Abstract
Rationale
The lung allocation score (LAS) was revised in 2015 to improve waiting list mortality and rate of transplant for patients with pulmonary arterial hypertension (PAH).
Objectives
We sought to determine if the 2015 revision achieved its intended goals.
Methods
Using the Standard Transplant Analysis and Research file, we assessed the impact of the 2015 LAS revision by comparing the pre- and postrevision eras. Registrants were divided into the LAS diagnostic categories: group A–chronic obstructive pulmonary disease; group B–pulmonary arterial hypertension; group C–cystic fibrosis; and group D–interstitial lung disease. Competing risk regressions were used to assess the two mutually exclusive competing risks of waiting list death and transplant. Cumulative incidence plots were created to visually inspect risks.
Measurements and Main Results
The LAS at organ matching increased by 14.2 points for registrants with PAH after the 2015 LAS revision, the greatest increase among diagnostic categories (other LAS categories: Δ, −0.9 to +2.8 points). Before the revision, registrants with PAH had the highest risk of death and lowest likelihood of transplant. After the 2015 revision, registrants with PAH still had the highest risk of death, now similar to those with interstitial lung disease, and the lowest rate of transplant, now similar to those with chronic obstructive pulmonary disease.
Conclusions
Although the 2015 LAS revision improved access to transplant and reduced the risk of waitlist death for patients with PAH, it did not go far enough. Significant differences in waitlist mortality and likelihood of transplant persist.
Keywords: lung allocation score, lung transplantation, pulmonary arterial hypertension, equity
At a Glance Commentary
Scientific Knowledge on the Subject
Of the different diagnostic groups, patients with pulmonary arterial hypertension are the least likely to receive a transplant and the most likely to die on the waiting list. In 2015, the lung allocation score (LAS) was revised to better allocate organs for patients with pulmonary arterial hypertension; however, the impact of this revision is unknown.
What This Study Adds to the Field
This study assesses the impact of the 2015 LAS revision on the equity of access to transplant in the United States. It is critically important to know the current landscape, given the recent 2021 change in the LAS calculation and the proposed continuous allocation model.
Pulmonary arterial hypertension (PAH) is a potentially fatal cardiopulmonary disease characterized by elevation in pulmonary vascular resistance (1). It primarily affects younger patients and disproportionately affects women (2, 3). Progression leads to dyspnea, physical disability, and limited life expectancy (4–7). Despite advances in pharmacologic therapies, lung transplant remains an important option for this progressive disease (8).
In 2005, the lung allocation score (LAS) was introduced by the United Network for Organ Sharing (UNOS) and changed allocation of donor lungs from one based on wait time to a system of urgency (9). The LAS reduced wait time and improved rate of transplant, primarily through enhanced allocation for pulmonary fibrosis (10). Unfortunately, PAH was the only diagnosis without reductions in waitlist mortality after the LAS (11). Subsequent efforts were made to determine if alternative methods to calculate the LAS might be more equitable for waitlisted patients with PAH (12, 13). In response to concerns of inequity, the Thoracic Committee developed the Lung Review Board, where an exception request for a higher LAS could be submitted. An automatic exception was also instituted to the 90th percentile of the LAS for patients with PAH who met certain criteria (14). Despite these efforts, recent studies demonstrate that patients with PAH continue to comprise the majority of exception requests, and survival is worse in patients with PAH when exceptions are not approved, a discrepancy not found in other diagnostic groups (15).
On February 19, 2015, the LAS was revised to include central venous pressure, change in bilirubin, and cardiac index in the calculation to better reflect severity of PAH and better predict waitlist survival (16). Preliminary data demonstrate that the 2015 LAS revision decreased the expected survival without transplant, as calculated by the waitlist urgency measure, and increased the post-transplant survival measure for patients with PAH, which led to an increase in the LAS for this diagnostic indication (17). Although an increase in the LAS is critically important, it remains unknown if the 2015 revision translated to decreased waitlist mortality and an equitable rate of transplant for patients with PAH when compared with other diagnostic groups. Furthermore, understanding the impact of the 2015 revision is critically important, because the LAS was updated again in September 2021. Some of the criteria included in 2015 specifically to help patients with PAH, such as rise in bilirubin, central venous pressure, and cardiac index, were removed from the waitlist urgency measure in 2021 (18). Consequently, the current LAS calculation has the potential to more negatively impact allocation for patients with PAH; thus, it is crucial to know if any gains were made in terms of equity after the 2015 LAS revision.
We sought to use the UNOS registry to assess the impact of the 2015 LAS revision on waitlist survival and rate of transplant. We hypothesized that the 2015 LAS revision would result in equitable access to transplant for patients with PAH compared with other diagnostic groups.
This work was previously presented in abstract form (19).
Methods
Study Population
This study was performed using data from the Organ Procurement and Transplantation Network, using the October 2021 Standard Transplant Analysis and Research file. We restricted our analysis to patients registered for lung transplant after the implementation of the LAS in 2005. To avoid including patients with observation time that overlapped before and after the 2015 LAS revision, we excluded any registrants listed on or before February 18, 2015 who had an end date of observation (i.e., transplanted, waitlist death, or censored) after February 18, 2015. We also excluded anyone listed for combined heart–lung transplant to minimize potential bias from including patients with congenital heart disease who may have been misclassified as having PAH.
Analytic Approach
We divided our study population into two cohorts: those actively listed under the LAS system before the 2015 LAS revision (i.e., registered between May 4, 2005, and February 18, 2015), and those actively listed after the 2015 LAS revision (i.e., registered on or after February 19, 2015). Of note, there were no participants included in this dataset registered after September 30, 2021, when the 2021 LAS revision was implemented. Participants were divided into diagnostic grouping based on LAS categories, where LAS category A represented registrants with obstructive lung diseases such as chronic obstructive pulmonary disease (COPD), LAS category B represented registrants with pulmonary vascular diseases such as PAH, LAS category C represented registrants with cystic fibrosis (CF) and bronchiectasis from immune deficiency syndromes, and LAS category D represented registrants with interstitial lung disease (ILD), primary graft failure, bronchiolitis obliterans syndrome, and sarcoidosis with an elevated pulmonary artery pressure (9). In the remaining text we will refer to the LAS categories by the predominant disease (COPD, PAH, CF, or ILD). We compared the LAS at listing and organ matching from before to after the 2015 LAS revision using Student’s t test among each individual LAS category. We compared the proportion of registrants transplanted in each diagnostic group and the proportion of registrants who died or were delisted for cause in each diagnostic group using the chi-square test.
As patients get sicker while waiting for transplant, their LAS tends to increase. We were also interested in determining if the increase in LAS over time was different among the varied diagnostic groups. To assess this, we calculated the delta LAS, which we defined as the change in the LAS from initial registration to the final score. We also divided the delta LAS by the observation time to determine the LAS points gained on average each day among the four diagnostic groups.
We used the competing risk regression to assess the mutually exclusive competing risks of waitlist death and transplant. As such, we ran two separate analyses, one with waitlist death as the outcome and transplant as the competing risk, and the other with transplant as the outcome and waitlist death as the competing risk.
To determine the impact of the 2015 LAS revision on all registrants, we first applied our competing risk regressions with era as the predictor. In this analysis we assessed the outcomes of death on the waiting list and transplant after the 2015 LAS revision, using the pre-LAS revision data as the reference group. We then ran the same analysis, restricted to individual LAS diagnostic categories, to determine the impact of the 2015 LAS revision on each category. We plotted cumulative incidence curves for the overall cohort and the individual LAS categories and applied the log-rank test. We also tested the interaction between era and LAS categories to determine if the period changes were different for the various disease states using standard Cox models.
We then aimed to determine if the 2015 LAS revision created an equitable approach, such that patients with PAH were no longer at an increased risk of death on the waitlist and had similar rates of transplant compared with other diagnostic groups. We assessed diagnostic group as a predictor in our competing risk regression, using LAS category B (PAH) as the reference, in the era before the 2015 LAS revision and then again in the era after the 2015 LAS revision. We also stratified these models by UNOS region to assess if there were regional differences in access to transplantation across diagnostic groups. We also plotted cumulative incidence curves assessing the cumulative incidence of waiting list death and cumulative incidence of transplant among LAS categories and applied the log-rank test.
We ran both unadjusted models and models adjusted for age, sex, race, height, type of transplant (unilateral vs. bilateral), blood type, calculated peak panel reactive antibody, and use of extracorporeal membrane oxygenation (ECMO) at listing. We chose these variables based on factors that we believed could influence the outcome of death on the waiting list and opportunity for transplant independently from the variables inherent in the LAS. Notably, although age is one of the LAS variables, we did include it in our adjustment variables because, in our experience, many centers are likely to not use an older donor on a young recipient, so older donors are likely to have a larger pool of potential donors. We chose to use sex and race in our adjustment variables to control for the social determinants of health that can impact access to transplantation. We chose to use height, blood type, and calculated peak panel reactive antibodies in our adjustment variables, because donors are matched on these variables. We chose transplant type because restricted listing for certain diagnoses impacts waitlist mortality when patients are candidates for a single-lung transplant (20). Finally, we also included ECMO at listing to ensure that any inequities in access to transplantation were not explained by differential use of ECMO.
We performed three sensitivity analyses on the competing risk regression models: in the first, we restricted our analysis to participants who were listed only for a bilateral lung transplant; in the second, we included the registrants listed for combined heart–lung transplant; in the third, we used the composite outcome of waitlist death or delisting for illness in lieu of waitlist death.
We do recognize that, although getting a transplant and dying on the waitlist are two mutually exclusive risks, which cannot happen in the same person, competing risk regression analyses may be subject to bias from dependent censoring (21–23). For this reason, we performed three analytic sensitivity analyses for our models comparing the different diagnostic groups. In the first, we performed cause-specific hazard modeling; in the second, we performed multistate modeling (24); and in the third, we used an inverse probability weighted regression adjustment fitted to a logistic regression model. Notably, we included the change in lung allocation score as one of the parameters in the inverse probability weighting as a marker of change in disease severity over time.
We also assessed post-transplant survival stratified by diagnostic group and era. This was done by looking at the proportion of patients alive at 30 days, 1 year, and 3 years after transplant. We also assessed post-transplant survival in unadjusted and adjusted Cox proportional hazard modeling.
For our time-to-event models, we assessed the assumptions of proportional hazards by visual inspection of the log minus log plots using the stphplot command. Analyses were conducted in STATA 15.1.
Results
A total of 46,173 participants were registered in UNOS to wait for a lung transplant or a heart–lung transplant during the study period (Table 1). Of these participants, we included the 39,675 who were registered for a lung transplant alone: 20,197 participants registered before the 2015 LAS revision, 19,478 after the 2015 LAS revision, and we excluded the 5,591 participants who were registered before the 2015 LAS revision but had an end date after the revision. We also excluded the 907 participants registered for combined heart–lung transplant in our primary analysis. Among the different diagnostic categories, those with PAH had the highest proportion of female registrants and the lowest proportion of White registrants, both before and after the 2015 LAS revision. The initial LAS increased the greatest after the revision in participants registered with PAH (Δ, +10.1 points; P < 0.001), as did the LAS at organ matching (Δ, +14.2 points; P < 0.001). Although other diagnostic categories had statistically significant differences in the LAS after the 2015 LAS revision, the magnitude of the changes were less substantial (Δ, −0.9 to +2.8 points). When assessing the delta LAS over time, registrants with PAH had a slope of change in the LAS that a was similar rate to registrants with COPD before the revision, but the slope of the rise was less steep than in patients with CF or ILD (Table 1). After the revision, registrants with PAH had a greater slope of rise than those with COPD but still at a lower rate than in patients with CF or ILD. PAH was the only diagnostic group to see an improvement in the slope of rise from before to after the 2015 LAS revision.
Table 1.
Demographics of Participants Listed for Transplantation
| Before the 2015 LAS Revision |
After the 2015 LAS Revision |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Overall | Group B (PAH) | Group A (COPD) | Group C (CF) | Group D (ILD) | Overall | Group B (PAH) | Group A (COPD) | Group C (CF) | Group D (ILD) | |
| No. (%) | 20,197 (100) | 997 (5) | 5,796 (29) | 2,572 (13) | 10,832 (54) | 19,478 (100) | 1,212 (6) | 4,522 (23) | 1,545 (8) | 12,199 (63) |
| Age, yr | 52.3 ± 15.6 | 38.8 ± 19.4 | 57.9 ± 9.0 | 28.6 ± 10.9 | 56.2 ± 12.9 | 56.3 ± 14.2 | 46.6 ± 17.6 | 60.2 ± 8.6 | 31.1 ± 11.8 | 59.0 ± 11.9 |
| Female | 8,885 (44) | 647 (65) | 2,951 (51) | 1,322 (51) | 3,965 (37) | 8,297 (43) | 802 (66) | 2,281 (50) | 818 (53) | 4,396 (36) |
| Race | ||||||||||
| White | 16,566 (82) | 703 (71) | 5,131 (89) | 2,381 (93) | 8,351 (77) | 14,670 (75) | 754 (62) | 3,845 (85) | 1,342 (87) | 8,729 (72) |
| Black | 1,802 (9) | 137 (14) | 479 (8) | 42 (2) | 1,144 (11) | 1,985 (10) | 193 (16) | 471 (11) | 52 (3) | 1,269 (10) |
| Other | 1,829 (9) | 157 (16) | 196 (3) | 149 (6) | 1,337 (12) | 2,823 (14) | 265 (22) | 206 (5) | 151 (10) | 2,201 (18) |
| Organ desired | ||||||||||
| Bilateral | 10,980 (54) | 896 (89) | 2,940 (51) | 2,467 (96) | 4,677 (43) | 11,642 (60) | 1,035 (85) | 2,714 (60) | 1,466 (95) | 6,427 (53) |
| Unilateral | 9,217 (46) | 101 (10) | 2,856 (49) | 105 (4) | 6,155 (57) | 7,836 (40) | 177 (15) | 1,808 (40) | 79 (5) | 5,772 (47) |
| Initial LAS | 40.7 ± 17.5 | 34.4 ± 9.5 | 32.3 ± 9.3 | 38.8 ± 16.2 | 46.3 ± 19.5 | 43.9 ± 16.8 | 44.5 ± 12.7 | 34.2 ± 7.8 | 44.2 ± 17.6 | 47.4 ± 18.0 |
| End LAS | 47.5 ± 21.6 | 34.2 ± 14.8 | 34.3 ± 12.9 | 48.5 ± 21.2 | 55.6 ± 21.8 | 49.7 ± 20.3 | 48.4 ± 16.3 | 35.6 ± 10.7 | 51.3 ± 21.8 | 54.7 ± 20.7 |
| Delta LAS | 6.8 ± 18.2 | 0.32 ± 13.2 | 2.0 ± 13.4 | 9.7 ± 20.1 | 9.4 ± 19.6 | 5.7 ± 14.5 | 3.9 ± 12.4 | 1.4 ± 10.0 | 7.1 ± 18.2 | 7.3 ± 15.3 |
| Delta LAS/day | 0.4 ± 2.8 | 0.1 ± 0.9 | 0.1 ± 1.6 | 0.4 ± 2.9 | 0.5 ± 3.4 | 0.3 ± 1.6 | 0.2 ± 1.3 | 0.1 ± 1.7 | 0.4 ± 2.8 | 0.3 ± 1.7 |
| Days observed | 195 ± 317 | 270 ± 395 | 297 ± 403 | 204 ± 299 | 132 ± 234 | 136 ± 224 | 175 ± 282 | 207 ± 285 | 156 ± 237 | 103 ± 178 |
| Days to death | 221 ± 340 | 262 ± 357 | 473 ± 493 | 219 ± 311 | 140 ± 237 | 143 ± 214 | 138 ± 212 | 297 ± 309 | 138 ± 166 | 116 ± 184 |
| Days to transplant | 153 ± 249 | 168 ± 220 | 222 ± 322 | 181 ± 266 | 109 ± 183 | 105 ± 174 | 119 ± 209 | 160 ± 226 | 116 ± 175 | 83 ± 141 |
| ECMO at listing | 279 (1) | 19 (2) | 38 (1) | 7 (0.1) | 215 (2) | 583 (5) | 75 (6) | 19 (0.4) | 81 (5) | 583 (5) |
| Die waiting | 1,923 (10) | 192 (19) | 331 (6) | 264 (10) | 1,136 (10) | 1,012 (5) | 93 (8) | 131 (3) | 69 (5) | 719 (6) |
| Die/delist | 3,180 (16) | 260 (26) | 630 (11) | 418 (16) | 1,872 (17) | 1,896 (10) | 162 (13) | 265 (6) | 104 (7) | 1,365 (11) |
| Transplanted | 15,609 (77) | 538 (54) | 4,592 (79) | 2,001 (78) | 8,478 (78) | 15,566 (80) | 770 (64) | 3,652 (81) | 1,277 (83) | 9,857 (81) |
Definition of abbreviations: CF = cystic fibrosis; COPD = chronic obstructive pulmonary disease; ECMO = extracorporeal membrane oxygenation; ILD = interstitial lung disease; LAS = lung allocation score; PAH = pulmonary arterial hypertension.
Categorical data presented as n (%). Continuous data presented as mean ± SD. Registrants stratified by LAS category. LAS category A represents registrants with COPD. LAS category B represents registrants with PAH. LAS category C represents registrants with CF. LAS category D represents registrants with ILD.
Of the patients registered before the 2015 LAS revision, 15,609 (77%) underwent transplantation, and 1,923 (10%) died on the waiting list. Before the 2015 LAS revision, 54% of patients with PAH underwent transplantation, and 19% died on the waiting list. This represents the lowest proportion of transplanted patients across all diagnostic groups (78–79% for others; P < 0.001) and the highest proportion of waitlist deaths across all diagnostic groups (6–10% for others; P < 0.001). After the 2015 LAS revision, there were 15,566 patients transplanted (80%), and 1,012 who died waiting (5%). The revision did improve allocation for patients with PAH, as the proportion who underwent transplantation increased to 64% and the proportion who died on the waiting list decreased to 13%. However, this still represented the lowest proportion of transplanted patients across all diagnostic groups (81–83% for others; P < 0.001) and the highest proportion of waitlist deaths across all diagnostic groups (6–11% for others; P < 0.001). Registrants with COPD had the longest time to waitlist death and longest time to transplantation, whereas registrants with ILD had the shortest time to waitlist death and shortest time to transplantation.
Among all participants, the risk of death on the waiting list was lower after the 2015 LAS revision (subdistribution hazard ratio [SHR], 0.68; 95% confidence interval [CI], 0.63–0.74) (Figure 1 and Table 2). When stratified by LAS category, the risk of death on the waiting list also improved across all diagnostic groups (all P < 0.001). The likelihood of transplant improved after the 2015 LAS revision (SHR, 1.19; 95% CI, 1.16–1.22) (Figure 2 and Table 2), and it also improved among each individual diagnostic category (all P < 0.001). Notably, among the diagnostic groups, registrants with PAH had the greatest reduction in waiting list mortality after implementation of the 2015 LAS revision (SHR, 0.50 for PAH vs. SHR, 0.54–0.70 for other diagnoses) and the greatest improvement in likelihood of transplantation (SHR, 1.38 for PAH vs. SHR, 1.15–1.22 for other diagnoses). Testing the interaction between LAS category and era in standard Cox models demonstrated that there was a significant improvement in transplantation across the period change for patients with PAH compared with the other diagnostic groups (all P < 0.001; see Table E1 in the online supplement). However, there was no improvement in waitlist mortality for PAH relative to COPD or CF when assessing the multiplicative HR for the interaction between era and diagnostic group (both P > 0.11). There was a nonsignificant trend for improved waitlist mortality in patients with PAH compared with ILD when testing the interaction between era and diagnostic group (P = 0.054).
Figure 1.
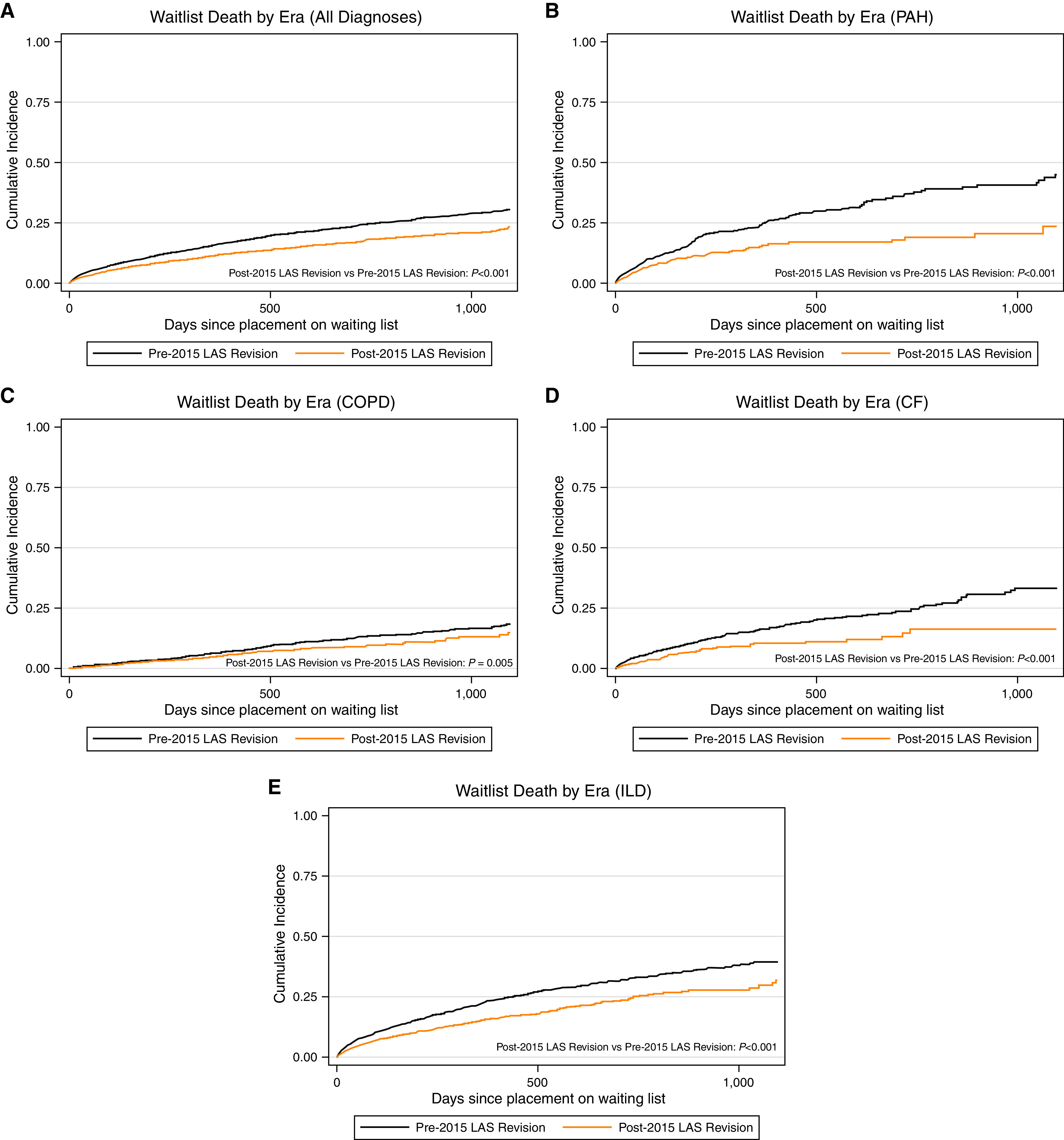
Cumulative incidence of waitlist death in (A) the whole cohort, (B) lung allocation score (LAS) category B–PAH, (C) LAS category A–COPD, (D) LAS category C–CF, and (E) LAS category D–ILD, stratified by era. Black lines indicate before 2015 LAS revision. Orange lines indicate after 2015 LAS revision. Log-rank test was performed to assess for statistical significance. CF = cystic fibrosis; COPD = chronic obstructive pulmonary disease; ILD = interstitial lung disease; PAH = pulmonary arterial hypertension.
Table 2.
Subdistribution Hazard Ratio for Post-2015 Revision Relative to Pre-2015 Revision with Competing Risk Regression
| Population | Outcome | Competing Risk | Subdistribution Hazard Ratio (95% CI) | P Value |
|---|---|---|---|---|
| Unadjusted | ||||
| All patients | Death | Transplant | 0.40 (0.31–0.52) | <0.001 |
| Group A (COPD) | 0.52 (0.43–0.64) | <0.001 | ||
| Group B (PAH) | 0.40 (0.31–0.52) | <0.001 | ||
| Group C (CF) | 0.42 (0.32–0.55) | <0.001 | ||
| Group D (ILD) | 0.56 (0.51–0.61) | <0.001 | ||
| All patients | Transplant | Death | 1.27 (1.25–1.30) | <0.001 |
| Group A (COPD) | 1.25 (1.20–1.31) | <0.001 | ||
| Group B (PAH) | 1.59 (1.43–1.77) | <0.001 | ||
| Group C (CF) | 1.32 (1.23–1.42) | <0.001 | ||
| Group D (ILD) | 1.22 (1.18–1.25) | <0.001 | ||
| Adjusted for age, sex, race, height, transplant type, blood type, panel reactive antibody, and ECMO at listing | ||||
| All patients | Death | Transplant | 0.68 (0.63–0.74) | <0.001 |
| Group A (COPD) | 0.65 (0.53–0.80) | <0.001 | ||
| Group B (PAH) | 0.50 (0.38–0.66) | <0.001 | ||
| Group C (CF) | 0.54 (0.41–0.70) | <0.001 | ||
| Group D (ILD) | 0.70 (0.64–0.77) | <0.001 | ||
| All patients | Transplant | Death | 1.19 (1.16–1.22) | <0.001 |
| Group A (COPD) | 1.19 (1.13–1.25) | <0.001 | ||
| Group B (PAH) | 1.38 (1.23–1.56) | <0.001 | ||
| Group C (CF) | 1.22 (1.13–1.32) | <0.001 | ||
| Group D (ILD) | 1.15 (1.11–1.19) | <0.001 |
Definition of abbreviations: CF = cystic fibrosis; CI = confidence interval; COPD = chronic obstructive pulmonary disease; ECMO = extracorporeal membrane oxygenation; ILD = interstitial lung disease; LAS = lung allocation score; PAH = pulmonary arterial hypertension.
Data presented as subdistribution hazard ratio with 95% CI and P value in unadjusted and adjusted competing risk regression models. The ratio represents the hazard of the outcome after the 2015 LAS revision with the prerevision hazard as the reference group. LAS category B represents registrants with pulmonary arterial hypertension. LAS category C represents registrants with CF. LAS category D represents registrants with ILD. Adjusted models included the following confounding and precision variables: age, sex, race, height, transplant type, ABO blood group, calculated peak panel reactive antibody, and ECMO at listing.
Figure 2.
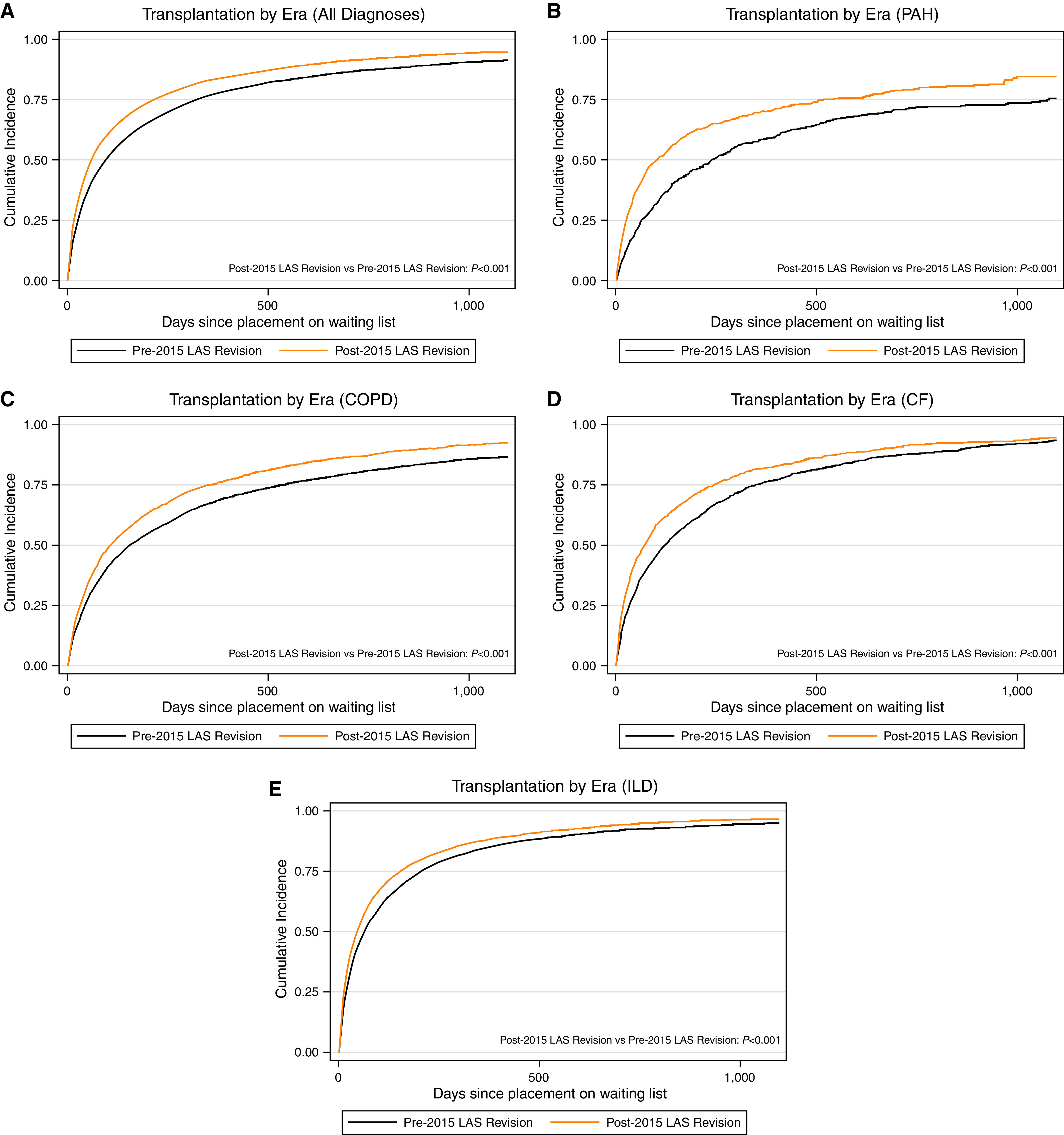
Cumulative incidence of transplantation in (A) the whole cohort; (B) lung allocation score (LAS) category B–PAH, (C) LAS category A–COPD, (D) LAS category C–CF, and (E) LAS category D–ILD, stratified by era. Black lines indicate pre-2015 LAS revision. Orange lines indicate post-2015 LAS revision. Log-rank test was performed to assess for statistical significance. For definition of abbreviations, see Figure 1.
Figure 3.
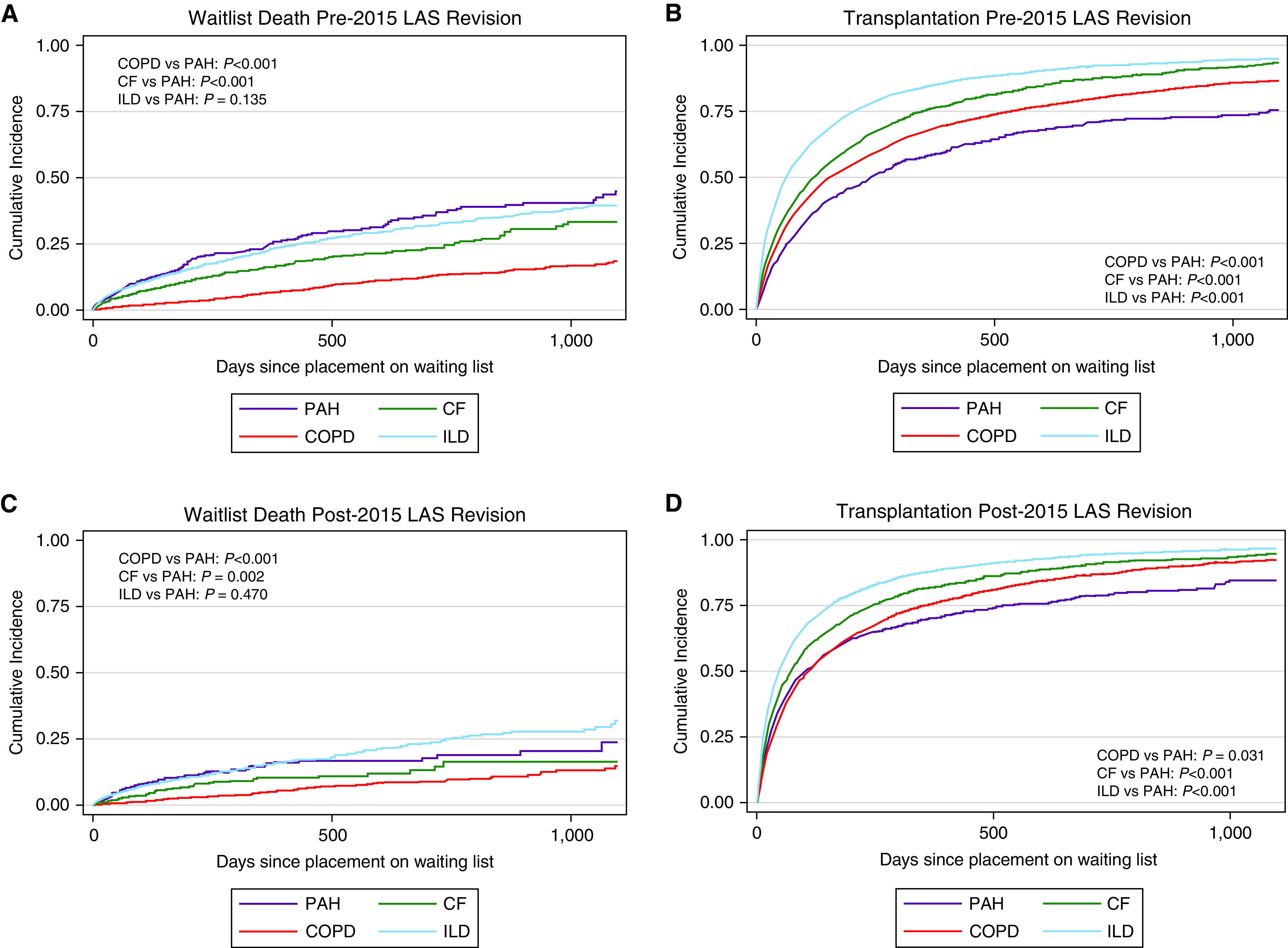
Cumulative incidence of (A) waitlist death, and (B) transplant before the 2015 lung allocation score (LAS) revision stratified by LAS category. Cumulative incidence of (C) waitlist death, and (D) transplant after the 2015 LAS revision stratified by LAS category. Purple lines denote LAS category B–PAH. Red lines indicate LAS category A–COPD. Green lines indicate LAS category C–CF. Blue lines indicate LAS category D–ILD. Log-rank test performed to assess for statistical significance. For definition of abbreviations, see Figure 1.
Relative to participants with PAH, the risk of death on the waiting list was lower in those registered with COPD (SHR, 0.37; 95% CI, 0.31–0.45), CF (SHR, 0.55; 95% CI, 0.45–0.68), and ILD (SHR, 0.77; 95% CI, 0.65–0.91) before the 2015 LAS revision (Figure 1 and Table 3). The point estimates for the SHRs for risk of death all moved closer to equitable allocation after the revision; however, the risk of death remained lower relative to PAH for COPD (0.43; 95% CI, 0.33–0.57) and tended to be lower for CF (SHR, 0.74; 95% CI, 0.53–1.03). Risk of waitlist death for those with ILD was similar to PAH after the 2015 LAS revision (SHR, 0.95; 95% CI, 0.76–1.20).
Table 3.
Subdistribution Hazard Ratio for Waitlist Death Relative to Lung Allocation Score Group B (Pulmonary Arterial Hypertension) with Competing Risk of Transplantation
| Before 2015 Revision | After 2015 Revision | |
|---|---|---|
| Unadjusted | ||
| Group A (COPD) | 0.25 (0.21–0.30) P < 0.001 | 0.34 (0.26–0.44) P < 0.001 |
| Group C (CF) | 0.49 (0.41–0.59) P < 0.001 | 0.53 (0.39–0.72) P < 0.001 |
| Group D (ILD) | 0.51 (0.44–0.60) P < 0.001 | 0.74 (0.59–0.92) P = 0.006 |
| Adjusted for age, sex, race, height, transplant type, blood type, panel reactive antibody, and ECMO at listing | ||
| Group A (COPD) | 0.37 (0.31–0.45) P < 0.001 | 0.43 (0.33–0.57) P < 0.001 |
| Group C (CF) | 0.55 (0.45–0.68) P < 0.001 | 0.74 (0.53–1.03) P = 0.079 |
| Group D (ILD) | 0.77 (0.65–0.91) P = 0.002 | 0.95 (0.76–1.20) P = 0.683 |
Definition of abbreviations: CF = cystic fibrosis; COPD = chronic obstructive pulmonary disease; ECMO = extracorporeal membrane oxygenation; ILD = interstitial lung disease; LAS = lung allocation score; PAH = pulmonary arterial hypertension.
Data presented as subdistribution hazard ratio with 95% confidence interval and P value in unadjusted and adjusted competing risk regression models. LAS category A represents registrants with COPD. LAS category B represents registrants with pulmonary arterial hypertension. LAS category C represents registrants with CF. LAS category D represents registrants with ILD. The ratio represents the hazard of waitlist death in each group relative to LAS category B (PAH). Adjusted models included the following confounding and precision variables: age, sex, race, height, transplant type, ABO blood group, calculated peak panel reactive antibody, and ECMO at listing.
Likelihood of transplant relative to participants registered with PAH was higher in those registered with COPD (SHR, 1.20; 95% CI, 1.09–1.31), CF (SHR, 1.75; 95% CI, 1.59–1.93), and ILD (SHR, 1.57; 95% CI, 1.43–1.72) before the 2015 LAS revision (Table 4). Similar to the point estimates for death, the SHRs for transplant moved closer to equitable allocation after the revision; however, the likelihood of transplant remained higher relative to PAH for registrants with CF (SHR, 1.31; 95% CI, 1.19–1.44) and ILD (SHR, 1.37; 95% CI, 1.27–1.48). The likelihood of transplantation was similar for COPD relative to PAH after the revision (SHR, 1.00; 95% CI, 0.92–1.08). There were regional differences noted, and patients with PAH seemed to have more equitable allocation in some UNOS regions both before and after the 2015 LAS revision (Tables E2 and E3).
Table 4.
Subdistribution Hazard Ratio for Transplant Relative to Lung Allocation Score Group B with Competing Risk of Death
| Before 2015 Revision | After 2015 Revision | |
|---|---|---|
| Unadjusted | ||
| Group A (COPD) | 1.62 (1.48–1.92) P < 0.001 | 1.19 (1.10–1.29) P < 0.001 |
| Group C (CF) | 1.75 (1.59–1.92) P < 0.001 | 1.39 (1.27–1.52) P < 0.001 |
| Group D (ILD) | 2.16 (1.98–2.35) P < 0.001 | 1.64 (1.52–1.77) P < 0.001 |
| Adjusted for age, sex, race, height, transplant type, blood type, panel reactive antibody, and ECMO at listing | ||
| Group A (COPD) | 1.20 (1.09–1.31) P < 0.001 | 1.00 (0.92–1.08) P = 0.977 |
| Group C (CF) | 1.75 (1.59–1.93) P < 0.001 | 1.31 (1.19–1.44) P < 0.001 |
| Group D (ILD) | 1.57 (1.43–1.72) P < 0.001 | 1.37 (1.27–1.48) P < 0.001 |
For definition of abbreviations, see Table 3.
Data presented as subdistribution hazard ratio with 95% confidence interval and P value in unadjusted and adjusted competing risk regression models. LAS category A represents registrants with COPD. LAS category B represents registrants with pulmonary arterial hypertension. LAS category C represents registrants with CF. LAS category D represents registrants with ILD. The ratio represents the hazard of transplant in each group relative to LAS category B (PAH). Adjusted models included the following confounding and precision variables: age, sex, race, height, transplant type, ABO blood group, calculated peak panel reactive antibody, and ECMO at listing.
We performed three sensitivity analyses where we 1) limited the cohort to registrants listed for a bilateral lung transplant; 2) included registrants listed for combined heart–lung transplant; and 3) used the combined outcome of waitlist death/delisting for illness. All three sensitivity analyses showed similar results to the primary model (Tables E4 and E5).
Our analytic sensitivity analyses using cause-specific hazard modeling and multistate modeling were also similar to the primary analyses (Tables E6–E8). It should be noted, however, that in adjusted, but not unadjusted, models, there was a slight benefit for patients with PAH relative to those with ILD in waitlist death after the 2015 revision, as well as relative to those with COPD in transplantation. Our analytic sensitivity analyses applying inverse probability weighted regression adjustment to a logistic regression model demonstrated that patients with PAH had the highest likelihood of waitlist death and lowest likelihood of transplantation compared with the other diagnostic groups both before and after the revision.
After transplant, patients with PAH had the lowest 30-day survival both before and after the revision (Table 5 and Figure 4). Notably, they had a relative improvement in longer-term survival, with a similar 1-year survival as ILD and a similar 3-year survival as all diagnostic groups. Cox proportional hazard modeling of post-transplant survival demonstrated similar results; furthermore, patients with PAH had a better survival relative to other diagnostic groups after the revision (Table E9).
Table 5.
Post-transplant Mortality by Diagnosis and Era
| Before the 2015 LAS Revision |
After the 2015 LAS Revision |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Overall | Group B (PAH) | Group A (COPD) | Group C (CF) | Group D (ILD) | Overall | Group B (PAH) | Group A (COPD) | Group C (CF) | Group D (ILD) | |
| Died at 30 d | 579 (4) | 40 (8) | 126 (3) | 38 (2) | 375 (4) | 357 (2) | 36 (5) | 77 (2) | 17 (1) | 227 (2) |
| Alive at 30 d | 14,740 (96) | 479 (92) | 4,386 (97) | 1,891 (98) | 7,984 (96) | 14,802 (98) | 712 (95) | 3,497 (98) | 1,231 (99) | 9,362 (98) |
| Died at 1 yr | 2,254 (15) | 87 (17) | 563 (12) | 204 (11) | 1,400 (17) | 1,378 (11) | 84 (14) | 281 (9) | 102 (9) | 911 (12) |
| Alive at 1 yr | 13,065 (85) | 432 (83) | 3,949 (88) | 1,725 (89) | 6,959 (83) | 11,402 (89) | 528 (87) | 2,813 (91) | 1,095 (91) | 6,966 (88) |
| Died at 3 yr | 4,730 (31) | 175 (34) | 1,262 (28) | 528 (27) | 2,765 (33) | 1,914 (25) | 85 (26) | 454 (24) | 179 (21) | 1,196 (26) |
| Alive at 3 yr | 10,589 (69) | 344 (66) | 3,250 (72) | 1,401 (73) | 5,594 (67) | 5,653 (75) | 242 (74) | 1,432 (76) | 658 (79) | 3,321 (74) |
For definition of abbreviations, see Table 1.
Data presented as number (%) of patients alive and number of patients dead at 30 days after transplant, 1 year after transplant, and 3 years after transplant. Registrants stratified by LAS category. LAS category A represents registrants with COPD. LAS category B represents registrants with PAH. LAS category C represents registrants with CF. LAS category D represents registrants with ILD.
Figure 4.
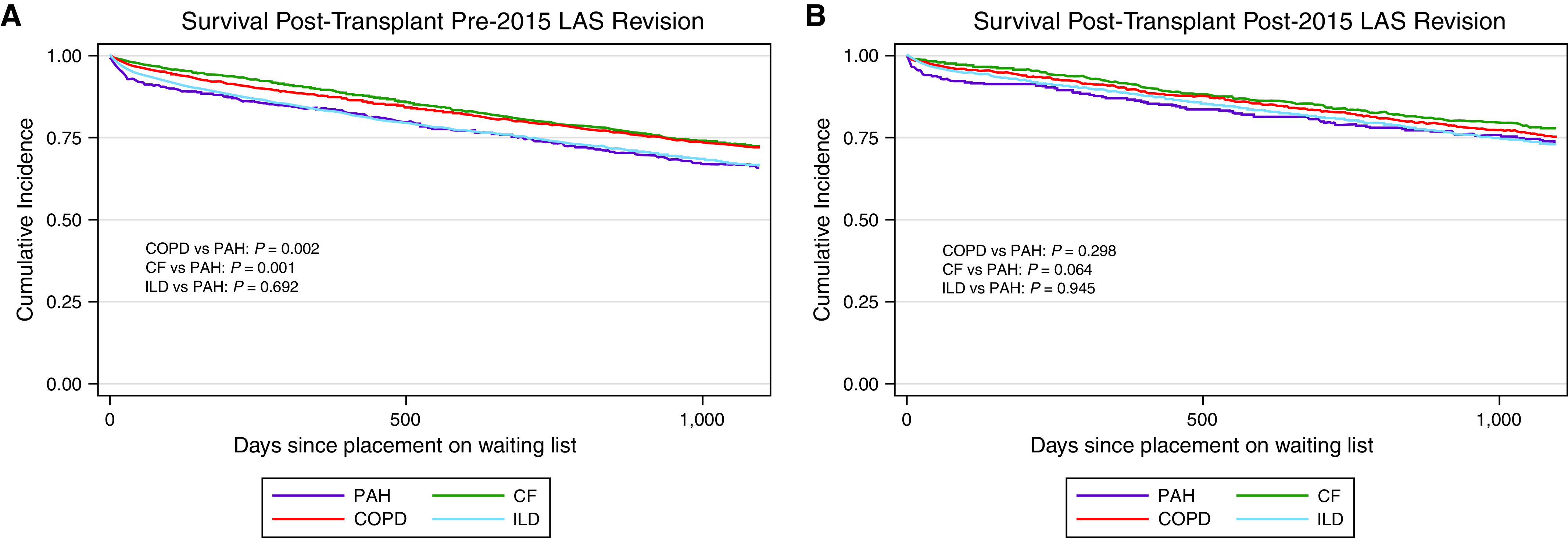
Kaplan-Meier plot of post-transplant survival (A) before the 2015 lung allocation score (LAS) revision stratified by LAS category, and (B) after the 2015 LAS revision stratified by LAS category. Purple lines denote LAS category B–PAH. Red lines indicate LAS category A–COPD. Green lines indicate LAS category C–CF. Blue lines indicate LAS category D–ILD. Log-rank test performed to assess for statistical significance. For definition of abbreviations, see Figure 1.
Discussion
In registry data from UNOS, we demonstrated that the 2015 LAS revision, which was intended to improve equitable transplant for patients with PAH, was a modest success. It improved waitlist mortality overall and across each individual LAS diagnostic category. It also improved rate of transplant overall and across each individual LAS diagnostic category. Moreover, the 2015 LAS revision did benefit patients with PAH the most, as they had the greatest increase in the LAS after the revision, and the point estimate of the SHR for improvement in rate of transplant was highest among those with PAH. This also led to a relative improvement in 3-year post-transplant survival relative to other diagnostic groups. Thus, the 2015 LAS revision did improve waiting list mortality and transplant for patients with PAH. Notably, however, the 2015 LAS revision did not fully achieve equitable access to transplant for patients with PAH. After the revision, we found that relative to other diagnostic indications, registrants with PAH still had the lowest likelihood of transplant, similar to those with COPD, and the highest waitlist death, similar to those with ILD. Participants with PAH still had the highest waiting list mortality and lowest likelihood of transplant in all three of our sensitivity analyses. Furthermore, we found similar results on our three analytic sensitivity analyses. Unfortunately, the LAS is still not an equitable way to allocate organs, as the likelihood of transplantation is not commensurate to the likelihood of waiting list death for patients with PAH.
The calculation of the LAS involves the use of multiple clinical variables to calculate two specific measures: 1) a waitlist urgency measure, which represents the expected days that any given registrant will live without transplant in the next year; and 2) a post-transplant survival measure, which represents the expected number of days a registrant will live in the year after transplant (9). Because the goal of the LAS is to minimize waiting list mortality and maximize post-transplant survival, the highest allocation scores go to patients with high expected waitlist mortality and high likelihood of recovery after transplantation. At the implementation of the LAS in 2005, the concept of multivariable risk assessment was not widely adopted in PAH, and, as such, the initial parameters included in the LAS calculation inadequately assessed the waitlist urgency measure in this population (4).
Indeed, the initial LAS calculation relied more heavily on parameters that impact patients with parenchymal lung disease, such as degree of hypoxemia, hypercarbia, and pulmonary function testing (9). Although including cardiac index, central venous pressure, and change in bilirubin in the 2015 LAS revision represents an attempt to include more relevant variables for patients with PAH (16), these variables are only abnormal in the presence of right ventricular failure, and the cut points chosen in the LAS model were not sensitive enough to discriminate risk in PAH to ensure equitable allocation. This is not entirely surprising, given the current knowledge regarding risk assessment in PAH. Within the last 10 years, multiple efforts have led to the development of well-validated risk assessment tools, such as the Registry to Evaluate Early and Long-Term PAH Disease Management (REVEAL) 2.0 risk calculator and the European Society of Cardiology/European Respiratory Society (ESC/ERS) risk stratification tool (25–28). Both of these risk assessment tools use multiple clinical parameters relevant to PAH, with REVEAL 2.0 having 13 variables (4 of which are similar to variables in the 2015 LAS calculation: renal function, 6-minute-walk distance, central venous pressure, and systolic pulmonary artery pressure) and ESC/ERS having 11 variables (3 of which are similar to variables in the 2015 LAS calculation: 6-min-walk distance, central venous pressure, and cardiac index). In fact, given the well-known difficulty expert physicians have in using clinical gestalt to assess risk in patients with PAH, the updated 2021 International Society for Heart and Lung Transplantation guidelines on the selection of lung transplant candidates moved to recommending using either the ESC/ERS or REVEAL risk assessment tools when deciding on who should be referred for and listed for transplant (29, 30). Incorporation of more PAH-relevant variables, in particular the ones used across both risk assessment tools, such as the B-type natriuretic peptide, functional class, and presence of a pericardial effusion, would be more likely to assess the waitlist urgency for patients with PAH.
Beyond the waitlist urgency measure, there is concern that patients with PAH are also negatively impacted by the post-transplant survival measure because, historically, patients with PAH have been the least likely to survive the first year after transplant (8). The mechanism of this is often attributed to their increased risk of primary graft dysfunction, for which pulmonary arterial hypertension is a known risk factor (31). However, it is also possible that the inherent inequities in the LAS could contribute to the increased risk of death after transplant, because patients with PAH need to attain a sufficient degree of right ventricular failure to develop an elevated bilirubin, high central venous pressure, or low cardiac index, which will allow them to achieve an LAS high enough to outpace/overcome competition from other diagnostic groups.
There is also significant cause for concern regarding the future of organ allocation for patients with PAH. The revision of the LAS implemented in October 2021 removed cardiac index and central venous pressure from the waitlist urgency measure based on a call to refit the LAS models using a more contemporary cohort of candidates and recipients. With this change, there are only 2 of 13 REVEAL 2.0 similar variables and only 1 of 11 ESC/ERS risk similar variables included in the 2021 LAS waitlist urgency calculation (18). Time will tell if the updated LAS calculation will be more equitable for patients with PAH; however, it appears that they may continue to face challenges. Furthermore, removal of the cardiac index only from the waitlist urgency measure, and not the post-transplant survival measure, can only negatively impact patients with PAH waiting for lung transplant, because having a cardiac index <2 L/min/m2 can only decrease their LAS via a lowering of the post-transplant survival measure, without any impact on the waitlist survival measure. Ultimately, it is highly concerning that more than 15 years after the implementation of the LAS calculation it is becoming more simplified and that patients with PAH continue to be disadvantaged by the LAS. With the latest changes in 2021, this group of patients may lose some of the benefits achieved by the 2015 LAS revision.
Beyond PAH, it is unclear how the updated 2021 LAS calculation will impact other diagnostic groups, as it was simplified via the removal of other relevant variables. The new calculation notably removed FVC from the waitlist urgency measure, making discrimination of severity of disease in other diagnostic groups more challenging, as lung function is an important prognostic marker for patients with parenchymal lung disease (32–34). It was also the only spirometry-based variable included in the 2015 LAS calculation, so removal of all spirometry data may impact discrimination of risk in patients with COPD, CF, and ILD. Rather than removing disease-specific variables, the LAS may provide better discrimination of risk by including more disease-specific variables. A previous study that combined data from the Cystic Fibrosis Foundation with the UNOS registry demonstrated that including CF-specific variables, namely, a decline in FEV1 over the preceding 12 months, the presence of any Burkholderia species, prolonged hospitalization in the preceding 12 months, and massive hemoptysis, improved the LAS and rank in patients with CF (35). This study also showed that including declines in FEV1 helped provide discrimination of waitlist mortality in patients with COPD. It is our belief that including disease-specific variables would be the best way to discriminate risk among these varied disease states, such as the parameters in the REVEAL calculator for PAH, the parameters described above for CF, the parameters of the gender, age, and physiology index (GAP) score for ILD, or the parameters of the body-mass index, airflow obstruction, dyspnea, and exercise index (BODE) index for COPD (25, 32, 35, 36). Better discrimination of risk among each individual diagnostic group is the only way to ensure the equity of transplantation using a system such as the LAS. Furthermore, it may be more beneficial for the post-transplant survival measure to include time points later than 1 year after transplant, as patients with PAH have the worst 1-year survival after transplant because of their inherent increased risk of primary graft dysfunction (37). Notably, however, patients with PAH who survive the perioperative period have excellent outcomes, as they have the second-best 10-year and 20-year survival, as well as the second-best conditional 3-month and conditional 1-year survival (8). Taking into account a survival measure that looks beyond 1-year after transplant would benefit patients with PAH and would likely maximize the overall benefit of transplant, rather than maximizing 1-year survival.
There is some hope that the current proposed changes to the allocation system in the United States may benefit patients with PAH. UNOS is currently proposing to move to a continuous distribution model of allocation in lung transplant recipients. In this new model, there will be an equal weighting of the waitlist urgency measure and the post-transplant survival measure. Although patients with PAH do have a low likelihood of survival on the waiting list, the current iteration of the waiting list urgency measure clearly underestimates their actual risk, so it is possible that deemphasizing the waiting list urgency measure will benefit patients with PAH. The bigger potential benefit of the new 1:1 ratio of urgency to survival, however, will likely be in how the post-transplant survival measure will be assessed. Under the new system, the post-transplant survival measure will try to predict 5-year survival instead of 1-year survival, which will likely benefit patients with PAH, given their improved long-term survival after transplant over those with COPD and ILD. Ultimately, future assessments need to be done to determine the impact of the proposed changes to the continuous distribution model of allocation after implementation.
There are limitations to our study. First, as our data come from the UNOS registry, it is inherently limited by the parameters collected. One particularly relevant related issue is that there is no centralized adjudication of diagnoses. Furthermore, patients may suffer from diseases that overlap groups, such as those with connective tissue–associated ILD–pulmonary arterial hypertension, which could be classified as either group B or group D, so it is possible that some patients may be misclassified. In addition, although the registry contains the LAS at listing and the LAS at censoring, it does not include longitudinal scores over time, so it was not possible to do modeling techniques including longitudinal scores. It should be noted that we also do not have data on the markers of disease severity used in the PAH risk assessment tools, so it is not clear if some of the increase in the LAS after the 2015 revision represents the impact of the revision itself or if providers are listing patients at later time points in the natural history of disease. Furthermore, because vasodilator prescribing practices are not reported in the registry, it is not clear how the availability of more advanced therapies and a better understanding of the use of up-front combination therapy contributed to our results. Another limitation is that although our study includes a large number of patients, it is limited to the United States. LAS calculation, so it is not clear if the same inequities exist for patients listed in other countries that use different allocation systems or variations of the LAS. Finally, we must also comment on the potential limitations of competing risk regression modeling, which can be subject to misinterpretation. We chose to report this modeling approach because we believe it best represents our data, where the outcomes are, by definition, mutually exclusive because the same individual cannot have a waitlist death and a transplant. However, competing risk regression models do potentially introduce bias of dependent censoring. We hope that some of this potential bias is mitigated by the fact that we found similar results in our analyses using the proportion of patients with an outcome, log-rank testing, cause-specific hazard modeling, multistate modeling, and inverse probability weighting of regression adjustment.
Our study also has several strengths. It is a large cohort with extended follow-up. It provides novel insight into the allocation of lungs in the United States and highlights the ongoing inequities among diagnostic groups. This is of critical importance, given that the LAS was recently revised again in September 2021, so understanding the baseline before this updated calculation is needed to assess the impact of this newest revision.
Six and one-half years after implementation of the 2015 LAS revision, which was meant to ensure more equitable allocation of lung transplants, patients with PAH are still disadvantaged, as they suffer from the lowest rate of transplant and the highest waitlist death compared with other diagnoses. Future studies need to assess the impact of the recently implemented 2021 LAS revision, and efforts to include more disease-specific risk factors in the LAS should be made, so that the waitlist urgency measures can adequately discriminate risk in the future.
Acknowledgments
Acknowledgment
The authors thank W. John Boscardin, Ph.D., of the University of California, San Francisco Clinical and Translational Science Institute Biostatistical Consulting service for statistical input.
Footnotes
Supported by National Institutes of Health grant UL1 TR991872.
Author Contributions: N.A.K., H.C., J.P.S., and T.D.M. made substantial contributions to the conception and design of the work. N.A.K., H.C., D.R.C., K.K., J.O., C.B., J.A.G., M.A.S., J.K., S.R.H., L.E.L., J.P.S., and T.D.M. made substantial contributions to the acquisition, analysis, or interpretation of data for the work. N.A.K. wrote the first draft of the manuscript. N.A.K., H.C., D.R.C., K.K., J.O., C.B., J.A.G., M.A.S., J.K., S.R.H., L.E.L., J.P.S., and T.D.M. revised the manuscript for important intellectual content.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202201-0217OC on September 12, 2022
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. McGoon MD, Benza RL, Escribano-Subias P, Jiang X, Miller DP, Peacock AJ, et al. Pulmonary arterial hypertension: epidemiology and registries. J Am Coll Cardiol . 2013;62:D51–D59. doi: 10.1016/j.jacc.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 2. Lau EMT, Giannoulatou E, Celermajer DS, Humbert M. Epidemiology and treatment of pulmonary arterial hypertension. Nat Rev Cardiol . 2017;14:603–614. doi: 10.1038/nrcardio.2017.84. [DOI] [PubMed] [Google Scholar]
- 3. Foderaro A, Ventetuolo CE. Pulmonary arterial hypertension and the sex hormone paradox. Curr Hypertens Rep . 2016;18:84. doi: 10.1007/s11906-016-0689-7. [DOI] [PubMed] [Google Scholar]
- 4. Chen H, De Marco T, Kobashigawa EA, Katz PP, Chang VW, Blanc PD. Comparison of cardiac and pulmonary-specific quality-of-life measures in pulmonary arterial hypertension. Eur Respir J . 2011;38:608–616. doi: 10.1183/09031936.00161410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shafazand S, Goldstein MK, Doyle RL, Hlatky MA, Gould MK. Health-related quality of life in patients with pulmonary arterial hypertension. Chest . 2004;126:1452–1459. doi: 10.1378/chest.126.5.1452. [DOI] [PubMed] [Google Scholar]
- 6. Fernandes CJ, Martins BC, Jardim CV, Ciconelli RM, Morinaga LK, Breda AP, et al. Quality of life as a prognostic marker in pulmonary arterial hypertension. Health Qual Life Outcomes . 2014;12:130. doi: 10.1186/s12955-014-0130-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Farber HW, Miller DP, Poms AD, Badesch DB, Frost AE, Muros-Le Rouzic E, et al. Five-year outcomes of patients enrolled in the REVEAL Registry. Chest . 2015;148:1043–1054. doi: 10.1378/chest.15-0300. [DOI] [PubMed] [Google Scholar]
- 8. Chambers DC, Cherikh WS, Goldfarb SB, Hayes D, Jr, Kucheryavaya AY, Toll AE, et al. International Society for Heart and Lung Transplantation The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: thirty-fifth adult lung and heart-lung transplant report-2018. Focus theme: multiorgan transplantation. J Heart Lung Transplant . 2018;37:1169–1183. doi: 10.1016/j.healun.2018.07.020. [DOI] [PubMed] [Google Scholar]
- 9. Egan TM, Murray S, Bustami RT, Shearon TH, McCullough KP, Edwards LB, et al. Development of the new lung allocation system in the United States. Am J Transplant . 2006;6:1212–1227. doi: 10.1111/j.1600-6143.2006.01276.x. [DOI] [PubMed] [Google Scholar]
- 10. Kozower BD, Meyers BF, Smith MA, De Oliveira NC, Cassivi SD, Guthrie TJ, et al. The impact of the lung allocation score on short-term transplantation outcomes: a multicenter study. J Thorac Cardiovasc Surg . 2008;135:166–171. doi: 10.1016/j.jtcvs.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 11. Chen H, Shiboski SC, Golden JA, Gould MK, Hays SR, Hoopes CW, et al. Impact of the lung allocation score on lung transplantation for pulmonary arterial hypertension. Am J Respir Crit Care Med . 2009;180:468–474. doi: 10.1164/rccm.200810-1603OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gomberg-Maitland M, Glassner-Kolmin C, Watson S, Frantz R, Park M, Frost A, et al. Survival in pulmonary arterial hypertension patients awaiting lung transplantation. J Heart Lung Transplant . 2013;32:1179–1186. doi: 10.1016/j.healun.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 13. Benza RL, Miller DP, Frost A, Barst RJ, Krichman AM, McGoon MD. Analysis of the lung allocation score estimation of risk of death in patients with pulmonary arterial hypertension using data from the REVEAL Registry. Transplantation . 2010;90:298–305. doi: 10.1097/TP.0b013e3181e49b83. [DOI] [PubMed] [Google Scholar]
- 14. Chan KM. Idiopathic pulmonary arterial hypertension and equity of donor lung allocation in the era of the lung allocation score: are we there yet? Am J Respir Crit Care Med . 2009;180:385–387. doi: 10.1164/rccm.200906-0976ED. [DOI] [PubMed] [Google Scholar]
- 15. Wille KM, Edwards LB, Callahan LR, McKoy AR, Chan KM. Characteristics of lung allocation score exception requests submitted to the national Lung Review Board. J Heart Lung Transplant . 2017;36:812–814. doi: 10.1016/j.healun.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 16. Valapour M, Skeans MA, Smith JM, Edwards LB, Cherikh WS, Uccellini K, et al. OPTN/SRTR 2015 annual data report: lung. Am J Transplant . 2017;17:357–424. doi: 10.1111/ajt.14129. [DOI] [PubMed] [Google Scholar]
- 17. Chan K, Robbins-Callahan L, Valapour M, Skeans M, Wozniak T, Edwards L. Early effects after the first major revision of the lung allocation score (LAS) in the United States [abstract] Chest . 2015;148:1079A. [Google Scholar]
- 18.OPTN Lung Transplantation Committee. Briefing to the OPTN Board of Directors on updated cohort for calculation of the lung allocation score (LAS). 2021https://optn.transplant.hrsa.gov/media/4244/updated-cohort-for-calculation-of-the-las.pdf [Google Scholar]
- 19.Kolaitis NA, Chen H, Calabrese DR, Leard LE, Singer JP, De Marco T. The lung allocation score remains inequitable for patients with PAH, even after the 2015 revision. Journal of Heart and Lung Transplantation (318) (Issue 4 Supplement) 2022;41:S144. [Google Scholar]
- 20. Hull TD, Leya GA, Axtell AL, Moonsamy P, Osho A, Chang DC, et al. Lung transplantation for chronic obstructive pulmonary disease: a call to modify the lung allocation score to decrease waitlist mortality. J Thorac Cardiovasc Surg . 2022;164:1222–1233.e11. doi: 10.1016/j.jtcvs.2021.11.086. [DOI] [PubMed] [Google Scholar]
- 21. Lesko CR, Lau B. Bias due to confounders for the exposure-competing risk relationship. Epidemiology . 2017;28:20–27. doi: 10.1097/EDE.0000000000000565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation . 2016;133:601–609. doi: 10.1161/CIRCULATIONAHA.115.017719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Andersen PK, Geskus RB, de Witte T, Putter H. Competing risks in epidemiology: possibilities and pitfalls. Int J Epidemiol . 2012;41:861–870. doi: 10.1093/ije/dyr213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Weibull CE, Lambert PC, Eloranta S, Andersson TML, Dickman PW, Crowther MJ. A multistate model incorporating estimation of excess hazards and multiple time scales. Stat Med . 2021;40:2139–2154. doi: 10.1002/sim.8894. [DOI] [PubMed] [Google Scholar]
- 25. Benza RL, Kanwar MK, Raina A, Scott JV, Zhao CL, Selej M, et al. Development and validation of an abridged version of the REVEAL 2.0 risk score calculator, REVEAL Lite 2, for use in patients with pulmonary arterial hypertension. Chest . 2021;159:337–346. doi: 10.1016/j.chest.2020.08.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Respir J . 2015;46:903–975. doi: 10.1183/13993003.01032-2015. [DOI] [PubMed] [Google Scholar]
- 27. Benza RL, Gomberg-Maitland M, Elliott CG, Farber HW, Foreman AJ, Frost AE, et al. Predicting survival in patients with pulmonary arterial hypertension: the REVEAL risk score calculator 2.0 and comparison with ESC/ERS-based risk assessment strategies. Chest . 2019;156:323–337. doi: 10.1016/j.chest.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 28. Boucly A, Weatherald J, Savale L, Jaïs X, Cottin V, Prevot G, et al. Risk assessment, prognosis and guideline implementation in pulmonary arterial hypertension. Eur Respir J . 2017;50:1700889. doi: 10.1183/13993003.00889-2017. [DOI] [PubMed] [Google Scholar]
- 29. Sahay S, Tonelli AR, Selej M, Watson Z, Benza RL. Risk assessment in patients with functional class II pulmonary arterial hypertension: comparison of physician gestalt with ESC/ERS and the REVEAL 2.0 risk score. PLoS One . 2020;15:e0241504. doi: 10.1371/journal.pone.0241504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Leard LE, Holm AM, Valapour M, Glanville AR, Attawar S, Aversa M, et al. Consensus document for the selection of lung transplant candidates: an update from the International Society for Heart and Lung Transplantation. J Heart Lung Transplant . 2021;40:1349–1379. doi: 10.1016/j.healun.2021.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shah RJ, Diamond JM, Cantu E, Flesch J, Lee JC, Lederer DJ, et al. Objective estimates improve risk stratification for primary graft dysfunction after lung transplantation. Am J Transplant . 2015;15:2188–2196. doi: 10.1111/ajt.13262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ley B, Ryerson CJ, Vittinghoff E, Ryu JH, Tomassetti S, Lee JS, et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann Intern Med . 2012;156:684–691. doi: 10.7326/0003-4819-156-10-201205150-00004. [DOI] [PubMed] [Google Scholar]
- 33. Cooper CB. Assessment of pulmonary function in COPD. Semin Respir Crit Care Med . 2005;26:246–252. doi: 10.1055/s-2005-869543. [DOI] [PubMed] [Google Scholar]
- 34. Vilozni D, Bentur L, Efrati O, Minuskin T, Barak A, Szeinberg A, et al. Spirometry in early childhood in cystic fibrosis patients. Chest . 2007;131:356–361. doi: 10.1378/chest.06-1351. [DOI] [PubMed] [Google Scholar]
- 35. Lehr CJ, Skeans M, Dasenbrook E, Fink A, Fernandez G, Faro A, et al. Effect of including important clinical variables on accuracy of the lung allocation score for cystic fibrosis and chronic obstructive pulmonary disease. Am J Respir Crit Care Med . 2019;200:1013–1021. doi: 10.1164/rccm.201902-0252OC. [DOI] [PubMed] [Google Scholar]
- 36. Celli BR, Cote CG, Marin JM, Casanova C, Montes de Oca M, Mendez RA, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med . 2004;350:1005–1012. doi: 10.1056/NEJMoa021322. [DOI] [PubMed] [Google Scholar]
- 37. Diamond JM, Lee JC, Kawut SM, Shah RJ, Localio AR, Bellamy SL, et al. Lung Transplant Outcomes Group Clinical risk factors for primary graft dysfunction after lung transplantation. Am J Respir Crit Care Med . 2013;187:527–534. doi: 10.1164/rccm.201210-1865OC. [DOI] [PMC free article] [PubMed] [Google Scholar]


