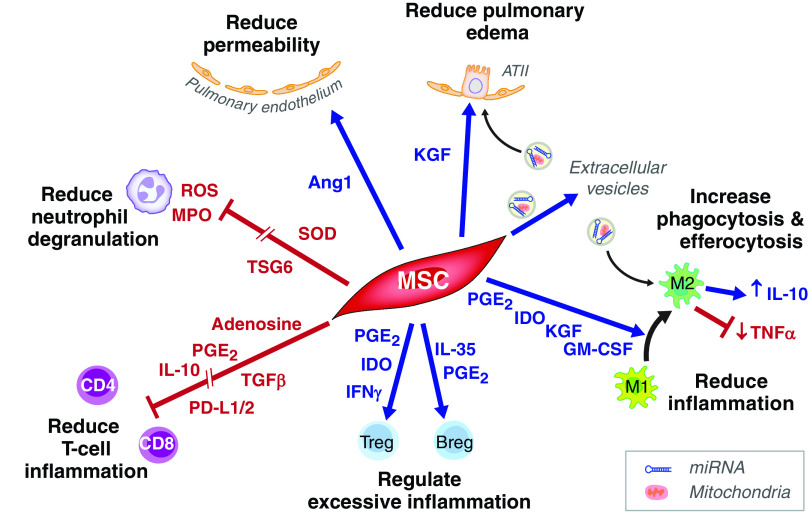
Considerable experimental evidence has indicated that allogeneic mesenchymal stromal cells (MSCs) isolated from bone marrow or umbilical cord might be effective for treating acute respiratory distress syndrome (ARDS). The preclinical studies have been done in mice, rats, sheep, and in an ex vivo perfused human lung model and reported that MSCs reduced lung endothelial and epithelial permeability to protein, increased the rate of alveolar fluid clearance, augmented bacterial killing, and also enhanced repair by favoring transition of monocytes and macrophages to an M2 resolution phenotype (1). In addition, much of the beneficial effects of MSCs appear to be mediated by the release of extracellular vesicles that carry biological cargo (mitochondria, microRNAs, and proteins) (2, 3) (Figure 1). The biological effects of MSC therapy in patients with ARDS are limited, although one recent study reported a significant decrease in the BAL concentration of total protein, a marker of pulmonary permeability, as well as a reduction in IL-6, angiopoietin-2, and soluble tumor necrosis factor receptor-1 in the MSC- versus placebo-treated patients (4).
Figure 1.
Cell interactions by which mesenchymal stem/stromal cells (MSCs) may reduce inflammation and lung injury in coronavirus disease (COVID-19)-related acute respiratory distress syndrome. MSC-derived keratinocyte growth factor (KGF) and mitochondria (delivered via extracellular vesicles [EVs]) drive ATII cell proliferation and ENaC-mediated fluid clearance, whereas Ang1 (angiopoietin-1) release by MSCs promotes pulmonary microvascular endothelial repair. SOD from MSCs inhibits neutrophil ROS, whereas TSG-6 inhibits neutrophil transepithelial migration and release of MPO. PG (prostaglandin) E2 and IDO–expressing MSCs drive a macrophage phenotype that is characterized by reduced inflammatory TNFα but increased antiinflammatory (IL-10) cytokine production, whereas KGF, GM-CSF, and EVs containing mitochondria and microRNA derived from MSCs, enhance macrophage phagocytosis and efferocytosis, leading to the resolution of infection and inflammation. MSC membrane-bound CD73 converts ATP into adenosine, which binds to lymphocyte A2a receptors suppressing T-cell proliferation and inflammation. IFN-γ, produced in response to viral infection, increases PD-L1 (programmed death ligand 1) expression on MSCs. PD-L1 interacts with its receptor (PD1) to inhibit T-cell activation. MSC-derived TGF-β (transforming growth factor), PGE2, and IL-10 further inhibit T-cell proliferation and inflammatory response, whereas IDO and PGE2 promote regulatory T-cell (Treg) expansion. In turn, MSC-derived IFNγ acts on Tregs to suppress effector B cells and antibody production. MSC-derived PGE2, together with IL-35, drive the production of IL-10–producing (antiinflammatory) Bregs. Breg = regulatory B cell; GM-CSF = granulocyte macrophage colony stimulating factor; IDO = indolamine-2,3-dioxygenase; MPO = myeloperoxidase; ROS = reactive oxygen species; SOD = superoxide dismutase; TSG-6 = TNF tumour necrosis factor stimulated gene 6 protein.
Most preclinical studies to date have been done in models of lung injury caused by endotoxin or bacteria. There is less information on the efficacy of MSCs in viral infection, although, in other diseases, they suppress T-cell proliferation and expand populations of regulatory B and T cells (5), which may reduce an overzealous inflammatory response, a feature of ARDS secondary to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Human MSCs do not express ACE2 receptors and resist infection (6). MSCs (7), or their extracellular vesicle-delivered microRNAs (8), reduce (SARS-CoV-2) proliferation in renal epithelial cell lines, but experimental studies of MSCs in COVID-19 pneumonia or ARDS are lacking. Experimental studies have tested the effects of MSCs on other viral pneumonia. Investigators showed no beneficial effect of MSCs on the degree of lung injury with H1N1 influenza in mice (9), but other studies reported beneficial effects of MSCs in cultured human alveolar type 2 cells exposed to more inflammatory influenza strains (H5N1 and H7N1) (10, 11) and reduced lung injury in H9N2 infection (12) and increased survival in older mice on H5N1 infection (10). On balance, the MSC data indicate potential biological benefits for ARDS, including the data from the MSC trial of ARDS (4), but as always, the challenge is how to translate biological signals to clinical benefit.
In this issue of the Journal, Bowdish and colleagues (pp. 261–270) report the results of a randomized, double-blind trial of bone marrow-derived MSCs for the treatment of moderate to severe COVID-19 ARDS with the primary endpoint of 30-day mortality. Patients were treated with two infusions of 2 million MSCs/kg at study entry and again 4 days later (13). The trial was stopped by data safety monitoring after 222 patients of the planned 300 patients had been enrolled because the prespecified mortality reduction from 40% to 23% was not likely to be achieved. The 30-day mortality numerically favored MSC therapy (37.5%) versus placebo (42.7%), but the P value was not significant (P = 0.43). There was a trend for MSC benefit in prespecified group analyses for 90-day mortality if the patients were younger than 65 years old.
At least six randomized controlled trials (RCTs) of MSCs have now been reported in COVID-19, together with multiple nonrandomized trials and case series. A recent systematic review and meta-analysis (14), including five RCTs and six nonrandomized trials of patients treated with MSCs for COVID-19 pneumonia until November 2021 (total of 403 patients: 207 receiving MSCs, 196 control subjects) reported a reduced relative risk of death (0.19; 95% confidence interval, 0.05–0.78) at Day 28 after MSCs. However, the studies were all small, recruited patients with differing severities of COVID-19 (not all had ARDS), and used different cell products with different doses at different times of illness. Furthermore, the studies were performed during different stages of the pandemic, and standard care has evolved. Therefore, it is difficult to draw any definite conclusions. A further small multicenter RCT in France published since the Kirkham meta-analysis (14) recruited only patients with mild to severe ARDS within 96 hours of onset (15). As with the trial by Bowdish and colleagues (13), there was no benefit in the MSC-treated cohort, but importantly, this study did include cytokine measurements with differential patterns emerging by Day 4 between the two groups (15).
The trial by Bowdish and colleagues (13) is an important contribution to testing cell-based therapies in ARDS, but there are some limitations. First, the trial was powered for an absolute 17% reduction in mortality, which was unrealistic; even the ARMA trial reported only a 9% absolute reduction in mortality in ARDS (16). In effect, the current study is really a phase II trial with a modest number of patients. Since there were no safety issues, it would have been preferable if the data safety monitoring board had allowed the trial to enroll the full 300 patients, so it would have maximized the information from this therapy, but the data safety monitoring board was constrained by the 30-day mortality endpoint. Second, whereas the MSC therapy was given at two time points, it is possible that the dose of 2 million MSCs/kg given at two time points is a submaximal dose for achieving an optimal therapeutic effect. Other MSC trials for ARDS have or are testing higher doses of MSCs (17), although there is no certainty about higher doses. The authors themselves speculate that higher doses might be needed for patients over the age of 65 years old. Third is the potential for an inhibitory interaction of corticosteroids on MSCs. Corticosteroids became part of standard care in moderate to severe COVID-19 during the course of this trial and were used in more than 80% of the recruited patients. Corticosteroids have been shown to reduce MSC viability and inhibit the effects of MSCs on T-cell proliferation in vitro, and inhibit the antiinflammatory and antifibrotic effects of MSCs in vivo (18). Finally, except for baseline concentrations of plasma IL-6 and IL-8 (not different between placebo and MSC-treated patients), we do not yet have the biology results from this trial, which is important to determine if there is a subgroup of patients with COVID-19 ARDS that might respond to MSC therapy, as was suggested in patients with COVID-19 treated with corticosteroids (19).
As the largest study to date of MSCs in ARDS secondary to COVID-19, the study by Bowdish and colleagues (13) adds to a growing body of evidence that MSCs are safe in critical illness, but many important questions about patient selection, MSC dose and timing, and their biological effects, including their interaction with other immunosuppressive therapies, remain unanswered.
Footnotes
Originally Published in Press as DOI: 10.1164/rccm.202209-1838ED on October 4, 2022
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Laffey JG, Matthay MA. Fifty years of research in ARDS. Cell-based therapy for acute respiratory distress syndrome. Biology and potential therapeutic value. Am J Respir Crit Care Med . 2017;196:266–273. doi: 10.1164/rccm.201701-0107CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Islam MN, Das SR, Emin MT, Wei M, Sun L, Westphalen K, et al. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med . 2012;18:759–765. doi: 10.1038/nm.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lee JW, Matthay MA. Is a part better than the whole for cell-based therapy for acute respiratory distress syndrome? Anesthesiology . 2019;130:683–685. doi: 10.1097/ALN.0000000000002653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wick KD, Leligdowicz A, Zhuo H, Ware LB, Matthay MA. Mesenchymal stromal cells reduce evidence of lung injury in patients with ARDS. JCI Insight . 2021;6:e148983. doi: 10.1172/jci.insight.148983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shaw TD, Krasnodembskaya AD, Schroeder GN, Zumla A, Maeurer M, O’Kane CM. Mesenchymal stromal cells: an antimicrobial and host-directed therapy for complex infectious diseases. Clin Microbiol Rev . 2021;34:e0006421. doi: 10.1128/CMR.00064-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Avanzini MA, Mura M, Percivalle E, Bastaroli F, Croce S, Valsecchi C, et al. Human mesenchymal stromal cells do not express ACE2 and TMPRSS2 and are not permissive to SARS-CoV-2 infection. Stem Cells Transl Med . 2021;10:636–642. doi: 10.1002/sctm.20-0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hussein MAA, Hussein HAM, Thabet AA, Selim KM, Dawood MA, El-Adly AM, et al. Human Wharton’s jelly mesenchymal stem cells secretome inhibits human SARS-CoV-2 and avian infectious bronchitis coronaviruses. Cells . 2022;11:1408. doi: 10.3390/cells11091408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Park JH, Choi Y, Lim C-W, Park J-M, Yu S-H, Kim Y, et al. Potential therapeutic effect of micrornas in extracellular vesicles from mesenchymal stem cells against SARS-CoV-2. Cells . 2021;10:2393. doi: 10.3390/cells10092393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gotts JE, Abbott J, Matthay MA. Influenza causes prolonged disruption of the alveolar-capillary barrier in mice unresponsive to mesenchymal stem cell therapy. Am J Physiol Lung Cell Mol Physiol . 2014;307:L395–L406. doi: 10.1152/ajplung.00110.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chan MC, Kuok DI, Leung CY, Hui KP, Valkenburg SA, Lau EH, et al. Human mesenchymal stromal cells reduce influenza A H5N1-associated acute lung injury in vitro and in vivo. Proc Natl Acad Sci USA . 2016;113:3621–3626. doi: 10.1073/pnas.1601911113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Loy H, Kuok DIT, Hui KPY, Choi MHL, Yuen W, Nicholls JM, et al. Therapeutic implications of human umbilical cord mesenchymal stromal cells in attenuating influenza A(H5N1) virus-associated acute lung injury. J Infect Dis . 2019;219:186–196. doi: 10.1093/infdis/jiy478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li Y, Xu J, Shi W, Chen C, Shao Y, Zhu L, et al. Mesenchymal stromal cell treatment prevents H9N2 avian influenza virus-induced acute lung injury in mice. Stem Cell Res Ther . 2016;7:159. doi: 10.1186/s13287-016-0395-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bowdish ME, Barkauskas CE, Overbey JR, Gottlieb RL, Osman K, Duggal A, et al. A randomized trial of mesenchymal stromal cells for moderate to severe ARDS from COVID-19. Am J Respir Crit Care Med . 2023;207:261–270. doi: 10.1164/rccm.202201-0157OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kirkham AM, Bailey AJM, Monaghan M, Shorr R, Lalu MM, Fergusson DA, et al. Updated living systematic review and meta-analysis of controlled trials of mesenchymal stromal cells to treat COVID-19: a framework for accelerated synthesis of trial evidence for rapid approval-FASTER approval. Stem Cells Transl Med . 2022;11:675–687. doi: 10.1093/stcltm/szac038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Monsel A, Hauw-Berlemont C, Mebarki M, Heming N, Mayaux J, Nguekap Tchoumba O, et al. APHP STROMA–CoV-2 Collaborative Research Group Treatment of COVID-19-associated ARDS with mesenchymal stromal cells: a multicenter randomized double-blind trial. Crit Care . 2022;26:48. doi: 10.1186/s13054-022-03930-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A, Acute Respiratory Distress Syndrome Network Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med . 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 17. Gorman E, Shankar-Hari M, Hopkins P, Tunnicliffe WS, Perkins GD, Silversides J, et al. Repair of acute respiratory distress syndrome by stromal cell administration (REALIST) trial: a phase 1 trial. EClinicalMedicine . 2021;41:101167. doi: 10.1016/j.eclinm.2021.101167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen X, Gan Y, Li W, Su J, Zhang Y, Huang Y, et al. The interaction between mesenchymal stem cells and steroids during inflammation. Cell Death Dis . 2014;5:e1009. doi: 10.1038/cddis.2013.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sinha P, Furfaro D, Cummings MJ, Abrams D, Delucchi K, Maddali MV, et al. Latent class analysis reveals COVID-19-related acute respiratory distress syndrome subgroups with differential responses to corticosteroids. Am J Respir Crit Care Med . 2021;204:1274–1285. doi: 10.1164/rccm.202105-1302OC. [DOI] [PMC free article] [PubMed] [Google Scholar]