Abstract
Background
Chimeric antigen receptor (CAR) T cells have achieved remarkable responses in patients with hematological malignancies; however, the potential of this therapeutic platform for solid tumors like glioblastoma (GBM) has been limited, due in large part to the targeting of single antigens in a heterogeneous disease. Strategies that allow CAR T cells to engage multiple antigens concomitantly may broaden therapeutic responses and mitigate the effects of immune escape.
Methods
Here we have developed a novel, dual-specific, tandem CAR T (TanCART) cell with the ability to simultaneously target both EGFRvIII and IL-13Rα2, two well-characterized tumor antigens that are frequently found on the surface of GBM cells but completely absent from normal brain tissues. We employed both standard immunological assays and multiple orthotopic preclinical models including patient-derived xenograft to demonstrate efficacy of this approach against heterogeneous tumors.
Results
Tandem CAR T cells displayed enhanced cytotoxicity in vitro against heterogeneous GBM populations, including patient-derived brain tumor cultures (P < .05). Compared to CAR T cells targeting single antigens, dual antigen engagement through the tandem construct was necessary to achieve long-term, complete, and durable responses in orthotopic murine models of heterogeneous GBM, including patient-derived xenografts (P < .05).
Conclusions
We demonstrate that TanCART is effective against heterogeneous tumors in the brain. These data lend further credence to the development of multi-specific CAR T cells in the treatment of GBM and other cancers.
Keywords: chimeric antigen receptor, glioblastoma, immunotherapy
Key Points.
Tandem CAR T (TanCART) cells were efficiently generated using a single lentiviral construct at high transduction efficiencies.
Simultaneous, dual-antigen targeting with TanCART cells achieved complete and durable cures in murine models of heterogeneous GBM.
Importance of the Study.
The most common primary malignant brain tumor, GBM, is also the most lethal, with few effective treatment options. Prior studies have shown that CAR T cells targeting the tumor-specific mutation of epidermal growth factor receptor (CART-EGFRvIII) have the capacity to accumulate in and target EGFRvIII-expressing glioma. However, CARs against single antigens are hampered by tumor heterogeneity, leading to eventual immune escape and progression. To mitigate these effects, we modified CART-EGFRvIII to simultaneously target IL-13ɑR2, another frequently expressed glioma-associated antigen, using a novel tandem CAR (TanCAR) that incorporates antigen-recognition moieties already shown to be safe in Phase I clinical trials. TanCART cells had superior activity compared to monospecific CARs and were able to achieve complete and durable tumor responses in murine models of heterogeneous GBM. This strategy warrants further investigation in patients and could be generalized to target alternative antigen combinations for both GBM and other solid tumors.
Glioblastoma (GBM) is the most common and most aggressive primary malignant brain tumor, remaining uniformly lethal despite multimodal therapy including maximal resection, radiation therapy, and chemotherapy.1 Immune-based therapy including checkpoint blockade with antibodies has demonstrated proven clinical benefit across several different liquid and solid cancer types, but has been less successful in GBM, which may be due to inadequate priming of T cells and a local immunosuppressive microenvironment.2
One way to bypass the limited T cell receptor (TCR) repertoire and to greatly enhance the activity of antitumor lymphocytes is to engineer T cells to express chimeric antigen receptors (CARs) and to infuse these cells as adoptive immunotherapy. Several CARs targeting different antigens in GBM have been recently described (eg, EGFRvIII,3 IL-13Rɑ2,4 HER2,5 EphA26,7), some of which are in early clinical development. Importantly, CAR T cells have been shown to localize to the brain and, in at least one case, were able to mediate the regression of late-stage, multifocal, bulky tumors. However, despite initial responses, CAR T cells against single antigens have been associated with disease recurrence, target-antigen loss, and escape in both GBM3,4 and in hematologic malignancies in which CD19 was targeted.8
Novel CAR T cells that target multiple antigens simultaneously have the potential to mitigate the impact of antigen heterogeneity and have emerged as a promising strategy for antigen-loss variants in hematological cancers.9 Several combinations of target antigens have been proposed with the goal of offsetting immune escape in GBM. However, prior efforts have involved targets with substantial expression in healthy tissues (eg, HER2),10 an approach that has been shown carry the potential risk of fatal autoimmune toxicity.11
Unlike other GBM-associated surface antigens, EGFRvIII and IL-13Rɑ2 are unique in that they are frequently present on the surface of GBM12 but completely absent or expressed at negligible levels in somatic tissues, thereby minimizing the risks of on-target, off-tumor toxicity. Recent work has also revealed that EGFRvIII and IL-13Rɑ2 can be co-expressed in the same cell and that intracellular crosstalk between these two molecules specifically confers a growth advantage to tumors,13 making them an especially favorable combination for concomitant targeting.
Here, we report preclinical testing and development of a novel tandem CAR T cell (TanCART) with dual specificity for EGFRvIII and IL-13Rɑ2, housed in a single transgene construct. We describe the design and the functional capacity of this therapy, demonstrating superiority of TanCART compared to monospecific CAR T cells in models of heterogeneous tumors and patient-derived GBM.
Materials and Methods
Cell Lines
The human glioma cell line U87MG and the chronic myeloid leukemia line K562 were purchased from the American Type Culture Collection (ATCC). The patient-derived glioblastoma neurosphere cell line, BT74, was provided by Dr. Santosh Kesari.14 This cell line was transduced to constitutively express green fluorescent protein. To isolate IL-13Rɑ2-positive cells, endogenous IL-13Rɑ2high U87MG cells were selected by flow cytometry. To create EGFRvIII-positive, IL-13Rɑ2-negative cells, the U87MG cell line was genetically modified by knocking out IL-13Rɑ2 through CRISPR-Cas9 with a single-guide RNA (sgRNA) from the Brunello library. This line was then engineered to express EGFRvIII by lentiviral transduction.15 The K562 cell line was genetically modified to express either IL-13Rɑ2 or EGFRvIII by lentiviral transduction. Cells were then sorted to collect a clonal population of either IL-13Rɑ2-negative, EGFRvIII-positive or IL-13Rɑ2-positive, EGFRvIII-negative. U87MG cells were cultured in Eagle’s Minimum Essential Medium (EMEM), supplemented with 10% heat-inactivated fetal bovine serum (FBS) as well as penicillin and streptomycin. K562 cells were cultured in Roswell Park Memorial Institute (RPMI) 1640 (1X) + GlutaMAX, supplemented with 10% FBS as well as penicillin and streptomycin. BT74 cells were cultured in Neurobasal medium, supplemented with L-Glutamine, B27 supplement, N2 supplement, heparin, penicillin, streptomycin, amphotericin B, human recombinant epidermal growth factor, and human recombinant fibroblast growth factor, as previously described.14
CAR T Cell Constructs
All CAR constructs incorporated a second-generation design containing either a hGM-CSF or CD8 leader sequence, a CD8 transmembrane (TM) domain, a 4-1BB costimulatory domain, and a CD3-zeta signaling domain. In addition, an mCherry reporter, separated by a T2A cleavage site, was inserted to allow for measurement of the transduction efficiency of the transduced cells. All experiments were performed with donor-matched activated untransduced (UTD) T cells, which served as a negative control. The CAR T cell targeting IL-13Rɑ2 is based on a previously described construct from Dr. Christine Brown and colleagues,16 which consists of a membrane tethered IL-13 ligand mutated at E13Y, which allows the zetakine to bind more specifically to IL-13Rɑ2 relative to the more widely expressed IL-13Rɑ1/IL-4Rɑ complex. CART-EGFRvIII contains a humanized scFv that is specific for EGFRvIII over wild-type EGFR.17 The tandem CAR construct was constructed using both the IL-13 zetakine and the humanized EGFRvIII scFv as described above.
CAR T Cell Production
Healthy donor leukopaks were purchased from the Massachusetts General Hospital blood bank under an IRB-approved protocol. Primary human T cells (Stem Cell Technologies, Cat. #15061) were purified and cryopreserved. To generate CAR T cells, primary T cells were thawed and activated using anti-CD3/CD28 Dynabeads (Life Technologies) at a 3:1 Dynabeads:T cells ratio on Day 0. On Day 1, activated T cells were transduced with lentivirus at a multiplicity of infection of 5 or 10. CART cells were de-beaded on Day 6–7 to allow for further expansion. Transduction efficiency was measured on Day 9–10 and cells were stored in liquid nitrogen on Day 10–15. Cells were cultured in RPMI 1640 (1X) + GlutaMAX with 10% FBS, penicillin, streptomycin, and supplemented with 20 IU ml−1 recombinant human IL-2. CAR T cells expressing different CAR constructs were normalized for CAR expression by adding UTD cells before cryopreservation and thawed prior to functional assays. All reported numbers of CAR T cell in the manuscript are based on CAR positivity.
T Cell Activation and Proliferation Assays
We conducted T cell activation assays where UTD cells, CART-EGFRvIII, CART-IL-13Rɑ2, or TanCART cells were co-cultured with K562 cells expressing either IL-13Rɑ2 or EGFRvIII for 16 h. The cells were then subjected to flow cytometry and assessed for CD69 expression. Long-term proliferation assays were performed using two iterations of irradiated (ie, 10 000 Rads in a Cesium-137 irradiator) K562 expressing the aforementioned targets of interest. Irradiated K562 cells were co-cultured with effector cells from three healthy donors at a 1:1 ratio. Media was replenished on Day 3–4. Every week, cells were counted to measure population doubling. Following counting, CAR T cells were re-stimulated with irradiated K562.
Cytotoxicity Assays
We measured cytotoxicity for the U87MG using the xCelligence Acea Biosciences RTCA Multi Plate reader (ACEA Biosciences). This instrument uses non-invasive electrical impedance monitoring to detect cell attachment in real-time. E-Plate View 96 plates were washed twice and plated with 20 000 U87MG cells per well. Cell index was measured for 27 h before the addition of effector cells. The assay was recorded over 96 h and measurements were taken every 15 min. Percentage specific lysis was calculated using the following equation: Percentage = [(cell index of target cells − cell index of CAR T cells)/(cell index of target cells)] × 100. For the BT74 neurosphere line, cytotoxicity was measured by IncuCyte Live Cell Analysis. This instrument measures cytotoxicity through the measurements of total green-fluorescent area. Proliferation of effector cells was measured by quantifying the total red fluorescence area. BT74 cells were cultured at 10 000 cells per well for three days prior to the addition of effector cells.
Flow Cytometry
The following antibody clones were used for each target: EGFRvIII (L8A4, Absolute Antibody), IL-13Rɑ2 (SHM38, BioLegend), CD69 (FN50, BioLegend), CD3 (UCHT1, BD Biosciences), CD4 (SK3, BD Biosciences), and CD8 (SK1, BD Pharmingen). DAPI was used to distinguish live cells from the dead. Cells were stained in the dark for 15 min at room temperature, washed twice in PBS with 2% FBS, and acquired on a Fortessa X-20 (BD Biosciences).
Animal Models
The mice used in these experiments were NOD.Cg-PrkdcscidIL2rgtm1Wjl/SzJ (NSG) purchased from the Jackson Laboratory. They were bred under pathogen-free conditions and in line with the protocols approved by the Massachusetts General Hospital Institutional Animal Care and Use Committee (IACUC). Mice were randomized to intraventricularly receive either UTD, CART-EGFRvIII, CART-IL-13Rɑ2, or TanCART cells suspended in PBS. For all intracranial implantations, tumor cells were loaded in a 10 μl syringe with a detachable 31 gauge needle (Hamilton). With the assistance of a stereotactic frame, tumor cells were implanted at 2 mm lateral to the bregma and a depth of 4 mm from the surface of the skull at the coronal suture. CAR T cells were loaded in an Ultra-Fine Insulin Syringe (3/10 mL 30G × 12.7 mm, BD Biosciences) and delivered intraventricularly, contralateral to the tumor implantation, infused at 2 mm to the left of and 0.3 mm anterior to bregma at a depth of 3 mm, as previously described.18 For all experiments, mice were treated with 1 × 106 CAR T cells, which has been previously reported to be in a typical “low” dose range for murine studies.19 Mice were monitored by trained, dedicated research personnel responsible for observing and evaluating animals. Euthanasia was determined by humane endpoints defined by deteriorating body condition, weight loss, inability to rise or ambulate, dehydration, and the presence of ulcerated, necrotic or infected tumors. Post-hoc power calculations were performed between TanCART-treated mice versus those treated with the UTD control utilizing log transformed bioluminescence measurements of tumor growth (BT74) as a continuous variable at day 70 post-treatment. Such an analysis with three mice per group had a 97.2% power to detect a difference between groups assuming ɑ = 0.05.20
Statistical Analysis
All analyses were performed with GraphPad Prism 7.0c software. Data were presented as means ± SD or SEM with statistically significant differences determined by tests as indicated in figure legends. All data presented in the manuscript, both in vitro and in vivo, reflect the results of multiple experiments (at least two), in some cases across several different model systems.
Results
Design and Generation of CAR T Cells With Single and Dual Antigen Specificity
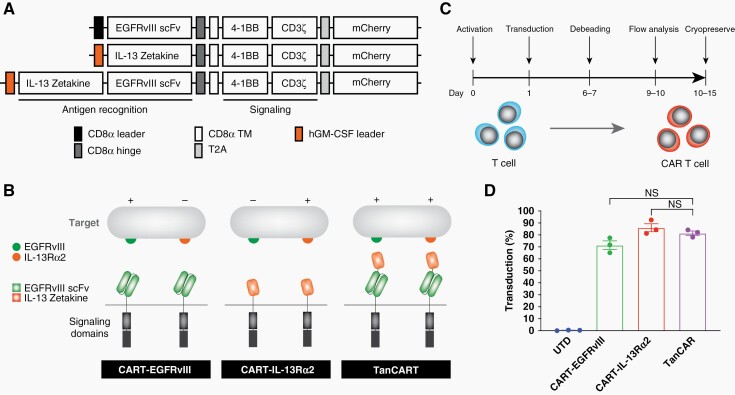
To target GBM, we designed several second-generation CAR constructs with single and dual specificity for IL-13Rɑ2 and EGFRvIII. We used two monospecific CAR T cells, CART-EGFRvIII and CART-IL-13Rɑ2, which employed the humanized anti-EGFRvIII scFv and IL-13 zetakine respectively, both of which have established safety profiles in prior clinical trials.3,16 In addition, we designed a TanCART construct with dual specificity consisting of both the anti-EGFRvIII scFv and IL-13 zetakine translated in tandem separated by a flexible glycine-serine linker (Figure 1A and B). We generated and tested the two possible orientations regarding the order of antigen-recognition domains (ie, humanized EGFRvIII scFv proximal to the membrane with IL-13 zetakine distal, and vice versa). The construct consisting of a proximal IL-13 zetakine and distal EGFRvIII scFv resulted in unforeseen effects on lentivirus production and yielded prohibitively low titers, precluding T cell transduction. Regarding the flexible linker, we selected a standard (Gly4Ser)4 sequence that we have optimized and used with success in our previously published work.21 In addition, this 20 amino acid Gly-Ser linker was employed in the original manuscript describing TanCAR, which also informed our design.22
Figure 1.
Design and generation of TanCART cells. (A) Single- and dual-specific CARs were designed to target EGFRvIII and IL-13Rɑ2. scFv, single-chain variable fragment; TM, transmembrane domain; T2A, 2A self-cleaving peptide. (B) Illustration of the second-generation constructs for CART-EGFRvIII, CART-IL-13Rɑ2, and TanCART. (C) Schematic depicting the timeline for production of CAR T cells. (D) Average CAR transduction efficiencies in primary human T cells from 3 healthy donors. Data are shown as mean ± SD.
All CAR T cells were generated via lentiviral transduction into primary human lymphocytes isolated from three healthy donors and manufactured according to the timeline depicted in Figure 1C. Using a third-generation, self-inactivating lentiviral vector system, comparable transduction efficiencies and mean fluorescence intensities (MFI) were achieved across all constructs as measured by flow cytometry for the mCherry reporter gene (Figure 1D, Supplementary Figure 1). Surface CAR expression using polyhistidine-tagged cognate antigen did not yield significantly different proportional expression when compared to mCherry alone (data not shown).
Single-antigen Engagement is Sufficient to Activate and Induce Proliferation of TanCART Cells
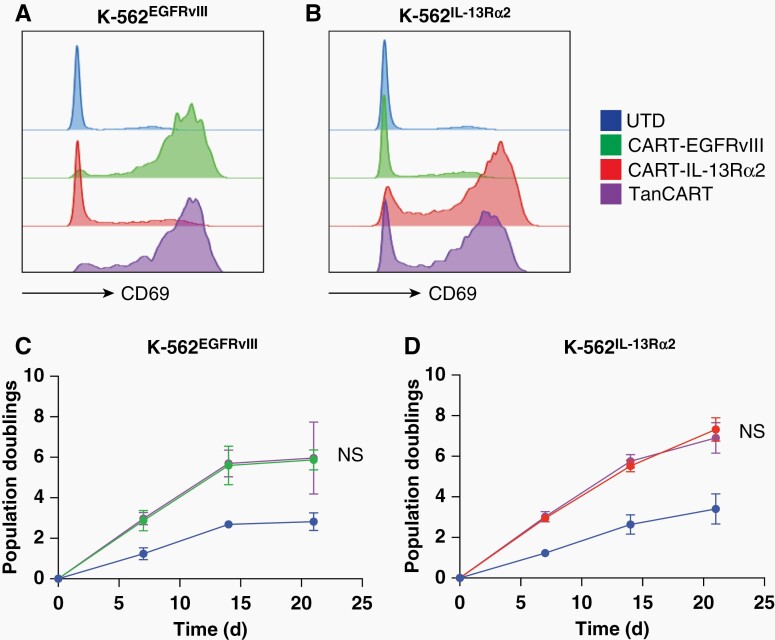
We studied the activation of TanCART cells compared to corresponding single-antigen specific CARs in the presence of either the EGFRvIII or IL-13Rɑ2 antigen. In response to stimulation with cells expressing EGFRvIII, we found that T cells transduced with TanCART and CART-EGFRvIII strongly upregulated CD69 surface expression in contrast to agnostic cells transduced with CART-IL-13Rɑ2 or otherwise untransduced (UTD) cells (Figure 2A). Conversely, in the presence of IL-13Rɑ2-expressing targets, only cells transduced with either TanCART or CART-IL-13Rɑ2 upregulated CD69 (Figure 2B). A more detailed analysis demonstrated that TanCART upregulated exhaustion markers (eg, PD-1, TIM-3, and LAG-3) and produced Th1 proflammatory cytokines (eg, IL-2, IFNγ, and TNFα) in response to both single-positive, pooled, and double-positive target cell lines in a similar fashion compared to their monospecific counterparts (Supplementary Figure 2). However, only TanCART cells responded as such in the presence of both single-antigen positive lines, unlike monospecific CAR T cells which did so only in the presence of their respective cognate antigen.
Figure 2.
TanCART cells activate and proliferate upon single antigen encounter. (A) Activation of UTD, CART-EGFRvIII, CART-IL-13Rα2, and TanCART cells in response to stimulation with K562-EGFRvIII and (B) K562-IL-13Rα2. CD69 expression was measured following 16 hours of co-culture at an effector-to-target ratio of 1:1. (C) Long-term proliferation of UTD, CART-EGFRvIII, CART-IL-13Rα2, and TanCART cells during weekly stimulations with irradiated K562-EGFRvIII and (D) K562-IL-13Rα2. Data are shown as mean total cell counts ± SD of three healthy donors. Statistical significance was calculated using unpaired two-tailed t-tests, NS = not significant.
Next, we investigated the proliferative capacity of TanCART in response to single-antigen stimulation. We performed long-term growth cultures using irradiated target cells. Upon stimulation with irradiated K562EGFRvIII we found that TanCART cells demonstrated logarithmic growth over three weeks. The difference between TanCART and the monospecific CART-EGFRvIII in this setting was not significant (Figure 2C). Likewise, when stimulated with K562IL-13Rɑ2, TanCART cells also proliferated with growth kinetics that were not significantly different when compared to monospecific CART-IL-13Rɑ2 T cells throughout the same interval (Figure 2D). Thus, we found that single-antigen engagement is sufficient to activate and induce proliferation of TanCART cells, and that these parameters do not appear to be significantly different when compared to monospecific CAR T cells targeted at each antigen in isolation.
TanCART Cells Demonstrate Superior Cytotoxicity Against Heterogeneous Glioma
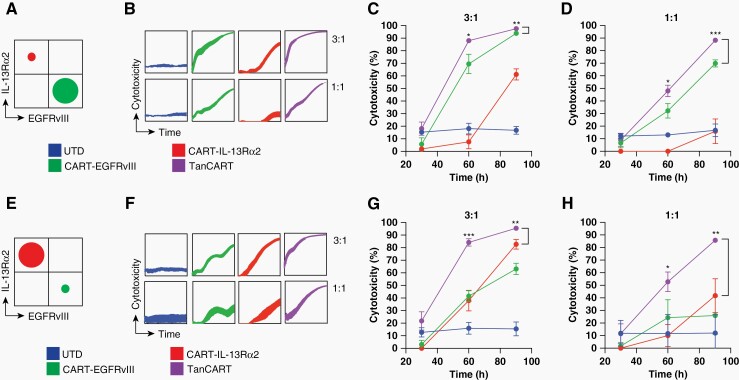
To model heterogeneous glioma and the effects of antigen escape, we generated GBM cell lines with expression of either EGFRvIII or IL-13Rɑ2 (ie, either EGFRvIII+/IL-13Rɑ2− U87MG or EGFRvIII−/IL-13Rɑ2+ U87MG). Using these newly generated GBM cell lines, we produced mixed target cell populations at different proportions. We used target ratios of 10:90 and 90:10, as this has been described previously to recapitulate heterogeneous EGFRvIII expression in preclinical models, and to provide a relatively stringent model that allowed us to test efficacy of the TanCART against monospecific CARs, even when the vast majority of the tumor expressed the monospecific target of interest. We evaluated the cytotoxicity of TanCART cells against these mixed cell populations using an impedance-based assay at various effector-to-target ratios. Using a predominantly EGFRvIII-positive target population (Figure 3A), real-time measurements demonstrated that while wells containing monospecific CAR T cells yielded only partial elimination of the mixed target cell population, TanCART cells mediated rapid tumor cytolysis with enhanced kinetics and potency (Figure 3B, Supplementary Figure 3). When displayed as percentage cytotoxicity at several time points, TanCART cells were more efficacious against mixed glioma populations, even at lower effector-to-target ratios (Supplementary Figure 3), compared to their monospecific counterparts (Figures 3C and D). These results were consistent in tests against a predominantly IL-13Rɑ2-positive population, wherein TanCART also exhibited more rapid and complete cytotoxicity when compared to either monospecific CAR T cell alone (Figure 3E–H). TanCART did not exhibit increased off-target activity against target cells compared to monospecific CAR T cells targeting EGFRvIII (Supplementary Figure 4).
Figure 3.
TanCART cells exhibit in vitro cytotoxicity against heterogeneous GBM cell populations. (A) Co-cultures of mixed target U87MG cells at 90:10 (EGFRvIII:IL-13Rα2) were utilized at effector-to-target ratios of 3:1 and 1:1. (B) Impedance-based cytotoxicity assays of UTD, CART-EGFRvIII, CART-IL-13Rα2, and TanCART cells against mixed U87MG target populations were performed. Effector cells were added to plated target cells at 28 h. (C) Individual time points were recorded at 30-, 60- and 90-hour timepoints at effector-to-target ratios of 3:1 and (D) 1:1. (E) Co-cultures of mixed target U87MG cells at 10:90 (EGFRvIII:IL-13Rα2) were also tested using (F) impedance-based cytotoxicity, again at ratios of (G) 3:1 and (H) 1:1. Data were measured in triplicates and are shown as mean ± SD. Statistical significance was calculated using unpaired two-tailed t-tests, *P ≤ .05, **P ≤ .01, ***P ≤ .001, and represent differences between bracketed groups.
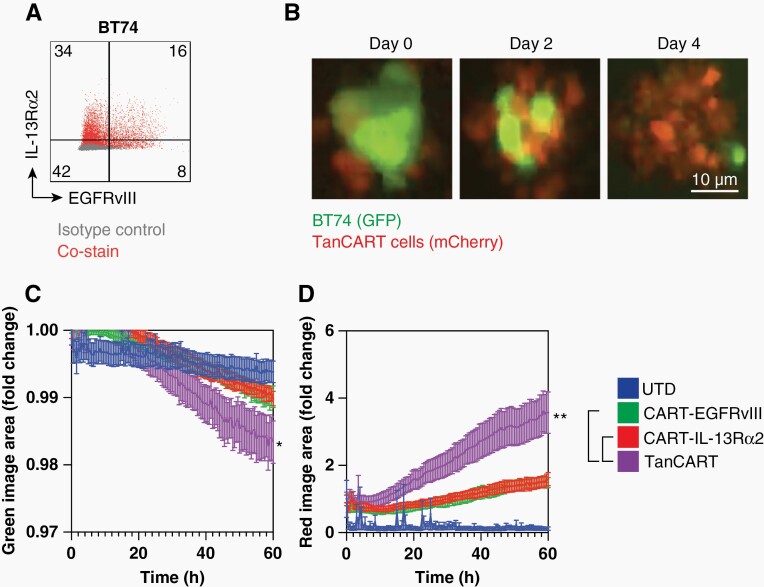
The GBM patient-derived xenograft (PDX) BT74 has been previously described as an intrinsically heterogeneous cell line that maintains physiologically relevant levels of EGFRvIII and faithfully recapitulates characteristics of the primary disease.23 BT74 is somewhat unique given that, in a study of more than 11 established GBM PDX neurospheres available to us, only one (ie, BT74, formerly GBM6) demonstrated suitable EGFRvIII expression for our purposes.23
We found that BT74 neurospheres express endogenous and heterogeneous levels of both EGFRvIII and IL-13Rɑ2, consistent with previous reports from human studies of GBM (Figure 4A).24 We used an image-based, live-cell assay to assess the antitumor effects of TanCART. We observed that TanCART cells tended to cluster and proliferate in response to BT74 neurospheres in culture (Figure 4B). We also demonstrated specific antitumor activity of TanCART against BT74 (Figure 4C). Additional evaluation revealed concomitant proliferation of TanCART cells that was enhanced compared to wells containing monospecific CAR or UTD T cell controls (Figure 4D).
Figure 4.
TanCART cells are effective against heterogeneous GBM PDX and proliferate in longitudinal assays in vitro. (A) Baseline surface expression levels of EGFRvIII and IL-13Rα2 in the GBM PDX, BT74. (B) Representative images from co-cultures with BT74 neuropsheres with TanCART cells during a cytotoxicity assay at an effector-to-target ratio of 1:1. (C) Live-cell analysis of BT74 neurosphere lysis by UTD, CART-EGFRvIII, CART-IL-13Rα2, and TanCART cells by quantification of total green image area. (D) Proliferation of effector cells measured by total red image area. Data were measured in triplicates and are shown as mean ± SEM. Experiments were repeated with at least two different normal donors showing similar results. Statistical significance at the 60-hour time point was calculated using unpaired two-tailed t-tests, *P ≤ .05.
TanCART Cells are Efficacious Against Heterogeneous Glioma in an Orthotopic Mouse Model
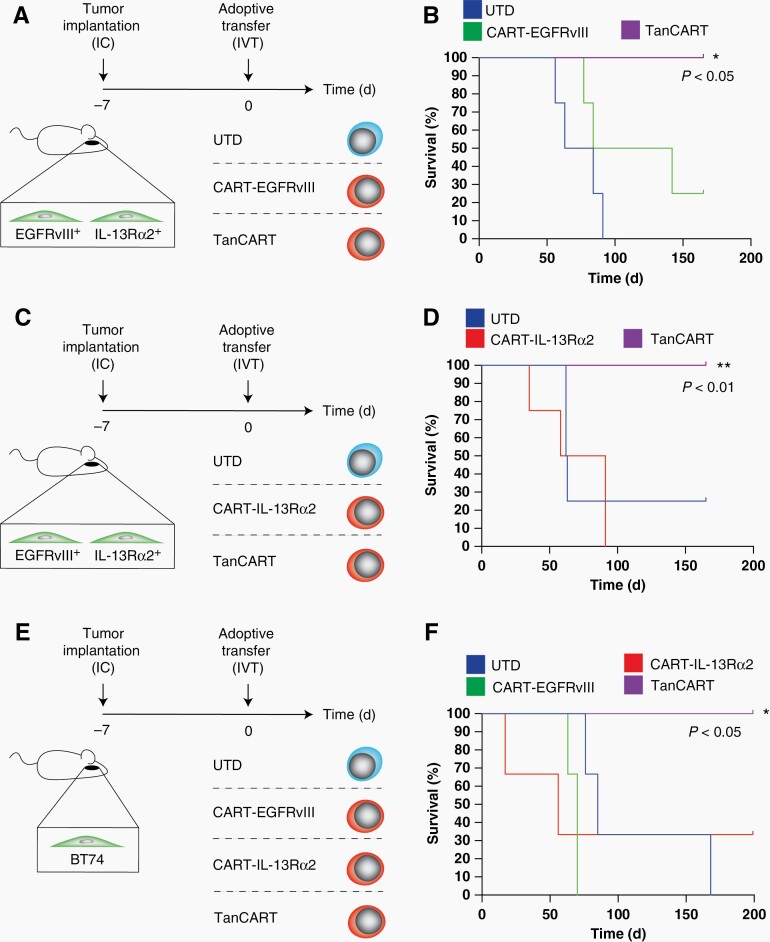
We assessed the anti-tumor activity of TanCART cells against mixed human glioma (U87MG) populations orthotopically implanted into the brains of NSG mice. Using this model, we demonstrated complete and durable treatment responses with universal long-term survival in all mice treated with TanCART cells. Conversely, mice treated with monospecific CAR T cells displayed incomplete responses and shorter survival times comparable to those receiving UTD T-cell control (Figure 5A–D).
Figure 5.
TanCART cells achieve complete and durable antitumor responses against heterogeneous brain tumors in an orthotopic animal model of GBM. (A) Schematic of the experimental design where mice were intracerebrally implanted with a mixed population of U87MG and treated with 1 × 106 effector cells intraventricularly seven days later (N = 4). (B) Kaplan-Meier survival analysis of the aforementioned experiment in which the tumor consisted primarily of IL-13Rɑ2-positive cells (10:90, EGFRvIII:IL-13Rα2). (C) Schematic of the experimental design where mice were alternatively engrafted with tumors consisting primarily of EGFRvIII-positive cells (90:10, EGFRvIII:IL-13Rα2) and (D) treated with monospecific or TanCART cells. (E) Mice were also engrafted intracerebrally with the GBM PDX, BT74 (5 × 105 cells) and treated with a single dose of 1 × 106 effector cells intraventricularly 7 days later (N = 3). (F) Kaplan-Meier survival analysis demonstrating survival of TanCART-treated animals compared to UTD and negative control, monospecific CAR T cell groups. Statistical significance was calculated by the log-rank test between TanCART and monospecific CAR T cell groups, *P ≤ .05.
Having demonstrated effective killing of an engineered model of heterogeneity, we extended our findings to test the in vivo treatment of the highly physiologic PDX, BT74. Mice were engrafted intracerebrally with BT74 cells and treated with effector cells by a single intraventricular infusion (Figure 5E). Compared to BT74-bearing mice treated with monospecific CAR T cells, only the group treated with TanCART cells exhibited universal, complete, and durable responses (Figure 5F). Brains from surviving mice treated with TanCART did not have detectable tumors by gross or histopathological examination. Mixed tumors (eg, either U87MG-based or BT74 PDX) maintained heterogeneous expression of both target antigens following engraftment. However, in mice treated with monospecific CAR T cells, treatment failure corresponded with loss of expression of the corresponding tumor antigen of interest (Supplementary Figure 5).
Discussion
CAR T cell therapy holds great promise for patients with GBM, but early clinical trials suggest that treating heterogeneous tumors using a single antigen approach may be limited by immune escape and tumor progression. Targeting multiple antigens simultaneously with a tandem CAR T cell has the potential to mitigate this effect. In the current study, we applied this strategy to generate a novel CAR that targets two GBM antigens (eg, EGFRvIII and IL-13Rɑ2). This CAR incorporates antigen-recognition domains identical to those that have been demonstrated to be safe in humans at the amino-acid level. An additional advance is the specific combination of antigens we used, which allows TanCART cells to target gliomas while minimizing on-target toxicity or cross-reactivity with normal somatic tissues. Finally, we found that TanCART was able to mediate complete and durable responses in murine models of GBM including PDX, with activity superior to that of their monospecific CAR counterparts against heterogeneous tumors.
Identifying suitable combinations of surface CAR targets can be difficult since most antigens associated with GBM are also widely expressed throughout the human body. Underscoring this challenge, dual-specific10 and trivalent25 CAR T cells for GBM consisting of a scFv against HER210 and intracellular CD28 costimulatory domains have been previously described; however, HER2 is known to be broadly expressed across several epithelial tissues, and CAR T cells targeting HER2 have resulted in the development of severe autoimmunity in humans—in one patient leading to fulminant and fatal on-target toxicity.11 A dual-specific CAR T cell targeting EphA2 has been recently described as well, although this target is also known to be widely expressed in healthy tissues.26 By contrast, we selected EGFRvIII for the TanCART platform based on our previous clinical experience demonstrating safety of CAR T cells targeting this mutation, and because it represents one of the few truly tumor-specific GBM-associated surface markers that has been characterized. IL-13Rɑ2 is also frequently detected in GBM and has been targeted safely by CAR T cells in humans, with negligible expression in normal brain and peripheral organs.27 It has been recently shown that expression of IL-13Rɑ2 and EGFRvIII in glioma tissues is significantly heterogeneous, suggesting that strategies to target these two antigens simultaneously could provide broader activity within individual tumors. In one study, five out of five EGFRvIII-positive tumors had detectable expression of IL-13Rɑ2, and two out of five of these tumors had levels of expression greater than 75%.28
We designed several second-generation CAR constructs with single and dual specificity for EGFRvIII and IL-13Rɑ2. We used monospecific CAR T cells directed against EGFRvIII (CART-EGFRvIII), which employed a humanized anti-EGFRvIII scFv and had been previously optimized for high specificity against EGFRvIII over wild-type EGFR.29 In addition, we used monospecific CAR T cells directed against IL-13Rɑ2 (CART-IL-13Rα2), which were constructed from a previously described zetakine binder, a membrane-tethered IL-13 ligand mutated at E13Y, to confer higher specificity for IL-13Rɑ2 relative to the more widely expressed IL-13Rɑ1/IL-4Rɑ complex.30,31 Importantly, both monospecific CAR T cells, CART-EGFRvIII and CART-IL-13Rɑ2, have previously established safety in prior early-phase clinical trials.3,32 Together, these CARs formed the basis of the TanCART construct and design presented here.
In our study, all mice in the TanCART group had durable and complete responses despite the fact that a sizeable portion of BT74 cells in culture was not found to express high levels of either antigen. The explanation for this finding is unclear, but may either stem from artifact associated with minor HLA mismatch between tumor and donor T cells, or may be due to fluctuations in target antigen expression within tumors themselves. We know, for instance that tumor antigen expressed naturally by gliomas—as opposed to transduced or modified cell lines—can change over time. It has been described, for instance that EGFRvIII present in primary tumor specimens may be absent at later times such as at recurrence.33 In the same vein, in a previous clinical trial of the IL-13Rɑ2 CAR T cell for glioblastoma—despite heterogeneous target antigen expression prior to treatment—complete responses were initially observed after administration of a monospecific CAR, although this was followed by eventual disease recurrence.4 The explanation for this outcome is currently unclear and is the subject of further investigation.
In a recent study, we reported that CAR T cells designed to secrete bispecific T cell engagers (BiTEs) against a broadly expressed antigen at very low concentrations could effectively circumvent antigen escape without detectable systemic toxicity.34 Both the TanCART and CART.BiTE platforms could potentially address shortcomings observed in our previous clinical trials of CART-EGFRvIII—namely the successful targeting of EGFRvIII but subsequent outgrowth and progression of tumors with underlying heterogeneity.3 Notably, rather than competing with each other, TanCART and bispecific antibodies are likely compatible, and combining these technologies could represent an opportunity to further expand the multivalent capacity of the fundamental CAR T cell approach.
Similarly, an alternative strategy to targeting multiple antigens simultaneously includes the concomitant infusion of monospecific CARs, which could ultimately mediate antitumor activity against heterogeneous tumors in a similar fashion to the TanCART approach. However, one of the main advantages of TanCART is the ability to manufacture cell therapy using a single genetic modification; that is to say, with insertion of a single transgene and infusion of a single gene-modified cell product, which greatly enhances its translational potential. This is especially true given that producing autologous CAR T cells for patients remains a financially challenging and technically laborious process.
The TanCART design creates a Boolean OR argument, which allows T cells to engage both single and double-positive populations. In the setting of a Boolean OR function, it might be hypothesized that the ability to engage both antigens, and the relative abundance of either target antigen, might lead to activation below the recognition threshold that would be expected when either antigen is encountered in isolation. CAR T cell potency has indeed been shown to be highly dependent on target antigen expression. This differentiates CAR T cells from native T cell receptors (TCR).35,36 That is to say, whereas a single peptide-MHC complex has been shown to be sufficient to elicit a cytolytic response, CARs often require a certain minimal threshold of target antigen expression before a meaningful antitumor response can be achieved. This was evidenced, for example, by immune escape related to the emergence of antigen low variants in a clinical trial of CD22 CAR T cells in patients with B-ALL.37 As aforementioned, the tandem CAR approach provides a Boolean OR argument, which, at least in theory, allows for target cell recognition at a threshold of antigen expression reflected by the sum of both target antigens combined. Thus, one advantage of tandem CARs may be that they widen a therapeutic window for an otherwise weakly expressed target antigen considered in isolation.
In our group, we typically administer CAR T cells directly into the cerebrospinal fluid to achieve high effector-to-target ratios in the intracranial space. Evidence from preclinical and clinical studies suggests that locoregional delivery may enhance treatment of tumors in the central nervous system, which are thought to be separated from the periphery to some degree by the blood-brain barrier.4,38,39 Administering cell therapies directly into cerebrospinal fluid may represent a feasible approach to maximizing localization of cell therapies to gliomas, since access into the lateral ventricle is performed routinely by neurosurgeons with ease, and because a substantial portion of cerebrospinal fluid is known to regularly cycle to reach the interstitial spaces of the brain parenchyma, where intrinsic tumors like GBM are located.40
We have consistently found intraventricular delivery of CAR T cells to be feasible and superior to systemic delivery when treating tumors in the brain in our preclinical models.31,41–43 This was also observed in a clinical trial for IL-13Rɑ2 CAR T cells where a complete response was observed only after repeated intraventricular infusion, as opposed to prior intracavitary administration in the same individual.4 Whether CAR T cells in the CSF simply traffic through CNS compartments by bulk flow or alternatively via regulated processes at the blood-cerebrospinal fluid barrier is currently unclear and requires further investigation. Ultimately, the potential mechanisms underlying survival and trafficking of CAR T cells in this setting may be best addressed in syngeneic models or even humans, which have the capacity to capture appropriate physiological interactions among infused cells, a replete immune system, and other endogenous tissues.
There is currently a relative dearth of adequate preclinical animal models that precisely reflect both the intact immunity and the profile of target antigen expression that would be encountered in patients with GBM. We chose the NSG mouse model as it permits the direct evaluation of human T cells in a format that is also translatable in terms of assessing efficacy against human glioma. A disadvantage of this system is the absence of a representative tumor microenvironment and endogenous immune system, which may modulate anti-tumor responses44 or could reveal additional relevant toxicities. Optimizing immune-competent models may greatly enhance future studies seeking to test novel constructs with maximum fidelity in the preclinical setting. To date, these models are unfortunately still not fully predictive of human toxicities from novel immunotherapies. If and when TanCART cells are translated to Phase I clinical trials, the treating physicians will be required to discuss potential toxicities as part of the informed consent process.
A limitation of our study is that certain cell lines were transduced to overexpress antigens of interest, apart from the BT74 neurospheres. It is likely that the BT74 PDX tumor line most closely recapitulated endogenous target expression in GBM, as the cells remained unmodified and had naturally heterogeneous patterns of expression.
To our knowledge, this is the first report of a tandem CAR T cell targeting EGFRvIII and IL-13Rɑ2 simultaneously. The results obtained with this construct address prior shortcomings in clinical trials of both EGFRvIII- and IL-13Rɑ2-targeted CAR T cells and warrant further investigation in patients with GBM.
Supplementary Material
Acknowledgments
The authors would like to thank Sonika Vatsa for technical assistance and Trisha R. Berger for assistance with editing the manuscript. The authors wish to thank the MGH Blood Transfusion Service for providing leukopacs and the MGH Flow Cytometry Core for flow sorting.
Contributor Information
Andrea Schmidts, Cellular Immunotherapy Program, Cancer Center, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Ambike A Srivastava, Cellular Immunotherapy Program, Cancer Center, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Rishab Ramapriyan, Department of Neurosurgery, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Stefanie R Bailey, Cellular Immunotherapy Program, Cancer Center, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Amanda A Bouffard, Cellular Immunotherapy Program, Cancer Center, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Daniel P Cahill, Department of Neurosurgery, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Bob S Carter, Department of Neurosurgery, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA.
William T Curry, Department of Neurosurgery, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Gavin P Dunn, Department of Neurosurgery, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Matthew J Frigault, Cellular Immunotherapy Program, Cancer Center, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Elizabeth R Gerstner, Department of Neurology, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA (E.R.G.).
Jack Y Ghannam, Department of Neurosurgery, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Michael C Kann, Cellular Immunotherapy Program, Cancer Center, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Rebecca C Larson, Cellular Immunotherapy Program, Cancer Center, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Mark B Leick, Cellular Immunotherapy Program, Cancer Center, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Brian V Nahed, Department of Neurosurgery, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Leland G Richardson, Department of Neurosurgery, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Irene Scarfò, Cellular Immunotherapy Program, Cancer Center, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Jing Sun, Department of Neurosurgery, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Hiroaki Wakimoto, Department of Neurosurgery, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Marcela V Maus, Cellular Immunotherapy Program, Cancer Center, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Bryan D Choi, Cellular Immunotherapy Program, Cancer Center, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA; Department of Neurosurgery, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Funding
This work was supported by German Cancer Aid Mildred-Scheel Post-Doctoral Research Fellowship (A.S.), NIH T32 CA009216 (S.R.B.), The Jenny Fund (W.T.C.), NIGMS T32GM007753 (J.Y.G.), T32GM144273 (J.Y.G.), NIH T32 #2T32CA071345-21A1 (M.B.L.), Lymphoma Research Foundation 612757 (I.S.), Stand Up to Cancer Innovative Research Grant (M.V.M.), Damon Runyon Cancer Research Foundation (M.V.M.), NIH R01 CA238268 (M.V.M.), NIH R25NS065743 (B.D.C.), NIH K12CA090435 (B.D.C.), A Shot for Life (B.D.C.), Neurosurgery Research & Education Foundation and B*Cured Research Fellowship Grant (B.D.C.), Society for Immunotherapy of Cancer—AstraZeneca Postdoctoral Cancer Immunotherapy in Combination Therapies Clinical Fellowship Award (B.D.C.), Swim Across America (B.D.C.), and Curing Kids Cancer (B.D.C.).
Authorship statement A.S., B.D.C., and M.V.M. designed the research. A.S., A.A.S., R.R., S.R.B., A.A.B., M.J.F., M.C.K., R.C.L., M.B.L., L.G.R., I.S., and J.S. performed experiments. D.P.C., B.S.C., W.T.C., G.P.D., E.R.G., B.V.N., H.W., M.V.M, and B.D.C. contributed reagents and analytic tools. A.S., A.A.S., J.Y.G., M.V.M., and B.D.C. analyzed and interpreted data. A.S., A.A.S., M.V.M., and B.D.C. wrote the paper.
Conflict of interest statement D.P.C. has consulted for the Massachusetts Institute of Technology, Advise Connect Inspire, Lilly, GlaxoSmithKline, Boston Pharmaceuticals, and Iconovir and serves on the advisory board of Pyramid Biosciences, which includes an equity interest, and has received honoraria and travel reimbursement from Merck for invited lectures. M.V.M. and B.D.C. are inventors on patents related to the use of engineered cell therapies for GBM and other cancers. M.V.M. holds equity in TCR2, Century Therapeutics, Genocea, Oncternal, and Neximmune, is on the Board of Directors of 2Seventy Bio, and has served as a consultant for multiple companies involved in cell therapies (unrelated to this work). B.D.C. received commercial research grants from ACEA Biosciences.
References
- 1. Akhavan D, Alizadeh D, Wang D, et al. CAR T cells for brain tumors: lessons learned and road ahead. Immunol Rev. 2019; 290(1):60–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Choi BD, Curry WT, Carter BS, Maus MV. Chimeric antigen receptor T-cell immunotherapy for glioblastoma: practical insights for neurosurgeons. Neurosurg Focus. 2018; 44(6):E13. [DOI] [PubMed] [Google Scholar]
- 3. O’Rourke DM, Nasrallah MP, Desai A, et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci Transl Med. 2017; 9(399): eaaa0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brown CE, Alizadeh D, Starr R, et al. Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N Engl J Med. 2016; 375(26):2561–2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ahmed N, Brawley V, Hegde M, et al. HER2-specific chimeric antigen receptor-modified virus-specific T cells for progressive glioblastoma: a phase 1 dose-escalation trial. JAMA Oncol. 2017; 3(8):1094–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yi Z, Prinzing BL, Cao F, et al. Optimizing EphA2-CAR T cells for the adoptive immunotherapy of glioma. Mol Ther Methods Clin Dev. 2018; 9:70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chow KK, Naik S, Kakarla S, et al. T cells redirected to EphA2 for the immunotherapy of glioblastoma. Mol Ther: J Am Soc Gene Ther. 2013; 21(3):629–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014; 371(16):1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shah NN, Johnson BD, Schneider D, et al. Bispecific anti-CD20, anti-CD19 CAR T cells for relapsed B cell malignancies: a phase 1 dose escalation and expansion trial. Nat Med. 2020; 26(10):1569–1575. [DOI] [PubMed] [Google Scholar]
- 10. Hegde M, Mukherjee M, Grada Z, et al. Tandem CAR T cells targeting HER2 and IL13Ralpha2 mitigate tumor antigen escape. J Clin Invest. 2016; 126(8):3036–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Morgan RA, Yang JC, Kitano M, et al. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010; 18(4):843–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Saikali S, Avril T, Collet B, et al. Expression of nine tumour antigens in a series of human glioblastoma multiforme: interest of EGFRvIII, IL-13Ralpha2, gp100 and TRP-2 for immunotherapy. J Neurooncol. 2007; 81(2):139–148. [DOI] [PubMed] [Google Scholar]
- 13. Newman JP, Wang GY, Arima K, et al. Interleukin-13 receptor alpha 2 cooperates with EGFRvIII signaling to promote glioblastoma multiforme. Nat Commun. 2017; 8(1):1913–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wakimoto H, Kesari S, Farrell CJ, et al. Human glioblastoma-derived cancer stem cells: establishment of invasive glioma models and treatment with oncolytic herpes simplex virus vectors. Cancer Res. 2009; 69(8):3472–3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Doench JG, Fusi N, Sullender M, et al. Optimized sgRNA design to maximize activity and minimize off-target effects of CRISPR-Cas9. Nat Biotechnol. 2016; 34(2):184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brown CE, Badie B, Barish ME, et al. Bioactivity and safety of IL13Rα2-redirected chimeric antigen receptor CD8+ T cells in patients with recurrent glioblastoma. Clin Cancer Res: Off J Am Assoc Cancer Res. 2015; 21(18):4062–4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ruella M, Levine BL. Smart CARS: optimized development of a chimeric antigen receptor (CAR) T cell targeting epidermal growth factor receptor variant III (EGFRvIII) for glioblastoma. Ann Transl Med. 2016; 4(1):13–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Taylor ZV, Khand B, Porgador A, Monsonego A, Eremenko E. An optimized intracerebroventricular injection of CD4(+) T cells into mice. STAR Protoc. 2021; 2(3):100725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhao Z, Condomines M, van der Stegen SJC, et al. Structural design of engineered costimulation determines tumor rejection kinetics and persistence of CAR T cells. Cancer Cell. 2015; 28(4):415–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kane SP. Post. ClinCalc. https://clincalc.com/stats/Power.aspx. Accessed April 12, 2022. [Google Scholar]
- 21. Ormhoj M, Scarfo I, Cabral ML, et al. Chimeric antigen receptor T cells targeting CD79b show efficacy in lymphoma with or without cotargeting CD19. Clin Cancer Res. 2019; 25(23):7046–7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Grada Z, Hegde M, Byrd T, et al. TanCAR: a novel bispecific chimeric antigen receptor for cancer immunotherapy. Mol Ther Nucleic Acids. 2013 Jul 9;2(7):e105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pandita A, Aldape KD, Zadeh G, Guha A, James CD. Contrasting in vivo and in vitro fates of glioblastoma cell subpopulations with amplified EGFR. Genes Chromosom Cancer. 2004; 39(1):29–36. [DOI] [PubMed] [Google Scholar]
- 24. Yin Y, Rodriguez JL, Li N, et al. Locally secreted BiTEs complement CAR T cells by enhancing killing of antigen heterogeneous solid tumors. Mol Ther. 2022; 30(7):2537–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bielamowicz K, Fousek K, Byrd TT, et al. Trivalent CAR T cells overcome interpatient antigenic variability in glioblastoma. Neuro Oncol. 2018; 20(4):506–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hafner C, Schmitz G, Meyer S, et al. Differential gene expression of Eph receptors and ephrins in benign human tissues and cancers. Clin Chem. 2004; 50(3):490–499. [DOI] [PubMed] [Google Scholar]
- 27. Debinski W, Gibo DM. Molecular expression analysis of restrictive receptor for interleukin 13, a brain tumor-associated cancer/testis antigen. Mol Med. 2000; 6(5):440–449. [PMC free article] [PubMed] [Google Scholar]
- 28. Yin Y, Rodriguez JL, Li N, et al. Locally secreted BiTEs complement CAR T cells by enhancing killing of antigen heterogeneous solid tumors. Mol Ther. 2022; 30(7):2537–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Johnson LA, Scholler J, Ohkuri T, et al. Rational development and characterization of humanized anti-EGFR variant III chimeric antigen receptor T cells for glioblastoma. Sci Transl Med. 2015; 7(275):275ra–27522.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kahlon KS, Brown C, Cooper LJ, et al. Specific recognition and killing of glioblastoma multiforme by interleukin 13-zetakine redirected cytolytic T cells. Cancer Res. 2004; 64(24):9160–9166. [DOI] [PubMed] [Google Scholar]
- 31. Brown CE, Aguilar B, Starr R, et al. Optimization of IL13Ralpha2-targeted chimeric antigen receptor T cells for improved anti-tumor efficacy against glioblastoma. Mol Ther. 2018; 26(1):31–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brown CE, Badie B, Barish ME, et al. Bioactivity and safety of IL13Ralpha2-redirected chimeric antigen receptor CD8+ T cells in patients with recurrent glioblastoma. Clin Cancer Res. 2015; 21(18):4062–4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. van den Bent MJ, Gao Y, Kerkhof M, et al. Changes in the EGFR amplification and EGFRvIII expression between paired primary and recurrent glioblastomas. Neuro Oncol. 2015; 17(7):935–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Choi BD, Yu X, Castano AP, et al. CAR-T cells secreting BiTEs circumvent antigen escape without detectable toxicity. Nat Biotechnol. 2019; 37(9):1049–1058. [DOI] [PubMed] [Google Scholar]
- 35. Sykulev Y, Joo M, Vturina I, Tsomides TJ, Eisen HN. Evidence that a single peptide-MHC complex on a target cell can elicit a cytolytic T cell response. Immunity. 1996; 4(6):565–571. [DOI] [PubMed] [Google Scholar]
- 36. Harris DT, Hager MV, Smith SN, et al. Comparison of T cell activities mediated by human TCRs and CARs that use the same recognition domains. J Immunol. 2018; 200(3):1088–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fry TJ, Shah NN, Orentas RJ, et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med. 2018; 24(1):20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Choi BD, Suryadevara CM, Gedeon PC, et al. Intracerebral delivery of a third generation EGFRvIII-specific chimeric antigen receptor is efficacious against human glioma. J Clin Neurosci. 2014; 21(1):189–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Choi BD, Yu X, Castano AP, et al. CRISPR-Cas9 disruption of PD-1 enhances activity of universal EGFRvIII CAR T cells in a preclinical model of human glioblastoma. J ImmunoTher Cancer. 2019; 7(1):304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med. 2012; 4(147):147ra–14111.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Priceman SJ, Tilakawardane D, Jeang B, et al. Regional delivery of chimeric antigen receptor-engineered T cells effectively targets HER2(+) breast cancer metastasis to the brain. Clin Cancer Res. 2018; 24(1):95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Donovan LK, Delaidelli A, Joseph SK, et al. Locoregional delivery of CAR T cells to the cerebrospinal fluid for treatment of metastatic medulloblastoma and ependymoma. Nat Med. 2020; 26(5): 720–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Theruvath J, Sotillo E, Mount CW, et al. Locoregionally administered B7-H3-targeted CAR T cells for treatment of atypical teratoid/rhabdoid tumors. Nat Med. 2020; 26(5):712–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sampson JH, Choi BD, Sanchez-Perez L, et al. EGFRvIII mCAR-modified T-cell therapy cures mice with established intracerebral glioma and generates host immunity against tumor-antigen loss. Clin Cancer Res. 2014; 20(4):972–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.