Abstract
Rationale
There are limited therapeutic options for patients with coronavirus disease (COVID-19)-related acute respiratory distress syndrome with inflammation-mediated lung injury. Mesenchymal stromal cells offer promise as immunomodulatory agents.
Objectives
Evaluation of efficacy and safety of allogeneic mesenchymal cells in mechanically-ventilated patients with moderate or severe COVID-19–induced respiratory failure.
Methods
Patients were randomized to two infusions of 2 million cells/kg or sham infusions, in addition to the standard of care. We hypothesized that cell therapy would be superior to sham control for the primary endpoint of 30-day mortality. The key secondary endpoint was ventilator-free survival within 60 days, accounting for deaths and withdrawals in a ranked analysis.
Measurements and Main Results
At the third interim analysis, the data and safety monitoring board recommended that the trial halt enrollment as the prespecified mortality reduction from 40% to 23% was unlikely to be achieved (n = 222 out of planned 300). Thirty-day mortality was 37.5% (42/112) in cell recipients versus 42.7% (47/110) in control patients (relative risk [RR], 0.88; 95% confidence interval, 0.64–1.21; P = 0.43). There were no significant differences in days alive off ventilation within 60 days (median rank, 117.3 [interquartile range, 60.0–169.5] in cell patients and 102.0 [interquartile range, 54.0–162.5] in control subjects; higher is better). Resolution or improvement of acute respiratory distress syndrome at 30 days was observed in 51/104 (49.0%) cell recipients and 46/106 (43.4%) control patients (odds ratio, 1.36; 95% confidence interval, 0.57–3.21). There were no infusion-related toxicities and overall serious adverse events over 30 days were similar.
Conclusions
Mesenchymal cells, while safe, did not improve 30-day survival or 60-day ventilator-free days in patients with moderate and/or severe COVID-19–related acute respiratory distress syndrome.
Keywords: mechanical ventilation, SARS-CoV-2, clinical trial, survival, stem cells
At a Glance Commentary
Scientific Knowledge on the Subject
Coronavirus disease (COVID-19)-induced acute respiratory distress syndrome (ARDS) remains a highly lethal and morbid condition with limited therapeutic options. Pre- and early clinical data have shown that mesenchymal stromal cells (MSCs) may improve lung injury and associated inflammation through a variety of mechanisms, including immune modulation, alveolar fluid clearance, bacterial clearance, regulation of pulmonary vascular endothelial permeability, and suppression of apoptosis.
What This Study Adds to the Field
In our trial, MSC therapy, compared with sham control, in patients with moderate to severe COVID-19–related ARDS did not produce the hypothesized reduction in 30-day mortality. During the pandemic, new insights have emerged into the existence of different inflammatory subtypes and the importance of age within the overall COVID-19–related ARDS population that might underlie a differential response to such immunomodulatory therapy. Future clinical trials are needed to identify the potential benefits of MSCs and differential dosing on the basis of immune status and age in susceptible phenotypes.
Although there have been advances in the treatment of hospitalized patients with coronavirus disease (COVID-19), a therapeutic gap persists for patients with acute respiratory distress syndrome (ARDS) secondary to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. SARS-CoV-2 directly infects epithelial cells and immune cells in the lung, leading to direct cytotoxic effects and lung injury with secondary inflammation (1). As the disease process progresses to respiratory failure, many patients develop a hyperinflammatory state characterized by increased concentrations of inflammatory mediators, including cytokines and chemokines such as IL-2, tumor necrosis factor, and macrophage chemoattractant protein, as well as elevated inflammatory biomarkers, including C-reactive protein (CRP) and ferritin (2). The hyperinflammatory state associated with COVID-19 has drawn comparisons with cytokine release syndrome, graft-versus-host disease, and hemophagocytic lymphohistiocytosis (1).
It has become increasingly clear that ARDS-related mortality in patients with COVID-19 is associated with increasing age and the accompanying dysregulated inflammatory response. Consequently, it has been postulated that targeted immunomodulation may mollify or reverse the hyperinflammatory state associated with COVID-19 and reduce ARDS-related mortality (3). Indeed, dexamethasone has been shown to reduce mortality in patients with hypoxemia hospitalized with COVID-19 receiving mechanical ventilation or oxygen (4, 5). However, other single-target immunomodulatory agents have demonstrated only mixed results (6, 7), accentuating the need to evaluate cellular-based therapies, which may have multiple targets.
Preclinical and early clinical data have shown that mesenchymal stromal cells (MSCs) may improve lung injury and associated inflammation through a variety of mechanisms, ranging from paracrine secretion of antiinflammatory cytokines and growth factors to production of antimicrobial peptides and restoration of epithelial bioenergetics through the transfer of mitochondria (8, 9). Adult-derived expanded MSCs have benefits in pediatric steroid-refractory acute graft-vs-host disease (SR-aGVHD) (10) and preclinical models of acute lung injury (11). MSCs have demonstrated safety in phase I/IIa trials of ARDS (12, 13). A small study of nonmechanically ventilated patients with COVID-19 pneumonia reported rapid recovery and improved inflammatory markers after treatment with MSCs (14). These data led to the design of a trial evaluating MSCs (using a lower dose of remestemcel-L than has been used clinically in pediatric SR-aGVHD) in patients with COVID-19–induced moderate or severe ARDS, the results of which are reported here.
Trial Registration: ClinicalTrials.gov (NCT04371393).
Methods
Study Design and Participants
This randomized, parallel, sham infusion-controlled trial evaluating MSCs in patients with SARS-CoV-2–related ARDS was conducted under an investigational new drug application. A coordinating center, independent event adjudication committee, and data and safety monitoring board oversaw trial progress. A central institutional review board approved the protocol, and informed consent was obtained from all patients or their legally authorized representatives.
The target population was adults with SARS-CoV-2, confirmed by real-time reverse transcription PCR assay, who required mechanical ventilation for moderate or severe ARDS (modified Berlin criteria) (15). Patients needed to have bilateral opacities on a chest radiograph or computerized tomographic scan, respiratory failure not fully explained by cardiac failure or fluid overload, and moderate to severe impairment of oxygenation assessed by the PaO2/FiO2 ratio. The severity of ARDS was defined as moderate if PaO2/FiO2 was >100 mm Hg and ⩽200 mm Hg and severe if PaO2/FiO2 was ⩽100 mm Hg, both with ventilator settings that included positive end-expiratory pressure ⩾5 cm H2O. In addition, patients were required to have a CRP concentration >4 mg/dl, an Acute Physiology and Chronic Health Evaluation (APACHE) score greater than 5, and creatinine clearance ⩾30 ml/min.
Patients were excluded if they were receiving extracorporeal membrane oxygenation, had evidence of bacterial pneumonia, were massively obese (body mass index above 55), had untreated HIV, malignancy within 12 months of active treatment, or elevated liver function tests (LFTs) (greater than 8× the upper limit of normal). In addition, patients were excluded if they had been intubated for more than 72 hours at the time of randomization or had a prior history of respiratory disease requiring supplemental oxygen.
Randomization and Masking
Patients were randomized in a 1:1 ratio using a web-based system, and randomization was stratified by clinical center and ARDS severity. A random permuted block design with block sizes of two and four was used. Patients and investigators were blinded, except for the site’s research pharmacist and one unblinded trial statistician. The infusion bags were masked to their content by the research pharmacist.
Procedures
Patients were randomized to receive intravenous infusion of MSCs (remestemcel-L) plus standard of care versus placebo (PlasmaLyte) plus standard of care. Patients received two infusions of the study product during the first week, with the second infusion 4 days after the first infusion (±1 d). The MSC dose was 2 × 106 MSC/kg of body weight. This dose regimen was adapted from that used in patients with SR-aGVHD in which 2 × 106 MSC/kg of body weight were infused twice weekly for 4 weeks; both aGVHD and COVID-19 ARDS have in common excessive T-cell proliferation and infiltration accompanied by damage to gut and pulmonary epithelial surfaces (10). The rationale for using a shorter dosing period with COVID-19–related ARDS was on the basis of a pilot trial that used this dosing regimen in a series of 11 adult patients with COVID-19 with moderate to severe ARDS on mechanical ventilation, 9 of whom were extubated and discharged from the ICU within 28 days of MSC initiation (16). In this trial, the MSCs were cryopreserved and stored at or below −135°C in liquid nitrogen vapor phase until use. It was held at distribution centers until requested by a treating hospital and transported to the hospital in controlled vapor shippers on a just-in-time basis to ensure the quality of the product at the time of treatment. The viability of the batches administered in the trial ranged from 78% to 93%. See the Appendix in the data supplement for manufacturing and cell viability.
The protocol included guidelines for SARS-CoV-2 ARDS management, such as ventilator settings, proning, sedation, pain management, and concomitant medications, including the use of off-label or investigational antiviral agents and investigational anti–IL-6 agents. The use of dexamethasone and remdesivir became the standard of care during the trial.
Outcomes
Patients were followed for 12 months after randomization. The primary endpoint was a reduction in all-cause mortality within 30 days after randomization. The key secondary endpoint was days alive off mechanical ventilation within 60 days after randomization. Secondary endpoints included mortality at 7, 14, 60, and 90 days and 12 months, resolution and/or improvement of ARDS, and clinical improvement at Days 7, 14, 21, and 30 after randomization. ARDS resolution or improvement was defined as being alive with a decrease in ARDS scale severity. Clinical improvement was defined as discharge or an improvement by two points on a seven-point ordinal scale that ranged from one (death) to seven (nonhospitalized status with the resumption of normal activities).
Total and ICU length of stay (LOS) after randomization for the index hospitalization, readmissions, and the total number of days in hospital within 60 days after randomization were assessed. Secondary safety endpoints included any infusion-related toxicity (hypersensitivity reaction within 2 h of administration) and the incidence of serious adverse events within 30 days after randomization. Pulmonary symptoms and vital status were assessed at 6 and 12 months.
Statistical Analysis
The trial was designed early in the pandemic when 30-day mortality for patients with ARDS was between 40% and 60% (17). The sample size was on the basis of a relatively high treatment effect because we needed a trial that could be accomplished in a tractable period of time, given the desperate need for new therapies for this high-mortality condition and the limited availability of cell products at the time. In addition, before the start of enrollment, the design was further modified from a phase II trial with a one-sided test of the primary endpoint to a phase II/III design with a two-sided test to facilitate registration and a more expeditious route to bring the therapy to patients, if efficacious. A sample size of 150 patients in each group ensured approximately 84% power to detect a difference of 17% from an assumed control rate of 40% mortality by 30 days after randomization using a two-sided, 0.05-level test of independent proportions. Three interim analyses for stopping accrual early for efficacy or futility when 30%, 45%, and 60% of patients had reached the primary endpoint were prespecified using Bayesian predictive probabilities (more detail on assumptions and operating characteristics are provided in the Supplemental Appendix).
The primary efficacy analysis compared the proportion of patients who died by 30 days after randomization between groups using a two-sided, 0.05-level test for independent proportions. Missing values because of early withdrawal were imputed as deaths. The key secondary efficacy endpoint was assessed using a two-sided, 0.05-level Wilcoxon rank-sum test. Patients surviving to Day 60 were ranked according to their number of ventilator-free days. Patients who withdrew or had an unknown extubation date were assigned ranks lower than the lowest observed rank, in order on the basis of the proportion of known days alive assessed for intubation and free from mechanical ventilation. Patients who died before Day 60 were assigned the lowest ranks, in order on the basis of the time of death. Differences in rank between groups were assessed using the Hodges-Lehmann estimate of location shift (18).
Survival at 7 and 14 days after randomization was assessed as above. Survival (60 and 90 d and 12 mo) was estimated using the Kaplan-Meier method, and comparisons were made with the log-rank test. Clinical improvement and resolution and/or improvement in ARDS were assessed using mixed effect logistic regression. LOS during the index hospitalization (including ICU d) and LOS through Day 60, including readmission days, were compared using Wilcoxon rank-sum tests. Deaths, withdrawals, and patients with incomplete data were ranked according to the worst-rank method described above. Group differences in readmission rates through Day 60 and serious adverse event rates through Day 30 were evaluated using Poisson regression with robust variance estimation.
Subgroup analyses of the primary endpoint in key clinical subgroups were prespecified when the number of patients in the strata was at least 20. With the exception of ARDS severity, for which a formal interaction test was prespecified, subgroup analyses are descriptive and presented as relative risks with 95% confidence intervals (CIs).
Safety endpoints (infusional toxicities and serious adverse events) were assessed in all randomized subjects who received any amount of study product. All other endpoints were evaluated in the intent-to-treat population. There was no formal correction of the type I error rate for multiple testing, as prespecified. As such, point estimates of treatment effects for secondary endpoints are presented with 95% CIs that have not been adjusted for multiplicity. Analyses were conducted using SAS version 9.4 (SAS Institute, Inc.).
Results
Patients
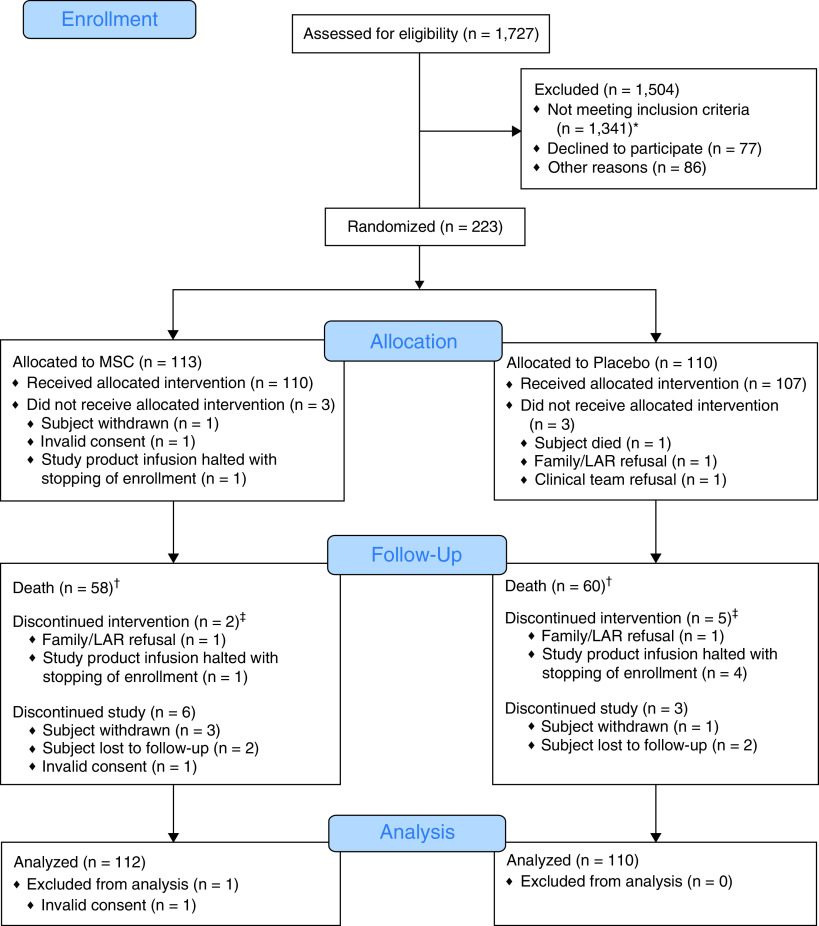
From April 30 to December 14, 2020, 223 patients were randomized across 20 U.S. sites (Figure 1). At the third interim analysis, randomization, but not follow-up, was halted by the data safety monitoring board because of a low predicted probability of observing any benefit of MSCs for the primary endpoint. The final analysis included 222 randomized patients (112 in the MSC and 110 in the control group). One patient was excluded from the study because the patient’s spokesperson, who signed the consent, was determined not to be a legally authorized representative. The mean age was 61.8 ± 13.0 years in the MSC group and 59.6 ± 13.8 years in the control group, with approximately 30% of patients categorized with severe ARDS (Table 1). Concomitant therapies included remdesivir in two-thirds of patients, corticosteroids in over 80% of patients, and anticoagulation in over 90% of patients at the time of enrollment. Median CRP was 13.0 in MSC recipients and 15.2 in control patients. Among MSC recipients, 107 (95.5%) received both infusions, 3 (2.7%) received only the first infusion, and 2 (1.8%) received no infusion. In the control group, 100 (90.9%) patients received both infusions, 7 (6.4%) received only one infusion, and 3 (2.7%) received none (Table E1 in the supplemental appendix).
Figure 1.
CONSORT (Consolidated Standards of Reporting Trials) diagram. *The most common reasons for not meeting inclusion/exclusion criteria included not having moderate and/or severe acute respiratory distress syndrome or requiring mechanical ventilator support (n = 533) and intubation greater than 72 hours (n = 208). †Three subjects discontinued intervention because of death (MSC = 1 and placebo = 2). ‡Discontinued intervention means the second infusion was not administered. LAR = legally authorized representative; MSC = mesenchymal stromal cell.
Table 1.
Patient Characteristics at Baseline
| Patient Characteristic | MSC (n = 112) | Placebo (n = 110) |
|---|---|---|
| Age (yr), mean ± SD | 61.8 ± 13.0 | 59.6 ± 13.8 |
| <45, n (%) | 12/112 (10.7) | 15/110 (13.6) |
| 45–65, n (%) | 46/112 (41.1) | 52/110 (47.3) |
| >65, n (%) | 54/112 (48.2) | 43/110 (39.1) |
| Female, n (%) | 33/112 (29.5) | 35/110 (31.8) |
| Race, n (%) | ||
| White | 46/112 (41.1) | 53/110 (48.2) |
| Black | 21/112 (18.8) | 15/110 (13.6) |
| Other* | 45/112 (40.2) | 42/110 (38.2) |
| Ethnicity, Hispanic or Latino, n (%) | 41/112 (36.6) | 47/110 (42.7) |
| BMI, n (%) | ||
| <30 | 47/112 (42.0) | 38/110 (34.5) |
| 30–40 | 49/112 (43.8) | 48/110 (43.6) |
| ⩾40 | 16/112 (14.3) | 24/110 (21.8) |
| Coexisting illness, n (%) | ||
| Hypertension | 65/110 (59.1) | 63/107 (58.9) |
| Diabetes mellitus | 46/109 (42.2) | 42/107 (39.3) |
| Renal disease | 14/109 (12.8) | 13/107 (12.1) |
| Prior pulmonary disease | 18/109 (16.5) | 15/106 (14.2) |
| Heart failure | 6/109 (5.5) | 4/108 (3.7) |
| Cancer | 12/109 (11.0) | 11/106 (10.4) |
| History of smoking/e-cigarette/vaping | 35/101 (34.7) | 31/101 (30.7) |
| COVID-19 medications, n (%) | ||
| Remdesivir | 76/112 (67.9) | 74/110 (67.3) |
| Convalescent plasma | 25/112 (22.3) | 30/110 (27.3) |
| IL-6 inhibitor | 6/112 (5.4) | 5/110 (4.5) |
| Glucocorticoid | 92/112 (82.1) | 96/110 (87.3) |
| Adjunctive medications, n (%) | ||
| Antibiotics | 92/112 (82.1) | 83/110 (75.5) |
| Anticoagulation | 103/112 (92.0) | 106/110 (96.4) |
| Antiplatelets | 22/112 (19.6) | 20/110 (18.2) |
| ACE inhibitor or ARB | 4/112 (3.6) | 1/110 (0.9) |
| Neuromuscular blockade or paralytic | 75/112 (67.0) | 71/110 (64.5) |
| Pulmonary vasodilators | 16/112 (14.3) | 15/110 (13.6) |
| Disease severity, n (%) | ||
| Moderate | 79/112 (70.5) | 76/110 (69.1) |
| Severe | 33/112 (29.5) | 34/110 (30.9) |
| SOFA score, n (%) | 6.6 ± 2.1 | 6.7 ± 1.9 |
| D on ventilation before randomization, median (IQR) | 1.0 (1.0–2.0) | 1.0 (1.0–2.0) |
| Use of prone ventilation, n (%) | 47/112 (42.0) | 50/110 (45.5) |
| Labs, median (IQR) | ||
| C-Reactive protein, mg/dl | 13.0 (6.6–21.5) | 15.2 (8.8–22.3) |
| IL-6, pg/ml | 58.2 (23.2–248.9) | 56.0 (22.1–205.2) |
| IL-8, pg/ml | 22.4 (16.5–36.5) | 24.9 (14.5–33.6) |
| Creatinine, mg/dl | 0.9 (0.7–1.3) | 1.0 (0.7–1.4) |
| Blood urea nitrogen, mg/dl | 26.0 (19.0–37.0) | 28.0 (21.0–40.0) |
| Lymphocytes, % | 7.0 (4.5–9.8) | 5.5 (3.7–9.5) |
| Neutrophil–lymphocyte ratio, % | 12.1 (8.5–19.3) | 15.3 (8.6–25.8) |
| Aspartate aminotransferase, U/L | 38.0 (29.0–61.0) | 32.0 (26.0–52.5) |
| Alanine aminotransferase, U/L | 30.0 (21.5–46.0) | 33.0 (22.0–55.0) |
Definition of abbreviations: ACE = angiotensin-converting-enzyme; ARB = angiotensin receptor blocker; BMI = body mass index; COVID-19 = coronavirus disease; IQR = interquartile range; MSC = mesenchymal stromal cell; SOFA = Sequential Organ Failure Assessment.
Categorical measures are presented as number/number observed (%), and continuous measures are presented as median (IQR) or mean ± SD.
“Other” race includes seven unknowns in the MSC group and six in the placebo group.
Mortality
At 30 days after randomization, 42 (37.5%) patients with MSC compared with 47 (42.7%) control patients had died (relative risk (RR), 0.88; 95% CI, 0.64–1.21; P = 0.43) (Table 2). One MSC patient withdrew before infusion and was imputed as a death. Figure 2A depicts survival at 60 and 90 days (hazard ratio, 0.89; 95% CI, 0.62–1.28).
Table 2.
Mortality, Hospitalizations, and Adverse Events
| MSC (n = 112) |
Placebo (n = 110) |
||||
|---|---|---|---|---|---|
| Mortality* | n (%) | n (%) | Relative Risk (95% CI) | ||
| Primary endpoint, died by D 30 | 42 (37.5) | 47 (42.7) | 0.88 (0.64–1.21) | ||
| Died by D 7 | 6 (5.4) | 5 (4.5) | 1.18 (0.37–3.75) | ||
| Died by D 14 | 21 (18.8) | 24 (21.8) | 0.86 (0.51–1.45) | ||
| MSC† (n = 44; Patient D = 1,604) |
Placebo† (n = 47; Patient D = 1,511) |
||||
| Patients, n (%) | Events (Rate per 30 Pt D), n | Patients, n (%) | Events (Rate per 30 Pt D), n | Relative Rate (95% CI) | |
| Readmissions at 60 d | 2 (4.5) | 4 (0.075) | 3 (6.4) | 3 (0.060) | 1.26 (0.22–7.29) |
| Serious AEs at 30 d | MSC‡ (n = 110; Patient D = 2,807) |
Placebo‡ (n = 107; Patient D = 2,646) |
Relative Rate (95% CI) | ||
| Patients, n (%) | Events (Rate per 30 Pt D), n | Patients, n (%) | Events (Rate per 30 Pt D), n | ||
| Neoplasm/tumorigenesis | 1 (0.9) | 1 (0.011) | 0 | 0 | — |
| Cardiac arrhythmias, sustained ventricular arrhythmia | 1 (0.9) | 1 (0.011) | 1 (0.9) | 1 (0.011) | 0.94 (0.06–14.81) |
| Cardiac arrhythmias, sustained supraventricular arrhythmia | 14 (12.7) | 14 (0.150) | 6 (5.6) | 6 (0.068) | 2.20 (0.86–5.60) |
| Cardiac arrhythmias, type not specified | 1 (0.9) | 1 (0.011) | 0 | 0 | — |
| Deterioration of respiratory status | 31 (28.2) | 31 (0.331) | 26 (24.3) | 30 (0.340) | 0.97 (0.62–1.53) |
| Hepatic dysfunction | 2 (1.8) | 2 (0.021) | 1 (0.9) | 1 (0.011) | 1.89 (0.17–20.63) |
| Major infection, localized | 16 (14.5) | 18 (0.192) | 21 (19.6) | 21 (0.238) | 0.81 (0.44–1.50) |
| Major Infection, sepsis | 16 (14.5) | 17 (0.182) | 17 (15.9) | 18 (0.204) | 0.89 (0.46–1.72) |
| Multisystem organ failure | 2 (1.8) | 2 (0.021) | 4 (3.7) | 4 (0.045) | 0.47 (0.09–2.56) |
| Myocardial infarction | 0 | 0 | 2 (1.9) | 2 (0.023) | — |
| Pleural effusion | 1 (0.9) | 1 (0.011) | 3 (2.8) | 4 (0.045) | 0.24 (0.02–2.30) |
| Psychiatric episode | 1 (0.9) | 1 (0.011) | 0 | 0 | — |
| Renal dysfunction, acute renal dysfunction | 26 (23.6) | 26 (0.278) | 25 (23.4) | 26 (0.295) | 0.94 (0.57–1.57) |
| Thromboembolic event, ischemic stroke | 2 (1.8) | 2 (0.021) | 1 (0.9) | 1 (0.011) | 1.89 (0.17–20.63) |
| Thromboembolic event, systemic thromboembolism | 1 (0.9) | 1 (0.011) | 2 (1.9) | 2 (0.023) | 0.47 (0.04–5.11) |
| Thromboembolic event, venous thromboembolism | 7 (6.4) | 7 (0.075) | 3 (2.8) | 3 (0.034) | 2.20 (0.59–8.22) |
| Vasodilatory state | 7 (6.4) | 7 (0.075) | 8 (7.5) | 8 (0.091) | 0.82 (0.31–2.21) |
| Other AE | 19 (17.3) | 28 (0.299) | 15 (14.0) | 18 (0.204) | 1.47 (0.72–2.99) |
| Pneumothorax | 6 (5.5) | 7 (0.075) | 6 (5.6) | 8 (0.091) | 0.82 (0.26–2.65) |
| All serious AEs | 68 (61.8) | 167 (1.785) | 70 (65.4) | 153 (1.735) | 1.03 (0.75–1.41) |
Definition of abbreviations: AE = adverse event; CI = confidence interval; MSC = mesenchymal stromal cell.
Per protocol, one patient in the MSC arm who withdrew before 30 days was imputed as a death.
A total of 129 patients was excluded from readmission analyses (66 in the MSC group and 63 in the placebo group) because they died during the index admission or were not discharged by Day 60. Two additional patients (both in the MSC arm) are excluded for early withdrawal or unavailable index hospitalization discharge data.
Safety endpoints are analyzed using the safety population, which is defined as all randomized subjects who received any amount of study product.
Figure 2.
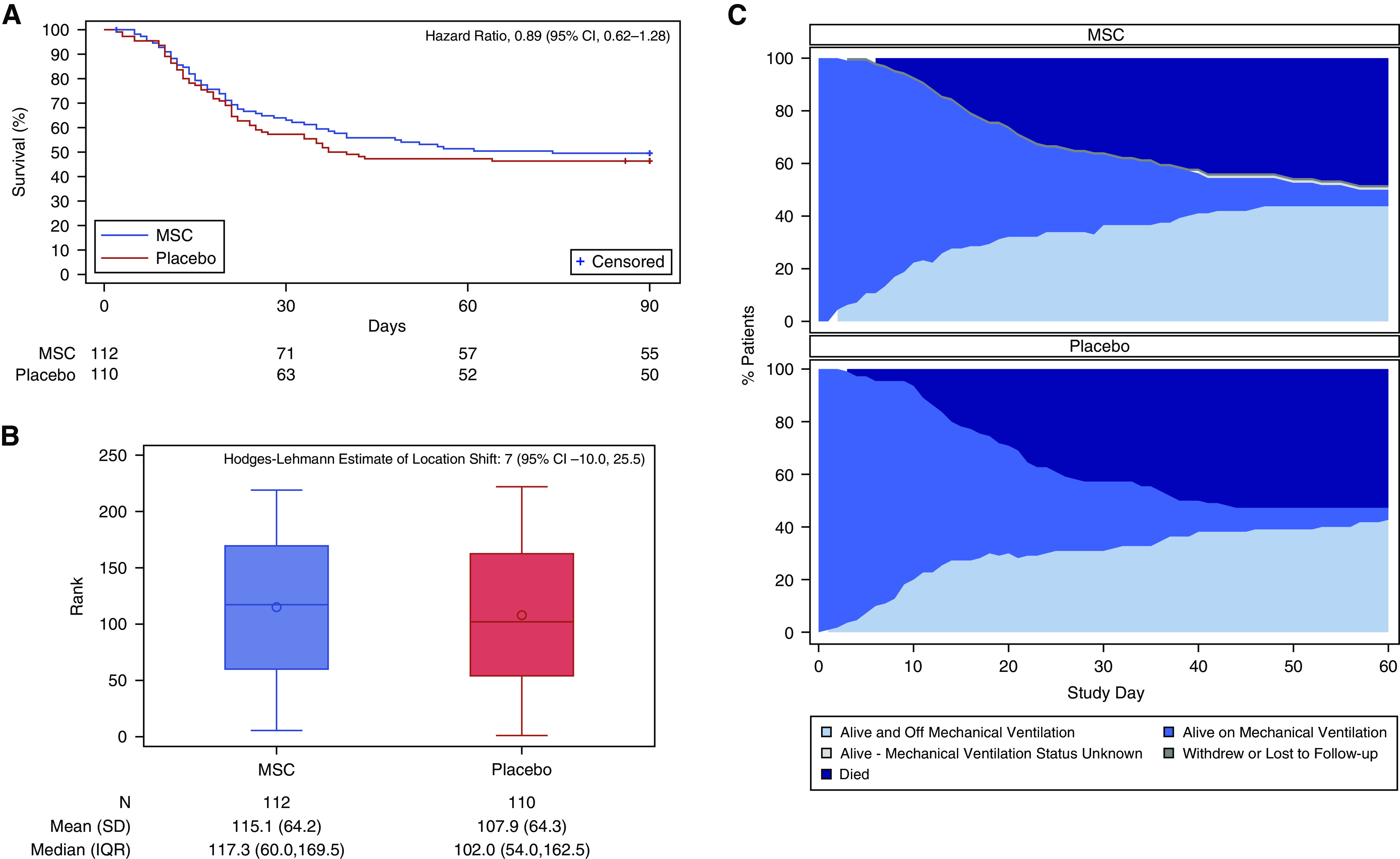
Survival and key secondary endpoint. (A) Depicts survival by randomization group. The tick marks show the censoring of data. (B) Depicts the distributions of rank by randomization group from the rank-based assessment of the key secondary endpoint, days alive, and free of mechanical ventilation at Day 60. Higher ranks correspond to better outcomes (i.e., fewer d on mechanical ventilation), and deaths were assigned the worst ranks in order of time of death. (C) Depicts the proportion of patients alive and off mechanical ventilation, alive on mechanical ventilation, alive with unknown ventilation status, withdrawn, or died, by randomization group over each study day from randomization through Day 60. CI = confidence interval; IQR = interquartile range; MSC = mesenchymal stromal cell.
Days Alive off Mechanical Ventilatory Support
At 60 days, the number of patients alive free from ventilator support was 49/110 (44.5%) in the MSC and 47/110 (42.7%) in the control group. Among survivors, the median days on the ventilator within 60 days was 13.5 (IQR, 7.5–35) in MSC and 14.5 (interquartile range (IQR), 9–37) in control patients. In a ranked analysis incorporating deaths and withdrawals, there were no significant between-group differences in days alive without ventilator support (Figures 2B and 2C).
Clinical Improvement and Resolution/Improvement of ARDS
At 30 days, 37/111 (33.3%) MSC patients and 34/110 (30.9%) control patients showed a two-point improvement from baseline on the seven-point ordinal scale or were discharged alive (OR, 1.17; 95% CI, 0.42–3.22) (Figure E1A, supplemental appendix). Resolution or improvement of ARDS at 30 days was observed in 51/104 (49.0%) MSC recipients and 46/106 (43.4%) in control patients (OR, 1.36; 95% CI, 0.57–3.21) (Figure E1B, supplemental appendix).
Adverse Events and Hospitalizations
There was no between-group difference in serious adverse events over 30 days (Table 2). No infusion-related toxicity events were observed during the course of the study. In a ranked analysis, there was no difference between groups in the index LOS or ICU days (Figures E2 and E3, supplemental appendix). Among survivors, median LOS was 25 days (IQR, 15–43) in the MSC group versus 26 days (IQR, 19–47) in the control group, with median ICU days of 18 (IQR, 11–30) and 21 (IQR, 12–36), respectively. There was no between-group difference in readmissions (Table 2) or the expanded total LOS through 60 days (Figure E4, supplemental appendix).
Twelve-Month Mortality and Pulmonary Symptoms
At 12 months, mortality remained similar between groups (hazard ratio, 0.91; 95% CI, 0.63–1.30) (Figure E5, supplemental appendix) and relatively unchanged from mortality at 90 days. By 12 months, the incidence of asthma, chronic obstructive pulmonary disease, emphysema, and pulmonary fibrosis was low across both groups (Table E2, supplemental appendix). At 12 months, none of the survivors was supported with invasive or noninvasive mechanical ventilation. At this time point, the use of supplementary oxygen was greater in the MSC group, but the numbers are small, and the confidence interval spans one (7/46 [15.2%] vs. 2/47 [4.3%]; RR, 3.58; 95% CI, 0.78–16.32) (Table E3, supplemental appendix).
Subgroups
In a subgroup analysis stratified by ARDS severity, the RR of 30-day death with MSCs compared with sham control was 0.87 (95% CI, 0.57–1.31) in patients with moderate ARDS and 0.91 (95% CI, 0.55–1.50) in those with severe ARDS. Figure 3 depicts descriptive prespecified subgroup analyses, such as age, ethnicity, and diabetes, of the primary endpoint (see Figure E6, supplemental appendix, for a similar analysis of 90-day mortality).
Figure 3.
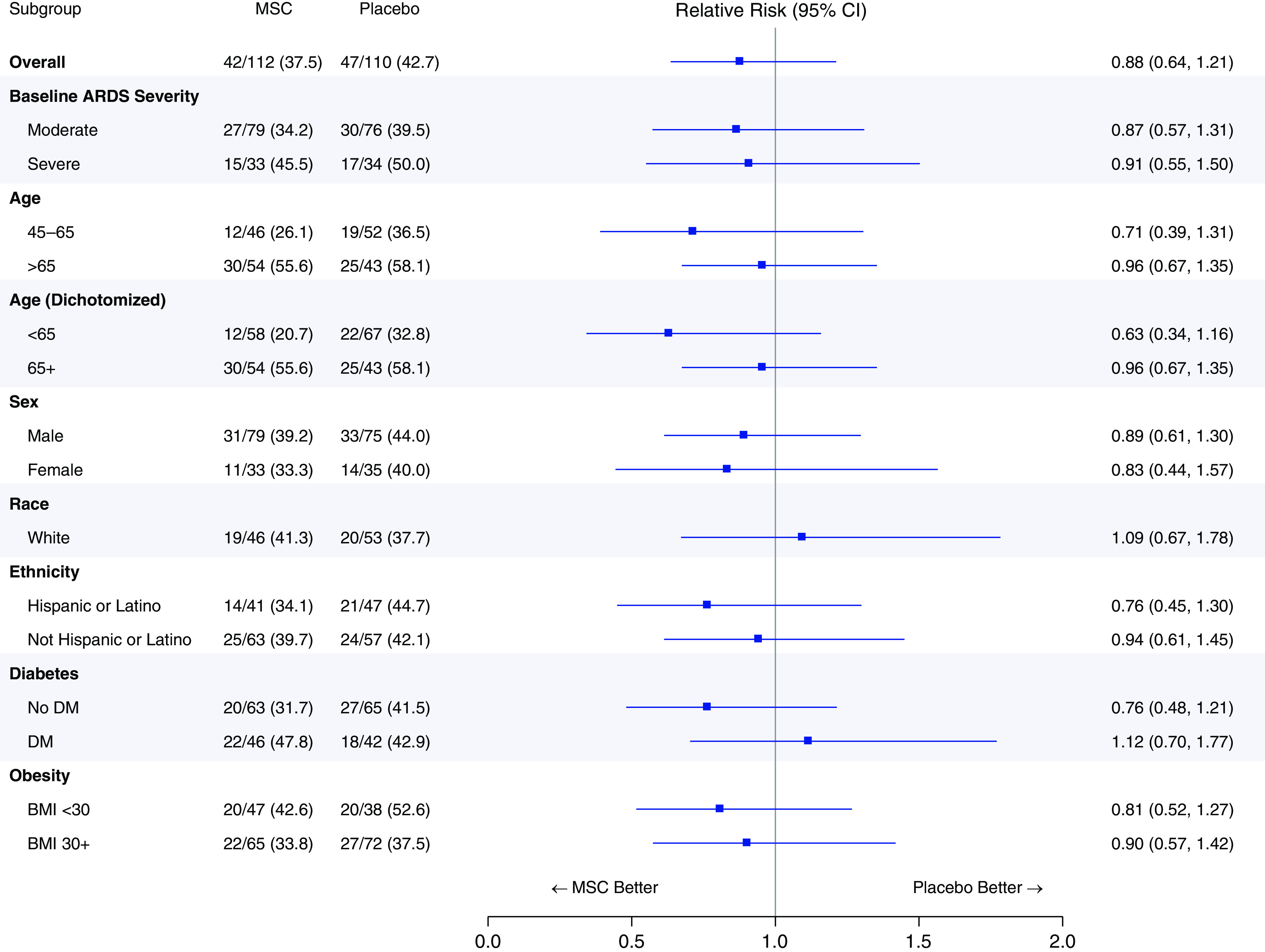
Subgroup analyses of the primary endpoint. Subgroup analyses of the primary endpoint in key clinical subgroups were prespecified. Per protocol, strata in which the number of patients assigned to a specific group is less than 20 were not considered. ARDS = acute respiratory distress syndrome; BMI = body mass index; CI = confidence interval; DM = diabetes mellitus; MSC = mesenchymal stromal cell.
Discussion
Patients with COVID-19–related ARDS have only a limited array of therapeutic options and, as this trial demonstrates, continue to experience very high mortality rates. This need has galvanized an intensive search for new candidate therapeutics and motivated the current effort to evaluate a fixed MSC dosing regimen for this condition. This trial that was halted at the third interim analysis for futility showed no significant difference in 30-day mortality with the use of MSCs versus sham control nor days off mechanical ventilation within 60 days of randomization. In terms of safety, there were no infusion-related toxicities or differences in serious adverse events over 30 days after randomization. Survival out to 12 months was not different between the MSC and sham control groups.
MSCs have potential therapeutic applications for the treatment of ARDS through a variety of mechanisms, including immune modulation, alveolar fluid clearance, bacterial clearance, regulation of pulmonary vascular endothelial permeability, and suppression of apoptosis (1). Preclinical models of ARDS have supported the safety and efficacy of MSC therapy for the treatment of lung injury (19–21); however, MSC therapy in patients with ARDS remains investigational. Two phase I trials have reported no safety concerns in administering MSCs to patients with ARDS (13, 22). In a randomized trial of 60 patients, Matthay and colleagues concluded that administration of MSCs was safe in patients with non–COVID-19–related moderate to severe ARDS (12). Overall 28-day mortality did not differ significantly between groups in that trial. However, those results may have been affected by the poor overall viability of MSCs used in that study (36–85%). Preliminary observational studies with MSCs in the setting of COVID-19 ARDS have demonstrated a signal of therapeutic benefit (23, 24).
There are several potential explanations for the results observed in this trial. These include an incomplete knowledge of COVID-19–related ARDS pathophysiology and the rapid evolution of COVID-19–related ventilatory and pharmacologic management practices during the trial. A key exclusion criterion concerned the duration of mechanical ventilation (>72 h) before enrollment. This stipulation was intended to ensure the administration of MSCs during the peak of the cytokine storm and before the development of end-stage, irreversible parenchymal lung damage. However, the timing of endotracheal intubation in the course of illness changed during trial conduct. In the first few months of the pandemic, guidelines recommended intubation and invasive mechanical ventilation when oxygen requirements on the nasal cannula reached 6 L/min (25). This guidance was driven largely by concern for aerosol generation with noninvasive respiratory support and the risk of rapid patient deterioration (26). As the COVID-19 pandemic progressed, concerns that high-flow nasal cannula could result in the dissemination of dangerous concentrations of infected aerosols receded (27). Clinicians also realized there were potential benefits to deferring or delaying mechanical ventilation, including avoidance of sedation, reduction in the time patients are immobilized, and minimization of patient–clinician communication challenges. Within 2–3 months of study initiation, increasing numbers of COVID-19 patients were managed with noninvasive respiratory support for days or weeks before intubation (28). This change in ventilatory management practices that affected a substantial number of enrolled patients meant that those who failed noninvasive ventilation and eventually required intubation may have progressed to the point of inflammatory parenchymal lung damage that was more developed and possibly less modifiable by the time of enrollment.
An additional factor to consider relates to the potential heterogeneity of the patient population enrolled. With regard to non–COVID-19–induced ARDS, researchers have described two distinct subphenotypes, hyperinflammatory or reactive and hypoinflammatory or uninflamed (29, 30). These subphenotypes differ in biomarker profiles, disease course, and, more importantly, the response to ARDS management strategies and outcomes. The hyperinflammatory subphenotype is characterized by increased concentrations of proinflammatory biomarkers, including IL-6, IL-8, and TNFR-1, decreased serum bicarbonate concentrations, vasopressor dependence, and the presence of sepsis (31). This subphenotype is also associated with higher mortality and fewer ventilator-free and organ failure-free days compared with the hypoinflammatory subphenotype (32). Recently, an exploratory study showed evidence that hyperinflammatory and hypoinflammatory subphenotypes may exist in COVID-19–induced ARDS (33). Chen and colleagues showed a significant survival benefit with corticosteroids in the hyperinflammatory subphenotype but no effect in the hypoinflammatory cohort (34). The proportion of such distinct subphenotypes among patients with COVID-19 enrolled in this trial, and any associated differential treatment effects may have contributed to the neutral findings. Serial inflammatory biomarkers were collected during the study, and planned analyses might help identify ARDS subphenotypes among enrolled patients, which require further study.
In defining these subphenotypes, age may also play a role as reflected in the signal of survival benefit seen in those under 65 years in the forest plot; see results section and 90-day mortality in Appendix. The absence of a signal in older patients is consistent with the fact that they have a longer duration of SARS-CoV-2 viral clearance because of age-related maladaptive immune responses that is associated with higher degrees of inflammation in the lungs (35–40). Consequently, it is possible that higher or more prolonged dosing with MSC would be required in older patients relative to younger patients to achieve similar survival benefits and may have affected the results of this trial. Although the lower dose may be sufficient in younger patients, future studies should explore age-related dosing.
This trial has several limitations. Disease management practices during the pandemic changed rapidly, and the protocol could not practically be modified at the same pace. Eligibility criteria reflected the state of clinical knowledge at the time of protocol design and did not account for prospective identification of subphenotypes of patients, which may have helped identify treatment responders. Moreover, as the timing of intubation changed from early in the cytokine storm to later in the disease course, the use of days on a ventilator as a marker of pulmonary parenchymal disease became imperfect. We did not collect information on the duration of noninvasive respiratory management before randomization to characterize this population further. Furthermore, the design of this phase II/III trial was on the basis of the expectation of a relatively high mortality reduction (similar to the one subsequently found in the Randomised Evaluation of COVID-19 Therapy [RECOVERY] trial) (4). Whereas a smaller relative mortality reduction would have been clinically meaningful, such a trial would have required the enrollment of several thousand patients, which would have been challenging within the constraints of the pandemic. Finally, because the trial was halted early, our ability to detect differences in secondary endpoints and subgroup analyses was reduced. Given that ARDS is a highly lethal disease with minimal treatment options, the signals identified in this trial deserve further exploration in future research.
Conclusions
In our trial, MSC therapy, compared with sham control, in patients with moderate to severe COVID-19–related ARDS did not produce the hypothesized reduction in 30-day mortality or improve ventilator-free days over 60 days after randomization. During the pandemic, new insights emerged into the existence of different inflammatory subtypes and the importance of age within the overall COVID-19–related ARDS population, which might underlie a differential response to immunomodulatory therapy. Future research is needed to identify the potential benefits of MSCs in susceptible phenotypes.
Acknowledgments
Acknowledgment
The authors are indebted to the generosity of the patients, who consented to participate in the trial.
Footnotes
A complete list of trial members may be found in the supplement.
Supported by Mesoblast, Inc. Biospecimen collection for the purposes of enabling mechanistic studies was supported by a cooperative agreement (U01 HL088942) funded by the National Heart, Lung, and Blood Institute. Mesoblast, Inc. was not involved in data collection and data analysis. Mesoblast reviewed the data and final manuscript, but the academic authors retained editorial control.
Author Contributions: M.E.B., C.E.B., J.R.O., R.L.G., K.O., A.D., M.E.M., J. Hupf, J.L.G., A.I., A.J.R., E.G.M., K.O’S., H.L.C., E. Burke, J. Hayes, F.G., S.I., M.G., P.T.O’G., M.J.M., P.K.S., E. Bagiella, A.J.M., and A.C.G. contributed to the protocol and design of the study. M.E.B., C.E.B., R.L.G., K.O., A.D., E.F., B.G.L., J.L.G., A.I., A.J.R., J.B.W., S.P., N.C.P., N.D.D., P.C.H., M.G., M.J.M., P.K.S., and F.D.P. are study site principal investigators. All authors (except E. Burke, J. Hayes, F.G., and S.I.) contributed to study conduct and data collection. J.R.O., E. Bagiella, and H.L.C. have accessed and verified the underlying data and conducted the statistical analysis. F.G. and S.I. were responsible for cell therapy manufacturing. All authors contributed to manuscript preparation or its critical review and approved the final version.
Data sharing statement: Mesoblast is interested in sharing clinical data from the trial with scientific researchers in the interest of advancing public health. Qualified external researchers can request deidentified participant data to conduct research. These deidentified data will only be available for request after all patient follow-up is completed and a period of 12 months has elapsed after U.S. Food and Drug Administration and European Medicines Agency approval. Proposals will be reviewed and approved by Mesoblast and the representative of the Coordinating Center. After approval of a proposal, data can be shared through a secure online platform after signing a data access agreement.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202201-0157OC on September 13, 2022
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Osuchowski MF, Winkler MS, Skirecki T, Cajander S, Shankar-Hari M, Lachmann G, et al. The COVID-19 puzzle: deciphering pathophysiology and phenotypes of a new disease entity. Lancet Respir Med . 2021;9:622–642. doi: 10.1016/S2213-2600(21)00218-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McGonagle D, Bridgewood C, Meaney JFM. A tricompartmental model of lung oxygenation disruption to explain pulmonary and systemic pathology in severe COVID-19. Lancet Respir Med . 2021;9:665–672. doi: 10.1016/S2213-2600(21)00213-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Angriman F, Ferreyro BL, Burry L, Fan E, Ferguson ND, Husain S, et al. Interleukin-6 receptor blockade in patients with COVID-19: placing clinical trials into context. Lancet Respir Med . 2021;9:655–664. doi: 10.1016/S2213-2600(21)00139-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al. RECOVERY Collaborative Group Dexamethasone in hospitalized patients with COVID-19. N Engl J Med . 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tomazini BM, Maia IS, Cavalcanti AB, Berwanger O, Rosa RG, Veiga VC, et al. COALITION COVID-19 Brazil III Investigators Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA . 2020;324:1307–1316. doi: 10.1001/jama.2020.17021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gordon AC, Mouncey PR, Al-Beidh F, Rowan KM, Nichol AD, Arabi YM, et al. REMAP-CAP Investigators Interleukin-6 receptor antagonists in critically ill patients with COVID-19. N Engl J Med . 2021;384:1491–1502. doi: 10.1056/NEJMoa2100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rosas IO, Bräu N, Waters M, Go RC, Hunter BD, Bhagani S, et al. Tocilizumab in hospitalized patients with severe COVID-19 pneumonia. N Engl J Med . 2021;384:1503–1516. doi: 10.1056/NEJMoa2028700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Matthay MA, Pati S, Lee JW. Concise review: mesenchymal stem (stromal) cells: biology and preclinical evidence for therapeutic potential for organ dysfunction following trauma or sepsis. Stem Cells . 2017;35:316–324. doi: 10.1002/stem.2551. [DOI] [PubMed] [Google Scholar]
- 9. Samsonraj RM, Raghunath M, Nurcombe V, Hui JH, van Wijnen AJ, Cool SM. Concise review: multifaceted characterization of human mesenchymal stem cells for use in regenerative medicine. Stem Cells Transl Med . 2017;6:2173–2185. doi: 10.1002/sctm.17-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kurtzberg J, Abdel-Azim H, Carpenter P, Chaudhury S, Horn B, Mahadeo K, et al. MSB-GVHD001/002 Study Group A phase 3, single-arm, prospective study of remestemcel-L, ex vivo culture-expanded adult human mesenchymal stromal cells for the treatment of pediatric patients who failed to respond to steroid treatment for acute graft-versus-host disease. Biol Blood Marrow Transplant . 2020;26:845–854. doi: 10.1016/j.bbmt.2020.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chan MCW, Kuok DIT, Leung CYH, Hui KPY, Valkenburg SA, Lau EHY, et al. Human mesenchymal stromal cells reduce influenza A H5N1-associated acute lung injury in vitro and in vivo. Proc Natl Acad Sci USA . 2016;113:3621–3626. doi: 10.1073/pnas.1601911113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Matthay MA, Calfee CS, Zhuo H, Thompson BT, Wilson JG, Levitt JE, et al. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): a randomised phase 2a safety trial. Lancet Respir Med . 2019;7:154–162. doi: 10.1016/S2213-2600(18)30418-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wilson JG, Liu KD, Zhuo H, Caballero L, McMillan M, Fang X, et al. Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical trial. Lancet Respir Med . 2015;3:24–32. doi: 10.1016/S2213-2600(14)70291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Leng Z, Zhu R, Hou W, Feng Y, Yang Y, Han Q, et al. Transplantation of ACE2- mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis . 2020;11:216–228. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moss M, Huang DT, Brower RG, Ferguson ND, Ginde AA, Gong MN, et al. National Heart, Lung, and Blood Institute PETAL Clinical Trials Network Early neuromuscular blockade in the acute respiratory distress syndrome. N Engl J Med . 2019;380:1997–2008. doi: 10.1056/NEJMoa1901686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Whittaker Brown SA, Iancu-Rubin C, Aboelela A, Abrahams A, Burke E, Drummond T, et al. Mesenchymal stromal cell therapy for acute respiratory distress syndrome due to coronavirus disease 2019. Cytotherapy . 2022;24:835–840. doi: 10.1016/j.jcyt.2022.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dennis JM, McGovern AP, Vollmer SJ, Mateen BA. Improving survival of critical care patients with coronavirus disease 2019 in England: a national cohort study, March to June 2020. Crit Care Med . 2021;49:209–214. doi: 10.1097/CCM.0000000000004747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hodges JL, Lehmann EL. Estimates of location based on rank tests. Ann Math Stat . 1963;34:598–611. [Google Scholar]
- 19. Asmussen S, Ito H, Traber DL, Lee JW, Cox RA, Hawkins HK, et al. Human mesenchymal stem cells reduce the severity of acute lung injury in a sheep model of bacterial pneumonia. Thorax . 2014;69:819–825. doi: 10.1136/thoraxjnl-2013-204980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Curley GF, Hayes M, Ansari B, Shaw G, Ryan A, Barry F, et al. Mesenchymal stem cells enhance recovery and repair following ventilator-induced lung injury in the rat. Thorax . 2012;67:496–501. doi: 10.1136/thoraxjnl-2011-201059. [DOI] [PubMed] [Google Scholar]
- 21. Gupta N, Su X, Popov B, Lee JW, Serikov V, Matthay MA. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol . 2007;179:1855–1863. doi: 10.4049/jimmunol.179.3.1855. [DOI] [PubMed] [Google Scholar]
- 22. Lanzoni G, Linetsky E, Correa D, Messinger Cayetano S, Alvarez RA, Kouroupis D, et al. Umbilical cord mesenchymal stem cells for COVID-19 acute respiratory distress syndrome: a double-blind, phase 1/2a, randomized controlled trial. Stem Cells Transl Med . 2021;10:660–673. doi: 10.1002/sctm.20-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Atluri S, Manchikanti L, Hirsch JA. Expanded umbilical cord mesenchymal stem cells (UC-MSCs) as a therapeutic strategy in managing critically ill COVID-19 patients: the case for compassionate use. Pain Physician . 2020;23:E71–E83. [PubMed] [Google Scholar]
- 24. Shetty AK. Mesenchymal stem cell infusion shows promise for combating coronavirus (COVID-19)-induced pneumonia. Aging Dis . 2020;11:462–464. doi: 10.14336/AD.2020.0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coronavirus disease 2019 (COVID-19) treatment guidelines. National Institutes of Health 2021https://www.covid19treatmentguidelines.nih.gov/ [PubMed]
- 26. Zuo MZ, Huang YG, Ma WH, Xue ZG, Zhang JQ, Gong YH, et al. Airway Management Chinese Society of Anesthesiology Task Force on Chinese Society of Anesthesiology Task Force on Airway Management. Expert recommendations for tracheal intubation in critically ill patients with novel coronavirus disease 2019. Chin Med Sci J . 2020;35:105–109. doi: 10.24920/003724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med . 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nasa P, Azoulay E, Khanna AK, Jain R, Gupta S, Javeri Y, et al. Expert consensus statements for the management of COVID-19-related acute respiratory failure using a Delphi method. Crit Care . 2021;25:106. doi: 10.1186/s13054-021-03491-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Calfee CS, Delucchi K, Parsons PE, Thompson BT, Ware LB, Matthay MA, NHLBI ARDS Network Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med . 2014;2:611–620. doi: 10.1016/S2213-2600(14)70097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Reddy K, Hardin CC, McAuley DF. COVID-19-related acute respiratory distress syndrome subphenotypes and differential response to corticosteroids: time for more precision? Am J Respir Crit Care Med . 2021;204:1241–1243. doi: 10.1164/rccm.202109-2213ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Matthay MA, Arabi YM, Siegel ER, Ware LB, Bos LDJ, Sinha P, et al. Phenotypes and personalized medicine in the acute respiratory distress syndrome. Intensive Care Med . 2020;46:2136–2152. doi: 10.1007/s00134-020-06296-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Reddy K, Sinha P, O’Kane CM, Gordon AC, Calfee CS, McAuley DF. Subphenotypes in critical care: translation into clinical practice. Lancet Respir Med . 2020;8:631–643. doi: 10.1016/S2213-2600(20)30124-7. [DOI] [PubMed] [Google Scholar]
- 33. Sinha P, Calfee CS, Cherian S, Brealey D, Cutler S, King C, et al. Prevalence of phenotypes of acute respiratory distress syndrome in critically ill patients with COVID-19: a prospective observational study. Lancet Respir Med . 2020;8:1209–1218. doi: 10.1016/S2213-2600(20)30366-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen H, Xie J, Su N, Wang J, Sun Q, Li S, et al. Corticosteroid therapy is associated with improved outcome in critically ill patients with COVID-19 with hyperinflammatory phenotype. Chest . 2021;159:1793–1802. doi: 10.1016/j.chest.2020.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bajaj V, Gadi N, Spihlman AP, Wu SC, Choi CH, Moulton VR. Aging, immunity, and COVID-19: how age influences the host immune response to coronavirus infections? Front Physiol . 2021;11:571416. doi: 10.3389/fphys.2020.571416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cunha LL, Perazzio SF, Azzi J, Cravedi P, Riella LV. Remodeling of the immune response with aging: immunosenescence and its potential impact on COVID-19 immune response. Front Immunol . 2020;11:1748. doi: 10.3389/fimmu.2020.01748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mathew D, Giles JR, Baxter AE, Oldridge DA, Greenplate AR, Wu JE, et al. UPenn COVID Processing Unit Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science . 2020;369:eabc8511. doi: 10.1126/science.abc8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rydyznski Moderbacher C, Ramirez SI, Dan JM, Grifoni A, Hastie KM, Weiskopf D, et al. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell . 2020;183:996–1012.e19. doi: 10.1016/j.cell.2020.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sette A, Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell . 2021;184:861–880. doi: 10.1016/j.cell.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stehlik P, Alcorn K, Jones A, Schlebusch S, Wattiaux A, Henry DA. Repeat testing for SARS-CoV-2: persistence of viral RNA is common, and clearance is slower in older people. Med J Aust . 2021;214:468–470. doi: 10.5694/mja2.51036. [DOI] [PMC free article] [PubMed] [Google Scholar]