Abstract
Neuronal and glial glutamate transporters remove the excitatory neurotransmitter glutamate from the synaptic cleft and thus prevent neurotoxicity. The proteins belong to a large and widespread family of secondary transporters, including bacterial glutamate, serine, and C4-dicarboxylate transporters; mammalian neutral-amino-acid transporters; and an increasing number of bacterial, archaeal, and eukaryotic proteins that have not yet been functionally characterized. Sixty members of the glutamate transporter family were found in the databases on the basis of sequence homology. The amino acid sequences of the carriers have diverged enormously. Homology between the members of the family is most apparent in a stretch of approximately 150 residues in the C-terminal part of the proteins. This region contains four reasonably well-conserved sequence motifs, all of which have been suggested to be part of the translocation pore or substrate binding site. Phylogenetic analysis of the C-terminal stretch revealed the presence of five subfamilies with characterized members: (i) the eukaryotic glutamate transporters, (ii) the bacterial glutamate transporters, (iii) the eukaryotic neutral-amino-acid transporters, (iv) the bacterial C4-dicarboxylate transporters, and (v) the bacterial serine transporters. A number of other subfamilies that do not contain characterized members have been defined. In contrast to their amino acid sequences, the hydropathy profiles of the members of the family are extremely well conserved. Analysis of the hydropathy profiles has suggested that the glutamate transporters have a global structure that is unique among secondary transporters. Experimentally, the unique structure of the transporters was recently confirmed by membrane topology studies. Although there is still controversy about part of the topology, the most likely model predicts the presence of eight membrane-spanning α-helices and a loop-pore structure which is unique among secondary transporters but may resemble loop-pores found in ion channels. A second distinctive structural feature is the presence of a highly amphipathic membrane-spanning helix that provides a hydrophilic path through the membrane. Recent data from analysis of site-directed mutants and studies on the mechanism and pharmacology of the transporters are discussed in relation to the structural model.
Eukaryotic, bacterial, and archaeal cells contain a large number of integral membrane proteins and protein complexes involved in solute transport across the membrane (48). In Escherichia coli as many as 10.8% of all chromosomal genes code for membrane transport proteins (65). All known and putative transport proteins have recently been classified in different families based on their sequence similarities. This led to the identification of 159 transporter families, of which secondary transporters represent the largest functional category, comprising 59 families (73).
Secondary transporters use the free energy stored in ion and/or solute gradients over the membrane to drive transport. Since three-dimensional structures of secondary transporters have not yet been solved, models for their structures are based on biochemical data and on (computational) analysis of their amino acid sequences. A typical secondary transporter consists of a single polypeptide component that forms a bundle of membrane-spanning α-helices connected by loops of various sizes. Many of these proteins contain 12 membrane-spanning segments, but different numbers are not exceptional (73). The 59 families of secondary transporters do not represent as many different global structures. It has been shown by hydropathy profile analysis that many of the families belong to the same structural class (53, 54). One of the families that forms a separate structural class is the glutamate transporter family, also called dicarboxylate/cation symporters (DCS; transporter classification 2.23) (73).
The glutamate transporter family includes transporters that are found in neurons and glial cells in the mammalian central nervous system and catalyze the reuptake of the excitatory neurotransmitter glutamate from the synaptic cleft (26, 38, 67, 81). Excessive amounts of extracellular glutamate, associated with several diseases, cause neuronal destruction via activation of the N-methyl-d-aspartate receptors (57, 60). The neuronal and glial glutamate transporters are believed to prevent excitotoxicity of glutamate (52, 71, 83) and may help to end the excitatory signal together with diffusion (58, 63). Since these proteins have been implicated in numerous neurological disorders, they have attracted much attention and have been well characterized.
The number of known transporters in the glutamate transporter family has grown rapidly during the last years, and many members of the family have now been characterized, including retinal glutamate transporters from vertebrates (3, 22), mammalian neutral-amino-acid transporters (5, 51, 75), and bacterial nutrient uptake proteins (23, 85, 86). Because of the variety of transporters that are being studied in the glutamate transporter family and the numerous techniques used to study them, the structural features of the transporter family are now rapidly being unraveled. This review focuses on the diversity of transporters in the family and on their structural properties. Physiological and pathological aspects have recently been reviewed elsewhere (37, 94).
THE FAMILY
Substrate Specificity
The members of the glutamate transporter family that have been functionally characterized can be classified in three groups based on their substrate specificity (Table 1): C4-dicarboxylate transporters (found in bacteria), glutamate/aspartate transporters (found in bacteria and eukaryotes) and neutral-amino-acid transporters (found in bacteria and eukaryotes). The bacterial C4-dicarboxylate carriers transport the tricarboxylic acid cycle intermediates succinate, fumarate, and malate (28, 29). In addition, the transporter from Rhizobium meliloti uses aspartate (106) and the transporters from Escherichia coli and Salmonella typhimurium use orotate as substrate (6). It is not known which transmembrane ion or solute gradient(s) provides the free energy to drive transport.
TABLE 1.
Substrate specificity of the members of the glutamate transporter family
| Subfamily | Kingdoma | Substratesb | Mode of energy coupling |
|---|---|---|---|
| C4-dicarboxylate transporters | B | Succinate, fumarate, malate (orotate, aspartate) | Unknown |
| Glutamate/aspartate transporters | B, E | Glutamate, aspartate, glutamate analogues | H+ symport; H+/Na+ symport; H+/Na+ symport-K+ antiport |
| Neutral-amino-acid transporters | B, E | Alanine, serine, cysteine, threonine (asparagine, glutamine) | Exchange; Na+ symport |
B, Bacteria; E, Eucarya.
High-affinity substrates are shown; substrates in parentheses are not accepted by all members of the subfamilies.
All glutamate transporters use l-glutamate and l-aspartate as high-affinity substrates (Km [Glu] < 100μM). In addition, several (nonphysiological) glutamate analogues are transported (38, 67, 81, 85, 88, 104). The bacterial proteins catalyze the electrogenic symport of glutamate with at least two cations (85, 87, 88). The GltP proteins from E. coli and Bacillus subtilis use protons to drive the uptake of glutamate, whereas the GltT proteins from Bacillus stearothermophilus and Bacillus caldotenax can replace one proton with a sodium ion (34). Na+ is preferentially used at elevated temperatures, but the selectivity for sodium ions is lost when these transporters are expressed in E. coli (87). These observations indicate that minor conformational changes may alter the cation specificity.
The eukaryotic glutamate transporters catalyze the electrogenic symport of glutamate with two or three sodium ions and one proton, whereas one potassium ion is antiported (2, 7, 24, 43, 112). The exact number of transported sodium ions is still a matter of debate (37, 39, 50, 112), but a stoichiometry of three sodium ions per glutamate is generally favored (50, 112). Transport of glutamate by the eukaryotic proteins is strictly dependent on sodium ions, but not all Na+ binding sites are equally selective. For the glutamate transporter EAAT2 of rats (also known as GLT-1), it was shown that only one Na+ binding site strictly requires sodium ions whereas lithium ions can replace Na+ in the others (33). Cesium, rubidium, and ammonium ions can replace K+ to various extents (44).
The high-affinity substrates for the mammalian neutral-amino-acid transporters are alanine, serine, cysteine, and threonine (Km < 100 μM), but some members (ACST2 from mice, humans, and rabbits) show a broader substrate specificity and accept glutamine and asparagine with high affinity and several other amino acids, including glutamate, with lower affinity (Km > 300 μM) (5, 45, 46, 82, 93). The human neutral amino acid transporter ASCT1 is an obligate exchanger that does not mediate a net flux of amino acids (111). Exchange of the amino acids is electroneutral and accompanied by a symmetrical exchange of one Na+. In contrast, mouse ASCT2 may exchange Na+ for K+ during turnover (93). Recently, a serine transporter from E. coli that is an Na+ symporter (61) was characterized.
Both the eukaryotic glutamate transporters and the eukaryotic neutral-amino-acid carriers mediate thermodynamically uncoupled ion fluxes. Besides substrate-independent cation leaks, substrate-dependent Cl− currents have been reported (3, 8, 22, 26, 39, 66, 79, 95, 101, 111). The extent of the substrate-dependent Cl− flux differs for the various members of the family but is very high in the glutamate transporters EAAT4 and EAAT5 from the human brain and retina and EAAT5A from the salamander retina (3, 22, 26). Thus, these proteins have a dual function as glutamate transporters and glutamate-gated chloride channels.
Diversity and Phylogeny
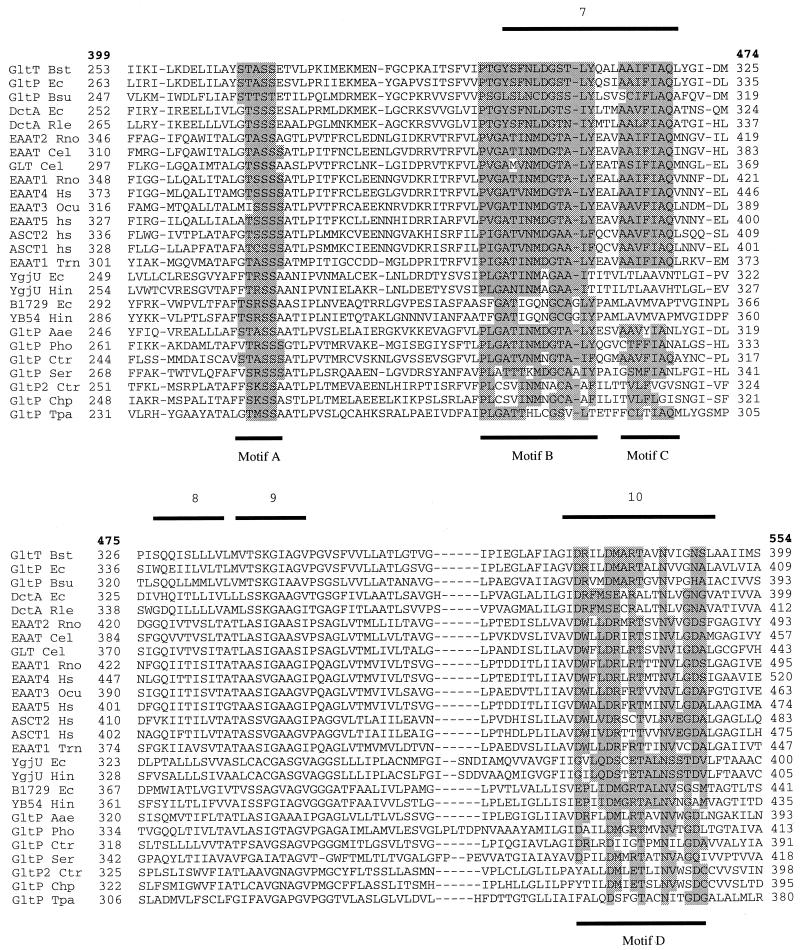
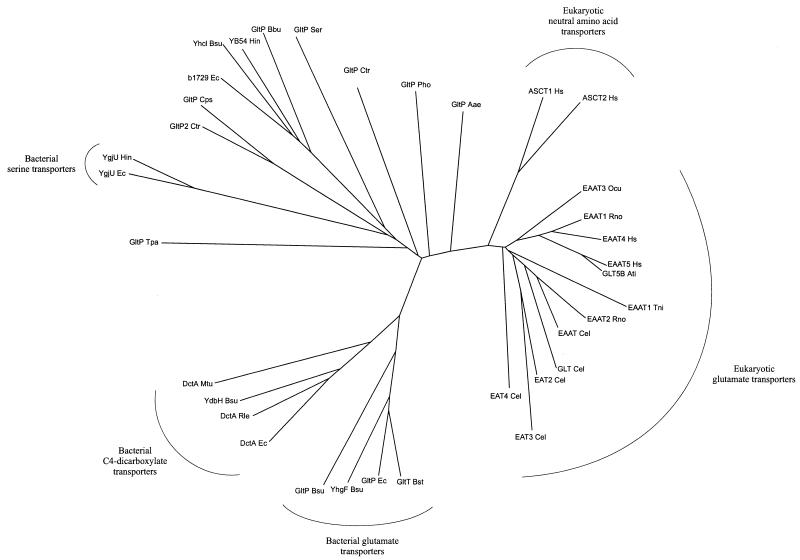
Sixty protein sequences from the Eucarya, Bacteria, and Archaea, belonging to the glutamate transporter family, were found in the protein and translated nucleotide databases by using the BLAST facility (1) (Table 2). The sequences vary in length from 396 to 581 residues, with the bacterial ones being significantly shorter (396 to 491 residues) than the eukaryotic ones (479 to 581 residues). Several organisms contain multiple paralogues of the family. The bacteria E. coli and B. subtilis have four paralogues each, whereas in eukaryotes (Homo sapiens) up to seven have been found so far. A multiple-sequence alignment was made with the CLUSTALX program (84). A stretch of about 150 residues in the C-terminal part of the sequences is better conserved than the N-terminal part (Fig. 1). This C-terminal part of the alignment, which is unambiguous and contains very few gaps, was used to construct the phylogenetic tree shown in Fig. 2.
TABLE 2.
Members of the glutamate transporter familya
| Name | Organism | Kingdomb | Description | No. of residues | Accession no. |
|---|---|---|---|---|---|
| ASCT2 Ocu | Oryctolagus cuniculus | E | Neutral-amino-acid transporter | 541 | O19105 |
| ASCT2 Hs | Homo sapiens | E | Neutral-amino-acid transporter | 541 | O15758 |
| ASCT2 Mmu | Mus musculus | E | Neutral-amino-acid transporter | 553 | P51912 |
| ASCT1 Hs | Homo sapiens | E | Neutral-amino-acid transporter | 532 | P43007 |
| ASCT1 Mmu | Mus musculus | E | Neutral-amino-acid transporter | 532 | O35874 |
| EAAT1 Tni | Trichoplusia ni | E | Glu/Asp transporter | 479 | AF003006 |
| EAAT3 Mmu | Mus musculus | E | Glu/Asp transporter | 523 | P51906 |
| EAAT3 Rno | Rattus norvegicus | E | Glu/Asp transporter | 523 | P51907 |
| EAAT3 Bta | Bos taurus | E | Glu/Asp transporter | 524 | O95135 |
| EAAT3 Ocuc | Oryctolagus cuniculus | E | Glu/Asp transporter | 524 | P31597 |
| EAAT3 Hs | Homo sapiens | E | Glu/Asp transporter | 524 | P43005 |
| EAAT1 Hs | Homo sapiens | E | Glu/Asp transporter | 542 | P43003 |
| EAAT1 Bta | Bos taurus | E | Glu/Asp transporter | 542 | P46411 |
| EAAT1 Rnoc | Rattus norvegicus | E | Glu/Asp transporter | 543 | P24942 |
| EAAT1 Mmu | Mus musculus | E | Glu/Asp transporter | 543 | 737473 |
| EAAT1 Ati | Ambystoma tigrinum | E | Glu/Asp transporter | 543 | AF018256 |
| EAAT4 Mmu | Mus musculus | E | Glu/Asp transporter | 561 | O35544 |
| EAAT4 Rno | Rattus norvegicus | E | Glu/Asp transporter | 561 | O35921 |
| EAAT4 Hs | Homo sapiens | E | Glu/Asp transporter | 564 | P48664 |
| EAAT5 Hs | Homo sapiens | E | Glu/Asp transporter | 560 | O00341 |
| GLT5A Ati | Ambystoma tigrinum | E | Glu/Asp transporter | 564 | AF018259 |
| GLT5B Ati | Ambystoma tigrinum | E | Glu/Asp transporter | 544 | AF018260 |
| EAAT2 Hs | Homo sapiens | E | Glu/Asp transporter | 572 | P43004 |
| EAAT2 Mmu | Mus musculus | E | Glu/Asp transporter | 572 | P43006 |
| EAAT2 Rnoc | Rattus norvegicus | E | Glu/Asp transporter | 573 | P31596 |
| GLT2A Ati | Ambystoma tigrinum | E | Glu/Asp transporter | 579 | AF018257 |
| GLT2B Ati | Ambystoma tigrinum | E | Glu/Asp transporter | 581 | AF018258 |
| EAAT Cel | Caenorhabditis elegans | E | Putative Glu/Asp transporter | 503 | O10901 |
| EAAT Ovo | Onchocerca volvulus | E | Putative Glu/Asp transporter | 492 | O25605 |
| EAT2 Cel | Caenorhabditis elegans | E | Putative Glu/Asp transporter | 532 | O21353 |
| EAT3 Cel | Caenorhabditis elegans | E | Putative Glu/Asp transporter | 575 | O21751 |
| GLT Cel | Caenorhabditis elegans | E | Putative Glu/Asp transporter | 491 | Z99277 |
| EAT4 Cel | Caenorhabditis elegans | E | Putative Glu/Asp transporter | 502 | O22682 |
| GltT Bca | Bacillus caldotenax | B | Glu/Asp transporter | 421 | P24944 |
| GltT Bst | Bacillus stearothermophilus | B | Glu/Asp transporter | 421 | P24943 |
| YhfG Bsu | Bacillus subtilis | B | Putative Glu/Asp transporter | 429 | Y14083 |
| GltP Ec | Escherichia coli | B | Glu/Asp transporter | 437 | P21345 |
| GltP Bsu | Bacillus subtilis | B | Glu/Asp transporter | 414 | P39817 |
| DctA Ec | Escherichia coli | B | C4-dicarboxylate transporter | 428 | P37312 |
| DctA Sty | Salmonella typhimurium | B | C4-dicarboxylate transporter | 428 | P50334 |
| DctA RleN | Rhizobium leguminosarum NGR234 | B | C4-dicarboxylate transporter | 449 | S38912 |
| DctA Sme | Sinorhizobium meliloti | B | C4-dicarboxylate transporter | 441 | P20672 |
| DctA Rle | Rhizobium leguminosarum | B | C4-dicarboxylate transporter | 444 | Q01857 |
| YdbH Bsu | Bacillus subtilis | B | Putative C4-dicarboxylate transporter | 421 | AB001488 |
| DctA Mtu | Mycobacterium tuberculosis | B | Putative C4-dicarboxylate transporter | 491 | Z81451 |
| b1729 Ec | Escherichia coli | B | Unknown | 463 | AE000268 |
| YB54 Hin | Haemophilus influenzae | B | Unknown | 440 | P45079 |
| YhcL Bsu | Bacillus subtilis | B | Unknown | 463 | P54596 |
| GltP Bbu | Borrelia burgdorferi | B | Unknown | 463 | AE001172 |
| YgjU Ec | Escherichia coli | B | Serine transporter | 414 | P42602 |
| YgjU Hin | Haemophilus influenzae | B | Putative serine transporter | 414 | P45246 |
| GltP Aae | Aquifex aeolicus | B | Unknown | 398 | AE000735 |
| GltP Ser | Saccharopolyspora erythraea | B | Unknown | 449 | S71005 |
| GltP Cps | Chlamydia psittaci | B | Unknown | 406 | AF017105 |
| GltP2 Ctr | Chlamydia trachomatis | B | Unknown | 415 | AE001296 |
| GltP Tpa | Treponema pallidum | B | Unknown | 396 | AE001231 |
| GltP2 Tpa | Treponema pallidum | B | Unknown | 407 | AE001262 |
| GltP2 Bbu | Borrelia burgdorferi | B | Unknown | 400 | AE001145 |
| GltP Ctr | Chlamydia trachomatis | B | Unknown | 412 | AE001313 |
| GltP Pho | Pyrococcus horikoshii | A | Unknown | 425 | AB009510 |
The BLAST search was performed at the BLAST Website (8a) using the Advance BLAST 2.0 mode. Only sequences from the databases that do not have obvious sequencing errors are shown; (splicing) variants of the same protein are not shown.
A, Archaea; B, Bacteria; E, Eucarya.
Rat EAAT1 and EAAT2 and rabbit EAAT3 are also named GLAST, GLT-1, and EAAC1, respectively. E. coli YgjU is also named SstT.
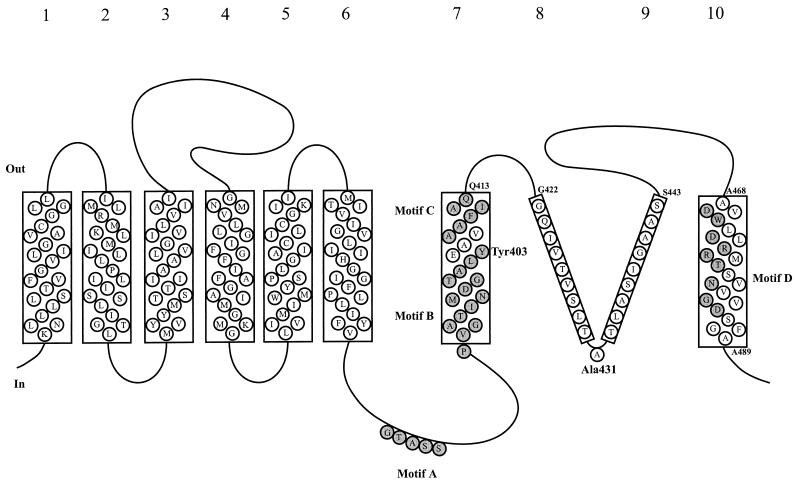
FIG. 1.
Multiple-sequence alignment of a stretch of approximately 150 residues near the C terminus of the transporters. The alignment was made with the program CLUSTALX (84). A representative set of 26 members of the glutamate transporter family is shown. Bold numbers refer to the positions in the multiple-sequence alignment and correspond to the numbers in Fig. 4. Other numbers refer to the residue numbers of the individual sequences. Bars below and above the sequences indicate the positions of the conserved motifs (motifs A to D, highlighted) and the positions of the transmembrane segments (as published by Grunewald et al. [32]), respectively. Abbreviated transporter names are taken from Table 2.
FIG. 2.
Phylogenetic tree of 35 members of the glutamate transporter family. The set of 35 members does not contain pairs of sequences with more than 70% identical residues. The tree is based on the part of the multiple-sequence alignment shown in Fig. 1. It was drawn with the DRAWTREE program from the PHYLIP package (27). Abbreviated transporter names are taken from Table 2.
The tree includes a subset of 35 members of the family that do not contain pairs of sequences with more than 70% identical residues. Since more similar pairs cluster closely together in the tree, they are less informative. Several distinct subfamilies can be recognized in the phylogenetic tree of the glutamate transporter family. All eukaryotic members cluster in one branch of the tree. They have at least 31% identical residues over the full length. The neutral amino acid transporters and the glutamate transporters clearly form separate groups within the eukaryotic branch, supporting the notion that substrate specificity correlates with phylogenetic classification (64). However, the eukaryotic and bacterial glutamate transporters that have similar substrate specificity do not cluster in the tree but belong to separate subfamilies. Likewise, the eukaryotic neutral-amino-acid transporters and the bacterial serine transporters are found in different branches of the tree. The analysis suggests that phylogenetic clustering of members of the family is determined hierarchically, first by the evolutionary position of the host organism and second by the substrate specificity (72).
The bacterial glutamate transporters are found in the same major branch as the bacterial C4-dicarboxylate transporters, but within this branch they form separate clusters. The bacterial glutamate transporters have more than 44% identical residues, whereas the C4-dicarboxylate transporters have at least 40% identical residues. The percentage of identical residues between the members of the GltP and DctA subfamilies varies from 27 to 42%. In both groups, a protein from B. subtilis that has not been functionally characterized is present (YhfG and YdbH, respectively). It is likely that these proteins have the same substrate specificity as the other members of the subfamilies to which they belong. The two bacterial subfamilies are more closely related to each other than to the eukaryotic subfamily. The bacterial and eukaryotic members have 18 to 28% identical residues.
The serine transporter YgjU of E. coli is only distantly related to the other characterized members of the family (with at most 20% identical residues). It is likely that the related protein YgjU of Haemophilus influenzae (56% identity) is also a serine transporter. The bacterial serine transporters are found in a cluster of proteins that branches off from a point near the center of the tree, indicating that they have diverged from the other sequences early in evolution (64). The cluster contains a large number of uncharacterized proteins that are only distantly related to the characterized members of the glutamate transporter family. The uncharacterized proteins are found not only in organisms like the chlamydiae (107), which are not closely related to the organisms in the clusters with characterized proteins, but also in organisms like E. coli and B. subtilis, which have well-characterized members. Therefore, it is likely that at least some of the uncharacterized members of the family are proteins with different substrate specificity from the transporters that have been characterized so far. Among the uncharacterized members, several subfamilies can be distinguished that are likely to consist of proteins with similar, currently unknown substrate specificity. Hence, b1729 of E. coli, YB54 of H. influenzae, GltP of Borrelia burgdorferi, and YhcL of B. subtilis probably are orthologues with the same function. The same applies to GltP2 of Chlamydia trachomatis and GltP of Chlamydia psittaci. Two other proteins, GltP2 of Treponema pallidum and GltP2 of B. burgdorferi, are not included in the phylogenetic tree (Fig. 2) because they have diverged so far that it is difficult to unambiguously align them (they have at most 19 and 17% of residues identical to those of other members of the family, respectively). Nevertheless, they are likely to belong to the family because of the (partial) presence of conserved motifs and characteristic features of the hydropathy profiles (see below).
Finally, two sequences occupy separate positions in the tree. The glutamate transporter homologue GltP Aae from the hyperthermophilic bacterium Aquifex aeolicus is more closely related to the eukaryotic transporters (32 to 39% identical residues) than to the bacterial members (22 to 32% identical residues). The separate position of GltP of A. aeolicus in the tree reflects the unique evolutionary position of this organism (16). The only archaeal sequence in the tree (GltP Pho) is equally closely related to the bacterial and eukaryotic sequences (approximately 30% of identical residues) and branches from a point near the center of the tree, reflecting the distinct phylogenetic position of the archaea (108).
STRUCTURAL ANALYSIS
Hydropathy Profile Analysis
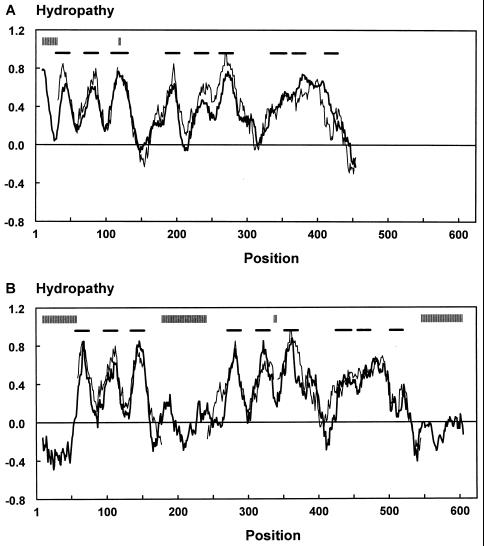
Although the amino acid sequences of the members of the glutamate transporter family have diverged considerably, their hydropathy profiles are remarkably similar (40, 53, 77) (Fig. 3). It is a general property of families of integral membrane proteins that the hydropathy profiles of the members are better conserved than the primary structures. The conservation of hydropathy profiles is a reflection of the conservation of the global structure of the members of a family (53, 54). Quantitative comparison of the hydropathy profiles of different families of secondary transporters has recently been used to group the transporters into four major structural classes. It was shown that the hydropathy profile of the glutamate transporter family is likely to reflect a global structure that is unique among secondary transporters (53).
FIG. 3.
Alignment of the average hydropathy profiles of the subfamily of bacterial glutamate transporters (thin line) and the subfamily consisting of b1729 of E. coli, YB54 of H. influenzae, GltP of B. burgdorferi, and YhcL of B. subtilis (thick line) (A) and the subfamilies of bacterial and eukaryotic glutamate transporters (thin and thick lines, respectively) (B). The profiles were aligned as specified by Lolkema and Slotboom (53) with a window of 19 amino acids. Vertical and horizontal bars above the profiles indicate the positions of gaps in the sequences and the positions of the transmembrane segments (32), respectively. The profiles are almost superimposable even though the sequences have considerably diverged. The bacterial glutamate transporters and the subfamily containing YB54 of H. influenzae have 18 to 24% identical residues (A), whereas the subfamilies of bacterial and eukaryotic glutamate transporters have 22 to 29% identical residues (B). The subfamily containing YB54 of H. influenzae has an extra hydrophobic segment at the N terminus.
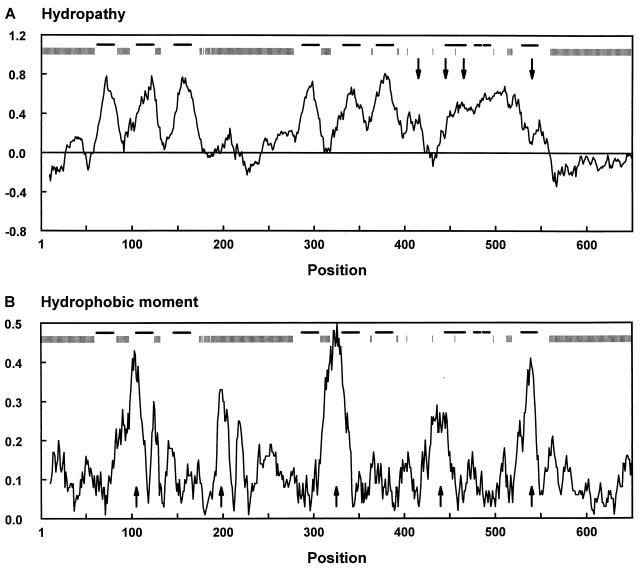
The average hydropathy profile derived from the multiple sequence alignment of a subset of 35 members of the family (Fig. 4A) emphasizes the conserved characteristics of the profiles, which include six alternating hydrophobic and hydrophilic regions in the N-terminal half and a large hydrophobic region in the C-terminal half. The subset of 35 members does not contain any pairs of sequences with more than 70% identical residues, so that the average profile is not biased toward the profile of a particular subfamily. The eukaryotic and prokaryotic members differ predominantly in the length of their hydrophilic regions. Three hydrophilic stretches are considerably longer in the eukaryotic proteins: the N-terminal and C-terminal extensions and the region between the third and fourth hydrophobic peaks, which is glycosylated in the eukaryotic members (13). As is commonly observed for families of integral membrane proteins, the gaps in the multiple-sequence alignment are found almost exclusively in the hydrophilic stretches (Fig. 4A).
FIG. 4.
Average profiles of hydropathy (A) and hydrophobic moment (B) of the glutamate transporter family. The set of 35 members (Fig. 2) which does not contain pairs of sequences with more than 70% identical residues was used. Vertical and horizontal bars above the profiles indicate the positions of gaps in the sequences and the positions of the transmembrane segments (32), respectively. Position numbers refer to positions in the multiple-sequence alignment and correspond to the bold numbers in Fig. 1. In panel A, a window of 19 residues was used and the arrows point to the positions of the conserved motifs A to D. In panel B, a window of 21 residues and a period of 3.6 residues, appropriate for α-helical structures, was used and the arrows point to the five conserved putative amphipatic helices (AH1 to AH5 from left to right).
Membrane Topology
The six hydrophobic segments in the N-terminal part of the glutamate transporters were predicted to be transmembrane helices in several reports (38, 67, 81, 88). Experimentally, the membrane topology of rat EAAT1 (GLAST) was determined by monitoring the glycosylation state of C-terminally truncated transporters fused to a reporter peptide with glycosylation sites (103) and the topology of rat EAAT2 (GLT-1) was assessed by labeling of single-cysteine mutants (32). Both studies confirmed the presence of six membrane-spanning segments in the N-terminal half of the transporters. Also, the results of trypsin digestion experiments on rat EAAT2 in membrane vesicles are consistent with the presence of six N-terminal membrane helices. These studies showed that the N terminus of the protein is located at the cytoplasmic side of the membrane whereas the large hydrophilic region between the third and fourth hydrophobic segments is extracellular (33), which is in agreement with the observed glycosylation of this loop in the eukaryotic transporters (13).
The C-terminal half of the proteins does not contain clear alternating regions of high and low hydrophobicity. Therefore, it is difficult to predict the position of transmembrane segments from the hydropathy profile. This has led to the proposal of several different models for the membrane topology of the proteins (35, 38, 67, 81, 88). The topologies of the C-terminal halves of rat EAAT1 and EAAT2, B. stearothermophilus GltT, and a small part of human EAAT1 were experimentally assessed (32, 74, 77, 103). The four studies gave conflicting results. In the bacterial transporter, four putative membrane-spanning helices were found (helices 7 to 10) when alkaline phosphatase was used as the reporter protein. Accessibility scanning of single-cysteine mutants of rat EAAT2 revealed two membrane-spanning helices at the same positions as helices 7 and 10 in the bacterial system. In addition, two short membrane-spanning segments of approximately 10 residues each were found in the region where bacterial helices 8 and 9 were proposed (Fig. 5). The two short membrane-spanning segments may form a reentrant loop or a loop-pore structure reminiscent of those found in ion channels (19, 55, 56). In the third study, on rat EAAT1, four membrane-spanning β-strands were proposed at significantly different positions from the membrane-spanning segments in the other studies. This model is not consistent with results of trypsin digestion studies on membrane vesicles containing rat EAAT2 (GLT-1). These experiments showed that the C terminus of the protein and the hydrophilic region following transmembrane segment 6 are located at the cytoplasmic side of the membrane (32, 33). The other two models are both consistent with these results and display several similar features, most importantly the presence of two membrane-spanning segments (numbered 7 and 10) that may form an important part of the substrate recognition site and translocation pore (see below). The only difference between the models is the position and length of membrane-spanning segments 8 and 9. At present, we tentatively favor the model with two helices and the loop-pore structure for the C-terminal part of the proteins, since it is based on the study of full-length and active carriers. Truncated transporters fused to reporter proteins may fold in a nonnative conformation (89). This may be especially the case when the protein does not consist of a conventional bundle of membrane-spanning α-helices.
FIG. 5.
Model for the membrane topology of the C-terminal part of the glutamate transporters comprising membrane-spanning segments 7 to 10 (based on the model of Grunewald et al. [32]). The sequence of rat EAAT2 (GLT-1) is shown. Membrane segments 7 and 10 are likely to be helices and were also proposed in the model of Slotboom et al. (77). Membrane segments 8 and 9 form a reentrant loop or loop-pore structure according to Grunewald et al. (32). Conserved motifs B and C and the conserved hydrophilic face of helix 10 (motif D) are shaded. Tyrosine 403, which is involved in potassium binding and is accessible from either side of the membrane depending on the presence of substrates, is located in the middle of helix 7.
A small part of the topology of human EAAT1 was also determined by accessibility scanning of single-cysteine mutants (74). The examined region includes the putative membrane-spanning segment 7 and its flanking sequences. It was suggested that rather than being a membrane-spanning helix, this region forms another reentrant loop with both ends positioned at the extracellular side of the membrane. This implies that an extra membrane-spanning segment must be present between the cytoplasmic end of helix 6 and the extracellular beginning of this putative reentrant loop. At present it is not possible to explain the discrepancies between the models for the membrane topology of rat EAAT2 and human EAAT1, since both studies are based on a similar experimental approach, the analysis of active single-cysteine versions of the transporters. Again we tentatively favor the model proposed by Grunewald et al. (32). Only their model is consistent with the trypsin digestion studies, discussed above, that demonstrated the intracellular location of the region between putative helices 6 and 7. Clearly, the topology of the C-terminal part of the transporters has to be further examined, not only because of the conflicts between the different models but also since loop-pore structures which are found in several ion channels (19, 55, 56) have not been found in secondary transporters before. A model for the membrane topology of the C-terminal half of the transporters is shown in Fig. 5. The positions of the membrane-spanning segments are indicated in Fig. 1 and 4a.
Periodicity Properties
The members of the glutamate transporter family were examined for patterns of residue hydrophobicity and residue conservation (or substitution) in their amino acid sequences (21, 77). Figure 4B shows the average periodicity profile of amino acid hydrophobicity of the family (amphipathy profile) with a period of 3.6 residues, appropriate for α-helical structures. The glutamate transporter family contains five regions with conserved hydrophobic moments that could form amphipathic α-helices (AH1 to AH5, Fig. 4B). AH1 to AH4 are found in loop regions connecting the putative transmembrane helices and may therefore form membrane surface helices with the axis parallel to the plane of the membrane and the hydrophilic residues exposed to the aqueous phase. AH2 and AH4 are found exclusively in the eukaryotic transporters. The hydrophobic moment of AH5 is conserved in all members of the family and has a particularly large value of 0.45 to 0.6/residue in the glutamate and C4-dicarboxylate transporters, which is larger than that of the peptide melittin, a considerably amphipatic peptide with a known α-helical structure (20). The hydrophobic moment is somewhat smaller in the mammalian neutral-amino-acid transporters and the bacterial serine transporters (0.3 to 0.4/residue). The putative amphipathic α-helix AH5 coincides with transmembrane segment 10 and thus provides a hydrophilic path through the membrane. It was suggested that this amphipathic membrane-spanning helix could provide part of the translocation pore (77). This suggestion recently gained experimental support in studies on chimeric transporters (see below).
Within transmembrane helices, the pattern of residue conservation (or substitution) can be used to discriminate between buried and lipid-exposed residues (18). The amphipathic membrane-spanning helix 10 has an exceptionally large substitution moment with α-helical periodicity (0.09/residue) (77). The hydrophobic face of the helix is less well conserved than the hydrophilic face, and it is likely that the membrane-spanning helix has a lipid-exposed hydrophobic face and a protein-buried, well-conserved hydrophilic face. The other putative membrane-spanning segments have considerably smaller substitution moments, but the moments of helices 1 and 3 (0.06 and 0.05/residue, respectively) may be significant.
Sequence Motifs
The glutamate transporter family does not contain residues that are conserved in all members. This is due to the inclusion of several distantly related sequences in the family that have emerged from recent genome-sequencing projects. For the same reason, most of the previously described “signature sequences” for the glutamate transporter family are not found in all the members listed in Table 2 (70). Only one sequence motif is conserved in all members of the family. This is a serine- and threonine-rich stretch in the hydrophilic region of the proteins following membrane-spanning helix 6 (motif A, Table 3 and Fig. 1 and 4A). It is located intracellularly in the topology model proposed by Grunewald et al. (32). Serine clusters were found in the ligand binding sites of the metabotropic glutamate receptors and in G-protein-coupled acetylcholine and biogenic amine receptors (62, 105). It was suggested that the stretch in the glutamate transporters may have a similar function (40), though there is as yet no conclusive experimental indication of such a function (59).
TABLE 3.
Sequence motifs in the glutamate transporter family
| Motif | Start positiona | Sequenceb | End positiona |
|---|---|---|---|
| A | 414 | (ST)(STARK)S(ST) | 417 |
| B | 443 | PxGx(TS)xN(ML)DGxx(LI)(FY) | 457 |
| C | 461 | Ax(IVL)F(LI)AQ | 467 |
| D (eukaryotic glutamate transporters) | 531 | DWxLDRxRTxxNVxGD | 546 |
| D (eukaryotic neutral-amino-acid transporters) | 531 | DWxVDRxxTxxNVEGD | 546 |
| D (bacterial glutamate transporters) | 531 | DRxxDMARTxxNxxG(NH) | 546 |
| D (bacterial C4-dicarboxylate transporters) | 531 | DRFMSExRxxxNxxGN | 546 |
| D (bacterial serine transporters) | 531 | GxLQDSxETALNSSTD | 546 |
Numbers refer to positions in the multiple-sequence alignment of Fig. 1.
When two or more residues are indicated in parentheses, either of the them is found; x refers to nonconserved residues.
A second motif that was suggested to be involved in substrate binding (67) is AX(I/V/L)F(L/I)AQ (motif C, Table 3 and Fig. 1), which is located in the putative membrane-spanning helix 7 (32, 77, 109). The motif is conserved in the glutamate, neutral-amino-acid, and C4-dicarboxylate carriers but not in most of the uncharacterized bacterial proteins. It may therefore be involved in binding of a carboxylate group of the substrate, which is the only functional group common to all substrates of the carriers that contain the motif. The motif is part of a stretch of 76 residues that is involved in the binding of dihydrokainate, a glutamate analogue that competitively inhibits glutamate transport (95) (see below). This observation is consistent with the hypothesis that the motif is involved in substrate binding.
The motif PXGX(T/S)XN(M/L)DGXX(L/I)(F/Y), located at the cytoplasmic interface of membrane helix 7, is present in all of the functionally characterized and most of the putative transporters (motif B, Table 3 and Fig. 1). This motif has been subjected to extensive mutagenesis studies and is involved in cation binding (see below). The stretch that forms the amphipathic membrane helix 10 (motif D) is conserved in most members of the family, but its exact amino acid composition varies along with the substrate specificity of the transporters (Table 3). The substrate-specific differences in this stretch are consistent with the hypothesis that the amphipathic membrane-spanning helix 10 may be part of the translocation pore (59, 77). Finally, the human glutamate transporter EAAT5 contains a C-terminal consensus motif for interaction with synaptic proteins that promote ion channel clustering (3). EAAT5 is one of the glutamate transporters that shows large substrate-dependent chloride currents (see above), and clustering with components of the signal transduction pathway may point to the function of the chloride currents.
FUNCTIONAL ANALYSIS
Catalytic Cycle
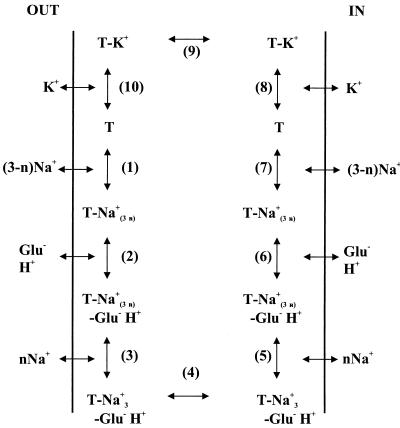
Extensive characterization of the eukaryotic glutamate carriers EAAT1 to EAAT3 has resulted in the model for the transport cycle that is shown in Fig. 6 (44). The transporters catalyze the uptake, efflux, and exchange of glutamate. In uptake and efflux, the complete cycle is followed in the forward and reverse directions, respectively, whereas during exchange, steps 1 to 7 (Fig. 6) are followed alternately in the forward and reverse directions. K+ is required only to reorient the empty carrier (steps 8 to 10). Exchange is not dependent on potassium ions, since reorientation of the empty carrier is omitted. The bacterial glutamate transporters also catalyze uptake, efflux and exchange, but none of the transport modes is believed to require potassium ions (87); the empty carrier reorients spontaneously.
FIG. 6.
Schematic representation of the transport cycle of the eukaryotic glutamate transporters (44). T, transporter; glu−, glutamate; n, the number of sodium ions that bind after glutamate binding. A description is given in the text.
The binding order of Na+, H+, and glutamate (steps 1 to 3) is a matter of debate. Kanner and coworkers (42, 68) showed that exchange catalyzed by rat EAAT2 does not require external Na+ at saturating glutamate concentrations whereas it does at nonsaturating glutamate concentrations. They concluded that all cotransported sodium ions bind to the transporter prior to glutamate binding. In contrast, Kanai et al. (39) found that binding of sodium ions is modulated by glutamate, and in their model one Na+ ion binds after the binding of glutamate (see also references 17 and 47). The observations indicate that the glutamate and Na+ binding sites intimately interact, which is in agreement with the results of recent mutagenesis studies (69, 114) (see below). The binding order may depend on the conditions used. The catalytic cycle of the neutral-amino-acid transporters may be similar to that of the exchange mode of the glutamate transporters. The human neutral-amino-acid transporter ASCT1 catalyzes electroneutral exchange of amino acids that is dependent on Na+ but not on K+, just like exchange catalyzed by the glutamate transporters (111). One difference is the Na+ stoichiometry, since only one sodium ion was found to interact with the neutral-amino-acid transporters (93, 111).
Substrate Binding Site
Several inhibitors of the glutamate transporters have been found, some of which have different specificities for different members of the family (4, 49, 96, 98). Noncompetitive inhibitors include oxidizing agents like ONOO−, which covalently interact with glutamate transporters and specifically inhibit the Vmax of transport (90, 91, 99, 100), and Zn2+, which is a partial inhibitor of the human and salamander glutamate transporters EAAT1 (80, 97). Arachidonic acid inhibits some glutamate transporter subtypes but stimulates others (92, 110).
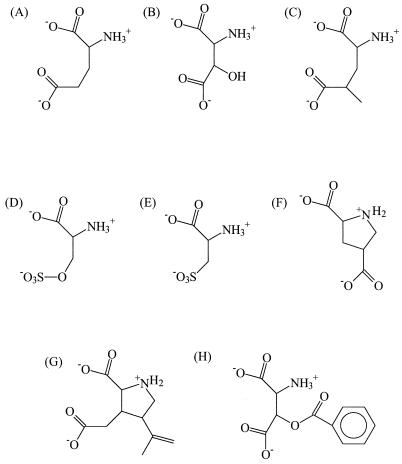
Most of the competitive inhibitors of the glutamate transporters were found to be substrates of the proteins that are transported with a high affinity (Km < 100 μM), e.g., d-aspartate, cysteate, and threo-β-hydroxyaspartate (Fig. 7) (38, 67, 81). Only a few competitive inhibitors that are not transported have been identified, such as threo-β-benzyloxyaspartate and kainate, a specific inhibitor of the EAAT2 subtypes (Fig. 7) (9, 49, 76, 96). The conformations of glutamate and aspartate in the substrate binding site have been modeled by comparing the structures of transported glutamate analogues and competitive blockers of the transporters (9, 10, 14, 49, 96). It was originally proposed that glutamate binds to the transporters in a folded form and aspartate binds in the extended form. In these conformations, the functional groups of the transported substrates (one amino group and two carboxylate groups) can be superimposed. However, more recent data from the analysis of conformationally constrained glutamate analogues showed that compounds that mimic the folded conformation as well as compounds that mimic a stretched conformation of glutamate bind to glutamate transporters but that only the “stretched” compounds can be transported (25).
FIG. 7.
Structures of some transported substrates (A, B, D, E, and F) and competitive inhibitors (G and H) of the glutamate transporters. 4-Methylglutamate (C) is a substrate of some transporters but a competitive inhibitor of others. (A) Glutamate; (B) β-hydroxyaspartate; (C) 4-methylglutamate; (D) serine-O-sulfate; (E) cysteate; (F) pyrrodiline-2,4-dicarboxylate; (G) kainate; (H) β-benzyloxyaspartate.
The nature and diastereomeric properties of the substituents at the β-carbon (C-3 position) of aspartate determine whether aspartate analogues are transported substrates or competitive blockers (49, 96). Thus, derivatives of aspartate with small groups at the β-carbon, such as β-hydroxyaspartate (Fig. 7B) and the related compounds β-acetoxyaspartate and β-propionyloxyaspartate, are transported by the bovine glutamate transporters EAAT1 and EAAT2 with high affinity (Km 10 to 100 μM). Derivatives with bulkier groups at the β-carbon, such as β-benzyloxyaspartate (Fig. 7H), are competitive blockers (Ki < 20 μM) (49, 76).
The transporters are less tolerant in accepting derivatives of glutamate as substrates. (2S,4S)-4-Methylglutamate (Fig. 7C) is not recognized by human EAAT1 and EAAT2, whereas its stereoisomer (2S,4R)-4-methylglutamate is a substrate for EAAT1 (Km = 54 μM) but a competitive blocker of EAAT2 (Ki = 3.4 μM). Both stereoisomers of 4-hydroxyglutamate are transported by the human glutamate transporters EAAT1 and EAAT2, but the affinity for erythro-4-hydroxyglutamate is very low (Km > 1 mM) (96). Substitutions at the C-3 position are even less well tolerated. None of the stereoisomers of 3-methylglutamate is recognized by EAAT1, while only the stereoisomer threo-3-methylglutamate is bound, but not transported, by EAAT2 (Ki = 2.3 μM).
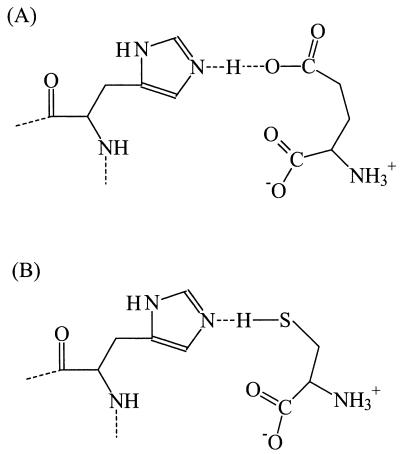
In the human glutamate transporter EAAT3, the stoichiometry of proton-substrate symport was found to depend on the charge of the transported substrate (112). Thus, transport of compounds which are anionic at physiological pH, like glutamate (pKa = 4.5) and cysteate (pKa = 1.5) (Fig. 7E), is associated with the symport of one proton. However, transport of cysteine, a low-affinity substrate for EAAT3 that is neutral at physiological pH (pKa = 8.3), is transported without a proton. Based on these observations, it was suggested that the anionic substrates are transported in the protonated state, i.e., as glutamic acid or cysteic acid (112). This mechanism of proton transport is feasible if the pKa of the anionic substrates is raised sufficiently in the substrate binding site of the transporter. This can be achieved by destabilizing the negative charge on the substrate in an apolar or negatively charged surrounding. However, this mechanism may be improbable since some substrates, like serine-O-sulfate (pKa < 0) (Fig. 7D), are so acidic that protonation is not likely to occur (98). Alternatively, the observed substrate-dependent proton stoichiometry could be explained if the formation of a hydrogen bridge between the substrate and a residue in the substrate binding site were essential (Fig. 8). Then, transport of anionic substrates requires a proton to bind in the substrate binding site to form the hydrogen bridge whereas uncharged substrates, like cysteine and glutamic acid, provide the proton for the hydrogen bridge themselves. This mechanism implies that the substrate binding site can exist in a protonated state and a unprotonated state, which can be accounted for by, for instance, the presence of a histidine residue, although other residues can also be envisaged (Fig. 8). The symported proton is shared by the substrate and the proton-accepting residue in the substrate binding site.
FIG. 8.
Model for hydrogen bridge formation between a residue in the substrate binding site of the glutamate transporters (histidine is used as an example) and the substrates glutamate (A) and cysteine (B). Hydrogen bridge formation can explain the observed substrate-dependent proton stoichiometry (112) (see the text).
Only at low pH are glutamate and cysteate also transported by the neutral-amino-acid transporters ASCT2 from mice, rabbits, and humans (46, 82, 93). The pH dependence of transport of the anionic compounds is caused by protonation of a residue on the transporter, possibly a histidine (82). Glutamate may therefore be transported by ASCT2 in a similar way to that by the glutamate transporters (93). However, unlike the glutamate transporters that may have a protonated substrate binding site at physiological pH, the neutral-amino-acid transporters are optimized for transport of neutral compounds and are normally not protonated. Nevertheless, since glutamate is a substrate for both the glutamate transporters and the neutral-amino-acid transporters, the substrate binding pockets of the transporters may have a similar spatial structure (93). A further indication for this suggestion is the observation that cysteine, a common substrate for the neutral-amino-acid transporters, is transported with low affinity by the human glutamate transporter EAAT3 (Km = 190 μM) (113).
Differences in inhibitor specificity as well as differences in Cl− permeability between the human glutamate transporters EAAT1 and EAAT2 have been used to identify domains that may be part of the substrate binding site and translocation pore. Chimeras were constructed, and it was shown that a stretch of 76 residues comprising the conserved motifs B and C and membrane-spanning segment 7 (Fig. 5) is involved in the binding of dihydrokainate, a glutamate analogue and nontransported competitive inhibitor of EAAT2 (95). The region comprising membrane-spanning segment 10, including motif D, is also involved in inhibitor recognition and accounts for differences in the chloride permeability as well (59). Hence, membrane-spanning segments 7 and 10 may form an important part of the substrate recognition site and translocation pore. Kanai et al. constructed chimeras between mouse EAAT2 and EAAT3 and showed that a region of 38 residues comprising conserved motif A (Fig. 5) is also involved in the recognition of dihydrokainate (41). However, dihydrokainate binds to an external site of the transporters (102), and in the topology model proposed by Grunewald et al. (32) this region is located intracellularly (Fig. 5). Therefore, it is unlikely that the region is directly involved in dihydrokainate binding, unless the topology model of the region is not correct (59).
Cation Binding Sites
Differences in the amino acid sequences of members of the glutamate transporter family have been used as a guide to find residues of the substrate binding site or translocation pore (12, 97, 115). A list of single and multiple amino acid substitutions that have been made in members of the glutamate transporters family is shown in Table 4. Mutations that completely abolish transport may be useful to delineate functionally or structurally important regions in the proteins. Thus, it was shown that two of the conserved hydrophilic residues in helical membrane-spanning segment 10 (Asp-470 in rat EAAT2 [69] and Arg-479 in rat EAAT1 [12]) are indispensable for transport activity. This is consistent with the suggestion that the hydrophilic face of segment 10 is part of the translocation pore (77). The exact function of residues that are indispensable for transport function is, however, difficult to assess.
TABLE 4.
Glutamate transporter mutantsa
| Mutated residue(s)b | Protein | Characteristic | Reference |
|---|---|---|---|
| H326(RKTN) | EAAT2 rno | Not functional | 115 |
| N396C | EAAT2 rno | Not functional | 109 |
| M397C | EAAT2 rno | Not functional | 109 |
| D398(CEGN) | EAAT2 rno | Not functional | 69 |
| G399C | EAAT2 rno | Not functional | 109 |
| T400(CSNA) | EAAT2 rno | Not functional | 114 |
| Y403(FWC) | EAAT2 rno | Cation binding affected | 114 |
| E404(CDGN) | EAAT2 rno | Cation binding affected | 44 |
| D470(EGN) | EAAT2 rno | Not functional | 69 |
| H146 | EAAT1 hs | Zn2+ binding | 97 |
| H156 | EAAT1 hs | Zn2+ binding | 97 |
| G394C | EAAT1 hs | Not functional | 74 |
| A395C | EAAT1 hs | Glutamate binding | 74 |
| N398C | EAAT1 hs | Not functional | 74 |
| M399C | EAAT1 hs | Not functional | 74 |
| D400C | EAAT1 hs | Not functional | 74 |
| G401C | EAAT1 hs | Targeting failure | 74 |
| T402C | EAAT1 hs | Not functional | 74 |
| F412C | EAAT1 hs | Targeting failure | 74 |
| I413C | EAAT1 hs | Targeting failure | 74 |
| Q415C | EAAT1 hs | Targeting failure | 74 |
| Y127F | EAAT1 rno | Not functional | 11 |
| N206T | EAAT1 rno | Glycosylation mutant | 13 |
| N216T | EAAT1 rno | Glycosylation mutant | 13 |
| L325H | EAAT1 rno | Targeting failure | 11 |
| E389F L390Vc | EAAT1 rno | Not functional | 103 |
| Y405F | EAAT1 rno | Like Y403C of EAAT2 rno | 109 |
| R479T | EAAT1 rno | Not functional | 12 |
Only mutants that are significantly different from the wild-type proteins are listed.
If a residue was replaced with multiple other amino acids, they are all indicated in parentheses.
Only the activity of the double mutant was tested.
The most informative mutants are those that alter a specific property of the transporters. Extensive mutagenesis studies of conserved motif B at the cytoplasmic end of membrane-spanning helix 7 (Fig. 5) in rat EAAT2 have shown that two residues (Y403 and E404) are involved in the binding of potassium ions (44, 114). Transporters in which these residues are conservatively mutated are unable to complete the transport cycle shown in Fig. 6 because they cannot reorient the empty carrier, which is due to a defect in K+ binding and/or translocation. These mutant transporters are, however, able to catalyze exchange that is K+ independent. Cysteine modification and protection studies on the Y403C mutant showed that this residue is alternately accessible from either side of the membrane depending on the presence of substrates or transport blockers (109). This is in agreement with the alternating accessibility of the K+ binding site from both sides of the membrane in the transport cycle (Fig. 6). Interestingly, E404 in rat EAAT2 is conserved in all eukaryotic glutamate transporters but not in the bacterial carriers and the neutral-amino-acid transporters (Fig. 1). This correlates with the observations that the neutral-amino-acid transporters are K+-independent obligate exchangers and that the bacterial transporters are glutamate cation symporters. Y403 of rat EAAT2 is not conserved in the neutral-amino-acid transporters either, but it is conserved in the bacterial carriers. Mutations of Y403 and E404 in rat EAAT2 also affect the Na+ and glutamate selectivity of the transporters, respectively, indicating that the three binding sites intimately interact (69, 114). The E404D mutant strongly prefers aspartate over glutamate, whereas the Y403W and Y403F mutants have an increased affinity for sodium ions but have lost the strict dependency on Na+ and also accept lithium and cesium ions. The proximate residues 396 to 400 in rat EAAT2 that are indispensable for transport were suggested to be part of the Na+ binding site(s) (109). These residues are also conserved in the bacterial glutamate transporters that do not use sodium ions. Furthermore, D304 of B. stearothermophilus GltT (the counterpart of D398 of rat EAAT2) is indispensable for function (78). Therefore these residues may be, more generally, involved in cation binding.
PROSPECTS
As long as a high-resolution structure of (one of) the members of the glutamate transporter family is not available, structural features must be obtained from biochemical and biophysical experiments. Mutagenesis studies and studies on chimeric transporters have so far proved to be very informative in providing information on the function of individual residues and domains, respectively. Mutagenesis is likely to remain useful as long as mutants with interesting properties can be found. One way to find interesting residues is to look for second-site revertants of mutant transporters that are inactive. For this, the bacterial members of the family can be used.
Cysteine-scanning mutagenesis is likely to become a major source of structural information. This technique has been extensively used in the study of the lactose transporter LacY from E. coli (30, 36), and the first results from this technique are now available for members of the glutamate transporter family. To fully benefit from the possibilities offered by cysteine-scanning mutagenesis, it will be necessary to use purified and reconstituted transporters. Such a pure system allows the use of a number of spectroscopic techniques to study both structural and dynamic properties of the transporters. The rat EAAT2 and B. stearothermophilus GltT glutamate transporters have been purified to homogeneity and reconstituted into proteoliposomes (15, 31). The bacterial system is suitable for the expression and large-scale purification of both wild-type and mutant transporters.
ACKNOWLEDGMENTS
This work was supported by the Ministry of Economic Affairs, the Ministry of Education, Culture and Science, and the Ministry of Agriculture, Nature Management and Fishery through the Netherland Association of Biotechnology Centres in The Netherlands (ABON) (to D.J.S.).
REFERENCES
- 1.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amato A, Barbour B, Szatkowski M, Attwell D. Counter-transport of potassium by the glutamate uptake carrier in glial cells isolated from the tiger salamander retina. J Physiol (London) 1994;479:371–380. doi: 10.1113/jphysiol.1994.sp020302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arriza J L, Eliasof S, Kavanaugh M P, Amara S G. Excitatory amino acid transporter 5, a retinal glutamate transporter coupled to a chloride conductance. Proc Natl Acad Sci USA. 1997;94:4155–4160. doi: 10.1073/pnas.94.8.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arriza J L, Fairman W A, Wadiche J I, Murdoch G H, Kavanaugh M P, Amara S G. Functional comparisons of three glutamate transporter subtypes cloned from human motor cortex. J Neurosci. 1994;14:5559–5569. doi: 10.1523/JNEUROSCI.14-09-05559.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arriza J L, Kavanaugh M P, Fairman W A, Wu Y N, Murdoch G H, North R A, Amara S G. Cloning and expression of a human neutral amino acid transporter with structural similarity to the glutamate transporter gene family. J Biol Chem. 1993;268:15329–15332. [PubMed] [Google Scholar]
- 6.Baker K E, Ditullio K P, Neuhard J, Kelln R A. Utilization of orotate as a pyrimidine source by Salmonella typhimurium and Escherichia coli requires the dicarboxylate transport protein encoded by dctA. J Bacteriol. 1996;178:7099–7105. doi: 10.1128/jb.178.24.7099-7105.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barbour B, Brew H, Attwell D. Electrogenic glutamate uptake in glial cells is activated by intracellular potassium. Nature. 1988;335:433–435. doi: 10.1038/335433a0. [DOI] [PubMed] [Google Scholar]
- 8.Billups B, Rossi D, Attwell D. Anion conductance behavior of the glutamate uptake carrier in salamander retinal glial cells. J Neurosci. 1996;16:6722–6731. doi: 10.1523/JNEUROSCI.16-21-06722.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8a.BLAST Website. 30 November 1998, revision date. [Online.] http://www.ncbi.nlm.nih.gov/BLAST. [30 November 1998, last date accessed.]
- 9.Bridges R J, Lovering F E, Koch H, Cotman C W, Chamberlin A R. A conformationally constrained competitive inhibitor of the sodium-dependent glutamate transporter in forebrain synaptosomes: l-anti-endo-3,4-methanopyrrolidine dicarboxylate. Neurosci Lett. 1994;174:193–197. doi: 10.1016/0304-3940(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 10.Bridges R J, Stanley M S, Anderson M W, Cotman C W, Chamberlin A R. Conformationally defined neurotransmitter analogues. Selective inhibition of glutamate uptake by one pyrrolidine-2,4-dicarboxylate diastereomer. J Med Chem. 1991;34:717–725. doi: 10.1021/jm00106a037. [DOI] [PubMed] [Google Scholar]
- 11.Choi I, Chiu S Y. Abolition of substrate-dependent currents by tyrosine mutation in the transmembrane domain of glutamate transporter. FEBS Lett. 1997;405:133–136. doi: 10.1016/s0014-5793(97)00155-5. [DOI] [PubMed] [Google Scholar]
- 12.Conradt M, Stoffel W. Functional analysis of the high affinity, Na(+)-dependent glutamate transporter GLAST-1 by site-directed mutagenesis. J Biol Chem. 1995;270:25207–25212. doi: 10.1074/jbc.270.42.25207. [DOI] [PubMed] [Google Scholar]
- 13.Conradt M, Storck T, Stoffel W. Localization of N-glycosylation sites and functional role of the carbohydrate units of GLAST-1, a cloned rat brain l-glutamate/l-aspartate transporter. Eur J Biochem. 1995;229:682–687. doi: 10.1111/j.1432-1033.1995.tb20514.x. [DOI] [PubMed] [Google Scholar]
- 14.Cooper B, Chebib M, Shen J, King N J, Darvey I G, Kuchel P W, Rothstein J D, Balcar V J. Structural selectivity and molecular nature of l-glutamate transport in cultured human fibroblasts. Arch Biochem Biophys. 1998;353:356–364. doi: 10.1006/abbi.1998.0626. [DOI] [PubMed] [Google Scholar]
- 15.Danbolt N C, Pines G, Kanner B I. Purification and reconstitution of the sodium- and potassium-coupled glutamate transport glycoprotein from rat brain. Biochemistry. 1990;29:6734–6740. doi: 10.1021/bi00480a025. [DOI] [PubMed] [Google Scholar]
- 16.Deckert G, Warren P V, Gaasterland T, Young W G, Lenox A L, Graham D E, Overbeek R, Snead M A, Keller M, Aujay M, Huber R, Feldman R A, Short J M, Olsen G J, Swanson R V. The complete genome of the hyperthermophilic bacterium Aquifex aeolicus. Nature. 1998;392:353–358. doi: 10.1038/32831. [DOI] [PubMed] [Google Scholar]
- 17.Donly B C, Richman A, Hawkins E, McLean H, Caveney S. Molecular cloning and functional expression of an insect high-affinity Na+-dependent glutamate transporter. Eur J Biochem. 1997;248:535–542. doi: 10.1111/j.1432-1033.1997.t01-1-00535.x. [DOI] [PubMed] [Google Scholar]
- 18.Donnelly D, Overington J P, Ruffle S V, Nugent J H, Blundell T L. Modeling alpha-helical transmembrane domains: the calculation and use of substitution tables for lipid-facing residues. Protein Sci. 1993;2:55–70. doi: 10.1002/pro.5560020106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doyle D A, Cabral J M, Pfuetzner R A, Kuo A, Gulbis J M, Cohen S L, Chait B T, MacKinnon R. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- 20.Eisenberg D. Three-dimensional structure of membrane and surface proteins. Annu Rev Biochem. 1984;53:595–623. doi: 10.1146/annurev.bi.53.070184.003115. [DOI] [PubMed] [Google Scholar]
- 21.Eisenberg D, Weiss R M, Terwilliger T C. The hydrophobic moment detects periodicity in protein hydrophobicity. Proc Natl Acad Sci USA. 1984;81:140–144. doi: 10.1073/pnas.81.1.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eliasof S, Arriza J L, Leighton B H, Kavanaugh M P, Amara S G. Excitatory amino acid transporters of the salamander retina: identification, localization, and function. J Neurosci. 1998;18:698–712. doi: 10.1523/JNEUROSCI.18-02-00698.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Engelke T, Jording D, Kapp D, Puhler A. Identification and sequence analysis of the Rhizobium meliloti dctA gene encoding the C4-dicarboxylate carrier. J Bacteriol. 1989;171:5551–5560. doi: 10.1128/jb.171.10.5551-5560.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Erecinska M, Wantorsky D, Wilson D F. Aspartate transport in synaptosomes from rat brain. J Biol Chem. 1983;258:9069–9077. [PubMed] [Google Scholar]
- 25.Esslinger C S, Koch H P, Kavanaugh M P, Philips D P, Chamberlin A R, Thompson C M, Bridges R J. Structural determinants of substrates and inhibitors: probing glutamate transporters with 2,4-methanopyrrolidine-2,4-dicarboxylate. Bioorg Med Chem Lett. 1998;8:3101–3106. doi: 10.1016/s0960-894x(98)00560-5. [DOI] [PubMed] [Google Scholar]
- 26.Fairman W A, Vandenberg R J, Arriza J L, Kavanaugh M P, Amara S G. An excitatory amino-acid transporter with properties of a ligand-gated chloride channel. Nature. 1995;375:599–603. doi: 10.1038/375599a0. [DOI] [PubMed] [Google Scholar]
- 27.Felsenstein J. PHYLIP (Phylogeny Inference Package) version 3.5c. Distributed by the author. Seattle: Department of Genetics, University of Washington; 1993. [Google Scholar]
- 28.Finan T M, Oresnik I, Bottacin A. Mutants of Rhizobium meliloti defective in succinate metabolism. J Bacteriol. 1988;170:3396–3403. doi: 10.1128/jb.170.8.3396-3403.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Finan T M, Wood J M, Jordan D C. Succinate transport in Rhizobium leguminosarum. J Bacteriol. 1981;148:193–202. doi: 10.1128/jb.148.1.193-202.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frillingos S, Sahin-Toth M, Wu J, Kaback H R. Cys-scanning mutagenesis: a novel approach to structure function relationships in polytopic membrane proteins. FASEB J. 1998;12:1281–1299. doi: 10.1096/fasebj.12.13.1281. [DOI] [PubMed] [Google Scholar]
- 31.Gaillard I, Slotboom D J, Knol J, Lolkema J S, Konings W N. Purification and reconstitution of the glutamate carrier GltT of the thermophilic bacterium Bacillus stearothermophilus. Biochemistry. 1996;35:6150–6156. doi: 10.1021/bi953005v. [DOI] [PubMed] [Google Scholar]
- 32.Grunewald M, Bendahan A, Kanner B I. Biotinylation of single cysteine mutants of the glutamate transporter GLT-1 from rat brain reveals its unusual topology. Neuron. 1998;21:623–632. doi: 10.1016/s0896-6273(00)80572-3. [DOI] [PubMed] [Google Scholar]
- 33.Grunewald M, Kanner B. Conformational changes monitored on the glutamate transporter GLT-1 indicate the existence of two neurotransmitter-bound states. J Biol Chem. 1995;270:17017–17024. doi: 10.1074/jbc.270.28.17017. [DOI] [PubMed] [Google Scholar]
- 34.Heyne R I, de Vrij W, Crielaard W, Konings W N. Sodium ion-dependent amino acid transport in membrane vesicles of Bacillus stearothermophilus. J Bacteriol. 1991;173:791–800. doi: 10.1128/jb.173.2.791-800.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jording D, Puhler A. The membrane topology of the Rhizobium meliloti C4-dicarboxylate permease (DctA) as derived from protein fusions with Escherichia coli K12 alkaline phosphatase (PhoA) and beta-galactosidase (LacZ) Mol Gen Genet. 1993;241:106–114. doi: 10.1007/BF00280207. [DOI] [PubMed] [Google Scholar]
- 36.Kaback H R, Wu J. From membrane to molecule to the third amino acid from the left with a membrane transport protein. Q Rev Biophys. 1997;30:333–364. doi: 10.1017/s0033583597003387. [DOI] [PubMed] [Google Scholar]
- 37.Kanai Y. Family of neutral and acidic amino acid transporters: molecular biology, physiology and medical implications. Curr Opin Cell Biol. 1997;9:565–572. doi: 10.1016/s0955-0674(97)80035-x. [DOI] [PubMed] [Google Scholar]
- 38.Kanai Y, Hediger M A. Primary structure and functional characterization of a high-affinity glutamate transporter. Nature. 1992;360:467–471. doi: 10.1038/360467a0. [DOI] [PubMed] [Google Scholar]
- 39.Kanai Y, Nussberger S, Romero M F, Boron W F, Hebert S C, Hediger M A. Electrogenic properties of the epithelial and neuronal high affinity glutamate transporter. J Biol Chem. 1995;270:16561–16568. doi: 10.1074/jbc.270.28.16561. [DOI] [PubMed] [Google Scholar]
- 40.Kanai Y, Smith C P, Hediger M A. A new family of neurotransmitter transporters: the high-affinity glutamate transporters. FASEB J. 1993;7:1450–1459. doi: 10.1096/fasebj.7.15.7903261. [DOI] [PubMed] [Google Scholar]
- 41.Kanai Y, Utsunomiya-Tate N, Endou H. Chimera analysis of glutamate transporter EAAC1 and GLT-1: a search for substrate binding sites. Soc Neurosci Abstr. 1998;23:1485. [Google Scholar]
- 42.Kanner B I, Bendahan A. Binding order of substrates to the sodium and potassium ion coupled l-glutamic acid transporter from rat brain. Biochemistry. 1982;21:6327–6330. doi: 10.1021/bi00267a044. [DOI] [PubMed] [Google Scholar]
- 43.Kanner B I, Sharon I. Active transport of l-glutamate by membrane vesicles isolated from rat brain. Biochemistry. 1978;17:3949–3953. doi: 10.1021/bi00612a011. [DOI] [PubMed] [Google Scholar]
- 44.Kavanaugh M P, Bendahan A, Zerangue N, Zhang Y, Kanner B I. Mutation of an amino acid residue influencing potassium coupling in the glutamate transporter GLT-1 induces obligate exchange. J Biol Chem. 1997;272:1703–1708. doi: 10.1074/jbc.272.3.1703. [DOI] [PubMed] [Google Scholar]
- 45.Kekuda R, Prasad P D, Fei Y J, Torres-Zamorano V, Sinha S, Yang-Feng T L, Leibach F H, Ganapathy V. Cloning of the sodium-dependent, broad-scope, neutral amino acid transporter Bo from a human placental choriocarcinoma cell line. J Biol Chem. 1996;271:18657–18661. doi: 10.1074/jbc.271.31.18657. [DOI] [PubMed] [Google Scholar]
- 46.Kekuda R, Torres-Zamorano V, Fei Y J, Prasad P D, Li H W, Mader L D, Leibach F H, Ganapathy V. Molecular and functional characterization of intestinal Na(+)-dependent neutral amino acid transporter B0. Am J Physiol. 1997;272:G1463–G1472. doi: 10.1152/ajpgi.1997.272.6.G1463. [DOI] [PubMed] [Google Scholar]
- 47.Klockner U, Storck T, Conradt M, Stoffel W. Functional properties and substrate specificity of the cloned l-glutamate/l-aspartate transporter GLAST-1 from rat brain expressed in Xenopus oocytes. J Neurosci. 1994;14:5759–5765. doi: 10.1523/JNEUROSCI.14-10-05759.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Konings W N, Kaback H R, Lolkema J S. Transport processes in eukaryotic and prokaryotic organisms. Amsterdam, The Netherlands: Elsevier Science B.V.; 1996. [Google Scholar]
- 49.Lebrun B, Sakaitani M, Shimamoto K, Yasuda-Kamatani Y, Nakajima T. New beta-hydroxyaspartate derivatives are competitive blockers for the bovine glutamate/aspartate transporter. J Biol Chem. 1997;272:20336–20339. doi: 10.1074/jbc.272.33.20336. [DOI] [PubMed] [Google Scholar]
- 50.Levy L M, Warr O, Attwell D. Stoichiometry of the glial glutamate transporter GLT-1 expressed inducibly in a chinese hamster ovary cell line selected for low endogenous Na+-dependent glutamate uptake. J Neurosci. 1998;18:9620–9628. doi: 10.1523/JNEUROSCI.18-23-09620.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liao K, Lane M D. Expression of a novel insulin-activated amino acid transporter gene during differentiation of 3T3-L1 preadipocytes into adipocytes. Biochem Biophys Res Commun. 1995;208:1008–1015. doi: 10.1006/bbrc.1995.1434. [DOI] [PubMed] [Google Scholar]
- 52.Lin C L, Bristol L A, Jin L, Dykes-Hoberg M, Crawford T, Clawson L, Rothstein J D. Aberrant RNA processing in a neurodegenerative disease: the cause for absent EAAT2, a glutamate transporter, in amyotrophic lateral sclerosis. Neuron. 1998;20:589–602. doi: 10.1016/s0896-6273(00)80997-6. [DOI] [PubMed] [Google Scholar]
- 53.Lolkema J S, Slotboom D J. Estimation of structural similarity of membrane proteins by hydropathy profile alignment. Mol Membr Biol. 1998;15:33–42. doi: 10.3109/09687689809027516. [DOI] [PubMed] [Google Scholar]
- 54.Lolkema J S, Slotboom D J. Hydropathy profile alignment. A tool to search for structural homologues of membrane proteins. FEMS Microbiol Rev. 1998;22:305–322. doi: 10.1111/j.1574-6976.1998.tb00372.x. [DOI] [PubMed] [Google Scholar]
- 55.MacKinnon R. Pore loops: an emerging theme in ion channel structure. Neuron. 1995;14:889–892. doi: 10.1016/0896-6273(95)90327-5. [DOI] [PubMed] [Google Scholar]
- 56.MacKinnon R, Cohen S L, Kuo A, Lee A, Chait B T. Structural conservation in prokaryotic and eukaryotic potassium channels. Science. 1998;280:106–109. doi: 10.1126/science.280.5360.106. [DOI] [PubMed] [Google Scholar]
- 57.Meldrum B, Garthwaite J. Excitatory amino acid neurotoxicity and neurodegenerative disease. Trends Pharmacol Sci. 1990;11:379–387. doi: 10.1016/0165-6147(90)90184-a. [DOI] [PubMed] [Google Scholar]
- 58.Mennerick S, Zorumski C F. Glial contributions to excitatory neurotransmission in cultured hippocampal cells. Nature. 1994;368:59–62. doi: 10.1038/368059a0. [DOI] [PubMed] [Google Scholar]
- 59.Mitrovic A D, Amara S G, Johnston G A, Vandenberg R J. Identification of functional domains of the human glutamate transporters EAAT1 and EAAT2. J Biol Chem. 1998;273:14698–14706. doi: 10.1074/jbc.273.24.14698. [DOI] [PubMed] [Google Scholar]
- 60.Nicholls D, Attwell D. The release and uptake of excitatory amino acids. Trends Pharmacol Sci. 1990;11:462–468. doi: 10.1016/0165-6147(90)90129-v. [DOI] [PubMed] [Google Scholar]
- 61.Ogawa W, Kim Y M, Mizushima T, Tsuchiya T. Cloning and expression of the Na+-coupled serine transporter from Escherichia coli and characteristics of the transporter. J Bacteriol. 1998;180:6749–6752. doi: 10.1128/jb.180.24.6749-6752.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.O’Hara P J, Sheppard P O, Thogersen H, Venezia D, Haldeman B A, McGrane V, Houamed K M, Thomsen C, Gilbert T L, Mulvihill E R. The ligand-binding domain in metabotropic glutamate receptors is related to bacterial periplasmic binding proteins. Neuron. 1993;11:41–52. doi: 10.1016/0896-6273(93)90269-w. [DOI] [PubMed] [Google Scholar]
- 63.Otis T S, Kavanaugh M P, Jahr C E. Postsynaptic glutamate transport at the climbing fiber-Purkinje cell synapse. Science. 1997;277:1515–1518. doi: 10.1126/science.277.5331.1515. [DOI] [PubMed] [Google Scholar]
- 64.Pao S S, Paulsen I T, Saier M H., Jr Major facilitator superfamily. Microbiol Mol Biol Rev. 1998;62:1–34. doi: 10.1128/mmbr.62.1.1-34.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Paulsen I T, Sliwinski M K, Saier M H., Jr Microbial genome analyses: global comparisons of transport capabilities based on phylogenies, bioenergetics and substrate specificities. J Mol Biol. 1998;277:573–592. doi: 10.1006/jmbi.1998.1609. [DOI] [PubMed] [Google Scholar]
- 66.Picaud S, Larsson H P, Wellis D P, Lecar H, Werblin F. Cone photoreceptors respond to their own glutamate release in the tiger salamander. Proc Natl Acad Sci USA. 1995;92:9417–9421. doi: 10.1073/pnas.92.20.9417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pines G, Danbolt N C, Bjoras M, Zhang Y, Bendahan A, Eide L, Koepsell H, Storm-Mathisen J, Seeberg E, Kanner B I. Cloning and expression of a rat brain l-glutamate transporter. Nature. 1992;360:464–467. doi: 10.1038/360464a0. [DOI] [PubMed] [Google Scholar]
- 68.Pines G, Kanner B I. Counterflow of l-glutamate in plasma membrane vesicles and reconstituted preparations from rat brain. Biochemistry. 1990;29:11209–11214. doi: 10.1021/bi00503a008. [DOI] [PubMed] [Google Scholar]
- 69.Pines G, Zhang Y, Kanner B I. Glutamate 404 is involved in the substrate discrimination of GLT-1, a (Na+ + K+)-coupled glutamate transporter from rat brain. J Biol Chem. 1995;270:17093–17097. doi: 10.1074/jbc.270.29.17093. [DOI] [PubMed] [Google Scholar]
- 70.Reizer J, Reizer A, Saier M H., Jr A functional superfamily of sodium/solute symporters. Biochim Biophys Acta. 1994;1197:133–166. doi: 10.1016/0304-4157(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 71.Rothstein J D, Dykes-Hoberg M, Pardo C A, Bristol L A, Jin L, Kuncl R W, Kanai Y, Hediger M A, Wang Y, Schielke J P, Welty D F. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 72.Saier M H., Jr Computer-aided analyses of transport protein sequences: gleaning evidence concerning function, structure, biogenesis, and evolution. Microbiol Rev. 1994;58:71–93. doi: 10.1128/mr.58.1.71-93.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Saier, M. H., Jr. 1998. Transport protein classification. [Online.] http://www-biology.ucsd.edu/∼msaier/transport/. [30 November 1998, last date accessed.]
- 74.Seal R P, Amara S G. A reentrant loop domain in the glutamate carrier EAAT1 participates in substrate binding and translocation. Neuron. 1998;21:1487–1498. doi: 10.1016/s0896-6273(00)80666-2. [DOI] [PubMed] [Google Scholar]
- 75.Shafqat S, Tamarappoo B K, Kilberg M S, Puranam R S, McNamara J O, Guadano-Ferraz A, Fremeau R T., Jr Cloning and expression of a novel Na(+)-dependent neutral amino acid transporter structurally related to mammalian Na+/glutamate cotransporters. J Biol Chem. 1993;268:15351–15355. [PubMed] [Google Scholar]
- 76.Shimamoto K, Lebrun B, Yasuda-Kamatani Y, Sakaitani M, Shigeri Y, Yumoto N, Nakajima T. dl-threo-beta-Benzyloxyaspartate, a potent blocker of excitatory amino acid transporters. Mol Pharmacol. 1998;53:195–201. doi: 10.1124/mol.53.2.195. [DOI] [PubMed] [Google Scholar]
- 77.Slotboom D J, Lolkema J S, Konings W N. Membrane topology of the C-terminal half of the neuronal, glial, and bacterial glutamate transporter family. J Biol Chem. 1996;271:31317–31321. doi: 10.1074/jbc.271.49.31317. [DOI] [PubMed] [Google Scholar]
- 78.Slotboom, D. J., J. S. Lolkema, and W. N. Konings. 1998. Unpublished work.
- 79.Sonders M S, Amara S G. Channels in transporters. Curr Opin Neurobiol. 1996;6:294–302. doi: 10.1016/s0959-4388(96)80111-5. [DOI] [PubMed] [Google Scholar]
- 80.Spiridon M, Kamm D, Billups B, Mobbs P, Attwell D. Modulation by zinc of the glutamate transporters in glial cells and cones isolated from the tiger salamander retina. J Physiol (London) 1998;506:363–376. doi: 10.1111/j.1469-7793.1998.363bw.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Storck T, Schulte S, Hofmann K, Stoffel W. Structure, expression, and functional analysis of a Na(+)-dependent glutamate/aspartate transporter from rat brain. Proc Natl Acad Sci USA. 1992;89:10955–10959. doi: 10.1073/pnas.89.22.10955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tamarappoo B K, McDonald K K, Kilberg M S. Expressed human hippocampal ASCT1 amino acid transporter exhibits a pH-dependent change in substrate specificity. Biochim Biophys Acta. 1996;1279:131–136. doi: 10.1016/0005-2736(95)00259-6. [DOI] [PubMed] [Google Scholar]
- 83.Tanaka K, Watase K, Manabe T, Yamada K, Watanabe M, Takahashi K, Iwama H, Nishikawa T, Ichihara N, Kikuchi T, Okuyama S, Kawashima N, Hori S, Takimoto M, Wada K. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science. 1997;276:1699–1702. doi: 10.1126/science.276.5319.1699. [DOI] [PubMed] [Google Scholar]
- 84.Thompson J D, Gibson T J, Plewniak F, Jeanmougin F, Higgins D G. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tolner B, Poolman B, Konings W N. Characterization and functional expression in Escherichia coli of the sodium/proton/glutamate symport proteins of Bacillus stearothermophilus and Bacillus caldotenax. Mol Microbiol. 1992;6:2845–2856. doi: 10.1111/j.1365-2958.1992.tb01464.x. [DOI] [PubMed] [Google Scholar]
- 86.Tolner B, Poolman B, Wallace B, Konings W N. Revised nucleotide sequence of the gltP gene, which encodes the proton-glutamate-aspartate transport protein of Escherichia coli K-12. J Bacteriol. 1992;174:2391–2393. doi: 10.1128/jb.174.7.2391-2393.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tolner B, Ubbink-Kok T, Poolman B, Konings W N. Cation-selectivity of the L-glutamate transporters of Escherichia coli, Bacillus stearothermophilus and Bacillus caldotenax: dependence on the environment in which the proteins are expressed. Mol Microbiol. 1995;18:123–133. doi: 10.1111/j.1365-2958.1995.mmi_18010123.x. [DOI] [PubMed] [Google Scholar]
- 88.Tolner B, Ubbink-Kok T, Poolman B, Konings W N. Characterization of the proton/glutamate symport protein of Bacillus subtilis and its functional expression in Escherichia coli. J Bacteriol. 1995;177:2863–2869. doi: 10.1128/jb.177.10.2863-2869.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Traxler B, Lee C, Boyd D, Beckwith J. The dynamics of assembly of a cytoplasmic membrane protein in Escherichia coli. J Biol Chem. 1992;267:5339–5345. [PubMed] [Google Scholar]
- 90.Trotti D, Rizzini B L, Rossi D, Haugeto O, Racagni G, Danbolt N C, Volterra A. Neuronal and glial glutamate transporters possess an SH-based redox regulatory mechanism. Eur J Neurosci. 1997;9:1236–1243. doi: 10.1111/j.1460-9568.1997.tb01478.x. [DOI] [PubMed] [Google Scholar]
- 91.Trotti D, Rossi D, Gjesdal O, Levy L M, Racagni G, Danbolt N C, Volterra A. Peroxynitrite inhibits glutamate transporter subtypes. J Biol Chem. 1996;271:5976–5979. doi: 10.1074/jbc.271.11.5976. [DOI] [PubMed] [Google Scholar]
- 92.Trotti D, Volterra A, Lehre K P, Rossi D, Gjesdal O, Racagni G, Danbolt N C. Arachidonic acid inhibits a purified and reconstituted glutamate transporter directly from the water phase and not via the phospholipid membrane. J Biol Chem. 1995;270:9890–9895. doi: 10.1074/jbc.270.17.9890. [DOI] [PubMed] [Google Scholar]
- 93.Utsunomiya-Tate N, Endou H, Kanai Y. Cloning and functional characterization of a system ASC-like Na+-dependent neutral amino acid transporter. J Biol Chem. 1996;271:14883–14890. doi: 10.1074/jbc.271.25.14883. [DOI] [PubMed] [Google Scholar]
- 94.Vandenberg R J. Molecular pharmacology and physiology of glutamate transporters in the central nervous system. Clin Exp Pharmacol Physiol. 1998;25:393–400. doi: 10.1111/j.1440-1681.1998.tb02221.x. [DOI] [PubMed] [Google Scholar]
- 95.Vandenberg R J, Arriza J L, Amara S G, Kavanaugh M P. Constitutive ion fluxes and substrate binding domains of human glutamate transporters. J Biol Chem. 1995;270:17668–17671. doi: 10.1074/jbc.270.30.17668. [DOI] [PubMed] [Google Scholar]
- 96.Vandenberg R J, Mitrovic A D, Chebib M, Balcar V J, Johnston G A. Contrasting modes of action of methylglutamate derivatives on the excitatory amino acid transporters, EAAT1 and EAAT2. Mol Pharmacol. 1997;51:809–815. doi: 10.1124/mol.51.5.809. [DOI] [PubMed] [Google Scholar]
- 97.Vandenberg R J, Mitrovic A D, Johnston G A. Molecular basis for differential inhibition of glutamate transporter subtypes by zinc ions. Mol Pharmacol. 1998;54:189–196. doi: 10.1124/mol.54.1.189. [DOI] [PubMed] [Google Scholar]
- 98.Vandenberg R J, Mitrovic A D, Johnston G A. Serine-O-sulphate transport by the human glutamate transporter, EAAT2. Br J Pharmacol. 1998;123:1593–1600. doi: 10.1038/sj.bjp.0701776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Volterra A, Trotti D, Racagni G. Glutamate uptake is inhibited by arachidonic acid and oxygen radicals via two distinct and additive mechanisms. Mol Pharmacol. 1994;46:986–992. [PubMed] [Google Scholar]
- 100.Volterra A, Trotti D, Tromba C, Floridi S, Racagni G. Glutamate uptake inhibition by oxygen free radicals in rat cortical astrocytes. J Neurosci. 1994;14:2924–2932. doi: 10.1523/JNEUROSCI.14-05-02924.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wadiche J I, Amara S G, Kavanaugh M P. Ion fluxes associated with excitatory amino acid transport. Neuron. 1995;15:721–728. doi: 10.1016/0896-6273(95)90159-0. [DOI] [PubMed] [Google Scholar]
- 102.Wadiche J I, Arriza J L, Amara S G, Kavanaugh M P. Kinetics of a human glutamate transporter. Neuron. 1995;14:1019–1027. doi: 10.1016/0896-6273(95)90340-2. [DOI] [PubMed] [Google Scholar]
- 103.Wahle S, Stoffel W. Membrane topology of the high-affinity l-glutamate transporter (GLAST-1) of the central nervous system. J Cell Biol. 1996;135:1867–1877. doi: 10.1083/jcb.135.6.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wallace B, Yang Y J, Hong J S, Lum D. Cloning and sequencing of a gene encoding a glutamate and aspartate carrier of Escherichia coli K-12. J Bacteriol. 1990;172:3214–3220. doi: 10.1128/jb.172.6.3214-3220.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang C D, Buck M A, Fraser C M. Site-directed mutagenesis of alpha 2A-adrenergic receptors: identification of amino acids involved in ligand binding and receptor activation by agonists. Mol Pharmacol. 1991;40:168–179. [PubMed] [Google Scholar]
- 106.Watson R J, Chan Y K, Wheatcroft R, Yang A F, Han S H. Rhizobium meliloti genes required for C4-dicarboxylate transport and symbiotic nitrogen fixation are located on a megaplasmid. J Bacteriol. 1988;170:927–934. doi: 10.1128/jb.170.2.927-934.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Weisburg W G, Hatch T P, Woese C R. Eubacterial origin of chlamydiae. J Bacteriol. 1986;167:570–574. doi: 10.1128/jb.167.2.570-574.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Woese C R, Kandler O, Wheelis M L. Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc Natl Acad Sci USA. 1990;87:4576–4579. doi: 10.1073/pnas.87.12.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zarbiv R, Grunewald M, Kavanaugh M P, Kanner B I. Cysteine scanning of the surroundings of an alkali-ion binding site of the glutamate transporter GLT-1 reveals a conformationally sensitive residue. J Biol Chem. 1998;273:14231–14237. doi: 10.1074/jbc.273.23.14231. [DOI] [PubMed] [Google Scholar]
- 110.Zerangue N, Arriza J L, Amara S G, Kavanaugh M P. Differential modulation of human glutamate transporter subtypes by arachidonic acid. J Biol Chem. 1995;270:6433–6435. doi: 10.1074/jbc.270.12.6433. [DOI] [PubMed] [Google Scholar]
- 111.Zerangue N, Kavanaugh M P. ASCT-1 is a neutral amino acid exchanger with chloride channel activity. J Biol Chem. 1996;271:27991–27994. doi: 10.1074/jbc.271.45.27991. [DOI] [PubMed] [Google Scholar]
- 112.Zerangue N, Kavanaugh M P. Flux coupling in a neuronal glutamate transporter. Nature. 1996;383:634–637. doi: 10.1038/383634a0. [DOI] [PubMed] [Google Scholar]
- 113.Zerangue N, Kavanaugh M P. Interaction of l-cysteine with a human excitatory amino acid transporter. J Physiol (London) 1996;493:419–423. doi: 10.1113/jphysiol.1996.sp021393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang Y, Bendahan A, Zarbiv R, Kavanaugh M P, Kanner B I. Molecular determinant of ion selectivity of a (Na+ + K+)-coupled rat brain glutamate transporter. Proc Natl Acad Sci USA. 1998;95:751–755. doi: 10.1073/pnas.95.2.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang Y, Pines G, Kanner B I. Histidine 326 is critical for the function of GLT-1, a (Na+ + K+)-coupled glutamate transporter from rat brain. J Biol Chem. 1994;269:19573–19577. [PubMed] [Google Scholar]