Abstract
Background:
First-line therapies based on immune-checkpoint inhibitors (ICIs) significantly improved survival of metastatic renal cell carcinoma (mRCC) patients. Cabozantinib was shown to target kinases involved in immune-escape and to prolong survival in patients pre-treated with tyrosine-kinase-inhibitors (TKIs). The impact of ICIs combinations in first line on subsequent therapies is still unclear.
Methods:
This is an open label, multicenter, single arm, phase II study designed to assess activity, safety and efficacy of cabozantinib in mRCC patients progressed after an adjuvant or first line anti-Programmed Death (PD)-1/PD-Ligand (PD-L) 1-based therapy. Primary endpoint was progression free survival (PFS), secondary endpoints were overall survival (OS), objective response rate (ORR) and safety.
Results:
31 patients were included in the analysis. After a median (m) follow-up of 11.9 months, mPFS was 8.3 months (90%CI 3.9-17.4) and mOS was 13.8 months (95%CI 7.7-29.0). ORR was 37.9% with an additional 13 patients achieving disease stability. Grade 3-4 adverse events occurred in 47% of patients, including more frequently creatine phosphokinase (CPK) serum level elevation, neutropenia, hyponatremia, diarrhea, hand-food syndrome, oral mucositis and hypertension.
Conclusions:
The BREAKPOINT trial met its primary endpoint showing that cabozantinib as second line therapy after ICIs was active in mRCC. Safety profile was manageable.
Trial registration number:
NCT03463681 - A Study of CaBozantinib in Patients With Advanced or Unresectable Renal cEll cArcinoma (BREAKPOINT) - https://clinicaltrials.gov/ct2/show/NCT03463681
Keywords: Renal cell carcinoma, TKI, immune-checkpoint inhibitor, treatment sequencing, anti-VEGF, combination immunotherapy
Introduction
For decades, the inhibition of the vascular endothelial growth factor (VEGF) pathway and of the mammalian target of rapamycin has been the milestone for treating metastatic renal cell carcinoma (mRCC).1 First-line treatment with VEGF-Receptor (VEGFR) tyrosine-kinase-inhibitors (TKIs) represented the standard of care for treatment-naïve patients, irrespective of prognostic risk group.2
Recently, anti-Programmed Death (PD)-1/PD-Ligand (PD-L) 1 immune-checkpoint inhibitors combined with TKIs (IO-TKI) (i.e. pembrolizumab plus axitinib, avelumab plus axitinib, pembrolizumab plus lenvatinib, nivolumab plus cabozantinib) and anti-PD-1 plus anti-Cytotoxic T-Lymphocyte Antigen 4 (CTLA-4) combinations (IO-IO) (i.e. nivolumab plus ipilimumab) significantly improved survival of untreated mRCC patients.3-7 The trials evaluating these new therapeutic strategies showed such terrific positive results that many of the combinations have been approved in USA and Europe and included also in treatment guidelines.4-10
Although these new approaches are changing the frontline standard of care for mRCC, some patients eventually develop relapse of disease due to primary or acquired resistance with unknown biological mechanisms.1,11,12 The impact of ICIs combinations in first-line treatments on subsequent therapies is still unclear. To date few trials prospectively tested second-line treatments based on anti-VEGF-TKIs for patients with ICI-refractory disease.13-18
Cabozantinib is an oral TKI targeting multiple kinases involved in angiogenesis, cellular growth, resistance to TKIs and ICIs and pathways determining RCC tumor growth and progression (i.e. VEGFRs, MET, AXL, RET, ROS1, TYRO3, MER, KIT, TRKB, FLT3, TIE-2).19 The METEOR trial showed a significant increase in overall survival (OS) and objective response rate (ORR) in the cabozantinib arm compared to everolimus in mRCC patients progressed after at least one VEGFR-targeted therapy, thus leading to the approval of cabozantinib in this disease setting.20 More recently, the primary analysis of the CANTATA trial provided evidence of cabozantinib efficacy in second- and third-line setting in mRCC patients who received at least one previous treatment, also including an IO-IO combination.13 Cabozantinib also improved patients’ outcome in the first-line setting. In particular, in the CABOSUN phase II trial, cabozantinib improved progression free survival (PFS) compared to sunitinib in untreated mRCC cases.21 Similarly, the combination of cabozantinib and nivolumab was shown to be superior to sunitinib monotherapy in terms of PFS, OS and ORR in mRCC naïve to therapies.3
Interestingly, some recent data suggested that continuing cabozantinib beyond radiological progression in heavily pre-treated patients could prolong post-progression survival compared to changing the subsequent therapy22 and that the drug significantly reduced bone reabsorption, leading to an important therapeutic effect on bone metastases.23
Different studies demonstrated that cabozantinib may be more effective after anti PD-L1 treatment suggesting its ability to overcome resistance to PD-L1.24 This mechanism has not yet been fully identified, however, it has been demonstrated that AXL, which is inhibited by cabozantinib, could be a factor of resistance to PD-1 blockade promoting tumor immune evasion.25,26
Considering the activity of cabozantinib in overcoming drug resistance and its well demonstrated efficacy and clinical benefit in different disease settings, it may represent an ideal TKI to be used in second line setting for mRCC pretreated with ICIs.
In order to prospectively assess activity, safety and efficacy of cabozantinib in mRCC patients progressed during or after an adjuvant or after a first line anti-PD-1/PD-L1-based therapy, we designed the multicenter, single arm, phase 2 BREAKPOINT trial (NCT03463681).
Here, we report the primary analysis of activity and safety outcomes. Results of the translational research will be presented elsewhere.
Patients and methods
Eligible patients were adults with mRCC with a predominant clear-cell component and any prognostic risk score by International Metastatic Renal-Cell Carcinoma Database Consortium’s (IMDC) criteria.27 Patients should have progressed after only one previous anticancer treatment received either as adjuvant or as first-line treatment for metastatic disease. In case of adjuvant immunotherapy, the recurrence should have occurred during or up to six months after therapy completion. Permitted treatments included a PD-1/PD-L1 inhibitor monotherapy (IO) or any combination with an angiogenesis inhibitor other than cabozantinib (IO-TKI combo) or anti-CTLA-4 (IO-IO combo). Patients with non-symptomatic brain metastases were eligible. Other key inclusion criteria were a Karnofsky performance status ⩾ 70%, life expectancy greater than three months and an adequate bone marrow, liver and renal function. The presence of measurable disease (as per Response Evaluation Criteria in Solid Tumors - RECIST 1.1 criteria)28 and documented radiological progression were mandatory. Patients with clinically significant cardiovascular disease, uncontrolled hypertension, history of human immunodeficiency virus (HIV) infection, active hepatitis B or C, symptomatic brain metastasis, and female patients pregnant or breast-feeding were excluded. Mandatory for inclusion was the availability of a formalin-fixed, paraffin-embedded (FFPE) tumor specimen or 20 serial, freshly cut, unstained slides from the primary tumor or any metastatic site.
Enrolled patients received cabozantinib 60 mg daily until progressive disease (PD) or unacceptable toxicity. Dose reductions and/or treatment interruptions within a cycle were permitted as recommended in the Summary of Product Characteristics.19 Common Terminology Criteria for Adverse Events version 4.0 was used to define and record all adverse events (AEs) and serious adverse events (SAEs).
The BREAKPOINT trial was conducted in accordance with the International Council for Harmonization Good Clinical Practice Guidelines and the principles of the 2013 Declaration of Helsinki. Independent ethics committees at each center approved the protocol (NCT03463681); all patients provided written informed consent.
Primary and secondary endpoints
Primary endpoint: PFS was defined as the time from start of treatment to disease progression or death, whichever occurred first.
Secondary endpoints: ORR was defined as the proportion of patients achieving a complete response (CR) or partial response (PR) to cabozantinib according to RECIST 1.1 criteria. OS was defined as time from start of treatment to death by any cause. To assess disease response, tumor assessments were performed by computed tomography (CT) or magnetic resonance imaging (MRI) at screening and every 12 weeks from the date of randomization thereafter. Bone-scan was performed if clinically indicated. Response and progression were evaluated by the investigators using the RECIST 1.1 criteria. To monitor AEs/SAEs, clinical evaluation and laboratory tests for hematology and chemistry were collected every four weeks throughout the treatment period. Study drug exposure was defined as the time from first cabozantinib intake to discontinuation.
Exploratory endpoints
Exploratory endpoints included the evaluation of PD-L1 expression by immunohistochemistry in tumor samples obtained from previous nephrectomy or archival/fresh biopsy, the monitoring of the immunological signature of tumor cells and the state of circulating immune cells to assess the modulating activity of cabozantinib on local and systemic tumor immunity. Finally, the trial aimed to explore bone formation markers (bone-specific alkaline phosphatase, procollagen type I N-terminal propeptide, osteoprotegerin) and bone reabsorption markers (carboxy-terminal collagen crosslinks, tartrate-resistant acid phosphatase 5b, receptor activator of nuclear factor kappa-Β ligand) in patients with or without bone involvement. Indeed, additional blood samples were collected at baseline, at day 1 of cycles 2 and 4 and at the end of treatment or disease progression to identify temporal patterns of changes on the bone turnovers biomarkers and to isolate peripheral blood mononuclear cell (PBMCs) for immune cell profiling.
Statistical analysis
By the methodology of Brookmeyer and Crowley, it was assumed that during an accrual period of 18 months and a minimum follow-up of 10 months, 49 patients would be necessary to detect an increment of the median PFS from 3.8 months (H0: median PFS < 3.8 months) to 7.4 months (H1: median PFS > 7.4 months) with a power of 90% and one-sided alpha of 5%. The large sample critical value detecting the increment of the PFS median survival time was assumed to be 5.5 months. The sample size calculation was performed using the SWOG sheet (https://stattools.crab.org/Calculators/oneNonParametricSurvival.htm).
Survival functions were estimated using the Kaplan-Meier method. Confidence intervals for the 50th percentile were based on the loglog-transformed confidence interval for the survival function. Length of follow-up was estimated using the reverse Kaplan-Meier method. ORR was estimated using exact methods (i.e. Clopper-Pearson confidence intervals). Descriptive statistics of patient demographics and clinical characteristics were reported as absolute and percentage frequencies for categorical variables and median with range for continuous variables.
To assess safety, all the AEs and SAEs reported throughout the treatment period were analyzed by type, grade and correlation to study-drug. All SAEs were also reported to the National Drug Agency, study sponsor and funder. Patients receiving at least one dose of study drug were included in the analysis of activity and safety. Each patient was counted once at the highest grade for each AE.
In order to investigate the heterogeneity of the study population in terms of drug activity and prognosis, IMDC prognostic risk group (good vs poor risk and intermediate vs poor risk) calculated at cabozantinib start and duration of first-line/adjuvant treatment were assessed as predictors. Univariable Cox and logistic regression models were used to detect and estimate their statistical association with response variables. Hazard Ratio (HR) and Odds Ratio (OR) were used as summary statistics, respectively. Fisher’s exact test was used to formally compare proportions.
When the planned length of accrual period was reached the database was locked before the primary analysis was performed. Primary analysis included all patients in the efficacy and safety population. All statistical analyses were done using the SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA). Survival curves were drawn using the Stata software version 15 (StataCorp, College Station, TX, USA).
At the beginning of 2021, due to the slowdown of the accrual rate secondary also to the outbreak of SARS-CoV-2 pandemic, the trial was amended to include also a retrospective cohort of patients treated with cabozantinib at the cancer centers part of the Italian Network for Research In Urologic-Oncology: Meet-Uro. Data collection was performed in the first four months of 2021 and included consecutive patients treated in the same time span as the prospective group until the pre-planned target population was reached. The analysis of the retrospective cohort of patients will be presented elsewhere.
Results
Between July 2018 and March 2021, 31 patients were enrolled at six Italian sites and 30 were included in the analysis. One patient was excluded due to study drop out. Baseline patients’ demographic and disease’s characteristics are summarized in Table 1. Median age was 62 years (range, 29-79), 23 patients (77%) were male and 16 (53%) had an intermediate IMDC risk score at study entry. Lung was the most prevalent site of metastases (70%, 21 patients). Nineteen subjects (63%) received the combination of ipilimumab and nivolumab as first-line therapy. Median duration of response to previous therapy with ICIs was 5.64 months (range 0.7-64.9); 24 patients (80%) discontinued first-line treatment due to disease progression, the remaining six due to AEs.
Table 1.
Patients’ demographic and disease characteristics at study entry.
| No. 30 (%) | |
|---|---|
| Median Age (years) | 62 (range, 29-79) |
| Sex | |
| Male | 23 (77) |
| Female | 7 (23) |
| Histology | |
| Clear Cell | 26 (87) |
| Papillary 1-2/Others | 4 (13) |
| Karnofsky Performance status | |
| 100% | 17 (57) |
| 80-90% | 7 (23) |
| ⩽70% | 2 (7) |
| Unknown | 4 (13) |
| IMDC score | |
| Good | 5 (17) |
| Intermediate | 16 (53) |
| Poor | 9 (30) |
| Previous therapy | |
| Pembrolizumab-Lenvatinib | 7 (23) |
| Avelumab-Axitinib | 1 (3) |
| Ipilimumab-Nivolumab | 19 (63) |
| Atezolizumab (adjuvant) | 3 (10) |
| Best response to previous therapy | |
| CR | 2 (7) |
| PR | 6 (20) |
| SD | 13 (43) |
| PD | 9 (30) |
| Site of metastasis | |
| Lung | 21 (70) |
| Liver | 4 (13) |
| Lymph nodes | 13 (43) |
| Bone | 4 (13) |
| Brain | 3 (10) |
| Adrenal | 10 (33) |
| Pancreas | 2 (7) |
| Kidney (contralateral) | 3 (10) |
| Others (peritoneum, spleen, pleura, soft tissues) | 7 (23) |
CR, complete response; IMDC, International Metastatic Renal-Cell Carcinoma Database Consortium’s criteria; PD, progressive disease; PR, partial response; SD, stable disease.
Primary and Secondary Endpoints
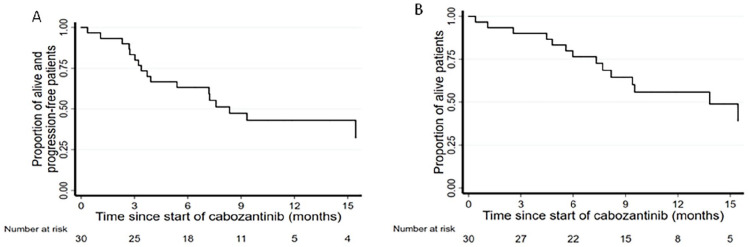
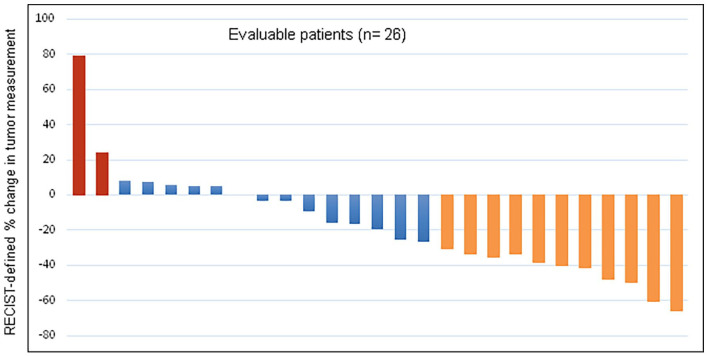
At data cut-off, 25 September 2021, after a median follow-up of 11.9 months (IQR 10.5-21.2), 47% of patients were alive (14), of whom 78% (11 subjects) were still on treatment. Estimated median duration of cabozantinib exposure was 7.3 months (range 1-21). Deaths from any causes were observed in 53% (16 patients) and 60% (18 patients) had progressed. mPFS was 8.3 months (95%CI 3.9-17.4 one-sided p-value = 0.024) (Figure 1A) and mOS was 13.8 months (95%CI 7.7-29.0) (Figure 1B). The confirmed ORR to treatment was 37.9% (95%CI: 20.7-57.7%), corresponding to 11 PR achieved with additional 13 patients (43%) with stable disease as best response (Figure 2).
Figure 1.
Kaplan-Meier curves of (A) progression free survival and (B) overall survival. Median follow-up 11.9 months.
Figure 2.
Waterfall plot of Best Response to Cabozantinib
The confirmed ORR was 37.9% (95%CI: 20.7-57.7%). 11 patients achieved a partial response to cabozantinib as best response, 13 disease stability, while 2 patients progressed. Of the four patients not evaluable, three patients died due to clinical disease progression before radiological staging.
In the subgroups analyses of survival, good- and intermediate-risk patients had a significantly longer PFS (intermediate vs poor: HR 0.19 [95%CI: 0.06-0.57]; good vs poor: HR 0.23 [95%CI: 0.06-0.95]; p-value for trend = 0.018) and significantly longer OS (intermediate vs poor: HR 0.17 [95%CI: 0.05-0.58]; good vs poor: HR 0.05 [95%CI: 0.005-0.47]; p-value for trend = 0.002). Similarly, PFS and OS were significantly longer in patients achieving a longer treatment duration with first-line/adjuvant treatment (Table 2).
Table 2.
PFS, OS and ORR according to IMDC prognostic risk group and duration of first line/adjuvant treatment.
| PFS | OS | ORR | |||||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | p-value for trend | HR (95% CI) | p-value for trend | OR (95% CI) | p-value for trend | ||
| IMDC score | Good | 0.23 (0.06-0.95) | 0.018 | 0.05 (0.005-0.47) | 0.002 | 12.00 (0.77-186.36) | 0.063 |
| Intermediate | 0.19 (0.06-0.57) | 0.17 (0.05-0.58) | 7.00 (0.69-70.74) | ||||
| Poor | 1 | ||||||
| Duration (months) of first line/adjuvant treatment | - | 0.84 ( 0.75-0.95) | 0.007 | 0.88 (0.78-1.00) | 0.049 | 1.00 (0.94-1.07) | 0.95 |
IMDC, International Metastatic Renal-Cell Carcinoma Database Consortium’s criteria; OS, overall survival; ORR, overall response rate; PFS, progression free survival.
Safety
The primary reason for study treatment discontinuation was radiographic PD (eight patients, 27%), seven patients (23%) discontinued for clinical PD or death, while two patients (7%) for reasons other than AEs or PD. One patient (3%) discontinued Cabozantinib for AEs. All the patients who discontinued therapy for radiological disease progression received subsequent systemic therapy.
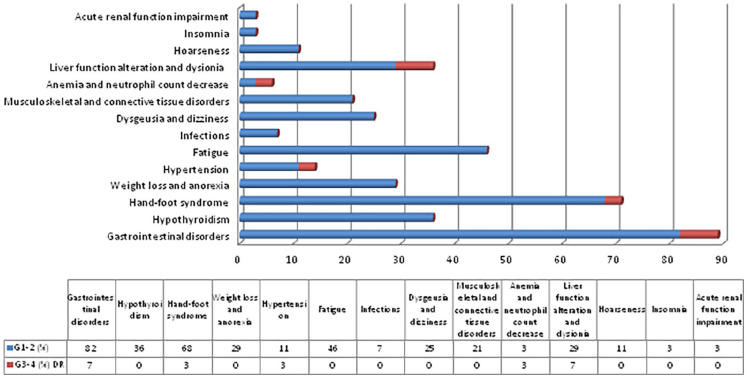
AEs of any grade were observed in 93% of patients (28/30) (Figure 3). At least one G1-2 AE was observed in 83% (25) patients, including in most of cases diarrhea, nausea, oral mucositis, dysgeusia, hand-foot syndrome, fatigue and hypothyroidism. G3-4 AEs occurred in 47% (14) patients, the 50% of which was classified as drug-related. More frequently drug-related G3-4 AEs included CPK serum levels elevation, neutropenia, hyponatriemia, diarrhea, hand-foot syndrome, mucositis and hypertension. Hyponatriemia G4 led to definitive discontinuation of drug in one patient. To manage all grade AEs, transitory withholding of Cabo was observed in 71% of patients (20 subjects of 28 presenting at least one AE) and for 14/28 patients (50%) dose reductions were needed.
Figure 3.
Drug-related adverse events observed during study drug administration.
AEs of any grade were observed in 93% of patients (28/30). G3-4 AEs occurred in 47% (14) patients, the 50% of which was classified as drug-related. (Biochemestry test alterations: serum bilirubin, calcium and sodium serum levels, CPK serum levels alterations; Gastrointestinal disorders: diarrhea, oral mucositis, nausea)
Discussion
For decades, the inhibition of angiogenesis was the main target of mRCC treatment since more than half of the cases of RCC exert the inactivation of the Von Hippel-Lindau (VHL) suppressor gene. More recent data highlighted a strong cross-talk and reciprocal influence between angiogenesis and microenvironmental immunity, redefining the therapeutic options in favor of combination strategies.29 Despite increased efficacy of novel first-line therapies,3-7 some patients still develop acquired resistance needing an effective subsequent therapy at disease relapse. To date, available data are insufficient to provide an answer to this unmet clinical need: do TKIs provide a survival benefit to patients affected by mRCC who progressed to a first-line based on ICIs?
The BREAKPOINT trial was designed prospectively to evaluate activity and safety of cabozantinib in second-line setting after ICIs. The study, planned to detect an increment of the median PFS from 3.8 to 7.4 months, met its primary endpoint: mPFS of 8.3 months (p 0.024). Our results are comparable to previous data from retrospective studies which reported mPFS ranging from 6.2 to 13.2 months with different TKIs (mainly axitinib, pazopanib, sunitinib and cabozantinib) used in second or further lines after immunotherapy.13-16,30-35
A retrospective trial reported a median time to treatment failure of 6.5 months with cabozantinib in patients who received a median of two previous line of therapy, including immunotherapy.36 Similarly, Iacovelli et al. observed that cabozantinib in third line after nivolumab achieved a mPFS of 11.5 months.37 In a prespecified subgroup analysis of the CANTATA trial presented at the 2021 ASCO Annual Meeting, cabozantinib plus placebo obtained a mPFS of 9.2 months in patients pre-treated with ICIs.13
Likewise, the mOS we also observed (13.8 months) was as expected from previous data.22-29
Within the overall population, we obtained an ORR of 37.9% with an additional 43% of disease stability, similarly to previous published data.13,30,31,33,34 In our series, three patients were not evaluable for response due to clinical PD occurrence before radiological examination and one for missing data.
Other authors reported longer survival outcomes and higher response rates within cohorts of patients receiving TKIs after IO-IO compared to TKIs after IO-TKI.33-35 In our series, a large majority of patients (63%) were treated in first line with IO-IO and only 26% received IO-TKI. Therefore, we could not use the type of previous treatment as predictor of primary and secondary endpoints. However, since the METEOR trial showed the efficacy of cabozantinib in mRCC patients progressed after at least one VEGFR-targeted therapy20 it could be of interest to further evaluate drug efficacy and activity after IO-TKI.
As expected, IMDC prognostic risk group was statistically associated with survival outcomes, but not ORR, thus confirming its role as clinical prognostic factor without any predictive value over response to treatment in this setting. Also the duration of previous ICIs-based treatment was statistically associated both to PFS and OS.
AEs observed were similar to previously established toxicity profile of the drug and were all manageable. Compared to the METEOR trial that reported G3 or higher-grade AEs in 71% of patients in the cabozantinib arm, of which a 39% were classified as drug related,20 we observed a lower rate of severe toxicity: G3-4 AEs in 47% of subjects, with drug-causality reported in 50% of cases. Therefore, the previous treatment with ICIs did not seem to modify the safety profile of cabozantinib that was confirmed to be manageable.
The ongoing prospective phase II Cabopoint trial, evaluating the efficacy and safety of cabozantinib as a second-line treatment in patients with advanced renal cell carcinoma progressed after a checkpoint inhibitor, will hopefully provide additional data for second line clinical decision-making post first line ICI treatment.17
Different from previous studies, the BREAKPOINT trial evaluated the role of cabozantinib after only one line of immunotherapy and included a prospective cohort of patients, thus showing prospectively the efficacy of cabozantinib in second line for mRCC. This is a strength of the present study. The small sample size and the slow accrual are limits of the present trial, both due to the unavailability of IO-TKI or IO-IO combinations in Italy at the time of BREAKPOINT study start and, then, to the outbreak of the SARS-CoV-2 pandemic in 2020, that strongly limited patients access to medical treatments. As a further consequence, the study was amended to include a retrospective cohort of patients, thus, the prospective cohort analysis was performed on 30 patients over the 49 pre-planned. This represents another limitation to the analysis.
However, to the best of our knowledge, the BREAKPOINT study is the first trial showing prospective activity and safety of cabozantinib after an adjuvant or first-line ICIs based immunotherapy in patients with mRCC. The study met its primary endpoint achieving a mPFS of 8.3 months. AEs were manageable with no new safety signals.
Acknowledgments
We deeply thank all the patients and their families who participated in the trial. A special thanks to all the investigators and their teams who contributed in each phase of study conduction. We thank also Ipsen as funder of the project.
Footnotes
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: GP has served as consultant/advisory board member for Astellas, AstraZeneca, Bayer, BMS, EISAI, Ipsen, Janssen, Merck, MSD, Novartis, Pfizer. He received research grants from Astellas, Ipsen and Novartis. UDG has served as consultant/advisory board member for Astellas, Bayer, BMS, Ipsen, Janssen, MSD, Novartis, Pfizer, Roche and Sanofi; has received travel support from BMS, Ipsen, Janssen and Pfizer; and has received research funding from AstraZeneca, Roche and Sanofi (Inst). SB received honoraria as a speaker at scientific events and advisory role by Bristol-Myers Squibb (BMS), Pfizer; MSD, Ipsen, Roche, Eli-Lilly, AstraZeneca and Novartis; he also received research funding from Novartis. RI reported participation to advisory board Astellas, BMS, Ipsen, Janssen, Merk, MSD, Pfizer, Sanofi. Consultancy: Astellas, Ipsen, Merk, MSD, Pfizer. Support to research activity (inst): BMS, Pfizer. CC has served as consultant/advisory board member for Janssen, Ipsen and BMS. CM received honoraria as advisory board member or speaker at scientific events by Astellas, BMS, Ipsen, Janssen, MSD, Pfizer, she also received travel support from BMS, Ipsen, Janssen. SS has served as consultant/advisory board member for MSD, BMS, Janssen, Ipsen, Novartis, Pfizer, Astellas. FDB received honoraria as advisory board member or speaker at scientific events by Roche, EMD Serono, NMS Nerviano Medical Science, Sanofi, MSD, Novartis, Incyte, BMS, Menarini, Healthcare Research & Pharmacoepidemiology, Merck Group, ACCMED, Nadirex, Pfizer, Servier, AMGEN, Dephaforum. EV reported receiving honoraria for consulting or advisory role from Janssen, Ipsen, MSD, Merck, Pfizer, Novartis MC, CP, LP, PS, VG, DB, FN, FC, CB, LD, AC, AG, SP declare no potential competing interests.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The trial was designed by academic investigators and was funded by Ipsen. Study drug was provided by the funder for the prospective cohort. The funder was given the opportunity to review the manuscript for scientific accuracy. The authors were free to accept or decline any feedback.
Ethics approval and consent to participate: The BREAKPOINT trial was conducted in accordance with the International Council for Harmonization Good Clinical Practice Guidelines and the principles of the 2013 Declaration of Helsinki. Independent ethics committees at each center approved the protocol (NCT03463681); all patients provided written informed consent.
ORCID iDs: Pierangela Sepe  https://orcid.org/0000-0002-0645-7293
https://orcid.org/0000-0002-0645-7293
Sebastiano Buti  https://orcid.org/0000-0003-0876-0226
https://orcid.org/0000-0003-0876-0226
Angela Gernone  https://orcid.org/0000-0002-8218-408X
https://orcid.org/0000-0002-8218-408X
References
- 1. Sepe P, Mennitto A, Corti F, et al. Immunotherapeutic targets and therapy for renal cell carcinoma. Immunotargets Ther 2020; 9: 273-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Motzer RJ, Hutson TE, Cella D, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med 2013; 369: 722-731. [DOI] [PubMed] [Google Scholar]
- 3. Choueiri TK, Powles T, Burotto M, et al. 696O_PR Nivolumab + cabozantinib vs sunitinib in first-line treatment for advanced renal cell carcinoma: First results from the randomized phase III CheckMate 9ER trial. Ann Oncol 2020; 31: S1159. [Google Scholar]
- 4. Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med 2018; 378: 1277-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 2019; 380: 1103-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 2019; 380: 1116-1127. [DOI] [PubMed] [Google Scholar]
- 7. Motzer R, Alekseev B, Rha SY, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med 2021; 384: 1289-1300. [DOI] [PubMed] [Google Scholar]
- 8. Escudier B, Porta C, Schmidinger M, et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-updagger. Ann Oncol 2019; 30: 706-720. [DOI] [PubMed] [Google Scholar]
- 9. Choueiri TK, Powles T, Burotto M, et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 2021; 384: 829-841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. NCCN. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Kidney Cancer - Version 3.2023 — September 22, 2022. 2022. [DOI] [PMC free article] [PubMed]
- 11. Rassy E, Flippot R, Albiges L. Tyrosine kinase inhibitors and immunotherapy combinations in renal cell carcinoma. Ther Adv Med Oncol 2020; 12: 1758835920907504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McKay RR, Bossé D, Choueiri TK. Evolving systemic treatment landscape for patients with advanced renal cell carcinoma. J Clin Oncol 2018; 36: 3615-3623. [DOI] [PubMed] [Google Scholar]
- 13. Tannir NM, Agarwal N, Porta C, et al. CANTATA: Primary analysis of a global, randomized, placebo (Pbo)-controlled, double-blind trial of telaglenastat (CB-839) + cabozantinib versus Pbo + cabozantinib in advanced/metastatic renal cell carcinoma (mRCC) patients (pts) who progressed on immune checkpoint inhibitor (ICI) or anti-angiogenic therapies. J Clin Oncol 2021; 39: 4501-4501. [Google Scholar]
- 14. Lee CH, Shah AY, Rasco D, et al. Lenvatinib plus pembrolizumab in patients with either treatment-naive or previously treated metastatic renal cell carcinoma (Study 111/KEYNOTE-146): a phase 1b/2 study. Lancet Oncol 2021; 22: 946-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ornstein MC, Pal SK, Wood LS, et al. Individualised axitinib regimen for patients with metastatic renal cell carcinoma after treatment with checkpoint inhibitors: a multicentre, single-arm, phase 2 study. Lancet Oncol 2019; 20: 1386-1394. [DOI] [PubMed] [Google Scholar]
- 16. Grande E, Gordoa TA, Torras OR, et al. INMUNOSUN-SOGUG trial: A prospective phase II study to assess the efficacy and safety of sunitinib as second-line (2L) treatment in patients (pts) with metastatic renal cell cancer (RCC) who received immunotherapy-based combination upfront. J Clin Oncol 2020; 38: 5060-5060. [Google Scholar]
- 17. Albiges L, Schmidinger M, Taguieva-Pioger N, et al. CaboPoint: a phase II study of cabozantinib as second-line treatment in patients with metastatic renal cell carcinoma. Future Oncol 2022; 18: 915-926. [DOI] [PubMed] [Google Scholar]
- 18. Navani V, Wells C, Boyne DJ, et al. CABOSEQ: The efficacy of cabozantinib post up-front immuno-oncology combinations in patients with advanced renal cell carcinoma: Results from the International Metastatic Renal Cell Carcinoma Database Consortium (IMDC). J Clin Oncol 2022; 40: 318-318. [Google Scholar]
- 19. CABOMETYX. INN-Cabozantinib, 2016, https://www.ema.europa.eu/en/medicines/human/EPAR/cabometyx
- 20. Choueiri TK, Escudier B, Powles T, et al. Cabozantinib versus everolimus in advanced renal-cell carcinoma. New Eng J Med 2015; 373: 1814-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Choueiri TK, Hessel C, Halabi S, et al. Cabozantinib versus sunitinib as initial therapy for metastatic renal cell carcinoma of intermediate or poor risk (Alliance A031203 CABOSUN randomised trial): Progression-free survival by independent review and overall survival update. Eur J Cancer 2018; 94: 115-125. 20180320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mennitto A, Zattarin E, Di Maio M, et al. Cabozantinib beyond progression improves survival in advanced renal cell carcinoma patients: the CABEYOND study (Meet-URO 21). Expert Rev Anticancer Ther 2022; 22: 115-121. 20211129. [DOI] [PubMed] [Google Scholar]
- 23. Ratta R, Verzoni E, Mennitto A, et al. Effects of cabozantinib on bone turnover markers in real-world metastatic renal cell carcinoma. Tumori 2021; 107: 542-549. [DOI] [PubMed] [Google Scholar]
- 24. Vano YA, Phan L, Gravis G, et al. Cabozantinib-nivolumab sequence in metastatic renal cell carcinoma: The CABIR study. Int J Cancer 2022; 151: 1335-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Son HY, Jeong HK. Immune evasion mechanism and AXL. Front Oncol 2021; 11: 756225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Terry S, Dalban C, Rioux-Leclercq N, et al. Association of AXL and PD-L1 expression with clinical outcomes in patients with advanced renal cell carcinoma treated with PD-1 blockade. Clin Cancer Res 2021; 27: 6749-6760. [DOI] [PubMed] [Google Scholar]
- 27. Heng DYC, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor–targeted agents: results from a large, multicenter study. J Clin Oncol 2009; 27: 5794-5799. [DOI] [PubMed] [Google Scholar]
- 28. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228-247. [DOI] [PubMed] [Google Scholar]
- 29. Mennitto A, Huber V, Ratta R, et al. Angiogenesis and immunity in renal carcinoma: can we turn an unhappy relationship into a happy marriage? J Clin Med 2020; 9: 20200328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Auvray M, Auclin E, Barthelemy P, et al. Second-line targeted therapies after nivolumab-ipilimumab failure in metastatic renal cell carcinoma. Eur J Cancer 2019; 108: 33-40. [DOI] [PubMed] [Google Scholar]
- 31. Shah AY, Kotecha RR, Lemke EA, et al. Outcomes of patients with metastatic clear-cell renal cell carcinoma treated with second-line VEGFR-TKI after first-line immune checkpoint inhibitors. Eur J Cancer 2019; 114: 67-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Graham J, Shah AY, Wells JC, et al. Outcomes of patients with metastatic renal cell carcinoma treated with targeted therapy after immuno-oncology checkpoint inhibitors. Eur Urol Oncol 2021; 4: 102-111. [DOI] [PubMed] [Google Scholar]
- 33. Barata PC, De Liano AG, Mendiratta P, et al. The efficacy of VEGFR TKI therapy after progression on immune combination therapy in metastatic renal cell carcinoma. Brit J Cancer 2018; 119: 160-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nadal R, Amin A, Geynisman DM, et al. Safety and clinical activity of vascular endothelial growth factor receptor (VEGFR)-tyrosine kinase inhibitors after programmed cell death 1 inhibitor treatment in patients with metastatic clear cell renal cell carcinoma. Ann Oncol 2016; 27: 1304-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dudani S, Graham J, Wells JC, et al. First-line immuno-oncology combination therapies in metastatic renal-cell carcinoma: Results from the International Metastatic Renal-cell Carcinoma Database Consortium. Eur Urol 2019; 76: 861-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McGregor BA, Lalani AA, Xie W, et al. Activity of cabozantinib after immune checkpoint blockade in metastatic clear-cell renal cell carcinoma. Eur J Cancer 2020; 135: 203-210. [DOI] [PubMed] [Google Scholar]
- 37. Iacovelli R, Ciccarese C, Facchini G, et al. Cabozantinib after a previous immune checkpoint inhibitor in metastatic renal cell carcinoma: a retrospective multi-institutional analysis. Targeted Oncol 2020; 15: 495-501. [DOI] [PubMed] [Google Scholar]