presence of circumferential calcification and luminal narrowing represents an obstacle to advancing large TAVI delivery systems. Non-calcified arteries may be stretched, and successful insertion can be achieved with a lumen as small as 0.75% of the TAVI sheath’s outer diameter. For calcified tortuous arteries it is recommended that the lumen is at least 1.25% of the sheath’s outer diameter for safe insertion because calcific vessels are unexpandable. The 14 or 16 Fr inner diameter sheaths of contemporary miniaturised delivery systems have external diameters that may exceed 8 mm, making circumferential calcifications with a narrow lumen an insurmountable obstacle to valve delivery25.
Peripheral IVL balloons may appear inadequate to treat large iliac arteries. However, 6-7 mm peripheral IVL balloons intended to be used in superficial femoral arteries appear large enough to convey energy to the arterial wall in vessels with severe calcified stenoses. The energy delivered via five electrodes by the Shockwave M5 40 mm long IVL balloon inflated at low pressure modifies the vessel rigidity with the creation of multiple longitudinal and transversal cracks, as shown by optical coherence tomography in coronary arteries26 and by histology and micro-CT in peripheral arteries.
The impression given by the repeat angiogram was that occasionally only a minimal gain was observed limited to the stenotic segments with greater luminal narrowing possibly due to the use of undersized IVL balloons. Anyway, the lumen enlargement obtained was always adequate to allow the insertion of the valve delivery system that was the primary aim of our study.
The various centres participating in this registry used slightly different practices, one of them consistently using larger non-compliant balloons at higher pressures and the others mainly relying on the IVL treatment. It is difficult to recommend one or the other strategy considering their universal success with a low rate of complications.
IVL was not only effective but also safe, with a low rate of major VARC 3 vascular complications (3.7%), including easily treatable vascular perforations and dissections at the treated sites. These percentages compare favourably with historical TAVI series27. Furthermore, in most cases the IVL balloon could be inserted without the need of predilatation. When predilatation was performed, often in an attempt to spare the use of IVL, a higher number of dissections were observed post procedure. Heavy calcifications and stenoses extending to the femoral puncture site remain a contraindication to transfemoral TAVI. Seven cases of combined surgical femoral preparation followed by iliac IVL were performed, suggesting that IVL also offers opportunities in these extreme cases. However, based on this limited series, and taking into account the occurrence of bleedings and transfusions, we cannot recommend this as a routine strategy in preference to alternative established routes.
Although transfemoral valve delivery was successful in all cases (100%), the rate of adequate valve implantation without complications was lower, with one annulus rupture and one valve embolisation that required immediate conversion to open cardiac surgery and a third post-operative death after a complicated procedure requiring ECMO. In the past, PAD was reported to be a negative prognostic predictor of events in TAVI patients but this finding was confused by the concomitant need of non-transfemoral access. Our paper suggests that safely solving the access problem in PAD patients is not sufficient to equalise their risk with the risk of the general TAVI population.
Recommendations shared by most of the operators in this registry include the importance of accurate preprocedural CT-imaging planning to evaluate the extension of iliofemoral calcification and to assess the complexity of the vascular anatomy, facilitated by three-dimensional CT reconstruction. IVL was used both electively and, in case of delivery failure, as a bail-out, in both instances with high success and a low rate of complications. Other recommendations include an adequate endovascular bail-out plan in case of vascular complication, especially related to the access site, i.e., use of endovascular treatment with balloon or covered/bare stent implantation.
The use of a protective wire from the contralateral femoral or, more recently, from the left radial artery has been almost abandoned as a routine procedure. However, most of the operators in this registry feel that the presence of this additional protection has proved very helpful in these severely diseased iliac arteries for rapid treatment of complications at the IVL sites or, more frequently, at the puncture site. The presence of an additional wire may also have helped in the achievement of a successful crossing, acting as a buddy wire in parallel with the very stiff primary wire.
Good procedural planning with measurements of the femoral and iliac vessels with angio CT and the smaller diameter of the most recent valve delivery systems contributed to a steady reduction of adverse events and resulted in an increased adoption of transfemoral TAVI, thereby reducing the need for alternative approaches28. We have probably reached a ceiling in the process of valve miniaturisation of delivery devices and the proposed new percutaneous approaches (transcaval, fully percutaneous transsubclavian and transaxillary) have not become standard of care outside selected centres29.
Limitations
This was not a randomised trial and it might be argued that some THVs may have crossed the iliofemoral axis with a conventional balloon PTA without the use of this expensive additional tool19. This is unlikely to be true, at least in a substantial number of these patients, because IVL was primarily used only for the most severely narrowed PAD lesions shown with CT while in less severe lesions and calcifications, IVL was kept as a standby and used only as bail-out after delivery failure. One may also argue that using non-compliant balloons or dedicated inflatable sheaths at high pressure can offer a cheaper alternative. The initial experience when aggressive PTA was used to force the passage of the TAVI delivery system showed catastrophic complications, suggesting a low threshold for alternative approaches. Confirmation of that was provided by the higher number of dissections observed post procedure when predilatation before IVL was performed.
The high number of procedures performed under general anaesthesia is different from the established practice and justified by the decision of one of the participating centres to proceed with initial patient intubation in order to have the possibility to convert to alternative approaches rapidly in case of delivery failure via a transfemoral route. We believe that this unusually high adoption of general anaesthesia will fall with increased operator experience and confidence with this novel technique.
Finally, caution must be exercised based on the incidence of non-IVL-related events, including a 2.8% rate of in-hospital mortality. This percentage is far from the <1% mortality observed in recent trials and also in the real-world experience of many high-volume centres, including those participating in this registry. IVL is very efficient in allowing valve delivery but does not eliminate the inherent increased risk of frail patients with PAD and multiple other comorbidities, often showing extreme calcifications extending to the aortic valve leaflets, ascending aorta and left ventricular (LV) outflow tract.
Conclusions
IVL safely and effectively expands the indications to transfemoral TAVI in patients with severe peripheral artery calcifications, allowing the use of a TF approach in >95% of an all-comers TAVI population and a reduction of alternative approaches to less than 3% when a liberal use of IVL is adopted in clinical practice. However, these patients continue to have a higher than average incidence of periprocedural complication.
Impact on daily practice
Modification of arterial compliance by peripheral IVL allows crossing heavily calcified iliofemoral axes with various TAVI delivery systems. This reduces the need for alternative, non-transfemoral TAVI approaches in patients with peripheral artery disease (PAD) and expands the indications for TF TAVI. Importantly, patients with severe calcific PAD have a high risk of non-IVL-related adverse events, including complications during valve deployment prompted by the severe calcification of aortic leaflets and periannular structures and access-site bleeding with failure of percutaneous closure devices.
Guest Editor
This paper was guest edited by Franz-Josef Neumann, MD; Department of Cardiology and Angiology II, University Heart Center Freiburg - Bad Krozingen, Bad Krozingen, Germany.
Supplementary data
Valve-related characteristics.
Acknowledgments
Acknowledgements
Contributors: Cedric Delhaye, Thibault Pamart, Flavien Vincent, Tom Denimal, Anais Gaul, Jonathan Sobocinski and Adeline Delsaux monitored data collection for the whole trial, drafted and revised the paper. Gintautas Bieliauskas, Vilhelmas Bajoras, Maarten Vanhaverbeke, Philippe Nuyens, and Fadi Sawaya contributed to this manuscript.
Conflict of interest statement
O. De Backer has received institutional research grants and consulting fees from Abbott, Boston Scientific and Shockwave Medical Inc. O. De Backer has received speaker fees from Shockwave Medical. F. Saia is a member of the Advisory Board for Edwards, Medtronic, Abbott, Bayer, Biotronik, Amgen, Boehringer-Ingelheim, and AstraZeneca, and has received lecture fees from Edwards, Medtronic, Abbott, Bayer, Amgen, Boehringer-Ingelheim, AstraZeneca, Boston Scientific, Volcano, and Chiesi, outside the submitted work. F. Meucci reports receiving speaker and consultation fees from Medtronic, Edwards and Boston Scientific. T. Palmerini has received lecture fees from Biotronik and Edwards. C. Di Mario has received research or educational grants from Abbott, Amgen, Asahi Intecc, AstraZeneca, Boston Scientific, Cardinal Health, CSL Behring, Chiesi, Daiichi Sankyo, Edwards, Medtronic, Menarini, Pfizer, Sanofi, Shockwave, Teleflex, and Volcano/Philips. The other authors have no conflicts of interest to declare. The Guest Editor reports lecture fees paid to his institution from Amgen, Bayer Healthcare, Biotronik, Boehringer Ingelheim, Boston Scientific, Daiichi Sankyo, Edwards Lifesciences, Ferrer, Pfizer, and Novartis, consultancy fees paid to his institution from Boehringer Ingelheim, and grant support from Bayer Healthcare, Boston Scientific, Biotronik, Edwards Lifesciences, GlaxoSmithKline, Medtronic, and Pfizer.
Abbreviations
- BMS
bare metal stent
- CT
computed tomography
- IVL
intravascular lithotripsy
- MLD
minimal lumen diameter
- PAD
peripheral artery disease
- PTA
percutaneous transluminal angioplasty
- SAVR
surgical aortic valve replacement
- STS
surgical risk score
- TAVI
transcatheter aortic valve implantation
- TF
transfemoral
- THV
transcatheter heart valve
- TL
target lesion
Contributor Information
Giulia Nardi, Structural Interventional Cardiology, Department of Clinical & Experimental Medicine, University Hospital Careggi, Florence, Italy.
Ole De Backer, The Heart Center, Rigshospitalet, Copenhagen University Hospital, Copenhagen, Denmark.
Francesco Saia, Interventional Cardiology Unit, Cardio-Thoracic Vascular Department, University Hospital of Bologna, Policlinico Sant’Orsola-Malpighi, Bologna, Italy.
Lars Sondergaard, The Heart Center, Rigshospitalet, Copenhagen University Hospital, Copenhagen, Denmark.
Francesca Ristalli, Structural Interventional Cardiology, Department of Clinical & Experimental Medicine, University Hospital Careggi, Florence, Italy.
Francesco Meucci, Structural Interventional Cardiology, Department of Clinical & Experimental Medicine, University Hospital Careggi, Florence, Italy.
Miroslava Stolcova, Structural Interventional Cardiology, Department of Clinical & Experimental Medicine, University Hospital Careggi, Florence, Italy.
Alessio Mattesini, Structural Interventional Cardiology, Department of Clinical & Experimental Medicine, University Hospital Careggi, Florence, Italy.
Pierluigi Demola, Structural Interventional Cardiology, Department of Clinical & Experimental Medicine, University Hospital Careggi, Florence, Italy.
Xi Wang, The Heart Center, Rigshospitalet, Copenhagen University Hospital, Copenhagen, Denmark; Department of Cardiology, West China Hospital, Sichuan University, Chengdu, Sichuan, China.
Anees Al Jabri, CNR Cardiovascular Centre, Massa, Italy.
Tullio Palmerini, Interventional Cardiology Unit, Cardio-Thoracic Vascular Department, University Hospital of Bologna, Policlinico Sant’Orsola-Malpighi, Bologna, Italy.
Antonio Giulio Bruno, Interventional Cardiology Unit, Cardio-Thoracic Vascular Department, University Hospital of Bologna, Policlinico Sant’Orsola-Malpighi, Bologna, Italy.
Alfonso Ielasi, Istituto Clinico Sant'Ambrogio, Gruppo Ospedaliero San Donato, Milan, Italy.
Eric Van Belle, Department of Interventional Cardiology for Coronary, Valves and Structural Heart Diseases, Cardiology, Institut Cœur Poumon, CHU de Lille, Université Lille, Lille, France.
Sergio Berti, CNR Cardiovascular Centre, Massa, Italy.
Carlo Di Mario, Structural Interventional Cardiology, Department of Clinical & Experimental Medicine, University Hospital Careggi, Florence, Italy.
References
- Patel JS, Krishnaswamy A, Svensson LG, Tuzcu EM, Mick S, Kapadia SR. Access Options for Transcatheter Aortic Valve Replacement in Patients with Unfavorable Aortoiliofemoral Anatomy. Curr Cardiol Rep. 2016;18:110. doi: 10.1007/s11886-016-0788-8. [DOI] [PubMed] [Google Scholar]
- Kurra V, Schoenhagen P, Roselli EE, Kapadia SR, Tuzcu EM, Greenberg R, Akhtar M, Desai MY, Flamm SD, Halliburton SS, Svensson LG, Sola S. Prevalence of significant peripheral artery disease in patients evaluated for percutaneous aortic valve insertion: Preprocedural assessment with multidetector computed tomography. J Thorac Cardiovasc Surg. 2009;137:1258–64. doi: 10.1016/j.jtcvs.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Arai T, Romano M, Lefèvre T, Hovasse T, Farge A, Le Houerou, Hayashida K, Watanabe Y, Garot P, Benamer H, Unterseeh T, Bouvier E, Morice MC, Chevalier B. Direct Comparison of Feasibility and Safety of Transfemoral Versus Transaortic Versus Transapical Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv. 2016;9:2320–5. doi: 10.1016/j.jcin.2016.08.009. [DOI] [PubMed] [Google Scholar]
- Guedeney P, Mehran R. Non-femoral TAVR: Time to stratify alternative vascular approaches. Catheter Cardiovasc Interv. 2018;92:1194–5. doi: 10.1002/ccd.27960. [DOI] [PubMed] [Google Scholar]
- Brodmann M, Werner M, Holden A, Tepe G, Scheinert D, Schwindt A, Wolf F, Jaff M, Lansky A, Zeller T. Primary outcomes and mechanism of action of intravascular lithotripsy in calcified, femoropopliteal lesions: Results of Disrupt PAD II. Catheter Cardiovasc Interv. 2019;93:335–42. doi: 10.1002/ccd.27943. [DOI] [PubMed] [Google Scholar]
- Brodmann M, Holden A, Zeller T. Safety and Feasibility of Intravascular Lithotripsy for Treatment of Below-the-Knee Arterial Stenoses. J Endovasc Ther. 2018;25:499–503. doi: 10.1177/1526602818783989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepe G, Brodmann M, Werner M, Bachinsky W, Holden A, Zeller T, Mangalmurti S, Nolte-Ernsting C, Bertolet B, Scheinert D, Gray WA Disrupt PAD III Investigators. Intravascular Lithotripsy for Peripheral Artery Calcification 30-Day Outcomes From the Randomized Disrupt PAD III Trial. JACC Cardiovasc Interv. 2021;14:1352–61. doi: 10.1016/j.jcin.2021.04.010. [DOI] [PubMed] [Google Scholar]
- Di Mario, Chiriatti N, Stolcova M, Meucci F, Squillantini G. Lithotripsy-assisted transfemoral aortic valve implantation. Eur Heart J. 2018;39:2655. doi: 10.1093/eurheartj/ehy021. [DOI] [PubMed] [Google Scholar]
- Gorla R, Cannone GS, Bedogni F, De Marco. Transfemoral aortic valve implantation following lithoplasty of iliac artery in a patient with poor vascular access. Catheter Cardiovasc Interv. 2019;93:E140–2. doi: 10.1002/ccd.27812. [DOI] [PubMed] [Google Scholar]
- Cruz-González I, González Ferreiro, Martín Moreiras, Trejo Velasco, Barreiro Pérez, Diego Nieto, Herrero Garibi, Rodríguez Collado, Sánchez Fernández. Facilitated Transfemoral Access by Shockwave Lithoplasty for Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv. 2019;12:e35–8. doi: 10.1016/j.jcin.2018.11.041. [DOI] [PubMed] [Google Scholar]
- Di Mario, Goodwin M, Ristalli F, Ravani M, Meucci F, Stolcova M, Sardella G, Salvi N, Bedogni F, Berti S, Babaliaros VC, Pop A, Caparrelli D, Stewart J, Devireddy C. A Prospective Registry of Intravascular Lithotripsy-Enabled Vascular Access for Transfemoral Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv. 2019;12:502–4. doi: 10.1016/j.jcin.2019.01.211. [DOI] [PubMed] [Google Scholar]
- Shahian DM, Jacobs JP, Badhwar V, Kurlansky PA, Furnary AP, Cleveland JC, Lobdell KW, Vassileva C, Wyler von, Thourani VH, Rankin JS, Edgerton JR, D'Agostino RS, Desai ND, Feng L, He X, O'Brien SM. The Society of Thoracic Surgeons 2018 Adult Cardiac Surgery Risk Models: Part 1—Background, Design Considerations, and Model Development. Ann Thorac Surg. 2018;105:1411–8. doi: 10.1016/j.athoracsur.2018.03.002. [DOI] [PubMed] [Google Scholar]
- Kellum , JA , Lameire N, Aspelin P, Barsoum RS, Burdmann EA, Goldstein SL, Herzog CA, Joannidis M, Kribben A, Levey AS, MacLeod AM, Mehta RL, Murray PT, Naicker S, Opal SM, Schaefer F, Schetz M, Uchino S. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney International Supplements. 2012;2:1–138. [Google Scholar]
- Moussa ID, Klein LW, Shah B, Mehran R, Mack MJ, Brilakis ES, Reilly JP, Zoghbi G, Holper E, Stone GW. Consideration of a new definition of clinically relevant myocardial infarction after coronary revascularization: an expert consensus document from the Society for Cardiovascular Angiography and Interventions (SCAI). J Am Coll Cardiol. 2013;62:1563–70. doi: 10.1016/j.jacc.2013.08.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Garcia HM, McFadden EP, Farb A, Mehran R, Stone GW, Spertus J, Onuma Y, Morel MA, van Es, Zuckerman B, Fearon WF, Taggart D, Kappetein AP, Krucoff MW, Vranckx P, Windecker S, Cutlip D, Serruys PW Academic Research Consortium. Standardized End Point Definitions for Coronary Intervention Trials: The Academic Research Consortium-2 Consensus Document. Circulation. 2018;137:W2635–50. doi: 10.1161/CIRCULATIONAHA.117.029289. [DOI] [PubMed] [Google Scholar]
- VARC-3 Writing, Généreux P, Piazza N, Alu MC, Nazif T, Hahn RT, Pibarot P, Bax JJ, Leipsic JA, Blanke P, Blackstone EH, Finn MT, Kapadia S, Linke A, Mack MJ, Makkar R, Mehran R, Popma JJ, Reardon M, Rodes-Cabau J, Van Mieghem, Webb JG, Cohen DJ, Leon MB. Valve Academic Research Consortium 3: updated endpoint definitions for aortic valve clinical research. Eur Heart J. 2021;42:1825–57. doi: 10.1093/eurheartj/ehaa799. [DOI] [PubMed] [Google Scholar]
- Rahman Z, Ullah M, Choudhury A. Coronary Artery Dissection and Perforation Complicating Percutaneous Coronary Intervention - A Review. Cardiovascular Journal. 2011;3:239–47. [Google Scholar]
- Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, Kaul S, Wiviott SD, Menon V, Nikolsky E, Serebruany V, Valgimigli M, Vranckx P, Taggart D, Sabik JF, Cutlip DE, Krucoff MW, Ohman EM, Steg PG, White H. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736–47. doi: 10.1161/CIRCULATIONAHA.110.009449. [DOI] [PubMed] [Google Scholar]
- Block PC, Mack M. If TAVR Cannot Be Transfemoral, Then What? JACC Cardiovasc Interv. 2016;9:2326–8. doi: 10.1016/j.jcin.2016.09.021. [DOI] [PubMed] [Google Scholar]
- Carroll JD, Mack MJ, Vemulapalli S, Herrmann HC, Gleason TG, Hanzel G, Deeb GM, Thourani VH, Cohen DJ, Desai N, Kirtane AJ, Fitzgerald S, Michaels J, Krohn C, Masoudi FA, Brindis RG, Bavaria JE. STS-ACC TVT Registry of Transcatheter Aortic Valve Replacement. J Am Coll Cardiol. 2020;76:2492–516. doi: 10.1016/j.jacc.2020.09.595. [DOI] [PubMed] [Google Scholar]
- Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, Capodanno D, Conradi L, De Bonis, De Paulis, Delgado V, Freemantle N, Haugaa KH, Jeppsson A, Jüni P, Pierard L, Prendergast BD, Sádaba JR, Tribouilloy C, Wojakowski W. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. EuroIntervention. 2022;17:e1126–96. doi: 10.4244/EIJ-E-21-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damluji AA, Murman M, Byun S, Moscucci M, Resar JR, Hasan RK, Alfonso CE, Carrillo RG, Williams DB, Kwon CC, Cho PW, Dijos M, Peltan J, Heldman AW, Cohen MG, Leroux L. Alternative access for transcatheter aortic valve replacement in older adults: A collaborative study from France and United States. Catheter Cardiovasc Interv. 2018;92:1182–93. doi: 10.1002/ccd.27690. [DOI] [PubMed] [Google Scholar]
- Kereiakes DJ, Di Mario, Riley RF, Fajadet J, Shlofmitz RA, Saito S, Ali ZA, Klein AJ, Price MJ, Hill JM, Stone GW. Intravascular Lithotripsy for Treatment of Calcified Coronary Lesions: Patient-Level Pooled Analysis of the Disrupt CAD Studies. JACC Cardiovasc Interv. 2021;14:1337–48. doi: 10.1016/j.jcin.2021.04.015. [DOI] [PubMed] [Google Scholar]
- Adams G, Shammas N, Mangalmurti S, Bernardo NL, Miller WE, Soukas PA, Parikh SA, Armstrong EJ, Tepe G, Lansky A, Gray WA. Intravascular Lithotripsy for Treatment of Calcified Lower Extremity Arterial Stenosis: Initial Analysis of the Disrupt PAD III Study. J Endovasc Ther. 2020;27:473–80. doi: 10.1177/1526602820914598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramlawi B, Anaya-Ayala JE, Reardon MJ. Transcatheter Aortic Valve Replacement (TAVR): access planning and strategies. Methodist Debakey Cardiovasc J. 2012;8:22–5. doi: 10.14797/mdcj-8-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali ZA, Brinton TJ, Hill JM, Maehara A, Matsumura M, Karimi Galougahi, Illindala U, Götberg M, Whitbourn R, Van Mieghem, Meredith IT, Di Mario, Fajadet J. Optical Coherence Tomography Characterization of Coronary Lithoplasty for Treatment of Calcified Lesions: First Description. JACC Cardiovasc Imaging. 2017;10:897–906. doi: 10.1016/j.jcmg.2017.05.012. [DOI] [PubMed] [Google Scholar]
- Zhang S, Kolominsky-Rabas PL. How TAVI registries report clinical outcomes—A systematic review of endpoints based on VARC-2 definitions. PLoS ONE. 2017;12:e0180815. doi: 10.1371/journal.pone.0180815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia DC, Benjo A, Cardoso RN, Macedo FY, Chavez P, Aziz EF, Herzog E, Alam M, de Marchena. Device stratified comparison among transfemoral, transapical and transubclavian access for Transcatheter Aortic Valve Replacement (TAVR): a meta-analysis. IntJ Cardiol. 2014;172:318–21. doi: 10.1016/j.ijcard.2013.12.162. [DOI] [PubMed] [Google Scholar]
- Young MN, Singh V, Sakhuja R. A Review of Alternative Access for Transcatheter Aortic Valve Replacement. Curr Treat Options Cardiovasc Med. 2018;20:62. doi: 10.1007/s11936-018-0648-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Valve-related characteristics.