Abstract
Background and Objectives:
We tested the feasibility of a simultaneous resection clinical trial in patients with synchronous colorectal cancer liver metastases to obtain the necessary information to plan a randomized trial.
Methods:
Multicenter feasibility single-arm trial enrolling patients with synchronous colorectal cancer liver metastases eligible for simultaneous resection. Prespecified criteria for feasibility were: proportion of eligible patients enrolled ≥66%, and the proportion of enrolled patients who completed simultaneous resection ≥75%. The prespecified 90-day major postoperative complication rate was 30%.
Results:
Of 61 eligible patients from February 2017 to August 2019, 41 were enrolled (67%; 95% confidence interval [CI], 55%–78%), 32 underwent simultaneous resection (78%; 95% CI, 63%–88%). Four patients were not enrolled due to the surgeon’s preference, three were due to the complexity of resection (right hepatectomy and low anterior resection). Intraoperative complications during liver resection (n = 4) and progression of disease (n = 4) were the main reasons for not undergoing simultaneous resection. The 90-day incidence of major complications was 41% (95% CI, 16%–58%) and the 90-day postoperative mortality was 6% (95% CI, 1.7%–20%).
Conclusion:
According to prespecified criteria, enrolling patients with synchronous colorectal cancer liver metastases to a trial of simultaneous resection is feasible; however, it is associated with higher than anticipated 90-day postoperative complications.
Keywords: clinical trial, colorectal cancer, colorectal liver metastases, feasibility, simultaneous resection, synchronous metastases
1 |. INTRODUCTION
Approximately 25% of patients who are diagnosed with colon cancer have synchronous liver metastases.1–3 These patients with the synchronous disease may still be candidates for a cure by resecting the primary tumor and the liver metastases with or without the addition of systemic chemotherapy.4,5 Patients able to undergo complete margin-negative resection are anticipated to have a 5-year overall survival of 50%.6,7
The appropriate timing of liver and colorectal resection among patients who present with the synchronous disease has not been standardized. Most patients undergo the more traditional staged resection pathway (i.e., resection of the primary and liver metastases on separate admissions with a period of recovery between the two operations), while others undergo simultaneous resection (i.e., resection of the primary and liver metastases on the same operative day). A simultaneous resection is an attractive option as it decreases the number of operations a patient will need, it may have an overall shorter length of hospital stay and thus lower healthcare costs, and it may avoid disease progression while waiting for a second surgery. However, staged resections may have lower rates of postoperative complications. Finally, a delay to liver surgery may demonstrate aggressive disease that avoids what would have proven to be a futile liver resection.
The decision to proceed with a simultaneous versus a staged approach is complex and depends on multiple factors, such as the location of the primary tumor, the extent of liver metastases, patient comorbidities, availability of hepatopancreatobiliary (HPB) surgeons, and local practice. Most studies informing the decision for simultaneous versus staged resections are retrospective and observational, and many are from single centers. Thus, there is great potential for bias favoring lower-risk patients who undergo simultaneous resection or possibly favoring patients who undergo delayed liver resection after demonstrating slow-growing disease. The only way to resolve such uncertainty is by evidence from well-designed and executed randomized clinical trials. The only randomized controlled trial to date comparing simultaneous versus staged resections was recently published.8 This multicenter French trial enrolled 105 patients, of which, 85 were analyzed, and suggested a similar postoperative complication rate between groups (49% vs. 46%, respectively), with a trend towards improved disease-free survival and overall survival in the simultaneous group that did not reach statistical significance. However, the trial was long (over 10 years to accrue) and included a small number of patients. Even in the absence of high-quality evidence, the use of simultaneous resection is increasing.9–11
We performed a pilot single-arm feasibility trial of simultaneous resection. Our primary aim was to determine potential enrollment numbers, rate of enrollment, and rate of simultaneous resection. Results, including potential clinical outcomes, will inform the design of a randomized trial comparing staged versus simultaneous resections.
2 |. MATERIALS AND METHODS
2.1 |. Study design and setting
This was a prospective single-arm feasibility trial at three HPB centers in Ontario, Canada, involving 11 HPB and 12 colorectal surgeons. Ontario is Canada’s largest province (population 15 million) that has centralized high-volume centers performing liver resection. Of the 10 dedicated HPB centers that perform liver resections, these three centers perform 50% of liver resections in the province.
2.2 |. Participants
Potentially eligible patients for the study included adult consecutive patients that presented with resectable primary colorectal cancer and resectable synchronous liver metastases.12 Patients were excluded from potential eligibility if they had the extrahepatic disease (other than resectable lung metastases), planned primary treatment with local transanal excision, liver metastases resection requiring a two-stage liver procedure, prior liver resection, or if the patient was pregnant. Patients were also excluded if they required resection of more than one additional pelvic or abdominal organ involved by direct primary tumor extension (i.e., duodenum, pancreas, bladder, prostate, or gynecological organs). If patients required neoadjuvant chemotherapy or radiation therapy, they were assessed for study eligibility after the planned neoadjuvant treatment was completed.
Eligible patients were approached to be enrolled once their surgeon(s) decided that a simultaneous resection was possible; however, the surgeon’s decision was not a requirement to meet eligibility criteria. The Research Ethics Board at each participating institution approved this study. All patients provided written informed consent before enrollment. This feasibility trial was registered with clinicaltrials.gov (NCT02954913).
2.3 |. Study intervention
Patients underwent resection of the primary tumor and liver metastases in the same anesthetic setting by one or two different surgeons (i.e., colorectal surgeon and HPB surgeon). The treating physician decided the type of colorectal and liver resection. The type of liver resection was described according to the Couinaud classification and the Brisbane terminology of liver anatomy.13,14 Resections of three or more segments of the liver were considered a major liver resection.15 The anesthetic technique and the order of liver resection or colorectal resection were determined by the clinical standard at each institution. It was recommended that a low central venous pressure be maintained to decrease intraoperative blood loss16,17 and that liver resection be performed before colorectal resection to keep a low central venous pressure during that part of the case. Any deviation from the intended intervention (i.e., colorectal or liver resection not performed at the same time of the index operation) was noted with a reason.
2.4 |. Surgery, follow up, and data collection
Patients attending the outpatient HPB clinics were screened for potential eligibility using the inclusion criteria. During the clinic visit, potentially eligible patients that did not meet any exclusion criteria were considered eligible patients for the trial. Patients that were eligible for participation were identified and approached for enrollment after confirming with the treating surgeon (s) that a simultaneous approach was possible at the next clinic visit. Following study enrollment, patients underwent a baseline assessment that included quality of life (QoL) questionnaires. Patients were then assessed the day of their surgery, every day during their hospital stay, at their first postoperative clinic visit, 4 weeks (±1 week) and at 12 weeks (±2 weeks) following the index operation. QoL questionnaire and health resource utilization forms were collected in each postoperative assessment. Operative data (i.e., surgical technique, type of colorectal and liver resection, operative time, and estimated blood loss for each procedure), pathological details, and postoperative complication data up to 90 days following surgery (including procedural reinterventions or reoperations and hospital readmissions or emergency room visits) were collected into case report forms that included deidentified source documentation and sent to the Coordinating Methods Centre in Hamilton, Ontario. Five-year overall survival information, obtained from Provincial Registries (Institute for Clinical and Evaluative Sciences), was a prespecified outcome to be obtained without active patient follow-up.
2.5 |. Outcomes
The goal was to gauge the feasibility of a future randomized controlled trial. Feasibility, the primary outcome was established by predefined criteria: 66% of eligible patients enrolled (enrollment fraction) and the proportion of patients who completed simultaneous resection of at least 75%.18 Baseline characteristics (including the location of the primary tumor and extent of metastases) of enrolled patients would be analyzed to define the inclusion/exclusion criteria of a larger trial. Secondary clinical outcomes included the incidence of major postoperative complications up to 90 days following surgery, which were classified as per Clavien–Dindo (CD) and the comprehensive complication index.19,20 Although not a component of the feasibility criteria, before study start-up, the steering committee agreed that a major complication rate of 30% would be the highest rate accepted for patients undergoing simultaneous resection for synchronous disease, a relative risk increase of 50% (from the baseline rate of 20% obtained from the literature).8,15,21,22 Other secondary outcomes included health-related QoL using the EORTC-QLQ-C30 and the EORTC-QLQ-LMC21.23,24
2.6 |. Statistical analyses
The sample size was based on the precision of the proportion of eligible patients being enrolled. Assuming an estimated enrollment of 66%, we would require 60 eligible patients to yield a 95% confidence interval (CI) of 54–77 around the estimated enrollment percentage. This would require more than 40 patients to be enrolled. Patient baseline characteristics and demographics, including operative characteristics, were presented using descriptive statistics. Categorical variables were presented in number and percentage and continuous variables as the median and interquartile range (IQR), as appropriate. The proportion with 95% CI of overall and major postoperative complications and the mortality at 90 days were calculated using the Wilson–Score method. QoL outcomes were summarized using means and corresponding standard deviations (SD). A change in the mean score of 10% or more was defined as a minimal clinically important difference (10-point difference in both scales).25,26 Statistical analyses were performed using R (R Foundation for Statistical Computing, version 3.5.0).
3 |. RESULTS
3.1 |. Patient demographics
The median age at the time of enrollment was 57 (IQR, 50–67). The most common location of the primary tumor was the rectum in 18/41 (44%) patients, followed by the right (12/41, 29%) and left (11/41, 27%) colon. The median number of liver lesions on imaging was 2 (IQR, 1–3) with 17/41 (42%) patients having bilateral liver lesions. Preoperative chemotherapy was administered to 27/41 (68%) patients (categorized as palliative chemotherapy in 9/41, 22%), with a median number of cycles of 6 (IQR, 5–8) (Table 1). All enrolled patients completed the follow-up schedule.
TABLE 1.
Baseline patient characteristics
| Variable | n = 41 |
|---|---|
| Age median (IQR) | 57 (50–67) |
| Female sex n (%) | 13 (32%) |
| Charlson comorbidity index n (%) | |
| 0 | 3 (7%) |
| 1 | 7 (17%) |
| ≥2 | 31 (76%) |
| ECOG performance status | |
| ECOG 0 n (%) | 19 (58%) |
| ECOG 1 n (%) | 13 (39%) |
| ASA physical status classification | |
| ASA 3 n (%) | 15 (38%) |
| ASA 4 n (%) | 25 (63%) |
| Primary n (%) | |
| Right and transverse colon | 12 (29%) |
| Left and sigmoid colon | 11 (27%) |
| Rectum | 18 (44%) |
| Number of liver lesions (radiology) (median, IQR) | 2 (1–3) |
| Max size liver lesions (radiology) (median, IQR) (mm) | 19 (14–31) |
| Bilateral liver lesions (radiology) n (%) | 17 (42%) |
| Preoperative CEA (median, IQR) (μg/L) | 4.7 (2.3–22) |
| Presurgical treatment (diversion) n (%) | 3 (7.3%) |
| Neoadjuvant therapy | |
| Chemotherapy n (%) | 27 (68%) |
| Number of cycles (median, IQR) | 6 (5–8) |
| Palliative intent n (%) | 9 (22%) |
| Radiation therapy n (%) | 16 (39%) |
Abbreviations: ASA, American Society of Anesthesiologists; CEA, carcinoembryonic antigen; ECOG, European Cooperative Oncology Group; IQR, interquartile range.
3.2 |. Patient flow and feasibility outcome measure
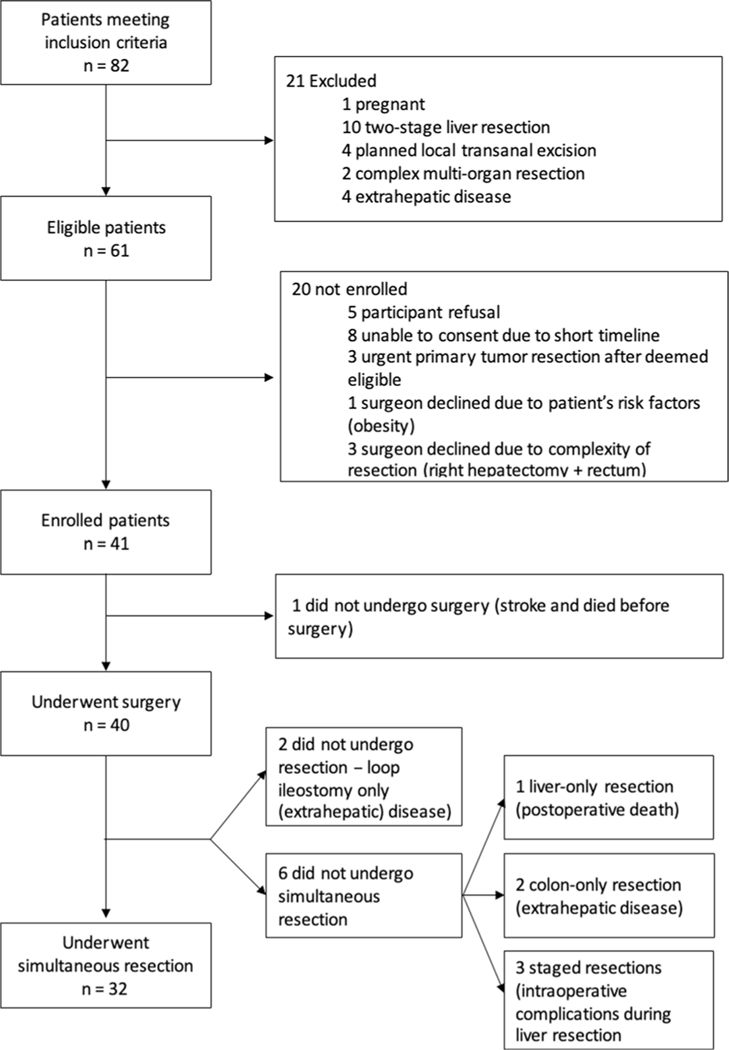
From February 2017 to August 2019, there were 82 patients who met the inclusion criteria and were deemed potentially eligible patients, of which, 21 met exclusion criteria, leaving 61 eligible patients (eligibility fraction 74%, 61/82). Of those, 41 (67%; 95% CI, 55%–78%) were enrolled (enrollment fraction, primary feasibility outcome). The reasons for nonenrollment were: eight patients were not approached with enough time before surgery, five patients refused to participate, four patients were not being enrolled due to surgeon’s choice (three due to complexity of the resection [i.e., right hepatectomy and low anterior resection] and one due to patient’s factors [i.e., obesity]), and three patients had an urgent primary tumor resection after being deemed eligible. The recruitment fraction was 50% (41/82). Of the 41 patients enrolled, 40 patients underwent surgery (one patient had a lethal preoperative stroke) and 32 underwent simultaneous resection (secondary feasibility outcome: 78%; 95% CI, 63%–88%). Reasons for not proceeding with simultaneous resection were: two patients not undergoing surgical resection (exploratory laparotomy/laparoscopy only) due to progression of metastatic disease found at the time of surgery; one patient undergoing liver-only resection due to intraoperative complications leading to death; two patients undergoing colon-only resection due to intraoperative findings of extrahepatic metastatic disease; and three patients undergoing staged resections due to intraoperative complications during liver resection (i.e., bleeding). The median time to staged resection in those three patients was 14 weeks (range 12–16) (Figure 1).
FIGURE 1.
Patient flow. Patients meeting inclusion criteria: Potentially eligible patients. Eligible patients: Patients meeting eligibility criteria
3.3 |. Clinical outcomes
Among patients who underwent simultaneous resection, the most commonly performed liver resection was a wedge nonanatomical resection (one or multiple wedges) in 10/32 (31%) patients followed by left lateral sectionectomy in 8/32 (25%) patients and right hemihepatectomy in 6/32 (19%) patients. The wedge resection were of the following segments: Segment 2 (n = 1), Segment 7 (n = 2), Segment 6 (n = 1), Segment 8 (n = 2), and multiple segments (n = 4). The most commonly performed colorectal resection was a low anterior resection in 14/32 (44%) patients, followed by right hemicolectomy in 10/32 (31%) patients (Table 2). On pathology report, the positive margin rate (i.e., R1—less or equal to 1 mm) was 9/32 (28%), mostly driven by the liver specimen (7/32, 23%—all seven margins ranging from 0.1 to 0.9 mm). There were no R2 resections performed.
TABLE 2.
Perioperative characteristics
| Variable | Patients who underwent simultaneous liver and colon surgery n = 32 |
|---|---|
| Operative approach n (%) | |
| Laparoscopic | 13 (41%) |
| Laparoscopic converted to open | 1 (3%) |
| Open | 18 (56%) |
| Midline incision | 18 |
| Subcostal incision | 1 |
| Liver as first organ resected n (%) | 24 (75%) |
| Multivisceral resectionan (%) | 6 (19%) |
| Liver resection type n (%) | |
| Right hemihepatectomy | 6 (19%) |
| Right posterior sectionectomy | 5 (16%) |
| Left hemihepatectomy | 3 (9%) |
| Left lateral sectionectomy | 8 (25%) |
| Wedge resections | 10 (31%) |
| Number of segments resected median (IQR) | 2 (2–4) |
| Colon resection type n (%) | |
| Right hemicolectomy | 10 (31%) |
| Left colectomy/sigmoidectomy | 2 (6%) |
| Low anterior resection | 14 (44%) |
| Abdominoperineal resection | 5 (13%) |
| Subtotal colectomy | 2 (6%) |
| Intraoperative blood loss (median, IQR) (ml) | 615 (395–1000) |
| Liver surgery | 400 (300–700) |
| Colon surgery | 200 (100–375) |
| Receipt of transfusion of blood products n (%) | 8 (25%) |
| Receipt of transfusion of pRBC n (%) | 5 (16%) |
| Number of pRBC transfused (median, IQR) | 2 (1–3) |
| OR time (median, IQR) (min) | 381 (269–425) |
| Liver surgery | 181 (95–252) |
| Colon surgery | 174 (124–226) |
Abbreviations: IQR, interquartile range; pRBC, packed red blood cells.
Multivisceral resection included: tail pancreas and spleen, duodenum, abdominal wall, ovaries, and diaphragm.
Major postoperative complications (CD ≥ 3) occurred in 16/40 (39%; 95% CI, 26%–54%) patients among those who underwent surgery, and in 13/32 (41%; 95% CI, 26%–58%) patients undergoing simultaneous resection (Table 3 and Table S1), respectively. The nonoperative reintervention rate was 14/40 (35%; 95% CI, 22%–51%) and 13/32 (41%; 95% CI, 26%–58%), the operative reintervention rate was 3/40 (8%; 95% CI, 2.6%–20%) and 3/32 (9%; 95% CI, 3%–24%), and the postoperative mortality rate was 4/40 (10%; 95% CI, 4%–23%) and 2/32 (6%; 95% CI, 1.7%–20%). The postoperative causes of death were: progression of cancer (patient did not undergo resection), postoperative bleeding (patient underwent liver-only resection), and for those who underwent simultaneous resection: posthepatectomy liver failure and colonic anastomotic dehiscence.
TABLE 3.
Postoperative outcomes
| Outcomes | Patients who underwent simultaneous liver and colon surgery n = 32 |
|---|---|
| Length of hospital stay (median, IQR) (days) | 10 (6–17) |
| Readmission n (%, 95% CI) | 6 (19%, 9%–35%) |
| 90-day all postoperative morbidity (Clavien–Dindo Grades 1–5), n (%, 95% CI) | 22 (69%, 51%–82%) |
| 90-day major postoperative morbidity (Clavien–Dindo Grade ≥ 3) n (%, 95% CI) | 13 (41%, 26%–58%) |
| Nonoperative intervention n (%, 95% CI) | 13 (41%, 26%–58%) |
| Operative reintervention n (%, 95% CI) | 3 (9%, 3%–24%) |
| 90-Day postoperative mortality n (%, 95% CI) | 2 (6%, 1.7%–20%) |
| Comprehensive complication index median (median, IQR) | 35 (29–51) |
Abbreviations: CI, confidence interval; IQR, interquartile range.
Of enrolled patients, there were 39/41 (95%) patients who completed the baseline QoL assessments, 35/38 (92%) the first postoperative assessment and 33/36 (92%) the second postoperative assessment. Of the patients who underwent simultaneous resection, 26/32, 81% completed all planned QoL questionnaires.
There was a decline in the mean global health QoL (EORTC-QLQ-C30) from baseline (mean, 68; SD, 24.8) to the 1-month (mean, 62; SD, 23; difference: −6.02) and 3-month evaluation (mean, 64; SD, 20; difference: −3.48). The physical functioning score had a clinically significant decline from baseline (mean, 86; SD, 17) to the 1-month evaluation (mean, 72; SD, 24; difference: −13.97), which recovered at 3 months (mean, 83; SD, 18; difference: −3.19), whereas role functioning declined from baseline (mean, 76; SD, 30) to the 1-month evaluation (mean, 52; SD, 33; difference: −23.68) and did not recover at 3 months (mean, 56; SD, 32; difference: −19.39). Social functioning declined from baseline (mean, 78; SD, 23) to the 1-month (mean, 67; SD, 30; difference: −10.96) and 3-month evaluation (mean, 67; SD, 27; difference: −10.96). The EORTC-QLQ-LMC-21 identified that fatigue remained an important symptom that did not improve from baseline (mean, 33; SD, 26) to the 1-month and the 3-month evaluation (mean difference: −12.12 and −14.24, respectively).
4 |. DISCUSSION
This study found that enrolling patients with synchronous colorectal cancer liver metastases to a trial of simultaneous resection is feasible, according to the prespecified enrollment fraction criteria and the proportion of enrolled patients undergoing simultaneous resection. Of the eligible patients, there were only five (5/61, 8%) patients that refused participation, four (4/61, 7%) that were not enrolled due to surgeon’s choice, and three patients (3/61, 5%) due to logistical reasons (primary tumor resected urgently elsewhere).
One of the goals of this study is to identify the patient population that surgeons would feel comfortable including in a randomized trial comparing simultaneous to staged liver resections. There were three eligible patients that were not enrolled due to the complexity of resection (i.e., right hepatectomy and low anterior resection), and of the patients enrolled there was only one that underwent a right hepatectomy simultaneous with low anterior resection. These findings suggest that patients who require a right hepatectomy and low anterior resection, may not be favored to participate in a trial including simultaneous resection. Although we collected the reasons for not enrolling patients that were eligible for the study, we did not collect information on patients that had their primary tumor removed before assessment of their liver metastases. Since patients were enrolled at tertiary care referral centers, it is possible that there were many other patients that would have been eligible for simultaneous resection if their primary were still in situ at the time of assessment, which may have made the enrollment fraction lower and the patient population different (i.e., older patients, more complex resections). In this study, there were three patients undergoing wedge resection of Segment 2 or Segment 6 combined with a rectal resection or a left hepatectomy. This may be another patient population not be suitable for a randomized trial (i.e., patients that require simple wedge resection of the liver, as surgeons may think that the added morbidity to any colorectal resection would be minimal.
While not defined as a limiting factor to determine feasibility, patients undergoing simultaneous resection experienced a higher than anticipated rate of major postoperative complications. Although this finding can seem disturbing, a recently published randomized trial comparing simultaneous to staged resections for colorectal cancer liver metastases found a major postoperative complication rate of 41% in the simultaneous group, suggesting a similar patient population between studies and implying that the rate of 20% obtained from previous studies was inaccurate.8 It is our belief that this rate although high, is still in the acceptable range for a complex procedure like this one, especially considering that the postoperative mortality rate, although higher compared to the mortality observed following liver resection alone (3%–5%),27 is similar to previously reported series of simultaneous resection.3,28 It is also reassuring that even though many QoL domains decreased significantly one month after surgery, most recovered to baseline levels by 3 months, consistent with previously published work on QoL in patients undergoing liver resection for colorectal cancer metastases.29 This is especially valid, considering the high compliance rate, suggesting that our QoL results did not overestimate the true QoL.
Some of the limitations of this study include its relatively small sample size, which decreases our ability to make generalizable conclusions, such as predictors of postoperative complications and mortality following surgery, although that was not the purpose of this feasibility trial. It is not clear with this study if patients undergoing complex rectal and liver resections experienced significantly worse postoperative complications compared to those who undergo less complex resections (i.e., left lateral sectionectomy and right hemicolectomy). Those analyses would have provided the surgeon a clearer picture when deciding to enroll patients in a simultaneous versus staged randomized trial. Most importantly, given that this was not a pilot randomized study, we did not answer the question of whether surgeons and patients were willing to enroll in a randomized trial of simultaneous versus staged resection. At the beginning of this study, we wanted to confirm that surgeons were capable of enrolling patients and that patients were willing to enroll in a simultaneous resection study, as the idea of this type of resection was relatively new and not fully embraced by the surgical community. With that in mind, we kept a record of patients that were eligible but not enrolled, including reasons for not being enrolled, and found only five patients who refused to participate in this trial. The recently published randomized trial from Europe may give us a glimpse of the struggles of including patients in such a trial since it took more than 10 years to enroll 85 patients.8 They cite the difficulty of finding eligible patients in tertiary care institutions since most resectable cases would be resected outside of the HPB center before referral. This situation may also be the case in our region as suggested by prior surgeon surveys in our area; however, the current study was not designed to prove that hypothesis.30 Moreover, this study is not able to answer the question of whether simultaneous resection of certain cases has already become standard of care. We do not know if surgeons would be willing to randomize a patient to a trial comparing simultaneous versus staged resection if the surgical community as a whole believes that a simple liver resection (i.e., a wedge of the left lateral sector) should be performed at the same time as a simple colon resection (i.e., right hemicolectomy).
5 |. CONCLUSION
It is feasible for surgeons to enroll patients in a trial of simultaneous resection for synchronous colorectal cancer liver metastases; however, surgeons may not be willing to enroll patients requiring complex procedures such as right hepatectomy and low anterior resection. Patients undergoing simultaneous resection have a high rate of postoperative complications, although this is not an impediment for a trial as the postoperative mortality rate is low and the decline in QoL seen at 1 month from surgery is transient, with most domains returning back to baseline at 3 months from surgery. Results from this study will be used to build upon a larger randomized trial comparing simultaneous to staged resections, providing relevant information that can be used to determine patient population, calculate sample size, and define outcomes of interest.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported in part by a grant from McMaster Surgical Associates and McMaster International Initiatives Micro-Fund.
Funding information
McMaster University, Grant/Award Numbers: McMaster International Initiatives Micro-Fund, McMaster Surgical Associates
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of the article at the publisher’s website.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Canadian Cancer Statistics Advisory Committee. Canadian Cancer Statistics 2019. Canadian Cancer Society; 2019. [Google Scholar]
- 2.Adam R, de Gramont A, Figueras J, et al. Managing synchronous liver metastases from colorectal cancer: a multidisciplinary international consensus. Cancer Treat Rev. 2015;41(9):729–741. [DOI] [PubMed] [Google Scholar]
- 3.Bogach J, Wang J, Griffiths C, et al. Simultaneous versus staged resection for synchronous colorectal liver metastases: a population-based cohort study. Int J Surg. 2020;74:68–75. [DOI] [PubMed] [Google Scholar]
- 4.Benson AB, Venook AP, Al-Hawary MM, et al. Rectal cancer, version 2.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16(7):874–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Engstrom PF, Arnoletti JP, Benson AB, et al. NCCN clinical practice guidelines in oncology: colon cancer. J Natl Compr Canc Netw. 2009; 7(8):778–831. [DOI] [PubMed] [Google Scholar]
- 6.Serrano PE, Gu CS, Husien M, et al. Risk factors for survival following recurrence after first liver resection for colorectal cancer liver metastases. J Surg Oncol. 2019;120:1420–1426. [DOI] [PubMed] [Google Scholar]
- 7.Kim HS, Lee JM, Kim HS, et al. Prognosis of synchronous colorectal liver metastases after simultaneous curative-intent surgery according to primary tumor location and KRAS mutational status. Ann Surg Oncol. 2020;27(13):5150–5158. [DOI] [PubMed] [Google Scholar]
- 8.Boudjema K, Locher C, Sabbagh C, et al. Simultaneous versus delayed resection for initially resectable synchronous colorectal cancer liver metastases: a prospective, open-label, randomized, controlled trial. Ann Surg. 2021;273(1):49–56. [DOI] [PubMed] [Google Scholar]
- 9.Vallance AE, van der Meulen J, Kuryba A, et al. The timing of liver resection in patients with colorectal cancer and synchronous liver metastases: a population-based study of current practice and survival. Colorectal Dis. 2018;20(6):486–495. [DOI] [PubMed] [Google Scholar]
- 10.Abelson JS, Michelassi F, Sun T, et al. Simultaneous resection for synchronous colorectal liver metastasis: the new standard of care? J Gastrointest Surg. 2017;21(6):975–982. [DOI] [PubMed] [Google Scholar]
- 11.Idrees JJ, Bagante F, Gani F, et al. Population level outcomes and costs of single stage colon and liver resection versus conventional two-stage approach for the resection of metastatic colorectal cancer. HPB. 2019;21(4):456–464. [DOI] [PubMed] [Google Scholar]
- 12.Serrano PE, Gafni A, Parpia S, et al. Simultaneous resection of colorectal cancer with synchronous liver metastases (RESECT), a pilot study. Int J Surg Protoc. 2018;8:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bismuth H.Revisiting liver anatomy and terminology of hepatectomies. Ann Surg. 2013;257(3):383–386. [DOI] [PubMed] [Google Scholar]
- 14.Strasberg SM. Nomenclature of hepatic anatomy and resections: a review of the Brisbane 2000 system. J Hepatobiliary Pancreat Surg. 2005;12(5):351–355. [DOI] [PubMed] [Google Scholar]
- 15.Reddy SK, Pawlik TM, Zorzi D, et al. Simultaneous resections of colorectal cancer and synchronous liver metastases: a multi-institutional analysis. Ann Surg Oncol. 2007;14(12):3481–3491. [DOI] [PubMed] [Google Scholar]
- 16.Chen H, Merchant NB, Didolkar MS. Hepatic resection using intermittent vascular inflow occlusion and low central venous pressure anesthesia improves morbidity and mortality. J Gastrointest Surg. 2000;4(2):162–167. [DOI] [PubMed] [Google Scholar]
- 17.Hughes MJ, Ventham NT, Harrison EM, Wigmore SJ. Central venous pressure and liver resection: a systematic review and meta-analysis. HPB. 2015;17(10):863–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wright JR, Bouma S, Dayes I, et al. The importance of reporting patient recruitment details in phase III trials. J Clin Oncol. 2006;24(6):843–845. [DOI] [PubMed] [Google Scholar]
- 19.Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slankamenac K, Graf R, Barkun J, Puhan MA, Clavien PA. The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg. 2013;258(1):1–7. [DOI] [PubMed] [Google Scholar]
- 21.Chua HK, Sondenaa K, Tsiotos GG, Larson DR, Wolff BG, Nagorney DM. Concurrent vs. staged colectomy and hepatectomy for primary colorectal cancer with synchronous hepatic metastases. Dis Colon Rectum. 2004;47(8):1310–1316. [DOI] [PubMed] [Google Scholar]
- 22.Serrano PE, Gafni A, Gu CS, et al. Positron emission tomography-computed tomography (PET-CT) versus No PET-CT in the management of potentially resectable colorectal cancer liver metastases: cost implications of a randomized controlled trial. J Oncol Pract. 2016;12(7):e765–e774. [DOI] [PubMed] [Google Scholar]
- 23.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376. [DOI] [PubMed] [Google Scholar]
- 24.Groenvold M, Klee MC, Sprangers MA, Aaronson NK. Validation of the EORTC QLQ-C30 quality of life questionnaire through combined qualitative and quantitative assessment of patient-observer agreement. J Clin Epidemiol. 1997;50(4):441–450. [DOI] [PubMed] [Google Scholar]
- 25.Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16(1):139–144. [DOI] [PubMed] [Google Scholar]
- 26.Ringash J, O’Sullivan B, Bezjak A, Redelmeier DA. Interpreting clinically significant changes in patient-reported outcomes. Cancer. 2007;110(1):196–202. [DOI] [PubMed] [Google Scholar]
- 27.Ercolani G, Grazi GL, Ravaioli M, et al. Liver resection for multiple colorectal metastases: influence of parenchymal involvement and total tumor volume, vs number or location, on long-term survival. Arch Surg. 2002;137(10):1187–1192. [DOI] [PubMed] [Google Scholar]
- 28.Capussotti L, Ferrero A, Vigano L, Ribero D, Lo Tesoriere R, Polastri R. Major liver resections synchronous with colorectal surgery. Ann Surg Oncol. 2007;14(1):195–201. [DOI] [PubMed] [Google Scholar]
- 29.Wei AC, Coburn NG, Moulton C, et al. A phase II multicenter study of metastasectomy of intra- and extra-hepatic metastases from colorectal cancer. J Clin Oncol. 2013;31(suppl 4):482.23248257 [Google Scholar]
- 30.Griffiths C, Bogach J, Simunovic M, et al. Simultaneous resection of colorectal cancer with synchronous liver metastases; a practice survey. HPB. 2020;22(5):728–734. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.