Abstract
It has been documented that Ca2+ overload and increased production of reactive oxygen species play a significant role in reperfusion injury (RI) of cardiomyocytes. Ischemia/reperfusion induces cell death as a result of necrosis, necroptosis, apoptosis, and possibly autophagy, pyroptosis and ferroptosis. It has also been demonstrated that the NLRP3 inflammasome is involved in RI of the heart. An increase in adrenergic system activity during the restoration of coronary perfusion negatively affected cardiac resistance to RI. Toll-like receptors are involved in RI of the heart. Angiotensin II and endothelin-1 aggravated ischemic/reperfusion injury of the heart. Activation of neutrophils, monocytes, CD4+ T-cells and platelets contributes to cardiac ischemia/reperfusion injury. Our review outlines the role of these factors in reperfusion cardiac injury.
Keywords: Heart, reperfusion injury, Ca2+ overload, oxidative stress, inflammation, apoptosis
1. INTRODUCTION
Despite all the achievements of cardiovascular medicine, the mortality rate in acute myocardial infarction (AMI) remains high [1]. Unfortunately, to date, no drug is available, which could effectively prevent reperfusion injury occurring after the restoration of coronary perfusion in patients with AMI and in patients with cardioplegic arrest [2]. Moreover, according to some investigators, reperfusion injury is responsible for up to 50% of the final size of infarction [3, 4]. Infarct size increased from 6 h (27%) to 24 h of reperfusion (41%) with no further increase at 48 and 72 h of reperfusion after a 60-min coronary artery occlusion (CAO) in dogs [5]. In our opinion, a more detailed understanding of the pathogenesis of myocardial reperfusion injury will greatly facilitate the development of more effective pharmacological interventions for the treatment of myocardial infarction.
Interventions to decrease infarct size will have no effect unless coronary blood flow is restored. It is generally accepted that infarct size can only be affected by the restoration of coronary blood flow. Thus, the death of cardiomyocytes in the ischemic zone is almost complete 6 h after CAO [6]. Therefore, reperfusion only results in positive effects if performed no later than 6 h after the onset of AMI. A few studies indicate that it is possible to limit infarct size without reperfusion [7]. These investigators suggest that the cardioprotective effect can be attributed to their anti-inflammatory action. After the restoration of blood flow in the ischemic myocardium, events develop very quickly. Intravenous administration of the selective P2Y12 receptor antagonist cangrelor before cardiac reperfusion reduces infarct size by approximately 50%, while injection of cangrelor 10 min after reperfusion does not affect infarct size in rabbits [8]. Administration of the κ-opioid receptor agonist U50,488 5 min prior to reperfusion can prevent reperfusion cardiac injury in rats [9]. However, U50,488 did not exhibit the infarct-reducing effect if this compound was injected 10 sec after reperfusion. It is our hypothesis that pharmacological agents should be administered prior to reperfusion so that they can penetrate into the ischemic zone before the restoration of coronary perfusion, relying upon collateral blood flow. However, it can also be hypothesized that drugs with the anti-inflammatory effect can initiate the infarct-reducing effect after the restoration of coronary blood flow. When NLRP3 inhibitor was administered 1 h after reperfusion it reduced infarct size in mice [10]. However, the NLRP3 inhibitor had no effect if it was administered after 3 hours of reperfusion. The NLRP3 inhibitor OLT1177 exhibited a strong infarct-reducing effect if it was administered 1 hour after reperfusion [11]. The infarct-limiting effect was markedly weakened if this inhibitor was administered 2 hours after the restoration of coronary blood flow. This effect completely disappeared when this compound was administered after 3 hours of reperfusion. The aforementioned study indicates the presence of early reperfusion injury of the heart which develops very rapidly (5 - 10 min after the onset of reperfusion) as well as delayed cardiac reperfusion injury which develops in the two - three hours of reperfusion.
2. CA2+ OVERLOAD
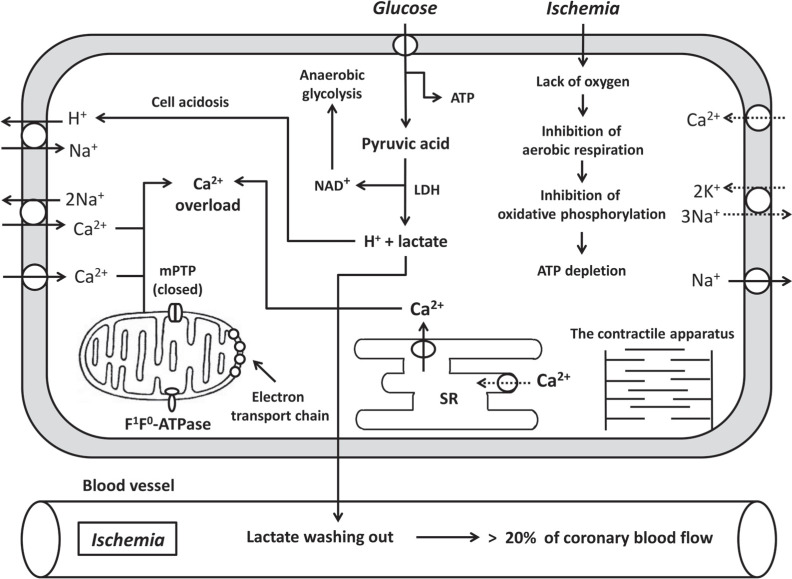
Reperfusion leads to rapid leaking of protons and lactic acid from the extracellular space. Rapid recovery of extracellular pH stimulates the Na+/H+ exchanger and the Na+/HCO3- symporter, which results in extrusion of protons from the cell and rapid normalization of intracellular pH, massive Na+ influx and intracellular Ca2+ overload due to the gain of 2Na+/Ca2+ exchange [12]. The importance of the Na+/H+ exchanger and the Na+/HCO3- symporter in RI of the heart has been confirmed by Cohen et al. [3] and Rodriguez-Sinovas et al. [13]. In studies in the isolated rabbit heart, a 90-min CAO and a 2 h-reperfusion were performed [3]. Hypercapnic buffer reperfusion (pH 6.9, 2 min) was demonstrated to decrease the infarct size/area at risk (IS/AAR) ratio by above 60% [3]. In a study that was performed in pigs with CAO and reperfusion [13], it was documented that acidic (Krebs solution at pH 6.4 for the first 3 min of reperfusion) cardiac reperfusion carried out after CAO, resulted in a decrease in the IS/AAR ratio by approximately 30%. These experiments indicate that the Na+/H+ exchanger and the Na+/HCO3- symporter may play an important role in reperfusion injury of the heart.
L-type Ca2+ channels also play an important role in Ca2+ overload (Fig. 1). Prevention of Ca2+ overload by intracoronary administration of the L-type Ca2+ channel inhibitor diltiazem during reperfusion resulted in the infarct-reducing effect in pigs [14]. The same effect is caused by the L-type Ca2+ channel blocker verapamil administered intravenously 5 min before reperfusion in rats [15]. In a study performed in the isolated perfused rat heart, it was found that hypoxic perfusion causes an increase in [Ca2+]i 10 min after the onset of hypoxia [16]. It has also been reported that L-type Ca2+ channel inhibitors diltiazem and nifedipine may prevent Ca2+ overload [16]. The β-adrenergic receptor (AR) blocker esmolol had the same effect. We demonstrated that β-adrenergic receptor antagonists propranolol and nadolol could induce the infarct-reducing effect when they were administered 5 min before reperfusion in rats [15]. The sarcoplasmic reticulum (SR) Ca2+-ATPase inhibitor thapsigargin did not affect Ca2+ overload [16]. These investigators concluded that the β-AR-stimulated L-type channel, which mediates Ca2+ entry in cardiomyocyte, contributes to hypoxic Ca2+ overload. However, the SR is not involved in Ca2+ overload during hypoxia of cardiomyocytes [16]. Calcium also can enter cardiomyocytes via 2Na+/Ca2+ exchange [12].
Fig. (1).
The main metabolic events that occur during ischemia in cardiomyocytes. SR, sarcoplasmic reticulum; MPTP, mitochondrial permeability transition pore; ROS, reactive oxygen species.
Recently, evidence has emerged that the cause of Ca2+ overload can be the opening of transient receptor potential (TRP) channels [17]. In mice with the deletion of the gene encoding TRPV4, infarct size was smaller than that in wild-type animals [18]. The TRPV4 agonist GSK1016790A, in contrast, increased infarct size. Other studies carried out in the culture of H9C2 cardiomyoblasts have demonstrated the existence of functionally active TRPV4 in cardiac cells [19]. The TRPV4 opener GSK1016790A caused [Ca2+]i elevation. This effect began at a GSK1016790A concentration of 100 nM. However, the TRPV4 channel blocker HC-067047 (1 μM) eliminated this effect. Hypoxia-reoxygenation of H9C2 cardiomyoblasts caused an increase in [Ca2+]i. Adding GSK1016790A to the incubation medium aggravated this effect, while the TRPV4 inhibitor HC-067047, reduced reoxygenation Ca2+ overload. The aforementioned data suggest that TRPV4 is involved in reoxygenation Ca2+ overload of cardiac cells.
During I/R Ca2+ overload is observed not only in the cytoplasm but also in the mitochondrial matrix via the mitochondrial calcium uniporter (MCU) [20]. Pretreatment with ruthenium red, an inhibitor of MCU, or with Ru360, a highly specific MUC inhibitor, increases tolerance of the isolated rat heart to I/R [21]. In a study performed in the isolated rat heart, a Ca2+ chelator BAPTA-AM was used at a concentration that abolished Ca2+ overload of both the cytoplasm and mitochondria and ruthenium red at the concentration that abolished Ca2+ overload of mitochondria but not the cytoplasm [22]. Both compounds enhanced cardiac resistance to I/R. The cardioprotective effect of ruthenium red and RU360 during I/R of the heart was confirmed by other investigators [23]. It should be noted that ruthenium red also is the transient receptor potential vanilloid (TRPV) channel antagonist [16]. It was documented that the mouse heart lacking MCU is more tolerant to I/R then the heart of wild-type mice [24]. Ruthenium red and RU360 were not used at reperfusion of the heart. Therefore, the role of Ca2+ overload of mitochondria in reperfusion cardiac injury remains to be clarified. However, it was documented that Ca2+ overload of mitochondria can promote mitochondrial transition pore (MPT pore) opening and cell death as a result of apoptosis [25].
Collectively, these reports indicate that reperfusion Ca2+ overload is likely mediated by both L-type Ca2+ channels and TRPV4 channel opening. The role of Ca2+ overload of mitochondria in reperfusion cardiac injury requires further investigation.
3. NO-SYNTHASE ACTIVATION
Ca2+ is required to activate endothelial NO-synthase (NOS3) and neuronal NO-synthase (NOS1), which can then further increase mitochondrial reactive oxygen species (ROS) production [25, 26]. Mitochondrial NO production is catalyzed by mitochondrial NOS (mtNOS), which has been identified as the α-isoform of nNOS [27]. Superoxide radical (O2•) can interact non-enzymatically with NO• to produce ONOO- [25]. It is believed that mitochondria are an important source of ROS in cardiomyocytes [26]. It was documented that NO• inhibits complex IV in mitochondria which in turn contributes to an increase in ROS production [28-30]. Complex I can be inhibited when NO• and the high level of matrix Ca2+ are present to activate O2• production and ONOO- synthesis which in turn causes S-nitrosylation of complex I [31]. Peroxynitrite anion by itself can also inhibit complex III and V which contributes to respiratory block and additional ROS production [32]. Increased ONOO- production promotes MPT pore opening [33]. MPT pore opening causes apoptosis and cell death.
Thus, excess NO• production could aggravate cardiac RI. However, moderate NO• formation contributes to increased cardiac tolerance to I/R since NO• stimulates guanylyl cyclase, which synthesizes cGMP activating protein kinase G. This kinase increases cardiac resistance to I/R [34].
4. OXIDATIVE STRESS
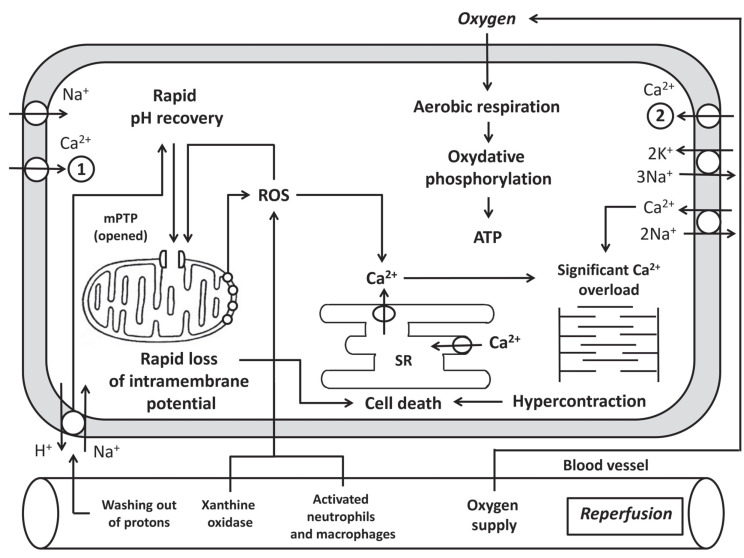
The sudden activation of aerobic metabolism upon reperfusion elicits a surge in ROS production. ROS are important mediators of cardiac reperfusion injury. When restoring perfusion, ROS have at least three main sources: the mitochondrial respiratory chain [35-37]; xanthine oxidase, which acts on hypoxanthine and xanthine [36, 37], and NADPH oxidase (Nox) from activated neutrophils and macrophages [38], which enter the myocardial tissue when blood flow is restored. In addition, the source of ROS may be Nox of cardiomyocytes that express Nox1, Nox2, Nox4 [35-37, 39]. It should be noted that xanthine oxidase is absent in the human myocardium [36]. In animals, xanthine oxidase generates O2• formation (Fig. 2).
Fig. (2).
The main metabolic events that occur during reperfusion in cardiomyocytes. SR, sarcoplasmic reticulum; MPTP, mitochondrial permeability transition pore; ROS - reactive oxygen species; 1. L-type Ca2+ channel; 2, TRPV4, transient receptor potential vanilloid channel 4.
Hypoxanthine + H2O + O2 → xanthine + H+ + O2•
xanthine + H2O + O2 → uric acid + H+ + O2•
NADPH oxidase also catalyzes O2• formation
NADPH + 2O2 → NADP+ + H+ + 2O2•
Mitochondria are an important source of O2∙. This free radical is formed by complex I and complex III of the respiratory chain [35, 36]. Superoxide radical enters the matrix and intermembrane space of mitochondria [35, 39]. Under normal conditions, NOS catalyzes nitric oxide (NO•) formation from L-arginine using tetrahydrobiopterin (BH4) as a cofactor. Under ischemia-reperfusion, BH4 synthesis is impaired. The lack of BH4 leads to uncoupling of NOS, which begins to synthesize O2• instead of NO (Fig. 2) [35, 40].
Under normal conditions, NOS catalyzes the reaction:
NADPH + O2 + arginine → citrulline + NO• + NADP+
Under ischemia-reperfusion, NOS catalyzes the reaction:
NADPH + O2 → O2• + NADP+ + H+
Dismutation of O2• occurs either spontaneously or by superoxide dismutase (SOD), causing hydrogen peroxide (H2O2) formation. H2O2 is relatively stable in vivo compared to other ROS molecules. This compound is lipid-soluble and can freely diffuse across membranes, acting as a physiological signaling molecule by selectively oxidizing target proteins [35, 36].
ROS cause lipid peroxidation, which leads to membrane damage, including sarcoplasmic reticulum membranes that in turn aggravate Ca2+ overload [41]. ROS activate and inactivate various enzymes [36]. They damage cellular structures, including DNA [35]. The recovery of neutral pH during reperfusion attenuates the inhibitory effects of H+ on MPT pore [4]. ROS and Ca2+, present in excess in the cytoplasm, cause MPT pore opening (Fig. 2) [4, 42]. Release into the cytoplasm of pro-apoptotic proteins cytochrome C and apoptosis inducing factor (AIF) activates apoptotic cascades [43, 44]. In addition, MPT pore inhibition during reperfusion protects the heart from RI [45, 46]. Cytochrome C and AIF are triggers of apoptosis. Therefore, their entry into the cytoplasm induces apoptosis [43, 44]. In addition, MPT pore opening causes a decrease in potential of the inner mitochondrial membrane (Δψ), which leads to a decrease in ATP synthesis by mitochondria. If this process affects all mitochondria, this can result in cell death due to necrosis [4, 43]. It should be noted that low ROS concentrations serve as intracellular signaling molecules which are involved in the formation of the cardioprotective effect of pre- and postconditioning [36]. It can be hypothesized that some ROS have myocardial damage, while other ROS provide the protective effect of pre- and postconditioning.
5. CALPAINS AND METALLOPROTEASES
Calpains are other targets of Ca2+ overload. Calpains are the family of Ca2+-dependent cysteine proteases [47]. Typically, calpains require Ca2+ in μM concentration range which is rarely observed in vivo [47]. A possible explanation may relate to the existence of microdomains which have a high Ca2+ concentration which is sufficient for calpain stimulation [47]. Pretreatment with a calpain inhibitor A-705253 before CAO and reperfusion reduced infarct size in pigs by 35% [48]. In a study performed in the isolated perfused rabbit heart it was demonstrated that the use of a calpain inhibitor A-705253 at the onset of reperfusion resulted in a decrease in the infarct size/area at risk (IS/AAR) ratio by 32% [49].
Matrix metalloproteinases (MMPs) are not Ca2+-dependent enzymes, but they can be implicated in cardiac I/R injury. However, data on their involvement in cardiac I/R damage is contradictory. It was reported that I/R of the isolated perfused heart caused a decrease in metalloproteinase-2 (MMP-2) activity in the myocardium [50]. According to other investigators [51], the activation of MMP-2 occurred after 15 min of ischemia, but following prolonged ischemia and reperfusion MMP-2 activity declined. Atrial biopsy samples were obtained before cardioplegia and within 10 min after removal of the aortic cross-clamp in patients with coronary artery bypass graft surgery [52]. There was a marked increase in MMP-2 and MMP-9 activity, and a decrease in TIMP-1, an endogenous inhibitor of MMPs, upon reperfusion in cardiac biopsies. It was documented that remote ischemic preconditioning decreases the IS/ARR ratio, reduces MMP-2 and MMP-9 expression, and increases the TIMP-1 level in the myocardium [53]. The broad-spectrum MMP inhibitor ilomastat prevented I/R cardiac injury [50, 54] and reperfusion damage of the heart [54, 55].
It should be noted that not all investigators could demonstrate the infarct-reducing effect of MMP inhibitors [56, 57]. It was found that MMP-8 is involved in the cardioprotective effect of bradykinin [56]. These investigators proposed that bradykinin triggers the protective signalling pathway through MMP-8-dependent transactivation of epidermal growth factor receptor (EGFR) at reperfusion [56].
The aforementioned studies indicated that calpains are involved in ischemic and reperfusion cardiac injury. Thus, it was demonstrated that the activation of MMP-2 and MMP-9 aggravates I/R cardiac injury. Stimulation of MMP-8 promotes an increase in cardiac tolerance to the impact of I/R.
6. DIFFERENT TYPES OF CELL DEATH AND THEIR MECHANISMS
There are three main forms of cell death, which morphologically differ significantly from each other: apoptosis, autophagy and necrosis [58].
6.1. Necrosis
It is possible that necrosis is the most common process of cell death during myocardial reperfusion. Morphologically, necrosis is characterized by an increase in cell volume (oncosis), swelling of mitochondria, activation of lysosomes, rupture of the plasma membrane and release of intracellular contents [58]. The released plasma proteins (troponin I, troponin T, creatine kinase-MB) are used to diagnose acute myocardial infarction. Released intracellular proteins stimulate a strong inflammatory (and immunogenic) reaction, which further enhances cellular damage [59, 60]. Usually, necrosis is understood as an unregulated form of cell death. Occurring at the same time is regulated necrosis which is caused by the MPT pore opening (MPT-driven necrosis) and necroptosis [58], both of which are different and will be detailed below.
A number of mechanisms are known to lead to cell necrosis during reperfusion. These include: (a) oxidative stress, which causes injury to the sarcolemma; (b) increased activity of destructive enzymes, for example, calpains, which are activated during Ca2+ overload of cardiomyocytes, cause proteolysis, increase fragility of cellular structures, injury of the cytoskeleton and the sarcolemma [61, 62]; (c) osmotic stress, which is caused by Na+-loading and Ca2+-loading, leads to an increase in the amount of water in the cell and, as a consequence, to their swelling. The aforementioned disruptions of normal cellular processes damage the cytoskeleton leading to osmotic fragility and rupture of the sarcolemma [63].
6.2. Necroptosis
By the 1990s, the consensus of investigators was that cardiomyocytes could die by an uncontrolled process (necrosis) or by programmed cell death (apoptosis). However, in 2000, it was discovered that tumor necrosis factor-α (TNF-α) can induce necrotic-like cell death of fibroblasts in the presence of caspase inhibitors [64]. The aforementioned data suggest the existence of programmed necrosis. This hypothesis was confirmed in 2005 when a group of investigators discovered controlled necrosis, which they called necroptosis [65]. This study was performed in U937 cell culture treated with TNF-α. Simultaneously, evidence was reported that the necroptosis blocker necrostatin-1 was the inhibitor of receptor interaction protein kinase 1 (RIPK1) [65]. Today it has been documented that necroptosis can be caused by the death receptor agonists, such as TNF-α, Fas-ligand and TNF-related apoptosis-inducing ligand (TRAIL) [65-67]. In addition, necroptosis may be induced by activation of Toll-like receptors 3 and 4 (TLR3/4) with bacterial lipopolysaccharide [66-68] or as a result of stimulation of interferon-γ receptors [66]. The activation of these death receptors is followed by autophosphorylation and activation of kinases: RIPK1 and RIPK3 [68]. A complex (necrosome) is formed, containing several phosphorylated molecules of RIPK1 and RIPK3. Necrosome catalyzes phosphorylation of pseudokinase MLKL (mixed lineage kinase domain-like) [68]. Phosphorylated MLKL molecules oligomerize, translocate into the plasma membrane, where they form a pore, which leads to damage to the cell membrane and eventually induces cell death as a result of necroptosis [68]. In 2007, it was demonstrated that intravenous administration of necrostatin-1 at the onset of reperfusion could decrease infarct size in mice [69]. Utilizing a pig model to investigate I/R cardiac damage, it was documented that intravenous administration of necrostatin-1 before reperfusion can reduce infarct size and preserve the function of the left ventricle [70]. The aforementioned studies emphasize the important role of necroptosis in cardiac RI.
6.3. Apoptosis
In 1972, Kerr et al. [71] discovered apoptosis, a regulated process of cell death mediated by internal or external stimuli. Apoptosis is regulated caspase-dependent cell death, which is accompanied by a decrease in cell volume, karyopyknosis and karyorrhexis [44, 71]. Cell fragmentation and chromatin condensation are completed by formation of apoptotic bodies surrounded by the cell membrane [71]. Apoptotic bodies are absorbed by phagocytes, thereby avoiding inflammation and indiscriminate cell injury. The aforementioned process prevents indiscriminate release of intracellular components and the subsequent inflammatory response, a hallmark of necrosis and necroptosis [44]. Apoptosis, in contrast to necrosis, is an ATP-dependent process that does not proceed if cellular ATP is severely depleted, as is the case under ischemic conditions. Therefore, apoptosis, which began during ischemia, is enhanced by reperfusion [40, 43]. Apoptosis proceeds by intrinsic and extrinsic pathways. The intrinsic pathway causing apoptosis is MPT pore opening with release to the cytoplasm of apoptotic inducers: apoptosis-inducing factor (AIF) and cytochrome C. The latter participates in apoptosome formation, which requires ATP [44]. The apoptosome includes in its structure cytochrome C, apoptosis protease-activating factor 1 (APAF-1) and procaspase 9, which is proteolytically activated to caspase 9, which, in turn, catalyzes the proteolytic activation of procaspases into caspases-3, -6, -7 [44]. It should be noted that collateral blood flow in the ischemic zone never drops to zero values and can reach 17% of the initial value in dogs [72]. However, some cells in the ischemic zone retain the ability to resynthesize ATP by glycolysis and, therefore, can undergo apoptosis. The extrinsic pathway of apoptosis may be triggered by the activation of death receptors or by the cessation of stimulation of growth factor receptors. Death receptors, which include TNF-α, Fas-ligand (CD95L) and TRAIL [44] play the key role in cell death during ischemia and reperfusion. The same ligands can cause necroptosis under pharmacological blockade of apoptosis [65]. It has now been documented that the apoptosis inhibitor Z-VAD-FMK increases tolerance of the isolated perfused guinea pig heart to I/R [73]. Consequently, apoptosis plays an important role in ischemic and especially in reperfusion injury of the heart.
6.4. Autophagy
Autophagy is another regulated process of cell death, which is characterized by lysosomal degradation of proteins [74]. Three types of autophagy are recognized. Macroautophagy involves the formation of vesicles which are called autophagosomes that bind cellular proteins, glycosides, lipids and organelles and then deliver them to lysosomes for degradation. Microautophagy refers to a process by which cellular elements are subjected to degradation and are directly absorbed by lysosomes [75]. Chaperone-mediated autophagy is characterized by the binding of proteins containing the KFERQ sequence to the Hsc70 chaperone which transports target proteins into lysosomes with the involvement of lysosomal membrane 2A protein (Lamp2A) [74]. Macroautophagy, which is usually referred to as autophagy, is crucial for the degradation of organelles and adaptation to cell stress, while the other two forms of autophagy are involved in specialized cellular functions [74, 75].
In its initial stage, autophagy is accompanied by phagophore formation, which represents a fragment of the endoplasmic reticular membrane with the involvement of the protein complex Beclin-Vps34 and the protein LC3 (microtubule-associated protein 1 light chain 3), a vesicle called an autophagosome is formed [74, 76]. Autophagy-related genes (Atg) encode more than 30 Atg proteins that participate in formation of autophagosomes [73, 75]. The proteins parkin and p62 play a key role in selective mitophagy [74, 77]. At the final stage of autophagy, autophagosome merges with the lysosome and the autolysosome is formed, in which digestion of intracellular structures takes place. Paradoxically, cell death as a result of autophagy can protect the heart from I/R injury [77]. For example, in pigs exposed to a 45-minute CAO and reperfusion, the putative autophagy inducer chloramphenicol reduced infarct size [78]. Data on the role of autophagy in myocardial RI in humans are contradictory. Autophagy reportedly is activated in the human myocardium after exposure to I/R [79]. However, there is also recent evidence that the cardioprotective phenomenon of remote preconditioning develops without the involvement of autophagy in humans [80].
6.5. Pyroptosis
Pyroptosis is a programmable form of cell death, which is characterized by DNA fragmentation, nuclear condensation and caspase dependence. This process resembles apoptosis but differs from the latter by breaking the cell membrane with subsequent activation of the inflammatory reaction [81]. The term “pyroptosis” was first proposed in 2001 [82]. Pyroptosis is accompanied by caspase-1-dependent pore formation in the cell membrane followed by the disappearance of the cellular ionic gradient, cell swelling and rupture of the cell membrane [83]. Caspase-1 is activated by multiprotein signaling complexes - inflammasomes, which in turn are activated by caspase activation and recruitment domains (CARD) [81].
Inflammasomes are oligomeric protein complexes that cleave to procaspase-1 and to caspase-1 [84, 85].
In addition, inflammasomes are involved in synthesis and secretion of proinflammatory cytokines: interleukin-1β (IL-1β) and interleukin 18 (IL-18) [84], which are involved in pyroptosis. A number of investigators have presented evidence that NOD-like receptor protein (NLRP3) and other components of the inflammasome are not constitutively expressed in cardiomyocytes [84]. A small amount of Nlrp3 mRNA was identified in the myocardium of wild-type mice [86]. NLRP3 inflammasome formation is regulated by nuclear factor-κB (NF-κB) [84]. Pro-inflammatory stimuli such as cytokines, cellular debris or microbial products that bind to TLRs can induce the expression of NLRP3 and other inflammasome proteins in cardiomyocytes, leukocytes, and other cells such as fibroblasts and endothelial cells [84]. Cytokines, cellular debris, and microbial products, collectively termed damage-associated molecular patterns (DAMPs) or pathogen-associated molecular patterns (PAMPS) bind to TLRs. The increased expression and activity of the inflammasome peaked after 1 day of reperfusion in the myocardium of mice [84]. Administration of the NLRP3 inflammasome inhibitor (NLRP3inh) at reperfusion did not reduce infarct size at 3 h after reperfusion, while it significantly reduced infarct size at 24 h after reperfusion [10]. It has been reported that OLT1177, a NLRP3 inflammasome inhibitor, administered 1 h after reperfusion dose-dependently reduced the IS/AAR ratio in mice [84]. The aforementioned studies indicate that the NLRP3 inflammasome is involved in reperfusion injury of the heart. This evidence indicates pyroptosis occurs primarily after the first 3 hours of reperfusion. However, there is evidence that stimulation of NLRP3 inflammasome formation may contribute to increased tolerance of the heart to I/R. Accordingly, NLRP3-deficient mice had larger infarctions than wild-type mice [87]. Pretreatment with the TLR2 agonist Pam3CSK4 reduced infarct size in wild-type but not NLRP3 deficient mice [87]. Sandager et al. [87] administered the TLR2 agonist before ischemia, while Tondo et al. [10, 84] administered the NLRP3 inflammasome inhibitors during reperfusion. It is possible that activation of the NLRP3 inflammasome before CAO enhances cardiac tolerance to I/R. The aforementioned data demonstrate that the NLRP3 inflammasome is involved in cardiac RI. Administration of the caspase-1/4 inhibitor VX-765 (16 mg/kg) 30 min prior to CAO and at reperfusion reduced the IS/AAR ratio by 47% [88]. The same investigators demonstrated that administration of VX-765 (32 mg/kg) prior to reperfusion reduced the IS/AAR ratio by 52% [89]. In addition, VX-765 decreased the serum interleukin-1β level and reduced caspase-1 activity. Consequently, pyroptosis is also involved in reperfusion death of cardiomyocytes.
6.6. Ferroptosis
Ferroptosis is a form of cell death, trigger of which is ferrous ion, which catalyzes the Fenton reaction:
Fe2+ + H2O2 → Fe3+ + OH• + OH−
The resulting hydroxyl radical induces lipid peroxidation which induces destruction of the cell membrane and cell death [90]. Thus, ferroptosis is essentially iron-mediated necrosis. Iron chelators, for example, deferoxamine, prevent the occurrence of ferroptosis [90]. It has been reported that deferoxamine increases cardiac resistance to ischemia during cold cardioplegia in rats [91]. Infusion of deferoxamine during coronary artery bypass grafting, which is accompanied by cardioplegic cardiac arrest and myocardial ischemia, reduced the level of lipid peroxidation products which are involved in RI of the heart in human [92]. In contrast, in a study performed on pigs with CAO and reperfusion, deferoxamine did not limit infarct size [93]. We found that deferoxamine did not affect the IS/AAR ratio in rats [unpublished data]. The reason for these ambiguous findings remains unclear.
Thus, reperfusion promotes cell death as a result of necrosis, necroptosis, apoptosis and, possibly, autophagy and pyroptosis. The role of ferroptosis in reperfusion injury of the heart requires further study.
7. ADRENERGIC SYSTEM
Catecholamines have positive inotropic and chronotropic effects mediated by cyclic AMP, which increases myocardial oxygen demand. Under conditions of limited oxygen delivery during CAO, excessive activation of ARs by catecholamines intensifies hypoxia of the ischemic myocardium, contributing to the expansion of the necrotic zone. The experimental data indicate an increase in the level of norepinephrine circulating in blood in response to CAO in rats and in dogs [94, 95] and an increase in the concentration of interstitial norepinephrine in the area of myocardial ischemia in rats and in rabbits [96, 97]. Fukui et al. found that after CAO in rats, the interstitial norepinephrine level increased 200-fold [96]. Circulating catecholamine concentrations were also increased in patients with AMI [98, 99]. The experimental data also indicate an increase in the level of catecholamines circulating in blood. High circulating epinephrine concentration is a predictor of mortality in AMI patients [100]. The ability of the β-AR antagonist propranolol to reduce infarct size in CAO in dogs was first demonstrated by Reimer et al. 1973 [101]. Later it was documented that the β-AR antagonists can reduce infarct size when administered just before reperfusion in rats [15]. Based on the aforementioned studies, we hypothesize that β-ARs are involved in RI cardiac injury.
8. HUMORAL FACTORS
The myocardium is impacted by dozens, if not hundreds, of circulating humoral factors. Let us consider only endothelin-1 and angiotensin II. Both of these peptides increase total peripheral resistance and blood pressure [102-104], thereby increasing myocardial afterload. The concentration of angiotensin II in blood and in myocardial tissue increases in response to CAO in rats and in dogs [95, 105]. Angiotensin II receptor blockade has proven to be cardioprotective in rats with CAO [106]. There is also evidence that AMI leads to an increase in the endothelin-1 level in blood plasma of patients with AMI [98, 107]. Endothelin-1 has been found to cause coronary artery vasospasm in rats [108], and its receptor antagonists are cardioprotective when administered at CAO and reperfusion in rats [109].
9. TOLL-LIKE RECEPTORS (TLRS)
TLRs are expressed by leukocytes and play an important role in innate immune responses [110]. These receptors recognize pathogen-associated molecular patterns, for example, TRL4 interacts with bacterial lipopolysaccharide, and TRL2 binds with bacterial lipoproteins [110]. In addition, these receptors interact with endogenous molecules that signal cell damage, for example, heat shock proteins (HSP70 and HSP60), as well as nuclear DNA-binding protein high mobility group box (HMGB-1) [110, 111]. Endogenous TLR’s activators belong to the DAMPs. TLRs have also been found in endotheliocytes [112] and cardiomyocytes [111, 113]. When activated, TLRs are involved in the inflammatory response acting through NF-κB translocation into the cell nucleus [111, 114]. Therefore, a number of investigators consider TLR inhibition as a viable approach for the treatment of AMI [111, 115, 116].
Studies in the isolated hearts of wild-type mice and the hearts of TLR2-deficient mice demonstrated that the latter fully restore their contractility after ischemia compared with the hearts of wild-type mice [117]. However, reperfusion creatine kinase (CK) release was the same in both groups. TLR2 deficiency protects coronary arteries from reperfusion endothelial dysfunction in mice [118]. Stimulation of TLR2 causes impaired contractility of murine cardiomyocytes [119]. A comparative study performed on wild-type and TLR4-defective C3H-Tlr4 (LPS-d) mice demonstrated that animals did not differ in the IS/AAR ratio, but in TLR4-defective mice, post-infarction remodeling was less pronounced [120]. Intravenous injection of anti-TLR2 antibodies reduced infarct size in mice by almost 50%, promoted a reduction of post-infarction scar, and improved cardiac contractility 28 days after infarction [117]. Administration of anti-TLR2 antibodies significantly reduced infiltration of neutrophils, macrophages and T-lymphocytes into the infarcted myocardium [117]. Knockout of the gene encoding TLR2 contributes to a decrease in the IS/AAR. Transplantation of bone marrow from wild-type mice to TLR2-null mice increased infarct size to control values [116]. This evidence indirectly indicates that TLR2 of leukocytes rather than TLR2 of cardiomyocytes play a crucial role in reperfusion injury of the heart. It has also been demonstrated that TLR4 is involved in post-infarction cardiac remodeling in pigs [121].
Currently, TLR2 and TLR4 are considered potential targets for the creation of new cardioprotective drugs [111, 116].
10. NEUTROPHILS AND MONOCYTES
Inflammation plays an important role in RI of the myocardium. The number of leukocytes in blood of patients with AMI was significantly correlated with infarct size, which was estimated by the CK, creatine kinase-MB (CK-MB), and troponin-I levels [122].
Neutrophil accumulation in reperfusion zone peaks 24 h after cardiac reperfusion in dogs [5]. Neutrophil adherence to left anterior descending coronary artery segments reaches a peak at 48 h of reperfusion [5]. Myeloperoxidase activity in the area at risk correlated with the extension of infarction during the first 24 h of reperfusion [5]. In 1983, Romson et al. [123] found that administration of anti-neutrophil antibodies to dogs before CAO reduced the IS/AAR ratio by 43%. Neutrophils and monocytes express integrin receptor (CD11b/CD18) [124], which enables these cells to adhere to vascular endothelium and invade the tissue parenchyma. Administration of antibodies to CD11b to dogs 45 min after CAO contributes to a 46% decrease in the IS/AAR ratio [125]. Similar findings were obtained by other investigators using monoclonal antibodies to CD11b/CD18 [126]. It was found that pretreatment with the selective adenosine A2 agonist CGS-21680 5 min before reperfusion of the heart decreases the IS/AAR by approximately 50% and reduces neutrophil accumulation in the AAR in dogs [127]. In addition, CGS-21680 reduced O2• production by activated neutrophils [127]. Neutrophils express adenosine receptors [128]. Therefore, it can be hypothesized that CGS-21680 limits accumulation of neutrophils in the myocardium due to the activation of adenosine receptors of these cells.
However, data on the role of neutrophils in RI of the heart is contradictory. It has been documented that neutrophils contain myeloperoxidase (MPO), which synthesizes cytotoxic aldehydes (formaldehyde, acrolein and others) that damage cardiomyocytes [129]. MPO activity in the reperfusion area was increased 5-fold in dogs [130]. Therefore, there is reason to believe that this enzyme is involved in reperfusion cardiac injury. However, a study performed on MPO-null mice demonstrated that infarct size in these mice does not differ from infarct size in wild-type mice [129]. However, postinfarction remodeling of the heart was less pronounced in MPO-null mice [130]. The use of the selective MPO inhibitor PF-1355 did not affect infarct size after transient CAO but prevented postinfarction remodeling in mice [131]. Consequently, MPO is not involved in RI of the heart but in postinfarction remodeling of the heart. The number of circulating neutrophils in AMI patients is significantly higher than in patients with stable angina [132]. In addition, the number of neutrophils correlated with an increase in the plasma CK level [132]. In a multicenter, placebo-controlled study in patients with STEMI, antibodies to CD11b/CD18 were administered before recanalization of the infarct-related coronary artery [133]. Infarct size was evaluated by the CK-MB level and by using SPECT with 99mTc-sestamibi. The study failed to detect the infarct-reducing effect of the antibodies to CD11b/CD18. The aforementioned data suggest that neutrophils do not play a significant role in RI of the human heart. It is still unclear why the data from animal experiments and the results of clinical studies differ.
Not only neutrophils, but also monocytes/macrophages migrate to the ischemia-reperfusion zone. Their migration is stimulated by chemokine monocyte chemoattractant protein-1 (MCP-1). Studies were performed on wild-type mice and mice deficient in chemokine receptor-2 [38]. Infarct size in mice with genetic deficiency of chemokine receptor-2 was smaller than in wild-type mice. By contrast, macrophage migration inhibitory factor (MIF) inhibits this process. It has now been documented that MIF contributes to a decrease in the IS/AAR ratio in mice with CAO and reperfusion [134]. In mice in which the gene encoding MIF was deleted, the IS/AAR ratio was higher than in wild-type mice [135]. These data clearly indicate that the restriction of migration of monocytes to the ischemic/reperfusion zone contributes to a reduction of RI. However, studies on the isolated hearts of MIF-/- mice have demonstrated that hearts of these mice are also less resistant to I/R than the hearts of wild-type mice [136]. Therefore, the cardioprotective effect of MCP-1 could not be directly associated with restriction of macrophage migration. It should be noted that M2 macrophages can protect isolated neonatal rat cardiomyocytes from the impact of H/R [137].
The aforementioned studies indicate that the migration of neutrophils and monocytes to the ischemic-reperfusion zone may be directly related to cardiac RI. However, strong evidence of involvement of these cells in reperfusion heart damage in humans has not yet been provided.
11. T-LYMPHOCYTES
In 2006, reperfusion was found to promote an increase in neutrophils and CD3+ T-lymphocytes in the murine myocardium [138]. The number of circulating lymphocytes, on the contrary, decreased, apparently due to their sequestration by the myocardium. It was found that the selective adenosine A2A receptor agonist ATL146e administered 5 min before reperfusion reduced T-lymphocyte accumulation and decreased infarct size. In Rag1 knockout mice lacking mature lymphocytes, infarct size was significantly smaller than in wild-type mice but increased to the level of wild-type mice following a transfer of 50 million CD4+ T-lymphocytes delivered from wild-type mice [138]. When mice were depleted of CD4+ T-lymphocytes by monoclonal antibodies, infarct size was significantly smaller than in control mice [138]. T-lymphocytes rapidly decline in the peripheral circulation over the first 90 min following reperfusion in patients with AMI [139] due to sequestration of T-lymphocytes by the myocardium. This fact suggests the possibility of T-lymphocytes’ involvement in RI of the human heart.
Consequently, CD4+T-cell accumulation may play an important role in cardiac RI.
12. PLATELETS
Platelets are activated in AMI [131, 132] in parallel with increased P-selectin expression by platelets [140, 141].
In 2002, Mirabet et al. tested the hypothesis that the effects of platelets on the myocardium depends on their activation [142]. Pig platelets were obtained 48 min before CAO, 10 min after reperfusion, and after a 60-minute sham operation. The expression of P-selectin platelets was higher in platelets isolated during reperfusion than in platelets isolated before ischemia or after a sham operation. The isolated perfused rat hearts were subjected to global ischemia and reperfusion. Five min before global ischemia, platelets were added to the perfusion medium. Lactate dehydrogenase (LDH) release during reperfusion was similar in hearts perfused with a solution containing platelets isolated before CAO or after a sham operation. LDH release was increased when platelets were isolated from the blood of pigs during reperfusion. Platelet activation by thrombin increased P-selectin expression and LDH release from the isolated rat heart. A close correlation was documented between P-selectin expression and LDH release and platelet accumulation in the myocardium. These investigators concluded [142] that the pathogenic effect of platelets in the reperfused myocardium depends on their activation, which is characterized by P-selectin expression. This expression is enhanced in response to ischemia/reperfusion. These results indicate that platelets play an important role in cardiac RI.
Platelets express GPIIb/IIIa receptor (integrin αIIbβ3) which is the receptor for fibrinogen, von Willebrand factor, fibronectin, and vitronectin [143]. The GPIIb/IIIa receptor seems to be also involved in reperfusion injury to the heart since blockade of this receptor with MK-0852 reduced the IS/AAR ratio after CAO and reperfusion. However, MK-0852 did not affect the AAR and blood flow in the ischemic zone in dogs [143]. Therefore, there is a reason to believe that MK-0852 not only inhibited platelet aggregation but also prevented the release of substances from platelets that aggravated I/R injury of the heart. With prolonged CAO and reperfusion, inhibition of GPIIb/IIIa receptor by tirofiban contributed to an improvement of microcirculation in the reperfusion zone, thereby preventing the formation of the microvascular obstruction area in dogs [144].
Purinergic P2Y12 (adenosine diphosphate agonist) receptor is another receptor which is also expressed in platelets. P2Y12 receptor antagonists inhibit platelet aggregation and are used to restore coronary perfusion and prevent recurrent ischemic events in AMI [145, 146]. In recent years, evidence has emerged that the P2Y12 receptor antagonist cangrelor cannot only inhibit platelet aggregation, but it can also reduce infarct size in rabbits [8]. Yang et al. detected that cangrelor limits infarct size when it is administered intravenously before reperfusion [8], in a situation very similar to the clinical setting. It has been reported that the infarct-reducing effect of cangrelor does not occur in isolated perfused rabbit hearts [8]. This provides indirect evidence of the involvement of platelets in cangrelor's cardioprotective effect. However, the protective effect of cangrelor does not appear to be associated with a change in platelet aggregation, since aspirin, which also inhibits platelet aggregation, did not affect infarct size [8]. The same infarct-limiting effect was exerted by the P2Y12 receptor antagonist ticagrelor, when administered to rats 5 min before reperfusion in rats [147]. These studies suggest that the most likely role of platelet P2Y12 receptor is regulation of cardiac resistance to reperfusion. In rats receiving the sphingosine kinase inhibitor dimethylsphingosine, cangrelor did not protect the rabbit heart from RI [148]. Thus, the protective mechanism of cangrelor seems to be associated with enhancement of sphingosine-1-phosphate synthesis, which is released from platelets [149] and results in the cardioprotective effect in experiments on the isolated perfused rat heart [150]. Moreover, sphingosine-1-phosphate is able to prevent cardiac RI in vivo in rats [151].
The aforementioned studies indicate that platelets play an important role in cardiac RI.
13. THE MAIN MANIFESTATIONS OF REPERFUSION INJURY OF THE HEART
In experimental studies and in the course of clinical observations, it is difficult to separate RI from ischemic injury. Therefore, in this section we discuss the manifestations of I/R heart’s damage. The most characteristic manifestation of I/R of the heart is necrosis and the appearance of markers of cardiomyocyte necrosis in blood: CK-MB, troponin I, and troponin T [152, 153]. It is also the appearance of coronary vascular dysfunction [5, 154-158], ventricular arrhythmias [16, 159-166], myocardial stunning [167], and the no-reflow phenomenon (Table 1) [168-173].
Table 1.
The main consequences of myocardial ischemia-reperfusion.
|
The Main Manifestations
of I/R Injury of the Heart |
The Mechanisms of Development of the Main Manifestations of I/R Injury | Refs. |
|---|---|---|
| Myocardial stunning | The Ca2+ overload of cardiomyocytes, activation of the Na+/H+ exchanger, 2Na+/Ca2+ exchanger and Na+/HCO3- symporter | Cohen M.V. et al., 2007 [3] Piper H.M. et al., 1999 [12] Rodriguez-Sinovas A. et al., 2009 [13] Herzog W.R. et al., 1997 [14] Lishmanov Y.B. et al., 2016 [15] Zhang H. et al., 2013 [16] Neri M. et al., 2017 [153] |
| Coronary vascular dysfunction |
Pyroptosis of endothelial cells, inflammation of endothelial cells, apoptosis and especially autophagy of endothelial cells, damage of the endothelial glycocalyx | Sun W. et al., 2019 [155] Gollmann-Tepeköylü C. et al., 2020 [156] Zhen W. et al., 2020 [157] Araibi H. et al., 2020 [158] |
| Ventricular arrhythmias | A decrease in repolarizing K+ currents, an increase in inward Ca2+ current, sympathetic nervous system activation, an increase in the cAMP level in cardiac cells, Ca2+ overload of cells, excess ROS production, neutrophil invasion in AAR, activation of 2Na+/Ca2+ exchange | Zhang H. et al., 2013 [16] Schwartz P.J., Stone H.L., 1980 [160] Bernier M. et al., 1986 [161] Lubbe W.F. et al., 1992 [162] Rosen M.R., 1995 [163] Dhein S. et al., 1995 [164] Antoons G. et al., 2012 [165] van der Weg K. et al., 2019 [166] |
| The no-reflow phenomenon | Swelling of endothelial cells, aggregation of blood cells in the microvessels, an increase in blood viscosity, impaired endothelium-dependent and endothelium-independent vasodilation | Boag S.E. et al., 2017 [139] Kloner R.A., 1974 [169] Loke K.E. et al, 1998 [170] Haiyun L. et al., 2004 [171] Cecchi E. et al., 2009 [172] Ming X. et al., 2012 [173] |
14. DIAGNOSIS AND TREATMENT OF ISCHEMIC AND REPERFUSION INJURY OF THE HEART
In clinical practice, I/R damage is observed in patients with AMI and also in cardiac surgery patients after cardio-pulmonary bypass. ST-segment elevation myocardial infarction (STEMI) can be easily diagnosed by the ST elevation on an ECG. However, it is more difficult to diagnose non-ST-segment elevation myocardial infarction (NSTEMI), which necessitates determination of the levels of myocardial necrosis markers: troponin I, troponin T, and creatine kinase-MB [167, 174, 175]. I/R injury in cardiac surgery patients is documented by increased serum troponin or CK-MB levels [176].
Determination of peak troponin or CK-MB levels allows one to indirectly assess infarct size [167, 174]. Magnetic resonance imaging (MRI) allows one to more accurately detect infract size [177, 178]. MRI and definition of the peak troponin or CK-MB levels are commonly used to assess the effectiveness of drugs in the treatment of AMI. In clinical practice, it is important to diagnose AMI and determine the localization of coronary artery thrombosis. Invasive coronary angiography permits not only correct localization of the site of coronary artery thrombosis but also to lead in stenting of the infarct-related coronary artery. Percutaneous coronary intervention (PCI) still remains the most effective treatment for AMI, since it restores coronary blood flow in 95% of cases [179]. PCI is effective in stopping ischemic damage to the heart, but it frequently results in reoxygenation injury to cardiomyocytes.
One of the most important aims of modern pharmacology is the development of drugs that can slow down the formation of ischemic injury of the heart before PCI and drugs that can prevent reperfusion injury of the heart.
The main cause of death in patients with AMI is cardiogenic shock [180]. The mortality rate is 30 - 70% in patients with AMI and cardiogenic shock [181, 182]. The occurrence of cardiogenic shock is directly dependent on infarct size [183]. Consequently, efforts of cardiologists should be directed towards infarct size reduction and improving cardiac contractility.
Another major problem is the no-reflow phenomenon which is observed in approximately 5% of patients with AMI and PCI [179]. However, even if we could restore coronary blood flow in the main coronary arteries, a problem of microvascular coronary obstruction occurs for which there currently is no effective treatment intervention [184, 185].
Glycoprotein IIb/IIIa inhibitors, P2Y12 receptor antagonist, aspirin (in 98% patients), statins (96%), beta-blockers (58%), angiotensin-converting enzyme inhibitors (9%), angiotensin receptor blocker (38%), aldosterone receptor antagonists (17%) are all used for the treatment of AMI in Handan First Hospital (Chine) [186]. The same drugs are used in other clinics for treatment of AMI. However, the ratio of use of these drugs can vary considerably. For example, in the San Francisco Veterans Affairs Health Care System (USA): the following ratio of drugs is used: aspirin (99%), beta-blocker (94%), renin-angiotensin-aldosterone system antagonist (75%), statins (96%), and thienopyridine (94%) [187].
CONCLUSION
Cardiomyocyte Ca2+ overload and increased production of ROS play pivotal roles in myocardial I/R injury. Necrosis, necroptosis, apoptosis and, possibly, autophagy and pyroptosis are involved in RI of the heart. The role of ferroptosis in I/R injury of the heart requires further investigation. An increase in activity of the adrenergic system during ischemia and the restoration of coronary perfusion negatively affect resistance of the heart to I/R. It has also been reported that neutrophils, monocytes, CD4+ T-cells and platelets play an important role in I/R cardiac damage. TLRs, angiotesin II and endothelin-1 are also involved in I/R injury of the heart. The main clinical manifestations of I/R cardiac injury are necrosis, ventricular arrhythmias, contractile dysfunction, and no-reflow phenomenon.
We hypothesize that there are early cardiac RIs that develop within a few minutes (5 - 10 min) after reperfusion, remote injuries that develop within a few hours and 24 to 48 hours after the restoration of coronary blood flow, and late reperfusion injuries that form within a few weeks after the restoration of coronary perfusion. The most studied of these RIs is early reperfusion damage of the heart. Less well documented is the mechanism(s) by which inflammation may play a major role in the remote development of myocardial injury. We know almost nothing about late RI and the extent that it contributes to pathological myocardial remodeling. Our hypothesis is that changes in gene transcription play an important role in the occurrence of post-infarction myocardial remodeling. Future studies should focus on the role of the receptor(s), signaling mechanisms, and inflammation in the pathogenesis of early and remote cardiac RI. The role of gene transcription alterations in the mechanism(s) of postinfarction remodeling also merits further investigation.
ACKNOWLEDGEMENTS
Declared none.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
The article was prepared with the support of Russian Foundation of Basic Research Grant 21-515-53003 and National Natural Science Foundation of China Grant 82111530058. The section dedicated to the main manifestations of reperfusion injury of the heart is framed within the framework of state assignments 122020300042-4 (A.V. Mukhomedzyanov).
CONFLICT OF INTEREST
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
REFERENCES
- 1.Olier I., Sirker A., Hildick-Smith D.J.R., et al. British cardiovascular intervention society and the national institute for cardiovascular out-comes research. Association of different antiplatelet therapies with mortality after primary percutaneous coronary intervention. Heart. 2018;104(20):1683–1690. doi: 10.1136/heartjnl-2017-312366. [DOI] [PubMed] [Google Scholar]
- 2.Maslov L.N., Barbarash O.L. Pharmacological approaches to limiting the infarct zone size in patients with acute myocardial infarction: Anal-ysis of clinical data. Eksp. Klin. Farmakol. 2018;81:34–41. [Google Scholar]
- 3.Cohen M.V., Yang X.M., Downey J.M. The pH hypothesis of postconditioning: Staccato reperfusion reintroduces oxygen and perpetuates myocardial acidosis. Circulation. 2007;115(14):1895–1903. doi: 10.1161/CIRCULATIONAHA.106.675710. [DOI] [PubMed] [Google Scholar]
- 4.Ong S.B., Samangouei P., Kalkhoran S.B., Hausenloy D.J. The mitochondrial permeability transition pore and its role in myocardial ischemia reperfusion injury. J. Mol. Cell. Cardiol. 2015;78:23–34. doi: 10.1016/j.yjmcc.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Zhao Z.Q., Nakamura M., Wang N.P., et al. Dynamic progression of contractile and endothelial dysfunction and infarct extension in the late phase of reperfusion. J. Surg. Res. 2000;94(2):133–144. doi: 10.1006/jsre.2000.6029. [DOI] [PubMed] [Google Scholar]
- 6.Reimer K.A., Lowe J.E., Rasmussen M.M., Jennings R.B. The wavefront phenomenon of ischemic cell death. 1. Myocardial infarct size vs duration of coronary occlusion in dogs. Circulation. 1977;56(5):786–794. doi: 10.1161/01.CIR.56.5.786. [DOI] [PubMed] [Google Scholar]
- 7.Eleawa S.M., Alkhateeb M., Ghosh S., et al. Coenzyme Q10 protects against acute consequences of experimental myocardial infarction in rats. Int. J. Physiol. Pathophysiol. Pharmacol. 2015;7(1):1–13. [PMC free article] [PubMed] [Google Scholar]
- 8.Yang X.M., Liu Y., Cui L., et al. Platelet P2Y12 blockers confer direct postconditioning-like protection in reperfused rabbit hearts. J. Cardiovasc. Pharmacol. Ther. 2013;18(3):251–262. doi: 10.1177/1074248412467692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peart J.N., Gross E.R., Reichelt M.E., Hsu A., Headrick J.P., Gross G.J. Activation of kappa-opioid receptors at reperfusion affords cardioprotec-tion in both rat and mouse hearts. Basic Res. Cardiol. 2008;103(5):454–463. doi: 10.1007/s00395-008-0726-z. [DOI] [PubMed] [Google Scholar]
- 10.Toldo S., Marchetti C., Mauro A.G., et al. Inhibition of the NLRP3 inflammasome limits the inflammatory injury following myocardial is-chemia-reperfusion in the mouse. Int. J. Cardiol. 2016;209:215–220. doi: 10.1016/j.ijcard.2016.02.043. [DOI] [PubMed] [Google Scholar]
- 11.Toldo S., Mauro A.G., Cutter Z., et al. The NLRP3 inflammasome inhibitor, OLT1177 (dapansutrile), reduces infarct size and preserves contractile function after ischemia reperfusion injury in the mouse. J. Cardiovasc. Pharmacol. 2019;73(4):215–222. doi: 10.1097/FJC.0000000000000658. [DOI] [PubMed] [Google Scholar]
- 12.Piper H.M., García-Dorado D. Prime causes of rapid cardiomyocyte death during reperfusion. Ann. Thorac. Surg. 1999;68(5):1913–1919. doi: 10.1016/S0003-4975(99)01025-5. [DOI] [PubMed] [Google Scholar]
- 13.Rodríguez-Sinovas A., Cabestrero A., García del Blanco B., Inserte J., García A., García-Dorado D. Intracoronary acid infusion as an alterna-tive to ischemic postconditioning in pigs. Basic Res. Cardiol. 2009;104(6):761–771. doi: 10.1007/s00395-009-0032-4. [DOI] [PubMed] [Google Scholar]
- 14.Herzog W.R., Vogel R.A., Schlossberg M.L., Edenbaum L.R., Scott H.J., Serebruany V.L. Short-term low dose intracoronary diltiazem adminis-tered at the onset of reperfusion reduces myocardial infarct size. Int. J. Cardiol. 1997;59(1):21–27. doi: 10.1016/S0167-5273(96)02883-5. [DOI] [PubMed] [Google Scholar]
- 15.Lishmanov Y.B., Maslov L.N., Mukhomedzyanov A.V. Role of β-adrenoceptors and L-type Ca2+-channels in the mechanism of reperfusion-Induced heart injury. Bull. Exp. Biol. Med. 2016;161(1):20–23. doi: 10.1007/s10517-016-3335-0. [DOI] [PubMed] [Google Scholar]
- 16.Zhang H., Shang W., Zhang X., et al. B-adrenergic-stimulated L-type channel Ca2+ entry mediates hypoxic Ca2+ overload in intact heart. J. Mol. Cell. Cardiol. 2013;65:51–58. doi: 10.1016/j.yjmcc.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Gorbunov A.S., Maslov L.N., Jaggi A.S., et al. Physiological and pathological role of TRPV1, TRPV2 and TRPV4 channels in heart. Curr. Cardiol. Rev. 2019;15(4):244–251. doi: 10.2174/1573403X15666190307112326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dong Q., Li J., Wu Q.F., et al. Blockage of transient receptor potential vanilloid 4 alleviates myocardial ischemia/reperfusion injury in mice. Sci. Rep. 2017;7(1):42678. doi: 10.1038/srep42678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu Q.F., Qian C., Zhao N., et al. Activation of transient receptor potential vanilloid 4 involves in hypoxia/reoxygenation injury in cardiomy-ocytes. Cell Death Dis. 2017;8(5):e2828. doi: 10.1038/cddis.2017.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Camara A.K., Bienengraeber M., Stowe D.F. Mitochondrial approaches to protect against cardiac ischemia and reperfusion injury. Front. Physiol. 2011;2:13. doi: 10.3389/fphys.2011.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang S.Z., Gao Q., Cao C.M., Bruce I.C., Xia Q. Involvement of the mitochondrial calcium uniporter in cardioprotection by ischemic precon-ditioning. Life Sci. 2006;78(7):738–745. doi: 10.1016/j.lfs.2005.05.076. [DOI] [PubMed] [Google Scholar]
- 22.Cao C.M., Yan W.Y., Liu J., et al. Attenuation of mitochondrial, but not cytosolic, Ca2+ overload reduces myocardial injury induced by is-chemia and reperfusion. Acta Pharmacol. Sin. 2006;27(7):911–918. doi: 10.1111/j.1745-7254.2006.00391.x. [DOI] [PubMed] [Google Scholar]
- 23.Salas M.A., Valverde C.A., Sánchez G., et al. The signalling pathway of CaMKII-mediated apoptosis and necrosis in the ischemia/reperfusion injury. J. Mol. Cell. Cardiol. 2010;48(6):1298–1306. doi: 10.1016/j.yjmcc.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luongo T.S., Lambert J.P., Yuan A., et al. The mitochondrial calcium uniporter matches energetic supply with cardiac workload during stress and modulates permeability transition. Cell Rep. 2015;12(1):23–34. doi: 10.1016/j.celrep.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hurst S., Hoek J., Sheu S.S. Mitochondrial Ca2+ and regulation of the permeability transition pore. J. Bioenerg. Biomembr. 2017;49(1):27–47. doi: 10.1007/s10863-016-9672-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alderton W.K., Cooper C.E., Knowles R.G. Nitric oxide synthases: Structure, function and inhibition. Biochem. J. 2001;357(Pt 3):593–615. doi: 10.1042/bj3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Litvinova L., Atochin D.N., Fattakhov N., Vasilenko M., Zatolokin P., Kirienkova E. Nitric oxide and mitochondria in metabolic syndrome. Front. Physiol. 2015;6:20. doi: 10.3389/fphys.2015.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brookes P., Darley-Usmar V.M. Hypothesis: The mitochondrial NO(*) signaling pathway, and the transduction of nitrosative to oxidative cell signals: An alternative function for cytochrome C oxidase. Free Radic. Biol. Med. 2002;32(4):370–374. doi: 10.1016/S0891-5849(01)00805-X. [DOI] [PubMed] [Google Scholar]
- 29.Brookes P.S. Mitochondrial nitric oxide synthase. Mitochondrion. 2004;3(4):187–204. doi: 10.1016/j.mito.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 30.Zaobornyj T., Ghafourifar P. Strategic localization of heart mitochondrial NOS: A review of the evidence. Am. J. Physiol. Heart Circ. Physiol. 2012;303(11):H1283–H1293. doi: 10.1152/ajpheart.00674.2011. [DOI] [PubMed] [Google Scholar]
- 31.Jekabsone A., Ivanoviene L., Brown G.C., Borutaite V. Nitric oxide and calcium together inactivate mitochondrial complex I and induce cytochrome c release. J. Mol. Cell. Cardiol. 2003;35(7):803–809. doi: 10.1016/S0022-2828(03)00137-8. [DOI] [PubMed] [Google Scholar]
- 32.Radi R., Rodriguez M., Castro L., Telleri R. Inhibition of mitochondrial electron transport by peroxynitrite. Arch. Biochem. Biophys. 1994;308(1):89–95. doi: 10.1006/abbi.1994.1013. [DOI] [PubMed] [Google Scholar]
- 33.Packer M.A., Scarlett J.L., Martin S.W., Murphy M.P. Induction of the mitochondrial permeability transition by peroxynitrite. Biochem. Soc. Trans. 1997;25(3):909–914. doi: 10.1042/bst0250909. [DOI] [PubMed] [Google Scholar]
- 34.Cohen M.V., Downey J.M. Cardioprotection: Spotlight on PKG. Br. J. Pharmacol. 2007;152(6):833–834. doi: 10.1038/sj.bjp.0707453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cadenas S. ROS and redox signaling in myocardial ischemia-reperfusion injury and cardioprotection. Free Radic. Biol. Med. 2018;117:76–89. doi: 10.1016/j.freeradbiomed.2018.01.024. [DOI] [PubMed] [Google Scholar]
- 36.Krylatov A.V., Maslov L.N., Voronkov N.S., et al. Reactive oxygen species as intracellular signaling molecules in the cardiovascular system. Curr. Cardiol. Rev. 2018;14(4):290–300. doi: 10.2174/1573403X14666180702152436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moris D., Spartalis M., Tzatzaki E., et al. The role of reactive oxygen species in myocardial redox signaling and regulation. Ann. Transl. Med. 2017;5(16):324. doi: 10.21037/atm.2017.06.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hayasaki T., Kaikita K., Okuma T., et al. CC chemokine receptor-2 deficiency attenuates oxidative stress and infarct size caused by myo-cardial ischemia-reperfusion in mice. Circ. J. 2006;70(3):342–351. doi: 10.1253/circj.70.342. [DOI] [PubMed] [Google Scholar]
- 39.Lesnefsky E.J., Chen Q., Tandler B., Hoppel C.L. Mitochondrial dysfunction and myocardial ischemia-reperfusion: Implications for novel therapies. Annu. Rev. Pharmacol. Toxicol. 2017;57(1):535–565. doi: 10.1146/annurev-pharmtox-010715-103335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alkaitis M.S., Crabtree M.J. Recoupling the cardiac nitric oxide synthases: Tetrahydrobiopterin synthesis and recycling. Curr. Heart Fail. Rep. 2012;9(3):200–210. doi: 10.1007/s11897-012-0097-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Monassier J.P. Reperfusion injury in acute myocardial infarction. From bench to cath lab. Part I: Basic considerations. Arch. Cardiovasc. Dis. 2008;101(7-8):491–500. doi: 10.1016/j.acvd.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 42.Halestrap A.P., Richardson A.P. The mitochondrial permeability transition: A current perspective on its identity and role in ischae-mia/reperfusion injury. J. Mol. Cell. Cardiol. 2015;78:129–141. doi: 10.1016/j.yjmcc.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 43.Halestrap A.P. A pore way to die: The role of mitochondria in reperfusion injury and cardioprotection. Biochem. Soc. Trans. 2010;38(4):841–860. doi: 10.1042/BST0380841. [DOI] [PubMed] [Google Scholar]
- 44.Kroemer G., Galluzzi L., Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol. Rev. 2007;87(1):99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- 45.Hausenloy D.J., Duchen M.R., Yellon D.M. Inhibiting mitochondrial permeability transition pore opening at reperfusion protects against is-chaemia-reperfusion injury. Cardiovasc. Res. 2003;60(3):617–625. doi: 10.1016/j.cardiores.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 46.Ong S.B., Subrayan S., Lim S.Y., Yellon D.M., Davidson S.M., Hausenloy D.J. Inhibiting mitochondrial fission protects the heart against ische-mia/reperfusion injury. Circulation. 2010;121(18):2012–2022. doi: 10.1161/CIRCULATIONAHA.109.906610. [DOI] [PubMed] [Google Scholar]
- 47.Sorimachi H., Ono Y. Regulation and physiological roles of the calpain system in muscular disorders. Cardiovasc. Res. 2012;96(1):11–22. doi: 10.1093/cvr/cvs157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khalil P.N., Neuhof C., Huss R., et al. Calpain inhibition reduces infarct size and improves global hemodynamics and left ventricular con-tractility in a porcine myocardial ischemia/reperfusion model. Eur. J. Pharmacol. 2005;528(1-3):124–131. doi: 10.1016/j.ejphar.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 49.Neuhof C., Fabiunk V., Speth M., et al. Reduction of myocardial infarction by postischemic administration of the calpain inhibitor A-705253 in comparison to the Na(+)/H(+) exchange inhibitor Cariporide in isolated perfused rabbit hearts. Biol. Chem. 2008;389(12):1505–1512. doi: 10.1515/BC.2008.172. [DOI] [PubMed] [Google Scholar]
- 50.Giricz Z., Lalu M.M., Csonka C., Bencsik P., Schulz R., Ferdinandy P. Hyperlipidemia attenuates the infarct size-limiting effect of ischemic preconditioning: Role of matrix metalloproteinase-2 inhibition. J. Pharmacol. Exp. Ther. 2006;316(1):154–161. doi: 10.1124/jpet.105.091140. [DOI] [PubMed] [Google Scholar]
- 51.Spániková A., Ivanová M., Matejíková J., Ravingerová T., Barancík M. Influence of ischemia/reperfusion and modulation of PI3K/Akt ki-nase pathway on matrix metalloproteinase-2 in rat hearts. Gen. Physiol. Biophys. 2010;29(1):31–40. doi: 10.4149/gpb_2010_01_31. [DOI] [PubMed] [Google Scholar]
- 52.Lalu M.M., Pasini E., Schulze C.J., et al. Ischaemia-reperfusion injury activates matrix metalloproteinases in the human heart. Eur. Heart J. 2005;26(1):27–35. doi: 10.1093/eurheartj/ehi007. [DOI] [PubMed] [Google Scholar]
- 53.Li S.J., Wu Y.N., Kang Y., et al. Noninvasive limb ischemic preconditioning protects against myocardial I/R injury in rats. J. Surg. Res. 2010;164(1):162–168. doi: 10.1016/j.jss.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 54.Bencsik P., Pálóczi J., Kocsis G.F., et al. Moderate inhibition of myocardial matrix metalloproteinase-2 by ilomastat is cardioprotective. Pharmacol. Res. 2014;80:36–42. doi: 10.1016/j.phrs.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 55.Bell R.M., Kunuthur S.P., Hendry C., Bruce-Hickman D., Davidson S., Yellon D.M. Matrix metalloproteinase inhibition protects CyPD knock-out mice independently of RISK/mPTP signalling: A parallel pathway to protection. Basic Res. Cardiol. 2013;108(2):331. doi: 10.1007/s00395-013-0331-7. [DOI] [PubMed] [Google Scholar]
- 56.Methner C., Donat U., Felix S.B., Krieg T. Cardioprotection of bradykinin at reperfusion involves transactivation of the epidermal growth factor receptor via matrix metalloproteinase-8. Acta Physiol. (Oxf.) 2009;197(4):265–271. doi: 10.1111/j.1748-1716.2009.02018.x. [DOI] [PubMed] [Google Scholar]
- 57.Griffin M.O., Jinno M., Miles L.A., Villarreal F.J. Reduction of myocardial infarct size by doxycycline: A role for plasmin inhibition. Mol. Cell. Biochem. 2005;270(1-2):1–11. doi: 10.1007/s11010-005-2540-3. [DOI] [PubMed] [Google Scholar]
- 58.Galluzzi L., Vitale I., Aaronson S.A., et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature committee on cell death 2018. Cell Death Differ. 2018;25(3):486–541. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arslan F., Smeets M.B., O’Neill L.A., et al. Myocardial ischemia/reperfusion injury is mediated by leukocytic toll-like receptor-2 and reduced by systemic administration of a novel anti-toll-like receptor-2 antibody. Circulation. 2010;121(1):80–90. doi: 10.1161/CIRCULATIONAHA.109.880187. [DOI] [PubMed] [Google Scholar]
- 60.Volz H.C., Buss S.J., Li J., et al. Autoimmunity against cardiac troponin I in ischaemia reperfusion injury. Eur. J. Heart Fail. 2011;13(10):1052–1059. doi: 10.1093/eurjhf/hfr098. [DOI] [PubMed] [Google Scholar]
- 61.Parameswaran S., Sharma R.K. Ischemia and reperfusion induce differential expression of calpastatin and its homologue high molecular weight calmodulin-binding protein in murine cardiomyocytes. PLoS One. 2014;9(12):e114653. doi: 10.1371/journal.pone.0114653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ruiz-Meana M., García-Dorado D. Translational cardiovascular medicine (II). Pathophysiology of ischemia-reperfusion injury: New thera-peutic options for acute myocardial infarction. Rev. Esp. Cardiol. 2009;62(2):199–209. doi: 10.1016/S0300-8932(09)70162-9. [DOI] [PubMed] [Google Scholar]
- 63.Okada T., Otani H., Wu Y., et al. Role of F-actin organization in p38 MAP kinase-mediated apoptosis and necrosis in neonatal rat cardio-myocytes subjected to simulated ischemia and reoxygenation. Am. J. Physiol. Heart Circ. Physiol. 2005;289(6):H2310–H2318. doi: 10.1152/ajpheart.00462.2005. [DOI] [PubMed] [Google Scholar]
- 64.Li M., Beg A.A. Induction of necrotic-like cell death by tumor necrosis factor alpha and caspase inhibitors: Novel mechanism for killing virus-infected cells. J. Virol. 2000;74(16):7470–7477. doi: 10.1128/JVI.74.16.7470-7477.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Degterev A., Huang Z., Boyce M., et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat. Chem. Biol. 2005;1(2):112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 66.Chen D., Yu J., Zhang L. Necroptosis: An alternative cell death program defending against cancer. Biochim. Biophys. Acta. 2016;1865(2):228–236. doi: 10.1016/j.bbcan.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang J., Zhang H., Li J., et al. RIP1-mediated regulation of lymphocyte survival and death responses. Immunol. Res. 2011;51(2-3):227–236. doi: 10.1007/s12026-011-8249-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Grootjans S., Vanden Berghe T., Vandenabeele P. Initiation and execution mechanisms of necroptosis: An overview. Cell Death Differ. 2017;24(7):1184–1195. doi: 10.1038/cdd.2017.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lim S.Y., Davidson S.M., Mocanu M.M., Yellon D.M., Smith C.C. The cardioprotective effect of necrostatin requires the cyclophilin-D compo-nent of the mitochondrial permeability transition pore. Cardiovasc. Drugs Ther. 2007;21(6):467–469. doi: 10.1007/s10557-007-6067-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Koudstaal S., Oerlemans M.I., Van der Spoel T.I., et al. Necrostatin-1 alleviates reperfusion injury following acute myocardial infarction in pigs. Eur. J. Clin. Invest. 2015;45(2):150–159. doi: 10.1111/eci.12391. [DOI] [PubMed] [Google Scholar]
- 71.Kerr J.F., Wyllie A.H., Currie A.R. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer. 1972;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vinten-Johansen J., Lefer D.J., Nakanishi K., Johnston W.E., Brian C.A., Cordell A.R. Controlled coronary hydrodynamics at the time of reper-fusion reduces post ischemic injury. Coron. Artery Dis. 1992;3(11):1081–1093. doi: 10.1097/00019501-199211000-00012. [DOI] [Google Scholar]
- 73.Koshinuma S., Miyamae M., Kaneda K., Kotani J., Figueredo V.M. Combination of necroptosis and apoptosis inhibition enhances cardiopro-tection against myocardial ischemia-reperfusion injury. J. Anesth. 2014;28(2):235–241. doi: 10.1007/s00540-013-1716-3. [DOI] [PubMed] [Google Scholar]
- 74.Sciarretta S., Maejima Y., Zablocki D., Sadoshima J. The role of autophagy in the heart. Annu. Rev. Physiol. 2018;80(1):1–26. doi: 10.1146/annurev-physiol-021317-121427. [DOI] [PubMed] [Google Scholar]
- 75.Shintani T., Klionsky D.J. Autophagy in health and disease: A double-edged sword. Science. 2004;306(5698):990–995. doi: 10.1126/science.1099993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ravikumar B., Sarkar S., Davies J.E., et al. Regulation of mammalian autophagy in physiology and pathophysiology. Physiol. Rev. 2010;90(4):1383–1435. doi: 10.1152/physrev.00030.2009. [DOI] [PubMed] [Google Scholar]
- 77.Huang C., Andres A.M., Ratliff E.P., Hernandez G., Lee P., Gottlieb R.A. Preconditioning involves selective mitophagy mediated by Parkin and p62/SQSTM1. PLoS One. 2011;6(6):e20975. doi: 10.1371/journal.pone.0020975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sala-Mercado J.A., Wider J., Undyala V.V., et al. Profound cardioprotection with chloramphenicol succinate in the swine model of myocardi-al ischemia-reperfusion injury. Circulation. 2010;122(11) Suppl.:S179–S184. doi: 10.1161/CIRCULATIONAHA.109.928242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Singh K.K., Yanagawa B., Quan A., et al. Autophagy gene fingerprint in human ischemia and reperfusion. J. Thorac. Cardiovasc. Surg. 2014;147(3):1065–1072.e1. doi: 10.1016/j.jtcvs.2013.04.042. [DOI] [PubMed] [Google Scholar]
- 80.Gedik N., Thielmann M., Kottenberg E., et al. No evidence for activated autophagy in left ventricular myocardium at early reperfusion with protection by remote ischemic preconditioning in patients undergoing coronary artery bypass grafting. PLoS One. 2014;9(5):e96567. doi: 10.1371/journal.pone.0096567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jorgensen I., Miao E.A. Pyroptotic cell death defends against intracellular pathogens. Immunol. Rev. 2015;265(1):130–142. doi: 10.1111/imr.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cookson B.T., Brennan M.A. Pro-inflammatory programmed cell death. Trends Microbiol. 2001;9(3):113–114. doi: 10.1016/S0966-842X(00)01936-3. [DOI] [PubMed] [Google Scholar]
- 83.Fink S.L., Cookson B.T. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell. Microbiol. 2006;8(11):1812–1825. doi: 10.1111/j.1462-5822.2006.00751.x. [DOI] [PubMed] [Google Scholar]
- 84.Toldo S., Mauro A.G., Cutter Z., Abbate A. Inflammasome, pyroptosis, and cytokines in myocardial ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2018;315(6):H1553–H1568. doi: 10.1152/ajpheart.00158.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zuurbier C.J., Abbate A., Cabrera-Fuentes H.A., et al. Innate immunity as a target for acute cardioprotection. Cardiovasc. Res. 2019;115(7):1131–1142. doi: 10.1093/cvr/cvy304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bracey N.A., Beck P.L., Muruve D.A., et al. The Nlrp3 inflammasome promotes myocardial dysfunction in structural cardiomyopathy through interleukin-1β. Exp. Physiol. 2013;98(2):462–472. doi: 10.1113/expphysiol.2012.068338. [DOI] [PubMed] [Google Scholar]
- 87.Sandanger Ø., Gao E., Ranheim T., et al. NLRP3 inflammasome activation during myocardial ischemia reperfusion is cardioprotective. Biochem. Biophys. Res. Commun. 2016;469(4):1012–1020. doi: 10.1016/j.bbrc.2015.12.051. [DOI] [PubMed] [Google Scholar]
- 88.Yang X.M., Downey J.M., Cohen M.V., Housley N.A., Alvarez D.F., Audia J.P. The highly selective caspase-1 inhibitor VX-765 provides addi-tive protection against myocardial infarction in rat hearts when combined with a platelet inhibitor. J. Cardiovasc. Pharmacol. Ther. 2017;22(6):574–578. doi: 10.1177/1074248417702890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Audia J.P., Yang X.M., Crockett E.S., et al. Caspase-1 inhibition by VX-765 administered at reperfusion in P2Y12 receptor antagonist-treated rats provides long-term reduction in myocardial infarct size and preservation of ventricular function. Basic Res. Cardiol. 2018;113(5):32. doi: 10.1007/s00395-018-0692-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xie Y., Hou W., Song X., et al. Ferroptosis: Process and function. Cell Death Differ. 2016;23(3):369–379. doi: 10.1038/cdd.2015.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dobsák P., Siegelova J., Wolf J.E., et al. Prevention of apoptosis by deferoxamine during 4 hours of cold cardioplegia and reperfusion: In vitro study of isolated working rat heart model. Pathophysiology. 2002;9(1):27–32. doi: 10.1016/S0928-4680(02)00054-8. [DOI] [PubMed] [Google Scholar]
- 92.Paraskevaidis I.A., Iliodromitis E.K., Vlahakos D., et al. Deferoxamine infusion during coronary artery bypass grafting ameliorates lipid peroxidation and protects the myocardium against reperfusion injury: Immediate and long-term significance. Eur. Heart J. 2005;26(3):263–270. doi: 10.1093/eurheartj/ehi028. [DOI] [PubMed] [Google Scholar]
- 93.Chatziathanasiou G.N., Nikas D.N., Katsouras C.S., et al. Combined intravenous treatment with ascorbic acid and desferrioxamine to reduce myocardial reperfusion injury in an experimental model resembling the clinical setting of primary PCI. Hellenic J. Cardiol. 2012;53(3):195–204. [PubMed] [Google Scholar]
- 94.Kawada T., Akiyama T., Li M., et al. Acute arterial baroreflex-mediated changes in plasma catecholamine concentrations in a chronic rat model of myocardial infarction. Physiol. Rep. 2016;4(15):e12880. doi: 10.14814/phy2.12880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Luo D., Hu H., Qin Z., et al. Stimulation of ganglionated plexus attenuates cardiac neural remodeling and heart failure progression in a ca-nine model of acute heart failure post-myocardial infarction. Auton. Neurosci. 2017;208:73–79. doi: 10.1016/j.autneu.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 96.Fukui Y., Nozawa T., Ihori H., et al. Nicorandil attenuates ischemia-reperfusion injury via inhibition of norepinephrine release from cardiac sympathetic nerve terminals. Int. Heart J. 2017;58(5):787–793. doi: 10.1536/ihj.16-391. [DOI] [PubMed] [Google Scholar]
- 97.Minatoguchi S., Uno Y., Kariya T., et al. Cross-talk among noradrenaline, adenosine and protein kinase C in the mechanisms of ischemic preconditioning in rabbits. J. Cardiovasc. Pharmacol. 2003;41(Suppl. 1):S39–S47. [PubMed] [Google Scholar]
- 98.Schäfer U., Kurz T., Jain D., et al. Impaired coronary flow and left ventricular dysfunction after mechanical recanalization in acute myocar-dial infarction: Role of neurohumoral activation? Basic Res. Cardiol. 2002;97(5):399–408. doi: 10.1007/s003950200049. [DOI] [PubMed] [Google Scholar]
- 99.Stubbs P.J., Laycock J., Alaghband-Zadeh J., Carter G., Noble M.I. Circulating stress hormone and insulin concentrations in acute coronary syndromes: Identification of insulin resistance on admission. Clin. Sci. (Lond.) 1999;96(6):589–595. doi: 10.1042/CS19980350. [DOI] [PubMed] [Google Scholar]
- 100.Johansson P.I., Bro-Jeppesen J., Kjaergaard J., Wanscher M., Hassager C., Ostrowski S.R. Sympathoadrenal activation and endothelial damage are inter correlated and predict increased mortality in patients resuscitated after out-of-hospital cardiac arrest. a post Hoc sub-study of pa-tients from the TTM-trial. PLoS One. 2015;10(3):e0120914. doi: 10.1371/journal.pone.0120914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Reimer K.A., Rasmussen M.M., Jennings R.B. Reduction by propranolol of myocardial necrosis following temporary coronary artery occlu-sion in dogs. Circ. Res. 1973;33(3):353–363. doi: 10.1161/01.RES.33.3.353. [DOI] [PubMed] [Google Scholar]
- 102.Ishikawa I., Hollenberg N.K. Blockade of the systemic and renal vascular actions of angiotensisn II with the 1-sar, 8-ala analogue in the rat. Life Sci. 1975;17(1):121–129. doi: 10.1016/0024-3205(75)90247-7. [DOI] [PubMed] [Google Scholar]
- 103.MacLean M.R., Randall M.D., Hiley C.R. Effects of moderate hypoxia, hypercapnia and acidosis on haemodynamic changes induced by endothelin-1 in the pithed rat. Br. J. Pharmacol. 1989;98(3):1055–1065. doi: 10.1111/j.1476-5381.1989.tb14638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yang X.P., Madeddu P., Micheletti R., et al. Effects of intravenous endothelin on hemodynamics and cardiac contractility in conscious Milan normotensive rats. J. Cardiovasc. Pharmacol. 1991;17(4):662–669. doi: 10.1097/00005344-199104000-00021. [DOI] [PubMed] [Google Scholar]
- 105.Ibarra-Lara L., Sánchez-Aguilar M., Sánchez-Mendoza A., et al. Fenofibrate therapy restores antioxidant protection and improves myocar-dial insulin resistance in a rat model of metabolic syndrome and myocardial ischemia: The role of angiotensin II. Molecules. 2016;22(1):E31. doi: 10.3390/molecules22010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hadi N.R., Al-Amran F.G., Hussien Y.A., Al-Yasiri I.K., Al-Turfy M. The cardioprotective potential of valsartan in myocardial ischaemia reperfusion injury. Cent. Eur. J. Immunol. 2015;40(2):159–166. doi: 10.5114/ceji.2015.52829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Di Pasquale P., Paterna S., Parrinello G., et al. Captopril does not affect plasma endothelin-1 during thrombolysis and reperfusion. Int. J. Cardiol. 1995;51(2):131–135. doi: 10.1016/0167-5273(95)02418-V. [DOI] [PubMed] [Google Scholar]
- 108.Homma S., Kimura T., Sakai S., et al. Calcitonin gene-related peptide protects the myocardium from ischemia induced by endothelin-1: Intravital microscopic observation and (31)P-MR spectroscopic studies. Life Sci. 2014;118(2):248–254. doi: 10.1016/j.lfs.2014.02.024. [DOI] [PubMed] [Google Scholar]
- 109.Singh A.D., Amit S., Kumar O.S., Rajan M., Mukesh N. Cardioprotective effects of bosentan, a mixed endothelin type A and B receptor antag-onist, during myocardial ischaemia and reperfusion in rats. Basic Clin. Pharmacol. Toxicol. 2006;98(6):604–610. doi: 10.1111/j.1742-7843.2006.pto_405.x. [DOI] [PubMed] [Google Scholar]
- 110.Matzinger P. The danger model: A renewed sense of self. Science. 2002;296(5566):301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 111.Vilahur G., Badimon L. Ischemia/reperfusion activates myocardial innate immune response: The key role of the toll-like receptor. Front. Physiol. 2014;5:496. doi: 10.3389/fphys.2014.00496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Faure E., Equils O., Sieling P.A., et al. Bacterial lipopolysaccharide activates NF-kappaB through toll-like receptor 4 (TLR-4) in cultured human dermal endothelial cells. Differential expression of TLR-4 and TLR-2 in endothelial cells. J. Biol. Chem. 2000;275(15):11058–11063. doi: 10.1074/jbc.275.15.11058. [DOI] [PubMed] [Google Scholar]
- 113.Frantz S., Kobzik L., Kim Y.D., et al. Toll4 (TLR4) expression in cardiac myocytes in normal and failing myocardium. J. Clin. Invest. 1999;104(3):271–280. doi: 10.1172/JCI6709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Brikos C., O’Neill L.A. Signalling of toll-like receptors. Handb. Exp. Pharmacol. 2008;183(183):21–50. doi: 10.1007/978-3-540-72167-3_2. [DOI] [PubMed] [Google Scholar]
- 115.Arslan F., Keogh B., McGuirk P., Parker A.E. TLR2 and TLR4 in ischemia reperfusion injury. Mediators Inflamm. 2010;2010:704202. doi: 10.1155/2010/704202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lin L., Knowlton A.A. Innate immunity and cardiomyocytes in ischemic heart disease. Life Sci. 2014;100(1):1–8. doi: 10.1016/j.lfs.2014.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sakata Y., Dong J.W., Vallejo J.G., et al. Toll-like receptor 2 modulates left ventricular function following ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2007;292(1):H503–H509. doi: 10.1152/ajpheart.00642.2006. [DOI] [PubMed] [Google Scholar]
- 118.Favre J., Musette P., Douin-Echinard V., et al. Toll-like receptors 2-deficient mice are protected against postischemic coronary endothelial dysfunction. Arterioscler. Thromb. Vasc. Biol. 2007;27(5):1064–1071. doi: 10.1161/ATVBAHA.107.140723. [DOI] [PubMed] [Google Scholar]
- 119.Boyd J.H., Mathur S., Wang Y., Bateman R.M., Walley K.R. Toll-like receptor stimulation in cardiomyoctes decreases contractility and initiates an NF-kappaB dependent inflammatory response. Cardiovasc. Res. 2006;72(3):384–393. doi: 10.1016/j.cardiores.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 120.Timmers L., Sluijter J.P., van Keulen J.K., et al. Toll-like receptor 4 mediates maladaptive left ventricular remodeling and impairs cardiac function after myocardial infarction. Circ. Res. 2008;102(2):257–264. doi: 10.1161/CIRCRESAHA.107.158220. [DOI] [PubMed] [Google Scholar]
- 121.Vilahur G., Juan-Babot O., Peña E., Oñate B., Casaní L., Badimon L. Molecular and cellular mechanisms involved in cardiac remodeling after acute myocardial infarction. J. Mol. Cell. Cardiol. 2011;50(3):522–533. doi: 10.1016/j.yjmcc.2010.12.021. [DOI] [PubMed] [Google Scholar]
- 122.Ferrari J.P., Lueneberg M.E., da Silva R.L., Fattah T., Gottschall C.A.M., Moreira D.M. Correlation between leukocyte count and infarct size in ST segment elevation myocardial infarction. Arch. Med. Sci. Atheroscler. Dis. 2016;1(1):e44–e48. doi: 10.5114/amsad.2016.60759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Romson J.L., Hook B.G., Kunkel S.L., Abrams G.D., Schork M.A., Lucchesi B.R. Reduction of the extent of ischemic myocardial injury by neu-trophil depletion in the dog. Circulation. 1983;67(5):1016–1023. doi: 10.1161/01.CIR.67.5.1016. [DOI] [PubMed] [Google Scholar]
- 124.Todd R.F., III, Nadler L.M., Schlossman S.F. Antigens on human monocytes identified by monoclonal antibodies. J. Immunol. 1981;126(4):1435–1442. [PubMed] [Google Scholar]
- 125.Simpson P.J., Todd R.F., III, Fantone J.C., Mickelson J.K., Griffin J.D., Lucchesi B.R. Reduction of experimental canine myocardial reperfusion injury by a monoclonal antibody (anti-Mo1, anti-CD11b) that inhibits leukocyte adhesion. J. Clin. Invest. 1988;81(2):624–629. doi: 10.1172/JCI113364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Simpson P.J., Todd R.F., III, Mickelson J.K., et al. Sustained limitation of myocardial reperfusion injury by a monoclonal antibody that alters leukocyte function. Circulation. 1990;81(1):226–237. doi: 10.1161/01.CIR.81.1.226. [DOI] [PubMed] [Google Scholar]
- 127.Jordan J.E., Zhao Z.Q., Sato H., Taft S., Vinten-Johansen J. Adenosine A2 receptor activation attenuates reperfusion injury by inhibiting neu-trophil accumulation, superoxide generation and coronary endothelial adherence. J. Pharmacol. Exp. Ther. 1997;280(1):301–309. [PubMed] [Google Scholar]
- 128.Xu X., Zheng S., Xiong Y., et al. Adenosine effectively restores endotoxin-induced inhibition of human neutrophil chemotaxis via A1 re-ceptor-p38 pathway. Inflamm. Res. 2017;66(4):353–364. doi: 10.1007/s00011-016-1021-3. [DOI] [PubMed] [Google Scholar]
- 129.Vasilyev N., Williams T., Brennan M.L., et al. Myeloperoxidase-generated oxidants modulate left ventricular remodeling but not infarct size after myocardial infarction. Circulation. 2005;112(18):2812–2820. doi: 10.1161/CIRCULATIONAHA.105.542340. [DOI] [PubMed] [Google Scholar]
- 130.Zhao Z.Q., Corvera J.S., Halkos M.E., et al. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: Comparison with ischemic preconditioning. Am. J. Physiol. Heart Circ. Physiol. 2003;285(2):H579–H588. doi: 10.1152/ajpheart.01064.2002. [DOI] [PubMed] [Google Scholar]
- 131.Ali M., Pulli B., Courties G., et al. Myeloperoxidase inhibition improves ventricular function and remodeling afterexperimental myocardial infarction. JACC Basic Transl. Sci. 2016;1(7):633–643. doi: 10.1016/j.jacbts.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.van Hout G.P., van Solinge W.W., Gijsberts C.M., et al. Elevated mean neutrophil volume represents altered neutrophil composition and re-flects damage after myocardial infarction. Basic Res. Cardiol. 2015;110(6):58. doi: 10.1007/s00395-015-0513-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Faxon D.P., Gibbons R.J., Chronos N.A., Gurbel P.A., Sheehan F. HALT-MI Investigators The effect of blockade of the CD11/CD18 integrin receptor on infarct size in patients with acute myocardial infarction treated with direct angioplasty: The results of the HALT-MI study. J. Am. Coll. Cardiol. 2002;40(7):1199–1204. doi: 10.1016/S0735-1097(02)02136-8. [DOI] [PubMed] [Google Scholar]
- 134.Luedike P., Hendgen-Cotta U.B., Sobierajski J., et al. Cardioprotection through S-nitros(yl)ation of macrophage migration inhibitory factor. Circulation. 2012;125(15):1880–1889. doi: 10.1161/CIRCULATIONAHA.111.069104. [DOI] [PubMed] [Google Scholar]
- 135.Koga K., Kenessey A., Powell S.R., Sison C.P., Miller E.J., Ojamaa K. Macrophage migration inhibitory factor provides cardioprotection during ischemia/reperfusion by reducing oxidative stress. Antioxid. Redox Signal. 2011;14(7):1191–1202. doi: 10.1089/ars.2010.3163. [DOI] [PubMed] [Google Scholar]
- 136.Qi D., Hu X., Wu X., et al. Cardiac macrophage migration inhibitory factor inhibits JNK pathway activation and injury during ische-mia/reperfusion. J. Clin. Invest. 2009;119(12):3807–3816. doi: 10.1172/JCI39738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dai Y., Wang S., Chang S., et al. M2 macrophage-derived exosomes carry microRNA-148a to alleviate myocardial ischemia/reperfusion injury via inhibiting TXNIP and the TLR4/NF-κB/NLRP3 inflammasome signaling pathway. J. Mol. Cell. Cardiol. 2020;142:65–79. doi: 10.1016/j.yjmcc.2020.02.007. [DOI] [PubMed] [Google Scholar]
- 138.Yang Z., Day Y.J., Toufektsian M.C., et al. Myocardial infarct-sparing effect of adenosine A2A receptor activation is due to its action on CD4+ T lymphocytes. Circulation. 2006;114(19):2056–2064. doi: 10.1161/CIRCULATIONAHA.106.649244. [DOI] [PubMed] [Google Scholar]
- 139.Boag S.E., Andreano E., Spyridopoulos I. Lymphocyte communication in myocardial ischemia/reperfusion injury. Antioxid. Redox Signal. 2017;26(12):660–675. doi: 10.1089/ars.2016.6940. [DOI] [PubMed] [Google Scholar]
- 140.Ault K.A., Cannon C.P., Mitchell J., et al. Platelet activation in patients after an acute coronary syndrome: Results from the TIMI-12 trial. Thrombolysis in myocardial infarction. J. Am. Coll. Cardiol. 1999;33(3):634–639. doi: 10.1016/S0735-1097(98)00635-4. [DOI] [PubMed] [Google Scholar]
- 141.Langford E.J., Wainwright R.J., Martin J.F. Platelet activation in acute myocardial infarction and unstable angina is inhibited by nitric oxide donors. Arterioscler. Thromb. Vasc. Biol. 1996;16(1):51–55. doi: 10.1161/01.ATV.16.1.51. [DOI] [PubMed] [Google Scholar]
- 142.Mirabet M., Garcia-Dorado D., Inserte J., et al. Platelets activated by transient coronary occlusion exacerbate ischemia-reperfusion injury in rat hearts. Am. J. Physiol. Heart Circ. Physiol. 2002;283(3):H1134–H1141. doi: 10.1152/ajpheart.00065.2002. [DOI] [PubMed] [Google Scholar]
- 143.Kingma J.G., Jr, Plante S., Bogaty P. Platelet GPIIb/IIIa receptor blockade reduces infarct size in a canine model of ischemia-reperfusion. J. Am. Coll. Cardiol. 2000;36(7):2317–2324. doi: 10.1016/S0735-1097(00)01016-0. [DOI] [PubMed] [Google Scholar]
- 144.Kunichika H., Ben-Yehuda O., Lafitte S., Kunichika N., Peters B., DeMaria A.N. Effects of glycoprotein IIb/IIIa inhibition on microvascular flow after coronary reperfusion. A quantitative myocardial contrast echocardiography study. J. Am. Coll. Cardiol. 2004;43(2):276–283. doi: 10.1016/j.jacc.2003.08.040. [DOI] [PubMed] [Google Scholar]
- 145.Cohen M.V., Downey J.M. Combined cardioprotectant and antithrombotic actions of platelet P2Y12 receptor antagonists in acute coronary syndrome: Just what the doctor ordered. J. Cardiovasc. Pharmacol. Ther. 2014;19(2):179–190. doi: 10.1177/1074248413508465. [DOI] [PubMed] [Google Scholar]
- 146.Cohen M.V., Downey J.M. The impact of irreproducibility and competing protection from P2Y12 antagonists on the discovery of cardiopro-tective interventions. Basic Res. Cardiol. 2017;112(6):64. doi: 10.1007/s00395-017-0653-y. [DOI] [PubMed] [Google Scholar]
- 147.Ye Y., Birnbaum G.D., Perez-Polo J.R., Nanhwan M.K., Nylander S., Birnbaum Y. Ticagrelor protects the heart against reperfusion injury and improves remodeling after myocardial infarction. Arterioscler. Thromb. Vasc. Biol. 2015;35(8):1805–1814. doi: 10.1161/ATVBAHA.115.305655. [DOI] [PubMed] [Google Scholar]
- 148.Cohen M.V., Yang X-M., White J., Yellon D.M., Bell R.M., Downey J.M. Cangrelor-mediated cardioprotection requires platelets and sphingo-sine phosphorylation. Cardiovasc. Drugs Ther. 2016;30(2):229–232. doi: 10.1007/s10557-015-6633-2. [DOI] [PubMed] [Google Scholar]
- 149.Knapp M. Cardioprotective role of sphingosine-1-phosphate. J. Physiol. Pharmacol. 2011;62(6):601–607. [PubMed] [Google Scholar]
- 150.Kelly-Laubscher R.F., King J.C., Hacking D., et al. Cardiac preconditioning with sphingosine-1-phosphate requires activation of signal trans-ducer and activator of transcription-3. Cardiovasc. J. S. Afr. 2014;25(3):118–123. doi: 10.5830/CVJA-2014-016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Fang R., Zhang L.L., Zhang L.Z., Li W., Li M., Wen K. Sphingosine 1-phosphate postconditioning protects against myocardial ische-mia/reperfusion injury in rats via mitochondrial signaling and Akt-Gsk3β phosphorylation. Arch. Med. Res. 2017;48(2):147–155. doi: 10.1016/j.arcmed.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 152.Ferrari R., Balla C., Malagù M., et al. Reperfusion damage - a story of success, failure, and hope. Circ. J. 2017;81(2):131–141. doi: 10.1253/circj.CJ-16-1124. [DOI] [PubMed] [Google Scholar]
- 153.Neri M., Riezzo I., Pascale N., Pomara C., Turillazzi E. Ischemia/reperfusion injury following acute myocardial infarction: A critical issue for clinicians and forensic pathologists. Mediators Inflamm. 2017;2017:7018393. doi: 10.1155/2017/7018393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kaljusto M.L., Stensløkken K.O., Mori T., et al. Preconditioning effects of steroids and hyperoxia on cardiac ischemia-reperfusion injury and vascular reactivity. Eur. J. Cardiothorac. Surg. 2008;33(3):355–363. doi: 10.1016/j.ejcts.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 155.Sun W., Lu H., Lyu L., et al. Gastrodin ameliorates microvascular reperfusion injury-induced pyroptosis by regulating the NLRP3/caspase-1 pathway. J. Physiol. Biochem. 2019;75(4):531–547. doi: 10.1007/s13105-019-00702-7. [DOI] [PubMed] [Google Scholar]
- 156.Gollmann-Tepeköylü C., Graber M., Pölzl L., et al. Toll-like receptor 3 mediates ischaemia/reperfusion injury after cardiac transplantation. Eur. J. Cardiothorac. Surg. 2020;57(5):826–835. doi: 10.1093/ejcts/ezz383. [DOI] [PubMed] [Google Scholar]
- 157.Zhen W., Hui D., Wenying S., Yulong S. MicroRNA-20b-5p regulates propofol-preconditioning-induced inhibition of autophagy in hypoxia-and-reoxygenation-stimulated endothelial cells. J. Biosci. 2020;45(1):35. doi: 10.1007/s12038-020-9998-8. [DOI] [PubMed] [Google Scholar]
- 158.Araibi H., van der Merwe E., Gwanyanya A., Kelly-Laubscher R. The effect of sphingosine-1-phosphate on the endothelial glycocalyx dur-ing ischemia-reperfusion injury in the isolated rat heart. Microcirculation. 2020;27(5):e12612. doi: 10.1111/micc.12612. [DOI] [PubMed] [Google Scholar]
- 159.Wit A.L., Janse M.J. Reperfusion arrhythmias and sudden cardiac death: A century of progress toward an understanding of the mechanisms. Circ. Res. 2001;89(9):741–743. doi: 10.1161/res.89.9.741. [DOI] [PubMed] [Google Scholar]
- 160.Schwartz PJ, Stone HL. Left stellectomy in the prevention of ventricular fibrillation caused by acute myocardial ischemia in conscious dogs with anterior myocardial infarction. Circulation. 1980;62(61):1256–65. doi: 10.1161/01.CIR.62.6.1256. [DOI] [PubMed] [Google Scholar]
- 161.Bernier M., Hearse D.J., Manning A.S. Reperfusion-induced arrhythmias and oxygen-derived free radicals. Studies with “anti-free radical” interventions and a free radical-generating system in the isolated perfused rat heart. Circ. Res. 1986;58(3):331–340. doi: 10.1161/01.RES.58.3.331. [DOI] [PubMed] [Google Scholar]
- 162.Lubbe W.F., Podzuweit T., Opie L.H. Potential arrhythmogenic role of cyclic adenosine monophosphate (AMP) and cytosolic calcium over-load: Implications for prophylactic effects of beta-blockers in myocardial infarction and proarrhythmic effects of phosphodiesterase in-hibitors. J. Am. Coll. Cardiol. 1992;19(7):1622–1633. doi: 10.1016/0735-1097(92)90629-2. [DOI] [PubMed] [Google Scholar]
- 163.Rosen M.R. Cardiac arrhythmias and antiarrhythmic drugs: Recent advances in our understanding of mechanism. J. Cardiovasc. Electrophysiol. 1995;6(10 Pt 2):868–879. doi: 10.1111/j.1540-8167.1995.tb00363.x. [DOI] [PubMed] [Google Scholar]
- 164.Dhein S., Schott M., Gottwald E., Müller A., Klaus W. The contribution of neutrophils to reperfusion arrhythmias and a possible role for antiadhesive pharmacological substances. Cardiovasc. Res. 1995;30(6):881–888. doi: 10.1016/S0008-6363(95)00131-X. [DOI] [PubMed] [Google Scholar]
- 165.Antoons G., Willems R., Sipido K.R. Alternative strategies in arrhythmia therapy: Evaluation of Na/Ca exchange as an anti-arrhythmic target. Pharmacol. Ther. 2012;134(1):26–42. doi: 10.1016/j.pharmthera.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 166.van der Weg K., Prinzen F.W., Gorgels A.P. Editor’s choice- reperfusion cardiac arrhythmias and their relation to reperfusion-induced cell death. Eur. Heart J. Acute Cardiovasc. Care. 2019;8(2):142–152. doi: 10.1177/2048872618812148. [DOI] [PubMed] [Google Scholar]
- 167.Dohi T., Maehara A., Brener S.J., et al. Utility of peak creatine kinase-MB measurements in predicting myocardial infarct size, left ventricular dysfunction, and outcome after first anterior wall acute myocardial infarction (from the INFUSE-AMI trial). Am. J. Cardiol. 2015;115(5):563–570. doi: 10.1016/j.amjcard.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 168.Heusch G. Coronary microvascular obstruction: The new frontier in cardioprotection. Basic Res. Cardiol. 2019;114(6):45. doi: 10.1007/s00395-019-0756-8. [DOI] [PubMed] [Google Scholar]
- 169.Kloner R.A., Ganote C.E., Jennings R.B. The “no-reflow” phenomenon after temporary coronary occlusion in the dog. J. Clin. Invest. 1974;54(6):1496–1508. doi: 10.1172/JCI107898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Loke K.E., Woodman O.L. Preconditioning improves myocardial function and reflow, but not vasodilator reactivity, after ischaemia and reperfusion in anaesthetized dogs. Clin. Exp. Pharmacol. Physiol. 1998;25(7-8):552–558. doi: 10.1111/j.1440-1681.1998.tb02250.x. [DOI] [PubMed] [Google Scholar]
- 171.Haiyun L., Yijia L., Honggang L., Honghai W. Protective effect of total flavones from Elsholtzia blanda (TFEB) on myocardial ischemia induced by coronary occlusion in canines. J. Ethnopharmacol. 2004;94(1):101–107. doi: 10.1016/j.jep.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 172.Cecchi E., Liotta A.A., Gori A.M., et al. Relationship between blood viscosity and infarct size in patients with ST-segment elevation myocar-dial infarction undergoing primary percutaneous coronary intervention. Int. J. Cardiol. 2009;134(2):189–194. doi: 10.1016/j.ijcard.2008.01.039. [DOI] [PubMed] [Google Scholar]
- 173.Ming X., Tongshen W., Delin W., Ronghua Z. Cardioprotective effect of the compound yangshen granule in rat models with acute myocardi-al infarction. Evid. Based Complement. Alternat. Med. 2012;2012:717123. doi: 10.1155/2012/717123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sundaram V., Rothnie K., Bloom C., et al. Impact of comorbidities on peak troponin levels and mortality in acute myocardial infarction. Heart. 2020;106(9):677–685. doi: 10.1136/heartjnl-2019-315844. [DOI] [PubMed] [Google Scholar]
- 175.Koechlin L., Boeddinghaus J., Nestelberger T., et al. Performance of the ESC 0/2h-algorithm using high-sensitivity cardiac troponin I in the early diagnosis of myocardial infarction. Am. Heart J. 2021;242:132–137. doi: 10.1016/j.ahj.2021.08.008. [DOI] [PubMed] [Google Scholar]
- 176.Schneider U., Mukharyamov M., Beyersdorf F., et al. The value of perioperative biomarker release for the assessment of myocardial injury or infarction in cardiac surgery. Eur. J. Cardiothorac. Surg. 2021:ezab493. doi: 10.1093/ejcts/ezab493. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 177.Leung S.W., Charnigo R.J., Ratajczak T., et al. End-systolic circumferential strain derived from cardiac magnetic resonance feature-tracking as a predictor of functional recovery in patients with ST-segment elevation myocardial infarction. J. Magn. Reson. Imaging. 2021;54(6):2000–2003. doi: 10.1002/jmri.27772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Jensch PJ, Stiermaier T, Reinstadler SJ, et al. Prognostic relevance of peri-infarct zone measured by cardiovascular magnetic resonance in patients with ST-segment elevation myocardial infarction. Int J Cardiol. 2021. S0167-5273(21): 01785-X. Online ahead of. [DOI] [PubMed]
- 179.McCartney P.J., Berry C. Redefining successful primary PCI. Eur. Heart J. Cardiovasc. Imaging. 2019;20(2):133–135. doi: 10.1093/ehjci/jey159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Basir M.B., Lemor A., Gorgis S., et al. Vasopressors independently associated with mortality in acute myocardial infarction and cardiogenic shock. Catheter. Cardiovasc. Interv. 2021 doi: 10.1002/ccd.29895. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 181.Garcia S., Schmidt C.W., Garberich R., et al. Temporal changes in patient characteristics and outcomes in ST-segment elevation myocardial infarction 2003-2018. Catheter. Cardiovasc. Interv. 2021;97(6):1109–1117. doi: 10.1002/ccd.28901. [DOI] [PubMed] [Google Scholar]
- 182.Sambola A., Elola F.J., Buera I., et al. Sex bias in admission to tertiary-care centres for acute myocardial infarction and cardiogenic shock. Eur. J. Clin. Invest. 2021;51(7):e13526. doi: 10.1111/eci.13526. [DOI] [PubMed] [Google Scholar]
- 183.Liakopoulos O.J., Schlachtenberger G., Wendt D., et al. Early clinical outcomes of surgical myocardial revascularization for acute coronary syndromes complicated by cardiogenic shock: A Report from the North-Rhine-Westphalia surgical myocardial infarction registry. J. Am. Heart Assoc. 2019;8(10):e012049. doi: 10.1161/JAHA.119.012049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Alekseeva Y.V., Vyshlov E.V., Pavlyukova E.N., Ussov V.Y., Markov V.A., Ryabov V.V. Impact of microvascular injury various types on function of left ventricular in patients with primary myocardial infarction with ST segment elevation. Kardiologiia. 2021;61(5):23–31. doi: 10.18087/cardio.2021.5.n1500. [DOI] [PubMed] [Google Scholar]
- 185.de Waha S., Patel M.R., Granger C.B., et al. Relationship between microvascular obstruction and adverse events following primary percutane-ous coronary intervention for ST-segment elevation myocardial infarction: An individual patient data pooled analysis from seven random-ized trials. Eur. Heart J. 2017;38(47):3502–3510. doi: 10.1093/eurheartj/ehx414. [DOI] [PubMed] [Google Scholar]
- 186.Xie Z.J., Xin S.L., Chang C., et al. Combined glycoprotein IIb/IIIa inhibitor therapy with ticagrelor for patients with acute coronary syndrome. PLoS One. 2021;16(2):e0246166. doi: 10.1371/journal.pone.0246166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Shitole S.G., Srinivas V., Berkowitz J.L., et al. Hyperglycaemia, adverse outcomes and impact of intravenous insulin therapy in patients pre-senting with acute ST-elevation myocardial infarction in a socioeconomically disadvantaged urban setting: The Montefiore STEMI Regis-try. Endocrinol. Diabetes Metab. 2019;3(1):e00089. doi: 10.1002/edm2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]