Abstract
Background
Retrievable stents and aspiration catheters have been developed to provide more effective arterial recanalisation in acute ischaemic stroke.
Aims
The aim of this analysis was to test the effect of mechanical thrombectomy on mortality and long-term neurological outcome in patients presenting with acute large-vessel anterior circulation ischaemic stroke.
Methods
A structured search identified randomised controlled trials of thrombectomy (using a retrievable stent or aspiration catheter) versus control on a background of medical therapy which included intravenous thrombolysis if appropriate. The primary endpoint was disability at 90-day follow-up as assessed by the modified Rankin scale (mRS). Secondary endpoints included all-cause mortality and symptomatic intracranial haemorrhage. A Bayesian mixed-effects model was used for analysis.
Results
Twelve trials met the inclusion criteria, comprising a total of 1,276 patients randomised to thrombectomy and 1,282 patients to control. Randomisation to thrombectomy significantly reduced disability at 90 days (odds ratio [OR] 0.52, 95% credible interval [CrI] 0.46 to 0.61, probability(control better)<0.0001). Furthermore, thrombectomy reduced the odds of functional dependence at 90 days, indicated by an mRS score >2 (OR 0.44, CrI 0.37 to 0.52, p<0.0001). Thrombectomy reduced all-cause mortality at 90 days (16.1% vs 19.2%, OR 0.81, 95% CrI 0.66 to 0.99, p=0.024). The frequency of symptomatic intracranial haemorrhage was similar between thrombectomy (4.2%) and control (4.0%) (OR 1.12, 95% CrI 0.76 to 1.68, p=0.72).
Conclusions
In patients with an acute anterior circulation stroke, modern device thrombectomy significantly reduces death and subsequent disability. The magnitude of these effects suggests that universal access to this treatment strategy should be the standard of care.
Introduction
The holy grail of treatment in acute ischaemic stroke caused by large vessel occlusion is prompt removal of the thrombus obstructing the cerebral artery to minimise permanent brain injury. This has become possible with the development of cerebral artery aspiration catheters and retrievable stents.
Acute ischaemic stroke accounts for 2.7 million deaths annually and is the leading cause of severe long-term disability in adults worldwide1. Administration of intravenous tissue plasminogen activator (IV-tPA) within 4.5 hours of symptom onset reduces subsequent disability, is first-line medical therapy, and is an established treatment target for healthcare systems globally2. However, IV-tPA does not achieve recanalisation in the majority of cases: success varies according to the length and composition of the occlusion, and long-term disability remains common3.
In stroke caused by large vessel occlusion, higher rates of revascularisation have been reported when mechanical thrombectomy is used in addition to thrombolysis4. The advantage arises because mechanical extraction is more effective at removing established thrombus which is resistant to enzymatic destruction through fibrinolysis. The addition of thrombectomy may therefore allow treatment to be effective over a broader time window from symptom onset, thereby providing effective therapy to patients who may otherwise achieve a poor result, or even be contraindicated, from thrombolytics.
Aspiration catheter techniques for acute ischaemic stroke apply negative pressure to suction the thrombus through or into a dedicated neurothrombectomy catheter. Retrievable stents consist of a self-expanding mesh which is deployed alongside the thrombus, ensnaring the clot within its struts, which is then retrieved into the catheter. Both techniques may be used with or without thrombolysis, and crossover between these technologies is common in clinical practice.
In 2016, the HERMES collaboration published a pooled patient-level analysis of the first five randomised controlled trials (RCTs) of thrombectomy using second-generation devices5. This confirmed that thrombectomy reduced disability at 90-day follow-up, albeit in a narrow patient population. We have conducted an updated meta-analysis which permits consolidation of subsequent RCTs which have covered a range of devices6, applied increasingly pragmatic protocols suitable for real-world service conditions7,8, and also provided focus on patients presenting late (up to 24 hours) after stroke onset9,10. By synthesising these data, we have been able to calculate a contemporary, real-world, estimate of the effect size of thrombectomy for stroke, and test whether any benefits extend to a reduction in all-cause mortality.
Methods
SEARCH STRATEGY
We performed a structured search of PubMed to identify all RCTs in any language which assigned patients to thrombectomy or control for the treatment of acute anterior circulation stroke, on a background of medical therapy, which could include intravenous thrombolysis. We searched for trials between January 2010 and July 2020. The search string is available in Supplementary Appendix 1.
The search was conducted independently by two investigators (C.A. Rajkumar and S. Ganesananthan) and the senior investigator (M. Shun-Shin) arbitrated any discrepancies.
INCLUSION AND EXCLUSION CRITERIA
We included any RCT which examined the effect of thrombectomy in addition to best medical therapy. This included the use of intravenous thrombolysis if appropriate. The use of any second-generation neurothrombectomy device (retrievable stent or aspiration catheter) was permitted. We excluded trials in which only a minority (<50%) of the thrombectomy group received thrombectomy with retrievable stents, aspiration catheters or a combination of the two (for example, trials which permitted wire manipulation in isolation as the thrombectomy technique).
ENDPOINTS
The primary efficacy endpoint for this meta-analysis was disability as assessed by the 90-day modified Rankin scale (mRS). The mRS is a validated 7-point scale of disability and dependence in activities of daily living for patients following stroke11. Higher grades indicate greater degrees of disability, where a score of 0 is equivalent to no symptoms at all, and 6 is equivalent to death. We additionally reported a dichotomised outcome from the mRS, which categorised an independent functional outcome (mRS ≤2) or a dependent outcome (mRS >2). The secondary efficacy endpoint was all-cause mortality at 90-day follow-up. The primary safety endpoint was the occurrence of symptomatic intracranial haemorrhage.
DATA ABSTRACTION AND ANALYSIS
Two authors (C.A. Rajkumar and S. Ganesananthan) independently abstracted the data from eligible studies and these data were verified by the senior author (M. Shun-Shin). For each trial, the number of patients in each mRS category at 90-day follow-up was abstracted from the intervention and control arms using the reported intention-to-treat analysis. This allowed us to construct, for each trial, a table with individual patient mRS scores that could be used to recreate the original analysis, rather than relying on a single summary effect size (such as a single odds ratio [OR] or mean difference) for each trial. We did not have access to the patient baseline covariates (e.g., time to reperfusion) that would allow the assessment of the impact of these on the effect size.
For the mRS endpoint, a Bayesian ordinal regression model was constructed for the individual patient’s mRS score. For the death and symptomatic intracranial haemorrhage endpoints, a Bayesian logistic regression model was constructed. Bayesian methods have multiple advantages over frequentist approaches. In addition to allowing external information or beliefs to be captured and made clear using prior distributions, they also allow direct probability statements to be made about parameters or their combinations12.
All models included a randomisation arm as a fixed effect and the trial as a random intercept, without random slope. The model family was binomial with the logit link function - four chains with 5,000 burn-in iterations and 5,000 post burn-in iterations. Effect sizes, 95% credible intervals, and probabilities that the effect was greater than 0 were calculated. Package default priors (normal distributions with a standard deviation of 100 on coefficients, and exponential distribution with a mean of 1 on the random effects) were used. For familiarity we present the probability that the control arm had a better outcome than the intervention. Forest plots were generated for the individual trials and summary effect.
For reader familiarity, we present an I2 equivalent statistic as a measure of heterogeneity. This was calculated from the effect sizes and standard errors from a Bayesian ordinal analysis of the individual trials and our model effect size.
Whilst the “number needed to treat” (NNT) has statistical and conceptual limitations13, we present the NNT for disability and mortality as the inverse of the absolute rate reduction of the raw data to allow comparison with other reports.
A sensitivity analysis was generated to assess the impact of the prior on the primary endpoint (Supplementary Table 1, Supplementary Table 2) and the secondary endpoint of all-cause mortality (Supplementary Table 3, Supplementary Table 4).
Publication bias for the primary endpoint was assessed with the construction of a funnel plot and asymmetry was assessed with Egger’s test14.
All analysis was performed on the statistical programming environment R15 using the “rms” package (with the “blrm” function) for modelling and the “tidyverse” set of packages.
Individual RCTs were critically appraised using the Cochrane risk of bias tool16 by two authors (C.A. Rajkumar and S. Ganesananthan), and results are reported in line with PRISMA guidance17.
Results
SEARCH RESULTS
The results of the search strategy are shown in Figure 1.
Figure 1. Consort diagram of search strategy.
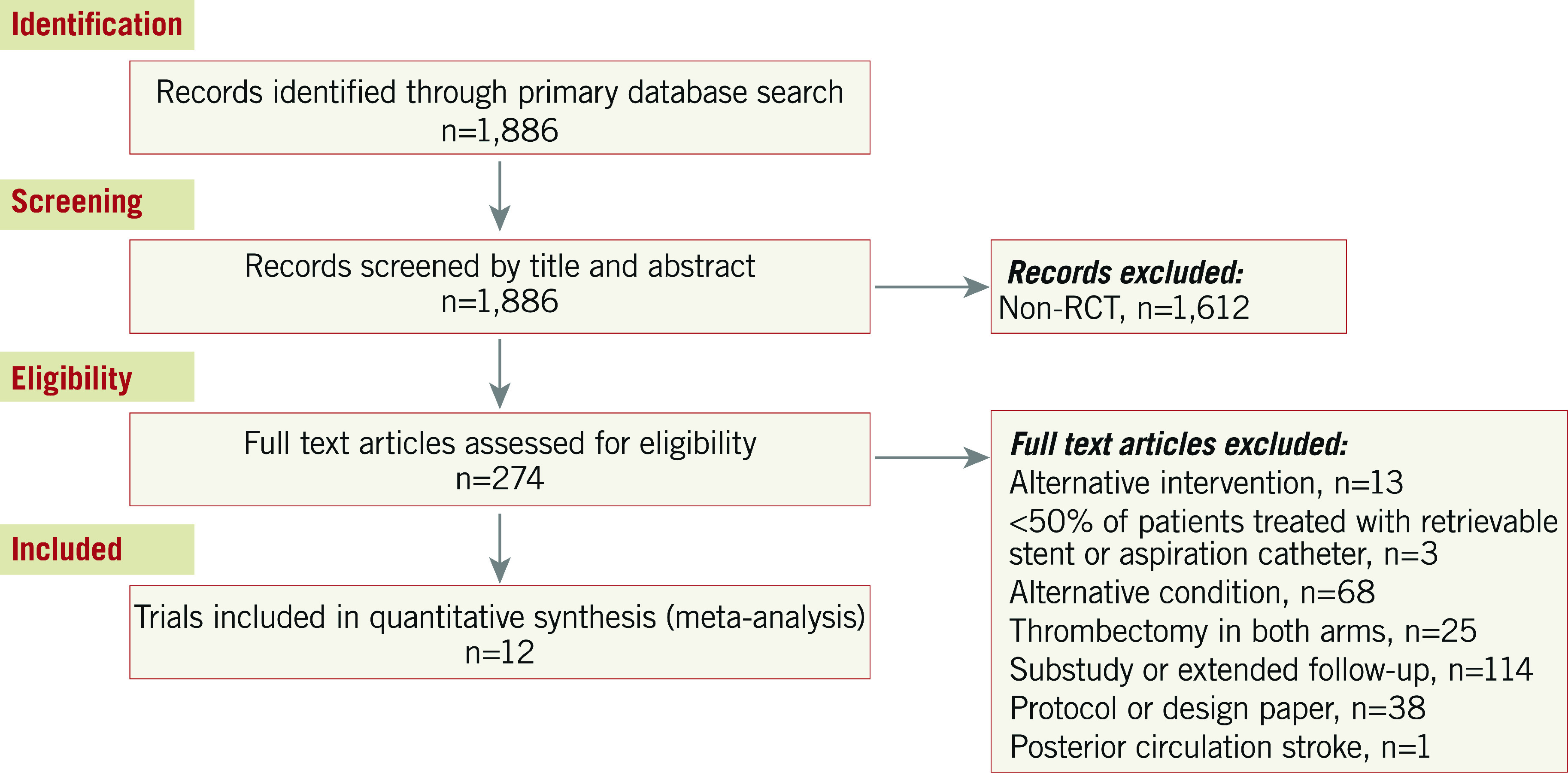
RCT: randomised controlled trial
Characteristics of eligible studies are summarised in Table 1.
Table 1. Summary characteristics of included trials.
|
TRIAL n MT/CON |
Journal | Year | Sites |
Median NIHSS MT/CON |
MT from symptom onset (hours) | Imaging modality for inclusion |
Proportion received IV thrombolytic therapy MT/CON |
Protocol mandated thrombectomy technique | Symptom onset to groin puncture (mins) | Attempt with any MT device | MT with stent retriever | MT with aspiration catheter | TICI 2b-3 at procedure end |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
ESCAPE20 165/150 |
NEJM | 2015 | 22 sites, worldwide | 16 / 17 | ≤12 | NCCT + CTA | 72.7 / 78.7 | Retrievable stent recommended | 241◊ (176-359) | 91.5% 151/165 | 86.1% 130/151 | Not specified | 72.4% 113/156 |
|
EXTEND-IA21 35/35 |
NEJM | 2015 |
10 sites, AUS + NZ |
17 / 13 | <4.5 |
CTA + CT perfusion |
100 / 100 | Solitaire | 210 (166-251) | 77.1% 27/35 | 100% 27/27 | 0.0% 0/27 |
86.2 25/29 |
|
MR CLEAN22 233/267 |
NEJM | 2015 | 16 sites, Netherlands | 17 / 18 | ≤6 | CTA / MRA / DSA | 87.1 / 90.6 | Any CE marked or FDA approved device | 260 (210-313) | 81.9% 193/233 | 98.4% 190/193 | Not specified | 58.7% 115/196 |
|
REVASCAT23 103/103 |
NEJM | 2015 | 4 sites, Spain | 17 / 17 | ≤8 | CTA / MRA / DSA | 68.0 / 77.7 | Solitaire | 269 (201-340) | 95.1% 98/103 | 100% 98/98 | 0.0% 0/98 | 65.7% 67/102 |
|
SWIFT PRIME24 98/98 |
NEJM | 2015 |
39 sites, USA, Europe |
17 / 17 | ≤6 | CTA / MRA | 100 / 100 | Solitaire | 224 (165-275) | 89.0% 87/98 | 100% 87/87 | 0.0% 0/87 | 88.0% 73/83 |
|
THRACE18 204/208 |
Lancet Neurology | 2016 | 26 sites, France | 18 / 17 | <5 | CTA / MRA | 100 / 100 | Any CE marked device | 250 (210-290) | 68.6% 140/204 | 90.0% 126/140 | 20.7% 29/140 | 68.8% 95/138 |
|
THERAPY6 55/53 |
Stroke | 2016 |
36 sites, USA, Germany |
17 / 18 | Not specified | NCCT + CTA | 100 / 100 | Penumbra | 227 (184-263) | 81.8% 45/55 | 15.5% 7/45 | 95.6% 43/45 | 73.3% 33/45 |
|
PISTE7 33/32 |
J Neurol Neurosurg Psychiatry | 2017 |
10 sites, UK |
18 / 14 | ≤6 | CTA / MRA | 100 / 100 | Any CE marked device | 209 | 97.0% 32/33 | 68%§ | 32%§ | 86.7% 26/30 |
|
EASI19 40/37 |
J Neurorad | 2017 |
1 site, Canada |
18 / 20 | <6 | NCCT | 57.5 / 62.2 | Any approved stent retriever | 245 (105-580) | 75.0% 30/40 | 96.7% 29/30 | Not specified | 76.7% 23/30 |
|
DAWN9 107/99 |
NEJM | 2018 | 26 sites, worldwide | 17 / 17 | 6-24 |
CTA / MRA + dwMR / CT perfusion |
4.7 / 13.1 | Trevo | 768◊◊ (636-1002) | 98.1% 105/107 | 100% 105/105 | 0.0% 0/105 | 84.1% 90/107 |
|
DEFUSE310 92/90 |
NEJM | 2018 |
38 sites, USA |
16 / 16 | 6-16 |
CTA / MRA + dwMR / CT perfusion |
10.8 / 8.9 | Any FDA approved device | 688* | 97.8% 90/92 | 82.2% 74/90 | 27.8%** 25/90 | 75.8% 69/91 |
|
RESILIENT8 111/110 |
NEJM | 2020 | 12 sites, Brazil | 18 / 18 | ≤8 | CTA / MRA / DSA | 68.5 / 71.8 | Solitaire FR / Penumbra | 259* | 94.6% 105/111 | 68.6% 72/105 | 66.7% 70/105 | 82.0% 91/111 |
| Values indicate mean±SD, unless indicated as a median (IQR). ◊Time from stroke onset to reperfusion. ◊◊ Time since last known to be well to arterial puncture. §Proportion of patients undergoing thrombectomy procedure in which a stent retriever was used as the first device. *Derived value from available values. **Number of patients who underwent aspiration alone. CON: control arm; CTA: CT angiogram; DSA: digital subtraction angiography; dwMR: diffusion weighted magnetic resonance; INT: intervention arm; MRA: magnetic resonance angiography; MT: mechanical thrombectomy; NCCT: non-contrast computed tomography; NIHSS: National Institutes of Health Stroke Scale; TICI: Thrombolysis In Cerebral Infarction score | |||||||||||||
Twelve trials6,7,8,9,10,18,19,20,21,22,23,24 were eligible, comprising 2,558 patients. A total of 1,276 patients were randomised to thrombectomy and 1,282 to control. Patients in both the thrombectomy and control arms were also allowed to receive thrombolysis if indicated. The majority of trials aimed to recruit patients with a time from symptom onset to delivery of thrombectomy of less than eight hours. Two trials recruited patients with delayed presentation (up to 1610 and 249 hours) from the time they were last known to be well; the majority of these patients were ineligible for IV thrombolysis. Excluding these trials, the median duration from symptom onset to groin puncture was 245 (224-259) minutes.
Median National Institutes of Health Stroke Scale (NIHSS) scores ranged from 16 to 18 in the thrombectomy arm and 14 to 20 in the control arm. The number of patients randomised to thrombectomy who received an attempt at thrombectomy with a dedicated device was 86.4% (1,103/1,276), in whom a retrievable stent (alone or in combination with another device) was used in 87.7% (967/1,103).
Three trials did not meet eligibility criteria for our main analysis, MR-RESCUE25, IMS III26 and SYNTHESIS Expansion27. In each case only a minority of patients were treated with a retrievable stent or aspiration catheter. We additionally performed a sensitivity analysis of the totality of the available data, in which these three trials were added (Supplementary Appendix 2).
RISK OF BIAS OF INCLUDED STUDIES
In all trials, patients were aware of treatment allocation. Two trials, THRACE18 and EASI19, did not blind the outcome assessor to treatment allocation. No other significant issues were identified. The risks of bias of the included studies are summarised in Supplementary Table 5.
PRIMARY EFFICACY ENDPOINT: MODIFIED RANKIN SCALE AT 90 DAYS
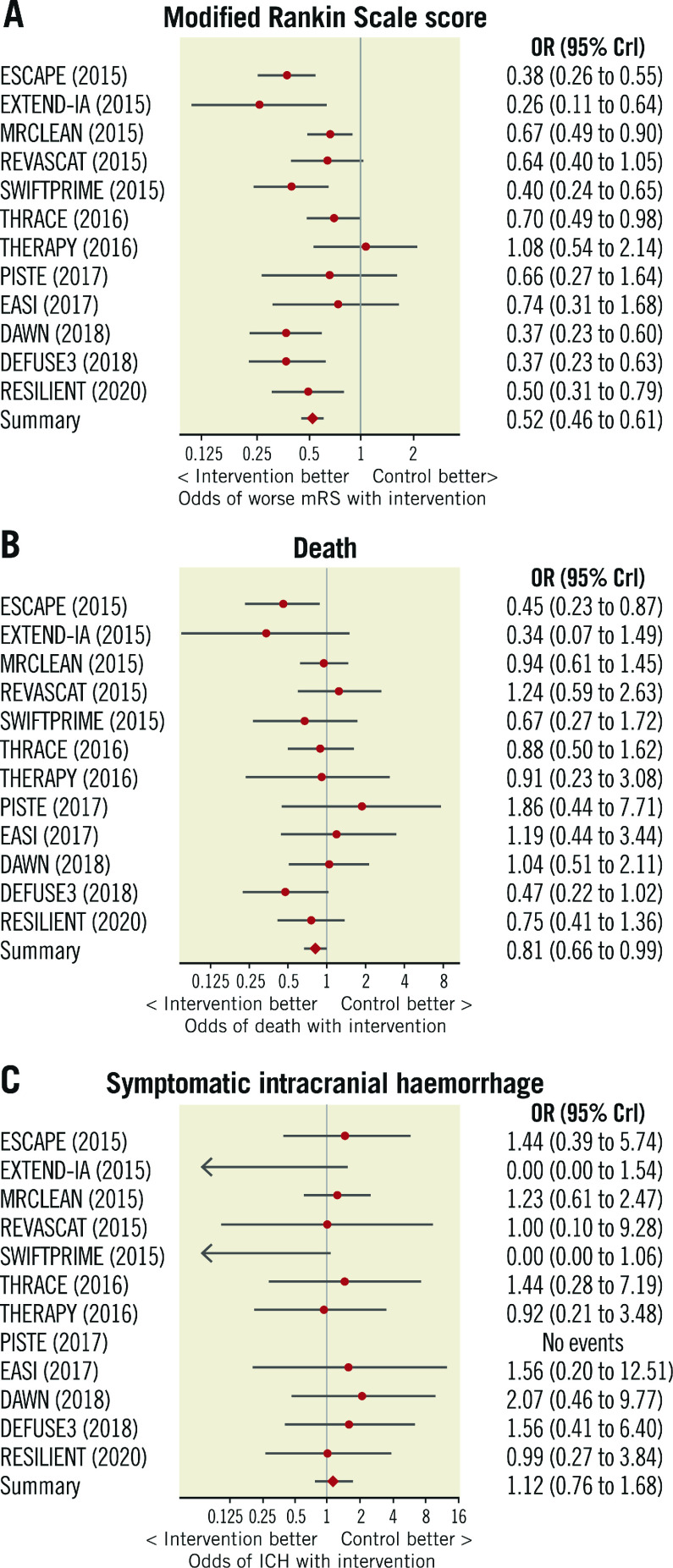
The distribution of mRS scores at 90-day follow-up is displayed in Figure 2. Thrombectomy reduced the level of disability at 90 days compared with medical therapy alone (OR 0.52, 95% credible interval [CrI] 0.46 to 0.61, p<0.0001, I2=48%) (Figure 3A). The median mRS at follow-up was 3 in the thrombectomy group and 4 in the control group (mean 2.8 vs 3.5).
Figure 2. Distribution of modified Rankin Scale scores at 90-day follow-up.
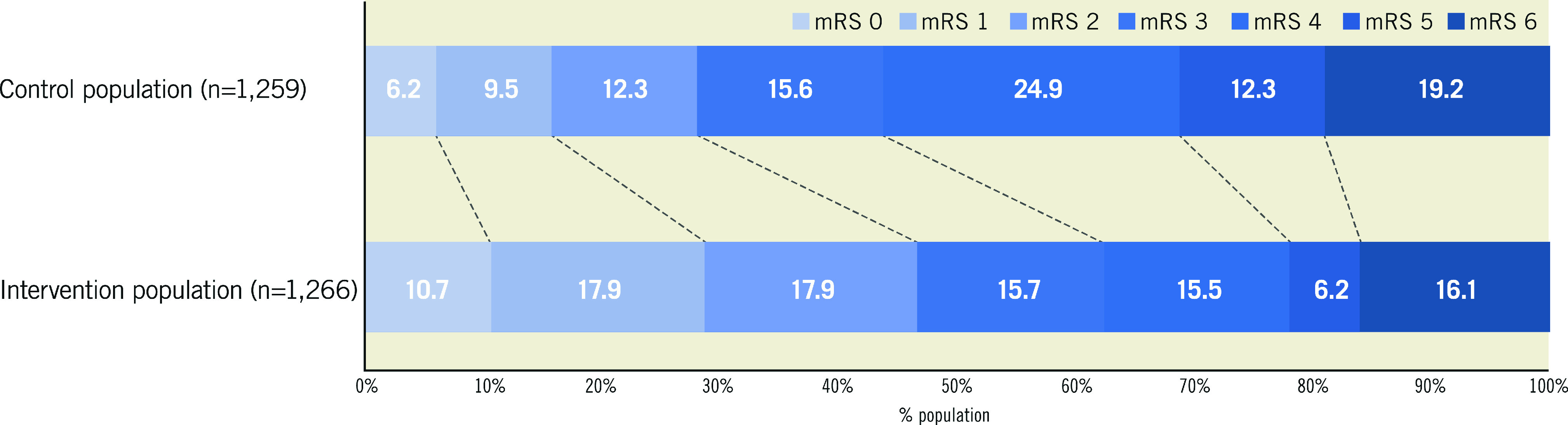
Higher scores indicate greater disability. mRS: modified Rankin Scale
Figure 3. Forest plots of outcome measures.
Forest plots indicating the effect of mechanical thrombectomy versus control for the treatment of acute ischaemic stroke on 90-day outcomes of (A) modified Rankin Scale score, (B) all-cause mortality, and (C) symptomatic intracranial haemorrhage. CrI: credible interval; ICH: intracranial haemorrhage; mRS: modified Rankin Scale score; OR: odds ratio
Thrombectomy reduced the odds of patients being functionally dependent (mRS >2) at 90 days (OR 0.44, CrI 0.37 to 0.52, p<0.0001). For every 5.4 patients treated with thrombectomy, one fewer patient will be dependent at 90 days (Central illustration).
Central illustration. Mechanical thrombectomy with retrievable stents and aspiration catheters for acute ischaemic stroke: a meta-analysis of randomised controlled trials.
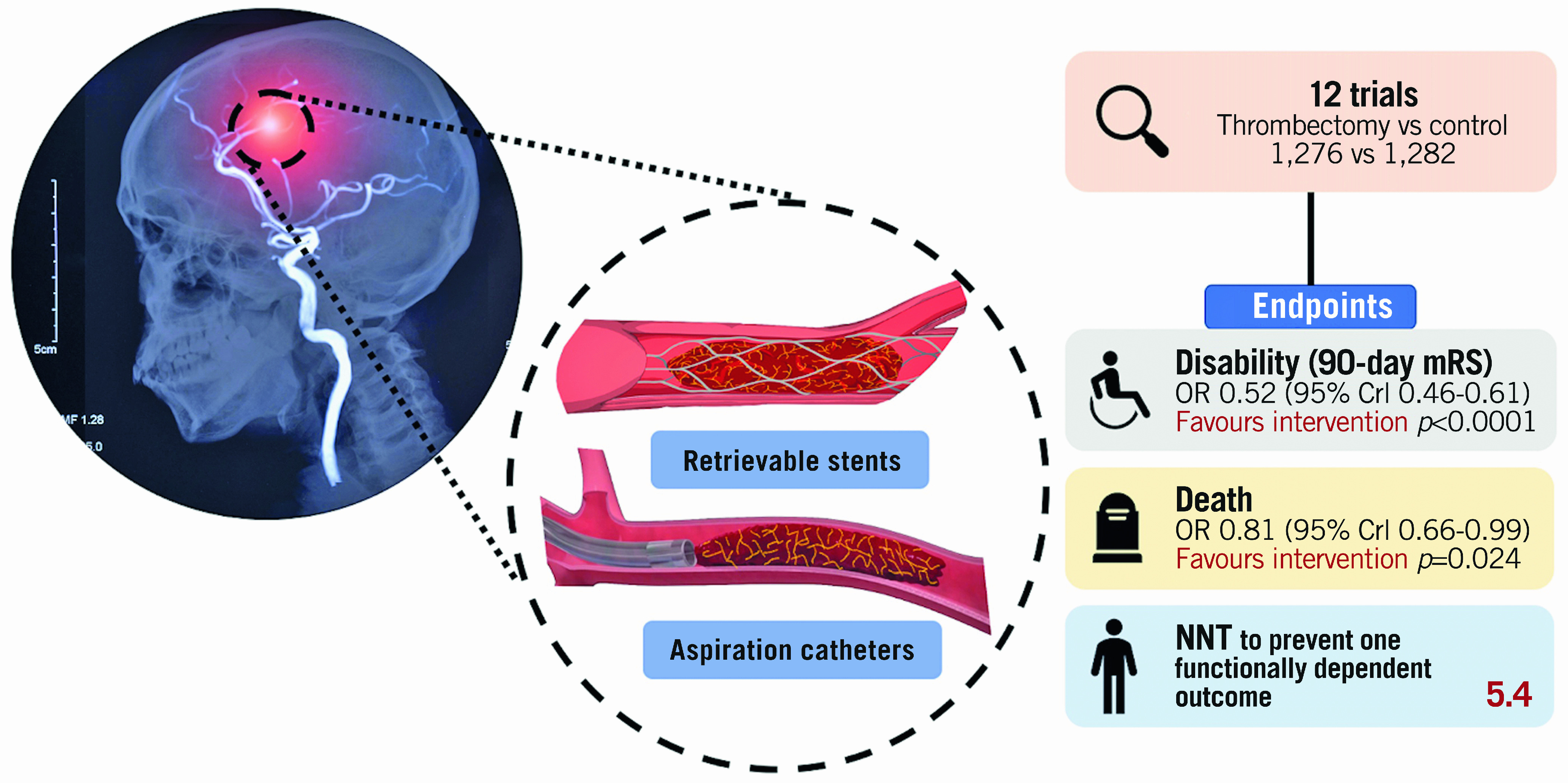
Crl: credible interval; mRS: modified Rankin scale; NNT: number needed to treat
SECONDARY EFFICACY ENDPOINT: ALL-CAUSE MORTALITY
At 90 days, death occurred in 16.1% (204/1,266) of patients randomised to thrombectomy and 19.2% (242/1,259) randomised to control. Thrombectomy reduced all-cause mortality (OR 0.81, 95% CrI 0.66 to 0.99, p=0.024, I2=0%) (Figure 3B). The NNT to prevent one death at 90 days was 32.
PRIMARY SAFETY ENDPOINT: SYMPTOMATIC INTRACRANIAL HAEMORRHAGE
Symptomatic intracranial haemorrhage occurred in a small proportion of patients randomised to thrombectomy (4.2%, 54/1,276) and control (4.0%, 51/1,282). The odds of symptomatic intracranial haemorrhage were similar between the arms (OR 1.12, 95% CrI 0.76 to 1.68, p=0.72, I2=0%) (Figure 3C); however, the small number of events limits certainty.
PUBLICATION BIAS
There was no strong evidence of publication bias in the trials included in this meta-analysis (funnel plot, Supplementary Figure 1, Egger’s test p=0.2).
SENSITIVITY ANALYSIS
Our sensitivity analysis extended the scope of eligible trials to those that did not routinely use retrievable stents or aspiration devices (Supplementary Table 6) but instead used wire manipulation, first-generation devices (such as the Merci retriever) or even attempted intra-arterial catheter-directed ultrasound. The results of this analysis are reported in Supplementary Figure 2.
SAMPLE DIAGNOSTIC PLOTS
Sample diagnostic plots (trace, density and autocorrelation) for each endpoint are shown in Supplementary Figure 3-Supplementary Figure 5.
Discussion
The RCTs of modern thrombectomy for acute ischaemic stroke (covering a diverse range of trial protocols, encompassing delays to treatment onset, differential use of thrombolytics, a variety of second-generation devices, and pragmatic treatment settings) show that thrombectomy (i) significantly reduces disability at 90-day follow-up, (ii) significantly reduces all-cause mortality at 90 days, and (iii) is not associated with an increase in the rate of symptomatic intracranial haemorrhage, although the small number of events limits certainty.
DISABILITY REDUCTION WITH THROMBECTOMY
A 1-grade better outcome in those randomised to thrombectomy is a substantial reduction in disability. It is the difference between being bedridden and not (mRS 5 vs 4). Alternatively, it is the difference between only being able to walk when someone is available to help and being able to walk whenever one wants to (mRS 4 vs 3).
The strength of our analysis is that it was sensitive across the full 7-point mRS scale, rather than draining statistical power by dichotomising at a particular threshold. However, some dichotomies are clinically important. For example, by dichotomising the results into independent (mRS ≤2) and dependent (mRS >2) categorisations, we can calculate that, for every 5.4 patients treated with the addition of thrombectomy to medical care, one fewer patient is dependent at 90 days. Put into context, the equivalent figure for functional independence with the addition of thrombolysis to medical therapy for stroke is 1828.
MORTALITY REDUCTION WITH THROMBECTOMY
This is the first complete meta-analysis to demonstrate a significant reduction in all-cause mortality following mechanical thrombectomy. Thrombectomy reduced mortality from 19.2% to 16.1%. The NNT to prevent one death at 90 days was 32. Not only has mortality reduction not been found with thrombolysis for stroke28, but this effect size of three absolute percentage points is similar to that of primary percutaneous coronary intervention (PCI) for ST-elevation myocardial infarction (STEMI) (which has been estimated at 2%)29.
IMPLICATIONS FOR SERVICE PROVISION
Timely intervention, to obtain the best clinical outcome, requires services that are available locally in many hospitals around a given country rather than isolated in small numbers of super-specialised centres to which the patient has to be transported. The lesson from primary PCI is that it was feasible and effective to have dozens of hospitals providing 24/7 emergency intervention, to which patients presenting anywhere in the country could be transported within tens of minutes.
In acute ischaemic stroke, as in acute myocardial infarction, early intervention is beneficial. Each 15-minute reduction in the time from stroke onset to tPA is associated with a 4% increase in the odds of walking independently at discharge30. Despite this, it is notable that the selected populations enrolled in the delayed presentation DEFUSE 310 and DAWN9 trials did not have the smallest effect sizes of trials of thrombectomy in this meta-analysis. Similarly, the EXTEND31 trial for thrombolysis for stroke found benefit up to nine hours. Whilst it must be stressed that the delayed presentation trials randomised a highly selected patient cohort in comparison to trials restricted to a six-hour time window, the RCT evidence for thrombectomy for stroke for delayed presentation exceeds that for primary PCI for STEMI9,10.
Services implemented for acute ischaemic stroke have mirrored those for acute myocardial infarction. First, there is thrombolysis made available locally. Then comes procedural intervention offered only in a small number of super-specialised centres. Finally, with recognition of mortality benefit, the health service moves to ensure universal access to prompt procedural intervention32,33.
For stroke intervention, the sites providing it need interdisciplinary teams with complementary expertise. Routine, 24/7 access to CT imaging, high dependency care and rehabilitation services are required. They also need 24/7 teams of proceduralists, catheter laboratory staff, and anaesthetists on-call for this intervention. Minimum practice standards for acute stroke care have been defined previously34. It is recommended that mechanical thrombectomy is available in all level 1 and level 2 stroke centres. However, routine access to thrombectomy remains limited in most European countries35.
No more RCTs are required on the general question of whether modern thrombectomy for acute ischaemic stroke is better than medical therapy alone for large artery acute ischaemic stroke. Although questions remain over the risk-benefit ratio of mechanical thrombectomy in certain subgroups, such as occlusions of the M2 segment of the middle cerebral artery, the overall benefit is seen across numerous trials with increasingly pragmatic protocols, performed in increasingly diverse healthcare systems. We can therefore be confident that the benefits of thrombectomy are both large and generalisable.
A mechanical thrombectomy service is a multidisciplinary effort. The next step for the healthcare system is rapid expansion of the pool of specialists able to perform the technique and an increase in the provision of 24/7 CT vascular imaging and interpretation, and advanced stroke care. For the provision of imaging, cost efficiency may be improved through artificial intelligence to screen large numbers of images for eligibility36.
Limitations
This meta-analysis addressed retrievable stent and aspiration catheter therapies. There have been previous technologies. The Merci device from 2004 used a retrievable coil, rather than a stent. It achieved Thrombolysis In Cerebral Infarction (TICI) 2b-3 recanalisation in only 25% of cases in the MRRESCUE trial25. There have been other trials in which the only mechanical intervention in most patients in the active arm was simple disruption of the thrombus with a guidewire. These trials also generally had lower standards of prior imaging confirmation of vascular occlusion amenable to thrombectomy. Those trials were not eligible for our analysis25,26,27. However, we have performed a sensitivity analysis in which they are added and found that the primary endpoint continues to show significant benefit of the intervention.
Furthermore, among the trials that did meet our inclusion criteria, there was substantial between-trial and within-trial heterogeneity in the specific devices used for thrombectomy. However, direct head-to-head RCTs comparing a strategy of direct aspiration versus retrievable stents as the first-line technique have been performed, with little evidence of difference between the two37,38.
There was significant variation in the inclusion criteria between trials. For example, the DAWN9 and DEFUSE 310 trials are distinct in design by their inclusion of patients presenting late after stroke onset. This is an important source of heterogeneity. However, whilst an attenuated benefit of thrombectomy might have been predicted in this setting, the effect sizes observed in these “late-presenter” trials were entirely consistent with trials restricted to patients with earlier presentation. The consistent effect seen across patient populations therefore gives confidence that the findings are generalisable. The HERMES collaboration have previously published predictors of response to thrombectomy based on individual patient data from a more limited number of trials39,40. Access to equivalent covariate data would be required for similar analyses to be performed for the expanded RCT data presented here.
Trials included in this meta-analysis typically recruited patients in centres with experienced interventional neuroradiologists in North America, Western Europe and Australasia. It could be argued that comparable results may not be achieved in less specialist centres. However, on account of the infancy of this therapy, the majority of operators in these trials would not have vast experience in thrombectomy. Furthermore, the purpose of the PISTE7 and RESILIENT8 trials was to assess whether the benefits of thrombectomy were reproducible in more pragmatic settings and public health systems. Reassuringly, they were found to be so.
Our conclusions are limited to patients presenting with acute large-vessel anterior circulation strokes as this is what was studied in the included trials. Vertebrobasilar occlusions are typically associated with more devastating neurological consequences, and intravenous therapy is limited by the fact that many patients present late with ill-defined symptoms or are delayed by diagnostic uncertainty41. Randomised trials of thrombectomy for vertebrobasilar occlusions have therefore been hampered by poor recruitment rates, and a high frequency of crossover from control to thrombectomy arms has been observed42. In addition, limited evidence suggests that mechanical thrombectomy in the posterior circulation may be more prone to complications than in the anterior circulation43.
Our primary endpoint, disability as assessed by the mRS, is subject to a number of limitations. First, there is substantial interobserver variability in scores awarded using the mRS, even by experienced researchers44. Second, its reporting is subject to bias in the absence of assessor blinding. Two trials18,19 included in this meta-analysis did not blind the assessor to treatment allocation; their results are therefore subject to this potential limitation.
Recruitment was halted prior to the planned randomisation target in 10/12 trials included in this meta-analysis. For seven trials6,7,19,20,21,23,24 this was because of evidence of efficacy from an external trial. Three trials were halted due to achievement of an internal efficacy margin on a pre-specified interim review8,9,10. A different result may have been obtained if these trials had run to completion.
Conclusions
Mechanical thrombectomy with retrievable stents and aspiration catheters significantly reduces disability at 90 days in anterior circulation ischaemic stroke. The available evidence now demonstrates that these benefits extend to a reduction in all-cause mortality. There is no significant increase in symptomatic intracranial haemorrhage. The absolute mortality effect is similar to primary PCI which has long been rolled out universally. Innovative action by healthcare policy makers could transform the disability and mortality outcomes for patients with acute ischaemic stroke, with limited ongoing cost if efficiently integrated with existing services.
Impact on daily practice
Contemporary trials of mechanical thrombectomy confirm a reduction in disability with a large effect size. For every 5.4 patients treated, one fewer patient is functionally dependent at 90 days. The available evidence now supports a significant reduction in all-cause mortality, a 3% absolute risk reduction at 90-day follow-up. The benefits of mechanical thrombectomy for acute ischaemic stroke are both large and generalisable. Urgent action is required to expand access to this life-saving therapy.
Supplementary data
Details of PubMed search terms.
Supplementary results.
Impact of setting increasingly flat priors for the β coefficients for the primary outcome (mRS score at 90 days).
Impact of setting increasingly flat priors for the random effect on primary outcome (mRS score at 90 days).
Impact of setting increasingly flat priors for the β coefficients for the endpoint of mortality.
Impact of setting increasingly flat priors for the random effect on the endpoint of mortality.
Cochrane risk of bias assessment tool for included studies.
Summary characteristics for trials added to the sensitivity analysis.
Funnel plot for risk of publication bias.
Forest plots inclusive of trials not restricted to the use of second-generation devices.
Sample diagnostic plots for modified Rankin Scale (mRS).
Sample diagnostic plots for death.
Sample diagnostic plots for symptomatic intracranial haemorrhage.
Acknowledgments
Funding
C. Rajkumar is a PhD Training Fellow at the Medical Research Council [Grant Number MR/S021108/1]. M. Foley is a PhD Training Fellow at the Medical Research Council [Grant Number MR/V001620/1]. J.P. Howard is a PhD Training Fellow at the Wellcome Trust [Grant number 212183/Z/18/Z]. A. Nowbar is supported by the NIHR Imperial Biomedical Research Centre (BRC).
Conflict of interest statement
I. Grunwald is a co-founder and a shareholder of Brainomix. R. Al-Lamee reports speakers’ honoraria from Philips Volcano and Menarini Pharmaceuticals. H. Seligman declares research funding from Amgen. The other authors have no conflicts of interest to declare.
Abbreviations
- CrI
credible interval
- ICH
intracranial haemorrhage
- mRS
modified Rankin scale
- NNT
number needed to treat
- RCT
randomised controlled trial
- tPA
tissue plasminogen activator
Contributor Information
Christopher A. Rajkumar, National Heart and Lung Institute, Imperial College London, London, United Kingdom; Imperial College Healthcare NHS Trust, London, United Kingdom.
Sashiananthan Ganesananthan, National Heart and Lung Institute, Imperial College London, London, United Kingdom.
Yousif Ahmad, National Heart and Lung Institute, Imperial College London, London, United Kingdom; Cedars-Sinai Medical Center, Los Angeles, CA, USA.
Henry Seligman, National Heart and Lung Institute, Imperial College London, London, United Kingdom; Imperial College Healthcare NHS Trust, London, United Kingdom.
George D. Thornton, University College London, London, United Kingdom; Barts Heart Centre at St Bartholomew's Hospital, London, United Kingdom.
Michael Foley, National Heart and Lung Institute, Imperial College London, London, United Kingdom; Imperial College Healthcare NHS Trust, London, United Kingdom.
Alexandra N. Nowbar, National Heart and Lung Institute, Imperial College London, London, United Kingdom; Imperial College Healthcare NHS Trust, London, United Kingdom.
James P. Howard, National Heart and Lung Institute, Imperial College London, London, United Kingdom; Imperial College Healthcare NHS Trust, London, United Kingdom.
Darrel P. Francis, National Heart and Lung Institute, Imperial College London, London, United Kingdom; Imperial College Healthcare NHS Trust, London, United Kingdom.
Thomas R. Keeble, Essex Cardiothoracic Centre, Basildon, United Kingdom; Anglia Ruskin School of Medicine, Chelmsford, Essex, United Kingdom.
Iris Q. Grunwald, Anglia Ruskin School of Medicine, Chelmsford, Essex, United Kingdom; University of Dundee, Dundee, United Kingdom.
Rasha K. Al-Lamee, National Heart and Lung Institute, Imperial College London, London, United Kingdom; Imperial College Healthcare NHS Trust, London, United Kingdom.
Iqbal Malik, Imperial College Healthcare NHS Trust, London, United Kingdom.
Matthew J. Shun-Shin, National Heart and Lung Institute, Imperial College London, London, United Kingdom; Imperial College Healthcare NHS Trust, London, United Kingdom.
References
- Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FV, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O’Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, VanWagner LB, Wilkins JT, Wong SS, Virani SS American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics – 2019 Update: A Report From the American Heart Association. Circulation. 2019;139:e56–528. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Leslie-Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV, Tirschwell DL. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019;50:e344–418. doi: 10.1161/STR.0000000000000211. [DOI] [PubMed] [Google Scholar]
- Bhatia R, Hill MD, Shobha N, Menon B, Bal S, Kochar P, Watson T, Goyal M, Demchuk AM. Low rates of acute recanalization with intravenous recombinant tissue plasminogen activator in ischemic stroke: real-world experience and a call for action. Stroke. 2010;41:2254–8. doi: 10.1161/STROKEAHA.110.592535. [DOI] [PubMed] [Google Scholar]
- Flottmann F, Leischner H, Broocks G, Nawabi J, Bernhardt M, Faizy TD, Deb-Chatterji M, Thomalla G, Fiehler J, Brekenfeld C. Recanalization Rate per Retrieval Attempt in Mechanical Thrombectomy for Acute Ischemic Stroke. Stroke. 2018;49:2523–5. doi: 10.1161/STROKEAHA.118.022737. [DOI] [PubMed] [Google Scholar]
- Goyal M, Menon BK, van Zwam, Dippel DWJ, Mitchell PJ, Demchuk AM, Dávalos A, Majoie CB, van der, de Miquel, Donnan GA, Roos YB, Bonafe A, Jahan R, Diener HC, van den, Levy EI, Berkhemer OA, Pereira VM, Rempel J, Millán M, Davis SM, Roy D, Thornton J, Román LS, Ribó M, Beumer D, Stouch B, Brown S, Campbell BCV, van Oostenbrugge, Saver JL, Hill MD, Jovin TG HERMES collaborators. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387:1723–31. doi: 10.1016/S0140-6736(16)00163-X. [DOI] [PubMed] [Google Scholar]
- Mocco J, Zaidat OO, von Kummer, Yoo AJ, Gupta R, Lopes D, Frei D, Shownkeen H, Budzik R, Ajani ZA, Grossman A, Altschul D, McDougall C, Blake L, Fitzsimmons BF, Yavagal D, Terry J, Farkas J, Lee SK, Baxter B, Wiesmann M, Knauth M, Heck D, Hussain S, Chiu D, Alexander MJ, Malisch T, Kirmani J, Miskolczi L, Khatri P THERAPY Trial Investigators*. Aspiration Thrombectomy After Intravenous Alteplase Versus Intravenous Alteplase Alone. Stroke. 2016;47:2331–8. doi: 10.1161/STROKEAHA.116.013372. [DOI] [PubMed] [Google Scholar]
- Muir KW, Ford GA, Messow CM, Ford I, Murray A, Clifton A, Brown MM, Madigan J, Lenthall R, Robertson F, Dixit A, Cloud GC, Wardlaw J, Freeman J, White P PISTE Investigators. Endovascular therapy for acute ischaemic stroke: the Pragmatic Ischaemic Stroke Thrombectomy Evaluation (PISTE) randomised, controlled trial. J Neurol Neurosurg Psychiatry. 2017;88:38–44. doi: 10.1136/jnnp-2016-314117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins SO, Mont’Alverne F, Rebello LC, Abud DG, Silva GS, Lima FO, Parente BSM, Nakiri GS, Faria MB, Frudit ME, de Carvalho, Waihrich E, Fiorot JA, Cardoso FB, Hidalgo RCT, Zétola VF, Carvalho FM, de Souza, Dias FA, Bandeira D, Miranda Alves, Wagner MB, Carbonera LA, Oliveira-Filho J, Bezerra DC, Liebeskind DS, Broderick J, Molina CA, Fogolin Passos, Saver JL, Pontes-Neto OM, Nogueira RG RESILIENT Investigators. Thrombectomy for Stroke in the Public Health Care System of Brazil. N Engl J Med. 2020;382:2316–26. doi: 10.1056/NEJMoa2000120. [DOI] [PubMed] [Google Scholar]
- Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, Yavagal DR, Ribo M, Cognard C, Hanel RA, Sila CA, Hassan AE, Millan M, Levy EI, Mitchell P, Chen M, English JD, Shah QA, Silver FL, Pereira VM, Mehta BP, Baxter BW, Abraham MG, Cardona P, Veznedaroglu E, Hellinger FR, Feng L, Kirmani JF, Lopes DK, Jankowitz BT, Frankel MR, Costalat V, Vora NA, Yoo AJ, Malik AM, Furlan AJ, Rubiera M, Aghaebrahim A, Olivot JM, Tekle WG, Shields R, Graves T, Lewis RJ, Smith WS, Liebeskind DS, Saver JL, Jovin TG DAWN Trial Investigators. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. N Engl J Med. 2018;378:11–21. doi: 10.1056/NEJMoa1706442. [DOI] [PubMed] [Google Scholar]
- Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, McTaggart RA, Torbey MT, Kim-Tenser M, Leslie-Mazwi T, Sarraj A, Kasner SE, Ansari SA, Yeatts SD, Hamilton S, Mlynash M, Heit JJ, Zaharchuk G, Kim S, Carrozzella J, Palesch YY, Demchuk AM, Bammer R, Lavori PW, Broderick JP, Lansberg MG DEFUSE 3 Investigators. Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. N Engl J Med. 2018;378:708–18. doi: 10.1056/NEJMoa1713973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks JL, Marotta CA. Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke. 2007;38:1091–6. doi: 10.1161/01.STR.0000258355.23810.c6. [DOI] [PubMed] [Google Scholar]
- Harrell FEJ. My Journey From Frequentist to Bayesian Statistics. [Last updated on 2021-08-11]. https://www.fharrell.com/post/journey.
- Hutton JL. Number needed to treat: properties and problems. J R Statist Soc A. 2000;163:403–19. [Google Scholar]
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Team RDC. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2010. [Google Scholar]
- Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracard S, Ducrocq X, Mas JL, Soudant M, Oppenheim C, Moulin T, Guillemin F. THRACE investigators. Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): a randomised controlled trial. Lancet Neurol. 2016;15:1138–47. doi: 10.1016/S1474-4422(16)30177-6. [DOI] [PubMed] [Google Scholar]
- Khoury NN, Darsaut TE, Ghostine J, Deschaintre Y, Daneault N, Durocher A, Lanthier S, Pope AY, Odier C, Lebrun LH, Guilbert F, Gentric JC, Batista A, Weill A, Roy D, Bracard S, Raymond J EASI trial collaborators. Endovascular thrombectomy and medical therapy versus medical therapy alone in acute stroke: A randomized care trial. J Neuroradiol. 2017;44:198–202. doi: 10.1016/j.neurad.2017.01.126. [DOI] [PubMed] [Google Scholar]
- Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, Roy D, Jovin TG, Willinsky RA, Sapkota BL, Dowlatshahi D, Frei DF, Kamal NR, Montanera WJ, Poppe AY, Ryckborst KJ, Silver FL, Shuaib A, Tampieri D, Williams D, Bang OY, Baxter BW, Burns PA, Choe H, Heo JH, Holmstedt CA, Jankowitz B, Kelly M, Linares G, Mandzia JL, Shankar J, Sohn SI, Swartz RH, Barber PA, Coutts SB, Smith EE, Morrish WF, Weill A, Subramaniam S, Mitha AP, Wong JH, Lowerison MW, Sajobi TT, Hill MD ESCAPE Trial Investigators. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372:1019–30. doi: 10.1056/NEJMoa1414905. [DOI] [PubMed] [Google Scholar]
- Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, Yan B, Dowling RJ, Parsons MW, Oxley TJ, Wu TY, Brooks M, Simpson MA, Miteff F, Levi CR, Krause M, Harrington TJ, Faulder KC, Steinfort BS, Priglinger M, Ang T, Scroop R, Barber PA, McGuinness B, Wijeratne T, Phan TG, Chong W, Chandra RV, Bladin CF, Badve M, Rice H, de Villiers, Ma H, Desmond PM, Donnan GA, Davis SM EXTEND-IA Investigators. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372:1009–18. doi: 10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- Berkhemer OA, Fransen PS, Beumer D, van den, Lingsma HF, Yoo AJ, Schonewille WJ, Vos JA, Nederkoorn PJ, Wermer MJ, van Walderveen, Staals J, Hofmeijer J, van Oostayen, Lycklama à, Boiten J, Brouwer PA, Emmer BJ, de Bruijn, van Dijk, Kappelle LJ, Lo RH, van Dijk, de Vries, de Kort, van Rooij, van den, van Hasselt, Aerden LA, Dallinga RJ, Visser MC, Bot JC, Vroomen PC, Eshghi O, Schreuder TH, Heijboer RJ, Keizer K, Tielbeek AV, den Hertog, Gerrits DG, van den, Karas GB, Steyerberg EW, Flach HZ, Marquering HA, Sprengers MES, Jenniskens SFM, Beenen LFM, van den, Koudstaal PJ, van Zwam, Roos YB, van der, van Oostenbrugge, Majoie CB, Dippel DW MR CLEAN investigators. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372:11–20. doi: 10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- Jovin TG, Chamorro A, Cobo E, de Miquel, Molina CA, Rovira A, San Román, Serena J, Abilleira S, Ribó M, Millán M, Urra X, Cardona P, López-Cancio E, Tomasello A, Castaño C, Blasco J, Aja L, Dorado L, Quesada H, Rubiera M, Hernández-Pérez M, Goyal M, Demchuk AM, von Kummer, Gallofré M, Dávalos A REVASCAT Trial Investigators. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372:2296–306. doi: 10.1056/NEJMoa1503780. [DOI] [PubMed] [Google Scholar]
- Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, Albers GW, Cognard C, Cohen DJ, Hacke W, Jansen O, Jovin TG, Mattle HP, Nogueira RG, Siddiqui AH, Yavagal DR, Baxter BW, Devlin TG, Lopes DK, Reddy VK, du Mesnil, Singer OC, Jahan R SWIFT PRIME Investigators. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372:2285–95. doi: 10.1056/NEJMoa1415061. [DOI] [PubMed] [Google Scholar]
- Kidwell CS, Jahan R, Gornbein J, Alger JR, Nenov V, Ajani Z, Feng L, Meyer BC, Olson S, Schwamm LH, Yoo AJ, Marshall RS, Meyers PM, Yavagal DR, Wintermark M, Guzy J, Starkman S, Saver JL MR RESCUE Investigators. A trial of imaging selection and endovascular treatment for ischemic stroke. N Engl J Med. 2013;368:914–23. doi: 10.1056/NEJMoa1212793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broderick JP, Palesch YY, Demchuk AM, Yeatts SD, Khatri P, Hill MD, Jauch EC, Jovin TG, Yan B, Silver FL, von Kummer, Molina CA, Demaerschalk BM, Budzik R, Clark WM, Zaidat OO, Malisch TW, Goyal M, Schonewille WJ, Mazighi M, Engelter ST, Anderson C, Spilker J, Carrozzella J, Ryckborst KJ, Janis LS, Martin RH, Foster LD, Tomsick TA Interventional Management of Stroke (IMS) III Investigators. Endovascular Therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med. 2013;368:893–903. doi: 10.1056/NEJMoa1214300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccone A, Valvassori L, Nichelatti M, Sgoifo A, Ponzio M, Sterzi R, Boccardi E SYNTHESIS Expansion Investigators. Endovascular treatment for acute ischemic stroke. N Engl J Med. 2013;368:904–13. doi: 10.1056/NEJMoa1213701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw JM, Murray V, Berge E, del Zoppo GJ. Thrombolysis for acute ischaemic stroke. Cochrane Database Syst Rev. 2014 Jul 29;:CD000213. doi: 10.1002/14651858.CD000213.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet. 2003;361:13–20. doi: 10.1016/S0140-6736(03)12113-7. [DOI] [PubMed] [Google Scholar]
- Saver JL, Fonarow GC, Smith EE, Reeves MJ, Grau-Sepulveda MV, Pan W, Olson DM, Hernandez AF, Peterson ED, Schwamm LH. Time to treatment with intravenous tissue plasminogen activator and outcome from acute ischemic stroke. JAMA. 2013;309:2480–8. doi: 10.1001/jama.2013.6959. [DOI] [PubMed] [Google Scholar]
- Ma H, Campbell BCV, Parsons MW, Churilov L, Levi CR, Hsu C, Kleinig TJ, Wijeratne T, Curtze S, Dewey HM, Miteff F, Tsai CH, Lee JT, Phan TG, Mahant N, Sun MC, Krause M, Sturm J, Grimley R, Chen CH, Hu CJ, Wong AA, Field D, Sun Y, Barber PA, Sabet A, Jannes J, Jeng JS, Clissold B, Markus R, Lin CH, Lien LM, Bladin CF, Christensen S, Yassi N, Sharma G, Bivard A, Desmond PM, Yan B, Mitchell PJ, Thijs V, Carey L, Meretoja A, Davis SM, Donnan GA EXTEND Investigators. Thrombolysis Guided by Perfusion Imaging up to 9 Hours after Onset of Stroke. N Engl J Med. 2019;380:1795–803. doi: 10.1056/NEJMoa1813046. [DOI] [PubMed] [Google Scholar]
- Kristensen SD, Fajadet J, Di Mario, Kaifoszova Z, Laut KG, Deleanu D, Gilard M, Guagliumi G, Goktekin O, Jorgova J, Kanakakis J, Ostojic M, Pereira H, Sabate M, Sobhy M, Vrints C, Wijns W, Widimsky P. Implementation of primary angioplasty in Europe: stent for life initiative progress report. EuroIntervention. 2012;8:35–42. doi: 10.4244/EIJV8I1A7. [DOI] [PubMed] [Google Scholar]
- Kristensen SD, Laut KG, Fajadet J, Kaifoszova Z, Kala P, Di Mario, Wijns W, Clemmensen P, Agladze V, Antoniades L, Alhabib KF, De Boer, Claeys MJ, Deleanu D, Dudek D, Erglis A, Gilard M, Goktekin O, Guagliumi G, Gudnason T, Hansen KW, Huber K, James S, Janota T, Jennings S, Kajander O, Kanakakis J, Kedev S, Kornowski R, Ludman PF, Merkely B, Milicic D, Najafov R, Nicolini FA, Noč M, Ostojic M, Pereira H, Radovanovic D, Sabaté M, Sobhy M, Sokolov M, Studencan M, Terzic I, Wahler S, Widimsky P European Association for Percutaneous Cardiovascular Interventions. Reperfusion therapy for ST elevation acute myocardial infarction 2010/2011: current status in 37 ESC countries. Eur Heart J. 2014;35:1957–70. doi: 10.1093/eurheartj/eht529. [DOI] [PubMed] [Google Scholar]
- Pierot L, Jayaraman MV, Szikora I, Hirsch JA, Baxter B, Miyachi S, Mahadevan J, Chong W, Mitchell PJ, Coulthard A, Rowley HA, Sanelli PC, Tampieri D, Brouwer PA, Fiehler J, Kocer N, Vilela P, Rovira A, Fischer U, Caso V, van der, Sakai N, Matsumaru Y, Yoshimura S, Anxionnat R, Desal H, Biscoito L, Pumar JM, Diaz O, Fraser JF, Linfante I, Liebeskind DS, Nogueira RG, Hacke W, Brainin M, Yan B, Soderman M, Taylor A, Pongpech S, Tanaka M, Karel T Asian-Australian Federation of Interventional and Therapeutic Neuroradiology (AAFITN), Australian and New Zealand Society of Neuroradiology (ANZSNR), American Society of Neuroradiology (ASNR), Canadian Society of Neuroradiology (CSNR), European Society of Minimally Invasive Neurological Therapy (ESMINT), European Society of Neuroradiology (ESNR), European Stroke Organization (ESO), Japanese Society for NeuroEndovascular Therapy (JSNET), The French Society of Neuroradiology (SFNR) Ibero-Latin American Society of Diagnostic and Therapeutic Neuroradiology (SILAN), Society of NeuroInterventional Surgery (SNIS), Society of Vascular and Interventional Neurology (SVIN), World Stroke Organization (WSO), World Federation of Interventional Neuroradiology (WFITN) Standards of practice in acute ischemic stroke intervention: international recommendations. J Neurointerv Surg. 2018;10:1121–6. doi: 10.1136/neurintsurg-2018-014287. [DOI] [PubMed] [Google Scholar]
- Aguiar de, von Martial, Abilleira S, Gattringer T, Kobayashi A, Gallofré M, Fazekas F, Szikora I, Feigin V, Caso V, Fischer U. Access to and delivery of acute ischaemic stroke treatments: A survey of national scientific societies and stroke experts in 44 European countries. Eur Stroke J. 2018;4:13–28. doi: 10.1177/2396987318786023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunwald IQ, Kulikovski J, Reith W, Gerry S, Namias R, Politi M, Papanagiotou P, Essig M, Mathur S, Joly O, Hussain K, Wagner V, Shah S, Harston G, Vlahovic J, Walter S, Podlasek A, Fassbender K. Collateral Automation for Triage in Stroke: Evaluating Automated Scoring of Collaterals in Acute Stroke on Computed Tomography Scans. Cerebrovasc Dis. 2019;47:217–22. doi: 10.1159/000500076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapergue B, Blanc R, Gory B, Labreuche J, Duhamel A, Marnat G, Saleme S, Costalat V, Bracard S, Desal H, Mazighi M, Consoli A, Piotin M, ASTER Trial. Effect of Endovascular Contact Aspiration vs Stent Retriever on Revascularization in Patients With Acute Ischemic Stroke and Large Vessel Occlusion: The ASTER Randomized Clinical Trial. JAMA. 2017;318:443–52. doi: 10.1001/jama.2017.9644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk AS, Siddiqui A, Fifi JT, De Leacy, Fiorella DJ, Gu E, Levy EI, Snyder KV, Hanel RA, Aghaebrahim A, Woodward BK, Hixson HR, Chaudry MI, Spiotta AM, Rai AT, Frei D, Almandoz JED, Kelly M, Arthur A, Baxter B, English J, Linfante I, Fargen KM, Mocco J. Aspiration thrombectomy versus stent retriever thrombectomy as first-line approach for large vessel occlusion (COMPASS): a multicentre, randomised, open label, blinded outcome, non-inferiority trial. Lancet. 2019;393:998–1008. doi: 10.1016/S0140-6736(19)30297-1. [DOI] [PubMed] [Google Scholar]
- Román LS, Menon BK, Blasco J, Hernández-Pérez M, Dávalos A, Majoie CBLM, Campbell BC, Guillemin F, Lingsma H, Anxionnat R, Epstein J, Saver JL, Marquering H, Wong JH, Lopes D, Reimann G, Desal H, Dippel DWJ, Coutts S, du Mesnil, Yavagal D, Ferre JC, Roos YBWEM, Liebeskind DS, Lenthall R, Molina C, Ajlan FS, Reddy V, Dowlatshahi D, Sourour NA, Oppenheim C, Mitha AP, Davis SM, Weimar C, van Oostenbrugge, Cobo E, Kleinig TJ, Donnan GA, van der, Demchuk AM, Berkhemer OA, Boers AMM, Ford GA, Muir KW, Scott Brown, Jovin T, van Zwam, Mitchell PJ, Hill MD, White P, Bracard S, Goyal M HERMES collaborators. Imaging features and safety and efficacy of endovascular stroke treatment: a meta-analysis of individual patient-level data. Lancet Neurol. 2018;17:895–904. doi: 10.1016/S1474-4422(18)30242-4. [DOI] [PubMed] [Google Scholar]
- Campbell BCV, Majoie CBLM, Albers GW, Menon BK, Yassi N, Sharma G, van Zwam, van Oostenbrugge, Demchuk AM, Guillemin F, White P, Dávalos A, van der, Butcher KS, Cherifi A, Marquering HA, Cloud G, Macho Fernández, Madigan J, Oppenheim C, Donnan GA, Roos YBWEM, Shankar J, Lingsma H, Bonafé A, Raoult H, Hernández-Pérez M, Bharatha A, Jahan R, Jansen O, Richard S, Levy EI, Berkhemer OA, Soudant M, Aja L, Davis SM, Krings T, Tisserand M, San Roman, Tomasello A, Beumer D, Brown S, Liebeskind DS, Bracard S, Muir KW, Dippel DWJ, Goyal M, Saver JL, Jovin TG, Hill MD, Mitchell PJ HERMES collaborators. Penumbral imaging and functional outcome in patients with anterior circulation ischaemic stroke treated with endovascular thrombectomy versus medical therapy: a meta-analysis of individual patient-level data. Lancet Neurol. 2019;18:46–55. doi: 10.1016/S1474-4422(18)30314-4. [DOI] [PubMed] [Google Scholar]
- Vergouwen MD, Algra A, Pfefferkorn T, Weimar C, Rueckert CM, Thijs V, Kappelle LJ, Schonewille WJ Basilar Artery International Cooperation Study (BASICS) Study Group. Time is brain(stem) in basilar artery occlusion. Stroke. 2012;43:3003–6. doi: 10.1161/STROKEAHA.112.666867. [DOI] [PubMed] [Google Scholar]
- Liu X, Dai Q, Ye R, Zi W, Liu Y, Wang H, Zhu W, Ma M, Yin Q, Li M, Fan X, Sun W, Han Y, Lv Q, Liu R, Yang D, Shi Z, Zheng D, Deng X, Wan Y, Wang Z, Geng Y, Chen X, Zhou Z, Liao G, Jin P, Liu Y, Liu X, Zhang M, Zhou F, Shi H, Zhang Y, Guo F, Yin C, Niu G, Zhang M, Cai X, Zhu Q, Chen Z, Liang Y, Li B, Lin M, Wang W, Xu H, Fu X, Liu W, Tian X, Gong Z, Shi H, Wang C, Lv P, Tao Z, Zhu L, Yang S, Hu W, Jiang P, Liebeskind DS, Pereira VM, Leung T, Yan B, Davis S, Xu G, Nogueira RG BEST Trial Investigators. Endovascular treatment versus standard medical treatment for vertebrobasilar artery occlusion (BEST): an open-label, randomised controlled trial. Lancet Neurol. 2020;19:115–22. doi: 10.1016/S1474-4422(19)30395-3. [DOI] [PubMed] [Google Scholar]
- Alonso de, Kawiorski MM, Ximénez-Carrillo Á, Cruz-Culebras A, García-Pastor A, Martínez-Sánchez P, Fernández-Prieto A, Caniego JL, Méndez JC, Zapata-Wainberg G, De Felipe-Mimbrera, Díaz-Otero F, Ruiz-Ares G, Frutos R, Bárcena-Ruiz E, Fandiño E, Marín B, Vivancos J, Masjuan J, Gil-Nuñez A, Díez-Tejedor E, Fuentes B Madrid Stroke Network. Mechanical thrombectomy for basilar artery thrombosis: a comparison of outcomes with anterior circulation occlusions. J Neurointerv Surg. 2017;9:1173–8. doi: 10.1136/neurintsurg-2016-012797. [DOI] [PubMed] [Google Scholar]
- Quinn TJ, Dawson J, Walters MR, Lees KR. Exploring the reliability of the modified rankin scale. Stroke. 2009;40:762–6. doi: 10.1161/STROKEAHA.108.522516. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Details of PubMed search terms.
Supplementary results.
Impact of setting increasingly flat priors for the β coefficients for the primary outcome (mRS score at 90 days).
Impact of setting increasingly flat priors for the random effect on primary outcome (mRS score at 90 days).
Impact of setting increasingly flat priors for the β coefficients for the endpoint of mortality.
Impact of setting increasingly flat priors for the random effect on the endpoint of mortality.
Cochrane risk of bias assessment tool for included studies.
Summary characteristics for trials added to the sensitivity analysis.
Funnel plot for risk of publication bias.
Forest plots inclusive of trials not restricted to the use of second-generation devices.
Sample diagnostic plots for modified Rankin Scale (mRS).
Sample diagnostic plots for death.
Sample diagnostic plots for symptomatic intracranial haemorrhage.