Abstract
Background:
Vaginal cancer is a rare disease for which prospective randomized trials do not exist. We aimed to assess survival outcomes, patterns of recurrence, prognostic factors, and toxicity in the curative treatment using image-guided radiotherapy (RT).
Methods:
In this retrospective review, we identified 53 patients who were treated at a single center with external beam radiotherapy and brachytherapy with or without concomitant chemotherapy from 2000 to 2021.
Results:
With a median follow-up of 64.5 months, the Kaplan-Meier 2-, 5-, and 7-year overall survival (OS) was found to be 74.8%, 62.8%, and 58.9%, respectively. Local and distant control were 67.8%, 65.0%, and 65.0% and 74.4%, 62.6%, and 62.6% at 2, 5, and 7 years, respectively. In univariate Cox proportional hazards ratio analysis, OS was significantly correlated to FIGO stage (hazard ratio [HR] 1.78, p = 0.042), postoperative RT (HR 0.41, p = 0.044), and concomitant chemotherapy (HR 0.31, p = 0.009). Local control rates were superior when an equivalent dose in 2-Gy fractions (EQD2) of ⩾65 Gy was delivered (HR 0.216, p = 0.028) and with the use of concurrent chemotherapy (HR 0.248, p = 0.011). Not surprisingly, local control was inferior for patients with a higher TNM stage (HR 3.303, p = 0.027). Minimal toxicity was observed with no patients having documentation of high-grade toxicity (CTCAE grade 3+).
Conclusion:
In treatment of vaginal cancer, high-dose RT in combination with brachytherapy is well tolerated and results in effective local control rates, which significantly improve with an EQD2(α/β=10) ⩾65 Gy. Multivariate analyses revealed concomitant chemotherapy was a positive prognostic factor for overall and progression-free survival.
Keywords: Squamous cell carcinoma of the vagina, vaginal neoplasms, EQD2, high-dose-rate brachytherapy, carbon ions
Introduction
Primary cancer of the vagina represents a rare tumor entity, which accounts for fewer than 1% to 2% of all gynecologic malignancies. It is primarily a disease of elderly women, with a mean age at diagnosis of 65 years.1,2 The most common vaginal tumor type is squamous cell carcinoma, but other rarer subtypes can be observed and include adenocarcinoma, adenoid cystic carcinoma, melanoma, and sarcoma.3 Squamous cell carcinoma is strongly associated with human papilloma virus and usually manifests from intraepithelial precursor lesion.4 Bleeding and vaginal discharge are the most commonly described clinical symptoms of vaginal cancer at the time of presentation.5,6 Risk factors and clinical presentation may differ depending on the histologic subtype, with pathogenesis and genetic and molecular features not being fully understood, especially in rare subtypes of melanoma or adenoid cystic carcinoma.7,8 Due to the rarity of the disease and the lack of randomized prospective data, the optimal treatment approach is unclear. Consequently, many of the recommended management approaches are extrapolated from more common gynecologic malignancies such as cervical, vulvar, and anal cancer.
Surgical resection is typically the treatment of choice for early stage vaginal cancers in favorable anatomical locations and size, if technically feasible.9 In contrast, definitive radiotherapy (RT) as an organ-sparing approach is preferred for locally advanced or medically inoperable early-stage tumors. Postoperative RT should be considered in cases of resected locally advanced, high-risk tumors or in the context of positive surgical margins.10,11 From a radiation standpoint, external beam RT (EBRT) in combination with intracavitary or interstitial brachytherapy is utilized, but regimens must be individualized based on patient and tumor characteristics.
Brachytherapy allows for a high-dose conformal radiation boost to the residual tumor and has been shown to result in superior overall survival (OS) compared to EBRT alone.12-15 Nevertheless, notable toxicities can be observed, including vaginal stenosis (in up to 46% of patients),16 dryness, and fistula. In addition, urinary and gastrointestinal toxicities such as mucositis, proctitis, obstruction, incontinence, and pain are frequently reported and are dose dependent.17
The use of radiosensitizing concurrent chemotherapy has been shown to provide superior local control (LC) and disease-free survival particularly for advanced stage tumors. This improvement in oncologic outcome after administering chemotherapy does not appear to dramatically worsen toxicity.17-19 Similar to the treatment of mucosal squamous cell carcinomas, cisplatin is commonly employed in vaginal tumors due to its radiosensitizing potential.20
Historically, retrospective studies describe a substantial risk of treatment failure in vaginal carcinomas distinct from cervical cancer, with a 2-year LC rate of only 68.8%21 and a 2- and 5-year OS of 55.3% and 45%–66%, respectively.19,21,22 FIGO stage and tumor size >4 cm are considered the most substantial prognostic factors.6,23
Given the lack of prospective data, the evidence for determining the optimal treatment strategy is low. Previous retrospective data are heterogenous and include a large variety of RT techniques, especially before 2000. The aim of the current study is to analyze clinical outcome, potential prognostic factors, and treatment-related toxicity following modern radiotherapeutic strategies with or without brachytherapy in the treatment of vaginal cancer.
Methods
Patient and treatment characteristics
In this single-center retrospective review, 53 patients (median age 66 ± 13.3 years) who were treated with RT or radiochemotherapy for biopsy-proven vaginal cancer between May 2000 and January 2021 were identified. The analysis was approved by the ethics committee of the University Hospital Heidelberg (S-453/2021). Patient and treatment characteristics are shown in Tables 1 and 2.
Table 1.
Patient characteristics.
| Characteristics | Values |
|---|---|
| Total | 53 |
| Age, y | 66 (31–92) |
| Karnofsky Performance Status, % | 80 (60–100) |
| FIGO stage | |
| I/II | 26 (49) |
| III/IV | 27 (51) |
| Nodal stage | |
| N0 | 17 (32) |
| N+ | 36 (68) |
| Tumor type | |
| Squamous cell carcinoma | 41 (77) |
| Adenocarcinoma | 7 (13) |
| Other | 5 (10) |
| Tumor size, mm | 31 (12–85) |
| BMI | 24.3 (17.9–43.6) |
| Previous hysterectomy | |
| No | 23 (43) |
| Yes | 30 (57) |
| Treated lesion | |
| Primary | 47 (89) |
| Recurrent | 6 (11) |
BMI: body mass index; FIGO: International Federation of Obstetrics and Gynecology.
Values are n (%) or median (range).
Table 2.
Treatment characteristics.
| Characteristics | Values |
|---|---|
| Concomitant chemotherapy | |
| RT only | 25 (47) |
| Radiochemotherapy | 28 (53) |
| RT concept | |
| Definitive | 30 (57) |
| Postoperative | 23 (43) |
| RT technique | |
| IMRT | 33 (62) |
| 3D | 15 (28) |
| AP/PA | 1 (2) |
| BT only | 4 (8) |
| Total dose in EQD2 (α/β = 10), Gy | 62.0 (28.4–81.6) |
| Total dose in EQD2 (α/β = 3), Gy | 64.4 (37.4–91.6) |
| Boost | |
| HDR-BT | 27 |
| C12 ions | 2 |
| No boost | 24 |
AP/PA: anterior-posterior/posterior-anterior; BT: brachytherapy; C12: carbon ion; EQD2: equivalent dose in 2 Gy fractions; HDR-BT: high dose rate brachytherapy; IMRT: intensity-modulated radiotherapy; RT: radiotherapy.
Values are n (%) or median (range).
Staging included gynecologic assessment with ultrasound, computed tomography (CT), or magnetic resonance imaging (MRI) of the abdomen/pelvis as well as X-ray or CT of the thorax. In addition, for cases of suspected bowel or bladder infiltration, cystoscopy, rectoscopy, or colposcopy was performed. Patients were classified according to the FIGO staging system (International Federation of Obstetrics and Gynecology)24 and American Joint Committee on Cancer 8th edition staging system. Squamous cell carcinoma was present in the majority of patients (n = 41 [77%]). The second most common tumor type identified was adenocarcinoma (n = 7 [13%]) with the remaining neoplasms being adenoid cystic carcinoma (n = 2 [4%]), malignant melanoma (n = 2 [4%]), and neuroendocrine carcinoma (n = 1 [2%]). From a staging standpoint, FIGO stage distribution was as follows: I (n = 14 [26%]), II (n = 12 [23%]), III (n = 14 [26%]), and IV (n = 13 [25%]).
Treatment
The majority of patients underwent definitive RT treatment (n = 30 [57%]), with the remainder treated in an adjuvant setting. For those patients who received upfront surgery, a radical colpectomy was most often performed (n = 23) with simultaneous bilateral adnexectomy (n = 4). The remaining surgeries included abdominal hysterectomy with upper vaginectomy and bilateral adnexectomy (n = 2) and anterior pelvic exenteration with complete colpectomy and colonic couch reconstruction (n = 2). Radical pelvic lymph node dissection was performed in 18 cases; sentinel biopsy was only utilized in one patient. Six patients (11%) were treated in a salvage setting following surgery at a median time to progression of 41.9 months (range 3.4–59.5 months).
For EBRT, a gross tumor volume (GTV) was delineated on available planning scans. The GTV was then enlarged by a 1–2 cm margin to form the clinical target volume (CTV), which further included the entire vagina and paravaginal tissue. A planning target volume (PTV) margin of 0.5 to 1.5 cm was added dictated by the radiation technique and image-guided RT. Total radiation dose was prescribed to the 95% isodose line encompassing the PTV. The distribution of radiation modalities observed in this trial mimicked the evolution of radiation modalities more broadly. As such, the following RT techniques were utilized: anterior-posterior/posterior-anterior field (n = 1), three-dimensional conformal RT (n = 15), intensity-modulated radiotherapy (IMRT) (n = 33), and brachytherapy alone (n = 4). For EBRT, a median dose of 50.4 Gy (range 40–63 Gy) in 15–35 once-daily fractions was employed. Dose constraints for adjacent organs at risk (OARs) were in accordance with the QUANTEC recommendations.25 All patients completed the treatment as planned.
Pelvic nodal irradiation was utilized in 43 patients (81%) with standard target volumes consisting of external and internal iliac, common iliac, obturator, and presacral regions. The inguinal lymph nodes were included in 32 patients (60%) due to a distal tumor location or large baseline tumor size. A median dose of 45 Gy (range 30.6–54.0 Gy) in 17–30 fractions was prescribed for pelvic lymph nodes. Of note, a lymph node boost was applied with a median dose of 55 Gy (range 50.0–59.4 Gy) in 25–28 fractions in 8 cases where pathologic lymph nodes were detected radiologically.
A high dose rate (HDR) brachytherapy boost was often delivered using Iridium-192 with intracavitary or interstitial techniques by a single or multichannel applicator for reasons of better coverage of the target volume and superior sparing of OARs. Because of the potential of tumor shrinking and downsizing during EBRT, the HDR brachytherapy boost was employed at the end of pelvic irradiation or shortly thereafter with a median boost dose to the D90 CTV of 20 Gy (range 10–47 Gy) in 2–13 fractions according to American Brachytherapy Society Consensus Guidelines. In addition, CT- and MRI-based imaging was used whenever feasible for optimization of individualized brachytherapy treatment plans. In two select adenoid cystic carcinoma cases, an additional carbon ion boost was applied with a median dose of 24 Gy relative biological effectiveness (range 18–24 Gy) in 6–8 fractions in lieu of brachytherapy.
For further comparison, EBRT doses, HDR brachytherapy, and carbon ion boost doses were converted to an equivalent dose in 2-Gy fractions (EQD2) using the linear quadratic model and tumor dose summations were calculated. An α/β ratio of 10 was assumed for the tumor, an α/β ratio of 3 for normal tissue.
A median total dose in EQD2 (α/β = 10) Gy of 62.0 Gy (range 28.4–81.6 Gy) and a median total dose in EQD2 (α/β = 3) Gy of 64.4 Gy (range 37.4–91.6 Gy) was measured for all patients.
Chemotherapy
Based on data from cervical cancer,19,26 concurrent chemotherapy with cisplatin was administered in 28 patients (53%) and omitted in 25 patients (47%) due to severe comorbidities, poor performance status, patient refusal, or early-stage tumors (FIGO I). Cisplatin 40 mg/m² weekly was administered intravenously for 5–6 cycles concurrently with EBRT. Total number of cycles and dose reduction was dependent on hematologic toxicity and renal function. A median of 5 cycles (range 2–6 cycles) were applied for the entire cohort, with 18 patients receiving full course chemotherapy (defined as 5–6 cycles) and 10 patients with a lower number of cycles (defined as 1–4 cycles) for medical reasons.
Oncologic and toxicity follow-up
Follow-up included clinical examinations, referring physician notes, and imaging (e.g. CT, MRI, or ultrasound), which were all utilized to evaluate treatment response. OAR toxicities were assessed according to Common Terminology Criteria for Adverse Events (CTCAE version 5.0) for urinary, gastrointestinal, or genital side effects.27 Clinical outcome including LC, OS, distant control (DC), and progression-free survival (PFS) was assessed with the time interval beginning from the last day of treatment until diagnosis of local or distant recurrence, death from any cause, or last contact with the patient. Local relapse was defined as any progression of tumor at the original tumor site or local pelvic lymph nodes. Distant recurrence was defined as progression occurring outside the pelvis. In the case of local or distant progression, subsequent treatment recommendations were reviewed in an interdisciplinary tumor conference.
Statistical analysis
LC, OS, DC, and PFS were analyzed during the follow-up period and calculated using the Kaplan-Meier method. Survival curves were compared between subgroups in univariate analysis by applying the log-rank test or Cox regression; a p value of less than 0.05 was considered statistically significant. Multivariate Cox models were used that included all significant variables from the univariate analysis. Statistical analysis was performed with IBM SPSS statistics (version 25).
Results
Outcome for the overall group of 53 patients with a median follow-up time of 64.5 months (range 0.3–247.4 months) is shown in Table 3.
Table 3.
Outcome.
| Overall group (n = 53) | OS | PFS | LC | DC |
|---|---|---|---|---|
| 2 years | 74.8 | 57.8 | 67.8 | 74.4 |
| 5 years | 62.8 | 43.4 | 65.0 | 62.6 |
| 7 years | 58.9 | 39.5 | 65.0 | 62.6 |
DC: distant control; LC: local control; OS: overall survival; PFS: progression-free-survival.
Values are percentages.
Overall and progression-free survival
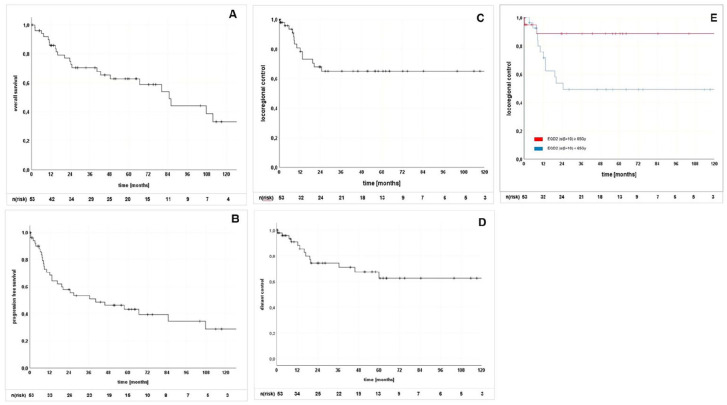
The 2-year, 5-year, and 7-year OS rates were 74.8%, 62.8%, und 58.9% (Figure 1 [A]), respectively. PFS 2-year, 5-year, and 7-year rates were 57.8%, 43.4%, and 39.5%, respectively (Figure 1 [B]).
Figure 1.
Kaplan-Meier curves for (A) overall survival, (B) progression-free survival, (C) locoregional control, (D) distant control, and (E) local control. EQD2: equivalent dose in 2 Gy fractions; n(risk): number at risk.
Squamous cell carcinoma and a baseline tumor size of <4 cm were significantly associated with improved PFS (hazard ratio [HR], 1.755 [confidence interval (CI), 1.038–2.967]; p = 0.036 and HR, 2.425 [1.132–5.194]; p = 0.023, respectively) on univariate analysis (Table 4).
Table 4.
Univariate analysis of prognostic factors influencing OS, PFS, LC, and DC.
| Factors | OS | PFS | LC | DC | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |
| Age | 1.015 (0.98–1.05) | 0.400 | 1.006 (0.98–1.04) | 0.660 | 1.000 (0.96–1.04) | 0.984 | 0.995 (0.95–1.04) | 0.807 |
| Tumor size, mm | 1.018 (0.997–1.04) | 0.093 | 1.015 (0.998–1.03) | 0.082 | 1.013 (0.99–1.04) | 0.334 | 1.013 (0.99–1.04) | 0.349 |
| Tumor size (⩾4 vs <4 cm) | 2.173 (0.95–4.99) | 0.067 | 2.425 (1.13–5.19) | 0.023 | 2.205 (0.76–6.37) | 0.144 | 2.984 (0.96–9.27) | 0.059 |
| Body mass index | 0.993 (0.92–1.07) | 0.843 | 0.976 (0.91–1.04) | 0.475 | 1.022 (0.94–1.11) | 0.594 | 1.011 (0.93–1.10) | 0.797 |
| Grading | 0.743 (0.31–1.77) | 0.462 | 0.668 (0.32–1.38) | 0.520 | 1.056 (0.35–3.19) | 0.805 | 1.405 (0.41–4.77) | 0.812 |
| Prior hysterectomy | 1.036 (0.45–2.40) | 0.933 | 0.830 (0.397–1.73) | 0.619 | 0.851 (0.29–2.43) | 0.762 | 1.155 (0.38–3.54) | 0.801 |
| FIGO (stage I/II vs III/IV) | 2.547 (1.00–6.48) | 0.042 | 1.734 (0.82–3.67) | 0.145 | 2.246 (0.75–6.71) | 0.136 | 1.271 (0.43–3.79) | 0.666 |
| TNM (T1/2 vs T3/4) | 2.767 (1.19–6.42) | 0.018 | 2.198 (1.06–4.56) | 0.035 | 3.303 (1.14–9.56) | 0.027 | 1.542 (0.50–4.74) | 0.450 |
| Nodal stage (N0 vs N+) | 1.142 (0.48–2.70) | 0.762 | 1.083 (0.50–2.33) | 0.838 | 1.128 (0.78–3.39) | 0.829 | 1.452 (0.49–4.33) | 0.501 |
| Tumor type (SCC vs AC vs other) | 1.413 (0.74–2.72) | 0.300 | 1.755 (1.04–2.97) | 0.036 | 1.441 (0.68–3.08) | 0.195 | 2.191 (1.11–4.33) | 0.024 |
| Primary vs recurrent | 1.320 (0.30–5.78) | 0.712 | 1.428 (0.43–4.79) | 0.561 | 0.042 (0–68.43) | 0.192 | 0.43 (0–284.59) | 0.280 |
| RT vs radiochemotherapy | 0.313 (0.13–0.75) | 0.009 | 0.302 (0.14–0.66) | 0.001 | 0.248 (0.08–0.79) | 0.011 | 0.244 (0.07–0.81) | 0.013 |
| RT concept (definitive vs postoperative) | 0.409 (0.17–1.00) | 0.044 | 0.534 (0.25–1.15) | 0.108 | 0.521 (0.17–1.56) | 0.244 | 1.237 (0.40–3.82) | 0.712 |
| EQD2 ⩾65 Gy (α/β = 10) | 0.582 (0.24–1.42) | 0.230 | 0.701 (0.32–1.52) | 0.368 | 0.216 (0.05–0.97) | 0.028 | 0.522 (0.16–1.70) | 0.281 |
AC: adenocarcinoma; CI: confidence interval; DC: distant control; EQD2: equivalent dose in 2 Gy fractions; HR: hazard ratio; LC: local control; OS: overall survival; PFS: progression-free survival; RT: radiotherapy; SCC: squamous cell carcinoma.
For continuous variables, the HRs represent a relative increase in risk according to unit change. A p value of <0.05 was considered statistically significant.
The use of concomitant chemotherapy was found to be a significant prognostic factor on both univariate and multivariate analyses (multivariate HR, 0.352 [CI, 0.136–0.910]; p = 0.009) for OS and PFS (multivariate: HR, 0.318 [CI, 0.136–0.747]; p = 0.009) (Table 5), respectively, whereas the number of cisplatin-based chemotherapy cycles did not significantly influence OS (HR, 1.015 [CI, 0.985–1.046]; p = 0.337) or PFS (HR, 1.000 [CI, 0.983–1.017]; p = 0.962). The diagnosis of nodal metastases did not significantly influence OS or PFS (HR, 1.142 [CI, 0.483–2.698]; p = 0.762 and HR, 1.083 [CI, 0.503–2.333]; p = 0.838).
Table 5.
Multivariate analysis of prognostic factors influencing OS, LC, PFS, and DC.
| Factors | OS | PFS | LC | DC | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95%CI | p | HR | 95%CI | p | HR | 95%CI | p | HR | 95%CI | p | |
| Tumor size (⩾4 vs <4 cm) | 1.905 (0.87–4.18) | 0.108 | ||||||||||
| FIGO stage (I/II vs III/IV) | 2.236 (0.53–9.37) | 0.271 | ||||||||||
| TNM (T1/2 vs T3/4) | 1.080 (0.26–4.52) | 0.916 | 1.960 (0.91–4.24) | 0.087 | 2.968 (1.01–8.71) | 0.048 | 1.094 (0.33–3.59) | 0.882 | ||||
| Tumor type (SCC vs AC vs other) | 0.930 (0.36–2.39) | 0.880 | 1.787 (0.52–6.17) | 0.358 | ||||||||
| Radiotherapy vs radiochemotherapy | 0.352 (0.14–0.91) | 0.009 | 0.318 (0.14–0.75) | 0.009 | 0.339 (0.10–1.12) | 0.076 | 0.297 (0.08–1.07) | 0.063 | ||||
| RT concept (definitive vs postoperative) | 0.694 (0.23–2.06) | 0.510 | ||||||||||
| EQD2 ⩾65 Gy (α/β=10) | 0.269 (0.06–1.24) | 0.092 | ||||||||||
AC: adenocarcinoma; CI: confidence interval; DC: distant control; EQD2: equivalent dose in 2 Gy fractions; HR: hazard ratio; LC: local control; OS: overall survival; PFS: progression-free survival; RT: radiotherapy; SCC: squamous cell carcinoma.
For continuous variables, the HRs represent a relative increase in risk according to unit change. A p value of <0.05 was considered statistically significant.
Local and distant control
Fourteen local failures were detected with a median time to relapse of 8.8 months (range 0.4–24.6 months) following radiotherapy. This resulted in 2-year, 5-year, and 7-year LC rates of 67.8%, 65.0%, and 65.0% (Figure 1 [C]) and DC rates of 74.4%, 62.6%, and 62.6% (Figure 1 [D]) after 2, 5, and 7 years, respectively.
Local or distant failure was significantly reduced with the use of concomitant chemotherapy in univariate analysis (HR, 0.248 [CI, 0.077–0.797]; p = 0.011 and HR, 0.244 [CI, 0.074–0.808]; p = 0.013), whereas the number of administered chemotherapy cycles had no significant effect on LC (HR, 1.015 [CI, 0.980–1.051]; p = 0.416) or DC (HR, 0.991 [CI, 0.963–1.019]; p = 0.510). Alternatively, FIGO stage could not be identified as a prognostic factor for LC (HR, 2.246 [CI, 0.751–6.711]; p = 0.136) or DC (HR, 1.271 [CI, 0.427–3.785]; p = 0.666). Furthermore, a higher TNM stage (T1/2 vs T3/4) was significantly associated with worse LC on univariate analysis (HR, 3.303 [CI, 1.142–9.556]; p = 0.027).
LC was significantly improved with the use of higher total doses in EQD2 (Figure 1 [E]). A cutoff dose of EQD2 ⩾65 Gy (α/β = 10) was identified for improved LC (HR, 0.216 [CI, 0.048–0.969]; p = 0.028) on univariate analysis.
This dose dependence held for patients treated in the definitive setting (HR, 0.103 [CI, 0.013–0.839]; p = 0.034), but not for those in a postoperative setting (HR, 0.459 [CI, 0.051–4.107]; p = 0.486). Of note, a total EQD2 (α/β =10) of less than 65 Gy was used (median 54.5 Gy, range 28.4–65.6 Gy) in all but one of the 14 local failures.
In total, distant metastases were diagnosed in 13 patients during the follow-up period, with the following organ distribution: pulmonary (n = 6), hepatic (n = 4), bone (n = 4), soft tissue (n = 3), and pleural (n = 2) lesions. Patients with squamous cell carcinoma had a significantly better DC (HR, 2.191 [CI, 1.108–4.330]; p = 0.024) on univariate analysis.
On multivariate analysis, only a higher TNM stage (T1/2 vs T3/4) could be identified as an independent prognostic factor for inferior local control (HR, 2.968 [CI, 1.012–8.705]; p = 0.048). The use of radiochemotherapy (p = 0.076) and an EQD2 ⩾65 Gy (p = 0.092) revealed a strong trend but was not significant.
Other prognostic factors and toxicity
Tables 4 and 5 display the results of the univariate and multivariate analyses of the most important assessed prognostic factors. Age, baseline tumor size, body mass index, grading, and previous hysterectomy for reasons not related to a malignancy were not identified as prognostic factors for LC, OS, DC, or PFS. Vaginal wall involvement (upper vs lower and anterior vs posterior vs circumferential), Karnofsky Performance Status, menopausal status, presence of dysplastic lesions, irradiation of the lymphatic drainage and overall treatment time, treatment decade, and treatment technique did not show any statistically significant influence on any of the assessed oncologic outcomes.
In general, RT and radiochemotherapy were well tolerated with no grade 3 or higher toxicity reported. The most common acute grade 1 and 2 toxicities were in the urinary tract in 22 patients (41.5%) and rectal proctitis developed in 10 patients (18.8%). Vaginal dryness or stricture was the most frequently identified late toxicity and was identified in 23 patients (43.3%) (grade 1+2). A correlation between the applied RT technique, addition of brachytherapy, or treatment with a simultaneous integrated boost and the extent of toxicity could not be demonstrated. In two patients, a pelvic insufficiency bone fracture was diagnosed on follow-up imaging and was treated conservatively (e.g. analgetic medication). Within the entire median follow-up time of 64.5 months, one patient was diagnosed with and died of an angiosarcoma of the bladder 5 years after radiochemotherapy, which was thought to be radiation-induced.
Discussion
In the modern era, the use of advanced RT techniques including dose escalation with individualized image-guided HDR brachytherapy and carbon particle therapy concomitantly with chemotherapy has made it possible to optimize the treatment of infrequent vaginal neoplasms at our institution. Due to the rarity of the disease and lack of randomized prospective trials, management guidelines for vaginal malignancies extrapolate many recommendations from those for cervical cancer. We explored oncologic and toxicity outcomes utilizing these modern approaches personalized to the individual patient and cancer.
In this study, tumor stage according to the FIGO classification represented a significant prognostic factor for OS (p = 0.042), which is consistent with prior studies. Hiniker et al.28 reported that both 2-year OS and 2-year LC were notably dependent on tumor stage with prognosis worsening for locally advanced tumors: stage I (96.2% and 80.6%), stage II (92.3% and 64.7%), stage III (66.6% and 44.4%), and stage IV (25% and 14.3%). In addition, a higher TNM stage was also predictive for inferior LC in both univariate (p = 0.027) and multivariate (p = 0.048) analyses in our study. Furthermore, tumor size ⩾4 cm was significantly associated with a worse PFS (p = 0.023). Tumor size ⩾4 cm is not included as a prognostic marker in the current TNM and FIGO classifications, but has been frequently reported to be prognostically relevant in the literature.2,28
Conflicting data have been reported for other prognostic factors including tumor de-differentiation based on histology, age, prior hysterectomy, involvement of the lower vagina, and pretherapeutic hemoglobin levels. Many of these clinical parameters have been associated with lower response rates; however, given their inconsistent importance in the literature, their clinical relevance as prognostic factors remains nebulous.1,2,23,29 Similarly, we were unable to identify any of these clinical parameters as critical prognostic markers in our cohort.
In contrast, the use of concurrent cisplatin-based chemotherapy has been consistently reported to be associated with superior LC and disease-free survival.17–19 In the present study, chemotherapy was significantly associated with improved LC, OS, PFS, and DC, especially for advanced tumor stages and at the cost of only moderate toxicity. Even if a full number of chemotherapy cycles could not demonstrate a significant benefit for superior outcome in our small cohort, it has been proven to be an important factor for risk reduction including improvement of DC.30 Hence, we advocate for the administration of full course concurrent chemotherapy in the form of cisplatin whenever clinically feasible.
The advent of highly conformal and precise RT techniques including IMRT, particle therapy, and MRI-guided HDR brachytherapy have allowed for a widening of the therapeutic window by personalizing RT plans to a specific patient and tumor.29 With these advancements, dosages can be escalated, minimizing surrounding normal tissue destruction. In the present study, we identified an improvement in LC when an EQD2 (α/β = 10) of at least 65 Gy or higher (p = 0.028) was utilized in the definitive but not the postoperative setting. This was consistent in the subgroup of patients treated in the definitive setting (n = 30, 75%) with gross tumor masses, but not for those in the postoperative setting (n = 23 [43%]). Nevertheless, due to the limited number of patients, our results should be interpreted with caution. Furthermore, prospective studies are needed to evaluate whether a similar dose–response relationship as known for cervical cancer exists for vaginal carcinoma.31,32
Previous studies advocated for radiation doses higher than 70 or 80 Gy to the primary tumor in the vagina,15,28 but reported increased treatment-related side effects with grade 3 and 4 toxicities in 10% of patients and two toxicity-related deaths6 as well as urogenital (8%), gastrointestinal (3%), and vaginal (8%) side effects in the study of Westerveld et al.,15 which could not be detected in our study. Westerveld et al.15 assessed the largest multicenter cohort of 148 patients with radiotherapy for vaginal cancer and found an improved benefit for LC with higher doses for locally advanced tumors, whereas this was not significant for the entire cohort and regardless of prior surgical treatment and interestingly without significant contribution by concomitant chemotherapy in univariate analysis.
Optimal dosage recommendations are lacking and previous studies with historical dose dependence might be confounded by older and less accurate forms of staging, radiology (modern CT, MRI, and positron emission tomography), and treatment planning. Modern RT utilizes high-definition imaging to identify all sites of gross disease and advanced radiation treatment planning ensures adequate doses are delivered to the tumor with conformal avoidance of normal structures. This is supported by the study of Laliscia et al.,33 where modern RT delivered with doses greater than 60 Gy resulted in excellent oncologic and toxicity outcomes.
A major strength of our study is the extended follow-up period of more than 5 years (median follow-up of 64.5 months). The LC rates reported in the present study are comparable to others in the literature, including Murakami et al.,21 who reported a 2-year LC of 68.8%. Our results indicate that the highest risk of LC is limited to the first 2 years following treatment. Major limitations of the present study include the limited patient number, a consequence of the rarity of the disease, and the retrospective nature of the review. Thus, subgroup analyses must be interpreted with caution. Due to the heterogeneity and variety in the radiation techniques applied to manage vaginal malignancies, further contributions to the sparse literature are greatly needed. Nevertheless, our cohort is one of the larger single-center cohorts reported for this rare tumor type in the literature and highlights outcomes using modern radiation techniques.
Conclusion
Radiochemotherapy with concurrent cisplatin achieved the best disease control rates and represents an effective treatment option for vaginal malignancies in the curative setting. Our results suggest that RT with an EQD2 of ⩾65 Gy is associated with improved locoregional control. For dose escalation, the use of HDR brachytherapy is a feasible and safe augmentation strategy with only moderate additional toxicity.
Acknowledgments
The authors thank the Cancer Registry of the National Center for Tumor Diseases for providing patient data.
Footnotes
Author contributions: E.M.: data curation, statistical analysis, investigation, validation, methodology, visualization, writing original draft, writing review, project administration, and editing. N.A., N.B., L.H., L.K., K.L., C.D., M.W., J.W.L., F.K.F.K., J.D.: validation, writing review, revision, editing. J.H.-R.: data curation, statistical analysis, investigation, validation, methodology, visualization, writing original draft, writing review, project administration, supervision, and editing.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial statement for research, authorship, and/or publication of the article: The authors acknowledge financial support by Deutsche Forschungsgemeinschaft within the funding program Open Access Publishing, by the Baden-Württemberg Ministry of Science, Research and the Arts, and by Ruprecht-Karls-Universität Heidelberg.
ORCID iDs: Eva Meixner  https://orcid.org/0000-0001-7087-9581
https://orcid.org/0000-0001-7087-9581
Kristin Lang  https://orcid.org/0000-0002-5355-3173
https://orcid.org/0000-0002-5355-3173
References
- 1. Gadducci A, Fabrini MG, Lanfredini N, et al. Squamous cell carcinoma of the vagina: natural history, treatment modalities and prognostic factors. Crit Rev Oncol Hematol 2015; 93: 211–224. [DOI] [PubMed] [Google Scholar]
- 2. Shah CA, Goff BA, Lowe K, et al. Factors affecting risk of mortality in women with vaginal cancer. Obstet Gynecol 2009; 113: 1038–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Creasman WT, Phillips JL, Menck HR. The National Cancer Data Base report on cancer of the vagina. Cancer 1998; 83: 1033–1040. [PubMed] [Google Scholar]
- 4. Alemany L, Saunier M, Tinoco L, et al. ; HPV VVAP study group. Large contribution of human papillomavirus in vaginal neoplastic lesions: a worldwide study in 597 samples. Eur J Cancer 2014; 50: 2846–2854. [DOI] [PubMed] [Google Scholar]
- 5. Adams TS, Cuello MA. Cancer of the vagina. Int J Gynaecol Obstet 2018; 143: 14–21. [DOI] [PubMed] [Google Scholar]
- 6. Kirkbride P, Fyles A, Rawlings GA, et al. Carcinoma of the vagina: experience at the Princess Margaret Hospital (1974-1989). Gynecol Oncol 1995; 56: 435–443. [DOI] [PubMed] [Google Scholar]
- 7. Gadducci A, Carinelli S, Guerrieri ME, et al. Melanoma of the lower genital tract: prognostic factors and treatment modalities. Gynecol Oncol 2018; 150: 180–189. [DOI] [PubMed] [Google Scholar]
- 8. Barcellini A, Gadducci A, Laliscia C, et al. Adenoid cystic carcinoma of Bartholin’s gland: what is the best approach? Oncology 2020; 98: 513–519. [DOI] [PubMed] [Google Scholar]
- 9. Stock RG, Chen AS, Seski J. A 30-year experience in the management of primary carcinoma of the vagina: analysis of prognostic factors and treatment modalities. Gynecol Oncol 1995; 56: 45–52. [DOI] [PubMed] [Google Scholar]
- 10. Tjalma WA, Monaghan JM, de Barros Lopes A, et al. The role of surgery in invasive squamous carcinoma of the vagina. Gynecol Oncol 2001; 81: 360–365. [DOI] [PubMed] [Google Scholar]
- 11. Kanayama N, Isohashi F, Yoshioka Y, et al. Definitive radiotherapy for primary vaginal cancer: correlation between treatment patterns and recurrence rate. J Radiat Res 2015; 56: 346–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Orton A, Boothe D, Williams N, et al. Brachytherapy improves survival in primary vaginal cancer. Gynecol Oncol 2016; 141: 501–506. [DOI] [PubMed] [Google Scholar]
- 13. Yang J, Delara R, Magrina J, et al. Management and outcomes of primary vaginal cancer. Gynecol Oncol 2020; 159: 456–463. [DOI] [PubMed] [Google Scholar]
- 14. Dimopoulos JC, Schmid MP, Fidarova E, et al. Treatment of locally advanced vaginal cancer with radiochemotherapy and magnetic resonance image-guided adaptive brachytherapy: dose-volume parameters and first clinical results. Int J Radiat Oncol Biol Phys 2012; 82: 1880–1888. [DOI] [PubMed] [Google Scholar]
- 15. Westerveld H, Schmid MP, Nout RA, et al. Image-guided adaptive brachytherapy (IGABT) for primary vaginal cancer: results of the International Multicenter RetroEMBRAVE Cohort Study. Cancers 2021; 13: 1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nanavati PJ, Fanning J, Hilgers RD, et al. High-dose-rate brachytherapy in primary stage I and II vaginal cancer. Gynecol Oncol 1993; 51: 67–71. [DOI] [PubMed] [Google Scholar]
- 17. Dalrymple JL, Russell AH, Lee SW, et al. Chemoradiation for primary invasive squamous carcinoma of the vagina. Int J Gynecol Cancer 2004; 14: 110–117. [DOI] [PubMed] [Google Scholar]
- 18. Miyamoto DT, Viswanathan AN. Concurrent chemoradiation for vaginal cancer. PLoS One 2013; 8: e65048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Samant R, Lau B, Choan E, et al. Primary vaginal cancer treated with concurrent chemoradiation using Cis-platinum. Int J Radiat Oncol Biol Phys 2007; 69: 746–750. [DOI] [PubMed] [Google Scholar]
- 20. Kunos CA. Therapeutic mechanisms of treatment in cervical and vaginal cancer. Oncol Hematol Rev 2012; 8: 55–60. [PMC free article] [PubMed] [Google Scholar]
- 21. Murakami N, Kasamatsu T, Sumi M, et al. Radiation therapy for primary vaginal carcinoma. J Radiat Res 2013; 54: 931–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rubin SC, Young J, Mikuta JJ. Squamous carcinoma of the vagina: treatment, complications, and long-term follow-up. Gynecol Oncol 1985; 20: 346–353. [DOI] [PubMed] [Google Scholar]
- 23. Hellman K, Lundell M, Silfverswärd C, et al. Clinical and histopathologic factors related to prognosis in primary squamous cell carcinoma of the vagina. Int J Gynecol Cancer 2006; 16: 1201–1211. [DOI] [PubMed] [Google Scholar]
- 24. FIGO Committee on Gynecologic Oncology. Current FIGO staging for cancer of the vagina, fallopian tube, ovary, and gestational trophoblastic neoplasia. Int J Gynaecol Obstet 2009; 105: 3–4. [DOI] [PubMed] [Google Scholar]
- 25. Bentzen SM, Constine LS, Deasy JO, et al. Quantitative Analyses of Normal Tissue Effects in the Clinic (QUANTEC): an introduction to the scientific issues. Int J Radiat Oncol Biol Phys 2010; 76: S3–S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rajagopalan MS, Xu KM, Lin JF, et al. Adoption and impact of concurrent chemoradiation therapy for vaginal cancer: a National Cancer Data Base (NCDB) study. Gynecol Oncol 2014; 135: 495–502. [DOI] [PubMed] [Google Scholar]
- 27. Kirchheiner K, Fidarova E, Nout RA, et al. Radiation-induced morphological changes in the vagina. Strahlenther Onkol 2012; 188: 1010–1017. [DOI] [PubMed] [Google Scholar]
- 28. Hiniker SM, Roux A, Murphy JD, et al. Primary squamous cell carcinoma of the vagina: prognostic factors, treatment patterns, and outcomes. Gynecol Oncol 2013; 131: 380–385. [DOI] [PubMed] [Google Scholar]
- 29. Westerveld H, Nesvacil N, Fokdal L, et al. Definitive radiotherapy with image-guided adaptive brachytherapy for primary vaginal cancer. Lancet Oncol 2020; 21: e157–e167. [DOI] [PubMed] [Google Scholar]
- 30. Schmid MP, Franckena M, Kirchheiner K, et al. Distant metastasis in patients with cervical cancer after primary radiotherapy with or without chemotherapy and image guided adaptive brachytherapy. Gynecol Oncol 2014; 133: 256–262. [DOI] [PubMed] [Google Scholar]
- 31. Tanderup K, Fokdal LU, Sturdza A, et al. Effect of tumor dose, volume and overall treatment time on local control after radiochemotherapy including MRI guided brachytherapy of locally advanced cervical cancer. Radiother Oncol 2016; 120: 441–446. [DOI] [PubMed] [Google Scholar]
- 32. Pötter R, Tanderup K, Schmid MP, et al. ; EMBRACE Collaborative Group. MRI-guided adaptive brachytherapy in locally advanced cervical cancer (EMBRACE-I): a multicentre prospective cohort study. Lancet Oncol 2021; 22: 538–547. [DOI] [PubMed] [Google Scholar]
- 33. Laliscia C, Gadducci A, Fabrini MG, et al. Definitive radiotherapy for primary squamous cell carcinoma of the vagina: are high-dose external beam radiotherapy and high-dose-rate brachytherapy boost the best treatment? Experience of two Italian institutes. Oncol Res Treat 2017; 40: 697–701. [DOI] [PubMed] [Google Scholar]