Abstract
In recent years, the influence of nutrition on the health and growth of children has become increasingly important. The relevance of nutrition is even greater for children who are facing cancer. Malnutrition, within the context of undernutrition and overnutrition, may impact not only the effectiveness of treatments and outcomes, but also the quality of life for patients and their families. In this article, we review nutritional assessment methods for children with cancer, focusing on the specific characteristics of this population and analyze the efficacy of nutritional interventions, which include enteral, parenteral, and nutritional education. From our analysis, two important conclusions emerged: i) there is a need to focus our attention on the nutritional status and the body composition of oncologic children, since these factors have a relevant impact on clinical outcomes during treatment as well as after their conclusion; ii) the support of skilled clinical nutrition personnel would be extremely helpful for the global management of these patients.
Keywords: Nutrition, paediatric oncology, nutritional assessment, parenteral nutrition, enteral nutrition, nutritional education
Introduction
Nutrition is the balanced source of energy and nutrients needed for cellular biochemical reactions and protection against damage.1 Our body can store nutrients in structural and functional pools (bone, muscle, adipose tissue, liver), that become available in times of need.1 A range of nutrients are also made available as products of the metabolic activity of the microbiome in significant, but undetermined quantities.2
At different ages and stages of life, energy and nutrients requirements change. During childhood, nutrition is essential to ensure linear growth, puberty, balanced body composition and finally health maintenance. An inability to access required energy and nutrients leads to abnormal body composition and ultimately to illness. At the other extreme, the consumption of foods that provide excessive calories leads to increased weight and fat mass. Metabolic dysregulation in overweight and obese people alters the body homeostasis and is related to increased morbidity and mortality.1
The presence of cancer represents an extremely stressful condition for the body, which requires adequate performance to cope with the disease. Malnutrition occurs frequently in children with cancer, with a reported prevalence varying from 5 to 48%3-6 and is related to many factors: tumour type and stage, intensity of treatment, host factors, as well as the socio-economic status of the family. Alteration of body composition during cancer therapy can result in increased infections, organ dysfunction, altered pharmacokinetics, poor quality of life and the occurrence of comorbidities.7
To improve outcomes in children with cancer, it is essential to focus attention on nutritional care. Understanding “what children are” (body composition), “what they eat” (quality of diet) and “what they can do” (performance status) are integral to the global care process.1
Assessment of nutritional status in children with cancer
Traditionally, nutritional assessment is based on anthropometric measures, biochemistry exams, clinical evaluation, and dietary intake. Assessment is a dynamic process for which there are no standard clinical guidelines and is required at each phase of the disease course: diagnosis, during treatment and follow-up post therapy.8
The Nutrition Working Group (NWG) of the International Society of Paediatric Oncology (SIOP), Committee on Paediatric Oncology in Developing Countries (PODC) recommends a standardized method of nutritional assessment for children with cancer, with an emphasis on ease of administration and cost-effectiveness.9
The NWG recommends that the minimum nutritional assessment includes weight, height, and mid-upper arm circumference (MUAC), calculation of body mass index (BMI), plotted on WHO growth charts to determine the appropriate percentile or Z-score for height for age (H/A), weight for age (W/A), weight for height (W/H), BMI for age (BMI/A), MUAC for age (MUAC/A), and triceps skinfold thickness (TSFT) for age (TSFT/A). The Z-score determines if the child is stunted, underweight, or acutely malnourished.10
While the classification of nutritional status based on weight and height is not reliable for children with cancer as measures of weight can be impaired by large tumour masses, hydration status, and organomegaly,11 several methods/tests are available. The first, dual energy X-ray absorptiometry (DXA) is considered the clinical gold standard to assess body composition,11-15 measuring proportion of fat mass, lean mass, and bone mineral content. However, this sophisticated method is not readily available in routine clinical practice. Secondly, MUAC is a rapid, easy and sensitive test for measuring musculature, available protein stores, lean body mass and at the same time it is independent of abdominal tumour masses, temporary gains in total body water and ethnicity.11-15 SIOP PODC recommends that MUAC should be used as an anthropometric measurement in children with malignancies.9,15 Moreover, many biochemical parameters can add information about a patient’s nutritional status.13,16 Certainly, the clinical evaluation of cancer patients remains essential to detect signs of malnutrition; these include the presence of muscle wasting, loss or excess subcutaneous fat, presence of oedema, mucous membrane dryness and hair changes. Clinicians should also consider conditions that may affect oral food intake, such as the inability to chew and swallow, loss of appetite, vomiting, diarrhoea, constipation, indigestion, or severe mucositis.8 Table 1 summarizes the anamnestic high-risk factors for malnutrition in children with cancer that must be routinely evaluated starting from the clinical history.
Table 1.
High-risk factors for malnutrition (undernutrition and overnutrition) in children with cancer.9,16,19,81,82.
| DIAGNOSIS |
|---|
| Solid tumours in advanced stages (neuroblastoma, Wilms tumour, rhabdomyosarcoma, Ewing sarcoma) |
| Central nervous system tumours (craniopharyngioma, medulloblastoma, astrocytoma, ependymoma) |
| High-risk and multiple relapsed acute leukaemia and lymphomas |
| Nasopharyngeal carcinoma |
| TREATMENT |
| Prolonged corticosteroid therapy with large doses |
| Hematopoietic stem cell transplantation (HSCT) |
| Graft Vs Host Disease |
| Major abdominal surgery |
| Irradiation to the gastrointestinal tract |
| High-dose cranial/craniospinal radiotherapy |
Children with cancer require a complete and varied diet, adequate in protein and energy content during treatment. Therefore, a complete dietary history is necessary for a nutritional assessment, including the intake of macro- and micro-nutrients, food aversions, allergies, or intolerances, current eating patterns, family behaviour as well as food hygiene at home.16,17 This full evaluation is best performed by expert personnel such as dieticians or clinical nutritionists, who should follow the patient during the entire treatment period and after its conclusion.
The recommended macronutrient intake for children is based on acceptable macronutrient dietary ranges (AMDR), which are based on percent ranges of total calories. For fat, 30% to 40% is recommended between the ages of 1 and 3 years, and 25% to 35% between the ages of 4 and 18 years; while 45% to 65% of energy should come from carbohydrates and 10% to 35% from proteins, between the ages of 1 and 3 years a 5% to 20% protein intake is recommended.8,18 There are no specific modified recommendations for nutrient intake in children with cancer, but diets should be tailored to meet the requirements of patients and modified during the different phases of therapy, in order to ensure adequate growth and development and provide appropriate interventions.8
Follow-up with a dietician should consist of a nutritional support strategy adapted to the individual patient nutritional needs, nutritional status, gastrointestinal function and current or expected treatment side effects. The dietician should also provide continuous nutritional education.8 Finally, it is recommended that patients at high-risk for malnutrition should be followed up very closely.9
Oral, enteral, and parenteral nutrition
Oral and enteral nutrition
The best way to nourish children with cancer is through the gastrointestinal tract, which has also the important benefit of maintaining mucosal gut integrity and a colonized gastrointestinal tract.19
The requirements of patients with cancer generally correspond with those of children of the same age and gender. Most children with cancer are not in a hypermetabolic condition, therefore do not require excess nutrient intake.20,21
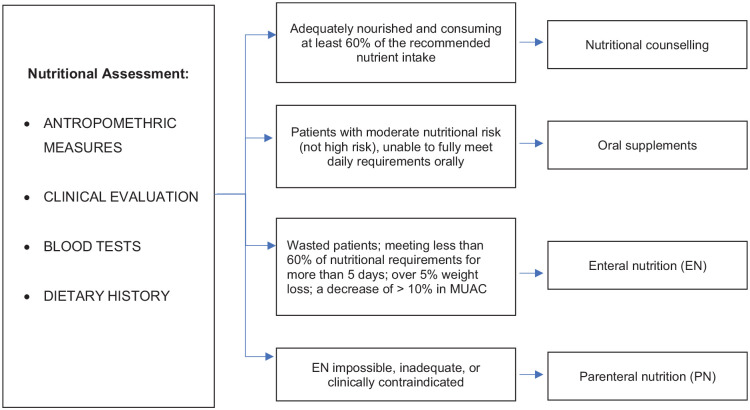
Nutritional counselling by an expert dietician is considered to be sufficient if a patient is adequately nourished, does not lose weight and is consuming at least 60% of the recommended nutrient intake.22-25 Counselling with the family should be provided as well regarding food hygiene, food shopping, cooking, and serving size. The use of a restrictive neutropenic diet has not proven to be superior to regular diets with respect to safe food handling.26-31
Oral supplements are indicated when the patient has a moderate nutritional risk or is unable to fully meet daily requirements orally. For this aim, commercial supplementation (liquid, semi-solid, or powder), energy dense foods or ready-to-use therapeutic foods may be suggested. Nutrient dense regional foods and homemade supplements may also be encouraged since they are less expensive, available, less processed, and usually better tolerated than commercial formulas.19
Enteral nutrition (EN) by feeding tube is indicated when energy and nutrient requirements are not met by oral intake in a patient with a functional gut. The generally accepted main indications for EN are severely wasted patients; meeting less than 60% of their estimated nutritional requirements orally for more than five consecutive days; over 5% weight loss since diagnosis; a decrease of > 10% in the mid upper arm circumference (MUAC) since diagnosis.21,24,32
EN is a safe and effective method to treat or prevent severe malnutrition. Often, the major barrier for the use of this treatment strategy is parental resistance.19 Complications of nasogastric tube placement and use are rare and include:27,32 misplacement, local infection and irritation, mucosal ulceration, aspiration with respiratory infections, mechanical obstruction, dysphagia and nausea.32-34
The type and size of tubes, methods of enteral feeding (bolus vs continuous) and type of formula (standard polymeric, concentrated, semi-elemental or elemental) must be tailored to the patients’ needs and clinical characteristics. For example, semi-elemental or elemental formulas are indicated for children with severely impaired gut digestion and absorption.19
Parenteral nutrition
Parenteral nutrition (PN) consists in the provision of intravenous solutions containing macronutrients (protein, carbohydrate, and fat) and micronutrients (vitamins and minerals) through a central venous catheter (CVC) or a peripheral access. Its scope is to provide adequate nutrition in children when EN is impossible, inadequate, or clinically contraindicated, such as in children with intestinal obstruction or paralytic ileus, intractable vomiting or diarrhoea, severe mucositis, intestinal graft versus host disease (GVHD), acute haemorrhage, severe pancreatitis, radiation enteritis or gastrointestinal perforation.35,36
Multiple conditions and treatment-related factors may contribute to intestinal insult and influence the nutritional status of children with cancer. This may result in alterations of absorptive and secretory functions of the intestinal mucosa, haemorrhages, intestinal dysmotility or intestinal failure.34
The possible complications related to the use of PN are: mechanical or equipment-related complications, such as CVC thrombosis, break, occlusion or dislodgement; infective complications, for example CVC-associated infections and metabolic complications, such as deficiency or excess of parenteral nutrition components (hypertriglyceridemia and hyperglycaemia), acid-base or electrolyte imbalance, drug interaction or compatibility problems, intestinal failure–associated liver disease, refeeding syndrome.34 For these reasons, patients undergoing PN should be closely monitored to immediately identify possible problems. Nutritional status needs continuous reassessment, including subclinical vitamins and trace elements deficiency assessment.34
Optimal management also includes the removal of precipitating or contributing factors of the intestinal failure (for example infections, drugs), especially in children following hematopoietic stem cell transplantation or pharmacological supportive care to reduce gastrointestinal symptoms (nausea, vomiting, diarrhoea, abdominal pain). An expert clinical nutritionist should be involved to determine specific targets of energy and protein requirements. Although an age-appropriate balance of carbohydrate, protein and fat should be maintained, metabolic changes in children with cancer may influence individual needs. Excessive supply of calories should be avoided, but it is also essential to guarantee adequate protein intake.34
Measures should be taken to avoid hyperglycaemia, prevent essential fatty acid deficiency,37 minimize the risk of intestinal failure-associated liver disease, for example avoiding excessive PN caloric intake, PN cycling through the administration of trophic feeds. Minimal enteral feeding, when available, may help restore a healthy microbiome, whose role seems essential for intestinal function and global health maintenance.36
Finally, PN should be discontinued as soon as the clinical condition allows it.
A possible approach from nutritional assessment to interventions is shown in Figure 1.
Figure 1.
Flow chart of nutritional assessment and possible interventions.
Cancer outcomes in children with malnutrition in high income countries
To understand the impact of malnutrition on outcomes of children with cancer, it is mandatory to use standardized methods for the assessment of nutritional status and repeated reassessments during and after treatment.1 As already mentioned, BMI may be misleading as it does not distinguish muscle from adipose. Furthermore, tissue and body weight may be influenced by tumoral masses and fluid retention. MUAC measurement is not yet routinely utilized in clinical practice and DXA has limited availability even in high-income countries (HICs).
These methodological issues underlie much of the variability reported in the prevalence of malnutrition in children with cancer in HICs.1 The lack of longitudinal data tracking nutritional status during or after treatment makes it difficult to draw conclusions about the impact of nutrition on clinical outcomes, as reported by a systematic review38 that identified 46 reports containing information from “developed” and “developing” countries. This review highlighted the need for high-quality, population-based, longitudinal studies using standard criteria to identify malnutrition.
Obese patients
In HICs, much attention has been focused on the impact of obesity on clinical outcomes in children with malignant disease.1 A systematic review by Orgel et al.39 demonstrated that a higher BMI was associated with a significantly increased mortality rate, poorer event free survival (EFS) and a statistically non-significant trend toward greater risk of relapse in children with leukemia.
Different studies have shown that obesity during treatment induction is an independent predictor of persistent positivity of minimal residual disease (MRD).40,41 There are also other associations between high BMI and treatment-related toxicities in children with acute lymphoblastic leukemia,42 including osteonecrosis,43 hepatotoxicity or pancreatitis40 and the incidence of adverse events during chemotherapy.41
Less data is available on children with acute myeloid leukemia (AML), although a high BMI at diagnosis has been associated with poorer EFS and overall survival.39 Furthermore, a link between obesity and higher rates of fatal infection and more treatment-related mortality has been suggested.44, 45
Underweight patients
Only a few studies have examined the relationship between undernutrition and outcomes in children with cancer in HICs. One study from the Netherlands6 reported an association between undernutrition, defined by BMI, identified in 5% of children at diagnosis, with a lower survival rate. The authors found that weight loss during treatment was associated with the increased presence of febrile neutropenic episodes with bacteraemia. The impact of undernutrition also continued as treatment progressed. Similar considerations were reported by Triarico et al.,46 who concluded that a personalized evaluation of nutritional risk at diagnosis and close monitoring of nutritional status are crucial for ensuring a timely and personalized nutritional intervention, which may potentially improve tolerance to chemotherapy and survival, in turn preventing prolonged hospitalization for infections. In a recent review, Paviglianiti45 underscored how being at either extreme of the index (underweight or obese) is associated with worse outcomes and toxicities in children with leukemia or undergoing hematopoietic stem-cell transplantation (HSCT).
Changes in body composition
Cancer treatments may affect not only BMI but may also cause changes in other body compartments. Sarcopenia (loss of muscle mass) has been reported particularly in children with ALL (Acute Lymphoblastic Leukemia), early in therapy and without restoration by the end of treatment.47-49 Sarcopenic obesity is well described in survivors of HSCT, after total body irradiation (TBI), as well as in long-term survivors of ALL.50,51 One consequence of this condition is that excess visceral fat and inadequate skeletal muscle mass, may lead to metabolic syndrome and cardiovascular disease in childhood cancer survivors.49
Bone mineral content is another compartment of the body that is adversely affected in paediatric cancer and its treatment.51 This is especially important because approximately 40% of bone mass accumulates during childhood and adolescence. Most of the studies in children with cancer have been performed in those with ALL. These patients often exhibit osteopenia at diagnosis, and this becomes more pronounced during treatment.52,53 It is associated with prevalent vertebral fractures, which are often clinically unrecognized.54 Osteopenia in children with cancer is not limited to those with ALL, but also to those with solid and brain tumours.52 This condition has a negative impact upon quality of life, as it involves pain, movement limitations and worse performance in physical activities.53
Effect of nutrition on drug disposition
The level of nourishment can affect drug disposition, in terms of absorption, distribution, metabolism and excretion. Chemotherapy may cause a loss of adipose tissue and lean tissue, while extracellular fluid volume may increase, modifying the drug distribution.55
In protein-caloric malnutrition, some oxidative functions of the liver are altered, especially those of cytochrome P450 1A2 and 2E1.56 Reduced protein intake and protein deficiency decrease renal blood flow, glomerular filtration, and renal tubular secretion.57 As a result, the clearance of some drugs, such as methotrexate and vincristine, may be reduced, increasing adverse effects.58-60 Additionally, there is a possible increased risk of cardiovascular toxicity in malnourished patients who receive anthracycline.60 Underweight patients also experience a significantly higher rate of chemotherapy-related toxicities, such as profound myelosuppression and febrile neutropenia, which may be a result of increased chemotherapy exposure.59 Appropriate nutrition intervention may mitigate some drug-metabolism alterations.61,62
Changes in body composition and organ function in obese patients may alter serum protein binding, metabolism, and renal function.63 For example, water soluble (hydrophilic) drugs have a lower total volume of distribution and lipophilic drugs have a higher volume of distribution.
Despite many possible methods (dosing by ideal body weight, by adjusted body weight and arbitrary dose capping), it is not easy to find the appropriate dose of chemotherapy in obese patients, but it should be considered case by case, based on the specific patient and drug characteristics (i.e., volume of distribution, lipophilicity, protein binding, renal elimination).55 Finally, higher body surface area is associated with an increased risk of anthracycline-related cardiotoxicity.61
Specific aspects in children with solid tumours
The comprehensive review of Joffe et al. evaluates the nutritional status and outcomes in children with solid tumours.64 These patients undergo multimodal therapy, including dose-intense antineoplastic agents, surgery, and radiotherapy. Often this multimodality therapy results in a constellation of serious adverse effects that further potentiate poor nutrition. The review indicates that up to 62% of paediatric solid tumour patients are either over-or undernourished at diagnosis. As we said the nutritional status affect survival and treatment-related toxicities, in both adult and paediatric malignancies.
The traditional methods of nutritional assessment, such as weight and BMI, are not reliable in case of large masses and do not accurately predict chemotherapy pharmacokinetics. This is more relevant for patients with solid tumours, who can develop sarcopenia and sarcopenic obesity, that are more sensitive indicators of toxicities, post-operative complication rates, hospital length of stay and survival.65,66
The role of body composition in paediatric solid tumours is a highly understudied area and is critical to improve our understanding of the mechanisms by which nutritional status may impact outcomes in this patient population.
Cancer outcomes in children with malnutrition in low- and middle- income countries (LMICs)
Malnutrition is prevalent in young population in LMICs, and undernutrition is very common in those diagnosed with cancer.67 Large studies in Central America demonstrated the influence of socio-economic disadvantage on malnutrition and showed how it is linked with poorer survival, higher morbidity, higher rate of treatment abandonment and a higher risk of relapse.15,68
In a report from Guatemala, children with ALL who were severely malnourished at diagnosis and remained so during treatment, had a five-year overall survival (OS) rate of 56.9%; on the contrary, the OS of children who were adequately nourished was 79.8%. For those who were severely undernourished at diagnosis but became adequately nourished within the first six months after diagnosis the 5-year-OS was 77.5%. This seems to be the first report of the efficacy of nutritional intervention in clinical outcomes for children with cancer.69,70
The Nutrition Working Group (NWG) of the Inter-national Society of Paediatric Oncology (SIOP), Committee on Paediatric Oncology in Developing Coun-tries (PODC) is focusing its attention on this issue. Priority areas for improving the nutritional management in LMIC include: improved nutrition education and assessment tools for health care professionals in order to develop a structured and standardized method of nutritional assessment; increased availability of nutrition education resources for patients; identification of the role of complementary and alternative therapies in symptoms management.71
Totadri et al. recently validated an SIOP-PODC algorithm for nutritional approach in children in India and they demonstrated that the applications of this algorithm result in significant improvement in nutritional status, measured by MUAC.35
Special considerations in this LMICs settings are the importance of infection screenings in children, such as TB, HIV, and parasitic infections, that could exacerbate malnutrition; dose modifications related to low weight to avoid excess of toxicity; the high prices of commercial nutritional supplements so that they become unaffordable in many LMICs. This problem leads to the engagement of nongovernmental organizations that can help the families, but it has also stimulated interest in ready-to-use local therapeutic foods (RUTF).
Nutrition, assessment, and quality of life in cancer survivors
The number of childhood cancer survivors has increased remarkably in the past few decades, reaching about 80%; this is a result of advances in treatments and better supportive care. Long-term health outcomes are now compromised due to late treatment-related effects, such as endocrinopathies, metabolic syndrome, cardiovascular disease, obesity, and hypertension.49
Overweight and obesity are reported to range from 40% to 50% after the conclusion of therapy in cancer survivors, especially those treated for ALL, lymphomas, sarcomas, and brain tumours. The major reported risk factors are cranial (18 Gy) and abdominal radiotherapy, total body irradiation (TBI), high-dose steroids during treatment,72 pancreatic insufficiency, diabetes mellitus, dyslipidaemia, poor dietary habits, sedentary lifestyle, changes in the gut microbiota and altered secretions of leptin, adiponectin, and ghrelin.73,74 A recent case-control study of survivors treated at the Children’s Hospital of Los Angeles also suggested that obesity might be associated with an increased risk of second malignant neoplasms.75
Cancer survivors often complain of a poor quality of life, that has a multifactorial origin and includes poor mental health, limited education, immobility due to a reduction in muscle strength, infertility, pain, anxiety, depression, and body dissatisfaction. Cohen et al.73 hypothesize that improving nutritional intake and dietary quality of survivors may improve not only metabolic and cardiovascular health, but also mental health and other domains of quality of life.
Survivors of childhood cancer have an increased risk of developing metabolic syndrome and reduced bone mass, exacerbated by vitamin D deficiency.76-79 Additionally, other nutritional risk factors, such as inadequate eating habits, smoking, sedentary lifestyle, and alcoholism may increase the cardio-vascular risk of the patients.8
This said, regular nutritional monitoring, during and after treatment, is essential to ensure adequate growth and provide appropriate interventions. Nutritional education and follow up should start soon after the oncological diagnosis and extend through survivorship to prevent or reverse nutritional deficiencies, preserve lean body mass, minimize nutrition-related side effects, reverse micronutrient deficiencies and finally improve quality of life.80
Parameters that should be frequently monitored include blood pressure, fasting blood sugar, lipids and cholesterol levels, renal function, liver function given the risk of development of steatohepatitis, bone density, micronutrients, and hormone levels. Additionally, the waist-to-height ratio, a proxy for central (visceral) adipose tissue, is considered better than BMI for obesity classification in young cancer survivors and relates well with the risk of cardiovascular disease.77 Viani et al.8 proposed a nutritional assessment for childhood cancer survivors differentiated by risk classes, suggesting for example monthly visits for undernourished patients, and a check-up every three months for obese patients.
Finally, efforts should be focused on improving physical activity in children with cancer, since exercise may improve many of the cited comorbidities and exercise has been associated with reduction in all-cause mortality in the general population.79 Given the increased risk of chronic diseases in cancer survivors, many experts and professional figures should be involved in the care of childhood cancer survivors, not only paediatric oncologists, but also nutritionists, endocrinologists, cardiologists, general paediatricians, internal medicine/family medicine physicians.
Conclusions
Balanced nutrition is an essential component for a healthy life. This is true not only in low-income countries where we face undernutrition, but also in high-income countries where we must also fight obesity.
Children with cancer require the best performance status during treatment, and this is possible only if they are well nourished. Hence, we need to focus greater attention on the nutritional assessment and body composition of children receiving treatment for cancer, to improve treatment efficacy when the equilibrium is broken, through comprehensive and timely nutritional support.
These efforts should not stop at the end of cancer treatment, but should continue for many years, as cancer survivors are fragile and susceptible to long-term comorbidities. Only with regular physical activity and a healthy diet we can assure our patients a good quality of life and life expectancy.
Acknowledgments
The authors would like to thank Dr. Laurene Kelly for the technical support.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from: Ricerca Corrente to Fondazione IRCCS Policlinico San Matteo [RCR 08045818, RCR 08069119], Regione Lombardia (project ID 2526393); Fondazione Regionale per la Ricerca Biomedica (Project nr. CP2_10/2018, PC).
ORCID iD: Marco Zecca  https://orcid.org/0000-0002-8818-1744
https://orcid.org/0000-0002-8818-1744
References
- 1. Barr RD, Stevens MCG. The influence of nutrition on clinical outcomes in children with cancer. Pediatr Blood Cancer 2020; 67: e28117. [DOI] [PubMed] [Google Scholar]
- 2. Valdes AM, Walter J, Segal E, et al. Role of the gut microbiota in nutrition and health. BMJ 2018; 361: k2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brinksma A, Roodbol PF, Sulkers E, et al. Changes in nutritional status in childhood cancer patients: a prospective cohort study. Clin Nutr 2015; 34: 66–73. [DOI] [PubMed] [Google Scholar]
- 4. Galati PC, Resende CM, Salomao RG, et al. Accurate determination of energy needs in children and adolescents with cancer. Nutr Cancer 2011; 63: 306–313. [DOI] [PubMed] [Google Scholar]
- 5. Murphy AJ, White M, Elliott SA, et al. Body composition of children with cancer during treatment and in survivorship. Am J Clin Nutr 2015; 102: 891–896. [DOI] [PubMed] [Google Scholar]
- 6. Loeffen EA, Brinksma A, Miedema KG, et al. Clinical implications of malnutrition in childhood cancer patients–infections and mortality. Support Care Cancer 2015; 23: 143–150. [DOI] [PubMed] [Google Scholar]
- 7. Rogers PC, Barr RD. The relevance of nutrition to pediatric oncology: A cancer control perspective. Pediatr Blood Cancer 2020; 67: e28213. [DOI] [PubMed] [Google Scholar]
- 8. Viani K, Trehan A, Manzoli B, et al. Assessment of nutritional status in children with cancer: A narrative review. Pediatr Blood Cancer 2020; 67: e28211. [DOI] [PubMed] [Google Scholar]
- 9. Ladas EJ, Arora B, Howard SC, et al. A framework for adapted nutritional therapy for children with cancer in low- and middle-income countries: A report from the SIOP PODC Nutrition Working Group. Pediatr Blood Cancer 2016; 63: 1339–1348. [DOI] [PubMed] [Google Scholar]
- 10. Das MK, Bhattacharyya N, Bhattacharyya AK. WHO child growth standards. Eur J Pediatr 2010; 169: 253–255; author reply 257–258. [DOI] [PubMed] [Google Scholar]
- 11. Barr RD. Nutritional status in children with cancer: Before, during and after therapy. Indian J Cancer 2015; 52: 173–175. [DOI] [PubMed] [Google Scholar]
- 12. Murphy AJ, White M, Davies PS. Body composition of children with cancer. Am J Clin Nutr 2010; 92: 55–60. [DOI] [PubMed] [Google Scholar]
- 13. Lifson LF, Hadley GP, Wiles NL, et al. Nutritional status of children with Wilms' tumour on admission to a South African hospital and its influence on outcome. Pediatr Blood Cancer 2017; 64. [DOI] [PubMed] [Google Scholar]
- 14. Barr R, Collins L, Nayiager T, et al. Nutritional status at diagnosis in children with cancer. 2. An assessment by arm anthropometry. J Pediatr Hematol Oncol 2011; 33: e101–104. [DOI] [PubMed] [Google Scholar]
- 15. Sala A, Rossi E, Antillon F, et al. Nutritional status at diagnosis is related to clinical outcomes in children and adolescents with cancer: a perspective from Central America. Eur J Cancer 2012; 48: 243–252. [DOI] [PubMed] [Google Scholar]
- 16. Ladas EJ, Sacks N, Meacham L, et al. A multidisciplinary review of nutrition considerations in the pediatric oncology population: a perspective from children's oncology group. Nutr Clin Pract 2005; 20: 377–393. [DOI] [PubMed] [Google Scholar]
- 17. Bazzan AJ, Newberg AB, Cho WC, et al. Diet and nutrition in cancer survivorship and palliative care. Evid Based Complement Alternat Med 2013; 2013: 917647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Arends J, Bachmann P, Baracos V, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr 2017; 36: 11–48. [DOI] [PubMed] [Google Scholar]
- 19. Trehan A, Viani K, da Cruz LB, et al. The importance of enteral nutrition to prevent or treat undernutrition in children undergoing treatment for cancer. Pediatr Blood Cancer 2020; 67: e28378. [DOI] [PubMed] [Google Scholar]
- 20. Braegger C, Decsi T, Dias JA, et al. Practical approach to paediatric enteral nutrition: a comment by the ESPGHAN committee on nutrition. J Pediatr Gastroenterol Nutr 2010; 51: 110–122. [DOI] [PubMed] [Google Scholar]
- 21. Viani K. Parenteral and enteral nutrition for pediatric oncology in low- and middle-income countries. Indian J Cancer 2015; 52: 182–184. [DOI] [PubMed] [Google Scholar]
- 22. Ball S, Brown TJ, Das A, et al. Effect of neutropenic diet on infection rates in cancer patients with neutropenia: a meta-analysis of randomized controlled trials. Am J Clin Oncol 2019; 42: 270–274. [DOI] [PubMed] [Google Scholar]
- 23. Rogers PC, Melnick SJ, Ladas EJ, et al. Children's Oncology Group (COG) Nutrition Committee. Pediatr Blood Cancer 2008; 50: 447–450; discussion 451. [DOI] [PubMed] [Google Scholar]
- 24. Sonbol MB, Jain T, Firwana B, et al. Neutropenic diets to prevent cancer infections: updated systematic review and meta-analysis. BMJ Support Palliat Care 2019; 9: 425–433. [DOI] [PubMed] [Google Scholar]
- 25. van Dalen EC, Mank A, Leclercq E, et al. Low bacterial diet versus control diet to prevent infection in cancer patients treated with chemotherapy causing episodes of neutropenia. Cochrane Database Syst Rev 2016; 4: CD006247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Boullata JI, Carrera AL, Harvey L, et al. ASPEN Safe Practices for Enteral Nutrition Therapy [Formula: see text]. JPEN J Parenter Enteral Nutr 2017; 41: 15–103. [DOI] [PubMed] [Google Scholar]
- 27. Irving SY, Rempel G, Lyman B, et al. Pediatric nasogastric tube placement and verification: best practice recommendations from the NOVEL Project. Nutr Clin Pract 2018; 33: 921–927. [DOI] [PubMed] [Google Scholar]
- 28. Bauer J, Jurgens H, Fruhwald MC. Important aspects of nutrition in children with cancer. Adv Nutr 2011; 2: 67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Taggart C, Neumann N, Alonso PB, et al. Comparing a neutropenic diet to a food safety-based diet in pediatric patients undergoing hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2019; 25: 1382–1386. [DOI] [PubMed] [Google Scholar]
- 30. Moody KM, Baker RA, Santizo RO, et al. A randomized trial of the effectiveness of the neutropenic diet versus food safety guidelines on infection rate in pediatric oncology patients. Pediatr Blood Cancer 2018; 65. [DOI] [PubMed] [Google Scholar]
- 31. Maia JE, da Cruz LB, Gregianin LJ. Microbiological profile and nutritional quality of a regular diet compared to a neutropenic diet in a pediatric oncology unit. Pediatr Blood Cancer 2018; 65. [DOI] [PubMed] [Google Scholar]
- 32. Seyedhejazi M, Hamidi M, Sheikhzadeh D, et al. Nasogastric tube placement errors and complications in pediatric intensive care unit: a case report. J Cardiovasc Thorac Res 2011; 3: 133–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee JH, Lim GY, Im SA, et al. Gastrointestinal complications following hematopoietic stem cell transplantation in children. Korean J Radiol 2008; 9: 449–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McGrath KH. Parenteral nutrition use in children with cancer. Pediatr Blood Cancer 2019; 66: e28000. [DOI] [PubMed] [Google Scholar]
- 35. Totadri S, Trehan A, Mahajan D, et al. Validation of an algorithmic nutritional approach in children undergoing chemotherapy for cancer. Pediatr Blood Cancer 2019; 66: e27980. [DOI] [PubMed] [Google Scholar]
- 36. Ohta K, Omura K, Hirano K, et al. The effects of an additive small amount of a low residual diet against total parenteral nutrition-induced gut mucosal barrier. Am J Surg 2003; 185: 79–85. [DOI] [PubMed] [Google Scholar]
- 37. Lapillonne A, Fidler Mis N, Goulet O, et al. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: Lipids. Clin Nutr 2018; 37: 2324–2336. [DOI] [PubMed] [Google Scholar]
- 38. Iniesta RR, Paciarotti I, Brougham MF, et al. Effects of pediatric cancer and its treatment on nutritional status: a systematic review. Nutr Rev 2015; 73: 276–295. [DOI] [PubMed] [Google Scholar]
- 39. Orgel E, Genkinger JM, Aggarwal D, et al. Association of body mass index and survival in pediatric leukemia: a meta-analysis. Am J Clin Nutr 2016; 103: 808–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Denton CC, Rawlins YA, Oberley MJ, et al. Predictors of hepatotoxicity and pancreatitis in children and adolescents with acute lymphoblastic leukemia treated according to contemporary regimens. Pediatr Blood Cancer 2018; 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Meenan CK, Kelly JA, Wang L, et al. Obesity in pediatric patients with acute lymphoblastic leukemia increases the risk of adverse events during pre-maintenance chemotherapy. Pediatr Blood Cancer 2019; 66: e27515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Eissa HM, Zhou Y, Panetta JC, et al. The effect of body mass index at diagnosis on clinical outcome in children with newly diagnosed acute lymphoblastic leukemia. Blood Cancer J 2017; 7: e531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Niinimaki RA, Harila-Saari AH, Jartti AE, et al. High body mass index increases the risk for osteonecrosis in children with acute lymphoblastic leukemia. J Clin Oncol 2007; 25: 1498–1504. [DOI] [PubMed] [Google Scholar]
- 44. Koskelo EK, Saarinen UM, Siimes MA. Skeletal muscle wasting and protein-energy malnutrition in children with a newly diagnosed acute leukemia. Cancer 1990; 66: 373–376. [DOI] [PubMed] [Google Scholar]
- 45. Paviglianiti A. A review on the impact of body mass index on outcomes in pediatric leukemia. J Blood Med 2020; 11: 205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Triarico S, Rinninella E, Cintoni M, et al. Impact of malnutrition on survival and infections among pediatric patients with cancer: a retrospective study. Eur Rev Med Pharmacol Sci 2019; 23: 1165–1175. [DOI] [PubMed] [Google Scholar]
- 47. Marriott CJC, Beaumont LF, Farncombe TH, et al. Body composition in long-term survivors of acute lymphoblastic leukemia diagnosed in childhood and adolescence: A focus on sarcopenic obesity. Cancer 2018; 124: 1225–1231. [DOI] [PubMed] [Google Scholar]
- 48. Mostoufi-Moab S, Ginsberg JP, Bunin N, et al. Body composition abnormalities in long-term survivors of pediatric hematopoietic stem cell transplantation. J Pediatr 2012; 160: 122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pluimakers VG, van Waas M, Neggers S, et al. Metabolic syndrome as cardiovascular risk factor in childhood cancer survivors. Crit Rev Oncol Hematol 2019; 133: 129–141. [DOI] [PubMed] [Google Scholar]
- 50. Halton JM, Atkinson SA, Fraher L, et al. Altered mineral metabolism and bone mass in children during treatment for acute lymphoblastic leukemia. J Bone Miner Res 1996; 11: 1774–1783. [DOI] [PubMed] [Google Scholar]
- 51. Sala A, Barr RD. Osteopenia and cancer in children and adolescents: the fragility of success. Cancer 2007; 109: 1420–1431. [DOI] [PubMed] [Google Scholar]
- 52. Baroncelli GI, Bertelloni S, Sodini F, et al. Osteoporosis in children and adolescents: etiology and management. Paediatr Drugs 2005; 7: 295–323. [DOI] [PubMed] [Google Scholar]
- 53. Odame I, Duckworth J, Talsma D, et al. Osteopenia, physical activity and health-related quality of life in survivors of brain tumors treated in childhood. Pediatr Blood Cancer 2006; 46: 357–362. [DOI] [PubMed] [Google Scholar]
- 54. Halton J, Gaboury I, Grant R, et al. Advanced vertebral fracture among newly diagnosed children with acute lymphoblastic leukemia: results of the Canadian Steroid-Associated Osteoporosis in the Pediatric Population (STOPP) research program. J Bone Miner Res 2009; 24: 1326–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wiernikowski JT, Bernhardt MB. Review of nutritional status, body composition, and effects of antineoplastic drug disposition. Pediatr Blood Cancer 2020; 67: e28207. [DOI] [PubMed] [Google Scholar]
- 56. Lee JH, Suh OK, Lee MG. Pharmacokinetic changes in drugs during protein-calorie malnutrition: correlation between drug metabolism and hepatic microsomal cytochrome P450 isozymes. Arch Pharm Res 2004; 27: 693–712. [DOI] [PubMed] [Google Scholar]
- 57. Murry DJ, Riva L, Poplack DG. Impact of nutrition on pharmacokinetics of anti-neoplastic agents. Int J Cancer Suppl 1998; 11: 48–51. [DOI] [PubMed] [Google Scholar]
- 58. Davis LE, Lenkinski RE, Shinkwin MA, et al. The effect of dietary protein depletion on hepatic 5-fluorouracil metabolism. Cancer 1993; 72: 3715–3722. [DOI] [PubMed] [Google Scholar]
- 59. Israels T, van de Wetering MD, Hesseling P, et al. Malnutrition and neutropenia in children treated for Burkitt lymphoma in Malawi. Pediatr Blood Cancer 2009; 53: 47–52. [DOI] [PubMed] [Google Scholar]
- 60. Tzioumis E, Adair LS. Childhood dual burden of under- and overnutrition in low- and middle-income countries: a critical review. Food Nutr Bull 2014; 35: 230–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Krischer JP, Epstein S, Cuthbertson DD, et al. Clinical cardiotoxicity following anthracycline treatment for childhood cancer: the Pediatric Oncology Group experience. J Clin Oncol 1997; 15: 1544–1552. [DOI] [PubMed] [Google Scholar]
- 62. Zhang FF, Parsons SK. Obesity in childhood cancer survivors: call for early weight management. Adv Nutr 2015; 6: 611–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Cheymol G. Effects of obesity on pharmacokinetics implications for drug therapy. Clin Pharmacokinet 2000; 39: 215–231. [DOI] [PubMed] [Google Scholar]
- 64. Joffe L, Dwyer S, Glade Bender JL, et al. Nutritional status and clinical outcomes in pediatric patients with solid tumors: A systematic review of the literature. Semin Oncol 2019; 46: 48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hopkins JJ, Sawyer MB. A review of body composition and pharmacokinetics in oncology. Expert Rev Clin Pharmacol 2017; 10: 947–956. [DOI] [PubMed] [Google Scholar]
- 66. Yip C, Dinkel C, Mahajan A, et al. Imaging body composition in cancer patients: visceral obesity, sarcopenia and sarcopenic obesity may impact on clinical outcome. Insights Imaging 2015; 6: 489–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Barr RD, Mosby TT. Nutritional status in children and adolescents with leukemia: An emphasis on clinical outcomes in low and middle income countries. Hematology 2016; 21: 199–205. [DOI] [PubMed] [Google Scholar]
- 68. Villanueva G, Blanco J, Rivas S, et al. Nutritional status at diagnosis of cancer in children and adolescents in Guatemala and its relationship to socioeconomic disadvantage: A retrospective cohort study. Pediatr Blood Cancer 2019; 66: e27647. [DOI] [PubMed] [Google Scholar]
- 69. Antillon F, de Maselli T, Garcia T, et al. Nutritional status of children during treatment for acute lymphoblastic leukemia in the Central American Pediatric Hematology Oncology Association (AHOPCA): preliminary data from Guatemala. Pediatr Blood Cancer 2008; 50: 502–505; discussion 517. [DOI] [PubMed] [Google Scholar]
- 70. Antillon F, Rossi E, Molina AL, et al. Nutritional status of children during treatment for acute lymphoblastic leukemia in Guatemala. Pediatr Blood Cancer 2013; 60: 911–915. [DOI] [PubMed] [Google Scholar]
- 71. Murphy AJ, Mosby TT, Rogers PC, et al. An international survey of nutritional practices in low- and middle-income countries: a report from the International Society of Pediatric Oncology (SIOP) PODC Nutrition Working Group. Eur J Clin Nutr 2014; 68: 1341–1345. [DOI] [PubMed] [Google Scholar]
- 72. Wilson CL, Liu W, Yang JJ, et al. Genetic and clinical factors associated with obesity among adult survivors of childhood cancer: A report from the St. Jude Lifetime Cohort. Cancer 2015; 121: 2262–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Cohen J, Collins L, Gregerson L, et al. Nutritional concerns of survivors of childhood cancer: A "First World" perspective. Pediatr Blood Cancer 2020; 67: e28193. [DOI] [PubMed] [Google Scholar]
- 74. Rosen GP, Beebe KL, Shaibi GQ. Vitamin D levels differ by cancer diagnosis and decline over time in survivors of childhood cancer. Pediatr Blood Cancer 2013; 60: 949–952. [DOI] [PubMed] [Google Scholar]
- 75. Moke DJ, Hamilton AS, Chehab L, et al. Obesity and risk for second malignant neoplasms in childhood cancer survivors: a case-control study utilizing the California Cancer Registry. Cancer Epidemiol Biomarkers Prev 2019; 28: 1612–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Halton JM, Atkinson SA, Fraher L, et al. Mineral homeostasis and bone mass at diagnosis in children with acute lymphoblastic leukemia. J Pediatr 1995; 126: 557–564. [DOI] [PubMed] [Google Scholar]
- 77. Karlage RE, Wilson CL, Zhang N, et al. Validity of anthropometric measurements for characterizing obesity among adult survivors of childhood cancer: A report from the St. Jude Lifetime Cohort Study. Cancer 2015; 121: 2036–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Selwood K, Ward E, Gibson F. Assessment and management of nutritional challenges in children's cancer care: a survey of current practice in the United Kingdom. Eur J Oncol Nurs 2010; 14: 439–446. [DOI] [PubMed] [Google Scholar]
- 79. Spreafico F, Murelli M, Timmons BW, et al. Sport activities and exercise as part of routine cancer care in children and adolescents. Pediatr Blood Cancer 2019; 66: e27826. [DOI] [PubMed] [Google Scholar]
- 80. Cohen JE, Wakefield CE, Cohn RJ. Nutritional interventions for survivors of childhood cancer. Cochrane Database Syst Rev 2016: CD009678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Schoeman J. Nutritional assessment and intervention in a pediatric oncology unit. Indian J Cancer 2015; 52: 186–190. [DOI] [PubMed] [Google Scholar]
- 82. Trumbo P, Schlicker S, Yates AA, et al. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J Am Diet Assoc 2002; 102: 1621–1630. [DOI] [PubMed] [Google Scholar]